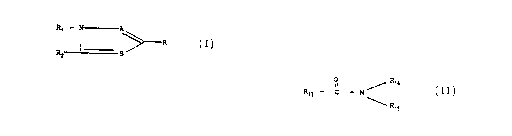Note: Descriptions are shown in the official language in which they were submitted.
WO 92/10098 ~ ~ ~ ~ PC1'/US91/09267
-1-
SAFENEI) HERBICIDAL SULFONAMIDE COMPOSITIONS
FIELD OF THE INVENTION
The field of the invention contemplatedi herein
pertains to the safening of herbicidal sulfonamide
compounds with antidotal or safener compounds. Parti-
cular herbicides involved are those generically charac-
terized as azolopyrimidine sulfonamides with or without
co-herbicidal compounds, e.g., a-haloacetamides, a-
haloacetanilides, thiocarbamates, and/or other classes
of herbicides.
BACKGROUND OF THE INVENTION
Mama herbicides injure crop plants at herbi-
cide application rates necessary to control weed growth.
Accordingly, many herbicides cannot be used for con-
trolling weed: in the presence of certain crops. Uncon-
trolled weed growth, however, results in lower crop
yield and reduced crop quality inasmuch as weeds compete
with crops fo~~ light, water and soil nutrients. Reduc-
tion of herbicidal injury to crops without an una.ccept-
able corresponding reduction of herbicidal action. on the
weeds can be ~iccomplished by use of crop protecta.nts
known as herbicide "antagonists", "antidotes" or
"safeners".
Weed control for crops, especially corn crops,
is one of the oldest and most highly developed areas in
weed science. For a herbicide product to be accepted
commercially :Eor corn crops, such herbicide product must
provide a rel~itively high level of control of both
grassy and broadleaf weeds in corn, in addition t.o
meeting sever~~l other criteria. For example, the herbi-
cide should possess relatively high unit activity so
that lower raises of herbicide application are feasible.
Lower application rates are desirable in order to
minimize expo~aure of the environment to the herbicide.
At the same tame, such herbicide must be selective in
herbicidal ef:Eect so as not to injure the crops.
Herbicidal se:Lectivity can be enhanced by use of
WO 92/10098 PCT/US91/09267
an appropriate antidote in combination with the herbi-
cide. But identification of an antidote which safens a
herbicide in crops is a highly complicated task.
Whether a compound or class of compounds provides
efficacious antidote or safening activity is not a
theoretical determination but must be done empirically.
Safening activity is determined empirically by observing
the complex interaction of several biological and chem-
ical factors, namely: the type of herbicide compound;
the type of weed to be controlled; the type of crop to
be protected from weed competition and herbicidal
injury; and the antidote compound itself. Moreover, the
herbicide and antidote must each possess chemical and
physical properties enabling preparation of a stable
formulation which is environmentally safe and easy to
apply to the field.
Among the various classes of compounds found
to be suitable for various herbicidal purposes are the
a-haloacetanilides and thiocarbamates. The former
herbicides, e.g., alachlor, acetochlor, metolachlor,
etc., are excellent preemergence or early post emergence
herbicides for controlling annual grasses and many
broadleaved weeds in corn, peanuts, soybeans and other
crops. The latter herbicides, exemplified by EPTC,
butylate, etc., are also used as selective preemergence
herbicides suitable for the control of many annual and
perennial weeds and some broadleaved species in a
variety of crops.
It is a common agronomic practice to use
various antidotal compounds to reduce the phytotoxicity
of some herbicides to various crops. For example,
fluorazole (active ingredient in SCREEN~ safener) is
used as a seed dressing to protect sorghum seed from
alachlor (active ingredient in LASSOm herbicide).
Similarly, cyometrinil (active ingredient in CONCEP~
safener) is a corn seed safener for use with metolachlor
and oxabetrinil (active ingredient in CONCEP II safener)
WO 92/10098 ~ ~ ~ ~ ~ PCC/US91/09267
-3-
is used to safen sorghum seed from injury by mete>1-
achlor. The compound N,N-diallyl dichloroacetam~.de
(common name ~3ichlormid is used to safen corn from
injury by the thiocarbamate 5-ethyl-N,N-dipropyl-
thiocarbamate (active ingredient in ERADICANE~
herbicide) and aceto- chlor (active ingredient in
HARNESS~ herbicide).
It :is an object of this invention to provide
compositions of azolopyrimidine sulfonamide herbicides
1o in combination with antidotes therefor, optionally con
taining one m- more co-herbicides, which compositions
are useful t~o reduce injury to crops, especially corn,
due to phytotoxicity of said herbicides.
Y OF THE INVENTION
The present invention relates to herbicidal
compositions comprising azolopyrimidine sulfonamides and
antidotal comF~ounds therefor to reduce injury to various
crops, particLilarly corn, from the phytotoxic effects of
said herbicide when used alone or in combination with
other compounds, particularly a-haloacetamides and a-
haloacetanilid,es, as co-herbicides. Except where noted
herein the term "a-haloacetamides" generically includes
a-haloacetanilides as a subgroup (which require a phenyl
or substituted phenyl attached to the acetamide nitrogen
atom) and acetamides which have substituents other than
a (un)substituted phenyl on the amide nitrogen.
In more particular, in a major aspect, this
invention relates to a composition comprising:
(a) a herbicidal compound according to Formula
I or an agriculturally-acceptable salt thereof:
R~ - ------A
I ~ R
RZ H
wherein
A an~3 B are independently N or CR3, provided
that at least ~cne of A or B is N;
R is -N (R4) SOZRS or -S02N (R6) R~;
WO 92/10098 PCT/US91/09267
4
R~ is hydrogen, alkyl, alkenyl, alkynyl, acyl,
acyloxy, cycloalkyl, cycloalkenyl, aryl,aralkyl, alkoxy,-
alkoxyalkyl, alkoxycarbonyl, aminocarbonyl, alkylsul-
finyl, alkylsulfonyl or heterocyclic group or where not
self inclusive any of these non-hydrogen radicals sub-
stituted with cyano, halogen, amino, mono- or di- C~_
alkylamino, C~_6 alkyl, haloalkyl, alkylthio, alkoxy,
alkoxyalkyl, alkoxycarbonyl, alkylthio, alkylsulphinyl
or alkylsulfonyl;
RZ is an R~ member, halogen, cyano, amino,
mono- or di- C~_4 alkyl amino, pyrrolyl or pyrrolyl
substituted with halogen, cyano, amino C~_4 alkyl or
alkoxy;
R~ and R2 may be combined to form a divalent
group which together with the N and C atoms to which
they are respectively attached form a heterocyclic ring
fused with the azolo ring, said heterocyclic ring
containing up to 10 ring members of which up to 4 are N,
S and/or O atoms and having saturated and/or unsaturated
bonds;
R3 is an RZ member or NOZ, S (O) ~C~_4 alkyl, where
n is an integer 0, 1, 2 or 3, C(O)RD, phenyl, phenoxy,
phenylthio, or these phenyl, phenoxy or phenylthio mem-
bers substituted with from 1 to 4 halogen, CN, CF3, NOZ
and/or C~_4 alkyl or alkoxy members; Rs is C~_6 alkyl,
haloalkyl, alkylthio, alkoxy, alkoxyalkyl, amino, mono-
or di-C~_4 alkylamino, phenyl or an R3 phenyl-substituted
member;
R4 and R6 are independently H or alkyl, acyl,
alkenyl, alkenyloxy, alkenyloxycarbonyl, alkynyl,
alkynyloxy, alkanoyl, alkoxy, haloalkoxy, haloalkylthio,
alkoxyalkyl, alkoxycarbonyl, or alkoxythiocarbonyl, each
having up to 10 carbon atoms; phenyl, benzyl, naphthyl-
phenylthio, phenoxy, phenoxythio, phenoxycarbonyl,
phenyl S (O) ~; phenyl S (O) ~C~_4 alkyl; phenyl S (O) ~Cm(K) ~I;
phenyl S(O)~CKm, where n is 0, 1, 2 or 3, m is 1-3 and K
is halogen; phenoxy- carbonyl, phenoxythio-
WO 92/10098 ~ ~ ~ PC1'/US91/09267
-5-
carbonyl, ami:nocarbonyl, or where not self-inclusive
said R4 and R6 members substituted with halogen, r:N, CF3,
NOz, OH and/or Ct_to alkyl, haloalkyl, alkoxy, alkoxy-
alkoxy, hydro:~cyalkoxy, alkylthioalkoxy, alkoxycarbonyl,
or polyalkoxynarbonyl, phenyl, halophenyl, benzyl,
benzyloxy, ph~anoxyalkoxy and agriculturally-acceptable
salts thereof when R4 and R6 are H and
RS and R~ are independently an aromatic
hydrocarbon ~on heterocyclic radical having up to l0 ring
members of wh:Lch up to four may be N, O and/or S in the
heterocyclic radical and said RS and R~ members sub-
stituted with one or more R4 members, 2-pyridyl,
pyridyloxy or 2-pyridylmethoxycarbonyl, dialkyl-
aminoalkoxycax~bonyl having up to 10 carbon atoms and the
radical C (O) Orf = C (Rq) 2, wherein R9 is H, phenyl, phenyl-
carbonyl, benz;yl, Ct_to alkyl, alkoxy, mono- or di-~Ct_6
alkylamino or -alkylaminocarbonyl, -S(O)~Rto, where n is
0, 1, 2 or 3 a.nd Rta is Ct_~ alkyl, haloalkyl, mono- or
di-Ct_~ alkylamino or alkylcarbonyl, said compound of
2o Formula I being used alone or in admixture with other
known herbicidal compounds as co-herbicides, preferably
an a-haloacetamide of the formula
O ~Rtt
IV C1CH2C - N
Rtt
wherein Rtt ands Rt2 are independently hydrogen; Ct_a alkyl,
alkoxy, alkoxyalkyl, acylaminomethyl, acyl-lower alkyl-
substituted aminomethyl; cycloalkyl, cycloalkylmethyl,
mono- or polyunsaturated alkenyl, alkynyl, cycloalkenyl,
cycloalkenylmethyl having up to 8 carbon atoms; phenyl;
or C'_to heteroc:yclyl or heterocyclylmethyl containing
from 1 to 4 ring hetero atoms selected independently
from N, S or O; and wherein said Rtt and Rt2 members may
be substituted with alkyl, alkenyl, alkynyl, alkenyloxy,
alkynyloxy, al:koxy, alkoxyalkyl, alkoxycarbomethyl. or
ethyl having u~p to 8 carbon atoms; nitro; halogen;
cyano; amino o:r Ct_4 alkyl-substituted amino; and wherein
R~i and Rt2 may be combined together with the N atom to
WO 92/ 10098 PCT/US91 /09267
which attached to form one of said heterocyclyl or
substituted-heterocyclyl members and
(b) an antidotally-effective amount of
(i) a compound of the formula
O /R~'
II R~3 - C - N/
Rt5
wherein R~3 can be selected from the group consisting of
haloalkyl; polyhaloalkyl; haloalkenyl; alkyl; alkenyl;
l0 cycloalkyl; cycloalkylalkyl; halogen; hydrogen; carbo-
alkoxy; N-alkenylcarbamylalkyl; N-alkenylcarbamyl; N-
alkyl-N-alkynylcarbamyl; N-alkyl-N-alkynylcarbamylalkyl;
N-alkenylcarbamylalkoxyalkyl; N-alkyl-N-alkynylcarba-
mylalkoxyalkyl; alkynoxy; haloalkoxy; thiocyanatoalkyl;
alkenylaminoalkyl; alkylcarboalkyl; cyanoalkyl; cyana-
toalkyl; alkenylaminosulfonalkyl; alkylthioalkyl; halo-
alkylcarbonyloxyalkyl, alkoxycarboalkyl; haloalkenyl-
carbonyloxyalkyl; hydroxyhaloalkyloxyalkyl; hydroxy-
alkylcarboalkyoxyalkyl; hydroxyalkyl; alkoxysulfono-
alkyl; furyl, thienyl; alkyldithiolenyl; thienalkyl;
phenyl and substituted phenyl wherein said substituents
can be selected from halogen, alkyl, haloalkyl, alkoxy,
carbamyl, vitro, carboxylic acids and their salts,
haloalkylcarbamyl; phenylalkyl; phenylhaloalkyl;
phenylalkenyl; substituted phenylalkenyl wherein
said substituents can be selected from halogen, alkyl,
alkoxy, halophenoxy, phenylalkoxy; phenylalkylcarboxy-
alkyl; phenylcycloalkyl; halophenylalkenoxy; halothio-
phenylalkyl; halophenoxyalkyl; bicycloalkyl; alkenyl-
carbamylpyridinyl; alkynylcarbamylpyridinyl; dialkenyl-
carbamylbicycloalkenyl; alkynylcarbamylbicycloalkenyl;
R~4 and R~5 can be the same or different and
can be selected from the group consisting of alkenyl;
haloalkenyl; hydrogen; alkyl; haloalkyl; alkynyl;
cyanoalkyl; hydroxyalkyl; hydroxyhaloalkyl; halo-
alkylcarboxyalkyl; alkylcarboxyalkyl; alkoxycarboxy-
alkyl; thioalkylcarboxyalkyl; alkoxycarboalkyl;
z~~zo
WO 92/10098 PCx'/US91/09267
alkylcarbamylcixyalkyl; amino; formyl; haloalkyl-N-
alkylamido; ha~loalkylamido; haloalkylamidoalkyl;
haloalkyl-N-al,kylamidoalkyl; haloalkylamidoalkenyl;
alkylimino; cycloalkyl; alkylcycloalkyl; alkoxyalkyl;
alkylsulfonylc~xyalkyl; mercaptoalkyl; alkylaminoa.:Lkyl;
alkoxycarboalk:enyl; haloalkylcarbonyl; alkylcarbonyl;
alkenylcarbamyloxyalkyl; cycloalkylcarbamyloxyalkyl;
alkoxycarbonyl; haloalkoxycarbonyl; halophenylcarbamyl-
oxyalkyl; cycloalkenyl; phenyl; substituted phenyl
wherein said substituents can be selected from alkyl,
halogen, haloalkyl, alkoxy, haloalkylamido, phthal-
amido, hydroxy, alkylcarbamyloxy, alkenylcarbamyloxy,
alkylamido, haloalkylamido or alkylcarboalkenyl;
phenylsulfonyl; substituted phenylalkyl wherein said
substituents can be selected from halogen or alkyl;
dioxyalkylene, halophenoxyalkylamidoalkyl; alkylthio-
diazolyl; piperidyl; piperidylalkyl; dioxolanylalkyl,
thiazolyl; alkylthiazolyl; benzothiazolyl; halobenzo-
thiazolyl; furyl; alkyl-substituted furyl; furyla~.kyl;
pyridyl; alkylpyridyl; alkyloxazolyl; tetrahydrofuryl-
alkyl; 3-cyano, thienyl; alkyl-substituted thienyl.; 4,5-
polyalkylene-t:hienyl; a-haloalkylacetamidophenylal.kyl;
a-haloalkylacetamidonitrophenylalkyl; a-haloalkylacet-
amidohalophenylalkyl; cyanoalkenyl;
2 5 R» and R~5 when taken together can form a
structure consisting of piperidinyl; alkylpiperidi.nyl;
pyridyl; di- o:c tetrahydropyridinyl; alkyltetrahydro-
pyridyl; morph~~lyl; alkylmorpholyl; azabicyclononyl;
diazacycloalkauyl; benzoalkylpyrrolidinyl; oxazoli.dinyl;
perhydrooxazolidinyl; alkyloxazolidyl; furyloxazol.i-
dinyl; thienyl~~xazolidinyl; pyridyloxazo.lidinyl;
pyrimidinyloxa;aolidinyl; benzooxazolidinyl; C3_~ spiro-
cycloalkyloxaz~~lidinyl; alkylaminoalkeny.l; alkylidene-
imino; pyrroli~iinyl; piperidonyl; perhydroazepinyl.;
perhydroazocim~rl; pyrazolyl; dihydropyrazolyl;
piperazinyl; p~arhydro-1,4-diazepinyl; quinolinyl;
isoquinolinyl; dihydro-, tetrahydro- and perhydro-
quinolyl- or -:isoquinolyl; indolyl and d:i- and
WO 92/ 10098 PCT/US91 /09267
-8-
perhydroindolyl and said combined R~~ and R~5 members
substituted with those independent R~4 and R~5 radicals
enumerated above; or
(ii) one of the following compounds
a-[(Cyanomethoxy)imino]benzeneaceto-
nitrile,
a-[(1,3-Dioxolan-2-yl-methoxy)-imino]-
benzeneacetonitrile,
O-[1,3-Dioxolan-2-ylmethyl]-2,2,2-tri-
l0 fluoromethyl-4'-chloroacetophenone oxime,
Benzenemethamine, N-[4-(dichloromethylene]-
1,3-diotholan-2-ylidene]-a-methyl,
hydrochloride,
Diphenylmethoxy acetic acid, methyl ester,
1,8-Naphthalic anhydride,
4,6-Dichloro-2-phenyl-pyrimidine,
2-Chloro-N-[1-(2,4,6-trimethylphenyl)-
ethenyl]acetamide,
Ethylene glycol acetal of 1,1-dichloro-
acetone,
1,3-Dioxolane, 2-(dichloromethyl)-2-
methyl-,
5-Thiazolecarboxylic Acid, 2-chloro-4-
(trifluoromethyl)-, (phenylmethyl)ester,
Phosphorothioic acid, O,O-diethyl O-(3-
methylphenyl)ester,
4-Pentenenitrile, 2-methyl-2-[(4-methyl-
phenyl)thio]-,
5-Chloro-8-(cyanomethoicy)quinoline,
1-Methylhexyl-2-(5-chloro-8-quinolinoxy)-
acetate or
O-(Methoxycarbonyl)-2-(8-quinolinoxy)-
acetamide oxime.
WO 92/10098
PCT/US91 /09267
-g-
P~~eferred herbicidal compounds according to
Formula I are those wherein A and B are both nitrogen; R
is -SOZN(R6) (R.r) ; R~ is phenyl, pyrimidinyl, triazinyl,
thiadiazolyl, pyrazinyl, pyridinyl, or any of said R~
radicals substituted with cyano, halogen, amino, mono-
or di-C~_~ alk~~lamino, C~_6 alkyl, haloalkyl, alkylthio,
alkoxy, alko~ralkyl, alkoxycarbonyl, alkylthio, alkyl-
sulfinyl or a~~kylsulfonyl; RZ is hydrogen, halogen,
cyano, amino, mono- or di-C~_4 alkylamino, C~_6 alk~,rl,
alkylsulfinyl, alkylsulfonyl, alkoxy, alkoxyalkyl,
alkoxycarbonyl., acyl, acyloxy or pyrrolyl optionally
substituted with C~_4 alkyl; R6 is hydrogen, C~_' al.kyl,
acyl, alkylsul.fonyl, alkoxy, alkoxycarbonyl, dialkyl-
carbamoyl or t>enzyl and R7 is furyl, thiophene or phenyl
or those rad:ic:als substituted independently with one or
more C~_' alkyl, haloalkyl, alkoxy, alkoxyalkyl, halo-
alkoxyalkyl, a~lkenyl, alkenyloxy, alkynyloxy, alkyl-
sulfinyl, alkylsulfonyl, alkoxycarbonyl, mono- or di-
alkylamino, amino, or vitro groups.
Ire the above embodiment of the invention,
preferred species include N-(2,6-dichloro-3-methyl-
phenyl)-1-(4-c:hloro-6-methoxypyrimidinyl-2-yl)-iH-
1,2,4-triazole:-3-sulphonamide; N-(2,6-difluorophenyl)-
1-(pyrimidin-2-yl)-5-methyl-1,2,4-triazole-3-sul-
phonamide; N-(2,6-dichloro-3-methylphenyl)-1(pyrimi-
din-2-yl)-5-meahyl-1,2,4-triazole-3-sulphonamide; N-(2-
methyl-6-nitrophenyl)-1-pyrimidin-2-yl)-5-methyl-1,2,4-
triazole-3-sulphonamide; N-(2,6-difluorophenyl)-1-(4,6-
dimethylpyrimi,din-2-yl)-5-methyl-1,2,4-triazole-3-
sulphonamide; N-(2,6-dichlorophenyl)-1-pyrimidin-2-yl)-
5-methyl-1,2,,4-triazole-3-sulphonamide; N-(2,6-
difluorophenyl.)-1-(4-methylpyrimidin-2-yl)-5-methyl-
1,2,4-triazole:-3-sulphonamide; N-(2,6-difluoro-
phenyl)-1-(4«a~ethoxy-6-methylpyrimidin-2-yl)-5-methyl-
1,2,4-triazole;-3-sulphonamide; N-(2,6-dichlorophenyl)-
1-(4,6-dimethaxy-1,3,5-triazin-2-yl)-5-methyl-1,2,4-
triazole-3-sulphonamide; N-(2,6-dichlorophenyl)-1-(4,6-
WO 92/10098 PCT/US91/09267
-10-
dimethyl-pyrimidin-2-yl)-1,2,4-triazole-3-sulphonamide;
N-(2,6-dichlorophenyl)-1-(4,6-dimethylpyrimidin-2-yl)-
5-methyl-1,2,4-triazole-3-sulphonamide; N-(2-methyl-6-
nitrophenyl)-1-(4,6-dimethylpyrimidin-2-yl)-5-methyl-
1,2,4-triazole-3-sulphonamide; N-(2,6-dichlorophenyl)-
5-(2,5-dimethylpyrrol-1-yl)-1,2,4-triazole-3-sulphon-
amide; N-(2,6-difluorophenyl)-5-(2,5-dimethylpyrrol-1-
yl)-1,2,4-triazole-3-sulphonamide; N-(2,6-dichloro-3-
methyl-phenyl)-1-(4,6-dimethylpyrimidin-2-yl)-1,2,4-
triazole-3-sulphonamide; N-(2,6-dichlorophenyl)-5-amino-
1-(4,6-dimethylpyrimidin-2-yl)-1,2,4-triazole-3-
sulphonamide; or N-(2,6-dichloro-3-methylphenyl)-5-
amino-1-(4,6-dimethoxy-1,3,5-triazin-2-yl)-1,2,4-
triazole-3-sulphonamide.
In other embodiments of the invention, more
preferred herbicidal compounds according to Formula I
are those wherein A and B are both nitrogen (N), R is -
SOZN (R6) (R~) and R~ and R2 are combined to form one of the
following divalent radicals:
Z Y X
(a) - N = C - C = C -
Z Y X
(b) _ ~ _ ~ _ ~ _
Z Y X
(C) -NH-C=C-CH-
Z Y X
(d) - N = C - CH - CH -
Z Y X
(e) - C = C - N = C -
WO 92/ 10098 PCT/US91 /092b7
11
Y X D
(f) - N = C - N - C -
Y X
(g) - S - C = C -
wherein R6 and R~ are as defined above: and X,
Y and Z are independently an R4 member, SO2, or adjacent
X and Y or Y and Z members may be combined to form a
saturated, partially unsaturated or unsaturated homo-
cyclic ring o:r heterocyclic ring containing up to l0
ring members ~cf which up to 4 may be oxygen, sulfur
and/or N and :D is oxygen or sulfur.
Preferred compounds containing the above
divalent structures are those wherein R~ is hydrogen,
alkyl, alkenyl, alkoxy, alkoxycarbonyl, alkoxythio-
carbonyl, alk~~lsulfinyl or alkylsulfony.l having up to 6
carbon atoms; amino, mono- or di-C~_4 alkylamino or
-alkylaminoca:rbonyl; phenyl, benzyl, benzoyl or an R6
member when not self-inclusive substituted with one or
more halogen, vitro, C~_' alkyl, haloalkyl or alkoxy
radicals; RT i.s unsubstituted phenyl or pyrazolyl or
optionally substituted independently with one or more
phenyl, halog~sn, vitro, trifluoromethyl, C~_6 alkyl,
alkoxy, alkox~alkyl, alkoxycarbonyl, -S(O)S alkyl,
-s (O) ~c,(R) Vii, -s (O) ~CK,~, amino, carbamyl, mono- o:r di-
C~_~ alkylamino or -alkyl carbamyl; X, Y and Z are
independently hydrogen, halogen, C~_~ alkyl, haloalkyl,
alkoxy, alkyl~thio or alkylsulfonyl, preferably sub-
stituted in one meta position and one or both ortho
positions; m ~3nd n are integers from o-:3 inclusive and K
is halogen.
Among preferred species of compounds wherein
R~ and RZ are combined to form the bivalent radical (a)
above, its te,trahydro analogs of bivalent radical. (b) or
WO 92/10098 PCT/US91/09267
~, a ~ ~ ~ ~ o -12-
their agriculturally-acceptable salts are the following
compounds:
5,7-di-methyl-N-(2,6-dichlorophenyl)-1,2,4-
triazolo[1,5-a]-pyrimidine-2-sulfonamide;
5-methyl-N-(2,6-dichlorophenyl)-1,2,4-triazolo[1,5-
a]-pyrimidine-2-sulfonamide;
5-methyl-N-(2-bromo-6-chlorophenyl)-1,2,4-
triazolo[1,5-a]-pyrimidine-2-sulfonamide;
5-methyl-N-(2,6-difluoro-3-methylphenyl)-1,2,4-
triazolo-[1,5-a]-pyrimidine-2-sulfonamide;
5-methyl-N-(2,6-difluorophenyl)-1,2,4-triazolo[1,5-
a]-pyrimidine-2-sulfonamide;
5,7-dimethoxy-N-(2,6-dichloro-3-methylphenyl)-
1,2,4-triazolo-[1,5-a]-pyrimidine-2-
sulfonamide;
5,7-dimethoxy-N-(2-methoxy-6-trifluoromethyl-
phenyl)-1,2,4-triazolo-[1,5-a]-pyrimidine-2-
sulfonamide;
5-methyl-7-methylthio-N-(2,6-dichlorophenyl)-1,2,4-
triazolo-[1,5-a]-pyrimidine-2-sulfonamide;
5-methyl-7-methylthio-N-(2-trifluoromethylphenyl)-
1,2,4-triazolo-[1,5-a]-pyrimidine-2-sulfonamide;
0
7-ethoxy-5-methyl-N-(2,6-dichloro-3-methylphenyl)-
1,2,4-triazolo-[1,5-a]pyrimidine-2-sulfonamide;
5,7-dimethyl-N-(2-chloro-6-phenylphenyl)-1,2,4-
triazolo[1,5-a]pyrimidine-2-sulfonamide;
5-methyl-N-(2-methyl-6-nitrophenyl)-1,2,4-tria
zolo[1,5-a]-pyrimidine-2-sulfonamide;
5-methyl-N-(2-chloro-6-methylphenyl)-1,2,4-tria-
zolo[1,5-a]-pyrimidine-2-sulfonamide;
Methyl-3-methyl-N-(5,7-dimethyl-1,2,4-triazolo-
[1,5-a]-pyrimidine-2-sulfonyl)anthranilate;
Methyl-3-methyl-N-(5-methyl-7-ethoxy-1,2,4-
triazolo-[1,5-a]-pyrimidine-2-sulfonyl)-
anthranilate;
Isopropyl-3-methyl-N-(5-methyl-1,2,4-triazolo-[1,5-
a]-pyrimidine-2-sulfonyl)anthranilate;
WO 92/10098 ~ ~ ~ ~ PCl"/US91/09267
-13-
6-Methyl-~N-(2-bromo-6-methylphenyl)-1,2,4-triazolo-
[1,5-2~]-pyrimidine-2-sulfonamide;
6-Methyl-~N- ( 2-f luoro-6-chlorophenyl ) -1, 2 , 4-
triazc~lo-[1,5-a]-pyrimidine-2-sulfonamide;
6-Methyl-~N-(2-chloro-6-methylphenyl)-1,2,4-tria-
zolo[1,5-a]-pyrimidine-2-sulfonamide;
6-Methyl-N-(2-methyl-6-nitrophenyl)-1,2,4-tr:iazolo-
[1,5-a]-pyrimidine-2-sulfonamide;
7-Ethoxy-5-methyl-N-(2-trifluoromethylphenyl;l-
l0 1,2,4-triazolo-[1,5-a]-pyrimidine-2-sulfonamide;
7-Methoxy-5-methyl-N-(2,6-dichloro-3-methylphenyl)
1,2,4-triazolo-[1,5-a]-pyrimidine-2-sulfonamide;
7-Ethoxy-5-methyl-N-(2-bromo-6-chloro-3-methyl
phenyl)-1,2,4-triazolo-[1,5-a]-pyrimidine-2-
sulfonamide;
5,7-Dimethoxy-N-(2,6-dibromo-3-methylphenyl)-~1,2,4-
triazolo-[1,5-a]-pyrimidine-2-sulfonamide;
5,7-Dimet:hoxy-N-(2,6-dichlorophenyl)-1,2,4-
triazolo-[1,5-a]-pyrimidine-2-sulfonamide;
7-Methyl-;K-(2,6-dichlorophenyl)-1,2,4-triazol.o-
[1,5-a]-pyrimidine-2-sulfonamide;
N-(2,6-Di~~hlorophenyl)-1,2,4-triazolo-[1,5-a]-
pyrimidine-2-sulfonamide;
7-Ethoxy-!5-methyl-N-(2,6-dibromo-3-methylphenyl)-
1,2,4-~triazolo-[1,5-a]-pyrimidine-2-sulfonamide;
6-Chloro-1J-(2,6-difluorophenyl)-1,2,4-triazol.o-
[1,5-a:)-pyrimidine-2-sulfonamide;
5-Methyl-'~-trifluoromethyl-N-(2-methoxy-6-tri-
fluoromethylphenyl)-1,2,4-triazolo-[1,5-a]-
pyrimidine-2-sulfonamide;
Methyl-3-~:luoro-N-(6-chloro-1,2,4-triazolo-[1,5-
a]-pyr:Lmidine-2-sulfonyl)anthranilate;
5,7-Dimethyl-N-(1,3-dimethyl-5-trifluoromethyl-4-
pyrazo~lyl)-1,2,4-triazolo-[1,5-a]-pyrimidine-2-
sulfonamide;
5-Methyl-rt-(1,3-dimethyl-5-trifluoromethyl-4-
pyrazo7.y1)-1,2,4-triazolo-[1,5-a]-pyrimidine-2-
sulfoneimide;
WO 92/10098 ° PCT/US91/09267
-14-
5,7-Dimethyl-N-(1-methyl-4-ethoxycarbonyl-5-
pyrazolyl)-1,2,4-triazolo-[1,5-a]-pyrimidine-2-
sulfonamide;
5,7-Dimethoxy-N-(2-chloro-1-napthyl)-1,2,4-
triazolo-[1,5-a]pyrimidine-2-sulfonamide;
5-Methyl-7-methoxy-N-(2-chloro-1-naphthyl)-1,2,4
triazolo-[1,5-a]-pyrimidine-2-sulfonamide;
5-Methyl-7-ethoxy-N-(2-chloro-1-naphthyl)-1,2,4
triazolo-[1,5-a]-pyrimidine-2-sulfonamide;
5-Methyl-N-(2-methylpropanoyl)-N-(2,6-difluoro-
phenyl)-1,2,4-triazolo-[1,5-a]-pyrimidine-2-
sulfonamide;
5-Methyl-N-acetyl-N-(2,6-dichlorophenyl)-1,2,4
triazolo-[1,5-a]-pyrimidine-2-sulfonamide;
5,7-Dimethyl-2-(N-[2-chloro, 6-propargyloxyphenyl]-
sulphamoyl)-1,2,4-triazolo[1,5-a]-pyrimidine;
5,7-Dimethyl-2-(N-[2-chloro-6-(2-ethoxyethoxy)-
phenyl]-sulphamoyl)-1,2,4-triazolo[1,5-a]-
pyrimidine;
5,7-Dimethyl-2-(N-[2-benzyloxy-6-chlorophenyl]-
sulphamoyl)-1,2,4-triazolo[1,5-a]-pyrimidine;
5,7-Dimethyl-2-(N-[2-allyloxy-6-fluorophenyl]-
sulphamoyl)-1,2,4-triazolo[1,5-a]-pyrimidine;
5,7-Dimethyl-2-(N-[2-chloro-6-(2-methoxymethoxy)-
phenyl]-sulphamoyl)-1,2,4-triazolo[1,5-a]-
pyrimidine;
5,7-Dimethyl-2-(N-[2-chloro-6-(2-hydroxyethoxy)
phenyl]-sulphamoyl)-1,2,4-triazolo[1,5-a]
pyrimidine;
5,7-Dimethyl-2-(N-[2-2-ethoxyethoxy)-6-fluoro-
phenyl]-sulphamoyl)-1,2,4-triazolo[1,5-a]-
pyrimidine;
5,7-Dimethyl-2-(N-[2-fluoro-6-(2-methylthio-
ethoxy)-phenyl]-sulphamoyl)-1,2,4-triazolo-
[1,5-a]-pyrimidine;
5,7-Dimethyl-2-(N-[2-chloro-6-(2-phenoxyethoxy)
phenyl]sulphamoyl)-1,2,4-triazolo[1,5-a]
pyrimid~.ne;
WO 92/10098 -.
PCf/US91 /092fi7
-15-
5,7-Dimethyl-2-(N-[2-chloro-6-(2-methoxyethaxy)
phenyl]-sulphamoyl-1,2,4-triazolo-[1,5-a~
pyrimidine;
5,7-Dimethyl-2-(N-[2-chloro-6-(2-n-propoxyethoxy)-
phenyl]-sulphamoyl)-1,2,4-triazolo[1,5-a]-
pyrimidine;
5,7-Dime~thyl-2-(N-[2-chloro-6-(3-methoxy-n-
propo:~cy) phenyl ] -sulphamoyl ) -1, 2 ,, 4-triazolo-
[1,5-a]-pyrimidine;
5,7-Dimet~hyl-2-(N-[2-chloro-6-(2-isopropoxy)-
ethox~tphenyl]-sulphamoyl)-1,2,4-triazolo-
[ 1, 5-~s ] -pyrimidine;
5,7-Dimel:hyl-(N-[2-fluoro-6-(2-n-propoxyethoxy)
pheny:l]-sulphamoyl)-1,2,4-triazolo-[1,5-a]
pyrim:Ldine;
5,7-Dimet:hyl-2-(N-[2-(2-ethoxyethoxy)phenyl]-
sulphi~moyl)-1,2,4-triazolo-[1,5-a]-pyrimidine;
5,7-Dimet:hyl-2-(N-[2,6-di(2-ethoxyethoxy)phenyl]
sulpha~moyl)-1,2,4-triazolo-[1,5-a]-pyrimi~dine;
5,7-Dimet:hyl-2-(N-[2-2-ethoxyethoxy)-6-metho:xy
phenyl.]-sulphamoyl)-1,2,4-triazolo[1,5-a]-
pyrimi.dine;
5,7-Dimet:hyl)-2-(N-[2-chloro-6-tetrahydrofurfur-
2-yl-c~xyphenyl]-sulphamoyl)-1,2,4-triazolo-[1,5-
2 5 a ] -pyx~imidine;
5,7-Dimet:hyl-2-(N-[2-(2-emthoxyethylamino)-phenyl]
sulpha,moyl)-1,2,4-triazolo[1,5-a]-pyrimidine;
5,7-Dimet.hyl-2-(N-[2-(2-methoxyethylthio)phenyl]-
sulpha.moyl)-1,2,4-triazolo-[1,5-a]-pyrimidine;
5,7-Dimet.hyl-2-(N-acetyl-N-[2-chloro-6-(2-ethoxy-
ethoxy~)phenyl]-sulphamoyl)-1,2,4-triazolo~-[1,5-
a]-pyrimidine;
5,7-Dimethyl-2-(N-acetyl-N-[2-chloro-6-(2-methoxy-
ethoxy)phenyl]-sulphamoyl)-1,2,4-triazolo~-[1,5-
a]-pyrimidine;
5,7-Dimethyl-2-(N-methyl-N-[2-chloro-6-(2-ethoxy-
ethoxy)phenyl]-sulphamoyl)-1,2,4-triazolo-[1,5-
a]-pyrimidine;
WO 92/ 10098 PCT/US91 /09267
-16-
5,7-Dimethyl-2-(N-[2-(2-ethoxyethoxy)-6-nitro-
phenyl)sulphamoyl)-1,2,4-triazolo-[1,5-a]-
pyrimidine; and
5-Methoxymethyl-N-(2-chloro-6-methylphenyl)-1,2,4-
triazolo-[2,5-a]-pyrimidine-2-sulfonamide.
