Note: Descriptions are shown in the official language in which they were submitted.
209'99"7
DIR 0518
Vitamin D comDOUnds and method of preparing these comuounds
The invention relates t~o new vitamin D compounds, to a method of
preparing these compounds and to their use in pharmacotherapy and
cosmetics. The invention further relates to valuable new intermediates.
It is generally known, that vitamin-D compounds or vitamin-D related
compounds ("vitamin-D compounds") have a strong biological activity and
may be used in all thoe;e cases in which problems with the calcium
metabolism play a part. A few years ago it was found that various active
vitamin-D compounds also have other pharmacotherapeutic activities and
may be used successfully, for example, for the treatment of certain skin
and bone diseases, for cosmetic applications and for treating diseases
which are related to cell differentiation, cell proliferation or
imbalance in the immune system, including diabetes mellitus,
hypertension and inflammatory diseases such as rheumatoid arthritis and
asthma. In addition, these compounds may be used in various veterinary
applications, and for diagnostic purposes.
Vitamin D compounds which are of interest for the above applications are
hydroxylated vitamin D compounds, in particular vitamin D compounds
hydroxylated in the la-, 24- and/or 25-positions. Recent developments in
the field of active vitamin D compounds are 19-nor-vitamin D compounds
(EP-A-0387077) and C,8-modified vitamin D compounds (EP-A-0521550),
preferably also hydroxylacted in the la-position and optionally in the
C,~-side chain. Other modifications of the C,~-side chain have been
proposed, likewise to improve the intended activity and to suppress
detrimental side-effects. Examples of modifications of the C,~-side chain
are chain elongations (homo compounds), 22-oxa modifications, fluor
substitutions, epo:Ky groups (e.g. WO 92/21695), etc. In addition certain
24-cyclopropyl-modified vitamin D compounds are disclosed in literature,
e.g. in WO 87/00834 (for- treating abnormal cell differentiation and
proliferation) anal in an article by Farach-Carson et al. in
Endocrinology 1991, 129, 1876-84. Generally, however, the above C,.,-side
chain modified vit~imin D compounds are still not completely satisfactory
as regards their scalectiv~=_ activity, i.e. the intended activity without
detrimental side-effects.
CA 02097997 2004-O1-27
27072-153
2
Further, the accessibility of the C,~ side chain modified vitamin D
compounds is often insufficient or unattractive. As an example, the
preparation of the above vitamin D compound disclosed by Farach-Carson
et al, seems very laborious, while the C,~ side chain build-up, described
in the above WO 87/00834, also requires various laborious synthetic
steps, using a not readily available ketone as a synthon. In this
connection there is a need for better accessible C,~-side chain modified
vitamin D compounds. As a matter of fact, both the starting compounds
for the preparation of such vitamin-D compounds must be easily available
or accessible, and the multistep preparation process must lead to the
intended purpose with sufficient selectivity and efficiency.
The present invention provides a new
class of vitamin D compounds, which is well accessible from readily
available or accessible starting materials.
According to the present invention there is provided
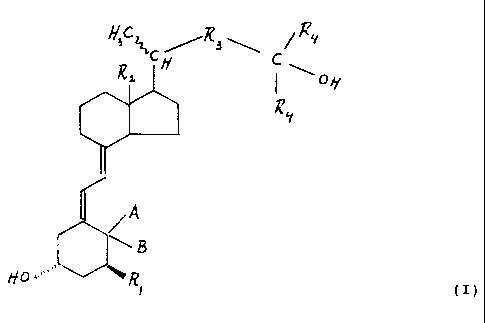
a new vitamin D compound of the general formula
Hac~~/~a\ ~/Ry
~oy
~y
(I)
wherein:
R, is a hydrogen atom or a hydroxy group;
RZ is a (C,-C3)alkyl groug, a hydroxy(C,-C3)alkyl group, a
(C,-Cz)alkoxymethyl group or a (CZ C3)alkenyl or alkynyl group;
R3 is a branched or non-branched, saturated or unsaturated aliphatic
3- to 5-membered hydrocarbon or oxahydrocarbon biradical,
having at least 3 atoms in the main chain and being optionally
substituted with one or more substituents selected from epoxy,
24'7997
3 DIR 0518
fluoro and hydroxy;
R4 is a sec. or tert. (C3-C6)alkyl group or a (C3-C6)cycloalkyl
group; and
A and B are each individually hydrogen atoms or methyl groups, or
A and B form together a methylene group.
The above new vitamin D compounds of the invention, presented by the
general formula I, are valuable substances. The biological results, as
illustrated in the Examples, indicate that these compounds are promising
as biologically active substances and may be used in all above-mentioned
pharmacotherapeuti.c indications, more in particular for the treatment of
osteoporosis, renal osteodystrophy, osteomalacia, skin disorders such as
psoriasis (and other hyperproliferative skin diseases), eczema and
dermatitis, myopathy, leukemia, breast and colon cancer, osteosarcomas,
squamous cell carcinomas, melanoma, certain immunological disorders, and
transplant rejections.
Furthermore, the new vitamin D compounds of the invention may be used
for wound healing .and may be incorporated in cosmetic compositions, such
as creams, lotions, ointments and the like, in order to preserve,
condition and/or protects the skin and to improve various skin
conditions, such as wrinkles, dry skin, skin slackness and insufficient
sebum secretion. The ne~a vitamin D compounds may also be used for
diagnostic purposes.
Suitable example:; of the above substituent R4 are: isopropyl,
cyclopropyl, tert.-butyl, thexyl (1,1,2-trimethylpropyl), 3-pentyl and
cyclopentyl.
