Note: Descriptions are shown in the official language in which they were submitted.
~~~:~92/0~347~
21~fi~9~ ~'4' ~~~r~~ t~~~
- 1 -
CONTROL OF SEA LICE IN SEAWATER FISH
Technical Field
This invention relates to the control of sea lice in
salmon and other seawater fish such as seabream.
Background Art
It is known commercially to treat salmon suffering from
infestation with sea lice by the use of the insecticide
dichlorvos. However dichlorvos is generally only effective
against mature lice and is considered not to affect juvenile
sea lice. Furthermore great care has to be taken with the
dichlorvos dosage as the insecticide is fatal to fish at
only 8 times the recommended dose for sea lice treatment.
Further there are indications that resistance to dichlorvos
is developing in sea lice. It is therefore desirable to
find alternative agents for treating sea lice on salmon and
other fish especially materials for which the dose
recommended can be very much less than the fatal (LD 50)
dose.
It is known to use the pyrethroid pesticides cyper-
methrin and its related compound alphacypermethrin for both
the control of pests in crops and against ectoparasites in
cattle and sheep including scab, lice and ked in sheep (see
The Pesticide Manual, 7th Edition, page 3690 ed C R
Worthing, The British Crop Protection Council).
Cypermethrin and alphacypermethrin are thus used in crop
sprays or cattle and sheep dips or sprays. However they and
other pyrethroids have not been proposed for use in treating
fish. Although it is stated in the Pesticide Manual that
the LD 50 (96 hours) for brown trout is 2.0-2.8 microgram
per litre (mcg/1), such tests are only to check that fish
are not at risk from normal agricultural usage. Further
data on the toxicity of cypermethrin to fish is to be found
in "Environmental Health Criteria 82: Cypermethrin",
published by World Health Organisation, Geneva 1989 as part
of IPCS International Programme on Chemical Safety. This
_, -~. ; ;-.,.f. ~ ~?~f~ce
~p. ;-~ ;~.,rinri ~.,_ a
1 . I ' ~ 1 ___ ~ _.. .. . .. ~. ~ ,
WO 92/16106 "~ PCT/GB92/00470
(r i
~~ ~ ~ .,..,
i
- 2 -
summarises work on the toxicity of cypermethrin and reports
in Table 8 that for technical cypermethrin, dispensed in
ethanol, at lOoC the LD 50 (96 hours) for atlantic salmon
having a weight of 5.3g is 2-2.4 microgram active
ingredient/litre. Because of these figures it has been
considered that cypermethrin is too toxic for use on fish.
However, we have found that pyrethroids, particularly
cypermethrin and alphacypermethrin, can be administered to
salmon and other seawater fish in a manner which is highly
effective in the control of sea lice in the salmon and other
fish while being much less toxic to the fish themselves than
dichlorvos.
s»m~ry of the Invention
Accordingly the present invention provides the use of
a pyrethroid pesticide, preferably cypermethrin or alpha
cypermethrin, in the treatment of salmon or other sea fish
suffering from sea lice infestation in a seawater
environment.
Further the invention provides use of a pyrethroid
pesticide, preferably cypermethrin or alphacypermethrin, for
the manufacture of a composition for treatment of sea lice
infestation in salmon or other sea fish in a seawater
environment.
It is not fully understood why cypermethrin is not
toxic to the salmon or other seawater fish in the
circumstances in which it is used in this invention. This
greater tolerance may be due to the presence of seawater
rather than freshwater. The use of alphacypermethrin as a
component of fish food for oral administration may also be
an important factor. Certainly, it is particularly
surprising that alphacypermethrin is highly effective when
administered orally. Good results at dosage rates equivalent
to 0.005mg/litre in water have been found whereas the dosage
rate for dichlorvos to give equivalent results is of the
3 5 oe~gyp f~ ~el,~c~ /-~ i~ze .
~~~~9 2 / 004~7~1
21~~~~~ 24_
MARCH 1993
- 3 -
The active ingredients used according to the present
invention are preferably administered to the salmon or other
fish in their feed but they could be added as a water or
bath treatment to the fish.
The active ingredient can be used in suspension or
emulsified concentrate form or as a solid formulation (e. g.
powder or granules) of a particle size typically in the
range 10 to 103 microns. The range of suspension
concentrate formulations is suitably 10 - 250 g active
ingredient per litre, and powder or granular formulations
typically being 1.0 to 2.5~ w/w pre-mixes added to finished
feed.
Accordingly the invention also provides salmon feeds
and treatment baths containing an active ingredient accord-
ing to the present invention and a method of treatment using
such active ingredient.
