Note: Descriptions are shown in the official language in which they were submitted.
2a~g~
SEPARATION OF UNSA~URATES
Field of the_Invention
The invention relates to the separation of unsaturated
organic compounds from other such compounds or from saturated
compounds.
Backaround
The separation of unsaturated compounds from others, or
more highly unsaturated compounds from less is important in
many fields, particularly for natural products containing
fatty acidsO Here the separation is often difficult to
achieve, compounds of importantly different nutritional or
general biological properties having only marginally different
physical properties. We have sought new approaches in terms
of both solvent systems and of overall separation processes.
~`
The Invention
The invention in one aspect lies in a process for
fractionating a diverse range of unsaturated compounds.
Examples are eicosanoids, tocopherols, tocotrienols etc. In
particular doubly, triply or more highly ethylenically
unsaturated compounds such as unsaturated fatty acids, fatty
alcohols and the like (polyunsaturates) are separated from
those with fewer ethylenic unsaturations or completely
; saturated, using a selective, liquid entrapment medium. The
unsaturated fraction is extracted from the entrapment medium
by contacting it with an immiscible release medium, and is
normally then recovered from the release medium, though in
principle the process can be two stage if for example the
unsaturate is not the primarily desired product. The
entrapment medium and normally also the release medium are
recycled.
, .. , , , , :, :
,: . ", :: ,, ~, . .: :
", . ..
, , , , ,, : , , : i.
, ,, , : . :
2 ~
In another aspect the inventlon lies in the entrapment
medium itself and its method of preparation. It also lies in
the separated products obtained by the process, of which the
unsaturates will normally be the more valuable product and
indeed may be novel produc~s in the sense of not having been
prepared before in puri~ied form. The fraction not selected
by the entrapment medium is however equally a product of the
process.
A particular application of the invention is to
fractionate mixtures of polyunsaturated fatty acids and
related compounds. The process can be applied successfully to
the fatty acids themselves and to their derivatives such as
salts, alkyl esters, mono-glycerides, di-glycerides, tri-
glycerides, phospholipids and amides as well as to other
compounds containing fatty acid carbon chains with
unconjugated double bonds, such as the fatty acid alcohols.
; Mixed glycerides can be fractionated, in particular for
example in recovery of the valuable triglyceride dilinoleoyl
monogamma linolenoyl glycerol (DLMG) from triglyceride
mixtures.
Considering entrapment media, the sulphones, particularly
sulpholane, have an unusual combination o~ properties.
Sulpholane, otherwise tetramethylene sulphone or
tetrahydrothiophene~ dioxide, is
~ 0~ S ~0
is the most common but other available sulphones are 3-
sulpholene, otherwise 2,5-dihydrothiophene-~ dioxide
0~ ~0
..... ..
,, .:
: '~
r
2~9~
and acyclic compounds such as dimethyl sulphone
ll .
CH ~ S CH3
3 ll
O
diiodomethyl p-tolyl sulphone
O
O
~ and di-(4-hydroxyphenyl) sulphone, otherwise Bisphenol S ~
:~ O ' '
~ HO~ 5 ~ , _ OH
~' O
of these, sulpholane itself is known broadly for use in
enriching the unsaturation level in fatty oils (Kirk Othmer,
section on "Sulpholanes and Sulphones" p. 964, with re~erences
to U.S. Patent 2 360 860 (1944) and WisniaX Br. Chem. Eng.
15Lll 76 (1970).) It is the preferred entrapment medium for
use in the process of the present invention.
The învention for the first time combines the selective
solvency of sulphones for unsaturated fatty acids with the
known ability of silver salts and other 'type-b' cations to
form reversible pi~complexes with the double bonds of
unsaturated compounds. This two-component entrapment medium
is the basis of a particularly valuable form of the current
invention which provides a continuous, efficient, flexible
process for the preparation of products of high quality and
purity suitable for the nutritional and pharmaceutical
industries.
,: , ,:
2~3~2X
Silver salts such as the nitrate dissolve readily in
sulpholane in the presenca of a proportion o~ water, for
example in commercial grade sulpholane containing 3 wt%
dionised water. Of metals forming pi-complexes with
unsaturated compounds silver is however only the best known,
and the use of salts of copper, gold or other metals with
incomplete electron shells capable of the required pi-
complexing is not excluded. Silver nitrate has a low
solubility in anhydrous sulfolane, but a solution of lOg
silver nitrate in 100 ml sulfolane water 95:5 by volume can
for example be obtained, and similarly a solution of 20g
silver nitrate in 100 ml sulfolane water 88:12.
