Note: Descriptions are shown in the official language in which they were submitted.
2101113
AUTOMATED FLUID PRE SSIJRE CONTROL SYSTEM
BACKGROUND
The invention relates generally to fluid control and, more particularly, to
automated control over fluid delivery pressure.
Infusion pumps are used to more precisely control the infusion of medical
fluids into the vascular system of a patient. Syringe pumps are one type of an
5 infusion pump in which the syringe plunger is moved into the syringe barrel at a
controlled rate to administer the ~yringe contents to the patient. Such pumps are
typically used to adn~in~ster the infusion fluid generally as opposed to locally. That is,
the medical fluid is placed into the bloodstrea n of the patient for general distribution
throughout the body rather than app~ing the medica1 fluid only to a 10calized delivery
10 site in the body. Consequentl~, such pumps are typically controlled to deliver the
medica1 fluid in accordance with a desired flow rate rather than at a precisely
controlled pressure.
While many such pumps include a pressure sensor, the sensed pressure is
typically used only to trigger pressure alarms that will shut off the flow from the
15 syringe pump if an occlusion or other undesirable condition is detected. The pressure
provided by the pump is often only controlled to reside in a particular pressure range,
~; which may be relatively wide, and to remain below a predetermined upper limit; the
range and upper limit being selected in dependence upon the particular application.
However, in the case of local drug delivery systems for ir~ecting medical
20 fluids into the walls of blood vessels, into body orgaDs or other interna1 delivery sites,
pressure control is a primary fluid delivery parameter. In some cases, it is desirable
to treat a disease by locally applying a medical drug in a high concentration. The
concentration may be so high that the drug could cause damage to other parts of the
body or even be life threatening if allowed to freely enter the blood stream of the
26 patient, yet the drug may have the desired effect if confined to a local application. In
. . ~ ,
-~ ;' ' I
2101113
such local applications, it has been found that pressure control is important.
Excessive pressure may cause a dissection of the vessel wall and too little pressure
will not force the medical fluid into the vessel wall but may aUow it to be swept away
by the bloodstream before it can be delivered to the desired location.
Studies have indicated that the application of particular medical fluid~ to the
vessel walls follow~ng the performance of an angioplasty procedure on those walls has
the potential of impeding restenosis at that site. For example, see Wolinsky et al.,
Local Introduction Of Dru~s Into The Arterial Wall: A Percutaneous Catheter
Techniaue, Journal of Interventional Cardiology, Vol. 2, No. 4, 1989, pgs 219-228.
These studies indicate that the application pressure of the medical fluid to the vessel
walls contributes greatly to the success or failure of the treatment. Thus, it would be
of value to precisely control the pressure of the medica1 fluid applied in such a
procedure.
The inflation baUoons used in such catheter based, local introduction
16 techniques typica~y include a certain number and size of apertures through which the
medical fluid wiU be delivered to the vessel walls. The balloon is positioned at the
desired location in the vessel and pressure is applied to inflate the baUoon. However,
the apertures tend to impede a rapid inflation because they aUow a part of the
inflation pressure to escape. AdditionaUy, the apertures may aUow some of the
inflation fluid to escape into the bloodstream during inflation. In the case where the
fluid to be app1ied to the vessel waUs has some higher level of toxicity to tbepatient if
the fluid should enter the bloodstream, a more rapid baUoon inflation is desired. One
means of achieviog a more rapid inflation is to apply a higher pressure during initial
inflation to force the baUoon into its operative conf~guration as soon as possible and
then lower the pressure to maintain the target pressure. An automated system would
be desirable to achieve this control over inflation.
Additionally, while the baUoon is inflated, and even when only partiaUy
inflated, the blood flow is interrupted or at least impeded. To avoid iruury to the
patient, a time limit on the inflation is imposed. Therefore, full inflation of the
baUoon should occur as rapidly as possible so that adm1nistration of the medical fluid
can be started as soon as possible so that all of the medical fluid can be applied to the
delivery site before expiration of the time limit.
- More precise pressure control is also desired where the medical fluid applied
to the vessel walls has some higher level of toxicity to the patient if the fluid should
enter the bloodstream. In this casej it is necessary to maintain the balloon at a
...... .. . . . . . . .
