Note: Descriptions are shown in the official language in which they were submitted.
W092/1~698 PCT/CA92/0010~
2 ~
MURINE MONOCLONAL ANTI~ODIES RECOGNIZING
POLYMOhPHIC DETERMINANTS OF HLA
2) Backqround of the Invention
fi) Field of the Invention
This invention relates to the production and use of
a set of monoclonal antibodi~es to HLA and its subtypes
and to the set of monoclonal antibodies so produced.
Such monoclonal antibodies are specific for antigens
coded by the HLA gene complex and therefore are useful in
the typing of human tissue that is to be used in organ
transplants.
(ii) Prior Art
The human system involves the production of serum
proteins, known as antibodies, by the lymphoid cell
series capable of reacting with antigenic determinants-
which trigger their production. Since the conventional
response of the immune system to an antigen with many
antigenic determinants is the production of antibodies to
each determinant, the anti~erum produced is heterologous
in nature and polyclonal, or produced by many different
cells each producing antibodies to a specific
determinant. Antigenic determinants may be referred to
as epitopes when more than one occurs on a single
molecule and particularly when each elicits an antibody
developing, immune response. A single antibody molecule
is specific for a unique antigenic determinant or
epitope.
The introduction of foreign material (antigenic
material) into the body of a vertebrate animal provokes
an immune reaction, the intent of which is to prevent the
antigenic material causing damage to the body and to
facilitate the rlemoval of such material from the body.
The immune system achieves this by producing
immunoglobulin molecules ~hereafter referred to as
antibodies) which have the property of selectively
. ": ''
,: .
'.
Wo92/l5698 PCT/CA92/OOlOS
2io~969 '.
recognising and binding to characteristic sites on the
antigenic material. These sites are known as
determinants and an antiyen may possess one or more such
determinants. Antibodies generated by the immune system
each have specificity to only one determinant but a
number of different antibodies may be produced if the
antigenic material against which antibodies are raised
possesses more than one determinant.
The primary function of antibodies is to protect the
body from harmful foreign material, by agglutinating it,
thereby assisting the normal body processes to remove the
material.
Antibodies are proteins that have the ability to
combine with and recognize other molecules, known as
antigens. Monoclonal antibodies are no different from
other antibodies and, except that they are very uniform
in their properties, recognize only one antigen or a
portion of an antigen known as a determinant.
In the case of cells, the determinant recognized is
an antigen on or in the cell which reacts with the
antibody. It is through these cell antigens that a
particular antibody recognizes, i.e. reacts with, a
particular kind of cell. Thus the cell antigens are
markers by which the cell is identified.
These antigenic markers may be used to observe the
normal process of cell differentiation and to locate
abnormalities within a given cell system. The process of
differentiation is accompanied by changes in the cell
surface antigenic phenotype, and antigens that
distinguish cells belonging to distinct differentiation
lineages or distinguish cells at different phases in the
same differentiation lineage may be observed if the
correct antibody iS available.
Human antibodies have been used both for diagnostic
and therapeutic purposes for a number of years.
Diagnostic techniques include blood typing for
' ' '
W092/15698 PCT/CA92/OOlOS
21~9g9
!
transfusion or transplantation. The oldest is isolation
from immune serum. However, the small concentration of
the antibody of desired specificity among those which are
generally present in serum presents a serious drawback.
Conventional antisera, produced by immuni~ing
animals with tumour cells or other antigens, contain a
myriad of different antibodies differing in their
specificity and properties. In 1975 Kohler and Milstein
introduced a procedure which lead to the production of
quantities of antibodies of precise and reproducible
specificity. The Kohler-Milstein procedure involved the
fusion of spleen cells (from an immunized animal) with an
immortal myeloma cell line. By antibody testing of the
fused cells (hybridomas), clones of the hybridomas were
selected that produced antibody of the desire
specificity. Each clone continued to produce only that
- one antibody, monoclonal antibody (mAb). As hybridoma
cells can be cultured indefinitely (or stored frozen in
liquid nitrogen), a constant supply of antibody with
uniform characteristics is assured.
The preparation of hybridoma cell lines can be
successful or not depending on such experimental factors
as nature of the inoculant, cell growth conditions,
hybridization conditions etc. Thus~-it is not always
possible to predict successful hybridoma preparation of
one cell line although success may have been achieved
with another cell line.
The usefulness of monoclonal antibodies has been
confirmed not only in the field of immunology but also in
many other ~ields. They are hence employed widely.
However, since these antibodies are produced primarily
with hybridoma originated from mouse cell, certain
inherent limitations are obviously imposed on their
application for the diagnosis and treatment of human.
Upon formation of a human monoclonal antibody, it is
necessary to obtain a cell which has been challenged by
WO92/15698 PCT/CA92/00l05
Z105969 4
a desired antigen and can produce a human antibody
specific to the antigen. The in-vivo antigenic
stimulation is not feasible in human except for a certain
class of antigens and there have not been established any
method applicable to various antigens.
It is attempted to obtain permanently-established
human cells by immortalizati.on of antibody-producing
cells, for example, by their fusion with human myeloma
cells, their transformation with an Epstein-Barr virus
(EBV) or the like and then to obtain a monoclonal
antibody from the permanently-established human cells.
Unlike in mouse lines, hybridomas or transformed cells
having the ability of stable production of antibody have
not been obtained in human lines for the time being.
15Monoclonal antibodies are uniform antibodies
directed to a single determinant or epitope on the
antigen molecule which may be repeated at several sites
of the molecule. Obviously, to produce such monoclonal
antibodies in vitro requires selecting a homogeneous
antibody having the desired specif.ications from numerous
antibodies elicited in a conventional polyclonal
response.
More recently, the production of human monoclonal
antibodies has become possible and these may serve as -
tools in diagnostic testing and in therapy. Two major
approaches are new possible for the production of mAB
direct immortalization of immunized lymphocytes with
Epstein-Barr Virus tEBV) and mab production by hybridomas
formed between immortalized human B cell lines (EBV~,
lymphoblastoid, or human or murine myelomas, and human B
lymphocytes from an immunized host. Neither of these
approaches has proved entirely satisfactory.
It is common,experience among practitioners in the
art that EBV transformation, while successful in forming
35 Mab-secreting cultures, will often fail to provide ~ -
antigen specific E~3V transfor~ed cells which have
W O 92/15698 PC~r/CA92/0010~
2~ Q5969
s
sufficiently long life spans to provide reliable sources
of the desired antibodies. This method fails to provide
reliably for antibody production over extended periods.
Previously produced hybridomas between immunized human B
cells and appropriately drug marked murine or human
myeloma or human lymphoblastoid cell lines have suffered
from low frequency of hybrid formation in the case of
human-human hybridizations. Mur.ine-murine hybridomas are
stable, but the antibodies produced are immunogenic if
used in passive therapy.
An immunized experimental animal can sometimes serve
as a source for specific antibody secreting B cells to
provide the immunized lymphoid member of the hybridoma.
This method cannot be used, however, to provide reagents
for HLA or other blood type testing since when human
antigens are injected, the plethora of antibodies
elicited is mostly immunoreactive to antigens common to
all humans, and the desired antigen-specific antibody is
formed only as a very small percentage of the total
response. Further, these non-human antibodies can
themselves result in an adverse immune response if
injected for human therapy.
There are two principal classes of lymphocytes
involved in the immune system of humans and animals. The
first of these (the thymus-derived cell or T cell) is
differentiated in the thymus from haemopoietic stem
cells. While within the thymus, the differentiating
cells are termed "thymocytes". The mature T cells emerge
from the thymus and circulate between the tissues,
lymphatics, and the bloodstream. These T cells form a
large proportion of the pool of recirculating small
lymphocytes. They have immunological specificity and are
directly involve~ in cell-mediated immune responses (such
as graft rejection) as effector cells. Although T cells
do not secrete humoral antibodies, they are sometimes
required for the secretion of these antibodies by the
: ~ .
WO92/1~698 PCT/CA92/00105
2~ 6
second class of lymphocytes discussed below. Some types
of T cells play a regulating function in other aspects of
the immune system. The mechanism of this process of cell
cooperation is not yet completely understood.
The second class of lymphocytes (the bone marrow- ;
derived cells or B cells) are those which secrete
antibody. They also develop from haemopoietic stem
c~lls, but their differentiation is not determined by the
thymus. In birds, they are differentiated in an organ
analogous to the thymus, called the Bursa of Fabricius.
In mammals, however, no equivalent organ has been
discovered, and it is thought that these B cells
differentiate within the bone marrow.
It is now recognized that T cells are divided into
at least several subtypes, termed "helper", "suppressor",
and "killer" T cells, which have the function of
(respectively) promoting a reaction, suppressing a
reaction, or killing (lysing) foreign cells. These -
subclasses are well understood for murine systems, but
they have only recently been described for human systems.
The ability to identify or suppress classes or sub-
classes of T cells is important for diagnosis or
treatment of various immunoregulatory disorders or
conditions.
2S Previous suppressor factors have been reported in
the literature. Such factors have potential use for
example, in the treatment of patients with cancer, graft
versus host diseases, autoimmune diseases and lympho-
proliferative malignancy disorders, e.g. leukemia. -
The discovery of the human major histocompatibility
complex (hereinafter "MHC") was when leukoagglutinating
antibodies were first found in the sera of multiply
transfused patie~ts in a pattern that suygested the
- antisera were detecting allo-antigens, antigens present
on the cells of some individuals of a given species which
are products of a polymorphic genetic locus. The role of
WO92/15698 PCT/CA92/00105
these antigens in determining the success of tissue and
organ transplants was soon appreciated and provided the
initial studies of genes that determine human leucocyte
antigens (hereinafter termed "HLA").
The HLA system is extremely polymorphic, having
multiple different alleles at each known genetic locus.
Based on their tissue distribution and structure, HLA
antigens have been divided into two broad classes: Class
I antigens which include the HLA A, HLA-B, and HLA-C
antigens found on virtually every human cell and which
have counterparts in other mammalian cells including the
murine system; and Class II antigens including the HLD-,
DR, DQ, and DP antigens found chiefly on the surface of
immunocompetent cells including macrophages/monocytes,
activated T-lymphocytes, and B lymphocytes. Class II
antigens also have counterparts in other mammalian
systems such as murine mammals. The presence of these
Class I and Class II antigenic molecules plays a major
role in the functional heterogeneity of p~ripheral T-
cells.
The different re~ulatory and effector functions ofT-cells are mediated by different subpopulation of cells
which can be distinguished by differences in their
phenotypes and antigenic determinants (identifiable by
different monoclonal antibodies). This has led to the
typing T cell functional subsets in accordance with the
expression of specific surface molecul~s which are
commonly designated by the letter "T" followed by a
number. Based on functional differences between T4 and
T8 cells, the peripheral blood T-cells can be broadly
divided into two populations: one population
constituting approximately 65% of peripheral blood T-
cells is T4+; thelother constituting approximately 35% of
all peripheral blood T-cells is T8+. The T8+ cell may be
activated to become a cytolytic T lymphocyte (hereinafter
termed "CTL cell") which functions as a cytotoxic
W092/1~698 PC~/CA92/00105
~9~ :
effector cell and plays an important role in the hosts'
defense against foreign bodies. In combination with
natural killer cells (hereinafter termed "NK cells") and
lymphokine activated killer cells (hereinafter tPrmed
"LAK cells"), these cells respond to protect the body
against invasion by foreign c:ells and substances. The
role of the T4~ cell has been traditionally viewed as an
inducer cell for the activation of other T-cell
subpopulation. This role is achieved in combi~ation with
an accessory cell or antigen presenting cell (hereinafter
termed "APC") which bears Class II MHc molecules on its
surface and is able to take up and process an
identifiable antigen. The antigen presented by an APC
bearing Class II molecules activates specific T4+ cells.
The activated T4+ cells in turn secrete a variety of
lymphokines to initiate the effector and cytolytic
functions of other T-cell lymphocytes. It is noteworthy
that with the increasing use of lymphocyte-mediated
immunotherapies including those directed against tumours,
all such immunotherapies utilize only those activated
lymphocytes equipped with cytolytic effector function,
e.g. CTL cells, NK cells, and LAK cells. T-cells of the
inducer phenotype are traditionally viewed as lacking the
necessary cytolytic activity and therefore have not been
considered useful for treatment of tumours as
immunotherapeutic lymphocytes.