Other preferred herbicidal compounds for use
herein wherein in Formula I R~ and RZ are combined to
form divalent radical (a) above, i.e.,
Z Y X
(a) - N = C - C = C -
and R is -S02N (R6) (RT) , are those wherein A is CR3, B is N
and R6, R~, X, Y and Z have the above-defined meanings
and
R3 is H, halogen, N02, CN, amino, phenyl,
phenylthio, phenoxy, C~_4 alkyl, mono- or di-C~_4 alkyl-
amino or alkoxy; -S (O) o_3-C~_4 alkyl; C (O) C~_~ alkyl,
-alkoxy, -alkylthio, mono- or dialkylamino or -phenyl;
or a substitutable R3 member substituted where not self-
inclusive with halogen, N02, CN, CF3 and/or C~_3 alkyl,
preferably methyl.
Preferred compounds according to the
foregoing embodiment are those wherein:
X and Z are independently H, CN, halogen,
amino, C~_~ alkyl, haloalkyl, alkylthio, alkoxy or mono-
or dialkylamino;
Y is H, CN, halogen, C~_~ alkyl, haloalkyl or
alkoxy;
R3 is halogen, N02, CN, C~_4 alkyl, haloalkyl,
C(O)alkyl or C(O)alkoxy;
R6 is H, benzyl, C (O) C~_4alkyl or -haloalkyl
and agriculturally-acceptable salts thereof when R6 is H
and
WO 92/ 10098 ~ ~ ~ ~ ~ ~ PCIf'/US91 /09267
-17-
is phenyl substituted in at least sine
ortho position with halogen, CN, NOZ, C~,.4 alkyl,
haloalkyl or ;~(O)~_3alkyl or haloalkyl; amino, mono- or
di-C~_~alkylami.no, optionally substituted phenyl,
phenylthio, phenoxy or benzyl, wherein said substituents
are from 1 to 4 of halogen, NOZ, CF3, CN or C~_3 alkyl,
preferably methyl; and at least one of the meta
positions of t:he RT phenyl group is substituted with a
_3 alkyl, preferably methyl.
Re:presentative species of the preceding
compounds include the following:
N-(2,6-di.fluorophenyl)-4,6-dimethylimidazolo[1,2-
a] -pyz~imidine-2-sulfonamide;
N-(2,6-di.fluorophenyl)-3-chloro-4,6-dimethylimida-
zolo ([ 1., 2-a ] -pyrimidine-2-sulfonamide;
N-(2,6-di.fluorophenyl)-3-bromo-4,6-dimethylimida-
zolo[1.,2-a]-pyrimidine-2-sulfonamide;
N-(2,6-di.fluorophenyl)-3-methylthio-4,6-dimethyl-
imidazolo[1,2-a]-pyrimidine-2-sulfonamide;
N-(2,6-di.chlorophenyl)-4,6-dimethylimidazolo[1,2-
a]-pyrimidine-2-sulfonamide;
N-(2,6-difluorophenyl)-3-cyano-4,6-dimethylimida-
zolo[1,2-a]-pyrimidine-2-sulfonamide;
N-(2,6-difluorophenyl)-N-benzyl-3-chloro-4,6-
dimeth.ylimidazolo[1,2-a]-pyrimidine-2-
sulfonamide;
N-(2-trifluoromethylphenyl)-4,6-dimethylimida-
zolo[1,2-a]-pyrimidine-2-sulfonamide;
N-(2-trif luoromethylphenyl)-3-chloro-4,6-dimethyl-
3o imidazolo[1,2-a]-pyrimidine-2-sulfonamide;
N-(2-carbomethoxy-6-methylphenyl)-3-chloro-4,6-
dimethylimidazolo[1,2-a]-pyrimidine-2-
sulfanamide;
N-(2,6-dichlorophenyl)-3-chloro-4,6-dimethyl~-
imidazolo[1,2-a]-pyrimidine-2-sulfonamide;
N-(2-chloro-6-methylphenyl-3-chloro-4,6-dimethyl
imidazolo[1,2-a]-pyrimidine-2-sulfonamide;
WO 92/ 10098 PCT/ US91 /09267
18
N-(2,6-difluorophenyl)-4-chloro-6-methylimida
zolo[1,2-a]-pyrimidine-2-sulfonamide;
N-(2,6-difluorophenyl)-4-methoxy-6-methylimida-
zolo[1,2-a]-pyrimidine-2-sulfonamide;
N-(2,6-difluorophenyl)-4,6-dichloroimidazolo-
[1,2-a]-pyrimidine-2-sulfonamide;
N-(2,6-difluorophenyl)-4,6-bismethoxyimidazolo-
[1,2-a]-pyrimidine-2-sulfonamide monohydrate;
Preferred and representative herbicidal
compounds according to Formula I wherein R~ and RZ are
combined to form the bivalent radicals (c) and (d) above
include tautomeric forms of the following compounds:
5,7-Dimethyl-N-(2-chloro-6-methylphenyl)-1,2,4-
triazolo-[1,5-a]-[4H,7H]-dihydropyrimidine-2-
sulphonamide,
7-Methyl-N-(2-chloro-6-methylphenyl)-1,2,4-tria-
zolo-[1,5-a][4H,7H]-dihydropyrimidine-2-
sulphonamide;
5,7-Dimethyl-N-(2-chloro-6-ethoxyphenyl)-1,2,4-
triazolo-[1,5-a][4H,7H]-dihydropyrimidine-2-
sulphonamide or
5,7-Dimethyl-N-(2-chloro-6-isopropoxyphenyl)-1,2,4-
triazolo-[1,5-a][4H,7H]-dihydropyrimidine-2-
sulphonamide.
Another group of preferred herbicidal
compounds of Formula I are those wherein R~ and RZ
combine to form the divalent radical (e) above, i.e.,
Z Y X
(e) - C = C - N = C -
wherein
X is H, CF3, C~_' alkyl, alkylthio or alkoxy;
Y and Z are independently H, CF3, CF3,
halogen or C~_4 alkoxy; provided that at least one of X,
Y or Z is Ci_4 alkoxy;
WO 92/ 10098 PCT/US91 /09257
-19-
R~, is H or C(O) Ci_4 alkyl or -haloalkyl and
agriculturall!r-acceptable salts thereof when R6 is H and
is phenyl. substituted in at least one
ortho position with halogen, CN, N02, C~"4 alkyl, rAalo-
alkyl or S(O)~_3 alkyl or haloalkyl; amino, mono- or di-
C~_4 alkylaminc~, optionally-substituted phenyl, phenyl-
thio, phenoxy or benzyl, wherein said substituents are
from 1 to 4 01: halogen, NOZ, CF3, CN or C~_3 alkyl,
preferably methyl; and at least one of the meta posi-
tions of the Ft~ phenyl group is substituted with a C~_
alkyl, prefereibly methyl.
One preferred compound according to those
defined in thE: preceding paragraph is 5-fluoro-7-
methoxy-N-(2,E>-difluorophenyl)-1,2,4-triazolo[1,5-c]-
pyrimidine-2~-:sulfonamide.
;5t:i11 another group of herbicidal sulfon-
amide compounds useful in combination with antidotal
compounds according to this invention are those
identified as (6,7)-dihydro-[1,2,4]-triazolo[1,5-.a]-
[1,3,5]-triazine-2-sulfonamides. Such compounds are
those accord.ir~g to Formula I wherein A and B are both N,
R is S02N (Rd) (1t~) and R~ and R2 are combined to form
divalent radical (f) above, i.a.,
Y X D
(f) - N = C ~- N - C -
wherein D is oxygen or sulfur;
X and Y are independently H, alkyl, alkenyl
or alkynyl haring up to 6 carbon atoms, phenyl, phenyl-
alkyl, pheny:la~lkenyl, phenylalkynyl or where not self-
inclusive an ?; or Y member other than H substituted with
one or more haclogen, C~_~ acyl, alkoxy, alkoxycarbonyl,
alkoxycarbony7.-C~_3 alkylene, carbamoyl, mono- or c3i-C~_6
carbamoyl or :~ (O) o_3C~_6 alkyl;
R6 is an X member or alkali metal atom or a
single metal s:quivalent of an alkaline earth, other
metal or ammonium anion, optionally substituted with
alkyl and
WO 92/10098 PCT/US91/09267
-20-
RT is preferably phenyl, naphthyl, pyridyl or
thienyl, optionally substituted with halogen, CN, NOZ,
S (O) o_3C~_6 alkyl, -CZ_6 alkenyl or alkynyl, amino,
carbamoyl, mono- or di-C~_' alkylamino or -alkylcarba-
moyl, C~_6 alkyl, acyl, alkoxy, alkoxyalkyl, alkoxy-
carbanoyl-C~_3 alkyl; phenyl or phenoxy optionally
substituted with one or more C~.4 alkyl, alkoxy, alkyl-
thio, halogen, N02 or amino, which substituents where not
self-inclusive and substitutable, substituted with
alkyl, alkenyl or alkynyl having up to 6 carbons, which
may optionally be substituted with one or more halogen,
OH, CN, N02 or C~_4 alkoxy or alkoxycarbonyl.
Exemplary preferred species according to the
structure defined in the preceding paragraph include
those wherein R~ is phenyl substituted in the ortho
positions independently with halogen, CF3, NOz, C~_3
alkyl, alkoxy or alkoxycarbonyl and substituted in the
meta and para positions with halogen, CF3 or C~_~ alkyl;
R6 is H, C~_i acyl or a single equivalent of a
metal ion and
X and Y are independently H, phenyl, alkyl,
alkenyl or alkynyl having up to 6 carbon atoms.
Preferred species according to the preceding
description include the following:
N-(2,6-Dichlorophenyl)-6,7-dihydro-N,5,6-trimethyl-
7-oxo[1,2,4]triazolo[1,5-a][1,3,5]-triazine-2-
sulphonamide;
N-(2,6-Dichlorophenyl)-6,7-dihydro-5,6-dimethyl-7-
oxo[1,2,4]triazole[1,5-a][1,3,5]-triazine-2-
sulphonamide;
N-(2,6-dichlorophenyl)-6,7-dihydro-5,6-dimethyl-7-
thioxo-[1,2,4]triazolo[1,5-a]-[1,3,5]-triazine-
2-sulphonamide;
N-(2,6-Dichlorophenyl)-6,7-dihydro-5,6-dimethyl-7
thioxo-[1,2,4]triazolo[1,5-a][1,3,5]triazine-2
sulphonamide;
WO 92/ 10098 ~ ~ ~ ~ PCT/ US91 /09267
-21-
N-(2,6-I)ichloro-3-methylphenyl)-6,7-dihydro-5,6-
dimet:hyl-7-thioxo-[1,2,4]triazolo[1,5-a][1,3,5]-
tria::ine-2-sulphonamide;
6,7-Dihydro-5,6-dimethyl-N-(2-methyl-6-nitro-
phenyl)-7-thioxo-[1,2,4]triazolo-[1,5-a]-
[1,:3,5]-triazine-2-sulphonamide;
N-(2-Chl.oro-6-fluorophenyl)-6,7-dihydro-5,6~-
dimet:hyl-7-thiaxo-[1,2,4]triazolo[1,5-a][1,3,5]-
triaz;ine-2-sulphonamide;
N-(2,6-Difluorophenyl)-6,7-dihydro-5,6-dimethyl-7-
thia~s:o-[1,2,4]triazolo[1,5-a][1,3,5]-triazine-
2-sulphonamide;
N-(2,6-L~ibromophenyl)-6,7-dihydro-5,6-dimethyl-7-
thiox:o-[1,2,4]triazolo[1,5-a]-[1,3,5]-tr.:iazine-
2-sulphonamide;
6,7-Dihydro-5,6-dimethyl-7-thioxo-N-(2-trif:luoro-
methylphenyl)-[1,2,4]triazolo[1,5-a]-[1,:3,5]-
triazine-2-sulphonamide;
6,7-Dihydro-5,6-dimethyl-N-phenyl-7-thioxo-x,1,2,4]
triazolo[1,5-a]-[1,3,5]-triazine-2-sulphonamide;
N-(2-Chlorophenyl)-6,7-dihydro-5,6-dimethyl--7
thiaxo-[1,2,4]triazolo-[1,5-a]-[1,3,5]-triazine-
2-sulfonamide.
A modification of the preceding 6,7-dihydro-
triazolotriazine sulfonamides includes compounds
according to Formula I wherein the only change is that B
is CR3, rathe~.~ than N; D, X, Y, R, R6, R~ and R~ combined
with R2 to fo~~m the divalent radical ( f ) , have the same
meanings as defined above and
R3 is defined to have the same members as X
and Y.
Preferred compounds according to this
embodiment of the invention herbicides include those
wherein
R.s is H, CN, No2, C~_4 acyl or alkoxycarbonyl;
carbamoyl, C.'~_' mono- or dialkyl carbamoyl or S (O) 0_3 Ci_4
alkyl;
WO 92/ 10098 PCT/US91 /09267
-22-
R~ is phenyl substituted in the ortho
positions independently with halogen, CF3, N02,
alkyl, alkoxy or alkoxycarbonyl and substituted in the
meta and para positions with halogen CF3 or Ci_4 alkyl;
R6 is H, C~_4 acyl or a single equivalent of a
metal ion and
X and Y are independently H, phenyl, alkyl,
alkenyl or alkynyl having up to 6 carbon atoms.
Preferred species according to the preceding
description include the following:
N-(2,6-Dichlorophenyl)-6,7-dihydro-5,6-dimethyl-3-
methoxycarbonyl-7-oxopyrazolo[1,5-a][1,3,5]-
triazine-2-sulphonamide;
N-(2,6-Difluorophenyl)-6,7-dihydro-5,6-dimethyl-3
methoxycarbonyl-7-thioxopyrazolo[1,5-a][1,3,5]
triazine-2-sulphonamide;
N-(2,6-Difluorophenyl)-6,7-dihydro-5,6-dimethyl-3-
methoxycarbonyl-7-oxopyrazolo[1,5-a][1,3,5]-
triazine-2-sulphonamide;
N-(2,6-Dichlorophenyl)-6,7-dihydro-5,6-dimethyl-3-
methoxycarbonyl-7-thioxopyrazolo[1,5-a][1,3,5]-
triazine-2-sulphonamide;
N-(2,6-Dichloro-3-methylphenyl)-6,7-dihydro-5,6
dimethyl-3-methoxycarbonyl-7-oxopyrazolo[1,5
a]-[1,3,5]-triazine-2-sulphonamide;
N-(2,6-Dichloro-3-methylphenyl)-6,7-dihydro-5,6-
dimethyl-3-methoxycarbonyl-7-thioxopyrazolo-
[1,5-a][1,3,5]-triazine-2-sulphonamide;
N-(2-Chloro-6-fluorophenyl)-6,7-dihydro-5,6
dimethyl-3-methoxycarbonyl-7-oxopyrazolo
[1,5-a][1,3,5]-triazine-2-sulphonamide;
N-(2-Chloro-6-fluorophenyl)-6,7-dihydro-5,6-
dimethyl-3-methoxycarbonyl-7-thioxopyrazolo-
[1,5-a][1,3,5]-triazine-2-sulphonamide;
N-(2-Chloro-6-methylphenyl)-6,7-dihydro-5,6-
dimethyl-3-methoxycarbonyl-7-oxopyrazolo-
[1,5-a][1,3,5]-triazine-2-sulphonamide;
WO 92/10098 PCT/US91/092fi7
-23-
N-(2-Chloro-6-methylphenyl)-6,7-dihydro-5,6~-
dimethyl-3-methoxycarbonyl-7-thioxopyrazolo-
[1,5-a][1,3,5]-triazine-2-sulphonamide;
N-(2,6-Dibromophenyl)-6,7-dihydro-5,6-dimethyl-
3-methoxycarbonyl-7-oxopyrazolo[1,5-a][1,3,5]-
triazine-2-sulphonamide;
N-(2,6-Dibromophenyl)-6,7-dihydro-5,6-dimeth.yl-3-
meth.oacycarbonyl-7-thioxopyrazolo[1,5-a][1,3,5]-
triazine-2-sulfonamide.
Another group of triazolosulfonamides
safened according to this invention are those according
to Formula I wherein R~ and R2 are combined to form the
divalent radi~~al (g) above, i.e.,
Y X
(g) - S - C = C -
and are characaerized as thiazolotriazole sulfonamides,
wherein:
X and Y are independently Hr OH, CN, N02,
halogen; alkyl, acyl, alkoxy, alkoxycarbonyl, alkenyl,
alkenyloxy, a:Lkynyl or alkynyloxy each having up to 6
carbon atoms; aryl, aralkyl or heterocyclic radical
having up to :~0 ring members of which up to 4 may be O,
S and/or N atoms; or X and Y may be combined to form an
alkylene chain of 3 or 4 carbon atoms; or said X and Y
substitutable members substituted with another X ~or Y
member when not self-inclusive;
R6 is H, acyl, alkyl, alkenyl or
alkoxycarbonyl. having up to 6 carbon atoms; aryl,
alkaryl or het:erocyclyl having up to 10 ring members of
which up to 4 may be O, S and/or N atoms; an alkali
metal ion, ammonium or C~_4 alkylammonium; or a
substitutable R6 member when not self inclusive
substituted with alkyl, alkoxy, acyl, alkenyl,
alkenyloxy, al.kynyl, alkynyloxy having up to 6 carbon
atoms and
R~ is an aromatic or heteroaromatic R6
member.
WO 92/10098 PCT/US91/09267
-24
Preferred members within the above
thiaazolotriazole sulfonamides are those wherein X and Y
are independently H or C~_6 alkyl, preferably methyl; R6
is H and RT is phenyl substituted with one or more
halogen, NOZ, C~_4 alkyl, alkoxy, alkoxycarbonyl or
alkylthio groups.
A preferred species according to the
preceding group of compounds is N-(2,6-difluorophenyl)-
thiazole[3,2-b][1,2,4)triazole-2-sulfonamide.
The preceding embodiments of triazolo- and
imidazolopyrimidine sulfonamide herbicides according to
Formula I used in this invention are characterized by
the R moiety -SOzN (R6) (R~) . In the following embodiments
analogous herbicides used herein are characterized by
the R moiety -N(R4)SOZ-RS. In these embodiments both A
and B are N, although it is within the purview of the
invention to replace either A or B with the CR3 moiety as
with the foregoing embodiments.
The first group of compounds according to
this embodiment of analogous compounds described in the
preceding paragraph are those wherein R~ and R2 are
combined to form the above bivalent radical (a), i.e.,
Z Y X
(a) -N = C - C = C -
or their tetrahydro analogs of bivalent radical (b)
above, i.e.,
Z Y X
( b ) - NH - CH - CH - CH -
wherein X, Y, Z, R4 and RS of Formula I have the same
meanings as those described earlier herein.
Preferred compounds within this embodiment
of herbicidal compounds are those wherein
Y and R4 are H;
X and Z are H or Ci_4 alkyl or alkoxy and
WO 92/10098 ~ ~ ~ PCf/US91/09267
-25-
Ri is phenyl substituted in a first o:rtho
position with halogen, NOZ, CF3, CN, carboxyl or C:~_4
alkoxycarbony:L; in the other ortho position in H,
halogen or C.i_,, alkoxycarbonyl and in the meta position
adjacent said first ortho position with H, halogen or
_' alkyl.
1?referrred species in the foregoing group of
compounds include the following:
N-5,7-din~ethyl-4,5,6,7-tetrahydro-1,2,4-triazolo-
[ 1, 5--a~ ] -pyrimidine-2-yl-2- ( 2 , 6-dichlorophenyl ) -
sulfonamide;
N-5-methyl-4,5,6,7-tetrahydro-1,2,4-triazolo[1,5-
a]-pyr~imidine-2-yl-2-(2,6-difluorophenyl)-
sulfon~amide;
N-(5,7-Dimethyl-1,2,4-triazolo[1,5-a]-pyrimidin-2-
yl)-2-thiophene sulfonamide;
N-Acetyl-2,6-dichloro-N-(5,7-dimethyl-1,2,4-tria-
zolo[1,5-a]-pyrimidin-2-yl)-benzenesulfonamide;
N-(5-Amino-1,2,4-triazol-3-yl)-2-nitrobenzenesul-
fonamide;
N-(5,7-Dimethyl-1,2,4-triazolo[1,5-a]-pyrimidin-
2-yl)-2-nitrobenzenesulfonamide;
N-(5-Amino-1,2,4-triazol-3-yl)-2,5-dichlorobenzene-
sulfanamide;
N-(5,7-ISimethyl-1,2,4-triazolo[1,5-a]pyrimidin-2-
yl)-2,5-dichlorabenzene-sulfonamide;
2-Chlora-N-(5-methyl-7-trifluoromethyl-1,2,4--tria-
zolo[1,5-a]pyrimidin-2-yl)benzenesulfonamide
2-Chloro-N-(7-methyl-1,2,4-triazolo[1,5-a]-
pyrimi~din-2-yl)benzenesulfonamide;
2-Chloro-:K-(1,2,4-triazolo[1,5-a]pyrimidin-2-yl)-
benzen~esulfonamide;
2-Chloro-,N-(6-Chloro-1,2,4-triazolo[1,5-a]-
pyrimidin-2-yl) benzenesulfonamide;
2-Chloro-1!1-(6-methyl-1,2,4-triazolo[1,5-a]-
pyrimi~iin-2-yl)benzenesulfonamide;
N-(5-Amino-1,2,4-triazol-3-yl)-2,6-dichloro-
benz en~=sul f onamide ;
WO 92/10098 PCT/US91/09267
-26-
A second group of preferred herbicides in
the class of those wherein in Formula I R is N(R4)SOZRS
includes compounds wherein R~ and R2 are combined to form
the bivalent radical (h), i.e.,
Y X
(h) - N = C - N = C -
wherein X, Y, R4 and RS have the same general meanings
and preferred members as described above in the first
group of compounds wherein R is N(R4)SOZ(RS).
Exemplary compounds within this group
include the following compounds wherein RS is a
substituted phenyl radical:
N-(5,?-dimethyl)-6,7-dihydro-[1,2,4]-triazole-
[1,5-a][1,3,5]-triazine-2-(2,6-difluorophenyl)-
sulfonamide;
N-(5-methyl)-6,7-dihydro-[1,2,4]-triazole[1,5-a]-
[1,3,5]-triazine-2-(2,6-difluorophenyl)-
sulfonamide;
N-(7-methoxy0-6,7-dihydro-[1,2,4]-triazole[1,5-a]-
[1,3,5]-triazine-2-(2,6-dichlorophenyl)-
sulfonamide;
N-(5,7-dimethoxy)-6,7-dihydro-[1,2,4]-triazole-
[1,5-a][1,3,5]-triazine-2-(2,3,6-trimethyl-
phenyl)sulfonamide;
N-(5-chloro)-6,7-dihydro-[1,2,4]-triazole[1,5-a]-
[1,3,5]-triazine-2-(2-acetyl-6-methylphenyl)-
sulfonamide and
N-(5-methoxymethyl)-6,7-dihydro[1,2,4]-triazole-
[1,5-a][1,3,5]-triazine-2-(2,6-difluorophenyl)-
sulfonamide.
Other preferred compounds are those wherein
RS is a substituted pyrazolyl, furanyl or thiophenyl
radical. Representative RS pyrazolyl members are the
pyrazol-4-yl sulfonamide compounds substituted in the 1-
position with H, Ci_4 alkyl or phenyl and in the 3- and
5-positions with H, halogen, CN, NOZ, CF3, phenyl,
benzyl, C~_4 alkyl, aminocarbonyl, mono- or dialkylamino
WO 92/10098 ~ ,~ ,~ ~ ~ PCT/US91/092~i7
-27-
carbonyl, alkoxycarbonyl, alkenyloxycarbonyl or alky-
nyloxycarbonyl, benzyloxycarbonyl or said phenyl and
benzyl members substituted with halogen, C~_' alkyl or
alkoxy.
Representative R5 furanyl and thiophenyl
members are the 2-yl and 3-yl isomers substituted in the
substitutable positions of the 2-yl radical with one or
more H, halogen or C~_~ alkyl and in the 3-yl radical
with one or more H, halogen or COO-alkyl, -alkenyl or
-alkynyl having up to 6. carbon atoms. Examples of such
compounds are:
N-(5,7-dimethyl)-6,7-dihydro-[1,2,4]-triazol.e-
[1,5-a][1,3,5]-triazine-2-thiophene sulfonamide;
N-(5-methyl)-6,7-dihydro-[1,2,4]-triazole-[1.,5-a]-
[1,3,5]-triazine-2-thiophenesulfonamide;
N-(5,7-dimethoxy)-6,7-dihydro-[1,2,4]-triazale-
[1,5-a][1,3,5]-triazine-2-furanesulfonami.de;
N-(5-met:hoxymethyl)-6,7-dihydro-[1,2,4]-tria.zole
[1,5-a][1,3,5]-triazine-furane sulfonamic~.e;
N-(5-met:hyl)-6,7-dihydro-[1,2,4]-triazole[1,3,5]-
triazine-2-(3-chloro-1-methyl-5-trifluoro-
methylpyrazol-4-yl)sulfonamide and
N-(5,7-dimethyl)-6,7-dihydro-[1,2,4]-triazole-
[1,3,5]-triazine-2-[4-chloro-5-methylsulfonyl)-
pyraz~~l-4-yl]sulfonamide.
A;s described herein, the aforementioned
azolopyrimidi~ne sulfonamides may be combined with.
antidotal compounds and used in that combined form or
further combined with other herbicides as co-herbicides.
Preferred herbicidal compounds useful as co-
herbicides herein are those according to Formula IV
wherein the R.i~ member is an alkoxyalkyl radical of the
structure -(E)-O-L, wherein E and L are linear or
branched-chain alkyl residues having a combined total of
up to 8 carbon atoms; or a substituted or unsubstituted
C4_~o heterocyclyl or heterocyclylmethyl radical con-
taining from :L to 4 ring hetero atoms selected indepen-
dently from N, S or O atoms and the R~2 member is also
WO 92/10098 PCT/US91/09267
~Q~5~ ~0
-28-
one of said heterocyclyl or heterocyclylmethyl radicals
or an optionally-substituted phenyl radical. Preferably
the phenyl radical is substituted with alkyl groups,
especially in the ortho positions. Similarly, some
preferred heterocyclic members are substituted with
alkyl or alkoxy radicals.
Among the more important heterocyclic R»
and/or R~2 members of Formula IV are mentioned indepen-
dently, the furanyl, thienyl, pyrazolyl, pyrrolyl,
isoxazolyl, isothiazolyl, triazolyl, imidazolyl, and
pyrimidinyl radicals and their analogs having a
methylene (-CH2-) moiety connecting the heterocyclic
radical to the acetamide nitrogen atom, e.g., pyrazol-
1-ylmethyl. When the heterocyclic radical is attached
directly to the amide nitrogen (with no intervening
methylene moiety), the attachment may be through a ring
carbon atom or a ring hetero atom as appropriate.
Other important R» and/or R~2 members include
the following: propynyl, alkoxycarbomethyl or -ethyl,
alkoxyiminoalkyl, benzyl, hydroxyalkyl, haloalkoxy and
-alkoxyalkyl, cyanoalkoxy and -alkoxyalkyl, methyl,
ethyl, propyl, butyl and their isomers, and the like.
Among preferred species of Formula IV are
mentioned N-(2,4-dimethylthien-3-yl)-N-(1-methoxyprop-
2-yl)-2-chloroacetamide, N-(iH-pyrazol-1-ylmethyl)-N-
(2,4-dimethylthien-3-71)-2-chloroacetamide and N-(1-
pyrazol-1-ylmethyl)-N-(4,6-dimethoxypyrimidin-5-yl)-2-
chloroacetamide.
Another important subgenus of preferred a-
haloacetamide compounds useful as co-herbicides are the
a-chloroacetanilides according to Formula V
O
II
C1CHZC - N - R~6
V ~ ~ ~ (R») p
WO 92/10098 PCT/US91/092ti7
-29-
wherein
R~6 is hydrogen, C~_6 alkyl, haloalkyl, alkoxy
or alkoxyalkyl, alkenyl, haloalkenyl, alkynyl or halo-
alkynyl having up to 6 carbon atoms, C5_~o heteroc,Yclyl or
heterocyclylmethyl having O, S and/or N atoms and which
may be substituted with halogen, C~_,~ alkyl, carbonyl-
alkyl or carb~~nylalkoxyalkyl, nitro, amino or cyano
groups;
R.i~ is hydrogen, halogen, nitro, amino, C
alkyl, alkoxy or alkoxyalkyl, and
p is 0-5.
Tlae most preferred species of Formula V are
2-chloro-2'-ethyl-6'-methyl-N-(ethoxymethyl)acetanilide
(common name '"acetochlor"), 2-chloro-2',6'-diethyl-N-
(methoxymethy.L)acetanilide (common name "alachlor"), 2-
chloro-2',6'-diethyl-N-(butoxymethyl)acetanilide (common
name "butachl~~r"), 2-chloro-2'-ethyl-6'-methyl-N-(1-
methyl-2-methoxyethyl)acetanilide (common name "metol-
achlor"), 2-chloro-2',6'-diethyl-N-(2-n-propoxyethyl)-
acetanilide (common name "pretilachlor") and 2-chloro-
2',6'-dimethy:L-N-(pyrazolylmethyl)acetanilide (common
name "metazachlor").
A larger group of preferred co-herbicides
includes the particular preferred species of Formulae IV
and V identif:Led above.
One group of preferred antidotal compounds
includes those according to Formula II wherein R~~ is C~_3
haloalkyl, Rig and R~5 are independently CZ_4 alkenyl or
haloalkenyl or 2, 3-dioxolan-2-yl-methyl and R~4 arid R~5
when combined form a C~_~o saturated or unsaturated
heterocyclic ring containing O, S and/or N atoms and
which may be :substituted with C~_5 alkyl, haloalkyl,
alkoxy, or al);oxyalkyl or haloacyl groups. The
preferred haloalkyl R member in formula II is
dichloromethy,'~. Preferred species in this group of
antidotal compounds are N,N-diallyl-dichloroacetamide
and N-(2-propE~nyl)-N-(1,3-dioxolanylmethyl)dichloro-
acetamide.
WO 92/ 10098 PCT/US91 /09267
~, ~ ~ ~ 2 t~ ~ 3
Still more preferred antidotal compounds
according to Formula II is a sub-group of substituted
1,3-oxazolidinyl dichloroacetamide having the formula
a 5 Rt7
O
I~
III C1ZCHC - N 3
-\ Z i
Rt9 RtaRta
wherein Rte is hydogen, Ct_~ alkyl, alkylol, haloalkyl or
l0 alkoxy, C2_6 alkoxyalkyl, a bicyclic hydro-
carbon radical having up to 10 carbon atoms,
phenyl or a saturated or unsaturated hetero-
cyclic radical having C4_to ring atoms and
containing O, S and/or N atoms, or said
phenyl and heterocyclic radical substituted
with one or more Ct_~ alkyl, haloalkyl,
alkoxy, alkoxyalkyl, halogen or vitro
radicals, and
Rta and Rt9 are independently hydrogen, Ct_4
alkyl or haloalkyl, phenyl or a heterocyclic
Rte member or together with the carbon atom
to which they are attached may form a C3-C~
spiro-cycloalkyl group.
Preferred members according to Formula III
are those wherein Rte is one of said heterocyclic members
and Rta and Rt9 are independently methyl, trif luoromethyl
or when combined with the carbon atom to which attached
form a CS or C6 cycloalkyl radical.
Preferred antidotal compounds according to
Formula III are the following compounds:
Oxazolidine, 3-(dichloroacetyl)-2,2,5-tri-
methyl-,
Oxazolidine, 3-(dichloroacetyl)-2,2-
dimethyl-5-phenyl-,
Oxazolidine, 3-(dichloroacetyl)-2,2-
dimethyl-5-(2-furanyl)-,
Oxazolidine, 3-(dichloroacetyl)-2,2-
dimethyl-5-(2-thienyl)-,
WO 92/10098 ~ ~ PCr/US91/09267
-31-
Pyridine, 3-[3-(dichloroacetyl)-2,2
dimethyl-5-oxazolidinyl]-,
4~-(dichloroacetyl)-1-oxa-4-azaspiro-(4,5)-
decane.
A~aother group of dichloroacetamide antidotal
compounds according to Formula II are the following
compounds:
4~-(Dichloroacetyl)-3,4-dihydro-3-methyl-2H-
2,4-benzoxazine,
E~thanone, 2,2-dichloro-1-(1,2,3,4-tet,ra-
hydro-1-methyl-2-isoquinolinyl)-,
N~-(Dichloroacetyl)-1,2,3,4-tetrahydro-
quinaldine,
1~-(Dichloroacetyl)-1,2,3,4-tetrahydro-
quinoline,
C:is/trans-piperazine, 1,4-bis(dichloro- 1,4-
acetyl)-2,5-dimethyl-,
1,5-Diazacyclononane, l,5bis-(dichloro-
acetyl,
1~-Azaspiro[4,4]nonane, 1-(dichloroacetyl),
P~,rrrolo [ 1, 2-a ] -pyrimidine- [ ~S ( 2H) ] -one, 1-
(dichloroacetyl)hexahydro-3,3,8a-
trimethyl,
2,2-Dimethyl-3-(dichloroacetyl)-1,3-oxazole
and
2,2-Dimethyl-5-methoxy-3-(dichloroacetyl)-
1,3-oxazole.
