Note: Descriptions are shown in the official language in which they were submitted.
W092/1~20 PCT/VS92/0123~ '
~ 2 1~
-~OL~EN- ~ R~ XODS--
BACKGROUND OF THE INVENTION
1. Field of the Invention
This invention relates to a collagen-hydrogel
material which contains a collagen-hydrogel for promoting
epithelial cell growth and which is adapted to be used to
fabricate artificial lens or contact lens which promotes
healing of corneal epithelium during implantation and more
particularly to a collagen hydrogel biomedical material
that is formed of a polymerized hydrophilic monomer which
is gelled and crosslinked to form a polymeric meshwork in
the presence of and anchoring collagen formed of a
constituent of ground tissue capable of promoting and
sustaining epithelial cell growth and regenerating growth
of the stroma and wherein an artificial lens, formed of the
collagen-hydrogel for promoting epithelial cell growth and
! 20 positioned over the pupillary zone of the eye contiguous
Bowman's membrane having a selected portion of corneal
epithelium removed therefrom, promotes epithelial cell
growth enabling corneal epithelium to attach to and cover
the artificial lens to implant the same in the eye between
Bowmann's membrane and a new layer of epithelial cells
forming corneal epithelium. Laid down in the layers of the
regenerated stroma are new keratocytes and collagen fibial
produced from keratocytes. This invention further relates
to a method for locating on a cornea an artificial lens
fabricated from a collagen-hydrogel for promoting
epithelial cell growth and regeneration of the stroma.
2. Description of the Prior Art
Before beginning a description of the prior art,
it would be helpful, in understanding the teachings of this
3~ invention, to define certain of the key terms that are used
in the teachings of this invention.
-' ~
~ . . . .~
W092/1~20 PCT/US92/01233
21~ 3 : - ~
Collagen, in its broadest sense, is a natural
; protein which serves as the ground substance or adhesive
substance between cells in living tissue. It is well known
in the art that collagen, as a substrate material, is
capable of promoting cell adhesion and growth. Other
j proteins are also known to be capable of supporting cell
growth of at least certain cell lines. In the present
invention, the preferred source of collagen, as a natural
protein, is derived from animal sources.
10It is also known that other macromolecules, that
is a molecule formed of a constituent of a ground substance
of tissue, can support cell growth. Typical of such
macromolecules, in addition to collagen, are
mucopolysaccnarides or fibronectin, which constituents of
ground substances of tissue are capable of promoting cell
growth.
One class of synthetic materials which have found
wide application as biomaterials is the class known as
; hydrogels. The term "hydrogel" refers to a broad class of
polymeric materials which are swollen extensively in water,
but which do not dissolve in water. Generally, hydrogels
are formed by polymerizing a hydrophilic monomer in an
aqueous solution under conditions where the polymer becomes
crosslinked so that a three dimensional polymer network is
formed which is sufficient to gel the solution. Hydrogels
are described in more detail in Hoffman, D. S., "Polymers
in Medicine and Surgery," Plenum Press, New York, pp 33-44
(1974).
Hydrogels have many desirable properties for
biomedical applications. For example, they can be made
nontoxic and compatible with tissue. In addition, they are
usually highly permeable to water, ions and small
molecules. ~s is noted herein below, despite these
favorable qualities, hydrogels have been found, in general,
to be unsuitable as substrates for cell attachment and
growth.
With the benefit of the above described
descriptions and definitions, the known prior art will now
be addressed.
.. . . . . .
. . . .: . . . . - ~ . - -
:: . ' ' .
.: `': ' ' . ' ~ . ' : . - ' . '
:' , : `: ~ .
. . . ' `' ' . ". : ~' ` .'
' ' ' ' '' " .. ' '...... ~ ~ " ' ' .':
W092/1~20 ~ 3 PCT/US92/01233
: It is known in the art to utilize a procedure
known as epikeratophakia for the correction of aphakia and
;,~,
high myopia in a human eye ~hereinafter referred to as the
` Epikeratophakia Procedurel'). In the Epikeratophakia
; 5 Procedure, human corneal tissue is used and the corneal
tissue is mechanically machined or polished to a specific
~i lens power to form a corneal tissue lens. The corneal
, tissue lens is then sutured to the anterior surface of the
cornea in the pupillary zone of the eye in order to change
the refractive power of the eye. The specific procedure
used for suturing the machined or polished corneal tissue
lens to the eye requires that a portion of corneal
~; epithelium be removed to expose a portion of Bowman's
membrane and corneal tissue lens then be placed directly
upon Bowman's membrane. During the healing process,
corneal tissue lens is covered by epithelial cells which
form the cornea epithelium implanting corneal tissue lens
r, between Bowman's membrane and corneal epithelium. This
i procedure depends on the availability of human cornea
~- 20 tissue.
; It is also known in the art to use frozen human
- corneal tissue, which is ground to a lenticular power, to
form a corneal tissue lens and to suture the same to
corneal stroma of a human eye to change the refractive
power of the eye. This procedure is known as the
; "keratomileusis" and is described in a published article
captioned "Keratophakia and Keratomileusis -Clinical
; Results" which appeared in August 1981, Volume 88, No. 8,
at pages 709 - 715 of American Academv of O~hthalmology by
Swinger, Casmir and Barraquer, Jose' (the
; "Swinger/Barraquer Publication").
It is also known in the art to use collagen-
, hydroxyethylmethacrylate hydrogels as substrates for
promoting cell growth in tissue culture. The material used
for the hydrogel is known as collagen-
hydroxeythylmethacrylic, and referred to as a HEMA
hydrogel, which was prepared in the presence of an aqueous
solution of native collagen. The resulting transparent
hydroge~ -ontaining collagen was evaluated as substrata for
.,~, ":, ,
, - . . . . . ...
.
,
~ - . . ~ . .
WO92/14420 210~113 PCT/US92/01233
growth of various cell iines in tissue culture. The
preparation and use of collagen-hydroxyethylmethacrylate
hydrogels for promoting cell growth in tissue culture is
described in a article entitled USE A COLLAGEN-
HYDROXYETHYLMETHACRYLATE HYDROGEL FOR CELL GROWTH whichappeared in Volume 77, Number 4, April 1980 at pages 2064-
2068 of the Proceedings of the National Academy of Science,
United States of America, wherein the authors were Linda
i Civerchia-Perez (the inventor herein), Barbara Faris, Gary
La Pointe, John Beldekas, Howard Leibowitz and Carl
~ Franzblau (the "Civerchia Publication"). The Civerchia
; Publication disclosed that the collagen-
hydroxyethylmethacrylate hydrogels for promoting cell
growth in tissue culture were prepared by polymerizing
monomeric hydroxyethylmethacylate in the presence of
various concentrations of soluble native collagen. The
resulting transparent hydrogels were used as substrate for
growth of IMR-90 human embryonic lung fibroblasts. It was
determined from these experiments that the growth of IMR 90
human embryonic lung fibroblasts was dose dependent upon
the amount of collagen contained within the hydrogel. The
resulting cell growth become intimately attached to the
hydrogel substrate, and could not be removed. It was also
noted during the experiments leading to the Civerchia
Publication that hydrogels containing albumin, gelatin
(denatured collagen) or collagenase-treated collagen do not
support cell growth. The results of this publication
provided a foundation for a relatively easy procedure for
experimentally probing mechanisms of cell adhesion and cell
differention. Substantially the same material is disclosed
in United States Patent 4,565,784 wherein the inventor
hereof is one of the co-inventors of the United States
Patent 4,565,784.
The use of hydrogels for the correction of
refractive error is well known in the art, and such
hydrogels are used as the base material for fabricating
soft contact lens. Soft contact lens are adapted to be
inserted into and removed from the eye. When soft contact
lens are placed in the eye of a user, the function thereof
. : ... : - :. . . . .
.. . ... : , ~: .. - , ... . -
.
-- .
. . . ', ~ ~ .` ~ ' . ,
. .
W092/14420 PCT/US92/01233
is to correct myopia, hyp~ropia, astigmatism, and aphakia.
, Typically such contact l~ are formed of a hydrogel
selected from the hydrophilic class of polymers, and the
hydrogel is molded or lathed to a specific lens power. The
soft contact lens, when placed over the pupillary zone of
the eye of a user, rests upon a tear film and corneal
epithelium and function to change the refractive power of
, the eye.
