Note: Descriptions are shown in the official language in which they were submitted.
WO92/19537 2-lo8q8~ PC~/CA92/~)l~t
,: ., .
Method for the Control of Sodium Oxala~e Levels in Sodium
Aluminate Solutions
Technical Field
This invention relates to a method for the removal o~
5 organic substances from a sodium aluminate solution in the
production of alumina from bauxite using the Bayer
process. More particularly, the invention relates to a
method for the efficient removal of sodium oxalate in the
sodium aluminate solution of the Bayer process.
Backqround Art
As is well known, the production of alumina by the
Bayer process comprises the steps of treating aluminous
ores such as bauxite (hereinafter referred to as bauxite)
with a hot caustic solution, such as a caustic soda
15 solution at a temperature generally above 130-C, to
extract the alumina portions contained in bauxite (the
digestion step); separating undissolved residues such as
iron oxide, silicates, titanium oxide and the like as red
mud from the slurry obtained in the digestion step (the
20 red mud sedimentation step); adding particles of aluminum
hydroxide as seed to a clarified sodium aluminate solution
after the separation of the undissolved residues
(hereinafter referred to as the Bayer solution) to
precipitate aluminum hydroxide at a temperature generally
25 between 85- and 50 C. (the precipitation step); separating
the precipitated aluminum hydroxide from the sodium
aluminate solution (the separation step): recycling a
portion of separated aluminum hydroxide precipitate as
seed to the precipitation step; and withdrawing the
30 remaining portion of separated aluminum hydroxide
precipitate as product, while recycling the sodium
aluminate solution after the separation of aluminum
hydroxide precipitate ~hereinafter referred to as the
spent liquor), as it is or after evaporation, to the
35 digestion step for bauxite.
The process solutions used in the Bayer process
contain among other constituents large concentrations of
sodium hydroxide, some sodium carbonate, and dissolved
W~92/19537 2 ~ Pcr/cAg2/ool8~
, . . .
alumina in the form of sodium aluminate. Solutions o~
sodium aluminate that are ~upersaturated at the
precipitation temperatures are re~erred to as pregnant
liquors or solutions; those of lower alumina c~ncentration
5 are the spent liquors or solutions. The concentrations o~
dissolved alumina and total caustic are usually expressed
in terms of the alumina/caustic ratio, in which the
alumina is expressed in g/L of Al203, while the caustic is
expressed as g/L of equivalent Na2CO3. Usually, pregnant
lO liquors are characterized by an alumina/caustic ratio
between 0~55 and 0.75, and spent liquors by an
alumina/caustic ratio between 0.25 and 0.50.
The starting bauxite contains various organic
substances and these tend to dissolve in the sodium
15 aluminate solution in the digestion step for bauxite.
Also, flocculating agents such as starch or a high
molecular weight coagulant, such as sodium polyacrylatè,
may be added to the slurry solution obtained in the
digestion step. As a result, organic substances gradually
20 accumulate in the circulated sodium aluminate solution of
the Bayer process or in the spent liquor of the Bayer
process.
The organic substances in the Bayer solution are
present in various forms and include sodium oxalate.
25 Among such organic substances, those having a low
solubility in the sodium aluminate solution of the Bayer
process, e.g. sodium oxalate, precipitate as crystals and
are attached to the surface of the aluminum hydroxide in
the precipitation step. When such aluminum hydroxide with
30 sodium oxalate attached is recycled as seed to the
precipitation step, the growth of aluminum hydroxide
crystals is hindered, so that aluminum hydroxide
precipitate having a large grain size and resistant to
particle breakdown (attrition) is very difficult to
35 produce. Also, aluminum hydroxide withdrawn as product is
contaminated with sodium oxalate, resulting in a lowering
~ of the purity. Moreover, the accumulated organic
WO g2/19~37 pcr/cAs2/ool 8X
~.~ 0 ~ 7~vl
substances hinder the sedimentation of red mud and the
separation of precipitated aluminum hydroxide ~rom ~odium
aluminate solution, thereby significantly lowering the
efficiency of alumina production.
In order to remove organic substances, such as sodium
oxalate, from aluminum hydroxide withdrawn as product or
aluminum hydroxide used by recycling as seed for the
precipitation step of the Bayer process, various methods
have been proposed.
