Note: Descriptions are shown in the official language in which they were submitted.
W093/14105 ~ 3'3 6 ;~ 5 7 PCT/EW3/0010S
DIFLUOROMETHYLENANDROSTENONE DERIVATIVES AND PROCESS FOR
` THEIR PREPARATION
.. ~.
The present invention relates to new 6-difluoromethylen-
androstenones, to a process for their preparation, to
~ pharmaceutical compositions containing them, and to thelr
,h~ use as th~;a~eutic -agents, in particular in the treatment
of hGrmone-dependent diseases in mammals.
Basic and clinical data indicate that aromatized metabol-
ites of ~-~d~ogens, i.e. the estrogens, are the hormones
in~olved in the pathogenic cellular changes associated
with the growth of some hormone-dependent cancers, such a_
breast, endometrial and ovarlan carcinomas.
Estrogens are also involved in the pathogenesls of benign
prostatic hyperplasia.
Endogenous estrogens are ultimately formed from either an-
. ~- drostenedione or testosterone as immediate precursors.
- The reaction Or central lmportance is the aromatization o~
,
20 the steroidic ring A, which is performed by the enzyme ; ~-
aromatase. As aromatization ls a unique reaction and the
last in the series of steps ln the biosynthesis of estro-
gens, lt has been envlsaged that an effective inhibitlon
Or the aromatase, resulting from compounds able to Interact
wlth the aromatizing steps, may have useful appllcation for
.--, . . .
.,- . .
:.
'~
~ SUBSTITUTE SHEET ~ ~
` 2~ ~6~5 ~ 2 -
controlling the amount of circulating estrogens, estrogen-
dependent processes in reproduction, and estrogen-dipend-
ent tumours.
-~ Known steroidal substances which have been reported to be
endowed with an aromatase-inhibiting action are, for ex-
; ample, ~ -testololactone (U.S. Pat. 2,744,120), 4-hydroxy-
androst-4-~ne-3,17-dione and esters thereof (see, for ex-
ample, U.S.Pa~. 4,235,893), 10-(1,2-propadienyl)-estr-4-ene-3,17- ~-
dione (U.S.Pat.4,289,762), 10-(2-propynyl)-estr 1 ene-3,17-dione (J. ~ -
Amer.Chem.~., 103, 3221 (1981) and U.S.Pat.4,322,416), 13-thioan-
drostene derivatives (~p.Pat.Appl. 100,566), androsta-4,6-diene-3, ~ --17-dione, androsta-1,4,6-triene-3,17-dione (G.B.Pat.Appl.2,100,60LA),
androsta-1,4-diene-3,17-dione tCancer Res. (Suppl.) 42,
3327 (1982)), 6-alkenylen-androsta-1,4-diene-3,17-diones
(U.S.Pat. 4,808,816 and U.S.Pat. 4,904,650) and 6-alkenyl-
en-androsta-1,4-dien-17-ol-3-one derivatives (U.S. Pat.
4,873,233). - -
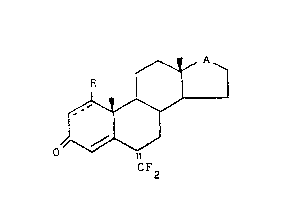
The present invention provides new compounds having the ~-
following general formula (I) - ~;
, ~ ~ ~ p (I)
~ ~ ' o~ ' :~," ' '.
-~ CF2
l~ wherein
~- the symbol = represents a double or single bond; ;
? ' ~ ' .
'_ '~' ' ' ' ',
. ~,`, ~. '
'.' ~'" ~ , " '
SUBSTITUTE SHEET ,
WO93/1410~ PCT/EP93/00105
F' J rr-~r r~
S
R is h~drogen or C1-C4 alkyl; and
A is a _C=~, ~ H~~OH or ~CH~~ORl group, in which Rl is
an acyl g-oup.
Compounds ralling within the scope of formula (I) above are
all the possible isomers, stereoisomers and their mixtures,
and th~ me~~lites and the metabolic precursors or bio-
prec~rsc-s ^~ the compound of formula (I). In the formulae
of this specification the heavy solid lines ( _ ) indicate
that a substituent is in the ~-configuratior., i.e. above the
plane of the ring, whereas a dotted line (.,....) indicates
that a substituent is in the ~-configuration, and a wavy
line (v__) indicates that a substituent may be either in the
~-configuration, i.e. below the plane of the ring, or in the
~-configuration or in both, i.e. a mixture thereof.
In particular when in the compounds of formula ~I) A is
-CH-~~H or --H -û~l the OH or ORl substituent may be either
in the X- o. in the B-configuration or in both, i.e. a mix-
ture thereof. Analogously, when the symbol ----- is a
2û single bond, the R substituent may be either in the ~- or
~-configuration or in both, i.e. a mixture thereof.
