Note: Descriptions are shown in the official language in which they were submitted.
WO 93/14096 PCr/~B93/00086
~ i u ~ ~ ~ 9
PHOSP~ONO DERIVATIVES OF AMINO AC~:)S AS MEIALU)PRal~EINASE
INHIBIIORS
FIELD QF THE INVENTION
This invention relates to a novel class of phosphonopeptidyl derivatives, to
processes for their preparation and to their use in medicine.
BACKGROUND TO THE INVENTION
. . .
In normal tissues, cellular connective tissue synthesis is offset by
extracellular matrix degradation, the two opposing effects existing in
dynamic equilibrium. Degradation of the matrix is brought about by the
action of proteinases released from resident connective tissue cells and
invading inflammatory cells, and is due, in part, to the activity of at least three
groups of metalloproteinases. These are the collagenases, the gelatinases
(or type-lV collagenases) and the stromelysins. Normally these catabolic
enzymes are tightly regulated at the level of their synthesis and secretion
and also at the level of their extracellular activity, the latter through the action
S of specific inhibitors, such as ~2-macroglobulins and TIMP (tissue inhibitor of
metalloproteinase), which form inactive complexes with metalloproteinases.
The accelerated, uncontrolled breakdown of connective tissues by
metalloproteinase catalysed resorption of the extracellular matrix is a feature
of many pathoiogical conditions, such as rheumatoid arthritis, corneal,
epidermal or gastric ulceration; tumour metastasis or invasion; periodontal
disease and bone disease. It can be expected that the pathogenesis of such
diseases is likely to be modified in a beneficial manner by the administration
of metalloproteinase inhibitors and numerous compounds have been
suggested for this purpose [for a general review see Wahl, R.C. .~I ~1 Ann.
Rep. Med. Chem. 25,175-184, Academic Press Inc., San Diego (1990)].
WO 93/14096 ~ 1 ~t ~J ~i i3 9 PCr/GB93/00086
Certain phosphonopeptides have been described as collagenase inhibitors
in European Patent Specification No. 320118 and International Patent
Specifications Nos. WO 91/15~16 and WO 91/15507.
SUMMARY OF THE INVENTION
We have a now found a new class of phosphonopeptidyl d~rivatives,
members of which are metalloproteinase inhibitors and which, in particular,
advantageously possess a potent and selective inhibitory action against
gelatinase.
.~ :
There is now much evidence that metalloproteinases are important in
tumour invasion and metastasis. Tumour cell gelatinase, in particular, has
been associated with the potential of tumour cells to invade and
metastasise. Tumour invasion and metastasis is the major cause of
treatment failure for cancer patients, and the use of a selective gelatinase
inhibitor such as a compound of the present invention which is capable of
inhibiting tumour cell invasion can be expected to improve the treatment of
this disease.
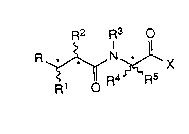
Thus according to one aspect of the invention we provide a compound of
formula (I)
R2 R3 O
~O R~
wherein R represents a-P(O)(X1R6)X2R7 group, where X1 and X2, which
may be the same or different, is each an oxygen or a sulphur atom, and R6
..
.
wo 93/t4096 pcr/GBs3/ooo86
2 i ~ U ~'1
-3-
and R7, which may be the same or different each represents a hydrogen
atom or an optionally substituted alkyl, aryl, or aralkyl group;
R1 represents a hydrogen atom or an optionally substituted alkyl, alkenyl,
aryl, aralkyl, heteroaralkyl or heteroarylthioalkyl group;
R2 represents an optionally substituted alkyl, alkenyl, cycloalkyl,
cycloalkylalkyl, aryl, aralkyl, aralkoxy, or aralkylthio group, or an amino (-
NH2), substituted amino, carboxyl (-CO2H) or esterified carboxyl group; :
R3 represents a hydrogen atom or an alkyl group:
R4 represents a hydrogen atom or an alkyl group;
R~ represents a group -lAlk]nR8 where Alk is an alkyl or alkenyl group
optionally interrupted by one or more -O- or-S- atoms or -N(R9)- groups
where R9 is a hydrogen atom or a C1 6alkyl group], n is zero or an integer 1,
and R8 is an optionally substituted cycloalkyl or cycloalkenyl group:
X represents an amino (-NH2), 0! substituted amino, hydroxyl or substituted
hydroxyl group;
and the salts, solvates and hydrates thereof.
It will be appreciated that the compounds according to the invention can
contain one or more asymmetrically substituted carbon atoms, for example
those marked with an asterisk in formula (I). The presence of one or more of
these aysmmetric centres in a compound of formula (I) can give rise to
stereoisomers, and in each case the invention is to be understood to extend
.
,
.. . .
wo 9~/14096 pcr/GBs3~ooog6
t ~ ~ 4
to all such stereoisomers, including enantiomers and diastereoisomers, and
mixtures, including racemic mixtures, thereof.
In the formulae herein, the ~line is used at a potential asymmetric centre to
represent the possibility of R- and S- configurations, the _ line and the ----
--- line to represent an unique configuration at an asymmetric centre.
When the groups R1 and/or R2 in compounds of formula (I) each represents
an optionally substituted alkyl or alkenyl group, it may be, for example, a
straight or branched C1 6 alkyl or C2 6alkenyl group, such as a methyl, ethyl,
n-propyl, i-propyl, n-butyl, i-butyl, s-butyl, t-butyl, n-pentyl, i-pentyl, n-hexyl,
ethenyl, 1-propenyl, 1-butenyl or 2-butenyl group optionally substituted by
one or more C~ 6alkoxy, e.g. methoxy, ethoxy, propoxy, C1 6alkylthio, e.g.
methylthio, ethylthio, propylthio, C6 ~2arylC~ 6alkoxy, e.g. phenylC1 6 alkoxy
such as benzyloxy, aralkylthio, e.g phenylC~ 6alkylthio such as benzylthio,
amino (-NH2), substituted amino, [such as -NHR1 0, where R1 0 is a C1 6 alkyl
e,g. methyl or ethyl, C6 12arylC1 6alkyl, e.g. phenylC1 6alkyl, such as benzyl,
C6 12aryl, e.g. phenyl, C3 8cycloalkyl, e.g. cyclohexyl, or C3 8cycloalkylC1
6alkyl, e.g. cyclohexylmethyl group], carboxyl (-CO2H) or -CO2R12 [where
R12 is as defined below] groups.
Aryl groups represented by R1 and/or R2 in compounds of formula (I) include
C6 12 aryl groups such as phenyl or 1- or 2-naphthyl groups.
Aralkyl groups represented by R1 and/or R2 include C6 12arylC1 6alkyl
groups such as phenylC1 6alkyl, or 1- or 2-naphthylC1 6alkyl, for example
benzyl, phenylethyl, phenylpropyl, phenylbutyl, phenylpentyl, 1- or 2-
naphthylmethyl, naphthylethyl, naphthylpropyl, naphthylbutyl or
naphthylpentyl groups.
When the group R1 in compounds of formula (I) is a heteroaralkyl group, it
. . . ' . . . , ' . -
.
- . ,. . ' . . '
WO 93/14096 PCT/GB93/00086
-5 -
may be for example a C3 6heteroarylC1 6alkyl group, such as an optionally
substituted pyrrolylmethyl, furanylmethyl, thienylmethyl, imidazolylmethyl,
oxazolylmethyl, thiazolylmethyl, pyrazolylmethyl, pyrrolidinylmethyl,
pyridinylmethyl, pyrimidinylmethyl, morpholinylmethyl, or piperazinylmethyl
group.
Heteroarylthioalkyl groups represented by R1 include C3 6heteroarylthioC1
6alkyl groups such as optionally substituted pyrrolylthiomethyl,
furanylthiomethyl, oxazolylthiomethyl, thiazolylthiomethyl,
pyrazolylthiomethyl, pyrrolidinylthiomethyl, pyridinylthiomethyl,
pyrimidinylthiomethyl, morpholinylthiomethyl, or piperazinylthiomethyl
groups.