U.S. Patent 3,372,985 describes a procedure in which
all of the alumina hydrate seed is filtered and washed in
two stages. In the second stage of washing, sodium
oxalate is dissolved in the washing liquor and removed,
and then caustic soda is recovered by treating the washing
15 liquor. But, as this method filters and washes great
quantities of solids, it requires equipment of large scale
and a vast volume of washing liquor.
U.S. Patent 3,899,571 teaches the addition of seed
crystals of sodium oxalate to promote the precipitation of
20 sodium oxalate from a spent Bayer liquor solution in which
the sodium oxalate solution is supersaturated. Prior to
use, these seed crystals are physically separated from the
slurry in which they are produced, and subjected to a
washing step to restore or improve their effectiveness as
25 seed. This is an uneconomical procedure with the washing
step requiring large scale equipment.
U.S. Patent 4,201,749 teaches a process to remove the
oxalate containing mother liquor surrounding the desired
aluminum hydroxide product. It was discovered that this
30 could be done by washing the product with first a solution
concentrated in sodium oxalate to remove the sodium
aluminate and second with hot water to dissolve the
oxalate retained by the aluminate hydroxide.
U.S. Patent 4,263,261 teaches the recycling of the
35 precipitated products as seed crystals to increase the
removal of dissolved sodium oxalate. This procedure is
distinguished by the step of dissolving 10 to 50% of the
WO92/19537 l'~l/CA92/(ol##
newly precipitated product in an unsaturated aqueous
solution of sodium oxalate to remove the impurities whlch
have been coprecipitated with the sodium oxalate, be~ore
using the remaining washed crystals as recycled seed in
5 the next cycle of the process.
Australian Patent Publication No. Au-A-35943/89,
published July 12, 1989, (Brown) describes a process for
removing sodium oxalate by adding sodium oxalate seed to a
spent liquor and causing the sodium oxalate to
10 precipitate. However, in order to do this, an additional
amount of suitable organic polymer must be added to the
saturated sodium aluminate solution to ensure that the
sodium oxalate does not precipitate during the
precipitation of aluminum hydroxide.
British Patent Publication No. 2,211,832, published
July 12, 1989, (Keeney et al) describes the removal of
sodium oxalate by crystallization in the presence of seed
crystals of spherulite morphology. This is achieved by
precipitating the seed crystals from a solution containing
20 a substance that causes the sodium oxalate to crystallize
in spherical form. The seed crystals of sodium oxalate
are added to the solution as a solid.
When sodium oxalate precipitated from spent liquor is
recycled as seed to precipitate sodium oxalate, there is
25 the disadvantage that the sodium oxalate seeds lose their
activity as seed during recycling due to the adsorption of
organics present in the Bayer solution. Washing with
water to reactivate the seed is not satisfactory because
this cannot be done without dissolving part of the oxalate
30 seed itself. As a result, it is difficult to maintain low
; oxalate levels in the treated solution and oxalate
precipitation can then occur in other parts of the
precipitation circuit with resulting detrimental effects.
It is the object of the present invention to find a
35 new and simplified procedure for solving the above
problems.
.
W092tl9537 2 ~ O ~ J ~ 5 Pcr/cAg2t~lxx
- 5
Disclosure of the Invention
The present invention solves khe problem o~
reactivating oxalate seed by avoiding the need to recycle
the oxalate seed. Instead, the present invention utilizes
5 a highly concentrated solution o~ dissolved oxalate to
precipitate new and very reactive seed. This new and
reactive seed is added to a process liquor or solution
circulating through the Bayer process and thereby
precipitates out any remaining dissolved oxalate, which is
10 then separated and the clarified liquor is returned to the
process. The result is low oxalate concentrations in the
liquor and, therefore, precipitation of oxalate in other
parts of the precipitation circuit can be avoided. In a
particular application of this invention, the coarse
15 aluminum hydroxide seed can be kept free from oxalate
contamination which would promote excessive nucleation of
alumina hydroxide fines.
Precipitation of sodium oxalate will occur when its
concentration is greater than that required for
20 autonucleation. The autonucleation concentration is a
function of temperature, concentration of stabilizers,
such as dissolved humates, organic compounds and polymers,
and the concentration of caustic. However, the
autonucleation concentration for most Bayer process
25 liguors is between 3 and 7 g/L sodium oxalate depending
upon the above variables, and generally around 4-5 g/L of
sodium oxalate.
