Note: Descriptions are shown in the official language in which they were submitted.
WO 92/08507 PCT/CA91 /00409
- 1 -
2U956~~
SYRINGE -
This invention relates to prefilled syringes for
use in medical or veterinary treatment.
In the applicant's published European Patent
Application 0298585, there is disclosed a syringe system
based on the concept of a "bottomless vial" in which the
conventional base of a glass vial is replaced by a rubber
piston which forms a hermetic seal with the walls of the
vial, and which has a downward extension within the vial
which can be coupled to a syringe plunger. When a plunger
is so coupled to the piston, and an adaptor is applied to
a cap of the vial, the vial is converted into a syringe.
An extremely important advantage of a syringe based upon
such a bottomless vial, as compared to conventional
prefilled syringe systems, is that it can be sterilized,
filled and capped utilizing conventional vial filling
equipment generally available and in use in the
pharmaceutical industry, rather than requiring specialized
capital intensive filling systems, and that the number of
clean room operations required in production of the product
is greatly reduced as compared with known syringe assembly
and filling systems. As described in more detail in the
above application, t.'~e system is also extremely versatile
in that the "bottomless vial" can form the basis of a wide
range of syringe delivery systems.
The very reason for the advantages of the above
system, namely that the vials can be sterilized, filled and
capped utilizing conventional vial filling machinery, is
also a limitation in certain applications, since the vials
must remain stable whilst passing through the machinery,
which in turn limits the permissible height of the vial
relative to its diameter. This problem is aggravated by
the necessity for providing a bead around the external
periphery of the bottom of the vial, both in order to
WO 92/08507 PCT/CA91 /00409
20JGGG> - 2 _
provide necessary strength to the glass and to provide
means for anchoring a component of the syringe such as a
combined piston retainer, plunger grip and plunger guide
which is applied after filling of the vial, by pressing it
over the bead. The projection of the bead, although kept
to a minimum, interacts with the beads of adjacent vials
and other obstructions in such a manner as to reduce the
stability of the vial. There are also constraints on the
shaping of the bead so as to prevent ramping effects as the
beads interact which tend to promote tipping.
Additionally, the application of the adaptor to the cap of
the vial is an additional clean-room operation which is not
required during the filling of conventional vials.
Although it is generally considered that the
inner side walls of syringes should be smooth and
unobstructed, it has now unexpectedly been found that
considerable advantages can be obtained by forming the
peripheral bead at the base of a "bottomless vial" at least
partially inwardly of an internal wall of the glass body of
the vial. This makes it possible for any external extent
of peripheral bead to be reduced or eliminated, which
improves the stability of the vial and enables its height
to diameter ratio to be somewhat increased. It is also
found that a versatile combined finger grip and plunger
guide of much simplified construction yet improved
performance can be utilized in conjunction with the
modified bead.
It is also found that in many applications it is
possible to dispense with the application of a separate
adaptor to the cap of the vial by incorporating an adaptor
into the cap structure in a manner which does not interfere
with normal operation of the conventional vial capping
machinery.
_ _. ,
WO 92/08507 PCT/CA91 /00409
- 3 - 2096662
These and other features of the invention are set
forth in the appended claims.
For a better understanding of the invention,
reference may be had to the following description of
preferred embodiments thereof, with reference to the
accompanying drawings in which:
Figure 1 is an exploded isometric view of the
components of a first embodiment the syringe;
Figure 2 is a vertical section through a vial
l0 portion of the syringe, ready for filling;
Figure 3 is a longitudinal section through an
assembled syringe, after discharge of its contents;
Figure 4 is a fragmentary longitudinal section on
an enlarged scale of a portion of the syringe shown in
Figure 3, showing a modification of the arrangement shown
in that Figure; and
Figure 5 is an enlarged vertical section through
the bead of a second embodiment of the syringe, also
showing adjacent parts of a modified piston retainer and
finger grip.
