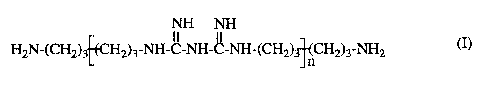Note: Descriptions are shown in the official language in which they were submitted.
CA 02098472 2003-02-20
21489-8690
-1-
Contact lens care product for hard and soft contact lenses
The present invention relates to a contact lens care product for hard and soft
contact
lenses, comprising a (poly)aminopropyl biguanide and a special buffer.
Contact lens care products comprising an aminopropyl biguanide are already
known. For
'example, GB 1432 345 describes ophthalmic compositions and compositions for
disin-
fecting contact lenses comprising an ophthalmologically acceptable polymeric
biguanide.
Only phosphate buffers are disclosed as suitable buffers in that context.
EP-A1-180 309 also discloses.disinfecting and preserving solutions for contact
lenses,
comprising a biguanide and a buffer. The biguanide is again of the aminopropyl
biguanide
type. As buffer especially borate buffers are proposed. Additional buffers
mentioned are
citrate buffers, bicarbonate buffers and mixed phosphate buffers.
In contrast, the present invention relates to contact lens care products
comprising an
aminopropyl biguanide or a salt thereof and as buffer the so-called tris
buffer (trometamol)
or a homolog thereof having up to 10 carbon atoms or a salt thereof. The
invention relates
also to the use of such a contact lens care product for cleaning and
disinfecting contact
lenses.
CA 02098472 2003-02-20
21489-8690
-1a-
According to one aspect of the present invention,
there is provided a contact lens care product comprising an
aminopropyl biguanide of formula
NH NH
HzN-(CHz)3 (CHz)3-N-C-N-C-N-(CHz)3 (CHz)3-NHz
H H H n
(I)
or a salt thereof and as buffer from 0.05 to less than 1.2
percent by weight of a compound of formula
(CHz) y OH
to H~ (CHz)X-i-NHz
(CHz) Z OH
(III)
or a salt thereof, wherein n is an integer from 1 to 500, x,
y and z are each independently of the others an integer, at
least 1, and the sum of x, y and z is from 3 to 9.
The aminopropyl biguanides to be used according to
the invention are especially those of formula I
NH NH
HzN- (CHz) 3 (CHz) 3 -H -C-H-C-H-(CHz) 3 n (CHz) 3' NHz
(I)
wherein n is an integer from 1 to 500; it is also possible
to use salts of compounds of formula I, especially
ophthalmologically acceptable salts thereof.
The compounds of formula I are known. Their
preparation is described, for example, in
-2-
C~B 702 268 and GB 1 152 24?.. In addition, those compounds are also
commercially
available, for example as Vant~xil~, Cosmocil~ or as Arlagard~E from ICI
Chemicals.
Depending on the manner in which they have been prepared, the compounds of
formula I
rnay comprise certain amounts of a secondary product of formula II
NH NH
H2N-(CH2)3~H2)3-NH-C-rTH-~-NH-(CH2)~(CH2)3-NH-C-NH-CN (II)
-J n
o:r salts thereof, wherein n is likewise an integer from 1 to 500. Mixtures of
compounds of
formula I with those of formuhr II can likewise be used according to the
invention. The
proportion of compounds of formula II, based on the total amount of compounds
of
formula I and compounds of formula II., is preferably less than 20 percent by
weight, more
preferably less than from 2 to 10 percent by weight and is especially
preferably zero
percent by weight.
The index n in formulae I and lI is preff;rably from 1 to 200, especially from
2 to 100,
more especially from 2 to 50 arid most especially from 3 to 12. Depending on
the value of
the index n in formula I or II, the molecular weight of the aminopropyl
biguanides that can
be: used is as low as the molecular weight of the monomer of formula I (n =
1), or in the
raurge of approximately 600 to 1600 if oligomers are used, i.e. when n is, for
example,
from 3 to 8, or alternatively in the range of from approximately 50 000 to
approximately
90 0100 if n is significantly higher, for e;cample approximately 270 to 500.
