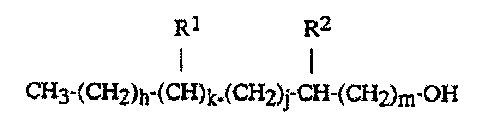Note: Descriptions are shown in the official language in which they were submitted.
2~.05t192
1::.,;.v0 92/15747 ~ P~.'TJUS92/01~91
Back rg~o and of the Invention
Field of the Invention
s o The present invention relates to highly processable aromatic
polyamide fibers, their production and use.
Description of the Prior A,rt
Research Disclosure, .Iuly 1980, No. 195, disclosure 19520, discloses
finishes useful for tieating industrial ~bers,such as polyamide and aramid
fibers,
which finishes include a lubricant, comprising esters composed of an
aliphatic,
saturated carboxylic acid and a polyhydric or aliphatic unbranched alcohol.
These
finishes also contain an emulsifier or emulsifying system, an anti-oxidant to
increase
the stability of the composition, polysiloxanes as a further thermostable
lubricant,
2 o and a sulfonated natural oil as an antistatic agent. Furthermore, these
finishes may
contain biostats, further emulsifiers, and lubricants.
However, the finishes according to the above reference are not
suitable for the purposes of the present invention in terms of surface
frictional
properties, scourability, the protection from depositing due to abrasion,
fibrillation
2 5 and antistatic properties of the resulting treated fibers.
High strength, high modulus fibers, have been proposed to reinforce
elastomeric and plastic materials.
Summary of the Invensi_on
3 o Most commercial fibers have a high rigidity, poor surface functional
characteristics leading to fibrillation, mainly caused by excessive friction
amonb
filaments, and poor surface affinity to most traditional elastomeric,
thermoplastic
and thermoset matrices which they reinforce.
These drawbacks and defects, which result in a degradation of
3 5 physical properties such as strength and modulus, have been driving a high
demand
~IU~UJ~
WO 92/15747 2 PC'T/U592/018~. ,.,~
for highly processable fibers which have to be easy to process through
knitting or
weaving operations a_nd do not lead to machine deposits.
According to this invention, the application of a particular
combination of surface treatment agents on the surface of previously dried
aramid
fibers using a finishing process already known; or the application of those
agents on
never-drawn never-dried aramid fibers yields a new surface treated fiber which
exhibits excellent processability characteristics in its use as a reinforcing
element for
rubber applications or as a yarn for fabric woven structures. The end use
performance of final products, using the fibers, is consequently significantly
improved.
The present invention accordingly relates to highly processable
aramid fibers of high modulus, improved surface frictional properties,
improved
scourability, low abrasion polymer depositing, low fibrillation and improved
longterm antistatic properties, having a coating of a lubricant, an
emulsifying system,
an antistatic agent, and other components, derived from a surface treatment
agent
which consists of
(a) 30 to 70% by weight of an esteroil lubricant, consisting of an ester,
composed of
(I) an alcohol component which is a branched, primary or secondary,
saturated monohydric alcohol of the general formula
R1 R2
CH3-(CHZ)h-(CH)k-(CH2)j-CH-(CH2)m-OH
z 5 wherein
R1 represents Cl-C16-alkyl,
R2 represents H, C1-C16-alkyl" if k=1 and
R2 represents Cl-C16-alkyl, if k=0,
h=OtoS
3o k=0or1
j = Oto4
m =0 to 16
and wherein the total number of carbon atoms
is below 25, and
3 ~ (II) a carboxylic acid component which is an
2i0~~~9:2,
'~".O 92!15747 ' 3 PCf/US92/0189I
unsaturated fatty acid of the general formula
R3-(COOH)s; s = 1-G
wherein
R3 represents C4-Clg-alkenyl, C4-Clg-alka_
dienyl, C4-C19-alkatrienyl, phenyl, naphthyl,
2-phenylethenyl, or which is an unsaturated
dicarboxylic acid of the general formula
HOOC-(CH = CH)n-COON
wherein n = 1 or 2,
1 o and said ester has a solidification point of
below +5°C, preferably below 0°C, a Idnematic
viscosity of 100 to 350 mm2/s (at 20°C) and an
iodine value between 30 and 140, preferably
between 30 and 80,
(b) 20 to 50% by weight of an emulsifying system which consists of
unsaturated ethoxylated fatty acids and/or unsaturated ethoxylated fatty
alcohols
and/or ethoxylated alkylamines of the general formula
R4-X-(EO)p(PO)q-OH
wherein
2 o R4 represents CS-C20-alkenyl, phenyl,
naphthyl, or Cg- or Cg-alkylphenyl,
X represents -COO-, -NH- or -O-,
EO represents an ethylene oxide unit,
PO represents a propylene oxide unit,
p =2to15and
q =OtolO,
(c) S to 15% by weight of an antistatic agent, consisting of alkali salts of
C4-C12-
alkyl sulfonates, C4-C12-alkyl phosphates or C4-C20-alkyl carboxylic acids,
(d) 0.2 to 2% by weight of a corrosion-inhibitor, and
3 0 (e) optionally additives,
and whereby the amount of said coating on said fibers is 0.05 to 2.0% by
weight,
preferably 0.2 to 1.0% by weight, of the fiber.