Referring to Figures 1 - 4 of the drawings, a
syringe comprises a syringe barrel in the form of a
somewhat elongated glass vial 2, of which the bottom wall
is absent apart from a slight inward projection of a
strengthening bead 6 formed at the bottom of a side wall 4
of the vial and best seen in Figure 4. In the example
shown the strengthening bead 6 also has a very slight
outward projection, but this is far smaller than would be
necessary if the bead were formed wholly externally of the
side wall 4, and may be entirely eliminated. In any event,
the outward extent of the proj ection should be insuf f icient
to prevent vials from standing very closely adjacent to one
another without sufficient space to tip. Typically the
projection will not exceed about one fifth of the total
thickness of the bead. The projection of the bead on the
i
WO 92/08507 PCT/CA91 /00409
20JG6fi~ - 4
inside should also be limited, both so that the head 10 of
a moulded rubber piston 8 can be inserted into the vial
past the projection (this is facilitated by the presence of
peripheral grooves 12 in the head between sealing lands
14), and so that a sleeve 18 of a combined finger grip,
piston stop and plunger guide 16 (henceforth referred to as
the finger grip) can be pushed past the projection whilst
remaining a snug fit within the side wall of the vial.
Insertion is facilitated by the slight flare provided at
the bottom entry to the vial body by the rounding of the
bead, and the insertion is readily mechanised.
The piston 8 is also provided with an integrally
moulded downward extension 20 which is formed with a
central cavity 23 to increase its flexibility relative to
the head 10 of the piston which is substantially solid.
The piston is dimensioned so that when it is inserted in
the vial 2, the lands 14 are compressed sufficiently to
form a hermetic seal against the interior of wall 4 whilst
permitting the piston to be moved longitudinally of the
vial. Initially, the piston is located at the bottom of
the vial (see Figure 2), with the bottom of extension 20
just within the vial so that it does not affect the ability
of the vial to stand upright on its base formed by the bead
6. The location of the fairly massive solid rubber piston
8 at the base of the vial helps stabilize the empty vial 2,
even when the height of the latter is somewhat greater
relative to its diameter than is normally required for
stability. The practical limit of the height to diameter
ratio is set entirely by the requirement that the vials can
be conveyed through a conventional vial filling and capping
machine in a sufficiently stable manner to permit reliable
operation of the machine at an acceptable speed. In the
example shown, the vial has an outside diameter of
approximately 3 cm and a height of 12.8 cm. For this
diameter a height of 14 centimetres is believed to approach
the practical limit for stability, but this ratio will vary
_. WO 92/08507 PCT/CA91 /00409
- 20966~~
somew~ ~t according to the relative wall thickness of the
vial :_~~d the weight of the piston. Provided that the
outward projection of the bead 6 is insufficient to affect
stability, so that -.~e vials can jostle without applying
5 tipping force to each other, and assuming use of a piston
generally as described, the maximum ratio attainable should
be greater than 4, but will be less than 5.
The stopper 22 and cap 24 applied by the
conventional vial filling and capping machinery may be of
conventional construction, although the stopper 22 is
preferably designed substantially to fill the neck of the
vial so as to minimize dead space above the piston when the
latter is pushed to the top of the vial (See Figure 3).
This ensures that as much as possible of the contents of a
syringe formed from the vial can be expelled by movement of
the piston.
The cap 24 is preferably modified as shown in
Figure 3 and Figure 5 . In Figure 3 , a conventional main
cap cooperates with a moulded plastic adaptor assembly
comprising an annular flange 26 within the cap, a
cylindrical extension 28 extending through the cap and a
thin diaphragm 30 closing a bottom end of the extension.
An internal thread 32, similar to that provided on
conventional syringe adaptors for receiving needles, such
as those sold under the trade-mark LUER-LOK, is formed
within the adaptor. A removable push on cap may be
provided to close the open end of the adaptor during
storage, being removed prior to use. In Figure 4, the
cylindrical extension 28 is formed integrally with the
aluminum cap, again with an internal thread 32. I have
found that the extension 28 can be accommodated by
conventional vial capping machinery, at any rate with no
more than minor modification, without interfering with the
capping process, whilst the provision of such an extension
2~9fi 66
- 6 -
enables the elimination of a separate adaptor cap, and the
additional assembly step required to apply it.
