Note: Descriptions are shown in the official language in which they were submitted.
CA 02108654 2002-06-12
- 1 -
ILLUMINATOR AND METHOD FOR PHOTODYNAMIC THERAPY
The invention directed to an illuminator for photodynamic therapy,
including diagnosis. Mare specifically, the invention is directed to an
illuminator for
photodynamic therapy which produces a high-powered beam at the appropriate
wavelengths which is highly uniform in both intensity and spectral
characteristics
(colour) throughout the illuminated area.
Photodynamic therapy ("PDT") or photochemotherapy is currently
being used to treat several types of aliments in or near the skin or other
tissues. For
example, PDT is being used to treat different types of skin cancer. In PDT, a
patient
is administered a photo-activable drug which accumulates in the tissue being
treated.
An area of the patient which includes the tissue being treated is then exposed
to light.
The light causes chemical and/or biological changes in the photo-activable
drug
which in turn selectively distinguishes, destroys or alters the target tissue
while at the
same time causing only mild and reversible damage to other tissues in the
treatment
area.
General background information on PDT using 5-Aminolevulinic acid
("ALA") can be found in U.S. Patent No. 5,079,262, entitled "Method of
Detection
and Treatment of Malignant and Non-Malignant Lesions Utilizing 5-
Aminolevulinic
Acid," and issued to James C. Kennedy et al. on January 7, 1992 and U.S.
Patent No.
5, 211,938, entitled "Method of Detection of Malignant and Non-Malignant
Lesions
by Photochemotherapy of Protoporphyrin IX Percursors," and issued to James C.
Kennedy et al. on May 18, 1993. ALA is a drug which functions as a prodrug in
the
body and metabolizes to protoporphyrin IX ("PpIX"). PpIX in cells may be
photoactivated with light of a certain
_2_
wavelength to either fluoresce, degrade or otherwise be
altered.
For therapeutic reasons' it is desirable to have a
large power output which is uniform in intensity and
color over a large area. Tn addition, it has been
discovered that use of light having wavelengths between
about 600 nm (nanometers) and about 700 nm is
particularly advantageous for certain forms of treatment.
Unfortunately, conventional illuminators produce a
relatively high percentage of light in the infrared
("TR") region. In order to prevent this high percentage
of infrared radiation from harming a patient, the overall
power output of the lamp must be limited. Moreover,
conventional illuminators do not produce a beam which is
uniform in intensity and color over a large area, e.g.,
greater than 10 cm (centimeters) in diameter, and do not
produce light virtually entirely in the 600 nm to 700 nm
wavelength range.
Accordingly, there is a real need for an improved
2o illuminator for photodynamic therapy.
It is an object of the invention, therefore, to
provide an improved illuminator far photodynamic therapy.
It is another object of the invention to provide an
illuminator beam for photodynamic therapy which includes
substantially no infrared light.
Another object of the invention is to provide an
illuminator for photodynamic therapy which produces a
very uniform beam in terms of both spectral
characteristics and intensity over a large area.
Yet another abject of the invention is to provide an
illuminator for photodynamic therapy which produces light
in a selected wavelength region, for example, almost
entirely in the 600 nm to 700 nm wavelength range.
According to a first aspect of the invention there
is provided an illuminator for photodynamic therapy which
.;
-3- ~~~i_~vv'~
includes a bulb and a filter assembly. The filter
assembly includes the following components in an optical
path: (1) a high-pass filter to filter out light having
wavelengths below a first wavelength value; (2) a low-
s pass dichroic filter to f_Llter out light having
wavelengths above a second wavelength value; and (3) a
dichroic mirror which reflects light having wavelengths
between the first wavelength value and the second
wavelength value and which transmits infrared light. An
exit lens assembly directs light transmitted through the
high-pass filter and the low-pass dichroic filter and
reflected by the dichroic mirror onto a patient for
photodynamic therapy.