The coating preferably consists of
50 to 60% by weight, most preferably SS to 60% by weight of the esteroil (a),
.. v~~U~U9~
wo 9z~ls~a~ 4 PCTlU592/018. ,.."
f
25 to 40% by weight, most preferably 29 to 35% by weight of the emulsifying
system
(b), S to 10% by weight, most preferably 5 to 7% by weight of the antistatic
agent
(c), 0.3 to 1% by weight, moss preferably 0.3 to 0.5% by weight of the
corrosion
inhibitor (d) and, if desired, optionally additives (e).
The aramid fibers of this invention are further characterized by a
specific breaking strength of 2.65 to 33.5 cN/dtex (3 to 38 g/den), a specific
modulus
off 8.83 to 2207 cN/dtex (10 to 2500 g/den), a fiber to metal dynamic friction
coefficient on a 1100 dtex aramid yarn of lower than 0.55, preferably below
0.50 at
200 m/min, a fiber to metal boundary friction coefficient on a 1100 dtex
aramid yarn
l o of lower than 0.10, preferably below 0.05 at 0.016 cm/s, an amount of
deposit due to
abrasion of lower than 0.5 mg/kg of yarn, a residual finish level of lower
than 15%
by weight of the initial finish level after washing.
The fiber of this invention provides an improved blend of properties
in terms of fume emission measured by weight losses, washability of the fiber
and
cohesiveness of the fiber compared with fibers using other finishes.
Within the scope of this invention, by "fibers" are meant continuous
filaments as well as a single yarn or cord, staple fibers, fiber tows (for
example from
stretch breaking processes), yarns or flat textile skeins, staple crimped
fibers, pulps,
industrial woven, twisted, knitted, braided, spiralled or wrapped textiles
from
2 o aramids with a fiber type structure.
Aramids are such polymers that are partially, preponderantly or
exclusively composed of aromatic rings, which are connected through carbamide
bridges or optionally, in addition also through other bridging structures.
T'he
structure of such aromatic polyamides can be elucidated by the following
general
2 5 formula of repeating units:
(-CO-NH~Al-NH-CO-A2-CO-)n
wherein A1 and A2 are the same or different and signify aromatic and/or
polyaromatic and/or heteroaromatic rings, that can also be substituted.
Typically
A1 and A2 may, independently from each other, be selected from 1,4-phenylene,
3 0 1,3-phenylene, 1,2-phenylene, 4,4'-biphenylene, 2,6-naphthylene, 1,5-
naphthylene,
1,4-naphthylene, phenoxyphenyl-4,4'-diylene, phenoxyphenyl-3,4'-diylene, 2,5-
pyridylene and 2,6-quinolylene which may or may not be substituted by one or
more
substituents which may comprise haloben, C1-C4-alkyl, phenyl, carboalkoayl, C1-
C4-
alkoxyl, acr,~loxy, nitro, dialkylamino, thioalkyl, carboxyl and sulfonyl. The
2~.U5092
~,'...~!O 92/15747 J PCT/U592/01891
-CONH-group may also be replaced by a carbonyl-hydrazide (-CONHNH-) group,
azo-or azoxy-group.
Fibers derived from wholly aromatic polyamides are preferred.
Examples of aramids are poly-m-phenylene-isophthalamide and poly-p-phenylene-
terephthalamide.
Especially suitable are poly-m-phenylene-
isophthalamide fibers according to US 3,287,324 and poly-p-phenylene-
terephthalamide fibers according to US 3,869,429 and DE 22 19 703.
