Note: Descriptions are shown in the official language in which they were submitted.
WO92/214~ ~ P~T/US92/04746
Devices and Methods For_Self~Contained,
Controlled-Release Mixinq
This application is a continuation in part of App.
Ser. No. 711,621 fîled June 5, 1991, from which priority
is claimed.
Field of the Invention
This invention is in the field of devices and methods
for the self-contained, time-released mixing of a liquid
solution. In particular, it relates to the mixing of a
reagent immersed in an aqueous solution in which the aque~
ous solution must be physically agitated to dissolve or
10 disperse said reagent within the aqueous solution.
Background of thç~ çn~i~n
Effervescence has been utilized for many years to
promote the mixing of a reagent within a solution. The
known methods, however, have several shortcomings, all of
15 which are overcome by the teachings of this invention.
Mixing is accomplished by the liberation of gas within a
solution from an element or chemical compound without the
application of heat. The effervescent chemical compound
is typically in the form of a tablet, which reacts with
~ 20 water to liberate carbon dioxide, and randomly floats
! throughout the solution as effervescent bubbles are
j ! liberated. As a result of random movement of the tablet,
dense reagents which are to be dissolved or dispersed in
the solution, remain at the bottom of ths solution con-
25 tainer and are never properly dissolved or dispersed.
Accordingly, there is a need for controlling of mixing
wit~ ef$ervescent bubbles.
~ The use of pure effervescent powder does not permit
3 one to control when the liberation of effervescent bubbles
~ 30 is to commence. Often, the reagent to be dissolved or
WO92/21434 PCT/US92/0~746
6~
dispersed takes the form of a lyophilized reagent which
has an associated reconstitution time. Many mixing
scenarios require that the lyophilized product partially
reconstitute before the effervescent mixing begins.
Another important control feature when u~il~izing pure
effervescent powder is the rate at which effervescent
bubbles are liberated. Uncontrolled bubble liberation
results in a foaming action which is detrimental to
complete mixing of most any solution system.
More recently, methods have been demonstrated whic~
control the rate at which reagents are delivered to a
solution system. In particular, European Patent Applica-
tion number 81401738.0 (Havey et al.) describes the use of
a solid organic binder carrier which is soluble or disper-
lS sible in water and which con~ains a measured ~uantity of
a water-soluble dispersible reagent whereby the protected
quantity of reagent contained within the solid organic
binder is released and dissolved concomitantly as the
binder is dissolved or dispersed by the aqueous solution.
In some situations, this method may be a satisfactory
alternative for controlling the rate at which the effer-
vescent powder interacts with water and therefore control-
ling the initiation of both effervescent bubbling and the
rate at which bubbling occurs. However, many common mix-
ing scenarios require that no additional constituents,such as solid organic binders, be dissolved or dispersed
into the reagent solution. Therefore, there exists a
continuing need for a device and methods capable of self-
contained, time-controlled mixing of solutions which does
not rely on the addition of extraneous reagen~s which may
be undesirable or even detrimental to the solution to be
mixed.
Brief Descri~tion of the Drawin~
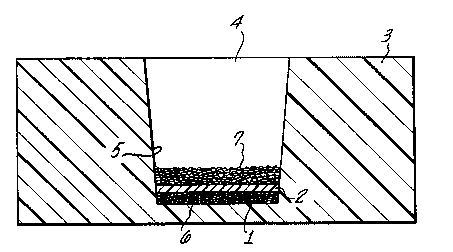
The ~igure is a cross-section of the device for use
: 35 with the present invention. A container 3 may have an
upper opening 4 defined by wall 5 and a bottom 6. The
WO92t21434 2 ~ 1 0 ~ ~ 8 PCT/US92/04746
.
container may be composed of plastic, glass, or other
suitable mat`~rials. As shown in the Figure, added to the
bottom of the container 3 is a thin layer of effervescent
reagent 1 and a solid, porous element 2 which is in
physical proximity to the effervescent reagent 1. The
diameter of the solid, porous element 2 is such that a
water-tight interference fit is made with wall 5 of
container 3.