Shill another preferred group of antidotal
compounds are the following which have a structure not
according to lFormula II:
a~-[(Cyanomethoxy)imino]benzeneacetoni.trile,
a~-[(1,3-Dioxolan-2-yl-methoxy)imino]benzene-
acetonitrile,
O~-[1,3-Dioxolan-2-ylmethyl]-2,2,2-tri.fluoro-
methyl-4'-chloroacetophenone oxi.me,
B~anzenemethamine, N-[4-(dichloromethylene)-
1,3-ditholan-2-ylidene]-cz-methyl,
hydrochloride,
WO 92/10098 PCT/US91/09267
) ~ ~ ~ -32-
Diphenylmethoxy acetic acid, methyl ester,
1,8-Naphthalic anhydride,
4,6-Dichloro-2-phenyl-pyrimidine,
2-Chloro-N-[1-(2,4,6-trimethylphenyl)-
ethenyl]acetamide,
Ethylene glycol acetal of 1,1-dichloro-
acetone,
1,3-Dioxolane, 2-(dichloromethyl)-2-
methyl-,
5-Thiazolecarboxylic Acid, 2-chloro-4-
(trifluoromethyl)-, (phenylmethyl)-
ester,
Phosphorothioic acid, O,O-diethyl O-(3-
methylphenyl)ester,
4-Pentenenitrile, 2-methyl-2-[(4-methyl-
phenyl)thio]-,
5-Chloro-8-(cyanomethoxy)quinoline,
1-Methylhexyl-2-(5-chloro-8-quinolinoxy)-
acetate or
o-(Methoxycarbonyl)-2-(8-quinolinoxy)
acetamide oxime.
The herbicidal and antidotal compounds of
Formulae I-V are known in the art. The sub-group of
1,3-oxazolidine dichloroacetamides of Formula III are
the subject of copending application, Serial No.
07/212,621, of common assignment herewith, priority
application for EP 304409 published February 22, 1989.
The term "haloalkyl" embraces radicals
wherein any one or more of the carbon atoms, preferably
from 1 to 4 in number, is substituted with one or more
halo groups, preferably selected from bromo, chloro and
fluoro. Specifically embraced by the term "haloalkyl"
are monohaloalkyl, dihaloalkyl and polyhaloalkyl groups.
A monohaloalkyl group, for example, may have either a
bromo, a chloro, or a fluoro atom within the group.
Dihaloalkyl and polyhaloalkyl groups may be substituted
WO 92/10098 ~ ~ ~ PCT/US91/092b7
-33-
with two or more of the same halo groups, or may have a
combination c~f different halo groups. A dihaloalkyl
group, for e~:ample, may have two bromo atoms, such as a
dibromomethyl. group, or two chloro atoms, such as a
dichlorometh~~l group, or one bromo atom and one d~hloro
atom, such a~~ a bromochloromethyl group. Examples of a
polyhaloalkyl. are perhaloalkyl groups such as trifluoro-
methyl and pe:rfluoroethyl groups.
~ihere in Farmulae III-V the halogen attached
to the acetyl. radical is the chlorine ion, it is con-
templated that the other halogens, i.e., bromo, iodo or
fluoro may be: substituted for the chloro.
F~referred haloalkyl R~3 members of Formula II
are dihalomet.hyl, particularly dichloromethyl, while the
preferred hal.oalkyl R~i member is a tri-halogenated
methyl radical, preferably trifluoromethyl.
lirhere the term "alkyl" is used either alone
or in compour.~d form (as in "haloalkyl"), it is intended
to embrace linear or branched radicals having up to four
carbon atoms, the preferred members being methyl and
ethyl.
E;y "agriculturally-acceptable salts" of the
compounds defined by the above formula is meant <i salt
or salts which readily ionize in aqueous media to form a
cation or anion of said compounds and the corresponding
salt anion ar cation, which salts have no deleterious
effect on the. antidotal properties of said compounds or
of the herbicidal properties of a given herbicide and
which permit formulation of the herbicide-antidote
composition without undue problems of mixing, suspen-
sion, stability, applicator equipment use, packaging,
etc.
E~y "antidotally-effective" is meant the
amount of antidote required to reduce the phytotoxicity
level or effect of a herbicide, preferably by at least
10% or 15%, taut naturally the greater the reduction in
herbicidal ir.~jury the better.
WO 92/10098 PCT/US91/09267
-34-
_ By "herbicidally-effective" is meant the
amount of herbicide required to effect a meaningful
injury or destruction to a significant portion of
affected undesirable plants or weeds. Although of no
hard and fast rule, it is desirable from a commercial
viewpoint that 80-85% or more of the weeds be destroyed,
although commercially significant suppression of weed
growth can occur at much lower levels, particularly with
some very noxious, herbicide-resistant plants.
The terms "antidote", "safening agent",
"safener", "antagonistic agent", "interferant", "crop
protectant" and "crop protective", are often-used terms
denoting a compound capable of reducing the phytotoxi-
city of a herbicide to a crop plant or crop seed. The
terms "crop protectant" and "crop protective" are
sometimes used to denote a composition containing as the
active ingredients, a herbicide-antidote combination
which provides protection from competitive weed growth
by reducing herbicidal injury to a valuable crop plant
while at the same time controlling or suppressing weed
growth occurring in the presence of the crop plant.
Antidotes protect crop plants by interfering with the
herbicidal action of a herbicide on the crop plants so
as to render the herbicide selective to weed plants
emerging or growing in the presence of crop plants.
Herbicides which may be used as co-
herbicides with the azolopyrimidine sulfonamides of
Formula I with benefit in combination with an antidote
as described herein include, preferably, thiocarbamates
(including dithiocarbamates), a-haloacetamides,
(described above) heterocyclyl phenyl ethers (especially
phenoxypyrazoles), imidazolinones, pyridines and
sulfonylureas. It is within the purview of this
invention that many other classes of herbicides, e.g.,
triazines, ureas, diphenyl ethers, nitroanilines,
thiazoles, isoxazoles, pyrrolidinones, aromatic and
heterocyclic di- and triketones, etc., the individual
members of which classes may be derivatives having one
WO 92/10098 PCl'/US91/09267
-35-
or more substituents selected from a wide variety of
radicals may ;suitably be used as co-herbicides. Such
combinations ~~an be used to obtain selective weec~l
control with Low crop injury in several varieties of
monocotyledon~~us crop plants such as corn, grain sorghum
(milo), and cereals such as wheat, rice, barley, oats,
and rye, as well as several varieties of dicotyledonous
crop plants including oil-seed crops such as soybeans
and cotton. :Particular utility for the antidotal.
compounds of 'this invention has been experienced with
various herbi~~ides in corn, sorghum and soybeans.
E:Kamples of important thiocarbamate
herbicides are= the following:
~,is-/trans-2,3-dichloroallyl-diisopropyl-
thiolcarbamate (common name "diallate");
Ethyl dipropylthiocarbamate (common name
"EPTC");
S~-ethyl diisobutyl (thiocarbamate) (common
name "butylate");
S~-propyl dipropyl(thiocarbamate) (common
name "vernolate");
2,3,3-trichloroallyl-diisopropylthiocarba-
mate (common name "triallate").
E:Kamples of important acetamide herbicides
are the following:
2~-chloro-N-isopropylacetanilide (common name
"propachlor");
2~-chloro-1',6'-diethyl-N-(methoxymethyl)-
acetanilide (common name "alachl.or");
2~-chloro-2',6'-diethyl-N-(butoxymethyl)-
acetanilide (common name "butach~lor");
2~-chloro-N-(ethoxymethyl)-6'-ethyl-Q-aceto-
toluidide (common name "acetochl.or");
Ethyl ester of N-chloroacetyl-N-(2,6-di-
ethylphenyl)glycine (common name
"diethatyl ethyl");
WO 92/10098 PCT/US91/09267
-36-
- 2-chloro-N-(2,6-dimethylphenyl)-N-(2-
methoxyethyl)acetamide (common name
"dimethachlor");
2-chloro-N-(2-n-propoxyethyl)-2',6'-diethyl-
acetanilide (common name
"pretilachlor");
2-chloro-N-(2-methoxy-1-methylethyl)-6'-
ethyl-Q-acetotoluidide (common name
"metolachlor");
2-chloro-2',6'-dimethyl-N-(1-pyrazol-1-yl-
methyl)acetanilide (common name "metaza-
chlor");
2-chloro-N-(2,6-dimethyl-1-cyclohexen-1-
yl)-N-(1H-pyrazol-lylmethyl)acetamide;
2-chloro-N-isopropyl-1-(3,5,5-trimethyl-
cyclohexen-1-yl)acetamide (common name
"trimexachlor");
2-Chloro-2'-methyl-6'-methoxy-N-(isopropoxy-
methyl)acetanilide;
2-Chloro-2'-methyl-6'-trifluoromethyl-N-
(ethoxymethyl)acetanilide.
N-(2,4-dimethylthien-3-yl)-N-(1-methoxyprop-
2-yl)-2-chloroacetamide;
N-(1H-pyrazol-2-ylmethyl)-N-(2,4-
dimethylthien-3-yl)-2-chloroacetamide and
N-(1-pyrazol-1-ylmethyl)-N-(4,6-dimethoxy-
pyrimidin-5-yl)-2-chloroacetamide.
Examples of important pyridine herbicides
include:
3-pyridinecarboxylic acid,-2(difluoro-
methyl)-5-4,5-dihydro-2-thiazolyl-4-
(2-methylpropyl)-6-(trifluoromethyl)-,
methyl ester;
3-pyridinecarboxylic acid, 2-(difluoro-
methyl)-4-(2-methylpropyl)-5-(1H-
pyrazol-1-ylcarbonyl)-6-(trifluoro-
methyl)-, methyl ester;
WO 92/10098 '~ ~ ~ ~ PCC/US91/092Ei7
-3~-
3,5-pyridinedicarboxylic acid, 2-(difluoro-
methyl)-4-(2-methylpropyl)-6-trifluoro-
methyl, dimethyl ester.
3,5-pyridine dicarbothioic acid, 2-
(difluoromethyl)-4-(2-methylpropyl)-6-
(trifluoromethyl)-, S,S-dimethyl ester.
E:Kamples of important heterocyclyl phenyl
ethers include:
l0 5-(trifluoromethyl)-4-chloro-3-(3'-[1-
ethoxycarbonyl]-ethoxy-4'-nitrophenoxy)-
1-methylpyrazole;
5-(trifluoromethyl)-4-chloro-3-(3'-methoxy-
4'-nitrophenoxy)-1-methy.lpyrazole;
5-(trifluoromethyl)-4-chloro-3-(3'-[1.-
butoxycarbonyl]-ethoxy-4'-nitrophenoxy)-
4-methylpyrazole;
5-(trifluoromethyl)-4-chloro-3-(3'-
methylsulfamoylcarbonyl propoxy-4'-
nitrophenoxy)-4-methylpyrazole;
5-(trifluoromethyl)-4-chloro-3-(3'-propoxy-
carbonylmethyloxime-4'-nitrophenoxy)-1-
methylpyrazole;
(.~) -2- [ 4- [ [ 5- (trif luoromethyl ) -2-pyri.dinyl ] -
oxy]phenoxy]propanoic acid.
Examples of important sulfonylureas include:
Henzenesulfonamide, 2-chloro-N-[[(4-methoxy-
6-methyl-1,3,5-triazin-2-yl)amino]-
carbonyl], (common name "chlorsul~:uron);
Benzoic acid, 2-[[[[(4-chloro-6-methoxy-2-
pyrimidin-2-yl)amino]carbonyl]amino]-
sulfonyl]-ethyl ester, (common name
"chlormuron ethyl);
2-Thiophenecarboxylic acid, 3-[[[[(4,6-di-
methoxy-1,3,5-triazin-2-yl)amino"~-
carbonyl]-amino]sulfonyl]-, methyl
ester (code No. DPX-M6316);
WO 92/ 10098 PCT/US91 /09267
~ '~ 9 2 0 - -38_
Benzoic acid, 2-[[[[(4,6-dimethyl-2-pyrimi-
dinyl)amino]carbonyl]amino]sulfonyl]-
methyl ester (common name "sulfometuron
methyl");
Benzenesulfonamide, 2-(2-chloroethoxy)-N-
[[(4-methoxy-6-methyl-1,3,5-triazin-2-
yl)amino]-carbonyl] (common name
"triasulfuron");
Benzoic acid, 2-[[[[(4-methoxy-6-methyl-
1,3,5-triazin-2-yl)amino]carbonyl]-
amino]sulfonyl]-methyl ester (common
name "metsulfuron methyl");
Benzoic acid, 2-[[[[[4,6-bis(difluoro-
methoxy)-2-pyrimidin-2-yl]amino]-
carbonyl]amino]sulfonyl]methyl ester;
(common name "primisulfuron")
Pyridine, 3-[[[[(4,6-dimethyl-2-pyrimidin-
2-yl)amino]carbonyl]amino]sulfonyl]-N,N-
dimethylcarbamoyl, (common name
"nicosulfuron");
Pyridine, 3-[[[[(4,6-dimethoxy-2-pyrimidin-
2-yl)amino]carbonyl]amino]sulfonyl]-
ethylsulfonyl, (code number "DPX E9636);
Benzenesulfonamide, 2-(methoxyethoxy)-N-
[[(4,6-dimethoxy-1,3,5-traizin-2-
yl)amino]carbonyl], (common name
"cinosulfuron")
Methyl-2-[[[[[(4,6-dimethoxy-2-pyrimidin-
2-yl)amino]carbonyl]amino]sulfonyl]-
methyl]benzoate, (common name "ben-
sulfuron methyl");
N-[(4,6-dimethoxypyrimidin-2-yl)amino-
carbonyl]-3-chloro-4-methoxycarbonyl-1-
methoxycarbonyl-1-methylpyrazole, (code
number "NC-319";
N-[(4,6-dimethoxypyrimidin-2-yl)amino-
carbonyl]-4-ethoxycarbonyl-1-methyl-
pyrazole, (code number "NC-311");
WO 92/10098 PCT/US91/0921i7
-39-
N-[(4,6-dimethylpyrimidin-2-yl)amino--
carbonyl]-1-(1-methylethyl)-iH-im:~dazole-
2-sulfonamide;
N-[(4-methoxy-6-methylpyrimidin-2-yl)-
aminocarbonyl]-1-(1-methylethyl)-~.H-
imidazole-2-sulfonamide;
N-[(4,6-dimethoxypyrimidin-2-yl)aminc>
carbonyl]-1-(1-methylethyl)-1H
imidazole-2-sulfonamide;
N-[(4,6-dimethylpyrimidin-2-yl)amino-
carbonyl]-1-ethyl-iH-imidazole-2-sul-
fonamide;
N-[(4-methoxy-6-methylpyrimidin-2-yl)-
aminocarbonyl]-1-ethyl-1H-imidazol.e-2-
sul f onamide ;
N~-[(4,6-dimethoxypyrimidin-2-yl)amino-
carbonyl]-1-ethyl-iH-imidazole-2-
sulfonamide;
N~-[(4,6-dimethoxypyrimidin-2-yl)amina-
carbonyl]-5-bromo-1-methyl-iH-imidazole-
4-sulfonamide.
E:Kamples of important imidazolinone
herbicides in~~lude:
3~-Quinolinecarboxylic acid, 2-[4,5-di.hydro-
4-methyl-4-(1-methylethyl)-5-oxo-1.H-
imidazol-2-yl]-;
3~-Pyridinecarboxylic acid, 2-[4,5-dihydro-
4-methyl-4-(1-methylethyl)-5-oxo-1.H-
imidazol-2-yl]-;
Benzoic Acid, 2-[4,5-dihydro-4-methyl-4-(1-
methylethyl)-5-oxo-iH-imidazol-2-yl]-
4(or 5)-methyl;
3~-pyridinecarboxylic acid, 5-ethyl-2-[4-
methyl-4-(1-methylethyl)-5-oxo-iH-
imidazol-2-yl]-;
WO 92/ 10098 PCT/US91 /09267
-40-
3-pyridinecarboxylic acid, 2-[4,5-dihydro-
4-methyl-4-(1-methylethyl)-5-oxo-iH-
imidazol-2-yl]-5-methyl-, ammonium
salt;
2-(5-Methyl-5-trifluoromethyl-1-H-imidazol-
4-on-2-yl)-pyridin-3-carboxylic acid;
2-(5-Methyl-5-trifluoromethyl-1-H-imidazol-
4-on-2-yl)5-(m)ethyl isonicotinic acid;
2-[5-(1-Fluoroethyl)-5-(m)ethyl-H-imidazol
4-on-2-yl]isonicotinic acid;
2-(5-(Difluoromethyl-5-(m)ethyl-1-H-
imidazol-4-on-2-yl]-5-(m)ethyl-
isonicotinic acid;
2-(5-(1-Fluoroethyl)-5-(m)ethyl)-imidazol-
4-on-2-yl]isonicotinic (m)ethyl ester;
Examples of important benzoic acid
derivative herbicides include:
3,6-Dichloro-2-methoxybenzoic acid (common
name "dicamba"),
2,5-Dichloro-3-aminobenzoic acid (common
name "amiben" and "chloramiben"),
5-(2'-Chloro-4'-trifluoromethylphenoxy)-2-
nitrobenzoic acid (common name
"acifluorfen"),
2,6-Dichlorobenzonitrile (common name
"dichlobenil"),
3,5,6-Trichloro-2-methoxybenzoic acid
(common name "Tricamba"),
2,3,6-Trichlorobenzoic acid, and
2,3,5,6-Tetrachlorobenzoic acid,
and salts, esters and amides of the above acids.
Examples of other important herbicides
include:
2-Chloro-4-(ethylamino)-6-(isopropylamino)-
s-triazine;
WO 92/ 10098 ~ ~ ~ ~ ~ PCr/US91 /09267
-41-
~t-Amino-6-tertbutyl-3-(methylthio)as-
triazine-5(4H)one;
~.~rifluoro-2,6-dinitro-N,N-dipropyl-p-
toluidine;
l3enzeneamine, N-(1-ethylprapyl)-3,4-
dimethyl-2,6-dinitro-;
:!-Pyrrolidinane, 3-chloro-4-(chloromethyl)-
1-[3-(trifluoromethyl)phenyl], traps-;
:~-Isoxazalidinone, 2-[(2-chlorophenyl)-
l0 methyl]-4,4-dimethyl-;
~;-Imidazolidinone, 3-[5-(1,1-dimethylethyl)-
3-isoxazolyl]-4-hydroxy-1-methyl-;
-Chloro-4-(1-cyano-1-methylethylamino)-6-
ethylamino-1,3,5-triazine;
methyl 5-(2,4-dichlorophenaxy)-2-nitro-
benzoate;
7.'-(Carboethoxy)ethyl-5-[2-chloro-4-(tri-
fluoromethyl)phenoxy]-2-nitrobenzaate;
p,mmonium-DrJ-homoalanin-4-yl(methyl)-
2 0 phosphinate;
1.-[(2-Fluoro-4-chloro-5-(2,3-dimethyl
butoxyphenyl0}tetrahydrophthalimide
and
2-(3,4-Dichlorophenyl)-4-methyl-1,2,4-oxa-
diazolidine-3,5-dione.
Z'he herbicides of particular and preferred
interest as c,o-herbicides with the azolopyrimidine sul-
fonamides of Formula I in compositions with antidotes
according to this invention include each of the above-
mentioned species from different chemical classes of
compounds exemplified as important herbicides, parti-
cularly those of current commercial interest and use and
those which m.ay be determined of commercial util:.ity.
Co-herbicidal compounds of preference
include the following:
alachlor,
acetochlor,
butachlor,
WO 92/10098 PCT/US91/09267
-42-
metolachlor,
pretilachlor
metazachlor,
2-chloro-2',6'-dimethyl-N-(2-methoxyethyl)-
acetanilide,
butylate and combinations thereof with the
commercial antidotes R-29148 or PPG-1292 and EPTC and
combinations thereof with the commercial antidotes 8-
25788, R-29148 or PPG-1292 any of which may further
contain an extender, e.g., dietholate.
All of the above specifically-named
antidotes and herbicides are known in the art.
As further detailed infra, while not
necessary, the composition containing the herbicide-
antidote combination may also contain other additaments,
e.g., biocides such as insecticides, fungicides,
nematocides, miticides, etc., fertilizers, inert
formulation aids, e.g., surfactants, emulsifiers,
defoamers, dyes, extenders, etc.
Combinations may be made of any one or more
of the described antidote compounds with any one or more
of the herbicide compounds of Formula I and co-
herbicides mentioned herein.
It will be recognized by those skilled in
the art that all herbicides have varying degrees of
phytotoxicity to various plants because of the
sensitivity of the plant to the herbicide. Thus, e.g.,
although certain crops such as corn and soybeans have a
high level of tolerance (i.e., low sensitivity) to the
phytotoxic effect of alachlor, other crops, e.g., milo
(grain sorghum), rice and wheat, have a low level of
tolerance (i.e., high sensitivity) to the phytotoxic
effects of alachlor. The same type of sensitivity to
herbicides as shown by crop plants is also exhibited by
weeds, some of which are very sensitive, others very
resistant to the phytotoxic effects of the herbicide.
WO 92/10098 ~ ~ ~ ~ ~ ~ PCT/US91/09267
-43-
When the sensitivity of a crop plant to a
herbicide is low, whereas the sensitivity of a wE_ed to
that herbicide is high, the "selectivity factor" of the
herbicide far preferentially injuring the weed while not
injuring they crop is high.
In an analogous manner, but more complex, an
antidotal campound may, and commonly does, have varying
degrees of crop protective effect against different
herbicides i.n different crops. Accordingly, as will be
appreciated by those skilled in the art, the..varalous
antidotes of' this invention, as with all classes of
antidotal campounds, will have greater or lesser crop
safening effects against various herbicides in various
crops than in others. Thus, while a given antidotal
compound may have no crop protective ability agaj~nst a
given herbicide in a given crop, that same antidotal
compound ma~~ have a very high crop protective ability
against the same given herbicide in a different crop or
against a different herbicide in the same crop. This is
an expected phenomenon.
DETp~ILED DESCRIPTION OF THE INVENTION
Antidote Compounds
As mentioned earlier, the antidotal
compounds used in the practice of this invention are
known compounds. The preferred compounds used herein
are the 1,3-oxazolidine dichloroacetamides according to
Formula III wherein the R» member is a heterocyc.lic
radical. Those compounds and synthesis methods therefor
are separately disclosed and claimed in the assignee's
said copendi.ng application, Serial No. 07/212,62:1 and
its corresponding EP 304409, published February ~?2,
1989.
Biological Evaluation
Effective weed control coupled with :Low crop
injury is a result of treatment of a plant locus with a
combination of herbicide compound and antidote compound.
By application to the "plant locus" is meant app:Lication
WO 92/10098 PCT/US91/09267
-44-
to the plant growing medium, such as soil, as well as to
the seeds, emerging seedlings, roots, stems, leaves, or
other plant parts.
The phrase "combination of herbicide
compound and antidote compound" embraces various methods
of treatment. For example, the soil of a plant locus
may be treated with a "tank-mix" composition containing
a mixture of the herbicide and the antidote which is "in
combination". Or, the soil may be treated with the
herbicide and antidote compounds separately so that the
"combination" is made on, or in, the soil. After such
treatments of the soil with a mixture of herbicide and
antidote or by separate or sequential application of the
herbicide and antidote to the soil, the herbicide 'and
antidote may be mixed into or incorporated into the soil
either by mechanical mixing of the soil with implements
or by "watering in" by rainfall or irrigation. The soil
of a plant locus may also be treated with antidote by
application of the antidote in a dispersible-concentrate
form such as a granule. The granule may be applied to a
furrow which is prepared for receipt of the crop seed
and the herbicide may be applied to the plant locus
either before or after in-furrow placement of the anti-
dote-containing granule so that the herbicide and anti-
dote form a "combination". Crop seed may be treated or
coated with the antidote compound either while the crop
seed is in-furrow just after seeding or, more commonly,
the crop seed may be treated or coated with antidote
prior to seeding into a furrow. The herbicide may be
applied to the soil plant locus before or after seeding
and a "combination" is made when both herbicide and
antidote-coated seed are in the soil. Also contem-
plated as a "combination" is a commercially-convenient
association or presentation of herbicide and antidote.
For example, the herbicide and antidote components in
concentrated form may be contained in separate con-
WO 92/ 10098 ~ ~ ~ '* PCT/US91 /0927
-45-
tainers, but such containers may be presented for sale
or sold together as a "combination" (composition). Or,
the herbicide and antidote components in concentrated
form may be in a mixture in a single container as a
"combinatian". Either such "combination" may be: diluted
or mixed with adjuvants suitable for soil applications.
Another example of a commercially-presented combination
is a container of antidote-coated crop seed sold, or
presented for sale, along with a container of herbicide
material. These containers may, or may not, be
physically attached to each other, but nonetheless
constitute a "combination of herbicide and antidote"
when intende~3 for use ultimately in the same plant
locus.
In the foregoing description of various
modes of application of the herbicide-antidote
combinations, it is inherent that each form of
application :requires that in some manner, the herbicide
and antidote will physically combine to form a
"composition'" of those agents.
'the amount of antidote employed in the
methods and ~~ompositions of the invention will vary
depending up~~n the particular herbicide with which the
antidote is ~amployed, the rate of application of the
herbicide, tile particular crop to be protected, and the
manner of app?lication to the plant locus. In each
instance the amount of antidote employed is a safening-
effective amount, that is, the amount which reduces, or
protects against, crop injury that otherwise would
3o result from t:he presence of the herbicide. The amount
of antidote ~amployed will be less than an amount that
will substani_ially injure the crop plant.
'.Che antidote can be applied to the crop
plant locus :in a mixture with the selected herbicide.
For example, where the crop seed is first planted, a
suitable mixi:ure of antidote and herbicide, whether in a
homogeneous :Liquid, emulsion, suspension or solid form,
WO 92/10098 PCT/US91/09267
Q ~9 ~ 0
-46-
can be applied to the surface of, or incorporated in,
the soil in which the seed has been planted. Or, the
herbicide-antidote mixture may be applied to the soil,
and then the seed thereafter "drilled" into the soil
below the soil layer containing the herbicide-antidote
mixture. The herbicide will reduce or eliminate the
presence of undesirable weed plants. Where the herbi-
cide would by itself injure the crop seedlings, the
presence of the antidote will reduce or eliminate the
injury to the crop seed caused by the herbicide. It is
not essential that the application of herbicide and the
antidote to the plant locus be made using the selected
herbicide and antidote in the form of a mixture or
composition. The herbicide and the antidote may be
applied to the plant locus in a sequential manner. For
example, the antidote may be first applied to the plant
locus and thereafter the herbicide is applied. Or, the
herbicide may be first applied to the plant locus and
thereafter the antidote is applied.
The ratio of herbicide to antidote may vary
depending upon the crop to be protected, weed to be
inhibited, herbicide used, etc., but normally a herbi-
cide-to-antidote ratio ranging from 1:25-to-60:1 (pre-
ferably 1:5-to-30:ij parts by weight may be employed,
although much higher rates of antidote may be used,
e.g., 1:100-1:300 parts by weight of herbicide-to-anti-
dote. As indicated above, the antidote may be applied
to the plant locus in a mixture, i.e., a mixture of a
herbicidally-effective amount of herbicide and a safen-
ing-effective amount of an antidote, or sequentially,
i.e., the plant locus may be treated with an effective
amount of the herbicide followed by a treatment with the
antidote or vice versa. In general, effective herbi-
cidal amounts are in the range of about 0.03 to about 12
kilograms/hectare, but rates as low as 0.004 kg/ha may
be used effectively. The preferred range of rate of
application is from about 0.1 to about 10 kg/ha. Pre-
WO 92/10098 ~ ~ °~ ~ y PCf/US91/092t~7
-47-
ferably, antidote application rates range from about
8-10 kg/ha down to about 0.05 kg/ha. It will be
appreciated that at times amounts either below or' above
these ranges will be necessary to obtain the best.
results. The selection of the herbicide to inhibit the
emergence and growth of weeds depends upon the species
of weeds to bra controlled and the crop to be protected.
The application of the antidote can be made
directly to the seed before planting. In this practice,
a quantity of crop seed is first coated with the anti-
dote. The co~3ted seed is thereafter planted. Th:e
herbicide may be applied to the soil before or after the
coated seed i;s planted.
I~z field applications, the herbicide, anti-
dote, or a mi:Kture thereof, may be applied to the: plant
locus without any adjuvants other than a solvent.
Usually, the herbicide, antidote, or a mixture thereof,
is applied in conjunction with one or more adjuva,nts in
liquid or solid form. Compositions or formulations
containing mi:rttures of an appropriate herbicide and
antidote usually are prepared by admixing the herbicide
and antidote with one or more adjuvants such as dilu-
ents, solvents, extenders, carriers, conditioning
agents, water, wetting agents, dispersing agents, or
emulsifying a~~ents, or any suitable combination of these
adjuvants. These mixtures may be in the form of parti-
culate solids, granules, pellets, wettable powders,
dusts, soluti~~ns, aqueous dispersions, or emulsions.
Avpplication of the herbicide, antidote, or
mixture there~~f, can be carried out by conventional
techniques utilizing, for example, hand-carried or
tractor-mounted spreaders, power dusters, boom arid hand
sprayers, spray dusters, and granular applicator:c. If
desired, application of the compositions of the ~.nven-
tion to plants can be accomplished by incorporating the
compositions in the soil or other media.
WO 92/ 10098 PCT/US91 /09267
~~5"~ ~Q ~~ 4s-
Evaluations of safening activity of a wide
variety of representative antidote compounds of.this
invention were carried out using the specific proce-
dure of Example 1 below in greenhouse testing. Measure-
ments of biological response as reported in Tables 1-3
were made in the following manner. A visual comparison
was made between a crop plant treated with a herbicide
alone and crop plant having no herbicide or antidote
treatment. A number was assigned to this visual
comparison indicating the percent injury or inhibition
to the herbicide-alone treated crop plant (column "WO"
in the tables indicating herbicide "without" antidote).
Also, a visual comparison was made between the crop
plant treated with herbicide + antidote combination and
the crop plant having no herbicide or antidote treat-
ment. A number was assigned to this visual comparison
indicating the percent injury or inhibition to the
herbicide + antidote treated crop plant (column "W" in
the tables indicating herbicide "with" antidote).
Observations of response by the weed species to
herbicide or herbicide + antidote were similarly
recorded. The degree of reduction of herbicide injury
provided by an antidote compound is indicated by the
magnitude that the plant inhibition number of column
"WO" exceeds the corresponding number of column "W".
Also reported in the tables are data in parenthesis
showing "safening effect" (defined below) for the
herbicide + antidote combinations calculated from the
plant inhibition numbers. These tables may show crop or
weed column headings under which there are no data. The
lack of such data is an indication of a failed test or
that the particular herbicide + antidote rate combina-
tion was not tested with any crop or weed.
Listed below are the names of the antidotal
compounds of preference herein and representative ones
WO 92/10098 ~ ~ ~ PCIf/US91/0926~7
-49-
therein for which data are reported in the table:a.
Antidote No. Nomenclature
1 4-Pentenenitrile, 2-methyl-2-[(~6-
methylphenyl)thio],
2 Acetic acid, (diphenylmethoxy)-, methyl
ester,
3 Benzenemethanamine, N-[4-(dichloro-
methylene)-1,3-dithiolan-2-
ylidene]-a-methyl-, hydrochloride,
4 Phosphorothioic acid, O,O-diethyl-O-
(3-methylphenyl) ester,
5 5-Thiazolecarboxylic acid, 2-chloro-4-
(trifluoromethyl)-, (phenylmethyl
ester),
6 Pyrimidine, 4,6-dichloro-2-phenyl-,
7 1H, 3H-Naphtho[1,8-cd]pyran-1,3-dione,
8 Henzeneacetonitrile, a-{[(1,3-dioxolan-
2-yl ) methoxy ] imino } -,
9 Acetamide, N,N-His(2-propenyl)-a,a-
dichloro, (also, N,N-diallyl
dichloroacetamide),
10 Oxazolidine, 3-(dichloroacetyl)-5-(2-
furanyl)-2,2-dimethyl-,
11 Cis/Trans-piperazine, 1,4-bis(di-
chloroacetyl)-2,5-dimethyl,
12 1-Oxa-4-azaspiro[4.5]decane, 4-
(dichloroacetyl)-, (also, 4-
dichloroacetyl-1-oxa-4-azaspiro-
(4,5)decane),
13 Oxazolidine, 3-(dichloroacetyl)-2,2,5-
trimethyl,
14 Oxazolidine, 3-(dichloroacetyl)-;Z,2-
dimethyl,
15 Acetamide, 2,2-dichloro-N-(1,3-
dioxolan-2-yl-methyl)-N-2-
propenyl,
WO 92/ 10098 PCT/US91 /09267
~~ g 5g 24
-50-
Antidote No. Nomenclature
16 Ethanone, 2,2-dichloro-1-(1,2,3,4-
tetrahydro-1-methyl-2-
isoquinolinyl,
17 1,3-Dioxolane, 2-(dichloromethyl)-2-
methyl-,
18 5-Chloro-8-(cyanomethoxy)quinoline,
19 1-Methylhexyl-2-(5-chloro-8-quino-
linoxy)acetate,
20 o-(Methoxycarbonyl)-2-(8-quinolinoxy)-
acetamide oxime,
21 4-(Dichloroacetyl)-2,3-dihydro-3-
methyl-2H-1,4-benzoxazine.
The following lists identify the herbicides
and co-herbicides used in tests, the data for which is
reported in the tables below:
Herbicide No. Nomenclature
1 5,5-Dimethyl-N-(2,6-dichloro-3-methyl-
phenyl)-1,2,4-triazolo[1,5-
a]pyrimidine-2-sulfonamide,
2 5-Methyl-N-(2,6-difluorophenyl)-1,2,4-
triazolo-[1,5-a]-pyrimidine-2-
sulfonamide,
3 5,7-Dimethyl-N-(2-nitrophenyl)-1,2,4-
triazolo-[1,5-a]-pyrimidine-2-
sulfonamide,
4 5,7-Dimethyl-N-[2-methoxy-6-(tri-
fluoromethyl)phenyl]-1,2,4-
triazolo-[1,5-a]-pyrimidine-2-
sulfonamide,
5 5-Methyl-7-ethoxy-N-(2,6-dichloro-
3-methylphenyl)-1,2,4-triazolo-
[1,5-a]-pyrimidine-2-sulfonamide,
6 N-(2,6-Difluorophenyl)-3-chloro-4,6-
dimethylimidazolo-[1,2-a]-
pyrimidine-2-sulfonamide,
WO 92/ 10098 ~ ~~" ~ ~ ~ ~' PCr/US91 /092fi7
-51-
Herbicide No., Nomenclature
7 N-(5,7-Dimethyl-1,2,4-triazolo-p1,5-
a]-pyrimidin-2-yl)-2,6-
dichlorophenyl sulfonamide,.