A vitamin D compound is preferred, having the above general formula I,
wherein:
R, is a hydroxy group;
Rz has the meaning given above;
R3 is a biradi.cal of the formula
- O - CHz - ( C:HZ ) o - ,, - CH~ - CHI - ( CH~ ) " - ,
3 5 - CH = CH - ( CHz ) ~ - Or - CH, - CHz - CH ( CH3 ) - ,
wherein n is J. or 2;
9 I DIR 0518
R, is an isopropyl group, a cyclopropyl group or a tert.-butyl
group; and
A and B are hydrogen. atoms or form together a methylene group.
Examples of pre-eminently suitable vitamin D compounds according to the
invention are vii:amin D compounds of the above general formula I,
wherein the symbo7_s R" Rz, R3, A and B have the above-defined meanings,
and R, is an isopropyl group;
because of their extreme7.y favourable biological properties.
It is a special merit of the present invention that the above new
vitamin D compounds of the invention can easily be prepared from readily
available startin<; materials. In particular, it has been found, that
the desired Cu-configuration, i.e. the attachment of the appropriate
substituents to C,S, can easily be achieved by starting from a readily
accessible ester compound.
Consequently, the invention also relates to a method of preparing a
vitamin D compound of then general formula I, as defined above, wherein
R, is a hydroxy group, which method is characterized according to the
present invention, in than an ester compound of the general formula
NC-~C ~R3~ C/°
~O Rb
30
. ,,.
nS
(IX)
wherein:
RZ, R3, A and 13 have 'the above-defined meanings,
RS is a protected hyclroxy group, and
R6 is a ( C,-C6) alkyl group;
is reacted with an organometallic compound of the general formula
RaM(X)P (III)
~os~ss~
DIR 0518
wherein:
R, has the above meaning,
X is C1, Br or I,
M is a metal selected from Li and Mg, and
5 p is, dependent on the valence of M, 0 or 1;
followed by deprot:ection.
In an equally attractive manner the C,~ - side chain can first be
finalized. Therefore the invention also relates to a method of preparing
a vitamin D compound as defined above, which method is characterized
according to the present invention, in that an ester compound of the
general formula
H3c~.n ~ ~3 ~ corn
RZ i H
°R6
(II)
wherein:
R2, R3 and R6 have the' above meanings, and
Rs' is an optionally protected hydroxy group;
is reacted with are organometallic compound of the general formula
R4M(X)P (III)
wherein the symbols have the above meanings;
after which the hy<irindane compound obtained, having the general formula
3o H3c ~~~ / '~a ~ ~ i Ry
R.s H I ~ o H
~y
IRh_
(IV)
is deprotected, if' RS' is a protected hydroxy group, and then oxidized
to the corresponding hydrindane-4-one compound of the general formula
2(l9'799'~
DIR 0518
Hjc;~C /~ R3 ' / Ry
H C
Rz I \ OH
~y
I
0 (v)
which compound of formula V, if desired after protection of the hydroxy
group, is then converted either
(a) with a Wittig reagent of the general formula
( q
;~ """~~~~
S 1 (VI)
wherein R,' is a hydrogen atom or a protected hydroxy group, and
the other symbols have the above meanings;
or (b), after enolization and derivatization of the enolic hydroxy
group, with an enyne compound of the general formula
R ,,,, ~?
(VII)
wherein the symbols have the above meanings, followed by hydrogenation
and isomerization, to produce a compound of the general formula I,
wherein A and B form together a methylene group;
followed by deprotection.
Aydroxy groups in 'the above intermediates or reactants may be protected
by a reaction with a suitable esterification or etherification agent. A
suitable esterification agent is an alkylchlorocarbonate having 2 to 5
carbon atoms, or an aromatic carboxylic acid or saturated aliphatic
carboxylic acid having 1 to 4 carbon atoms such as benzoic acid, or a
derivative of such acids suitable for the esterification reaction. In
order to protect hydroxy groups in the form of an ether, in principle
any etherification agent: known for this purpose is suitable: for
zo~~~~~
DIR 0518
example, a trialkylsilylimidazole, a trialkylsilylhalide, a trialkyl-
silyltriflate (-trifluoromethanesulfonate), a diphenylalkylsilylhalide,
or a diphenylalkylsilylt.riflate, or a derivative thereof, the alkyl
groups of which have 1 to 6 carbon atoms.
Particularly suitable for this purpose are trimethylsilylchloride,
tert.-butyldimethylsilylc:hloride, dimethyl-(1,1,2-trimethylpropyl)-
silylchloride, tert.-butyldimethylsilyl triflate, or trimethylsilyl-
imidazole, because these etherification agents readily react with the
hydroxy group to tie protected to form an ether function, which on the
one hand is sufficiently stable under the conditions of the reaction or
reactions in view, but on the other hand can easily be removed
[deprotection] to recover the original hydroxy group; tert.-butyldime-
thylsilylchloride or triflate is to be preferred, because the tert.-
butyldimethylsilyl group has been found to be excellently suitable as a
protective group.
The enolic hydroxy group is preferably derivatized by a reaction with N-
phenyltriflimide to produce a triflate.
The starting compounds of formula II can conveniently be prepared from
readily available substances, e.g. for the synthesis of vitamin D
compounds with the above-defined preferred C,~ side chains as follows:
r,,,
vitamin DZ \d H 1
/. oion. ~ ~ ~oo(~xafr'on
x. ted,
_~C I I a )
chain extension: O N
r,, o G, ~OH
H
Z
-a .,, I
.\CN
nil off .,od..~
/,T sCC pyz~:.G~.e 1. DIBAL o ff
(XIIb)
z. NacN(Dl~lso~ z. N~,BHy
0 ~r
209"x'99"?