The reagent 7 to be dissolved or dispersed in the
aqueous liquid sample is typically added to the upper
surface of the solid, porous element 2. It should be
noted that generally, the p~re size of element 2 will be
smaller than the particle size of reagent 7 so as to mini-
mize the loss of the reagent to the underside of element
2 through the pores of ~. It should also be noted that
the porous element has been modified to exhibit a pre-
determined des~red surface hydrophilicity so as to control
the initial bubble formation, size of the bubbles, and
rate of bubble production. A liquid sample containing
water, such as blood, serum, or urina, would then be added
to container 3 by introduction through container opening
4. After the liquid sample permeates element 2, either by
capillary action-or by hydrostatic pressure, it contacts
the effervescent reagent 1 and chemically reacts forming
effervescent bubbles. As bubbles form, they begin to
migrate through the pores of component 2 to the upper
surface of 2, achieving a dynamic state with the movement
of the liquid in the oppos~ite direction, from the upper
surface to the lower surface of component 2 through the
pores of component 2. The movement of effervescent
bubbles through the fluid in contact with component 2
mechanically agitates reagent 7, dissolving or dispersing
reagent 7 throughout the solution. After a suitable
period of time, reagent 7 will have completely dissolved
or been dispersed in the liquid sample without the aid of
additional outside mechanical intervention.
.
WO92/21434 PCT/US92/0~7~6
2 i 1~8~ --
summary of the Invention
The present invention is devices and apparatus for
mixing an aqueous solution, having three major components:
: a. reagent capable of libera~ing gas ~ he form of
effervescent bubbles without the application of heat;
b. porous means of predetermined surface hydro-
philicity comprising pores generally transverse in a
controlled manner thereto, so as to permit li~uid or gas
to transverse therethrough in a controlled manner when
said aqueous solution is added to said porous means;
c. reaction well of sufficient volume to containsaid aqueous solution, said reagent and said porous means,
positioned such that upon addition of said aqueous solu-
tion, said aqueous solution permeates said porous means,
.~ l5 contacts said reagent, resulting in the formation of
effervescent bubbles which are released through said
porous means to mix said aqueous so1ution.
, The pxesent invention provides a device and method
for the self-contained, time-controlled mixing of an
aqueous solution by the controlled release of effervescent
~ bubbles without additional other solid organic binders
!~, which can dissolve or disperse in the solution with poten-
tial adverse effects. In general, the teachings of this
invention are applicable for use in all liquid~liquid and
liquid/solid solutions where it is desired to control the
mixing of solid reagents by dissolution or dispersion.
However, there is a particular need for such a device and
method in ~the area of clinical chemical analysis. ~o
illustrate the present invention, it is hereafter
described as utilizod in the field of clinical chemical
analyæis.
Detailed ~escription of Preferred Embodiments
The devices and apparatus of this invention employ a
. powdered reagent which can liberate effervescent bubbles
when in contact with water. In addition, the device has
.~. ~, ,, ~ . . .
-W092/21434 2 ~ 1 0 ~ S i PCT/US92/04746
a porous means, for example, a porous plastic, polyethy-
lene or ceramic disc, an artificial membrane, a filter,
including cellulose, glass fiber and cellular acetate
filters and a screen including plastic an~ metallic
screens which has pores generally transvers~o its upper
and lower surfaces. The surface of the porous means is
modified by plasma treatment or treatment with a surfac-
tant to result in the exhibition of a desired surface
hydrophilicity. Many reagents are naturally hydrophillic,
such as cellulose acetate and glass fiber. Naturally
hydrophillic materials have an affinity for attracting,
adsorbing or absorbing water. On the other hand,
naturally hydrophobi~ materials include polyethylene and
polyproplyene. Such materials have a tendency to repel or
to fail to adsorb or absorp water. The surface
hydrophilicity can be selected or predetermined to exhibit
desired characteristics using a variety of methods as
described herein and which are known to those skilled in
the art. The pores permit a liquid or gas to traverse
from one surface to the other surface of the second
member. The pore size may be microporous, for example in
the preferred embodiment using a polyethylene disc.
Generally, pore size may range from 40-50 mi~rons.
However, a plastic sheet having holes of approximately
.030 inch in diameter may also be used. In a preferred
embodiment, the porous member is located above and in
physical proximity (which-may include physical contact) to
the reagent and serves as a means of both physically
separating the reagent from the aqueous liquid which will
be brought into contact with the upper surface of the
porous member and of controlling the migration of gases
from the area below the lower surface of the porous means
to the upper surface of the porous member. In some
embodiments, the reagent may be embedded in the porous
means and the porous means may be immersed in aqueous
solution to liberate the effervescent bubbles.
