Note: Descriptions are shown in the official language in which they were submitted.
WO 93/01147 PCI/USg2/0~16
2103~ lS~
ALUMINUM NITRIDE DENSIFICATION
WITH MINIMAL GRAIN GP~OWTH
Aluminum nitride has many structural and electrical application~s. Aluminum
5 nitride articles that have high density and high thermal conductivity are generally produced by
densifying a powder compact that i ncludes al umi num nitride powder and at I east one
densifying additi~e.
One method of densifying aluminum nitride powder includes forming a powder
compact of aluminum nitride and a densifying additive that includes one or more oxides ~f a
10 Group lla or a Group ll~a element. However, such compactstypic311y must be heated to a
temperature above 1800~C in order to form a dense, high thermal condu~tivity aluminum
nitridearti~le. Aluminumni~ridedensifiedattemperaturesabovel800Coftenhasagrainsize
that is significantly larger than that of aluminum nitride in the powder compact. Grain size
influences physical properties of dense aluminum nitride articles. A relatively large grain size
15 can adversely affect properties such as thermal shock resistance, strength and fracture
toughness.
In another method, the densifying additive includes at least one fluoride of a
Group lla or a Group Illa element. These densifying additives generally have lower melting
points than do Group lia and Group Illa oxides~ However, they often volatilize at temperatures
20 which are lowerthan are required to obtain sufficient densification of aluminum nitride. Also,
~iroup lla and Group Illa fluorides typically exhibit poor wetting of aluminum nitride powder
and are often relatively hygroscopic, thereby limiting their usa~e in densification of aluminum
nitride powder.
Thus, a need exists for an improved method for densifying aluminum nitride
25 which overcomes or minimizes the aforementioned problems.
One aspect of the present invention is a method of forming a sin~ered body of
aluminum nitride that comprises heating, following removal of binder in.an atmosphere that
favors subsequent densification by sintering to at least 90 percent of theoretical density, a
powder compact formed from a binder and a homogeneous powdered admixture of Al N,
-1-
WO93/01147 ~ 3~ Pcr/usg2/058l6
A1203, YF3 and Y203 to a sintering temperature within a range of from 1 500C to 165ûC at a
heating rate sufficient to form a densi~ying amount of a high temperature yttrium-aluminum
oxyfluoride si ntering liquid and maintaining that temperature for a period of time sufficient to
yield a sintered body having a grain si~e that is less than one order of magnitude larger than
5 that of the alumi num nitride powder, a density greater than or equal to 90 percent of
theoretical density and a thermal conductivity greater than or equal to 100 W/m K and
- containing, when cooled, aluminum nitride and a grain boundary phase that includes yttrium
oxyfluoride and yttrium aluminate, the admixture containing, based upon admixture weight,
AIN in an amount within a range of from 88 to 99 percent by weight and, as sintering additives,
10 a combined total amount of Al2O3, YF3 and Y2O3 within a range of from 1 to 12 percent by
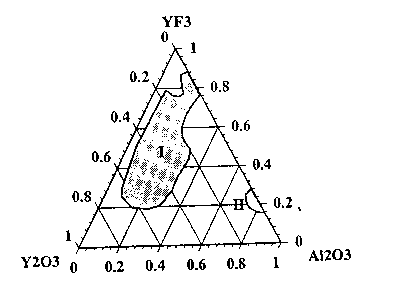
weight, the sintering additives having a composition defined and encompassed by areas I and ll
of Figure 1 wherein, based upon total weight of sintering additives with all weight fractions
totaling 1.0, the A12O3 weight fraction ranges from 0.04 to 0.36 for area I and from 0;72 to 0.84
for area tl, the Y203 weight fraction ranges from greater than 0 to 0.68 for area I and from
15 greater than 0 to 0.06 for area ll, and the Y F3 weight fraction ranges from 0.18 to 0.88 for area
and from 0.16 to 0.28 for area ll.
