Note: Descriptions are shown in the official language in which they were submitted.
~ 21~ U~
O.Z. 0050/42360
1,3.4-Trisubstituted Pi~eridine derivatives, the
, . .
~ preparation and use thereof
.: .
.~ The present invention relates to 1,3,4-
tri~ubstituted piperidine derivatives, to a process for
their preparation and to their use as drugs.
It is known that butyrophenone derivatives with
~- basic substituents have neuroleptic and cerebroprotective
: effects (US 4,605,655, EP 410 114). It appears in this
,~ connection that the observed affinities for a receptors
. 10 are particularly important.
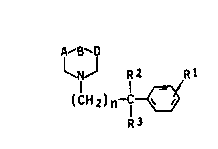
We have now found that 1,3,4-trisubstituted
. piperidine derivatives of the formula I
A-B~
(CH~
.~ R~ .
'~ where
R1 is hydrogen, fluorine, chlorine or bromine,
R2 is hydroxyl, or phenyl which is unsubstituted or
sub~tituted by fluorine, chlorine or bromine,
~: R3 is hydrogen, or
R2 and R3 together are oxygen, and
A-B-D is
~i --CH2--CIy~ - q~ ,
~ R5 or
i R7
R '~ R ~
. i --CH 2~ R 6
.i~ R 7
~j .
where
"
.` :
.,
-
- .:
~, ,
:: .
-~ . 2 ~
.. . .
: - 2 - O.Z. 0050/42360
.; ., .- . .
;` R~ is C~3-alkyl or is phenyl or thienyl which can be
sub~tituted.by fluorine or chlorine,
- Rs is hydrogen or hydroxyl,
. Rc is hydrogen,
- 5 R' is hydroxyl, or
.. R6 and R7 together are oxygen,
and their ~alts with physiologically tolerated acids have
.~ valuable pharmacological propertie~.
.- Rl to R' and n in the formula I preferably have
.: 10 the following meanings:
R1: hydrogen, fluorine, chlorine
.~ R~: hydroxyl, p-fluorophenyl
-~.q R3: hydrogen or together with R2 oxygen
i R': methyl, ethyl, phenyl, p-fluorophenyl, 2-thienyl
;~. 15 R5: hydrogen, hydroxyl
. R6: hydrogen
l............... R7: hydroxyl or with R6 oxygen.
The following compounds are particularly.
:. preferred:
.. 20 1-(4-Fluorophenyl)-4-[4-hydroxy-tran~-3-phenyl-
. (hydroxy)methyl-4-phenyl-1-piperidinyl]-1-butanone, 1-(4-
' fluorophenyl)-4-[trans-(3-phenyl-hydroxymethyl-4-phenyl)-
4-hydroxy-piperidin-1-yl]-butan-1-ol, 1-(4-fluorophenyl)-
4-(3-benzoyl-4-phenyl-~-dehydro-piperidin-1-yl)-butan-1-
~` 25 one,1-(4-fluorophenyl)-4-(3-benzoyl-4-phenyl-~3-dehydro-
.: piperidin-l-yl)-butan-1-one, 1-(bis-4-fluorophenyl)-4-.
~. ttran~-(3-phenyl-hydroxymethyl-4-phenyl)-4-hydroxy-
.~ piperidin-1-yl]-butane, 1-(4-fluorophenyl)-4-[trans-(3-p-
` fluorophenyl-hydroxymethyl-4-p-fluorophenyl-4-hydroxy-
piperidin-l-yl]-butan-1-one, 1-~4-fluorophenyl)-4-[tran~-
~: (3-p-fluorophenyl-hydroxymethyl-4-p-fluorophenyl-4-
iy hydroxypiperidin-l-yl]-butan-l-ol~ 1-(4-fluorophenyl)-4-
[trans-(3-acetyl-4-methyl)-4-hydroxy-piperidin-1-yl]-
^~ butan-1-one, 1-(4-fluorophenyl)-4-[tran~-(3-acetyl-4-
methyl)-4-hydroxy-piperidin-1-yl]-butan-1-ol, 1-(4-
.. ~. fluorophenyl)-4-(3-acetyl-4-methyl-~3-dehydropiperidin-1-
.yl)-butan-l-one~ 4-fluorophenyl)-4-(3-acetyl-4-methyl- ..
~3
., .
~.,
-. .: : .
...
- ~, 21Gr~
~ 3 - O.z. 0050/42360
. . .
~3-dehydropiperidin-l-yl)-butan-l-ol, 1-(bis-4-fluoro-
phenyl)-4-(3-acetyl-4-methyl-~3-dehydro-piperidin-l-yl)-
- butane.
The compounds of the formula I according to the
5 invention can be prepared by reacting a compound of the
formula II
,:. ,;,.,
;, R 2
" ~U--(CH2)3~< Il,
;: .............................. R 3 R 1
..-
" where Rl, R2, R3 and n have the stated meanings, and Nu is
a nucleofugic leaving group, with a 3,4-disubstituted
piperidine derivative of the formula III
:~".
B`II
~'.' ~ ~Ir,
- H
; 10 where A, B and D have the meanings stated for formula I,
- and converting the resulting compound where appropriate
Y,~ into the addition salt with a physiologically tolerated
'~ acid.
.- A suitable and preferred nucleofugic leaving
group for Nu is halogen, especially bromine or chlorine.
~- The reaction is expediently carried out in the
presence of an inert base such as triethylamine or
pota~3ium carbonate to trap acid in an inert solvent such
a~ a cyclic saturated ether, especially tetrahydrofuran
or dioxane, or an alkylbenzene such a~ toluene or xylene.