!~
~, .
WO92/21434 2 1 1 0 6 ,~ 8 PCT/US92/0~6
The size of the pores is selected so as to initiate
contact between the aqueous liquid and the reagent by
induci~g flow either via capillary action or by forcing
liquid through the pores via hydrostatic precsure. The
pore size al50 dictates the size of the~e~fervescent
bubbles which will be liberated. The hydrophilicity of
the surface is selected so as to control the rate of flow
of the aqueous liquid to the effervescent reagent and,
thus, modulate the evolution of gas which is released
through said porous means. ~or some applications, a buf-
fer can be integrated into the porous member to control
the final pH. For example, sodium bicarbonate and citri~
acid liberate gas in an acidic media. One may embed a
basic solution such as tribasic sodium in the pores of the
porous means to raise the pH of the medium in which the
bubbles evolve prior to dispersion to the bulk aqueous
solution. ~or other desired purposes other reagents may
be included in the pores of the porous means.
The third component of the devices according to this
invention is a reaction well which is of sufficient volume
to contain the reagent and the porous member, as well as
additional liquid and powder reagents (sample, buffers,
etc.) The inside diameter of the reaction well and the
outer diameter of ~he porous means are positioned such
that a water-tight seal between the wall of the reaction
well and the perimeter of the porous means is accom-
plished. ~his may be achieved through an inter~erence fit
or bonding. As one skilled~in the art will rPcognize~ the
amount of reagent needed to sufficiently mix the sol~tion
is dependent on the surface area of the reagent well and
the amount of reagent used.
The method of the present invention comprises adding
an aqueous solution to the upper surface of the porous
means element and allowing the liquid to initiate contact
with the effervescent powder of the reagent via either
capillary action or hydrostatic pres ure. Due to the
watertight seal between the reaction well wall and the
~ wo g2/2.434 2 1 1 0 ~ 8 8 PC~US92/04746
porous means, the only path through which the liquid may
travel is through the pores of the porous means. As the
liquid permeates the porous means, the water in the liquid
comes in contact with the effervescent reagent and the
chemical reaction is initiated resulting in~the formation
of effervescent bubbles. A dynamic state is then
achieved, in which there is a movement of liquid through
the porous means to the reagent, the water in the liquid
interacts with the effervescent reagent, effervescent
bubbles are produced and small bubbles are released
through the porous means.
The timing of the initial bubble formation, size of
the bubbles, and rate of bubble production all can be
readily adjusted by various changes in the porous-means
including pore size, thickness, pore density, various
chemical additives to the porous means and hydrophilicity
of the surface. The desired result of these adjustments
is to achieve desired modulation of the evolution of gases
by the selection of the reagents and materials such as the
porous means.
As noted above, the device comprises a powdered rea-
gent which will liberate gas without the application of
heat. Those skilled in the art will appreciate that there
jare many such compounds in use today, by way of example
~25 but without limitation, a preferred embodiment of the
t~present invention is a reagent which liberate carbon
dioxide when in contact with water, such as a blended
powder consisting of citric acid and sodium bicarbonate.
As previously described, devices according to this
invention contain a solid, porous means having pores gen-
erally transverse to its upper and lower surfaces. The
selection of the material to be used is important in
controlling the timing of the initial bubble formation,
the size of the bubbles, and the rate of bubble produc-
tion. Those skilled in the art will appreciate the largeselection of materials available for use, including with-
out limitation artificial membranes, porous plastics,
~,, .
W092/21434 PCT/~S92/0~6
2l 106~8
porous ceramics, and solid materials which have been
modified to contain very small capillary holes. Those
materials which are naturally hydrophobic can be modified
to a predetermined or desired hydrophilicity.by plasma
treatment, corona discharge treatment, or tkeatment with
a surfactant.
The following examples are presented to further
illustrate particular embodiments of the present
in~ention.