It is also known in the art to experimentally
implant high water content, intracorneal implants
fabricated from a Vistamarc hydrogel in the eye of rhesus
monkeys and to develop keratometric data therefrom.
Typical of publications describing this procedure are (i)
an article captioned HYDROGEL KERATOPHAKIA: A FREEHAND
POCKET DISSECTION IN THE MONKEY MODEL which appeared in the
1986 Volume 70 issue, at pages 187-191 of the British
Journal of Ophthalmology by Bernard E. McCarey et al (the
"McCarey Publication"), and (ii) an article captioned
HYDROGEL KERATOPHAKIA: A MICROKERATOME DISSECTION IN THE
MONKEY MODEL which appeared in the 1986 Volume 70 issue, at
pages 192-198 of the British Journal of Ophthalmology by W.
Houdijn Beekuis et al (the "Beekuis Publication"). These
publications disclose that hydrogels can be implanted into
~: the cornea of a monkey and that the hydrogel materials are
compatible with the cornea tissue of a monkey.
U. S. Patent 4,126,904 to Dennis D. Shepard, M.
` D. discloses artificial lenses, which are hard contact
lenses, which are adapted to be placed in the eye of a
user. In addition, U. S. Patent 4,126,904 discloses a
method of locating the same on the cornea of the eye. The
disclosed artificial lens has an optical portion, which
preferably is circular in shape and dimensioned to overlie
the pupillary zone of an eye, and a non-optical portion,
termed the "haptic" portion, which is used as a means for
permanently affixing the lens to the eye. As taught by U.
S. Patent 4,126,904, the artificial lens can be affixed to
the anterior surface of the cornea by suturing, stapling or
like attachment means for securing the lens to adjacent
W092/l~20 ; ~ , PCT/US92/0l233
2 ~ 6
structure of the eyeball so that the lens will move with
the eyeball.
Biologically stablized compositions comprising
collagen as the major component with ethylenically
unsaturate compounds used as contact lens is known in the
art and are disclosed in United States Patent Nos.4,452,925
and 4,388,428. In these compositions, the ratio of
collagen by weight to the composition is very high and use
of high collagen levels in contact lens may make the lens
cloudy and affect the transparency thereof and are of such
high levels that the same are incapable of significantly
promoting epithelial cell growth and regeneration of the
stroma. These United States Patents do not disclose, teach
or suggest that the concentrations levels of collagen
shoild be controlled to low levels which are capable of
promoting epithelial cell growth and regeneration of the
stroma.
It is also known in the art that Chiron
Ophthalmics, Inc. has isolated a epithelial growth factor
molecule and has used this molecule to apply to the human
eye to produce a more rapid resolution of corneal abrasion.
SUMMARY OF THE INVENTION
Disclosed herein is a collagen hydrogel for
promoting epithelial cell growth and regeneration of the
stroma formed from a stock solution of collagen which is
produced by centrifuging and concentrating a soluble
collagen and adding the stock solution of collagen to a
hydrogel polymer formed by the free radical polymerization
of a hydrophilic monomer solution gelled and crosslinked to
; form a three dimensional polymeric meshwork for anchoring
collagen from the stock solution of collagen forming a
collagen-hydrogel for promoting epithelial cell growth and
regeneration of the stroma to produce keratocytes including
collagen fibial growth, the collagen-hydrogel being capable
of promoting and supporting growth of epithelial cells
enabling corneal epithelium to attach to and cover the
anterior surface of and to regenerate the stroma to produce
. . ~ .
.
- . . :. - ~ : .
-: . ,
- . ~ :
- WO92/1~20 '~ L 13 PcT/vs92/ol233
~7
keratocytes including collagen fibial growth enabling the
corneal epithelial to adhere to and cover the collagen
hydrogel and to regenerate the stroma in the vicinity of
the corneal epithelial, the collagen-hydrogel material
having a ratio by weight of collagen-to-hydrogel in the
range of about .6-to-lO00 and less than .6-to-lOO0 but at a
level wherein sufficient collagen is present by weight to
at least one of promote epithelial cell growth and
regeneration of the stroma.
Also disclosed herein is a method of fabricating
a collagen~hydrogel comprising the steps of forming a
radical free polymer of a hydrophilic monomer; mixing the
hydrophilic monomer with a stock solution of collagen
forming a clear viscous monomer solution wherein said
collagen-hydrogel material has a ratio by weight of
collagen-to-hydrogel in the range of about .6-to-lOO0 and
less than .6-to-lO00 but at a level wherein sufficient
collagen is present by weight to at least one of promote
epithelial cell growth and regeneration of the stroma; and
heating said viscous monomer solution in the presence of a
: crosslinking agent to polymerize the same into a three
dimensional polymeric meshwork having collagen from the
stock solution of collagen interdispersed within the three
dimensional polymeric meshwork.
None of the prior art discloses, teaches or
suggests a collagen-hydrogel which is capable of promoting
epithelial cell growth when fabricated into an artificial
lens which is positioned over the pupillary zone of the eye
'~ contiguous with Bowman's membrane to promote and support
epithelial cell growth enabling corneal epithelium to
become attached to and implant the artificial lens between
Bowman's membrane and corneal epithelium.
; This invention relates to the use of a
transparent collagen-hydrogel, as a biomedical material,
which is capable of being molded to a given lenticular
power as in the preparation of a contact lens, to produce
an artificial lens having a collagen-hydrogel for promoting
epithelial cell growth. Such an artificial lens is adapted
to be sutured, glued, or held in place with bandage or
.
`:
~ W092/l~20 2 1 0 4 1 1 3 PCT/U592/~233
therapeutic contact lens until the epithelium growth occurs
directly to the anterior surface of the cornea directly on
Bowman's membrane and functions to correct refractive
errors of the eye. The collagen-hydrogel, referred to
sometimes herein as a "collagen-hydrogel for promoting
epithelial cell growth," will be covered by corneal
epithelium during the healing process. The growth of the
epithelial cells to form corneal epithelium on the anterior
surface of the eye during the healing process is very
similar to that experienced in the Epikeratophakia
Procedure.
In the present invention, a hydrogel polymer is
~- disclosed that is formed by the free radical polymerization
of a hydrophilic monomer solution gelled and crosslinked to
form a three dimensional polymeric meshwork anchoring
` macromolecules. The macromolecules comprise a constituent
of a ground substance of tissue, such as a native collagen,
`~ interspersed within the polymeric meshwork forming a
collagen-hydrogel for promoting epithelial cell growth. An
optical lens for the eye fabricated from the collagen-
hydrogel, when attached to Bowman's membrane of the cornea
; of an eye, is capable of supporting and promoting
epithelial cell growth enabling corneal epithelium to
attach to and cover an artificial lens formed of the
collagen-hydrogel for promoting epithelial cell growth
.;5 during the healing process.
Also disclosed herein is an artificial lens,
which preferably is a contact lens having a predetermined
shape and power, which is fabricated from the collagen-
hydrogel biomedical material and which is adapted to beaffixed to Bowman's membrane of the cornea of an eye. When
the artificial lens formed of the collagen-hydrogel for
promoting epithelial cell growth is so affixed to the eye,
it promotes and supports growth of epithelial cells across
the surface thereof to produce corneal epithelium formed of
several layers of epithelial cells. In the preferred
embodiment, the contact lens comprises a lens body having
anterior and posterior surface and formed of a collagen-
hydrogel for promoting epithelial cell growth. The
~ : . : . .
W092/l~20 ~ PCT/US92/01233
. ~,, g
hydrogel comprises a hydrogel polymer formed by the free
radical polymerization of a hydrophilic monomer solution
gelled and crosslinked to form a three dimensional
polymeric meshwork anchoring macromolecules. The
macromolecules comprise a constituent of a ground substance
of tissue interspersed within the polymeric meshwork
; forming a collagen-hydrogel for promoting epithelial cell
growth. The collagen-hydrogel is capable of promoting and
supporting growth of corneal epithelium formed of several
layers of epithelial cells which implant the artificial
lens between Bowman's membrane and corneal epithelium. The
lens body is adapted to have the posterior surface thereof
positioned over the pupillary zone of the eye, and is
affixed to Bowman's membrane in an area substantially equal
to the shape of the lens body having corneal epithelium
removed therefrom. When the lens body is so affixed, it is
capable of supporting and promoting epithelial cell growth
enabling corneal epithelium to attach to and cover the
anterior surface of the lens body.