A second, related aspect of the invention is a method of forming a sintered bodyof aluminum nitridethatcomprises heating, following removal of binder in an atmosphere
that favors subsequent densification by sintering to at least 90 percent of theoretical density, a
20 powder compact formed from a binder and a homogeneous powdered admixture of AIN,
Al2O3, Y F3 and CaF2 to a si nteri ng tem peratu re withi n a range of f rom 1 500C to 1 650C at a
heating rate sufficient to form a densifying amount of a high temperature
yttrium-calcium-aluminum oxyfluoride sintering liquid and maintaining that temperature for a
period of time sufficient to yield a sintered body having a grain size that is less than one order
25 of magnitude larger than that of the AIN powder, a density greater than or equal to 90 percent
of theoretical density and a thermai conductivity greater than or equal to 100 W/m K and
~- ~ containing, when cooled, aluminum nitride and a grain boundary phase that includes yttrium
oxyfluoride and yttrium aluminate or calcium aluminate or both, the admixture containing,
- based upon admixture weight, Al N i n an amount within a range of from 88 to 99 percent by
30 weight and, as sintering additives, a combined total amount of Al2O3, YF3 and CaF2 within a
range of from 12 to 1 percent by vveight, the sintering additives having a composition defined
~ and encompassed by areas I and ll of Figure 2 wherein, based upon total weight of sintering
:~ additives with all weight fractions totaling 1.0, the Al2O3 weight fraction ranges from 0.08 to
0.30 for area I and 0.08 to 0.68 for area 11, the CaF2 weight fraction ranges from greater than 0
35 to 0.24 for area I and from greater than 0 to 0.68 for area ll, and the YF3 weight fraction ranges
from 0.57 to 0.92 for area I and from 0.01 to 0.56 for area 11.
This invention has many advantages. The invention allows densification of the
powder compact without significantly increasing the aluminum nitride grain size. The rate of
-2-
2103048
densification can also be increased at a given sintering temperature. In addition, the sintering
temperatures suitable for use in the present invention allow sintering in conventional alurnina
. sintering furnaces. Also the combination of high thermal conductivity and relatively small
grain size increases the performance of aluminum nitride articles in rnany applications such as
5 in high-wattage, high-circuit density electronic packaging.
Figure 1 is a ternary composition graph showing combinations of YF3, Al203 and
Y203 that, when used as sintering additives for aluminum nitri~e, provide a density greater
than or equal to 90 percent of theoretical density and a thermal conductivity of greater than or
equal to 100W/m K at sintering temperatures within a range of from 1500C to 1650C,
10 preferably 1525Cto 1625C. Thecombinationsareidentified asregionsordomainsl and ll.
The grapn represents total Al203 content rather than Al203 added as a sintering additive.
Figure 1A presen~sthe same information as Figure 1 save for replacing total Al203
content with Al203 added as a sintering additive.
Figure 2 is a ternary composition graph showing combinations of YF3, Al203 and
CaF2 that, when used as sintering additives for aluminum nitride, provide a density greater
than or equal to 90 percent of theoretical density and a thermal conductivity of grea.ter than or
equaltolOOW/mKatsinteringtemperatureswithinarangeoffrom 1500Cto1650C,
preferably 1525C to 1625C. The com bi n~ti ons are identified as regions or domai ns I and l l .
The graph represents total Al203 content ratherthan Al203 added as a sintering additive.
20 . Figure 2A presentsthe same information as Figure 1 save for replacing total Al203
content with Al203 added as a sintering additive.
The above features and other details of. the invention will now be more
particularly described and pointed out in the claims. The particular embodiments of the
invention are shown by way of illustration and not as limitations of the invention. The
25 principle features of this invention may be empl oyed i n various embodi ments without
departing from the scope of the invention.
A powdered admixture suitable for purposes of the present invention is
homogeneous and comprises AIN powder and a densifying composition or combination of
sintering additives. Suitable AIN powder includes AIN powder that, when combined with a
30 suitable densifying composition and heated to a temperature, and for a period of ~ime,
sufficient to densify the AIN powder, will densify without a significant increase of the average
grain size of the powder compact. "Significant increase", as used herein, means less than one
order of magnitude.
- The AIN powder desirably has a particle size within a range of from 0.1 to 2.511m,
35 preferably from 0.1 to 1.5 ~m, a specific surface area within a range of from 1.5 to 7 m2tgm,
. preferably 2.0 to 5.0 m~!gm, most preferably 3.0 to 5.0 m2/gm and an oxygen content within a
- SUBSTITUl~ EET
21030~8
range of from 0.2 to 2.5~,/o by weight, preferably 0.8 to 1.8'~ Dy weight. Oxygen can be present
in the AIN powder as impurities within the crystal lattice of AIN powder or in a metal oxide,
- such as aluminum oxide (Al2O3) or aluminum oxynitride, at ~he surface of AIN powder grains.
Examples of suitable aluminum nitride (AIN) powder incluc,e: Electronic Grade AIN powder,
5 commercially availaole from The Dow Chemical Company; and Grade F AIN powder,
- commercially available from Tokuyama Soda Company.
Densifying compositions suitable for use with the present invention include
combinations of three sintering aids. The sintering aids are Al2O3, YF3 and eitner CaF2 or Y2O3.
....
-3a-
WO 93/01147 2~ ~3~ PCI/U~92/05Xl~i
The relative amounts of each sintering aid are shown in regions I and ll Figures 1 and 2 wherein
thethird sintering aid is, respectively, calcium fluoride and yttrium oxide. The amounts are
expressed in weight fractions so that the sum of all weight fractions equals 1Ø The
combinationsallowthealuminum nitride powdertodensifyand form adensified aluminum5 nitride article or body without significantly increasing aluminum nitride grain size.