Suitably the active ingredient is administered orally,
for example in feed to achieve a dose range of~between 0.025
- 5:0 mg/kg fish/daily preferably 0.8-1.5 mg/kg, more
preferably about 1.0 mg/kg for salmon, and is added to baths
at a range between 0.001 and 0.5 ppm.
It is believed that the active ingredient when
administered orally is taken up by the fish and passes
through to the skin where the lice exist as topical
ectoparasites. Because of the residual concentration of
pyrethroids in the fish it is desirable to leave sufficient
time after the last application for the concentration of
pyrethroid to have fallen to an acceptable level before
killing and consuming the fish.
Brief Description of the Drawincrs
The invention is further illustrated by way of example
with reference to the following Examples and the
accompanying drawings, in which Figures 1 to 8 are graphs
illustrating the Examples.
-'t~Y~t L:~i;C2 ~~~~....~.a~~ . ....
r "~'tOtl ~v a : ~ -,., y ? _ .';. ~:r". s
... .. . _.. ,_ r~,.'C~.;
WO 92/16106 PCT/GB92/00470
4
Bxam~les illustrating. the Invention
Bxam~le 1
In vitro acute toxicit at different concentrations to sea
lice
Sea lice for these trials were collected from Marine
Harvest's Strontian site, Loch Sunart, Scotland.
The lice were collected from freshly killed salmon.
Salmon were killed with a blow to the cranium and were not
to be subjected to anaesthesia (e.g. benzocaine.C02) or any
such compounds as they may affect the lice. The lice were
removed from the salmon with forceps and placed in plastic
bags of fresh sea water (FSW) and transported in closed
insulated containers immediately to the experimental
facility.
The lice were separated into three groups of fifteen
into glass 200 ml containers each containing 150 ml of the
appropriate working solution to be tested. Concentrations
will depend on the solution tested, in addition to a
control. Sea water used for the trial was drawn from Loch
Ailort, Scotland. Once in the containers the lice are
placed into an incubator at 10°C.
After 1 hour the mortality of the lice was recorded and
the solution changed with FSW. Mortality of lice was
recorded according to the following scale:
A - Alive, ability to swim
M - Moribund) inability to swim, twitching/response to
stimulus.
D - Dead, no movement or response to stimulus.
After observations on the lice after 1 hour have been
made samples are placed back into the incubator and mor-
tality was recorded at hourly intervals until approximately
~~Q% of the controls were dead or moribund, both being
WO 92/16106 PCT/GB92/U0470
- 5 -
considered as respondent to the test and grouped together
for analysis.
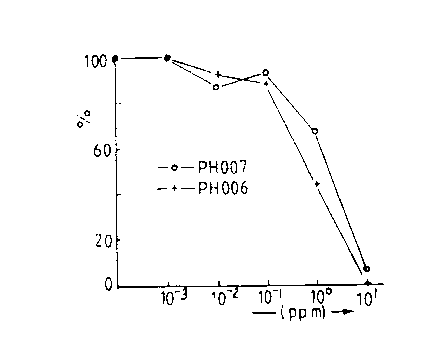
Solutions of 0.001, 0.01, 0.1, 1 and 10 ppm of cyper-
methrin (PH006) and alphacypermethrin (PH007) in sea water
were freshly prepared and used immediately in this test.
The solutions were prepared by adding 1 ml of a 10% w/w
emulsified concentrate of each of PH006 or PH007 to 24 ml
absolute ethyl alcohol and mixing with 975 ml sea water to
produce a 100 ml stock solution. The stock solutions were
diluted 1 to 10 (100 ml stock solution to 900 ml sea water)
to produce: 10, 1.0, 0.1, 0.01 and 0.001 ppm working
solutions
Temperatures and salinities during the trials were
11.5°C and 16-18 ppt (parts per thousand). It was noted that
the salinity of the water from the harvest site was 12.5 ppt
at the time of lice collection.
It was found that 30% or more of the controls were dead
within 8 hours. The results for the response of the lice
after 1 hour after treatment are shown in Figure 1 and
Figures 2 and 3 summarize the acute toxicities for the
individual compounds over time.
B~ ale 2
In vitro toxicity test to different states of sea lice
Sea lice for these trials were selected from McConnell
~ 25 Salmon's Laga Bay site, Loch Sunart, Scotland using the
method described in Example 1.
The lice were separated into g-~oups used in triplicate
(PH006, PH007 and a control) and each group consisted of 15
adult females, 15 adult males and 15 preadult 1 females.
