Note: Descriptions are shown in the official language in which they were submitted.
C ~
SK/K-19399/A
Thermosettin~ compositions based on epoxY resins. dicyandiamide and a curin~
accelerator havin~e a ~ood ratio between stabilit~ and reactivitv
The present invention relates tO the use of compounds of the formula 1 shown below as
curing accelerators for therrnoset~ng compositions comprising at least one epoxy resin
and dicyandiamide, corresponding therrnosetting compositions and two advantageous
processes for the preparation of compounds of the formula 1.
Dicyandiamide is a known hardener which is often employed for epoxy resins (Henry Lee,
Kris Neville "Handbook of Epoxy Resins", McGraw-Hill Book Company, New York,
1967, pages 10-16). In fact, it has only a very low reactivity at room temperature or
moderately elevated temperatures up to about 40C, and thus allows prolonged handling
and/or storage of compositions of epoxy resins and dicyandiamide at these temperatures,
without par~al curing rendering processability of the compositions more difficult or even
impossible, for example because of increased viscosity. Nevertheless, dicyandiamide also
requires relatively high temperatures for cuIing~ at least above 145C, especially if rapid
curing is necessary, as is often the case, for example, for applications as adhesives.
To increase ~e reactivity of such composi~ions, cuIing accele~ators are therefore usually
added. l~udazole derivatives, and in particular
N'-(3-chloro4-methylphenyl)-N,N-dirnethylurea ~chlorotoluron), are known to be
especially good curing accelerators. Chlorotolu~on is capable of increasing the reactivity
of epoxy resin/dicyandiarnide compositions quite considerably. Thus, the gelling time of
chlorotoluron-accelerated epoxy resin/dicyandiamide mixtures at lS0C is only a few
minutes, while without the curing accelerator about half an hour to an hour passes before a
corresponding composition gels at the same temperature. Chlorotoluron and other
comparably good accelerators have the disadvantage, however, tha~ they also increase the
reactivity of the reaction mixtures at room temperat Lre and moderately elevatedtemperature so drastically that the shelf-life of these compositions is no longer
satisfactory.
The object was therefore to provide a curing accelerator for therrnosetting compositis)ns
. , : - , . .
., . . - ~
;,. ~ ~, . , , ;
. ~ - ~ : : : , , ,,: .
'` s~
- 2 -
based on epoxy resins and dicyandiamide, which although allowing suring rates
comparable to those of chlorotoluron at elevated temperature, shows only a low reactivity
at room temperature or moderately elevated temperature, so that the processability of the
compositions is not changed or changed only insignificandy at these temperatures, even
during a relatively long standing time or storage.
Surprisingly, it has now been found that this object can be achieved by using a compound
of the formula 1 as the curing accelerator for a thermose~ting composition comprising at
least one epoxy resin and dicyandiamide
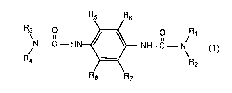
N--e_ HN~ llH e~
in which, in formula 1,
the radicals Rl and R3 independently of one another are each an aLkyl, cycloalkyl or aryl
group,
the radicals R2 and R4 independently of one ano~er are each an aLkyl or aryl group, or
the radical Rl together with the radical R2 and/or the radical R3 together with the radical
R4 are each a 1,4-tetramethylene or a 195-pent~unethylene group, and finally
the radicals Rs, R6, R7 and R8 independently of one another are each a hydrogen or
halogen atom or a Cl-C4aLtcyl or Cl-C4aL~coxy group.
ALkyl radicals Rl to R4 in fo~mula 1 are preferably alkyl groups having 1 to 6 carbon
atoms, which can be straight-chain or branched, for exarnple methyl, e~hyl, propyl,
iso-propyl, n-butyl, sec-butyl, tert-butyl, iso-butyl or straight-chain s~r correspondingly
branched pentyl or hexyl groups. Cycloalkyl radicals Rl ~o R4 are, in particular,
cyclopentyl or cyclohexyl groups, and aryl radicals Rl to R4 are, in particular, phenyl
groups or radicals which are derived from the cycloaL~cyl mentioned and from phenyl
groups by replacing one or more, for example two or three, hydrogen atoms by halogen
atoms, in parlicular chlorine or bromine atoms, or by straight-chain or branched alkyl
groups having 1, 2, 3 or 4 carbon atoms or by aLtcoxy groups corresponding to these aL~cyl
groups.
~: ,; , . .
. .
, . ... ,- ~ .
.; . ':: '
, , .
., ~ , , ,' ~ , ,:,
,............................... .
- 3 -
The compounds of the formula 1 are known in at least some cases and can be obtained, for
example, in a manner which is likewise known per se by simultaneous reaction of a
diamine of the formula --
R'5~R6
H2N ~NH2
R8 R7
with an amine of the fonnula HNRlR2 and an amine of the formula HNR3R4 in the
presence of carbon monoxide and sulfur in an autoclave. The radicals Rl to R8 in the
abovementioned formulae have the same meaning as in fonnula 1. The amounts of the
reactants are not cridcal, but the reactants should in general be present at least in the
stoichiometric amounts. This process is described in detail with the aid of a number of
examples in US-A-2,993,M4.