The present invention includes all
the possible isomers, e.g. the single 1~,1?~ ,1?~;
- l~,l?q and 1~,17~ epimers, as well as ail possible mix-
25 tures therec~, e.&. 1(~,~),17~ ),17~ ,17(~,~); s
.
' '- :' '
-- '
., - :.... .
~ SUBSTITUTE SHEET
WO93/14105 PCT/EP93/00105
~6'~7 - 4 _
lB,17(~,~) and l(q,~),17(q,B)-isomers of the compounds of
formula (I). Hence a compound of the invention herein spe-
cifically mentioned, without any indication of its stereo-
chemistry, represents all the possible single isomers or
mixtures thereof.
A Cl-C4 alkyl group is preferably a methyl or ethyl group,
more preferably a methyl group. The alkyl radical ma~ be a
branched or straight chain radical.
Rl as an acyl group may be residue of any physiologically --
tolerable acid. Preferred examples of said acids are
Cl-C4 alkanoic acids, in particùlar acetic, propionic and
butyric aci~s.
: .,
As stated above, the present invention also includes with-
in its scope pharmaceutically acceptable bio-precursors
..
lS (otherwise known as pro-drugs) of the compounds oS formula
(I), i.e. compounds which have a different formula to for-
¦ mula (I) above but which nevertheless upon administration
¦~ ~ to a human being are converted directly or indirectIy in
vivo into a compound of formula (I).
20 Prererred compounds of the invention are the compounds Or !
I formula (I) wherein
A is a -C=O or -CH__OH group;
- R is hydrogen; and
I the symbol --- represents a single or.double bond.
Examples Or specific compounds of the invention are the
` '' '
1-~ ~,.. ,-.
1~ .... . .. .
r`- .
I .,~
I . I .
l .,~
.~ " ' .
'` SUBSTITUTE SHEET ~ ~
W093/l4105 PfCT/EP93/~105
f!~ J ~
(
; followin~ coirfounds which, when appropriate, may be either
~- or ~f-epimers or ~,r~-mixtures of said epimers:
6-difluorome~hylenandrost-4-ene-3,17-dione;
6-difluoromethylenandrost-4-en-17f~-ol-3-one;
6-difluorome hylenandrost-4-en-17r~-ol-3-one;
6-àifiuo-omf~-nylena drosta-1,4-diene-3,17-dione;
6-difluorome'hylenandrosta-1,4-dien-17~-ol-3-one; and
f 6-d fluo~o.~.e ~lenandrosta-i,4-dien-17r~-ol-3-one.
The compounrfs of the invention can be obtained by a process
comprising:
a) dehydrofluorinating a compound of formula (II)
~ (II)
~ l . L ~ -
o/ l ~
¦ wherein 2
R is as defined above, thus obtaining a compound of for-
mula (I) wherein the symbol --- is a single bond and
A is a ~CO group; or ~ .
b) dehydrogenating a compound of formula (III) ~ I
~y .'-.'
``- ~ '':,
' ' '
SUBSTITUTE SHEET
W093/14105 PCT/EP93/0010S
hl ~5~7 - 6 -
wherein
R is as cefined above, thus ob~aining a compound of fo~-
mula (I) wherein the symbol --- is a double bond and
A is a ~CO group; or
c) reducing selectively a compound of formula (IV)
O ,~'
~ (IV~ ~
O ' '''-' ' , .
CF2 : '
wherein
R and the symbol --- are as defined above, thus obtain- .
~ ing a compound of formula (I) wherein R and the symbol
-~ 10 --- are as defined above and A is a _ CH~-OH group; or
d) acylating a compound of formula (V)
OH
(V)
~ 11 '
CF2 --
. : wherein . ~ :
R and the symbol --- are as defined above, thus obtain-
ing a compound of formula (I) wherein R and the symbol
.
~. , ,... ~ '
SlJBsTlTuTE~ JFFT : ~
WO 93/14105 ~ ~ r~ PCT/EPg3/OOtO5
,- .L j~ jJ ;~
7 --
--- are as defined above and A is a ~ CH~ORl group in
which R1 is an acyi group; and, if desired. converting
a compound of formula (I) into another compound of for-
mula (I), and/or, if desired, separating a mixture of
isomers of compounds of formula (I) into the single
isomers.
The dehydrofluorination of a compound of formula (II) can
be carried out by using basic dehydrohalogenation a~ents
such as pyridine or a LiBr-Li2CO3-DMF mixture. Preferably
the dehydrofluorination is carried out by chromatographing
the raw steroid on neutral or basic alumina and using as
eluant an inert soIvent such as benzene, dichloromethane
or ethyl acetate.