In the contact lens care products according to the invention, the compound of
formula I is
used preferably in an amount, based on the total amount of the contact lens
care product,
which is advantageously formulated as .an aqueous solution, of from 0.1 to 100
ppm
(0.00001 - 0.01 percent by weight), especially in an amount of from 0.5 to 50
ppm
(Ci.OC1005 - 0.005 percent by weight) and more especially in an amount of from
1 to
ppm (0.0001 - 0.001 percenr. by weight), for example 1, 2 or 5 ppm.
S~rlts of compounds of formula I and formula II that are suitable within the
scope of the
present invention are water-soluble salts that are advantageously
ophthalmologically
acceptable. Suitable salts are those with inorganic or organic acids, for
example hydro-
chlorides, hydrobromides, borates, acetates, gluconates, sulfonates, maleates,
ascorbates,
2Q~84'~2
_3_
tartrates or citrates.
T'he trometamol and its homologs having up to 10, preferably from 5 to 7,
carbon atoms
used according to the invention preferably are the compounds of formula III
( i H2 Iy-OH
HO-(CH2)X-C-NH2 (III)
(CH2 )Z OH
v~~herein x, y and z are each independently of the others an integer, at least
1, and the sum
of x, y and z is from 3 to 9, preferably from 3 to 6, or a salt thereof.
Special preference is
given to 2-amino-2-hydroxymethyl-1,3-propanediol (trometamol), which
corresponds to a
compound of formula III wherein each of x and y and z is 1. The compound
trometamol is
a:~so referred to as tris buffer.
Trometamol and its homologs leaving up to 10 carbon atoms are already known.
Their use
in medicaments for the treatment of inf7,ammations of the eye has already been
disclosed
in EP-A2-242 328. Compositions for the disinfection of contact lenses,
comprising 1.2
tromethamine/tromethamine hydrochloride and 1 ppm polyhexamethylene biguanide
hydrochloride, are already kno~,vn from WO 92/11876. Compounds of formula III
are,
rr,,oreover, also commercially available.
The amount of trometamol used as buffer in the contact lens care products
according to
the invention, based on the total amount of the contact lens care product, is
preferably
from 0.05 to 5 percent by weiglht, preferably from 0.1 to 2.5 percent by
weight and
especially preferably from approximately 0.2 to approximately 1.5 percent by
weight. The
amount of trometamol used as huffer in the contact lens care products
according to the
invention, based on the total amount of the contact lens care product, is more
especially
from 0.05 to less than 1.2 percent by weight or from 0.05 to less than 0.8
percent by
weight, very especially from 0.1 to 0.6 percent by weight, especially from 0.1
to less than
0.6 percent by weight, for example up to 0.5 percent by weight.
2-Amino-2-hydroxymethyl-1,3-propane.diol or a homolog thereof having up to 10
carbon
atoms, or a salt thereof, can be used alone as buffer. Alternatively, one of
the above-
mentioned compounds can be used together with a salt thereof. Again,
preference is given
20984'2
-4-
here to the use of ophthalmolo;gically acceptable salts, such as the salts
mentioned above
in connection with compounds of formula I. Especially suitable are the
hydrochlorides or
maleates of compounds of formula III, i.e., for example, a combination of 2-
amino-
2-hydroxymethyl-1,3-propanediol with the hydrochloride of 2-amino-2-
hydroxymethyl-
1,3-propanediol, or a combination of 2-amino-2-hydroxymethyl-1,3-propanediol
with the
maleate of 2-amino-2-hydroxymethyl-1,3-propanediol or 2-amino-2-hydroxymethyl-
1,3-propanediol, to which smaa.l amounts of hydrochloric acid are added.
T'he contact lens care products according to the invention preferably do not
comprise
buffers other than compounds of formula III. They may, however, comprise other
buffers
in addition to one or more compounds of formula III.