8 5-Fluoromethyl-7-methoxy-N-(2,6--di-
chlorophenyl)-1,2,4-triazolo-[1,5-
a]-pyrimidine-2-sulfonamide
,
9 5-Methoxy-7-fluoro-N-(2,6-difluoro-
phenyl)-1,2,4-triazolo-[1
~~-c]-
,
pyrimidine-2-sulfonamide.
Co-Herbicide L~omenclature
A 2-Chloro-N-(ethoxymethyl)-6'-ethyl-o-
acetotoluidide ("acetochlor")
,
B 2-Chloro-N-(2-methoxy-1-methylethyl)-
6'-ethyl-o-acetotoluidide
("metolachlor"),
C S-Ethyl-bis(2-methylpropyl)carba-
mothioate ("butylate"),
D S-ethyl dipropylcarbamothioate, (common
name "EPTC"),
E N,N-Dimethyl-N'-[4-(1-methylethyl)-
phenyl]urea, (common name
"isoproturon"),
F Benzoic acid, 2-[[[[[4,6-bis(difluoro-
methoxy)-2-pyrimidin-2-yl]amino]-
carbonyl]amino]sulfonyl]met,hyl
ester, (common name
"primisulfuron"),
G 2-Chloro-1',6'-diethyl-N-(methoxy-
methyl)acetanilide, (common. name
"alachlor"),
H 2-Chloro-1',6'-diethyl-N-(butoxy-
methyl)acetanilide, (common name
"butachlor").
WO 92/10098 PCT/US91/09267
-52-
In the foregoing lists, azolopyrimidine
sulfonamide No. 2 in combination with Antidote Nos. 9,
10, 12, 13, 15 and 18-20 and with or without Co-
herbicides A, B or C are particularly preferred.
Example 1
The following procedure shows interaction between
herbicide and antidote when both are incorporated in a
soil cover layer before emergence of crop and weed
species. Containers were filled and compacted with a
fumigated silt loam top soil to a depth of about 1.3 cm
from the top of the container. A first container was
designated as an untreated control, a second container
was designated as a herbicide control, and a third con-
tainer was designated as a herbicide + antidote test
container. Each of the containers was seeded with a
crop species. A measured amount of each herbicide
dispersed or dissolved in acetone or water was applied
to a measured quantity of soil. To this same quantity
of soil treated with herbicide, there was added a
measured amount of antidote dispersed or dissolved in
acetone or water. The quantity of soil treated with the
herbicide and antidote was thoroughly mixed to incor-
porate the herbicide and antidote in the soil uniformly.
The seed bed in the third container of soil was covered
with the soil treated with the herbicide and antidote
and the container was leveled. For each test series,
the seed beds of the first and second containers were
likewise covered by soil layers. The cover layer of the
first container was not treated with herbicide or
antidote. The cover layer of the second container had a
measured quantity of herbicide alone incorporated
therein. Each container received 0.6 mm of overhead
irrigation. The containers were then placed on a bench
in a greenhouse and sub-irrigated as required for the
duration of the test. Plant response was observed about
three weeks after initial treatment.
WO 92/10098 ~ PC'f/US91/092b~7
-53-
:Ln the tables below, the rates of appli-
cation of heck>icides and antidote3 are given in k.ilo-
grams/hectare and, as noted above, the following symbols
have the indicated meaning:
W = % Plant Inhibitian caused by combination
of herbicide a.nd antidote.
WC~ _ % Plant Inhibition caused by herbicide
alone.
L)ata reported in parentheses = % Safening
Effect.
( _) - WO - W X 100
WO
In this test, Antidote Nos. 1-12 were tested
against Herbicide Nos. 1.-5 in a plurality of crops,
i.e., corn, grain sorghum, soybeans, wheat and rice, in
the presence of the weeds, yellow foxtail and velwet-
leaf, having the symbols "YEFT" and "VELE", respec-
tively. Results are reported in Table 1.
WO 92/10098 PCT/US91/09267
-54-
0
a ~ ~ ~ _ _ _ _
W ~ a! ~ N V' ~ N
,a O O ~~ O N ~ O N
3 wn o ~n ~- ~n ~- o ~- w -- ,n --
a r~ ~ e~ e~ a'
3 ~ ~ N _
E _ _ _ _ _ _ _
w
W O O O N O O CD O
O ~ O ~ O " ~n ~ O v O ~- ~1 ~ O
3 vo u~ N ~ v' ~w vn
O O ~n O ~ ~
rf N .i _ _ _ _ _ _
W o O O ~ O O r o
w W n ~~ .1 rwo .i
W "'~ ui .. ,n ... p .. o ... o ... o ... ~ .. u~ ...
a
r1 ri N n-1 N
H
x
w o o m o
e~ r~ .-, ~
... ~ o ...
r ~ o O .-r --. ~ ov
W
u1 ~ O O ~ w O O O w
N ~ .~ .i .a ~n N
O
m W .i ~. .. r,
o .-r O O .-1 O O ~i O
E O ~- o -- o ~- o -- O ~ ~n ~- o ~ o ~-
ac m ao ao m r ao ao
x ~ wn O
W r N
~ ~ ~ ~ ~. ~. ~ ~
a ~ N ~ o N ~ u°,
o ~ o -- u, -- o .- o -- o .- o -- o .- o --
o ... 3 vo ~ N vo N .i r
0 0
"' .. ~ ,. ., ,. ... ~ ..
~ o 0 0 0 0 ~ o ~ o 0 0 o v o 0
N r1 r1 N rl .-1 r1
V' V' d' d' ~ ~ d'
N N N N N N N N
N N N N N N N N
1
N W
D n-i .i ~-i N N N N1 t~1
et t0 V' d' v0 d' ~ t0 d' ~0' ~D
E N Il1 r1 N t!1 rl N In ri N 1f1
N O O N O O N O O N O
O ri ri r1 ri .1 ~-1 r1 n-1 r1 ri ri
WO 92/ 10098 ~ 9 ~ ~ ~ PCT/LJS91 /092117
-55-
0
3
W
a -~ .- .=, ~~; .. ..~ ~
0 0 .~ a o ..,
0 0
3 ~ ~ ~ n ~ ~ ~ n
O
3
H
.... ... .. .... ....
m
O ~ O ~ O ~ o N
H
U _
w r
W _ _ _ O
~" ~ ~ O O O cOn ~ ~ O
W fx O -mn ~mn ... O ,- of r. O ..- O ~ O -- w ~.
O 3 ~ N ~ .1 .yn ,-i
Z
N
Z
3
H
W ,t .. .~. ,-. ... p n ... ...
W N d'?, O C? O p ~ p ~ y ~ V O O
O ~ 3 ~ ~ v' .-~ .-~ .-~ ~ ~ cO.i
Z
O
'~ H ~ 3
o-r W _
Eb,, Z v01 3 r O ago V n v n O m aOD ~ ~ aOD
H
y .,
O Z
.~ ...
O C! m ~ O ~ O Wn ~ O ~ ~ ~ O ~ ~ ~ O O
N ~ 3 N t~ f1 N t'1 1-1 ~-1
p~ 3 ... "' .'~ .. .-. .~ .. .~ .-.
S O °~' N O ~ O ~ O .-1
~"'1 N
d' V ~ V V' d' V Q
N N N N N N N N N
N N N N N N N N N
i
N W
H H
A O ~ ar ~ v uwn uwo vo
WI ~ wo .r wD ~ wo
.1 N V1 .1 N 1l1 ri N tt1
O N O O N O O N O
rl ri ri N r-1 N rl ri ~-1
WO PCT/US91/09267
92/10098
-5 6-
0
3
W .,
1 1
a d ~ M O~ . r ~
,
W rl rl f' N r1 f~1 ~ O O
O " O O ~ O O O ~ ~ O '-'
"' .. '.' "' r. r. '_'
M d' ~'1 N V t'1 N d Q' ~"f
O
_
3
_ _ _ _
F O
ts. _ A1 _ O O O W
W O O -~ O i!1 of .-1 N O O
O ~ O ut O O O O 1!1 O O ~-
~- ~ ~ ~ ...
N f~ f'1 N P~1 N ~ s! N
E' O
V 3 ~. ~. ..
W
U O O O O
~'' O
N .1 O ~ ~"r O 1D rl O O O
w
a o -- ,n o o ,n o o ,r, o u,--
-- -- .- -- - .- .. --
O 3 ~ ~ .~ M M .,
z
z
W o
w 3
... p .. ... .-.
~C n o ~ e~
W o o v v c v o
O o w O O m o v ~. o --
~ O in O
3 .~ r~ .-~ e~ .-i .~ N N .1
A
z
0
.1 E'
H W
dl m _ _ _ _
~' ~ O m O of O O v0 O O
O O ~- ui O O ~n O O w ui O --
- r ~
cn r ao ao t~ ao ao ao aC ao ao
3
E
a z
~,
~' o
z
o
~ -~ O O - O O - .-. -.
3
~t _ ~ ~ ~ ~
O
a O a
G D
aG
O 0 ~- o o O O O O O ~n ~n--
c9 ,- ~ ~-
t~ N eh .i ~ ef .1
~-
3
.. .. .... ~. e~ ~. .~ .... ~. .,
O O O O ~ O O o 0 0
O ...,~ p p O p p ,n O
.. ... ... r. .,. .. ...
d ~ v
N N N N N N N N N N
N N N N N N N N N N
~D !~ n r m O OD O~ O~ O~
O
W O ~ d' ~D ~ V' v0 Q
r1 N lf1 rl N 1!1 ~-1 N U1 .1
O N O O N O O N O O
r1 ~ r1 r1 r1 ri ri rl r1 r-1
W O
~ Z
WO 92/ 10098 ~ ~ PCT/US91 /092~i7
-57-
0
3
W
a -. N ~ .. ~. .,
o v .-, o ~ ~ o ; r,
~ m n o u, o -- o m
--
3 ~ rf r~ ~ ~ et ~ ~ M
O
3
_
E
w 'n N O v!f
W ~ O O V~ ut N O O O
Yi O ~- O ~f1 u1...O .. ~ O
.. ... ...
3 1f1 V' f'1 f'1 N ri h 10 N
E O
U 3
O r1 O o
a o 0 0 0 ~n o o ~n o -
-
3 Q N ~ ~'f N .-1
Z
H
Z
W O
3 -.
4
Er ...... O .. ... p
h h O ,..... p .H O
W ri u1 ri
O O O ~ ~ -i
~ u1....tn O O O ~ u1
.. .r
p 3 N ~-1 '~ V ri .-~
Z
O
.1 ~ 3
E'
W
,..~ ... ... ,-... ... .... .,.
~ O O
~ N ~ o n ~ v p g o
Z '
~" 3 a a a a ~ a a
" D o D D o o
E
t
x N
3
,..... p .-, ...,-, ... ... p
~
fx h 41 !~ t0 U1 ~ W
O U' O w u1 O anr O ~ O u1
~- .. ~' O ...
~! N v-i N ~'1 ni ~, 0
'~'
3
O
....~ ~. ~ .~ ....
O O O O O O O O
~n O O O O O ~n o O
.i .i ~ ~ .i .1
~s v a v~ v~ v v~ a
N N N N N N N N N
N N N N N N N N N
1
H W
E~ O O O
(~
~ H H W -1 .-1 .1 N N N
O
C7 .-~ .1 .i .1
Z
W V' ~O d' ~0' v0 d' W D d'
I N lf1 ri N ll1 r1 N U'1 H
N O O N O O N O O
G.'
e~ r-i .-1 n-1 r1 rl '-1 r-1 .-1
W
x z
WO 92/10098 PCT/US91/09267
~ _5s_
3 p
a ~ ri
o
W
a ~ o
0 0 o v v .-~ .-,
0 0 0 0 0 0 ~n .-
--
3 ao ul rw c ~ m n
3 er
, ~
p
H _ _
G4 u1 M
W O O N O t~ f~1 O
O 1!1 U1 U1 O Qv
~ ~ ~
3 ov ov ~ aw o a~ o,
U
_
W
W O
_ _ _ _ _ ~
N O O O In O O
a o - u, o o o o
- - .- .- --
3 ov ~ .1 av M ov
Z
H
z
N r1
G4
WC ~ O
W O O O ~ O O
d1 u1 O ~f1 O O O O
. ~
.
P t'1 rl ~O N fD
O N
Il1 O 1f1 N
r
...
V' M ~O M
i~ O = = v O .1
b ~ W , ~ . '~'
~
o . u
e~ i
H
o
x
~
o o a~ o
x
~, x o a~ ~o
~
o .-, ~ _ ., ..
o 0 0 0 o 0 0
~
0 ~ 0
vm- o o o~ o a~ ~ o
3
o o o o
.i .i .~ r.,
0 o o
_ _
n M .-~
_ _
o 0
.-. o el o~ o w
0 0 .-, wo .1 M
o p o o o
-. ..- -.-
ao M v .1
v ~r ~ a~ ~ sr
N N N N N N N
N N N N N N N
1
H
6~7
rl r-1 .-1 N
A
N N M
N 47 P N O P N W f~ N
ri N O ~ N O r1 N O
.-1 O O 1 O O ~ O O .-1
a
N N N N N N N N N N
w o
x z
WO 92/10098
PCT/ US91 /092117
-59-
0
3
W
a o -. -. -- - -- ~. ._ ,:,
0 0 0 0 0 0 0 ~ m
~n.- ~n o o w r, o o - o -- ~n
3 ~ ~ ao .n u~ w o sr w o
O
3
_
H
_
G4 u1
W O N O O O O O O O c7
am ~- w -.-~ o~~.-u~....... ~ ,n.r ,n....O
-.~ ... ,.,.
3 ao v o~ o~ n ~o o~ 00
.i .,
H O
U
3
W W O
' ~
O ~ o 0 0 0 0 0 o c7
W
a o o-- o o o ~n o o u, o.
V, 3 st O CO W Ow D ov n
z
z
W o
w 3
H ~ .
~. .. .
,~ ~t ... .,...~ ... ~
en W O ~ O O O C O ~ O
~t1 O of O v O O O O in~ O
. ~ ~
3 v .1 aD n ~ .-w o m
x
0
_ _ _
4G ri ~ ~ ~ ~ ~. P1 r1
H '~ ~ O v O ~ O r1 c
O ~ O O O m O O n of...,n
~ y
cn .r ~ aw o w w o w o u~
3
x
H
O
O O O O O O O O O
~ n ~ O
3e: o o o o O ~ n o 0
0 o O o o 0 0
.1 .~ .i .i .i ,1 r,
y . o ... ... ..,.vo o o ~....
In 0 O 0 ino v ~n .-~ o c~
~ O o~ m
--
~ n O ...,n.~ ,~
N 0~ O~ d' d' ... ~ t'1
.-1
t ~ ~ v v ar v ~ a v
N N N N N N N N N N
N CV N N N N N N N N
I
~'1 ~'f V' d ~ Il1 ll1 tf1 ~O vD
W m n N ~ n N m n N W
N O -~ N O .1 N O .i N
O O .i O O ..r O O .-i O
N N N N N N N N N N
I
x
Z
WO 92/ 10098 PCT/ US91 /09267
-60-
0
3
... ~
... p ... th p ~ ... ~ ...
o O :: o ~ - :W o o
m o m w O O O m n m
,
O er ~ r ~ e~ t~
O
3
_ _ _ _ _ _
F
_ _ _ _
fs m n O ~ O O O
(c~ P rl N If1 O Il1 1l1 O O O
in..-O w - O n .w n O -, o~~- O
... -w v r.
3 .-i a0 ~n ~ O w '1 e~1 O ov
o
U O
G J
r' " H O ~ O O In O O O O O
LY O - O O - O O - u1 O 00 O - u1
- - - - - ...
3 .1 av ~ 1 o w o ~ o~ o~
O
Z
H
2
O
3
~ ~ ~
W O O o v O O v n o 0
W w n O O O O w ui ~n
-
3 ~ .-i ~ ~ .~ ao v .w o ~r ..,
0
x
0
'' ~ ~
_ _ _ _
d m
H .~ ~ N ~ ~ ,~ .~ ~
'~ H
~ ~1 O O N O ~ .-1 ~O f~ O
0 0 -- m o - m o - o o o u,- u,
- - - - - --
v~ 3 v w o r~ ao t ~ w o v
x .-
o z
z '"'' ~ ~. -.
.,.... ~, ~ ...~
a 0 0 0 ;o o ~ ao 0 0 0
i~
~ g N ~ ~
3 a O ~ O O
o
o o 0
.1 .i .1
0 o o o 0 0
O ~ ~ O n O O ~ o o
o .,.~ of- O O ...O O p o - O
... .. ... - ... ..-
f'~) r1 N r1
er ~ ~ a~ ~ a a
N N N N N N N N N N
N N N N N N N N N N
H
~7
E~
) ~ 1~ 1~ c~ ~ fx1 47 Q~ O~ O~
O
Z
I~ N ~ l~ N ~ ~ N ~ t~
O ~ N O ~ N O ~ N O
O .-~ O O ~ O O 1 O O
N N N N N N N N N N
I
Z
WO 92/ 10098 ~ ~ ~ !'' PC1'/US91 /0926.7
-61-
0
3
W ,
a M o ao 0
W .1 ~ p .-i ~ .-v O O
O '- ~n O O ~n O in
-r ...
r d' t'~1 elf ~ rf ~O ~D d'
O
3
_ _
H
G4 .~ ~ N ... ... .~ ...
W O r- y' O O O CO O cf
O U1 W p ~. p Q p ._.
~ r .. ... r
3 a w o ei o~ ao vo o~ r
H O
U 3
W w ~.
_
w
H O O O ~!1 N O O O O
w
a ao .- o ,n o u, ,n o
... _. ., ... ~ ,.r Q "
O 3 a m n .-~ tr .1 .~ p p
,
Z
N
x
G" 3
H
w ,~ ., .. ~:, v
O O O O
x 'n N ~ ~ ~ ~ V
~
p 3 a e ~ ,~ N
o
x
0
'-rH ~
3
_ _ _
w
.a
a -~ rf ~ O o en o e~
D
x
O
s ~ ..,. .-,
ri
3
_
C~ v O O O O O O
~ p ~ p
v~ o ao vo o~ ao
r-
3
Q
.1 ~ .a
... .-, .-, p ... ...p
~ W''1 O ~'1
O O 1~O
'i
O O O U1 O O N O O
v
N N rl M N e'~ ,.i
~ v .r er .r .r ~ v ~ a~
as
H N N N N N N N N N
O
~
D
N N N N N N N N N
OI O O O .-i .-1 .1 N N N
x ~ .., .-, .-, .-, ~ ~, .,
W N O f~ N O P N O t~
I r-1 N O -1 N O -1 N O
.-1 O O .-.iO O '-1 O O
N N N N N N N N N
l
~
z
WO 92/ 10098 PCT/US91 /09267
_.
W
a
ao 0 0 0 0 0 0 0
y nn u, o ~n o o m
y lf d' tf O r1 r W O r7
O O w O
3 V' N N
H
_ _ _ _ _ _ _ _
G4
W O O O O O O O O
... O O O ~ O ~ O ...p
.. ... ... .~. ...
3 vo vo ~ ao ~ w o
E
V O w n u~
W 3 h r1
W ~ .,
N N O O ~ O O O O
W
m O O ~ ~ O ,~v O
.. ... ... ... .r
o y o m w o vo ~ av
z
z
w
o u, u, o
3 N N N
~ ~
O 1f1
W O ~ O O O ~ O O
sv O O O ~n O ~f1-. u1 u1
f~1 N N ~'1 f~1 r1 f~ N
z
N
''' E'' ~ ~ ~ O
3
ri
W
01 m ~ - -
m .., .-. . .-, ... , .~ ,
, ,
O
~ m ~0 W U7 h 1~1
/7
~
a
o an ui
p h t!1f'1
Z
~
x" ~ .. ... ~ ... ~ ~ ...
C' Q) O~ Uf N
p: O ~ N h ~f1 h O O
0 1f1 1f1 y. ~ ~ O r O ~ u7
U' .~.. .r .~. .r
V7 h V' N ~D N r1 O~ tD
',~
K1 1I1O
rf rl r1 ~
O
... .-. O .-. .-. ... .-.
O O .1 O O O O O
O O O It1 O tf7...~I1...
... .r v ~ ~
t0 N f'~1 N ~-1 ~ N
d' d' V' V ~ V d' d' ~?
N N N N N N N N N N N
N N N N N N N N N N N
I
N
W
.-1 ~-1 rl N N N ~'1 f'1
I
O
Z
W OD N G7 O N O O N (>0 OD N
I d' n-1N d' rl N tT ri N V' r1
'd'.1 O d' .~ O Q .1 O d' .1
m r1 c~1c~ ~1 r1 M c~1 r1 ~ r1 c~7
~
W 0
Z
WO 92/ 10098 ~ ~ PC1'/US91 /09267
-63
0
3
W
a -- -- .. ., ~. .. ..
O O ~ O o O O O O O
O u1 O O O m tW n o ,n .r O r.
3 N h r'f N t0 U1 N ~D ~0' ct
H
fa, .~~.. ~. ... ~. .....
W O O O O O O O O O O
i~ ~n u1 O O M
O tn of O w
3 N w o r) n e) ew o w
H
U
Ww
V ~ ~ ~. ..
~ n ~ .
rr d o 0 0 ~ p d
~ ~ ~ ~ '~ u~ ,~ ~ '-
~
O
3 a ,
D
x
z
3
H
~ .,. .~ .-, _ ~ ~.
W O O O O O _ vn O O N
O
,
O tn tn tn p o .,.
O 3 N Ifl r'1 N A1 N ~ f'~1 N
Z
.i ~ ~
H
_
m
ad
H ~ O O v O O N O
O u1 O O ~1 O O O ~ O ...
tn er ov tw o ao t~ u~ ao vo m
3
a x ~
o z
o
~ H ~ .., .....
3
~ ~
a
O O O ~ ~o
o -- o~ a o o - o ~n u, - r,
cn r~ o o~ m ~o N ~ ,~ ,o
-
0
_.~ ~ .. ~ ~ p
0 0 0 o c ~ o
0
' ~ ~ o 0 0 o - o r.
'
~ . t0 N
o ,
,
V ~ V' ~ d' ~ ~ d' d'
N N N N N N N N N N
N N N N N N N N N N
I
N (s7
H H
r) ~ a' of u~ tn w 0 vD vO
O Ca N fD O N p p N p
N d' ~1 N d ~-i N ~ ,1 N
o ~ ~-, o a~ '., o a .~ o
r1 r1 ~ M rW 1 r1 N M ~7
w O
m z
WO 92/10098 PCT/US91/09267
~64-
0
3
W ,_,
t'~1 .~, .~.!I1 ... .~.... .. .~. N
0 0 ~ O o ao o o
O w o m O O w o o w
3 V' r1 N ~ N r1 tf1 f'W -1 f"1
O
3 p
H ... ... ...
t., ~ O w o w
W O O O -t N N O O rl N
...tl1 O If1 lf1 O O O
r ~ ...
3 d' ~fl N t'1 N .-1 d' ~' tn
H O
U 3
W W
' ' ~ o o o 0 o o o O o o
w a o ,n ,n.. u, o ", _.u, o o o
,. r. .- r. .r _. ~ _.
O 3 0~ ~ .r ap vo N h vo v ao
Z
N
z
W O
w 3
_ _ _ _
d O ~n O w
W o O ~ ~ o o o O
x ~n O in O O of of O O ~n
.
,~ ~ N N rt ~1 N r1 P'1 N1 N ~"1
v
H ~ 3
01 m m ~. .~ .~ ~ .... ea .~ .~ n
r. ~ ° ;. ~. ° V ~~ o
b x o o ~n ~n o ~n o ~mn o -- o
H V~ W vo vo W vo ~ 47 vD N CD
D Z
O
ao
a o '' ~ ' ~ v o o
~
O ~n , O O O O O ~n O O
C9 O
h e~f .-1 V' N .~-1 h ~ h
O
... .. p~ .-. .~. h n1O --
O O N O O v v -i O
O If1 O Il1 O O u1 O O ll1
r
N rl rl N N rl r1 .1 N
d' ~ d ~' d ~ V ~ V' d'
N N N N N N N N N N
N N N N N N N N N N
t
N
w
H
H
h h h a0 CD cD o~ o~ T O
D
Z
N ~ ~ N (D ~ N ~ CO
~-i N tT ~ N d' ~ N tT
a .i o a' .-~ o ~ .~ o a
~ rt r1 r1 r1 r1 nt r1 M eh
,
x
w O
x z
WO 92/10098 PCT/US91/092G7
-65-
0
3
W
a
0 0 0 0 0 0 0 0
u~ ~n ~n u~ o u, o u,
3 N N v0 N N vD ~'~1 .H
O
N
G'~ ~ w O m
W O N O O v O O N
i~ O ~- ~ u~ u~ O m O ui
~-
f') rl L~1 N .-1 h f1 .-1
O
3
_
W
_ _ _
U
H O r1 O O O O O O
a ~,.- o u, ,~ o - ,n o o
.- -- .- -- .- --
3 ~ .-~ ao vo N m h
O
3
_ _ _
E~
0 0 0 o
w yn o v V o 0 0
N ~ ~ N ~ ~
3 ~ n
.r
'i H
H W _ _ _ _
m v~ .~ .., o, o
N 3
z -.
,T, H ~ o
t9 ~ O - aD .-i .-,
~
~
~ Nv 0 ~ ~
3 a r
o
O
.. .. Q,
O O N O O .-~ O o
m o ~ o o O w n
--
.-i .-~ N N .-~ ~ ~ .1
a' ~ ~e a d ~ d
N N N N N N N N
N N N N N N N N
I
H
(~H
O O
G H H H ri r1 H
z
0~7 N ~ ~ N ~ ~ N
.1 N ~' .~ N d' .-1 N
O d' .-1 O ~ r1 O
m
, r1 t"1 r1 t'~ r1 t'1 A1 t~
a
w o
x z
WO PCT/US91/09267
92/10098
6
Q ~ ~
~
3
~ N
W .-.
0 0 0 0 o o o
~ 0 0 0 ~ ~ ~
', y 0 N r1 ~O N .-1 d
3 N
~ ~
_ _ _
~
vo r
W O d O O ~1 O
>. eo w n o u~ O ,n
-- m - .- ~- r.
3 ov eh N W d ~ r
H ~ ~ N
U 3
W
W ,-. .~ ~. ...
w
c W n aD a
r-~ O O N e1 d O O r
W aG O 1n 1n O O O u1 O
- ~- r --
O 3 r rf .1 d N N eG
Z
N
m O w
w 3 r m
... ~.
O O O O O O
W ~ ~ ~ ~ r
en ,
O O m n ow n O w
t0 N r1 d .1 .-1 D
Z
O
u1 O O
,.., E, ~ r d en
3
CO W
... .., ~. ... .., .~ ...
'"a ""'~ am, O o O O O O r O
'.
'~ "
o ~ o a ~ d r
o o o
'a 7C o u, u,
--
O~ N
... ..
... ~o o ... ~. " ... ._
0 r ~ o ~ o
o o o ~n ~n o
.
v~ o ao N o a~ ~ a~ o~
--
o o
.i
o wn o
O ~ d
0 0 0 0~ d o O~ d
~n o o ,n O
O ..- .~
'
d O~ N O~ N
3 O
d d d d d d d d
N N N N N N N N
N N N N N N N N
I
N
W
D .1 e1 .-1 N N N r"1 ~1
00 d ~ m d 1 O d '1 t~ d
W .-1O O .-~ O O .-1 O O .-1 O
O O O O O O O O O O O
O O O O O O O O O O O
W d d d d d d' d d d d V'
I
~
Z
WO 92/10098 ~ "~ ,~ PC~f'/US91/0926~7
-67-
0
3
~
a _ .. ~, ~ ~, .,. .. ...._
,
W O O N O ~ O O O O O
O p ~ ~ N p ~ ~ " p ~' ~
" - " "' "' " "' ~'
3 .W D .-1 .W '1 N .-r t0 N1 .-1
O
3
_ _
~
y n ao . o
W ' O o O o v o .-i o w
y n o m O u~ O O O - O o
--
0, r N a~ d e~ p ao .-r
O
_ _
3
fs7 o O
U O t'1 u~ ~ ~ O
H .1 aD ~ N O r .-i O O O
a o o o ~n ~n o o ~n- ~n-- o
- - - - - - - -
W D N .-I ~O ~i ~O t'1 N
3
_ _ _ _ _ _
H
_ _ _ _
r1 tf O O O O
r~ . wn O vo r O vo d O
,n ,n C, p w m p .. o ...,n
.. .. - .. - ... ~.
w o N N ~ .-~ .-~ r en
v
H
,,., ~ 3
d m pp ~ ~ ,. ~ e~~ ~. r ~. .. r~~
H >1 0 10 O O ~~ O .~1 O O r1
O O ~- O ~.' O "' ~ '~' ~ " p " ~ r' p - O " O
N 3 d r d ~'1 vo ~! N aD r N
Z ~
O Z
Z H
O ~ O O yD O O ~O
1 ~ p ~ p ~ ~ o
3 a a
o o
~. N ~. .. ~. ~.
Q O O O d N O O O O
O O o p o ~ ~
- - "" "' "' '' - ~ o
'-'
U o r rt ov M ,.i ~
d d d d d d d ~! d d
N N N N N N N N N N
N N N N N N N N N N
1
H
W
H
E
A )~ ~ d d w w ~o vD v0
Z
.-1 Op d H ~ d r1 O d .1
W O ~ O O ~ O O .-'1O O
O O O o O O O O O o
O O O o O O O O o 0
O d d d d d d d d d d
l
z
s
WO 92/10098 PCf/US91/09267
-f,$_
0
3
W
_
a ~ a0 u~
W ~ O OO O O r1 N O O
1f1 O '~'1n ~In N O ~ ~ O ...O
..r r r. .r ... .,.
r1 N .-1 of t~1 N N .-1 w D
O
3
_ __ _
H
_ _
w ~ r m~ o .-i
W ~ r Nr1 ~ O O ~ O O
O U1 ~ M ~O O U1 01 U7
~ ~ ~.
O ri .-i ~O ~ N 01 d' N Q~
H O
U 3
W ~ ~ ~ ~ .-. ...
fr. U -- o w n... mn ... r m
W
H O N NO ~ N O In N O
W
fx O r u1 ~ In vO .r O in O tn In ._.,n
v v r
r N .~ r N .1 r .-~ .- w o
x
H
x
W o
w 3
_ _ _
o rn o r o
W ~ ~ o
O ~ O m ..u1 O u1 O O ui O
3 r t~f ~-1 v0 N ~-1 ~p N H r
v
'1 E' ~ 3
d ai pa'~p ~ .. ~ ~ ,,
'~ ~r O O v O O v O O O o
O o ~n ~n O wn O O wn
v1 3 co vo N ao ~ N co m M r
D Z O
H 3
C9 -- .i O -. r1 O -. .1 O
~
a ~ v v ~ v ' o v ~0 0
d
~ uw n in ~n in o - w o
o - -
O
c~ ov r .1 0~ u~ .1 O r .-~ o
3
O O
O _ _ _ _ _
~ N ~ .~. CO ~ ~ r ~ Q~
a~ N O o~ r O V~ ~o O
p p p N ~ ...o p
... ... ... ... ... ...
O~ r1 N O~ .-1 u7 .-1 H v0
d' ~ ~ V' d' ~ V' V' V'
N N N N N N N N N N
N N N N N N N N N N
HW
r r r o7 a0 ap o~ O~ o~ O
D
O ~ rl O e! H m ~' .~ O
r-1 O O r1 O O 1 O O .-1
O O O O O O O O O O
O O O O O O O O O O
d' d' d' V' ~f V' V ~ ~' tf
I
x
Z
WO 92/ 10098 PCr/US91 /092ti7
-69-
0
3
W
_ _ _ _
a ~n
W O O O O O N O O
.. ... ..... ~. ._. .. r,.
N ~1 ~' N N N ~7 N
3
_ _ _ _ _ _
E
G4 N tl1 .-1N 1(1f'~
~
iW l1 1~1 O M 1f1~ O O
...
N .-1 Q N n-1 r1 A1 N
E O
U
... ..
w U rt .,. .-~... ...
w ~
H v O ~ O O y . p
-i
a o o ul- u~ o u,
v
V, ~ N N V' M A1 V' r1 N
Z
h~
x
0
~'' 3
~C E .~ ....... ... ..., ...
V1 .~ ..,
W ~ a ~
O O ao
O m u w n
~ ~n
3 N rl ~ N N n-1 rl
p
2
O
fr
'-' ~ 3
f, w
m -- r e~ ~i .. ~ ...
ca a~ o ~ v .~ o o .~ o
z
. ~ ~ ~ ~ ~ n ~
ro '
z
3 , ~ n
E ,
Z ..
a z o
rr O
3
a :
~
a ~ o o o vi,
~
0 ~ n Q d pV Q
~ v
v O a O
1 O
3
O O
O _ _ _ _
a a' ao -. o r .-.
~ O ~ = o : ~ O
S ul O . O O o . O
m _. .
m
N v-i 10 rl V' ri
~r at a' a .r ~ ~ a
N N N N N N N N
N N N N N N N N
1
N [i~
E E
Z O O O m1 rl 'i N N N
OI
~ O .w .1 .-i .i ~ ri .~ .-i
Z
rl O Q '-1 CD V' .-~
O O O O O O O O
O O O O O O O O
V~ V' ~ ~T cf
W O
Z
WO 92/ PCT/US91
10098 /09267
-70-
0 0 o ui
3 r vo N
W
_ _ _ _ _ _ _
a o
W O O N O O O O O
O ,n'-'p ~ "' ~ p r' p
'-' "' r' '.'
3 0~ ao N m ~ ~ o~
p o ~
a a c
o o :i
o o
_ _ _ _ _
G4 f'1O O
W O ~ ~ O vo .i O ~o
o o -- o ,~-- ,n o ~- ,n ,n--
-- r- ~- --
3 0~ c~ ao r o~ c~
E, w O m
U 3 n ~o N
W W ..
U O O
N O O ~O O O d' O O
W
p; p p .,.p p ...~ ~ ...~
.,. ... ... ...
O 3 ao c~ ~ aD ~u .a r ~o
x
N
x
W ~ o
3 a
G4 o
H
_ _
W 10 O ~O O O O O
dv ~n O ~n O O of O O
3 m a0 .~ o~ T rf T O
0
x
O O
m m m
r, w
d m .~ ..... .. ~.
m
'"'. o 0 0 0 0 o 0 o
a
N ~ ~
3 a0 aD c0 0~ O~ GD
a
' ~ o
x
o --
p
.. ~ ..