Also disclosed herein is a method of fabricating
a collagen-hydrogel for promoting epithelial cell growth.
The method comprises the steps of forming a radical free
polymer of a hydrophilic monomer; mixing the hydrophilic
monomer with a diluted solution of macromolecules
comprising a constituent of ground substance of tissue in
the presence of a weak solution of ammonium persulfate and
sodium metabisulfate forming a clear viscous monomer
solution; and heating the polymer mixture in the presence
of a crosslinking agent to polymerize the same into a three
dimensional polymeric meshwork having macromolecules
comprising a constituent of ground substance of tissue
interspersed within the three dimensional polymeric
meshwork.
The hydrogel used in the prior art for lenses
which are placed onto the cornea of the eye or implanted on
the eye have serious disadvantages which are overcome by
the teachings of this invention.
The Epikeratophakia Procedure and the procedure
described in the Barraquer Publication require the use of
`
.,
' :
..
W092/1~20 , PCT/US92/01~33
, 21~'~1'13'" lo ¢~
human corneas as the source of corneal tissue. The corneal
tissue must be processed into a predetermined shape and
- power to fabricate an implantable corneal tissue lens. The
source of human corneal tissue is limited, and the cost
` 5 thereof is controlled, thereby limiting the availability of
the corneal tissue for the Epikeratophakia Procedure and
the use of the Epikeratophakia Procedure itself as a
readily available alternative.
None of the prior art which disclose the use of
collagen-hydrogel for fabricating artificial lens disclose,
; teach, or suggest the use of a collagen-hydrogel which has
been gelled and crosslinked to form a three dimensional
polymeric meshwork anchoring macromolecules wherein the
;macromolecules comprise a constituent of a ground substance
~; 15 of tissue interspersed within the polymeric meshwork
forming a collagen-hydrogel for promoting epithelial cell -~
growth when the hydrodel is attached to Bowman's membrane
of the cornea of an eye. As a result of collagen-hydrogel
for promoting epithelial cell growth, corneal epithelium is
cabable of attaching to and covering the co'lagen-hydrogel.
The prior art Civerchia Publication discloses the
experimental use of a collagen-hydroxyethylmethacrylate
hydrogel as tissue growing substrate for promoting tissue
cell growth of IMR-90 human embryonic fibroblasts, which
are cells harvested from the lungs of a human fetus, as an
experimental means to probe the mechanism of cell adhesion
and cell differention. Thus, the teachings of the
;Civerchia Publication are limited to experimental tissue
culture applications in that the Civerchia Publication did
not recognize, teach, suggest, or disclose either the
concept of or the use of a collagen-hydrogel for promoting
epithelial cell growth as a basic material for fabrication
of an artificial lens which, when implanted on, or into,
the eye, would result in overcoming rejection of the
artificial lens and the promotion of and support of the
growth of epithelial cells to enable corneal epithelium to
attach to and cover the anterior surface of the artificial
lens with several layers of epit:helial cells to form
corneal epithelium resulting in the artificial lens being
: . . . . . ..................... . . .
- . : ,
: '
W092/1~20 ~ 1 U ~ 1 1 3 PCT/US92/01233 `
( ' ~
implanted between Bowman's membrane and corneal epithelium.
i The McCarey Publication and Beekuis Publication
disclose the implantation of artificial lens, using the
freepocket dissection method and the Barraquer method,
respectively, wherein the artificial lenses were fabricated
from hydrogels with high water content. The results
disclosed by both the McCarey Publication and Beekuis
Publication were that the hydrogels were well tolerated
within the corneal tissue. The Beekuis Publication
disclosed that the implantation of hydrogels had interface
problems along the edge of implant, apparently from tissue
buildup at the boundary layer between the lens/corneal
;~ epithelium interface. The Beekuis Publication noted that
implants with abruptly cut edges versus a fine wedge tended
to have more light scattering collagen at the implant
margin. The collagen referred to is the native corneal
'~ collagen located within the corneal tissue of the monkey,
and to native collagen. There is no collagen interspersed
within the hydrogel moleculer structure that was used to
fabricate the artificial lens implanted within the monkeys
as described in both the McCarey Publication and Beekuis
Publication.
The artificial lens and method for implanting the
same disclosed in U. S. Patent 4,126,904 relates to so
called "hard contact lens", and the lens are formed of
standard plastics or known hard plastics, such as
polymethylmetacrylate (PMMA), none of which contain a
collagen-hydrogel for promoting epithelial cell growth.
The concept of surgically postitioning the artificial lens
over the pupillary zone of the eye is applicable to this
invention, it being noted, however, that the artificial
lens attached to the eye using the teachings of U. S.
Patent 4,126,904 results in the lens being affixed to the
anterior surface of corneal epithelium.
Thus one aspect of the present invention is that
the collagen-hydrogel material for promoting epithelial
cell growth can be used as the base material for
fabrication of artificial lens of a reproducable power and
quality as is well known in the art for producing contact
' .
,
. ~
' ~ '
W092/1~20 ~ 3 i! 12 PCTtUS92/01233
. lens. The collagen-hydrogel artificial lens can be
reproduced reliably in the laboratory and is not dependent
upon the availability of human tissue as is the case in the
production of a corneal tissue lens as described by the
: 5 prior art.
Another aspect of the present invention is that
the artificial lens fabricated from the collagen-hydrogel
', for promoting epithelial cell growth of the present
; invention and implanted on the eye results in the
elimination of rejection of the artificial lens by the
cornea and promotes and supports growth of epithelial cells
during the healing process to actually implant the lens
between Bowman's membrane and a new layer of corneal
epithelium grown from the epithelial cells.
Another aspect of the present invention is that
; the artificial lens can be fabricated to any selected
geometrical shape or diopter power from the collagen-
hydrogel for promoting epithelial cell growth using any one
' of molding, lathing or freezing processes.
Another aspect of the present invention is that
the collagen-hydrogel for promoting epithelial cell growth
enables corneal epithelium to attach to and cover the
anterior surface of an artificial lens implanted within the
eye because of the growth of epithelial cells which produce
a corneal epithelium having several layers of cell
thickness resulting in the artificial lens being implanted
between Bowman's membrane and corneal epithelium.
Another aspect of the present invention is that
the implantation of the artificial lens requires only the
removal of corneal epithelium from the surface of the
cornea and the formation of a small "V" shaped slot and
corneal wing which does not disturb the integrity of the
cornea any more than a corneal abrasion or a superficial
corneal laceration. The artificial lens is covered with
epithelial cells during the healing process.
Another aspect of the present invention is that
the necessity of maintaining a "tear layer" between the
posterior surface of a soft contact lens and corneal
epithelium is eliminated.
- . : :
'' ~' ,:
'
W092/l~20 ~1U 11 ~ 3 PCT/US92/O1233 - ~
t-'- 13
Another aspect of the present invention is that
when the epithelial cells attach to and cover the anterior
surface of an artificial lens fabricated from the collagen-
hydrogel for promoting epithelial cell growth, if it ever
; 5 becomes surgically necessary to remove and replace the
, artificial lens, such as for example in an accident
- damaging the eye, the collagen-hydrogel can be stripped
from Bowman's Membrane and corneal epithelium can regrow
over a defect, or a new collagen-hydrogel can be placed
which will support regrowth of corneal epithelium. This is
one of the basic aspects of the Epikeratophakia Procedure.
That advantage is that a corneal overlay is less invasive
to the eye than a corneal inlay.
Another aspect of the present invention is that
the collagen-hydrogel for promoting epithelial cell growth
disclosed herein is formed by the free radical
polymerization of a hydrophilic monomer solution gelled and
crosslinked to form a three dimensional polymeric meshwork
anchoring macromolecules, comprising a constituent of a
ground substance of tissue, which are interspersed within
the polymeric meshwork forming a collagen-hydrogel for
promoting epithelial cell growth.
Another aspect of the present invention is that
the macromolecules may be a native collagen derived from
animal sources and capable of promoting and supporting
growth of epithelial cells.