Example 1
A porous die cut high density polyethyle~e disc which
is approximately 0.265l' in diameter, 0.035" thick, and
with a pore size range of 40-50 microns (Porex Technology3
is placed in an l.SM potassium phosphate tribasic a~ueous
solution containing 0.1% Triton X-lO0 and 5% ethanol. The
solution and disc is first placed under reduced pressure
for 5 minutes to evacuate all air which may have been
trapped in the porous disc and to ensure that all disc
surfaces are in contact with the solution and then an
additicnal five minutes under atmospheric pressure. The
treated discs are then dried by placing them in a vacuum
oven at reduced pressure for 60 minutes at 70 degrees C.
Approximately lS mg of a citric acid and sodium bicarbo-
nate b~ended powder (CI~A Labs) is then placed in the
bottom of a reaction well 0.250" in diameter and 0.~90"
high and the dry, treated disc is press fit over the top
of the powder; covering th~ powder in such a way so as to
ensure that the only path which is readily available for
liquid migration is through the disc pores. Two lyophil-
ized reagent beads are then placed in the reaction well.The first bead consists of a lyophili~ed metal sol and the
second bead consists of a lyophilized protein. A 140
microliter sample of human urine containing 25 ng/mL of
phencyclidine is pipetted into the reaction well and is
allowed to incubate for 5 minutes. The liberation of
effervescent bubbles is delayed for approximately lO
-~WO92/21434 ~ 11 OiJ~ PCT/US9~04746
seconds, thus allowing the lyophilized beads to partially
reconstitute. After approximately lO seconds, initial
effervescent bub~les measuring approximately O.OlO" in
diameter are produced at rate of approximatqly 3 bubbles
per secondr The production of the bubbles ~continues for
approximately l.5 minutes, but a~ a steadily decreasing
rate. At the end of the 5 minute incubation period, the
liquid sample is transferred to a l.2 micron, Biodyne &
nylon membrane (Pall Biosupport) upon which has been
immobilized antibody against phencyclidine in a discret~
zone. After the liquid sample has been completely
absorbed, the membrane is washed with an aqueous solution
containing borate buffered saline and Lubrol at 0.02~ w/v.
The resultant colored di~crete zone has a color intensity
within the range of samples which are.mechanically mixed.
Example 2
A die cut solid polystyrene disc which is approxi-
mately 0.265" DA, 0O020~ thick, and contains a series of
four 0.030~' in diameter holes located at 0.050" from
center and spaced 90 degrees apart is placed in an aqueous
solution of 0.01% Triton X-lO0 and 5% ethanol. The
treated discs are then dried by placing them in a vacuum
o~en for 5 minutes at 70 degrees C. Approximately 15 mg
of a citric acid and sodium bicarbonate blended powder
(CIM~ Labs) is then placed in the bottom of a reaction
well 0.265" in diameter and 0.259'1 high and the dry,
treated disc is press fit'on to the top of the powder,
co~ering the powder in such a way so as to ensure that the
only path which is readily available for liquid migration
30 i5 through the 0.0301' diameter holes. Two lyophilized
reagent beads are then placed in the reaction well. The
first bead consists of a lyophilized metal sol and the
second bead consists of a lyophilized protein. A 140
microliter sample of human urine containing 25 ng/mL of
phencyclidine is pipetted into the reaction well and is
allowed to incubate for 5 minutes. The liberation of
WO92~21434 PCT/US9~/0~6
211~88 lo
effervescent bubbles is delayed or approximately lo
seconds, thus allowing the lyophilized beads to partially
reconstitute. ~fter approximately lo seconds, initial
effervescent bubbles measuring approximately o.olo" in
diameter are produced at rate of approximat~ly 3 bubbles
per second. The production of the bubbles continues for
approximately 1.5 minutes, but at a steadily decreasing
rate. At the end of the 5 minute incubation period, the
liquid sample is transferred to a 1.2 micron, Biodyne C
nylon membrane ~Pall Biosupport) upon which has been
immobilized antibody against phencyclidine in a discrete
zone. After the liquid sample has been completely
absorbed, the membrane is washed with an a~ueous solution
containing borate buffered saline and Lubrol at 0.02~ w/v.
The resultant colored discrete zone has a color intensity
within the range of samples which are mechanically mixed.
Example 3
The porous polyethylene discs used in this invPntion
were made hydrophilic by plasma treatment of the sur~ace.