The human system of multiple T-cell subpopulation
has ~ direct counterpart in the murine system. There are
two major functional subsets of T-lymphocytes in the
murine system. The L3T4+ subset of T-lymphocytes has
inducer or helper functions and is generally activated by
APCs that bear exogenous antigen and express Class II
molecules (Ia) of the MHC. This subset is equivalent to
the T4+ lymphocyte subpopulation in humans. The second
major T-cell subset expresses Lyt-2 determinants and
possesses either suppressor or cytolytic functions.
.
.
r
WO92/15698 PCT~CA92/00105
21 0~969
These are equivalent to T8+ lymphocytes in human~. When
activated, Lyt-2 cells become cytolytic T-lymphocytes
(CTL cells) which generally lack the L3T4+ antigenic
marker and which recognize Class I molecules of the MHC.
SAs in the human system, it has been traditionally
viewed that L3T4+ inducer T cells help initiate the
effector functions of other T-lymphocytes, but do not
demonstrate any cytolytic effect themselves. Very
recently however, several investigators observed an
effector function for selected L3T4~, antigen-specific,
Ia-restricted T-cell clones. These investigations
comprised in-vitro experiments in which selected L~T4+ -
clones appeared to be cytolytic in short-term (less than
6 hours) chromium release assays for Ia-bearing B-cell
hybridoma targets in the presence of antigen. The
primary thrust of each report dealt with the specificity
and the killing mechanisms for the observed cytotoxicity.
To date, therefore, there is little knowledge or ; `
appreciation as to: whether inducer T-cells generally in
murine and human systems are able to express cytolytic
effector function; whether all major types of antigen
presenting cells are sensiti~e to such cytolytic
activity; whether such cytolytic activity can be ~`
maximally expressed and, if so, under what conditions; ~ `
25 and whether such inducer T-cell cytolytic activity can be "
utilized in-vivo for any therapeutic purpose.
Numerous methods have been already proposed for
detection of HLA antibodies. Among these methods, -
techniques measuring the release of radioisotopes, e.g. ;
5ICr from labelled target cells or of fluorescent
compounds were said to have a high degree of accuracy.
Unfortunately, they require too large a number of cells `-
and serum and areltoo time consuming to be considered for
routine or emergency HLA typing.
3SThe presently most frequently used method for
determination of complement-dependent cytotoxicity ~ "
WO9~i/lS698 PCTiCA92/00105
2`~9~
mediated by anti-HLA antibodies is the microcytotoxicity
test (designated by the abbreviation MCT) which was first
proposed by Mittal et al. This method, which consists in
measuring the uptake of supraYital dyes by lysed target
cells, has been found to be a simple, reliable and '
accurate method for performing routine HLA typing or
cross-match test and has been universally accepted by
most histocompatibility laboratories. These laboratories
have standardized the originally described process with
only minor changes from one to another.
Most of the successive technical steps in the MCT
process have now been automatised with the exception of
the final step which consists in the re~ding of the
percentage of dead cells. Since this reading should be
performed under microscope, it depends on the use of the
technician to appreciate the uptake of the supravital
dyes and consequently could not be automatised. In
addition, this MCT process is often considered to be
inadequate for precisely measuring the extent of cell
damage. Thus, for these two reasons, it could be of a
great interest for histocompatibility centres, to provide
a more precise and easily quantifiable method for
measuring anti-HLA mediated complement-dependent
cytotoxicity. Many attempts have been made in order to
25 find such a method. -
Transplantation of human organs, particularly the
kidney, has become a relatively common procedure.
Ideally, the donor organ is obtained from an identical
twin since the antigens of the donor and recipient in
such a case are identical and no histoincompatibility
exists. Therefore, no immune response to the graft
occurs in such a transfer, known as an isograft.
However, most tra!nsplants are between two less closely
related inclividuals of the same species and
histocompatibility differences in such an allograft may
be strong or weak, depending on the individuals. The
W092/15698 PCT/CA92/00l0~
21~96~
11
fate of transplanted tissues and org~ns depends on a
number of factors, but the recipient's immune response to
graft antigens is the central event. Definition of
antigenic systems which serve as strong barriers to
transplantation has therefore become a major
investigational interest, having both practical
application in clinical transplantation and theoretical
value in understanding the natural role of the
histocompatibility antigens in immunobiology.
A single chromosomal gene complex codes for the
major histocompatibility antigens in each vertebrate
species investigated so far. In humans, the
- histocompatibility antigens are produced by the HLA gene
complex. This complex occupies a portion of the short
arm of the human C6 chromosome and consists of several
series of paired alleles which are inherited from
generation to generation in a dominant fashion,
segregating randomly from other important antigens such
as the ABH red blood cell type groups.
Antigens of the HLA system are divided into two
classes. Each class I antigen consists of an 11.500-
dalton ~2-microglobulin sub-unit and a 44.OCO-dalton heavy
chain which carries the antigenic specificity. Three
gene loci (A, B and C) are recognized for the class I
antigens. There are over sixty clearly defined A and B
specificities while 8C locus specificities are known.
Evidence that this gene complex plays the major role in
the transplantation response comes from the fact that
haplotype-matched sibling donor-recipient combinations
show excellent re~ults in kidney transplantation, in the
vicinity of 85% to 90~ long term survival.
A non-serologically defined antigen, responsible for
the mixed lymphocyte response r is caused by a distinct
locus called D. Although D-locus antigens are not as yet
clearly identifiable by serotyping techniques,
serologically defined specificities closely related to
-- . .... , ~,,
WO92/15698 PCT~CA92/00105
'2105969 12
the D-locus have been defineclO These have the special
property of that being expressed on platelets or
unstimulated T lymphocytes. These specificities are
termed class II having two glycoprotein chains of 29.(XX)
(~) and 34.(XX~ (~) daltons and lacking ~2 globulin.
These antigens are also termed HLA-DR (D-related) and are
important in tissue typing. -
Tissuè typing is currently being carried out using
sera obtained from multiparous women. A major problem
exists because of the unreliability of this source. only
a limited amount of antibody is available from any one
woman. Accordingly, it is necessary continually to
replace standard antibodies with new antibodies which
must be standardized and checked against the previously
existing ones. Furthermore, because of the heterogeneous
nature of antibodies obtained in this fashion, cross-
reactivity is a major problem. Accordingly, there have
been many attempts to produce antibodies more suitable
for crossmatching by immunological techniques. Several
such attempts involve xenoimmune (cross-species) sera to
detect allospecificity in the antigen-donor species.
Examples include rabbit anti-human antibodies and monkey
anti-human antibodies. One specific ~nti-HLA serum
appears to be a rabbit anti-A9 serum prepared by
immunization with A9 antigen purified from human serum or
urine. ~owever, A9 is actually a common determinate of
A23 and A24, and thus possibly the allele-specific major
epitope is not the only target with which this antibody
reacts.
The adven~ of hybridomal techniques has brought
about the possibility of producing homogeneous
populations of highly specific antibodies against a
variety of antigèns. Antibodies have been produced by
somatic cell hybrids between myeloma cells and spleen or
lymph cells that are specific for malignant tumours.
Continuous cell lines have been produced of genetically-
W092/15698 PCT/CA92/00105
2~.as969
stable fused-cell hybrids capable of producing large
amounts of IgG antibodies against specific viruses.
Hybridomas have been provided which produce
monoclonal IgG antibodies against tetanus toxin.
Monoclonal antibodies have also been described against
human tumour cells. ~n acldition, there have been
disclosures of the use of rat-mouse hybridomas and their
application to studies of the histocompatibility complex
in various species. Several rat-mouse hybridomas
producing rat, anti-rat antibodies have been found which
were reactive with determinants on cells from other
species, e.g. humans. Some hybridoma antibodies found
which were reactive with peripheral blood lymphocytes
from randomly chosen humans in a complement-mediated
cytotoxicity assay. However, such antibodies were not
useful in identifying private determinants of the HLA
locus since no correlation between specific HLA antigens
and the hybridomally produced antibodies occurred.
Matching for antigens determined by the HLA genes is
an important component of the whole process of
transplantation of organs and tissues. In the case of
bone `marrow transplantation this tissue typing and
matching becomes critical; certain mismatches may lead to
the death of the patient. Genes determine the production
of at least six types of molecule of immunogenetic
importance which fall into two classes, I and II. Class
I molecules are called H~A-A, HLA-B and HLA-C while Class
II molecules are called HLA-DR, HLA-DQ and HLA-DP, with
some further subdivisions within these. All the
molecules are extraordinarily polymorphic and so typing
patients and donors is complex. Finding matches outside
of family groupings is usually difficult, since fully
matched pairs in the general population are rare.
Typing for Class I molecules and for HLA-DR is well
35 advanced. HLA-DQ is less well served with reagents and ! ~' '
HLA-DP is for all practical purposes not typed by routine
~..
..
WO92/15698 PCT/CA92/00~05
2~05 g~9 14
tissue typing laboratories. The reason for the lack of
DP typing is the unavailability of antibodies against the
H~A-DP polymorphism.
The patent literature is replete with patents which
5 relate to monoclonal antiboclies and cell lines and
methods of use thereof for therapy and typing. For
example, U.S. Patent No. 4,314,026 patented February 2,
1982 to B. Descamps-Latscha provided a process for
determining the complement-dependent cytotoxicity
10 mediated by anti-HLA antibodies by means of ~TP
determination and device for ATP determination. That
patent provided such a process which included measuring
the loss of intra-cellular ATP after addition of
complement to anti-HLA-coated target cells. Cytolysis
15 was determined by measuring the intracellular ATP content
of human lymphoid cells (target cells) after these latter
had been incubated with antiserum (anti-HLA antibody) and
complement (rabbit serum). When both target cells and
serum shared the same HLA specificities, cell lysis was
20 observed and exteriorised by a dramatic loss of its
intracellular ATP content. r
U.S. Pater.t No. 4,517,289 patented May 14, 1985 by
E. L. Milford et al, provided monoclonal antibodies for
human tissue cross-matching. That patent provided an
25 immortal, antibody-producing, hybridomally-produced clone
and an antibody produced thereby. The antibody was an
immunoglobulin specific for an antigenic determinant
encoded by an HLA gene complex in humans. The clone was
produced by an immortal cell line fused with a lymphocyte
30 obtained from a first rat immunized against cells
obtainDd from a second rat having a different
histocompatibility antigen. That patent therefore also
provided novel hybridoma cell lines, novel monoclonal
antibodies against a human HLA antigen, the antibody
35 having been produced by a novel hybridoma cell line and
wo92/ls69B PCT/CA92/00105
21~9~9
a tissue-crossing assay kit, including a monoclonal
antibody produced by a novel cell line and a dye.
U.S. Patent No. 4,634,66~ patented January 6, 1987
by E. G Engleman et al, provided an ideal fusion partner
for specific B-lymphoid cell lines, producing triomas
that secreted specific antibodies of human character.
The immortalizing, non-secreting hybridoma having human
characteristics was prepared by fusing mouse myeloma
cells with human B lymphocytes and selecting the fusion
product for stable immunoglobulin secretion and HLA
surface antigen production, followed by treating the
selected fusion product with mutagen and selecting the
mutated product for non-secretion of immunoglobulin but
retention of ~LA antigen production. That invention also
provided the products of fusing the immortalizing
hybridomas with suitable human immunized lymphoid cells.
Such triomas are useful sources of desired Mab's. That
invention also provides human monoclonal antibodies which
are produced by the triomas and their diagnostic and -
therapeutic compositions and uses. Thus, the above
patent provided a specifically recited immortalizing
fusion partner for use in producing a trioma cell line
capable of secreting a human monoclonal antibody specific
against a selected antigen, when fused with a non-
malignant b-lymphoid cell derived from a human donor
exposed to such antigen. It also provided a trioma cell
line capable of secreting a normal human monoclonal
antibody specific against a selected antigen. The cell
line was the fusion product of a mouse myeloma/non-
malignant human B-lymphocyte hybridoma fusion partner
which expressed HLA surface antigens, did not secrete
immunoglobulins, and was deficient in hypoxanthine
phosphoribosyl transferase, as evidenced by the inability
of the fusion partner to grow in hypoxanthine-
35~ aminopterin-thymidine or azaserin-hypoxanthine medium,
WO92/15698 PCT/CA92/00]05
2~59~9
16
and a non-malignant B-lymphoicl cell derived from a human
donor exposed to the selected antigen.
