Note: Descriptions are shown in the official language in which they were submitted.
WO 92/19682 PCr/~JS9~1~)3671
~90~9
i BLENDS OF COPOLY(ARYLENE SULFIDE) AND
.
The invention relates to blends of a copoly(arylene
sulfide) and a polyamideq
~ Poly(arylene sulfide) resins are thermoplastic
polymeric materials with good thermal stability, unusual
: insolubility, resistance to chemical environments and
in~erent flame resistance. Poly(arylene sulfide) resins
additionally h~ve ~ood electrical insulative properties
which make them ideal f~r electrical and electronic
applica~ions. Their:exGellent resistance to chemical
degradation makes them ideal for use in chemical
environments which in~olve organic solvents ~nd strong
mineral acids, such as~;coatings for pipes, tanks, pumps and
l5~ ~;oth~r equipment. These polymers can be prepared by reacting ~;
p-dichloro-benæene with~sodium sulfide in a polar organic
sol~ént to produce poly~pheny~ene sul~ide) and the by-
product sodium chloride~in accordance with U~.S. 2,513~188
and~U.S.~2,538,941. ~An improvement on:this procedure
2;0~ involv~s ~ddin~ M-haloamides as:catalysts.
Recently:copoly(arylene sulfides) have been discovered.
Thes~ polymers can be described as having repeating units
correspondin~ to t~e:~tructure
:::
[ ( A--S ) l_X (--A--S--S~) x ] n
wh rein x is in th~ range of 0.5 to 0~001, A is a divalent
: : aromatic radical and n is at least 200 and- is preferably in
the range of 500 ~o 5,000.
It has:now bee~ disco~ered that the rate at which the
copoly(arylene sulfide) crystallizes can be increased by
: :
:
WOg~/19682 P~T/US92/03671
~39~79
blending a polyamide with the copoly(arylene sulfide). This `~
blend can be broadly described as an admixture of
::
(A~ from 99.5 to 50 weight percent, based on the
weight of the admixture, of a copoly(arylene
sulfide) corresponding ~o the structure -~:
[ (--A--S~ x (--A--S--S--) x ~ n
~:~
wherein A is a divalent substituted or
unsubstitu~ed aromatic radical, x is in the range
of 0.5 to 0.001 and n is at least 25, and
~B) from 0.5 to 50 weight percent, based on the weight
of the admixture, of two particular types of ~-
polyamides.
:~ .
: The copoly(arylene sulfide) polymers useful in this
invention are identical to the copoly(aryiene sulfide) ::
polymers disclosed in U.5. 4,786,713 and U.S~ 4,855,3g3,
: hereln incorporated by reference, PXGept that the minimum
~alue of n of the copoly(arylene sulfide) polymers useful in
this invention is lower than the minimum value of n for the
cop~ly(arylene sulf~de) p~lymers which is disclosed in these
references. The copoly(arylene sulfide) polymers useful in
this in~ention are therefore inherent in the disclosure of
hese references because as the molecular weight builds up
toward the miminum value of n of at least ~00 which is
disclosed in these references the molecular weight passes
through a molecular.weight associated wi~h the lower minimum
: ~alue of n o~ 25 o~ the cop~ly(arylene sulfide) pol~m~rs of
this invention. The copoly(arylene sulfide) polymers use~ul
i~ this invention can be prepared by those skilled in the
art by following the teachings of these references and
controlling the stoichiometery, time, temperature and other
WO92/19682 PCT/US92/03G71
9 0 7 9
variables of the reaction to achiefe a molecular weight
associated with a value of n which is at least 25.
The diiodoaromatic compounds which can be utilized to
prepare the copoly(arylene sulfide) useful in this
5 invention, include unsubstituted or substituted aromatics
which have two iodine substituents. Preferred
diiodoaromatic compounds are the diiodobenzenes, --
diiodonaphthalenes and diiodobiphenyls which may be
un~ubstituted or substituted. More preferably the
: I0 diiodoaromatic compounds suitabla for the present invention
" ~
:: : include p-~iiodoben2ene, m-diiodobenzene, p,p'-
diiodobiphenyl, p,p'-diiodobiphenyl, p,p'-diiododiphenyl
ether and ~,6-diiodonaphthalene. Most pr ferably the diiodo ~-
compound is p-dii~dobenzene.