2~1113
predetermined pressure throughout the application of the drug to lessen its chances
of entering the bloodstream. The baUoon must be kept at a high enough pressure so
that the apertures formed in the baUoon are in extremely close contact with the
vessel walls. That is, the balloon cannot be permitted to deflate to an extent where
5 the apertures are exposed to the bloodstream 90 that the medical fluid would be
taken from the delivery site by the blood flow. By this means, drugs which may be
somewhat toxic if applied to the patient through the bloodstream may be locaUy
applied to the walls of a blood vessel to perform a post angioplasty or other function
while not adversely affecting the patient.
In one type of prior inflation/deflation system, a syringe is attached to the
proximal end of a catheter contau~ing the baUoon and a pressure gauge is locatedadjacent the syringe to measure the pressure of the medical fluid in the catheter.
The plunger of the syringe is manually moved into the syringe barrel to expel the
fluid contents from the syringe through the catheter and into the vessel walls through
15 the baUoon apertures. The pressure indicated on the pressure gauge is monitored by
the operator during the movement of the syringe plunger and the operator varies the
movement of the syringe plunger in an attempt to maint~in the desired pressure. It
has been found that manual methods such as this typicaUy do not adapt quickly
enough to compensate for pressure variances as the fluid is being delivered into the
20 vessel waUs. Pressure variances may be caused by various factors inc1uding site
geometry, blood pressure, the number of apertures in the baUoon, catheter geometry,
and the viscosity of the medical fluid being applied, for example.
A drop in the pressure indicated on the pressure gauge may stimulate the
operator to accelerate the movement of the plunger into the sgringe barrel which may
25 result in a pressure spike. High pressures have been found to result in necrosis of
the imner media of the vessel (Wolinsky et al., id) and if too high, dissection of the
vessel. Low pressures have the effect of transmitting the drug into the bloodstream,
as discussed above. Thus, it is desirable to provide a system which permits a more
rapid response time with more precise control over the pressure.
A further consideration in such systems is deflation and removal of the
delivery device. In the case where the delivery device comprises an inflatable baUoon
having apertures for applying a medical fluid, deflation should occur so that the drag
remaining in the balloon is captured by the catheter, rather than being released into
the blood skeam. Applying a pressure below the pressure of the delivery site, which
36 in some cases may require a negative pressure, should occur relatively rapidly so that
2101113
the medical fluid is not released into the bloodstream. The pressure m the system
may change as the balloon is collapsed, thus monitoring the pressure and correcting it
as it varies during removal of the balloon is desirable. Applying a negative pressure
which is too large will unnecessarily draw blood or other body fluids into the catheter
5 while too little negative pressure may allow the medical fluid to enter into the
bloodstream.
In an angioplasty system in which a catheter having an inflatable balloon is
positioned at a delivery site for applying pressure to the vessel walls by inflating the
balloon, more precise pressure control may also provide a benefit. Although this is a
10 closed system in that the inflation fluid is not continuously leaving the system as in
the drug delivery system, pressure must be monitored for proper performance of the
procedure. Excessive pressure may cause damage to the vessel walls while
insufficient pressure will not expand the vessel walls enough to successfully
accomplish the angioplasty.
It has also been found desirable in many drug delivery systems to more
precisely control the volume of the medical fluid delivered along with more precise
pressure control. In some applications, the volume of the drug to be applied to the
delivery site, such as blood vessel walls, iB very small (less than five milliliters).
Present fluid in~ection sy6tems have not permitted the precise control over pressure
in the delivery of such a small amount of fluid.
Hence, it has been recogni~ed by those skilled in the art that a more
accurate fluid pressure control system is desirable. It has also been recognized that a
system which provides automated control over pressure during the delivery of fluid iB
also desirable. It has been further recognized that a system whuch accurately controls
pressure during the local application of medical fluid while avoiding infusion of the
medical fluid into other areas is desirable. The present invention fulfills these needs ?
and others.
SUMMARY OF THE INVEN'rION
The present invention provides for the local adlninistration of fluid at a
predetermined pressure through a delivery device positioned at a site internal to the
body of a patient. Delivery is achieved by an apparatus which comprises a reservoir - -
containing the fluid to be delivered and a conduit such as a catheter, connectedbetween the reservoir and the delivery device. A pressure sensor senses pressureindicative of the fluid pressure and provides a sensed pressure signal representative
... . . .