Substitutions of another alkaline earth fluoride for CaF2 or a rare earth oxide or a
rare earth fluoride for, respectively, Y2O3 and YF~ should provide some combinaticlns that yield
satis~actory results in terms of thermal conductivity and density. The substitutions would,
however, lead to development of ternary composition diagrams with regions or domains of
10 satisfactory thermal conductivity and density that differ from those of Figures 1 and 2. In
addition, the use of aluminum nitride powder with a substantially greater oxygen content
would lead to modification of domains I and ll in Figures 1 and 2.
The densifying compositions designated as regions or domains I and l! in Figures 1
and 2 typically provide a density of at least 95 percent of theoretical densit;y and a thermal
15 conductivity of at least 100 W/mK (watts/(meter) (degrees Kelvin)) when they are combined
with aluminum nitride and heated to 1 625C at a rate of 2.5C/minute and maintained at that
temperature for 6 hours. As the temperature decreasesto 1 550C, or even 1 500C, thermal
conductivity values of at least 100 WlmK may be attained, but the density decreases to at least
90 percent of theoretical density. Further decreases in temperature lead to reductions in both
20 thermal conductivity and density and yield materials that may be suitable for uses that do not
require high density, high therrnal conductivity or both. Conversely, an incr~ase in sintering
temperature may broaden the regions wherein thermal conductivity values of at least 150
WlmK may be attained. Similar effects are observed with variations in heating rate and
sintering time.
An example of a suitable calcium fluoride (CaF2) is reagent grade CaF2,
commercially available from Fisher Scientific Company. An example of a suitable aluminum
oxide (Al2O3~is reagent grade Al2O3, commercially available from Johnson Matthey Alfa
Products. An example of a suitable yttrium fluoride (YF3) is 99.9% pure powder, commercially
available from Aldrich Chemical Co.. An example of a suitable yttrium oxide (Y2O3) is 99.99%
30 pure powder, commercially available from Unocal-Molycorp.
The Al2O3 portion of the sintering aid combination is in addition to any oxyç~en-
containing speciesthat may be present on aluminum nitride powder surfaces~ High resolution
transmission electron microscopy of such surfaces shows they are typically amorphous. Energy
dispersive spectroscopy in an analytical transmission electron microscope using a focused
35 electron beam that is localized within the coating shows they typically contain Al-O-N or Al-O
species or both.
The powdered admixture of AIN, Al2O3, YF3 and either Y2O3 or CaF2 is suitabJy
prepared by conventional procedures. The admixture contains, based upon admixture weight,
-4-
~ ~ k`~
WO 93/01147 2 1 0 3 0 1 8 PCIIUS92/05816
AIN in an amount within a range of from 88 to 99 percent by weight. The range is preferably
from 91 to 97 percent by weight. On the same basis, a combined total amount of Al2O3, YF~
and either Y2O3 or CaF2 within a range of from about 12 to about 1 percent by weight. The
range is preferably from 9 to 3 percent by weight.
A binder can be combined with the powdered admixture to help form a powder
compact. An example of a suitable binder is a mixture of Cimarec~ binder (XUS 40303.00),
commercially available from The Dow Chemical Company, and polyethylene glycol. The binder
can be mixed with a suitable solvent to form a binder solution that is then combined with the -
powder mixture. Examplesof suitable solvents include ethanol and trichloroethane. More
10 than one binder or solvent can be used to form the binder solution. The amount of binder is
preferably within a range of from 3 to 10% by weight of binder solution. A suitable amount of
binder solution iswithin a range of from 1.0 ml/gm to 2 ml/gm of powder mixture.- The binder solution can also include a dispersant that is suitable for reducing
agglomeration of the powdered admixture. Examples of suitable dispersants include natural
15 fish oil and fatty acids. The amount of dispersant is preferably in a range of from 0.1 to 0.005
gm/ml of bindersolution.
Conventional grinding or miliing media may be used to blend the powdered
admixture and binder solution. High density aluminum nitride grinding media, commercially -
available from U.S. Stoneware Corp., provides satisfactory results. Suitable amounts of
20 grinding media are within a range of from 1 to 4 grams/gram of the combined powder mixture
and binder solution.
The powdered admixture and binder solution can be blended and milled using
conventional apparatusand procedures. The milling apparatus issuitably made from, or lined
~ with, a material, such as polyethylene, that will not rea~t with powdered admixture
; 25 components or the binder solution. The milling apparatus, such as a bottle, is typically rotated
for a period of 1 to 12 hours at a rate of from 10 to 100 revolutions per minute to com/ert the
, powdered admixture and binder solution into a milled slurry. The milled slurry is then
recovered from the milling media, dried and converted to a powder by conventional
- ~ procedures, such as spray drying.