0 ~ ~
~
a o o o o 0 0
4
O o ~n o ul o ,n u~ ~n--
c~ -- --
m ao ~ ov n N aw n
--
3
m an
~l1N .-~1 '~' .-
O
~
O O 01 O O Q~ N
~!f p ...O O v of an...O p ...
~ v ...
M P1 1I1 N r1 ~f1 N
d' d' Q t? ~ ~ d
N N N N N N N N
N N N N N N N N
1
N
W
rl r1 rl N N N t'1 c'1
EC
A
x
N O f~ N ~ P N 41 I~ N Cp
rl N O ~ N O ~ N O ~ N
r1 O O .~ O O -i O O ~ O
m If11f1tf1 If1 ~fW f1 Il1 1!1 1l1 11W f'f
l
z
~
WO 92/10098 ~ PC'1/US91/09267
-71
0
3
W
a i~ -. o ~ o
0 o V o -r o o c;
o 0 0 0 u1 0 v o ~n o
~ -- o
3 N o~ r N r u1 N ao ,o r.,
O
_
3
_ _
Ea O
G4 c7 -- ~ O p
W N O -1 .-~ O O d O vo c'
Y~ o u1 O O O O ~n o u1- p
~ r.- ~ ~ ~ .- ...
3 N a1 r ao ao .~ a~ r ~r
W .~. ~. O ... ..
U O - mn O r u~ O O'
H V' O N .-1 N r .-~ O O vD
CY ~f1 !1 tlf O 1lf 1ff O ~!1 O O
-W ~ ~ .. ~ ...
3 .-~ r d u1 .i r .n .-,
O
3
(~ .. ..,
', .~ ... .., f'~1 N O ~ r-1A1
w a o o ~o N v
v v
~ V
3 d 01 W r1 d N 0~ u1 .-,
v
..i ~ 3
H
"' ~ a o 0 0 ;u o 0 0 0 0
0 0 0. 0 0 0 ~n o 0 0 0
E.b., H m 3 ao 01 0~ are aD aD ao ov ao ao
D Z O
~ H 3
O O O O V O O O O o
O C~ O u1 tr1 ~l1 O C O O u1 O
~
VJ .1 O~ d ~~f u1 d N Ov r
~
3
O
O
... .-. p ui O e1 .~. ....
~ ~ ~ ' ~ ~ O o
u1 u1 O O u1 u1 O O O ~
~-
n-1 If1 P1 N H ri 1I1 N n-1
d d d d d d d d d V
N N N N N N N N N N
N N N N N N N N N N
I
H W
H
I ~ d d d w u1 uw o vo ~D
A Z
r N CC r N ~ r N Cb r
O H N O r1 N O r1 N O
O .1 O O .-I O O .-i O O
W
!1 u1 111 Lf1 tf1 It1 U1 Il1 tt1 1f1
S Z
WO 92/ 10098 PCT/ US91 /09267
~~ ~ !9 ~ ~ _~2_
0
3
W ;
_
a .. ~.. .. .. ., ~ , ., ...
~
~ =
o o u, m o w n w u, o
tb N r ~O N m r'1 N
O
_ _
E O O
_ _ _
r o o~ o r~ o ~,
W O O ~ O ~ ~ O ~ V'
y, ,n O O O ,n p ,n O .. ,n ..O .r
... ... ... r. .. ... ...
3 ao ao au vo ov r .~ r
H O
W 3
W .~. ... .. .~ p ...
fs. U O t'~1 O n1 O r7
~r O o vo .i O vo O r'1
W OG u~ m O ui u~ O O O ...O .-.m ..
... .. .. ... ... ... ,.
O 3 r vo .r vo vo .-~ ao w o
x
N
x
W O
G4 ....
3
"~ H O
O w .1 r~
W O o v o v0 ' O " W O
O O O O an O w n -- w n
-
~ 3 r ao o~ r el ow .-i m
p
x
0
.1
H
r-r W
O O O O O O O O ~o O
p ~ ,n O ,n in p O O ...,n ...,n ...
... .. .,. r. ... ... ...
v~ ao ao ao ao ao ao o, ao r ao
3
H
a
~ z
o
i
a 0 0 0 0 0 0 0 0
d
o CW n ,~ ,n o ,n ,n o ,n.r O .r,n .r
-- - - ~ ... -- .r
v1 aD r .~ o~ ~ 1 0~ ~ r
--
3
3
_ _ _ _ _ _ _ _
~ ~
O o O O O O O O
W t!1 O O O y ~n N .. p .~.~n ..
.. .r .. .. ..
y f1 N ~ ~'1 .-1 Iff ~'~1 vf'f
V V' V V' ~ ~ d' ~ d
N N N N N N N N N N
N N N N N N N N N N
1
H H
) r r r ao aD a0 0~ o~ a O
O Z
N ~ r N ~ r N ~ r N
e-1 N O rl N O ~ N O r-I
ri O O i O O i O O 1
a
1f1 It1 II1 U7 ~f1 1f7 V1 1I1 UI tf7
w o
x x
WO 92/10098 ~ PCC/US91/092~07
_73_
0
3
W .~ ... ~
a _ ~
N O O w
W O O O
O
O O If1 O ~ ~ O
.r
3 vo st c0 ~1 N r1 ~ et
O
3
W .. .,.
f n ~ ~ ~ ..
O o o~
w ~ o 0
~ o
O-- o 0 o w u, ,n
3 cW O O~ d' .-1 N N
N O
U 3
W W
~ ~~
w .~ ~ ,.. ~
G4 ~ O r1 N ~
w H d' ~ o ~ ' o
a o .r, o u, ~ v ' o
~. ... ~ ~
Z 3 ~ .1 ao ~
H
Z
O
u'' 3
H ~
,~ ~. ,~ .. c Wn a, .'
= ~ ~ ~ ~ o
~ ~ ~ ~ o ~. v
um n
p 3 W ~o .r
Z
O
3
C! ~"~
m
_ _ _
n-1 m _
N
_
;p ~ o 0 o O O O O o
x ~ ~" ~' '~'
H 3 a a a a w a o o
o o o o o ~ o
x
~
~ o
z
eo x
a ., ~ ., .. ._ ~, ~ ~
~
o w ns u, 0 0 o V o 0
o .
fA " ~ N ~ O O
" ,
3 N rl C~ V1
O
_
o rf ... .~ ._, p .-.
n V o 0 o v o o
o o ui o
m n ,n o
N 1
. !n t') ~-1 ~ ~ N
d' ~! ~ d V' Q' ~ V'
N N N N N N N N
N N N N N N N N
I
N
W O O ~ .i N N
E
Er
?
O
O
a N
O .-I .-1 ri r1 n-1 ri r1 ,..I
N
N O rl N O .-
. I N O
O O .1 O O ~ O O
r-1
tt1 1n tI1 u1 V1 v!1 t11 u7
W O
x z
WO 92/10098 PCT/US91/09267
-74
Example 2
Following the same procedure described in Example
1, Antidote Nos. 1, 3-11 and 13-17 were tested for their
efficacy against Herbicide No. 6. The same crop and
weed species used in Example 1 were used in this test.
Results are reported in Table 2.
A AA
WO 92/10098 PCr/US91/092ti7
-75
C~ ~n O w O m O
.-in ~I n ~ n
E _
w
W N n
i~ O C' O O O O O O O ~- O O
3 n ao w w n n N
C w O w O u~ o
n .1 n 'r n
W _ _
n n
O C O O O O O O O O O
3 a v a ~ ~ u~ N
3 ~
~
W ~
w U rn e.~
~
o o O ~ O o ~ o O O o
G
O 3 ~ ~
,'
Z
N
z
O m in
3 ~ .-~ ,..,
3 o M r O O N O O O
O 3 ~ ~
2
O
N N ~ o 0 ~ o
3
v a ~ a a v
N ~ o o o
O
roz ~ 0 0 ~ 0 0 0 0 0 0 0
3 ~ ~ ~0
N
a
N T N 01 N O~ N
_
O
w ~ ~ O ~ T O er o
3 c ~ p
,
o u~ o ~n o m
O N ~D N t0 N ~O N
~
_ _ O
O O .~.
O
O
an O 1n O O O O O tn O O
n-1tY1O~ r1 N N n
a~ ~r v d ~ ~ ~ a er
N N N N N N N N N N N
N N N N N N N N N N N
I
N
W
E
I ~ ~ d~ O~ O~ o~ r1 r1 r1 n n
O
Z
W n 0.7N n O N n GO N n CO
N r1 O N ri O N rl O N
O O .1 O O -~ O O ~-1 O O
CL
~D u7 ~D ~D t0 ~D t0 ~D ~O ~D ~D
W O
Z
WO 92/10098 PCT/US91/09267
-76-
o ~, o ~, o ~ o ~,
.
3 r -i r .-1 r ~ r
_ _ _
H
G O d' o
Q.
W - ~
. O o o O O O o O o o o
a ~
r rw o w n w o
3 ~
r ~ ~ r
W
a o ~ r
W .,
0 0 0 0 0 0 0 0 0 0 o
vo r~ ~ t~1 V' d' u1
H
V 3 ~ v
W
.. ,.. ...
w y
n O w
H O N 1I1 N
W
fx o O O o O O O o 0 0 o
O 3 ~ eh N
Z
H
Z
W
w 3
W ~ O o
O O N O O O O O O O
N
O
x
Q
N O d' m ~ m ~ O
QI W
m
r-1 m ~ ~ ~ .~
h~
ro o o o o ~n o o ~n o 0 0 o
x -
v~ ao ~ ao v ao m ao
3
E
H
C~ N O~ N 0~ N C~
.. .. ...
p4 O O O N rl rl
~ O O O O ~ o
tn O~ N 0~ N m V
3 '
1l1 O 1f1 O 1l1 O lli
Q ~0 N ~D N ~D N ~D
_ _ _ ~ _
... O N O ~ O tf
O .-1 ~O ~I1 O ~ Q~
u1 O O u1 O O In O O in O
~o N ~ ~o
d ~
N N N N N N N N N N N
N N N N N N N N N N N
1
N W
E
r .-v.~ ,.'~,~i~ m ~ ~ r1 r1
p
W N r lD N r O N r m N r
ri O N rl O N ~ O N rl O
.-1 O O .~ O O ~ O O ri O
~p ~O ~O ~O t0 ~D ~D ~D ~O ~D ~D
0.~'
WO
x x
WO 92/10098 ~ ~ PCr/US91/09267
-77-
O ~ O O ,n
~ r
t., n t~
W N O et r
O u1 O O O O O O ~-
- ~ O
3 N u~ .1 ~ ,.~ r
O O w O m
O w
3 .1 r ~ r .1 r
w
a v n
~ ~
~ ~ O O O O O w -
~- -
O
3 ~ N ~D
3
w
U a0 ~p
N
w ~
a o N o o an 0 0 0 o
3
x
x
w
t" 3
~ ~
E o
w ~ p
w
O O O ~- O o O o
O
x
O
N
N
Z d' W tf 4~ ~f m
H
CI
CD
.-1 ~ ..~.
1-1
o
ro 0 ~ 0 0 0 ~ O p
z
E. v o
, 13 0
a
p, 5E w y u, uw n
~
5 N 01 N O~ N O~
Z
O
~ .. ~. ~ ..
H
3
y o n
O o ~ O
o ui O In
3 N ~o N ~ N
~
~ o
o O ui
r ~ ~o
o o o m n o o ~n o
-
N .-1 N N
v .t ~ a ~ ~ v ~ ~ a a~
N N N N N N N N N N N N
N N N N N N N N N N N N
I
N
w
H
H
OI d' ~ N N N W O W tf1If1 t!1~p
O n-1 rl ~1 ri ~-1r-1
z
N r a7 N r ~ N r W N r
N .1 O N .1 O N .-1 O N rl O
o 1 o a ~ o o ~ o o r, o
~ ~o ~o ~o .o ~c ~c ~o ~o ~o ~o ~o
,
x
w o
s z
WO 92/ 10098 PCT/US91 /09267
_7s_
0 0 ~ a ~ o ~ o
3 ri h n-i h ri h ~-i h
~
H O _
_ _ _
Gta O d
W r1 Y~1 h h h
O O O O O O O O O O O
-- -r ~ -~ --
If1 d h N h N h
O O O
h h h h
W
a
a o ~ o
;
0 0 0 0 0 0 0 0 0 0 0
.1 M d ~(1 N v0 N 111
U
3
W
W ... ... ... ...
'
U O O u1 O
W
r .r v
a o .n o o u, 0 0 0 0 0 0
-
V, ,'~ N N N ~"1 N
Z
N
Z
N
3 .i .i .1 .~-~
o
H _ _ _
W ~ O d d d
~ ~
O O O O O O O O O O o
D
Z
O
N d 07 d 07 d W d OD
~
~r O O O O
O O O
W l1 Q) ~O W t0 ~D t~1
W
x N C~ N O~ N 01 N O~
N
O
, _ _ _ _
3
~ N 10 v0 O .-.
QS O N rl r-1 N v0
O u1 of O O O O O O O O O
(~ .- -r -- --
f/1 N h ~D ~ U1 a1 N T
',~
O 1f1 O If1 O 1I1 O u1
N ~D N ~D N ~D N ~D
~ ~
O _ _ _ O _
O N vf1 U1 O d
~-i ~D N W N ~ .-1 N
O u1 O an O O u1 O O O O
- r ~ ..
N rl t0 r1 10 t!1
d d d d d d d d d d d
N N N N N N N N N N N
N N N N N N N N N N N
H
W
E
H
~ ~ ~ ~ ~ W O vD .i ~
D .
Z -~
N h G1 N h CD N h ~ N
N ~ O N ri O N .-1 O N rl
O .~ O O 1 O O r-1 O O r1
W ~O ~O ~D ~D ~O ~D ~O ~D ~D t0 ~O
,
W 0
WO 92/ 10098 ~ ~ ~ PCr/US91 /09267
-79-
3
a r
E~
_
G4
W n
?~ o t7 O
3 r n
O ~ ~n
3 ' n
r
W
a
o c) o
3 ca n
H
V
W
W
~''
U w
N N
W
p: O C) O
O 3
x
N
x
W ~
3 '
.,
d E ~
d
'p ~ O CI O
3 .-
3
O
x
0
N
N G O
H W b'
d m p
x 0 ~i m
H t
17
3
m u~
x m ov
H
~
..
a
~
i o
0 0 ~r~ o
0 - -
W Ni ao
--
3
Cw n
_ ~o
Ni
p .-,
Z o
O
p p C p
.,. ...
a a'
N Ni N
N N N
1
1-1 W
on. zl ° 0 0
~~ O N ~1
O O a-1
~O v0 ~D
x
WO 92/10098 PCT/US91/09267
-80-
Example 3
Additional tests were conducted according to
the procedure in Examples 1 and 2 in order to evaluate
the antidotal effect of various antidotes against
invention Herbicide No. 1 alone and in combination with
co-herbicides A, B and C. Also, combinations of these
co-herbicidal compounds and invention Herbicide No. 2
were also tested. In this test the crops were corn and
sorghum and eight (8) weeds having the following
identification by symbol:
MOLL - Morningglory
COBU - Cocklebur
VELE - Velvetleaf
BLNS - Black nightshade
YEFT - Yellow foxtail
YENS - Yellow nutsedge
BYGR - Barnyardgrass
SHCA - Shattercane
Results of this test are shown in Table 3.
WO ~ ~ ~
9
2/10098
PCT/US91/09267
-81-
o o o
o o Q ~
3
h vo ,p
x O O O
o ~ ~ N
v~ _
3
O O o
0 0
h U1 ~f1 r'1
a
1 ~ O o 0
a~0 '~ 'd' N
3
0 0 0 0
0 0 0
0 O o
.. .. r.
3
O O ~ m 0 ~:i
~ u t _
1
' W o ao
r1 O .1 N
3 ~ ~ o ; ...
"' " '-
E a
G4
I
W c0 O u1 O u1 u1m
O
s. a, v~ o, o. h ~ N
3
O O p
N O N
3 O O ~n c~
.. r. ... .-.
V, Q~ Q h
O O O
1
E ~ O 0 1!1N .1
3
U ~ ~
_ _
W
Gs, Il1 h
~ ~ O O
W O O tn m
rf N N .-1
O
w O O O w O O
2 U Il7v0 N n-1r1 _
3
W ~ .i
w O O rl
If) Ifl 1!1 C7
v
v~ 3 0~ Cn ao co
,
dP Z
1
"i m3 m ~ ~ ~ o
Ga o a a ~ ~ '
a , o o
. _ O
b ~
E O O
3 ~ ~
O W
a
1
E ~ 3 ~ '~ o un o an~n b
a7 ao r h voas N
N
~ ~ ~
G O O O - O
3 ao ao ao h
D
ro
V m aooaoo n ~ ~ovo
a ., ... ~. ...
0 0 0 0 '
ca
0 0 0 0
an 3 aD h h vv
a
0 0 0 0 0 0 ~n
,/ O h t0 ~O V' N 'i
'
, 'O
-.1
C
I
N
I~ O O O
) ~
O
Z
~' N ~O Q V~ h ~ ~? N VD Op
E N ~ t!'fN .-1 O O N .1 If1 CV
N ~ O O O O O N ri Q
Q
a
W 1 rl rl n-irl rirl rl .-1 ni ri
O
W
E
i
)
U
x
Z
t I I I I I I I I I a
WO 92/10098 PCT/US91/09267
~-82-..
4
O O O t~ ~O M O O O
O o O u1 u1 0 O o
.r ... t~1 11
N ~ ~ r1 r1 N
._.
U
O
3
O N
O O O N N O O O O
3 o 0 0 0 ~n o 0 0 0
--
N ~ N1 f'1 N N
a
C9
O
i
p _
p
3
et ~ O
O v ~ vp d.
~ ~
3 ao O ~ ~ o w n ~ O
~ "
Q, w n a~ o ao r r
.a
w
3
a. _ _
p ... ~ ao .i O .-. ... ...
O O ~ ~ ~ ~ O O
3 O O O O ,n ~ ~
~ .
v0 vD V~ ~ ~ m ~ a0 V'
1
3
O r1 O w ... ...
3 ~ ~ ~ r
.
O ~ ~ o 0
, a
-, o
3
E
U
W ... ... ... ... ...
O O W r'1
~ o O o ~ ~ O ~ 0
G3 0 O o ,~ ,n O o o
~. ~
~ .i .~ ~ e~ .-~ .1 .i
1
Z8
3
rr
_
Z .. m ~ .. .. .. .,
3
o v v o o o o o
p ~ ~ ~ ~ ~ ~ ~
a ~ ~o o. o~ o~ o~ o~ o~
1
a
3
G1 eno a
0
.i O
.r' ~. .. .~ .~. .. ~. .~ ....
' 0 0 o ao m
E W 0 0 0 0 0 m ~n O O ... .,
3 n w O ,n u~ ... ... ...
... .~ ...
Z w o vo o~ ov o~ t~ ac vo
~"~ '
a ro
w
m .,~ ... ... .. ... .-, ... .-,
'' c
= o
a ,n .n o u, o o o o m -
r ~n u~ o~ o~ o~ a~ o. ~o a~
U
E,~
U -..I
.r
.-. .~ ... .~ ... ... ~ CL
O O O O O O O O O LL
O w O O O vf1 O O O
av3 ui en ~ o~ ac ~ n
o
x w
...,
c
a
I
O O O N N N fV N N
e-1 r1 e-1 .-1 rl n-1 ri .-1 r1 #
o
G
O
rl O O N r1 1l1 N ~ O
E
O O O N 1 O O O o
OD
a ., ~.I ~.I ., .~ .a ~.I .~ .,
w
O
Z
m
I
a
I
Z 1 1 i
U 1 I I I I I
~
o o ~ o
o
WO
92/10098
PC r/US91/09267
_ 3_
0 0 0 0 0 0 0 0
3 o O o ~ p
~ o
,4 ~ ~ ..
,
U
O
1
m
3
~o O O O O O o O
o tn O O O tn ,n p
r r m ~ ,.1 ,.,
~'
o
~'
3
m
er ~. ~. ., .. ... ..
1 O O O O O rt
3
O O
O
m ~"~ of
Z .1 ,..,
W
H
~ o m o ~ . o o
i
E., O o O tn p p .. O O
a~ a~ o, ao ao r tn
W
O tn of O O en
c
3 a ~ ~ ~ ~ ' 0
O o o ~n o o o o o
v
O (D tf1 N .-1 ,-~
N
U
_ _
W
G -~ rf r tn rf
"
3 ; : ; ~ 0
W o . . , o o 0 o
u, o tn tn
~ 'i N e1 .1 n-1 .-1
Z
Z _
d ~ ' ' O O O O
,"~ rn a o~ ov o, o~ o, o r r
m
O
H a c 0 0 0 o ao o m
o ~ o 0 o
~
0 ~ o, a~ of a
o
a m
m
a o 0 0 0 o c"'i ao c
Z O U1 U1 tf1 lit y~ tit tf1
v v v
3
to o In l r to .r tn .a
U
... ~ .-a
O O O O O O _ O
O
N p O ~ ~ o
at a~ r tn v i m
a l
m
0
b
.,.,
c
I
*
N tT 0t tT pt pt
V' ~i N tD 41 V' r d'
O N 'i tl1 N ~-1 O O
O N .-1 O O O O O
m
~i ~-1 ri ni r1 ~1 ,t ,1
Z
m
Z)
I I I 1 I I 1 I
WO / PCT/US91
92 10098 /09267
-84-
o o o o o o o o o
3 O ~ ~ p 0 p p p p
n tl1 U1 V' N N Q~ Cb
U
O
x
3
o O O O O O O O O
O O O O ~ o o
o o o~ o~ o~ v~ o, 0 0
.i .d
O
~'
3
00
O O O O O O O O O
o O O O ~ ~ ~ p p
p~ O o
t/~ O o O O
~ .,
w
o O o O o O O o 0
H O O O o O O O o 0
.1 .i .~ .i .~ .~ .1 r,
O u~ .-i O ,.. ... .-. ...
,.-v c ~r O O O o .i
v O
O o in ' ui O w o 0 0~
- ~ r- ~
~n
a O w n w e~ .i .~ ~ av
E1
U
n ,-. ... ,-. .. an N
~o o O o O o 0
0 ~ ~ o an O o
0
N N N N r1 rl rl N ~'~1
O U
O O O O O O O O O
N O O
p~ O O O O ~ O ~O
a 0 ~ .~ .r .-r .-
ro w o
o
3
o
O
.Q ~ .... ~. ~. .. ...
ro O O O O O O O O O
E" to CD m O O O O CO e0
p,~ o~ o~ o~ o~ o~ O n o~ o~ d
O a
ro
w
a
0
O O O ~n of O o O O ..a
~ ~ ~
o~ o~ v v~ m ao n o~ a~
ro
a
w
0 0 0 0 0 0 0 0 0 '
ro
o ~ ~ p o p o N p
~ v v ~ v ~ v ~ ~
o~ ao ao ao w o m ao ao y
0
w
.a
a
N
~ O O O O O O O N N
rl rl rl rl rl ri rl rl ri
D
N ~o ao ar n ~ v N
N ..i of N .i O O N
N .~ O O O O O N
a' rl r1 ri ri ri r) ri ei ri
wo
d' d' d' d' d' d' ~'
ri ~ ri r-1 ri rl n-1 ri r1
O O O O O O O O O
~
U ~C ~C ~ fC ft ~C ~C ~ ~C
~
o ~ ~
~s o
WO 2/10098
9
PCT /US91/092.67
_ -85-
_ _ _
C: O O O O o 0 0 0
3 ~n o O ~n in O 0 0 0
d v0 vo ~ r1 N C~ p r r
U
O
x
3
c; o o 0 0 0 0 0 0
~ ~ ~ ~ o ~' o o ao
o~ o, o, a~ o o~ o
o o,
O ~ ~
m
0 0 0 0 ~" ~ "'
O o o
o- ~ ~ o
O p O
O p O o
,., ,"~ .~ ~ .~ ~.
0 0 0 0 0 0 0
O ~ O O O
,
O O O r
.i .i .-~ .-~ .1 .~ .r
i~
O~ O O O O O
O O
a o ~ ~ O vm n o
.r
m a ~ o~ ~n v
o o
0
U
..
''3 O O o O O v ,~ N
o
o of in o o o m in in
N rl rl N N .H .-1
N
~r
O O O O G O O C O
x ~ o ~ 0 0 0 0 0 0
e.~ O O O O O O
~ ~
m ~
O
~ ~ ~ ~ O O C -.~
o ~ O ~ O ~ ~
O b0 0~ 0~ Q~ ~ m O~ ri 0~ O~ m
a "
m
.. o
0
0 0 0 0 0 0 0 0 0
an o o ~m n ~w in O -.a
o~ a~ ao r r ao ao ao o,
.r
..
~ ~ ~ ~ O 0 0
O o O O O O ~ o o
a~ ao ao r m ao ao a a
o o
m
a
0
c
d
i
~
H N N N N N 0~ pv p~ p~ t
Iff N rl O O N rl 1f1 N
O O O O O N ~-1 O O
ri r~ ri ri '1 ri ri '-1
d' V' V' d' d' d' d' d'
r-1 r-1 r1 r1 r1 r1 r1 .1 rW
O O O O O O O O O
U
~
~
d d d d d d d d d
~~~~5~ 20
WO 92/10098- ~ PCT/US91/09267
-86-
0 0 0
O ,. u, w .r
--
U
O
S
~
N
O O O
,n o -.
...
o~ o, o
0
3
m
0 o O
O ~ ~
0
W
o o O
o ... ~ ...
E .r p
p O p
~,
~
O
N O O
O -~ ~n O
-~
N ~1 N
0
U
O O O
0 v ~ p v
v
N
O O O
v ~ v
C~
a
m m ~o
w
.. ., .. o
' 0 0 0
O v Il1 p 'r
~
p4 O~ O P ..1
O a
m
a
w
0 0 o m
o m o
o~ e~ ao
b
0
.a
w
= .. a
o o ..
- o
~o m m
''' o~ o~ v~
p
0 0
0 0 0
~ r,
.i
r, ~ .1
0 0 0
ao
~
U ~ ~ ~C
x
9 2
p
WO
92/10098
P~ T/US91/09267
rn r; ~ ao .;
m = o o
= ~
. a
D
g ~ g
a a
o o
0 o v. rn o
o o -- ~ ... ...
.-. ...
o ., r.,
0 0 ~n ao 0 0
0 0 0~ o~ o~ 00
.~ r,
0 0 0
O O O o~ ~n
~ ' c.
~ O O a ~ ''~ o ..
i
w o o
.., .1 ..~
0 0
O o
O O
v
Cr, O C ri .-1 Q~
p ~ p
r1 ri ri ei
~ O O
O O
O p ~ p
~
N O D O O O O
rl ri ri ri '-1 .-1
U
..
O O op
C7
an ~,n O O O m
~1 .:V 0) N ~ r1
.. ..
0 o o o o
0 ~ ~ ~ ~
o v~ v~ o~ o~
N
41
O~ iT O~ 0) 0t 0v N
.G
_ _
O v O O CD
tf1 O Il1
~ ...
0 ~9 O~ ~ ~ ~ ~ y
a
~n ~~ o u, a7 0
o~ ~T aD n o~ ap
.. .. C
' ~ ~
in O O of
ao o~ ao n ao
~
a o ~ ..
a
ia a a a ~
o o
G1
o O o o ,p w
p o b
v p
a ao a a
u o
v
m .n u, o m o
aD n n n aD n 'd
C
s
E, ov ov o~ o~ o~
s
N ~D N ~ N ~ N ~ N ~ N
rl 1 rl N rl N rl N rl N ,.y
V
n-1 t7 rl O ~-1 O n1 O ~-1 O r1
N cV N N N N N N N N
N
~O ~0 N N N N ~O ~O N N N
m w .1 .~ .~ .1u n .~ .i .i
O c7 .-1.-r~ .-~O O .i
00
WC it ~0 fn U U rC it fa to U
" VSO PCT/US91
~2% /09267
10098
~ "
.
~$8_
.. .. ~. .. .. .. ., .,
o w N ..~ ~ ~ o
!l1 O y U1 Il1 O Il1 Iff O
N O ri .-1 e-~ N .-1 .-1
h... ~ m 47 h O~' ~p"' m'~ O"'
N
f'~ ri C~
~ ~
O O O ~f1 ~f1 O u1 O~ O
~
N7 O O 0~ m m ~D O~ O
'~ .1
C9
m
C O O O 1 O O O O
~ O O O "'
O O ~ O O
T O O O ~ O ~ O O
ei rl ri rl ri rl
CO O O v ~ 1 O O O
O~ O O
0~ 0~ 0~ O O O
v O v O r O ~ O O
Ov O Iff O U1 O ~. O O
1f1
.
a o~ ~, o~ ..~ o~ ., a~ ..~ .,
0
m
U ..
e~f of a0 ~"~
p v O N O v O V O
O O ~ O O O ~1 O
f'~1 V N v0 A1 1f1 rl V N
O
O v O O O O O O O
~ ~ p ~ ~ ~ ~ p p "
Z , 0~ 0~ O O~ 0~ 0~ Q~ O O
11 m N
r1 ri ~. i. ~. ~.
ri O O V O v v -a
tf1 1~1 O If1 O U1 Iff O Iff
h O Q7 01 O O~ C~ 0~ ~D m
a
b
C
O G O ~ O O ~ O O
O ~ O O ~ O
p~ ~ 0~ O o~ O a0 0~
O b
...
~ ~ ~ ~ ~ p W
of n O ~e1 O of O O O
W
h h ~ ~ ~ m ~ ~ m
b
H 0v O O O O O O cr N
.a .i .i .~ .1 .i .-r s
O N m N ~i1 !V ~ N Q7
N ri H ri H r1 N n1 N
O ,~ O 1 O ei O .1 O
H N N N N N N N N
N ~p ~p N N N N ~O ~O
ri In Il1 '1 ri '-1 '1 IA If1
wl O O rl rl rl 1-1 O O
a
i
U ,d ,~ 00 00 U U ~ ~C
WO 92/10098 PCT/US91/09267
-a9-
r~ N U7 1D
p vf1 yll p v
rl r v
r
v
U
O
3
m
O~ O O ~O
p p ~n ...
.. ..
o~ o, o~ n
O
O
3
m
. - o~
3 0 . o
~
~ ~
v
v~ o a
o
~
O
3
~,
3
E., O aD aD m
~ O T o~ C1
W .i
3 O v O
o O
a ~ rn
1
0 0
t~ 3
o ~?
u~ O e, O
u~ m ~ O
d' ~"1 V' N
OO
U 3
o~ O O O
Z ~"'~ ~~ ~"' O
~
p
a
N
.r N
G
H ~ ~ V O ..a
o ~ m o
p n O~
a
a
H
C
D u1 ~O p O
p p p .. ..a
... ...
v~ ov ao a7
O
U
m
.a
rr
L1
O O O b
m u~ u1 O
n n aD n
a
0
x '
..,
H ~ N N N N 1
~ rt ri rt ri #
D
N p N m
n-1 N rl N
rl O ~ O
m
N N N N
N N N N
ri rt r1 ra
ri
m
U ~
~
m m U U
WO 92/10098 PCT/US91/09267
-90-
Example 4
Following the same procedure described in
Example 1, eighteen (18) different antidotes were tested
for their efficacy against Herbicide No. 9 in corn,
grain sorghum, soybean and rice crops in the presence of
velvetleaf and yellow foxtail weeds. Observations were
taken eleven days after treatment. The test results are
shown in Table 4, wherein the following symbols are
used:
GRSO (grain sorghum);
SOBS (soybean);
WH (wheat);
VELE (velvetleaf) and
YEFT (yellow foxtail).
Plant injury is recorded as percent inhibition of
growth.
WO 92/ 10098 PCT/US91 /09267
-91-
t
, o 0 0 0 0 00 O o ln o 0 o c. o 0
Cn tD~'1 ~' ~OC>\ r1 h 00 ~O01 ~'00
w o o 0 0 0 0 0 0 0 0 0 0 0 0
oD a~N ~ srco r~h Inao h o0
w
al
0
.,.r
U tn tf1c7 O If1Cn O O O O O O O If1CO
.a ~) Cn 00vD h COCn If1 Cn01 t0 COCn ~D o0Cn
C
H
1~ ~ In O O O In00 O O O O O O O O O
G ~ CO l'1~~ N ~001 N 10 rlh ~ 10
fly
L~.
W
O) IftIf1C7 O O In O O O O O O O O
t'1N .-1 .-1N r1N eh O N f"1
d'
O O C) tf1 O 01 tn O CO O 111If1 O O GO
~O OICA OD CnCn eh C!1Cn r1 00CI t0 GOCI
f0
It1O O O O II1 O O IL1 O O 1f1 O O O
tn .-1 d' tl1O m -1~-1 e'1 N
Cn
O 01 C~ Cn O1 O1
IC
x ~ ~ o o ~ a~ o ~ ~ o ~r~ o
0 0 0 0 ..~ o o rl o o ~ o 0
.iio o ci o 0 0 0 0 0 0 0 0 0 0 0
N
x
a
i~ N N N N N N N N N N N N
b I i I
Q', N N N N N N N N N N N N
1
-~
Cl
~ 1 I I ~ ~ ~r ov ov o~ ch e~e~ h h h
+~
In o In o In
ri r-1 N N
WO 92/10098 PCT/US91/09267
-92-
t~, o o In o o In o 0 0 0 0 0 0 0 0
wl a o, r a, ~ coov ~ o w n o~
w
a o 0 0 0 0 0 0 0 In o o In o o In
M Q~ InCO M 10Cn M ~ tnCO
C
O
te
-. U O O In In InO O O O O O ap O O a0
a
~Q l ~'~ 0~ N ~OC1 10 01O 00 O~O~ N ~ C~
a
C
H
O O O O O O If1O O In O O O O O O
~~ r-1 r-1M N ~ N
'd
41 C4
O
C
O O O O O O O InO O O In O O O
N N M M M rl
O
U
st
O
p1 c~ o In co O O In O O ao O Inao O O ao
0001 01 OCO~ M 0101 d' 00O~ N Q~01
E
0 o In o o In o o In o 0 0 0 0 0
O M rlrl ."1N .-1
O~
01 C1 01 O~ 01
x o ~ ~ o ~ ~ o ~ ~ o ~ ~ o o
0 0 ~ o o ~ o o ~ o o .~ o
.G o 0 0 0 0 0 0 0 0 0 0 0 0 0 0
a~
x
a~ ~ ~ ~ ~ ~ ~ ~ ~r~ .~ ~ ~ ~ ~ ~r
- N N N N N N N N N N N N N N N
Qi N N N N N N N N N N N N N N N
1
-.i
y) ~ ,~,~ 111 !f1In M M M e! ~'~' N N N
y -1 e~-1e-1 rl rlri ri e-)rl
p ~ O If1
,~ ,-1 N N
WO 92/ 10098 PC~/US91 /09267
-93-
0 o c~ 0 0 0 0 0 0 0 0 00 o m
o
N ~0'(T ~D00 h C1 N 01 pp
W
a
w
> o o un o 0 0 0 0 0 0 0 0 0 o
In
C'1 N 00 N 11700 d'h C1 h
pp
U O O tt1 O O 1f1 O O tf1 O O O o O II1
sr h O w0' OG01 ~O 0001 N1 COO c't O~G1
t
N
O O O O O O O tL1O O O O O O O
tl1 N N Il1
41W
G
W
i~ ~ O O 111 O O O O O O O O O O O O
O N ~ N r-1N ~ N N
U
4) ~ O vcfao O apao O O oo ~c1 O O O O O
~ 0~ ~o o,O~ ao c~v>, tn o,o ~o o~o
ro ~
H
0 0 0 0 0 0 0 0 0 0 0 0 0 o w
rl N e~!C1
?.