U.S. Patent No. 4,657,760 patented April 14, 1987 by
P.C. Kung, provided methods and compositions using
monoclonal antibodies to human T cells. This patentee
provided a novel hybridoma which was capable of producing
a monoclonal antibody against an antigen found on
essentially all normal human peripheral T cells. The
antibody so produced was monospecific for a single
determinant on normal human T cells and contained
essentially no other anti-human immuneglobulin. The
patentee also provided a novel hybridoma producing
antibody to an antigen found on essentially all normal
human T cells, the antibody itself, and diagnostic and
therapeutic methods employing the antibody.
U.S. Patent No. 4,681,760 patented July 21, 1987
provided a method of conferring immunotolerance to a
specific antigen. That patent provided a method for
suppressing undesired immune responses, e.g. allergic
reactions, to antigens whose administration to the
subject was either desired or inevitable but otherwise
harmless. It also provided a method for inducing
tolerance to tissue transplants. The patented method
involved the co-administration of the antigen for which
immunotolerance is sought and an antibody which is
specific for the "L3T4-equivalent" differentiation
antigen on T cells, thus preventing these helper T cells
from participating in the immune response otherwise
concurrently mounted against the particular co-inject~d
or co-administered antigen.
U.S. Patent ~o. 4,692,405 patented September 8, 1987
by A. Freedman et al, provided monoclonal antibodies to
antigen on activated human B-cells and assays therefor,
protein antigenic determinants therefor and methods of
making same. That invention provided a monoclonal
antibody recognizing an antigenic determinant on
.. "~.
WO92/15698 PCT/CA92/OOlOS
2~9~9
17
activated human B-cells. That invention also provided a
substantially pure protein having an antigenic
determinant or determinants substantially identical to
determinants of a single-chain polypeptide having an
apparent molecular weight of approximately 75,000 daltons
under reducing conditions and 67,000 daltons under non-
reducing conditions, the single-chain polypep~ide being
a protein on the surface of activated human B-cells.
That invention also provided a specifically recited
10 process for preparing the antigenic protein. That `
invention also provided kits useful for assaying a '~
biological sample for the presence of cells expressing
the antigen of the invention and for assaying a
biological sample for the presence of antibody to the
cells expressing the antigen of the invention. These
kits contained one or more containers, each holding
separately detectably labelled or unlabelled antibody or
antigen of the invention, and in another compartment, a
means for detecting the formation of immunocomplexes.
U.S. Patent No. 4,708,930 patented November 24, 1987
to K. H. Kartright et al. provided murine, monoclonal
antibodies specific to a unique antigenic determinant on
the surface and in the cytoplasm of human neoplastic`
tissue are produced. The unique antigenic determinant ',~
25 was designated the "KC-4 antigen" which was capable of '
eliciting an antibody which bonded selectively only to
neoplastic carcinoma cells and not to normal human
tissues. ''~
U.S. Patent No. 4,710,4S7 patented December 1, 1987 -
by Bo Dupont e_ al, provided monoclonal antibodies for
human haematopoietic glycoproteins. The patentee also
provided a specifically recited method for
differentiating between human B cells and T cells in a
human haematopoietic specimen.
U.S. Patent No. 4,743,681 patented May 10, 1988 by
P.C. Kang et al, provided a hybrid cell line for
W092/15698 PCT/CA92/OOlOS
2~05~g~ ,
18
producing monoclonal antibody to a human T cell antigens
and antibodies. The patentee provided a hybridoma
(designated OICTll) which was capable of producing
monoclonal antibodies against an antigen found on
essentially all normal human peripheral T cells and on
approximately 95% of normal human thymocytes, but not on
normal human B cells or null cells. The antibody so
produced was monospecific for a single determinant on
essentially all normal human peripheral T cells and
contained essentially no other anti-human immune
globulin.
U.S. Patent No. 4,843,004 patented June 27, 1989 by
C. Platsoucas provided a specifically recited method for
the production of human T-T cell hybrids and production
suppressor factor by human T-T cell hybrids. The
patented method was developed for the production of human
haematopoietic cell hybrids especially T-T cell hybrids
as determined by HLA typin~. Some of these T-T cell
hybrids pr~duce factors useful for biotherapy or
exhibiting specific-immunological functions. This is
accomplished by fusing cells from human T cell lines with
appropriately sensitized or induced human T cells
exhibiting specific immunological function or producing
the desired factors.
U.S. Patent No. 4,861,589 patented August 29, 1989
by S. T. Ju, provided a method for therapeutically
treating abnormal cells expressing a major
histocompatibility complex class II antigen using
cytolytic inducer T4 cells. That patent provided a
specifically recited method for treating a subject
afflicted with tumour cells expressing a major
histocompatibility complex Class II antigen either
constitutively or~inductively.
U.S. Patent No. 5,009,995 patented April 23, 1991 by
A. Albino, provided monoclonal antibodies to melanoma
cells. The patent related to monoclonal antibodies
r
.- . ' ~. : . , ' ~:............ . , . . : .
- : . : : . . .
WO92/15698 PCT/CA92/0010~ ~
19
recognizing the gpl30 antigen of human cells. Monoclonal
antibodies which recognize di~;tinct determinants on this
antigen and methods of detecting the determinants by
immunoassay with the monoclonal antibodies which
recognize them are also disclosed. Hybridoma cell lines
which produced such monoclonal antibodies were also
disclosed. The monoclonal antibodies are useful in the
detection of the gpl30 antigerl and human cells including
melanoma which contain this antigen.
lo In spite of the technical literature and the patents
described above there still exists a need ~or monoclonal
antibodies suitable for tissue matching.
It is common knowledge that attempts to prevent
unwanted immune responses have not been particularly
successful. For example, efforts are made to match
transplant recipients with donors so as to minimize the
amount of immunogenic response to foreign materials.
Only in the case of identical twins c~n reasonable
success be certain. The limitations of such an approach
are so apparent as to warrant no further comment.
Alternatively, brute force efforts to suppress the immune
system in general, such as administration of anti-mitotic
agsnts may prevent rejection at the expense of the
recipient's life due to the resulting susceptibility to
infection.
An alternate approach applicable only to preventing
tissue rejection is passive immunization of recipients
with antibodies directed against the histocompatibility
antigens. Other approaches also applicable only to the
transplant rejec$ion problem have employed treatment of
the donor tissue. These are based on the assumption that
the rejectiGn response is caused by the
histocompatibilitly antigens on the surface of passenger
leukocytes carried on the transplant which leukocytes are
not an essential part of the desired tissue Per se. In-
vivo culture of the donor transplant tissue has been used
, ,," '"',
WOg2/l5698 PCT/CA92/00105
S9~
to eliminate passenger leukocytes. The donor tissue
has also been treated directly with suitable antibodies~
the use of immunotoxins formed by conjugating antibodies
with a cytotoxic moiety has also been suggested for
pretreatment of donor tissue.
Methods to prevent imm~me responses to soluble
antigens have been largely ~onfined to avoidance of
exposure. Patients allergic to certain drugs are treated
with alternative formulations when available; hay fever
sufferers attempt to stay away from the immunogenic
pollen. If avoidance is impossible, one must resort to
treating the symptoms.
The major histocompatability complex (MHC) of human
is a cluster o~ genes occupying a region located on the
sixth chromosome. This complex, denoted HLA (Human
Leukocyte Antigen), is currently divided into five major
gen loci, which according to World Health organization
nomenclature are designated HLA-A, HLA-B, HLA-C, HLA-D,
and HLA-DR. The products of the HLA genes are commonly
called ~'antigens~. The genes of the A, B, and C loci
encode the classical transplantation antigens whereas the
genes of the D and DR loci most probably encode antigens
that control immune responsiveness. HLA antigens are
present in the membranes of human cells. Some are
present in most cells of the body whereas others are
present only in specific kinds of cells. For instance,
HLA-DR antigens have been identified in B cells but not
in resting T cells.
The HLA antigens are categorized into types that
vary from individual to individual. HLA typing is used
in paternity determinations, transplant and transfusion
compatibility testing, blood component therapy,
anthropological studies, and in disease association
correlation to diagnose diseases or to predict
susceptibility to disease. Current HLA-DR typing
techniques consist of two basic methods. One involves
, ~, .. . ' .. , , ' ' . , .. , .' '~ . , ' '
WO92/1~698 PCT/CA92/00l05
2~ ~3~
21
separating B cells from a tot:al lymphocyte sample, e.g.
peripheral blood lymphocytes (PBL), treating the B cells
with anti-DR sera and complement, and reading the
resultant cytotoxicity as an index of reactivity. The B
cells are separated from the total lymphocyte population
because DR antigens are present only in B cells and B
cells constitute only a small proportion, typically 10%
to 25%, of PBL. cytotoxicity of such a small proportion
of cells would be difficult to discern accurately.
Numerous methods have been used previously to separate B
cells from PBL. The most common method takes advantage
of the reaction of T cells with sheep erythrocytes (SRBC)
to form rosettes that can be centrifuged through a layer
of Ficol-Hypaque, leaving the B cells at the top of the
gradient. Other methods take advantage of the affinity
of B cells for various materials such as nylon wool,
Degalon beads, and anti-human F(ab')2 reagent. These
methods suffer from various combinations of being time
consuming or technically dif~icult, yielding impure
preparations ti.e. contamination with non-B lymphocytes),
providing poor absolute yields of testable B cells or,
yielding separated B cells that have poor viability.
Monoclonal antibodies against HLA-DR antigens have been
used to separate B cells from PBL for use in HLA-DR
typing tests.
The second basic HLA-DR typing method s the two
colour fluorescence technique. In this method, to label
B cells with an immunofluorescent marker, a PBL -
preparation is incubated with a fluorochrome labelled
anti-human Ig, washed, and then dispensed in tissue
typing trays. Following sequential incubations with DR ~- -
antisera and complement the test results are read by --
determining the percent of viable B cells remaining by
adding a fluorescent vital dye and measuring percent
35 viability only of those cells having ring -
immunofluorescence. Although this method avoids a B cell
',
W092/15698 PCT/CA92/00105
r~
22
separation step, it requires that the cells be stained
with anti-human Ig. It also is practical only when read
under high power microscopy and, therefore, has a more
demanding reading step than the B cell separation method.
The serologically-defined HLA-DR4 specificity is
complex and has recently been reported to have eight
allelic variants or subtypes. These subtypes have been
defined mainly by T cell recognition methods and have
been confirmed by DNA typing techniques. From analysis
10 of sequence data it is apparent that the serologically !
-defined DR4 specificity can be attxibuted to amino acid
differences in the first and second hypervariable regions
of the first domain of the DR4 molecule, whereas the
subtypic differences are all located in the third
hypervarible region. Some subtypes vary by as little as
one amino acid and at the most by three; yet these
differences are enough to be recognized by T cells.
It has been known for some time that DR4 is
associated with susceptibility to developing Rheumatoid
Arthritis (RA) and that this association is mainly with
the subtypes Dw4 and Dw14 in caucasians and ~lacks.
These two subtypes and the Dw15 subtype which is
associated with RA in the Oriental populàtion, vary by
only one to three conservative amino acid substitutions
at positions 71 and 86 for Dw4 and Dw14, and at positions
57 and 71 for Dw4 and Dw15. What is most intriguing is
that two non-DR4 alleles, DRl and DR14 (subtype Dw16)
which have major differences in the first and second
hypervariable regions from each other and from DR4, have
almost complete sequence homogology with Dw14 in the
third hypervariable region (1). Both these specificities
have been shown to be associated with predisposition to
RA in different ethnic groups, for example, DR1 in the
Jewish population and DR14 (Dw16) in the Yakima Indians
(7,8). Although one can sperulate on the role of a
putative epitope formed by these residues, which is
- ' : "~ ,, ,,." .~ ,.." ,, ",, .,~ ", "
.. . , .,. ." . , .. , . .~
WO92/15698 PCT/CA92/0010~
23 2~
located on the alpha helix of the peptide binding site,
in antigen presentation to T-cells in RA individuals, the ;
relevance of the association still remains a puzzle.