~ The dehydrogenation of a compound of formula (III) may be
¦ 15 performed according to known methods, e.g. by treatment - -
~- with DDQ, according to D. Wrlker and J.D. Hiebert: Chem. -
~ Rev. 67, 156 (1967), or by treatment with selenium dioxide,
¦ chloranil or benzeneseleninic acid. Preferably the reaction
is performed by treatment with benzeneseleninic anhydride
`- 20 in an inert organic solvent, such as chlorobenzene or car- -
bon tetrachloride. at a temperature ranging from about
60C to about 120C and reaction times varying from about
2 to about 48 hours.
~ The selective reduction of a compound of formula (IV) may ~ -
¦ 25 be carried out by a well known method, for example as des-
'"': ~ ' ' ''; , :
.,~ , ' '.
! .;
".: ' , ' .
'-~. SUBSTiTUTE SHEET
W093/14105 PCT/EW3/0010~
7 - 8 -
cribed by C. Djerassi in Steroid Reactions (1963) or by
J. Fried in Organic Reactions in Steroid Chemistry Vol.I -~ --
(1972). Preferably the reductlon is carried out with a com-
j plexed metal hydride, in particular with sodium borohydr-
ide in an inert organic solvent, in particular in ~ethanol
solution a- te-~eratures ranging from about O to about
50C. -~
The acviat.c~ c. a compound of formula (V) can be perform- ;
ed by reac.ion with a reactive derivative of a suitable
carboxylic acid, such as an anhydride or halidè, in the
presence of a basic agent, at temperatures ranging from
~ about O to abbut 50C. Preferably the acylation is carried -
I out by reaction with the respectlve anhydride in the pres-
¦~ ence of an organic base, such as pyridine.
15 The separation of a mixture of isomers into the single ~;
T ~ isomers as well as the conversion Or a compound of formula
(I) into another compound of formula (I) may be carried
out according to known methods. For example a 17~-hydroxy ,
derivative of a compound of formula (I) may be converted
- 20 into the respective 17~-hydroxy derivative by basic cata-
¦ lysis, e.g. with O.lN sodium hydroxide in an aliphatic al-
cohol, e.g. ethanol.
~- ~ Slmilarly, processes bl, c~ and d) can be regarded as op-
tional conversions of a compound of formula (I) into an-
other compound of ~ormula (I). In fact, a compound Or for-
~'' ' ,,'','"''
' ~ . ~ ':'' ~ ."'
~ -1 SUBSTITlJTE SHEET -:
. . ~ ." ,; ~ ~ ; ",~ . ., ~, 1, .,!,
'J" ' .~`' '' '' ~'~,~ ' ,`~ .,, :` "~t . ~ , ;. ,;~, ~: :j, , ... .. ,~
W093/14105 PCT/EP93/00105
g _
mula (III) is a compound of formula (I) wherein the symbol
--- is a single bond, A is _CO and R is as herein defined.
A compound of formula (IV) is a compound of formula (I)
wherein A is CO and the symbol _ and R are as herein
defined; and a compound of formula (~') is a compound of
formula (I) wherein A is ~CHw~OH æ~ the sym,bol = and
R are as herein defined.
A compound of formula (II) can be ob~ained by oxidizing a
compound of formuia (VI)
OH
~ ~ J ~ ~ (VI) ~ ;
HO ~F ~
CF2 ~ ~ ,
wherein R is as defined above.
The oxidation of a compound of formula (VI) can be per- -
formed according to known methods, e.g. by treatment with
the Jones reagent as described by Fieser and Fieser in
lS Reagents for Organic Synthesis ~, 142 (Wiley 1967). Jones
reagent is a solution of chromic acid and sùlfuric acid
1 in water. The oxidation may be carried out by titrating a
¦ stirred solution of the alcoholic compound in acetone at
temperatures ranging from about -20C to about 30C with
the Jones reagent.
., ~ :
- .'' .'.'.. :.'
.~`.': .
,r
~ ~ SUBSTITUTE SHEET
~ ~ . " ~
~ ''~W'' ' ' t ~'r ;. '' ~:. ' . -' ,".i ; ~ ':~ .; ;:~ ;:; .
W093/14105 PCTtEP93/OOlOS
r ~ 10 --
~i~oi,~
A compound of formula (VI) wherein R is as defined above
may be obt2in~ by saponification of a compound of for-
mula (VII)
OR
(VII)
R 1 C r
' 2 ~ .
5 wherein R is as defined above and Rl, being as herein de- 1~ .
fined,is preferably a lower alkanoyl group, in particular -
acetyl.
j The saponification may be performed by a conventional :.
method, e.g. by treatment with an alkali metal hydroxide in
alcoholic solution, preferably with potassium hydroxide in
.
methanol or ethanol at temperatures ranging from about 20C
to reflux temperature.