,sax
d o ~ a~ o a ~r o ~ ~a~ o a a~ o a a~
0 0 .-i o o ~ o o ~-~ o o ~ o o
O O O O O O O O ~' O O O O O O
4) ~ ~'V' d' srd' d' d'~V' d' ~ e! d' d'
N N N N N N N N X11 N N N N ~
N
N
Lli N N N N N N N N ~V N N N N N
N
1
41
CO ODOD N N N 1f1 tf1Il1 1D 1p~p h h
~i e-~r1 ri r-1~-~ r-i ~-1e-1 e-~ h
ri
r1
u1 O u1 O
e-1 r-1 N N
WO 92/10098 PCT/US91/09267
-94-
0 0 0 0 0 00 0 0 0 0 0 ~n
V' O~ N h O~ N h O~ h C~
O O O O O O O O 111 O O O
N pp ~D 01 ~D CO 10 01
U o u1 ~r1 o 0 0o O O w O o co
,Q~ N 1f1Q~ d' ~O 01 e! 0101 t0 O~ G1
Fi
O O O O O O O O O O O O
i.!0 p h
'd
G
.,
O O O O O O O O O O O O
e~iN ~ N
O
U
.
r
O O O O o in in ino in O vc1
h 01 C1 N O~ O~ r1 000~ eh 01 C1
O O O O O O O O O O O
~D rl h
O
b
Z
x
.A Ov
O
d O d' d' O ~' d' O d'~ O d' d'
Z . p ~ O O .-~ O O r-1 O O r1
O , ,
O O O O O O O O O O O
b
C1 d' d' d d' at d' '~' '~''~'d' e!d'
N N N N N N N N N N N N
~!,'N N N N N N N N N N N N
v0 .-1 .-1 O O O CO COOO
t0 .-i
vD
.-i rl rl rl rl~
C
lI1 O If1 O
N
WO 92/10098 PCT/US91/092~7
-95-
A1:1 antidotes at 2.24 kg/ha, except Antidote
No. 4, provided safening to Hert~icide No. 9 at 0~~14
kg/ha to one or more of the tested crops. Antidote Nos.
and 12 were the most active corn safeners, reducing
5 injury from 55% to 0%. Antidote No. 5 (flurazole~)
exhibited the best sorghum safening, reducing injury
from 90% to 0%. Antidote No. 18 was the most effica-
cious safener for corn and soybeans; this compound
reduced soybean injury from 35% to 0%. Antidote No. 4
10 anomalously enhanced corn injury from Herbicide N'o. 9.
However, that safener did reduce injury to soybeans from
25% (commercially unsatisfactory) to 10% at 0.04 kg/ha.
Example :5
The test procedure, crops and weeds and anti-
dotes used in this example were the same as those used
in the test o~° Example 4, but the herbicide in this
example was Hearbicide No. 8. Observation of test
results was m~~de thirteen days after treatment. Test
data are reported in Table 5.
WO 92/10098 PCT/US91/09267
_96_
E,I o 0 0 0 0 0 0 0 0 In o o In o 0
fir f~ c'1 tp l!7 00 lf7 00 d' CO N
W
W
al 0 0 0 0 0 0 0 0 0 0 In o 0 0
01 1f1 N t~ t11 O O~ 10 O~ f~ N 01 d'
O
U If1If1O O O O O O O O tt1If1O O
O
. a1 I~ sr C~ CO N1 O~ CO N1 C1 f~ N C~ t~
I d'
t11O O O O O O O O O If1O O O
O
CO 1n r-1 Cv I~ 01 t' 01 tn Op d'
W tf7 tf1O O O O If1 O O In O O 111 O
O
t' tf1r1 O~ ~D ~-1C1 ~D 01 d' I~ If1
I
111 fly tn O O 00 O O 00 IffO 111 tI1O Il1 It1
O
I ~ O~ 10 01 00 N 0~ f~ N C1 n N 01 10
N
N C7
r~
.G
H
00 O O u1 O O If1 Il1If1O If1If1In O
O
I~ 10 N 00 ~' N t~ f'1e-iO~ f"1rl (~ d'
O
O
fa
z=
o~ o~ o, rn o~
~ o~ ~ o ~ ~ o ~ ~r o ~ ~ o ~r ~
x o
0 0 ~ o o rl 0 0 rl 0 0 .~ 0
0
x o 0 0 0 0 0 0 0 0 0 0 0 0 0
0
b
a~ m
~J N N N N N N N N N N N N
ro I I I .
(Y., x N N N N N N N N N N N N
O
I
-.1 d 1 1 I d~ ~' d' Ov Qv Gv r~ N1 cn n t~ t~
!~ O
a ~a
In o u1 o In
-i ~ N N
WO 92/10098 PCT/US91/09267
_g7_
W
n o o ~ 0 0 0 0 o w a o 0 0 0
W) '
10 f 00 r-I 00 f"1 00 N 00 ~0'
1
O ~ O O O O O O O O 1f1 O O
' G1 lf1 01 M OI U7
O
U
O t11O In tf1O O O O O O O u1 0 o
A '~ ~
. 01 f C1 C1 ~Dd' G1 ODd' O~ t0~' O~ 07N
.,i
O 1f1O O 1f7O O O O O 1I1O O C)O
00 tD O~ c'~1 O~ tl1 O~ 10 C~ tI1
h
'W--1
f~ ~ O O O u1 O O u1 u1In O O tf1 O O O
O ov t~
o~ ~ o wlN ov ~ ~ Q, m
G
U
v
Il1 pl, tf1O tl1 0 ~f1O tn O O In O O 1f1 C~O
01 C1ri 01 C1
01 l~N 01 00N O~ t~e!
r~
.L~
ro
H
o ~ o ino w n o w n o
~
o
ro a ~ o a ~ro ~ ~ o o
x ~
x
~ o o .-a o o ~ o o a ~ o ~r ~r0
..~ o o ~ 0
.fix o 0 0 0 0 0 0 0 0 0 0 0 0 0 0
v
x
ro
a~ .
x N
N N N N N N N N N N N N N N
ro ~
. .
L1S N N N N N N N N N N N N N N N
rY.
O
z
~ e-1v-ie-1 tf1 1l1!fl P'1 f"1M d' d'd' N N N
1~ l i -1
~
r r e ~i '-1e-~ e-1 rle-1
~
'L3
to O ~ O
rl e-i N N
WO PCT/US91/09267
92/10098
~98~
H
tz, Ino 0 0 0 0 0 0 0 00 In o 0 0 0
w oor~ rn vo av~ o o 0o w
l
w Ino o w o o m o 0 00 0 o m o 0
G~N1 O~ t~ N C1t~ l'~ Q1 CO N 00 I~
W
O
V ao0 o w o o inu1 o u1 0 o m u1o
0100 ~!'01 Cn N) 0100 f'1 01 00 rl 01 01N
I-I IL1O O tl1 O O 111O O tf1tf1O O O O
OWO d' Q~ d' O~d' r-1 O~ d' 01 f~r-1
b r-)
Cl
C4
O
--.i ~ O O O O O tf1 O O O In If1O CI If1O
~
O ~ O~ ~ r'1 01t' N 01 t~ rt ~Or1
O ~
O
U
w InO In ao O O aoIn O ao In o In u1O
C9 01CD N C~ 01 ~O 0101 ~0 O~ 01 N 01 O~rt
41
r~
H
lf1If1O lf1 tf1O O If1O O lI1O O tI1O
O~d' ~ d' r-1 01d' O~ 1f1 CO tf1ri
O
O o~ o~ o~ o~ o~
~C
$~ d'd' O d' d' O d'd' O d' ~ O ss d~O
v-1O O r-1 O O rlO O ~ O O ~ O O
,L~ O O O O O O O O O O O O O O O
4l
Z
Gl erd' ~ ~ d' ~ ~ra ~ d~ w ~ d~
T
i~ N N N N N N N N N N N N N N N
b
a, N N N N N N N N N N N N N N N
,Y,
O
x
wi CO00 00 N N N lf1In tW O 10 ~O l~ I~t~
GI
iJ r-1 ri ~ r-1e-1r-1 r-1ri ri ri rir1
~.1
G
O
~
'C3
1f1 O tf1 O t11
e-1 e-1 N N
WO 92/10098 ~ PCT/US91/092ti7
_99_
H
0 0 0 0 0 o cn o o cn o 0
w co ~ oo ~ oo ~ oo ~
wl 0 0 o w o 0 0 0 0 0 0 0
01 ~ON f~ M N Ca f~ C1 CO tpt1
O
U
--i o o W n o 0 0 0 o w o 0
.,.,
ov o0 ov cot~ rn co ~r a, ov~
G
H y cna o 0 0 o m o w o 0
0o t~ o~ ~ o, w c~ ..
b
a~
~ w
- a 0 o a in o 0 0 o c> m o 0
O o ~ n W O~ I~ 01 tf1~I 01 N N
~
O
U
O
in ~ yn o a w n o cn o m ao 0 0
t9 ov o~r, o~ o~~r o~ ov N ov o
a
.a
ro
H
c
0 0 0 0 0 0 o cn c m w n
CO lL7rl CO stN CO N rl
00
O it C~ o~ rn O
~.r 'S. 'a' d' O s!~ ~' O t! d' O tr d' O
~ O O r-i O O ri O O .-1 O O
I~x o 0 0 0 0 0 0 0 0 0 0 0
s
ro
a~ x
i~ N N N N N N N N N N N
N
!d
Qi x N N N N N N N N N N N
N
O
x
~o Two ,-~ .-i 0 0 o ao 0o
,.-~ co
~o
m o m o
~i r-i N
WO 92/10098 PCT/US91/09267
-100-
In this test Antidote No. 10 was the most
active to safen Herbicide No. 8 to corn, although all
antidotes did provide some corn safening. At 2.24 kg/ha
Antidote No. 10 reduced corn injury from 60% at 0.04
kg/ha to 25%. Antidote No. 5 was the most efficacious
for safening Herbicide No. 8 to sorghum. Most safeners
except No. 15 and No. 12 provided sorghum safening to
some extent. At 2.24 kg/ha, Antidote No. 5 reduced
sorghum injury by the herbicide at 0.009 kg/ha from 60%
to 0%. Antidote No. 18 at 2.24 kg/ha reduced wheat
injury and soybean injury from Herbicide No. 8 at 0.04
kg/ha from 50% to 10% and 55% to 20%, respectively.
Example-6
In this example, a test was conducted to
determine the antidotal efficacy of Antidote Nos. 10 and
18 against Herbicide No. 2 in corn, soybean, sorghum,
wheat and rice crops in the presence of yellow foxtail
and velvetleaf weeds. Test results are shown in Table
6.
WO 92/10098 ~ ~ ~ ~ ,~ ~ PCT/US91/09267
-101-
tE.yn o a o 0 0 0 0 0 0 0 0 o w u,
w ~D t~ f'f 1~ d' t~ f'7 I~ t', t~ r1 f~ r1
w
al 0 0 0 ~n o ~n o 0 0 0 0 0 0 0 0
d' f~ ~r N m r.wo r~i r. vo
U
. o 0 0 u,ir,O in 0 o u1C 0 0 o 0
-1 'a' e"1c"1f"1N ll1t',tl1l1110d' ('~lf1
Ili
I
!;
N
tf1O O tC1O O O O O O O O O O O
N r~ N e-1e-1ri
C4
O 1f1O O O O O O O InO O O InO
fO ~' r'1r~ N v-1V' N N ~0l'~1f~N l~~O
0
O O O O O O If1
OG O~ 01er Il~ri O O O O 00lC1O O
O~~O Q1Il1Q1~O 00e!'
.Ja
ro
O O O ~~1f1O O O O O O 1nO tf1O
N N ~-1N e-i
4l
ro
O 00 Z
'C~ ri N
r-1 OD 00 ~' V' N N 10 ~C OC 00
Q Q x 1 I I 1 sr e! N N rl r-I In In N N
'~ z ( ~P ~' N N .-1 r-~ O O O O
0o ro
'L~ r'1 iI f N
i N V
'~I 1 rl r~
I
I 1 I I 1 1 1 I 1 1
rl r~ r~
~z
v
b
U ro
'rl N ;L;
N f'~iO N N CO N CO N C10 N
00 OD
~-1 r~l r~ N ri -1 N ri N ri v-1
N ~-1 N
v ox . 1 1
,'S', z r-~) r~ CI r~ r~i O r~l O r~ C
O v-1 O
p ~ p
r"1 '~ N
WO 92/10098 PCT/US91/09267
-102-
In this test, Antidote No. 10 was twice or
greater as active as Antidote No. 18 as a corn safener
against Herbicide No. 2. At 2.24 kg/ha, Antidote No. 18
reduced corn injury by the herbicide from 30% to 10% and
at 4.48 kg/ha sorghum injury by the herbicide was
reduced from 55% to 15% by Antidote No. 18.
Example 7
Another test was conducted according to the
procedure described and used in the foregoing examples
in which six (6) different antidotes were tested against
four (4) different herbicides according to Formula I.
Test results were observed twelve days after treatment
and are shown in Table 7.
WO 92/ 10098 ~ ~ ~ ~ PCT/US91 /09267
-103-
w
a yn o c mno o w o o m o 0 0 0 0 o e~o
t~ ~DN GOd' N 01 00ri C100 tf1CO V' N l~ ~DN
0 O O O O 1t1O O O C7O
1 0110 N 00 V' t"1~ ~'N
C
O
+~U o 0 0 ~no O o ~r1o O o O tn o o o t7o
l
rlr l N N C1f~ 10f~ N 1~N O~ GO ~OC7
.ACx
C
H
O O O O O O if1O O o0O O if1u1 O O C7O
M OGN 01 00~-1 0100 N n ri ~-1
h
H
C4
w o 'n~ ~ ~ ~ o w n o 0 0 0 o a o
l 00 00t~ ~Oc'1N Ct G100 C101 t~~D ~O e"7CO t~t~
Cl U71 O O O i70O O 00 O O 00O O O In O O O O
~ M N ~T01 10C1 1D.-1 01~O .-i01 CO d'N
.CZ
ro
(1
0 0 o w o 0 0 0 0 ~mn o w o o u~ c.o
N lf1rl CO f"1 U1N r-1 .-I~I
ro
01 ~' ~Ds! ~V00 I~N 00!~ N 00 I~N 00 n d' ~C~~'
x:
N Ine-1.~N O .~ N O .-~1N O H N O N lfm-1
ro
cx c~ o o .~o o ,-io o ,-io o .-io o co c o
x
nr'
.,.,
U
.,i
.Ja .-1.-1ri fVN N to tf1lfl In111lt1N N N rl r-!e-1
>N
ro
d ~ ~ ~ ~ ~r a
x
N N N N N N N N N
ro 1 I I I I 1 I I I
N N N N N N N N N
J~
O
'~
''i M C1 M M f"1(~'1f'1M c'7
O 1 1 1 1 1 I I I I
~
x
.-1r-Irlri rl ,..~,..1.
s~
a
w o w o m
ri rl N N
WO 92/10098 PCT/US91/09267
-104-
w
,.a In o o InIn o 0 0 0 0 0 o InIn o m o 0
001D 0000 N 00 ~Of'1O~ Q~lf1COlflC700 f~N
(.Hz, O O O If7It1O O O O O O O O O O 1f1InO
W I 01 ~ C1 0000 N t~ errl O~ 00M G~d' t'fI~ If1N
O
O
r-1G )
+~ U O o o u1In O o o o u1 Ino o o 0 0 0 o
r1 r i 00 M rl 0~01 lflsr rl 1~ tf)e-i41I~ d'ItlN
~
.O ~ r
H
.-. 00 It1O O O O O O O !f1 u1O O O O O O O
~ ~ 00C7 0000 rl O~ COC'1I~N ~O
Gl~
b
r-1
rl(1a
~J W IllO O O O tf7O O O 1f1 toO O O O O O O
O I C~ Q100 ~O1C M CO l~f~ O~ 01O ~'N riCO GOf~
O
U V1
n
O
fn 00 u1O O O u1O O O O u1O CDtf1u1O O O
,~ a ,, p W' 01C1 N N O t0rl O~f~ N 00 r-1
H
b
E
o Ino 0 0 0 0 0 o In InIn o 0 0 0 0 0
O ri rl 00 ll1rl N
Id
G) N 00I~ N 00 I~d' 1Dd' N 00P N CO I~d' t0d'
PI N O rlN O N Illrl r-1 N O H N O N lf1H
~!',C, rl O O rlO O N O O .-I O O .-1O O N O O
,1
rO
U
.,i
tn tIlll1N N N e~-1rirl tll lf1ll1N N N r-1ri~i
f-1Z
4l
x
N N N N N N N N N N N N N N N N N N
N N N N N N N N N N N N N N N N N N
N
O
O er d'~ sr~ ~r~ ~ W Inu wlmn m n u'wn
,n o w o m
,1 e-1 N N
WO 92/10098
PCT/US91 /09267
-105-
w
a ~n o 0 0 0 0 0 0 o u, 0 0 0 0 0 ~n o 0
I
W Cn h N CO~ N CO h N Cn COc~fOptp N CO h d'
G41 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
W CJ110N Casr c"'1~ tDN 01 CON CO~D N O~ eDt!'
O
O
Cl
U O o ti O o o 0 o o ~r~o o u~O o 0 o o
..I rl h e'1 C~t0 r7if1N h t0 CnCO ~ h V'r-1
O
H
tf1O !f1O O O O O O If1tf1O O O O O O O
01 00C1 h N 10 O~ COt"11CN tt1rl
O
""r~1
rl
1~ In Its1t1O O O O O O tryInO lf1O lf1O O O
O 01 O~O~ ~OV' In00 00h O'~C101 N d' r-1C1 i70h
O
U
O
v V m ino u1u1 O o o o o O O tnO vc1O o O
~
cx ow o ~ ovh w o ,~ o ao rnow o o~ m .-a
t9
ro
0 0 o co0 0 0 0 0 0 o m ~no o m o 0
w eh~ ~ ~ ~ ~o er.-iN ~ c~ N
ro
Cl N COh N 00 h d' t0d' N COh N 00 h ~'
rl N O ~iN O N lf1ri ~-1N O .~1N O N U e-1
'1
ro ,
AG L~ r"1O O e~~O O N O O ~-1O O e-1O O N C7O
d
'L1
U
O It1tf1If1N N N ~-1v-1ri In lf1tL1N N N '-1~~r1
4l
x
ro
d a ~ ~r~ ~ a~ ~r~ ~r d ~r ~r~ ~ ~r a ~
.1~ N N N N N N N N N N N N N N N N I'JN
ro . . . . .
Qi N N N N N N N N N N N N N N N N e~JN
dl
O
rl o ~O 1010 ~~O10 10~D 1D~O h h h h h h h r h
a
w o W n o
v-1 rl N N
WO 92/ 10098 PCT/US91 /09267
-106-
0 0 o w o o w o 0
o~ r ~ oo ~ N oo ~ M
o w o u»n o w o 0
W O~ I~ C'1 CO d' N 00 Wit' N
O
~r1
yJ ~ O O O tf1 tf1 O O O O
~.1 .-1 00 ~D r-I C1 ~ l1~ ~O rl
.a
G
1d O O 1f1 tf1 O O O O
C1 CO N rl ~ C1
O ~i
yJ If1 O O O O O O O O
O O 01 0~ O~ I~ N N O~ t~ 00
U
v
O N O O O 00 O O O O O
r~ C1 l~ r1 C1 00 d' 00
H
O If1 O O O O O O O
I~ d' N M
G1 ',L' N 00 ~ N 00 ~ d' 10 d'
rl N O rl N O N tf1
p'" ~ O O r1 O O N O O
41
'O
U
,Q lL1 l11 lf1 N N N rl r-1 rl
O
fd
Q) d' 'd' d' d' d' d' d' d' d'
~J N N N N N N N N N
b
(1,' N N N N N N N N N
O
i~
O
CO 00 00 00 00 CO CO CO 00
~z
c
a
,ri o ~n
WO 92/10098 ~ ~ ~ ~ ~ PCr/US91/092fi7
-107-
Example ~3
The same procedure described in Example 7 was
conducted using the same antidotha to safen the same
crops against Herbicide No. 4. c.~bservations were taken
thirteen days after treatment. '.;Cest results are shown
in Table 8.
WO 92/10098 PCT/US91/09267
-108-
W
a o 0 0 0 0 0 0 0 0 0 0 0 0 0
0
l M ~G er N I~ rl
O O O O O O O O O O O O O O
O
W oo u1 to M ao 00 ~ ov
U O o o 0 0 o 0 o O o o 0 0 0
0
- ~ d' 10 ~ CO M I~ M ~ d'
rl
I
O O O O O O O O O O O O O O
O
1p rl M I~ 1C M rl
w
t~ 1f1 O O 00 O O 00 O O 00 O O CO O
O
~ O~ 1r1et OW O d' O~ ~O vG 01 V'd' O~ 00
O
Q) fly If1 O O O O O O O O O O O tf1 O
O
C~ d'N C~ If)N 01 d' O~ If1v-1 01 ~'
N
.Q
ro
C
O O O tf1 O O 1f1O O If1 O O If1 O
O
~ ri v-i d' M M rl
U
d~
oro
z
N O O N O O N O O N O O N O O
O O O O O O O O O O O O O O O
11 O O O O O O O O O O O O O O O
d
x
ro
a~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~r
x
N N N N N N N N N N N N
ro ~ i ~ . .
(Y, N N N N N N N N N N N N
d
O
.O
-ri M M M ~' d' ~' lt1In lfl t0 10 1p
O
+~ ~ ~ ~ ~ ,~ ~
z
m o w o m
e-I e~ N N
WO 92/10098 ~ ~ ~ ~ ~ PCT/US91/092~57
-109
w
al o 0 0 0 0 0
W r~f r~ M N
Grr O O O O O O
I
W 01 C1N O~ 00
d
UI o ~na o 0 0
. CO N f~ lf1
Gx
-~ ~n o a o 0 0
M N ri
wl W
C01 O O O 1f1 O O
O O cnr~ 0 0
~
O
C1 fn In O O O O O
01 InN O~ 1C
.a
ro
H
tf1 O O O O O
t~ ~O N
d'
o ro
zx
N O O N O
O
O O O O O
O
.a
x
0 0 0 0 0
0
x
ro
vx
1~ N N N N N
N
ro
W' N N N N N
r~G N
J~
O
'L1
w r 1~!~ a0 0o
O co
ri e-W-i v-1 ri
ri
a
w o
WO 92/10098 PCT/US91/09267
-110-
Referring to the data in Tables 7 and 8, the
test results indicated that the more readily safened
herbicides were Nos. 2 and 4. Antidote Nos. 14 and 15
were the most active to safen corn against Herbicide No.
2, although all safeners were active to varying degrees
against that herbicide. At 2.24 kg/ha, Antidotes 14 and
reduced corn injury by Herbicide No. 2 at 1.12 kg/ha
from 55% to 0%. For safening Herbicide No. 4 to corn,
Antidote No. 13 at 2.24 kg/ha was the most active'
10 safener followed by Antidote Nos. 15 and 16. Antidote
No. 13 reduced corn injury by Herbicide No. 4 at 0.02
kg/ha from 70% to 15%. Antidote Nos. 13, 16 and 17 at
2.24 kg/ha reduced corn injury from Herbicide No. 5 at
1.12 kg/ha from 80% to 50-60%.
15 Antidote No. 18 at 2.24 kg/ha reduced wheat
injury from Herbicide No. 2 at 1.12 kg/ha from 80% to
15%, and injury by Herbicide No. 4 at 0.02 kg/ha from
60$ to 40%.
Antidote Nos. 16 and 18 at 2.24 kg/ha reduced
soybean injury from Herbicide No. 4 at 0.001 kg/ha from
40% to 0%.
Finally, Antidote Nos. 13 and 14 reduced
sorghum injury by Herbicide No. 2 at 1.12 kg/ha from 60%
to 25-40%. Unexpectedly, injury by Herbicide No. 1 at
2.24 kg/ha was enhanced noticeably by Antidote Nos. 15,
16, 17 and 18. This reflects the unpredictability of
antidotal action by various antidotes against various
herbicides, at least under some test conditions.
Exam 1P a 9
In this example, the safening action by
Antidote Nos. 10 and 21 was tested against Herbicide
Nos. 1-6 and 8 in the same plant species as in the
preceding examples. The procedure described in Example
1 was followed in this test. Test results were observed
twelve (12) days after treatment and are shown in Table
9.
WO 92/ 10098 ~ PCT/ US91 /092b7
-111- s
0 0 0 0 0 0 0 0 0 ~n c:. o w o 0 0 0 0 0 0 0
~ 00 ~D C~ t~ N CO N C1 1'~ C~ O~ 10 C1 ~ G~ st
O 1f1 O O O O O O O O C:~ O II1 O O O O O O O O
W O~ 00 tl' 01 t0 d' 47 N e-9 C~ 01 00 ~D v-1 10 N
G
O
d
1~ U off O o O O O 1l1 O 1l1 O C~ O 111 O O O O ~D if1 O O
~1-1 rl O~ G1 N 01 00 10 01 r~ ~D O~ OC 01 01 01 01 t~ 01 01 OD
,O al
O
N
O lf1 O O O O 1l1 In O O C O O O O O O r0 O O O
i~ ~ WO ~D ~d' 111 ri o0 ~ Ow0 tn
C
ro
a~
y m a m o 0 0 0 0 0 0 0 0 ~c1 0 ~n o o ~c1 0 0
01 ~ W N OD ~f' !~ Q1 ~ t~ 10 N v0 ri
0D 1f1 Ili In O O If1 IL1 O Il1 1l1 O O It1 O O O O Op O O
ri O~ C~ ~" 01 01 N 01 !~1 N 01 Ilb CO ~t7 Ot ~' C~ 00
.a
ro
E~
u1 m a m o 0 0 0 o u1 ~n o ~c1 0 0 0 0 o mn o
~D f'1 M 1D sf' r-4 CO h ~ ~' OD
ro d' ~-1 f~ O~
O at 10 ~' N CO !~ N N 00 N O O N CD ~ N 00 O e! ~' O
i~ N lW-1 rl N O ef' ~-1 N O O O ~-1 N O e1 N O rl O O
b
LY, N O O r~i O O d' rl O O O O rl O O e~ O O O O O
N
.d
.,.
U
.,.
.a O e-1 r-I r-1 N N N M f"1 C7 d' d' d' lf1 In ll1 10 ~O 10 t0 CO 00
4!
x
a~
i i ~ i i ~ ~ i ~ i ~ i i i ~ ~ i
a~
0
.'.,
u1 o w o ir1
eW -~1 N N
WO 92/ 10098 PCT/US91 /09267
-112-
In o 0 0 0 0 0 0 0 0 0 0 o In u10 0 0 0 0
01 t~ t0 t000 ~ 01 d'~D d'CO O W O CO N
fs. O O O O O O O O O O O O O O tnO O O O O
WI ~ ~ app N ~O N ~O I~C~ O~ N N M
G
O
a1
~,JV O tf1O O tf1LL1O O u1O O lf1O O tf1O O O O O
rl r1 O Ot I~ N1 00O~ COO~ C~ M t~ d'01 O~ h CO 1~01
~
.>aLx
... O O O O O O O O O O O O O O O O O O O O
~ ~ ~ N t0 C1 rld' d'C~ 01 lL1 N
O
O b
C
..1W
+! W O O O O O O O O O O O O O O tf1O O O O O
G CO N rl tf1 N 1f1IL1C~ GO ~O d'
I
U N
If1O O O If1O O O tf1O O 111O O II1O O O O O
01 Ca d' t"1O P 01 N CO d' l~ N C~ N
E
0 0 o 0 o 0 0 0 Ino 0 0 0 In Ino 0 0 0 0
N N 10 M f~110 rl
O~ O~ O~ '"'
O ~ er O O V'd' O ~' d'!~ CGN I~00 N O O N CON
rl O O O O r1 O O r-1O N ~-1O N rlO O O N rl
b
(Y, O O O O O O O O O O O rl O O rlO O O O r-I
rd
U
.17 O~ O~ T 01 O~01 00CO CO10 ~G10 Il1lf1111~f'd'st M r'1
d
x
~a
N N ~ ~r~ ~ ~r ~r~ ~ ~r ~ ~ ~rN N N
N N N N N N N N N N N N
1 1 I
N N N N N N N N N N N N N N N N N
O
'-) e-1ri e-1r) r-1rl rIri r)ri r W r-1rl .-1rl
I 1 I N N N N N N N N N N N N -1 N N N N
N
C
O If1 O If1
,~ ,-1 N N
WO 92/10098 PC'~'/US91/09267
-113-
w
a l 0 0 0 0 0 0 0 0 o c~0 0 0 0 o w o m n c~
w ao ~aao w o ov r, ao ~~r, h a, ~ o wop~ o~
0 0 0 0 0 0 0 0 o cno 0 0 0 0 0 o cn cnc.
w h .a~co 0000 ov r~ h ca r,oo c-~o woc, ov
0
...,a~
i~U u~O u1O O O O O O O O u1 O O u1 O O u1O
I
C w a o w o co co C~ c~h o~o~ ~ Cn ~ Cn Cn
.D0.
.,1
G
.-. O O O O O O O O If16.'1O O O O O O If1O !nO
cn N ~ r o .~-~.r coov h ~ ov o~
~ ro
C
-.c
w cno o cn o o cn o 0 0 0 0 0 o cncn cno 0 0
C: ~ h N h CO O~ N h ~ h ~D00 C~
U
.r
IA O O O 1f10 ll1o O O COO ~f1CO O O O O O O O
y
n N h vo C~ ~ rn CnCn M Cn ~ Cn
ro
C
O o O O O cn O O O o O u1cn O O O O O u1O
er M h M h
ro o, rn r.,
Gl COh CCN d'v0 er O er yrO d'0' h OpN h 00 N O
x
Q'O N ." W1lflN O O rIO O r-iO N ~-1O N .-1O
ro
OG d'O C7r-1O O N O O C~O O O O O ~-~1O O rlO
.Y.
41
.O
-,
U
,G !"1N N N e-1e-i'-1O~ C~ C!1~ Cbc~ ~D\D10 Il1In ll1d'
Z
d
x
ro
d ~ ~ r ~r ~ ~ ~ ~ ~ .r~r r r ~r~ ~ ~ ~ a~~
1~ N N N N N N N N N CVN N N N N N N N N N
ro . . . . . . . .
~1,' N N N N N N N N N fJN N N N N N N N N N
0l
O
-ri ~ rl rW-i .~-~.-~1.-~1O O C7O O O O O O O O O O
Q
Z N N N N N N N ~-1 rl ~V.-~1~is~ ~ r-1,..~
C:
tn O tf1 O tn
r-~i v-~1 N N
WO 92/10098 PCT/US91/09267
-114-
w
,al 0 0 0 0 0 0 0 ul 0 0 0
W N 00 M 00 1C CO (~ CO Q1
fEs,l O O O O O O O ll1 O O O
W N N N1 00 e~i d' 00 00 01 O~
C
O
~r-1 Cl
y V o o O O ao o O u'f o O u1
~.-1 .'1 N CO d' I~ O~ C~ O~ 00 C1
~.'~
G
.~ ~~ O O O O 1f1 O O O O O O
p ~ ~ ~G d' O7
G1 t',
"~ 1C
C~.
~.-1 Ar
W O 1f1 O O O O O O 111 O O
d' u1 00 r1 v0 CO O~
U
can u1 O O o ul O o irl O O ul
~ t~ .~ u1 ~ O~ girl of
.4
G
OI O O O O o O O O O O O
U
d o N ao N ao t~ co N a ~o
J~ O O N rl d' O N ~-1 r-1 tC1 N
pC, O O O ~ d' O O r1 0 0 N
d
.O
U
~ r'1 t'1 C'1 N N N
G1
x
N N N N N N N N N N N
b , . . . . . . . .
p'" N N N N N N N N N N N
4l
i~
O
O O O O O O O O O O O
~ rl e-1 e-i e-1 ~ ri ri e-1 e-1
O
ri
~~ 9 2 ~
WO 92/ 10098 PCT/US91 /09267
-115-
In t:he test of this ex~imple, Antidote Nos. 10
and 21 safeneci corn against all lest herbicides. At
2.24 kg/ha theae antidotes safened corn to Herbicide No.
2, reducing :injury by 35%; reducing sorghum injury by
50-70%; injure to wheat by 20-40~k and rice by 60%. Both
antidotes sl:ic~htly reduced the phytotoxicity of Herbi-
cide No. 2 to the test weeds. Antidote No. 15 exhibited
higher activ:it.y than No. 21 in reeducing corn injury by
Herbicide Nos. 1 and 6.
Example 10
The example describes t:he results of tests
with Antidote Nos. 18 and 20 to ~:afen Herbicide Nos. 2
and 4 in corn, grain sorghum, soybean and wheat chops in
the presence of green foxtail (GF;FT); seeding johnson-
grass (SEJG); barnyardgrass (BYGF:); morningglory a;MOGL)
and velvetleaf weeds. The preplant incorporation
("PPI") procedure used in this example was the same as
in preceding examples. Observations were made sixteen
days after treatment. Test results are shown in Z'able
10.
