Note: Descriptions are shown in the official language in which they were submitted.
i
WO 92/18665 ~ PCT/US92/02862
_~_ ~~.fl~~~~1
METHOD AND COMPOSITION FOR INHIBITING GENERAL
AND PI aC~~Oll R~O~~~e Invest ~yING TOWER 'CATER
1. Field of the Invention
The present invention relates to a method of inhibiting corrosion in cooling
tower
. systems and, more specifically, for lowering the pitting rite associated
with tuberculation of
carbon steel and other corrosion-prone materials to less than the general
corrosion rate.
Cooling towers are widely used in the industry to cool water used in heat
exchangers,
refrigeration units, etc. Commonly, the cooling tower systems employed in such
environments are of the recirculating type; that is, the water used for
cooling purposes is
recycled to the cooling tower for chilling via evaporation. It is common for
the cooling
tower water to become corrosive from time to time, regardless of the level of
sophistication
of chemical addition and treatment. During these occurrences, tuberculation
can form on the
surface of the metal which provides sites for pitting co~xosion. The
subsequent pitting
beneath the tuberculation is the most serious form of corrosion and the
primary cause of
corrosion-induced equipment failure in cooling systems.
Specifically then, there are two types of corrosion which .must be controlled.
General, or uniform, corrosion and pitting, or localized, corrosion. General
corrosion rate
is the measure of the thickness of metal lost. It is measured in thousands of
an inch of metal
loss per year, referred to as mils per year (mpy). Pitting corrosion is also.
expressed as mils
per year, but refers to depth at a specific site.
Typically, an untreated water system my have a general (uniform) metal loss of
0.060
inches per year (60 mpy). By the addition of corrosion inhibitors, the general
corrosion rate
can be reduced. In a properly treated cooling system the general corrosion
rate will normally
be measured at less than 5.0 mpy. The pitting rate is considered to be
properly controlled
a if it is three to five times the general corrosion rate. Both the general
and pitting rates can
be measured either via metal coupons, or with electrical corrosion measuring
instruments.
CA 02107791 2001-05-02
-2-
2 Descr~tion of the Back r~ ound
Historically, a wide assortment of anti-corrosion compositions have been used
for
corrosion inhibition. For example, heavy metals, such as water-soluble
chromium and zinc
compounds have been used to virtually eliminate general corrosion and to a
certain extent
control pitting corrosion. Pitting corrosion, however, is still a serious
problem. Since
environmental considerations have progressively eliminated the use of toxic,
heavy metals,
such as chromate and zinc, less effective or more expensive corrosion
inhibitors have come
into extensive use. For example, it is known that water-soluble molybdates are
effective in
controlling corrosion and do not present environmental problems. However,
molybdates are
relatively expensive to use.
As disclosed in U.S. Patent No. 4,867,944 issued September 19, 1999, effective
corrosion inhibition in cooling tower systems can be accomplished by the use
of a
composition which includes a water-soluble zinc compound, a water-soluble
molybdate and
an orthophosphate. Similar corrosion inhibitors are also disclosed, for
example, in U.S.
Patents Nos. 4,217,216 issued August 12, 1980; 4,176,059 issued November 27,
1979;
4,017,315 issued April 12, 1977; DE No. 2850925 dated May 31, 1979; and Japan
Kokai JP
No. 52/38438 (77/38437) dated March 25, 1977. Additionally, an article
entitled "Molybdate
As A Pipeline Corrosion Inhibitor For Co-Water Slurry Systems", Phys. Metall.
Res. Lab.
1986, discloses a composition comprised of molybdate, zinc sulfate and
potassium phosphate
as an erosion-corrosion inhibitor for steel used in cold water slurries.
Although the use of molybdates, alone and in combination with other corrosion
inhibitors such as phosphates, provide more effective general corrosion
inhibitors in the sense
that certain environmental problems can be alleviated if the molybdates are
used without
toxic, heavy metals, there is still no known method of effecting control of
pitting corrosion to
CA 02107791 2001-05-02
-3-
the point where it can be virtually eliminated or at least reduced to a point
less than or equal
to the general corrosion rate.