Thus, the present invention relates to a process for
removing sodium oxalate from a sodium aluminate liquor
30 produced through bauxite digestion using the Bayer
process, the liquor including aluminum hydroxide and
sodium oxalate therein. The sodium aluminate liquor
enters an aluminum hydroxide precipitation circuit for
removing the aluminum hydroxide, leaving a spent liquor,
35 which is typically recycled to the bauxite digestion step.
The sodium oxalate removal procedure of the present
invention comprises the steps of (a) contin.uously
'' .
. . .
W092/19~37 PCI/CA92/0018
~1Q~!iQ ~ 6
generating new and reactive seed particles of sodium
oxalate by mixing together an aqueou~ solution o~ sodium
oxalate and a portion of a Bayer procesc sodium aluminate
liquor to achieve a concentration of sodium oxalate within
5 a range to generate spheroidal particles of sodium
oxalate, (b) adding a suspension of the above spheroidal
particles of sodium oxalate as seed to a second portion of
the Bayer pr~cess sodium aluminate liquor, to thereby
precipitate the remainder of dissolved sodium oxalate and
10 (c) separating the sodium oxalate precipitate, leaving a
liquor free from sodium oxalate contamination.
The Bayer process sodium aluminate liquor to which
the present invention relates may be any sodium aluminate
solution or liquor of the Bayer process, but is usually a
15 spent liguor from the final stages of classification and
typically has an alumina/caustic ratio (Al20JNa2CO3) of
about 0.25 to 0.50. The spent liquor may be entirely free
of aluminum hydroxide particles, or may include suspended
aluminum hydroxide particles which were not removed during
20 classification and filtration.
As stated hereinabove, autonucleation concentration
for most Bayer process liquors is between about 3 and 7
g/L sodium oxalate. However, it has been found according
to the present invention that there is concentration range
25 of sodium oxalate above this at which spheroidal particles
are generated. Typically, the concentration should be at
least about 5% above the autonucleation concentration.
There is also an ùpper limit beyond which spheroidal
particles are no longer produced and fine needles are
30 formed which are difficult to filter. Thus, the maximum
concentration is in the order of about 75% abave the
autonucleation concentration. However, it is generally
preferred to operate in the range of about 10 to 25% above
the autonucleation concentration.
The best results are obtained with the process of the
present invention when the freshly prepared seed particles
of sodium oxalate are used directly as seed without any
W092/19537 2 ~ O ~, 7 ~ ~ PCI/CA92/OU18X
in~ermediate storage or any intermediate treatment, ~uch
as washing.
The spheroidal particles o~ sodium ox~late may be
conveniently separated from their production ~olution by
5 filtration, sedimentation, hydrocloning, etc. to ~orm a
- concentrated suspension. This concentrated suspension,
without any kind of intermediate treatment, is fed as seed
directly into the Bayer process liquor. Alternatively,
the entire seed production suspension, including
lO spheroidal particles of sodium oxalate and their mother
liquor may be added directly to the main stream of Bayer
process liquor.
While the benefits of the present invention can be
achieved by adding the sodium oxalate seed crystals to
15 spent liquor circulating through any of the stages of the
Bayer process, it is particularly effective at the
tertiary classification stage, and at the final
precipitation stages.
It is also preferable to utilize a two stage washing
; 20 to separate the sodium oxalate for further treatment.
Brief Descri~tion of the Drawinqs
The invention is described with respect to the
attached drawings in which:
Figure l is a schematic flow diagram showing the
25 relationship of the oxalate removal system of the
invention with respect to the precipitation and
classification system of the Bayer process, and
Figure 2 is a schematic flow diagram showing an
alternative procedure.
Best Modes For CarrYina Out The Invention
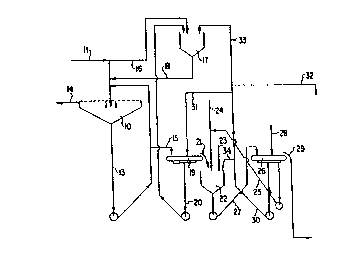
As shown in Figure l, the numeral lO represents a
tertiary classifier of a traditional Bayer process with a
Bayer liguor feed ll of fine aluminum hydroxide seed and
spent liquor from a previous stage. An underflow stream
35 13 is withdrawn from the bottom of classifier lO and is
recycled to feed line ll. Spent liquor is drawn off at
14.