~ The reaction is usually carried out at from 80 to
A~'.. i 150C and is generally complete within from 1 to
10 hours.
The products of the formula I can be converted by
~ubsequent reaction~ as indicated in the examples.
These reaction~ comprise oxidations of the 3-
hydroxymethylpiperidine structure (R~ = OH in formula I)
~ to the corresponding carbonyl derivative3 with Jones
^~ reagent (chromium(VI) oxide in 25 % strength sulfuric
.
. .
" , . ' ',
' ~":
':
2 1 ~
- 4 - o.Z. 0050/42360
~` acid), reduction of the 2-butanone moiety (R2 + R3 =
oxygen) to the corresponding 2-butanol derivative with
sodium boranate, elimination of H2O from the 4-hydroxy-
piperidine moiety (Rs = OH) to give ~4-dehydropiperidine
derivative with concentrated sulfuric acid, and the base-
catalyzed double-bond shift to give the ~3-dehydro-
~ i piperidine compound.
; The compounds of the formula I according to the
invention are usually obtained in the form of yellowish
or yellow crystals and can be purified byrecrystallization from the conventional organic ~olvents,
preferably from a lower alcohol, such as ethanol, or by
column chromatography.
The free 1,3,4-trisubstituted piperidine deriva-
tive~ of the formula I can be converted in a conventionalway into the addition ~alt~ with a phy~iologically
tolerated acid, preferably by adding one equivalent of
the appropriate acid to a solution. Examples of pharma-
ceutically tolerated acids are hydrochloric acid, phos-
phoric acid, eulfuric acid, methanesulfonic acid,
` ~ulfamic acid, maleic acid, fumaric acid, oxalic acid,
~ tartaric acid and citric acid.
J` -' ' ~he compounds according to the invention have
-~ valuable pharmacological properties. They can be used as
- 25 neuroleptics, antidepressants, sedatives, hypnotics or
i~j cerebroprotectivec. It is possible for a plurality of the
said propertie~ to be combined in one compound according
~1 to the invention.
They are therefore suitable for the treatment of
p~ychoses, preferably ~chizophrenia, and anxiety states,
for the treatment and prevention of strokes or distur-
bances of cerebral function with an organic cause, and
for the treatment of sleep disturbance~.
The present invention accordingly also relates to
a therapeutic composition which contains a compound of
the formula I or its physiologically tolerated acid
;i addition salt a~ active substance in addition to
~;
:~.
~ - :
.,~ . ,. . , - :
;s - 5 - O.z. 0050/42360
~ conventional carriers and diluents, and to the use of the
- novel copounds for controlling diseases.
The compound according to the invention can be
'r. administered in a conventional way orally or parenteral-
ly, intravenously or intramuscularly.
The dosage dependR on the age, condition and
weight of the patient and on the mode of administration.
As a rule, the daily dose of active ~ubstance i~ about 1-
100 mg/kg of body weight on oral administration and 0.1-
`;- 10 2 mg/kg of body weight on parenteral administration.
; The novel compounds can be used in conventional
solid or liquid pharmaceutical forms, e.g. as uncoated or
(film-~coated tablets, capsules, powders, granules,
~ suppositories, solutions, ointments, creams or spray~.
; 15 These are produced in a conventional way. The active
sub~tances can for this purpose be processed with conven-
tional pharmaceutical aids ~uch as tablet binders,
fillerc, preservatives, tablet disintegrant~, flow
i' regulators, plasticizers, wetting agents, disper~ants,
emulsifiers, solvents, retardants, antioxidants and/or
propellant gases tcf. H. Sucker et al.: Pharmazeutische
Technologie, Thieme-Verlag, Stuttgart, 1978). The forms
- obtained in this way normally contain the active sub-
;~ ~ stance in an amount of from 0.1 to 99 % by weight.
The substances of the formula II which are
required a~ starting materials for synthesizing the novel
compounds are known.
The substances of the formula III have not
previously been described (but see DE 41 12 352). They
';'!' 30 are prepared, for example, by reacting 2 mole equivalents
of an a,~-unsaturated ketone of the formula IV
CH2=CH-C0-~4 IV
;!
; or a ~-halo ketone of the formula V
Hal-C~2-CH2-C0-R~ V,
where R' ha~ the abovementioned meanings, with an amine
,~ I
,
,
.~
. Y
... .
- . . , , . : - ~
. -' .~ ' ~' \ ' ' :
~: :
.. . . ~ ~
-` 2~ ~7~5
.~
6 - o.z. 0050/42360
~- H2N-Ra where R8 is benzyl which is unsubstit-lted or
substituted by halogen, methoxy or nitro, or is allyl, in
- the presence of 1 mole equivalent of sodium hydroxide
solution in methanol at 50C. This reaction iR usually
diastereoRelective to give the compound VI with di
equatorial trans configuration with respect to R4
. ' ,,~
HO. R4
-R4
J v r,
R8
,:
which can be converted by elimination of R~ and, where
appropriate, subRequent elimination of water and pos~ible
rearrangement and reduction as described above for the
; 10 final products into the compounds of the formula III.