According to a second aspect of the invention there
is provided an illuminator for photodynamic therapy which
includes a bulb to produce non-coherent light and a
spherical condensing mirror located behind the bulb. A
lens assembly is located along an optical path to produce
an image of light from the bulb and the spherical
condensing mirror. A dichroic filter, located along the
optical path, filters out light having wavelengths above
approximately 700 manometers. A dichroic mirror, located
along the optical path, reflects light having wavelengths
below approximately 700 manometers and transmits infrared
light. A filter, located along the optical path, filters
out light having wavelengths below approximately 600
manometers. An exit lens assembly having at least one
Fresnel lens is located along the optical path to direct
light having wavelengths between 600 manometers and 700
manometers onto a patient. The illuminator also may
include a heat dissipator located to receive infrared
light transmitted by the dichroic mirror to dissipate
incident infrared light as heat.
According to a third aspect of the invention there
is provided a method of photodynamic therapy which
includes the steps of: (a) providing light in a selected
wavelength region, for example, between 600 manometers
and 700 manometers; (b) energizing a bulb; (c) passing
light through a high-pass filter having a cutoff at a
wavelength of, for example, about 600 nanometers and
through a low-pass dichroic filter having a cutoff at a
wavelength of, for example, aibout 700 nanometers; (d)
removing infrared wavelengths using a dichroic mirror
which reflects non-infrared light and which transmits
infrared light; and (e) directing light transmitted
through the high-pass filter and the low-pass dichroic
filter and reflected by the dichroic mirror to the
patient to activate a light activable drug, including its
prodrugs and metabolites.
According to a fourth aspect of the invention there
is provided an illuminator for providing a uniform beam
to a patient for photodynamic therapy. The illuminator
includes a bulb having a filament, a condenser lens
assembly, and an exit lens assembly. The condenser lens
assembly images light from the bulb onto a plane lying in
a region which includes the exit lens assembly and the
space between the exit lens assembly and the patient but
excludes the patient. The exit lens assembly images a
virtual image of the condenser lens assembly onto the
patient.
Other objects, features, and advantages of the
invention will become apparent from the detailed
description of preferred embodiments of the invention set
forth below.
Preferred embodiments of the invention will be
described in detail below with reference to the
accompanying drawings, wherein:
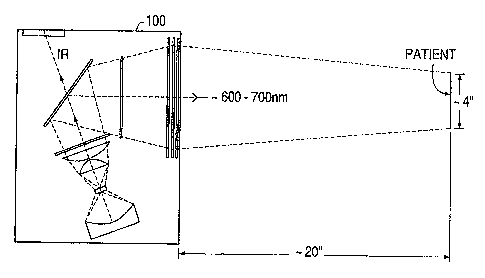
Figure 1 illustrates an overall arrangement for
conducting photodynamic therapy in accordance with a
preferred embodiment of the invention;
Figure 2 illustrates a detailed design for the
illuminator of Figure 1;
-5-
Figure 3 is a graph showing the spectral content of
bulb 10 of Figure 2;
Figure 4 illustrates the image produced by mirror
20 of Figure 2;
Figure 5 illustrates combined transmission/
reflection versus wavelength characteristics for filters
45 and 60 and mirror 50 of Figure 2;
Figure 6 illustrates percent transmission versus
wavelength characteristics for filter 45 of Figure 2;
Figure 7 illustrates percent transmission versus
wavelength characteristics for mirror 50 of Figure 2;
Figure 8 illustrates percent transmission versus
wavelength characteristics for filter 60 of Figure 2;
Figure 9 illustrates overall output versus
wavelength characteristics for illuminator 100 of Figure
2;
Figure to illustrates output versus wavelength
characteristics at different positions within the
illuminated area for illuminator 100 of Figure 2; and
Figure 11 illustrates total light intensity measured
at different positions along the vertical diameter of an
illuminated area for the illuminator 100 of Figure 2.
The invention provides an illuminator for
photodynamic therapy which permits radiation treatment
including diagnosis of a large region of a patient with
a high-powered uniform beam. The invention can be used,
for example, to treat an area between 10 cm and 20 cm in
diameter with light having wavelengths between about 600
nm and about 700 nm with high intensity and spectral
uniformity. The illuminator conveniently provides
approximately 1 to 60 mW/cmz of light which results in
doses in the 600 nm to 700 nm region on the order of 100
J/cm2. At the same time, the illuminator is small and
lightweight.