In order to convert the vial into a syringe,
either a double ended needle 34 of the blood collecting
type may be applied directly to the extension 28 (See
Figure 4 ) or an adaptor 3 6 ( See Figures 1 and 3 ) may be
provided for any needle or alternative delivery device
equipped with a standard syringe coupling so as to provide
the latter with the capability of penetrating the stopper
22, as well as the diaphragm 30 if present. The adaptor 36
has a needle 38 and external thread 40 at one end, the
needle providing the penetration function and the thread 40
engaging the thread 32, while its other end provides an
internally threaded socket 42 and coaxial spigot 44 for
forming a fluid-tight coupling to the needle or the like.
Prior to fitting the double ended needle 34, or
needle and adaptor 36, a plunger 46 is applied to the
extension 20 of the piston. The plunger has a shaft 48, of
cruciform cross-section in the example shown, an internally
threaded sleeve 50 at its one end, and an end flange 52 at
its other end. The sleeve 50 has internal multistart
threads 54, complementary to external multistart threads 56
on the extension 20. The lands between the threads 54 on
the sleeve 50 and the threads 56 on the extension 20~ both
stop short respectively of the outer end of the sleeve 50
and the inner end of the extension 20 so as to form
abutments which prevent the sleeve 50 from being screwed
tightly against the underside of the head 10 of the piston.
This means that any tilting forces applied to the plunger
are applied to the relatively flexible extension 20 and not
directly to the head 10, thus minimizing the risk of
breaking the hermetic seal between the head 10 and the
vial.
SUBSTITUTE SHEET
21, .12. 92 ~9
i
CA 02096662 2002-09-19
The plunger is formed of a hinge-forming
synthetic plastic such as a pharmaceutical grade
polypropylene, and a generally semicircular peripheral
portion 62 of the flange and is separated from the
remainder by a slot 64, remaining connected only by thin,
hinge-forming connections 66. This portion 62 provides a
f finger loop which can be pulled rearwardly, as shown by
broken lines in Figure 1, to facilitate handling of the
plunger. As a supplemental or alternative feature, a
notch 71 may be formed in the shaft 48 of the plunger, to
provide a hook by means of which the syringe may be
suspended when used in certain infusion applications.
In order to provide the various functions of
preventing total withdrawal of the piston, forming a
guide for the plunger and restricting its tilting
movements, and providing a finger grip for the user, the
combined finger grip 16 and retainer is pressed into the
bottom of the vial 2 after filling and capping of the
latter. It comprises the sleeve 18 and a peripheral
flange forming oppositely extending finger tags 68. It
is also moulded from pharmaceutical grade plastic such as
polypropylene. The sleeve 18 is a resilient press fit in
the open end of the vial 4 so that it is slightly
compressed by the internal projection of the bead 6
during insertion. Insertion of the retainer 16 may be
facilitated by moderate warming of at least the retainer
and the slight flare provided by the rounding of the bead
6 also facilitates insertion. Beneath the grips 68 of
the sleeve has shallow arcuate grooves 70 in which a
portion of the bead 6 snaps as the sleeve is pressed
home. Forces applied to the grips 68 tending to pull the
sleeve 18 away from the vial in turn tend to deform the
sleeve 18, in such a manner as to increase the grip of
the
i
CA 02096662 2002-09-19
grooves 70 on the portion of the bead thus resisting
withdrawal of the sleeve 18.
During the manufacture, the empty vials 4 are
conveyed through a conventional sterilizing station, the
piston 8 is inserted in each vial 4, and the latter is
filled and capped utilizing conventional vial filling and
capping machinery (but preferably using a modified cap as
shown in Figures 1 and 3 or Figure 5). The guide and
finger grip 16 is then pressed into the base of the vial,
which is shipped with the plunger 46 unattached. Prior
to use, the plunger 46 is screwed onto the piston, and a
needle or the like is applied to the extension 28,
utilizing an adapter 36 if necessary so as to penetrate
the stopper 22, at which point the syringe is ready for
use.
