Note: Descriptions are shown in the official language in which they were submitted.
210~62~o
PI/5-19237/A
Method of controllin~ insects
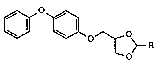
The invention relates to a method of controlling insects, in which compounds of the
fo~mula
[~ ~~[~ R
in which R is ethyl or propyl, are used,
characterised in that insects from the order Homoptera or from the order Lepidoptera are
controlled,
to the use of these compounds for ~is purpose, to insecticidal compositions for Ihis
purpose whose acdve ingredient is selected from these compounds, and to a proeess for
the preparation and the use of these compositions, with the proviso that (2R,4S)-2-ethyl-
4-(4-phenoxy)phenoxymethyl- 1 ,3-dioxolane in fully enantiomerically pure form and in
predominandy enantiomerically pure form is excepted from the scope of all subject
matters of the invention.
Within the scope of the present invention, the abovementioned (2R,4SJ enantiomer is "in
predominarltly enantiomerically pure form", in which case, inter alia, the abovementioned
proviso applies, when a m~xture of compounds of the formula I comprises at least 90%, in
particular at least 88%, more particularly at least 85%, preferably at least 80%, especially
at least 70%, most preferably more than 50%, of this enantiomer relative to the total
number of the molecules in this mixture.
US-4,097,581 proposes compounds of the formula
~ ~o~ XR' (II),
in which Rl is hydrogen, Cl-C7allcyl, C3-C6cycloaLlcyl, C2-C3aLlcenyl, C2-C3alkynyl,
C2-C4methoxyaLkyl, chloromethyl or benzyl and
R2 is hydrogen or Cl-C6aLlcyl or
. ~
.
';
;~
210462~
Rl and R2 together are the group -(CH2)n-, in which n is 4 or 5, or together with the carbon
atom to which they are bonded are the group of the formula
CH3
(III),
CH(cH3)2
as active ingredients in compositions for enhancing fruit abscission and as acdve
ingredients in pesticidal composidons, such as insecdcidal composidons, especially
composidons for controlling insects from the order Diptera and especially from the order
Coleoptera.
Unexpec~edly and, bearing in mind the above described disclosure of US4,097,581,entirely surprisingly, it has now been found that the compounds of the formula I are highly
suitable for controlling insects of certain families and genera from the order Homoptera
and of certain families from the order Lepidoptera, viz for controlling insects of the
families Aleyrodidae, Cicadellidae, Coccidae, Margarodidae and Psyllidae, all of which
belong to the order Homoptera,
insects of the genera Aonidiella, Aspidiotus, Aulacaspis, Chrysomphalus, Lepidosaphes,
Parlatoria, Pseudaulacaspis, Quadraspidiotus, Selenaspidus and Unaspis from the family
Diaspididae, which belongs to the order Homoptera,
insects of the genus Planococcus f~om the family Pseudococcidae, which belongs to the
order Homoptera, and
insects of the families Gracilariidae, Lyonedidae, Olethreuddae, Psychidae and
Tortricidae, all of which belong to the order Lepidoptera
The reason why this outstanding suitability of the compounds of the formula I for
controlling insects of certain families and genera from the order Homoptera and of certain
families from the order Lepidoptera is so surprising is that, although the compounds of the
fonnula I fall within the scope of the compounds of the formula II disclosed in
US-4,097,581 and are even disclosed specifically, in the form of diastereomer mixtures, in
US-4,097,58 1 in the table in columns 9 and 10, US-4,097,58 1 makes no mendon what-
soever of the outstanding acdvity of the compounds of the formula I according to the
present invendon, neither of a specifically marked acdvity of the compounds of the
formula II against the insects, mentioned according to the invention, of certain families
and genera from the order Homoptera and of certain families from the order Lepidoptera,
- ~ -
.. ....
, .
. ~
.
210~62~P
nor of a corresponding specifically marked activity of the present compounds of the
formula I, which can be regarded as a specific sub-group of the compounds of the formula
II which, however, is not disclosed in US-4,097,581.
Controlling insects of the type mentioned according to the invention is highly irnportant
for the user in the field of insect control, since, for exarnple, enormous economical losses,
~or example due to the damage caused by these insects on agricultural produce, occur if
these insects are not controlled in a targeted manner.