Additives can be used with the aramid and, in fact, it has been found
1 o that up to as much as 10 percent by weight of other polymeric material can
be
blended with the aramid or that copolymers can be used having as much as 10
percent by weight of other diamine substituted for the diamine of the aramid
or as
much as 10 percent by weight of other diacid chloride substituted for the
diacid
chloride of the aramid.
Additional suitable aromatic polyamides are of the following structure
(-NH-Ar 1-X-Ar2-NH-CO-Ar I-X-Ar2-CO-)n
in which
X represents O, S, S02, NR, N2, CR2, CO,
R represents H, CI-C4-alkyl, and
2 o Arl and Ar2 which may be same or different are selected from 1,2-
phenylene, 1,3-
phenylene and 1,4-phenylene and in which at least one hydrogen atom may be
substituted with halogen and/or Cl-C4-alkyl.
The finish formulation of this invention comprises a lubricant, an
emulsifying system, an antistatic agent and a corrosion inhibitor, and if
desired,
2 5 water and/ar other additives.
The lubricant is an esteroil which is characterized as stated above.
Examples for the alcohol compound (I) of the ester can be 2-methyl-1-propanol,
2-
butanol, 2-pentanol, 2-methyl-I-butanol, 3-methyl-3-1-butanol, 3-methyl-2-
butanol,
2-methyl-I-pentanol, 4-methyl-1-pentanol, 4-methyl-2-pentanol, 2.pentanol, 3-
3 o heptanol, 2-octanol, 2-ethyl-1-hexanol, 3,5-dimethyl-1-hexanol, 5-nonanol,
2-6-
dimethyl-4-heptanol, iso-hexadecyl alcohol or iso-tridecyl alcohol. Examples
for the
carboxylic acid component (II) can be lauroleic acid, myristoleic acid,
palmitoleic
acid, oleic acid, gadoleic acid, erucic acid, ricinoleic acid, tallow acid,
linoleic acid.
linolenic acid, fumaric acid, malefic acid, cinnamic acid, naphthaline
carboxylic acid.
3 ~ benzoic acid, terephthalic acid, isophthalic acid, trimellitic acid or
pyromellitic acid.
21.050J2
WU 92115747 6 PCT/US92/Ol$~,
The kinematic viscosity of the esteroil preferably is in the range of 200
to 300 mm2/s (20°C).
The emulsifying system of this invention is as defined above.
Examples of unsaturated fatty acids are lauroleic acid, myri.stoleic acid,
palznitoleic
acid, gadoleic acid, erucic acid or ricinoleic acid, preferably oleic acid
(with 3-15
moles ethylene oxide). Examples of unsaturated fatty alcohol are elaidyl
alcohol,
erucyl alcohol, brassidyl alcohol, preferably oleyl alcohol and/or tallow
alcohol
(with 3-10 moles of EO). Further examples are Cg- or Cg-
alkylphenolethoxylates,
preferably octylphenol-or nonylphenolethoxylates (5-5 moles of EO).
t o The antistatic compounds are alkali salts, preferably sodium salts of
alkyl sulfonates (e.g. lauryl or oleyl sulfonate), alkyl phosphates like C4-
C12-alkyl
phosphates (mono/diester mixture) and salts of fatty acids, e.g. oleic acid.
The
sodium chloride content should be below 0.1%. It is also possible to use
alkylsulfates, however, they are not preferred because they hydrolyze easily
and
therefore loose their antistatic efficiency.
Useful corrosion inhibitors are diethanolamine salts of C4-C12
alkylphosphate-esters (mono/di) or amine salts of fatty acids or benzoic acid.
The formulation may optionally contain water for stabilization
reasons even before it is diluted with water in order to obtain the
concentration at
z o which it is applied to the fibers.
Additives can optionally be incorporated in the formulation if specific
properties or process conditions are required, for example adhesion, specific
cross-
linkage, UV-protection, antioxidation, pigmentation or rheological adjustment.
These additives may further comprise fungicides, bacteriocides and biocides.
2 5 A formulation for the treatment of aramid fibers can be prepared by
mixing all components at an elevated temperature, preferably at a temperature
between 30° and 40°C, in order to obtain a homogeneous and clear
oil. Thus, for
example, a mixture consisting of 550 g of isobutyl oleate, 350 g of
emulsifying system
which consists of 200 g nonylphenol ethoxylate (8 EO) and 150 g of oleic acid
3 o ethoxylate (I0 EO), 70 g of sodium decylsulfonate and 5 g of the
diethanolamine
salt of benzoic acid can be prepared. If necessary, about 25 g of water is
added to
the mixture to eliminate any turbidity. The addition of water may also be
necessan~
to obtain a stable clear oil. If required, the pH value can be adjusted to be
within 6
and 8, preferably 7, using diethanolamine or acetic acid.