Summary of the Invention
It is therefore an object of the present invention to provide an improved
method and
composition for reducing the pitting corrosion in cooling tower systems.
Another object of the present invention is to provide a method and composition
for
reducing the pitting corrosion in cooling tower systems which eliminates the
use of toxic,
heavy metals.
Still a further object of the present invention is to provide a method and
composition
for reducing the pitting corrosion in cooling tower systems to a point less
than, or equal to,
the general corrosion rate.
The above and other objects of the present invention will become apparent from
the
description given herein, the accompanying drawings, and the appended claims.
In one aspect of the present invention, there is provided a method of
inhibiting the
pitting corrosion rate of carbon steel in a cooling tower system comprising:
adding to a
cooling tower liquid used in an open, recirculating cooling tower system an
effective amount
of a corrosion-inhibiting composition comprising from about 1 to about 10 ppm
of a water-
soluble molybdate, calculated as molybdate, and from about 5 to about 25 ppm
of a stabilized
orthophosphate, calculated as phosphate, said phosphate stabilizer being
capable of
preventing precipitation and/or crystallization of insoluble calcium phosphate
under
conditions that would result in precipitation of such phosphates if said
phosphate stabilizers
were not present, said corrosion-inhibiting composition being substantially
free of active
zinc, said cooling tower liquid containing a calcium compound, the liquid of
said cooling
CA 02107791 2001-05-02
-4-
tower liquid consisting essentially of water, and circulating said cooling
tower liquid in said
system.
In another aspect of the present invention, there is provided a method of
inhibiting the
pitting corrosion rate of carbon steel in a cooling tower system comprising:
adding to a
cooling tower liquid containing a calcium compound an effective amount of
corrosion
inhibiting composition consisting essentially of from about 1 to about 10 ppm
of a water-
soluble molybdate, calculated as molybdate, from about 5 to about 25 ppm of a
stabilized
orthophosphate, calculated as phosphate, from about 1 to about 30 ppm of a
phosphate
stabilizer capable of preventing precipitation and/or crystallization of
insoluble calcium
phosphate under conditions that would result in precipitation of such
phosphates if said
phosphate stabilizers were not present, and an additive selected from the
class consisting of
iron sequestrants present in an amount of from about 1 to about 20 ppm, copper
corrosion
inhibitors present in an amount of from about 1 to about 20 ppm and mixtures
thereof, said
corrosion-inhibiting composition being substantially free of active zinc, and
circulating said
liquid in said system.
Brief Description of the Drawings
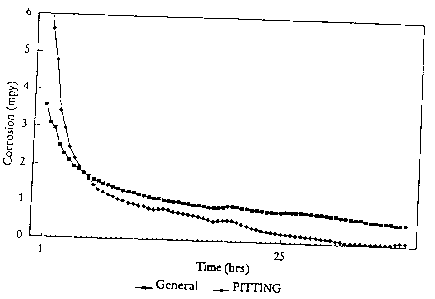
Fig. 1 is a graph showing a comparison of general and pitting corrosion rates
using
stabilized phosphate without any molybdate.
Fig. 2 is a graph similar to Fig. 1 showing a comparison of general and
pitting
corrosion rates using stabilized phosphate and molybdate.
Fig. 3 is a graph showing a comparison of general and pitting corrosion rates
using
stabilized phosphate and molybdate in which the molybdate has been added
incrementally
over time.
CA 02107791 2001-05-02
-4a-
Fig. 4 is a graph showing a comparison of general and pitting corrosion rates
for a
refinery cooling system using stabilized phosphate and molybdate.
Fig. 5 is a graph showing a comparison of general and pitting corrosion rates
in a
petrochemical cooling system using stabilized phosphate, molybdate and zinc
chloride.
Description of the Preferred Embodiment
The present invention is based upon the unexpected finding that the use of a
corrosion
inhibiting composition containing a water-soluble molybdate and a stabilized
orthophosphate
results in a pitting corrosion rate which is less than, or equal to, the
general corrosion rate.