Preferred within the scope of the present invention is, on the one hand, a method of
controlling insects of the type mentioned according to the invention, in which compounds
of the formula I in which R is ethyl are employed.
Preferred within the scope of the present invention is, on the other hand, a method of
controlling
(1) insects of the family Coccidae,
in particular of the genera Ceroplastes, Pulvinaria and Saissetia,
preferably of the species Ceroplastes floridensis, Ceroplastes sinensis, Pulvinaria psidii
and Saissetia oleae,
in particular of the species Ceroplastes floridensis, Ceroplastes sinensis and Saissetia
oleae,
particularly of the species Ceroplastes floridensis and Saissetia oleae,
especially of ~e species Ceroplastes floridensis,
especially of the species Saissetia oleae;
(2) insects of the species Ceroplastes rubens from the genus Ceroplastes from the family
Coccidae;
(3) insects of the genera Aonidiella, Lepidosaphes, Parlatoria, Chrysomphalus,
Aulacaspis, Aspidiotus, Selenaspidus, Pseudaulacaspis, Quadraspidiotus and Unaspis from
the farnily Diaspididae,
preferably of the species Aonidiella aurantii, Lepidosaphes beckii, Lepidosaphes ulmi,
Parlatoria pergandei, Parlatoria b1anchardii, Parlatoria ziziphi, Chrysomphalus aonidum,
Aulacaspis tubercularis, Aspidiotus hederae, Selenaspidus articulatus, Pseudaulacaspis
pentagona, Quadraspidiotus perniciosus, Unaspis citri and Unaspis yanonensis,
210462~o
- 4 -
in particular of the species Aonidiella aurantii, Lepidosaphes beckii, Parlatoria pergandei,
Parlatoria blanchardii, Parlatoria ziziphi, Chrysomphalus aonidum, Aulacaspis
tubercularis, Selenaspidus articulatus, Pseudaulacaspis pentagona, Quadraspidiotus
perniciosus, Unaspis citri and Unaspis yanonensis,
more par~icularly of the species Aonidiella aurantii, Lepidosaphes beckii, Parlatoria
pergandei, Pseudaulacaspis pentagona, Quadraspidiotus perniciosus, Unaspis citri and
Unaspis yanonensis,
especially of the species Aonidiella aurantii and Lepidosaphes beckii,
very particularly of the species Aonidiella aurantii,
very particularly of the species Lepidosaphes beckii;
(4) insects of the family Margarodidae,
in particular of the genus Icerya,
preferably of the species Icerya purchasi;
(5) insects of the genus Planococcus from the family Pseudococcidae, of the genus Psylla
frorn the farnily Psyllidae and of the genera Trialeurodes and Bemisia from the farnily
Aleyrodidae,
preferably of the species Planococcus ficus, Planococcus c;tri, Psylla pyri, Psylla pyricola,
Trialeurodes vaporariorum and Bemisia tabaci,
especially of ~e species Planococcus ficus, Psylla pyricola, Trialeurodes vaporariorum
and Bemisia tabaci,
more especially of the species Psylla pyricola, Trialeurodes vaporariorum and Bemisia
tabaci,
in particular of the species Psylla pyricola;
(~) insects of the family Olethreutidae,
in particular of the genera Cydia and Laspeyresia,
preferably of the species Cydia pomonella, Laspeyresia molesta and Laspeyresia
funebrana,
in particular of the species Cydia pomonella and Laspeyresia molesta,
very particularly of the species Laspeyresia molesta,
very particularly of the species Cydia pomonella;
(7) insects of the genus Eupoecilia from the family Olethreutidae,
in particular of the species Eupoecilia ambiguella;
~' '
,:
210~2~
(8) insects of the family Tortricidae,
in particular of the genera Adoxophyes, Pandemis, Cacoecia and Eulia,
preferably of the species Adoxophyes reticulana, Pandemis heparana, Cacoecia costana,
Cacoecia pronubana and Eulia sphaleropa,
in particular of the species Adoxophyes reticulana, Pandemis heparana and Cacoecia
pronubana.
very particularly of the species Adoxophyes reticulana;
(9) insects of the family Lyonetiidae,
in particular of the genera Leucoptera and Lyonetia,
preferably of the species Leucoptera scitella and Lyonetia clerkella,
in particular of the species Leucoptera scitella;
(10) insects of the fanuly Gracilariidae,
in particular of the genus Lithocolletis,
preferably of the species Lithocolletis blancardella and Lithocolletis corylifoliella,
in par~cular of the species Lithocolletis blancardella
The compounds of the formula I which are used according to the invention are known and
are described, for example, in US-4,097,581.