The following examples illustrate the invention: ~
EXAMPLE 1
:.
a) Preparation of the starting material 4-hydroxy-
trans-3-phenyl(hydroxy)methyl-4-phenylpiperidine
600 g (1.62 mol) of trans-3-benzoyl-4-phenyl-4-
hydroxy-l-benzylpiperidine in 1.4 l of ethyl acetate
- mixed with 1.4 l of methanol were catalytically hydro-
: genated with the addition of 20 g of palladium llO %) on
; carbon at 70C under a hydrogen pre~3ure of 100 bar for
3 20 12 h. The mixture was filtered at 50C to remove cata-
-~ lyst, evaporated to dryness, taken up in 0.6 l of acetone
and stirred while cooling. The precipitated product was
~''1 filtered off with ~uction, the filtrate was concentrated,
the re~idue was ~tirred in 300 ml of acetone again while
cooling, and a ~econd product fraction was filtered off
~ with ~uction.
i~ Yield: 345 g ~75 %) of the pure diastereomer of
the product, melting point 167-169C.
b) Preparation of the final product 1-(4-fluorophenyl)-
4-[4-hydroxy-trans-3-phenyl(hydroxy)methyl-4-phenyl-
1-piperidinyl]-1-butanone
20.0 g (70.7 mmol) of 4-hydroxy-trans-3-phenyl-
(hydroxy3methyl-4-phenylpiperidine in 210 ml of xylene
,
;
~ .
.... ~ . . . . -. . .
'. ~. . . : ' ~ :
~,~ , - .
I . .. ........ .. . .
2 1 ~
: - 7 - o.z. 0050/42360
were mixed with 26.7 ml (162-mmol) of ~-chloro-4-fluoro-
butyrophenone and with 18.8 g (136 mmol) of finely
powdered potas~ium carbonate in addition to 1.0 g of
potassium iodide and refluxed while stirring vigorously
for 16 h. After cooling, 200 ml of toluene were added and
the mixture was ~tirred vigorously while the pale solid
precipitated. The crude product was filtered off with
suction, washed with toluene and dried at 50C under
reduced pre3sure. The ~olid was then digested in l 1 of
water at 50C and finally the product wa~ filtered off
with ~uction (melting point 182-183C), yield: 22.6 g
(72 %).
The following can be prepared in a Yimilar way:
- 2. 1-(4-Fluorophenyl)-4-[4-hydroxy-trans-3-phenyl-
(hydroxy)methyl-4-phenyl-1-piperidinyl]butane
Alkylating reagent: l-(p-fluorophenyl)-4--
chlorobutane
3. 1-Phenyl-4-[4-hydroxy-trans-3-phenyl(hydroxy)methyl-
4-phenyl-1-piperidinyl]-1-butanone,
Melting point 173-175C
4. 1-Phenyl-4-[4-hydroxy-trans-3-phenyl(hydroxy)methyl-
4-phenyl-1-piperidinyl]butane
5. 1-(4-~romophenyl)-4-[4-hydroxy-trans-3-phenyl-
(hydroxy)methyl-4-phenyl-1-piperidinyl]-1-butanone,
- 25 Melting point 186-187C
EXAMPLE 6
a) Preparation of the starting material
4-Hydroxy-trans-3-p-fluorophenyl(hydroxy)methyl-4-p-
fluorophenylpiperidine
30.0 g ~67.6 mmol) of4-hydroxy-1-benzyl-trans-3-
p-fluorobenzoyl-4-p-fluorophenylpiperidine hydrochloride
in 1 1 of methanol were catalytically hydrogenated with
the addition of 4.0 g of palladium (10 %) on carbon at
, 50C for 8 h. After filtration and washing with methanol,
the filtrate was evaporated to dryne~s. The residue wa~
taken up in a mixture of 140 ml of methanol and 500 ml of
water at 70C, concentrated ammonia was added until the
.
: . :
~ ~ ,
~ ~:
`~ 2 1 O ~ ~ ~ 5
- 8 - O.Z. 0050/42360
mixture was alkaline, and the precipitated product was
filtered off with suction and washed thoroughly with
water. Drying at 60C under reduced pressure resulted in
20.2 g (94 %) of product of melting point 215-217C.
b) Preparation of the f inal product
1-(4-Fluorophenyl)-4-[4-hydroxy-tran~-3-p-fluoro-
phenyl(hydroxy)methyl-4-p-fluorophenyl-l-
` piperidinyl]-1-butanone
; 20.0 g (63.0 mmol) of 4-hydroxy-tran~-3-p-fluoro-
phenyl(hydroxy)methyl-4-p-fluorophenylpiperidine in a
-~ mixture of 250 ml of toluene and 25 ml of dimethyl-
formamide were mixed with 20.6 ml (125 mmol) of ~-chloro-
4-fluorobutyrophenone and with finely powdered potas~ium
carbonate in addition to 1.0 g of potas~ium iodide and
refluxe~ while stirring vigorou~ly for 8 h. After cool-
ing, the mixture wa~ evaporated in a rotary evaporator,-
the residue was taken up in a little dimethylformamide,
and the solution was poured into vigorously stirred
ice-water. The precipitated solid was filtered off with
- 20 suction, thoroughly washed with water and recrystallized
- from ethanol to give 23.5 g (78 %) of product of melting
~- point 175-177C.
'~7 The following can be prepared in a similar way:
;~ 7. 1-(4-Fluorophenyl)-4-[4-hydroxy-trans-3-p-fluoro-
'~, 25 phenyl(hydroxy)methyl-4-p-fluorophenyl-1-
~'1 piperidinyl]butane, melting point 170-171C.
8. 1-Phenyl-4-[4-hydroxy-trans-3-p-fluorophenyl-
'j (hydroxy)methyl-4-p-fluorophenyl-1-piperidinyl]-1-
butanone
;~ 30 9. 1-Phenyl-4-[4-hydroxy-trans-3-p-fluorophenyl-
(hydroxy)methyl-4-p-fluorophenyl-1-piperidinyl]-
~, butane
~ EXAMPLE 10
i; a) Preparation of the starting material 4-hydroxy-cis-
3-acetyl-4-methylpiperidine
20.1 g (81.0 mmol) of 4-hydroxy-1-benzyl-ci~-3-
acetyl-4-methylpiperidine in 700 ml of methanol were
.'