° DIR 0518
c/ O
CND CHCOVR~ ~~,yb
XII
(2,, ~ ~'u r~ Svmc'Ca t c'o-h~
OH
wherein n is 1 or 2.
The introduction of a C,g modification (RZ) into the vitamin D compound
of the invention can convE:niently be achieved as described in the above-
mentioned EP-A-0521550.
Suitable examples of orc~anometallic compounds of the above general
formula III are lithium compounds, such as isopropyllithium,
cyclopropyllithium and tert.-butyllithium, and Grignard reagents, such
as isopropylmagnesium chloride, cyclopropylmagnesium chloride and tert.-
butylmagnesium chloride, as well as the corresponding bromides.
The intermediate eater compound of the general formula IX, presented
above, is new. Therefore the present invention also relates to this
intermediate, as wall as to a method of preparing this compound.
The ester compound of the general formula IX, wherein A and B form
together a methylene group, can conveniently be prepared by reacting an
ester of the general formula
"t~ /H
Rz ,~ ~ ~R6
(VIII)
wherein:
Rz, R3 and R6 have the above meanings, and
R~ is a derivatized hydroxy group,
is reacted with an enyne compound of the general formula
3 5 F~~ ~~ ~s ( V I I -A )
wherein the symbol:a have lthe above meanings;
followed by hydrogenation and isomerization.
~0~~9J'?
DIR 0518
This reaction is preferably carried out in two reaction steps, viz. by
first reacting the ingredients under the influence of an organic base
such as triethylamine, arid in the presence of a palladium catalyst such
as (PPh3)ZPdClz, send by then subjecting the product obtained to a
hydrogenation with hydrogen under the influence of a suitable catalyst
such as Lindlar catalyst (Pd on CaCO3, poisoned with lead), followed by
an isomerization of the previtamin configuration obtained to the vitamin
structure of the general formula IX.
Alternatively, said ester compound of the general formula IX can easily
be synthetized by reacting a modified Windaus Grundmann ketone of the
general formula X or XI with a Wittig reagent as follows:
H3~~ ~ R3~ , ~o
I~z CH ~ ~'p R6 I4 ~'~~~ ~z.
~'-. $ ~ ( I X )
I ~ ',,,"
VI-A
p (X) S S' ( )
or:
,~~ ~ '~'I
usC'~.,aG ~~~
+ VI-A
de zoftcf.
~al~'~a.f~Qx
~ (XI)
RS
,,, ~~ B
Rs Rs
(XIII)
CH1-CH~:OOR~
XIII ----~ IX, wherein R3 = (CHZ)3 or (CHz)4.
~Zr~ ~ Cu .z~ -~ohncc ~-i'° ~c~
The symbols in thsa above formulas are defined hereinbefore.
The intermediate hydrindane-4-one compound of the general formula V,
presented above, is new. 'therefore the present invention also relates to
this intermediate and to a method of preparing this compound, viz. by
oxidizing a hydrindane compound of the general formula IV, as defined
above, with an oxidizing agent, preferably selected from a chromium-
CA 02097997 2004-O1-27
27072-153
to
containing oxidant such as pyridinium chlorochromate or pyridinium
dichromate, and ruthenium tetroxide.
The intermediate hydrindane compound of the general formula IV,
presented above, is also new. Consequently, the present invention
relates in addition to this intermediate and to a method of preparing
this compound, viz. by reacting a compound of the general formula II, as
defined above, with a metal-organic compound of the general formula III,
as also defined above, in an inert organic solvent.
To improve the applicability of the new vitamin D compounds of the
invention for the above-described pharmacotherapeutic indications, the
compounds are usually processed to pharmaceutical compositions,
comprising an effective amount of said vitamin D compound as the active
ingredient in addition to a pharmaceutically acceptable carrier and/or
at least one pharmaceutically acceptable auxiliary substance. Such a
composition may be delivered in a dosage unit form for oral, topical
(dermal) or parenteral administration, comprising approx. O.1 Ng to
approx. 0.1 mg active ingredient per dosage unit.
A composition for diagnostic purposes may comprise, in addition to the
vitamin D compound of the present invention, a compatible, non-toxic
carrier and/or at least one auxiliary substance.
A cosmetical composition may comprise, in addition to an effective
amount (in the range of approx. 0.1 pg to approx. 0.1 mg per dosage unit
in.a dosage unit form) of the vitamin D compound of the present
invention, a cosmetically acceptable, non-toxic carrier and/or at least
one auxiliary substance.
The invention also relates to a method for the treatment and
prophylaxis of a number of disease states including autoiummune diseases
(including diabetes mellitus), acne, a,lopecia, skin aging (including
photo-aging), imbalance in the immune system, inflammatory diseases such
as rheumatoid arthritis and asthma, as well as diseases related to
abnormal cell differentiation and/or proliferation, in a warm-blooded
living being, comprising administering to said being or treating said
being with a pharmaceutical composition as defined above in a quantity
effective for the intended purpose. Examples of such diseases are
CA 02097997 2004-O1-27
27072-153
11
psoriasis and other hyperproliferative skin diseases.
The present invention also relates to the use of
the above pharmaceutical compositions for the treatment of
solid, skin and blood cancers, in particular of blood
cancers such as leukemia, of breast cancer, and of skin
cancers such as melanoma and squamous cell carcinoma.
The above-defined cosmetical compositions, in
particular selected from the group consisting of creams,
lotions, ointments, liposomes and gels, can be used for the
treatment and prevention of a number of skin disorders, such
as inadequate skin firmness or texture, insufficient skin
hydration, wrinkles and insufficient sebum secretion.