The compounds of the formula I which are used according to the invention are valuable
active ingredients even at low rates of concentration when used preventively andlor
curatively in the field of insect control while being well tolerated by warm-blooded
species, fish and plants. The active ingredients which are used according to the invention
are active against all or individual development stages of normally sensitive, but also of
resistant, insects of the abovementioned type. The insecticidal activity of the active
ingredients which are used according to the invention can become apparent directly, i.e.
by a destruction of the insects, which happens immediately or only after some time has
elapsed, for example during molting, or indirectly, for example by reduced oviposition
and/or hatching rate, the good activity corresponding to a mortality of at least 50 to 60%.
The active ingredients which are used according to the invention can be used forcontrolling, i.e. containing or destroying, pests of the abovementioned type which can be
found in particular on plants, especially on useful plants and ornamentals in agriculture,
210462~
horticulture and in forests, or on parts of such plants, such as fruit, flowers, foliage, staLks,
tubers or roots, and in some cases the protection against these pests even extends to paTts
of the plants which are formed at a later point in time.
Suitable target crops are, in particular, crops of pome fruit, for example apples or pears,
stone fruit, for example peaches, citrus fMit, for example lemons, oranges or grapefruits,
vegetables, for example potatoes, beans, tomatoes or cucumbers, pepper, olives, mangoes,
grapes, ornamentals, nuts, guavas, tea or avocados,
especially pome fruit, stone fruit, citrus fruit, vegetables, olives, mangoes, grapes,
ornamentals or nuts,
preferably pome fruit, stone fruit or citrus fruit.
Other fields of application for the acdve ingredients which are used according to the
invendon are the protecdon of stored p~oducts or stores, the protection of material and, in
the hygiene sector, in particular the protection of domestic animals or productive livestock
against pests of the abovementioned type.
The invendon therefore also relates to insecticidal composidons for use against pests of
the abovementioned type, such as emulsifiable concentrates, suspension concentrates,
ready-to-spray or ready-t~dilute solutdons, spreadable pastes, dilute emulsions, wettable
powders, soluble powders, dispersible powders, dusts, granules or encapsulations in
polymeric substances, all of which comprise - at least - one of the acdve ingredients which
are used according to the invention and which are to be selected depending on the
intended aims and the prevailing circumstances.
In these composidons, the active ingredient is employed as pure acdve ingredient, for
example a solid active ingredient in a specific particle size, or, preferably, together with -
at least - one of the auxiliaries conventdonally used in the art of formulatdons, such as ex-
tenders, for example solvents or solid carriers, or surface-acdve compounds (su~factants).
The following are examples of suitable solvents: partially hydrogenated or unhydrogena-
ted aromatic hydrocarbons, preferably ~e fracdons C8 to Cl2 of aL~cylbenzenes, such as
xylene mixtures, aLlcylated naphthalenes or tetrahydronaphthalene, aliphatic or cycloali-
phatic hydrocarbons, such as paraffins or cyclohexane, alcohols, such as ethanol, propanol
or butanol, glycols and their ethers and esters, such as propylene glycol, dipropylene
glycol ether, ethylene glycol, ethylene glycol monomethyl ether or ethylene glycol
,
210~62~
monoethyl ether, ketones, such as cyclohexanone, isophorone or diacetone alcohol,
strongly polar solvents, such as N-methylpyrrolid-2-one, dimethyl sulfoxide or
N,N-dimethylformamide, water, epoxidized or unepoxidized vegetable oils, such as epoxi-
dized or unepoxidized rapeseed oil, castor oil, coconut oil or soya oil, and silicone oils.
Solid carriers which are used, for example for dusts and dispersible powders, are, as a rule,
ground natural minerals, such as calcite, talc, kaolin, montmorillonite or attapulgite. To
improve the physical properties, it is also possible to add highly-disperse silicas or
highly-disperse sorptive polymers. Possible particulate, adsorptive carriers for granules are
either porous types, such as pumice, brick grit, sepiolite or bentonite, and possible
non-sorptive canier materials are calcite or sand. Moreover, a large number of granulated
materials of inorganic or organic nature, in particular dolomite or comminuted plant
residues, can be used.