The contact lens care products according to the invention are preferably
formulated in
such a manner that they are isotonic wish lachrymal fluid. They may in general
comprise
additives that are customary fo:r contact lens care products. Those include,
for example,
compounds that influence tonicity, surface-active compounds, compounds that
influence
viscosity, or complex formers. The contact lens care products according to the
invention
comprise those or other customary additives in amounts that vary within the
range of the
values familiar to the person skilled in the art.
A. solution that is isotonic with lachrymal fluid is generally understood to
be a solution the
concentration of which corresponds to the concentration of a 0.9 % sodium
chloride
solution. Departures therefrom are entirely possible, provided that the
contact lenses to be
treated are not damaged thereby. Isotonicity with lachrymal fluid or a
different desired
tonicity can be established by t:he addition of organic or inorganic compounds
that
influence tonicity. The former can be used, for example, in amounts of
approximately 1 to
4.5 percent by weight, the latter in amounts of approximately 0.1 to 1.3
percent by weight.
In general, the amount of the compound that influences tonicity that is added
is such that
the tonicity of the composition according to the invention is especially in
the range of
from 200 to 450 milliosmols, preferably in the range of from approximately 270
to
approximately 330 milliosmols. Typical organic compounds of that type are, for
example,
glycerol, urea, propylene glycol or sugars, such as mannitol or sorbitol, and
typical
inorganic compounds of that type are especially potassium chloride and sodium
chloride.
Mixtures of those compounds with one another can also be used according to the
invention.
-5-
Suitable surface-active compounds are mentioned, for example, in EP-A2-180
309.
Examples of especially suitable representatives that can be used according to
the invention
are poloxamer types and miranol types,. Other representatives are known to a
person
skilled in the art. Those compounds can be used, for example, in amounts of up
to
20 percent by weight, espe:ciahly in amounts of from 0.4 to 5 percent by
weight.
Suitable compounds that influence viscosity are also known to a person skilled
in the art.
Examples of especially suitabhe representatives that can be used according to
the invention
are polyvinyl alcohol, hydroxyethylcellulose, hydroxypropylmethylcellulose and
poly-
a~~rylic acid. Typical amounts for those: compounds are from 0.1 to 2 percent
by weight.
A,n especially suitable complex; former is especially
ethylenediaminetetraacetic acid,
ahbreviated to EDTA, and salts thereof, such as sodium salts. Typical amounts
for those
c~~mpounds are from 0.01 to 1 percent by weight.
The contact lens care products according to the invention are suitable for all
types of
contact lenses. These include capeciall;y so-called hard and soft contact
lenses, and also
so-called hard-flexible or highly gas-permeable contact lenses. The contact
lens care
products according to the invention exhibit an antimicrobial action and, also,
a cleaning
acaion. Depending on the specific intended use, the contact lens care products
according
to the invention can be used as cleaning; solutions, disinfectants, or, for
example, as
solutions for storing, rinsing, abetting or soaking contact lenses. All those
solutions are
distinguished by good cleaning; and disinfecting action and a high degree of
tolerability in
a single solution.
In addition, while exhibiting better antvnicrobial activity, the contact lens
care products
according to the invention surprisingly exhibit significantly better
cytotoxicity properties
than do the care producta known from the prior art, for example Bausch &
Lomb's Kombi
solution. The overall spectrum of properties of the contact lens care products
according to
the invention is therefore considered to be significantly superior to the
properties of the
contact lens care products known from the prior art.
The contact lens care products according to the invention are prepared in a
manner known
Q~r se, especially by conventional mixing of the constituents with water or
dissolving of
the constituents in water.
2(~984'~2
-6-
T'he compositions according to the invention are especially suitable for
cleaning and disin-
fecting contact lenses. The contact lens; care products according to the
invention are used
in a manner known her se, for example by bringing a contact lens into contact
with the
contact lens care product for a period o:f time sufficient for cleaning or
disinfection.
>r~epending on the type of lens and the degree of contamination, a period of
from several
rr~inutes to about 24 hours, prei:erably up to approximately 4 to 12 hours, is
sufficient.