The invention also provides for use of the
compounds and compositions of the invention in the treatment
and prophylaxis of the noted diseases and disorders as well
as use of the compounds and compositions for preparing
medicaments.
The invention also provides commercial packages
comprising the compounds or compositions of the invention
and associated therewith instructions for the use thereof in
the treatment and prophylaxis of the noted diseases and
disorders.
The invention will now be described in greater
detail with reference to the following specific Examples.
CA 02097997 2004-O1-27
27072-153
lla
Examples
Example I
Pre aration of vitamin ester
reaction equation:
OH
(a) (b) ---;
------;
..-----;
C02CH 3
1 0 (e)'
(c) (d)
-'
TBSO''~~ (6~OTB5
~09"?9~1'7
12 DIR 0518
C o GH
OC3
7~ s ''
to °''~ ,.~~ ~,~ TBSo~'
m i,~,~
(a). Ph3P (6.5 g) and imidazole (4.8 g) are added to a solution of diol
(1) (5.0 g) in THE' (100 ml). The suspension is cooled to -20°C, and IZ
(6.28 g) is added in portions. After being stirred for 15 min, the
reaction mixture is warmed to room temp., further stirred for 15 min.,
cooled to 0°C, an~9 poured into saturated aqueous NaHC03 ( 50 ml ) .
The
mixture is extracted with Et2o and the extract is washed with saturated
aqueous NazS~o3 anc~ H~O, dried and filtered. Concentration affords a
residue which is purified. by flash chromatography (8$ EtOAc/hexane) to
give 7.32 g of iodide (2). After crystallization from EtOAc/hexane, the
product has a melting point of 51°C; identification by NMR and elem.
analysis.
(b). Pyridinium dichromate (8.66 g) is added to a solution of compound
( 2 ) ( 3. 99 g ) in 50 ml CEf~Cl2. The mixture is stirred for 6 h at room
temp. Etzo (60 ml) is added, and the resulting suspension is stirred for
15 min and filtered. The :filtrate is washed with brine, dried, filtered
and concentrated. Purification by flash chromatography (10~
EtOAc/hexane) gives the desired iodo ketone (3) in a yield of 3.57 g;
crystallization from EtiO,/hexane: m.p. 65°C. Identification by NMR and
elem. analysis.
(c). Lithium diisopropyleunine is prepared by addition of i-Pr~NH (3.9
mmol) to a cooled [-78°C) solution of n-BuLi in hexane (3.5 mmol in
1.43
ml). After stirring for 10 min, the mixture is diluted with THF (4 ml),
stirred at 0°C for 30 min, and cooled to -78°C. A solution of
ketone (3)
(1.0 g) in 14 ml THF is slowly added, followed by a solution of N
phenyltriflimide (1.225 g) in 4 ml THF. The mixture is stirred for 2 h
2U~'~99'7
13 DIR 0518
at -78°C. After being warmed to 0°C, the reaction is quenched by
addition of a few drops of MeOH and water. Concentration gives a crude
product which is diluted with EtOAc/hexane (30 ml), washed with brine,
dried, filtered a.nd concentrated. The resulting residue is purified by
flash chromatography (2%. EtOAc/hexane), affording 1.29 g of the iodo
triflate (4) as a colourless oil. Identification by NMR and elem.
analysis.
(d). A suspension of CuI (201 mg) and Zn (161 mg) in EtOH/Hz0 (6 ml 7:3;
deoxygenated) is aonicated for 5 min. Methyl acrylate (637 girl, freshly
distilled), and a solution of the iodide (4) (160 mg) in EtOH/H20 (1 ml
7:3) are successively added, and the resulting mixture is sonicated for
40 min. Dilution with Et20 (15 ml) and filtration gives a solution that
is washed with brine. The aqueous phase is extracted with Et~O (30 ml)
and the combined organic extracts are dried, filtered and concentrated.
Flash chromatography of 1=he residue (6$ EtOAc/hexane) affords 96 mg of
the methyl ester t5) (co:Lourless oil). Identification by NMR and elem.
analysis.
(e). The palladium-catalyzed coupling between vinyl triflate (5) and the
enyne (6) is performed as follows:
A mixture of enyne (6) (507 mg), triflate (5) (500 mg), Et3N (4.85 mmol)
and (Ph3P)ZPdCl2 (1.6 mg) in 21 ml DMF is heated at 75°C for 1 h. The
mixture is cooled to room temp., diluted with EtoAc/hexane (50 ml 1:3),
and washed with brine. lJrying, filtration and concentration gives a
residue which is p~urified~ by flash chromatography (2-4$ Et20/hexane) to
afford 675 mg of dienyne (7) (viscous liquid). Identification by NMR.
(f). Product (7) is hydrogenated by Lindlar catalyst as follows:
A solution (0.2 m:L) of 50 pl quinoline in 10 ml hexane is added to a
solution of dienyn,e (7) (305 mg) in 12 ml hexane. Lindlar catalyst (50
mg), previously dried, is added and the resulting solution is exposed to
hydrogen gas at ~3tmosph~eric pressure. After stirring for 8 h, the
reaction mixture is filtered and concentrated. The residue is purified
by flash chromatography (1-3$ Et~o/hexane) to give 295 mg of the
protected previtamin D compound.
Isomerization of previtamin D compound to vitamin D compound (8):
20979'7
14 DIR 0518
The previtamin D compound obtained (295 mg) is dissolved in 15 ml
isooctane and refluxed in the dark for 5 h. Concentration gives a
residue which is purified by flash chromatography (2-4~ Etzo/hexane) to
afford 290 mg of c~ompouncl (8). the product is identified by 'H-NMR, "C-
NMR and elem. analysis.