Suitable surface-active compounds are, depending on the nature of the active ingredient to
be formulated, non-ionic, cationic and/or anionic surfactants or surfactant mixtures which
have good emulsifying, dispersing and wetdng properties. The surfactants listed below are
only given by way of example; a large number of surfactants which are conventionally
used in the art of formuladon and suitable according to the invention are described in the
specialist literature.
Suitable non-ionic surfactants are mainly polyglycol ether derivatives or aliphatic or
cycloaliphatic alcohols, saturated or unsaturated fatty acids and aLI~ylphenols, which can
have 3 to 30 glycol ether groups and 8 to 20 carbon atoms in the ~aliphadc) hydrocarbon
radical and 6 to 18 carbon atoms in the alkyl radical of the alkylphenols. Other suitable
non-ionic surfactants are water-soluble polyethylene oxide adducts with polypropylene
glycol, ethylene diaminopolypropylene glycol and aL~yl polypropylene glycol which have
1 to 10 carbon atoms in the aLkyl chain and 20 to 250 ethylene glycol ether groups and 10
to 100 propylene glycol ether groups. The abovementioned compounds customarily have 1
to S ethylene glycol units per propylene glycol unit. Examples which may be mentioned
are nonylphenolpolyethoxyethanols, castor oil polyglycol ethers, polypropylene/-polyethylene oxide adducts, tributylphenoxypolyethoxyethanol, polyethylene glycol and
octylphenoxypolyethoxyethanol. Other substances which are suitable are fatty acid esters
of polyoxyethylene sorbitan, such as polyoxyethylene sorbitan trioleate.
The cationic surfactants are mainly quaternary ammonium salts which have, as
,
210~62~
substituents, at least one aL~cyl radical having 8 to 22 C atoms and, as further substituents,
lower halogenated or unhalogenated aL~yl, benzyl or lower hydroxyalkyl radicals. The
salts are preferably in the form of halides, methylsulfates or ethylsulfates. Examples are
stearyltrimethylammonium chloride and benzyldi(2-chloroethyl)ethylammonium brornide.
Suitable anionic surfactants can be either so-called water-soluble soaps or water-soluble
synthetic surface-active compounds. Soaps which are suitable are the aL~cali metal salts,
aL~caline earth metal salts or substituted or unsubstituted ammonium salts of higher fatty
acids (C10-C22), such as the sodium salts or potassium salts of oleic or stearic acid, or of
natural rnixtures of fatty acids which can be obtained from, for example, coconut oil or tall
oil; mention must also be made of the fatty acid methyltaurinates. However, so-called
synthetic surfactants are used more frequently, in particular fatty sulfonates, fatty sulfates,
sulfonated benzimidazole derivatives or alkylarylsulfonates. The fatty sulfonates and fatty
sulfates are, as a rule, in the forrn of aL~ali metal salts, aL~caline earth metal salts or
substituted or unsubstituted ammonium salts and have in general an alkyl radical having 8
to 22 carbon atoms, aL~cyl also including the alkyl moiety of acyl radicals; examples which
may be mentioned are the sodium salt or calcium salt of ligninsulfonic acid, of the
dodecylsulfuric ester or of a fatty alcohol sulfate mixture prepared with natural fatty acids.
This group also includes the salts of the sulfuric esters and sulfonic acids of fatty
alcohoVethylene oxide adducts. The sulfonated benzimidazole derivatives have preferably
2 sulfonyl groups and a fatty acid radical having approxirnately 8 to 22 C atoms.
Alkylarylsulfonates are, for example, the sodium salts, calcium salts or
triethanolarnmonium salts of dodecylbenzenesulfonic acid, of dibutylnaphthalenesulfonic
acid or of a naphthalenesulfonic acid/formaldehyde condensation product. Suitable
phosphates, for example salts of the phosphoric ester of a p-nonylphenol/(4-14)ethylene
oxide adduct, or phospholipids, are also suitable.