- . ,
.
2101113
-- 6 --
thereof. A controller automatically receives the sensed pressure signal, automatically
compares the sensed pressure to the predetermined delivery pressure and
automatically provides a pressure error signal representative of the difference. A
driving means automatically varies the pressure of the fluid being administered to the
5 delivery site in response to the pressure error signal from the controller to
automatically attain the predetermined pressure.
In a method for locally administering a fluid at a predetermined pressure to
a patient at a site internal to the body of the patient, the fluid to be delivered is
contained in a reservoir and pressure is applied to move the fluid from the reservoir
10 through a fluid conduit and through a delivery device to the delivery site. Pressure is
automatically sensed to indicate the pressure of the fluid and a sensed pressure signal
representative of the sensed pressure is automatically compared to the predetermined
pressure. A pressure error signal is provided in the event that the two pressures are
different and the pressure of the fluid is automatically varied in accordance with the
15 pressure error signal to attain the predetermined pressure.
In another aspect of the invention, a pneumatic driving means varies the
volume of the reservoir to control the fluid pressure. In the case where the fluid
reservoir comprises a syringe having a barrel containing the fluid to be administered,
the barrel is coupled to the catheter and the pneumatic driving means forces the20 syringe plunger into the barrel to control the fluid pressure. The driving means
moves the plunger more rapidly or more slowly in accordance with the error signal.
In another aspect of the invention, a volume detector is provided which
detects the volume of the fluid delivered and provides a volume signal representative
of the detected volume. The controller automatically compares the volume signal to
25 the volume selected for delivery and controls the driving means to cease delivery of
the fluid when the selected volume has been delivered. The volume detector may
comprise a &placement detector to detect the movement of the driving means or the
syringe plunger. The position of the selected movable device indicates the amount of
fluid delivered. This may then be compared to the amount de~ired to be delivered30 and the driving means stopped at the time that the selected amount has been
delivered. A linear resistor may be used as the displacement detector and may detect
the position of the syringe plunger through a linkage attached to the driving means.
The voltage from the linear resistor c~n be used to indicate the absolute position,
including the end-of-travel position, of the driver.
? . `
.....
,, ~
2101~3
-- 7 --
Another feature involves detecting the end of travel of the driving means. In
the case where a linear resistor is used to detect displacement, failure of the
resistance to change over a period of time may be used to indicate end-of-travel.
In yet another feature, the controller monitors the sensed pressure signal
6 while monitoring the volume of fluid delivered. In the case where the pressuredecreases below a predetermined threshold while the volume delivered continues to
increase, an alarm is provided and the delivery of fluid may be stopped.
In another aspect of the invention, the operator may select reverse
movement of the driving means, so that the fluid is not delivered through the delivery
10 device to the site but is drawn back into the catheter or syringe. This feature would
allow deflation of the balloon and would prevent the delivery of the medical fluid to
the bloodstream during removal of the catheter.
In a further aspect, the controller automatically times the sensed pressure
signal for the amount of time the sensed pressure exceeds a predetermined pressure
16 threshold and upon reaching a predetermined time limit, for example forty-five
seconds, automatically controls the driving means to cease delivery of the fluid. In the
case where a balloon is used, the driving means may also be commanded to deflatethe balloon.
Other aspects and advantages of the invention will become apparent from
20 the following detailed description and the accompanying drawings, illustrating, by way
of example, the features of the invention.