'H-NMR ( b, CDC13) : 6.24 and 6.02 (d, 2H) , 5.18 (m, 1H) , 4.87 (m, 1H) ,
4.37 (m, 1H), 4.1E~ (m, 1H), 3.67 (s, 3H), 0.93 (d, 3H), 0.88 (s, 18H),
0.53 (s, 3H), 0.07 ( s, 1.2 H).
'3C-NMR (b, CDC13): 173.1, 148.4, 141.0, 135.0, 132.2, 118.0, 111.2, 72.1,
67.5, 56.3, 51.3, 46.0, 45.7, 44.8, 40.6, 35.8, 35.3, 34.4, 31.5, 28.8,
27.6, 25.8, 25.7, 23.4, 22.6, 22.1, 21.5, 18.7, 18.1, 18.0, 14.0, 11.9,
-4.8, -4.8, -4.9, -5.2.
Elem. anal.: Calcd.. for C:~H~04Siz: C, 70,75; H, 10.62. Found: C, 70.42;
H, 10.43.
In a corresponding manner the following ester compounds are prepared:
general formula
3---' C oz RC
25 iaso~~.,
vi r~J
compound no . R~ R, R6
( 9 ) CHI ( CFi2 ) 4 CZHs
( 10 ) C~HS ( CH~ ) 3 C,HS
Compd. (9): This 24-homo compound is prepared by using in the above step
(a) as the starting substance a homologue of compd. ( 1 ) , having a 1-
methyl-3-hydroxypropyl side chain; in step (d) ethyl acrylate is used as
the olefin.
'H-NMR (b, CDC13): 0.08 (s, 12H), 0.52 (s, 3H), 0.87 (s, 18H), 0.90 (d,
3H), 1.25 (t, 3H), 4.11 (q, 2H), 4.20 (m, 1H), 4.37 (m, 1H), 4.87 (d,
1H), 5.18 (d, 1H), 6.01 (d, 1H), 6.24 (d, 1H).
Compd. (10): This, 18-homa compound is prepared by using in the above
~o~~9s~r
15 DIR 0518
step (a) as the starting compound a homologue of compd. (1), prepared as
described in published European patent application 521550 (compound no.
(64)].
~H-NMR (b, CDC13): 0.87 (s, 6H), 0.88 (t, 3H), 1.01 (d, 3H), 1.25 (t,
3H), 1.98 (t, 1H), 2.25 (m, 2H), 2.44 (dd, 1H), 4.13 (q, 2H), 4.18 (m,
1H), 4.37 (m, 1H),. 4.86 (s, 1H), 5.17 (s, 1H), 6.01 (d, 1H), 6.23 (d,
1H).
Example II
Preparation of vitamin D compound from vitamin ester
reaction equation:
CD CN3
20 T~S~ viOJ ~Q~''~' vn
Compound (11) is prepared as follows:
Cyclopropyl bromide (0.51 mmol) is slowly added to a cooled (-20°C)
solution of t-BuLi in Et20 (0.51 mmol in 0.602 ml), to produce
cyclopropyllithium. The resulting mixture is warmed to room temp. and
diluted with 2.4 ;ml Et,O. 1 ml of this solution is slowly added to a
cooled (-78°C) solution of compd. (8) (50 mg) in 3 ml Etzo. The
reaction
mixture is allowed to come to -40°C and quenched with a few drops of
water. The resulting solution is diluted with Et,O, washed with brine,
dried, filtered and concentrated. The concentrate is filtered through a
flash chromatography column (2$ Etzo/hexane), affording a product (46 mg)
which is dissolved in 7 ml THF and stirred in the dark at room temp.
with tetrabutyl ammoniumfluoride in THF (0.36 mmol in 0.36 ml) for 24 h.
Concentration gives a residue which is diluted with EtOAc (20 ml),
dried, filtered, concentrated and flash chromatographed (60$
EtOAc/hexane) to give 23 mg of the desired compound (11) as a white
solid.
~H-NMR (S, CDZC1=): 6.44 a.nd 5.99 (d, 2H), 5.27 (br-d, 1H), 4.95 (br-d,
209'99"?
16 DIR 0518
1H), 4.35 (m, 1H), 4.15 (m, 1H), 0.92 (d, 3H), 0.81 (m, 2H), 0.53 (s,
3H), 0.34 (m, 8H).
'3C-NMR (S, CDZC12): 148.6, 143.5, 133.9, 125.1, 117.6, 111.8, 71.2, 71.0,
67.2, 57.2, 56.8, 45.8, 43.5, 43.4, 41.0, 37.1, 36.6, 29.4, 28.0, 24.0,
22.6, 20.8, 19.0, 12.1, C1.8, -0.5.
In a corresponding manner the following vitamin D compounds are
prepared:
general foraula
~y
off
Ry
20
compound no. RZ R3 R4
( 12 ) CH3 ( CFiz C ( CHs
) 3 ) 3
(13) CH3 (CHz)3 CH(CH3)~
(14) CzHS (CH,)3 CH(CH3)=
(15) C.,HS (CH~)3 cyclopropyl
( 16 ) CH3 ( CH~ ) cyc lopropyl
a
( 17 ) CH3 ( CH~ ) CH ( CH3
4 ) 2
Compd. (12) is prepared by using t-butyllithium instead of
cyclopropyllithium.
Compounds (13), (7.4) and (17) are prepared by using isopropyllithium
instead of cyclopropyllit:hium.
