Note: Descriptions are shown in the official language in which they were submitted.
W092/18540 PCT/US92/~297~
, . 1-- .
- 210732~
DescriPtion
.: .
Anti-Human Immunode~iciency
Virus Reco~binant Antibodies
Backqround of the Invention
This invention relates to recombinant hybrid
molecules for use in therapy and prevention of viral
in~ections.
There are a wide variety o~ foreign substances or
organisms which can enter the body to cause illness.
Mammals including man respond to such an invasion with
an "immune response" which is the result of many
complex interactions between a variety of cells and
humoral ~actors. Although many different cells
participate, lymphocytes are the primary cells involved
in generating an immune response so as to protect an
individual from foreign substances such as bacteria,
viruses and foreign cells.
There are two principal classes of lymphocytes, B
cells and T cells. ~oth classes are derived from
progenitor hematopoietic stem cells. Mature T cells
have been classified into thre~ subpopulations based on
the different tasks they ~erform. Helper T cells (Th)
are required for promoting or enhancing B cell antibody
production. Cytotoxic killer T cells (T~), otherwise
~nown as cytotoxic T lymphocyt~s ~CTL) directly kill
their target cells by cell lysis. Suppressor T cells
; (T,) suppress or down-regulate immunological reactions.
These differént subpopulations of T cells express
a variety of cell eurface proteins some of which are
termed "marker proteins" because they are
characteristic of the particular subpopulations. For
example, most of the Th cells express the cell surface
CD4 protein, whereas most CTL and Ts cells expre~s the
: -. :
WO92/18~0 PCT/US92/02975
r~
3 ~ 3 2~ 9
cell surface CD8 protein. Swain, "Evidence for two
Distinct Classes of Murine B Cell Growth Factors with
Activities in Di~ferent Functional ~ssays", J. Exp.
Med., 158:822 (1983). Additionally, matllre T cells can
be distinguished from immature T cells (t:hymocytes) by
the presence of the cell surface T cell receptor (TCR),
a transmembrane protein complex found on mature T cells
which is capable of recognizing antigen in association
with self-antigens encoded by MHC yenes.
As it is now understood, initiation and
maintenance of immune responses involve cell to cell
interactions and depend on the recognition of and
interactions between particular proteins or protein
complexes on the surface of B cells, T cells, foreign
substances, foreign cells and infected cells.
There are at least two separable aspects of the
immune response, cell-mediated and antibody-mediated
responses. Both begin when a T cell recognizes a
foreign antigen. The cell-mediated response involves
the lytic activity of CTL activated by exposure to
antigen and proceeds in the absence of B cells. CT1
can also be nonspecifically activated to lyse any cell
in close proximity by having an antibody bound to a
cell-surface protein such as CD3. For the
antibody-mediated response to occur, the Th cell which
has been activated by exposure to a foreign antigen
interacts with a B cell to stimulate B cell production '
of humoral proteins known as immunoglobulins or
antibodies.
Although T cells directly participate in the ;
cell-mediated immune responses to foreign antigens, B
cell production of antibodies is the most important
aspect of immunity. The requisite variety of
antibodies is pro~ided by the diversity of
immunoglobulin gene~. Genatic rearrangement further
increases their Yariety. Each set of mature
:,
.
~:.' ,. ' .:
:
WO92/18~0 PCT/~S~2/0297
~ 3
2~329 ~
immunoglobulin genes is the result of a further genetic
rearrangement. Providing yet more diversity, there are
saveral immunoglobulin classes with varying feat~res.
For a review of immunoglobulin genetics and protein
structure see Lewin, "Genes III", ~ohn Wiley and Sons,
N.Y. (1987).
The developing techniques of genetic engineering
have been employed in various approaches to assist the
natural immune system and to provide reagents for
performing diagnostic tests. For instance, protein
sequences corresponding to the antigenic determinants
of various organisms suitable for use as vaccines have
been prepared both synthetically and by recombinant DNA
techniques.