A. preferred solution according to the invention comprises, for example,
a~ninopropyl biguanide 0.0005 to 0.05 mg/ml
2-amino-2-hydroxymethyl-1,3~-propanediol 0.67 to 4.03 mg/ml
2-amino-2-hydroxymethyl-1,3--propanediol . HCl 2.64 to 4.02 mg/ml,
the total amount of buffer being preferably less than 8 mg/ml and especially
less than
6 mg/ml,
and may preferably also comprise:
NaCI or KCl 3 to 9 mg/ml
especially 5.5 to 9 mg/ml
surface-active compound 5 to 30 mg/ml
EDTA 0.1 to 2 mg/ml.
A solution according to the invention that is likewise preferred comprises,
for example,
aminopropyl biguanide 0.0005 to 0.05 mg/ml
2-amino-2-hydroxymethyl-1,3-propanediol 1.00 to 4.00 mg/ml
especially about 2.5 mg/ml
and small amounts of HCl to establish the desired pH range.
The desired pH range of the compositions according to the invention is
especially approx-
imately 7.0 to 7.5, preferably from 7.1 to 7.4 and especially preferably 7.3.
T:he following Examples serve to illustrate the invention. They are not,
however, intended
to limit the scope of the invention in any way, and especially not to the
subject-matter of
the Examples.
2~~~~"~2
_7-
F;xample 1: Formulation for soft contact lenses
1 ml of solution comprises:
aminopropyl biguanide (Arlagard~) 0.005 mg
2-amino-2-hydroxymethyl-1,3-propanediol 0.97 mg
2-amino-2-hydroxymethyl-1,3-propanediol ~ HCl 6.61 mg
1'TaCI 6.6 mg
poloxamer 407 10 mg
>=;DTA 1 mg.
>=;xample 2: Formulation for herd contact lenses
1 ml of solution comprises:
aminopropyl biguanide (Arlag;ard~) 0.005 mg
2-amino-2-hydroxymethyl-1,3-propanediol 0.97 mg
2-amino-2-hydroxymethyl-1,3-propanediol 6.61 mg
~ HCl
rfaCl 6.6 mg
poloxamer 407 10 mg
EDTA 1 mg
polyvinyl alcohol 14 mg.
Example 3: Formulation for soft contact lenses
1 ml of solution comprises:
aminopropyl biguanide (Arlag~~rd E~) 0.005 mg
2-amino-2-hydroxymethyl-1,3--propanediol 1.39 mg
2-amino-2-hydroxymethyl-1,3-propanediol ~ HCl 6.06 mg
I~ aCI 5.5 mg
p~~loxamer 407 10 mg
EDTA 1 mg.
Example 4: Antimicrobial acti'r~
The formulation of Example 3 is tested against the following test organisms:
Escherichia
coli, Staphylococcus aureus, Pseudomonas aeruginosa, Candida albicans,
Aspergillus
niger. The following Table shows the initial inoculum as well as the number of
organisms
20984'2
_g_
still to be found after the formulation of Example 3 has been allowed to act
for a period of
4. hours and of 6 hours:
7.'est organism initial number of number
of
inc~culum organisms organisms
after after
4 hours 6 hours
Facherichia coli 6.8 - 105 0 0
Staphylococcus aureus8.9' ~ 105 0 0
F'seudomonas aeruginosa1.0' - 106 0 0
C:andida albicans 1.1 - 106 2.7 - 105 1.8 - 105
~cspergillus niger 1.1 - 106 b.2 - 105 1.1 - 106
I:n contrast, the following values are determined for Bausch & Lomb's Renu
solution
comprising aminopropyl bigu~~nide (0..'i ppm) and a borate buffer:
Test organism initial number of number
of
inoculum organisms organisms
after after
4 hours 6 hours
F,scherichia coli 6.8 - 105 9.5 - 101 4.3 - 101
Staphylococcus aureus8.9 - 105 5.7 - 101 3.8 - 101
Pseudomonas aeruginosa1.0 ~ 106 0 0
C'andida albicans l . l - 106 4.9 - 105 6.2 - 105
A,spergillus niger 1.1 - 106 5.5 ~ 105 4.3 - 105
Example 5: Cytotoxicity test
In order to determine the cytot~~xic potential, the formulation of Example 3
is subjected to
the growth inhibition test and compared with Bausch & Lomb's Kombi solution.