Thus, the composition of the present invention can comprise, consist of, or
consist essentially
of the molybdate and the stabilized orthophosphate. In particular, it has been
found that if
there is no zinc present in a form and a level which would normally allow it
to act as a
corrosion inhibitor (hereafter referred to as "active zinc"), the pitting
corrosion rate is less
than the general corrosion rate. Such active zinc compounds are usually
inorganic, water-
WO 92/18665 PGT/US92/02862
_5.
~~~~~~
soluble compounds such as zinc halides. Thus, there its provided a corrosion
inhibiting
composition which is environmentally safe since it eliminates toxic, heavy
metals such as
zmc.
The two main components used in the method and composition of the present
invention are a water-soluble molybdate and a stabilized phosphate
(orthophosphate). The
water-soluble molybdate can be virtually any molybdate" usually an inorganic
molybdate,
which has sufficient water solubility for the particular cooling tower water
system. Alkali
metal molybdates are preferred, sodium molybdate being especially preferred
because of its
relative high solubility. The molybdate compound will be: present in the
compositions in an
amount of from about 1 to about 10 ppm, calculated as molybdate (MoO; ) as the
active
component, amounts of molybdate of from about 3 to about 6 ppm being
especially desirable.
The second major component used in the compositions and method of the present
invention is a "stabilized" phosphate. The word "stabilized", as used herein;
refers to a
condition under which orthophosphate in the water being treated will remain in
solution
despite a level of calcium or similar metal ions and system pH which would
normally result
in precipitation of generally insoluble metal (calcium) phosphate. In this
regard, it is known
that phosphate has a limited solubility in water when calcium and other
alkaline earth metals
are present, phosphate solubility following the equation:
26 logo (pH) + logo (oPO,) + 1.5 logo (CaCO,) = 25.5
It is also known that corrosion protection improves as phosphate levels are
raised. Indeed,
general corrosion rates are reduced most effectively when the phosphate level,
calcium level
and the system pH are such that the solubility of calcium phosphate is
exceeded in
. accordance with the equation shown above. In order to achieve the benefits
of using high
phosphate levels in corrosion protection but prevent unwanted precipitation of
calcium or
CA 02107791 2001-05-02
-6-
other similar metal phosphates, it is known to employ what are known as
"stabilized"
phosphates. Stabilized phosphates, as is known to those skilled in the art,
are achieved by
incorporating in or adding to the orthophosphate-containing cooling water one
or more
polymeric materials which by various proposed theories prevent the
precipitation of calcium
or other metal phosphates. Stabilization of phosphates and polymers used
therefore are
disclosed in U.S. Patent No. 4,711,725, and other patents mentioned therein.
In general, there
are a myriad of dispersants or materials, which are generally polymeric in
nature, e.g.
homopolymers, copolymers, terpolymers, which will prevent precipitation or
crystallization
of calcium or similar metal phosphates.
Non-limiting examples of materials (phosphate stabilizers) used to form
stabilized
phosphates include polymers derived from (meth)acrylic acids and salts as well
as mixtures
of such polymers with other compounds and polymers, such as phosphonic acids,
copolymers
of (meth)acrylic acids and vinyl esters, such as hydroxyethyl methylacrylate
and hydroxy
propylacrylate, and copolymers of (meth)acrylic acids and salts with
acrylamide alkyl or aryl
sulfonates or unsubstituted acrylamides. Additionally, polymers, e.g.
homopolymers,
copolymers and terpolymers, formed from acrylic acid, 2-acrylamido-2-methyl
propane
sulfonic acid (AMPS) and unsubstituted acrylamides have also been proposed for
use. Still
other materials which are disclosed in the aforementioned U.S. Patent No.
4,711,725 can be
employed as phosphate stabilizers. It is to be understood that the phosphate
stabilizers which
can be employed include any compound, polymer, whether synthetic or natural,
or mixtures
thereof, which can perform the function of preventing precipitation and/or
crystallization of
insoluble metal phosphate under conditions, e.g. pH, which would result in
precipitation of
such phosphates if the phosphate stabilizers were not present. In general, the
phosphate
WO 92/18665
PC'T/US92/02862
_7_
stabilizers will be present in an amount ranging from about 1 to about 30 ppm.