Antibodies are extremely important in diagnostic
and therapeutic applications due to their diversity and
specificityO Molecular biology techniques have been
used to increase the availability of antibodies for
scientific applications. For instance, a single
20 antibody producing B cell can be immortalized and -
expanded to provide an in vitro source of antibodies of
a single specificity known as a "monoclonal antibody"
(mAb). Such an immortal B cell line is termed a
"hybridoma".
Until recently, the source of most mAb has been
murine ~mouse) hybridomas. Although they have been
used extensively in diagnostic procedures, murine mAb
àre not well suited for induction of passive immunity
or other therapeutic applications in mammals including
humans and nonsyngeneic mice. ~oreover, murine
antibodies are recognized as foreign by other mammalian
specie~ and elicit an îmmune response which may itself
cause illness. Human mAb would therefore be extremely
useful in the treatment of a wide variety of human
35 di~eases. However, production of human mAb has proven -
to be much more difficult than that of murine mAb.
:
:
W092/18~0 PCT/US92/0~975
-4-
1 3~9
Consequently they are not yet available in sufficient
quantities or varieties to be used as therapeutics
To overcome the problems of immune responses to
foreign mAb and the lack of suitable humaln mAb, at
least in part, genetic engineering techniques have been
used to construct hybrid immunoglobulin molecules which
contain the antigen binding region of the murine
antibodies and the remainder of the molecule is
composed of human antibody sequences which are not
recognized as foreign. Jones et al., "Replacing ~he
Complementarity-DeteL ;n;ng Regions in a Human Antibody
With Those From a Mouse", Nature, 321:522-525 tl986).
These hybrid antibodies eventually elicit an immune
response in human therapy, and they often do not
function as effectively as the parent murine
antibodies. For a review of the use and drawbacks of
murine and human mAb see Carlsson et al. 'IMonoclonal
Antibodies into the 90's: the All Purpose Tool",
Bio/Technology, 7:567-573, tl989).
Summary of the Invention
The present invention provides novel therapeutic
agents and methods which combine the cell-mediated and ;
antibody-mediated aspects of the immune response in a ;
single agent for use in human therapy, particularly
viral therapy and in tumor therapy. In therapy oP
viral infections, thesa novel agents act to block virus
lnfection by focusing cytotoxic T lymphocytes (CTL) to
virally infected cells thus causing lysis of the
30 infected cells~ -
This invention provi~es hybrid antibodies which
comprise a base portion corresponding to the constant
portion of human immunoglobulin G, a combining site
selected for specificity to the particular target
antigen, and a combining site which binds to and
activates human CTL. If the hybrid antibodies are to
,. .. .
.
WO92/18~0 P~ S92/02975
,~ -5-
2~Q7~2~
be used in therapy o~ viral infections, preferred
hybrids have a target antigen combining site which
binds to the protein responsible for virus infection,
the viral "cell-recognition" protein, thus neutralizing
- 5 infectivity of the virus. Since the cell-recogni-tion
protein .is often expressed on the surface of virally
infected cells, the hybrid antibody can also bind to
these infected cells. Binding of the hybrid antibodies
to both a virus infected cell and CTL causes activation
of the CTL and subsequent lysis of the infected cell.
A hybrid antibody according to the invention for
treatment of infections by human immunodeficiency virus
(HIV), which causes the acquired immune deficiency
syndrome tAIDS) disease~ preferably includes a
combining site which binds to the protein CD3 so as to
activate CTL, and a combining site specific for both
HIV antigens budding from the surface of infected
cells. For example, the antigen-recognition combining
site might be the variable portions of an antibody
20 specific for HIV coat proteins. :
!
Brief DescriPtion of the Drawinqs
Figure 1 is an illustration of an immunoglobulin
molecule illustrating its Y shape, combining sites,
hinge regions, light and heavy chains and their
corresponding variable and constant domains.