Optional substituents which may be present on heteroaralkyl or
heteroarylthioalkyl groups represented by R1 include those discussed below
in relation to R1 and/or R2 when these groups are for example aralkyl or
aralkylthioalkyl groups.
Cycloalkyl groups represented by the group R2 in compounds according to
the invention include C3 8cycloalkyl groups such as cyclopentyl or
cyclohexyl groups.
When R2 is a cycloalkylalkyl group it may be for example a C3 8cycloalkylC1
6alkyl group such as a cyclopentylC1 6alkyl or cyclohexylC1 6alkyl group, for
example a cyclopentylmethyl, cyclopentylethyl, cyclopentylpropyl,
cyclopentylbutyl, cyclohexylmethyl, cyclohexylethyl, cyclohexylpropyl, or
cyc!ohexylbutyl group.
When R2 is an aralkoxy or an aralkylthio group it may be for example a C6
12arylC1 6alkoxy or C6 12arylC1 6alkylthio group such as a phenylC1
6alkoxy or phenylC1 6alkythio group. e.g. a benzyloxy, phenylethoxy,
WO 93/ l 4096 PCT/G B93/00086
3 -6-
phenylpropoxy, phenylbutoxy, benzylthio, phenylethylthio, phenylpropylthio
or phenylbutylthio group.
The cycloalkyl, cycloalkylalkyl, aryl, aralkyl, aralkoxy or aralkylthio groups
represented by R1 and/or R2 in compounds of formula (1) may each
optionally be substituted in the cyclic part of the group by one, two or more
substituents [R11] selected from halogen atoms, e.g. fluorine, chlorine,
bromine or iodine atoms, or C1 6alkyl, e.g. methyl or ethyl, C1 6alkoxy e.g.
methoxy or ethoxy, C2 6alkylenedioxy, e.g. ethylenedioxy, haloC1 6alkyl, e.g.
tri-fluoromethyl, C~ 6alkylamino, e.g. methylamino or ethylamino, C1
dialkylamino, e.g. dimethylamino or diethylamino, amino (-NH2), nitro,
cyano, hydroxyl (-OH), carboxyl (-CO2H), -CO2R9, where R9 is as defined
above, C1 6alkylcarbonyl, e.g. acetyl, sulphonyl (-SO3H), C1
6alkylsulphonyl, e.g. methylsulphonyl, aminosulphonyl (-SO2NH2), C1 6
alkylaminosulphonyl, e.g. methylaminosulphonyl or ethylaminosulphonyl,
C1 6dialkylaminosulphonyl e.g. dimethylaminosulphonyl or
diethylaminosulphonyl, carboxamido (-CONH2), C1 6alkylaminocarbonyl,
e.g. methylaminocarbonyl or ethylaminocarbonyl, C1 6dialkylaminocarbonyl,
e.g. dimethylaminocarbonyl or diethylaminocarbonyl, sulphonylamino (-
NHSO2H), C1 6alkylsulphonylamino, e.g. methylsulphonylamino or
ethylsulphonylamino, or. C1 6dialkylsulphonylamino, e.g.
dimethylsulphonylamino or diethylsulphonylamino groups. It will be
appreciated that where two or more R11 substituents are present, these need
not necessarily be the same atoms and/or groups. The R11 substituents
may be present at any ring carbon atom away from that attached to the rest
of the molecule of formula (1). Thus, for example, in phenyl groups any
substituents may be present at the 2-, 3-, 4-, 5- or 6- positions relative to the
ring carbon atom attached to the remainder of the molecule.
When the group R2 in compounds of formula (1) is a substituted amino group,
this may be for example a group -NHR1 0 where R1 0 is as defined above.
. . . . . . . . . . ... . .
Wo 93/14096 ~, PCr/Gs93/~oox6
;~, 1 il ~ .i U
-7-
Esterified carboxyl groups represented by R2 include groups of formula -
CO2R1 2 where R1 2 is a straight or branched, optionally substituted C1 8alkyl
group such as a methyl, ethyl. n-propyl, i-propyl, n-butyl, i-butyl, s-butyl or t-
butyl group; a C6 12arylC1.8alkyl group such as an optionally substituted
benzyl, phenylethyl, phenylpropyl, 1-naphthylmethyl or 2-naphthyimethyl
group; a C6 12aryl group such as an optionally substituted phenyl, 1-
naphthyl or 2-naphthyl group; a C6 12aryloxyC1 8alkyl group such as an
optionally substituted phenyloxymethyl~ phenyloxyethyl, 1
naphthyloxymethyl or 2-naphthyloxymethyl group; an optionally substituted
C1.8alkanoyloxyC1.8alkyl group, such as a pivaloyloxymethyl,
propionyloxyethyl or propionyloxypropyl group; or a C6 12aroyloxyC1 8alkyl
group such as an optionally substituted benzoyloxyethyl or
benzoyloxypropyl group. Optional substituents present on the groups R12
include for example one or more halogen atoms such as fluorine, chlorine,
bromine or iodine atoms, or C1 4alkyl, e.g. methyl or ethyl, or C1 ~alkoxy, e.g.methoxy or ethoxy, groups.
When the groups R3 and R4 in compounds of formula (I) are alkyl groups,
they may be for example C1 6alkyl groups such as methyl or ethyl groups.
The groups R6 and/or R7 in compounds of formula (I) may eac.h be a
hydrogen atom or an optionally substituted straight or blanched C1 6alkyl,
e.g. methyl, ethyl, n-propyl, i-propyl, n-butyl or i-butyl, C6 12aryl, e.g. phenyl,
or C6 12arylC1 6alkyl, e.g. benzyl, phenylethyl or phenylpropyl group.
Optional substituents present on alkyl groups of this type include one or
more C1 6alkoxy, e.g. methoxy, ethoxy, or C1 6alkylthio, e.g. methylthio, or
ethylthio groups or an optionally substituted C6 12aryloxy, e.g. phenyloxy,
C6 12arylthio e.g. phenylthio, C6.~2arylC1.6alkoxy e.g. benzyloxy or C6
12arylC1 6alkylthio e.g. benzylthio. Optional substituents present on the
group R6 or R7 when it is an aryl or aralkyl group or an alkyl group
substituted by an aryloxy or arylthio group include R11 groups present on the
.
.
Wo 93/1~096 pcr/GBs3/ooo86
cyclic part of R6 or R7 as defined above.
When the group Alk is present in compounds of formula (I) it may be a
straight or branched C1 6alkyl. e.g. methyl, ethyl, n-propyl i-propyl, n-butyl, i-
butyl, n-pentyl or n-hexyl or C2 6alkenyl e.g. ethenyl or 1-propenyl group
optionally interrupted by one or more -O- or -S- atoms or -N(R9)- groups
where R9 is a hydrogen atom or a C1 6alkyl group such as a methyl group.
- The group R8 in compounds of formula (I) may represent a C3 8cycloalkyl,
e.g. cyclopentyl or cyclohexyl, or C3 8cycloalkenyl e.g. cyclopentenyl or
cyclohexenyl, group optionally substituted by one, two or more C1 6alkyl,
e.g. methyl or ethyl, C1 6alkoxy, e.g. methoxy or ethoxy, C1 6alkylthio, e.g.
methylthio, or hydroxyl groups.
When X in the compounds of formula (I) represents a substituted amino
group it may be for example a group of formula -NR13R14, where R13 and
R14, which may be the same or different, is each a hydrogen atom (with the
proviso that when one of R13 or R14 is a hydrogen atom, the other is not) or
an optionally substituted straight ot branched alkyl group, optionally
interrupted by one or more -O- or-S- atoms or -N(R9)- or aminocarbonyloxy
[-NHC(O)O-] groups or R13 and R14~ together with the nitrogen atom to which
they are attached, may form an optionally substituted C3 6cyclic amino group
optionally possessing one or more other heteroatoms selected from -O- or -
S-, or-N(R9)- groups.