WO PCT/US91/09267
92/10098
- 116-
w o 0 0 0 ~n o 0 0 0 0 0 0 0 0
1~ M M I~ ~!' N r-1 01 d' N
a
0 0 0 0 0 0 0 0 0 0 0 0 0 0
lf1 M 1C 00 d' M
x
0 0 0 0 0 0 0 0 0 0 0 0 0 0
\O M 00 f~ V' M O~10 (~ M f~ lf7
h~ O O O O O O O O O O O O O O
W M N C~ 00 ~' 00ll1 ~D N f~ d'
O
-rl (r., O O O O O O O O O O O O O O
,Q ~~ 01 ~O d' I~M t~ N ~O d'
C
H
O O If1 O O O O O O O O O O O
N pp 1G N N N d' M r1
~i
L1~
W
O O O O O O O O O O O O O O
O ~~ N O~ I~ ~O N 00~0 1f1 d' 00 d'
O
'~ t4 O O CO O O O tf1O O O 1f7 O O O
~!' N Q~ 00 00 ~O 01t~ I~ l~ 01 10
H
O O O O ~ O O O O if11f1 O O O
M e~iC1 ~O M 00~' N ."~00 Ill
Id
d' 'd' d'd' 'd' s! d' d' d' V'
~J N N N N N N N N N N
~ I I I I
x N N N N N N N N N N
O
1 I 1 1 Op 00 CO00 O O O O o0 O
H r-I v-~Irl N N N N H N
sr d' 'a'
N GO N O N 00 N O N 00 N O
rl N O O H N O O .-1 N O O
I 1
p4 ~ 0 0 0 rl 0 0 0 .~ 0 0 0
x
b
U
.~
O N N d' d' N N Q'd' N N d' d' 1 I
y.a
,'Z,
x
,n o m o w
H e-i N N
Q ~~ 5g ~ 0
WO 92/ 10098 PCT/US91 /09267
-117-
Antidote Nos. 18 and 217 in this test were
shown to reduce injury to wheat by Herbicide No. 4 at
0.02 kg/ha from 85% to 20-30% whE~n the antidote rate was
2.24 kg/ha. 'These antidotes also provided some corn
protection against Herbicide No. 4.
Still other tests were conducted to determine
the efficacy of a variety of antidotal compounds against
herbicides according to Formula 7: above as the primary
herbicide in combination with various other compounds as
co-herbicides in various crops. The results of those
tests are described in Examples 7.1-14 below.
Example 1:1
This example describes tests with Antidote
Nos. 9, 10 and 12 to safen corn a.nd grain sorghum
against combinations of Herbicide No. 6 with acetochlor,
metolachlor and EPTC, i.e., Co-herbicide Nos. A, B and
D, respectively. The PPI test procedure used in this
test was the same as described in Example 1. Obse~rva-
tions were taken two weeks after treatment. Test
results are sh~~wn in Table 11 in which test plants. (not
previously identified above) are identified by abbre-
viated symbols as follows:
Shat~tercane (SHCA);
Yellow nutsedge (YENS);
Blaclt Nightshade (BLNS) and
Cocklebur ( COBU) .
WO PCT/US91
92/ /09267
10098
1 18-
-
a
of 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
f 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
o
U
W
O O O O O O O O tf1O O O O O o O O O
W ~OM 1DM 10 M f~ d'1~ d'~O t~I CO N ~G
O O O O 1t1tftO tf1O O to O 111O O O O O
O~CO 0100 00 l~ C1 00C1 001~ tI1 01 Op COt~ 00ll1
O
fn If1lf1If1O O tC1lt1 O 00 O 1f1O O tf1117O O O
C~00 d'00 O 00 O~ ~ C~ 1~01 l~ G1 N 00.-1O~I~
~i
fsr O O 0000 O t11O O O O tf1O O O O O O O
W O O C~O~ C~ CO O O O O CO t~ O O O O COt~
l
.-1r-I e-1 ~ rl ri ~ e-I.-1r-I
!f'tO O O O O O 111O O O O O 1f1O O O O
W O~O O~O~ O~ t~ O C~O O 01 t~ O CO O~O 00t~
O O O O 1f1O O O O O t11O O O O O O O
~r O O O O 0~ C~ O O O O t~ 1~ O O O O CO00
e-Irl rirl r1 rlrl e-i e-1v-i~ ri
O O tf1In O Il11f1 Il1O O If1O O O O O 1l1O
O~C~ 0000 O~ d' 01 t~01 00aG 10 01 e"1!~N t~~O
4l
r~
1f11f1IffO O O O O In O If1O tn O lC1O O O
1~ 10~ ~D N f"1 N ri rl e-1
E
N N N N N N N N N N N N
p'., 1 1 1 1 1 1
x N N N N N N N N N N N N
I
O
O 1 1 1 1 1 I 01 01p1 O1C1 01 O O O O O O
~z
c
a
a~
~o 1010 N N N N ~O ~ON N N N 10 1D N N N N
!!f 1f1Iflr-1rl rl r-1Ifl ll1r~ ~-1r-iri ll1In rlr-1riv-~1
~ o o ~ ,~ ~ ~ o o .-~~ .-~~ o o ~
x
a~
1
U
O
.a
U
~ a a m m o o a a m m o o a a m m o 0
0
',I, ' N 00 N O N 00 N 00N 00N CO N 00 N 00 N tp
b rIN ~ N rl N r1 N rl N rl N rl N rlN riN
~ ~-iO .~O r1 O rl O .~ O ~ O .-1O rlO rlO
x
G1 t0~D t010 ~O 10 10 ~O~D ~O~O 10 ~O 10 ~D~O 10t0
O I
T.
Z
O tf1 O lf1
,.. p 1 N N
WO 92/ 10098 PCT/US91 /092b7
-119-
a
c 90 0 0 0 0 0
a o0 0 0 0 0 0
I
U
W
a lo 0 0 0 0 0
ao vor w oo r
0 o m o w o
a~
o, a,ov ooao co
0
c mn o ~r wnm n
Q' Q,rn o,a~ o~
H
r~ o o a o o w
l
w 0 0 0 0 0 00
b
co o c mno 0
G
w cv rno~ ao0o r
.,.,
0 o c o 0 0
0 o c o 0o vo
V
rl r~e-dr-1
.r
111 If1O O o O
- O~ 000~ m o~ 00
N o O O Ifto tf1
E~
u1 a .-I
d
~r er~r a ~
N N N N N N
N N N N N N
O
'd
O N N N N N N
i~ ~ .-I~ .~~.-I.-a
Z
Itf
~ ~ ~ON N N N
Ti
ro
x
0 o r.;r-i,-i,-i
.r.,
I
U
O
U
.fa
a a oa ~~ao 0
i~ N 00N 00N CO
T,
rl N rl N rl N
a4 .~-1O r-1W ~ O
~ ' ~o~o ,a~o ~o
~
z
w o
WO 92/ 10098 PCT/ US91 /09267
-120-
In the above test all of combinations of
Herbicide No. 6 applied at 1.12 kg/ha with Co-herbicides
A, B and D were safened to corn with all of the test
antidotes. Antidote No. 10 was the most active safener,
followed by Antidote No. 9. At 2.24 kg/ha Antidote No.
reduced corn injury from 60-75% to 10-15% and also
reduced sorghum injury and shattercane activity when
mixed in Herbicide No. 6 plus Co-herbicides A and B.
10 Example 12
In this example, tests were conducted to
evaluate the safening effect of Antidote Nos. 3 and 18
against herbicidal combinations of Herbicide No. 2 and
isoproturon (Co-herbicide E) in wheat, again using the
test procedure as in the foregoing examples. In this
test, the weed catchwood bedstraw (Galium aparine),
commonly associated with wheat cultures, was the test
weed. Observations were made sixteen days after
treatment of the plants with the test chemicals. Test
results are shown in Table 12, wherein the above weed is
identified by the abbreviation "CWBS"; "WH" symbolizes
wheat.
WO 92/ 10098 ~ ~ PCT/US91 /0927
-121-
c
ao 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
31 0 0 o c~ 0 0 0 0 0 0 0 0 0 0 0 0 0 o 0
U ~-1.-i.~ ~-1~ ~ ~ ~1 ~ ~a ~ .~ ~-1~ ~ .-a co
C
N
!;
h
r-i 1t1tf1O O O It1tn O 111O C~ O 1f1O tt1O 1f1O tf1
01 f~N rl 01l~t~ I~01 I~fV ~ 01 0110 t~M c1O
r-i
ro d' ~--I er rl O N CO N
d' e~ d' rl
O Z . . . . I I I I ~ . . . I 1 1 1
1 I N 1 d' ~--I d' rl
ro
xx
N '~
ri 1"~
4l
x w 1 I I 1 w w w w 1 I I 1 w w w w 1 1 I I
'~ o
a
ro
N
ro
a~ ~ ~c anN .~w o vc ~rvc a~N ~ ~r vovo w o 0o
i~ N lf1~'~-1N N lf1If1N ll1efr~ N N ll7Il1N tf1N
~
ri
L1r' N O ~'~ N N O O N O Crrl N N O O N O d'
.-1
Y
Ol
1
N N W W N N N N N N W W N N N N N N W
W
sr ~' V' d' sr d'
N N IV N N N N N N N N N
ro I I I I 1 I I I
11r' DG N N fV N N N N N N N N N
d
i~
O
'C O
w Z
i.~ I 1 I I I I 1 1 cn M c~, r1 en ri cn r1 pp pp pp op
ri ri ri r-1
a
m o ~r, o m
r-1 ~-1 N N
WO 92/ 10098 PCT/ US91 /09267
-122-
c
m 0 0 0 0 0
0
) 0 0 0 0
..aU
H
C
r-1 O O O lflO
O
h,~~ d' N d'N
00 N CON
O It3 d' r-1d'H
O . . I
I
O . . d'H
yl d' H
O
Lx
O
U
f'r
W W W W 1
1
O ~
O
U
b
E~
ro
N d'
~J N N tf1~t1
1
I
(Y, N N O O
4l
x
,.
I
O N N N N I
I
U
v
4) d' d' d~ d' d' d'
~J N N N N N N
p~,, ,Y, N N N N N N
O
'Lf O
w Z
O O O O M O
,!~", H r-1 r-1 '.~I ri
tn O
~i
WO 92/10098 PCT/US91/092~7
-123-
In the above test Antidote No. 18 at 2.24
kg/ha reduced wheat injury by t',~e combination of
Herbicide No., 2 (at 2.24 kg/ha) and Co-herbicide E at
4.48 kg/ha f~~om 90-95% to 35-40%. Antidote No. 3 was
not shown to be particularly ef:Eective against t:he above
herbicide/co--herbicide combination in wheat under the
test conditions.
Example ~3
This example describes the results of tests to
evaluate the antidotal efficacy of Antidote Nos. 1, 2
and 6 to safe:n rice against the combined herbicidal
effects of Herbicide No. 2 and butachlor (Co-herbicide
H). The test. procedure was the same as in preceding
examples. Harnyardgrass (HYGR) was the test weed.
Observations were made thirteen days after treatment.
Test results are shown in Table 13.
WO 92/ 10098 PCT/US91 /09267
-124
~
c ~n ~no 0 0 0 0 0 0 0 0 0 0 0 0 0 ~n o 0 0
o ~ a, I~0 0 0 0 0 o v~ I~0 0 0 0 0 0 0~ r o 0
~
ao ~ r,,-
.r.,
.Q
.'.,
x
c
H
c
ro
a~
G4 U O O O InO O O O In O O O 1f1O O lf1lf1111O O
rl 01 ~'lflC'100 Cad' lf7CO 1~N l~ 0~f~ l!1O~ Ca
H
ro
G1 CO N 00 N CO N 00 N
1~ d' rier H ~' e-1~' H
ro I I 1 I I 1 I 1 I I I I
ay'rl~' ri d' ri~' ri
c1
H
I I I 1 x x x x I I I 1 x x x x i I 1 I
a~
ro
ro
Q) N ~ 00 N N N 00 CO N 0~OD N N N 00 OpN 0000 N
1~ rl N d' rle-1rlN N ~-iN d' r-Ie-1r-'1N N H N d' rl
ro
(Y, rl O d' rie-1rlO O riO d' ri rlrlO O rl O d' rl
.ra
41
x
N N x x N N N N N N x x N N N N N N x x
O
U
a~~r~ ~ ~ a~a
1~ I 1 I ( I 1 I I N N N N N N N N N N N N
ro
(l,' N N N N N N N N N N N N
O
r0
W O
1~ Z 1 I 1 I I I I I N N N N N N N N ~D 10 1p ~O
c
lf1 O to O u1
rl H N N
WO 92/10098 PCT/US91/092b7
-125-
O O G O 1f1 O O O O 1~O O O O O
O O O ;~ O 01 10 01O O 17O O
. m rir-Iv-1r-1v--)
J~
.Q
.,..1
Hi
ro
r~
U O o~u1 O u1 O O O In c7In O O O O
O o wclu1 w oo c~t~o~ t~r t~
b
!~
ro
QI Cn N CO N OO fVCn N
O
i~ d' r-1d' rl d' ~ad' v-1
V ro . . . . 1 1 1 1 . . . . 1 1 1
x
f
ro~ .a x x x x I 1 1 I x a"x x 1 1 I
a~
ro
H
ro
C1 N N 00 00 N 00 00N N N 00 Op
~ r-1N N r-i N d'e-ie~ ~~N N
b I 1
I
a4 ,-i r.io o ,-i o erri~-i~;0 0
.,
a~
x
I N N N N N N x x N leiN N I I I
O
U
v
GI d' ~'sn'd' d' d' stsfi~' ~"d' d'
i~ N N N N N N N N N l~JN N
ro I I I
Qi N N N N N N N N N N N N
d
O
'>7
w O
~ Z to vD ~cmo ~ ~ .-i ,~ ,-1 ~a ~ ~ N vo
O
'"1 r-1 N
WO 92/10098 PCT/US91/09267
-126-
The data in the above test indicates that
Antidote Nos. 2 and 6 reduced butachlor injury from 50%
to 0-20% at 4.48 kg/ha of butachlor. However, combi-
nations of Herbicide No. 1 at 0.28 kg/ha with or without
butachlor at 1.12 kg/ha were not effectively safened by
the test antidotes under the conditions of this test.
Example 14
In this example Antidote Nos. 10 and 2l~were
tested for their efficacy against the combined herbi-
cidal effects of Herbicide No. 2 and Co-herbicide B
(metolachlor) to protect corn in the presence of yellow
foxtail (Yeft), barnyardgrass (Bygr) and shattercane
(Shca), under PPI conditions, the procedure used in the
foregoing examples. Observations were made eight days
after treatment. Test results are shown in Table 14.
WO 92/10098
PCT/US91 /09267
-127-
U
O O o If7O O O O O W O O O O O O O O u1
y o ~ ~ ovo o,c, o In ~~~h h O o v~h vo cn
,>a
A:
.C~ O O O O O O O O O O O O O O O O O O O
C~ tD~ O O O O O 111 O O O O O C~ 1D N O
N m ~~ rl.-~Ie-1r-1r-1 r-1v-1r-1v-1r-1 ~.t
i~
>;~ O O c~ O O O O O O t~O O O O O O O O O
b W tT ~Do O C1 O O O ~O tVO O O O O O
h N O
~-1r-1ri e-1.-1ri e-1r-1r-1r-1rirl
O O o O In O O O O o O O O If1O O u1 If10
OG d'C1 00 h h ~D ~' e~ h ll1~' N
U
'~a~ aw o er voet vo ~ to w e ~,,~ ~r
N l11N If1N If1 N If1N 111N lf1 N
1 I I I
I 1
x N O N O N O N O N O N O N
ro
I
s,J
a~
v~
x 1 1 m m m m m m 1 g m m m m m m I I a1
~
U
o-
U
,G
GI 1p d' t0 1Cetrd' t0 s! ~01p erd' 10 d'
lf1i
r 1f1tf1r1 r1 In ~ lf1u1 v-1.-1 ll1 i
ro . . a 1 I 1 r
1
x o 0 0 0 0 0 o ci c~0 0 0 0 0
a~
b
.'.,
U
.,.1
7 N N 1 I N N N N N f'JI 1 N N N N N N NI
>
x
ro
a~ ~ ~r~
x
N IVN N N N N N N N N'
1 1 1 I 1 I f I . . ,
N fVN N N N N N N N N
O
'Z7
o .~ .-1~i r1 .~iW -1.-1 O O O
1~ 1 I I I I I 1 I
Z
N N N N N N N N .-i ,.-1
a
In o m o
e-i .-1 N N
WO 92/10098 PCT/US91/09267
-128-
c
0
.,.,
.'.,
,s~ 0 0 o m o 0 0
>~~ o o a~a~ r
H tJ) rl rl
:; O O O O O O O
f0 O O O O O
H H ~i ~iH H
'O
O O O O O O O
O O O O O
O
H H H H H
C
O O O O O O O
O
10 ~f'N
U
U
v
H ~
ae 1~ 'a'~Dd' 10
If1N !ftN In
I 1
1 O N O N O
ftf N
E O!
1
O
O
'd
U 00 a1 a1m Ca 1 I
U
to
G1 ~o ~Dat ~'
In lL1e-1H
b I 1 I
L1G O O O O
.d
.,
U
.,
.Q N N N N N I 1
x
G) et ~' e!d' d~ cf'sr
;J N N N N N N N
IIS
a, N N N N N N N
al
O
'1~
W O O O O O .-1O
O
i~ ri ri e-1r-1e-1 N e1
Z
O
1f1 O
ri
WO 92/10098 PCT/US91/09267
-129-
Reference to the data in Table 14 will show
that Herbicide No. 2 alone or ire combination with Co-
Herbicide B ~~as significantly moderated in phytot~oxicity
to corn by bcth Antidote Nos. 10 and 21, the former
antidote being more active than the latter. At ~>..24
kg/ha Antidote No. 10 reduced corn injury by the Herbi-
cide No. 2/Co-herbicide B combination at rates of:
0.14/2.24 kg/ha from 70% to 20%. Both antidotes reduced
activity of Herbicide No. 2 against the narrowleaf weeds
yellow foxtail and barnyardgrass, but did not reduce the
activity of the Co-herbicide B.
Examdle 15
This example was designed to evaluate t:he
antidotal effectiveness of representative antidote
compounds against herbicidal compounds according to
Formula I eitlher alone or in combination with co-
herbicidal compounds when the antidotes are applied as a
crop seed coating on crop seeds, e.g., corn and sorghum
seeds, according to the procedure described below.
The following procedure was used to determine
the interaction between a herbicide and antidote when
the herbicide is topically applied to the soil surface
and the antidote is applied to crop seed. Crop plant
seed may be t~~eated with the antidote either by con-
tacting the seed with antidote i:n powder form or by
contacting t:h~~ seed with a solution or suspension of
antidote compound dissolved or suspended in a suitable
solvent, typically methylene chloride or toluene.
Relative amounts of antidote compound and seed are used
to provide an antidote-on-seed concentration, on a
percent weighl:/weight basis, typically within the range
of about 0.03 to 0.13%. Containers were filled and
compacted with fumigated silt loam type soil to a depth
of about 1.3 c:m from the top of vhe container. A first
container was designated as an untreated control, a
second container was designated ~3s a herbicide control,
WO 92/10098 PCT/US91/09267
-130-
and a third container was designated as a herbicide +
antidote test container. Untreated crop seed was placed
in the first and second containers. Antidote-treated
crop seed was placed in the third container. Then, each
of the second and third containers was filled and
leveled with a cover layer of soil having incorporated
therein the selected herbicide at a pre-determined
concentration. The first container was filled and
leveled with soil containing no herbicide. All
containers were given about 0.6 cm of overhead water to
simulate an activating rainfall. The containers were
placed on a greenhouse bench and sub-irrigated as
required for the duration of the test. Plant response
is typically observed within about three weeks after
initial treatment; in this example the observation was
made twelve days after treatment.
In this example, Antidotes No. 5 (common name
"flurazole"), No. 10 and No. 8 (common name "oxabetri-
nil") were coated onto sorghum and corn seeds for
testing with Herbicide No. 2, the herbicides alachlor
(Co-herbicide G) or metolachlor (Co-herbicide B) and
mixtures thereof. Yellow foxtail (YEFT), wild proso
millet (WIPM), velvetreaf (VELE) and morningglory (MOGL)
were present as the test weeds. Test results are shown
in Table 15. The percent injury or inhibition values
resulting from the herbicide treatments are shown under
each test plant.
~~'~~9 ~0
WO 92/10098 PCT/US91/09267
-131-
a
0 0 0 0 0 0
d' ch1 I I I I I 1!'1N 1 1 1 1 I i N 1 I
W
a l l11O O O O O
W f~ t0I I I 1 I I I~ d'I 1 1 I 1 I !~ N I 1
G
O O O O !f1 O O 1
H O~ O1I 1 I I I I O~ C1I I 1 I I I OD I
.,..1
O O O 1f1 O O
C W O~ CvI' I I I I 1 01 011 I 1 I I I 00 I 1
H
rocn o o ~c~0 0 0 o In In Ina m o o m o w o 0 0
C~ 0001 t'1f~ ~'~Or1 01 f~f'N lf1N r7N C1 10C1r1
C
m n o a o w n o w o o ~r~u~ o o w o w o
f'7ri1p N ri e~e-1e1 c'~1 e1~rl rle-1r1 r-II 1
ro
Gl co co0o coca ao000o ao 00ayac o00o ao00
H 1~ N N N N N N N N N N t11N N N N N
ro
I I I I
v A4 o 0 0 0 0 0 0 0 0 o ceo 0 0 0 0
x
v
b b
H
U
I
.
U C9 C7C.~C7C9 C9C7C9 cG CDaCCG CCIa1 00CD I I I I
.tl
4l
ro
0l vo awo w o w o er vo w o w 0 w o w D a vD~
T
d
ro
G4 o O o O o O o O O o o o o o o o O c7o o
'C!
.,i
U
.,.I
N N N N N N N N N N N N N N N N N fVN N
h
T
ro
a~ ao aoao 000000 000o coco co0o aoco
~
N N N N N N N N N N N N N N
ro I I . . . . . . I 1 1
I
fx O O O O O O O O O O O O O O
'b
1~ I I tf1u700 COO O I I W f1 opCO O O I o 1f1If1
Z
H H H H
Lf1 O u1 O u1
r-1 r-1 N N
WO 92/10098 PCT/US91/09267
-132-
a
0 0 0 0 0
I 1 1 1 1 1 1 I I I
w
a o 0 0 0 0
w I I I I I I I 1 I 1
)
>
0
w o 0 0 0 0
,a ~ 1 I 1 I C~I 1 1 01 1 1 I
r
c
H
O O O O O
I I 1 I O~I 1 I O~ 1 1 I
ro
0
w m nno w o 0 0 0 0 0 0 0 0
t~ N l~ N Inrl d'
,d U'
N
O G
y,.~ O O O O O
I I 1 I r1I I I I I 1
!;
O
U
ro
d aoao ao aoao 0oao ao
N N N N N N N N
ro 1 1 1 1 1 I I
O a, O O O O O O O O
r~
O
ro ,o
N
U
I.
I 1 1 1 C~C~ C~ U'CG Oc~f~ flaI 1 I
U
01
d to
y u1 r1u1 H
ro . 1 I I 1 I I I I I I 1
a o 0 0 0
d
~o
U
..a
,Q N N N N I 1 I 1 1 I I I 1 I I
O
ro
41 t0 a00o aC a0 oG o0 0000 0oao0o c0
N N N N N N N N N N N N N
ro I I
(Y, O O O O O O O O O O O O O
4l
i~
O
'L~
rl
O
+7 Op 00O O I lI100 O I lf100 O ll1CO O
j; H e-1 r-1 r1 ri
If1 O ~f1 O
H e-1 N
Z~j ~ 5~ ~ Q
WO 92/10098 PCT/US91/092h7
-133-
The data in Table 15 indicate that Antidotes 8
and 10 provide=d higher safening of corn and sorghum
against Herbi~~ide No. 2 than did Antidote No. 5. At
0.28 kg/ha An~~idote Nos. 8 and 10 reduced injury by
Herbicide No. 2 at 0.56 kg/ha in combination with Co-
herbicides B or G at 0.28 kg/ha from 30-35% to 0% to
15%. Antidotes No. 5 at 0.25 kg/ha appeared to enhance
corn injury (an occasional anomaly) by the same
combination off' herbicides.
At 0.28 kg/ha Antidotes 5 and 10 reduced
sorghum injur5r by Herbicide No. ;2 at 0.56 kg/ha with and
without Co-herbicides B and G also at 0.56 kg/ha (20-
55%), while Antidote No. 5 at 0.25 kg/ha did not reduce
sorghum injur5r in this test. At the rate of 0.14 kg/ha
for Herbicide No. 2 with and without Co-herbicides B and
G at 0.28 kg,/tia, all of the antic3otes reduced injury to
sorghum from E>0-80% to 20-40%.
Since sorghum is normally safened by each of
the test antidotes to commercial:Ly-acceptable levels
against alach7.or and metolachlor,, it is believed that
excess injury to sorghum was due to Herbicide No. 2.
Accordingly, it is suggested that: lower rates of that
herbicide and~'or higher concentr<itions of one or ;more of
the antidotes as a seed coating would further reduce
sorghum injure by the above mixtures of herbicides/co-
herbicides. ,P~djustment of relative ratios of
herbicide (s) and/or antidotes fon~ maximum safety is
standard practice.
Her>icide No. 5 at 0.56 kg/ha enhanced
herbicidal activity against the broadleaf weed
velvetleaf in combinations with alachlor or metolachlor.
WO 92/10098 PCT/US91/09267
-134-
Examvle 16
TEST A. In other tests with safener-coated
crop seeds, e.g., corn seeds, following the procedure
described in Example 16, Antidotes 9, 10 and 13 were
used to evaluate their efficacy against Herbicide No. 2
alone and in combination with primisulfuron (Co-herbi-
cide F) in corn with velvetleaf as the weed.
Test data for observations in one test made at
nine days after application (DAA) of the chemicals are
shown in Table 16A. For informational purposes, plant
injury observations were made for solvents and a surfac-
tant present used in the seed coating procedure
WO 92/10098
PCT/US91 /092b7
-135-
an o 0 o co~n o 0 0 0 0 0 0
.
o ~ ~o ~ ~ a~~ o
.a
0
o,
O t11O If100If1111 O O tf1O O O
1p N ID f"1N N1 ~ N N ri N N
U
to
ro
G1 4) N N N N N
r~ ro ~ ,~ ...~~ ~
. 1 I 1 1
ro x ,-,,-~ 1 ,-a...I,..,
1 1 firI'4 1 w fs,w 1 1 I
0
U
r ~ a~it ef~
N tV N N N N
I I 1 I
N tV N N N N
N I tV N N N N 1 I 1
dP
lt1
O
4l d' d'd' d' ~'d'
T,
N N N N N N N
ro
ro 1 I r
Qi
N N N N N N N V
ro I
IJ \
O
O O CT N1 O 01 e"7,+O,J n y
1 I I ~-1 W -1 ,..
a
a x a
w o m o m
e-1 ,-I N N
WO 92/10098 PCT/US91/09267
-136-
TEST B. In a similar test, observations
were taken at the time of seven days after application
of the chemicals instead of nine days as in Test A.
Primary interest in this test was to determine the
effect, if any, on corn injury of a shortened
observation period, together with test readings with
three each of the antidotes and their combinations with
the herbicide and/or co-herbicides instead of twa as
used in Test A. Test B results are shown in Table 16B;
no weed was present in that test.
WO 92/10098 PCT/US91/09267
-137-
0
,o .,
mn ~c~ o wn In o 0 o In In
t~ f"1 e-1 !'~i N rl N rl N .-1 rl r-1
v
~O
ro
4) N N N N N N N N
ro 1 '~'~ I '-'~' 1 '''~ ~"'.-,
I
tY ri~ ~-1v-~1 r-1rl r-1ri
I
1 firW I i~ Lrr I W G4 1 firG4
rtt ~ ~r a ~ .a~ ~ d,
N 1 N N I N N I ;V N N
1~ . . .
1
N N N N N V N N
.0
N I N N I N N 1 'V N I N
x
N N N N N IV N N N
ro 1 I I
N N N N N IV N N N
ro x
o ro
O O O 01 01 f t"1C'1f"1 >;
'~ 1 I I I1 O
O r-1e-1e-~ ~i r~ r-1
i~ O
O U
,~
u1 O W O
r-I ''~ N
WO 92/10098 PCT/US91/09267
-138-
In' '~he~ aboi~e tests, the data in Table 16A show
that unsafened corn had 60% injury due to Herbicide No.
2 applied at 2.24 kg/ha. However, when the corn seed
was coated the antidotes, also at 2.24 kg/ha, corn
injury was reduced to 35%-45%. Still further reduction
in corn injury by Herbicide No. 2 is shown in Table 16B,
wherein under the Test B conditions, each of the
antidotes at 2.24 kg/ha reduced corn injury by Herbicide
No. 2 at 2.24 kg/ha with or without co-herbicide F at
1.12 kg/ha from 35% to 10-20%.
It appears that the higher corn injury due to
Herbicide No. 2 with no safener present in Table 16A may
be due to the longer exposure time of the herbicide to
the unsafened corn seed.
As will be apparent, the data in the above
tables reflect the fact that azolopyrimidine sulfonamide
herbicides are susceptible of having their phytotoxicity
to crops reduced by various antidotal (safener) com-
pounds, while still providing control or suppression of
various narrowleaf and broadleaf weeds. The data also
reflect the common occurrence that the safening effect
on various herbicides by various safeners will have
different degrees of effect in different crops and weeds
depending upon a variety of factors, including, relative
concentrations of herbicides and/or co-herbicides and/or
antidotes, weather and soil conditions, water content,
etc., as well appreciated in the art.
The herbicidal compositions of this invention,
including concentrates which require dilution prior to
application, may contain at least one active ingredient
and an adjuvant in liquid or solid form. The composi-
tions are prepared by admixing the active ingredient
with an adjuvant including diluents, extenders,
carriers, and conditioning agents to provide composi-
tions in the form of finely-divided particulate solids,
granules, pellets, solutions, dispersions or emulsions.
Thus, it is believed that the active ingredient could be
WO 92/10098 PCT/US91/09267
-139-
used with an adjuvant such as a finely-divided solid, a
liquid of organic origin, water, a wetting agent,, a
dispersing agent, an emulsifying agent or any su~~table
combination of these.
Suitable wetting agents are believed to
include alkyl benzene and alkyl naphthalene sulfonates,
sulfated fatty alcohols, amines or acid amides, long
chain acid esters of sodium isothionate, esters of
sodium sulfos~uccinate, sulfated or sulfonated fatty acid
esters, petroleum sulfonates, sulfonated vegetable oils,
ditertiary ac~stylenic glycols, polyoxyethylene
derivatives o:E alkylphenols (particularly isooctylphenol
and nonylphenc~l) and polyoxyethylene derivatives of the
mono-higher fatty acid esters of hexitol anhydrides
(e. g., sorbitan). Preferred dis;~ersants are methyl
cellulose, polyvinyl alcohol, sodium lignin sulfonates,
polymeric a1k5,1 naphthalene sulf~~nates, sodium
naphthalene sulfonate, and polymc=thylene bisnaphthalene
sulfonate. WE~ttable powders are water-dispersible
compositions containing one or mare active ingredients,
an inert sol:i~l extender and one car more wetting and
dispersing ag~:nts. The inert so::id extenders are
usually of mir.~eral origin such aac the natural clays,
diatomaceous earth and synthetic minerals derived from
silica and the. like. Examples oi' such extenders :include
kaolinites, attapulgite clay and synthetic magnes:ium
silicate. The wettable powders compositions of this
invention usually contain from above 0.5 to 60 parts
(preferably from 5-20 parts) of active ingredient" from
about 0.25 to 25 parts (preferably 1-15 parts) of
wetting agent" from about 0.25 tc~ 25 parts (preferably
1.0-15 parts) of dispersant and from 5 to about 95 parts
(preferably 5-50 parts) of inert solid extender, all
parts being by weight of the total composition. Where
required, from about 0.1 to 2.0 Farts of the solid inert
extender can be replaced by a corrosion inhibitor or
anti-foaming a~~ent or both.
WO 92/10098 PCT/US91/09267
-140-
Other formulations include dust concentrates
comprising from 0.1 to 60% by weight of the active
ingredient on a suitable extender; these dusts may be
diluted for application at concentrations within the
range of from about 0.1-10% by weight.
Aqueous suspensions or emulsions may be
prepared by stirring a nonaqueous solution of a water-
insoluble active ingredient and an emulsification agent
with water until uniform and then homogenizing to give
stable emulsion of very finely divided particles. The
resulting concentrated aqueous suspension is charac-
terized by its extremely small particle size, so that
when diluted and sprayed, coverage is very uniform.
Suitable concentrations of these formulations contain
from about 0.1-60%, preferably 5-50% by weight of active
ingredient, the upper limit being determined by the
solubility limit of active ingredient in the solvent.
Concentrates are usually solutions of active ingredient
in water-immiscible or partially water-immiscible
solvents together with a surface active agent. Suitable
solvents for the active ingredient of this invention
include dimethylformamide, dimethylsulfoxide, N-methyl-
pyrrolidone, hydrocarbons, and water-immiscible ethers,
esters, or ketones. However, other high strength liquid
concentrates may be formulated by dissolving the active
ingredient in a solvent then diluting, e.g., with
kerosene, to spray concentration.
The concentrate compositions herein generally
contain from about 0.1 to 95 parts (preferably 5-60
parts) active ingredient, about 0.25 to 50 parts
(preferably 1-25 parts) surface active agent and where
required about 5 to 94 parts solvent, all parts being be
weight based on the total weight of emulsifiable oil.
Granules are physically stable particulate
compositions comprising active ingredient adhering to or
distributed through a basic matrix of an inert, finely-
divided particulate extender. In order to aid leaching
WO 92/10098 PCT/US91/092~67
-141-
of the active: ingredient from the particulate ex~ender,
a surface ac:~t.ive agent can be present in the compo-
sition. Natural clays, pyrophyl.lites, illite, and
vermiculite are examples of opez~able classes of
particulate mineral extenders. The preferred extenders
are the porous, absorptive, preformed particles such as
preformed and screened particulate attapulgite or' heat
expanded, particulate vermiculite and the finely-
divided clays such as kaolin clays, hydrated attapulgite
or bentonitic clays. These extenders are sprayed or
blended with 'the active ingredient to form the
herbicidal gr~3nules.
The granular compositions of this invention
may contain from about 0.1 to abut 30 parts by weight
of active ingredient per 100 parts by weight of clay and
0 to about 5 ~~arts by weight of surface active agent per
100 parts by weight of particulate clay.
The compositions of this invention can also
contain other additaments, for e:Kample, fertilizers,
other herbicides, other pesticid~ss, safeners and the
like used as adjuvants or in comJ~ination with any of the
above-descri:bead adjuvants. Chemicals useful in
combination w~Lth the active ingr~adients of this
invention inc7.uded, for example, triazines, ureas,
sulfonylureas, carbamates, acetamides, acetanilides,
uracils, acetic acid or phenol d~arivatives, thiol-
carbamates, t=viazoles, benzoic acid and its derivatives,
nitriles, biprienyl ethers, nitrobenzenes, etc.
Fertilizers useful in combination with the
active ingredients include, for example, ammonium
nitrate, urea, potash and superptrosphate. Other useful
additaments include materials in which plant organisms
take root and grow such as comport, manure, humus, sand
and the like.
Herk~icidal formulation:. of the types described
above contemplated as within the purview of this
invention are exemplified in several illustrative
embodiments below.