BRIEF DESCRIPTION OF THE DRAWINGS
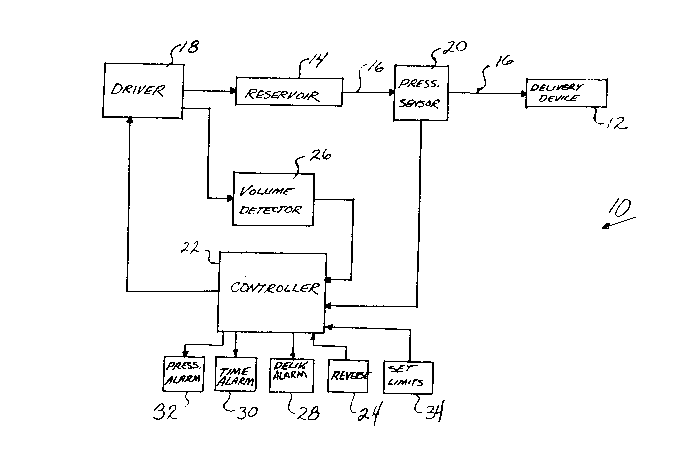
FIG. 1 is a block diagram of an automated fluid pressure control system
26 having both pressure and volume control features;
FIG. 2 is a schematic/block diagram of an automated fluid pressure control
~ystem supplying a medical fluid to an inflation b~alloon using a pneumatic driver and a
syringe as the fluid reservoir and having a volume detector;
FIG. 3 is a flow chart presenting a method of automated fluid pressure
30 control in an automated fluid pressure control system such as that shown in FIG. 2;
FIG. 4 is a schematic/block diagram of an automated fluid pressure control
system supplying a medical fluid to an inflation balloon using a pneumatic dl~iver, a
syringe as the fluid reservoir, an air-in line detector, a pressure detector in the
catheter line, and a volume detector; and
,~ . .
.
.. '
210111~ -
-- 8 --
FIG. 6 is a flow chart presenting a method of automated fluid pressure
control in an automated fluid pressure control system such as that shown in EIG. 4.
DETAILED DESCRIPTION OF PREFERRE~D EMBODIMENTS
Referring now to the drawings with more particularity, wherein like
reference numerals designate like or corresponding elements among the several views,
there is shown in FIG. 1 an automated fluid pressure control system 10 which
provides a fluid to a fluid delivery device 12. The fluid delivery device 12 maycomprise an inflatable balloon having apertures through which the supply fluid is
locally administered to a delivery site internal to the body of a patient. The delivery
site may comprise the walls of a blood vesseL
A reservoir 14 containing the fluid to be delivered is connected to the
delivery device 12 by a conduit 16, such as a lumen of a catheter. A driver 18
provides pressure to move the fluid from the reservoir 14 through the catheter 1~ to
16 the delivery device 12. The pressure provided by the driver 18 is selected to maintain
the balloon in the inflated state and force the fluid through the apertures of the
balloon mto the vessel waDs. The pressure is maintained high enough so that the
fluid leaving the balloon will only be administered to the walls of the b100d vessel and
will not flow to any significarlt extent into the bloodstream. At the same time, the
pressure is not 80 high as to cause damage to the vessel. The size of the balloon is
selected such that when inflated, some pressure will be applied by the balloon to the
vessel walls to cor~;ne the apertures to intimate contact with those vessel walls. A - -
balloon having a size too small will allow the medical fluid leaving the apertures to be
swept away by the bloodstream while a baDoon with a size too large may cause
26 damage to the vessel when inflatedL
A pressure sensor 20 in this embodiment senses the pressure in the conduit
between the reservoir 14 and the delivery device 12 and provides a sensed pressuFe
signal to a controller 22. The controller 22 compares the sensed pressure to a desired
administration pressure and outputs an error signal in the event that they difFer. The
30 error signal is provided to the driver 18 which alters the pressure of the medical fluid
to attain the desired administration pressure.
In the embodiment shown in FIG. 1, the driver 18 alters the volume of the
reservoir 14 thereby controDing the pFessure of the fluid in the system. Because the
delivery device 12 permits the outflow of the fluid provided by the reservoir 14, the
3~ driver 18 must continuaDy empty the reservoir 14 to maintain the desired
. .
:
- ' .
.- :-.:, . .
210il~3
administration pressure. In the event that the sensed pressure is less than the
desired admimstration pressure, the rate at which the reservoir 14 is emptied by the
driver 18 wiU be increased to raise the pressure. In the event that the sensed
pressure is above the desired administration pressure, the rate at which the reservoir
6 14 is emptied by the driver 18 will be decreased to lower the pressure.
In another feature, the controller 22 may also receive a reverse control signal
24 from an operator in which case the controller 22 wiU control the driver 18 to stop
the flow of the medical fluid. In the case where an infiatable baUoon is used as the
delh~ery device, the driver may reverse the flow to deflate the baUoon and to assure
10 that none of the medical fluid leaves the delivery device 12 during withdrawal from
the patient. The driver 18 may accomplish this by increasing the volume of the
reservoir thereby creating negative press~re.