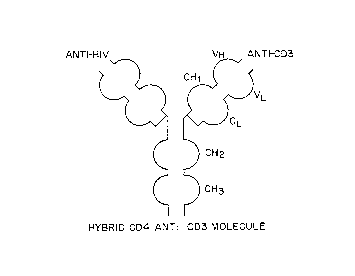
Figure 2 is an illustration o~ a protein complex
containing a single immunoglobulin combining site
capable of recognizing a virus, virus infected cell or
viral antigen, a single immunoglobulin combining site
capable of recognizing and binding to CD3 so as to
activate CTL, an immunoglobulin hinge reg.ion separating
the combining sites from the immunoglvbulin constant
domain CH2 and CH3 regions.
Figure 3 is a schematic diagram of a DNA construct ..
containing the VH D-J gene.
,
W092/18~0 PCT~US92/0~97~
?~Q~9 ~
Figure 4 is a flow diagram showing a cloning
scheme of the VH-D-J region.
Detailed Description of the Invention
It has now been found that~the antibody-mediated
and the cell-mediated immune responses can be combined
in a singlP recombinant protein complex so as to offer
novel therapeutic advantages for diseases such 3iS viral
infections. The invention relates to hybrid antibodies
engineered by recombinant DNA techniques which are
useful in therapy and prevention of viral infections in
humans.
Central to the hybrid antibody of the invention is
a base portion comprising at least a part of human
immunoglobulin G (IgG). As shown schematically in
Figure 1, IgG is a tetrameric protein complex formed
from two identical heavy chains H and H' and two
identical light chains L and L'. These chains are -~
joined by disulfide bonds into a Y-ihaped complex. In
20 solution however, the molecule takes on a more globular ~
shape. -
Protein sequence analysis of immunoglobulins has
led to the definition of specific regions or functional
domains within each of these chains. Each chain has a
variable region (VL and VH) located at its amino
terminus. The variable domains created by the pairing
of the VL and VH regions constitute the
àntigen-recognition portion or "combining site" of the
molecule. There are two combining sites per molecule.
The ~ariable domains of these chains are highly
variable in sequence and provide the diversity for ~ -
antibody combining sites to be highly specific for a
variety of antigens. Each of the chains also includes
essentially constant regions, which do not vary in
resiponae to the nature of the antigen recognized by the
combining sites. The light chains have a single
: '- .. - :' ,.
~ : .
WO92/18~0 PCT/US92/02975
-7-
21~732~
constant region (CL), while the hea~y chains possess
three separate constant regions (CHl, CH2 and CH3).
The pairing o~ CL and CHl produce the ~irst constant
domains, Cl, while the pairing of the CH2 regions
produces the second constant domain, C2 and the pairing
of the CH3 regions produces the third constant domain,
C3. The four constant domains, two Cl's, C2 and C3,
constitute the Y shaped base portion of the
immunoglobulin molecule. In addition, the h~avy chains
also have a hinge region separating Cl and C2 from the
remainder of the molecule. The hinge imparts
flexibility to the tetramer.
In a preferred embodiment, the protein complexes
of the invention have a Y shaped base portion which is
l~ the same as some or all of the constant regions of
human immunoglobulin. This use of human immunoglobulin
avoids the problem of the modified immunoglobulin being -~
recognized as a foreign species itself, and thus
facilitates its use in human therapy. Additionally the
20 base portion may confer effector functions on the -
molecule such as in vivo stability, Fc receptor
binding, protein A binding, complement fixation, and
placental transfer. It will thus be understood that
modified sequences based on immunoglobulin molecules
are within the scope of the present invention so long
as the modification does not give rise to immune
rejection problems.
To the base portion, there is added a combining
site which binds to and activates CTL, and a combining
site which binds to antigen. A particularly suitable
antibody combining site for CTL activation is the
combining site of an antibody specific to the CTL cell
surface protein CD3. Antibodies specific to other CTL
surface proteins which also function to activate CT~
are also encompassed within the scope of this
invention. These combining sites are affixed via
W092/18~0 ~CT/~S92/02975
8 ~ !
3~9
peptide bonds to the amino terminal ends of the base
portion on the arms of the immunoglobulin-like Y.
The anti~en-recognition combining site is selected
to provide specificity to a particular target organism.
For example, the combining site of~an antibody speci~ic
to the target organism can be af~ixed to the amino
terminal end of an arm of the base portion.