.
,; .
'' .
. . . ~
.~. :. , .. ':, `
~:'~ . .- : ,
, ' ` ~ - ` ., ~ , .
, ~, .
- ,
-
~ 21 ~7~5
- . ,.. -
_ g - o.z. 0050/42360
catalytically hydrogenated with the addition of 2.5 g of
palladium (10 %) on carbon at room temperature for 8 h.
- After filtration and wa~hing with methanol, the filtrate
was evaporated to dryne~s. 11.9 g (94 %) of product were
isolated, melting point 91-93C. The hydrochloride melts
at 118-199C.
.:;,; .
- The tran~ isomer can be prepared in a ~imilar
way: decomposition above 133C (hydrochloride).
b) Preparation of the final product
` 10 1-(4-Fluorophenyl)-4-[4-hydroxy-cis-3-acetyl-4-
`; methyl-1-piperidinyl)-1-butanone
20.5 g (131 mmol) of 4-hydroxy-ci~-3-acetyl-4-
methylpiperidine in a mixture of 250 ml of toluene and
25 ml of dimethylformamide were mixed with 21.4 ml
(131 mmol) of ~-chloro-4-~luorobutyrophenone and with
18 g (131 mmol) of finely powdered potassium carbonate in
addition to 1.0 g of potassium iodide and refluxed while
stirring viyorously for 3 h. After cooling, the mixture
~' was evaporated in a rotary evaporator, and the residue
wa~ partitioned between methylene chloride and water. The
aqueous pha~e wa~ extracted with methylene chloride, and
; then the organic phase was dried with sodium ~ulfate and
~: evaporated. The crude product was purified by column
s~ chromatography (~ilica gel, methylene chloride/methanol
98/~2). 9.5 g (22 %) of product were isolated, de-
compo~ition above 90C (tartrate).
The following can be prepared in a ~imilar way:
(4-Fluorophenyl)-4-[4-hydroxy-trans-3-acetyl-4-
methyl-1-piperidinyl]-1-butanone,
i~ 30 Melting point 174-175C (hydrochloride)
12. 1-~4-Fluorophenyl)-4-[4-hytroxy-cis-3-acetyl-4-
methyl)-1-piperidinyl]-1-butanol,
Alkylating reagent: l-hydroxy-1-(p-fluorophenyl)-4-
` chlorobutane
~' 35 13. 1-(4-Fluorophenyl)-4-[4-hydroxy-trans-3-acetyl-4-
methyl-l-piperidinyl]-l-butanol~ melting point 97-99C,
Alkylating reagent: 1-hydroxy-1-(p-fluorophenyl)-
~ . .
``3
. ~ .
~,'' ., `~ ' `
" ' ' , `
. ~ ~
~ 2~7~5
.
- 10 - O.z. 0050/42360
4-chlorobutane
- 14. 1-(4-Fluorophenyl)-4-[4-hydroxy-ci~-3-acetyl-4-
methyl-l-piperidinyl]butane, melting point 85-88c
(hydrochloride)
.: 5 15. 1-(4-Fluorophenyl)-4-~4-hydroxy-trans-3-acetyl-4-
. methyl-1-piperidinyl]butane, melting point 105-107C
; (hydrochloride)
- 16. 1-(4-Phenyl)-4-[4-hydroxy-cis-3-acetyl-4-methyl-1-
. piperidinyl]-l-butanone
17. 1-(4-Phenyl)-4-[4-hydroxy-trans-3-acetyl-4-methyl-1-
piperidinyl]-1-butanone
; 18. 1-(4-Phenyl)-4-[4-hydroxy-cis-3-acetyl-4-methyl-1-
piperidinyl]butane
19. 1-(4-Phenyl)-4-[4-hydroxy-tran~-3-acetyl-4-methyl-1-
piperidinyl]butane
; 20. 1-(4-Fluorophenyl)-4-[4-hydroxy-trans-3-propionyl-4-. .
ethyl-l-piperidinyl]-l-butanone
. EXAMPLE 21
~ 4-fluorophenyl)-4-[4-hydroxy-trans-3-phenyl-
20 (hydroxy)methyl-4-phenyl-1-piperidinyl]butane
.^~ 22.8 g t80.6 mmol) of 4-hydroxy-trans-3-phenyl-
.~ (hydroxy)methyl-4-phenylpiperidine (Example la) in400 ml
.S- of xylene mixed with 40 ml of DMF were mixed with 22.6 g
(80.6 mmol) of 1,1-bis(4-fluorophenyl)-4-chlorobutane and
25 with 18.6 g (132 mmol) of finely powdered pota~sium
:1 carbonate in addition to 0-3 g of potassium iodide and
refluxed while stirring vigorously for 8 h. The mixture
-. was ~ooled and then evaporated to dryness. The residue
' was disaolved in DMF, and the solution was poured into
`~ 30 2.5 1 of vigorously stirred ice-water. After stirring for
. 1 hour, the precipitated solid was filtered off with
: suction to give 40.1 g (95 %) of product of melting point
~ 160-162~C.
,1 The following can be prepared in a similar way:
22. 1,1-~is~4-fluorophenyl)-4-[4-hydroxy-trans-3-p-
fluonq~Ysyl~ xxy)m~yl~t~~KrqpYIyl-l-l4yr~lyl]b~ne
23. 1,1-~is(4-fluorophenyl)-4-[cis-3-acetyl-4-hydroxy-1-
~ .