When R13 and/or R14 is an alkyl group it may be for example a C1 6alkyl
group such as a methyl, ethyl, n-propyl, i-propyl, n-butyl, i-butyl, s-butyl, or t-
butyl group, optionally interrupted by one or more -O- or -S- atoms, or -
N(R9)- or aminocarbonyloxy groups and may be for example a
methoxymethyl, ethoxymethyl, methoxyethyl, ethoxyethyl or
. . . . , . ~ . . ~
. : .
wo 93/14096 ~ ) U ~3 pcr/GB93/ooo86
ethylaminocarbonyloxymethyl group. The optional substituents which may
be present on such groups include hydroxyl (-OH), carboxyl (-CO2H),
esterified carboxyl (-CO2R12), carboxamido (-CONH2), substituted
carboxamido, e.g. a group -CoNR13R14 where NR13R14 is as defined
herein, amino (-NH2), substituted amino, for example a group of formula -
NR12R13, or aryl, e.g. C6 12 aryl such as phenyl, optionally substituted by
one, two or more R11 substituents selected from those listed above in
relation to the group R2.
Particular examples of cyclic amino groups represented by -NR13R14
include morpholinyl, imidazolyl, piperazinyl, pyrrolyl, oxazolyl, thiazolyl,
pyrazolyl, pyrrolidinyl, pyridinyl and pyrimidinyl groups.
When the group X is a substituted hydroxyl group it may be for example a
group oR1 3 where R1 3 is as defined above, other than a hydrogen atom.
Salts of compounds of formula (1) include pharmaceutically acceptable
salts, for example acid addition salts derived from inorganic or organic acids~
such as hydrochlorides, hydrobromides, hydroiodides, p-toluene
sulphonates, phosphates, sulphates, acetates. trifluoroacetates propionates,
citrates, malonates, succinates, lactates, oxalates, tartarates and benzoates.
Salts may also be formed with bases. Such salts include salts derived from
inorganic or organic bases,. for example alkali metal salts such as sodium or
potassium salts, alkaline earth metal salts such as magnesium or calcium
salts, and organic amine salts such as morpholine, piperidine,
dimethylamine or diethylamine salts.
The group R in compounds of formula (I) may in particular be a -
P(O)(OR6)0R7, e.g. a -P(O)(OH)OR7 group, or a -P(O)(SH)OR7 or -
P(O)(OH)SR7 group. Examples of such groups include -P(O)(OCH3)0CH3,-
wo 93/14096 pcr/GBs3/ooo86
,t ~ U ~ ,
-1 O-
P(O)(OCH2CH3)OCH2CH3, -P(O)(OH)OH, -P(O)(OH)SH, -P(O)(SH)OH,
P(O)(OH)OCH3, -P(O)(OH)SCH3, -P(O)(OH)OCH2CH3, -P(O)(OH)OPh,
P(O)(OH)SPh,-P(O)(OH)OCH2Ph or-P((:))(OH)SCH2Ph, where Ph is a
phenyl group optionally substituted by one or more substituents R~
In the compounds of formula (I) the group R1 may in particular be a C1 6alkyl
group such as a methyl group, an aralkyl group such as benzyl group, an
arylthioalkyl group such as a phenythiomethyl group or a heteroarylthioalkyl
group such as thienylthiomethyl, pyridinylthiomethyl or pyri.midinylthiomethyl
group or is especially a hydrogen atom.
The group R2 may be in particular an optionally substituted C1 6alkyl, C3
8cycloalkyl, C3 8cycloalkylC1 6alkyl, C6 12aryl, C6 12arylC1 6alkoxy or C6
12aralkylthio group and, especially, a C6 12arylC1 6alkyl group. Particular
types of these groups are optionally substituted C3 6 alkyl, such as n-propyl,
i-propyl, n-butyl, i-butyl, s-butyl, t-butyl, n-pentyl or i-pentyl; cyclopentyl;cyclohexyl; cyclopentylC1 6alkyl, such as cyclopentylC3 6alkyl, e.g.
cyclopentylpropyl, cyclopentylbutyl, or cyclopentylpentyl; phenyl; ~- or ~-
naphthyl; phenylC1 6alkoxy, e.g. phenylethoxy, phenylpropoxy or
phenylbutoxy; phenylC1 6 alkylthio, e.g. phenylethylthio, phenylpropylthio or
phenylbutylthio; and, especially, phenylC1 6alkyl such as phenylC3 6alkyl
e.g. phenylpropyl, phenylbutyl or phenylpentyl; or 1- or 2-naphthylC1 6alkyl
such as 1- or 2-naphthylC3 6alkyl, e.g. 1- or 2-naphthylpropyl, naphthylbutyl
or naphthylpentyl. Each of these cycloalkyl or aryl groups may be
substituted, by one two or more substituents R~1 described above.
The groups R3 and R4 in compounds of formula (I) may each in particular be
a methyl group, or, especially, a hydrogen atom.
The group R5 in compounds of formula (I) rnay be in particular a group -
AlkR8, where R3 is an optionally substituted cycloalkyl or cycloalkenyl group.
.
: . : . ,
. . ... . .
.
WO 93/14096 PCT/G1~93/OOn86
.i ,J V 9
Thus, the group R5 in compounds of formula (1) may be an optionally
substituted C3 8cycloalkylC1 6alkyl [e.g. cyclopentylC1 6alkyl such as
cyclopentylmethyl or cyclopentylethyl. or cyclohexyC1 6alkyl such as
cyclohexylmethyl or cyclohexylethyl], C3 8cycloalkenylC1 6alkyl [e.g.
cyclopentenylC1 6alkyl such as cyclopentenylmethyl or cyclohexenylC1
6alkyl such as cyclohexenylmethyl], cycloalkylC1 3alkoxyC1 3alkyl [e.g.
cyclopentylmethoxymethyl, cyclohexylmethoxymethyl] C3 8cycloalkenylC1
3alkoxyC1 3alkyl [e.g. cyclopentenylmethoxymethyl or
cyclohexenylmethoxymethyl] C3 8cycloalkylC1 3alkylthioC1 3alkyl [e.g.
cyclopentylmethylthiomethyl or cyclohexylmethylthiomethyl] or C3
8cycloalkenylC1 3alkylthioC1 3alkyl [e.g. cyclopentenylmethylthiomethyl or
cyclohexenylmethylthiomethyl], C3 8cycloalkyC~ 3alkylaminoC1 3alkyl [e.g.
cyclopentylmethylaminomethyl, or cyclohexylmethylaminomethyl3 or C3
8cycloalkenylC1 3alkyaminoC1 3alkyl [e.g. cyclopentenylmethylaminomethyl
or cyclohexenylmethylaminomethyl] group.
The group X in compounds of tormula (I) may be in particular an amino (-
NH2) or-NR13R14 group. Particular -NR13R14 groups are -NHR14 groups.
Groups of this type include those where R14 is a C1 6alkyl group. for
example a methyl, ethyl, or n-propyl group, optionally interrupted by one or
more -O- or-S- atoms or-N(R9) [e.g. -NH- or-N(CH3)-] or aminocarbonyloxy
groups, and optionally substituted by a hydroxyl, carboxyl, carboxyalkyl, e.g.
carboxymethyl, carboxamido, amino, -NR13R14, [for example di-C
6alkylamino such as dimethylamino, C~ 6alkylamino such as methylamino~
or C3 6 cyclic amino such as morpholinyl, pyrrolidinyl or pyridinyl] or phenyl
optionally substituted by one, two or more R11 substituents.
A particularly useful grol~p of compounds according to the invention is that of
formula (I) wherein R5 is an AlkR8, group, where Alk is a C1 6 alkyl and R8 is
a C3 8 cycloalkyl or C3 acycloalkenyl group.
WO 93/14096 PCI/GB43/00086
Another particularly useful group of compounds according to the invention is
that of formula (I) where R2 is an optionally substituted alkyl. cycloalkyl,
cycloalkylalkyl, aryl, aralkoxy or aralkylthio group.
A further particularly useful group of compounds of formula (I) are those -
wherein X is an amino or substituted amino group.