In the preferred embodiment of the present
invention, the anti~en-recognition combining site is
affixed via peptide bonds to one arm of the Y shaped
base portion and the antibody combining site specific
for CTL is affixed via peptide bonds to the other arm
of the Y shaped base portion.
The hybrid immunoglobulins of the present
invention are useful in the treatment of a wide variety
of viral infections. They are particularly well suited
for treatment of in~ections by viruses which upon
infection of the host cell cause expression of viral
coat proteins prior to cell death. In most cases this
cellular expression of viral coat proteins leads to a
cell surface form of such proteins. Examples include
but are not limited to the hemagglutinin protein
complex of influenza virus, the env proteins of murine
leukemia virus, the env proteins o~ Rous sarcoma virus -
25 and the env proteins of HIV. Often the viral protein ~:
expressed by infected cells is the same viral coat
protein which recognizes and binds to the cell receptor
protein to initiate infection. This is true in the -
case o~ HIV.
It is well known that anti-idiotype antibodies
carryinq the internal image of microbial antigens as
well as antibodies against TCR of T cells can stimulate
humoral and cellular antimicrobial immunity.
A preferred embo~ nt to create such novel
35 antibodies is to incorporate antigenic sequences -
; ~ directly into the antibody by genetic manipulation. A ~ -
W09~/18~0 PCT/U~92/02975
_ g _
2107329
method is described herein whereby such antibodies are
produced by genetic engineering to replace a segment of
immunoglobulin molecule with a sequence corresponding
to HIV antigenic determinants recognized by ~ or T
cells.
Exemplifying the present invention, the D seyment
o~ the heavy chain of an antibody has now been replaced
by influenza virus nucleoprotein (NP) epitope which is
capable o~ being recognized by T cells. The construct
was expressed in the SP2/0 myeloma cell line. Such
transfected SP2tO were killed by T cells specific for
the NP epitope.
In the Examples provided below, two DNA expression
vectors pSV2gpt-9lA3VH-CIgG2b and pSV2neo-9lA3L, both
carrying a heavy and a light chain gene of an
anti-arsenate antibody called 91A3. The
pSV2gpt-9lA3VH-CIgG2b carries an IgG2b constant region
gene inserted in the HindIII restriction endonuclease
site and the rearranged 5.5 kb VHD~ gene of the 91A3
antibody inserted in the ~EcoRI restriction endonuclease
site as shown in Figure 3. The 5.5 kb fragment also -
contains the heavy chain Ig promoter and enhancer. The
pSV2neo-9lA3L carries the rearranged VL and CL genes
and the necessary regulatory elements inserted into the
EcoRI and BamHI restriction endonuclease sites. It has
now been shown that cotransfection o~ these vectors
into the nonsecreting myeloma cell linP, SP2/0 leads to
the expression of a functional 91A3 antibody.
This antibody derives its ~H from the J5$8 family
and its D segment is probably involved in antigen
binding. These observations suggest that these D
segments are surfa~e exposed. In fact, the
hydrophilicity profile o~ the 91A3 VH also predicts ;-
that its D segment is surface exposed. For these
reasons the 91A3VHDJ was chosen to construct the Ig
chimera carrying the NP epitope. The goal of this
-
-~ .
:: .
w092/l8~0 ~0~ 3~9 -lo- PCT/US92/~2~S
study was to replace the 9 amino acid D segment with a
15 amino acid NP CTL epitope as illustrated in
Figure 3.
This epitope corresponds to amino acid residues
147-161 within the NP of PR8 virus and is known to
induce virus specific CTLs in Balb/C but not C57BL/6
mlce.
The molecules of the present invention are the
product of recombinant DNA engineering or chemical
cross-linking. Methods of fusing genes in the proper
orientation, transforming the genes into a suitable '
host cell and expressing and purifying the proteins are
known in the art and examples are provided below.