In another aspect shown in FIG. 1, a volume detector 26 determines the
amount of fluid delivered to the delivery device 12 and provides a volume signal to the
lo controller 22. The volume signal i9 compared by the controller 22 to a signalrepresenting a desired volume to be administered and, in the event that the two are
equal, the controller 22 controls the driver 18 to cea~e delivery of the fluid to the
delivery device 12. The volume detector 26 may take different forms, one of which is
a monitor of the movement of the driver 18. Where certain positions of the driver 18
20 are correlated with certain volumes of fluid expelled from the reservoir 14, the
amount of fluid delivered can be determined from the position of the driver 18.
Additionally, the volume detector 26 may also determine the end-of-travel
position of the driver 18 and, when the driver reaches that position, may output a
signal to the controller 22 which in turn may then automatically control the driver 18
26 to cease delivery of the fluid. An embodiment of an alternate end-of travel
arrangement is discussed in greater detail below.
In accordance with another aspect of FIG. 1, the controller 22 compares the
pressure sensed by pressure sensor 20 to the volume of fluid delivered as detected by
the volume detector 26. In the event that the rate of fluid delivered remains the
30 same but the pressure sensed decreases below a predetermined minimum, for
example two atmospheres, and stays at that predetermined pressure for a selectedperiod of time, for example two seconds, the controller 22 will provide a delivery
alarm 28. Ihis delivery alarm 28 is intended to indicate a fluid delivery problem.
In accordance with another feature of the invention, the controller 22
35 monitors the time of the delivery of the fluid from the reservoir 14. Upon receiving
.
210111~
-- 10 --
from the pressure sensor 20 an indication that the desired administration pressure
has been reached, the controller 22 may then begin to time the administration of the
fluid to the delivery site and upon reaching the time limit, provide a time alarrn 30
and/or cause the driver 18 to cease delivery. For example, where a dilatation balloon
5 is used for applying the fluid to the walls of a blood vessel, the predetermined time
limit may be set at a safe period of blood flow interruption so as not to harm the
patient, for example, ninety second~. Upon reaching that time limit, the controller 22
may automatically issue the time ahrm 30 and automatically contsol the driver 18 to
defl~te the balloon.
10The controller 22 may also include a maximum pressure runit. In the event
that the pre~sure exceeds that limit, the controller 22 will provide a pressure alarm
32 and immediately lower the pressure such as by controlling the driver 18 to cease
operation. For example, a pressure limit of eight atmospheres may be input to the
controller 22. If the pressure of the fluid should exceed eight atmospheres, deIivery :
15 would be immediately stopped and the pressure alarm 32 provided.
The desired administration pressure, pressure alarm limit and time alarm
limit may be set into the controller 22 via front panel controls 34, a keyboard or other
means. The front panel may also contain a sv,litch for reverse drive 24 of the driver
18 to accomplish deflation of the delivery device 12, when delivery device 12 i8 an
20 inflatable balloon fitted with apertures.
Referring now to FIG. 2, a more detailed drawing of an embodiment of an
automated fluid pressure control system 10 in accordance with the invention is
presented. In this figure, the reservoir take3 the form of a syringe 36 and the syringe
plunger 38 operates to vary the volume of the syringe 36 by moving farther into or
25 out of the syringe barrel. The syringe barrel is rigidly mounted by means of a
mounting member 40. A dilatation catheter 42 is connected to the output port 44 of
the syringe and has a dilatation balloon 46 mounted at its distal end.
The driver comprises in this case a slide mechanism 48 coupled to the
syringe plunger 38 for moving the syringe plunger 38 in relation to the syringe b~rrel,
30 an air cylinder 50 and an air controller 52 comprising a solenoid valve (not shown) and
a control circuit (not shown). A pneumatic power source, in this case a source of air
pressure, is supplied to the air controller 52 via an input line 54. The air controller 52
receives the air pressure from the air pressure line 54 and controls that air pressure
in accord~mce with the desired administration pressure set mto the air controller 52
35 along electrical line 56.
.. : . : . : . ~ .
-: -.
. ., ~ ",
..