Another aspect of the present invention is that
the sources of native collagen can be harvested from
tissues of human cornea, livestock cornea or calf's or
livestock skins, all of which are widely available in an
almost unlimited supply and at a reasonable cost.
Another aspect of the present invention is that
the hydrogel can be formed from a hydrophilic monomer such
as hydroxyethyle- methacrylate.
Another aspect is that hydrogel can be
polymerized in the presence of a crosslinking agent to form
a three dimensional polvmeric meshwork having controlled
spacings between the molecules thereof to anchor the
macromolecules which have a known size and to insure that
,; :
- '
:
.
.
WO92/1~20 ~ 1~4i1~ ~ PCT/US92/01233
A 14
; the macromolecules will be substantially uniformly
interspersed within the polvmeric meshwork of the
polymerized hydophilic monomer.
Another aspect of the present invention is that
; 5 the step of forming the crosslinking of the hydrogel can be
performed with a crosslinking agent which may be external,
;~ such as for example ultraviolet radiation, or a
crosslinking agent added to the hydrogel clear viscous
monomer solution, which crosslinking agent may be, for
10 example, ethylene glycol dimethacrylate or
methymethacrylate.
p~ Another aspect of the present invention is that
the artificial lens can be formed of the collagen-hydrogel
for promoting epithelial cell growth of the present
15 invention wherein the artificial lens has an optical
portion configured for placement over the pupillary zone of
the eye and on the central anterior surface of Bowman's
membrane of the cornea having corneal epitheleum thereof
removed. The optical portion of the artificial lens is
20 dimensioned to substantially cover the total anterior
surface of the pupillary zone of an eye.
Another aspect of the present invention is that a
; method of fabricating a collagen-hydrogel for promoting
epithelial cell growth which, when positioned contiguous to
25 Bowman's membrane and corneal epithelium of the cornea of
an eye, promotes and supports epithelial cell growth to
form a corneal epithelium is disclosed herein.
Another aspect of the present invention is that a
method for locating on the cornea an artificial lens having
30 a preselected geometric shape and power and including an
optical portion having an outer edge, a posterior surface
and an anterior surface is disclosed herein.
Another aspect of the present invention is that
the method for locating on the cornea an artificial lens
35 having a preselected geometic shape and power includes the
steps of removing from Bowman's membrane over the pupillary
zone of the eye a portion of corneal epithelium on an area
slightly greater than the generalized shape of the
artificial lens; forming on Bowman's membrane and corneal
- . .
. .; , . .
,
... . . .
W092/1~20 2 1 ~ PCT/US92/01233
~- 15
stroma a "v" shaped annular groove having a diameter
substantially equal to the maximum geometrical dimensions
-: of the artificial lens and defining therearound a
peripheral and medial edge and having a preselected depth
which is less than the thickness of the corneal stroma;
dissecting the peripheral edge of the groove forming a wing
of corneal tissue having a preselected length; separating
the medial edge of the groove from the corneal groove;
1 placing the posterior surface of the artificial lens on the
anterior surface of the cornea and positioning the outer
edge of the artificial lens under the corneal wing, and
. affixing the artificial lens to the cornea over the
.- pupilary zone of the eye to maintain the same on the cornea
with the posterior surface in contact with Bowman's
membrane and the corneal wing overlying the edge of the
artificial lens enabling corneal epithelium to touch and
interact with the collagen-hydrogel for promoting
: epithelial cell growth and to respond to the cell growth
promoting constituent in the collagen-hydrogel for
promoting epithelial cell growth over a healing period
where epithelial cells grow over and adhere to the
artificial lens implanting the same in the cornea under a
new growth layer of corneal epithelium.
Another aspect of the present invention is that
the artificial lens formed of a collagen-hydrogel for
promoting epithelial cell growth can be implanted by using
surgical procedures similar to those used in corneal
overlays and corneal inlays which are easier to perform
than the implantation of a lens within the corneal stroma
as taught by the Swinger/Barraquer Publication.
Another aspect of the present invention is that
the step of suturing the artificial lens to Bowman's
membrane can be accomplished using suturing techniques
presently in the art which include removable suturing
: 35 material, such as nylon, mersilene, or prolene, or
removable devices, such as staples, or biodegradable
suturing material, such as pos, vicrylor dexon, by use of
the suturing techniques disclosed in U. S. Patent 4,126,904
cited above, suturing through openings formed in the lens,
., - ~- . , .
:
W092/1~20 ~ PCT/US92/01233
~ 16
by suturing around the edge thereof by use of a running
stitch suturing method known as the "running shoe lace"
stitch, suturing by use of individual or "interrupted"
sutures, or by use of a ~iodegradable adhesive which is
applied to the posterior surface of an artificial lens
formed of the collagen-hydrogel for promoting epithelial
cell growth disclosed herein. Also, the artificial lens
could be held in place with presently available
"therapeutic" contact lenses.
BRIEF DESCRIPTION OF THE DRAWINGS
These and other advantages of this invention will
be readily apparent when considered in light of the
detailed description hereinafter of the preferred
embodiment and when considered in light of the drawing set
forth herein which includes the following figures:
Fig. l is a block diagram of the method for
producing the collagen-hydrogel for promoting epithelial
cell growth of the present invention;
Fig. 2 is a pictorial representation of an eye
showing the relationship between corneal epithelium,
Bowman's membrane and the corneal stroma and a
representation of an artificial lens formed of the
collagen-hydrogel for promoting epithelial cell growth
which is adapted to be implanted with the eye using the
surgical procedures set forth herein;
Fig. 3 is a pictorial representation of an eye
showing the relationship between an implanted artificial
lens shown in Fig. 2 implanted between Bowman's membrane
and corneal epithelium after the eye has healed and the
epithelial cells have grown to several layers in thickness
and form corneal epithelium which is attached to and covers
the anterior surface of the artificial lens;
Fig. 4 is a cross sectional view of an eye
illustrating that the implanted artificial lens illustrated
pictorially in Fig. 2 overlies the pupillary zone of the
eye and that the same is in the form of a corneal inlay
after the healing process;
., . , . ~ . .
..
. .
W092/14420 2 1 ~ ~ 1 1 3 PCT/US92/01233
Fig. 5 illustrates pictorially the first steps of
the surgical procedure of removing a portion of corneal
epithelium to expose a portion of Bowman's membrane and
forming an annular shaped "V" groove wherein the "V" shaped
groove has a peripheral edge and medial edge;
Fig. 6 illustrates pictorially that the area of
removed corneal epithelium is generally circular in shape
and that the "V" shaped groove is located peripherally
within the area of removed corneal epithelium;
Fig. 7 illustrates pictorially the corneal wing
that is formed in the peripheral edge of the annular "V"
shaped groove;
Fig. 8 illustrates the insertion of the edge of
the artificial lens under the corneal wing;
Fig. 9 illustrates pictorially the relationship
of the edge of the artificial lens under the corneal wing
after completion of the surgery and before the healing
process;
Fig. lO illustrates pictorially the relationship
of the edge of the artificial lens under the corneal wing
after completion of the surgery and after the healing
process wherein the epithelial cells have grown to form
corneal epithelium implanting the lens between Bowman's
membrane and corneal epithelium;
Fig. 11 is a partial cross section showing the
use of a removable suturing material for affixing the
artificial lens to the cornea after the lens has been
positioned in place as illustrated in Fig. lO;
Fig. 12 is a representation of a circular shaped
lens having two openings formed therein to permit the means
for performing the suturing step illustrated in Fig. 11;
Fig. 13 is a pictorial representation of a
complete eye illustrating the sutured lens on the cornea;
Fig. 14 is a representation of a rectangular
shaped artificial lens having an optical portion and edges
which can be used to suture the lens in position over the
pupillary zone of the eye;
Fig. 15 is a representation of a circular shaped
artificial lens formed of the collagen-hydrogel for
: , - . .. . :, :, , . . . . -
,,. . : .. . .. . ..
.
'' -' , ' ' ' . ' ' ' " ' ~ ' ~ ,'' ~'' '
~I . . .
.. . . . .
