Note: Descriptions are shown in the official language in which they were submitted.
W O 92/18494 ~ 1 ~ a 1~ ~ PC~r/EP92/00776
METHYL ESTERS OF ALDIMIN0- OR KETIMIN0-0XY-0RTH0T0LYLACRYLIC ACID,
MANUFACTURING PR0CESS AND FUNGICIDES CONTAINING THEM
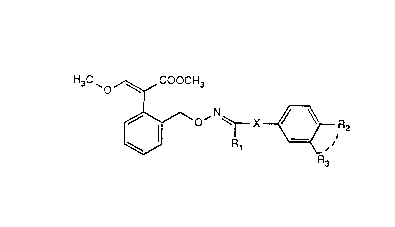
The presem in~emion relates to oxime ethers of ihe general îorrnula I in Ihc forrn of
racemic mixtures thereof or isomers thereof
H~C~ ~ COOCH3
R~
R '
! 3
wherein
Rl is hydrogen, Cl4aLIcyl, C~ 3alkoxymethyl, methylthio, cyano, cyclopropyl
or trifluoromethyl,
R2 and R3 together with the t vo carbon atoms to which they are bonded form an
unsubslituted or substituted five- to seven-membered ring comprisin~ 1 or '
oxygen atoms, and :~
X is a single bond, -C(R)2-. CO, -CR=CR-, C(R).O. C(R)2S or C(R)~C(Ri.,
in which
R is hydrogen and/or Cl 3alkyl, with the proviso that X is one of the groups
-C(R)2-, -CR=CR-, C(R)20, C(R)2S and C(R)2C(R)2. if
Rl is methyl and R2 and R3 together are methylenediox~.
} The compounds according to the invention have fungicidal properties and are suitable as
. fungicidal active ingredients, especially for use in agriculture and horliculture.
The invention relates also to a process for the preparation of the compounds according to
the invention, ~o funoicidal compositions comprising such compounds as active
. ` ~ ingredients, and to the use of such compounds and compositions for controlling
ph~ topa~hogenic fungi in agriculture and horticulture.
. .
.. . . . .
. ,~ . .
Wo 92/18494 PCr/EP92/00776
,~ u ~ 1 ~ 8
in the above formula I and hereinafter, alkyl or aL'coxy ~roups arc, dc~endinc on ~h~
number of carbon atoms, strai~ht-chained or branched.
Substituents of the oxy~en-containing ring are, for example, C~alkyl, Cj 4alkoxy and
fluorine; there may be several such substituents which may be identical or different.
Oxime ethers in which the five- to seven-membered rinc formed by R~ and R3 is mono- or ~ -.
pol~ -substituted by Cl-C.~alk~ l, Cl-C4alkoxy and/or by fluorine are therefore preferred.
(Sub-group IA). ~ -
If as~mmetric carbon atoms are present in the compounds of formula I, the compounds
occur in optically active form. The compounds in any case occur in the [E] or Ihe [Z] ~c)rm
simply as a resul~ of the presence of the aliphatic and the imino double bond. In addition,
atropic isomerism may occur. Formula I is to include all those possible isomeric forms and
mixtures thereof, for example racemic mixtures and any [EIZ] mixtures.
The process according to the invention for the preparation of the compounds according to ~ . .
the invention comprises reacting an oxime of the general formula II
C = N-OH :
R2 ~X II - ~
.:" ~ .
R3 .
wherein Rl, R2, R3 and X are as defined above, ~ .
with a benzyl alcohol derivative of the general formula III . .
CH30HC~c ,COOCH3
UCH2 ~ III ~,
. ~ "'
'. ' ."' .
~ ~ wherein U is a leavino group. ...
' ~
., ~".:,.. '
. .
Wo 92/18494 pcr/Ep92/oo776
~, ~ r ~
~, 1 'u
-3-
This rcaction is a nuclcophilic subs~itution which can be carried out under rcaction
conditions that arn customarv in this respect. The leaving oroup U present in the b~nz~ l
alcohol derivati~ e of forTnula III is preferably to be understood as bcin_ chlorine, brominc,
iodine, methanesulfonvk)xy, benzenesulfonyloxy or p-toluenesulfonyloxy. The reaction is
ad~antaoeously carried out in a basic medium in an inert orcanic diluen~ which may bc,
especially. aliphatic and aromatic, unsubstituted or halogenated hvdrocarbons, such as
pentane, hexane. heptane, cyclohexane, petroleum ether, petroleum, lioroin, benzene,
toluene. methylene chZoridc. ethvlene chloride. chloroform, carbon tetrachloride. chloro-
benzene, o-dichlorobenzene, also ethers, such as diethyl and dibutyl ether, olvcol dimethyl
ether and diolvcol dimethy! ether. tetrahydrofuran and dioxane, also kelones, such as
acetone, methvl e~hyl ke~one, meth~ l isoprop~ l ketone and meth~ l isobutyl ketone, also
nitriles, such as, for example, acetonitrile and propionitrile. benzonitrile, olutaric acid
dinitrile, and, in addition, amides, such as, for example, dimethylformamide, dimethyl-
acetamide and N-methylpyrrolidone, as well as dimethyl sulfoxide, tetramethylenesulfone
and hexamethylphosphoric acid triarnide.
. . .
There may be mentioned as bases: hydroxides, hydrogen carbonates, carbonates andalcoholates of alkali and aL~caline earth metals (Li, Na, K, Ca, Mg), sodium hydride,
tertiary amines, such as trimethylamine and triethylamine, pyridine, picolines, N-methyl-
,i morpholine. N-ethylpyrrolidine, diazabicyclo[4,3,0]undecane (DBU), 1,4-diazabicyclo- -
'~,2,2-octane (DABCO), diazabicyclo[3,2,0]nonane (DBN). and or~anolithium
compounds, such as n-butyllithium.