WO92~l9537 PCI/CAg2/0~1~
~ 8 r~
A part of recycle stream 13 is drawn o~ via line 15
and is fed to a primary seed filter 19. This ~ilter ls
designed for efficient washing of a ~ilter cake. The
filter 19 is washed by a wash stream 31 which compri~e~ a
5 filtrate from secondary seed filter 26. This wash i5 rich
in oxalate and low in soda and removes the sodium
aluminate liquor retained by the filter cake without
removing oxalate crystals. The primary filtrate 20 is fed
as a component to oxalate seed generating vessel 17.
The primary filter cake 21 from primary filter 19 is
transferred to reslurry tank 22 and agitated for a period
of 1/2 to 1 1/2 hours where it is reslurried in a stream
of secondary filter wash filtrate 25 plus, if necessary,
hot condensate make up 23, the flow of which controls the
15 concentration of sodium oxalate in the reslurry liquor.
The reslurry tank level is maintained by a draw off pump.
The wash filtrate 25 is heated to 65-100~C by steam 24
whereby solid sodium oxalate present in the vessel 22 is
dissolved. The concentration of aluminum hydroxide solids
20 in the reslurry tank 22 is normally uncontrolled, but may
be adjusted separately from the oxalate concentration or
by the use of the strong filtrate recycle 34 from
secondary filter 26. Normally this is necessary only
during start up of the process.
After a sufficient residence time, a flow of slurry
27 is withdrawn from the bottom of reslurry tank 22 and is
filtered by the secondary seed filter 26. This filter is
also adapted for washing a filter cake.
In filter 26, the oxalate rich liquor in the filter
30 cake is displaced by a hot condensate wash 28. It is
advantageous to take two filtrates from filter 26. The
strong filtrate 30 is used in streams 31, 32, 33 and 34.
The more dilute later wash filtrate 25 is in part recycled
; to reslurry tank 22 via line 25 and the washed filter cake
35 29 is used as fine seed in the main aluminum hydroxide
precipitation circuit.
The main filtrate stream may be split.in varying
.
, .
WO92/19537 PCT/CA92/~1XX
2 ~ ~ ~, 7 '~J 5
,.
proportions between streams 25 and 30 for fine tuning o~
the process and stream 33 is fed to oxalate seed
generating vessel 17, and ultimately returns to the
tertiary classifier 10 via line 18. In the reactor 17
5 which may have a short residence time, the oxalate rich
secondary filtrate stream 33 is reacted with Bayer liquor
feed 16 and primary filtrate 20 to produce fresh finely
divided oxalate crystals which move via line 18 to become
part of the feed stream 11 to tertiary classifier 10.
10 These fresh finely divided oxalate crystals effectively
strip the dissolved oxalate from the spent liquor in the
tertiary classifier.
A stream of oxalate rich secondary filtrate 32 may be
directed to the oxalate disposal system the flow rate of
15 which is varied according to how much oxalate should be
directed to 17 and this stream may be treated by any
technique appropriate to the location, e.g. lime
precipitation of calcium oxalate, biological decompo-
sition, etc. In the case of a plant operating on a
20 relatively high silica bauxite, which necessarily has a
large requirement for make up caustic, an appropriate
technique is to salt out the oxalate in a relatively pure
form using concentrated (about 50% w/w) caustic. The
sodium oxalate crystals may then be filtered off, and high
25 caustic filtrate along with some spent liquor is used to
prepare the oxalate seed. The oxalate crystals may be
sold as a by-product, calcined in the liquor purification
unit, etc.
The stream 34, which recycles strong oxalate liquor
30 to the reslurry tank 22, may be used during start up, or
as an additional control on the aluminum hydroxide solids
concentration in reslurry tank 22. The stream 31 is used
as a displacement wash on primary filter 19 to displace
spent liquor from the filter cake 21 whilst leaving its
35 oxalate concentration unchanged.
In the flow sheet shown in Figure 2, the numeral 40
represents a Bayer liquor stream from anywhere in a Bayer
21 V~ ,; PCJ/CA92/()OlX8
10 ' "
cixcuit, preferably a spent liquor after precipitation o~
the aluminum hydroxide. A side stream ~1 oP this ll~uor
is cooled in cooler 42 to a temperature betwe~n 40 and
70~C, preferably 50~C.