The lice were subjected to a one hour bath treatment
WO 92/16106 PCT/GB92/00470
_ 6 _
using a 0.01 ppm solution (prepared using the method of
Example 1) of each of PH006 and PH007 and a control solution
as described in Example 1 and then followed by a recovery
period in fresh salt water.. Temperatures and salinities
during the trials were 11.5°C and 18.5 ppt respectively.
As in Example 1 responses to the treatment were
recorded at hourly intervals until 30~ or more of the
control samples were either dead or moribund.
The results are summarized in Figure 4. In all groups
male survival was poorest followed by preadult 1 females.
Compound PH007 was found to be marginally more toxic than
compound PH006. Both compounds were found to have sig-
nificant toxicity to the smaller stages of lice.
Bxamvle 3
In vivo toxicity to sea lice
Two trials were conducted to assess the toxicities of
PH006 and PH007 to sea lice in vivo.
A total of 150 juvenile salmon (Salmon salary were
obtained from Marine Harvest's Inver Ailort hatchery, Loch
Ailort, Scotland. The fish were transferred to fibreglass
tanks (measuring 145 cm x 145 cm) with a water depth of 30
cm and given a 24 hour acclimatisation period. All tanks
were supplied with flow-through sea water and were aerated.
These fish were maintained as stock and used for the trials
listed below.
Trial 1
Adult and pre adult (male and female) sea lice were
collected from Marine Harvest's Strontian site, as in
Example 1. The lice were collected from harvested fish as
above. The lice were given a 2 hour acclimatisation period
at the sea water facility prior to being released and
WO 92/16106 PCT/GB92/00470
_ 7 _
allowed to infect salmon.
Salmon were infected with lice by anaesthetizing the
fish (0.075% benzocaine solution) and soaking them in a
small mesh lined container with large numbers of lice. The
fish were removed from the infection bath when a minimum of
lice could be seen attached to them. All fish were
placed into a single tank (as above) and allowed 24 hours to
recover.
Concentrations tested for bath treatments were: 0.01,
10 0.05, 0.1 ppm of PH006 and of PH007 in addition to two
control groups (containing absolute alcohol but no active
ingredient).
Individual groups of fish were randomly selected and
placed into separate tanks (105 cm x 105 cm with adjustable
depth) supplied with flow-through sea water and an ap-
propriate amount of compound stock solution (either 100 ppm
stock solution prepared by adding 100 ml of 10% w/w
concentrate to 100 ml absolute alcohol or 1000 ppm stock
solution prepared by adding 1 ml of 10% w/w concentrate to
99 ml absolute alcohol) added to achieve the desired
treatment concentration. During treatments, water supply to
the tanks was shut off and a constant volume maintained.
Aeration was used during all treatments. Treatments were
one hour in duration after which the water in the tanks was
flushed out and sea water supplies reinstated. Two control
groups were used, a pre treatment control and a treatment
control. Nine fish were used in the pre treatment group and
eight fish were used, per group, for the remaining groups.
The pre treatment control was enumerated for lice before the
beginning of the experiment to establish the parasite load
24 hours following infection. The second or treatment
control was enumerated along with the experimental groups
(48 hours after infection) for parasite load to determine
the number of lice lost due to handling or to poor water
quality. Mean numbers of parasite per fish for the
different groups were compared with a students t-test.
W'O 92/16106 ~ PCT/GB92/0047(1
v
_ 8 _
Temperatures and salinities during treatments were 11°C and
17.5 ppt respectively.
After 24 hours, fish were enumerated for mortalities
and sacrificed with a blow to the cranium; weighed, and the
number of lice per fish was recorded. Feed was withheld
from the fish throughout the entire experiment.
A total of 65 fish were used for the trial with a mean
weight of 457.01 gms (std. dev. - 83.04). None of the fish
died during any of the treatments, except one fish in the
PH007 0.01 ppm group which had jumped out of its holding
tank overnight. In the PH007 0.01 ppm group, some of the
fish showed signs of stress for a couple of hours after
treatment (see Example 4 for characterisation of stress
behaviours). The mean number of lice per fish in the pre
treatment control group was 26.78 (std. dev. - 15.79). The
mean number of lice per fish in the treatment control group
was 14.88 (std. dev. - 7.10). The means were found not to
be significantly different (alpha - 0.05; t statistic -
1.96). In the three PH006 treatments only one louse was
found (0.01 pgm treatment). There were no live lice found
in any of the PH007 treatments.
Trial 2
The same protocols were used as in the previous trial
using bath treatment concentrations of 0.001, 0.005 and 0.01
ppm. The lice used for the trial were collected from the
McConnell Salmon's Laga Bay site, Loch Sunart. Eight fish
were used in the pre treatment group, nine fish were used in
the treatment control and seven fish were used in each of
the experimental groups. Temperatures and salinities during
the trials were 11°C and 22.5-24.0 ppt (27.0 ppt at the lice
collection site).