Plasma treatment systems can be purchased by, for example,
Plasma Science, Inc., Belmont, California. The plasma
treatment derivatizes the surface with functional groups
which create a hydrophilic surface. ~hose skilled in the
art will recognize that the plasma treatment of plastic is
performed in a controlled atmosphere of a specific gas in
a high frequency field. The gas ionizes~ generating free
radicals which react with tAe surface. For example, oxy-
gen gas in a high fre~uency field results in positively
and negatively charged monoatomic and diatomic oxygen
ions, ozone, ionized ozone, oxygen atoms, metastably
excited oxygen molecule~ and free electrons. The active
species created are capable of reacting with the polymer
carbon-carbon bonds to provide a variety of oxygen moie-
ties on the surface. Analysis of the surfaces shows the
i35 presenc of carbonyl, carboxyl, hydroxyl and hyperperoxide
~groups. The carboxyl and hydroxyl groups cause the sur-
~WO92/214~ 2 1 1 0 ~ ~ ~ PCT/US92/W746
11
face of the polyethylene to become hydrophilic. The
duration of the plasma treatment in the chamber of the
device affects the degree of hydrophilicity of the sur-
face. For example, treatment times ranging from 1 min. to
60 min. creates surfaces of varying hydrophil rcity. Gen-
erally, the power used is between 100 and 1,000 watts and
the pressure is between 1 and 800 mtorr. The degree of
hydrophilicity furthermore affects the degree of bubbling
of the reaction mixture in the reaction cup; that is, the
greater the hydrophilicity (the longer plasma treatment
times, the greater the current and the greater the gas
pressure), the greater the bubbling ability of the discs.
Example 4
The porous polyethylene discs were made hydrophilic
by adsorption of detergents to the surface. For example,
the discs were placed in an ethanol solution containing 1%
(w/v) Triton X-100 at room temperature for 30 min. The
discs were removed and dried. The resulting discs were
hydrophilic as evidenced by their ability to create bub-
bles in a reaction mixture in the reaction cup. Thedegree of hydrophilicity of the discs was also changed by
varying the Triton X-100 concentration in the ethanol
solution from about 0.01% to 10% (w/v). Empirical
adjustments may be made as desired for particular
reagents, materials and purposes. The greater the Triton
X-100 concentration, the greater the hydrophilicity of the
discs as evidenced by their bubbling ability.
Example 5
Porous die cut high density polyethylene discs which
are approximately 0.265 inches in diameter, 0.035 inches
thick, and with a pore size range of 40-50 microns (Porex
Technologies) zre placed in a 0.1% or 10% Triton X-100 and
5% ethanol solution. The solution and discs are first
placed under reduced pressure for 5 minutes to evacuate
all air which may have been trapped in the porous disc and
Wos2/2l434 PCT/US92/~ 6
h ~ 8
to ensure that all disc surfaces are in contact with the
solution. Thereafter, they are placed for an additional
five minutes under atmospheric pressure. The treated
discs are then dried by placing them in a vacu~m oven for
60 minutes at 70 degrees Centigrade. ApproXimately 15 mg
of citric acid and sodium bicarbonate blended powder (CIM~
Labs) is then placed in the bottom of a reaction well
0.250 inches in diameter and 0.290 inches high and deep.
The treated disc is press fi~ted over the top of the
10 powder in such a way so as to ensure that the only path
which is readily available for liquid migration is through
the disc pore. A series of assemblies are then completed
with each of the two treated discs types and then the
assemblies are labelled. 1.40 microliters of deionized
15 water is added to each of the reaction well assemblies and
the observed results are noted below:
~1) the assemblies which were treated with the 0.1%
tTriton solutions began to bubble approximately 5 seconds
after the deionized water was added. The bubbles were
20 large, uniformly liberated one at a time, and no foam was
;generated;
2) the assemblies which were treated with the 10%
Triton solution began to bubble immediately after the
deionized water was added. The bubbles were very small
25 and formed a layer of foam.
This best mode example demonstrates that control of
¦the timing of the initial bubble formation, size of the
bubbles, and rate of bubble production can be achieved by
selecting the hydrophilicity of the surface by appropriate
30 treatment.
While the invention has been described above in terms
of specific embodiments, these have been provided for
;illustrative purposes only and are not to limit the scope
of the invention which is defined by the claims.
~ . .