2105092 ,
~''!O 92/15747 7 PCT/US92/01891
(,",.~ ,
The finish formulation of this invention is further characterized by a
viscosity of 150 to 500 mm2/s, preferably of 150 to 300 mm2/s (at
20°C), a weight-
loss of less than 25%, preferably less than 15%, after 2 h at 200°C, a
surface tension
of a 1% emulsion of less than 3S mN/m, preferably less than 32 mN/m at
20°C.
The invention further relates to a process for the production of a
highly processable aromatic polyamide fiber coated on the surface with a
surface
treatment agent.
Coating of aramid fibers with the surface treatment agent of this
invention can take place in various ways and, more specifically, for example,
1 o according to the following processes (a) and (b).
According to process (a), the application of the surface treatment
agent is made on never-dried never-drawn aramid fiber; and according to
process
(b) the application of the surface treatment agent is done on previously dried
azamid fiber, in each case using any known coating device. The finish
formulation is
15 used neat or in a diluted aqueous form, which is in a concentration of as
low as 1%
by weight.
In the preferred route for process (a), the finish formulation is applied
in a concentration of about 30% by weight in water (this means 30 parts by
weight
finish formulation + 70 parts by weight water) on a wet aramid fiber. The
emulsion
2 o treated fiber is then dried during the fiber stretching drying step at a
temperature
between 150 and 190°C, preferably at around 170°C for few
seconds (5-10 s) while
the yarn speed is around 630 m/min (workable range 120-1200 m/min).
In the preferred route of process (b) yarns and cords of aramid fibers
are passed through a dip of the finish formulation in a dipping unit to coat
them and
25 then are dried in an air heated chamber at 80 to 190°C, preferably
at 110 to 130°C
with a predetermined tension of 6 N for an untwisted 1670 dtex yarn. The most
preferred temperature for this step is about 120°C. Depending on the
dip
concentration for the finish formualtion, which may be from 1% to 100% by
weight
in water, the speed is adjusted to be from 15 to 100 m/min. By a~finish
formulation
3 0 of 100%, is meant that the finish is neat.
The finish levels for both processes, (a) and (b), are in the range of
0.05 to 2% by weight, preferably 0.2 to 1.0% by weight.
If desired, processes (a) and (b) can be conducted as a multi-step
process in which the fiber may be several times immersed in a surface
treatment
3 5 agent and in turn dried. For example, the treatment agent can be applied
an the
~1U50J2 ,
wo gzits~a; ~~ pcrius9aio~s. ',
never-dried wet fiber, then the fiber can be dried and thereafter the surface
treatment agent can be applied once more or even several times more with or
without intermediate drying.
Fibers of this invention can be used in the reinforcement of hoses,
belts, ropes and cables including optical cables, rubber goods and composite
structures (e.g. spouting goods, medical supplies, building and acoustic
material,
transport and protective equipment for civil and military applications ).
to ExamRl~
rn this example, aramid fiber in a yarn of 1100 dtex and coated by the
finish of this invention was compared with commercially-available aramid yarn
of
the same dtex coated by a standard fuiish.
The aramid fiber of this invention shows superiority, in terms of
~5 friction, especially dynamic friction F/M (200 m/min), deposit measured in
mg/kg
of yarn, and fibrillation compared to the control aramid fiber (Comparison)
which is
commercially available.
For antistatic evaluation, a generally good performance starts at -6
kV, consequently the measured value of -2.5 kV for the fiber of this invention
is
2 o excellent in terms of staticity.
The scourability (wash-off property) is a very important factor since
the residual finish level after a washing-step (measured in %) impacts any
subsequent finishing operation. Scourability values mentioned in the Table
below
were obtained on an industrial scale using fabrics made of the yarn of this
invention
2 5 and compared with a control yarn which was a commercial product of the
same
denier treated with a standard finish. The values were confirmed in the
laboratory
by washing the yarns two times with soft water at SO°C using 100 inl of
water for 10
g of yarn.