Compd. (12): 'H-NMR (5, CDC:Ij): 6.38 and 6.01 (d, 2H), 5.33 (m, 1H), 5.00
(m, 1H), 4.43 (m, 1H), 4.23 (m, 1H), 1.00 (s, 18H), 0.93 (d, 3H), 0.54
( s, 3H) . "C-NMR ( ~~, CDC13) : 148.7, 143.4, 134.0, 125.0, 117.6, 111.8,
80.0, 71.1, 67.1, fi0.6, 5fi.9, 56.8, 46.3, 45.8, 43.4, 42.8, 40.9, 37.0,
209'99?'
17 DIR 0518
36.5, 34.2, 29.4, :?8.0, 24.0, 23.3, 22.7, 19.2,
28.8, 12.1.
Compel. (13): (5, C:D30D): 0.55 (s, 3H), 0.90 2.22(dd,
'H-NM:R (m, 15H),
1H), 2.48 (dd, 2.83(dd, 1H), 4.09 (m, 1H), 4.31 4.86(b?,
1H), (t, 1H),
1H), 5.25 (b, 6.05(d, 1H), 6.29 (d, 1H).
1H),
Compel. (14): (b, CDC13): 0.84 (t, 3H), 0.95 (m, 1.00(d,
'H-NNIR 12H),
3H), 2.32 (dd, 2.60(dd, 1H), 2.83 (dd, 1H), 4.24 4.44(m,
1H), (m, 1H),
1H), 5.01 (b, 5.33(b, 1H), 6.01 (d, 1H), 6.39
1H), (d, 1H).
Compel. (15): (5, 0.91(t,
'H-NMR CD3oD):
0.70-0.40
(m,
8H),
0.76
(m,
2H),
3H), 1.01 (d, 2.00(m, 1H), 2.22 (dd, 1H), 2.29 2.48(dd,
3H), (b, 1H),
1H), 2.83 (dd, 4.09 5.25(b,
1H), (m,
1H),
4.31
(t,
1H),
4.85
(b,
1H),
1H), 6.04 (d, 6.29(d, 1H).
1H),
Compel . (16): 'H-NMR(b, 0.79(m,
CCi30D):
0.20-0.40
(m,
8H),
0.57
(s,
3H),
2H), 0.95 (d, (dd, 1H), 2.51 (dd, 1H), 2.86 4.12(m,
3H), 2.25 (dd, 1H),
1H), 4.35 (t, 4.89(b, 1H), 5.28 (b, 1H), 6.08 6.32(d,
1H)" (d, 1H),
1H).
Compel. (17): 'H-NMR (S, C:D3oD): 0.53 (s, 3H), 0.90 (m, 12H), 2.22 (dd,
1H), 2.48 (dd, 1H), 2.83 (dd, 1H), 4.09 (m, 1H), 4.31 (t, 1H), 4.86 (b,
1H), 5.25 (b, 1H), 6.05 (d, 1H), 6.29 (d, 1H).
Example III
Preparation of 1-(1-methyl-5-hvdroxv-5 5-diisopropvl-pentvll-
hvdrindanol-4 (19)
Reaction equation:
2 5 ~. ~, ' ~z Cz.NS
a., - - c3H v J p~
35 (a). Starting compound (1) is converted to the corresponding iodide (2)
as described in Example I(a).
(b). The compound (2) obtained is converted in a corresponding manner as
DIR 0518
described in Example I(d), using ethyl acrylate as the olefin, to
produce ester compound (:18).
(c ) . Ester compound ( 18 ) is converted to compound ( 19 ) by a reaction
with an excess of isopropyllithium, in a corresponding manner as
described in Example II. The product is identified by 'H-NMR.
In a corresponding manner the following compounds are prepared:
general formula
R
to
~ joy
Ry
is
off
compound no. R~ R3 R4
( 20 ) CH == CH~ ( CHZ ) 3 cyc lopropyl
( 21 ) CH == CH, ( CHZ ) 3 isopropyl
(22) CEi3 (CH2)4 isopropyl
20 (23) CEi3 (CHZ), cyclopropyl
The products are i_dentifi_ed by 'H-NMR.
Example IV
Preparation of 1-(1-methyl-5-hydroxy-5 5-diisopropyl-oentyll-
25 hvdrindanone-4 (2~1
Reaction equation
0
Oxidation of compound (19) by using pyridinium dichromate as the
oxidant, in a corresponding manner as described in Example I(b), affords
the desired ketone~ (24) in a yield of 84~. The product is identified by
' H-NMR .
19 DIR 0518
In a corresponding manner the following ketones are prepared:
general forrula
~3~
C
I woy
~y
0
compound no. RZ R3 R4
(25) CH -= CHI (CHz)3 cyclopropyl
( 2 6 ) CH - CHI ( CHz ) 3 i sopropyl
(27) CH3 (CH,)4 isopropyl
(28) Cli3 (CHz)4 cyclopropyl
The products are :identif.ied by ~H-NMR.
Example V
Prevaration of vii:amin D compound (131 from ketone (241
reaction equation:
- ''~~.
~'oTMS
,a ~z y~ --
0
2 5 f~ ~Q ~ OT~
,;, ~
0 'z '~~ ~07
~d~
i. zed.
z, r'so~ne r.
3. d~ptot~
H o ,,,...
~H
TBSO~''~~~ arss %$s0'"1 ~'oTr~s
2~~"~99'?
20 DIR 0518
(a). The free hydroxy group is protected by a reaction with
trimethylsilyltriflate (~TBS-triflate) in the presence of triethylamine
and in methylene <:hloride as the solvent; temp. -78°C -- 0°C;
yield of
compound (29) is .30%.
(b) The enolisation is carried out in a corresponding manner as
described in Example I(c), producing compound (30) in a yield of 71%.
(c). In a corree3ponding manner as described in Example I(e), the
coupling reaction with enyne (6) is performed, affording compound (31)
in a yield of 94%.