WO92/14420 ~ 113 ; PCT/US92/01233
18 ~ ~;
promoting epithelial cell growth of this invention and
having an implanted ring of material having different
optical properties than that of the collagen-hydrogel for
promoting epithelial cell growth and which provides
differential passage of an image to the retina and which is
of a size and shape to be implanted on the cornea using the
teachings of this invention;
Fig. 16 is a representation of a circular shaped
artificial lens formed from the collagen-hydrogel for
promoting epithelial cell growth disclosed herein having
tabs extending therefrom which may be used by a surgeon in
implanting the artificial lens in the eye using the
teachings of this invention;
Fig. 17 is a representation of a circular shaped
artificial lens formed from the collagen-hydrogel for
promoting epithelial cell growth disclosed herein having
two aligned circular support members extending opposite
direction therefrom which may be used by a surgeon in
implanting the artificial lens in the eye using the
teachings of this invention; and
Fig. 18 is a representation of a circular shaped
artificial lens formed from the collagen-hydrogel for
promoting epithelial cell growth disclosed herein having
three circular tabs spaced equidistantly around the
periphery of an optical lens which may be used by a surgeon
in implanting the artificial lens in the eye using the
teachings of this invention.
DESCRIPTION OF THE PREFERRED EMBODIMENT
The block diagram of Fig. l illustrates the
various steps of the preferred method of fabricating a
collagen-hydrogel for promoting epithelial cell growth when
positioned contiguous to Bowman's membrane and corneal
epithelium of the cornea of an eye. The method comprises
, the step of forming a free radical pol,vmerization of a
hydrophilic monomer which is illustrated by block 20 of
Fig. l. The so formed hydrophilic monomer solution used in
the step of mixing with an aqueous solution of
,
~': :~.
.. !
W092/14420 ~ 3 PCT/US92/01233 ~'
macromolecules comprising a constituent of ground substance
of tissue in the presence of a weak solution of ammonium
; persulfate and sodium metabisulfate forming a clear viscous
monomer solution as illustrated by box 22 of Fig. l. If
the crosslinking agent is to be used to crosslink the
polymer during this step, the crosslinking agent is added
during the mixing step to insure that the viscous monomer
solution had a crosslinking agent therein such that the
step of heating will cause the crosslinking to occur to
form the polymerized meshwork. The addition of the
crosslinking agent to the monomer solution is illustrated
by block 26 of Figure l.
The next step of heating the viscous monomer
solution is illustrated by block 28 of Figure l. The
heating occurs in the presence of a crosslinking agent to
polymerize the same into a three dimensional polymeric
meshwork having macromolecules, which are constituent of
ground substance of tissue interspersed within the three
dimensional polymeric meshwork. If the crosslinking agent
was added to the monomer solution during the mixing step as
illustrated by blocks 22 and 26, then the crosslinking and
interspersing of the macromolcules with the polymeric
structure occurs during the heating. By controlling the
temperature and heating time of the heating step, the
macromolecules are substantially uniformally interspersed
with the three dimensional polymeric meshwork.
Alternatively, the crosslinking can be obtained
without the heating step, and without the crosslinking
agent being in the viscous monomer solution, as is discused
hereinbelow.
If a crosslinking agent is not added to the
monomer solution during the mixing phase as described
above, the crosslinking can be performed by irradiating the
monomer solution during the heating phase with gamma or
ultraviolet irradiation. The gamma or ultraviolet
irradiation causes the polymerized solution to crosslink
and form a three dimensional polyermic meshwork wherein the
spaces between the crosslinked molcules of the polymerized
.
.. . . ~ : . : . . . . . .
, .. . - . : , ,
. .
- : - , . , ~-
,- . , . : ~ ,
. .
.
, . . ~ . : . .
WO92/lM20 2 ~ O ~1~ 3 PCT/US92/01233
~ 20 ~
hydrophilic monomer contain the macromolecules interspersed
therein.
The collagen-hydrogel of this invention differs
from those known in the prior art because of the
crosslinking of the hydrogel into a three dimension
- meshwork for anchoring macromolecules capable of supporting
anchor-dependent cell growth. Generally hydrogels per se
are formed by forming a crosslinked polymer in an aqueous
solution to gel the solution. This can be done by free
radical polymerization of hydrophilic monomers, such as
hydroxyethylmethacrylate (HEMA). This process is well
known in the art and is described in Refojo, M. J. (1956),
Journal Ap~lied Polymer Science, 9, pages 3416-3426, and
, Holly, H. and Refojo, M. J. (1975), Journal of Biomedical
Material Res., 9, page 315. Many other hydrophilic
monomers in addition to HEMA can be employed.
As noted hereinabove, the preferred macromolecule
added to support cell growth is native collagen, a known
substrate for good cell growth. Soluble collagen can be
prepared by art-recognized techniques. In addition, other
proteins are satisfactory as long as they will support cell
attachment and growth. One example of an additional
protein known to support cell growth is fibronectin.
Macromolecules in addition to proteins can also
be added to these hydrogels as long as they are capable of
supporting growth of epithelial cells. Polysaccharides and
;~ mucopolysaccharides are one class of such macromolecules,
and those skilled in the art will know others.
,~ Small molecules are not employed because they can
diffuse throughout the three dimensional meshwork of the
crosslinked hydrogel. Since one of the requirements is that
the cell growth supporting molecules must be anchored in
the meshwork of the hydrogel, only macromolecules are used
for promoting growth of epithelial cells. The suitable
macromolecules can be water soluble or insoluble, with the
former being preferred.
Hydrogel polymers formed by free radical
polymerization of monomer solutions, which is the case for
HEMA hydrogel, require crossllnking to form the three
: ' ' ' .
' ' - ' ' , ' - . . ~ '' ' .. ~ . ~
. .
. .
'
.. " '.
~ W092/14420 ~ 3 PCT/US92/01233
~ 21
dimensional polymeric structure of meshwork to gel the
aqueous solution. HEMA monomer solutions normally contain
some dimethacrylate which can crosslink the gel structure.
The addition of crosslinking agents such as ethylene glycol
dimethacrylate to the polymerization process can change the
resultant hydrogel. Generally, the addition of
crosslinking agents tend to increase the rigidity and
mechanical strength of the hydrogel. Addition of
crosslinking agents, such as ethylene glycol dimethacrylate
and methymethacrylate, to the polymerization mixture in
the presence of native collagen, still changes the physical
properties of the hydrogel, and such additions to the
polymerization mixture are compatible with the native
collagen, and result in the collagen-hydrogel which support
growth of epithelial cells. Other known crosslinking
agents that can be used satisfactorily in producing the
collagen-hydrogel include diacrylates and dimethacrylates
or other divalent molecules.
The following examples are of methods for
producing the collagen-hydrogel for promoting growth of
epithelial cells of the present invention.
EXAMPLE I
Polymers of hydroxyethyl methacrylate (HEMA) are
prepared by the method of Refojo, described hereinbefore.
Pepsin soluble collagen is prepared by stirring the
ground shaved skin from a one week old calf in 0.5 M acetic
acid at 4`C. The residue, after centrifugation, is
resuspended in 0.5 M acetic acid containing porcine pepsin
at a final enzyme-tissue ratio of 1:50 (wet weight) and
allowed to stir overnight. The stabilized collagen is then
precipitated by addition of solid NaCl to a concentration
of 5%. The resulting precipitate is resolubilized in 0.5 M
acetic acid, then dialized exhaustively versus 0.02 M
Na2HPO4, pH 7.44 at 4`C. Following dialysis, the
precipitate is subjected to differential NaCl precipitation
at pH 7.44 as described in Chung, E. and Miller, E.J.
(1974), Science, 183, pages 1200-1201. These precipitates
are then lyophilized and suspended in 0.5 M acetic acid at
.: - ' ' ~:' ' . ". '.: ; ~ .'.'; '' ', ' :.: .
.
':
:. i . .
WO92/1~20 , PCT/US92/01233
3 22
a concentration of 1.2-1.4 mg/ml as determined by
hydroxyproline content, and allowed to stir overnight at
` 4`C. The resulting solution is dialyzed against 0.15 M
NaCl-0.05 M Tris, pH 7.44, overnight at 4`C. This solution
is used as a stock solution of collagen.