Friction coefficients were determined according to the following
3 o method: A package of yarn is threaded through a tensioning device, between
a
guide roll and two strain gauges, and onto a take-up roll driven by a variable
speed
motor. The two strain gauges record Ti and T2 input and output tension
respectively. The coefficient of friction is computed according to the
formula:
Tl/T' = e~ (a ' ~
2105092
~0 9zns~4~ 9 ~crius9ziots9'
...
where a is the friction angle and f the friction coefficient (fiber to fiber,
fiber to
metal or fiber to ceranic, depending on whether a polished chrome or ceramic
pin
was used). The Rothsci~ild friction meter R-1182 has been used according to
the
standard procedure known in the art.
The deposit due to abrasion was measured on a "Staff-Tester G 555"
(Zweigle, West Germany) with which the weight of the abraded fiber-material
arising from fiber to fiber friction was determined.
The fibrillation index was determined on a "G 566" apparatus
(Zweigle, West Germany).
Comparison of yvs_ic a~~R .rtiPt
Com- ThlS
(1100 dtex Fiberl narison Invention
1. Friction
. Fiber/Fiber (0.016 cm/s) 0.22 0.215
( 128 cm/s) 0.28 0.265
Fiber/Metal (0.016 cm/s) 0.12 0.045
(128 cm/s) 0.30 0.265
(200 m/min) 0.70 0.55
2 0 2. Deposit (mg/kg) 10 0.5
3. Fibrillation index 21 2-5
4. Scourability 46% 9%
(Residual knish level)
x m 1 2
In this example, a fabric woven from the yarn of this invention and a
comparison fabric woven from commercially available yarn having a standard
finish
were tested for ballistic performance.
The fabrics were made of 1111 dtex (1000 denier) yarns.
3 0 Usually in the area of high tenacity fiber the weaving operation of
ballistic fabrics leads to strength losses usually quantified by extracting
the yarn out
of the fabric and measuring the tenacity according to the standard procedures
known in the art. The following Table shows that the product of this invention
yields a significant advantage since, in a heavy fabric construction
(typically 12 ends
3 5 per cm), the strength loss is reduced by half (7 vs. 14%). The ballistic
performance
WO 92/i5747 2o PCT/US92/Oi89
21009 ~~2
(VSp: see test procedure) is also improved by 8% at the greige fabric level
and S to
8% at the finished level (meaning after final fabric treatment).
In the case of light weight fabric, typically 8 ends per cm, the ballistic
performance is also increased by 4.5% at the greige fabric level.
Strerneth Conve;lion and ,$~~j,~~ir pPrt'r,rmance
Fabric Grade Strength Strength Percentage
Loss, Loss, improvement
This Com- in Ballistic
1 o Invention parison Performance
V50 of This
Invention vs.
om arp icon
15 HEAVY FABRIC of the
state of the art:
1. greige 5%-9% 14%-18%
2. ballistic perform.
(greige fabric) + g%
2 0 3. ballistic perform.
(finished fabric) + 5-8%
LT('',T~T FARRTC" of thA
stat~g of the art
2 5 1. greige 0-2% 0-2%
2. ballistic perfornn.
(greige fabric) + 5%
Ballistic tests
3 0 The ballistic test method for determining V50 was carried out
according to the 1\TATO standardization agreement STANAG 2920.
The V50 ballistic limit velocity for a material or armour is defined as
that velocity for which the probability of penetration of the chosen
projectiles is
exactly 0.~, using the Up and Down firing method and calculation described
below.
210~09~
':';U 92/15747 1 ~ I PCT/U592/0189!
The Up and Down firing method:
The first round shall be loaded with the amount of propellant
calculated to give the projectile a velocity equivalent to the estimated VSO
ballistic
limit of the armour. If the first round fired produces a complete penetration,
the
second round shall be loaded with a fixed decrement of propellant calculated
to
produce a velocity about 30 m/s lower than the first. If the first round fired
results
in a partial penetration, the second round shall be loaded with a fixed
increment of
propellant calculated to produce a velocity about 30 m/s higher than the first
round.
Upon achieving the first set of penetration reversals, the propellant charge
should
1o be adjusted with the fixed amount to yield an increment or decrement of
velocity of
about 15 m/s. Firing will then continue in accordance with a given
procedure'to
obtain an estimate of the VSO (BLP) [Ballistic Limit Protection].
VSO calculation:
After a number of projectiles have been fired the VSO is calculated as
the mean of the velocities recorded for the fair impact the fair impacts
consisting of
the three highest partial velocities for partial penetration and the three
lowest
velocities for complete penetration provided that all six velocities fall
within a
bracket of 40 m/s.