2~01113
In one embodiment, the air controller 52 comprises an electro-pneumatic
regulator having the model designation of "QB" and manufactured by Proportion-Air,
Inc. of McCordsville, Indiana. This particular regulator incorporates a closed loop
pressure control system where the pressure output of the regulator is sensed by a
5 pressure transducer and is compared to the pressure signal set in the electronic
control circuit of the regulator. In the event that a difference is sensed between the
two pressures, the internal control circuit of the regulator changes the pressure
output. The output pressure from the regulator is coupled to the air cylinder 50which applies force against the syringe plunger 38 to move it relative to the syringe
10 barrel thereby affecting the pressure of the fluid in the syringe. Because of thi~
direct physical link, the pressure sensed at the output of the regu1ator 52 is therefore
indicative of the pressure of the fluid in the syringe 36.
The solenoid valve (not shown) of the air controller 62 may be switched to
port the output air pressure from the regulator to one side or the other of the air . -
15 cylinder to cause the syringe plunger 38 to either move farther into the syrmge barrel
or more farther out of the syringe b~rrel.
A displacement detector 58 is also shown in FIG. 2 and detects the position
of the slide mechanism 48. In this case, the displacement detector 68 comprises a
linear resistor having a wiping contact connected to a linkage 60 which is connected to
20 the slide mechani~m 48 for moving the syrin~e plunger 38. The wiping contact varies
the voltage across the linear resistor 58 in dependence upon the position of the slide
mechanism 48. Because the slide mechanism 48 is directly connected to the syringe
plunger, the position of the slide mechanism 48 corresponds to the position of the
syringe plunger 38. Thus, the amount of fluid provided from the syringe 36 can be
25 determined by the voltage of the linear resistor 68.
The controller G2 of FIG. 2 comprises two main parts, an interface unit 64
which comprises an analog-to-digital converter (A-to-D converter) and a digital-to-
analog converter (D-to-A converter), a bus 63, and a processor 66. The processor ô6
in this case comprises a micro-computer but may comprise other types of processors.
30 The output dgnal of the linear resistor 68 is analog in form and is provided to the
interface unit 64 for conversion to a digital signal before forwarding it on to the
processor 66. The desired delivery pressure of the syringe fluid is input in theprocessor 66 through the keyboard 68 in this case. The processor 66 provides a
digital output signal representative of the pressure which is converted to an analog
36 signal by the interface unit 64. That analog pressure signal is provided to the electro-
.
:
: .,.- :, ,:: ,
: - : :.:: -.,,. :
2101113
- 12 -
pneumatic regulator 52 to control the air cylinder 50 and the slide mechanism 48. In
this embodiment, the pressure controller would include the main controller 62 which
provides the control signal for the desired pressure and also the pressure controller
which is built into the QB electro-pneumatic regulator.
An A-to-D converter found usable is the DASCON-l, from Metabyte, Inc.
which is a twel~re-bit converter, although other converters may be used. A
displacement detector which may be used i9 the LT-103 from Waters Manufacturing.Referring now to FIGS. 2 and 3, an embodiment of a method of automated
fluid pressure control is presented. The syringe size is input 70 and either a full
stroke delivery or a measured volume delivery is selected 72. The desired
administration pressure PlnpUt, the delivery volume Vinput, and the maximum time of
inflation TlnpUt are entered 74, although a default time value such as ninety seconds
may be used. The processor 66 converts 76 the delivery pressure PlnpUt to the
pressure to be provided by the reg~lator 52 Preg~ The processor 66 also converts 78
16 the delivery volume Vlnput to plunger travel distance dinpUt according to the syringe
size. Next, the air cy]inder 50 is vented 80, the solenoid activated 82 and the syringe
and slide mechanism 48 are manually aligned 84 with the syringe plunger 38 beingengaged. The delivery sequence is initiated 86 and the starting voltage from thelinear resistor B8 is read 88 as the zero travel distance. The timer is started 90 and
the pressure Preg to be maintained by the regulator 52 is sent to the regulator. The
travel distance "d" of the slide mechamsm 48 and the time "t" are continually
monitored 94 and 96 and when either d = dinpUt or t = tinput, the timer is stopped
98 and the cylinder retracted 100 for removal or further disposition of the catheter
42.