A total of fifty nine fish were used for the trial with
a mean weight of 483 . 83 gms ( std. dev . - 68 . 21 ) . None of
the fish died during any of the treatments except one fish
WO 92/16106 ~ ~ ~ ~, PC'T/GB92/00470
_ g _
in the PH007 0.005 group which had jumped out of its holding
tank overnight. In the PH007 0.01 ppm group, some of the
fish showed signs of stress for a couple of hours after
treatment (see Example 4 for a characterisation of stress
behaviours). The mean number of lice per fish in the pre
treatment control group was 26.88 (std. dev. - 11.38). The
mean number of lice per fish in the treatment control group
was 26.67 (std. dev. - 8.5). The means between the two
control groups were not different (alpha = 0.05; t statis-
tic - 0.043). Lice were only found in the 0.01 ppm treat-
ment groups with a mean number of lice per fish of 2.14
(std. dev. - 1.86) for PH006 and 1.14 (std. dev. - 0.89) for
PH007. The results for this trial are summarized in Figure
5.
Examy~le 4
Three groups of eight fish taken from the same stock as
for Example 3 were placed into three separated tanks and
given three 1 hour treatments with compounds PH006 and PH007
at 0.5 ppm at 24 hour intervals. The first treatment was in
105 x 105 cm tanks. For the second and third treatment fish
were moved to 145 cm square tanks. The same treatment
protocols were used as in the in vivo trials of Example 3.
Results for this trial are summarized in Figure 6. The
mean weights for the fish used in the trial were not
recorded but are estimated at 469.77 gms as calculated from
the combined weights (n - 124) of fish from the first two
trials of Example 3 which represent 84$ of the stock fish
which were used for all. three trials. No control fish were
lost during the entire experiment. In the PH006 one fish
died of ter the third treatment. Ir. the PH007 group one fish
died after each of the first two treatments. Although very
few fish were lost in each of the treatments, they were
notably stressed by the treatment displaying a repeated
recovery behavioural pattern. During treatment fish would
i~
WO 92/16106 PCT/GB92/00470
- 10 -
become lethargic until the tank was flushed and filled with
FSW. Immediately after treatment and during the period when
tanks were filling, fish displayed a marked loss of co-
ordination with occasional convulsive fits as marked by
sideways shaking of the head. Alternatively, fish would lie
on the bottom of the tank slowly ventilating or would swim
slowly with mouths wide open. In several instances several
fish were observed lying on the bottom of the tanks
motionless) slowly ventilating, for several hours eventually
recovering by the next day. The control fish did not
display any of these behaviours.
Consistent with in vitro tests, compound PH007 was
found to be marginally more toxic than compound PH006 to
both lice and salmon in vivo. Both compounds showed good
efficacy in removing lice from salmon at a concentration of
0.005 ppm. Compound PH006 was the most efficacious since
salmon could tolerate repeated doses at 100 times the dose
rate required to remove all the lice from infected fish.
Low salinities of the sea water at the facility may have
stressed the lice as indicated by a slight decrease in the
number of lice between the two control groups in the first
experiment than in the second where salinities were higher.
It was interesting to note that higher salinities during
Trial 2 of Example 3, which should have reduced the en-
vironmental stress on the lice, did not reduce the toxicity
of the compounds.
R~cam~le 5
Two separate trials were run and are referred to as
Trial 1 and Trial 2.
Atlantic salmon smolts were obtained from Marine
Aquaculture's loch Fyne grow-out site and transferred to
holding tanks at the Sea Life Centre. Fish were held for 4
weeks during which time they were offered pelleted salmon
feed ad libitum. Prior to each of the trials, fish were
divided into several groups of thirteen (depending on the
WO 92/16106 ~ PCT/GB92/00470
- 11 -
concentrations tested) and infected with sea lice collected
at the Kerrera fisheries grow-out site near Oban.
Techniques used for collecting lice are those outlined in
previous Examples. To infect fish, approximately 225-300
lice were added to each of the experimental tanks, where the
volume had been temporarily reduced to 200 litres, and
allowed to stand static for four hours, of ter which water
supplies were reinstated.
Twenty four hours following infection, three fish were
removed from each of the groups, sacrificed, weighed and
enumerated for lice. Based on the average weight of the
fish, fish were fed a medicated diet containing compound
PH007 at dose rates of: 0.025, 0.05 and 0.1 (Trial 1)and
0.1. 0.5 and 1.0 (Trial 2) mg/kg for three consecutive days.