~ he entrapment medium is thus particularly suitably made
up of sulpholane, water and silver nitrate. Sulpholane is a
viscous, high-boiling, non-toxic, dipolar, aprotic solvent
which has selectivity for fatty acids and fatty acid esters
depending upon the molecular weight and degree of
unsaturation. Silver has been used in the chromatographic
separation of unsaturated compounds. However no free solvent
system which dissolves silver salts whilst having low
solubility for saturated compouncls has hitherto been found,
and the technical and commercial demands associated with
recovery, re-use and re-circulation ol silver have remained
unsatisfied.
The successful application and degree of selectiVity of
the process depends upon the partition coefficients of the
tarqet substance between the feed mixture and the entrapment
medium and between the entrapment medium and the release
medium. Favourable partition ratios ~or dif~erent taryet
extractives can be obtained by adjusting the entrapment
medium, ~or example as to the amount o~ water and silver salt
used to make up the pre~erred silver containing sulpholane
medium, and hy selecting an appropriate release medium with
the required solubilising and polarity properties.
.. . . ..
' ' , .' ',' ~
' ' :, , ,
, . .
: , :: ~ ~,.
209~26
The silver/sulpholane entrapmen$ medium is very
satisfactory in that it allows the separation of fatty acids
containing two, three or more double bonds from those that are
dienes, monoenes or saturates. Furthermore, a similar level
of selectivity is obtained when the fatty acids are present in
the much more complex and heterogeneous triglyceride form, of
which natural vegetable-seed oils, marine fish oils and fungal
biomass oils are composed. For example, in evening primrose
oil mixed triglycerides such as DLMG containing one or more
triply unsaturated gamma-linolenic acid moeities are
selectively concentrated at the expense of triglyceride
species containing various permutations of saturated,
monoenoic and dienoic acyl groups.
An integral part of the preferred separation process is
the use of the release medium to extract the target
polyunsaturated substancas from the liquid entrapment medium.
The release medium has to be largely immiscible with the
entrapment medium and has of course to dissolve the target
polyunsaturated fraction; it should also desirably have a low
boiling point for ready subsequent separation from the target
fraction. Hydrocarbon solvents such as hexane, or petroleum
hydrocarbon mixtures, or olefins such as cyclohexene, are
suited to many applications of the process and can be selected
to give enhanced extraction for a particular extractive by
manipulation of partitioning behaviour. Extraction of
polyunsaturated fatty acid species with hexane for example i5
essentially quantitative and the hexane is readily removed and
recovered ~or re-use. The sulpholane/silver medium is also
easily ~reed from excess hexane and is suited to be re-cycled
directly.
Although sulpholane has been quoted as the solution
component of the entrapment medium because it is commercially
available and inexpensive, other sulpholane derivatiVes can
also be used. ~ixtures of sulphonas with standard organic
:
,
- : . . .: . ., , . ::
,~
, ,, : .
.
; ' , , , , :~ : : . . : .:
2~ '3~2~
reayents such as acetone, ethanol and ethyl acetate can also
be used successfully as the entrapment medium in the process.
The salt component of the entrapment medium need not
necessarily be a nitrate. Other soluble salts, for example
silver tetrafluoroborate and silver trifluoroacetate, can be
used. The entrapment medium can also include other cations
which form pi-complexes with unsaturated compounds.
Conveniently the process of this invention in preferred
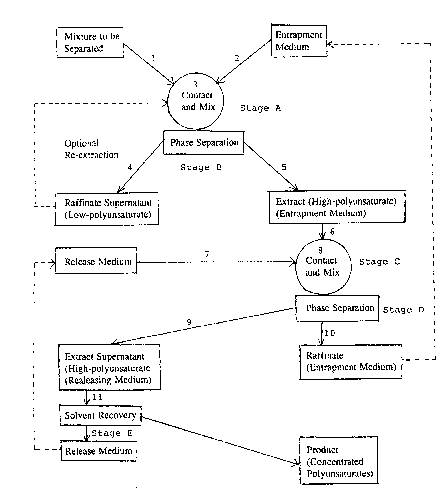
form may be set out as comprising the following stages:
A. Contacting a mixture to be separated with an entrapment
medium in a multiphase system and allowing a target
polyunsaturate from the mixture to migrate into the entrapment
medium to form reversihle pi-complexes therein;
B. Separating from each other a raffinate phase (containing
non-polyunsaturates) and an extract phase containing the
entrapment medium and target polyunsaturate;
C. Contacting the extract phase containing the entrapment
medium and the target polyunsaturate with a releas~ medium
immiscible with the extract phase but taking up the target
polyunsaturate;
D. Separating the release medium containing the target
polyunsaturate from the entrapment medium, which is re-cycled;
and
E. Recovering the release medium, which is re-cycled,
leaving the polyunsaturate product.