In general, in compounds of formula (I) the groups R1, R3 and R4 is each
preferably a hydrogen atom.
An especially useful group of compounds according to the invention has the
formula (la)
R2 H O
R ~ .J~ X '
O R5 (la)
wherein R, R2. R5 and X are as defined for formula (I); and the salts, solvates
and hydrates thereof.
: -
A particularly useful group of compounds of formula (la) are those wherein R
represents a P(o)(oH)oR7 group; R2 represents an optionally substituted
alkyl, alkenyl, cycloalkyl, cycloalkylalkyl~ aryl, aralkoxy or aralkylthio group;
R5 represents a group -AlkR8, where Alk is a C1 6 alkyl group and R8 is a
cycloalkyl or cyc!oalkenyl group;
X is an amino (-NH2) or substituted amino group; and the salts, solvates and
hydrates thereof.
: .
,
WO 93/14096 PCr/GB93/OOOX6
-1 3-
Particularly useful compounds of formula (la) are those wherein R5 is a
group -AlkR3, and R8 is an optionally substituted cyclohexyl group.
Compounds of this type in which R5 is a cyclohexylC1 6alkyl group,
particularly a cyclohexylmethyl group, are especially useful.
Other useful compounds of formula (la) include those wherein R2 represents
a C3 6alkyl group, particularly an iso-butyl or n-pentyl group, or a
cycloalkylC3 6alkyl group, particularly a cyclohexylpropyl, cyclohexylbutyl or
cyclohexylpentyl group, or especially an optionally substituted phenylC2
6alkyl group particularly an optionally substituted phenylethyl phenylpropyl,
o phenylbutyl or phenylpentyl group. Optional substituents on the phenyl
group may be one, two or more R11 groups as defined for compounds of
formula (I).
In the compounds of formula (la) X may be a -NH2 group or a group -
NR1 3R14 as defined for compounds of formula (I).
An especially useful group of compounds according to the invention has the
formula (la) wherein R2 is an optionally substituted phenylC3 6alkyl group,
especially an optionally substituted phenylpropyl or phenylbutyl group, R5 is
a cyclohexylmethyl group; and X is a amino (-NH2) or NR13R14 group.
Compounds of this type wherein X is -NH2 or -NHR13 are particularly useful.
In general, compounds of formula (I) wherein R is a -P(O)(OH)OH group are
particularly preferred.
In the compounds of formulae (I) and (1 a), when the group R5 is a
cycloalkylC1 6alkyl group then the chiral centre to which this group is
attached preferably has a S-configuration.
- : - , - . .
, ' ~ '
'
.
wo 93/14096 pcr/GBs3/ooo86
The compounds according to the invention may be prepared by the
following processes. In the description and formulae below the groups R,
R1, R2, R3, R4, R5 and X are as defined above, except where otherwise
indicated. It will be appreciated that functional groups, such as amino,
hydroxyl or carboxyl groups, present in the various compounds described
below, and which it is desired to retain, may need to be in protected form
before any reaction is initiated. In such instances, removal of the protecting
group may be the final step in a particular reaction. Suitable amino or
hydroxyl protecting groups include benzyl, benzyloxycarbonyl or t-
butyloxycarbonyl groups. These may be removed from a protected
derivative by c'atalytic hydrogenation using for example hydrogen in the
presence of a metal catalyst, for example palladium on a support such as
carbon in a solvent such as an alcohol e.g. methanol, or by treatment with
trimethylsilyl iodide or trifluoroacetic acid in an aqueous solvent. Suitable
carboxyl protecting groups include benzyl groups, which may be removed
from a protected derivative by the methods just discussed, or alkyl groups,
such as a t-butyl group which may be removed from a protected derivative
by treatment with trifluoroacetic acid in an aqueous solvent. Other suitable
protecting groups and methods for their use will be readily apparent. The
formation of the protected amino, hydroxyl or carboxyl group may be
achieved using standard alkylation or esterification procedures, for example
as described below.
Thus according to a further aspect of the invention a compound of formula (I)
may be prepared by coupling an acid of formula (Il)
R2
R ~,OH
R1 o (Il)
., . , . . ~ -
' ., ' - .
- . . .. ~ . .~ .
.
.
WO 93/14096 PC r/GB93/ooos6
J~V3
-1 5-
or an active derivative thereof. with an amine of formula (111)
R3 O
HN
~ X
R4 R5 (111)
followed by removal of any protecting groups~
Active derivatives of acids for formula (Il) include for example a~id
anhydrides, or acid halides, such as acid chlorides~
The coupling reaction may be performed using standard conditions ~or
amination reactions of this type~ Thus, for example the reaction may be
achieved in a solvent, for example an inert organic solvent such as an et~er,
e.g. a cyclic ether such as tetrahydrofuran, an amide e.g. a substituted arnide
such as dimethylformamide, or a halogenated hydrocarbon such as
dichloromethane at a low temperaturs, e.g. -30C to amibient temperature,
such as -20C to 0C, optionally in the presence of a base, e.g. an organic
base such as an amine, e.g. triethylamine or a cyclic amine such as N-
methylmorpholine~ Where an acid of formula (Il) is used, the reaction r~3ay
additionally be performed in the presence of a condensing agent, ~tor
example a diimide such as N,N'-dicyclohexylcarbodiimide, advantageousiy
in the presence of a triazole such as l-hydroxybenzotriazole Alternatively,
the acid may be reacted with a chloroformate for example
ethylchlorotormate, prior to reaction with the amine of formula (111)~
Free hydroxyl or carboxyl groups in the starting materials of formulae (Il) and
(1ll) may need to be protected during the coupling reaction. Suita~le
protecting groups and methods for their removal may be those men~ion~d
above~
. ~ . : . ~ : . . . .: .
, . , . ~ . . ,,, -
WO 93/14096 PCT/GB93/00086
w ' ;~ ~ ~ V V~ -16-
Compounds of formula (Il) for use in this reaction are preferably those
wherein at least one of R6 or R7 in the group R is other than a hydrogen
atom. Conveniently, each of R6 and R7 is an optionally substituted alkyl, aryl
or aralkyl group. Such groups, when present in compounds of the invention
may be cleaved as described below to yield other compounds of the
invention wherein R6 and/or R7 is each a hydrogen atom.
It will be appreciated that where a particular steroisomer of formula (I) is
required, this may be obtained by resolution of a mixture of isomers
following the coupling reaction of an acid of formula (Il) and an amine of
formula (111). Conventional resolution techniques may be used, for example
separation of isomers by Chromatography e.g. by use of high performance
liquid chrormatography. Where desired, however, appropriate homochiral
starting materials may be used in the coupling reaction to yield a particular
stereo isomer of formula (1). Thus, in particular process a compound of
formula (la) may be prepared by reaction of a compound of formula (lla)
R ~OH
o (lla)
with an amine of formula (Illa)
HN~X
R5 (Illa)
as described above.
' : .. ,-,, . ~ ,~
.
~. ~ , , .
wo 93/14096 Pcr/GB93/ooo86
1 7
Compounds of formula (I) wherein R is a group -P(o)(X1R6)X2R7 and R6
and/or R7 is a hydrogen atom may be prepared from a corresponding
compound of formula (I) wherein R6 and/or R7 is an optionally substituted
alkyl, aryl or aralkyl group by a cleavage reaction, using for example a
reagent such as trialkylsilyl halide, e.g. a trialkylsilyl bromide such as
bromotrimethylsilane, in an inert solvent such as a halogenated
hydrocarbon e.g. dichloromethane, or an aqueous acid or alkali; or, when R6
and/or R7 is an aralkyl group by hydrogenolysis using reagents and
conditions as described below for the preparation of intermediates of
formula (Il).
In the following description of the preparation of intermediate compounds
the groups R6 and/or R7 in the group R are preferably other than a hydrogen
atom.
Intermediate acids of formula (Il) may be prepared from a corresponding
ester of formula (IV)
R2
R~,OR1C
R1 (IV)
where R15 is an aralkyl group, such as a benzyl group, by hydrogenolysis,
for example by reaction with hydrogen in the presence of a metal catalyst,
e.g. palladium, on a support such as carbon. The reaction may be
performed in a solvent such as an alcohol, e.g. methanol optionally at an
20elevated pressure and temperature.
i
.. . . . -
., . .