In an alternate embodiment, the volume of fluid to be administered may be
entered by means of a cassette identifier. Entry of the cassette identifier will inform
the processor 66 of the end-of-travel position for that particular cassette and may also
include the maximum pressure Pm~X permitted with that cassette. This informationmay be provided by means of a bar code placed on the cassette which may be read by
a bar code reader (not shown) attached to the processor 66. Use of the term
"cassette" is not meant to be limiting. The cassette referred to may take the form of
a syringe or other device which performs the function of a fluid reservoir. A look-up
table containing information corresponding to each of the po~sible cassettes usable in
the system may be inchlded with the processoJ 66.
.
,
210111~
-- 13 --
Referring now to FIG. 4, a second embo&ent of an automated fluid
pressure control system is presented. As in FIG. 2, a fluid reservoir comprising a
syringe 36 and a pneumatic driver for moving the syringe plunger 38 are used to
control the pressure of the fluid in the syringe 36. The fluid is forced out of the
syringe 36, into the catheter 42 and into the delivery device which, in this case, is an
inflatable balloon 46. However, the system of FIG. 4 also includes a pres~ure sensor
102 for monitoring the pressure of the fluid. The pressure sensor 102 may take the
form of a silicon piezo-resistor which is responsive to the pressure of the fluid
between the output port 44 of the syringe 36 and the catheter 42. A commonly
available pressure sensor is the MP~-700D from Motorola The pressure sensed is
provided to the interface unit 64 on line 104 for conversion to a digital signal and for
forwarding to the processor 66. Additionally, a pressure display 106 is provided for
monitoring the pressure of the fluid being delivered.
The processor 66 compares the digital pressure signal to the desired
administration pressure and outputs on the bus 63 an error signal. The interface unit
64 converts that digital error signal to an analog signal for forwarding to the air
pressure controller 52. However, in the embodiment where a pressure regulator isused such as the QB electro-pneumatic regulator which converts the input signal to a
pressure, the error signal m~st be added to or subtracted from the desired pressure
signal provided to the regulator. In this embodiment, there are two pressure controls.
The pressure regulator 52 itself monitors its own output while the additional pressure
sensor 102 mounted at the output of the 8yringe 36 also monitors pressure.
A further feature incorporated in the system of FIG. 4 is an air-in-line sensor
108 for detecting air bubbles. The output of the air-in-line sensor is transmitted on
line 110 to the interface unit 64 for conversion to a digital signal and is thentransmitted to the processor 66. In the event that an air bubble of a particu1ar size is
detected, the processor 66 may stop delivery of the fluid by the pneumatic driver and
provide an alarm. Such air-in-line sensors are available from Zevex, Inc. in Murray,
Utah. A1arms, such as those for air-in-line and pressure, may be provided by a display
114 connected to the processor 66 and may also be given audibly.
Known techniques for increasing the accuracy of the volume detection of
fluid delivered to the delivery device 46 ma~r be employed. For example, slope, offset
and scale factors may be stored and made available to the processor 66 for each
displacement detector 58 u8ed. Different syringes or cassettes may be used with the
system, each of which has a look up table made available to the processor 66.
2101113
-- 14 --
Techniques other than look-up tables may be used such as interpolation between
beg~mung-of-travel and end-of-travel points. The operator may be prompted for a
cassette code and upon entering that code, the processor may then create a look up
table for that cassette based on characteristic data of the cassette previously stored.
6 Processing techniques for smoothing pneumatic driver 60 and regulator 52
responses may be included in the processor 66. For example, a classical proportional,
integral, derivative control system may be used.
Referring now to FIGS. 4 and 6, an embodiment of a method for automated
fluid pressure control is presented. The syringe size is input 70 and either a full
stroke delivery or a measured volume delivery is selected 72. The desired
administration pressure PinpUt~ the maximum pressure Pm,l", the delivery volume
Vlnput~ and the maximum time of inflation TinpUt are entered 74, although a default
time value such a3 ninety seconds may be used. The processor 66 converts 76 the
delivery pressure PinpUt to the pressure to be provided by the regulator 62 Pr~q. The
16 processor 66 also converts 78 the delivery volume Vinput to plunger travel distance
dinpUt according to the ~yringe size. Next, the air cylinder 50 is vented 80, the
solenoid activated 82 and the syringe and slide mechanism 48 are manually aligned 84
with the syringe plunger 38 being engaged. The delivery sequence is initiated 86 and
the starting voltage from the linear reBistor 58 is read 88 as the zero travel distance.