The milled dry powder isthen formed into a precursor pellet having a suitable
shape by conventional technology. Suitable shapes include disks, right cylinders, spheres and
briquettes
~- ~ If desired, milled dry powder can be sifted through a suitable screen to form a
~ dried, milled mixture of substantially uniform consistency before it is formed into a shape~
-~; 35 Suitablescreenmeshsizesareinarangeoffrom40to120~425llmto125l1mscreenopening)~
; ~he mesh size is preferably 60 (250~1m screen opening). Suitable screen materials include
stainless steel, copper, and synthetics, such as nylon.
-5-
,
WO 93/01 147 PCr/~JS92/05816
q~
Precursor pellets or greenware may be formed by die pressing, injection molding,tape casting, slip casting or by some other conventional method. The pellets are exposed to
conditions sufficient to remove the binder and dispersant therefrom. In one conventional
procedure, precursor pellets are loaded into a suitable furnace for binder removal. The
5 temperature in the furnace isthen raised to between 50ûC and 700C for a period of 1 to 6
hours, or as long as is needed to remove at least a substantial portion of ~he binder. The
- precursor pellets are preferably exposed to a temperature of abou~ 550C for about one hour in
air or for about 5 hours in a nitrogen atmosphere. Removal of the binder and dispersant
converts each precursor pellet to a powder compact.
Removal of the binder, also known as binder burn-out, may occur either in air orin a nitrogen atmosphere when the weight fraction of Al203 is intermediate between its upper
and lower limits respectively for Figures 1 and 2. As the weight fraction of Al203 approaches its
lower limit, ai r is a preferred binder burn-out atmosphere. At or near the lower Al20~ limit, a
nitrogen atmosphere may lead to a thermal conductiYity or density or both that is lower than
15 desired. Conversely, asthe Al203 weight fraction approaches its upper limit, nitrogen is a
preferred binder burn-out atmosphere. At or nearthe upper limit, air may yield the same
results as nitrogen at the lower limit.
When using sintering aid compositions shown in Figure 1, the burn-out
atmosphere can be either air or nitrogen when the Al203 weight fraction is from 0.08 to 0.32
20 for area I and from 0.76 to 0.80 for area ll. The atmosphere is air when the Al203 weight
fra~tion is from 0.04 to less than 0.08 for area I and from 0.72 to less than 0.76 for area ll. The
atmosphere is nitrogen when the A1203 weight fraction is from greater than 0.32 to 0.36 for
arealandfromgreaterthanO.80toO.84forareall. Whenusingsinteringaidcompositions
shown in Figure 2, the burn-out atmosphere can be either air or nitrogen when the Al203
25 weight fraction is from 0.12 to 0.26 for area I and from 0.12 to 0.64 for area l l . The atmosphere
is air when the Al203 weight fraction is from 0.08to less than 0.12 for areas I and ll. The
atmosphere is nitrogen when the Al20~ weight fraction is from greater than 0.26 to 0.30 for
area I and from greater than 0.64 to 0.68 for area ll.
A quantity of powder compacts is loaded onto a suitable tray which is !oaded into
30 a suitable crucible. Examples of suitable trays and crucibles are those formed of boron nitride.
The crucible is desirably capped with a lid, preferably formed of boron nitride. The capped
crucible and its contents are loaded into a furnace, such as a refractory-type furnace with
graphite ortungsten heating elements.
The capped crucible and its contents are exposed to conditions sufficient to
35 convert the powder compacts into a densified or sintered aluminum nitride article without
substantially increasing the grain size of aluminum nitride over that of the aluminum nitride
powder. "Without a significant increase of grain size," as that term is used herein, means that,
during densi~lcation to about 90% theoretical density, the average grain size of AIN in the
WO 93/01147 2 1 0 3 0 1 ~ PCJ/US92/05816
powder compact increases by no more than one order of magnitude. The average Al N grai n
size in the sintered article is in a range of from 1 to 5 llm.
The sintered aluminum nitride article has a density that is desirably at least 90%,
preferably at least 95%, of the theoretical density of aluminum nitride. The si ntered article has
5 a thermal conductivity that is desirably at least 100, pref~rably at least 150, WlmK.