The cooled outlet stream 43 i5 divided into two
streams 44 and 45. The stream 44 feeds into a reaction
vessel 47 which optionally connects to a second reaction
vessel 48. The stream 45 feeds into a reaction vessel 46
for the production of sodium oxalate seed.
Also feeding into the reaction vessel 46 is a stream
60 of oxalate-rich solution which has been produced in
vessel 58. The oxalate-rich stream 60 and the process
stream 45 are mixed in vessel 46 in the proper proportions
to produce the required spheroidal particles of sodium
15 aluminate to be used as seed. A stream 49 carries a
suspension of these particles to reactor vessel 47 where
; this comes in contact with stream 44 of process liquor.
The discharge 50 from reactor vessel 48 flows to a
filter 51 with the filtr~te from this filter being
20 recycled to combine with process stream 40 and the filter
cake 53 being deposited on a conveyor belt system 54 which
; can move in either direction. This conveyor belt 54 may
deliver the solids 53 (1) into vessel 47, or (2) into
vessel 58 via discharge 57 or (3) via discharge 55 into
25 disposal area 56.
When the solids product 53 from filter 51 is being
used as solids feed 57 to reactor vessel 58, side stream
61 is operated such that solid product 53 is subjected to
a displacement wash whereby residual spent liquor is
30 displaced by an oxalate-rich stream from discharge 60.
The procedure shown in Figure 2 is of particular
interest for use with Bayer liquor which is free of
particles of aluminum trihydrate. The oxalate-rich stream
60 is used to raise the oxalate concentration in reaction
35 vessel 46 to 0.25-3.0 gpl, preferably 0.5-1.00 gpl over
the critical oxalate concentration in reactor 46. The
autonucleation concentration is, of course, that
- W092/19537 ~ O~r(~ ~ pcr/~As2/
11 ,
concèntration above which sodium oxalate spontaneou~ly
crystallizes from unseeded Bayer liquor at the
temperature, caustic and impurity concentratlon exlsting.
This value is normally about twice the absolute solubility
5 of sodium oxalate under the specified conditions.
Additional preferred embodiments of this invention
are described in the following non-limiting examples. In
these examples the spent Bayer liquor contains:
Caustic, (expressed as Na2CO3) 265 g/L
1o Alumina 111 g/L
Sodium Oxalate (NazC204) 3.2 g/L
Temperature 50~C
The oxalate washing solution contains:
Caustic (expressed as Na2CO3) 10 g/L
Sodium oxalate (Na~C204) 30 g/L
Temperature 50'C
Exam~le 1
100 mL of oxalate washing solution was added at the
rate of 100 mL/min. to 1000 mL of spent Bayer liquor. The
20 mixture contained 5.64 g/L dissolved sodium oxalate. It
was held at 50 C, and stirred continuously. The
concentration of dissolved sodium oxalate was measured at
intervals, and found to decrease as follows.
Time Elapsed (h) Conc'n Dissolved Oxalate g/L
0 3.2
1/4 1.7
1/2 1.4
1 1.4
2 1.4
There was good removal of dissolved sodium oxalate
from the mixture.
The precipitated sodium oxalate was found to be in
the form of spheroidal particles. Such spheroidal
particles, unlike needle-like particles, are easy to
35 filter or separate by other means from the liquor. It is
also noteworthy that the total concentration of dissolved
sodium oxalate in the combined solution was 5.64 g/L,
~;
W092/19537 ~ P~l/CAg2/~l~
12
which is above the concentration at which autonucleation
and spontaneous precipitation occurs.
Example 2
The same procedure described in Example 2 was
5 repeated, but 200 mL, i.e. double the amount of Example 1,
of oxalate washing solution was added. This mixture
contained 7.6 g/L of dissolved sodium oxalate. The
concentration of dissolved sodium oxalate with time varied
as follows:
Time Elapsed (h) Conc'n Dissolved Oxalate g/L
0 3.2
1/4 1.5
1/2 1.4
1 1.4
2 1.5
Again there was good removal of dissolved sodium
oxalate from the solution. However the precipitated
sodium oxalate was in the form of fine needles, which are
difficult to filter, and are unsatisfactory for easy
20 separation from the liquid.