Two novel one-stage processes ~ur~ermore have proved to be suitable for the preparation
of compounds of the formula 1. The present invention likewise relates to these processes.
The two processes render the use of a pressu~ized autoclave unnecessary and do not lead
to the formation of hydrogen sulfide, as does the already known preparation process
described above, so that absorption of hydrogen sulfide is unnecessary.
In the first of these one-stage processes, a diisocyanate compound of ~he formula
Rs R6
OCN~ NCO
)Y,
R8 R7
in which
Rs, R6, R7 and R8 have the sarne meaning as already mentioned abovet
is reacted with in each case at least approximately stoichiometric amounts of the amines
HNRlR2 and HNR3R4
in which
the radicals Rl to R4 likewise have the meaning already mentioned above,
in a suitable solvent which is inert towards isocyanates, for example in ethyl acetate,
dioxane or a suitable hydrocarbon, under anhydrous conditions and with heating, and the
, .. . . , , .-, ... . .. .
'3
- 4 -
product of the formula 1 is isolated. For isolation, for example, the solvent can be stripped
off. However, some of the compounds of the formula 1 are so sparingly soluble in the
solvents mentioned that they can be isolated in an outstanding yield merely by filtration.
This particularly applies to the compounds of the formula 1 in which the radicals Rl to R4
are all an allcyl radical, preferably the same aL~cyl radical, having 1 to 3 carbon atoms. In
general, the process described leads to yields of the desired product of approximately a
hundred per cent and produces this in a high purity.
Like the autoclave process described above, the second process starts from a diamine of
the formula
Rs R6
H2N ~ N~12
~ '.
R8 R7
which is reacted with at leas~ approximately stoichiometric amounts of the carbamoyl
chlorides of the formulae
O Rl R3
Cl--C--N\ and Cl--C--N\
R2 R4
in a suitable solvent. The product of the formula l is then isolated. The reaction in general
proceeds as a slightly exo~ermic reaction.
The invention furthermore relates to thermosetting compositions comprising at least one
epoxy resin, dicyandiamide and a curing accelerator, wherein a compound of the formula
1 as de~med above is employed as the curing accelerator.
The compositions according to the invention display a similar reactivity to compositions
based on chlorotoluron as the curing accelerator at elevated temperature, but at the same
time have an unexpectedly greatly increased stability at room temperature compared with
the compositions mentioned, this being comparable to the stability of corresponding
compositions without a curing acclerator.
Particularly preferred compositions are those based on those curing accelerators of the
formula 1
in which
r,.. . ,, -: , : .'
;;' ~ '
. . . ' .
.
.
..'. .
~' . ,
the radicals Rl, R2, R3 and R4 in formula 1 independently of one another are each an alkyl
group having 1 to 3 carbon atoms or phenyl,
and compositions based on compounds of the formula 1
in which
the radicals R5, R6, R7 and R8 are all hydrogen atoms.
Finally, the most preferred compositions according ~o the invention are those comp~ising
the compound of the formula 1
in which
the radicals Rl, R2, R3 and R4 are all methyl and
the radicals R5, R6, R7 and R8 are all hydrogen atoms.
All the customary di- and polyepoxides and epoxy resin prepolymers are suitable epoxy
resins for the invention. The di- and polyepoxides can be aliphatic, cycloaliphatic or
aromatic compounds. Examples of such compounds are the glycidyl ethers and
~-methylglycidyl ethers of aliphatic or cycloaliphatic diols or polyols, for example those
of ethylene glycol, propane-1,2-diol, propane-1,3-diol, butane-1,4-diol, diethylene glycol,
polyet~lylene glycol, polypropylene glycol, glycerol, trimethylolpropane or
1,4-dimethylolcyclohexane, or of 2,2-bis(4-hydroxycyclohexyl)propane, the glycidyl
ethers of di- and polyphenols, for example resorcinol, 4,4'-dihydroxydiphenylme~ane,
4,4'-dihydroxydiphenyl-2,2-propane, novolaks and
1,1,2~2-tetrakis(4-hydroxyphenyl)ethane. Other examI~les are N-glycidyl compounds, for
example the diglycidyl compounds of ethyleneurea, 1,3-propyleneurea or
S-dimethylhydantoin or of 4,4'-methylene-5,5'-tetramethyldihydantoin, or those such as
triglycidyl isocyanurate.