An example of conditions sufficient to densify the alumi num nitride i n the
- powder compact includes heating the powder compact at a rate between 2 and 30C per
minute from ambient temperature to a sintering temperature. Heating preferably occurs at a
rate of 2.5 to 25C per minute begi nning at 1 200C when the sintering additives are YF3, Al2O3
10 and Y2O3, and at 1000C when the sintering additives are YF3, Al2O3 and CaF2. A satisfactory
heating schedule begins at 24C per minute from 20C to 1 OOO~C. This is followed by heating at
20C per minute up to 1 575C. If higher temperatures are used, the heating rate is reduced to
1 S~C per minute. Heating desirably occurs at atmospheric pressure.
The powder compact is heated to a temperature sufficient to cause the Al N to
15 densify to at least ninety percent of the theoretical density of aluminum nitride without a
substantial increase of the grain size of the powder compact. TheAlN is preferably densified to
at least 95 percent, more preferably to 98 percent or more, of theoretical density.
The sintering temperature is desirably within a range of from 1 500C to 1 650C,
preferablyfrom 153ûCto 1625C, morepreferablyfrom 1550Cto 1600C. Temperaturesless
20 than 1 500C typically do not lead to densities of at least 90 percent of theoretical density i n the
absence of uneconomical sintering times. Temperatures in excess of 1 650C may be used, but
produce no substantial advantage other than a possible greater latitude in sintering aid
combinationsthat may allow one or more components to be eliminated. Such higher
temperatures may, however, require the use of specially designed sintering furnaces rather
25 than conventional alumina sintering furnaces.
The sintering temperature is maintained for a period of time sufficient to attain a
density of at least 90 percent of theoretical density and a thermal conductivity of at leas~
100W/mK. Suitabletimesrangefrom2to 16hours,desirablyfrom4to 12hours~preferably
from 6 to 8 hours.
After maintaining the sintering temperature for such a period of time, the
temperature within the furnace is desirably 1 owered at a rate from 1 to 50C per minute to a
temperature of 1 200C. The temperature is preferably 1 owered at a rate from 5 to 30C per
minute.
After cooling to ambient, a sintered body containi ng aluminum nitride and a
grain boundary phase results. When the sintering additives are YF3, Y203 and A1203, the grain
boundary phase includes yttrium oxyfluoride and yttrium aluminate. When the sintering
additives are YF3, CaF2 and Al2O3, the grain boundary phase includes yttrium oxyfluoride and
yttrium aluminate or calcium aluminate or botli.
-7-
WO 93/01147 ~ 3a ~ PCr/US92/OSB16
Although the mechanism of the invention is not cornpletely understood, it is
believed that the components of the densifying composition react to form a sintering liquid,
such as a eutectic or a peritectic liquid. When the sintering additives are YF3, Y2O3 and Al2O3,
the sintering liquid is a yttrium-aluminum oxyfluoride. When the sintering additives are YF3,
5 CaF2 and Al2O3, the sintering liquid is a yttrium-calcium-aluminum oxyfluoride.
The invention will now be further and specifically described by the following
- exampies. All parts are by weight (PBW) and all percentages are by weight un!ess othenNise
stated.
ExamDle I
A series of admixtures were prepared that contained AIN powder and a
densifying composition that included Y2O3, YF3, and Al2O3. Three different electronic grade
aluminum nitride powderswere used. The powders were: Ai N- 1, a materi,al commercially
available from The Dow Chemical Company under the designation XUS 35'i44 and having an
oxygen content of 1.07% and a surface area of 3.42 m2tg; AIN-2, an experimental material
15 developed by The Dow Chemical Company with an oxygen content of 1.0% and a surface area
of 4.00 m2/g; and AIN-3, an experimental material developed by The Dow Chemical Company
with an oxygen content of 1.7% and a surf3ce area of 4.35 m2/g. The aluminum nitride powder
and the densifying compQsition contained in each admixture are shown in Table I together
with the burn-out atmosphere, either air or nitrogen (N~), the percent of theoretical density
20 and the thermal conductivity.
Additional components were combined with the mixtur(?s to form slurries. All of
the slurries included 100 parts of a combination of AIN and the densifying combination, 0.5
parts of fish oil as a dispersant, 2.3 parts Cimarec~ binder, 4.2 parts polyethylene glycol, and 120
parts of a 50/50 blend of ethanol and chlorothene.
The slurries were milled for two hours using 1.5 parts of high density AIN milling
media, commercially available from U.S. Stoneware Corp, per part of aluminum nitride powder.
The milled slurries were recovered from the milling media and dried to yield a milled dry
powder. The powder wasthen dry-pressed into peliets using a die set and a hydraulic press
withapressureof 15,000psi(103.41~APa).
The binder used in forming the pellets was rernoved by firing or heating the
pellets in an oven under a stream of compressed ai r or nitrogen at a temperature of 550C for a
period of one hour. The ramp rate during cooling and heating was 2 Vminute.