In this
teat the decrease in cell growth in the presence of toxic compounds is
determined by
c~~mparing the protein content of treated cell cultures with the protein
content of untreated
call cultures after 72 hours' incubation. The test solutions are serially
diluted with the cell
culture medium (DMEM-FCS j. L 929 cell cultures are incubated for 72 hours in
the
presence of solutions of various concentrations. It is found that the solution
according to
20984'2
-9-
presence of solutions of various concentrations. It is found that the solution
according to
the invention induces cytotoxic effects only at concentrations of 20 % v/v,
while the
Kombi solution already induces cytotoxic effects at concentrations of 5 % v/v.
In the test carried out, the protein content is a measure of the cell growth
or the inhibition
of growth induced by toxic substances. Growth inhibition of more than 30 %
compared
with untreated cultures is considered to be a clear cytotoxic effect.
Solution A): Formulation according to Example 3
Concentration
of the solution (% v/v] 20.0 10.0 5.0 2.5 1.3 0.6
Growth inhibition [%] 32 11 4 4 2 0
Solution B): Kombi solution from Bausch & Lomb
Concentration
of the solution [% v/v] 20.0 10.0 5.0 2.5 1.3 0.6
Growth inhibition [%] 92 68 40 25 11 7
These results demonstrate that the Kombi solution already induces cytotoxic
effects at a
dilution lower by a factor of 4, namely at 5 % v/v, compared with the
formulation accord-
ing to Example 3 with which such effects occur only with 20 % v/v solutions.
Example 6: Formulation fo:r hard contact lenses
1 ml of solution comprises:
polyaminopropyl biguanide: (Cosmocil~)0.002 mg
2-amino-2-hydroxymethyl-1,3-propanediol2.5 mg
NaCI 7.5 mg
poloxamer 407 10 mg
hydroxyethylcellulose 3.2 mg
EDTA 1 mg
HCl to adjust pH
20984'~~
- 10-
Example 7: Formulation fo:r soft contact lenses
1 ml of solution comprises:
polyaminopropyl biguanide: (Cosmocil~) 0.001 mg
2-amino-2-hydroxymethyl-1,3-propanediol2.45 mg
NaCI 7.4 mg
poloxamer 407 1.0 mg
EDTA ~ 0.25 mg
HCl to adjust pH
Example 8: Antimicrobial activity
The formulation according to Example 6 is tested against the following test
organisms:
Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, Candida
albicans,
Aspergillus niger. The following Table indicates the initial inoculum and the
number of
organisms still to be found .after the formulation of Example 6 has been
allowed to act for
a period of 6 hours:
Test organism initial number of
inoculum organisms
after 6 hours
Escherichia coli 1.1 106 0
-
Staphylococcus aureus1.4 106 0
-
Pseudomonas aeruginosa1.3 106 0
-
Candida albicans 1.2 i06 4.1 ~ 105
-
Aspergillus niger 5.7 i05 2.8 - 105
-
Example 9: Cytotoxicit~te;~t
Analogously to Example 5 'the formulation according to Example 6 is subjected
to the
growth inhibition test. The test shows that the solution according to the
invention induces
only extremely slight cytotoxic effects even at concentrations of 20 % v/v,
while the
Kombi solution already induces significant cytotoxic effects at concentrations
of 5 % v/v
(see Example 5).
209$4'2
-11-
Solution according to Exar~iple 6
Concentration
of solution [% v/v] 20.0 10.0 5.0 2.5 1.3 0.6
growth inhibition [%] 12 S 0 0 0 3