_6_
Figure 1 illustrates an overall arrangement for
conducting photodynamic therapy in accordance with a
preferred embodiment of the invention. As illustrated in
Figure 1, an illuminator 100 is located approximately 20
inches away from a patient who has been administered a
photo-activable drug which accumulates in the target
tissue. In this embodiment, the illuminator 100 is
approximately 16 inches by 12 inches (41 cm to 30 cm,
respectively) in cross-section. The illuminator 100
produces light having wavelengths between approximately
600 nm and 700 nm and shines this light on the patient
over an area approximately 10 cm to 20 cm in diameter.
Light in this wavelength range is reddish-orange to dark
red in color. The light in turn causes chemical and/or
biological changes in the drug to selectively activate,
destroy or alter the drug in target tissue.
Figure 2 illustrates a detailed design for the
illuminator 100 of Figure 1. As illustrated in Figure 2,
the illuminator 100 includes a 1000 watt quartz tungsten-
halogen (QTH) bulb 10, held in a holder 11, as a non-
coherent unpolarized light source. The bulb 10 has a
type CC-8 filament, is approximately 18 mm (millimeters)
x 7 mm, and is powered by a standard 110 VAC power
source. The output power of the bulb 10 is controlled by
controlling the power delivered to the bulb using a
rotary switch 12.
A tungsten-halogen bulb is used because it can
operate on standard AC power and is inexpensive. Because
bulb 10 can operate on standard AC power, additional
power supplies are not required. Additionally, use of
this bulb permits the illuminator to start up
instantaneously and produce a continuous output with no
sharp spectral lines. Bulb 10 essentially emits as a
black body. Figure 3 illustrates theoretical curves for
the spectral content of bulb 10 and indicates that most
of the energy of the bulb is emitted in the infrared
region above 700 nm.
-r-.
A spherical condensing mirror 20 is placed behind the
bulb 10 to produce an image of the filament parallel and
beside the filament, as shown in Figure 4, to create a
bright, approximately square source of light, to increase
the amount of light directed at the patient, and to
increase lamp life for a given output. The bulb 10 is
positioned slightly off-axis with respect to the optical
axis of the mirror 20 so that the bulb and its image are
symmetrically positioned about the optical axis of the
mirror. Bulb 10 and mirror 20 together produce an
uncollimated beam.
High boron content borosilica glass condensers 30 and
40 image the light from bulb 10 and mirror 20 onto the
exit lens assembly (to be described below) or between the
exit lens assembly and the patient, but not onto the
patient. The first condenser 30 is an aspheric lens and
the second condenser 40 is a piano-convex lens. This
arrangement minimizes the size of the illuminator and
provides lens diameters necessary to achieve the desired
illumination area on the patient.
Radiation from the near ultraviolet ( "W" ) region to
the near infrared region that is emitted by the bulb 10
is conditioned by filters 45 and 60 and mirror 50 which
together ensure that the light provided to the patient is
between about 600 nm and about 700 nm. Figure 5
illustrates the combined percent of transmission or
reflection to the patient versus wavelength
characteristics for filters 45 and 60 and mirror 50. In
this figure the horizontal axes corresponding to 0% are
displaced a small amaunt to allow a better view of the
characteristics for each component. As illustrated in
Figure 5, the combined effect of these elements is to
create a window P between 600 nm and 700 nm and to filter
out light outside of this window P. As indicated by
Figure 5, transmission in the 600 nm to 700 nm region is
essentially 100%, except for reflection losses. Thus,
virtually all of the light generated in this region by
bulb 10 goes onto the patient for treatment purposes.
The detailed properties and characteristics of each of
these elements will be described below in turn.
Dichroic filter 45 in the beam path transmits light
below about 720 nm, and thus transmits 600 nm to 700 nm
light, while filtering out light between 720 nm and
approximately 1100 nm. This filtering is accomplished by
reflecting the unwanted wavelengths. Figure 6
illustrates percent transmission versus wavelength
characteristics for filter 45. Filter 45 determines the
high wavelength cutoff (about 700 nm).