In this example, knitting processability evaluation was carried out
under the following conditions: ELHA Circular Knitting Machine (Model RRU),
test duration 4 hours, machine speed 6 7 0 rpm, knitting speed 1 S m/min ;
knitting
2 5 construction 3 stitches /cm .
WO 92/1547 ~ ~ ~ ~ ~ ~ ~ I .
~'CT'/US92/018. .,~tg. ,
Fnd-Llee rmance of renyYarn T~
Perfo Diffe 8g
Yarn Curt- Com- .,
This Invention
TYPe parison parison Process
0 T/m 120 T/m (a) and (b)
0 T/m
Fibril- high none none
lation
Knit not
Design uniform uniform uniform
Deposit build-up, slight no
deposit deposit deposit
Coverage not low optimum
Factor uniform
2 o A.s can be seen in the table, above, optimum productivity levels and
maximum value in use could be obtained using yarns of this invention versus
the
Comparison yarns. The state of the art product is used twisted. The results
clearly
.show the advantage related to the possibility of avoiding the twisting
operation by
using yarns of this invention.
'' am 1 4
:~'s In this example, fatigue trials on hoses, made using yarn of this
> ~ invention, were carried out to the Ford specification with pressures of I-
3.5 bar at 0.5
;i
Hz according to the most severe trapezoid' waveform.
r
3 o With Comparison yarn, 50,000 cycles to failure are generally obtained
and are sufficient to pass the test. However, a result of 80,000 cycles has
been
obtained for five hose samples containing yarns of this invention. This shows
a
significant superiority of the yarns of .this invention in terms of fatigue
resistance.
J J
210'5092
~:;;'"O 92/15747 ~ ... PCT/US92/01891
Example ~
In this example, the strength e~ciency conversion of cords made
using yarns of this invention was compared with that of cords made using
Comparison yarns.
Compared with commercially available aramid based construction, up
to 30% better suength efficiency conversion was obtained by using yarn of this
invention for cord construction. If a cord is made of several yarns, the
strength of
the cord theoretically should be equal to the strength of each yarn,
multiplied by the
number of yarns, which is never the case in practice. However, the finish of
this
1 o invention helps to overcome this problem.
In a laboratory test, the strength of a parallel construction made of
three commercial 1100 dtex (1000 filaments) aramid yarns with a final twisting
of
140 T/m (twists per meter) was determined to be 524 N. This was compared with
a
parallel cord construction made of three 1100 dtex yarns which were treated
with
15 the finish of this invention (0.8% by weight finish level). The finally
obtained
strength of a yarn with a twist level of 140 T/m was 592 N which corresponds
to a
30% increase.
m 1
2 o In this example, several qualities of yarn of this invention were tested
and compared with those qualities as exhibited by Comparison yarn.
Test conditions:
Weight loss is measured by the percentage of finish material lost after
25 exposing the fibers at 230°C for 8 hours. The finish percentage is
determined by
solvent extraction before and after the heat exposure.
The percentage of residue after scouring is also determined by solvent
extraction of the residual finish remaining on the fiber after washing
(scouring) the
fiber according to washing procedures known and applied in the industry. The
3 o percentage is calculated versus the initial finish level determined prior
to the
scouring step.
The fiber to metal (F/M) friction coefficient is measured at 150
m/min using the Rothschild equipment and method as described previously.
i
WO 92/1579 i PCi'/U592/O18 ,;:; ,.
2105~g~ 4
Finish weight Finish remaining F/M
loss on after friction
Fiber heatinl;r_ couri ~~i 'g~
This invention 5 8 0.50 '
{"non-fuming")
B
This invention 11 14 0.47 i
to
Comparison 23 45 0.65
All yarns were 1000 denier, 670 filament and were coated in the dried
state using a neat finish formulation at a rate of 750 m/minute to a level of
0.8%.
The finish of A included a 70/30 mixture of benzene tricarboxylic acid
and benzene dicarboxylic acid as the carboxylic acid component for the
esteroil.
The finish of B included a 70/30 mixture of C-18/C-16 alkenyl monocarboxylic
acid
component for the esteroil. '
2 0 The finish of the Comparison was C-12/C-15 mineral oil-based as
disclosed in Research Disclosure No. 195, disclosure 19520, July, 1980.
By this example, it is noted that finish formulations of this invention in
which a carboxylic acid component for the esteroil is used having more than
one
carboxylic acid group, that is, where n=2-6, the finish formulation yields
2 5 considerably less weight loss on heating. Less weight loss on heating
means less
fuming in use and operation at elevated temperatures.