. A compound of formula (VII), wherein R and Rl are as de- ~ ` -
fined above, may be obtained from a compound of formula
-,
lS (VIII)
~- R ~ .;
'' ~
,'''""
, 1 1 (VIII)
R O ~ F
~` 1 o
~, .
. .
..
',:~
~; SUBSTITUTE SHEE~ :
~ ; e
W093/14105 ~ J ~ ~ t ~ r PCT/EP93/~105
~.i'J~
-- 11 --
wherein R and Rl are as defined above by applying e.g. the
method described in U.S. Pat. 3,504,002. Accordingly, the
6-ketosteroid of formula (VII) is treated with at least
equimolar amounts of tributylphosphine and sodium chloro-
difluoroacetate, at a temperature in the range of 150 -
200C,Using as solvent disubstituted hydrocarbon amide.
It is viewed as involving the in situ production of tri-
butylphosphinedifluoromethylene, which reacts with the
6-keto grou?. Alternativelj, the method mentioned in U.S.
lO Pat. 4,567,000 can be used. Accordingly, a solution of the
protected 6-ketosteroid in an inert solvent such as THF,
dioxane or diglyme is added to a cold solution of (di- ~
ethylphosphinyl)difluoromethyllithium at about -60C to -
about -80C. This reagent may be prepared by treatment of
diethyl difluoromethylphosphonate with l.l equivalent of
lithium diisopropylamide in THF at -7~C.
A compound of formula (VIIIj wherein R and Rl are as de-
fined above may be obtained by oxidation of a compound of
formula (IX)
R 1
~ (IX)
P.lO
OH
SUBSTITUTE SHEET
WO93/14105 PCT/EP93/00105
- 1~
in which R and R~ are as defined above. This conversion may '
be performed according to a well known oxidation method in
steroid chemistry, e.g. according to the method described
above. ~ ,
A compound of formula (IX) wherein R and R~ are as defined
above may be obt2ir,ed by cle2vage of the 5~,6~-epoxide ring
in a compound of formula (X) ~- ,
OR1
~10~,1 (X~
wherein R and Rl are as defin,ed above. ' ,
The cleavage may be carried out, e.g., accordingly to
H.B. Henbest et al. (J. Chem. Soc. 1957, 4765) by reaction - -
with the boron trifluoride-ether complex in an inert or-
,- ganic solvent such as benzene or ethyl ether at tempera- '
tures ranging from about O to SOC. In the case of 5,6~- -
lS epoxide, the cleavage affords the diaxial 5~,6n-fluoro-
' hydr~n as desired. ,,
,,~ A compound of formula (X), wherein R and Rl are as defin-
, ed above, may be obtained from a compound of formula (XI)
.
SUBSTITUTE SHE-cT
. ~ ~ .
WO93/1410S 2 1 v ~ PCT/EP93/~105
(XI)
Br
wherein R an~ R1 are as defined above;
by dehydrobro,,,ination, e.g. according to J.F. Templeton
et al. in J. Chem. Soc. Perkin Trans. I, 1361 (1987). Thus
a solution of the 5~,6~-bromohydrin in an lnert organic
solvent, such as dichloromethane or THF, is stirred with
. . .
basic alumina at room temperature. Alternatively, the
¦ 5~,6~-bromohydrin can be treated with a weak base such as
sodium acetate in alcoholic solution, e.g. in methanol,
at temperatures ranging from about 20C to reflux tempera-
ture, e.g. as described in ~elg. Pat. 625,669.
¦ A compound Or formula (XI), wherein R and R1 are as defin-
ed above, may be obtained from a compound of formula (XII)
~ (XII)
R l
~ SUBSTITUTE SHEET
W093/14105 PCT/EP93/OOlOS
~ l a ~n ~ ,7 14 -
',
wherein R and Rl are as defined above;
by treatment with a N-bromoamide such as N-bromoacetamide
;or N-bromosuccinimide in the presence or not of perchloric
acid in an aqueous solvent mixture s~ch as aqueous dioxan
or aqueous i,2-dimethoxyethane at temperatures ran~ing
from about 0C to room temperature, e.g. as described in
J. Chem. Sor. l95a, 4108.
The com?ou~a^ of ,ormula (XII) are kno~n compounds or may
be obtained by known methods from known compounds.
When in the new compounds of the present invention and in
the intermediate products thereof groups are present which
need to be protected before submitting them to the here-
above illustrated reactions, they may be protected before
the reactions take place and then deprotected at the end
of the reaCtio-l, according to well known methods in organ-
ic chemistry.