The unit operations or stages in this process are common
to many standard solvent extraction processes and may be
carried out in any convenient n,anner, but the accompanying
: . ,:: ,,: "
! ~. "
'
2~526
general flow diagram provides an lllustrative example of the
process.
The mixture to be separated is introduced through ~eed
line 1 and admixed vigorously with the entrapment medium,
introduced through a separate line 2, in the contact zone 3.
Conveniently the ratio of medium to mixture is between 1:2 and
20:1 and the contact time is between 20 seconds and 100
minutes at temperatures between -80 C and 90 C. Suitable
equipment for carrying out this liquid-liquid extraction may
comprise an impinging jet mixer, an agitation vessel, a
centrifugal extractor, etcO and co-current and counter-current
systems can be used.
In the contracting zone the mixture and the entrapment
medium are caused to produce a supernatant raffinate phase and
an extract phase which are separately withdrawn respectively
through lines 4 and 5. The separation of these two phases may
be effected by settling, decantation or centrifugation. The
raffinate phase can be fed bacX to the contacting zone for re-
partitioning to separate residual polyunsaturates from non-
polyunsaturate species.
The extract phase containing the polyunsaturate fraction
complexed with the entrapment medium is transferred through
line 6 and partitioned against release medium (supplied via
line 7) in the contact zone 8. The same sort of equipment as
is indicated above can be used in this second extraction stage
;and also in the associated phase separation, although the
operating conditions may have to be varied. Conveniently the
ratio of releasing medium to entrapment medium/polyunsaturates
complex is between 20:1 and 1:5 and contact time is ~etween 20
seconds and 60 minutes at ambient temperature.
~,
; The extract phase and the raffinate phase are separately
withdrawn respectively through lines 9 and 10. The raffinate
from this second extraction stage is the entrapment medium and
';
,
2 ~ 2 6
a
is fed directly back to process with no further treatment
required, since the process can tolerate the small amount of
release medium required to saturate the entrapment medium.
The extract phase containing the low boiling release
medium and the product is transferred through line l to
;solvent recovery system where the release medium is removed,
condensed and collected. The product can be treated by short
path distillation to remove all traces of the release medium.
It may be necessary to treat the release medium with brine
occasionally to remove silver ions, easily reco~rered as
silver chloride by filtration with subsequent removal of the
water by centrifugation.
~ .
- The following examples of particular separations
illustrate the invention, Examples l and 2 being to
preparation of entrapment media, the rest to separation
;~processes of various kinds.
EXAMPLE 1
:~ :
Silver nitrate (lOg) was dissolved with heating to 70 C
in water (5 ml) and sulpholane (95 ml~ added with stirring to
form a clear solution from which silver nitrate did not
crystallise at room temperature. This stoc~ solution is
suitable for most separations and may be re-used more than
twenty times in the following examples without degeneration or
cross contamination. It is referred to as 95 SAg.
EXAMPLE 2
Silver nitrate (20g) was dissolved in 12 ml of water at
40C and sulpholane (88 ml) added with stirring. This
solution is referred to as 88 SAg, and is very stable. It is
capable of being recycled many times with little fall off in
selectivity and separation performance.
, ~ ; , ~ , ~ . ,
:, : "
, . .: . .