As a rule, the compositions comprise 0.1 to 99%, in particular 0.1 to 95%, of active
ingredient and 1 to 99.9%, in particular 5 to 99.9%, of at least one solid or liquid auxiliary,
it being possible as a rule, for 0 to 25%, in particular 0.1 to 20%, of the compositions to be
surfactants (% in each case meaning percent by weight). While concentrated compositions
are more preferred as commercially available goods, the end consumer uses, as a rule,
dilute compositions whose active substance concentrations are considerably lower.
Preferred compositions are in particular composed as follows (% = percent by weight):
2104628
g
Emulsifiiable concentrates:
Active ingredient: 1 tO 90%, preferably S to 20%
Surfactant: 1 to 30%, preferably 10 to 20 %
Solvent: 5 to 98%, preferably 70 to 85 %
Dusts:
Active ingredient: 0.1 to 10%, preferably 0.1 to 1%
Solid canier: 99.9 to 90%, preferably 99.9 to 99%
Suspension concentrates:
Active ingredient: 5 to 75%, preferably 10 to 50%
Water: 94 to 24%, preferably 88 to 30%
Surfactant: 1 to 40%, preferably 2 to 30%
Wettable powders:
Active ingredient: 0.5 to 90%, preferably 1 to 80%
Surfactant: 0.5 to 20%, preferably 1 to 15%
Solid carrier: S to 99%, preferably 15 to 98%
Granules:
Active ingredient: 0.5 to 30%, preferably 3 to 15%
Solid carrier: 99.5 to 70%, preferably 97 to 85%
By adding other insecticidal active ingredients, the spectrum of action of the compositions
according to the invention can be broadened considerably and adapted to prevailing
circumstances. Suitable active ingredients which can be added are, for example,
representatives of the following active ingredient classes: organophosphorus compounds,
nitrophenols and derivatives, formamidines, ureas, carbamates, pyrethroids, chlorinated
hydrocarbons and Bacillus thuringiensis preparations. The compositions according to the
invention can also comprise other solid or liquid auxiliaries, such as stabiliærs, for
example epoxidiæd or unepoxidized vegetable oils (for example epoxidiæd coconut oil,
rapeseed oil or soya oil), antifoams, for example silicone oil, preservatives, viscosity
regulators, binders and/or tackifiers, and fertiliærs or other active ingIedients for
aehieving specific effects, for example bactericides, fungicides, nematicides,
molluscicides or selective herbicides.
, . .
. .
;
~. , ;
2104628
- 10-
The compositions according to the invention are prepared in a known manner, for example
in the absence of auxiliaries, grinding, screening and/or compressing of a solid active in-
gredient or active ingredient mixture, for example to give a specific particle size, and in
the presence of at least one auxiliary for example by intimately mixing and/or grinding the
active ingredient or active ingredient rnixture with the additive(s). The invention also re-
lates to these processes for the preparation of the compositions according to the invention
and to the use of the compounds of the formula I for the preparation of these compositions.
The invention also relates to the methods of application for the compositions, that is to say
the methods of controlling pests of the abovementioned type, such as spraying, atomizing,
dusting, painting on, dressing, scattering or pouring, which are to be selected depending on
the intended aims and the prevailing circumstances, and to the use of the compositions for
the control of pests of the abovementioned type. Typical rates of concentration are
between 0.1 and 1000 ppm, preferably between 0.1 and 500 ppm, of active ingredient.
Spray mixtures are employed, in particular, at active ingredient concentrations of 50, 100,
150 or 200 ppm. The rates of application per hectare are generally 1 to 2000 g of acdve
ingredient per hectare, in particular 10 to 1000 g/ha, preferably 20 to 600 g/ha. Rates of
application of 300, 400 or 450 g of active ingredient per hectare are preferred. Rates of
application of 0.25, 0.75, 1.0 to 2.0 g of active ingredient per tree are preferred.
A preferred me~od of application in the field of crop protection is application to the
foliage of the plant (folia application), where frequency and rate of application can be
adapted to suit the danger of infestation with the pest in question. However, the active
ingredient can also reach the plants through the root system (systerr;ic action) by
drenching the locus of the plants with a liquid composition or incorporating the active
ingredient in solid form into the locus of the plants, for example into the soil, for example
in the form of granules (soil application).