, ., ~
- . .
~ 2~ L~ 7~
..... .
;'`-"' '~- - 11 - O.Z. 0050/42360
~, .
:.- piperidinyl]butane, melting point 116-ll9~C
(hydrochloride)
~', 24. 1,1-Bist4-fluorophenyl)-4-[tran~-3-acetyl-4-hydroxy-
1-piperidinyl]butane, melting point 93-96C
' , 5 (hydrochloride)
EXAMPLE 25
~" 1-(4-Fluorophenyl)-4-[4-hydroxy-tran~-3-phenyl(hydroxy)-
, methyl 4-phenyl-1-piperidinyl]-1-butanol
5.0 g (11.6 mmol) of 1-t4-fluorophenyl)-4-[4-
' , 10 hydroxy-trans-3-phenyl(hydroxy)methyl-4-phenyl-1-
' piperidinyl]-1-butanone (Example lb) were dis~olved in a
',, mixture of 80 ml of methanol and 100 ml of tetra-
hydrofuran, and 0.6 g (16 mmol) of sodium boranate was
added. The mixture was stirred at room temperature for
' 15 2 h and then concentrated in a rotary evaporator. The
~ residue was partitioned between methylene chloride and,
;',, water at pH 10, and the organic phase waR dried with
'i sodium ~ulfate and evaporated to give 4.6 g (88 %) of, product of melting point 90-92C (decomposition).
.,~ 20 The following can be prepared in a similar way: -
26. 1-Phenyl-4-t4-hydroxy-tran~-3-phenyl(hydroxy)methyl-
4-phenyl-1-piperidinyl]-1-butanol
Melting point: 90-92C
,~ 27. 1-(4-Fluorophenyl)-4-[4-hydroxy-trans-3-p-fluoro-
'', 25 phenyl(hydroxy)methyl-4 -p-f luorophenyl-1-
', piperidinyl]-1-butanol
;,j 28. 1-~4-Fluorophenyl)-4-[4-hydroxy-tran~-3-p-fluoro-
'~, phenyl~hydroxy)methyl-4-p-fluorophenyl-1-
,~, piperidinyl]butane
,~' 30 29. 1-Phenyl-4-[4-h'ydroxy-trans-3-p-fluorophenyl-
~ (hydroxy)methyl-4-p-fluorophenyl-1-piperidinyl]-
``^, 1-butanol
'~' 30. 1-(4-Fluorophenyl)-4-[4-hydroxy-cis-3-a-hydroxy-
'`~ ethyl-4-methyl-1-piperidinyl]-1-butanol
31. 1-~4-Fluorophenyl)-4-~4-hydroxy-trans-3-a-hydroxy-
ethyl-4-methyl-1-piperidinyl]-1-butanol
32. 1-(4-Fluorophenyl)-4-[4-hydroxy-trans-3-a-hydroxy-
'; .
~'1
.
i,.
.
':~
' ' ' ~ ' ' ~
2 ~ ~ 7 ~
.`.. i . ...
- - 12 - O.Z. 0050/42360
ethyl-4-methyl-1-piperidinyl]butane
33. l-(4-Bromophenyl)-4-[4-hydroxy-tran~-3-phenyl-
(hydroxy)methyl-4-phenyl-1-piperidinyl]-1-butanol,
Melting point 92-93C
- 5 34. 1,1-si~(4-fluorophenyl)-4-[4-hydroxy-ci~-3-a-
~, hydroxyethyl-4-methyl-l-piperidinyl]butane
35. 1,1-Bi~(4-fluorophenyl)-4-[4-hydroxy-trans-3--
hydroxyethyl-4-methyl-1-piperidinyl]butane
EXAMPLE 36
1-(4-Fluorophenyl)-4-[4-hydroxy-tran~-3-benzoyl-4-phenyl-
1-piperidinyl]-1-butanone
22.6 g (50.7 mmol~ of 1-t4-fluorophenyl)-4-[4-
hydroxy-tran~-3-phenyl(hydroxy)methyl-4-phenyl-1-
piperidinyl)-l-butanone (Example lb) were ~uspended in
500 ml of acetone and then, while stirring vigorously,
21 ml (56 mmol) of JoneY reagent were added dropwise. The-
temperature rose to 32C during thi~. The mixture was
stirred at room temperature for 12 h and then evaporated
in a rotary evaporator. The mixture was then poured into
ice-water, methylene chloride was added, dilute sodium
hydroxide solution was added until alkaline, and the
precipitated chromium oxide was filtered off with
suction. The aqueous phase was extracted with methylene
chloride and then the organic phase was dried with ~odium
sulfate and concentrated to give 21.1 g (95 %) of product
of melting point 128-130C.
The following can be prepared in a similar way:
37. 1-(4-Fluorophenyl)-4-[4-hydroxy-trans-3-p-
fluorobenzoyl-4-p-fluorophenyl-1-piperidinyl]-
1-butanone,
Melting point 111-113C
38. 1-Phenyl-4-[4-hydroxy-trans-3-p-fluorobenzoyl-4-p-
fluorophenyl-1-piperidinyl]-1-butanone
EXAMPLE 39
1,1-Bis(4-fluorophenyl)-4-[4-hydroxy-trans-3-benzoyl-4-
phenyl-1-piperidinyl]butane
4.0 g (7.6 mmol) of 1,1-bi~(4-fluoroph-nyl)-4-[4-
.