The debindered pellets were disposed in refractory crucibles and loaded in a high-
temperature sintering furnace. The pellets were densified by heating the crucibles and their
contents in the furnace at a rate of 30Clminute to a temperature of 1200C, followed ~y
heatingata rateof 2.5C/minuteto 1625Cand maintained atthattemperaturefora period of
time of about six hours under a stream of nitrogen gas. The densified pellets were then cooled
to ambient temperature at a rate of about 5C~minute.
-8-
-
210304~
_ . . _ .,
,.
r I r ~ ~ ~ r- ¦
... . E~.~o
~)
I ~ .
~ ~1 1` 0 0 IJ'l U l r' ~ a~ ~o ~ ~r Ir ,--1 G
d~ o c~ O oo ~ ~ 0 0 cn ~1 r~ a) u~
E~ a , a: c~ co a~
~ C
.,, v ~r ~ ~ ~ ~r ~ ~ ~ ~, ,~ ~1 ,~ ~ ~
:~ 3 o o o o o o o o o
.U C
s o r~
C ~ ~ ~ I`
3 ~ o o o o o o o o o o o o o o ~,
. ..
.
~-'
E~ ¢ ~ o o o o o o o o o o O O O O
~ ~ z
I v O a) ~ . N
1: ' . m ¢
æ ' r t~
¢ V ~ 0 r~ 0 a~ co O
3 . G~ ._
I~'
Z C' O ~ ~ ~ I I I I ~ ~ ~ ~ ~ ~ ~ .C
-'~ Z Z Z Z Z Z Z Z Z Z Z Z Z Z QJ
Q C ¢ ¢ ¢ ¢ ~ ¢ ¢ ¢ ¢ ¢ ¢ ¢ ¢ ¢ O
_l ~ ~ ~r er ~ ~ a~
E
n~ X
~) Z ~ E ~
)~ ~
Q~ '~ Z
U~ Z _
WO 93/01147 PCI/US92/OSX16
2l~3~48
~ _
U
d~ I~ d'
E~ a ~ ' ` '~ ~ ~ ~ ~ ~ O~ a; a~ a
0 ~ c~ o
.~ ~ ~ ~ O O O C~
3 o o o o o o o o o o o o ~ o o
v~
E4.~ O O~ o o ~r
~ :~ u O O o o o o O ~ O o o o o o
H E4
Ql O ~ ~ o o ~ .~
~ .~1 . ~I r--~ O O O O ~ N ~ ~ t~l ~ ~ ~ ~
¢ ~ U O O O O O O O O O O O O O O C:~
~ . O
~: V o ~ ~ ~ A N ,~ Z ~ 1 Z --1
~ O ~ Z ~ ¢ ;I Z ¢ ¢
¢
Z ~ o a~ o o o
~ ~ o~ ~ x er ~ ~
~ ~
~ N ~IC~ ~ ~ N ~ I t~ ~ ~
Z~,~.'C) I I I I I I I i I I I I I I I ._
z z z æ æ z z z z z z z z æ æ a.
~ ~ ¢ ~ ¢ ~ ¢
Q~ ~ a~
O O ~t r-l N ~1 ~ ~ 15~ ~ ~ U~ t~ r~ C~ t~
E3 E~ I I I I I I I I I I I I I I ~ 1
10 :~ U t.) ~,) V C.) O U ~ U C~ X
0 5~: a~
I N N N N N N N r~ ~ N ¦ Z
~r.
~ ? ~, s - l o -
- . ` ' . . .
WO 93/01 147 2 1 0 3 0 ~ 8 PCr/US92/05816
. _
V .~ ,~ O
,, C cr~
~ r~ u ol
E~l l O~ rO~
o E ~" Z " z Z
æ~ o o O
¢~ X~ ,o
I C N N ~ C
~ ~ .
~i " o u E
Q~ ~O ~ N . Z
, ,~nz , _ , , ll
WO 93/01 147 PC~/~JS92/05816
c~o~3o 4~
ExamPle 11
A second series of samples was prepared by the method described above in
Example 1, with the exception that CaF2 was used in place of Y20~ and the cooling rate was
5 increased to 50C/minute. The composition, theoretical density and thermal conductivity are
shown in Table ll.
.... .
WO 93/01147 PCI /USg2/05X16
2lO3~ g~'~
,
o~
,., V e o ~ ~ ~ o o~ o Ln Ln ., Ln ~ O O
~ 3 ,~
U ,
I :>.
~ ~ V a~ Ln Ln Cl~ ~ Ln ~ r Ln 1~ r
d~ o U U~ ~ Ln ~ co
~ D 00 0 a~
J~ :
S O o O ~ O O
~ O O O O O C; C:~ C~ C~ O
~J O er ~ L~ Ln ~ 1-- 0 0 C~ 0 a~ ~
O O o o o o O O O O
H~1 . ~
~ ~ O r~ C10 a~ ''' ~ .