The compounds of the present invention are inhlbitors of
the biotransformation of androgens into estrogens, i.e.,
`they are steroidal aromatase inhibitors.
¦ 20 The aromatase inhibitory activity of these compounds was
~i' demonstrated by employing the in vitro test described by
Thompson and Siiteri (E.A. Thompson a,nd P.K. Silteri, J.
Biol. Chem. 249, 5364, 1974) which utilizes the human pla-
cental microsomal fraction as enzyme source. In this test
the aromatization rate~of androstenedione into estrone was
,4'~ ~ :
~ ~ " '. '
`.~` SUf3STlTUTE SHEET
~ ~ ,
r ; ; ` . - ; ~ , . .
. ; '~. ? ~ t~ i ? ~
WO93/14105 PCT/EP93/00105
f ~ .L U ~
- 15 -
evaluated by incubating (lB- H)androstenedione (50 nM) in
the presence of NADPH with the enzyme preparation and by
measuring the amount of H20 formed during 20 min incuba-
tion at 37C.
The compounds, incubated at various concentrations, showed
a relevant aromatase inhibitory activity.
By virtue of their ability to inhibit aromatase and, con-
sequently, to reduce estrogen levels, the compounds of the
invention are useful in mammals, including humans, in the
treatment and prevention of various estrogen-dependent dis-
eases, i.e. breast, endometrial, ovarian and pancreatic
cancers, gynecomastia, benign breast disease, endometriosis,
polycystic ovarian disease and precocious puberty. Another
application of the compounds Or the invention is in the
therapeutic and/or prophylactic treatment of prostatic
hyperplasia, a disease of the estrogen-dependent stromal
tissue.
The compounds of the invention can find also use for the ~ -
treatment of male infertility associated with oligospermia
20 and for female fertility control, by virtue of their abil- ~
ity to inhibit ovulation and eg8 nidation. ;
In view of their low toxicity the compounds of
the invention can be used safely in medicine. For example,
the approximate acute toxicity (LD50) of the compounds of
~ 25 the invention in the mouse, dete-mined by single adminis- ~ ~
.. ~ : ' .
~,'~,;, ' ' ~
... . .
... . .
`~ SUBSTITUTE SHEc~
WO93/14105 PCT/EP93/00105
~ 16 -
tration of increasing doses and measur~d on the seventh
day after the treatment was found to be negligible.
- The compounds of the invention can be administered in avariety of dosage forms, e.g. orally, in the form of tab-
lets, capsules, sugar or film coated tablets, liquid solu-
tions or suspensions; rectally, in the form of suppositories;
parenterally, e.g. intramuscularly, or by intravenous in-
jection or infusion.
The dosage depends on the age, weight, conditions of the
patient and admininistration route; for example, the dos-
age adopted for oral administration to adult humans may
range from about 10 to about 150-200 mg pro dose, from 1
to 5 times daily. -
The invention includes pharmaceutical compositions com-
prising a compound of the invention in association with a
,.~ . . .
- pharmaceutically acceptable excipient (which can be a car-
- rier or diluent?.
The pharmaceutical compositions containing the compounds
I of the invention are usually prepared following conven-
~, , .
-- 20 tional methods and are administered in a pharmaceutically
suitable form.
~or example the solid oral forms may contain, together with
the active compound, diluents, e.g. lactose, dextrose, -~
saccharose. cellulose, corn starch or potato starch; lu-
; 25 bricants. e.g. silica, talc, stearic acid, magnesium or
`;~'" , '
~, ~ ~ . ,-, .
~'`;.,,'~.; '
:~ SUBSTITUTE SHEET
i~. ;~ -.. - ` .. - . . .... , . . - . .. ., - . ., ~ -.. -.. . - .i, `.. - . -
",.~, .. . ., , . .. .. ... . . . .;, ,. . ., .;., . ., ., . , ,; , .. .. - - .. , ` .. . .. .... .. .. . . . . .. . . . .
W093/14~05 PCT/EPg3/~10S
~C~ 5'~
calcium stearate, and/or polyethylene glycols; binding
agents, e.g. starches, arabic gums, gelatin, methylcel-
lulose, carboxymethylcellulose or polyvinyl pyrrolidone;
disaggregating agents, e.g. a starch, alginic acid, al-
ginates or sodium starch glycolate; effervescing mixtures;dyestuffs, sweeteners; wetting agents, such as lecithin,
polysorbates, laurylsulphates; and, in general, non-toxic
and pharmacologically inactive substances used in pharma-
ceutical formulations. Said pharmaceutical preparations
10 may be manufactured in known manner, for example, by means ~-
of mixing, granulating, tabletting, sugar-coating, or
film-coating,processes. The liquid dispersions for oral
administration may be e.g. syrups, emulsions and suspen-
sions.