Furthermore, silver oxide can be used as a catalytically active weak base. The reaction
`, temperature is advantageously in the range of from -20C to +80C, preferably from 0C
to +60C.
Alternatively, the reaction can be carried out with phase transfer catalysis in an organic
solvent, such as, for example, methylene chloride, in the presence of an aqueous basic
solution, for example sodium hydroxide solution, and a phase transfer catalysl, such as, for
example, tetrabutylammonium hydrogen sulfate, at room temperature.
The isolation and purification of the compounds of forrnula I so obtained can be effected
in accordance with methods known er se. Any isomeric mixtures ob~ained, for example
EtZ isomeric mixtures. can be separated into the pure isomers likewise by methods known
~ '
E~F.VQN:EPA-Munchen 04 ;23- 4-93 ; 10'49 ; +41 61 696 79 76 49892~994~O5;# 3
-4~
p~ se, for example by chromatog~aphy or f~tclional cry!ttallisation.
Thc oximcs of ~onnula 1~ used as staning materials in Ihe process according to thc
invention cither are l~nown or cQn be plrp3rcd in accordance wilh m~,ods hlown ~r se,
for example by reacttng the corre~ponding carbollyl compound R~R2C=O with hydroxyl-
flminc hydrochloride ir. ul)c preser)ce of a b t,e, s~or examplc sodium or po~tssium
hydroxide or pyridine. O~h~r metl~ods Can bc found in Houbet7-Weyl, "~Icth~en der
anischcn Chemlc"~ ~,'o!. X14, pa~es 3-308 (1~) ("Hcrstellung u.ld Umwandlung vonOximen" (Prepar~ion and conversion of oximes)). The pre~ent invenuon rel~ses llicewisc
to those c~nmpound~ of fiormu]a n that are no-iel.
The s~arsing ma;cria;s of formula 111 can also be prepDred in A manncr l;nown ~ ~c, îor
example as descrlbed in European Patens Publication No. 203 606 and in the literalure
c,tlsd thercin.
~, . . .
Ce~ in fungicid~! spocies of thc fiosmula I aro disclosed in ~O-A-90/07493. . .-
An important group of co;npound~ wi~mn fonnul~ I or the a~ove-mentioned ~u~-group IA
comprises tho~c oxime c~hcrs in ~vhich R2 and R3 toelher are a m. thylcnedioxy or : . .
.hylenedioxy bridge bonded by way of oxy~en lo thc pher.yl ring, which bndge may bc
mono- or poly-sub~titut~d by snclh~l, me~hoxy andfor by f'uorinc, while ~, X ~nd R are
as dcfined. (Sub-groop lB).
Within ~at group, prcferr~d compounds are tnosc in which X is a single bond or one of
thc ~roups -C(R~2-, CO-, CR10 ~d C~2CR " and especi~lly thos~ ~n whicb Rl is methyl, .:
isopropyl"ne~hoxymethyl, cyclopropyl or CF3.
Anothcr pr~ferre~ group within gub-~roup IB comprises those compounds in which X is
one of the group~ CR=CR- and -C(R)2S~ nd espceially those in which R~ is me~hyl, iso-
propyl, methoxyme~hyl, cyclopropyl or CF3. (Sub-group Ib).
Another important group of compounds comp~ises oxims ethers of formula 1 or ~A in
which R2 and R3 to~c~her with the two c~rbon atoms lo vvhich they are bondcd form a
five-n~mbcted rin comprisin~ one oxygen alom or a sc~n-membcred ring ~ompndn .two oxygen ~oms, which rings ~ro unsubsti~u~cd or mono- or poly-substitutcd by melhyl,
SUBS'~O, UTE SHE~T - -
~ .: -. .. . . .. . .. , - ~ .. . - . . . . .. . ... . ... . . , .- . . . . . . . .
E.~F.V~:EPA-Munc~en 04 ;23- 4-93 ; 1~:5~ ; t41 61 699 79 73~ 493923994455;~ 4
- 4a-
methoxy andJor by fluo~ne, whi]c R~, X and R arc as defincd. (Sub-group IC.)
Oxime cshers of forrnula I or of sub-group IA in which R2 and R3 togc~l-er with thc phenyl
'' - .
.,', - ~
.,. :
.: :
.c~
.~j '
. - '.
--' ~.
. ' ~
'' .,
. ~'.
~'- ' '
.~
~ -
,, ., ~
i i,
',.:~' ' ' " ',"' - .
- .. ..
,,
,x ~
~:. , ::,
~ Wo 92/18494 PCr/EP92/00776
~l~o~a8
,.:, 5
ring tn which thcy arc bonded form a bcnzo-1,3-dioxane ring. while Rl, X and R are as
dcfined, also constitute an imponant ranoe of compounds. (Sub-group ID.)
Compounds nos. 2, 3, 4, 15, 16, 17, 21, 23, 37 and 38 listed in the following Table 1 may
be mentioned among the especially preferred compounds. ~ -
.,
Typical examples of the novel compounds of ~he presen~ invemion are givcn in ~hefollowing Table 1.
Table I
Comp. Rl X ~ physical data [incl.
R
No. massspectrum] - ~ ;
:
, CH3 - ~ oil
381(M+,1%),145(100)
2 CH3 - ~ m.p. 97-98
. ~ O :,. . .
3 CH3 ~ ~ oil
O ' .~ .',.
41 l(M+,6%),145(100)
4 CH3 CH=CH ~J m.p. 121-1''2
~:~ - O
; 5 CH3 CH.CH3 ~J
~ '.' .:
'~ ~ .~;''' .-
Wo 92/18494 PCI/EP92/00776
' 'J .1 ~ ~
- 6 -
Table I (continualion)
:,, ~R
.~ Comp. Rl X \=~ 2 physical data [incl.