. . ,
WO 93/14096 PCT/GB93/00086
~ i u .~ ~ ~ 9 -1 8-
The intermediates of formula (IV) may be prepared by reaction of an acrylate
of formula (V)
~,R15
R1 o (V)
with a phosphite:P(OR16)(X1R6)X2R7 where R16 is a leaving group, for
example a silyl group such as a trialkylsilyl group e.g. a trimethylsilyl group,at an elevated temperature.
Acrylates of formula (V) may be prepared by reaction of an mono-ester of
formula (Vl)
O O
HOJ~oR15
R2 (Vl)
with an aldehyde R1CHO or polymer thereof e.g. paraformaldehyde or
paraldehyde in the presence of a base, for example an organic base such
as piperidine. The reaction may be performed in a solvent, such as pyridine,
optionally at an elevated temperature.
Mono-esters of formula (Vl) may be prepared by hydrolysis of the
corresponding di-ester of formula (Vll)
,, . ., . . . ~ .
, . . - - -
. . . . - ,
.
, . -
wo 93/14096 ~- PCT/GB93/00086
~ i iJ ~ù 9
, g
o o
R15OJ~oR1s
R2 (Vll)
using a base, for example an alkali hydroxide such as potassium hydroxide,
in an inert solvent such as dioxan at a low temperature e.g. around OC.
Diesters of formula (Vll) may be prepared by alkylation of the corresponding
malonates of formula R15OCoCH2CO2R15 with a halide R2Hal, where Hal is
a halogen atom such as a chlorine or bromine atom in the presence of a
base, e.g. a hydride such as sodium hydride in a solvent such as
tetrahydrofuran at ambient temperature.
Malonates of formula R15OCoCH2CO2R15 are either known compounds or
may be prepared by methods analogous to those used for the preparation of
the known compounds.
Intermediate phosphites of ~ormula :P(oR16)(X1R6)X2R7 for use in the
preparation of intermediates of formula (IV) may be prepated by react!on of a
phosphite HP(o)(X1R6)X2R7 with an appropriate amine (R15)2NH e.g. a
silazane, at an elevated temperature, e.g. the reflux temperature.
.
Phosphites of formula HP(o)(X1R6)X2R7 are either known compounds or
rnay be prepared by methods analogous to those used for the preparation of
the known compounds.
In an alternative process, intermediate acids of formula (Il) may be prepared
by reaction of an acid R2CH2CO2H with a phosphonate
P(O)(X1R6)(X2R7)CH2OR16 where R16 is a leaving group, for example a
- . . ..
- : . ~: ,, . . . .,~ . .
. .. . - . . . . . .
WO 93~140~6 PCr/GB93/00086
-20-
trifluoromethylsulphonyloxy group in the presence of a base such as n-
butyllithium in a solvent such as tetrahydrofuran. Phosphonates for use in
this reaction may be prepared from the corresponding compound
P(O)(X1R6)(X2R7)CH20H by reaction with paraformaldehyde in the presence
of a base such as triethylamine at an elevated temperature followed by
reaction with a halide R16Hal in the presence of a base such as sodium
hydride in a solvent such as an ether. Phosphonates
P(O)(X1R6)(X2R7)CH20H are either known compounds or may be prepared
by methods analogous to those used for the preparation of the known
compounds.
Intermediates-~f formula (111) and acids of formula R2CH2CO2H are either
known compounds, or may be prepared from known starting materials by
methods analogous to those used for the preparation of the known
compounds.
The homochiral acids of formula (lla) may be prepared according to another
feature of the invention by oxidation of an oxazolidinone of formula (Vlll)
,1~
R 1~ N O
O
~ Ph (Vlll)
(where Ph is a phenyl group)
using an oxidising agent such as peroxide, e.g. hydrogen peroxide in a
solvent such as an ether e.g. a cyclic ether such as tetrahydrofuran, at a low
temperature, e.g. around 0C followed by treatment with a base. such as
lithium hydroxide, at an elevated temperature.
-
` ' ' . '. ' '
W093/t4096 `. . ~ I PCr/GB93/00086
The compounds of formula (Vlll) are novel, particularly useful, intermediates
for the preparation of stereoisomers of formula (la) and form a further aspect
of the invention.
The compounds of formula tVIII) may be prepared by reaction of an acyl
halide RCH2CH(R2)COHal (where Hal is a halogen atom such as chloride,
bromine or iodine atom) with a solution of (S)-4-(phenylmethyl)-2-
oxazolidinone in the presence of a base such as n-butyl lithium in a solvent
such as tetrahydrofuran at a low temperature, e.g. around -78C.
Acyl halides RCH2 CH(R2)COHal may be prepared by treatment of the
corresponding known acids RCH2CH(R2)CO2H with conventional
halogenating agents for example thionyl halides under standard reaction
conditions.
The compounds according to the invention are potent and selective
inhibitors of gelatinase. The activity and selectivity of the compounds may
be determined by the use of appropriate enzyme inhibition test for example
as described in Example A hereinafter. In our tests using this approach,
compounds according to the invention have been shown to inhibit
gelatinase with Ki values in the picomolar-nanomolar range and to have
particularly useful selectivity for gelatinase over stromelysin and
collagenase.
The ability of compounds of the invention to prevent tumour cell invasion
may be demonstrated in a standard mouse model. Thus, briefly, nude mice
may be inoculated with a tumour cell line showing gelatinase - dependent
invasion and the ability of compounds according to the invention to reduce
subsequent lung tumour colonisation may be evaluated in accordance with
standard procedures. In out tests, compounds according to the invention,
when administered intravenously at 1mg/kg to mice in the above model
- . . . . .
.
.
wo 93/14096 pcr/GB93/ooo86
; 9 -22-
have reduced lung tumour colonisation to negligable levels, and do not
cause any adverse effects at this dose.
The compounds according to the invention can be expected to be of use to
prevent tumour cell metastasis and invasion. The compounds may therefore
be of use in the treatment of cancer, particularly in conjunction with
radiotherapy, chemotherapy or surgery, or in patients presenting with
primary tumours, to control the development of tumour metastasises. Thus,
according to a further aspect of the invention we provide a compound of
formula tl) for use in the treatment of cancer to control the development of
tumour metastasises. Particular cancers may include breast, melanoma,
lung, head, neck or bladder cancers.
For use according to this aspect of the invention, the compounds of formula
(I) may be formulated in a conventional manner, optionally with one or more
physiologically acceptable carriers, diluents or excipients.
Thus according to a further aspect of the invention we provide a
pharmaceutical composition comprising a compound of formula (I) and a
pharmaceutically acceptable diluent, carrier or excipient.
In a still further aspect the invention provides a process for the production ofa pharmaceutical composition comprising bringing a compound of formula
(1) into association with a pharmaceutically acceptable diluent, carrier or
excipient.
Compounds for use according to the present invention may be formulated
for oral, buccal, parental or rectal administration or in a form suitable for
nasal administration or administration by inhalation or insufflation.
For oral administration, the pharrnaceutical compositions may take the form
of, for example, tablets or capsules prepared by conventional means with
wo 93/1~096 pcr/GB93/ooo86
~it3~iJ9
-23-
pharmaceutically acceptable excipients such as binding agents (e.g.
pregelatinised maize starch, polyvinylpyrrolidone or hydroxypropl
methylcellulose); fillers (e.g. Iactose, microcrystalline cellulose or calcium
hydrogen phosphate); lubricants (e.g. magnesium stearate, talc or silica);
disintegrants (e.g. potato starch or sodium glycollate); or wetting agents (e.g.sodium lauryl sulphate). The tablets may be coated by methods well known
in the art. Liquid preparations for oral administration may take the form of,
for example, solutions, syrups or suspensions, or they may be presented as
a dry product for constitution with water or other suitable vehicle before use.