The stabilized orthophosphate will be present in the method and composition of
the
present invention in an amount of from about 5 to about 20 ppm, calculated as
phosphate
(PO,). The orthophosphate can be any water-soluble orthophosphate and can
include, without
- limitation, compounds such as monosodium phosphate,, disodium phosphate,
trisodium
phosphate, phosphoric acid, etc. It will be recognized that the
orthophosphates, generally
the most hydrated form of phosphate, are to be distinguished from
polyphosphates which can
also be used in the composition and which exhibit some lower degree of
hydration together
with being comprised of multiple PO, groups.
Although an effective corrosion inhibitor which will reduce pitting corrosion
to a level
equal to or below that of general corrosion can be obtained using only the
molybdate
compound and the stabilized phosphate as described above and provided there is
no added
active zinc as described hereafter; it is to be understood tlhat other,
conventional agents or
additives normally employed in corrosion inhibiting compositions can be
employed. For
example, polyphosphates can be employed with advantage, the polyphosphates,
when
employed, normally being present in amounts ranging from about 1 to about 30
ppm,
calculated as phosphate. Thus, non-limiting examples of useful water-soluble
polyphosphates
include tetrapotassium pyrophosphate, sodium hexametaphosphate, sodium
tripolyphosphate,
tetrasodium pyrophosphate, etc. It will be appreciated that when placed in a
water solution,
polyphosphates can, to some extent, convert to orthophosphates. Accordingly,
it is within
the scope of the present invention to form the stabilized phosphate by adding
only a
polyphosphate compound in an amount which will provide the required amount of
orthophosphate as set out above.
The corrosion inhibiting composition and method of the present invention can
also
WO 92/18665 PCT/US92/02862
x r~ g _
contain, with advantai~p ~ cants, such as polycarboxylic acids, e.g.
polymaleic
anhydride, various other homopolymers and copolymers, organic phosphonates,
etc., which
serve as iron sequestrants. When employed, such dispersants or sequestrants
will generally
be present in amounts generally ranging from about 1_ to about 20 ppm in the
cooling tower
water.
When copper components are present in the cooling tower system, it is also
desirable
to incorporate copper and copper alloy corrosion inhibitors such as
mercaptobenzotriazole
(MBT), benzotriazole (BZT), tolyltriazole (TTA), etc. When employed, such
copper
corrosion inhibitors will generally be present in an amount of from about 1 to
about 20 ppm
of the cooling tower water.
If desired, the compositions can also contain microbiocides, anti-foulants,
and other
such additives.
In carrying out the method of the present invention, the corrosion inhibiting
composition will be introduced into the cooling tower water in an effective
amount, i.e., an
amount which takes into the account parameters such as the degree of
contamination of the
cooling tower water, the pH, etc., which can be determined by well known
methods.
Generally, an amount of from about 20 to about 100 ppm of the inhibitor
composition,
calculated as the total of the active components, is employed. It wilt be
recognized,
however, that smaller or greater amounts can be employed depending on the
condition of the
cooling tower water.
In carrying out the method of the present invention, the components of the
composition can be added in virtually any manner. It is convenient to add the
water-soluble
molybdate in conjunction with the stabilized phosphate and any other
additional corrosion
inhibiting additives to the cooling water as a combined mixture by
conventional, well known
WO 92/186b5 PCT/US92/02862
_9_
methods. However, the individual components can be added separately if
desired.
As noted above, the present invention is buttress~.,d on the finding that if
molybdate
and stabilized phosphate are used together in the substantial absence of water-
soluble zinc
compounds or other sources of active zinc, the pitting rate can be maintained
at a level equal
to or below the general corrosion rate. For some reason, nnot totally
understood; the presence
of active zinc, which is generally regarded as a highly eiffective general
corrosion inhibitor
interferes with the combined action of the molybdate and t:he stabilized
phosphate. The term
"substantially free of active zinc", as used herein, refers to a level of zinc
below which the
zinc does not act to any significant extent as a corrosion inhibitor.