.Q O .,~ ~ 1 . . . o o ~ ~ .
E~ ~. ~ o o o o o o C; ' O O o o ~ '
. ~ o
O ~ N ~ ~ ~ ~ ~ ~ l l
~ o ~ s ¢ æ ¢ z ,~ æ ¢ Z ~S Z .~ Z .
m ~, Q.
d~ Ln Ln Ln Ln L~ ~ C~
~: 3 Ln L ~ CO C~ ~O ~ Ln ~ ~ ~r
~ . C
Z~O I ~
Z Z Z Z Z Z Z Z Z Z Z ~Z; Z Z ~
a ~ ~0
U~ r L L r-l _I
¦ E E w w w w ~ b w w w w ~ ~ w ¦ ~
~ ~ * ~ ~ r Z
,Ut Z _ _ _~ *
--1 3--
WO 93/01147 ~ ~30 4~ PCr/US92/05XI6
, . ~
E ,,, ~ ~ o ~ o o~ ~D ~ ~,
o
I :~
a) ~ ~ ~ ~ In
~P o ~ U
s ~
E~
O ~ ~ ~ ~ ~ r~ ~ ~
.~ I-v ~ ~ ~ ~ ~ ~ .
3 ~ o o o o o o o o
~ ~ ~ ~r ~ ~ ~ o o
r~
~3 ~a o o o o o o o o
H
a~ ~o ~ O O l_ ~ ~,
Q .,~
El ¢3 o o ~ o o o ~ o o
Z
~ ~ O ~ . z z ,¢ z ~ z
m e~ ~ .
z o~ O O O O O O O Q
--I v ~ r s~
~ 3 o~ o ~ O
.
Z.~.o z :zs Z Z Z Z Z Z ~
"~ ~n v ,~ ,, ,, _, ,-, ~1 _I ~1
~ C ~¢ ¢ ¢ ¢ O
a~ ~ ~ ~
t`S~ N ~ t~ ~1 ~ Q
~ E
~0 ~ H H 1-1 H 1-1 H H H X
U~ Z H H H H H H 1--1 H Q~
~ . ~ _
~1 ~.D 1~ CO 0~ N ~1 Z
Ul Z
- 1 4 -
WO 93~01147 2 t 0 3 ~ 4 8 PCr/US92/05816
The data in Tables I and ll illustrate two points. First, the burn-out atmosphere
can make a substantial difference in density as shown in samples 1 1, 12, and 17-20 of Table I
and 3, 4, 7, 8, 15 and 16 of Table ll. Second, it illustratesthe significance of the upper and lower
5 limits for Al2O3-
ExamDle 111
A third series of samples were prepared by a modification of the method
described in Example I using the sintering aids of Example 11. The modifications were a decrease
in sintering temperature to 1 535C, an increase in the heating rate to 25~C/min. and an increase
10 in the cooling rate to 50~Clmin. The results are shown in Table 111 in the same manner as in
Tables I and 11.
....
WO93/01147 ~ 30 ~8 PCr/U~92/05816
E ,,~ u~ , o ,, , , , , ~ , ,,
E~o
I ~ r 0 ~ n
~ o ~ N O ~r N 0 0 0
_ ~ ~ ~ ~ 1 'n
h .,~ o o _I ~ o o . , . . c~
~) 3 v o o o o c~ o o o o o
14
l h ~ o o o o o o ¦
~1 ~ co u7 ~,
r-J N .rl ~ er ~ ~1 N ~ ~ . O u~
E~ ~3 (~S O o o o o o
~ Z
I V o N ~ æ . Z Z ¢ Z ¢
m ¢~
~ N ~ ~
~ 3 ~ X a~ O
''Z ;~. Z Z Z Z Z Z X Z Z ~ Z .~
~ U~ ~ ~1 _I ~ ~1 ~ --I ~ ~ ~ '~ ~ ~
a ~ ¢ ~ c o
Q
~ H ~ H ~ H H H H ~ H ~ X
a) ~ ~
~ * ~ ~ Z
cn Z
-16-
WO 93/01147 2 1 ~ 3 0 ~1 ~ Pcr/uss2/ossl6
The data in Table lll demonstrate that a density of 90 /O of theoretical and a
thermal conductivity of 1 00W/mK are attai nable within the composition ranges specified for
- Al2O3, CaF2 and YF3. If a density of 95% of theoretical density with the same thermal
5 conductivity is desired, the composition limits must be narrowed. Suitable results should be
attainable with an Al2O3 weight fraction of 0.20 to 0.45, a CaF2 weight fraction of 0.06 to 0.38
and a YF3 weight fraction of 0.40 to 0.60.