Such liquid preparations may be prepared by conventional means with
pharmaceutically acceptable additives such as suspending agents,
emulsifying agents, non-aqueous vehi¢les; and preservatives. The
preparations may also contain buffer salts, flavouring, colouring and
sweetening agents as appropriate.
Preparations for oral administration may be suitably formulated to give
controlled release of the active compound.
For buccal administration the compositions may take the form of tablets or
lozenges formulated in conventional manner.
The compounds of formula (I) may be formulated for parental administration
by injection e.g. by bolus injection or continuous infusion. Formulations for
injection may be presented in unit dosage form. The compositions for
injection may take such forms as suspensions, solutions or emulsions in oily
or aqueous vehicles, and may contain formulatory agents such as
suspending, stabilising and/or dispersing agents. Alternatively, the active
ingredient may be in powder form for constitution with a suitable vehicle, e.g.
sterile pyrogen-free water, before use.
The compounds of formula (I) may also be formulated in rectal compositions
such as suppositories or retention enemas, e.g. containing conventional
-. . ~
- . .
.. - .. . . . ..
: - ~
. . . . . . .
. . . . . . .
Wo 93/14096 pcr/GBs3looo86
ù 3 -24-
suppository bases such as cocoa butter or other glycerides.
In addition to the formulations described above the compounds of formula (I)
may also be formulated as a depot preparation. Such long acting
formulations may be administered by implantation or by intramuscular
injection.
For nasal administration or administration by inhalation the compounds for
use according to the present invention are conventiently delivered in the
form of an aerosol spray presentation for pressurised packs or a nebuliser,
with the use of suitable propellant, e.g. dichlorodifluoromethane,
trichlorofluoromethane, dichlorotetrafluoroethane, carbon dioxide or other
suitable gas.
The compositions may, if desired, be presented in a pack or dispenser
device which may contain one or more unit dosage forms containing the
active ingredient. The pack or dispenser device may be accompanied by
instructions for admininstration.
The doses of compounds of formula (I) used to control the development of
tumour metastasises will vary depending on the condition of the patient to be
treated but in general may be in the range around 0.5mg to ~Omg/kg body
weight, particularly from about 1 mg to 40mg/kg body weight. Dosage units
may be varied according to the route of administration of the compound in
accordance with conventional practice.
. - . ~ -
- - ~ ......
WO 93/14096 PCT/GB93/00086
- ~2~ il 9
DescriDtion of S~eCi~ic Embo~imen~
The invention is further illustrated in the following non-limiting Examples.
In the Examples, the following abbreviations are used:
RT - room temperature
DCCI- N,N'-dicyclohexylcarbodiimide
DMF - dimethylformamide
THF - tetrahydrofuran
TFA - trifluoroacetic acid
RPHPLC reverse phase high performance liquid chromatography
HOBT- N-hydroxybenzotriazole
Et20 - diethylether
EDC - 1-(3-dimenthylaminopropyl)-3-ethylcarbodiimide,
hydrochloride
All temperatures are in C
.. . . -
- . . - . : .:
. - , . ~ . . . . ~ .
- . ~ ' , .
WO 93/14096 PCI`/GB93/00086
ù 9 -26-
lntermediate 1
E~nzyl (2-benzvloxvcarboxvl-5-phenyl! eentanoate
To a suspension of NaH (5.049) in dry THF (200ml) was added dropwise
over a period of 1 hour, dibenzyl malonate (~6.869, 50ml) and the
temperature allowed to rise to 43. 1-Bromo-3-phenylpropane (39.8g,
30.4ml) was added and the reaction kept at RT for 3 hours. The mixture was
refluxed for 4 hours, cooled, poured into H2O/NaCI/NH4CI (400ml) and
extracted with CH2CI2 (4 x 100ml). The organic layer was dried (MgSO
and concentrated to give a yellow oil. The ~!~ cQmpound (63.6g) was
purified on s~lica gel (Merck 938~) using Et2O/hexane (5:95)
Et2O/hexane (25:75).
1 HNMR (CDCI3) ~ 7.0-7.40 (15H, m), 5.15 (4H, s), 3.45 (1 H, d, d), 2.60 (2H,
t),1.85-2.0~ (2H, m),1.55-1.65 (2H, m)
Intermediate 2
2-Benzyloxycar~ -ehenvleentanoic acid
To Intermediate 1 (63.69) dissolved in 1,4-dioxan (400ml) was added KOH
(8.879) in H2O (110ml) at 0. The mixture was allowed to rise to RT and
stirred overnight. The solvent was removed in vacuo and H2O (lOOml)
added. Extraction with Et2O removed any undesired diester and the
aqueous phase was acidified (pH~1) with 1.0M HCI. The product was
extracted into Et2O (3 x 100ml), dried (MgSO4) and concentrated to give the
title comDound (29.0g).
1HNMR (CDCI3) ~ 10 (1H, bs) 7.10-7.60 (10H, m), 5.20 (2H, s) 3.48 (1H, t),
2.~2 (2H, t),1.86-2.10 (2H, m),1.60-1.80 (2H, m)
WO 93/1~096 i` PCl`/GB93/0~086
j~ 2 V ~ ~ ~) 9
-27-
lntermediate 3
O-Benzvl-2-(3-Dhenvl~proeylproD-2-enoate
Paraformaldehyde (380mg) was added to a stirred solution of the
Intermediate 2 (2.69) dissolved in anhydrous pyridine (25ml) containing
s piperidine (118mg, 137111). The reaction was heated at 60 for 2.1/2 hours,
cooled, poured into 1.0 MHCI and extracted with Et2O (3 x 50mL). The Et2O - ^
layer was dried (MgSO4), and concentrated to give the title compound
(1.3g).
1HNMR (CDCI3) â 7.12-7.36 (10H, m), 6.21 (1H, d), 5.55 (1H, d), 5.20 (2H,
s), 2.62 (2H, t),2.37 (2H, t),1.76-1.88 (2H, m).
Intermedi~ 4
Dimethvltrimethvlsilvl~hosDhite
A mixture of dimethylphosphite (9.18ml) and hexamethyldisilazane
(25.32ml) was heated under reflux (bath temperature 140) for 3h. The
` 15 crude mixture was distilled in vacuo lapproximately 5mm Hg) through a
12cm vigueux column and the traction distilling at bath temperature 95-
11 0 collected to give the ~!~ compound (7.20g)
HNMR (CDCI3) O/ppm 3.50 (s,3H),3.46 (s, 3H),0.26 (s,9H).
Intermediate 5
BenaLl (~-~imethoxvQh~hinylmethvl-5-Qhenvl)r~entanoate
A mixture of Intermediate 4 (1.877g) and Intermediate 3 (1.044g) were
stirred at 70 (N2 atom) for 18h. Water (10ml) and chloroform (10ml) were
.. . . . - ;
. . . . . .
-
WO 93/14096 ~ PCT/GB93/00086
w ~ 3
-28-
added and the mixture stirred vigrously for 0.5h. The mixture was partitioned
between water (10ml) and chlorofom (15ml) and the aqueous layer further
extracted with chloroform (20ml). The organic layers were dried (MgSO4)
and concentrated in vacuo to a crude oil 1.559. This was chromatographed
on silica 60 ('Merck' 9385) eluting with ethyl acetate-methanol (2%) to give
the title comDound as an oil (0.34239)
HNMR (CDCI3) ~ 7.35 (5, 5H), 7.77 (t, 2H), 7.17 (t, 1H), 7.08 (d, 2H), 5.115
(s, 2H), 2.83 (m,1H), 2.57 (t, 2H), 2.29 dd and 2.23 dd (-together 1 H), 1.87
dd and 1.83 dd (together 1 H) and 1.76-1.50 ppm (br in 4H).
o Intermediate 6
2-DimethoxvehosDhinvlmethvl-5-Dhenylpentanoic acid
A mixture of Intermediate 5 (0.33979) and 10% palladium on carbon catalyst
(50mg) in methanol (20ml) was stirred under an atmosphere of hydrogen for
18h. Tlc silicagel 60 (chloroform-methanol 10% eluent) showed conversion
to one product with RfO,23. The mixture was filtered and concentrated in
vacuQ to give the ~ com~ound as a colourless oil (0.24989)
1HNMR (CDCI3) ~ 7.30-7.19 (m, 3H), 7.16 (d, 3H) 3.76 (s, 3H), 3.72 (s, 3H),
2.79 (m, 1 H), 2.63 (t, 2H), 2.30 (dd) and 2.24 (dd)- together 1 H,1.88 (dd) and1.82 (dd) - together 1 H and 1.76-1.66 (m, 4H) ppm.