The use of a dichroic for filter 45 presents several
advantages over an absorption type filter. Since the
dichroic does not absorb light, it can tolerate much
higher intensities than absorption type filters. This is
important in this case because filter 45 directly
receives a large percentage of the bulb output. Bulk
absorption filters which eliminate infrared exist, but,
if used in this position would break due to excessive
heat absorption. A bulk absorption filter could be put
in the exit assembly (where filter 60 is) for high
wavelength cutoff, however, because it would still absorb
a significant portion of the bulb output at this
position, it would still heat up considerably and either
break or melt the plastic Fresnel lenses in the exit
assembly unless very aggressive cooling was provided.
More importantly, bulk absorption filters which are
designed for high wavelength cutoff have a very gradual
decrease in transmission as a function of wavelength.
Filters strong (opaque) enough to fully absorb the
infrared would cause a significant loss of power in the
spectral region of interest (600 to 700 nm). Filters
which provide enough power in the spectral region of
interest would leak too much infrared to the patient.
The sharp cutoff of the dichroic allows the illuminator
to filter out virtually all (greater than 90%) of the
. infrared while at the same time transmit virtually all
(greater than 90%) of the light in the 600 nm to 700 nm
region.
--1 U c1
=9-
Ideally, filter 45 should have a very sharp cutoff
at 700 nm without attenuating any light below 700 nm. As
illustrated in Figure 6, filter 45 starts transmitting
again at around 1100 nm. All dichroic filters have this
property of retransmitting at a wavelength longer than a
certain wavelength. The dichroic mirror 50 ensures that
radiation above 1100 nm does not reach the patient.
~ichroic mirror 50 operates in the reflecting mode
to transmit infrared radiation to a heat dissipater 55
(to be described below) and to reflect radiation between
600 nm and 700 nm tc~ the exit lens assembly. Thus,
mirror 50 is used to '°dump°' infrared radiation away from
the patient. Figure 5 illustrates the percent of light
which is reflected to the patient by mirror 50 fox
various wavelengths. As illustrated in Figure 5, light
in the 600 nm to 700 nat region is reflected to the
patient. At about 750 nm mirror 50 begins to dump the
light to heat dissipater 55. Figure 7 illustrates
transmission versus wavelength characteristics for mirror
50 near the low cutoff point. As can be seen from Figure
7, at about 500 nm most of the incident light is not
transmitted to heat dissipater 55 but is instead
reflected to the patient.
A long-wavelength-pass acrylic filter 60 filters out
violet and ultraviolet light. Filter 60 is a bulk
absorption-type filter which absorbs short wavelengths
and transmits long wavelengths. In this embodiment,
filter 60 is a number 2226 filter manufactured by Rohm
and Haas. Figure 8 illustrates the transmission versus
wavelength characteristics for filter 60. Ideally filter
60 should have a very sharp cutoff at 600 nm without
attenuating light above 600 nm. Filter 60 has a 50%
cutoff point at 590 nm and a half-width (10 to 90%
transmission) of approximately 20 nm. Thus, at 600 nm
90% of the incident light is transmitted.
Correct matching of filter 45, mirror 50, and filter
60 is important. In the illuminator 100 the light is not
collimated, i.e., not parallel. When light reaches a
-10-
dichroic surface (e.g., filter 45 or mirror 50) the
cutoff wavelength between reflection and transmission
depends slightly on the angle of incidence when the
surface is roughly perpendicular to the incoming light,
as is the case for filter 45. This dependence is much
greater when the dichroic surface is not perpendicular to
the central ray, as is the case for mirror 50. This is
one reason why the cutoffs of mirror 50 are not used to
delimit the 600 nm to 700 nm band. If mirror 50 were
used to delimit the desired band, there would be color
variation in the light provided to the patient due to
slight changes in the angle of incidence with respect to
mirror 50.
Figures 9 and 10 illustrate output versus wavelength
characteristics for illuminator 100. Figure 9
illustrates overall output versus wavelength
characteristics. Figure 10 illustrates spectral
characteristics measured at different positions within an
illuminated area. The different curves in Figure 10
correspond to five positions with respect to a 10 cm
circle on the patient: one at the center and the other
four at the edges in a square pattern. Both high and low
wavelength cutoff total variations are within
approximately 3 nm. This illustrates the excellent
spectral, or color, uniformity obtained with the filter
set described above (i.e., on the order of 0.5% of the
total range) . As will be appreciated from these figures,
illuminator 100 produces a very uniform output over the
desired range of 600 nm to 700 nm while projecting
virtually no radiation outside of the desired range.