13 - O.z. 0050/42360
hydroxy-trans-3-phenyl(hydroxy)methyl-4-phenyl~
piperidinyl]butane (Example 21) were di~olved in 50 ml
of glacial acetic acid and then, while stirring vigorous-
ly, 4.3 ml (11.4 mmol) of Jones reagent were added
dropwi~e. The mixture was ~tirred at 60C for 12 h and'
; then the ~olution was decanted off the precip'itated
chromium salts. The mixture wa~ then poured into ice-
water, methylene chloride was added, and sodium hydroxide
~olution was added until alkaline. The aqueous phase was
extracted with methylene ~hloride and then the organic
' phase was dried with sodium ~ulfate and concentrated. The
' crude product was purified by column chromatography
'~' (silica gel, methylene chloride/methanol 98/2) to give
~ 1.0 g (50 %) of product ~oil).
;~`' 15 'The following can be prepared in a ~imilar way:
40. 1,1-Bis(4-fluorophenyl)-4-t4-hydroxy-trans-3-p-
; fluorobenzoyl-4-p-fluorophenyl-1-piperidinyl]butane
'' EXAMPLE 41
~ a) Preparation of the starting material
i` 20 1. 4-Hydroxy-trans-3-benzoyl-4-phenylpiperidine
'~ 8 ml (21.4 mmol) of Jones reagent were added
dropwise to 6.0 g (21.2 mmol) of 4-hydroxy-trans-3-
phenyl(hydroxy)methyl-4-phenylpiperidine (Example la) in
~ ' 150 ml of acetone. The temperature rose to 30C during
'~ 25 this. The mixture was stirred at room temperature for
:j 1 h, and the solution was decanted off the precipitated
i~ chromium oxide and evaporated to half the volume in a
"~ rotary evaporator. The mixture wa~ then poured into ice-
water, dilute sodium hydroxide solution was added until
`' 30 alkaline, the mixture was extracted several times with
methylene chloride, and the organic phase was dried with
odium sulfate and evaporated. 5.2 g (87 %) of product
were isolated and, after recrystallization from ethyl
acetate, melted at 128-129C.
1 35 2. 3-Benzoyl-4-phenyl-~'-dehydropiperidine
'i 400 ml of methylene chloride and then 112.6 g
3 (304 mmol) of 4-hydroxy-tran~-3-benzoyl-4-phenyl-
~,
.j . . . .
. ~ , , .
; . ,
~'` 2~7~
`~ - 14 - O.Z. 0050/42360
piperidine dissolved in 350 ml c.ff methylene chloride were
added dropwise to 186 g (1.9 mol) of concentrated ~ul-
furic acid while cooling in ice to 0-5C. The mixture was
~tirred while cooling in ice for 2-3 h and then poured
into ice-water, concentrated sodium hydroxide solution
waff~ added (to pH 10). The mixture was partitioned between
methylene chloride and water, and the organic phase was
dried over sodium sulfate and concentrated. The crude
product was purified by column chromatography (fqilica
~- 10 gel, methylene chloride + 5 ~ methanol) to fgiVe a yield
of 61 g (57 ~) of melting point 118-119C.
-- b) Preparation of the final product
1-(4-Fluorophenyl)-4-[3-benzoyl-4-phenyl-~-dehydro-
1-piperidinyl]-1-butanone
15.4 g (59 mmol) of 3-benzoyl-4-phenyl-f~4-
dehydropiperidine in 90 ml of xylene were mixed with-
~-;; 11.5 ml (70 mmol) of ~ff-chloro-4-fluorobutyrophenone and
with 12.1 g ( Iff~-ff8 mmol) of finely powdered potaRqium
,~, carbonate tngether with 0.5 g of potassium iodide and
re~luxed while stirring vigorouqly for 15 h. The mixture
was concentrated in a rotary evaporator and then the
' ~ residue was partitioned between ice-water and methylene
chloride, making alkaline with dilute sodium hydroxide
solution. The aqueous phase wa~ extracted with methylene
chloride and then the organic phase wa~ dried with sodium
sulfate and concentrated. The crude product was purified
f by column chromatography (silica gel, methylene
chloride/methanol 98/2) to give 5.2 g (21 ~ of product
' of melting point 89-91C (hydrochloride).
The following can be prepared in a similar way:
42. 1-(4-Fluorophenyl)-4-[3-benzoyl-4-phenyl-f~'-dehydro-
l-piperidinyl]-l-butanol
. f~ EXAMPLE 43
1-(4-Fluorophenyl)-4-[3-benzoyl-4-phenyl-~-dehydro-1-
`~ 35 piperidinyl]-l-butanone
;~ 100 ml of methylene chloride and then 10.0 g
(22.4 mmol) of 1-(4-fluorophenyl)-4-[4-hydroxy-trans-3-
., :
.
.; : . ~ - . . . .
r~
t' ~. ' ' '
: : :: . , ,
. . .
`~ 21076~
; ~ - 15 - O.Z. 0050/42360
~enzoyl-4-phenyl-1-piperidinyl]-1-butanone (Example 36)
dissolved in 50 ml of methylene chloride were added
dropwise to 18.0 ml (336 mmol) of concentrated ~ulfuric
acid while cooling in ice to 0-5C. The mixture wa~
~tirred while cooling in ice for 2 h and then poured into
ice-water, adjusted to pH 10 with concentrated sodium
,7 hydroxide solution and partitioned between methylene
chloride and water, and the organic phase was dried over
sodium ~ulfate and concentrated. The crude product was
purified by column chromatography (silica gel, methylene
chloride 99/1) to give a yield of 4.2 g (44 %) of pale
oil. The hydrochloride melts at 89-91C.