Separate control groups were used for each of the trials.
The compound was incorporated into the feed in a precooled
(<40oC) 5% gelatin solution added to the feed at a rate of
lml/12.5g of feed. Fish were fed the medicated feed at a
rate of 1.5% body weight/day for three days and then fed
unmedicated feed ad libitum for the remainder of the
experimental period. The compound used for the experimental
groups (ref PH/RDL/007H) was a 5% w/w fine beige powder.
Controls were given doses, corresponding to the highest
doses used in the experimental groups, with a placebo (ref
PH/RDL/007A) which was also a fine beige powder.
Fish were sampled for lice 4, 7 and 14 days following
the last medicated feed treatment. To enumerate fish for
lice, fish were sedated in a mild benzocaine solution (2 ml
10%, in acetone, per 1 litre FSW) and the number of
pre/adult stages of lice recorded. On the third and last
sampling fish were sacrificed) weight and number of lice
recorded and then destroyed.
Results
Temperatures and salinities throughout the experimental
period were 12.0 + 1.OOC and 31 - 36 ppt. The mean weights
i.
WO 92/16106 PCT/GB92/00470
12
and lice burdens (number lice/fish) in both trials for the
pre treatment counts are given in Tables 1 and 2. Mean
numbers of lice following treatments for each of the
experimental groups for Trials 1 and 2 are given in Figures
7 and 8 respectively.
There were no fish mortalities in either of the trials
throughout the experiment and no unusual behaviours or
reactions were noted in any of the fish. However, fish were
notably agitated following infection as indicated by a
significant increase in jumping and flashing activity in the
tanks. It is likely that lice were knocked off fish as they
came into contact with objects such as screening material
covering the tanks and the standpipes.
Table 1. Trial 1 - Mean weights and lice burdens in
pretreatment samples (N=three fish in each group).
Grou Mean weight (g) Mean No Lice
(mg/~g) (std.dev.) (std.dev.)
0.0 162.3 (40.1) 10.3 (2.9)
0.025 160.7 (36.1) 15.0 (3.0)
0.05 146.7 (38.7) 15.3 (5.1)
0.1 159.3 (22.1) 13.0 (7.0)
Table 2. Trial 2 - Mean weiqhts and lice burdens in
pretreatment samples (N=three fish in each group).
Grou Mean weight (g) Mean No Lice
(mg/~g) (std.dev.) (std.dev.)
0.0 147.0 (45.9) 20.3 (10.0)
0.1 210.0 (42.6) 2?.7 (4.2)
0.5 167.7 (4.5) 34.0 (16.1)
1.0 169.0 (66.3) 29.3 (23.2)
Conclusions
Although these results are based on artificial
laboratory conditions, they clearly indicate that compound
WO 92/16106 PCT/GB92/00470
2~~~~9~
- 13 -
PH007 has potential as an orally administered sea lice
chemotherapeutant. Overall reductions in the number of lice
present, as seen in the differences between pretreatment and
the successive control samples were probably caused by the
high activity of the fish following infection. Maximum
efficacy was achieved with four days from treatment with no
further, significant, reduction in lice numbers 1 or 2 weeks
following treatment. However, the dose rate of 1.0 mg/kg
may not necessarily be indicative of a true therapeutic dose
due to the small sample sizes of the groups and the
artificial system used. In addition, the variable feeding
response in the experimental system may explain the
difference in efficacy between Trial 1 & 2 in the 0.1 mg/kg
groups. However, reduced efficacy in the second experiment
may have, in part been due to slightly lower water
temperatures. Overall it was established that the compound
achieved good efficacy without any ill effects to the
treated fish.
A number of other pyrethroid pesticides have been
tested in accordance with the invention and the following
table gives the % mortality in sea lice following 1 hour
bath treatments in-vitro with 6 different pyrethroid
compounds each administered at 2 different concentrations.
Hours post Treatment
2 5 COI~OUND 1 6
Negative control 0 0
Solvent (ABS) control 10 10
Cypermethrin 0.1 ppm 0 70
10 40
Cypermethrin 0.01 ppm
Deltametrin 0.1 ppm 20 90
70
Deltametrin 0.01 ppm 20
Resmethrin 0.6 ppm 50 70
Resmethrin 0.06 ppm 50 50
Permethrin 0.5 ppm 0 30
Permethrin 0.05 ppm 0 10
Cyhalothrin 0.2 ppm 60 40
Cyhalothrin 0.02 ppm 0 10
Tetramethrin 0.8 ppm 10 100
Tetramethrin 0.08 ppm 0 70