The exit lens assembly includes two acrylic Fresnel
lenses 72 and 74. The exit lens assembly images the
virtual image of lens 30 and lens 40 onto the patient.
The optical system formed by mirror 20, aspheric lens 30,
lens 40 and Fresnel lenses 72 and 74 is designed to
produce light having a uniform intensity within the
illuminated area. One of the objects of this invention
is to provide uniform illumination using a filament type
N
°11- ~~~d~c~'t3ei
light bulb such as a tungsten halogen lamp because of the
advantages of low initial and maintenance costs and easy
starting and operation associated with these types of
bulbs. For this type of light source, simply collimating
the beam is not adequate to achieve uniformity as is the
case for sources with small emitting areas, such as arc
lamps. The large size of the filament makes it virtually
impossible to provide perfect collimation. A pseudo
collimated beam (i.e., one where the center rays of the
cones of light at any point in the beam are parallel)
would be very non-uniform and have a very large
divergence, like a poorly designed flashlight.
Figure 11 illustrates the total light intensity
measured along the vertical diameter of an illuminated
area on the patient (at a distance of 52 cm). These
measurements demonstrate that the optical system of
illuminator 100 produces a beam highly uniform in total
intensity. This highly uniform intensity results from
the optics scrambling the image of the filament such that
an image of the filament is not reproduced on the
patient. In other words, the spatial variation of light
on the patient is much more uniform than the spatial
variation of light from the bulb.
A mechanical assembly 90 and a spot diameter control
knob 92 are provided to move lenses 72 and 74 over a
range of about 8 cm to vary the spot diameter between 10
cm and 20 cm. The exit lens assembly provides a very
uniform beam within a well-defined illuminated area at
the position of focus on the patient. A tempered glass
exit window 78 pro-cects the Fresnel lenses, seals the
housing, and shields the operator and the patient from
hot components. In this embodiment, all of the optics
other than the bulb 10 are coated with a conventional
anti-reflection material to minimize reflection losses.
The infrared light which is transmitted through
mirror 50 strikes internal heat dissipator 55, which
converts the infrared light into heat. Heat dissipater
55 is provided with holes which serve as the exhaust for
~~~8~~~~
' -12-
cooling air drawn into the il7.uminator housing by a fan
14 (illustrated diagrammatically in Figure 2) through
holes 9 and foam filter 15. The passage of cooling air
through the holes in heat dissipator 55 removes the heat
generated in heat dissipator 55.
The bulb 10 produces a relatively large amount of
light. At such power levels even a small percentage of
infrared radiation would burn the patient if not
virtually totally eliminated. The above design ensures
that the patient is tr~aated with high-powered light which
is virtually free of infrared components.
The fan 14 cools the optics and the housing for the
optics. Foam filter 15 on the input side of the fan
minimizes the accumulation of particles on the optical
elements. Internal tunnels and baffles (not shown)
direct cool. air to the high temperature areas and prevent
the temperature of the housing itself and the exhaust a.ir
from exceeding 30°C over ambient temperature. A
temperature sensor (not shown) ensures that the fan
operates when the illuminator is hot.
Medical instrumentation which uses light can cause
eye damage if maximum radiance levels are exceeded and
can cause hyperthermia if maximum radiant flux density
levels are exceeded. The invention ensures that there is
no danger from either. The maximum radiance at any
position is approximately 5 W/[cm2*Sr], which is more
than an order of magnitude lower than ophthalmologic
lamps. The maximum flux density at any position is 100
mW/cm2, which is low enough to prevent hyperthernia.
If a bright, very small spot source on the order of
a few millimeters (e. g., a Xe lamp) were used with this
optical layout, levels of optical radiation intensity
would occur in some regions outside of the illuminator
which would be dangerous to both the operator and the
patient. This illuminator is specifically designed to be
used with a large, extended light source such as a
filament. This type of danger also exists with 7_aser
-13-
sources unless the illuminator is arranged to diffuse the
beam without losing too much radiation.