The syrups may contain as carrier, for example, saccharose
or sacchzrose with glycerine and/or mannitol and/or sor-
, bitol.
.
¦ The suspensions and the emulsions may contain as carrier,
- for example, a natural gum, agar, sodium alginate, pectin,
~- 20 methylcellulose, carboxymethylcellulose, or polyvlnyl al-
cohol.
^ - The suspensions or solutions for intramuscular inJections
may contain, together with the active compound, a pharma- ,,
ceutically acceptable carrier, e.g- sterile water, olive
Z5 oll, ethyl oleate, glycols, e-g- propylene glycol, and ir
SIJB~STITUTE SHEET
WO93/14105 PCT/EP93/00105
h~ 7
-- 18 --
desired, a suitable amount of lidocaine hydrochloride. The
solutions for intravenous injections or infusions may
contain as carrier, for example, sterile water or preferably
they may be in the form of sterile, aqueous, isotonic saline
5 solutions.
The suppositories may contain together with the active
compound a pharmaceutically acceptable carrier, e.g.
cocoa-butter, polyethylene g'ycol, a polyoxyetnylene
sorbitan fatty acid ester surfactant or lecithin.
lO The present invention also provides a compound of formula
(I) for use in a method of treatment of the human or animal
body by therapy, especially for use as an aromatase
inhibitor.
The present invention additionally provides the use of a
lS compound of formula (I) in the manufacture of a medicament
for use as an aromatase inhibitor. ~ -
The present invention further provides a method of
inhibiting aromatase in mammals, including humans, which
¦ comprises applying thereto an effective amount of a compound
¦ 20 of formula (I).
¦ The present invention still further provides an aromatase
inhibiting agent comprising a compound of formula (I).
The following examples further illustrate the invention:
Example l (I, A= `C=0, R=H, _ single bond)
,~ 25 First Jones reagent is prepared by dissolving 2.672 g of
chromic trioxide in 2.3 ml of conc.sulfuric acid and di-
- ~ lutlng with water to a volume of lO ml. Then to a stirred
solution of 6-dlfluoromethylene-5~-fluoroandrostan-3,17-
~,. .
~`` SUBSTITUTE SHEET
WO93/14105 ~ J O ~ 7 PCT/EP93~00105
diol (358 mg, 1 mmol) in acetone (30 ml) is added portion-
wise under cooling 2 ml Jones reagent. After stirrlng for
~2 h at 10-15C and another~ h at 20-25C, methanol (1 ml)
is added to destroy excess reagent. The resultant green
solution is filtered to remove the chromium salts and then
carefully diluted with water in order to precipitate raw
6-difluoromethylene-5~-fluoroandrostan-3,17-dione. The
precipitate lS filtered off, desiccated, diss~lved in
.;
'
!
i, '
~'~',".
'."' .,
. :: .. . ..
. . . ~
1~ , . .
,''' ~ :'','."' ''
SUBSTITUTE SHEET
W093~14105 PCT/EP93/~105
benzene (10 ml) and adsorbed onto a basic alumina column
(20 g). Finally elution with benzene-ether affords pure
6-difluoromethylene-androst-4-ene-3tl7-dione (167 mg, 50%
yield).
C20H24F22 calcd C 71.83 H 7.23 F 11.36
found: C 71.71 H 7.11 F 11.42
IR cm : 1740 (17-CO), 1725 (C=CF2), 1670 (3-CO), 1620
(~4
MS (m/z) 334
Example 2 (I, A= ~C=O, R=H, _ double bond)
A solution of 6-difluoromethylenandrost-4-ene-3.17-dione
(334 mg, 1 mmol) and benzen~ selenic anhydride (360 mmol) in
chlorobenzene (30 mol) is heated for 4 h at 90-100C. Then
the solvent is removed in vacuum and the residue
15 chromatographed on silica gel with benzene-ether mixture as
eluant thus giving pure 6-difluoromethylenandrosta-1,4-
diene-3,17-dione (266 mg, ~0~ yield).
C20H22F202 calcd: C 72.27 H 6.67 F 11.43
found: C 72.15 H 6.55 F 11.35
IR cm : 1735 (17-COj, 1720 (C=CF2), 1650 (3-CO), 1610 (~ )
MS (m/z): 332.
SUBSTITUTE SHEET
.. ... ... .. . . ~ ,..... . . .. . , ...... . . . . . . . , . - -
~ . .. . . ` ,,- . . . . . - . . ;.. .. ; . . , . ,. , .. . . ~ ~ . . . . .