5 3 ! Summary of the Invention
(i) Aims of the Invent:ion -: -
What is desired is a specific immuntolerance with
respect to a particular antigen, leaving the general
competence of the immune system intact. None of the
foregoing approaches, described in the technical and
patent literature above, achieved such a selective
immunosuppression of the subject. Treatments employed to
prevent transplant rejection which are directed toward
the host Der se generally depress the entire system;
15- treatments of the donor tissue alter the nature of the
foreign material introduced. In the case of allergic
responses to drugs or to environmental antigens,
alteration of the foreign material is either undesirable
or impractical. In the present invention, the immune
system of the host is selectively and specifically
suppressed with respect to a particular immunogen without
impairing general immunocompetence.
It is also desirable to provide a process which is
suitable for HLA typing of human lymphoid cells, and
which is also appropriate for anti-HLA antibodies
detection in ~he serum from subjects sensitized against --
histocompatiblity antigens(-polytransfused patients,
multiparous women, organ graft recipients~.
Accordingly, it is an object of this invention to ~ -~
provide a hybridoma cell line and an antibody produced
thereby useful for the tissue typing of human tissues.
It is a further object of this invention to provide
a method of prod~cing such antibodies in an efficient
manner.
A principal object of the present invention is to
provide a simple and effective HLA-DR typing technique
WO92/15698 PCT/CA92~00105
~59~9
24
that: (1) does not involve a B cell separation step or a
lymphocyte staining step; and ~2) is based on
cytotoxicity function such that the sera and Gomplement
used in available lymphocytotoxicity tests may be used in
the invention method.
Another object of this invention is to provide
murine monoclonal antibodies recognizing polymorphic
determinants of HLA-DP.
rii~ ~Statements of Invention
lo since a substantial body of work has already been
done using T cell cloning techniques to try to understand
the role of DR4 in RA, it was decided to put efforts intG
producing monoclonal antibodies (moab) to XLA-DR4 and its
subtypes. Such antibodies would enable the testing of
whether non-DR4 individuals who develop RA have
conformationally-equivalent epitopes to Dw4. If proven
correct, antibodies that recognize such epitopes would be
useful in identifying individuals at risk for RA and
additionally, may also prove useful in specifically
blocking peptide presentation to T cells. At the very
least such antibodies would simplify DR4 subtyping for
tissue matching for organ transplantation.
The DR4 specificity was originally defined by
alloantisera derived from multiparous females but
attempts to subtype with such reagents have been mostly
unsatisfactory. Attempts to make murine monoclonal
antibodies to HLA antigens generally proved to be more
difficult than had been anticipated. This is thought to
be due to the type of immunogen, usually whole cells,
which express an enormous array of different molecules
including at least six different HLA antigens= The
murine immune system recognizes most of these molecules
as foreign and ev~n when purified HLA molecules are used,
the bulk of the antigen-specific cells will be against
~5 the species-specific or monomorphic determinants present
on the histocom~atibility molecules.
WO92tl~698 PCT/C~92/00~05
2~
The development of mousQ transfectant cell lines
expressing human histocompati~ility molecules seemed to
- be a tremendous advance in this technology, particularly
with respect to an anti-DP moab made using a transfectant
as an immunogen. Theoretically, the only foreign
molecule expressed on the surface of the transfectant
should be a HLA molecule. Therefore, the bulk of the ~;~
antigen-specific B cells should be directed to HhA
molecules and some of these should be directed to
polymorphic determinants. The ability to differentially
screen hundreds of hybridoma supernatants for specific
antibody in a short time as was provided by
transfectants, was also an important advance.
The present invention provides a set of monoclonal
antibodies that react with epitopes on DR4 molecules.
Specifically the present invention provides monoclonal
antibodies which are specific for HLA-DR4 molecules.
The present invention also provides the use of the
murine monoclonal antibodies to detect subtypes of DR4.
It also provides the use of the murine monoclonal
antibodies which react with putative RA-susceptibility
determinants for the study of rheumatoid arthritis.
The present invention also provides for the
production of 14 such monoclonal antibodies, and for the
25 characterization of the properties thereof. -
The present invention also provides for the
producing and of analyzing the specificities of moabs to
the subtypes of HLA-DR4 using transfectants.
The present invention also provides two other
antibodies with DR4 subtypic specificity, that were
produced from mice immunized with human molecules.
(iii) Other Features of the Invention
Embodiments of such antibodies include the
following: NFLD.Dl which binds to all DR4 molecules;
NFLD.D12, wh:ich binds only to the Dw4 subtype of DR4;
NFLD.D14, which binds to Dw4 and Dw14 subtypes; NFLD.D7,
WO92/15698 PCT/CA92/00105
2~ ~963 26
which binds to all DR4 and DR2 molecules but also, less
strongly with several non-DR4 molecules; NFLD.D2,
NFLD.D3, NFLD.D4, NFLD.D8 and NFLD.D9, which bind
strongly to Dw4 and Dw14, but not at all to the subty~e
of DR4 called DwlO, which give moderate to low reactions
with some other DR4 subtypes, and also which react with
DRl, DR2, and DRl4 (Dw16); and NFLD.DlO which reacts with
the Dw9 subtype of DRl4 as well as binding weakly to
some of the DR3-, DR7-, and DR9- typed B cell lines.
4) Brie~ Description of the Drawinqs
In the accompanying drawings,
Figure l is a histogram which shows the reactions in
CELISA of the antibody NFLD.DlO. Each bar represents the
reaction against a particular transfectant line, whosa
specificities are shown at the bottom of the figure. The
heights of the bars represent the adjusted optical
densities, which have (a) had the background subtracted
and then (b) been converted into a percentage figure with
reference to a positive control antibody, in this ca~e
L243, a~ antibody-reactive with all DR molecules;
Figure 2 is a histogram which shows the reaction in
CELISA of the antibody NFLD.D7; and
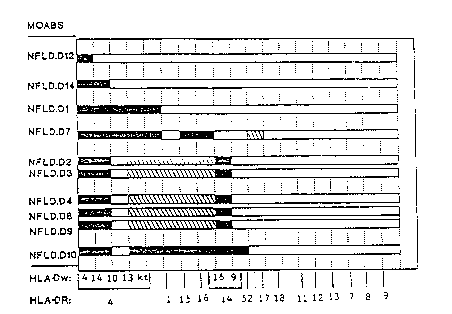
Figure 3 is a diagram which shows the di~fering
specificities of this series of antibodies. Each bar
represents the reactions of one antibody, which identity
is given on the vertical axis. On the horizontal axis
the relevant DR subtypes are each assigned one interval;
below these are given the DR grouping in which the
subtypes are contained; thus DR4 contains Dw4, Dw14,
DwlO, Dwl3, and Kt. Filled parts of the bars indicate
strong reactions of an antibody with the DR or Dw type
shown; latch~ed ~ndicates smaller but still significant
reactions. White indicates negative reactions.
:
WO92/15698 PCT/CA92/00105
27 :
5~ Descrl~tion of Preferred Embodiments
The following describe various procedures according
to the present invention.
Transfectants as Immunoqens for Producinq Monoclonal
Antibodies
Transfectants:
The DR4 transfectants used for the immunizations and ~.
most of the analysis are now included in the
transfectants distributed by the organizers of the 11th
IHW (see Table l below).
TABLE 1 HLA-DR Expressing Transfectants Obtained fr~m
the 11th International Histocompatibility ~ :
Workshop and Used for Specificity Analysis.
: .. - .
.: . ..
11th IHW HLA
Number Specificity Local Name Contributor
' .:
8103 DRl DAP3 DRl~ R. Sekaly/E. Long
8104 DR Bon DR BON P. Claude~C. Thomaaen
B107 DR2aDw2 DAP-3DR2a* D.Jaraguemad/E. Long
8109 DR2bDw2 DAP-3DR2b~ D. Jaraquemad/E. Long
B110 DR2aDwl2 L-DR2-Dw12 T. Saaazuki
8111 DR2bDw12 LARBl H. Inoko
8112 DR3 L168.2~ R. Karr/J. Silver
8115 DR4Dw4 DAP-3DR4~ R.Sekaly/E.Long/R.Karr
8116 DR4Dw10 L164.11~ R. Karr/J. Silver
8118 DR4Dw14 L165.6~ R. Karr/J. Silver
8122 DR4DwKT2 B18 R. Lechler '
B123 DR4DwrAS L89.2~ R. Xarr/J. Silver
B124 DR4Dw~AS Bl9 R. Lechler
B125 DRwllDw5 L91.7*~ R. Karr/J. Silver
B126 DRw14Dw9 L167.2~' R. ~arr/J. Silver
B127 DR14Dw16 LlB2.1~ R. Karr/J. Silver
B131 DRw10 29ØC.27 H. Peter
B132 DR52aDw24 LR6.2/52a B. Mach
B134 DRw52bDw25 DAP-3DR52b~ P.. Sekaly/E. Long
B13B DRw53 ~DR4Dw15) L17.B~ R. Karr/J. Silver
WO92/lS698 PCT/CA92/00105
2~
The transfectants as described in the Table 1 above
include L89.2. (Dw13); L164.11 (Dwlo); L165.6 (Dw14); Dw4
transfectant (DAP3DR4); two other transfectants, L24306
(DR4Dw4) and L259.1 (DR4Dw13). All transfectants were
grown in Dulbecco's modified Eagles medium (DMEM)
containing 10% fetal bovine serum (FBS~, 5 x 103 mM 2-
mercaptoethanol, penicillin and streptomycin (Flow
Laboratories). The cells were grown on either 10 cm
dishes (FALCONTM) or 75 cm flasks (LINBROTM) and were
harvested in log phase using trypsin (Flow Laboratories)
and left in standard type bacteriological petri dishes
for one to three days. Expression was assayed by CELISA
or FACS analysis using ~he moabs Tu39 or GSP4.1 prior to
immunization.
Immunizations: ,
Cells expressing high levels of HLA were washed
three times with phosphate-buffered saline (PBS) and in
all immunization procedures 1 x 107 cells were injected.
Essentially three strategies were tried (as shown below
in Tables 2 - 4).
W092/15698 PCT/CA92/00105
S96~ '''
29
TA~LE 2 Summary of Data o~l Fusions Derived from C3H .-~`
Mice given Indiscriminate Immunizations With
HLA-Class II Transfectants
i,' ,. ;.
~ ~,,,.,",.
::.
IMMW~L~ATIo~ SCHF~LE
~wks) zations ~ Hybri~s~
: ~008t splenocyte9) h Ab ~d ific ~.
__ . . _ .. _ ... . :
10 1~ DRl IP DRl-IP 75
R5 M 12 2~ DRl IP 5 week8
14 3~ DRl IP after
last boost ( 214) 1 DR
monomorph
2 1~ DR4~CFA
3 o R12 M 16 2~ Dw4 IP 4 week~ 1 DR
32 3 Dw4 IP last boost 1478) monomorph
polymorphs
:
35 R13 F 28 2~ DwlO IP DwlO-IV 1015 2 DR
monomorphs
- after (423)
last boost
1~ DW14 IP Dw14-IV 204 1 DR
R14F 28 2~ Dw14 IP 3 wk~ aft~r monomorph
last boosS
R15F 2'3 2~ Dw14 IP 3 wks after ~230) NONE
'
~ Total numb~r of hybrid from the fusion.
~ Number of hybrids per 101 spleen cell~.
wo 92/1~698 PCJ/CA92/00105
'21~S~9
TAsLE 3 Res~lts of Fusions Done Using Neonatally
Tolerized C3H Mice and a Control Group
IMMUNIZAI~ON~ LkS
Fusion Sex Age Toleri- Immuni- ~oost ~ Hybri~ds~ Selected
zat~on zation Iper 10 Hybri'ds
splenocytes)~
R6 M <24hrs non-
2 wks Dw4 IP
6 wks Dw4IP 412 polymorph
~ CFA (25a) 1 DR
14 wks Dw4 IP polymorph
Same as Dw14
R7 M 8 wks for R6 . ~ CFA Dw4 IS 273 2 weak
14 wks Dw14 IP ~273)
. _ _
NONE 1' Dw4
R8 16 k ~CFA Dw4 IP 758 1 monomorph
w 8 2 Dw4 IP ~ 20 wks ~469~1 weak
polymorph
NONE 1~ Dw4
R9 M 8 wks ~ CFA Dw4'IP 750 1 monomorph
1 wks 2 Dw4 IV Q 20 wks ~444~1 weak
polymorph
- 35 R10 M 24 hrs Dw10 Dw13
- IP ~ CFA Dw13 - 7
16 wks ~ 28 wks 1250
- 20 wks Dw13 IP . ~28a~
NONE
R11 M 16 wks Dw13 Dw 13 boost 600 1 anti-
~ CFA ~ 2a wks DR4
20 wks Dw13 IP ~462)3 mono-
morphs
Total number-of hybrid from the fusion.