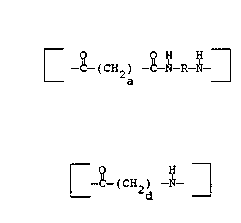
15 :~ The polyamides useful in this invention and the method
of their preparation:~are~ well known in the art. One
:polyamide useful in this invention corresponds to the ~-~
: structure
(CHz) ~ R-~
25 ; ~ wherein
a is~n integer in:the range of 4 to 12 and
i5 preferably 4,
~R corresponds to the structure
:; : (-~H2-)b
30 ; . wherein b is an in~eger in the
range o~ 4 to 13 and is preferably
: ~ 6
:~
:: :
WO~2/196~2 PCT/US92/03671
c~ 19 4
or to the structure
. _ . .
--( CH2 ) -- \ / ( CH2 )
c c- c
wherein
c is 0, l, 2 or 3 and is preferably 0 -~-~
and
n is at least 2S, preferably 50. ~.
Examples Qf these polyamides include
~5 ~ poly(hexamethylene~adipamide, p~ly(hexamethylene -:~
sebac~amide), poly(hexamethylene dodecane diamide),
poly(tridecane biassylamide), and poly(hexamethylene
terephthalamide. In the preferred embodiment wherein a is 4
: and b is 6 the polyamide is often called p~ly(hexamethylene
20~:;adipamide3. In the preferred embodiment wherein a is 4 and :~
is:0 the polyamide is often called poly~cyclohexylene
adlpamide)~
Another polyamide useful in this invention corresponds
; ~ ~
to the structure
(CH2)
wherein~
d~is an integer in the range of 5 to ll,
: : :
~ and is preferably 5,
:; ;
m~is at least 50 and preferably is at
least ~5.
Examples o~ these polyamides include poly~e-
:~ - h : caprolactam); poly(enantholactam), poly(w-undecanaamide)~
: poly(capryllactum), and poly(w-pelar~onamide) 7 In the
:: .
WO92/19682 PCT/US92/03671
21 ~t~û7~
preferred embodiment where d is 5 the polyamide is often
called poly(e-caprolactam).
The blends of this invention can be prepared by known :.
techniques for blending polymers. For example, the polymers
can be coextruded in convention twin screw extrusion
equipment. Also, polymers of both polymers may be admixed
and the admixed powders extruded in a single screw extruder.
Preferably, an admixture of powdered polym~r is prepared and
the admixture powder is extruded in a single screw extruder.
~he amount of copoly(arylene sulfide) is in the range ~--
of 99.5 to 50 weight percent, preferably ~8 to 75 weight
percent, based on the weight of the admix~ure. The amount
of polyamide is in the range of 0.5 to 50 weight percent,
preferably 2 to 25 weight percent, ~ased on the weight of
the:a~mixture.~
The compositions of this invention can be used f~r
preparation of various shaped articles such as pellets,
fib~rs and molded articles. The polymer can be prepared
~ into these shaped articles by conventional processes, such
;~ 20 as injection molding, melt spinning, and melt extrusion.
: The compositions of this invention can additionaIly
contain:fillers, nuc:leating agents and~ reinforcing materials
in~the form~o:f fibers,- minerals, powders or mats. For
examp:le,:the omposi~tions can contain glass fibers, aluminum
25~ oxide, calcium oxide,~silicon dioxid ,~Titanium dioxide,
~: copper, kaolin~ and the like.
:The compositions of this inven~ion are normaIly solid
in the sense that at typical room temperatures and pressures
the compositions are in a solid state as compared to a
liquid:state. The soIid character of the composition
results from ~oth polymers having a~sufficiently high
molecular weight to be~a solid.