W093/14105 PCT/EP93/00105
~ 1 3 ~ i 7
- 21 -
Example 3 (I, A= `CH-OH, R=H, --- single or double bond)
To a stirred solution of 6-difluoromethylenandrost-4-ene-
3,17-dione (334 mg, 1 mmol) in methanol (20 ml) is added
sodium borohydride (57 mg, 1.5 mmol) over a period of 10
min at 0C and stirring is continued for 1 h at 0C. After
addition o~ few drops of acetic acid, the mixture is con-
centrated under vacuum, diluted with water and then ex-
tracted w,th ethyl acetate. The combined organic phases
are washed with saline solution, dried over sodium sulfate
, 10 and then evaporated in vacuum. The residue is 6ubmitted
; to column chromatography on silica gel. Gradient elution.
with benzene ether mixtures affords pure 6-difluora~ethylen-
androst-4-en-17~-ol-3-one (202 mg, 60-% yield).
C20H26F202 calcd: C 71.40 H 7.79 F 11.30
found: C 71.31 H 7.68 F 11.15
IR cm : 3410 (OH), 1720 (C=CF2), 1~75 (3-CO), 1625 (~ ) -
MS (m/z): -36.
According to the above described procedure and starting ;
from the appropriate compound of formula (~V)
respectively one can prepare the following products as
single isomers, as well as their 17~,17~-mixtures:
6-difluoromethylenandrost-4-en-17~-ol-3-one;
6-dlfluoromethylenandrosta-1,4-dien-17~-ol-3-one; and
6-difluoromethylenandrosta-1,4-dien-17~3-ol-3-one.
' ,'', ': ' '
' ' ~' :
~ - . .
'` ' ' ' " ~ :'S ' ' '
~ ' ~ ''.
SUBSTJTUTE SHEET ` :`
WO93/14105 PCT/EP93/00105
~ 3 7
Example 4
A solution of 3~,17~-diacetoxy-6-difluoromethylene-51~-
fluoroandrostane (442 mg, 1 mmol) in methanol (15 ml) con-
taining potassium hydroxide (336 mg, 6 mmol) is refluxed
for 4 h. Tne reaction mixture is concentrated under vacuum,
diluted with water and extracted with ethyl acetate. The
combined o~ganic layers are washed with saline solution,
dried over magnesium sulfate and evaporated under reduced
pressure. The residue is crystallized from acetone-he~ane
giving pure 6-difluoromethylene-5d-fluoroandrostane-3,17-
diol (323 mg, 90 % yiel~).
C20H29F3O2 calcd: C 67.G2 H 8.15 F 15.90
found: C 67.10 H 8.05 F 15.81
MS (m/z): 358.
15IR cm : 3350 (OH), 1720 (C=CF2)
.,,:',
` SUBSTITUTE SHEET
~J ~ 7 : ~ j, `
W093/14105 ~1;JU ~ 7 PCTtEP93tO0105
Example 5
A solution of 3B,17~-diacetoxy-5~-fluoroandrostan-6-one
(408 g, l mmol) and tributylphosphine (344 mg, 1.7 mmol)
in N-methyl-2-pyrrolidone (2 ml) is heated to 180C. To
this solution is added dropwise during 40 min a solution
of sod u~ _hlo-odifluoroacetate (401 mg, 3.3 mmol) in
N-methyl-2-pyrrolidone (6 ml). The reaction mixture is
dissolved in benzene after cooling and the solution sub-
m~tted to column chromato3raphy. Gradient elution with
benzene-ether mixture affords besides starting material
the desire~ 3B,17~-diacetoxy-6-difluoromethylene-5~-fluo-
roandrostane (133 mg, 30 % yield).
c24H33F304 calcd: C 65.14 H 7.52 F 12.88
found: C 65.05 H 7.44 F 12.75
15 MS (m/z): 442.
IR cm : 1740 (-OCOCH3), 1725 (C=CF2).
~.' ''
'. ' .
.
... ~ ,.. -, . ..
i ~
,'".; ,,
'~' '''.
I' ....
1' , "'
SUBSTITUTE SHEET : -
WO93/14105 PCT/EPg3/00105
~ L ~ 7 _ 24 -
Example 6
To a solution of 3~,17~-diacetoxy-5~-fluoroandrostan-6~-
ol (408 mg, l mmol) in acetone (20 ml) is added portion-
wise Jones reagent (1 ml) under cooling at 10-15C. The
5 stirring is continued for ~ h at lO-15C and another X2 h
at 20-25C. After addition of methanol (1 ml), the green
mixture is filtered and the filtrate carefully diluted
with water i~ order to precipitate the raw product. This
is filtered off and chromatographed on silica gel using
i lO gradient elution with benzene-ether mixtures. Thus pure
3~,17~-diacetoxy-5~-fluoroandrostan-3-one is obtained
s (327 mg, 80 ~ yield).