~ Number of hybrids per 10; spleen cells. ~ '
5 0 ;
W092/15698 PCT/CA92tO0105
9 ~ ~ :
3:L
TAB~E ~ Results of Fusio3ls Done to Compare I3nmunization
-
.:
,
' . '
Po3t- IMMUNIZATION l! Hybrid~ Number of
(per 103 ~ybrid~ Fusion
splenocytes3~Selected Serum
Titer
Time
Fusion Primary Interval Boost
(Weeks)
1128(434) 7 <1/100
336 (480~ 2 1/1600
R16 IP - 4 IS238(170~ 5 1/400
R27 65 467 ~275~ 3 1/800
R29 7 X 542~3403 X = 4.3
R18 IP 4 IP864(455~ 0 1/800
R20 5 41 41 1 1/800
R26 6 546~455~ 1 1/800
X s 483~317~ X 0.
R17 4
R23 CFA 5 IS
R25 S/C 6 75B (237) 1 >1/1600
R30 7 38 (23) 3 >1~1600
409(340) ` 4 1/1600
592 (423) 4 1~3200
X = 449(-258) X = 3
R19 4 812(427) 18 1/3200
R21 CFA 5 IS672(354~ 0 >1/3200
R24 S~C 6 560~467~ 9 >1~800
R28 7 7041440) 7 >1/32Q0
X = 68~(422~ X = 11
-
Total number of hybrid from the fu~ion.
Number of hybrid3 per 103 spleen cell~.
:~
IP = Intraperitoneal. CFA = Complete Freunds Adjuvent
S~C = Subcutaneou~. IS = Intranpleinic.
X Average.
W092/15698 PCT~CA92/OOloS
2~96~
32
The first approach consisted of indiscriminate
standard-type immunizations where young adult C3H mice
were immunized twice intraperitoneally ~IP~ followed by
a final boost intravenously (IV) or IP three days prior
to fusion. In the second approach, neonatal tolerization
was attempted. Finally, the third group consisted of 10
wk old femal mice which were primed either IP in saline
- or subcutaneously in complete Freund's adjuvant ~CFA),
left for 4 to 6 weeks and then boosted either IP or
intraspenically (IS) three days prior to fusion. In
several of the experiments serum was collected before
and/or at the time of fusion.
Fuslon:
All fusions were done three days after the final
boost and were carried out using the fusion partner
SP2/0-Ag~4, (see Shulman M, Wilde CD & Kohler G. "A
better cell line for making hybridomas secreting specific
antibodies". Nature 1978: 276: 269.). Hybridization was
done according to a previously described method, (see
20 Drover S, Marshall WH & Younghusband HB. "A mouse `
W092/15698 2 ~ ~ 5 9 6 gPCT/CA92/00105
monoclonal antibody with HLA-DR4 associated specificity''O
Tissue Antigens 1985: 26: 340-343.). Cells were seeded
at various concentrations and using different feeder
cells in order to establish optimal conditions.
Selection ~as done using standard HAT (hypoxanthine and
thymidine reagents) in DMEM containing 20% FBS and ;
supplements as described above.
Screeninq & S~ecif icity Testlng:
All supernatants were tested approximately lO days
after fusion using CELISA as previously described (see
Morris RE, Thompson PT & Hong R. "Cellular enzyme-linked
immunospecific assay (CELISA) I. A new micromethod that
detects antibodies to cell surface antigens". Hum
Immunol 1982: 5: 1-19; and Drover S & Marshall WH.
"Glutaraldehyde fixation of target cells to plastic for
ELISA assays of monoclonal anti-HLA antibodies produces
artefacts". J Immunol M~thods 19~6: 90: 275-2~1.).
For the first screen, the supernatants were tested
against the immunizing cells and all positive were
differentially screened on the following day against the
immunizing cell and non-transfected L cells. Those that
were positive only with the immunizing cells were
selected for further testing against a small panel of
transfected cells, including those expressing DP, DQ and
Z5 informative DR. Hybridomas were then selected for ---
cloning and further analyzed on both transfectants and B
cell lines. ~ -
Use of Human B Cell Lines as Immunoqens
The production of NFLD.D12 and NFLD.D13:
This was achieved in a fusion made from Balb/c -
spleen cells. The mice had been primed with affinity
purified HLA molecules extracted from a lysate of the B
cell line SAVC' (lOth Workshop ~9034), using beads
(DYNAL~M) that had been coated with two antibodies: first,
anti-mouse IgG had been coated by the manufacturer;
secondly the beads wer~ coated with a mouse IgG1
WO92/lS698 PCT/CA92/00105
,9~5 34
monoclonal antibody made in t:his laboratory (NFLD.M67)
that detects a monomorphic determinant on HLA-DP
molecules. Beads loaded with two antibodies and class II
molecules absorbed onto them from the lysate were
injected subcutaneously in CFA in four sites. The boost
injection given three days bef~re splenectomy and fusion
consisted of lo million SAVC cells injected
intravenously.
Transfectant Cell Lines
The lines used for these experiments are listed
below in Table 5.
~A~LE 5. ~LA-DP Transfectant Cell Lines
11th w/S Number Other N~me DP Type Provided bY:
8301 L3.6.2 DPal~0201 J. G. 3Odmer
8302 DAP-3 DPw2 DP31'0201 R. SekalyiE~. Long
8303 L11.3 DP31'0401 J. G. aodmer
- 8304 LPP 3-6 DPw4 H. Inoko
8305 L25.4 DP31~0402 R. Karr
8306 LAP 4108-6 Cp H. Inoko
~DPal~osol)
IDPAl'0201)
A 1
: . ., ::
.
~';' ~'
: , . ..
'
~ ~ .
-
W O 92~15698 2 ~ ~/CA92/00105
flasks (LINBROTM) and were harvested when in log phase
using trypsin. Following harvest, they were cultured in
standard bacteriological PETRI dishes (to which they do
not stick) for one to three days. Culture medium
consisted of Dulbecco's modified Eagles medium (DMEM)
containing 10% fetal bovine ~erum (FBS), 5 x 10-5 mM 2-
mercaptoethanol, penicillin and streptomycin. Expression
of HLA-DP was assessed by flow cytometry or by OELISA
assay, using either the monoclonal antibody B7/21 or one
of our own antibodies against monomorphic DP
specificities.
Immunization
Protocols varied from experiment to expériment, but
a typical protocol is as follows: 107 transfectant cells
were injected subcutaneously, dividing the dose between
four sites on the back, together with Freunds complete
adjuvant, 0.1 ml per site. After a wait of 4-8 weeks,
the mice were boosted by 107 cells, either gi~en
introperitonealy or intrasplenically, and the spleen
removed three days later. In some experiments, a primary
immunization with human B cell line cells or with DP
transfectants was made by intravenous injection; three
days later the spleen was removed and a fusion performed.
Fusions
Fusions were performed three days after the last
injection of antigen and were carried out with the fusion
partner SP2/0-Agl4 (Shulman M, Wilde CD, Kohler G. Nature
1978: 276: 289.). Fusions in the presence of
polyethylene glycol were done according to a standard
method, (Drover S, Marshall WH, Youghusband HB. Tissue
Antigens 1985: 26: 340.). Usually 2 x 105 cells per well
were plated in a-96 well plate with flat bottomed wells.
.
.,: : . ~ . .. .. . .
- : - .. . .. ~ . .. . . ~
WO92/15698 PCT/CA92/0010~
~ ~5~9
36
Screeninq and Specificity Testinq
All supernatants were tested approximately ten days
after fusion, using the CE`LISA assay, (Morris RE,
Thompson PT, Hong R. Hum Immunol 1982: 5: 1; Drover S,
Marshall WH. J Immunol Met l9El6: 90: 275.).
In the first screen, the :immunizing cell was used as
a target. In a second screen of positive derived from
the first screen, differential testing was done on the
transfectant that had been used as immunogen and on L
cells. In the case of experiments where human B cell
lines were the immunogen, the second screen was done on
several B cell lines plus a human T cell line that fails
to express class II HLA molecules (Molt/4).
Later studies of specificity were done using human
B cell lines in CELISA assays. The majority were the
homozygous cell lines collected during the 10th IHW, (see -~
Yang SY, Milford E, Hammerling ~, Dupont B. In
"Immunobiology of HLA, Vol. 1, Histocompatibility Testing
1987" B Dupont Editor 1989: p. 11.) and these were `
supplemented by a few others obtained from other
laboratories ~here DNA sequencing had been done on them.
6! _ Operation of Preferred Embodiments
Results
In some of the earlier fusions, hybrids were lost
through what appeared to be overcrowding and lysis due to
cytotoxic T cells. This problem was partially alleviated
by plating the fused cells at a maximum densi`ty of 2 x 105
cells per wall and eliminating spleen cells or thymocytes
as feeder cells.
In the first set of experiments, summarized in Table
6, five fusions were derived from mice that were randumly
immunized. Over 2500 hybrids were assayed on the
immunizing cells; after differentially screening the
positives, six were fuxther analyzed for polymorphic
activity. As can be seen from the data, no hybrids made
WO92/1~698 PCT/CA92/00105
6 9
37
antibody with a short specificity to polymorphic
epitopes; only two antibodies reacted to a polymorphism
and 5 hybrids produced antibody to class II monomorphic
determinants.
An attempt was made at neonatally tolerizing C3H
mice by injecting them with non-DR4 transfeatants at
various times from age 24 hours to 6 weeks. Prior to
immuni~.ation, serum samples were obtained from these mice
as well as from non-tolerized litter mates. The sera
were titered in CELISA on the tolerizing cells and on
non-transfected L cells. The CELISA data showed evidence
of antibody activity to the tolerizing cells, indicating
that tolerance to DR had not been achieved. It was
decided to use some of the mice for fusions and, at age
8 to 16 weeks, they and some of their non-tolerized
litter mates were immunized with DR4-expressing
transfectants. It is obvious from the data shown~in
Table 6 that very few hybrids were selected for further
studies despite screening over 4000 hybrids. After
limited specificity analysis, it appeared that one of the
hybrids was making an antibody specific for DR4. This
antibody derived from the Rll fusion and now designated
NFLD.D1, is described in more detail below.
Fifteen fusions done to compare immunization
schedules were very productive. The data summarized in
Table 7 clearly show that the worst immunization strategy
was an IP primary followed by an IP boost. The best
strategy was immunizing subcutaneously along with CFA
followed by an IP boost, while the other two strategies
immunizing IP in saline or subcutaneously with CFA
followed by an IS boost were equally good. Titering of
sera was not always predictive of the number of specific
hybrids. For example, the mouse (fusion R21) that
produced the highest titer against the immunizing cells,
although producing a large number of hybrids, did not
yield a single one making antibody specific for the
, .. , ... . . ~ , .. ~ . . - . ~ . .
WO92/1~698 PCT/CA92/00l0~
2~ ~59~9
38
immunizing cell. On the other hand the mouse (fusion
R16) which had the lowest titer produced 7 hybrids making
specific antibody.
From the above experiments in which a total of
fifteen fusions were done, yielding over 8000 hybrids,
sixty-four hybrids were selected for further analysis. -
Thirteen produced antibody to polymorphic determinants;
eight of these are described below. `
".
NFLD.D Monoclonal Antibodies
The monoclonal antibodies derived from mice
immunized with transfectants were all isotyped as IgG1.
This is a non-complement fixing subclass so all
specificity analysis has been done using CELISA.
Homozygous B cell lines from the 10th IHW were used for
specificity analysis on eight monoclonal antibodies
produced from mice immunized with DR4-expressing
20 transfectants. These tests were done using optimally- -
diluted supernatants from cloned hybridomas. In addition
two monoclonal antibodies from uncloned hybridomas
resulting from mice immunized with human B cell lines
were also studied (see below). The antibodies were also
25 tested on a panel of L-cell transfectants expressi~g ~ -
various DR4 and non-DR4 molecules. A summary of the data
presented below in Tables 6 and 7 and Figure 3 shows
there are six different patterns of reactivity.
Preliminary- testing shows that the antibodies are
reactive with B lymphocytes from some individuals and a
full analysis will be done when the subtyping-of the DR
specificities is available.