The examples which follow are intended to illustrate the invention. They do not restrict
the invention. Temperatures are given in degrees centigrade.
Formulation Examples (% = percent by weight)
Example Fl: Emulsion concentrates a) b) c)
Activeingredient 25 % 40% 50%
Calcium dodecylbenænesulfonate 5 % 8 % 6 %
210462~
11 -
Castor oil polyethylene glycol ether
(36 mol of EO) 5 %
Tributylphenol polyethylene glycol ether
(30 mol of EO) -12 % 4 %
Cyclohexanone -15 % 20 %
Xylenemixture 65 % 25 % 20%
Emulsions of any desired concentration can be prepared with such concenlrates by dilution
vith water.
Example F2: Solutions a) b)c) d)
Active ingredient 80 % 10 %5 % 95 %
Ethylene glycol monomethyl
ether 20 %
Polyethylene glycol MW 400 - 70 %
N-Methyl-2-pyrrolidone - 20 %
Epoxidized coconut oil - - 1% 5 %
Petroleum ether (boiling range
160-190) - - 94 %
The solutions ale suitable for use in the form of microdrops.
Exam~le F3: Granules a) b)c) d)
Active ingredient 5 % 10 %8 % 21 %
Kaolin 94 % - 79 % 54 %
Highly-disperse silica 1 % - 13 % 7 %
Attapulgite - 90 % - 18 %
The active ingredient is dissolved in dichloromethane, the solution is sprayed onto the
carrier, and the solvent is subsequently evaporated in vacuo.
ExarnPle F4: Dusts a) b)
Active ingredient 2 % 5 %
Highly-disperse silica 1% 5 %
Talc 97 %
Kaolin - 90 %
: . ~
,, ~. ..
210~62~;
Ready-tc~use dusts are obtained by intimately mixing the carriers with the active
ingredient.
Example F5: Wettable powders a) b)c~
Activeingredient 25 % 50%75 %
Sodium ligninsulfonate 5 % 5 %
Sodium lauryl sulfate 3 % - 5 %
Sodium diisobutylnaphthalene-
sulfonate - 6 %10 %
Octylphenol polyethylene glycol-
ether (7-8 mol of EO) - 2 %
Highly-disperse silica 5 % 10 %10 %
Kaolin 62% 27 %
The active ingredient is mixed with the additives and the mixture is ground thoroughly in
a suitable mill. This gives wettable powders which can be diluted with water to give
suspensions of any desired concentration.
Exarnple F6: Emulsion concentrate
Active ingredient 10 %
Octylphenol polyethylene glycoleether
(4-5 mol of EO) 3 %
Calcium dodc,cylbenænesulfonate 3 %
Castor oil polyglycol ether
(36 mol of EO) 4 %
Cyclohexanone 30 %
Xylene mixture 50 %
Emulsions of any desired concentradon can be prepared from this concentrate by dilution
with water.
ExampleF Dusts a)b)
Acdve ingredient 5 %8 %
Talc 95 %
Kaolin - 92 %
:'' ' -'
.
..
21~4628
Ready-t~use dusts are obtained by mixing the active ingredient with the carrier and
grinding the mixture on a suitable mill.
Example F8: Extruder granules
Active ingredient 10 %
Sodium ligninsulfonate 2 ~o
Carboxymethylcellulose 1 %
Kaolin 87 %
The active ingredient is rnixed with the addidves, and the rnixture is ground and moistened
with water. This mixture is extruded and granulated, and the granules are subsequently
dried in a stream of air.
Example F9: Coated ~eranules
Active ingredient 3 %
Polyethylene glycol (MW 200) 3 %
Kaolin 94 %
In a rnixer, the finely ground active ingredient is applied uniformly to the kaolin which has
been moistened with polyethylene glycol. Dust-free coated granules are thus obtained.
Example F10: Suspension concentrate
Active ingredient 40 %
Ethylene glycol 10 %
Nonylphenol polyethylene glycol ether
(15 mol of EO) 6 %
Sodium ligninsulfonate 10 %
Carboxymethylcellulose 1 %
37% aqueous formaldehyde solution 0.2 %
Silicone oil in the form of a 75%
aqueous emulsion 0.8 %
Water 32 %
The finely ground active ingredient is mixed intimately with the additives. This gives a
suspension concentrate from which suspensions of any desired concen~ation can beprepared by dilution with water.