Intermediate 7
L ~-cyclohexylalanine-N-(2-phenvlethvl~ amide
tBoc-,B-cyclohexyl-L-alanine (1.359, 5mmoL) was dissolved in dry CH2C12.
4-Nitrophenol (695mg, 5mmoL) was added followed by DCCI (1.03g,
5mmoL). After 1 hour at room temperature the reaction was concentrated m
vaçuo, ether was added and the solution filtered. The residue was
Wo 93/14096 pcr/Gs93/ooo86
ù 9
-29-
concentrated in vacuo, dissolved in CH2CI2 (10ml) and phenethylamine :
(690111, 5.5mmoL) was added. The reaction was poured into NaHC03 and
extracted with CH2CI2 (3 x 20ml), was dried (Na2S04) and concentrated in
~Q. Purification on silica gel (Merck 93~5) using
CH2CI2~CH2CI2/MeOH 85:15) gave a clean oil (9OOmg) which was
dissolved in CH2C12/TFA (9:1) and left a RT for 30 min. The reaction was
concentrated in~L~o~ dissolved in CH2C12 (~Oml) and poured into
Na2C03 (aq). The organic layer was separated, dried (Na2S04) and
concentrated in vacuo to give an oil which was purified on silica gel (Merck
9385) using CH2CI2/methanol/triethylamine 96:3:1 to give the title
comDound as an oil (500mg).
1 H NMR (CDCL3) ~ 0.95 (m, 2H),1.25 (m, 6H),1.55 (bs, 2H),1.65 (m, 5H),
2.8 (t, 2H, J=6HZ), 3.4 (dd,1 H, J=3 and 1 OHZ), 3.5 (dd, 2H, J=6 and 12HZ),
7.2 (m,5H)
Intermediate 8
O-(Diethyl)hvdroxvmethvlohosphonate
A mixture of diethyl phosphite (209) paraformaldehyde (4.44g) and
triethylamine (2ml) was heated to 60C for 1 hour. The reaction was then
cooled and partitioned between chloroform and aqueous citric acid. The
organic layer was separated, washed with brine, dried (MgSO4) and
evaporated to give the title compound as a clear colourless oil. (20.73g).
HNMR (CDCI3)
4.2(4H, m); 3.9 (2H, d); 1.35 (6H, t).
~ .
.
. , ~ . ' ' : :
.
.
.
wo 93/14096 pcr/GB93/ooo86
, 3 -30-
lntermediate 9
O-(Diethvl)trifluoromethvlsuleh~nyloxvmethylehosDhonate
To a suspension of sodium hydride (3.85g) in dry ether (200ml) at -30C
was added by syringe trifluoromethanesulphonyl chloride (25g). A solution
of Intermediate 8 (20.739) in dry ether (20ml) was added dropwise over 20
minutes maintaining the internal temperature at -20C. After stirring the `
suspension for 2 hours at -20C, the excess hydride was removed by
filtering through Celite. The filtrate was diluted with dichloromethane,
washed twice with 10% aqueous sodium bicarbonate, separated, dried
(MgSO4) and evaporated to give the title compound as a pale yellow oil.
The Ii~L~. comeound was purified on silica, eluting with 35% ethyl acetate in
hexane (10.59).
H NMR (CDCI3)
~ 4,60 (2H, d); 4.25 (4H, m); 1.40 (6H, t)
Intermediate 1 0
2-DiethoxvDhosDhinylmethyl-5L~-methylehenyl~Dentanoic acid
A solution of n-butyllithium in hexane (13ml) was added to a solution of di-
isopropylamine (2.94ml) in dry THF (30ml) at 0C. After stirring for 30
minutes, a solution of p-toluylvaleric acid (2.0g) in dry THF (15ml) was
added and the reaction mixture was warmed to 35C for 30 minutes. After
cooling to room temperature, hexamethylphosphorus triamide (1.89ml) was
added, followed by Intermediate 9 (3.12g) as a solution in THF (15ml). The
reaction was stirred for 3 hours at room temperature then quenched with
aqueous citric acid, extracted into ~t2O, dried (MgSO4) and evaporated. The
residue was chromatographed on silica, eluting with 3 - 10% CH30H in
wo 93/ 14096 Pcr/ G s93/00086
~ i ù ~ , 9
-31 -
CH2CI2~ to give the title compound as a clear gum (1.26g).
HNMR (CDCI3)
7.05 (4H, m); 4.05 (4H, m); 2.5-2.85 (3H, m); 2.3 (3H, s); 2.25 (1 H, m); 1.~-
1.9 (5H, m); 1.25 (3H, t).
Exam~ 1
. . .
3-PhosDhono-2R S-~henvlproDvl-1 -oxoeroovl]-L-,B-cyclohexvlalanine-N-~2-
~henylethvl) amide. dimethvlester
A solution of Intermediate 6 (151.7mg) in dry tetrahydrofuran (3.0ml) was
cooled to -30 and treated with N-methylmorpholine (59.1~L1) and ethyl
chloroformate (49.0111). The mixture was stirred at -30 for 1 h and a solution
of Intermediate 7 (240.8mg) and N-methylmorphorpholine (59.1ml) in dry
DMF (2.0ml) added dropwise at -30C. The mixture was allowed to warm up
to RT overnight and the solvent removed in vacuo. The residue was
partitioned between water (15ml) and ethyl acetate (20ml) and the aqueous
layer further extracted with ethyl acetate (2 x 20ml). The combined organic
layers were dried (MgSO4) and concentrated in ~uo to give an oil
(304.6mg) which was chromatographed on silicagel 60 ('Merck' 9385)
eluting with ethyl acetatemethanol (2%) to give the title comeound as a
mixture of diastereoisomers and as an oil (189.4mg)
1 HNMR (CDCI3) ~ 7.64 (br, t, 0.5H), 7.40-7.10 (br, m,10H), 6.31 (br, t,0.5H~
5.17 (d, 0.5H), 5.78 (d,0.5H), 4.5 (br, dt) and 4.14 (dd) -together 1H, 3.72 ~s,3H), 3.69 (s, 3H), 3.45 (m, 2H), 2.83 (t) and 2.69 (t) - together 2H, 2.60 (br, q,
2H), 2.45 (br, m,1 H) 2.35-2.05 (m,1 H) and 2.0-0.8 (br, m,18H) ppm.
.. . .
WO 93/14096 PCT/G~93/00086
3 2-
Example 2
[3-Phos~hono-2R-~henyleropvl-1 -oxo~ro~yl]-L-~-cvclohexvlalaninç-N-(2-
Dhenvlethyl) amide
A solution of the compound of Example 1 (189.4mg) in dry dichloromethane
(5.0ml) was treated with bromotrimethylsilane (1.0ml) and the mixture stirred
overnight at room temperature. Methanol (15.0ml) was added and the
mixture stirred at room temperature for 0.5h. The solvent was removed in
vacuo, the residue azeotroped with ethanol (40ml) and then dissolved in
ethanol water (1 :1) (30ml). The solution was stirred at room temperature for
0.5h and the solvent removed in vacuo to give a mixture of RS and SS
diastereoisome`~s as an oily solid. This was separated by preparative HPLC
on a Dynamax C18RP column (21.4mm dia) using 0.1% TFA-H2O (A) and
0.1% TFA-CH3CN(C) and gradient elution between (70:30) and 5:95)
respectively. Peak 1 (elution time 14.71 mins) yielded the RS isomer
(20 0mg) as a white solid.