(d). The final reaction step is carried out in a corresponding manner as
described in Example I(f), followed by deprotection (desilylation), as
described in Example II,, with tetrabutyl a.mmoniumfluoride. The final
vitamin D compound (13) is obtained in an overall yield of 65%. The
product is identical with the product obtained according to Example II.
In a corresponding manner the following vitamin D compounds are
prepared.
general formula
R3~C~~y
~ ~ off
Ry
,,
H 0 \h,
v h.
compound no. R, R3 R4
( 32 ) CH :- CH= ( CH,) 3 cyclopropyl
(33) CH =- CHz (CHz)3 isopropyl
( 17 ) CH3 ( CH~ ) 4 isopropyl
( 16 ) Cfi3 ( CHz ) 4 cyc lopropyl
The products are identified by 'H-NMR. The last two vitamin D compounds
are identical with the corresponding vitamin D compounds prepared
according to Example II.
_. ~09"T99'7
21 DIR 0518
Example VI
Preparation of 19-nor-vitamin D compound (351 from ketone (291
Reaction equation:
r
rMS
\ ~..
i s s o' o TB s
~~ ~3 ~J
0
x. dcpzGE.
,,,.
Hog, ~~ c~~~
A solution of 1.14 g (2 nvnol) of phosphine oxide (34) in 15 ml of dry
THF is cooled to -78°C. n-Buthyllithium (BuLi), as a 2.5 M
solution in
hexane, is added d~copwise until the red colour persists. Then 0.8 ml of
a 2.5 M solution of BuLi is added. Stirring is continued for 15 min,
followed by the dropwise addition of 0.73 g (1.8 mmol) of ketone (29) in
5 ml THF. After another hour of stirring, the reaction mixture is
allowed to reach 0°C and then quenched by the addition of 50 ml of a
saturated NH,C1-solution. lExtractive work-up and flash chromatography (2$
EtOAc in hexane) then affords the protected diene compound. Desilylation
by reaction with 10 eq. of tetrabutylammonium fluoride (TBAF.3aq) in THF
(10 ml) during 48 hours gives compound (35), which is purified by flash
chromatography using EtOAc as an eluent, followed by recrystallization
from MeOH/EtOAc. The overall yield is 53$. Identification by 'H-NMR.
In a corresponding manner the following vitamin D compounds are
prepared:
general formula ~3~. ~ ~ ay
~ ~ off
Ry
y°\ UH
209'7~9'~
22 DIR 0518
compound RZ R3 R4 A B
no .
(36) CH := (CHZ)3 isopropyl H H
CH2
( 3'1 ) CH := ( cyc H H
CHz CHZ lopropyl
)
3
( 38 ) CFizOH ( isopropyl H H
CHI
)
3
The products are i_dentif~Led by 'H-NMR.
Example VII
Affinity to intracellular vitamin D receptor
Vitamin D compounds according to the invention are dissolved in ethanol
in concentrations ranging from 10-'3 to 10-' M. The affinity towards the
calf thymus intra<:ellular vitamin D receptor (VDR) is determined in a
biological assay. In this: assay, 3H-1a,25-dihydroxycholecalciferol (3H
1a,25-DHCC), which is specifically bound to the VDR, is replaced by the
tested compounds. Especially the tested compounds 11, 13 and 14 have a
very high VDR-ai:finity.. A high VDR-affinity is indicative for
biologically active substances.
Example VIII
Affinity to vitamin D binding protein
Vitamin D binding protein (DBP) is the specific carrier for vitamin D
and its metabolites in :blood. The biological activity of vitamin D
compounds depends nn the it binding to DBP, because strong binding to DBP
will reduce the intracellular access to the VDR. Binding to the DBP may
also influence t:he ha7Lf-life of the vitamin D derivatives in
circulation. Weak binders are rapidly metabolized, which is a favourable
aspect in topical application.
In the assay, DBP :s incubated with 3H-1a,25-DHCC and 1a,25-DHCC or with
several vitamin D <:ompounds according to the invention. To this purpose,
the vitamin compounds are dissolved in ethanol in concentrations ranging
from 10'x' to 2. 5 x 10'6 M. The percentage bound/unbound 'H-la, 25-DHCC is
then calculated. DBP is purified from total human serum. The results are
shown in the appended Figures 1 and 2. Figures 1 and 2 show the binding
of vitamin D compounds to human vitamin D binding protein.
['H]1a,25(OH)~D~ _ '13-1a,25-DHCC; in both Figures ~ - 1a,25-DHCC (known
compound); in Fig. 1 ~ = compound 13 and D - compound 14; in Fig.
2 Q = compound 11.
_.. 20~'~00:
23 DIR 0518
Compounds 14 and 11 bind rather weakly to the DBP, compared to the known
1x,25-DHCC. Compound 13 is a very weak binder.
Example IX
Cell differentiation
Vitamin D compoundls according to the invention are dissolved in ethanol
in concentrations ranging from 10-'2 to 10-6 M and tested for their
capacity to induce cell differentiation in a HL-60 assay. In this assay,
morphologic and biochemical examination of the human leukemic cell line
HL-60 is done, in order to establish whether cell differentiation has
taken place.
Differentiation is expressed as the maturation parameters nitroblue
tetrazolium (NBT;~ reduction, non-specific esterase, and as the
percentage of mature cells beyond the myelocyte stage which is visible
after staining with May-Grunwald Giemsa. After culturing with the known
1a,25-DHCC or with vitamin D compounds of the invention, the percentage
of cells containing black formazan deposits is determined. An increase
in the percentage of NBT reducing cells indicates an increase in cell
differentiation.