.
WO92/15698 PCT/CA92/0010~
2 ~ 0 ~
39
TA~LE 6 Reactivity of NFLD.D Monoclonal An~ibodies
Produced to HLA-DR4 with Human B Cell Lines.
HLA-CLASS II~ REACTIVI~Y FOR NFLD-~
Cell
Line~ DR Dw DQ DP Dl D2 D3 D4 D7 D8 D9 D10 D12 D14
9034 4,53 4 8 10 8 10 6 3 a 6 8 10 10 10
0 9029 4,53 4 8 2.1 10 10 6 3 8 8 10 10 10 10
9031 4,53 4 a 4.1 10 10 6 8 6 8 10 10 10 10 -
9025 4,53 4 7 4 10 10 6 6 6 0 10 10 10 10
9027 4,53 4 7 4 10 10 6 6 4 0 10 10 10 8
9028 4,53 14 ~ 4 10 10 6 8 6 0 10 10 ~ 4
S098 4,53 14 8 ? 8 10 6 8 6 6 8 10 4 2
LS40 4,53 14 3 3.6 10 1 1 1 4 1 1 1 1 2
9026 4,53 10 8 4.1 10 1 1 1 6 0 1 1 2 4
TS10 4,53 10 3 ? 8 4 4 6 4 6 6 8
9030 4,53 13 7 3 8 6 4 6 6 6 6 8 0 0
9024 4,53 XI 8 5 1 4 4 6 1 6 8 10
9002 1 20 5 4.1 1 4 4 4 1 6 6 8
9003 1 1 5 13 1 4 6 4 6 0 6 10
2 0 9014 15 2 6 4.1 1 6 8 6 8 4 6 8
9010 15 2 6 4.2 0 2 0 4 6 1 2 6 0 0
9013 15 2 6 4.2 1 6 6 6 6 6 6 10 4
9011 15 12 6 ? 1 8 6 6 6 6 6 10 0 0
9015 16 21 5 3 1 6 6 4 6 6 6 10 0 0
9009 16 21 1 4.1,14 1 6 6 6 6 6 8 10 0 0
9016 16 22 7 4.2 1 1 1 1 2
9023 17,52 3,24 2 1 1 1 1 1 1 1 1 2
9022 17,52 3,24 2 3 1 1 1 1 2 1 1 4 4 2
9018 17,52 3,25 2 3 1 1 1 1 1 1 1 1 1 2
9019 17,52 3,25 2 2.2
9021 18.52 NE~,24 4 3
9037 11,52 5.25 7 4.2
9043 11,52 5,25 7 10 1 1 1 1 1 0
3 9032 11,52 DB2 25 5 2 1 1 1 1 1 1 1 1 0
9038 12,52 Da6 25 7 2.1 1 I 1 1 l l 1 1 o
9060 13,52 18,25 5 19 1 1 1 1 1 1 1 1 0 0
9059 13,52 19,2~ 6 3 1 1 1 1 1 1 1 1 1 4
9055 13,52 19,26 6 5
9063 13,52 19,26 6 16
35 9056 13;14,52 9/19,25 5 2.1,13 1 1 1 1 1 0 1 8 1 2
9054 14,52 9,25 5 4.2 0 1 0 1 1 1 1 2 0 0
9057 14,52 9,25 1 4 1 1 1 1 1 1 1 6 0 0
9064 14,52 16,24 7 13 1 8 6 8 1 6 8 10
9052 7,53 11 9 4.1 0 1 0 1 1 1 ~ 1 0 0
9049 7,53 17 2 1 1 1 1 1 1 1 1 2 2
9047 7,53 DB1 2 17 l 1 1 1 1 0 1 2 1 0
4 0 9096 7,53 Dal 2 15 1 1 1 1 1 1 1 6
9069 8,52 8.1 4 4.1 0 1 0 1 1 1 1 1 0 0
9071 8,52 8.2 4 3 1 1 1 1 1 0 1 1 1 4 r-
9066 8,52 8.3 6 3
9074 9,53 23 9 ? 0 1-- 0 1 1 1 1 1 0 0
9075 9,53 23 9 4.1 1 1 1 1 1 1 1 2 0 0
45 9076 9-,53 ? 3 1 1 1 1 1 1 1 4
Refers to the 10th International Histocompatibllity numbers
designated forlthe homozygous B cell lines Ireference 22).
~ The HLA cla~s II types and splits were obtained from
references 23 to 26.
The CELISA data converted to conventional serology scores: 1
0-10% binding: 2, 11-20%; 4, 21-40%; 6, 41-80% 8, 81-100%,
10, > 100%; 0, not done.
. ' ' . ' : ,:
.
W092/15698 PCT/CA92/00105
S 9 ~ 9
~ABLE 7 Reactivity' of NE'LD Monoclonal Anti~odies
Produced Ayainst HLA-DR4 Using HLA-DR
Expressing Transfe~tants.
. :.
Trans- NF1D MONOCLONAL ANTI~ODIES
DR Gene
ExpressedD1 D2 D3 D4 D7 DB D9 10
- -- - . . __ ,:
Dap3 DR4118~ 45 38 7D 93 121 92 125
DR4 ~w4) (.90)~1) (1) ~1) ~1) tl~
L243.6128 58 34 71 96 12a 94 142
DR4 ~w4~ ~.94) ~1.28~ ~.89~ ~l.Q1~ ~1.03~ ~1.06) ~1.02) ~1/14)
L165.6111 51 40 7292 112 98 117 .
DR4 ~w14) ~.84) ~1.13) ~1.05) ~1.03) ~.99) ~.93) ll.06)~.94)
L259.1131 6 10 40100 73 47 141
DR4 ~w13) ~1.0) ~.13) ~.26) ~.53) ~1.08~ l.60) ~.51)~1.13)
L164.11 118 0 0 2 25 0 0 0
DR4 ~w10) ~.90~ ~.03~~.27)
L167.20 0 13
L182.1O 4 21 49 0118 73 192
DR14 ~w16) ~.09)~.55)~.70) ~.97) ~.79~1.54~
Dap3DR1 0 27 19 48 0 87 57 132
DRl ~wl~ 1.60~~.50~~.69~ ~.72l ~.62~1.06~
Dap3 DR2a 0 7 44 28107 68 3a 14
DR2 ~w2) t.l6~ 11.16~ t.40) ~1.15) ~.56~ ~.41~
Dap3 DR2b 0 0 0 0 0 0 0 0
.
' Reactivity OD value of test calculated as a percentage of the
pos1t ve control.
The numbers in brackets refers to the ratio of ~ ~eactivity
for cach cell divided by the ~ reactivity for the immunizing
substype DW13 for NFLD.Dl and DW4 for all the other ~oabs.
,
:: ~ .
~ ~. .....
. - . ,. , . . .. : - . , - ~ . . . . . .. . -; , . .. .. . , .: , .. .... .
: :
WO92/l~S9~ PCT/CA92/00105
~l 21~59~9
NFLD.D1:
This moab was d~rived from a mouse (R11) immunized
with DR4-Dw13 expressing transfectants as shown in Table
3. It appears completely monospecific for the DR4
specificity since it reacts with all the subtypes,
although Dw15 has not so far been tested (Table 6). This
specificity was confirmed ~y testing on a small panel of
transfectants as is shown in Table 7. In addition,
testing supernatant from the uncloned hybrid against
additional transfectants provided by the 11th I~ (data
not shown) revealed no extra reactivity.
NFLD.D2. NFLD.D3, NFLD.D4. NFLD.D8, and NFLD.D9:
All exsept NFLD.D3 were obtained from different
microculture plates of the same fusion (R19, see Table 4)
and are believed to be derived from different clones
although their specificities are similar. The
specificity of NFLD.D3 which was derived from a
completely different fusion (R17, Table 4) is remarkably
similar. It is apparent from the data presented in ~able `
that all five moabs react most strongly with DR4
subtypes Dw4 and Dw14, to a lesser extent with Dw13 and
KT, and not at all with DwlO. In addition they also
react with DR1, DR14 (Dw16) and DR2 (all` its subtypes)
but with no other DR molecules that were tested. The
pattern of reactivity for the cell lines has been
confirmed by testing transfectants (Table 6) with a few
exceptions. The weaker pattern for NFLD.D2 with the DR4
(Dw13), DRl, DR2 and DR14 (Dw16) compared to the DR4 (Dw4
and w14) homozygous, typing cells is striking on the
transfectant cells, particularly for those expressing
Dw16 and DR2. NFLD.D3 also gives reduced binding to the
DR1 and Dw16 transfectants, but reacts quite well with
the DR2A trans'fectant, suggesting that these two
antibodies are different. The other three are extremely
similar and may have been derived from one clonally
expanded B celi in the spleen of the R19 mouse.
W092/15698 PCT/CA92/00105
2~9~ 42
NFLD.D10:
This antibody, derived from a different fusion, R23
(Table 4), has a similar activity to those derived from
R19 but reacts more strongly with DW13, DRl, DR2 and DR14
(w16). Unlike the preceding antibodies, it also reacts
with the DR14 subtype Dw9 and weakly with some DR17, DR7
and DR9 cells. From data obtained by testing undiluted
supernatant on the 11th IXW transfectants (Figure 1),
this pattern ~as essentially confirmed. However, the
weak reactivity observed with DR3 was not apparent when
the appropriate DR3 transfectant was tested. No DR7
transfectant with good expression was available for
testing. In addition the antibody reacted weakly with
the DRlO transfectant.
NFLD.D7:
It is obvious from the data presented in Tables 6
and 7 that this moab has a different pattern of
reactivity than all of the above. In addition to
reacting with all the DR4 subtypes, it reacts strongly
with all the DR2 subtypes but not with DR1. It also
reacts weakly with some DR3 lines. From the CELISA data
on the 11th IHW transfectants (Figure 2) in which
undiluted supernatant was used it appears to crossreact
with DR52 specificity.
NFLD.D12 and NFLD.D13:
These two antibodies, obtained from the same mouse,
were selected out in preliminary screening as they were
reactive with the SAVC cell line that had been used for
the immunizations but negative with a contrasting human
B cell line H0301, (lOth Workshop number is 9055). Both
were isotyped as IgM. They were then tested on a minimum
of 33 B cell lines, most beiny from the well
characterized col'lection of homozygous cell lines studied
in the 10th International HLA Workshop. The CELISA
results are summarized in Table 4.
.. , . . ,, .,, , . ., - ., ~.. .. .. . , ...... . :
W092/15698 PCT/CA92/OOlOS
21~9~9
43
It can be seen that NFLD.D12 give positive results
with five cell lines that express the Dw4 subtype of DR4.
Reactions with other types are small except for modest
binding to the Dw12 variant of DR15. In the case of
NFLD.Dl3, Dw4 is again a target molecule but with this
antibody there are significant reactions also with one of
the four lines expressing the Dw14 subtype of DR4. Minor
reactions are also noted with the remaining two members
of the Dw14 subtypes as well as with the DwlO subtype.
Reactions with the remaining cells in the panel were
mostly either negative or trivial.
Some 76 fusions have been performed using a variety
of experimental designs and with C3H mice, Balbtc mice
and C3H x Balb/c F1 hybrids. Mice have been immunized
with and without adjuvant, by various routes
(subcutaneous, intraperitoneal, intravenous,
intrasplenic) and with varying antigen doses. The
antigenic material has mostly been in the form of L-cell
transfectants expressing HhA-DP molecules, but some
immunizations have been done with EBV-transformed human
B cell lines; a few immunizations have been done with
affinity-purified HLA-DP molecules and with synthetic
peptides designed to reproduce small polymorphic parts of
the HLA-DP molecules. About the only clear conclusion
reached in terms of immunization protocols is th~t
immunization with peptides has been a fruitless
procedure.
The antibodies described here and summarized in
Tables 8a and 8b, represent those selected after
exhaustive screening and testing of many thousands of
hybridomas, typically on the order of 1000 per fusion
(one fusion means one mouse spleen).
:, , . : ~ - , . ,
WO 92/15698 PCr/CA92/00105
2i~9~ 44 -
U~ _ = _ . :,
C ~ U ~ ~ U
:1~ I -~ _C ~- O _C ,~ ~ ~ X '3
CO ~
1-
3 ~ -- ~ -- ~
~ ~ ._.
U~ U) >~ C~l (::1 _ G _ ~ _ _ ~J : .