1 HNMR (CDCI3) ~ 7.27-6.95 (br, m,10H, 4.25 (t, 1 H), 3.27 (m, 2H)2.75-2.40
(m, 5H), 2.03 (dd) and 1.95 (dd) -together 1 H and 1.80-0.68 (br, m,18H) ppm
Peak 2 (elution time 15.98 mins) yielded the SS isomer (58.4mg) as a white
solid.
1HNMR (CDC13) ~ 7.35-7.0 (br, m,10H), 4.25 (dd,1 H), 3.30 (br, t,2H), 2.80-
2.40 (br, m,5H), 2.15-1.94 (m,1H) and 1.85-0.6 ppm (br, m,18H)
The following compounds w-ere prepared in a similar manner to the
compound of Example 2 by reaction of Intermediate 6 with an appropriate
amide, and subsequent hydrolysis of the resulting dimethyl ester followed by
separation of diastereoisomers, as described above.
WO 93/14096 PCI`/GB93/00086
ù 9
Example 3 ~-
[3-PhosDhono-2S-Dhenvlpro,~1-1-oxoeroDy~l-L-~~clohe~y!~lanine-~-
alanine
1HNMR (CDCI3) ~7.15-6.90 (m, 5H), 4.19 (dd,1H), 3.14 (m~ 2H), 2.65-2.40
s (m, 3H), 2.34 (t, 2H),1.99 (dd) and 1.96 (dd) -together 1 H and 1.70-0.52 ~r,
m,18H)
Examl~le 4
~-Pho~hono-2R-phenvlDroDvl-1 -oxoDropyl]-L-,B-cvclohexylalanine
HNMR (CDCI3) ~ 7 30-7.10 (m, 5H), 4.26 (br, t,1 H), 3.28 (m, 2h), 2.74-2.46
(m, 3h), 2.35 (t, 2H), 2.03 (dd) and 1,97 (dd) together 1 H and 1 90-0 54 (br,
m,18H
Example 5
~,-Pho ~ ho~Q ~ Qhenv~QQyl-1-oxQQ ~ yl ~ ~ ~ Lohe ~l~l~ine-~-
alanine. methvl ester
1HNMR (CDCI3) ~ 7 22-7.0 (m, 5H), 4.25 (dd, 1 H), 3.56 (s~ 3H), 3.32 ~d, tr,
2H), 2.70-2.46 (m, 3H), 2.44 (t, 2H), 2.14 (br, h, 1H) and 1.80-0.65 ppm (br,
m,18H)
wo 93/1~096 pcr/Gss3/ooo86
J t v
Example Ç
[3 -Pho spho no-2 R.S- phQ~Qropv 1-1 -oxop ropy 11- L-~-cycl ohexvlal anin e-N_
~4(3-a~noeroDvl)morpholine] amide. bromine salt
ISOMER i 1H ~ (DMSO db) 8.48 (br, t, ~ 0.5H), 8.30 (d, ~ 0.5H),7.34-7.20 (m,
2H), 7.20-7.10 (br, d, 3H), 4.16 (m, 1H), 3.95 (m, 2H) 3.80-3.0 (br, m)
overlapped with H2O,2.60 (m,1H) 2.0 (br, q, lH) and 1.80-0.60 (br, m,18H).
ISOMER 2 1HNMR ~ (DMSO db) 8.57 (br, t, - 0.5H) 8.27 (d, ~ 0.5H), 7.30-
7.20 (m, 2H), 7.20-7.10 (br, d, 3H) 4.14 (m,1H), 3.92 (m,2H), 3.80-3.0 (br, m)
overlapped with H2O, 2.65 (m, lh), 1.95 (br, q, 1H) and 1.75-0:60 (br, m,
18H) ppm
Example 7
~3:phos~hQnQ:2B~ 4 mQ~hvl~hQn~ QcQ~ QXQ~Q~Q~
cyclohexylalanine-N-(2-phenylethyl!amide. diethylester
A solution in dry DMF (5ml) of Intermediate 10 (250mg), triethylamine
(101.4~L1); N-hydroxybenzotriazole (98.6mg), Intermediate 7 (200mg) and
EDC (140mg) was stirred at room temperature for 5 hours under nitrogen.
The reaction mixtur0 was partitioned between Et2O and aqueous citric acid,
the organic layer separated, washed with aqueous NaHCO3, dried (MgSO4)
and evaporated to give the ~L~!Q compound (320mg) as a 50:50 mixture of
diastereomers.
H NMR (CDCI3)
7.9(0.5H,t); 7.0-7.3 (9H, m); 4.5 (0.5H, m); 4.5 (0.5H, m); 4.35 (0.5H, m); 4.0
(4H, m); 3.40 (2H, m); 2.70-2.90 (2H, m); 2.40-2.10 (8H, m); 0.80-2.0 (23H,
m).
,,
WO 93/1~096 ~ PC'r/GB93/00086
-35-
Example 8
~3 PhQs,QhQno 2B,S ~4 meIh~!~hen~lLQ o~
cvclohexvlalanine-N-(2-phenylethvl!-amide
A solution of ~he compound of Example 7 (320mg) in dry CH2CI2 (20ml) was
treated with trimethylsilylbromide (2ml) at room temperature for 2.~ days
The reaction was quenched with methanol (5ml) and volatile material was
removed. The two diastereoisomers were separated by reverse phase
HPLC (Dynamax 60A). Eluent: 50 - 100% acetonitrile in 0.1% TFA/water,to
yield the R-isomer (49mg).
1H NMR (CD30D)
7.15-7.30 (5H, m); 7.0 (4H, m); 4.35 (1 H, t); 3.35 (2H, m); 2.0-2.75 (1 OH, m);
0.80-1.85 (1 7H, m).
EXAMPLE A
The activity and selectivity of the compounds of the invention may be
determined as described below.
All enzyme assays to determine Ki values were performed using the peptide
substrate Dnp-Pro-Leu-Gly-Leu-Trp-Ala-D-Arg-NH2. [M. Sharon Stock and
Robert D. Gray. JBC 264, 4277-81, 1989). The enzymes cleave at the Gly-
Leu bond which can be followed fluorimetrically by measuring the increase
in Trp fluorescence emission associated with the removal of the quenching
dinitrophenol (Dnp) group.
Essentially, enzyme (e.g. gelatinase, stromelysin. collagenase) at 0.08-2nM;
a range of inhibitor concentrations (0.1-50 x Ki) and substrate (approx.
20~1m) are incubated overnight in G.1M Tris/HCI buffer, pH 7.~, containing
0.1M NaCI, 10mM CaCI2 and 0.05%. Brij 35 at either room temperature or
37C depending on the enzyme. The reaction is stopped by adjusting the
- . ., . ~ ~
... . .
wo 93/14096 pc~r/GBs3/ooo86
9 -36-
pH to 4 using 0.1M sodium acetate buffer and the fluorescence read at an
excitation wavelength of 280nm and emission wavelength of 346nm.
Kj values can be established using the equation for tight-being inhibition:-
--~/(Kj(app) + [1]2 + 2 (Kj(app) - [I])[E] + [E]2 (Kj(app)+ [I] - [
2[q ~ J
where V0 is the initial rate of reaction in the absence of inhibitor, Vj is the
initial rate in the presence of inhibitor, [E] is the total enzyme concentrationand [~] the total inhibitor concentration in the reaction mixture.
For stromelysin and collagenase, Kj (app) was assumed to approximate to
the true Kj as [S] ~< Km for the substrate hydrolysis. For gelatinase the K
was determined by performing the analyses at several substrate
concentrations. A plot of Kj(app) vs. [S] then gave the tnue Kj as the value of
the y-axis intercept.
The following results were obtained with compounds according to the
invention:
. Ki(nM)
Compound of Collagenase Stromelysin-1 Gelatinase-72KD
Examele No.
2 25~1M 701 18.9
4 22.1 ~M 1 .7311M46.5
8 1 7~1M 277 2.5
., : . , . . . . : .
. ~. ..
,. . . .. . . .