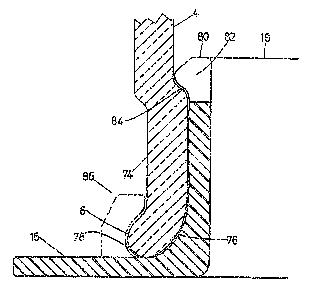
A modified configuration of the bottom end of
the vial body is shown in Figure 5, in which an
alternative approach is utilized to bringing the bead at
the bottom end substantially within the diameter of the
cylindrical vial body. Peripheral beads around the
openings of glass bodies of this type are conventionally
formed by flame softening the glass and adjusting the
positioning and profile of the bead by rolling the body
against suitable forming surfaces. In the Figure 5
embodiment, a bottom portion 74 of the body 4 is flame
softened and rolled so as slightly to reduce its diameter
over about a length of typically 5-6 mm, with a fairly
conventional out-turned rounded bead 6 formed by flaring
the bottom of this reduced diameter section. The
reduction in diameter is such that at least the greater
part of the bead is within the general diameter of the
body, but is not so great as to require excessive force
in inserting the piston. In the example shown, the
outside diameter of the bead is very slightly greater
I
CA 02096662 2002-09-19
_ g _
than the general outside diameter of the body but this
need not be so. In a typical example, the inside and
outside diameters of the main portion of the vial body
are 27 mm and 30 mm respectively, providing a wall
thickness of 1.5 mm, and the reduction in diameter at the
bottom is about 1 mm. The bead can then be formed by
flaring the bottom end of the vial without increasing the
outside diameter of the bead significantly beyond that of
the main portion of the vial, typically by no more than
0.5 mm. A significant flare 76 can be provided which
facilitates insertion of the piston despite the reduction
in diameter of the bottom of the body, and, because of
the flare, the bottom contact line 78 of the vial when
free-standing on a plane surface is substantially
coincident with the outside diameter of the main body 4
of the vial, thus maximizing stability. Juxtaposition of
the vial bodies in the event of jostling on a line will
prevent any ramping tendencies which might otherwise
occur with a flared bottom configuration of this type.
Whilst the presence of the piston after its
insertion in the vial body acts to introduce a
substantial mass which tends to stabilize the vial, the
mass of the piston relative to that of the vial body will
decrease as the height of the latter increases.
Nevertheless it will result in a smaller elevation of the
center of gravity of the assembly as the vial becomes
higher than would otherwise be the case. It is also
desirable that the vial bodies be stable without the
piston present so that they may be conveyed through a
sterilizer prior to insertion of the pistons. The
present invention is particularly valuable in this
respect since the disturbing influence of a bead at the
open end projecting beyond the diameter of the main
i
CA 02096662 2002-09-19
- 9a -
portion of the body is particularly severe under such
conditions.
In order to cooperate with the modified vial
body profile, the finger grip/retainer 16 must also be
modified. The groove 70 is replaced by a bead 80 at the
upper end of the cylindrical portion 18, which bead may
be moulded with slots 82 if necessary to facilitate
insertion, and/or the component 16 may be warmed to
facilitate insertion. The bead must retain the component
with sufficient tenacity to withstand pressures from the
piston which may be developed through pressure build-up
in the vial during normal
WO 92/08507 PCT/CA91 /00409
- 10 -
storage, although it should be noted that contact of the
piston on the bead may actually help retain it by forcing
it against the shoulder 84. Alternatively or additionally,
claws 86 may be moulded onto the component 16 to retain it
by engagement with the bead 6. If the portion 18 of the
component 16 is dimension so as to abut the lower face of
the piston, it will further assist in stabilizing the latter
and maintaining an hermetic seal.