OC~ ~1 . _ : -'
~ C ~ O O ~
E~ u~ o ~ ~o ~D 0 '1:) ~D ~ .
E 1~ J o 1~ o z _ .
''~
S~5BSTITUTE SHEET: `
WO 92/15698 PCr/CA92tO0105
9 ~ 9
44A
i~ ~
b - L~l `
~ L~ ~- ~ ~ . h. I
SUB~ITUTE SHEE~
~ .. .. .. .. .....
WO 92/l~i698 PCr/CA92/0010~ ~ .
2~0ri969 -` .
. . 45
~ ~ r~
O ~ X ~ O ~ ~ a
H ~ C O ~ ._ ~ _ O C C X ~ .
_ n _ v _ n
) ~ Ln _ N _ C . ~ _
. I ~ _ C ~ _ ~ QJ ._ O ~ . : ' .
~c- -~u ~ ,~d ,_ .~
u ~ v. . _ 9 L N . '
o ~ c c c o c ~ ,~, 3 N
O O O O O--~O = ~ - O C~ -_
v~t~l v~ ~ r3 O o 3 ~ c~l C ;
o c o al c~ c ~ c ^-- z o
O C~ .__ _ _ - _
~7 ~ _ _ o~ cn ~ :.,
D ~ N ~ ~S o ~ ~ t s ~ ~ : ~
o ~o o o C~ o .
= = L~ ~ ~
. .. ~ .
~ . . .
SU~STilrlJTE SHEET. -:
-
WO92/15698 PCT/CA92/0010~
21~969
45A
Antibodies Recoqnizina M onomorphic Determinants
Four antibodies recognizi~g monomorphic determinants
have been selected for listing, from a larger number. OL
those which appear to recognize a monomorphic de~erminant
that is only found on H~-DP molecules, one is IgG1
subclass (NFLD.M67), one ls 1g~2 (~FLD.M68) and a third
is IgM (NFLD.m6O), thus ma~ing an interesting set for use
in a variety of experiments. The four~h, IgG1
(NFLD.M~s), appears to recognize all D~ and all DR
molecules but is ne~ative Cor those DQ molecules Ihat
have so far ~een tested, na~.ely the DQ t~ansfec~ants
distributed to participantc OI the 11th International
U~st~Co~D2t~ t`~ W~r'cc~.o~ !T~W! -
Antibodies Recoqnizinq Polvmorphic Determinants
One antibody, N~LD.M5~, was produced in an earlyexperiment. This antibody showed a striking resemblance
to two other published antibodies. In our laboratory the
same specificity has been found again, either with a
,
SUE~ST~TUTE SHEET
WO92/1~698 PCT/CA9~/00105
, .
210~969 46
strong resemblance (NFLD.M60 and NFLD.M64) or with
relatively minor variations (NFLD.M73, NFLD.M74 and
NFLD.M75). NFLD.M58 is considered to be recognizing an
epitope requiring the amino acid sequence DE at positions
55 and 56 on the HLA-DP beta c:hain. Tests to prove this
conclusion are planned, using mutated beta chain
molecules.
Anothar specificity found in our collection of
antibodies is shown by NFLD.M63. It is sufficiently
different from M58 to warrant description separately. It
binds best to HLA-DPw3 molecules as well as to HLA-DRwll
molecules (including a DRwll transfectant). There are
weaker reactions with other DP molecules, as shown in the :~;
table, which may be possibly lost if the antibody
concentration is reduced by dilution.
A different specificity is displaced by NFLD.M66.
This antibody reacts with virtually all HLA-DP nolecules
except for the DPw2 and DPw4 molecules (coded by
DPBlI0201, DPB1*0202, DPB1*0401 and DPBl*0402 genes).
This pattern suggested that it might be recognizing a
polymorphism due to the amino acid sequence DEAV at
positions 83-86 on the beta chain. Further testing
supported this hypothesis because it failed also to
recognize molecules coded by the DPB1*1501 gen which
carries VGPM at these positions (DPw2 and DPw4 molecules
are GGPM at that location. This hypothesis is currently
being tested using beta chains deliberately mutated at
these four positions. -~
A further specificity is showed by NFLD.M70. This
cytotoxic antibody has been tested on most of the
homozygous celi lines from the loth IHW and only -
recognizes tho~e which carry the less common alpha chain,
coded by the DPAl*0201 gene. This chain, which we can
call the alpha 2 chain for short, is found almost
exclusively with beta chains coding for DPwl and DPw5, as
well as with molecules (not yet officially named by WHO
WO92/1~698 PCT/CA92/00105
47 2i~ 969
nomenclature committee) coded by DPBl*0901, DPBl*1001 and
DPBl*l901. That NFLD.M70 is truly dependent on the
presence of the alpha 2 chain is shown by its failure to
bind to transfectant molecules containing the alpha 1
chain in combination with a DPB1*0901 coded chain
(marshall et al., unpublished observation); a similar
transfectant made with the alpha ~ chain was fully
reactive.
A fourth antibody, different from all the others is
NFLD.M770 This antibody binds to cells that Pxpress DP
molecules containing the amino acid sequence "QL" at
positions 10 and 11 on the beta chain. The single
exception tG this is that cells expressing the DPB1*1301
gene are not recognized by this antibody. Since there
are no available examples of homozygous cell lines
expressing DPB1.1101, the antibody has not been evaluated
for its reaction to the product of DP81*1101; according
to the present interpretation it should bind, unless the
DP molecular structure is influenced by polymorphic
sequences in the adjacent chain, which may be the case
for the non-binding DPBl*1301 product. In preliminary
studies the NFLD.M77 antibody binds also, as predicted,
to transfectant cells expressing the DPB1*0901 gene.
These results make it clear (Figure 3) that there
are six main patterns of reactivity. The first two
patterns show that two monoclonal antibodies (NFLD.D12 &
13) have the potential to differentiate between Dw4 &
Dw14 at least on B cell lines since NFLD.D12 only reacts
with Dw4 cells whereas NFLD.D13 reacts with Dw4 and some
but not all Dw14 cells. In the case of the second
antibody Dw14 specificity is not totally clear since we
do not know whether the Dw14 cell lines are 14.1 or 14.2.
Both variants differ from Dw4 by an arginine substitution
for lysine at position 71 and they differ from each other
by a glycine for valine substitution at position 86. Dw4
has glycine at this position. It is also interesting to
,: . , , , . . . : .: ::, - :, : - , , - ., : , :.
WO92/1~698 PCT/CA92/00l05
2 1 0 5 9 6 9 48
note that neither of these antibodies reacted with DR1
(Dw20) cell line, (9002) which is identical to Dw14.2 in
the third hypervariable region, nor with D~l (Dwl) cell
line, (9003) which is identical to Dw14.1 in the same
region. The DR14 (Dw16) cell line (9064) which is
identical to DR1 (Dwl) and ~)w14.1 in this region was -
clearly negative. This suggests that if indeed the
NFLD.D14 antibody can differentiate between Dw14.1 and
Dw14.2, the apitope must also be influenced by amino
acids outside the third hypervariable region; otherwise
one would expect it to react with either DR1 or Dw16 in
the same way as does the CCCL20 moab (Dejelo CL, Braun
WE, Zachary AA, Teresi, GA, Smerglia AR & Clark LV. Hum
Immunol 1986: 17: 135-136.) which reacts mainly with
Dw14, Dw4, DR1 and DR14 (Dw16). The epitope for this
moab has recently been mapped to positions 67, 70 and 71
and its activity does not appear to be influenced by -
residues in the rest of the molecule (Hiraiwa A, Yamanaka
K, Kwok WW, Mickelson EM, Masewicz S, Hansen JA, Radka SF
& Nepom GT. "Structural requirements for recognition of
the HLA-DW14 class II epitope: a key HLA determinant
associated with rheumatoid arthritis". Proc Natl Acad
Sci USA 1990: 87: 8051-8055.). To our kno~ledge, this is
the first example of monoclonal antibodies with the
ability to discriminate between two conservative amino
acid substitutions in a manner similar to T cell ~-
recognition.
An interesting and unexplained finding in the case
of NFLD.D12 and NFLD.D13, is that these-two antibodies,
used at the same concentration as for B cells lines,
faiIed to bind significantly to L cell transfectants
expressing Dw4 or other DR4 subtypes. It is speculated
that this could b~ due either to a low molecular density ;
on the L cells which could prevent multiple attachments
of the IgM antibody binding sites, or an altered
glycosylation pattern coded by the mouse cell, or perhaps
- WO92/15698 PCT/CA92~00105
9:
it is due to the presence of a different peptide in the
class II groove than is found in human B cell lines.
The moab NFLD.Dl shown as the third pattern
recognized all DR4 cells tested and no other cells. It
5 is expected that this moab r~acts with an epitope found ~ -
on all DR4 subtypes but not on non-DR4 molecules.
Whether or not the epitope is the same as that recognized
by the HLA-DR4 moab, GS359-13FlO, (Alber CA, Watts R,
Xlohe EP, Drover S, Marshall WH, Radka SF & Karr W.
"Multiple regions of HLA-DR~l chains determine
polymorphic epitopes recognized by monoclonal
antibodies". J Immunol 1989: 143: 2248-2254.) will be
determined by epitope mapping at a later time.
The reactivity pattern for D7 is considerably more
complex (Table ~, Figures 2 and 3~. In addition to
reacting with all DR4 cells tested, it also reacts
moderately with DR2 cells (all subtypes). At the
dilution used for specificity analysis on the cell lines
(Table 6) it reacted weakly ox not at all with numerous
cells expressing DR52. However, testing on transfectants
using undiluted supernatant from an uncloned culture was
positive for the two D~52 transfectants tFigure 2).
Since the DRB8 gene, which encodes the DR52 specificity,
is constitutively expressed at lower levels than the DRB1
gene, this simply may be a dilution problem. It is also
possible that the culture from which D7 was~derived was
not clonal. More testing on the transfectants using
supernatant from a cloned culture, as well as testing the
cell lines with antibody in excess, should clarify this.
The fifth pattern shown in Figure 3 is for five
moabs, but the specificities are not quits as simple as
portrayed in the figure, due to graduations of
reactivity. Thi~ is particularly apparent in the data in
Table 6. Both NFLD.D2 and NFLD.D3 are considerably less
35 reactive with the Dw13 expressing transfectant (L259.1)
than are NFLD.D4, NFLD.D8, and NFLD.D9 moabs, all of
-
WO92/15698 PCT/CA92/00105
2105969 .
which show the same degree of reactivity. However,
NFLD.D2 differs from the others in that it reacts poorly
with the Dw16 transfectant tLl82.1) and with the DR2a
transfectant lines.
The final pattern shown in Figure 3, produced by
NFLD.Dlo is similar to the pattern produced by NFLD.D4,
NFLD.D8, and NFLD.D9 but it binds Dw13 more strongly. In
addition it also binds to DR14 (Dwg) molecules and gives
weak reactions with some DR3, DR7, and DRs molecules.
When used undiluted on the transfectants, it is also
weakly bound to DRwlO (Figure 1).
The fact that antibodies react with DR2 is an
interesting feature. The DR2 specificity is even more
complex than DR4 in that there are two serologically-
defined specificities (DR15 and DR16), each with twosubtypes. Also each DR2 haplotype contains two expressed
genes (DRBl and DRB5). Analysis of the sequences and
transfectant data (Figure 2) suggest that in the DR15
speci~icity the positive molecules are coded for by DRB1,
whereas in the D~16 specificity the positive molecules
are coded for by DRB5. Another interesting feature is
that all the reactive molecules for pattern 5 have
glutamine at position 70 and all except DR2 have lysine
or aryinine at position 71, both considered to be the --
critical amino acids involved in the susceptibility to
RA. However, not all molecules with these residues
confer susceptibility to RA. A final interesting point
is that NFLD.D10 which also reacts with molecules
containing these residues, cross reacts with other
molecules containing arginine at position 70, such as
DRl4 (Dw~), DRs and DRlo. It has been speculated by
Gregerson et al. (see Gregersen PK, Silver J & Winchester
RJ. "The shared epitope hypothesis. An approach to
understanding the molecular genetics cf susceptibility to
rheumatoid arthritis". Arth ~ Rheum 1987: 30 1205-
1213.) that this residue in some specificities, e.g. DR53
- . , - ;,, ., ,., , . . ~ ~ .