.
2104628
14 21~89-8738
B oaical Examples (percentages aré by weight, unless stated
otherwise)
The active ingredient is used in each case in three different
isomer ratios A, B and C:
A = 29% 2R,4S-isomer, 29% 2S,4R-isomer, 21~ 2S,4S-isomer and
21% 2R,4R-isomer
B = 28% 2R,4S-isomer, 28% 2S,4R-isomer, 22% 2S,4S-isomer and
22~ 2R,4R-isomer
C = 27.5% 2R,4S-isomer, 27.5% 2S,4R-isomer, 22.5% 2S,4S-
isomer and 22.5~ 2R,4R-isomer
Example Bl: Activity against Adoxophyes reticulana ~ovicidal)
Adoxophyes reticulana eggs which have been deposited on filter
paper are briefly immersed into a test solution of 400 ppm of
active ingredient in acetone/water. After the test solution has
dried on, the eggs are incubated in Petri dishes. After 6 days,
the percentage hatching rate of the eggs is evaluated by
comparison with untreated control batches (% reduction in hatching
rate).
In this test, compounds of the formula I are very effective. In
particular the compound of the formula I in which R is ethyl has
an activity of over 80%.
Exam~le B2: Activity aaainst Aonidiella aurantii
Potato tubers are populated with Aonidiella aurantii crawlers.
After about 2 weeks, the potatoes are immersed into an aqueous
emulsion spray mixture or suspension spray mixture which comprises
400 ppm of active ingredient. After the tubers have dried, they
. ., -
, .
,
21046~
14a 21489-8738
are incubated in a plastic container~ To evaluate the experiment
after 10 to 12 weeks, the survival rate of the crawlers of the
first subsequent generation of the treated population is compared
with that of untreated control batches.
In this test, compounds of the formula I are very effective. In
particular the compound of the formula I in which R is ethyl has
an activity of over 80%.
Example B3: Activity aqainst Aonidiella aurantii
Citrus trifoliata cuttings are populated with Aonidiella aurantii
crawlers. After about 2 weeks, the cuttings are sprayed to drip
point with an aqueous emulsion spray mixture comprising 50 ppm oE
active ingredient. To evaluate the experiment after 10 to 12
weeks, the survival rate of the crawlers of the first subsequent
generation of the treated population is compared with that of
untreated control batches.
In this test, compounds of the formula I are very effective. In
particular the compound of the formula I in which R is ethyl has
an activity of over 80%.
Exam~le B4: Activity aqainst Bemisia tabaci
Dwarf bean plants are placed into gauze cages and populated with
Bemisia tabaci adults. After oviposition, all adults are removed.
10 days later, the plants and the nymphs thereon are sprayed with
an aqueous emulsion spray mixture comprising 400 ppm of active
ingredient. After a further 14 days, the percentage hatching rate
of the eggs is evaluated by comparison with untreated control
batches.
,,.: :
...
-' . :~,' .-. ,:
210~62~ `
In this test, compounds of the formula I are very effective. In particular the compound of
the formula I in which R is ethyl has an activity of over 80%~
Example B5: Activitv a ainst Bemisia tabaci
Dwarf bean plants are placed into gauze cages and populated widh Bemisia tabaci adults.
After oviposition, all adults are removed. 2 days later, the plants and the nymphs dhereon
are sprayed widh an aqueous emulsion spray mixture comprising 400 ppm of active
ingredient. After a further 10 days, dle percentage hatching rate of the eggs is evaluated by
comparison with untreated control batches.
In dhis test, compounds of the formula I are very effective. In particular dhe compound of
dhe formula I in which R is ethyl has an activity of over 80%.
Exarnple B6: Activitv a~ainst Cvdia pomonella (ovicidal)
Cydia pomonella eggs which have been deposited on filter paper are briefly immersed into
a test solution of 400 ppm of active ingredient in acetone/water. After dhe test solution has
dried on, dhe eggs are incubated in Petri dishes. After 6 days, the percentage hatching rate
of dhe eggs is evaluated by comparison with untreated control batches (% reduction in
hatching rate).
In dhis test, compounds of the formula I are very effective. In particular the compound of
the formula I in which R is ethyl has an activity of over 80%.