One ml of commerical HEMA, 1.0 ml of ethylene
glycol, 1.0 ml of stock solution of collagen (properly
diluted), 0.1 ml of 6% ammonium persulfate and 0.1 ml of
12% sodium metabisulfate are added in sequence. A
(quantity 0.1 ml of ethylene glycol dimethacrylate), a
crosslinking agent, is added to the solution. After
mixing, the resulting clear viscous monomer solution is
heated for two hours at 38`C, in a mold, as used in the
production of a contact lens. The resulting clear flexible
collagen-hydrogel is then dialyzed exhaustively versus the
; Tris-NaCl buffer, pH 7.44, to remove residual monomer and
ethylene glycol. During dialysis, the collagen-hydrogel
membranes become opaque, but transparency returns once the
ethylene glycol has been exchanged for water.
EXAMPLE II
~! A collagen-hydrogel monomer viscous solution is
prepared as in EXAMPLE I except that the ammonium
persulfate and sodium metabisulfate are not added to the
solution. The collagen-hydrogel is exposed to gamma
radiation or ultra violet radiation for two hours to
polymerize the monomer solution. The resulting collagen-
hydrogel, is sterilized in Puc~'s Ca++Mg++ free saline
containing 1,000 units penicillin, 50 ml Aureomycin, and
0.25 ml Fungizoine per ml of medium and placed under an
ultraviolet light for two hours. The collagen-hydrogel is
then transferred to a Puck's saline containing penicillin
and streptomycin and stored at 4`C prior to use.
';
3s EXAMPLE III
The White Cat Study
A White Cat Study was performed on a normal cat
which was white in color. The purpose of the experiment
and study was to implant an artificial lens using the
, ' ~'
- " ~ '' ' ' : : . :'.
WO92/1~20 ~ ~ 4 ~ ~ 3 PCT/US92/01233
23
teachings of the collagen-hydrogel invention into one eye
of the subject cat to determine to what extent that the
collagen hydrogel enhanced epithelial cell growth.
' ' .
(A) Preparation of~ L~len hYdroael material and
artificial lens from the collaaen hydrogel
material
: The artificial lens utilized in the White Cat
Study was fabricated from a collagen hydrogel material
prepared in accordance with the method of Example I above.
; l0 The collagen-hydrogel material was prepared using
l0 milligrams of collagen per milliter of collagen stock
solution. The collagen used was a type 8 placentia
collagen. The mixture was polymarized between two glass
slides to form a generally planar rectangular sheet having
a thickness of about .5 millimeters to about l.0
millimeters. One artificial lens was cut from the
generally planar rectangular sheet having a generally
rectangular shape. The dimensions of the artificial lens
cut from the sheet of material was approximately 6
millimeters in length, approximately 3 millimeters in width
; and about .5 millimeters to about l.O millimeters in
thickness. The physical characteristics of the lens were
~ that it had the appearance of a clear plastic sheet, was
- smooth, transparent and optically clear.
,:
` 25 (B) Method of suraerv for affixina an artificial
lens fabricated from the collaaen hydroqel
material to one eye of a white cat
The surgical procedure for affixing the
artificial lens fabricated from the above described
collagen hydrogel material to the subject white cat was
performed in a standard operating room. The subject white
cat was anesthetized using standard procedures. After the
anesthesia became effective, the initial step performed was
to remove the epithelium layer from the cornea. This step
was performed by scraping off the epithelial cells located
in the center of the eye forming a generally square area of
about 8 millimeters by 8 millimeters. A rectangular shaped
. ' ' . . , : ~' .. ,:: . . . :,
~W092/l~20 ~ld~i13 24 PCT/US9~/0~23~ 1
artificial lens was prepared from the sheet of material and
the dimensions of the artificial lens was approximately 6
millimeters in length, approximately 3 millimeters in width
and about .5 millimeters to about 1.0 millimeters in
thickness.
~- The cornea was then prepared surgically using the
steps of the method described in the connection with Figs.
5 through 9 hereof. The artificial lens was inserted
under the corneal wing and sutured in place using a 10-
nylon suture forming 8 uninterrupted stitches. The third
eyelid or nictating membrane of the subject cat was pulled
over the cornea a stitched in the closed position to
:prevent the cat from subsequently traumatizing the eye. The
subject white cat was then placed in a cat pound for a
;15 period of about one week to permit the lens to become
implanted by the growth of epithelial cells. After the
expiration of about one week, the subject cat was
sacrificed and the one eye having the artificial lens
affixed thereto was surgically removed, as a nucleared eye,
for an eye histologic procedure.
(C) Results of eye histoloqic procedure of an
artificial lens fabricated from the collaqen
hydroqel material affixed to one eye of a
white cat
The one eye having the artificial lens affixed
thereto that was surgically removed for the eye histologic
procedure was placed into a standard foramalin solution.
The analysis of the nucleared eye was performed using
standard pathological procedures for the fixation of the
eye. The global area of the eye disclosed that the cornea
was curved, that the artificial cell was in place, that the
artificial lens was clear and that the cornea was clear.
An examination of a tissue sample of the cornea disclosed
that the epithelial cells had grown over and implanted the
artificial lens. Also, it further noted that the stroma hd
also regenerated and had grown over the the edge of and
became attached to the artificial lens. The regeneration
of the stroma was unexpected. The examination of the
,
~ - ~
WO92/]~20 2 ~ PCT/US92/01233
stroma further disclosed that the eye had hematoxyalineosin
present in the stroma. An examination of the layers of the
stroma disclosed that new keratocytes, i.e. cells that
produce collagen, were present in the laminated layers of
` 5 the stroma. As a result of the presence of the
keratocytes, collagen fibial, i.e. molecules produced by
keratocytes, were laid down in the laminated layers of the
stroma. The results clearly showed that: (a) epithelial
cell growth occurred over and implanted the artificial lens
into the cornea; and (b) the stroma was regenerated and the
keratocytes were growing over and attaching to the edge of
the artificial lens and producing collagen fibial or
collagen molecules.
EXAMPLE IV
The Black Cat StudY
' The Black Cat Study was performed on a normal cat
which was black in color. The purpose of the experiment
and study was to implant an artificial lens using the
teachings of the collagen-hydrogel invention into one eye
of the subject cat to determine to what extent that the
collagen hydrogel enhanced epithelial cell growth.
~A) Pre~aration of collaaen hydroqel material
' and artificial lens from the collagen
hvdrogel material
The artificial lens utilized in the Black Cat
Study was fabricated from a collagen hydrogel material
prepared in the same manner as that described above for the
White Cat Study of Example III above.
The sheet of collagen hydrogel material likewise
had a thickness of about .5 millimeters to about l.0
millimeters. The dimensions of the artificial lens cut
from the sheet of material was approximately 6 millimeters
in length, approximately 3 millimeters in widtli1 and about
.5 millimeters to about l.0 millimeters in thickness. The
physical characteristics of the lens were that it had the
- . , - ~: ,
.
W092/1~20 PCT/US92/01233
3 26
appearance of a clear plastic sheet, was smooth,
transparent and optically clear.
(B) Method of surgery for affixina an artificial
lens fabricated from the collaaen hvdroqel
- 5 material to one eye of a black cat
The surgical procedure for affixing the
artificial lens fabricated from the above described
` collagen hydrogel material to the subject black cat was
substantially the same as that described hereinbefore for
the white cat in The White Cat as described in Example III
above. The initial step of removing the epithelium layer
from the cornea was performed by scraping off the
epithelial cells located in the center of the eye forming a
generally square area of about 12 millimeters by 8
millimeters. A rectangular shaped artificial lens was
prepared from the sheet of material and the dimensions of
the artificial lens was approximately 6 millimeters in
length, approximately 3 millimeters in width and about .5
millimeters to about l.0 millimeters in thickness.
The cornea was then prepared surgically, the
artificial lens was inserted under the corneal wing and
sutured in situ in substantially the same manner as
described above for the white cat in The White Cat Study of
Example III above. The subject black cat was then placed
in a cat pound for a period of about one month to permit
the lens to become implanted by the growth of epithelial
cells. After the expiration of about one month, the
subject cat was sacrificed and the one eye having the
artificial lens affixed thereto was surgically removed, as
a nucleared eye, for an eye histologic procedure.