R
.' I~o. mass spectrum]
.,
CH3 OJ
0
`, 7 CH3 CH(CH3)\=< J
;~ ... .
3 8 CH; c(CH3)3 ~3
~ ~o
g CH3CH2 ~ ~O ~ oil . .
- 411(M',4%),190(100)
.Q~ 0 ' , ~ ~
Ct`,~ 0 CH3CH2CH2 ~ \=~ ~ oil
' 425(Mi,1%),145(100) . :'
~ ~0 ' . '
11 (CH3)2CH ~ oil
O
` ~ ~ ~ 425tM~,1%),145(100)
O
1~ CH3OCH2 ' \=~ oil
": ~ : . O ~ : .
427(M+,7%),145(100)
13 CH3 CH2O \=< J oil
` 113(M+,25~o)145(100)
WO 92/18494 ~ 3 8 PCr/EW2/00726
Table I (continualion)
~R2 , :
; Comp. Rl R3 physlcal da~a [incl.
No. mass spectrum]
,` ~0 ~ .,
-^ 15 CF3 ~ oil
:~ -: . .. 451(~+~4~c).145(100)
~. ~ o - :. .
; ~ 16 ~ oil
:~ O ~:
'f~' 4'3(M+.'~'o).202(100)
~'~ 17 CH3 ~ ~,~, F oil
419(M+,2%),145(100) .~. -
, ~, . -.
18 ~ F
19 CH3 CH2 {~,~ F . -
O F
,~
, 20 CH3 -O l CH3
. 21 CH3 - \=< J~CH3 m.p. 65-69
22 A - ~ CH9 ~ '
~. . . .
wo 92/18494 pcr/Ep92/oo776
~l~o ~5~ ~
Table I (continuation)
: Comp. R~ ~; ~ R2 physical data [incl.
No. massspectrum]
. .
23 CH3 ~ ~,1 oil
O OCH3
413(I~I+,45'c),14S(100)
2~ CH3 - ~ 451(~+,7$c),145(100)
.` o F
.
CH3 -CH2CH~- ~" F
. F
, ~0
26 CH3 CH=CH ~<o,l, ~
~ ~0 : -" ,
- 27 CH; CH=CH \=( ~
F
28 CH3 ~ \=~ ~< 433(M+,5%),145(100)
'' ' ~0 ''. ':' ,
~ 29 CH3 CH=CH \=~ ~k F
; _~_ '; '' ' '
. ~ 30 CH3 CH=CHJ~ CH3 : .
, r~:'i_ ' ~,, ` ,; . : ~ ~
WO 92/18494 " ' ~ PCr/EP92/00776
g
Tablc 1 (conlinu~ion)
~R2
Comp. R~ r)hy~iical da~ [incl.
R3
No. mass spectrum]
31 CH3 CH=CH ~
o 1 OCH3
32 CH3 CH2-S-
~ ,'," ,
33 CH3 CH~-S- ~O ~F
~o ' ':
34 CH3 CH2-S- ~ ~ -
O ..
~~0
(CH3)2CH CH=CH ~ J
.
, ~_ ,.''. ',:' '
36 CH3CH2 ~ ~ J
o :, .. .
397(M+,4%),145(100)
~0 .','' ' ~:
37 CF3 - ~ J oil
o .
437(M+,1~o),145(100)
38 ~ il
O .,' '
188(91~c),145(100)
~0 . :''-:' ' ''
39 CH3OCH2 - ~ J
-: ~ '
: 413(M+,1%),145(100)
', '
.~ . .. .
~'' ' ' .: .' ' ,.,.. , , ' 'i , , ~, ' ;' ' '' . :
~, , : . .... ., , , , : ' , .' .. '.:;, '' .', ,. ', ; :: . ' ' : '
Wo 92/18494 PC~/EP9t/00776
- 10 -
Tablc I (conlinua~ion)
~ R2
Comp. R~ physical dat~ ~incl.
R3
No. mass spectrum]
H - ~J oil
369(1~1+,1~c),145(100)
11 ~ CH.,CH~CH - \=< J oil
411(M+.l~c).1 ~S(lO0)
~0
42 (CH3)2CH - \=< ~ oil
190(55%),145(100)
~0 .,
43 H ~ F oil
405(M+,1%),145(I00)
44 H CH(CH3)-CH2-~J
~ ,.
4S CH3S ~ J 415(M+,2.5%)145(100)
~_0 ~-~' ' '
46 H C(CH3)2- \=< J
.
0 " '
47 CH3 CH(CH3)-O- \=( j
. . .
48 H C(CH3)=CH- ~J
: ` .. : .
1~; ' .
! ~
Wo 92/18494 f~ 1 a s ;~ ~ ~ PCl/EP92/00776
.. .
Table I (continu~ion) ~I~~
~R2
Comp. Rl X ~ physic~l d~ [incl.
R3
No. m~ss spccLrum]
~ ". .
49 CH3 CH(CH3)CH2 \=< J~ F
F
~ ''" .
CH3 C(CH3)=CH ~--,~ F
F
~0 .~ .
~1 CH3 CH=C(CH3) \=< ~1~ F
F
~~0 : ' ,,'
52 CH3 CH=C(CH3) \=< J
O '.
~ .
53 CH3 CH(CH3)CH2 \=< J
O : -
54 CH3 CH.. CH(CH3) --Q J . ( ~.
O -
CH3 C(CH3)2CH2 \=< J
O ', '- ':
~_0 : .
S6 CH3CH2CH(CH3~ - \=< J oil
- , O '
425(I~1+,2~7o),14:~(100)
O
!` 57 CH3CH2CH(CH3) - \=< ~ oil
O
: . . 439(M~,2~c),145(100)
,,-,
- i
~,,
~ .