Generally speaking, a
level of zinc of 0.5 ppm or less, calculated as zinc, would be considered
substantially free
of active zinc. Amounts of about 0.5 ppm or greater of active zinc results in
increased
pitting corrosion, i.e. a pitting corrosion rate equal to or greater than the
general corrosion
rate. It will also be understood that substantial levels of zinc in the
corrosion inhibitor can
be tolerated if the zinc is in some form; e.g. chelated, which does not
normally allow it to
act as a corrosion inhibitor.
The present invention has proven to be particularly effective in preventing;
or
inhibiting, pitting corrosion associated with tuberculation. As carbon steel
is the metal that
is most commonly used in cooling system piping and in heat exchanger
construction, pitting
of carbon steel is of major interest to the industry. The present invention
can be used on
various types of cooling tower systems, such as forced draft towers, induced
draft towers,
and hyperbolic towers. Tower flow may be counterflow or crossflow. The method
and
composition find equal application to atmospheric cooling towers and natural
draft towers,
but find particular application in open, recirculating cooling tower systems.
To more fully illustrate the present invention, the folllowing non-limiting
examples are
WO 92/18665 ~; ~ PCT/US92/02862
-10-
presented. Amounts are calculated on a per weight basis of the active agent,
e.g. PO"
Mo0" etc.
Example 1
Clarified Brazos river water was concentrated to five cycles and the
mAlkalinity
adjusted to 100 ppm. To a sample of this water was added a stabilized
phosphate corrosion
inhibitor having the following composition:
Table 1
COMPONENT ACTIVE PPM
Monosodium phosphate 20
Tetrapotassium pyrophosphate (TKPP) 7
Hydroxyethylidenediphosphonate (HEDP) 3
Tolytriazole (TTA) 4
Polymaleic anhydride (PMA) 5
AMPS (Copolymer) l0
The data on general and pitting corrosion rates was acquired using a Rohrback
Cosasco
Model 9030 Corrator. Both general and pitting corrosion rates were measured
and computer
logged every 15 seconds. Every thirty minutes the previous 120 sample points
were
averaged and added to the database for graphic presentation. Thus, every
twenty-four hours
it was possible to plot 48 data points representing the averages of 5,760
discreet readings.
The resulting general and pitting corrosion rates are shown in Fig. 1. As can
be seen from
Fig. 1, after an initial brief passivation period. The general corrosion rate
leveled out at 1.0
mpy and the pitting rate at 2.8 mpy. These results closely mirror data which
workers in the
field have generally observed using stabilized phosphate alone.
WO 92/18665 PCT/US92/02862
_11_ ~:
Examl 1~ a 2
To a second sample of the Brazos River water used in Example 1 was added the
corrosion inhibiting composition shown in Table 1 wilh the exception that the
composition
contained sufficient sodium molybdate to provide 6.0 ppm active molybdate
(Mod,). The
data was obtained in the manner described in Example l:, the results being
shown graphically
in Fig. 2.
As can be seen from reviewing Fig: 2, the addition of molybdate to the
stabilized
phosphate improves the general corrosion rate to 0.6 mpy. However, and
dramatically, the
pitting corrosion rate lowered to only 0.1 mpy, a rate heretofore thought
unobtainable vis-a-
vis the general corrosion rate.
WO 92/18665 PCT/US92/02862
~~~~~-12-
Exam 1
To a third sample of the Brazos River water used in Examples 1 and 2 was added
the
corrosion inhibiting composition shown in Table 2. Subsequent to the initial
addition of the
corrosion inhibiting composition, sodium molybdate was incrementally added to
provide an
active level of molybdate of 0.5 ppm. The results, measured as per the method
of Example
1, are shown graphically in Fig. 3 which demonstrates that as the molybdate
level increases,
pitting corrosion rates dramatically decrease and eventually fall below
general corrosion
rates. The results of Fig. 3 also demonstrate that at a level of about 3.5 ppm
of moIybdate,
maximum inhibition of pitting corrosion is obtained.