3 C23H33FO5 calcd: C 67.62 H 8.14 F 4.65
¦ found: C 67.45 H 8.05 F 4.57
15 MS (m/z): 408. ~-
IR cm : 1735 (acetate), 1705 (6-ketone).
..
:: ": ' '
, ,.
. .
.
,,
; ' ~,
s;~
.j
, .
SUBSTITUTE SHEET
.. . ..
WO93/14105 2 ~ ~ V ~ PCTtEP93/0010S
Example 7
A solution of 3~,17B-diacetoxy-5,6B-epoxyandrostane (389
mg, 1 mmol) in benzene (20 ml) is treated with boron tri-
fluoride etherate (227 mg, 1.6 mmol) at 20-25C for X2 h.
The solution is washed with water, dried over potassium
carbonate a~d the solvent evaporated under vacuum. The
residue is chromatographed as usually to give pure 3~,17~- -
diaeeto~y-5~-fluoro-androstan-6~-ol (308 mg, 75 % yield).
C23H35F05 calcd: C 67.29 H 8.59 F 4.63 ~-
found: C 67.15 H 8.46 F 4.55
MS (m/z): 408
IR cm : 33,50 (hydroxy), 1735 (acetate) - ~ -
Example 8 -
A solution of 5~-bromo-3~,17~-diacetoxyandrostan-6~-ol
15 (471 m~, 1 mmol) in dichloromethane (25 ml) containing
basic alumina (3 g) is stirred for 2 h at 20-25C. The
alumina is filtered off, the residue washed with dichloro-
methane and the combined filtra'e evaporated under vacuum.
The residue is crystallized fro" dichloromethane-acetone
20 to afford pure 3~,17B-diacetoxy-5,6~-epoxyandrostane
(233 mg, 60 % yield). ~ -
C23H3405 calcd: C 70.74 H 3.78
found: C 70.55 H 8.65
MS (m/z): 390
` 25 IR cm : 1735 (acetate).
`' ~ .. ' .
' ': . , '
;
".; , . .
URSTITUTE SHEET
W093/1410~ PCT/EP93/00105
LJ~ ~ ~ 7
- 26 -
Example 9
A solution of 3~,17B-diacetoxyandrost-5-ene (379 mg, -
1 mmol) in dioxan (5 ml) is treated with N-bromoacetamide --
(193 mg, 1.4 mmol), perchloric acid 70 % (0.1 ml) and
water (1 ml) for ~ h at 20-25C. The mixture is diluted
with water and then extracted with benzene. The organic
layer is separated, washed with water, dried over sodium
sulfate and evaporated in vacuum. The residue is crystal-
lized from chloroform-hexane to give pure 5~-bromo-3B,17~-
diacetoxy-androstan-6B-ol (306 mg, 65 % yield).
BrO5 calcd: C 58.60 H 7.488r 16.95
found: C 58.30 H 7.35Br 16.60
MS (m/z): 471
IR cm : 3350 (OH), 1735 (acetate).
.
` 15 Example 10
Tablets each weighing 0.150 g and containing 25 mB of the
active substance, were manufactured as follows:
Composition (for 10,000 tablets):
6-difluoromethylenandrost-4-ene-3,17-dione 250 g
20 Lactose 800 g
Corn starch 415 g
~-~ Talc powder 30 g
-~ Magnesium stearate 5 g ~ ;
~ SUBSTITUTE SHEET : - -
WO93/14105 ,~ 5 7 PCT/EP93/~10
- 27 -
The 6-difluoromethylenandro5t-4-ene-3,l7-dione, the lact-
ose and half ~he corn starch were mixed; the mixture was
then forced through a sieve of 0.5 mm mesh size. Corn
starch (10 g) was suspended in warm water (90 ml) and the
resulting p2ste was used to granulate the powder.
The granul~te was dried, comminuted on a sieve of 1.4 mm
mesh size, tnen the remaining quantity of starch, talc
and magnesium stearate was added, carefully mixed and pro-
cessed inls ~le~s.
.. . .
"~
Example 11
¦ Capsules, each dosed at 0.200 g and containing 20 mg of
I the active substance were prepared.
Composition for 500 capsules:
~ 6-difluoromethylenandrost-4-en-i7~-ol-3-one 10 g
¦ ~ 15 Lactose 3o 8
f Corn starch 5 g
Magnesium stearate 5 g
This formulation was encapsulated in two-piece hard ge-
latln capsules and dosed at 0.200 g for each capsule.
' .'" ' ' '
".','
1 ~,"' ' .
.-'; ' , '~ ',
. . . :
SUBSTITUTE SHEET