Detailed DNA cloning methods are provided in a variety
of sources. See e.g. Sambrook et al., "Molecular
Cloning A Laboratory Manual", Cold Spring Harbor
Laboratory Press, NY (1989). -~
Once the fused genes have been cloned, they are
transfected into a ~uitable host for expression of the
20 encoded protein. The cloned gene may be first inserted ~ -
into an appropriate expression vector or may be
transfected into the cell as linear DNA for
recombination with the host genome. Suitable
expression vectors include but are not limited to
plasmids, viruses and retroviruses. Choice of a
suitable vector will be determined in part on the
choice of the host used for protein expression.
Sùitable hosts include but are not limited to bacteria,
mammalian cell lines, whole animals such as transgenic ~
mice and insect cell iines. Although insect cell lines
have not heretofors been used for the expression of
immunoglobulin proteins it is thought that the -
difference in glycoprotein patterns compared to the
products of mammalian cell lines may produce more
effective proteins. Insect cell lines are less
expensive to maintain and produce more protein compared
,
..
: ~;
~ ~ .
W092/18~ PCT/US~2/02975
--1 1 ~
2107329
to mammalian cell lines and are thus more suitable to
large-iicale protein production. Genes expressed by
insect cell lines do not contain exons therefore the
exons should be excised in genes prior to thPir
expreission in insect cell lines. Excision is
relatively straightforward and can be accomplished for
instance directly by oligonucleotide directed
site-specific mutagenesis or indirectly by cDNA
cloning.
Transfer of the gene into the host can be done by
any of the well known means in the art. For example,
methods of gene transfer include but are not limited to
CaCl2 mediated transfection in the case of bacteria and
in the case of eukaryotic cells, CaPO4 mediated
transfection, viral infection including retroviral
latent infection, electroporation, liposome mediated
DNA transfer and microinjection among others.
Any suitable method of purifying proteins produced
by the host may be used in the practice of the present
invention. See e.g. Webb et al., "Cell-surface
Expression and Purification of Human CD4 Produced in
Baculovirus-infected Insect Cells", Proc. Natl. Acad.
Sci. USA, 85:7731-7735 (1989); and Moran et al.,
"Characterization of Variable-Region Genes and Shared
Crossreactive Idiotypes of Antibodies Specific for
Antigens of Various Influenza Viruses", Vir. Immunol.,
12 (1987).
The present invention is useful in directing the
cell-mediated immune response against virally in~ected
cells. HIV infected cell~ are used here as an example
of the utility of the present invention but it should
be understood that other diseases could be treated and
are considered to be within the scope of the invention.
As with all pharmaceutical compositions, the
effective amounts of the antibodies of the invention
must be determined empirically. Factors to be
.
W092/l~iO PCT~US92/02975
-12-
3~9
considered include the condition to be treated, whether
or not the antibody will be complexed with or
covalently attached to a toxin, route o~ administration
for the composition, i.e. intravenous, intramuscular,
subcutaneous, etc., and the number of doses to be
administered. Such factors are known in the art and it
is well within the skill of physicians to make such
determinations without undue experimentation.
The following examples are meant to illustrate but
not limit this invention.
Example 1 - DNA Cloninq
The procedure for deleting the 27 nucleotides
coding for the D segment of IgG, and the insertion of
45 bases corresponding to the NP epitope, is summarized
15 in Fig. ~. All enzymes were used according to the
manufacturer's instructions (New England Biolabs,
Beverly, MA). Unless otherwise spPcifically mentioned,
DNA cloning was performed according to the methods
described in Maniatis et al. (19~2).
Using this method the D segment of VHi region of
91A3 anti-arsonate antibody is replaced with one of:
(a) The consensus sequence of the B cell epitope
of the cysteine loop of gpl20. The se~juencP of this
epitope varies, however, a consensus sequence deduced
from 245 HIV isolate sequences borne by 241 isolates
was established. The amino acid sequence of the
consensus corresponds to residues 301-319 of gpl20 and
is ~iS follows:
Arg-Lys-Ser-Ile-His-Ile-Gly-Pro-Gly-Arg-Ala-Phie-Tyr-
30 Thr-Thr-Gly-Glu-Ile-Ile ;
(b) The T cell epitope of residues 12-35 of gag
of HIV-1 HxB2 isolate: -
Glu-Leu-Asp-Arg-Trp-Glu-Lys-Ile-Arg-Leu-Arg-Pro-Gly-
Gly-Lys-Lys-Lys-Tyr-Lys-~eu-Lys-His-Ile-Val
~c) A T cell epitope of HIV-l reverse
transcriptase; residues 325-349
: '' ' '
' ': , ' :
: ' . '
. .