The timer is started 90 and the pressure Prog to be maintained by the regulator 62 is
sent to the regulator. In other embodiments, the timer may be automatically started
upon reaching a predetermined minimum pressure, as measured by the pressure
3ensor 102; for example, two atmospheres.
The fluid line is monitored 114 for the existence of air and if an unacceptably
high amount of air is detected, an alarm is provided 116. The delivery pre~sure is
continuously monitored 118 and if that pressure equals or exceeds Pm~ 120, an alarm
is provided 116. If the delivery pressure is not equal to PinpUt 122, the delivery
pressure is increased or decreased 124 until PinpUt is reached. The travel distance of
the slide mechanism 48 is continually monitored 04 and 90 and when either the
distance traveled "d" equa]s dinpUt or the time expired "t" equals tinput, the timer is
stopped 98 and the cylinder retracted 100 for removal or further disposition of the
catheter 42.
An end-of-travel determination of the syringe plunger may be made in the
above embodiments. In one technique, the end of travel displacement may be
programmed into the processor 66 as a characteristic of each cassette used. This data
. ~
210111~
- 15 -
may reside in a look-up table in the processor or be input to the processor 66 in other
ways. In another technique, the displacement sensor may be monitored for change
and in the event that no further change occurs, the end of travel of the ~;yringe
plunger may be assumed. In the case of FIGS. 3 and 6 where step 72 requires a
6 selection of full stroke delivery or measured volume delivery, the full stroke delivery
may be determined by end of travel.
Additionally, a higher initiat pressure may be programmed into the processor
66 for starting the fluid delivery. This initial pressure would be greater than PlnpUt
and would cause rapid inflation of the delivery device so that a minimum loss of10 inflation fluid occurs. After inflating, the pressure would then be reduced to P1npUt.
A pneumatic power source typicaUy available in most hospital rooms is the
100 psi air provided at a waU fittmg A connecting hose 64 may be coupled betweenthe waU fitting and the air pressure controUer 52 to provide a pneumatic energy
source for the air cylinder 60. The use of pneumatic power rather than electrical
16 power to pressurize the delivery fluid reduces the electrical hazard to the patient.
It was found that a pneumatic system provided the needed ramp-up speed to
quickly inflate the balloon with the fluid to be administered while allowing losing only
an insignificant amount of the fluid to enter the bloodstream during inflation. Other
driver systems may be used, such as a motor system using a stepper motor.
20 However, it was also found that a pneumatic system offered a cost effective system
which may be contained in a relatively small and light weight case. Pneumatic energy
is readily available in most hospital rooms; hence, no portable source of power, such a~
an air cylinder, need be included m one embodiment. In another embodiment where
air pressure is not available from the room, a portable air cylinder may be used. The
26 embodiment using pneumatic power provides a rapid response and accurate control.
In the case where a stepper motor is used to provide the driving means, a more
complex, heavier and more expensive system may result because of the addit,ion of the
stepper motor itself.
AdditionaUy, the driver 18 may take forms other than a volume controller of
30 the reservoir 14. A device which draws the fluid from the reservoir without varying
the volume of the reservoir may be used.
The systems and methods described above may be used with an angioplasty
catheter to accurately control the pressure of the inflated baUoon, although the air-in-
line sensor 108 and the displacement detector 68 would have limited value.
36 AdditionaUy, the systems and methods of the ~IGS. 1-6 are not limited to applications
' ' ' ' '~
:. , ' ' '' '': . .
., : - : . .
2101113
involving only the blood vessel3. They may be used in procedures with organs such a3
the kidney, the liver; and used in other procedures such as with the prostate and in
other ducts.
Although specific embodiments of the invention have been deæribed and
illu3trated, it is clear that the invention i3 3uæeptible to numerous modification3 and
embodiment3 within the ability of those 3killed in the art, and without the exercise of
the inventive faculty. Thus, it should be understood that various changes in form,
detail and application of the present invention may be made without departing from
the spirit and æope of the invention.