Table 2
COMPONENT ACTIVE PPM
Monosodium phosphate 13
Tetrapotassium pyrophosphate (TKPP) 4.5
Hydroxyethylidenediphosphonate (HEDP) 2.5
Tolytriazole (TTA) 2
Polymaleic anhydride (PMA) 3
AMPS (Copolymer) 6.5
yWO 92/18665 PCT/US92/02862
-13-
. x 1 4
E amp a
The composition and method of the present invention was tested in an open,
recirculating cooling tower system used in a refinery. 'The corrosion
inhibiting composition
was as follows:
Table 3
COMPONENT ACTIVE PPM
Monosodium phosphate 10
Sodium molybdate
TKPP 3
HEDP 1.6
TTA 1.5
AMPS (Terpolymer)
AMPS (Copolymer] 5.2
PMA 1. 6
Pitting and general corrosion measurements were made l;enerally according to
the procedure
of Example 1. The results are shown graphically in Fig. 4 which plots
corrosion rates over
a 240 hour time period. As can be seen from Fig. 4, the same characteristic
passivation
curve was followed by the general corrosion rate leveling at 1.1 mpy and the
pitting
corrosion rate at 0.2 mpy. Data collected over a six-month period has
consistently shown
0.5 mpy general and 0.1 mpy pitting corrosion rates demonstrating that the
method and
composition of the present invention achieved the remarkable result of
maintaining the pitting
corrosion rate at a level below the general corrosion rate.
WO 92/18665 PCT/US92/0286_2
-14-
Ex m 1
The procedure of Example 4 was repeated on an open, recirculating cooling
tower
system in a petrochemical facility. The corrosion inhibiting composition
employed was as
shown in Table 4.
Table 4
COMPONENT ACTIVE PPM
Sodium phosphate
Sodium molybdate 4
Zinc chloride
TKPP 2.5
I-IEDP ~ 2
TTA 1.5
AMPS (Terpolymer) 1.5
AMPS (Copolymer) 2.4
p~ 3.1
In all cases, pitting and general corrosion rates were measured as per the
same general
method of Example 1 but without computer logging. The data for general and
pitting
corrosion rates are shown in Fig. 5 which is a graph of data accumulated over
a 150-day
period during which molybdate, the stabilized phosphate and, in addition, a
water-soluble
zinc compound were employed. As can be seen from Fig. 5, the pitting corrosion
rate was
always above the general corrosion rate. Indeed, and as is generally
experienced by other
workers, spiking of the pitting corrosion rate was noticeable and frequent
throughout the test
period.
WO 92/18665 PCT/US92/02862
-IS- 1~~'~~.
A comparison of the results from Examples 4 and s (Figs. 4 and s) shows that
when
water-soluble zinc compounds are present; and for some unexplained reason, the
pitting
corrosion rate remains above the general corrosion rate. In this regard, it
can be stated that
the cooling system water of both Examples 4 and s was essentially comparable
and that the
s corrosion inhibiting compositions were essentially the carne, the primary
difference being that
the composition used in Example s contained zinc chloride sufficient to
provide 2 ppm
calculated as zinc.
It has thus been demonstrated that using the mefhod and composition of the
present
invention, pitting corrosion rates equal to or Iess than general corrosion
rates can be obtained
using a combination of a water-soluble molybdate with a stabilized phosphate
in the ranges
discussed above and provided that active zinc is substantially excluded from
the composition,
i.e. zinc containing compounds or materials in which the zinc can act as an
active corrosion
inhibitor are kept below about O.s ppm. Generally speaking, water-soluble zinc
compounds
such as zinc halides, e.g. zinc chloride, are considered :sources of active
zinc.
is The foregoing disclosure and description of the invention is illustrative
and
explanatory thereof, and various changes in the method and composition may be
made within
the scope of the appended claims without departing from the spirit of the
invention.