.,
~ .
~, :, ' ': r ;' S
wo 92/18494 pcr/Ep92/oo776
h i :,,i :J 1.~ j ,3
- 12 -
Table I (con~inuation)
~ R2
Comp. R~ ~ ~ physic~l data [incl.
R3
No mass spectrum]
~,_o
58 H ~ oil
o
383(1~-,59c)~ 145(100)
~ '' -.
59 CH~OCH~ oil
411(I\1~ c).145(100)
~o .. ' ~. '
CF3 - \=<~ oll
435(M+,16%)145(100) ~ "
61 CH3CH2 ~ {~J oil
395(M ' ,5%),145(100)
62CH~iCH2CH2 ~ ~G oil
- 409(1~i,8C~c),188(100) - ~ '
63CH3CH2 ~ \=< ~I~ F oil
F ~ -
The compounds according to the invention have fungicidal action and can accordingly be
used to control or prevent fungus infestation in agriculture, in honiculture and in wood
protection. They are especially suitable for inhibiting the growth of or for destroying
phytopathogenic fungi on pD~S of plants, for example leaves, stems, roots, tubers, fruit or
blossom, and on seed, as well as noxious fungi occurring in the soil. The compounds
according~to the invention can also be used to control fungi that cause wood to decomposc
or to became discoloured. The compounds according to the invention are effective, for
examplei in *e control of fungi of the classes Deuieromycetes, Ascomycetes, Basidio-
mycetes and Phycomycetes~
Y.,~
WO 92/18494 h ~ Pcr/Ep92/00776
- ]3 -
The compounds accordin~ to thc in~cmion arc especially suilable for conLrollin~ th~
followin;, pathoeens: -
True mildew fun,i (for example Erysiphe ~raminis. Erysiphc cichoracearum. Podosphacra -.
leucotricha, Uncinula necator. Sphaerotheca spp.) ;
Rust fungi (for example Puccinia tritici, Puccinia recondita. Puccini.~ hordci Puccinia
coronata, Puccinia striiformis, Puccinia arachidis, Hemileia astatri~. Uromyces fabae) ; ~. -
Scab funoi (for example Venturia inaequalis)
Cercospora spp. (for example Cercospora arachidicola. Cercospora b~ ola
Mycosphaerella spp. (for example Mycosphaerella ft jiensis)
Alternaria spp. (for example Alternaria brassicae, Alternaria mali) :
Septoria spp. (for example Septoria nodorum)
Helminthosporium spp. (for example. Helminthosporium teres, Helminthosporium oryzea~
Plasmopara spp. (for example Plasmopara viticola) -~
Pseudoperonospora spp. (for example Pseudoperonospora cubensis)
Phytophthora spp. (for example Phytophthora infestans)
Pseudocercosporella spp. (for example Pseudocercosporella herpotrichoides)
Piricularia spp. (for example Piricularia oryzae)
The compounds are also effective against, for example. fungi of the ~enera Tilletia,
Ustilago, Rhizoctonia, Verticillium, Fusarium, Pythium, Gaeumannomyces, Sclerotinia,
Monilia. Botrytis, Peronospora, Bremia, Gloeosporium, Cercosporidium, Penicillium,
Ceratocystis, Rhynchosporium, Pyrenophora, Diaporthe, Ramularia and Leptosphaeria.
Certain representatives of the compounds accordin~ to the invention are also effective.
- ' -.
WO 92/18494 PCI'/EP92/00776
~ J ~ r ~-
-S. V '~
- 14-
aoainst wood-des~ruc~i~e fun~ i, such a~, for example. of the oenera Coniophora, Gloeo-
phyllum, Poria, Merulius Trametcs. Aureobasidium. Sclerophoma and Trichoderma.
The compounds according to the invemion are distinouished by a prophylactic and
curative action, but especially by a marked systemic aclion.
The compounds according to the in~ention are effecti-e aoainst phytopathooenic fungi
under greenhouse condi[ions at concenLrations as low as from O.a mo to 500 mg of active - -
in~redient per litre of spra~ mixture. Under field conditions it is ad~,antaoeous to use
concentra~ions of from ~0 o to l kg of compound of formula I per hectare and treatment.
In order to control seed-borne and soi!-borne fun~ i hy the seed-dressino process, it is
advanta_eous to use concemra~ions of from O.OOl g to l.O _ of compound of formula I p.,r
kg of seed.
The compounds according to the invention can be formulated into a variety of compo-
sidons, for example solutions, suspensions, emulsions, emulsifiable concentrates and
pulverulent preparations. The fungicidal compositions according to the invendon comprise
an effective amount of at least one compound of the general formula I. as defined above, ~ -
and formulation auxiliaries. The compositions advantageously comprise at least one of the
following formulation auxiliaries: -
'. . '
Solid carriers; solvents or dispersion agents; surfactants (wetting a. ents and emulsifiers);
dispersion agents (without surfactant action); and stabilisers.
.
Suitable solid carriers are essentially: natural mineral substances, such as kaolin,
argillaceous earths, kieselguhr, talcum, bentonite, chal~;, for example prepared chalk,
magnesium carbonate, limestone, quartz, dolomite, attapulgite, montmorillonite and
diatomaceous earth; synthetic mineral substances, such as highly dispersed silicic acid,
aluminium oxide and silicates; organic substances, such as cellulose, starch, urea and
synthetic resins; and fertilisers, such as phosphates and nitrates, it being possible for such
carriers to be preænt, for example, in the form of granules cr powders.
Sui~able solvents and dispersion agents are essentially: aromatic compounds, such as
toluene, xylenes, benzene and alkylnaphthalenes; chlor;.nated aromatic compounds and
chlorinated aliphatic hydrocarbons, such as chlorobenzenes, chloroethylenes and
methylene chloride; aliphatic hydrocarbons, such as cyclohexane and paraffins, for
`: ' . "'; . ' '
,
wo 92~18494 pcr/Ep92/oo776
example crude oil fractions; alcohols, such as butanol and glycol, as well as Ihe e~hcrs an~
esters thereof; kemnes. such as acetone, methyl ethyl ke~one. methyl isobutyl ketone and
cyclohexanone; and stronoly polar solvcnts and dispersion a~ents, such as dimethyl-
formamide, N-methylpyrrolidone and dimethyl sulfoxide, such solvents and dispersion
agents preferably havin flash points of a~ least 30C and boiling points of at least 50C,
and water. Other suitable solvents and dispersion agents are so-called liquefied gaseous
extenders or carriers, which are products that are gaseous al room tempemture and under
normal pressure. Examples of such products are especially aerosol propellants, such as
(halo)hydrocarbons. If water is used as the solvent. it is also possible tO use, for example,
organic solvents as auxiliary solvents.
The surfactants (wening agents and emulsifiers) may be non-ionic compounds, such as
condensates of fatty acids, fatty alcohols or fatty-substituted phenols wi~h ethylene oxide;
fatty acid esters and ethers of sugars or polyhydric alcohols; the products ~hat are obtained
from sugars or polyhydric alcohols by condensadon with ethylene oxide; block
copolymers of ethylene oxide and propylene oxide; or alkyl dimethyl aminoxides.
.
The sur~factants may also be anionic compounds, such as soaps; fatty sulfate esters, for
example dodecylsodium sulfate, octadecylsodium sulfate and cetylsodium sulfate; aLlcyl
sulfonates, aryl sulfonates and fatty aromadc sulfonates, such as alkylbenzenesulfonates,
for example calcium dodecylbenzenesulfonate, and butylnaphthalenesulfonates; and more
complex fatty sulfonates, for example the amide condensates of oleic acid and N-methyl-
taurin and the sodium sulfonate of dioctyl succinate.
Finally, the surfactants may be cationic compounds, such as alkyldimethylbenzyl-ammonium chlorides, dialkyldimethylammonium chlorides, alkyltrimethylammonium
chlorides and ethoxylated quaternary arnmonium chlorides.
Suitable dispersion agents (without surfactant acdon) are essendally: lignin, sodium and
ammonium salts of lignosulfonic acid, sodium salts of maleic acid anhydride-diiso-
butylene copolymers, sodium and ammonium salts of sulfonated polycondensates of
naphthalene and formaldehyde, and sulfite waste liquors. ~
There may be used as dispersion agents that are especially suitable as thickeners or
anti-settling agents, for example, methylcellulose, carboxymethylcellulose, hydroxyethyl-
cellulose, polyvinyl alcohol, alginates, caseinates and blood albumin.
,,~ -..... ..... ~ ,
:. - :-. - . . ,: ;: - . - : . : ~ . . :
: .- - .. ,, . ~ ., - ;;: - . - ' . ~ -
wo 92/18494 pcr/Ep92/oo776
2 ~ v ;~ 16 -
Examples of suitable st~bilisers are acid-formin~ aoenLs, for examl~le epichlorohydrin,
phenyl ~ lycidyl e~hers nnd so-a epoxides; ami-oxid;mts. for example p~allic acid es~ers and
butylhydroxytoluene; UV-absorbers, for example substituted benzophenones, diphenyl-
acrylonitrilic acid esters and cinnamic acid es~ers; rLnd deac~ivators ~or example sal~s of
ethylenediaminetetraacetic acid and polyglycols.
The fungicidal composi~ions accordin.o to the invention may, in addition to the compounds
of formula I. also comprise ~ther active inoredients. for example oiher t-pes of funoicidal
composition, insecticidal and acaricidal composi~ions, bactencides. plant orowthre; ulators and fertilisers. Such combined compositions are sui~b]e for broadenin~ the
activity spectrum. or for influencing pl.qnt growth in a specific manner. ~ -
The fungicidal compositions according to the invention, dependino on their type, generall~
comprise from 0.0001 to 95 7Q by weight of compound(s) according to the inven~ion as
active ingredient(s). They may be in a form suitable for storage and transport. In such
forms, for example emulsifiable concentrates, the acdve ingredient concentration is
norrnally in the upper region of the above concentration range. Those forms can then be
diluted with the same or different formuladon auxiliaries to give active ingredient
concentrations suitable for pracdcal use, and such concentrations normally lie in the lower
region of the above concentration range. Emulsifiable concentrates generally comprise
from 5 to 85 % by weight, preferably from 25 to 75 ~c by wei_ht, of the compound(s)
according to the invention. Suitable forms of application are, inter alia, ready-for-use
solutions, emulsions and suspensions, which are suitable, for example, as spray mixtures.
The concentration of active ingredient in such spray mixtures may be, for exarnple, from
0.0001 to 20 % by weight. In the ultra-low-volume process, spray mixtures may beformulated in which the active ingredient concentradon is preferably from 0.5 to 20 % by
weight, while the spray mixtures formulated in the low-volume process and in the high-
vo1ume process preferably have an active ingredient concentration of from 0.02 to 1.0 5
by weight and from 0.002 to 0.1 % by weight, respectively.
- The fungicidal compositions æcording to the invention can be prepared by mixing at least
one compound according to the invention with formula~ion auxiliaries.
The compositions can be prepared in known manner, for example b~ mixing the aclive
ingredients with solid carriers, by dissolving or suspendin._ in suitable solven~s or
., i,~ .., - .
. .
wo 92/18494 pcr/Ew2/oo776
h ~
dispersion aoents, optionally wilh the use of surfactants as wetting agents or emulsifiers or
of dispersion agents, by dilutino pre-prepared emulsifiable concentrates with solvents or
dispersion agents, etc..
In the case of pulverulent compositions. the active inoredient can be mixed with a solid
carrier, for example by grinding them with one another; alternatively the solid carrier can
be impregnated with a solution or suspension of the active ingredient and then the solvent -~ -
or dispersion agent can be removed by evaporation, heating or by filtering with suction
under reduced pressure. By the addition of surfactants or dispersion aoents, such
pulverulent compositions can be rendered readily wettable with water so that the~ can be
converted into aqueous suspensions which are suitable. for example, as sprayino
compositions.
The compounds according to the invention can also be mixed with a surfactant and a solid
carrier to form a wettable powder that is dispersible in water, or they can be mixed with a
solid pre-granulated carrier to form a product in granule form.
If desired, a compound according to the invention can be dissolved in a water-immiscible
solvent, such as, for example, an alicyclic ketone, which advantageously contains
dissolved emulsifier, so that the solution hæ self-emulsifying action when added to water.
Alternatively, the active ingredient can be mixed with an emulsifier and the mixture can
then be diluted with waur to the desired concentration. In addition, the active ingredient ~.
can be dissolved in a solvent and then mixed with an emulsifier. Such a mixture can also
be diluted with water to the desired concentration. In that manner, emulsifiableconcentrates or ready-for-use emulsions are obtained. . - -
The composidons according to the invendon can be used in accordance with the methods
of applicadon customaly in plant protecdon or in agriculture. The method according to the ~ ~ -
invention of controlling fungi comprises treadng the material to be protected, for example
plants, parts of plants, or seed, with an effective amount of a compound according to the -
inwntion or of a composition according to the invendon.
The following Examples illustrate the invention.
~' ` ` '
. ; '' ' .
;.'~ ' . ~ .
~`~ ' ` ' ,
wo 92/18494 pcr/E~92/oo776
2 '.Q6'1~ 18-
Preparation of the coml~ol~nds of formula I:
Example 1 59.92 g (0.21 mol) of 2-(a-bromo-o-tOlyl)-3-meLhoxyaCrylic acid methyl ester
and 40.6 g (0.21 mol) of 3,4-ethylenedioxyacetophenoneoxirne in 210 ml of methylene
chloride are stirred intensively at room temperature for 10 minutes with 210 ml of 2.2N
sodium hydroxide soluhon and 92.3 g of tetrabutylammonium hvdro!en sulfate. Then the
same amounts of methylene chloride, 2.2N sodium hydroxide solution and tetrabutyl-
ammonium hydrooen sulfate are added and the batch is shirred for a further 10 minutes.
The same amounts of 2.2N sodium hydroxide solution and tetrabutylarnmonium hydro~en
sulfate are then added aoain, followed, after a further 10 minutes' stilTing, hv saturated
sodium hydrogen carbonate solmion.
The mixture is extracted three times with 150 ml of ethyl acetate each time, and the
combined organic phases are washed with saturated sodium chloride solution and dried
over anhydrous sodium sulfate. After removing the solvent by distilladon, the oil that - -
reimains is purified by chromatography on silica gel using n-hexaneldiethyl ether (3:2) as
eluant.
Recrystallisation from diethyl ether/n-hexane yields [y-3-methoxy-2-[a-~ l(a-methyl-
3,4-e~ylenedioxybenzyl)imino]oxy)-o-tolyl]-acrylic acid methyl ester in the form of
colourless crystals, m.p. 97-98C. [Comp. No. 2]
.
The compounds listed in Table I can be prepared in an analooous manner.
- Formuladon ExamDles
; ~ , : ,
Fl:
An emulsifiable concentrate has, for example, the following composition:
ollitre ~
compound of Table l 100 j s
nonylphenol-(lO)ethoxyla~e
(non-ionic emulsifier) 50
-~~ calcium dodecylbenzenesulfonate
(anionic emulsifier) 25
N-rnethyl-2-pyrrolidone (solubiliser) 200
~ , ,
- ' , ' " .
: ' ,' ' ' ' ` ' . "' ' . ,'' : ' ' ' ' , . - ' :' ' ' ' . . '. '- ' '. .. ' ' ` . . . ' '".'` '. : . '
wo 92/l8494 pcr/Ep92/oo776
- 19-
mixture of alkylbenzenes (solvent) ad 1 litre
':
The acdve incredient and the emulsifiers are dissolved in ~he solvent and in thesolubiliser. A ready-for-use spray mixture of any desired dilution can be prepared by
emulsifying this concentrate in water.
F~:
A wettable powder has, for exarnple, the following composition:
~7c bv weioht
compound of Table 1 25.0
silicic acid (hydrated; carrier) 20.0
sodium laurylsulfate (weuing agent) 2.0
sodium lignosulfonate (dispersion agent) 4.0
kaolin (carrier) 49.0 , . -
The components are mixed with one another and finely ground in a suitable mill. A
suspension that is suitable as a ready-for-use spray mixture is produced by dispersing the
mixture in water.
BioloQical Exam~les:
ExamDle B 1: Action aoainst Puccinia oraminis in wheat
a),Residual-orotective action
Wheat plants are sprayed 6 days after sowing with a spray mixture (0.02 % activeingredient) prepared from a wettable powder formulation of the test compound. After 24
hours the treated plants are infected with a uredospore suspension of the fungus. The
infected plants are incubated for 48 hours at 95-100 % relatdve humidity and about 20C
and then stood in a greenhouse at about 22C. Evaluation of rust pustule development is
made 12 days after infecdon.
b) Svstemic action
Wheat plants are watered 5 days after sowing ,with a spray mixture (0.006 % active
ingredient, based on the volume of the soil) prepared from a wettable powder formulation
of the test compound. After 48 hours the treated plants are infected with a uredospore - ' ' ',
suspension of the fungus. The infected plants are then incubated for 48 hours at 95-100 ~ -
.
. .- ~, :,. . . -: : ~ -
;... : . . . . .
wo 92/18494 pcr/Ep92/oo776
~t ~S~
- 20 -
relativc humidity and about 20C and then stood in a oreenhousc al ~boul "C.
Evaluation of rust pustule development is madc 12 d~ys afler infec~ion.
Puccinia attack is 100 % on untreated and infected conuol plants. Compounds of Table I
exhibit good activity aoainst Puccinia funoi. The followino compounds inhibit Puccinia
infestation to less than 20 %: nos. 1, ', 3, 9, 10, 13, 15, 16, 21, 23, ,6. 37, 38, 39, 41, 4',
43 and 4~1.
Examr)le B2: Action aoainst Cercosrora arachidicola on
.oroundnut plants
a) Residual-protecti~e aclion
Groundnut plants I0-IS cm in height are sprayed with a spray mixture (~).02 ~o active
ingredient) prepared from a wettable powder forrnulation of the test compound, and
infected 48 hours later with a conidia suspension of the fungus. The infected plants are
incubated for 72 hours at about 21 C and high humidity and then stood in a greenhouse
until the typical leaf specks occur. Evaluation of the fungicidal action is made 12 days
after infecdon and is based on the number and size of the specks.
b) Svstemic action
Groundnut plants 10-15 cm in heighl are watered with a spray mixture tO.06 % active :
ingredient, based on the volume of the soil) prepared from a wettable powder formulation
of the test compound. The treated plants are infected 48 hours later v. ith a conidia .:
suspension of the fungus and then incubated for 72 hours at about 21 C and high humidity. ~ :
The plants are then stood in a greenhouse and evaluation of fungus infestation is made 11 : ~ -
days later.
Compared with untreated and infected control plants (number and size of the specks =
100 %), Cercospora infestation on groundnut plants treated with compounds of Table 1 is
substantially reduced. The Cercospora infestation is inhibited to-less than 20 % with
compounds nos. 1, 2, 3, 10, I l, 12, 13, 15, 16, 21. 23, 36, 37, 38. 39. 41, 42, 43, 44, 57, 58 ~ - .
and S9.
ExamDleB3: ActionaoainstErvsiPhe-raminisonbarlev
a) Residual-Protective action :
Barley plants about 8 cm in heip,ht are sprayed wi~h a spray mixture (0.02 ~G ac~ive
ingredient) prepared from a wettable powder forrnulation of the test compound. The
. .. .
.... ..
WO 92tl8494 PCr/EP92/00~76
21~6~3
- 21 -
treated plants are dusted with conidia of thc funcus after 3 to 4 hours. The infectcd barle
plants are stood in a creenhouse at abou~ 22C. The funcus attack is evaluated after 10
days.
b) Svstemic ac~ion
A spray mixture (0.006 SO active ingredient, based on ~he volume of the soil) prepared
from a wettable powder formulation of the test compound is used to water barley plants
about 8 cm in heioht. Care is taken that the spray mixture does not come into contact with
the parts of the plants above the soil. The treated plants are dusted 48 hours la~er w ith
conidia of the funous. ll~e infected barley plants are then stood in a ~reenhouse at about
22C and evaluation of fungus infestation is made after 10 da-s.
Compounds of formula I exhibit good activity against Erysiphe fungi. The Erysiphe
infestation is 100 % on untreated and infected control plants. The fungus infestation in
barley is substantially inhibited by compounds of Table 1. The fungus infestation is
inhibited to 0-5 % by compounds nos. 1, 3, 4, 10, 11, 13, 15, 16, 21, 37, 38, 39, 41, 42, 43,
44, 59 and 61. -
Examole B4: Residual-~rotective acdon aoainst Venturia
:~ inæqualis on aDple shoots
Apple cuttings with 10-20 cm long fresh shoots are sprayed with a spray mixture (0.06 %
active ingredient) prepared from a wettable powder formulation of the test compound. The
ueated planls are infected 24 hours later with a conidia suspension of the fungus. The
plants are then incubated for 5 days at 90-100 % relative humidity and stood in a green-
house for a further 10 days at 20-24C. Scab infestation is evaluated 15 days after
infection. Compounds of Table 1 inhibit the Venturia infestation to a substantial degree.
Compounds nos. 1, 3, 21, 36, 37, 38, 39, 41, 42, 43, 4~ and others limit the infestation to
less than 20 %.
Example B5: Action aoainst Botrvtis cinerea on beans.
Residual-protective action
` Bean plants about 10 cm in height are sprayed with a spray mixture (0.02 ~o active
ingredient) prepared from a wettable powder formulation of the lest compound. After
48 hours the ueated plants are infected with a conidia suspension of the fungus; The
infected plants are incubated for 3 days at 95-100 qo relative humidity and 21 C and then
evaluated for funeus infesta~ion.
"
. -:
.,~ . .
WO 92/18494 PCI /EP92/00776
r- J ~ r~ ~3 22 -
The Botrytis infestation on untreated and infected bean plants is 100 5c. After tre:ltmem
with one of compounds nos. 4, 13, 17, 21, 23, 21, 28, 36, S9 and 61 Or formula 1. ~he
infestation is less than 20 %.
,
Example B6: Action aoainst Rhizoctonia solani (soil funous on rice DlanLs)
a) Protective-local soil anr~lication
A spray mixture prepared from a formulation of the test compound (0.006 ~c active
ingredient) is used to waier 12-day-old rice plants without wettino the parts of the pl~ms
above the soil. A suspension of mycelium and sclerotia of R. solani is applied to the
surface of the soil in order to infect the treated plants. The plants are incuba~ed for 6 da- s
at 27C (day) and ~f3C (night) and 100 7O relative humidity (humidity box) in a clima~i.
chamber and then evaluation is made of the funeus infestation on the leaf sheath, leaves
and stems.
b) Protective-local foliar a~Plicadon
12-day-old rice plants are sprayed with the spray mixture mentioned under a). The treated
plants are infected one day later with a suspension of mycelium and sclerotia of R. solani.
The plants are incubated for 6 days at 27C (day) and 23C (night) and 100 % relative ~ -
humidity (humidity box) in a climatic charnber and then evaluation is made of the fun_us
infestation on the leaf sheath, Ieaves and stems.
Compounds of Table l exhibit good activity by inhibiting the Rhizoctonia infestation. By
contrast, infestation is 100 % on the untreated and infected control plants. The fungus
infestation is inhibited to substantially below 20 qc by compounds nos. 2, 9, 17, 23, 24, S9,
61 and 63.
.'
Example B7: Action aQainst Phvtophthora infestans on tomatoes
a) Curative action
Tomato plants of the "Roter Gnom" variety are reared for three weeks and then sprayed
with a zoospore suspension of the fungus and incubated in a chamber at 18 to 20C and
saturation humidity. Humidity is discontinued after 24 hours. When the plants have dried
they are sprayed with a mixture comprising a wettable powder forrnulation of the tes~ .
compound at a concentration of 200 ppm. After the spray coating has dried, the plants are
placed in the humidity chamber again for 4 days. The number and size of the typical le~f
specks that have occurred after this period are used as a standard for evaluating the
, .~'.
. .... ~., .. -,
: .,.,:
wo 92/18494 pcr/Ep92/oo776
2 ~
activity of lhe test compounds.
b) Preventive ~vstemic action
A wet~able powder formulation of the test compound is applied at a concentration of 60
ppm (based on the volume of the soil) to the soil surface of three-week-old tomato plan~s
of the "Roter Gnom" variety in pots. After a waiting period of three days, the undersides
of the leaves are sprayed with a zoospore suspension of Phytophthora infestans. They are
then kept in a spray chamber for S days at 18 to 20C and saturation humidity. At the end
of this period typical leaf specks forrn, the number and size of which are used tO evaluate
the activity of the test compounds.
Compounds of Table 1, for exarnple compounds nos. 4, 9, I 1, 13, 15, 16, 17, 23, 24, 2~,
36, 57, 59, 61, 62 and 63, inhibit the Phytophthora infesta~ion to less than 20 ~c.
Exam~le B8: Action aoainst Plasmopara viticola (Bert. et Curt.) (Berl. et Detoni) on vincs
a) Residual-~reventive action
Vine cuttings of the "Chasselas" variety are reared in a greenhouse. At the 10-leaf stage, 3
plants are sprayed with a mixture (200 ppm acdve ingredient) prepared from a wettable
powder formulation of the test compound. After the spray coating has dried, the
undersides of the leaves are uniformly infected with a spore suspension of the fungus. The
plants are then kept for 8 days in a humidity chamber. At the end of this period clear
symptoms of the disease appear on the control plants. The number and size of the infected -
sites on the treated plants are used as a standard for evaluating the activity of the test
compounds.
b) Curative acdon
Vine cuttings of the "Chasselas" variety are reared in a greenhouse and, at the 10-leaf
stage, the undersides of the leaves are infected with a spore suspension of Plasmopara
viticola. After 24 hours in the humidity charnber, the plants are sprayed with an active
in8redient mixture prepared frorn a wettable powder formulation of the test compound
(500 ppm active ingredient). The plants are then kept for a further 7 days in the humidity
chamber. At the end of this period, the symptoms of the disease appear on the control
plants. The number and size of the infected sites on the treated plants are used as a
standard for evaluating the activity of the test compounds.
The compounds of formula I, especially compounds nos. 1, ~, 3, 4, 9, lO. I l. 13. 1~, 16,
WO 92/18494 PCI'/EP92/00776
r..; ~ r
24 -
17, 23, 24, 28, 36, 37, 3~, 41, 42, ~3, 44, 56, 57, 58, 59, 60, 61, 62 and 63, strongly inhibi
the Plasmopara infestation ~o, in some cases, less than 10 '-7O
Example B9: Action aoainst Pvthium debarvanum on suoarbeet
(Beta vulgaris)
a) Action after soil al~Plication
The fungus is cultivated on sterile oat grains and added to a mix~ure of earth and sand. The
eanh so infected is introduced into flowerpots and sown with sugarbeet seeds. Imme-
diately after sowing, an aqueous suspension (20 ppm active ingredient ba~sed on the
volume of the earth) prepared from a wettable powder formulation of the lest preparation i ~ - -
is poured over the earth. The pots are then stood in a greenhouse at '0-'~C for ~-3
weeks. The earth is kept constantly and uniformly moist by light spraying with water. The
test is evaluated by de~ermining the rate of emergence of the sugarbeel plants and the
proponion of healthy and diseased plants.
b) Action after dressino a~plication --
The fungus is cultivated on sterile oat grains and added to a mixture of earth and sand, The
ear~ so infected is introduced into flowerpots and sown with sugarbeet seed which has
been dressed with a dressing powder formuladon of the test preparadon (1000 ppm active -
ingredient based on the weight of the seed). The pots containing the seeds are stood in a ~ ~
greenhouse for 2-3 weeks at 20-24C. The earth is kept uniformly moist by light sprayin_ -
with water. The ~est is evaluated by determining the rate of emergence of the sugarbeet
plants and the proportion of healthy and diseased plants.
A rate of emergence of more than 80 % is achieved with compounds of Table 1. Thecorresponding control plants have a rate of emergence of less than 30 % and an unhealth~ , -
appearance.
' ~,' ,'"' . .'.
.~.. .....
"
: ' '" ' , .
- ' .,: ' ~ '.
`: :