- ~ ; ' ;,
,:
., ,::;, ,
2 ~
~AMPLE 3
Refined evening primrose oil was taken, containing 8.2%
gamma-linolenic acid in terms of its fatty acid composition
and 13.8% of it5 triglyceride in the form of the isomers of
DLMG, and lOg was added to 95 SAg (100 ml) and vigorously
shaken for a total contact time of 1 minute. It was then
allowed to separate into two distinct layers over a period of
5 minutes. The supernatant raffinate phase consisting of
saturated and monoeroic fatty acid containing triglycerides
was decanted. The bottom phase consisting of triglycerides
containing polyunsaturated fatty acids in the entrapment
medium was then contacted with 20 ml of the releasing medium
hexane and shaken for 2 minutes. The two phases were allowed
to settle for 10 minutes. The sup~rnatant Pxtract phase
containing the oil was decanted~ A second 20 ml of hexane was
added to the 95 SAg phase and the mixture shaken for 2 minutes
before allowing to settle for phase separation. The
supernatant hexane extract was combined with the first extract
and transferred to a solvent recovery vessel where the hexane
was removed. The evening primros~e oil remaining (2.05g) was
enriched in the target polyunsaturated fatty acid gamma-
linolenic acid by a factor of 2 . 9 i . e . the product contained
23.8% gamma-linolenic acid and 50% of its triglycerides as
DLMG.
EXAMPLE 4
, .
Unrefined sardine oil was converted into its fatty acid
ethyl esters by transesterification with sodium ethoxide in
ethanol at 60 C. The crude ethyl esters were purified by
thin-film evaporation at 130C and 0.03mm pressure to yield a
colourless mixture of ethyl esters. A sample of this (lOg)
was contacted with 88 SAg (80ml) and shaken vigorously for 5
minutes and was then allowed to settle for 20 minutes. The
ïraffinate was decanted and contacted a second time with the
;!:;
", ;: ' : ~ :
, ~ '`"' ' ;, , ''~," , ' ;
': ' ' ' . " ' ' " , ~
2 ~ 2 6
entrapment medium (40 ml), vigorously shaken for 5 minutes and
allowed to settle for 20 minutes. Once the supernatant
raffinate was decanted and discarded, the bottom layer was
combined with the bottom layer from the first partitioning and
separation. Cyclohexene (30 ml) was contacted with this
combined entrapment media and shaken for 8 minutes. After a
subsequerlt period of 15 minutes the two phases had completely
separated and the supernatant releasing medium layer was
decanted. The remaining bottom layer was then extracted twice
again with 2 x 30 ml aliquots of cyclohexene. The three
cyclohexene extracts were transferred to the solvent recovery
vessel, where the solvent was removed under vacuum to yield a
mixture of ethyl esters (2.42g) enriched in the target
polyunsaturated fatty acids. Eicosapentaenoic acid had been
concentrated from 14.8% to 33.4% and docosahexaenoic acid from
7.6% to 13.8%. The total of omega-3 polyunsaturated fatty
acids had been lncreased from 24.8% to 52.9%.
EXAMPLE 5
An oil (125g) extracted from the cultured biomass of the
fungus Mortierella alpina containing arachidonic acid (15%) in
both phospholipid and triglyceride forms was vigorvusly mixed
with one litre of 95 SAg containing 5% ethyl acetate. After
10 minutes shaking the two phases were allowed to separate for
30 minutes and the supernatant raffinate oil was decanted and
discarded. The lower phase was contacted with 50 ml petroleum
spirit as the releasing medium, mixed thoroughly for 20
minutes and allowed to settle for 60 minutes. The supernatant
extract phase containing the polyunsaturated enriched oil was
decanted and the petroleum spirit removed by vacuum
evaporation. The fungal oil product was enriched in the
target quadruply unsaturated arachidonic acid by a factor of
2.5 i.e. the oil contained 37.5% with a yield of 28% w/w.
, , i~ ~ .
.
,, ''~ . :' '' ,
2~9,~2~
11
EXAMPLE 6
; Palm oil residue i.e. the material removed during the
deodorisation stage of the refining operation was treated with
acetone at low temperature to remove the major proportion of
fatty acids and triglycerides as a crystal fraction by
filtration. The ~iltrate contained largely sterols,
tocopherols and tocotrienols and lOg of this was contacted
with 95 SAg (120g) and shaken vigorously for 5 minutes. A~ter
allowing 15 minutes for the two phases to form, the upper
raffinate phase was decanted. The lower phase was contacted
with 250ml hexane, shaXen for 10 minutes and allowed to settle
for 20 minutes before decanting off the upper extract layer.
This was then trans~erred to a rotary vacuum evaporator where
hexane, the release medium was removed. The product (2~2g~
contained 60% tocotrienols, compounds containing three double
~onds on the phytyl side chain, of which the gamma species was
the predominant. Furthermore, the product recovered from the
raffinate above contained 50% tocopherols, similar compounds
but containing no double bonds on the phytyl side chain.
~ .
, .
,:~
~'
., , . . ,,: . : " , ..
: , . .. . .