Proliferation and vitality of the cell cultures are established by
counting the number of cells and by the trypan blue exclusion method.
The vitality and proliferation of the cells in the HL-60 cultures are
good in all conditions tested. 1a,25-DHCC (known), compound 12, compound
11, compound 13 and compound 14 all induce differentiation and
maturation of the HL-60 cells. In the cytological test (non-specific
esterase and May-Grunwal~d Giemsa) especially compounds 13 and 11 are
good differentiators. The optimum effect is found at concentrations in
the range of 10-e to 10-' M.
The NBT-reduction inducing capacity of compounds 13 and 14 is about 10
x stronger than that of 'the known 1a,25-DHCC. Compounds 11 and 12 are
about 5 x more potent in inducing NBT-reduction than 1a,25-DHCC (Figures
3 and 4).
The above implies that the tested new vitamin D compounds of the
invention display a higher cell differentiating activity than the known
1a,25-DHCC.
zos~s9~
24 DIR 0518
Figures 3 and 4 (appended) show the differentiating effect of the tested
vitamin D compounds on human leukemia cells of the HL-60 line. In both
Figures ~ = 1a,25-DHCC; in Fig. 3 O is compound 13 and O is com:..pound
14; in Fig. 4 d =- compound 11 and D = compound 12.
Example X
Calciotropic effects
The most well-known efect of la, 25-DHCC is its action on the calcium
metabolism, the ca.lciotropic effect. Calciotropic target organs are the
intestine, the bone and t:he kidney.
The vitamin D compounds according to the invention are dissolved in
ethanol and tested in the so-called Caco-2 assay for intestinal calcium
(Ca) transport. In this ~3ssay, the vitamin D - induced influx of 'SCaZ+
is measured in monolayers of the intestinal cancer cell line Caco-2.
This influx is corrected for the concentration-driven Ca'-+ influx and is
a measure for the Ca transport across the intestinal wall. The Caco-2
cells are known to have vitamin D receptors.
Increased intestinal calcium transport can be the first step leading to
a rise in blood calcium levels (and eventually to hypercalcemia).
In Table A below 'the effects of vitamin D compounds of the invention,
compared with the known 1a,25-DHCC, on the Ca'+ influx in intestinal
Caco-2 cell cultures are presented. The values in the table represent
the relative increase in Ca=+ influx tthe va7uP fnr ~n_~S-nu~r ;
arbitrarily fixed at 100).
The results in Table A demonstrate that compound 11, compound 13 and
compound 14 are we.3ker at:Lmulators of intestinal calcium absorption than
1a,25-DHCC.
Table A
experiment 1:
compound 7.0-9 M
1a,25-DHCC 100
compound 11 79
compound 12 99
209"997
25 DIR 0518
experiment 2:
compound 1.0-9 M
1a,25-DHCC 100
compound 13 65
compound 14 78
Example XI
Calciotropic effect
Together with the :intestine and the bone, the kidney is one of the major
target organs of 1a,25-D1HCC. The kidney plays an extremely important
role in calcium hc~meosta:ois, since about 98~ of the calcium has to be
reabsorbed in the kidneys in order to prevent calcium loss and
hypocalcemia.
The vitamin D compounds according to the invention are dissolved in
ethanol and tested in the rabbit kidney cell assay. In this assay,
reabsorption of 'SCa'' is measured in monolayers of rabbit kidney cells.
The cells are isolated by immunodissection of connecting tubules with
the aid of monoclonal antibodies.
In Table B below the effE~cts of vitamin D compounds of the invention,
compared with the known 1.x,25-DHCC, on the Ca'+-reabsorption in rabbit
renal cell cultures are presented. The values in the Table represent the
increase in Ca=+-reabsorpt.ion in nmol/cm~/h.
The results in Table B demonstrate that compound 13 and compound 11 are
weaker stimulators of renal Ca-reabsorption than compound 12 and 1a,25-
DHCC itself.
Table B
compound ~D-9 M
1a,25-DHCC ~~----13.4
compound 11 2.4
compound 12 12.8
compound 13 ~-3,7
Example XII
Cell differentiation versus calciotropic effect
209'?99'7
26 DIR 0518
One of the set-bac:ks of t:he highly active vitamin D compounds, such as
the well-known 1a,25-DHCC, is its calciotropic effect, which may lead to
toxic hypercalciuria, hypercalcemia and urolithiasis. Therefore, it
should be very advantageous to develop compounds with a high selectivity
of biological action. In other words, compounds in which the ratio
between the induction of cell differentiation and calciotropic effects,
e.g. the stimulation of intestinal Ca transport, is changed compared to
1a,25-DHCC.
The ratio between differentiation-inducing capacity and the stimulation
of intestinal Ca transport is defined as the fraction between the
concentration at which 50~ NBT reduction in HL-60 cells is obtained, and
the concentration at which a half-maximal increase in intestinal Caz+
transport is reached. The smaller the ratio, the higher the relative
cell differentiating capacity.
The results are presentecl in Table C.
Table C
compound fraction ~ ratio relative to DHCC
1a,25-DHCC 107 ~ 1.00
compound 12 29,7 0.2g
compound 11 14.4 0.13
compound 14 2.6 0.02
compound 13 2.5 0.02
Table C shows, that the tested new vitamin D compounds of the invention
have better rela;.ive cell differentiating properties than the known
1a,25-DHCC. Compound 14 and compound 13 have 50 x more selective actions
than 1a,25-DHCC. C:ompouncl 11 has a 8 x better ratio. Compound 12 has a
4 x better ratio. This makes the new vitamin D compounds of the present
invention extremely suitable for applications where the differentiation
of cells (such as in hyperproliferative conditions) is desired.