WO 92/ 10098 PCT/US91 /09267
-142-
I Emulsifiable Concentrates
Weight Percent
A. Herbicide No. 1 11.0
Antidote No. 9 10.0
Free acid of complex organic phosphate
or aromatic or aliphatic hydrophobe
base (e. g., GAFAC RE-610, registered
trademark of GAF Corp.) 5.59
Polyoxyethylene/polyoxypropylene block
l0 copolymer with butanol (e. g., Tergitol XFi,
registered trademark of Union Carbide Corp.)
Phenol 5.34
Monochlorobenzene 66.96
100.00
B. Herbicide No. 2 25.00
Antidote No. 10 15.00
Free acid of complex organic phosphate
of aromatic or aliphatic hydrophobe
base (e. g., GAFAC RE-610) 5.00
Polyoxyethylene/polyoxypropylene block
copolymer with butanol (e. g., Tergitol
) 1.60
Phenol 4.75
Monochlorobenzene 48-6565
100.00
C. Herbicide No. 3 12.0
Antidote No. 1 12.0
Free acid of complex organic phosphate
or aromatic or aliphatic hydrophobe
base (e. g., GAFAC RE-610, registered
trademark of GAF Corp.) 6.0
Polyoxyethylene/polyoxypropylene block
copolymer with butanol (e.g., Tergitol XH,
registered trademark of Union Carbide
Corp.) 1.5
Phenol 5.5
Monochlorobenzene 63.0
100.00
WO 92/10098 PCT/US91/09267
-143-
Weight= Percent
D. Herbicide: No. 4 20.0
Antidote No. 2 15.0
Free ac:icl of complex organic: phosphate
of aromatic or aliphatic hyctrophobe
base ( a " g' . , GAFAC RE-610 ~5 . 00
Polyoxyet.hylene/polyoxypropylene block
copolymer with butanol (e. g., Tergitol
a?.. 0
Phenol ci . 0
Monochlarobenzene 5.1 . 0
100.00
E. Herbicide No. 5 13..0
Antidote No. 3
Free acid of complex organic phosphate
or aromatic or aliphatic hydrophobe
base (e. g. GAFAC RE-610, registered
trademark of GAF Corp.) 5.59
Polyoxyetlzylene/polyoxypropylene block
copolymer with butanol (e.g., Tergitol XH,
registered trademark of Union Carbide
Corp.) 1.11
Phenol 5.34
Monochlorobenzene 7.96
100.00
F. Herbicide No. 6 15.00
Antidote tTo. 4 10.00
Free acid of complex organic phosphate
of aromatic or aliphatic hyd::ophobe
base (e. g., GAFAC RE-610 5.00
Polyoxyethylene/polyoxypropy:lene block
copolymer with butanol (e. g., Tergitol XH) 1.60
Phenol 4.75
Monochlor~~benzene 6365
100.00
WO 92/ 10098 PCT/US91 /09267
-144-
Weiqht Percent
II. Flowables
A. Herbicide No. 7 15.0
Antidote No. 5 10.0
Methyl cellulose 0.3
Silica Aerogel 1.5
Sodium lignosulfonate 3.5
Sodium N-methyl-N-oleyl taurate 1.0
Water 677
100.00
B. Herbicide No. 8 30.0
Antidote No. 6 15.0
Methyl cellulose .3
Silica aerogel 1.5
Sodium lignosulfonate 3.5
Sodium N-methyl-N-oleyl taurate 1.0
Water 47.7
100.00
C. Herbicide No. 9 20.0
Antidote No. 7 10.0
Methyl cellulose 0.3
Silica Aerogel 1.5
Sodium lignosulfonate 3.5
Sodium N-methyl-N-oleyl taurate 3.0
Water 620
100.00
D. Herbicide No. 1 20.0
Antidote No. 8 25.0
Methyl cellulose 0.5
Silica Aerogel 2.0
Sodium lignosulfonate 3.5
Sodium N-methyl-N-oleyl taurate 2.0
Water 47.0
100.00
a ~ 9 ~
WO 92/10098 PCT/US91/09267
-145-
Weight: Percent
E. Herbicide: No. 2 40.0
Antidote No. 10 20.0
Methyl c:e:llulose .3
Silica aerogel ~
5
.,
Sodium l:ignosulfonate _i . 5
Sodium M-methyl-N-oleyl taurate 1..0
Water 3 y, . 7
lOCi.00
III. Wettable Powders
A. Herbicide No. 2 1~~.0
Antidote :No. ZO 10.0
Sodium li~~nosulfonate 3.0
Sodium N-~aethyl-N-oleyl-taurate 1.0
Amorphous silica (synthetic) 71.0
100.0
B. Herbicide No. 4 60.0
Antidote 1Jo . 1l 2 0 . 0
Sodium dioctyl sulfosuccinate 1.25
Calcium l:Lgnosulfonate 1.75
Amorphous silica (synthetic) 17.0
100.0
C. Herbicide No. 5 10.0
Antidote rfo. 12 10.0
Sodium l:ic~nosulfonate 3.0
Sodium N-methyl-N-oleyl-taurr~te 1.0
Kaolinite clay 86.0
100.00
D. Herbicide No. 6 20.0
Antidote N'o. 13 20..0
Sodium ligwosulfonate 3 , 0
Sodium N-methyl-N-oleyl-taurate 1.,0
Amorphous silica (synthetic) 66..0
100., 0
9109 ~ PCT/US91/09267
L~ '~ =146-
Weicxht Percent
E. Herbicide No. 7 65.0
Antidote No. 14 10.0
Sodium dioctyl sulfosuccinate 1.25
Calcium lignosulfonate 1.75
Amorphous silica synthetic 22.0
100.00
F. Herbicide No. 8 15.0
Antidote No. 15 15.0
Sodium lignosulfonate 3.0
Sodium N-methyl-N-oleyl-taurate 1.0
Kaolinite clay 66.0
100.00
IV. Dusts
A. Herbicide No. 9 2.0
Antidote No. 16 4.0
Attapulgite 940
100.00
B. Herbicide No. 1 50.0
Antidote No. 10 20.0
Montmorillonite 40.0
100.00
C. Herbicide No. 3 10.0
Antidote No. 11 10.0
Ethylene glycol 1.0
Bentonite 69.0
100.00
D. Herbicide No. 3 10.0
Antidote No. 12 12.0
Attapulgite 78.0
100.00
E. Herbicide No. 4 50.0
Antidote No. 13 10.0
Montmorillonite 40.0
100.00
WO 92/ 10098 PCT/ US91 /09267
-147-
Weight Percent
F. Herbicide: No. 5 30.0
Antidote No . 14 3 c) . 0
Ethylene glycol .L.O
Bentonit~ 35).0
100.00
G. Herbicide No. 6
Antidote No. 15 r;,0
Diatomaceous earth c,., 0
100.0
V. Granules
A. Herbicide No. 7 15.0
Antidote No. 16 15.0
Granular attapulgite (20/40 mesh) _7C~_.0
100.0
B. Herbicide No. 8 30.0
Antidote l!Jo . 1 2 0 . 0
Diatomaceous earth (20/40) 70,0
100.0
C. Herbicide No. 9 1.0
Antidote 1Jo . 4 2 . 0
Ethylene c~lycal 5.0
Methylene blue 0.1
Pyrophyll:Lte g 1~ 9
100.0
D. Herbicide No. 1 15.0
Antidote Zio. 1.0 5.0
Pyrophyll~.te (20/40) gp~
100.0
E. Herbicide No. 2 15.0
Antidote Dfo. 9 15.0
Granular a~ttapulgite (20/40 mesh) 70.0
100.0
F. Herbicide No. 3 20.0
Antidote hfo. 11 10.0
Diatomace~~us earth (20/40) 70.0
100.0
WO 92/10098 PCT/US91/09267
-148-
Weig ht Percent
G. Herbicide No. 4 5.0
Antidote No. 12 5.0
Ethylene glycol 5.0
Methylene blue 0.5
Pyrophyllite 890
100.00
H. Herbicide No. 5 10.0
Antidote No. 13 10.0
Pyrophyllite (20/40) 80.0
100.0
VI. Suspension Concentrates
A. Herbicide No. 1 16.0
Antidote No. 18 15.0
Nonylphenol ethoxylate 9.5 mole
EO Sterox NJ 13.8
Sodium lignosulfonate (Reax 88B) 12.2
Water 43.0
100.0
B. Herbicide No. 2 30.0
Antidote No. 19 10.0
Potassium salt of napthalene sulfonate
formaldehyde condensate (DAXAD aag) 9.0
Nonylphenol ethoxylate 10 mole EO
(Igepal CO-660) 90
Water 320
100.0
C. Herbicide No. 3 10.0
Antidote No. 20 10.0
Sodium dioctyl sulfosuccinate Aerosol
OTB 11.0
Castor oil + 36 Ethylene oxide
(FloMo 3G) 11.0
Methanol 60.0
100.0
WO 92/10098 PCT/US91/09267
-149-
Weicrht Percent
D. Herbicide No. 4 x.5.0
Antidote No. 6 5.0
Nonylph.enol ethoxylate 9.5 mole
EO St~~rOX NJ
1.4.8
Sodium l.ignosulfonate (Reax 88B) 1.1.2
Water 54.0
100.0
E. Herbicid~a No. 5 30.0
Antidote No. 7 30.0
Potassiu rn salt of napthalene sulfonate
forma7.dehyde condensate (DAXAD aag) g.0
Nonylphenol ethoxylate 10 mole EO
(Igepa~l CO-660) 7.0
Water 25.0
100.0
F. Herbicide: No. 6 1:8.0
Antidote No. 8 2:2.0
Nonylphen,ol ethoxylate 9.5 mole
EO Sterox NJ 14.0
Sodium lignosulfonate (Reax 88B) 12.0
Water 34.0
10t).0
G. Herbicide No. 7 2c~.0
Antidote No. 9
Potassium salt of napthalene sulfonate
formaldehyde condensate (DAXAD aag) 8.0
Nonylphenol ethoxylate l0 mole EO
(Igepal CO-660) 10.0
Water 48.0
lOCl.O
H. Herbicide No. 8 14..0
Antidote lJo. 10 14.0
Sodium dioctyl sulfosuccinate Aerosol OTB 12.0
Castor oi:l + 36 Ethylene oxide
(FloMo 3G) 12.0
Methanol 48.0
100.0
WO 92/10098 PCT/US91/09267
_ -150-
Weight Percent
VII. Susuoemulsions
A. Herbicide No. 9 15.0
Antidote No. 1 15.0
Calcium dodecylbenzene sulfonate/-
polyoxyethylene ethers blend
(e. g., Atlox 3437F) 13.0
Calcium dodecylbenzene sulfonate
(FloMo 60H)
Sodium salt of a polymerized alkyl
napthalene sulfonic acid (Daxad 1G) 3.0
Water 45.0
100.0
B. Herbicide No. 1 20.0
Co-Herbicide - acetochlor 20.0
Antidote No. 1 20.0
Calcium dodecyl sulfonate/alkylaryl
polyether alcohol blend 9.0
Sodium Lignosulfonate (Marasperse
N-22) 4.10
Water 37.0
100.0
C. Herbicide No. 2 20.0
Co-Herbicide - alachlor 20.0
Antidote No. 10 15.0
Calcium dodecylbenzene sulfonate/-
polyoxyethylene ethers blend
(Atlox~ 3437F) 6.0
Sodium dioctyl sulfosuccinate
Aerosol OT 5.0
Water 34.0
100.0
WO 92/10098 P('f/US91/09267
-151-
Weight: Percent
D. Herbicide No. 3 15.0
Co-herbicide - acetochlor 15.0
Antidote No. 18 5.0
Atlox 3437F 9.0
Sodium s~3lt of a condensed napthalene
sulfonic acid (Tamol SN) 6.0
Water 70.0
100.0
E. Herbicide. No. 1 20.0
Co-Herbic:ide - butachlor 20.0
Antidote No. 10 20.0
Monochlorobenzene 10.0
Atlox 34=~7F 1D.0
Sodium :lignosulfonate (Reax 88H) 5,0
Water 5 ~5 . 0
100.0
F. Herbicide: No. 2 35.0
Co-Herbic:ide - pretilachlor 2c).0
Antidote No. 10 2c).0
Calcium d,odecylbenzene sulfonate/-
polyaxyethylene ethers blend
(e. g., Atlox 3437F) 11.0
Calcium dodecylbenzene sulfonate
(FloMo 60H)
Ei . 0
Sodium salt of a polymerized alkyl
napthalene sulfonic acid (Daxad iG) a.0
Water
loC~ . o
G. Herbicide No. 3 30.0
Co-Herbicide - trimexachlor 15.0
Antidote No. 10 20.0
Calcium d~decyl sulfonate/alkylaryl
polyet:her alcohol blend 11.0
Sodium Li.~3nosulfonate (Marasperse
N-22) 2.0
Water 32.0
100.0
WO 92/ 10098 PCT/US91 /09267
~~ ~ ~ r
_. _ -152-
Weight Percent
H. Herbicide No. 1 28.0
Co-Herbicide - EPTC 20.0
Antidote No. 10 20.0
Calcium dodecylbenzene sulfonate/-
polyoxyethylene ethers blend
(Atlox~ 3437F) 5.0
Sodium dioctyl sulfosuccinate
Aerosol OT 3'.0
10Water 24.0
100.0
VIII. Liquid Concentrates
A. Herbicide No. 2 20.0
Co-Herbicide - EPTC 15.0
15Antidote No. 10 15.0
Xylene 50.0
100.0
B. Herbicide No. 3 30.0
Co-Herbicide - butylate 20.0
20Antidote No. 10 20.0
Dimethyl sulfoxide 30.0
100.0
C. Herbicide No. 1 10.0
Co-Herbicide - acetochlor 15.0
25Antidote No. 9 20.0
N-methylpyrrolidone 55.0
100.0
D. Herbicide No. 2 15.0
Co-Herbicide - metolachlor 15.0
30Antidote No. 9 10.0
Ethoxylated castor oil 15.0
Rhodamine B 1.5
Dimethylformamide 43.5
100.0
o ~~ o
WO 92/10098 PCT/US91/092b7
-153-
Weicxht Percent
E. Herbicide No. 3 lk).0
Co-Herbicide - butachlor 1~).0
Antidote No. 10 10.0
Atlox 3437F Ci.O
Xylene 65.0
100.0
F. Herbicide No. 4 25.0
Co-Herbi.cide - pretilachlor 1_~..0
Antidote No. 7 1C).0
Xylene 50.0
lOCl.O
G. Herbicide No. 5 2~.0
Co-Herbicide - metolachlor 1~~.0
Ant idote :No . 8 2 c'. . 0
Dimethyl ,sulfoxide 38.0
lOCi.O
H. Herbicide No. 6 25.0
Co-Herbicide - butylate 25.0
Antidote 1!10. 9 30.0
N-methylp~,~rrol idone 2 0 . 0
100.0
I. Herbicide No. 7 15.0
Co-Herbic:ide - acetochlor 15.0
2 5 Ant idote 1Jo . 10 2 0 . 0
Ethoxylated castor oil 15.0
Rhodamine B 1.5
Dimethylformamide 34.5
100.0
J. Herbicide No. 8 15.0
Co-Herbicde - alachlor 15.0
Antidote Zto. 11 15.0
Atlox 34:3.'F 5.0
Xylene 30.0
100.0
WO 92/ 10098 PCT/ US91 /09267
-154-
Weight Percent
IX. Microcapsules
A. Herbicide No. 1 encapsulated
in a polyurea shell wall 15.0
Reax~ C-21 5.0
Co-Herbicide - acetochlor 10.0
Antidote No. 10 10.0
Water 60.0
10 0..
0
B. Herbicide No. 2 encapsulated
in a polyurea shell wall 15.0
Co-Herbicide - acetochlor 10.0
Antidote No. 10 10.0
Treax, LTM~ 5.0
Water 60.0
100.0
C. Herbicide No. 2 encapsulated
in a polyurea shell wall 20.0
Co-Herbicide - alachlor 10.0
Antidote No. 10 10.0
Reax C-21 3.0
Water 470
100.0
D. Herbicide No. 2 encapsulated
in a polyurea shell wall 22.0
Co-Herbicide - butachlor 13.0
Antidote No. 18 10.0
Reax 88~B 2.0
Water 43.9
100.0
E. Herbicide No. 2 encapsulated
in a polyurea shell wall 16.0
Co-Herbicide - pretilachlor 10.0
Antidote No. 19 15.0
Reax~ C-21 4.0
Water 55.0
100.0
~Q 95Q ~Q
WO 92/ 10098 P(T/US91 /09267
-155-
Weicxht: Percent
F. Herbicide No. 2 encapsulated
in a polyurea shell wall I,g,p
Co-Herbicide - EPTC 5.0
Antidote No. 20 15.0
Treax, I~I'~i
5.0
Water 57.0
100.0
G. Herbicide No. 1 encapsulated
in a ~?olyurea shell wall 10.0
Co-Herbic:ide - butylate 10.0
Antidote No. 10 15.0
Reax C-2:L 5.0
Water _60.0
100.0
H. HerbicidE: No. 1 encapsulated
in a F>olyurea shell wall 10.0
Co-Herb:ic;ide - metolachlor 10.0
Antidote No. 10 20.0
Reax 88~EI dZ . 0
Water 58.0
lOC).0
#~2~1~9~ ~ PCT/US91/09267
-156
As will be appreciated by those skilled in the
art, the practice of this invention comprises the use of
the antidotal compounds disclosed and claimed herein
with any herbicidally-active azolopyrimidine sulfonamide
or derivative compound which may optionally be combined
with co-herbicides from many different classes of
chemistry. Obviously, the above listings of exemplary
compounds is not intended to be exhaustive, but
representative. Again, as noted earlier herein, .it is
expected that not every combination of herbicide and
antidote will result in safening of all crops, but it is
within the skill of the art to test any given herbicide
with an invention antidote in plant screens of any
spectrum of plants and note the results.
The foregoing embodiments illustrate that the
combinations of herbicide and antidote of this invention
are useful in controlling weeds while reducing herbici-
dal injury to crop plants under greenhouse test condi-
tions.
The herbicide, antidote, or a mixture thereof,
may be applied to the plant locus without any adjuvants
other than a solvent. These mixtures may be in the form
of emulsifiable concentrates, microencapsulates, parti-
culate solids, granules of varying particle size, e.g.,
water-dispersible or water-soluble granules or larger
dry granules, pellets, wettable powders, dusts,
solutions, aqueous dispersions, or emulsions.
Examples of suitable adjuvants are finely-
divided solid carriers and extenders including talcs,
clays, pumice, silica, diatomaceous earth, quartz,
Fuller's earth, sulfur, powdered cork, powdered wood,
walnut flour, chalk, tobacco dust, charcoal, and the
like. Typical liquid diluents include Stoddard's
solvent, acetone, methylene chloride, alcohols, glycols,
ethyl acetate, benzene, and the like. Liquids and
wettable powders usually contain as a conditioning agent
one or more surface-active agents in amounts sufficient
to make a composition readily dispersible in water or in
WO 92/10098 PCT/US9l/09267
-157-
oil. The term "surface-active agent" includes wetting
agents, dispersing agents, suspending agents, and
emulsifying agents. Typical surface-active agenta are
mentioned in U.S. Patent No. 2,547,724.
Compositions of this invention generally
contain from..3bout 5 to 95 parts herbicide-and-ar,~tidote,
about 1 to 50 parts surface-active agent, and about 4 to
94 parts solvent, all parts being by weight based on the
total weight ~~f the composition.
The crop may be protected by treating the crop
seed with an e=ffective amount of antidote prior to
planting. Generally, smaller amounts of antidote are
required to treat such seeds. A weight ratio of as
little as 0.5 parts of antidote per 1000 parts of seed
may be effective. The amount of antidote utilized in
treating the geed may be increased if desired.
Generally, howrever, a weight ratio of antidote-to-seed
weight may range from 0.1 to 10.0 parts of antidote per
1000 parts of seed. Since only a very small amount of
active antidote is usually required for the seed
treatment, the. compound preferably is formulated as an
organic solution, powder, emulsifiable concentrate,
water solutian, or flowable formulation, which can be
diluted with water by the seed treater for use in seed
treating apparatus. Under certain conditions, it may be
desirable to dissolve the antidote in an organic :solvent
or carrier far use as a seed treatment or the pure
compound alone may be used under properly controlled
conditions.
For antidote seed-coating for antidotes
applied to soil in granular or liquid formulations,
suitable carriers may be either solids, such as talc,
sand, clay, di~3tomaceous earth, sawdust, calcium
carbonate, and the like, or liquids, such as water,
kerosene, acetone, benzene, toluene, xylene and th.e
like, in which the active antidote may be either
dissolved or d:~spersed. Emulsifying agents are used to
achieve a suitable emulsion if two immiscible liquids
WO 92/10098 PCT/US91/09267
a -158-
are used as a carrier. Wetting agents may also be used
to aid in dispersing the active antidote in liquids used
as a carrier in which the antidote is not completely
soluble. Emulsifying agents and wetting agents are sold
under numerous tradenames and trademarks and may be
either pure compounds, mixtures of compounds of the same
general groups, or they may be mixtures of compounds of
different classes. Typical satisfactory surface active
agents which may be used are alkali metal higher-.
alkylarylsulfonates such as sodium dodecylbenzene-
sulfonate and the sodium salts of alkylnaphthalene-
sulfonic acids, fatty alcohol sulfates such as the
sodium salts of monoesters of sulfuric acid with n-
aliphatic alcohols containing 8-18 carbon atoms, long-
chain quaternary ammonium compounds, sodium salts of
petroleum-derived alkylsulfonic acids, polyethylene
sorbitan monooleate, alkylaryl polyether alcohols,
water-soluble lignin sulfonate salts, alkali casein
compositions, long-chain alcohols usually containing 10-
18 carbon atoms, and condensation products of ethylene
oxide with fatty acids, alkylphenols and mercaptans.
The invention herein has been specifically
exemplified with the herbicidal compounds identified
above as Herbicide Nos. 1-9 as representative of the
compounds of Formula I, by the commercial herbicides
acetochlor and metolachlor as representative of the co-
herbicidal compounds of Formula V and by butylate and
EPTC as representative of the thiocarbamate class of
herbicides and by various dichloroacetamide antidotes as
representative of the compounds according to Formulae II
and III, as well as a multiplicity of other antidotes
having a variety of chemical structures. It is to be
understood that other compounds within the scope of the
above formulae and other chemical classes are specifi-
cally contemplated as within the scope of this inven-
tion. For example, other triazolopyrimidine - and
imidazolopyrimidine sulfonamides and their derivatives
VI~O 92/10098 ~ ~ "~ ~ P('f/US91/09'G7
-159-
contemplated herein include the compounds described in
the following U.S. patents and EP applications as
relevant to the compounds of Formula I:
A. Compound: wherein R is the -SOzN(R6) (R~) moiety A and
B are both N and
1. R~ and R2 are discrete, uncomb:ined radicals:
4,889,553
4,959,094
2. R~ and RZ are combined to form substituted
arid/or unsub~~tituted bivalent radicals which may contain
one or more hetero atoms and saturated, partially
saturated or unsaturated bonds:
4,740,233 4,E54,964 5,041,157
4,741,764 4,960,455 EP Appln. 0 375 076
4,755,212 4,$59,231 EP Appln. 0 343 752
4,818,273 4,795,483 AU Appln.AU-A-68391
4,886,883 4,910,306
4,~>54,163 4,979,981
4,959,473 5,013,351
B. Compounds analogous to those in A2 above, except
that in Formula I only one of A or B is N while the
other is CR3 z~s defined above:
4,731,446
4,799,952
4,892,5'76
C. Compounds wherein R is the -N(R~)SOzRS moiety, A and B
are both N and R~ and R2 are combined to form a bivalent
radical as in A2 above:
4,638,0'75 4,822,404
4,650,892 4,685,958
The above specifically mentioned herbicidal
compounds used as co-herbicides herein are intended
merely as exemplary of the classes of herbicides which
they represent. However, it is expressly contemplated
that many other herbicidal compounds analogous to those
represented h.=rein having a variety of equivalent radi-
WO 92/ 10098 I'Crl US91 /092E7
f -160-
cals substituted on the central nucleus may similarly be
safened to various crop plants to a greater or lesser
extent with the antidotal compounds of. this invention.
For example, other a-haloacetamide and a-haloacet-
anilide compounds useful as herbicides are described in
U.S. Patent Numbers 3,442,945, 3,547,620, 3,574,746,
3,586,496, 3,830,841, 3,901,768, 4,249,935, 4,319,918,
4,517,011, 4,601,745, 4,657,579 and 4,666,502 and
Australian Patent No. AU-A1-18044/88.
Herbicidally-useful thiocarbamate compounds
are described in U.S. Patent Nos. 2,913,327, 3,330,643
and 3,330,821.
Other herbicidal pyridine compounds are
described in U.S. Patent 4,6,92,184 and U.S. Patent
4,826,532, of common assignment herewith.
Herbicidally-useful heterocycyl phenyl ethers
(especially pyrazolyl aryl ethers) are described, e.g.,
in U.S. Patent 4,298,749.
Herbicidal diphenyl ethers and nitrophenyl
ethers include 2,4-dichlorophenyl 4'-nitrophenyl ether
("nitrofen"), 2-chloro-1-(3'-ethoxy-4'-nitrophenoxy)-4-
trifluoromethylbenzene ("Oxyfluorfen"), 2',4'-dichloro-
phenyl 3-methoxy-4-nitrophenyl ether ("Chlormethoxy-
nil"), methyl 2-[4'-(2", 4"-dichlorophenoxy)-phenoxy]-
propionate, N-(2'-phenoxyethyl)-2-[5'-(2"-chloro-4"-
trifluoromethylphenoxy)-phenoxy]-propionamide, 2-
methoxyethyl 2-[vitro-5-(2-chloro-4-trifluoromethyl-
phenoxy)-phenoxyl-propionate and 2-chloro-4-tri-
fluoromethylphenyl 3'-ox3zolin-2'--yl-4'-nitroPhenyl-
ether.
Another generic class of agrichemically-
important herbicidal~compounds specifically contemplated
for use as co-herbicidal compounds in combination with
the antidotal compounds of this invention are the ureas
and sulfonylurea derivatives. Important herbicidal
ureas include 1-(benzothiazol-2-yl)-1,3-dimethylurea;
WO 92/10098 PCC/US91/092b~7
-161-
phenylureas, Eor example: 3-(3-chloro-p-tolyl)-1,1-
dimethylurea ("chlorotoluron"), 1,1-dimethyl-3-(a,a,a-
trifluoro-m-tolyl)urea ("fluometuron"), 3-(4-bromo-3-
chlorophenyl)~-methoxy-1-methylurea ("chlorbromuron"),
3-(4-bromophenyl)-1-methoxy-1-methylurea ("metobro-
muron"), 3-(3,,4-dichlorophenyl)-1-methoxy-1-methylurea
("linuron"), :3-(4-chlorophenyl)-1-methoxy-1-methylurea
("monolinuron"), 3-(3,4-dichlorophenyl)-1,1-dimethyl-
lurea ("diuron"), 3-(4-chlorophenyl)-1,1-dimethylurea
("monuron") .and 3-(3-chloro-4-methoxyphenyl)-1,1-
dimethylurea i;"metoxuron");
Important herbicidal sulfonylureas and
sulfonamides :specifically contemplated as useful as co-
herbicides in compositions with the antidotal compounds
of this invention include those disclosed in the
following patents: U.S. Patent Numbers 4,383,113,
4,127,405, 4,979,821, 4,481,029, 4,514,212, 4,420,325,
4,638,004, 4,,E~75,046, 4,681,620, 4,741,760, 4,723,123,
4,411,690, 4,,i'18,937, 4,620,868, 4,668,277, 4,592,776,
4,666,508, 4,,E~96,695, 4,731,446, 4,678,498, 4,786,314,
4,889,550, 4,931,081 and 4,668,279; EP Numbers 08x4224,
173312, 87780, 190105, 256396, 264021, 264672, 142152,
244847, 176304, 177163, 187470, 187489, 184385, 232067,
234352, 189069, 224842, 249938, 246984 and 282613, and
German Offen« DE 3,618,004.
Among other herbicidal sulfonylureas disclosed
in one or more: of the above patents which are of parti-
cular interest, are mentioned the species N-[(4-methoxy-
6-methylpyrimidin-2-yl)aminocarbonyl]-3-chloro-4-
methoxy-carbonyl-1-methylpyrazole-5-sulfonamide; N-[(4-
methoxy-6-methylpyrimidin-2-yl)aminocarbonyl]-3-chloro-
4-ethoxycarbanyl-1-methylpyrazole-5-sulfonamide; N-
[(4,6-dimethoxypyrimidin-2-yl)aminocarbonyl]-3-chloro-
4-ethoxycarbanyl-1-methylpyrazole-5-sulfonamide; N-[(4-
methoxy-6-methylpyrimidin-2-yl)aminocarbonyl]-3-bromo-
4-ethoxycarbanyl-1-methylpyrazole-5-sulfonamide; N-
[(4,6-dimethaxypyrimidin-2-yl)aminocarbonyl]-3-bromo-4-
WO 92/10098 PCT/US91/09267
-162-
ethoxy-carbonyl-1-methylpyrazole-5-sulfonamide and N-
(methoxy-carbonyl-1-phenyl sulfonyl-N'-(bis-
difluoromethoxy-pyrimidin-2-yl)urea.
other herbicidal imidazolinone or imidazolidi-
none or -dione compounds within the purview of this
invention as co-herbicides which may be safened for use
in various crops include the compounds disclosed in the
following exemplary publications: EP Numbers 041623,
133310, 198552, 216360 and 298029; JA 1109-790,
JA 1197-580A, J6 1183-272A and J6 3196-750A;. and
Australian published Application No. AU 8661-073A,
GB 2 172 886A and U.S. Patent Numbers 4,188,487,
4,297,128, 4,562,257, 4,554,013, 4,647,301, 4,638,068,
4,650,514, 4,709,036, 4,749,403, 4,749,404, 4,776,619,
4,798,619 and 4,741,767.
Still other classes of herbicidal compounds
contemplated for combination with azolopyrimidine sul-
fonamide derivatives and the antidotes of this invention
include the following representative species:
Triazines and triazinones: 2,4-bis-(isopropylamino)-6-
methylthio-1,3,5-triazine ("prometryn"), 2,4-bis-(ethyl-
amino)-6-methylthio-1,3,5-triazine ("simetryn"), 2-
(1',2'-dimethylpropylamino)-4-ethylamino-6-methyl-thio-
1,3,5-triazine ("dimethametryn"), 2-(chloro-4,6-bis-
(ethylamino)-1,3,5-triazine ("simazine"), 2-tert-butyl-
amino-4-chloro-6-ethylamino-1,3,5-triazine ("terbuthyl-
azine"), 2-tert-butylamino-4-ethylamino-6-methoxy-1,3,5-
triazine ("terbumeton"), 2-tertbutylamino-4-ethylamino-
6-methylthio-1,3,5-triazine ("terbutryn"), 2-ethylamino-
4-isopropylamino-6-methylthio-1,3,5-triazine ("ametryn")
and 3,4-bis-(methylamino)-6-tert-butyl-4,4-dihydro-
1,2,4-triazin-5-one.
Oxadiazolones: 5-tert-butyl-3-(2',4'-dichloro-5'-iso
propoxyphenyl)-1,3,4-oxadiazol-2-one ("Oxadiazon").
NO 92/10098 ~ ~ ~ ~ PCT/US91/09267
-163-
Phosphates: S-2--methylpiperidinocarbonylmethyl O,O-di-
propyl phosphorodithioate ("Piperophos").
Pyrazoles: L,3-dimethyl-4-(2',4'-dichlorobenzo:Lyl)-5-
(4'-tolylsul:Eonyioxy)-pyrazole; aryl- and heterocyclic-
substituted pyraz oles, e.g. , as exemplified in 3~P No.
0361114; Jap;~nese Kokai No. JP 50137061 and U.S. Patent
4,008,249. ;?referred species of such substituted-
pyrazole com~~ounds include 4-chloro-3-(4-chloro-2-
fluoro-5-(2-~~ropynyloxy)phenyl)-1-methyl-5-(methyl-
sulfonyl)-1F; pyrazol_e and analogs thereof, e.g., where
the substitu~~nt .in the 5-position of the pyrazole ring
is a haloalkyl radical, preferably CF3.
Alao a--(phenoxyphenoxy)-propionic acid
derivatives and a-pyridyl-2-oxyphenoxy)-propionic acid
derivatives.
Ot::;~er herbicidal compounds useful as co-
herbicides with the azolopyrimidine sulfonamide
compounds of' Formula I include aromatic and heterocyclic
di- and triketones exemplified in U.S. Patent Nos.
4,797,147, 4,853,028, 4,854,966, 4,855,477, 4,938,796
and 4,869,748.
Still other co-herbicidal compoundB contem-
plated herein are pyrrolidinones, e.g, the 1-phenyl-3-
carboxyamidopyrrolidinonea discloBed in U.S. Patent
4,874,422, and the 1-phenyl-4-haloalkylpyrrolidones
discloBed i.n U.S. Patent 4,515,627, etc.
;till other herbicidal compounds useful as co-
herbicides herein include benzoic acid derivatives of
the type exemplified by 5-(2'-chloro-4'-trifluoromethyl-
phenoxy)-2--nitrobenzoic acid ("Acifluorfen"), 2,6-
dichlorobenzonitrile ("dichlobenil"), 3,6-dichloro-2-
methoxybenzoic acid ("dicamba"), etc. and compounds
disclosed in U.S Patents 3,013,054, 3,027,248 a.nd
3,979,437, ~~tC.
WO 92/10098 PCf/US91/09267
-164-
In addition to the antidotal compounds
exemplified herein, other representative antidotal
compounds according to Formula II or other structure
expressly contemplated herein are disclosed in various
patents, e.g., U.S. Patent Nos. 3,959,304, 4,072,688,
4,137,070, 4,124,372, 4,124,376, 4,483,706, 4,636,244,
4,033,756, 4,493,726, 4,708,735, 4,256,481, 4,199,506,
4,251,261, 4,070,389, 4,231,783, 4,269,775, 4,152,137,
4,755,218, 4,964,893, 4,623,727, 4,822,884, 4,851,031,
4,902,340, 4,749,406, 4,758,264, 4,785,105, 4,785,106,
4,294,764, 5,028,256 and 5,037,468; EP Nos. 159,287,
159,290, 258,184, 94,349, 2,121,403, 0253291, 0007588,
0190105, 0229649, 0430004 and 16618; and South African
Patent No. 82/7681.
Although this invention has been described with
,;: respect to specific embodiments, the details of these
embodiments are not to be construed as limitations. Various
equivalents, changes and modifications may be made without
departing from the spirit and scope of this invention, and
it
is understood that such equivalent embodiments are part of
this invention.