- The following can be prepared in a similar way:
44. 1-(4-Fluorophenyl)-4-[3-benzoyl-4-phenyl-~-dehydro-
l-piperidinyl]butane
45. 1-(4-Fluorophenyl)-4-[3-p-fluorobenzoyl-4-fluoro-
phenyl- ~4 -dehydro-l-piperidinyl]-l-butanone
46. 1-(4-Fluorophenyl)-4-[3-p-fluorobenzoyl-4-fluoro-
phenyl-~-dehydro-1-piperidinyl]butane
- 20 47. l-Phenyl-4-[3-benzoyl-4-phenyl-~4-dehydro-1-
piperidinyl]-l-butanone
48. 1-Phenyl-4-[3-p-fluorobenzoyl-4-p-fluorophenyl-~'-
- dehydro-1-piperidinyl]-1-butanone
49. 1,1-~is(4-fluorophenyl)-4-[3-benzoyl-4-phenyl-~
dehydro-l-piperidinyl]butane,
Melting point 85-87C (hydrochloride)
~' 50. 1,1-Bis(4-fluorophenyl)-4-[3-p-fluorobenzoyl-4-p-
:~ fluorophenyl-~'-dehydro-l-piperidinyl]butane
1 EXAMPLE 51
:3 30 l-(4-Fluorophenyl)-4-[3-acetyl-4-methyl-~3-dehydro-l-
piperidinyl]-1-butanone
200 ml of methylene chloride and then 22.0 g
3~ (68.5 mmol)ofl-(4-fluorophenyl)-4-[4-hydroxy-cis,trans-
3-acetyl-4-methyl-1-piperidinyl]-1-butanone (Example 10,
11) dissolved in 100 ml of methylene chloride were added
dropwi~e to 40 ml (747 mmol) of concentrated sulfuric
acid while cooling in ice to 0-5C. The mixture was
.
,
.3' '
':
.'.' ' ' ~ '
2 L~7 ~ ~ 5
16 - o.z. 0050/42360
- stirred while cooling in ice for 2 h and then at 30-35C
~- for l h, and was then poured into ice-water, adjuYted to
:. pH 10 with concentrated sodium hydroxide solution and
^i partitioned between methylene chloride and water, and the
organic phase wai3 dried with sodium sulfate and con-
~; centrated. The crude product wa~ purified ~y column
chromatography (silica gel, methylene chloride 99/1) to
yield 9.8 g (45 %) of melting point 67-70C (tartrate).
The following can be prepared in a i~imilar way:
: 10 52. 1-(4-Fluorophenyl)-4-[3-acetyl-4-methyl-~3-dehydro-
l-piperidinyl]butane
53. 1-Phenyl-4-[3-acetyl-4-methyl-~3-dehydro-1-
piperidinyl]-1-butanone
54. 1-Phenyl-4-[3-acetyl-4-methyl-~3-dehydro-1-
piperidinyl]butane
EXAMPLE 55
1-(4-Fluorophenyl)-4-[3-phenyl(hydroxy)methyl-4-phenyl-
-dehydro-l-piperidinyl]-1-butanone
4.0 g (9.0 mmol) of 1-(4-fluorophenyl)-4-[3-
' 20 benzoyl-4-phenyl-~-dehydro-1-piperidinyl]-1-butanone
(Example 41, 43) were suspended in 50 ml of methanol and,
at 30C, 0.34 g (9.0 mmol) of sodium boronate wa8 810wly.
added. The mixture was stirred at room temperature for
2 h and then evaporated in a rotary evaporator. The
residue was partitioned between methylene chloride and
water at pH 10, and the organic phase wa~ dried with
sodium sulfate and concentrated to give 3.8 g (95 %) of
`~ product of melting point 191-192C (hydrochloride).
EXAMPLE 56
i~ 30 a) Preparation of t~e starting material
; 3-3enzoyl-4-phenyl-~'-dehydropiperidine
12.0 g (66 mmol) of 30 % sodium methylate ~olu-
tion were added to 4.2 g (16.0 mmol) of 3-benzoyl-4-
phenyl-~-dehydropiperidine (Example 41 a) in 60 ml of
methanol, and the mixture was refluxed for 8 h and then
stirred at room temperature overnight and evaporated to
; dryness in a rotary evaporator. The residue was poured
. '. .
~ ~ .
~, .
, ~ ~
... . .
~,
,~ 2 ~ r~
.....
.. ........
: ~ - 17 - o.Z. 0050/42360
into ice-water, the mixture was extracted several times
with methylene chloride, and the organic phases were
,~ dried with sodium sulfate and evaporated. The crude
product was purified by column chromatography (silica
; 5 gel, methylene chloride + 1 % methanol) to yield 1.9 g
(45 %) of melting point 189-192C.
b) Preparation of the final product
1-(4-Fluorophenyl)-4-[3-benzoyl-4-phenyl-~3-dehydro-1-
piperidinyl]-1-butanone
2.8 g (10.6 mmol) of 3-benzoyl-4-phenyl-~3-
dehydropiperidine in 50 ml of xylene were mixed with
~`~ 2.6 ml (15.4 mmol) of ~-chloro-4-fluoro~utyrophenone and
with 2.2 g (16 mmol) of finely powdered potassium car-
~ bonate together with 0.5 g of potassium iodide and
; 15 refluxed while stirring vigorously for 13 h. The mixture
was partitioned between methylene chloride and water, and-
the organic pha~e was dried with ~odium sulfate and
concentrated. The crude product was purified by column
chromatography (silica gel, methylene chloride + 5 %
methanol) to yield 1.4 g (31 %) of product with melting
point 171-172C.