WO92/15698 PCT/CA92/00105
21 0~969
51
and DRlo may be conformationally equivalent to the RA-
susceptibility epitope on Dw4. It has also recently been
reported that DR10 is highly associated with RA in the
Spanish population.
The HLA-DP system, discovered in a remarkable series
of experiments by Shaw et al. (see Shaw S, Johnson AH,
Shearer GM. J Exp Med 1980: ~52: 565.) was revealed by a
primed lymphocyte test (PLT) procedure. In a PLT, the
polymorphism ~s recognized by T-lymphocytes and not by
antibodies. By 1984, PLT had revealed six probable
alleles. An uncertainty wlth the DP system was that it
might not be accessible to classical serology. However,
various observations have contributed to showing that it
is accessible. A monoclonal antibody was made by Heyes
et al. (see Heyes J, Austin P, Bodmer J, et al. Proc Natl
Acad Sci 1986: 83: 3417.) using a transfectant cell line
as immunogen, that reac~ed primarily with DPw4 and to a
lesser extent with DPw2 cells. Second, Johnson et al.
(see Johnson AH, Thorsby E, Nakatsuji T, et al. Hum
Immunol 1986: 17: 21.) found an antiserum that had been
raised by planned immunization that reacted with DPwl
positive cells once a confounding anti-DR2 antibody had
been removed by absorption. Third, Park et al. (Park MS,
Tonai R, Terasaki PI, et al. Abstract for ASHI (American
Society for Histocompatibility and Immunogenetics meeting
in 1986) found, by screening 5000 pregnancy sera, a few
which reacted reasonably well with either DPwl, DPw3 and
DPw4. So by 1986 it was becoming clear that the DP
system was accessible to serology.
The antibodies provided by the present invention
takes this conclusion further. There is now no reason to
think there should not be other monoclonal antibodies
produced, eithe~ to other polymorphisms that have been
revealed by DNA sequencing of DP alleles (see Bodmer JG,
35 Marsh SGE, Albert ED et al. Tissue Antigens 1991: 37:
1.) or to reciprocal epitopes ~rom those for which
W092/15698 PCT/CA92/OOlV5
~ ~ .
2los969 .-
52
antibodies have been producecl e.g. non-DE at position 55-
56, GGPM at positions 83-86, or to the common alpha chain
(DPAl*O101), or to sequences at positions 10 and 11 other
than QL.
Eventually there should be a series of anti-DP
antibodies available that will allow DP typing to be
carried out by serological methods in a useful, valid
fashion to compare the matching of donor and recipient in
critical situations such as in bone marrow
transplantation. The monoclonal antibodies may not be
good at recognizing alleles, since there is ~o much
sharing of polymorphic portions of the molecule between
alleles, bllt they should be excellent at detecting
epitopes, which after all is what are important in
provoking immune responses, either of graft rejection or
of sraft versus host disease.
Finally, it should be noted that two of the
monoclonal antibodies described here are of the IgGl
subclass and cannot be used in complement-dependent
cytotoxicity assays. If these can be converted to IgG2
or IgG3 by the heavy chain "switchingl' procedure then all
of them may be available for use in routine hospital
tissue typing laboratories; those laboratories rely most
heavily upon complement dependent cytotoxicity as a
tissue typing technique.
Fourteen murine monoclonal antibodies have been
provided herein. These were chosen because -of their
specificity for HLA-DR4 molecules. There are 6 patterns
of reactivity among these ten antibodies (5 are very
-30 similar). NFLD.D1 binds to all DR4 molecules, whilst
others bind only to subtypes of DR4. The shortest,
NFLD.D12, binds only to the Dw4 subtype of DR4; NFLD.D14
binds to Dw4 and~Dwl4 subtypes; NFLD.D7 binds to all DR4
and DR2 molecules but also, less strongly with several
non-DR4 molecules. NFLD.D2, D3, D4, D8 & D9 have
approximately the same pattern as each other, they all
WO92/15698 PCT/CA92/00]05
2~05969
53
bind strongly to Dw4 and Dw14, but not at all to the
subtype of DR4 called DwlO; they give moderate to low
reactions with some other DR4 subtypes; they also react
with DR1, DR2, and DR14 (Dw16). The final pattern, that
of NFLD.DlO resembles the one just described; in
addition, it reacts with the Dw9 subtype of DR14 as
well as binding weakly to some of the DR3-, DR7-, and
DR9-typed B cell lines.
These antibodies, in combination, can be used to
detect subtypes of DR4 and will dc this more quickly and
simply than is possible with T cell technology. In
addition, they have relevance to studies of rheumatoid
arthritis, particular those which react with putative RA-
susceptibility determinants.
As used in the present invention,"cell line" refers
to various embodiments including, but not limited to
individual cells, harvested cells and cultures containing
cells so long as these are derived from cells of the cell
line referred to. By "derived" is meant progeny or
issue. It is, further, known in the art that spontaneous
or induced changes can take place in karyotype during
storage or transfer. Therefore, cells derived from the
cell line referred to may not be precisely identical to
the ancestral cells or cultures, and any cell line
referred to includes such variants.
WO92~15698 PCT/CA92/00105
-.~
i21 0~969
54
REFERENc2s
1. Bodmer JG, Marsh SGE, Parham P, et al
Nomenclature For Factors of the HLA system", 1989
Tissue Antigens 1990: 35: 1-8.
:. .
2. Stastny P. "Mixed Lymphocyte Cultures in Rheumatoid
Arthrltls". J Clin Invest 1976: 57: 1148-1157.
3. Nepom GT, Seyfried CE, ~olbeck SL, Wilske KR ~ Nepom
BS. "Identification of HLA-Dw14 Genes in DR4+
Rheumatoid Arthritis". Lancet 1987: I: 1002-1004.
. ~
4. Karr RW, Rodey GE, Lee T, & Schwartz BD. ~. ;
"Assoclation of HLA-DRw4 With Rheumatoid Arthritis
ln Black and Whit~ Patients". 1980: 23: 1241.
5. Martell RW, Stein M, Davis P, West G, Emmanuel J &
du Toit ED. "The Association Between HLA and
Rheumatoid Arthritis in Zimbabwean Blacks". TissUe
Antigens 1990: 36: 125-126.
6. Ohta N, Nishimura YK, Tanimoto R, Horiuchi Y, Abe C
Shiokawa Y, Abe T, Katagiri M, Yoshiki T & Sasazuki
T. "Association Between HLA and Japanese Patients
~ith Rheumatoid Arthritis". Hum Immunol 1982: 5:
123-132.
7. Christiansen FT, Kelly H ~ Dawkins RL. I'Rheumatoid
Arthritis". In: Albert ED, Baur MP, Mayr WR, eds.
'Hlstocompatibility Testing" 1984. Heidelberg:
Sprlnger Verlag, 1984: 378-379.
.
8. Willkens RF, Nepom GT, Marks CR, Nettles JW ~ Nepom
BS. "Association of HLA-Dw16 With Rheumatoid
Arthri*~s in Yakima Indians". Arth & Rheum 1991:
34: 43-47.
9. Duquesnoy RJ,, Marrari M, Hackbarths S & Zeevi A
"Serological and Cellular Definition of a New HLA-D~
Associated Determinant, MC1, and its Association
With Rheumatoid Arthritis". Hum Immunol 1984: 10:
165-176.
WO92/1~698 PCT/CA92/00105
21~a9~ -
lO. Weyand CM, Goronzy J & Fathman CG. "Human T-cell
Clones Used to Define ~Functional Epitopes on ~LA
Class II Molecules". Proc Natl Acad Sci USA 1986-
83: 762-766.
11. Winchester RJ & Gregersen PK. Ilrrhe Molecular Basis
of Susceptibility to Rheumatoid Arthritis: The
Conformational Equivalence Hypothesis". Springer
Semin Immunopathol 1988: 10: 119-139.
12. Heyes J, Austin P, Bodmer J, Bodmer W, Madrigal A,
Mazzilli MC & Trowsdale J. "Monoclonal Antibodies
to HLA-DP-Transfected Mouse L Cells". Proc Natl
Acad Sci 1986: 83: 3417-3421.
13. Shulman M, Wilde CD & Kohler G. "A Better Cell Line
For ~aXing Hybridomas Secreting Speci~ic
Antibodies". Nature 1978: 276: 269.
,
14. Drover S, Marshall WH & Younghusband HB. "A Mouse
Monoclonal Antibody With HLA-DR4 Associated
Specificity~'. Tissue Antigens 1985: 26: 340 343.
15. Morris ~E, Thompson PT & Hong R. "Cellular Enzyme-
linked Immunospecific Assay (CELISA) I. A New
Micromethod That Detects Antibodies to Cell Surface
Antigens". Hum Immunol 1982: 5: 1-19. ~ -
: -"
16. Drover S. & Marshall WH. "Glutaraldehyde Fixation
of Target Cells to Plastic For ELISA Assays of
Monoclonal Anti-HLA Antibodies Produces Artefacts".
J Immunol Methods 1986: 90: 275-281.
17. Dejelo CL, Braun WE, Zachary AA, Taresi, GA
Smerglia ~R ~ Clark LV. Hum Immunol 1986: 17: 135-
136.
18. Hiraiwa A, Yamanaka K, Kwok WW, Mickelson XM,
Masewicz S, Hansen JA, Radka SF & Nepom GT. ~
"Structural Requirements for ~ecognition of the HLA- . -
Dw14 Class' II Epitope: A Key HLA Determinant
Associated With Rheumatoid Arthristis". Proc Natl
Acad Sci USA l99O: 87: 8051-8055. ~;
~' '
. : .
:., ,
WO92/15698 PCT/CA92/00105
21~5969
56
19. Alber CA, Watts R, Klohe EP, Drover S, Marshall WH,
Radka SF & Karr W. "Multiple Regions of HLA-DR~l
Chains Determine Polymorphic Epitopes Recognized By
Monoclonal Antibodies". J Immunol 1989: 143: 2248-
20. Gregersen PK, Silver J & Winchester RJ. "The Shared
Epitope Hypothesis. An Approach To Understanding
th~ Molecular Genetics of Susceptibility to
Rheumatoid Arthritis". Arth & Rheum I987: 30: 1205-
. .
21. Sanchez B, Moreno I, Magarino R, Garzon M, Gonzales ~.
MF, Garcla A ~ Nunez-Roldan A. "HLA-DRwlO Confers
the Hlghest Susceptibility to Rheumatoid Arthritis
n a Spanlsh Population". Tissue Antigens 1990: 360
22. Young Yank S, Milford E, Hammerling U ~ ~upont B.
'Description of the Reference Panel of ~-
Lymphoblastoid Cell Lines for Factors of the ~A
System: The B Cell Panel Designed For the Tenth
Internatlonal Histocompatibility Workshop" In-
Dupont 8, ed. "Immunobiology of HLA. Vol.
Hlstocompatibility Testing" 1987. New York- -
Springer-Verlag 1989: 420-425.
23. Knowles R~, Adluri V, Xilaru S, Sirotina A and Small
TN. "Binding Patterns of the Monoclonal Antibodies
in the 10th Workshop 2-D Gel Analysis of Class II
Antlgens". In: Dupont ~, ed. "Immunobiology of
HLA. Vol. 1. Histocompatibility Testing" 1987
New York: Springer-Verlag 1989: 420-425.
24. Bugawan TL, Horn GT, Long CM, et al. "Analysis of
HLA-DP Allelic Sequence Polymorphism Using the In
Vitro Enzymatic DNA Amplification of DP-~ and DP-
~
Locl'. J Immunol 1988: 141: 4024-4030. `
2S. Angelini G, Bugawan TL, Delfino L, Erlich HA &
Ferrara GB. "HLA-DP Typing by DNA Amplification and
Hyrldization With Specific Oligonucleotides". Hum
Immunol 1989,: 26: 169-177.
: ~:
26. ~ernandez-Vina M, Moraes ME & Stastny P. "DNA
Typing For Class II HLA Antigens With Allele- `
Specific or Group-Specific Amplification III.
'
W092/1~698 PCT/CA92/00~0~
21~59~
.
57
26. Fernandez-Vina M, Moraes M~ & Stastny P. ~'DNA
Typ~ n~ For Class II HLA Antigens With Allele-
Speclfic or Group-Specific Amplification III. Typing
or 24 Alleles of HLA-DP". Hum Immunol l99l: 30: