Note: Descriptions are shown in the official language in which they were submitted.
WO92/17148 2~ g5 PCr~F~91~00090
Equipment for manufacturing of subcutaneous capsules
The present invention relates to an e~auipment for manu-
facturing of capsules filled with a medical substance
essentially in powder form, which ~Arcl~lPc are intended
to be implanted under the skin and from which the medical
substance will diffuse lnto the blood circulation through
the wall material of the capsules. Intended uses are i . a
dosage of hormones, PcpP~ 1 y contraceptive h~ ~nPc as
well as of antabus agents.
A problem in the dosage of contraceptive hoL ^~ has
been above all the small amount of hormone substance to
be portioned accurately in powder form dosages into the
smallsized capsules. The amount of substance diffusing
from the capsule is proportional to the amount of sub-
stance in the capsule and therefore, to assure a correct
and in all cases a truly predictable liberation, the
capsules should include exactly the same amount of subs-
tance. In the said h~ -7 use, the tolerance's allowed
are ~ 596, which accuracy is extremely difficult to reach
with the prior used manual dosing. The manual doslng
method is, naturally, also a very expensive stage in th~
pIoduction of the capsules because of the great need of
huma n work f orce .
The invention relates to providing a totally automatic
manufacturing process for subcutaneous capsules filled
with a medical :~ub~Lallc~, whereby the uniformity of the
end result is essentially better than that of correspond-
ing prior art processes, lnvolving partly manual stages.
In capsule production the starting point is a tube for
forming the casing of the capsule, and for which a suit-
able material is a c~ 1 ~ r~nP plastic . The diameter of the
tube for manufacturing of hormone capsules is l . 5 mm. The
tube is fed to the capsule manufacturing line as con-
WO92/17148 ,,~ 35~ PCI/F191/000911
tinuous lengths from a suitable apparatus. At the star-
ting point of the capsule manufacturing line, the tube is
cut into capsule blanks o~ a suitable length, e. g . to
lengths of about 34 mm.
The said blanks are then arranged into a row formed by
several blanks. One row may comprise e.g. 12 blanks. The
position of the blanks is fidv.lnt 3eo~cly chosen in con-
formity with the h~nfll in~ positions of the tube in the
prPc~P~in3 cutting device. One of such rows is advanta-
geously joined together and it will then form a hfln~l in~
unit for the next process stages. In order to assemble
this h~n~l ~n~ unit row it is posC~h1e to use a means to
keep the blanks in their prescribed positions and with
the help of which the h;~n-ll i n~ unit row is transferred
from one stzge to another ln the process, as well as
positioned for each process stage. The said means can
2dvantageously be a cassette of a clamping claw structure
where the claws are in a mutual spring loaded clamping
contact and where mutually co-operating indent2tion slots
have been formed on the contact faces oi the claws ~n
order to form through holes for receiving the capsule
blanks . The claws are made to retract f rom each other
against the said spring load to receiv~ the capsule
~5 bl~nks. The operation of the said cassette is advanta-
geously synchronized with the capsule blank cutt~ng devi-
ce.
To make the capsule blank fillable, one of its ends has
to be sealed. In the apparatus according to the applica-
tion, this stage is carried out so that one end of the
tubes forming the capsule blanks receives a small amount
of glue, e.g. a silicone glue, that adheres well to the
tube material forming the capsule wall. The glueing is
carried out by using a very thin nozzle needle inserted
to a depth of about l. 6 mm in the capsule blank . The glue
is discharged from the nozzle simultaneuously when the
_ _ _ _ _ _
WO 92/t7148 ~ 1 0 ~ PCrJFI9lJD0091~
nozzle needle is rotated and extracted. By ad~usting the
speed by which the nozzle needle is extracted it is pos-
sible to regulate the amount of glue remaining in the
capsule blank. This possibility is utilized in the pro-
cess A~rr~rCl 1 ng to the application to eliminate the im-
pncts of the viscosity fluctuations of the glue, by moni-
toring the load on the glue pump and regulating the speed
by which the nozzle needle is extracted in relation to
the load. A high viscosity glue, discharged slowly from
the needle, will load the dosing pump more than a low
viscosity glue. Whenever the load on the dosiny pump is
rising, the speed by which the needle is extracted is
slowed down.
The glue fed to the end of the capsule blank is hardened
by a suitable accelerator, e.g. using humidity or heat-
ing. After the hardening of the glue there is the possi-
bility, whenever desired, to use a finishing treatment of
the sealed end by cutting out a part of the end of the
capsule blank, from the area sealed by glueing. After
sealing the capsule blanks, the blank row is turned so
that the ends still left open are facing upwards. In this
position the capsule blanks are forwarded to the filling
stage .
The filling stage invplves as an essentlal element the
dosing of the mass of material to be fed into the capsu-
le blank. In the process according to the application, a
device is used in which the dosing is based on a disc
glidable along a plane surface and having through apertu-
res determining the dose quantities. The material to be
dosed is fed to the apel Lu~s closed by the lower plane
surface, and the correct quantity is fin~l i7c~rl by sweep-
ing any extra material away at the respective apertures
to the upper surface level of the disc. The quantities
thus dosed are delivered, individually to each capsule
blank, by moving the disc along the plane surface to such
_ _ _ _ , . .... . . , _
WO 92/17148 Pcr/FI91/0009n
2~ o~g35: 4 ~
a position where the lower surfaces of the apertures will
be f reed
In the process according to the application the doseo
quantity of materlal are dropped from each aperture to
its respective transportation groove made in the surface
of a disc, operating as a vibrating Cu~lv~:yul. When the
a~eL LUL~:~ are emptied the dosing disc is transferred back
to its filling position. The dosed material quantlties
proceed in the grooves of the vibrating .:u,-v~y~ further
to feeding funnels under which the capsule blanks, car-
ried by the clamping claw u~ssetL~:, have been conveyed.
In order to promote the flow of the material quantities
into the capsule blanks, a special spiral f ~eder is used
The said feeder comprises a spiral spring that has a
diameter less than the inner diameter of the capsule
blank, and a straight steering wire therein This spring
is rotatable in the forward direction of its spiral.
2û As far as the feeding process itself is rnnf~prnpd~ the
procedure is carried out so that the spiral spring is
inserted into the capsule blank and made to rotate. Si-
multaneously with the activation of the rotative movement
the raising of the spiral spring from the capsule blank
is initiated. When the rotation direction is as said
above, the spring acts as a feeder screw. When the fill-
ing of the capsule blank advances, the spiral spring ~is
e~LLdu~d, still in rotation, from the capsule blank.
During the said operational stages the feeding funnels
3û are kept under vibration. When the head of the spiral
spring has risen to the desired material filling level
its rising r v. L is stopped, but vibration, and rota-
tion of the spring are continued. Thus, the spiral spring
determines the height of the material column fed to the
capsule:
After the filling stage, it is advisable to clean the
;
WO 92/17148 2 ~ 8 ~ PCr/F191/00 l91
inner surfaces of the open ends of the capsule blanks to
remove any adhered filling material to ensure the se~ling
of the ends of the capsule blanks without problems. The
~1 e~n~ ns may be carried out on the inside of the mouth
area of the capsule blank by uæing a scraping rotating
mandrel or a ~LLr~ n~ brush. The ends of the capsule
blanks are sealed by using similar ~L~,eduL~s as in con-
nection with the above described closing of the f irst
ends of the capsule blanks . Af ter the glue used in the
sealing process has hardened, the capsule ends may be
similarly finished by cutting parts away from their seal-
ed portions.
After these stages the completed capsule~ are conveyed
through as such known process stages, whereby the capsu-
les are washed, dried, inspected, packaged and sterilized
to ready-to-use products.
In the following, the invention will be described re~er-
ring to the annexed drawing where:
Fig . l shows a f low chart of the process according to
the application in principle;
Fig. 2 shows an ~mh~rlir-nt of an apparatus in princi-
ple, for cutting the capsule blanks and their arran-
gement in hAnrl 1 i n~ unit rows,
Fig. 3 shows a dosing and filling apparatus seen in
the forward direction of the process;
Fig. 4 shows a dosing and filling apparatus seen from
above,
r
Fig. 5 shows a dosing and fiiling apparatus seen from
the side with respect to the forward direction of the
process;
WO 92/17148 21~ ~ 9 8 ~ pcr/Fls1/ooosn
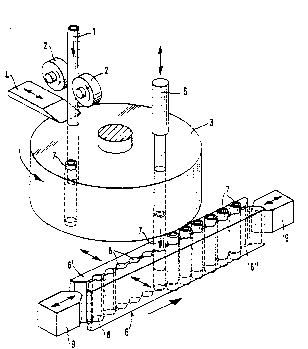
Fig . 6 shows a dosing and f illing apparatus axonomet -
rically;
Fig. 7 shows a simplified flow chart according to
Fig. 6, and
Fig. 8 shows a magnified detail from the area of the
feeding funnels.
-
In the basic flow chart ~ccording to Fig. l the process
stages are shown in the advancing order principally as
described above.
The first stage of the process, i e. the cutting of the
capsule blanks and their a~ lcln~ ~rt to rows forming the
handling unit, is described in more detail in the draw-
ing Fig. 2.
The silicone tube l forming the casing of the capsule
blanks is fed as a continuous length from a coil, guided
by two feeder rolls 2, to an aperture made in a disc 3
rotatable around a vertical axle. The aperture is a
through hole and its diameter is clest3nPcl to match the
outer diameter of the tube so that the cut tube stays in
the aperture without any separate supporting means, part-
ly due to the distortion tendency caused by the material
memory of the tube. In connection with the tube feeding
station there is a cutter device 4 by which the tube is
cut to the upper surface level of the disc. The length of
the capsule blank to be cut may be detPrmi nPd by the
duration of the rotation movement of the feeder dlscs 2.
..
By rotating the disc around its vertical axle, it is
possible to move the capsule blank, formed by the cut
tube, to a discharge station where the capsule blank is
taken from its aperture by a punch-through mandrel 5.
From the point-of-view of the flexible operation of the
WO 92/17148 2 ~ ~ ~ 58 ~ PCr/Fl9l/00090
apparatus, it is advantageous to have several through
apertures in the disc 3 whereby the cutting of a new
blank and the discharging of the previous blank may be
carried out as simultaneous operations.
The operation of the cutting device is advantageously
~y~ u--ized with the device arranging the cut capsule
blanks. As such a device arranging the capsule blanks it
is advantageous to use a spring-loaded l ~rin~ claw
cassette 6 consisting of two opposite co-operating halves
6 ' and 6 ' ' . The halves are separated from each other by a
vertical dividing plane, the separating surfaces being
furnished with essentially semi-circular opposite Qrooves
traversing the dividing plane, that, when operating to-
gether, form the through apertures 8 receiving the capsu-
le blanks 7. The apertures 8 have been placed at an even
distance from each other, and the clamping claw cassette
6 has been arranged to be moved stepwise with steps of
respective length in the direction of the row of apertu-
res to receive the capsule blanks 7 coming from the cut-
ting device.
To facilitate the reception of individual capsule blanks
7 from the cutting device, the clamping claw cassette 6
~ has been arranged to be opened, to a limited extent, for
each receiving operation. This opening capability has
been provided by two wedges 9, actin3 in the division
plane at the ends of the cassette, which wedges can be
pushed towards each other in the division plane.
By means of these wedges, the clamping claw cassette 6
ha~ves, may be retracted f rom each other against the
springloaded (not shown) force pressing them against each
other. The springload may be provided for, e . g . in con-
nection with screw joints ~oining the clamping claw cas-
sette halves, by spring washers or similar devices, as
the opening movement does not need to be very big in
WO 92/1714~ 210 6 9 ~ ~ PCI /FI91/00090
relation to the diameter of the capsule blank.
Deviating from the embodiment shown in the drawing figu-
res 1 and 2, the disc 3 of the cutting device may also be
arr2nged to rotate around a horizontal axle and, respec-
tively, the rl 2 , in9 claw cassette 6 arranged to receive
the cut capsule blanks in horizontal positions.
The capsule blanks are conveyed, carried by the said
rl 1 1 nr claw cas2,et ~ 6, to the next processing stages
and through them, of which a general description concern-
ing the sealing of the second ends and their trimming has
been given above. These operati~ons may be carried out_
when the capsule blanks are either in a vertical positi-
on, as shown in Fig. 1, or alternatively, in a horizontal
position. An alternative trimming of the ends may also be
'in~l with the trimming following the sealing of the
filling end, to be ti~crr~h~A later, to a single stage
only .
As an important partial staç,2e in the process, the dosing
of the material to be packaged into the capsules is de-
scribed, as well as the equipment relating thereto, re-
ferring to the drawing figures 3 to 8. The said equipment
is used to dosage the material for each capsule blank and
to direct it to the respective capsule along its own
pzth .
The dosing eguipment is based on the so-called aperture
disc tPrhnrlogy, whereby the material mass is dosed by
the means of a plate of even thickness movable on a plane
surface and provided with through apertures defining the
dosage quantities. When the dosage guantities are meter-
ed, the disc rests on the said plane surf ace and the
plane surface closes the bottom part of the apertures_
The material to be dosed is made to f low into the apertu-
res and the exact quantity is ~etermined by sweeping ~ he
WO92/17148 21~ 5 PCr~F191~00090
excessively fed heap of material away, to the upper sur-
face level of the disc. Then the disc is moved along the
said plane surface to a discharging station, where the
lower surface of the apertures is opened to a receptacle
for the material.
In the device according to Fig . 3, the material used f or
filling the dosage aye, ~u~s is fed from a storage con-
tainer 10 by means of a compartment feeder 11, performing
a rough dosing, to a trough-like vibrating conv yu~ 12.
This vibrating Cullv-:yul ~L~Insrels the material to be
dosed further to a distribution through 13 at a lower le-
vel, the open lower part of which is def ined by the
dosing disc 14. The dosing disc 14 is glidable on the
plane surf2ce 15. The number of through apertures in the
dosing disc 14 in the described embodiment is half of
that of the capsule blanks to be filled in the clamping
claw cassette 6. The parts of the device may naturally
also be tli~ nP~ so that the number of the apertures
and the number of the capsule blanks to be filled are the
same. The diameter of the apertures 16 is dimensioned so,
taking the thickness of the aperture disc 14 into ac-
count, that the apertures, when filled up to the upper
surface level of the disc, will define the volume of the
dose intended for each capsule, or so that a dosage volu-
me is formed by a multiple of aperture fillings.
In the device described in the drawing f igures 3, 4, and
5 the material to be dosed and transported by the vibrat-
ing uu--v~yu~ 12 is spread in the distribution through 13
by means of 2 device movable by a cylinder-piston device
18. An optical control device 19 is 2rr2nged in connec-
tion with the through by means of which the surf ace level
in the distribution through 13 of the material to be
dosed is monitored, and on the basis of the information
of which the number of the operation cycles of the com-
partment feeder 11, which functions as a rough dosing
-
WO 92/17148 PCr/FI91/0009(~
~ , . . .
21~9~5 lo ~
device ~ is determine
The plane surface 15, on which the dosing aperture disc
14 is glidable and upon which it rests duriny filling o~i
the dosing apertures, is ~ ~ inPd with a suitable vib-
rating device in order to direct a vibrating effect on
the plane surface during the filling stage Of the apertu-
res 16. ThiS vibrating effect Will act on the r;~rk~gi n~
density of the material to be f illed lnto the dosing
0 c-~eL~ult:s 16. It is possihle to regulate the packaging
degree by regulating the duration and/or the intensity of
the vibration. Another fector infl~nr~nr the packaging
degree is the filling level in the distribution through
3 of the material to be filled monitored by the said
optical control device 19
After filling of the apertures, the dosing disc 14 is
pushed along the plane surface 15 to the discharge sta-
tion of the apertures. The upper surface of the dosing
disc 14 is in gliding contact with the lower surface of
the distribution through 13 whereby the lower edge of the
through levels the dose volumes in the apertures to the
upper surface leYel of the dosing disc 14, whereby the
excess material remains in the distribution through 13
closed by the dosing disc 14.
In the level surface 15 there is formed a row of dischar-
ge apertures 20 corrF~srnn~in~ to the dosing apertures 16,
above which row the apertures 16 are glided. Arranged
below the discharging apertures 20, there is a vibrating
~Gnv~y~ formed by a groove disc 21. In the groove disc
21 there is the same number of grooves 22 as there are
discharging apertures 20, and the grooves and the dis-
charging apertures are aligned so that the material in
the dosing apertures can be discharged through the dis-
charging apertures 20 to their respective groove 22 in
the ~roove di c 21 The ~roove di_c 21 is pref~
WO 92/1714~ ~ I Q ~ ~ 8 ~ PCr/F19~ ~0009h
1 i ni n~ and equipped w$th a vibration device, whereby 2
groove 22 forms a ~:O~V~yul for a single dosage.
The dosaged material is transferred on the groove disc 21
to fill$ng funnels 23 arranged in a transverse row be-
neath the fail edge of the groove disc 21. Below these
filling funnels there has in turn been transferred a row
of capsule blanks 7 carried by the t~lr, in~ claw cassette
6, the lower ends of the c~psule blanks having been seal-
ed and advantageously trimmed according to the measures
described above.
The equipment according to the figures 3 to 8 also comp-
rises a filling apparatus 24, comprising a number of
feeding devices corresponding to the number of filling
funnels 23. Each feeding device comprises a rotating
motor 25, a spindle 26, and a feeder screw 27 attached to
the the lower end of the spindle. To operate the feeder
screw 27 within the conditions determined by the small
.li -ionc of the capsules to be filled, the filling
screw is formed by a thin spiral spring. For better cont-
rol of the movements of the spiral spring, a thin
straight guiding wire is placed inside the spring,
extending substantially along the whole length of the
spiral spring.
The feeder screw 27 formed by the spiral spring is di-
rected through each feeding funnel 23 to the capsule
blank below it and is made to rotate. The rotative move-
ment is naturally chosen in the feeding direction of the
spiral spring. The feeder screw rotating in the capsule
blank feeds the material fed into the feeder funnel effi-
ciently into the capsule blank. In order to avoid unne-
cessary compacting of the material in the capsule blank,
the feeder screw is raised as filling is advancing. After
the dosed batch has been completely fed, the feeder screw
is removed completely from the capsule blank, and the
WO 92/17148 PCr/F191/00090
21(~gg~ 12
blanks are transferred to be sealed. Before sealing, it
is possible to clean the inside of their mouth areas to
remove any L~ ining filling material. Sealing is carrled
out by glueing in the same way as the sealing of the
$ opposite end of the blank before the filliny stage.
After sealing, the ends of the filled capsules can be
trimmed by cutting. After trimming, the final capsules
will be removed from their respective ~ ,rin~ claw cas-
settes nfter which operation they will be processed indi-
vidually according to prior technology fl~ sc~ee~l briefly
above .