. ~
The following can be prepared in a similar way:
57. 1-(4-Fluorophenyl)-4-[3-benzoyl-4-phenyl-~3-dehydro-
piperidinyl]-l-butanol
.~ 25 EXAMPLE 58
3 l-(4-Fluorophenyl)-4-[3-benzoyl-4-phenyl-~3-dehydro-l-
piperidinyl]-1-butanone
5.7 g (32 mmol) of 30 ~ sodium methylate solution
were added to 4.5 g (10.5 mmol) of 1-(4-fluorophenyl)-4-
t3-benzoyl-4-phenyl-~4-dehydro-l-piperidinyl]-l-butanone
(Example 41, 43) in 60 ml of methanol, and the mixture
j was refluxed for 1.5 h, then stirred at room temperature
overnight and evaporated to dryness in a rotary
, evaporator. The re~idue was poured into ice-water, the
; 35 mixture was partitioned between methylene chloride and
water, the pH was adjusted to 10, and the organic phase
was dried over ~odium sulfate and concentrated. The crude
,, ~
"`1
... .
~x.
;~.......................................... ~:
':,.~:
.
2~7~
` - - 18 - O.Z. 0050/42360
product was purified by column chromatography (silica
gel, methylene chloride ~ 2.5 % methanol) to yield 2.2 g
~ (48 %) of product whose maleate melt~ at 171 172C.
- The following can be prepared in a ~imilar way:
59. l-(4-Fluorophenyl)-4-~3-benzoyl-4-phenyl-~3-dehydro-
~ 1-piperidinyl]butane
; 60. 1-(4-Fluorophenyl)-4-[3-p-fluorobenzoyl-4-p-fluoro-
phenyl-~3-dehydro-l-piperidinyl]-1-butanone,
Melting point 211-213C (hydrochloride)
61. 1-(4-Fluorophenyl)-4-[3-p-fluorobenzoyl-4-p-fluoro-
phenyl-~3-dehydro-l-piperidinyl]butane
-~ 62. 1-Phenyl-4-[3-benzoyl-4-phenyl-~3-dehydro-1-
piperidinyl]-l-butanone
63. l-Phenyl-4-[3-p-fluorobenzoyl-4-p-fluorophenyl-~3-
lS dehydro-1-piperidinyl]-1-butanone
64. l,1-Bi~(4-fluorophenyl)-4-[3-benzoyl-4-phenyl-A3--
dçhydro-1-piperidinyl~butane
Melting point 85-87C (hydrochloride)
- 65. 1,1-Bis(4-fluorophenylJ-4-t3-p-fluorobenzoyl-4-p-
-- 20 fluorophenyl-~3-dehydro-l-piperidinyl]butane.
- EXAMPLE 66
-~1
-) a) Preparation of the starting material
Ci3- 3-phenyl(hydroxy)methyl-4-phenylpiperidine
7.8 g (22.1 mmol) of 3-benzoyl-4-phenyl-1-benzyl-
~-dehydropiperidine (prepared as in Example 41 a) in
, 300 ml of ethanol were catalytically hydrogenated with
; the addition of 1.6 g of palladium (10 ~) on carbon at
t room temperature under atmospheric pressure for 48 h. The
-~ mixture was filtered to remove the catalyst, evaporated
to dryne~s, taken up in 40 ml of acetone with heating,
r" and cooled while stirring. The precipitated product waq
~l filtered off with suction and washed with acetone. Yield:
,~ , 2.3 g (39 ~); the hydrochloride melt~ at 239-240C.
b) Preparation of the final product
1-(4-Fluorophenyl)-4-tcis-3-phenyl(hydroxyjmethyl-4-
I phenyl-l-piperidinyl]-l-butanone
~ 4.0 g (15.0 mmol) ofcis-3-phenyl(hydroxy~methyl-
, ..
~,
,
~i 23 ~75~5
9 - O ~ Z,~ 0050/42360
- 4-phenylpiperidine in 50 ml of toluene were mixed with
- 3.8 ml (23 mmol) of ~-chloro-4-fluorobutyrophenone and
with finely powdered potas~ium carbonate in addition to
1.O g of potassium iodide and refluxed while stirring
; 5 vigorou~ly for 25 h. After cooling, the filtrate was
concentrated, the reRidue wa~ partitioned at pH 10
between methylene chloride and water, and the organic
phase was dried and concentrated. The crude product wa~
purified by column chromatography (~ilioa gel, methylene
chloride + 5 % methanol) to i~olate 3.5 g (54 ~) of
product of melting point 113-114C.
~; The following can be prepared in a ~imilar way:
67. 1-(4-Fluorophenyl)-4-~ci~-3-phenyl(hydroxy)methyl-4-
phenyl-l-piperidinyl]butane
- 15 68. 1-(4-Fluorophenyl)-4-[cis-3-p-fluorophenyl(hydroxy)-
~ methyl-4-p-fluorophenyl-1-piperidinyl]-1-butanone -
;,~,?,, 69. 1-(4-Fluorophenyl)-4-[ci~-3-acetyl-4-methyl-1-
.i piperidinyl]-l-butanone
70. 1-(4-Fluorophenyl)-4-tci~-3-acetyl-4-methyl-1-
~ 20 piperidinyllbutane
.~ :
"-'i~
. `~ .
~,js
~ ' .
....
~;,1, ~
.,;;
'
.
. ~ ,
:i: , .
:. .