This Example shows that an increase of the dissolved
sodium oxalate concentration to 7.6 g/L, 90% above the
concentration for autonucleation creates an excessive
amount of nuclei, which results in the precipitation of
25 many fine crystals. This experiment showed that the
morphology of the precipitated crystals is affected by the
concentration of dissolved sodium oxalate at the beginning
of the crystallization.
ExamDle 3
According to the procedure of Example 1, 25 g/L of
oxalate washing solution was added to 1000 mL of spent
Bayer liquor. The resulting mixture contained 3.85 g/L of
dissolved sodium oxalate. The concentration of dissolved
sodium oxalate with time varied as follows:
WO 92/19537 PCr/CAg2/0018~
- 2 ~ ~3 ~ r~
i"~ . .
13
Time Elapsed (h) Conc'n Dis~olved Oxal~te g/L
0 3.8
1/4 3.~
1/2 3.8
1 3.8
2 3.8
There was no removal of sodium oxalate from the
solution. This is because the concentration of dissolved
sodium oxalate was below the concentration at which
10 autonucleation occurs.
Exam~le 4
The same solutions used in Example 3 were again
reacted, but in two steps.
In the first step, 25 mL of oxalate washing solution
15 was added to 250 mL of spent Bayer liquor. The
concentration of dissolved sodium oxalate was 5.64 g/L.
The combined solution was held at 50 ' C for 15 minutes with
stirring.
In the second step, the remaining 750 mL of spent
20 Bayer liquor was added to the solutions prepared in step
l. This gave a solution in which the total sodium oxalate
concentration was 3.~5 g/L, equivalent to that of Example
3. Thè variation of dissolved sodium oxalate
concentration with time was as follows:
Time Elapsed (h)Conc'n Dissolved Oxalate g/L
0 3.2
1/4 2.6
1~2 2.4
1 2.3
2 1.5
Under these conditions, there is good removal of
dissolved sodium oxalate. The precipitated crystals were
~pheroidal in shape, and filtered easily.
The difference between Examples 3 and 4 is that in
35 the first step, the concentration of dissolved sodium
oxalate at 5.64 g/L was sufficient for autonucleation to
occur sc that there were nuclei on which crystals could
,~
:':
.; .
W092/19537 PCT/CA92/~1
~ g 3 14
grow during precipitation.
In the second step, the addition o~ more ~pent liquor
reduced the sodium oxalate concentration below the ox~late
concentration required for autonucleation. Neverthele~s,
5 crystallization continued, because nuclei were already
present in the combined solutions.
This examples demonstrates that nuclei must be
present in order to precipitate easily filterable
crystals.
Example 5
The oxalate precipitate obtained in Example 4 was
filtered from the liquor. The calculated weight of the
sodium oxalate solids was 2.4 g.
This wet unwashed precipitate was added to 1000 mL of
15 spent Bayer liquor. The mixture was held at 50-C with
stirring for two hours. The concentration of dissolved
sodium oxalate at the end of the time was 2.9 g/L, only
0.3 g/L lower than the initial concentration.
This example showed that aged sodium oxalate crystals
20 present at about 2.4 g/L and which had been precipitated
on a preceding experiment and separated by filtration,
were not as effective in precipitating the dissolved
sodium oxalate as the freshly prepared sodium oxalate
crystals.
ExamDle 6
Another batch of sodium oxalate crystals were
prepared according to the procedure of Example 4. These
crystals were washed thoroughly with 300 mL of cold water,
in order to remove any occluded liquor and impurities.
30 The calculated weight of the washed sodium oxalate was
2.40 g.
This washed seed was added to 1000 mL of spent Bayer
liquor, according to the procedure described in Example 5.
The mixture was held at 50 C with stirring for two hours.
35 At the end of this time, the concentration of dissolved
sodium oxalate was 2.7 g/L. This concentration is higher
than that obtained in Example 4, and indicates less
: .,. ~ , , ,~
;.
W092~19537 2 ~ I'C~/C~92/001~
.~ . . .
removal of oxalate occurred.
This example shows that water washed aged sodlum
oxalate is not as effective a seed for precipitating
dissolved sodium oxalate as ~reshly prepared sodium
5 oxalate seed.
,
.~' '
;. .