WO92/18540 PCT/~S~2/02975
f ~ -13-
2~7329
Ala-Ile-Phe-Gln-Ser-Ser-Met-Thr-Lys-Ile-Leu-Glu-Pro-
Phe-~rg-Lys-Gln-Asn-Pro-Asp-Ile-Val-Ile-Tyr-Gln
Briefly, cloning was done by subcloning the 5.5 kb
91A3VHDJ fragment into the EcoRI restriction
- 5 endonuclease site of the pUCl9 plasmid. Two unique
restriction endonuclease sites (NcoI and ApaI, 638 bp
apart) surrounding the D region were identified. The
primers P1 and P3, shown in Fig. 4, are exactly
complementary to their corresponding strands. However
P2 matches with its complementary strand down to the
last nucleotide 5' of the D reyion (filled part of the
bar). The remaining 30 nucleotides (hatched part of
the bar) are those of the NP epitope. Primer P4
contains nucleotides complementary to the corresponding
strand down to the last nucleotide 5' of the D region.
The remaining unmatched nucleotides correspond to 30
bases of the NP epitope. An S~eI restriction
endonuclease site was created within the overlapping
nucleotides between P2 and P4.
Using polymerase chain reaction, two fragments are
produced. In one set of reactions, the annealing of
the P3 and P4 primers to the plasmid results in the
production of 570 bp fragment. In another set of
reactions, the annealing of P1 and P2 to plasmid ;
provides a 326 bp fragment. To delete the MP
overlapping sequences, both fragments are digested with
_I. The ligation of fragments, sharing each half o~
thé NP epitope, generates an 870 bp fragment containing
the 45 bp NP epitope inserted in-frame. The following
steps consist of digesting both the original
-~ pUC19-VHDJ9lA3 and the 870 bp fragment with the
restriction endonucleases NcoI and APaI. The ligation
of the 656 bp fragment into the digested plasmid
provides a vector possessing the coding region of the ; ;~
NP~epitope instead of the D segment. The 5.5 kb EcoRI
~:: : ' ':''' .,
W092/~8~0 PCr/US9~/02975
3~9 -14- ~
VH-NP.J fragment is then subcloned into the EcoRI
restriction endonuclease site of the expression vector.
Cotransfection was done using the gene pulsar
transfection apparatus according to the manufacturer's
instructions (Biorad). Cotransfection of the plasmid
pSV2gpt-9lA3-VHNPJ-CIgG2b and the pSV2neo-9lA3L plasmid
into the non-secreting myeloma cell line SP2/0 and
selection with mycophenolic acid and geneticin (G418)
allows the synthesis and secretion of th~e 91A3-NP
chimeric antibody.
SP2/0 are contransfected with heavy chain bearing
HIV epitopes together with parental light chain to
create transfectomas. Antibodies produced by these
t~ansfectomas are used to induce humoral or cellular
anti-HIV immunity.
Example 2 - ~ctivity of Chimeric Antibodies
NP-specific cytotoxic T cell clones have been
generated from Balb/c mice ; un; zed with PR8 influen2a '~
virus and expan*ed in vitro with irradiated spleen
cells coated with 5 ~g NP. The cytotoxicity assay was
carried out by incubating 5~Cr-labeled target cells and
NP-specific CTL at lO:1 E/T ratio for 4 hours. The
coating of target cells with NP was performed by
incubating 106 cells with 5 ~g peptide for 30 minutes,
washing and then labeling with 5~Cr as previously
described by Ito et al., J. Immunol. Met., 103:229
(1987). NP peptide tTYQRTRALVRTGMDP) is a T cell
epitope recognized in as~ociation with H-2Kd whereas the
peptide (IASNENMDAMESSTS) is a T cell epitope
recognized in association with H-2Db antigen.