(C) Results of eye_histoloaic procedure of an
artificial lens fabricated from the collaaen
hydroael material affixed to one eye of a
black cat
The one eye having the artificial lens affixed
thereto, that was surgically removed for the eye histologic
procedure, was placed into a standard foramalin solution
:,
.
WO92/1~20 ~ PCT/US92/0~233
27
and a standard pathological procedure for the fixation of
- the eye was performed in substantially the same manner as
described above for the white cat in The White Cat Study as
descibed above in Example III. The analysis disclosed that
the in the global area of the eye that the cornea was
curved, that the artificial cell was in place, that the
artificial lens w~s clear and that the cornea was clear.
The examination disclosed that the eye had been subject to
some trauma in that the third eyelid or nictating membrane
of the subject cat, which initially had been pulled over
,( the cornea a stitched in the closed position to prevent the
cat from subsequently traumatizing the eye, had been
ruptured and the third eyelid permitted the cornea to be
; exposed in a sufficient manner for the subject black cat to
i 15 traumatize the eye.
However, an examination of a tissue sample of the
cornea disclosed that the epithelial cells had grown over
and implanted the artificial lens. Also, it further noted
that the stroma had also regenerated and had grown over the
the edge of and became attached to the artificial lens.
The examination of the stroma further disclosed that the
eye had hematoxyalineosin present in the stroma and that
regeneration of the stroma had also occurred in the black
cat. An examination of the layers of the stroma disclosed
; 25 that new keratocytes, i.e. cells that produce collagen,
were present in the laminated layers of the stroma. As a
, result of the presence of the keratocytes, collagen fibial,
i.e. molecules produced by keratocytes, were laid down in
the laminated layers of the stroma. The results clearly
: 30 showed that: (a) epithelial cell growth occurred over and
implanted the artificial lens into the cornea; and (b) the
stroma was regenerated and the keratocytes were growing
; over and attaching to the edge of the artificial lens and
producing collagen fibial or collagen molecules.
EXAMPLE V
A collagen-hydrogel monomer viscous solution is
prepared as in EXAMPLE I except that an epithelial cell
growth enhancer is added to the collagen-hydrogel monomer
.: , . . , - - . - ~ . . ~, . . : -
' - ~ ''' ' ~''' : . ':, ~
- - - ~-
: '' ' '
~ W092/1~20 PCT/US92/01233
i ~U~113; '` 28 ~
viscous solution before heating and polymerization. The
amount of epithelial cell growth enhancer that could be
added by weight could be as low as a trace of epithelial
cell growth enhancer or could be in the order of the weight
of collagen as determined by the ratio by weight of the
collagen in the collagen-to-hydrogel weight or higher
depending on the material. The maximum amount of
epithelial cell growth enhancer that could be added is that
amount which would make the collagen-hydrigel material
cloudy and, thus impair the transparency of the collagen-
hydrogel material or optical lens made therefrom. This
would result in the epithelial cell growth enhancers being
interspersed throughout the polymereized material.
Examples of epithelial cell growth enhancers
are: (a) the epithelial growth factor molecule such as, for
example an epithelial growth factor molecule isolated by
Chiron Ophthalmics, Inc. and used for to produce a more
rapid resolution of corneal abrasion; or (b) Fibronectin, a
macromolecule which enhances epithelial cell growth.
EXAMPLE VI
A collagen-hydrogel monomer viscous solution is
prepared as in EXAMPLE I. If desired, an optical lens or
artificial lens can be fabricated from the collagen-
hydrogel material as described herein either before or
after treatment with an epithelial cell growth enhancers.
Either the collagen-hydrogel material, prior to fabrication
of an optical lens or artificial lens therefrom or the
optical lens or artificial lens fabricated from the
collagen-hydrogel material is soak in a solution containing
the epithelial cell growth enhancers in sufficient
concentration to cause the molecule of the epithelial cell
growth enhancers to permeate the outer layer of material
forming a thin layer of epithelial cell growth enhancer on
the exterior outer surface or to enable molecules of the
epithelial cell growth enhancers to attached to and be
supported by the outer surface of the collagen-hydrogel
material.
.
W092/]~20 PCT/US92/01233
~ 2~113 ~ :
EXAMPLE VI
The preferred embodiment of the collagen-hydrogel
material for practising this invention is to prepare the
collagen-hydrogel material as set forth in Example I
wherein the volumes of the components comprise:
1 millimeter of a stock solution of
collagen;
1 millimeter of HEMA;
1 millimeter of ethylenically unsaturated
monomeric units;
.1 millimeter of ammonium persulfate; and
.1 millimeter of metabisulfate
wherein the ammonium persulfate and metabisulfate are
catalysts.
(End of examples).
Based on the above examples, a calculation of the
weight of collagen in the collagen-hydrogel material has a
ratio by weight of collagen-to-hydrogel in the rane of
about .6-to-1000. This is the upper limit of the ratio by
weight in that a greater ratio by weight of collagen-to-
hydrogel may result in the material becoming cloudy thereby
rendering the material undesireable for fabricating an
artificial lens or optical lens therefrom. Thus, the
collagen-hydrogel material can have a ratio by weight which
is less than .6-tO-1000, but at a level wherein sufficient
collagen is present in weight to at least one of promote
epithelial cell growth and regeneration of the stroma to
30 produce keratocytes including collagen fibial growth. -
A person skilled-in-the-art could, likewise,
calculate the mole percent of each of the components in the
composition.
Collagen-hydrogel which contain HEMA alone, or
HEMA, ethylene glycol dimethacrylate and methymethacrylate,
and all combinations thereof, in strata, support various
other cell growth lines in tissue culture. Specifically,
W092/1~20 ~ ~ Q ~ 't l 3' '' ; '; ' ' PCT/US92/01233
the so formed collagen-hydrogels successfully supported
growth of the following cell lines:
(l) Rabbit smooth muscle cells;
(2~ Calf smooth muscle cell;
(3) Lung endothelial cells;
(4) Lung epithelial cell;
(5) Epithelial Cells; and
(6) Regeneration of the stroma to produce
keratocytes including collagen fibial
growth.
It is likely that the collagen-hydrogel disclosed
herein can serve as strata for growth of all cells of all
classes, epithelial, endothelial and mesothelial, which
appear to be compatible with cells of all tissues,
including corneal epithelial cells.
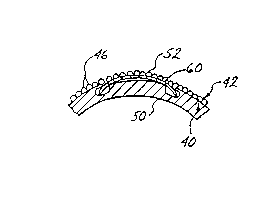
Fig. 2 illustrates pictorially the method of
positioning an artificial lens, fabricated from the
collagen-hydrogel as described above, and formed of a
predetermined geometrical shape and lenticular powe.r, such
as a contact lens, to the cornea. The eye, shown generally
as 40, has a corneal epithelium 42 formed of layers of
epithelial cells illustrated graphically as humps 46. Below
corneal epithelium 42 is Bowman's membrane 48, which
supports corneal epithelium. Below Bowman's membrane is
the corneal stroma 50. An artificial lens, such as for
example a contact lens having an optical portion, 60 is
positioned above corneal epithelium to illustrate the size
thereof.
Fig. 3 illustrates pictorially the preferred
location of the contact lens 60 in the eye after the
healing process. The contact lens 60 is located between
Bowman's membrane and corneal epithelium after new
epithelial cells 52 have grown during the healing process
to cover the anterior surface of the lens 60.
Fig. 4 illustrate that the contact lens is
positioned over the pupillary zone 62 of the eye and
implanted between Bowman's membrane 48 and corneal
epithelium 42.
WO92/1~20 X~ 3 PCT/US92/01233
31
Figs. 5 through 10 disclose a method for locating
on the cornea an optical lens, which may be an artificial
lens such as a contact lens, having a preselected geometric
shape and lenticular power wherein the optical lens
comprises an optical portion having an outer edge 66, an
anterior surface 70 and a posterior surface 72, the
elements 66, 70 and 72 being shown in Fig. 2. For purposes
of the steps illustrated in Figs 5 through 10, the
artificial lens has been fabricated from the collagen-
hydrogel, and the specific contact lens has been formed by(i) a contact lens mold, or (ii) frozen collagen-hydrogel
which has been lathed, so as to form a contact lens of a
predetermined shape and power. Prior to placement of the
lens on the cornea, the contact lens is sterilized by
exposure to ultraviolet light.