The enhanced rate of crystallization of the composition
of this inventi~n is extremely significantO High rates of
crystallizatîon enables crystalline-shaped articles, such as
W092/~96X2 ~C~/US92/03671
21~Y(~79
molded parts, extruded fibers or drawn film, to be more
easily prepared because processing time is saved, thereby
increasing production efficiency.
In this invention the crystallization rate of the
composition of the invention is measured by the difference
in the ~SC transitions of Tcc - Tch, often referred to by
those skilled in the art as the quantity "delta". If two
polymers were blended together one would expect the delta
value of the blend to be the linear weighted a~eragel of the
two component polymers. This relationship can be expressed
: by the equation
,
deltablend - deltal x weight fractionl + delta2 x weight -~
fraction2
Thus, for example, where equal weights of the
5:~: copoly(arylene sulfide) and another polymer are employed, ::~
~ . ~
the resul~ant deltablend would:be expected to be the average
of deltal and delta2.:
: The transitions~Tcc and Tch are defined by heating the
polymer sample in~a Differential Scanning Calorimeter ~DSC)
20~ instrument: at a scan rate of 20C~min. the Tcc is
determined by heating the polymer to a molten state, usually
: 300C and then cooling at 200c~min. The peak of the
exotherm observed is~defined as the Tcc~ The Tch is
.
determined by heating a sample of polymer to the melt again
~: ::25 and quenching the sample snto a metal block cooled in dry
; ice. The thus produced glassy sample is then heated from
room temperature up~to a~melt. The peak of the exotherm
obs rved in this heating sequence is defined as the Tch.
As will be recognized by those skilled in the art,
there are numerous cases where either Tcc or Tch will be: ~: missing in the DSC trace because o~ the polymer system
crystallization rate;being so slow~ This is normally a
result of higher disulfide levels in the polymer. In such
,.
,
WO92/~96g~ PCT/US92/~3671
21~87~
cases, delta becomes undefined and the means of comparison
become the transition that does remain, such as Tch. Thus,
comparing Tch's of the components and the final blend is
also a valid means of assessing the expected transition vs. ;.
the one actually observed for the blend. The equation used
for compari.son is the same as above except substituting Tcc
for delta.
In the examples given below, differential scanning
calorimetry (DSC~ is performed using a Du Pont 951
Thermoanalyzer instrument and employing a scanning rate of
20C~min.
Example 1
This example illustrates the slow crystallization rate
of copoly(phenylene sulfide).
lS Copoly(phenylene-sulfide) was prepared according to the
procedure described in U.S. Patent No. 4,786~713. The
: copoly(phenylene sulfide) had a melt viscosity of about
~ 74,000 poise at 320C at a shear rate of 2~, sec-1. The
:~ ~ ~al~ue of x for the copoly~phenylene sulfide) was estimated
: to b,e about 0.094 based on elemental analysis. The polym~r
: had~a glass transition temperature of about 94C as measured
: by Differential Scanning Calorimetry. The glass transition
temp,erature were determined as follows: ~he sample was
first heated from room temperature to 330 at a heating rage
2~ of ~0C~min (first heating cycle). The sample was held at
330C ~or one minute and subsequently quenched to room
temperature at 320OC~mln. In the second reheat cycle, it
was heated at 20Oc~min to 330C, held for one minute at
330C, and then cooled at 200C~min. The temperaturç of
crystallization, Tch, was obtained from the exothermic peak
temperature during the second reheat cycle. The temperature
of cryst~ zation upon cooling, Tcc was obtained during the
cooling scan from the melt. The copc,ly~phenylene sulfide)
has a melting polnt of 265C as measured by 1st cycle
,
W092/196~2 PCT/US92/03671
~ 9 ~,3 r~ ~3
heating scan on DSC. The copoly(phenylene sulfide) did not
show any significant crystallization exotherm during second
heating scan or subsequent cooling scan from the melt, thus
establishing that the copoly(phenylene sulfide) has a very
: 5 slow crystallizing material.
:`
Exam~le 2
: This example illustrates the enhanced rate of
crystallizati~n of the cvmpositions of this inventio~ :
composed ~f copoly(phenylene sulfide) and poly(hexamethylene
adipamide).