The illuminator 100 outputs between 55 and 65 mW/cm2
when the spot diameter is 10 cm and between 17 and 20
mW/cm2 when the spot diameter is 20 cm. Because the
total light throughput is constant, the intensity is
inversely proportional to the square of the illuminated
spot diameter. At the same time, the distance of the
most uniform illumination area from exit window 78 varies
with the spot diameter. The following Table 1 shows the
optimum distance between the patient and the exit window
78 to achieve the most uniform illumination at the
patient as a function of spot diameter.
TABLE 1
Optimum Illuminator -° Patient Distance
for the Most Uniform Illumination
Illuminated Distance Between
Diameter (cm) Illuminator and
Patient (em)
10 52
11 53
13 57
15 61
17 66
20 76
The following Table 2 sets forth detailed
construction data for one implementation of the
invention. This implementation delivers 7 W (Watts) of
power in a 10 cm spot at a distance of 52 cm.
~~~~~J~
-14-
TABLE 2
Detailed Construction Data
Bulb 10
Power 1000 W
Filament Length 18 mm
Filament Width
Power 600-700 nm 47 W
Mirror 20
Radius 60 mm
l0 Thickness at the center 4
Lens 30
Distance from Filament 25 mm
Diameter 58 mm
Focal Length 39 mm
Thickness 27.5 mm
Lens 40
Distance Between Lens 30 and Lens 40 5 mm
Diameter 89
Length Focal 308 mm
Center Thickness 8.4 mm
Edge Thickness 3
Filter 45
Distance From Lens 40 10 mm
Thickness 4-6 mm
Refraction Index 1.47
Square Side 100 mm
Filter 50
Distance to Lens 40 100 mm
Thickness
Refraction Index 1~47
Height 116 mm
Angle of Normal to Beam Center 40°
Width 151 mm
-15-
Filter u0
Path Distance From Lens 40 170 mm
Diameter 139 mm
Fresnel Lens 72
Path Distance from Lens 40 310 mm
Focal Length 354 mm
Diameter 226 mm
Thickness 2~8
Fresnel Lens 74
Distance Between Lens 72 and Lens 74 5 mm
Focal Length 610 mm
Diameter 226 mm
Window 78
Distance Petween Lens 74 and Window 78 5 mm
Diameter 226 mm
Thickness
To prevent damage due to thermal stress in the
situation where the illuminator is unplugged while it is
operating at full power, the optics and filters near the
bulb 10 are made of heat resistant low-thermal-expansion
glass and are mounted with high temperature plastic
fasteners or high temperature flexible adhesive to
minimize thermally induced mechanical stresses. The
acrylic components, i.e., filter 60 and Fresnel lenses 72
arid 74, have a long term maximum operating temperature of
80°C arid a short term maximum temperature of 96°C. To
ensure that these temperatures are not reached even when
the lamp is unplugged while in operation, these
components are mounted fax from the bulb and are
protected by internal baffles. Thus, even the heat soak
from an unplugged system will not raise the temperature
of the acrylic components above their allowable limits.
Although the invention has been described above by
reference to certain preferred embodiments of the
invention, the invention is not limited to the preferred
embodiments set forth above. Other designs, variations,
--,
-16-
applications, and modificatians will occur to those
skilled in the art after receiving the above teachings.
By way of example, it is understood that the
arrangement of the filters can be varied. For example,
light could first be passed through a reflecting dichroic
mirror (e. g., mirror 50), then through a long pass bulk
filter (e. g., filter 60), then through a transmitting
dichroic filter (e.g., filter 45). Or, light could be
passed through a long pass bulk filter (e.g. , filter 60) ,
then through a transmitting dichroic filter (e. g., filter
45) , and then through a reflecting dichroic mirror (e.g. ,
mirror 50). The Fresnel lenses can be replaced with a
standard glass lens or lenses. A collimated beam may be
acceptable in certain applications even though use of a
collimated beam would result in a less uniform radiation
pattern and a worse depth of focus. Alternatively, the
illuminator could be used to direct light of a wavelength
that causes the drug to fluoresce and then be detected by
conventional means. The scope of the invention is
therefore defined by reference to the following claims.