Fig, 5 illustrates the first step of the surgical
method, that step being the removing from Bowman's membrane
40, over the pupillary zone of the eye, a portion of
corneal epithelium on an area slightly greater than the
generalized shape of the optical lens, which area is
represented by area 76. This step is similar to the removal
of corneal epithelium from the anterior surface of the
cornea in the Epikeratophakia Procedure.
Thereafter, the next step is that of forming on
Bowman's membrane 40 a "V" shaped annular groove 78 having
a diameter substantially equal to the maximum geometrical
dimensions of the optical lens 60 and defining therearound
a peripheral edge 80 and a medial edge 82. The "V" shaped,
annular groove 78 has a preselected depth which is less
than the thickness of the corneal stroma 50. Typically,
the groove is formed to have a depth of about 0.3 mm, and
the depth is prepared in the cornea utilizing a 7mm
trephine.
Fig. 6 illustrates, by means of a front view, the
cornea showing the area 76 of corneal epithelium 46 that
has been removed from and to expose the area of Bowman's
membrane 40 from which corneal epithelium 46 has been
removed. Also, the annular shape of the "V" groove 78 is
illustrated.
,
- ~ . . : . . . ~ . -
W092/1~20 ; i PCT/US92/01233
~o~3 32 ~ !
~ Fig. 7 shows the next step of dissecting the
peripheral edge 80 of the groove 78 forming a wing 88 of
corneal tissue having a preselected length. This step is
performed by the surgeon in the following manner. As in an ~ -
Epikeratophakia Procedure, a corneal-spreading instrument
is used to dissect the peripheral edge 80 of the groove 78
forming a corneal wing, preferably of about l.5 mm in
length. The edge 66 of the optical lens 60 is to be
located under the corneal wing 88. The medial edge 82 is
cut free of the globe, ie. the curved surface of Bowman's
membrane 40.
Fig. 8 illustrates the next step of placing the
posterior surface 72 of the optical lens 60 on the anterior
surface of Bowman's membrane and positioning the outer edge
66 of the optical lens 60 under the corneal wing 88.
Fig. 9 illustrates the final position of the lens
60 over the pupillary zone of the eye before the optical
lens is attached to or affixed to the eye. The attachment
can be performed in any number of procedures, one of which
is illustrated in Fig. ll. Fig. 9 is then a representation
of the optical lens in the eye at the end of the surgical
procedure, and before the healing process. It is pointed
out that the anterior surface of the lens 60 is free of any
cell growth. As illustrated in Fig. 9, the edge 66 of the
lens 60 is positioned relative to and in contact with
corneal epithelium 42 and the corneal wing 88 lies flush
with the anterior surface of the optical lens 60.
Fig. lO is a representation of the condition of
the eye at the end of the healing process.
30As shown in Fig. lO, the edge 66 of the lens 60
is positioned so as to enable the epithelial cells to touch
and interact with the collagen-hydrogel lens 60 to promote
epithelial cell growth over a healing period. During the
healing period, new epithelial cells 52 grow over and
adhere to the anterior surface 70 of the optical lens 60,
implanting the same in the cornea under a new growth of
corneal epithelium 42 formed from several layers of new
epithelial cells.
' '' -'
' ~
.
W0 92/14420 210 ~ Pcr/uss2/nl233
Fig.11 illustrates one method of suturing a lens
to Bowman's membrane wherein the optical lens includes at
least two openings therein adjacent the outer edge thereof.
Such a lens is llustrated in Fig. 12 as lens 90 having
openings 92 and 94. As illustrated in Fig. 11, the lens 90
is affixed to Bowman's membrane by the step of suturing the
optical lens so to Bowman's membrane through the openings
92 and 94. The suture material is shown as a single loop
stitch 100 in Fig. 11.
In the alternative, the lens 60, illustrated in
Figs. 9 and 10, could be affixed to Bowman's membrane by
the step of bonding with a biodegradable adhesive the
posterior surface 72 of the optical lens to Bowman's
membrane 40.
The method of affixing the lens to Bowman's
membrane can be accomplished with either a removable or
biogradable suturing material, staples or the like. One
preferred method for insuring that the lens 90 does not
separate from Bowman's surface resulting in the edge 96
moving from under the corneal wing 88 is to utilize the
i~ step of suturing the optical lens 90 to Bowman's membrane
with a biodegradable suturing material in the form of a
running "shoe lace" stitching which passes through the
outer edge of the optical lens 90 and Bowman's membrane 40.
Fig. 13 illustrates in a front view, after
completion of locating the lens on the cornea of the eye
and before beginning the healing process, the relationship
of the eye 90 to the cornea wherein the lens 90, of Figs.
11 and 12, i5 sutured to Bowman's membrane through openings
92 and 94 of the lens 90.
Fig. 14 illustrates a possible lens configuration
for an artificial lens 110 having an optical portion 112
confiaured for placement over the pupillary zone of the eye
and on the central anterior surface of Bowman's membrane of
the cornea having corneal epithelium thereof removed. The
optical portion terminates in end tabs 114 and is formed
such that the optical portion is dimensioned to
substantially cover the total anterior surface of the
pupillary zone of an eye. The entire lens 110 including
' . . ~ , ., ~ '
- . :
W092/1~20 ~ Ll3 PCT/US92/0~233
34
the optical portion 112 and tabs 114 is formed of a
collagen-hydrogel for promoting epithelial cell growth.
Fig. 15 is a representation of a circular shaped
artificial lens 120 formed of the collagen-hydrogel for
promoting epithelial cell growth and having implanted
therein a ring 122 of material having different optical
properties than that of the collagen-hydrogel for promoting
epithelial cell growth used in the lens 120. The ring 122
functions to focus at the center thereof while the outer
edge of the ring 122 passes light to the retina. This
results in a differential passage of an image to the
retina. The lens 120 is of a size and shape to be
implanted on the cornea using the teachings of this
invention.
Fig. 16 is a representation of a circular shaped
optical portion 124 of an artificial lens formed from the
collagen-hydrogel for promoting epithelial cell growth
disclosed herein having tabs 126 extending therefrom which
may be used by a surgeon in implanting the artificial lens
in the eye using the teachings of this invention. The
optical portion 124 and the tabs 126 are formed of the
collagen-hydrogel.
Fig. 17 is a representation of a circular shaped
artificial lens having an optical portion 130 formed from
the collagen-hydrogel for promoting epithelial cell growth
disclosed herein and two aligned circular support members
132 extending in opposite directions from the optical
portion 130 which may be used by a surgeon in implanting
the artificial lens in the eye using the teachings of this
invention. The optical portion 130 and the tabs 132 are
formed of the collagen-hydrogel.
Fig. 18 is a representation of a circular shaped
artificial lens formed from the collagen-hydrogel for
promoting epithelial cell growth disclosed herein wherein
the optical portion 140 has three circular tabs or support
members 142 having apertures formed therein spaced
equidistantly around the periphery of an optical lens
portion 140. The support members 142 may be used by a
surgeon in implanting the artificial lens in the eye using
'
. :
.
.
WO92/14420 2 1 ~ PCT/US92/01233
~ 35
- the teachings of this invention. The optical portion 130
and the tabs 132 are formed of the collagen-hydrogel.
It is envisioned that the collagen-hydrogel of
the present invention, al~d artificial lens formed from the
collagen-hydrogel, can be used for epicorneal, corneal or
transcorneal lenses which are capable of promoting and
supporting epithelial cell growth during the healing
period. During the healing process, a bandage contact lens
may be placed on the eye until the anterior surface of the
lens is covered by corneal epithelium.
The collagen-hydrogel biomedical material
disclosed herein has, in its preferred embodiment,
application in the artificial lens field because of the
properties of the collagen-hydrogel promoting the growth of ~-
epithelial cells. It is envisioned that such collagen-
hydrogel could be used as substrata for support of growth
of other cells in the human body wherein the hydrogel could
be formed of any one of a number of monomers of the
hydrophilic class of polymers, and that other so formed
hydrogels when used in a collagen-hydrogel with appropriate
, macromolecules as described herein could be used to enable
the growth of other classes of human tissue other than
epithelial cells.
, ' ' ~ : ' -