E~ample IV
A series of samples were prepared using the compositions of samples 1 1-20 from
10 Table 1, but two different modifications of the sintering conditions. One modification
maintained the same sirotering temperature, but increased the heating rate from 2.5C/min to
15C/min. The other modification maintained the same heating rate, but lowered the sintering
temperatureto 1550C. Thecoolingratewas50C/minuteasin Exampleslland lll. Thermal
conductivity and percent theoretical density values are presented in Table IV together with the
15 values from Table 1.
3Q
. .
, ~ .
WO 93/01147 PCrIU~;92/05816
.:
e U s o ~
C~ N ~ --
~-~ ~ ~ Ul * I a~
CP O U U~
a) ~ D
E~ Q
7 ~ ~ ~
~ ~ In u~
E~ C~ ~ 3 ~
C~ ~ ~ , ,,............ _ ~
Q~_~ V ~r ,n ~ ~ ~ ~ ~ 1` ~ -I _
d~ O ~ ~ v~
~ ' a ~ D ~
~ ~lV ~
a) E :I v ~ I ~ r` ~ r` r` ~ ~ , ~ ,
_, L~ ~, .,, e
Q ~: a) ~: ~ ~ ,~
E~ .e Ou
C~ U~
.~1 v ~ *
~ d~OC~ C
E~ ~ ~ :
, o E,~ æ ~S Z ,~C æ ~ z ,~ z .'
~ o
Q a~ N ~ ~:
"'' ' E~ U C ) c~ U ~ ~x
U~Z C
Q~ O O
~ e ~ z
~n z ._ _
18-
WO 93/01147 PCI/US92/05816
2 1 0 ~
Example V
A series of samples were prepared usi ng the compositions of samples 7-12 from
Table ll, buttwo different modifications of the sintering conditions. One modification
5 maintained the same sintering temperature, but increased the heating rate from 2.5~C/min to
1 5C/min. The other modification increased the heating rate from 2.5C/min to 1 5C/min and
lowered the si nteri ng temperature to 1 600C. Thermal cond ucti vity and percent theoreti cal
density values are presented in Table V together with the val ues from Table ll.
.. .
-19-
WO 93/01 147 P~/lJS92/05X16
` i .;
~ ~ ~o~
~ ~ ~ ~ ~ ~ U~
C ~ C :~ ~ ~ ~ ~ ~ ~ ~1
~U ~0-~- __
o N , ~
~n~1 d~ o u e ~ co
E~ ~
^
E3 :~ ~ o
L~ O
C S 0 ~
n E~ U , . . . _ _ _
d~ O ~ ~ ~,
E~ ~ O
Q~ ~V~ l Z
~ E~ o
D C e ~ 3 r~ I ~ I ~ I t
o~ . _-
I U~V~
:1 0 E .C ~ Z ,~, Z , ~ Z .C
m ~ o
,
~I Q~
E~ e ~ E
~ ~ H H H H H H X
U~ Z H H H H H H a~
a~
~ Z
U~ Z I~
. _
~ 2 0 ~
WO 93/01 1 47 210 3 (31 ~- 8 PCI / I,'S92/OSX 1 6
The data presented in Tables IV and V demonstrate that some sintering airJ
combi nations yield acceptable thermal tonductivity values and densities at tem peratures as low
as 1 550C. The data also show that a faster heating rate to a sintering temperature of 1 625Cc
5 improvesthermal conductivity, density or both. A similar effect is expected at 1 550C. As such,
samples 5, 7, 9 and, perhaps, 2 and 4 would have a density of 90 percent of theoretical density
- or greater at an increased heating rate such as 1 5C/minute or higher.
Example V~
A sample was prepared using a modification of the procedure of Example I with a
10 compositionthatcontained 100partsAlN(9Ov~rt%)and 11 partsofsinteringaids. Thesinteringaidsandtheirweightfractionswere: 0.27AI203;0.55YF3;andO.18Y203. The
modified procedure altered the binder formulation to form a tape cast sample. The binder
removal procedure by using nitrogen ratherthan air, a tirne of 4 hours rather than 2 hours ànd
a temperature of 600C rather than 550C. Sintering occurred at a set temperature of t 650C
15 ratherthan 1625C. The sintered material had a density of 3.26 g/cc (98.5 % theoretical density)
and a thermal conductivity of 145 W/mK. Analysis of the phase chemistry by x-ray powder
diffraction revealed the presence of Y3AI5012 and YOF.
In the absence of Al203, yttrium aluminate does not form and the density and
thermal conductivity values are lower. Similar results are expected with other compositions
20 disclosed herein.
Eauivalents
Those skilled in the art will recognize, or be able to ascertain using no more than
routine experimentation, many equivalentsto the specific em~odiments of the invention
described specifically herein. Such equivalents are intended to be encompassed in the scope of
2S the following claims.
.
;
-21 -
. . _ .