Powder of the copoly(phenylene sulfide) prepared in
Example l~was admixed with powdered poly(hexamethylene
adipamide), to make a blend containing 80% by weight
copoly(phenylene sulfide~ and 20% by weight o~
I5 poly(hPxamethylene adipamide). The admixture was dried at
90~ for 12 hours in a for~ed air-circulated oven. The
polymer admixture was extruded thru an extruder. The
temperature at th~ end of the die was kept at about 300C
and;~he rpm of the screw was about 90. The ex~ruded
2~0 material was analyzed by DSC for thermal transitions. The
blended material had:a Tch (temperature of crystallizati~n
during seco~d heating)~::of about 112C. DSC experiment was
performed as follows~. The sample was:first heated from room
: temperature to 330C at:a heating rate of 20C~min (first
5~ heating cycle)~ The sampIe was held at 330C for one minute
and subse~uen~ly ~uenched to room temperature at 320~C~min.
In the second reheat cycle, it was heated at 20C~min to
: 330C~ held for`one minute at 330C, and then cooled at :~
:20~c~min. The temperature of crystallization, Tch, was
obtained from the~exothermic peak temperature~:;during the
second r heat cycle. The temperature of crystallizati~n
upon cooling, Tcc was obtained during the c~oling scan ~rom
the melt. Tcc for the blend was about 222C. These data
illustrate that the ompositions of this inve~tivn exhibits
:
WO92/19682 PCT/US92/03~71
~lo!~n7~ ;
an enhanced rate of crystallization composed to that of only ~.
copoly(phenylene sulfide).
xamPle 3
This example illustrates the faster crystallization
rate of the composition of this invention.
Example 2 was repeated except that the amount of
poly(hexamethylene adipamide) was 5~ by weight. The melt
blended material h~d a Tch of 130.3C and Tcc of 202.5C.
The crystallization rate is much higher as compared to 100%
copoly(phenylene sulfide~ of Example l.
Example 4
This example illustrates the faster Grystallization
rate of the composition of this invention.
Example 2: is repeated except that ~he amount of
poly(hexamethylene adipamide) is reduced to 1% ~y weight.
The melt blended material had a Tch of 134C and tcc of
208C. These data establish that the compositio~ of the
: ~ invention exhibits an enhanced rate of crysta1liza~ion
: compared to~100% copoly(phenylene sulfide) of Example l.
2~0 ~Exam~Ie 5
: This example illustrates the faster crystallizati~n
;rate of the composition of this invention.
: :Example 2 i5 repeated @xcept that the amount o~
: : poly~(hexamethy-ene adipamide) is reduced ~o 0.5% by weight.
The melt blended material had a Tch of 167C and Tcc of
153.4C. These data establishes that the composition ~f
;this invention exhibit an enhanced rate of crystallization
compared to 100% copoly(phenylene sulfide) of Example l.
WO92/19682 PCT/US92/03671
210g~79
Exam~le 6
This example illustrates the faster crystallization
rate of the composition of this invention comprises
copoly(phenylene sulfide) and poly(e-caprolactam).
Five weight ~ poly(e-caprolactam) was melt blended with .
95 weight % of the copoly(phenylene sulfide~ used in Example
1. DSC transitions showed a Tch of 123C and Tcc of
lg5.7C. These data establish a much faster rate of
: : crystallization of the composition of the invention than
~:: 10 that for 100% copoly(phenylene sulfide) of Example 1.
:~ .,
Example 7
This example illustrates the faster rate of
crystallization;of the composition of this invention.
~ : 5 wt ~ of poly(w-undecaneamide) was melt blended with
: : lS 95 wt % of copoly(phenylene sulfide). The procedure was the
: ~ same as in Example 2.~ DSC transitions showed a Tch
: (temperature of crystalli~ation upon heating) of lll~C and
Tcc (temperature of crystallization upon cooling) of 206C.
~ This again illustrates faster crystallization behavior of
:~ ~ 20 copoly~(phenylene sul~ide) in the blend.
: :
:~::: :
'~