The results presented in Tables 1 and 2 show that
chimeric Ig bearing the in~luenza virus epitope bound a
rabbit anti-NP antibodies and lost its binding to
arsonate since the D segment which plays an important
role in the binding of arsonate was replaGed with viral
peptide. ;
, .~
. ..: :
: :.
.::
W092/l8540 P~T/US92/02975
-15-
210~329
Table 1
Immunochemical Properties of Immunc~globulins
Produced by SP2/0 Coinfected wikh
5PSV2qpt-91A3qpt-9lA3VH and Psv2neo-glA3L
Binding to Binding of 91A3:Cg produced by
T14-10 (in cpm)
Ars BS~ 15~445 t 101
Rabbit Antimouse IgG2b 42,724 t 127
Binding to arsonate was determined by incubation
of lOng of antibody on a microtiter plate coated with
either arsonate BSA or BSA alone and bound antibodies
were revealed with l25I rat antimouse K antibody.
~inding to anti-isotype antibody was performed by
incubation of lOng of antibodies on plates coated with
rat antimouse ~ mAB and bound antibody was revealed
using ~25I goat antimouse IgG2b antibodies.
Table 2
~ .
Bindinq ProPerties of 91A3 Chimeric Immunoqlobulin
~ (in cpm)
: Binding to 91A3-NP 91A3
(chimeric) ~native)
Arsonate BSA 792 ~ 22 15,445 + 101
Anti-NP antibodies 5,616 + 217 1,246 + 76 .
~ Binding to arsonate-BSA was carried out as
previously described in Example 1. Binding to rabbit
anti~NP antibodies was assessed by incubating
transfectoma supernatants on microtiter plates coated
with affinity chromatography purified anti-NP -
antibodies and bound antibodies were revealed using l25I
goat antimouse IgG2b. ;.
NP-specific CTL were able to kill SP2/0 :~
45 transfected with:chim.eric Ig gene indicating that NP ;~
:- :
WO92J18~0 ~3~9 -lS- PCT~US92/02975
epitope is expressed on cell-surface as in cells
infected with the virus.
The data in Table 3 (panel A) show that the CT~
clone is able to kill PR8 and X31 influenza virus
infected P815 cells (H-2d) as well as P815 cells coated .
with NP. No significant killing was seen with P815 :.
cells coated with irrelevant NP known to be recognized
in association with H-2Db by C57BL/6 CTL. Panel B shows
the ability of NP specific CTL to kill SP2/0 cells,
expressing chimeric Ig genes, or coated with NP. No
killing was observed with cells expressing V~w, VLW or
both genes. However, significant killing is observed
with SP2/0 VHC_VLW transfectomas.
Table 3
Killing of SP2/0 cells transfected
with plasmid carrying the VH-NP : :
chimeric gene (Vl~r), bY NP-specific CTL
Target Cells % Specific 5ICr release -.
(1) (2)
.: .
A P815 14 12 :
P815-NP H-2d 77 49 : :
P815-NP H-2d 14 10 -::
P815 infected with PR8 59 51
P815 infected with X31 77 64 : .
P815 infected with B Lee 19 9 .
30 B SP2/0 ND 2
SP2/0 / VHW_V~W 9 ND -
SP2/0 / VHW VLW
coated with NP-H2d30 ND .-:.
SP2/0 / V}~ VLW
coated with NP-H2b 7 ND
SP2/0 / VHW 2 ND .
SP2/0 / VLW ND :~.
SP2 / O / VHC
SP2/O / VHC VLW 28 21
- :.
~ :.:: ..;.:
ND = not done
, '' ,' '
', :-~ ,
~; '~ '~'' ' ''
WO9~/18~40 2 1 0 7 3 2 9 PCT/~S92/02975
17
These results clearly show that cells transfected
with chimeric i~unoglobulin genes bearing an epitope
of influenza virus recognized by CTL are killed by CTL
as are influenza in~ected cells or cel].s artificially
(in vitro) coated with peptide.
' ~
.
.
.
:
,