Other glycidyl compounds which are of industrial importance are the glycidyl esters of
carboxylic acids, in particular di- and polycarboxylic acids. Examples of t'nese are glycidyl
esters of succinic acid, adipic acid, azelaic acid, sebacic acid, phthalic acid, terephthalic
acid, tetra- and hexahydrophthalic acid, isophthalic acid or trimellitic acid, or of dimerized
fatty acids.
l~xamples of polyepoxides other than glycidyl compounds are the diepoxides of
vinylcyclohexene and dicyclopenta(liene,
3-(3',4'-epoxycyclohexyl)-8,9-epoxy-2,4-dioxaspiro[5.5]undecane, ~he
3',4'-epoxycyclohexylmethyl ester of 3,4-epoxycyclohexanecarboxylic acid, butadiene
~',;' ' ' ~ ' ~ ' ' " ' " ' ' ' ' ' "
~ 1 3 ~
diepoxide or isoprene diepoxide, epoxidized linoleic acid derivates or epoxidized
polybutadiene.
Preferred epoxy resins are diglycidyl ethers, which may be advanced, of dihydric phenols
or dihydric aliphatic alcohols having 2 to 4 carbon atoms, in particular the diglycidyl
ethers, which may be advanced, of 2,2-bis(4-hydroxyphenyl)propane and
bis(4-hydroxyphenyl)methane, or a mixture of these epoxy resins.
Although precisely compositions based on a resin component of only epoxy resins
represent an important embodiment of the invention, the curable compositions furthermore
can also comprise polyisocyanates, in addition tO the epoxy resins.
Suitable polyisocyanates here are all the polyisocyanates capable of crosslinking, for
example hexamethylene diisocyanate (HDI), ~imethylhexamethylene diisocyanate
(TMDI), cyclohexane diisocyanate (CHDI), isophorone diisocyanate
(3,5,5-trimethyl-1-isocyanat~3-isocyanatomethylcyclohexane; IPDI),
methylene-dicyclohexyl isocyanate (E~DI)l), p-phenylene diisocyanate (PPDI),
diisocyanatotoluene ~IDI), for example 2,4-diisocyanatotoluene, 2,6-diisocyanatotoluene
and industrial mixtures of the ~vo isomers, naphthylene diisocyanate (NDI), in particular
1,5-naphthylene diisocyanate, dianisidine diisocyanate (I)ADI), methylene-diphenyl
diisocyanate (MDI), in par~icular the 4,4'-isomer, but also industrial mixtures of va~ious
isomers, for example the 4,4'- and 2,4'-isomers, or polymedlylene-polyphenyl isocyanates
(PAPI). Polyisocyanates which are obtainable by reaction of polyisocyanates withthemselves via isocyanate groups, such as uretdiones or carbodiirnide compounds, which
are formed by reaction of two isocyanate groups, or such as isocyanurate or biuret
compounds, which are formed by reaction of three isocyanate groups, are likewise also
particularly suitable. Polyisocyanate prepolymers which contain on average more than one
isocyanate group per molecule and are obtained by preliminary reaction of a molar e~ccess
of, for example, one of the abovementioned polyisocyanates with an organic material
which contains at least two active hydrogen atoms per molecule, for example in tlle form
of hydroxyl groups, as in the case of polyaLkylene glycols, a~e also suitable for the
invention. Isocyanates such as those mentioned are generally available, and a wide range
are commercially obtainable.
Preferred polyisocyanates are methylene-diphenyl diisocyanate (MDI), isophorone
diisocyanate (IPDI), hexamethylene diisocyanate, diisocyanatotoluene (TDI), ~or example
: - , , ,
, . . ; .
,,; , ,~: . :
, ~ .: , ,
~,,,. ~
,:': ,, :' :
';~J, ~ ~ ~ fi ~ 1
2,4-diisocyanatotoluene, 2,6-diisocyanatotoluene and industrial mixtures of the two
isomers, and prepolymers of the isocyanates mentioned with polyalcohols and reaction
products which are obtainable by reaction of the polyisocyanates with themselves via
isocyanate groups.
The free isocyanate groups of the polyisocyanates can also be blocked in the customary
manner, for example with phenols, oximes~ caprolactams, imidazoles, pyrazoles orindazoles. Pyrazole-blocked polyisocyanates are preferred, because they already release
isocyanate again and start to cure at relatively low temperatures, from about 80C.
Pyrazole-blocked polyisocyanates and ~heir preparation are described in US-A-4,976,837
and EP-A-0 500 495. The preparation can be carried out by quantitative reaction of
suitable pyrazoles with tne polyiss)cyanates under an inert gas. The reaction is preferably
ca~ied out at elevated temperature and in a suitable inert solvent (for example toluene), if
appropriate in the presence of a catalyst (for example dibutyltin dilaurate). As a result of
the exothermic reaction of the two compounds, cooling may be necessary. The pyrazoles
used for blocking preferably have tne formula
N R
H~ ~
~\R2
R3
in which
E~" R2 and R3 independently of one another are hydrogen, hydroxyl, aLkyl, in particular
having up to S carbon atoms, alkyloxy, in particular having 1 to 5 carbon atoms, aL~cylthio,
in particular having 1 to 5 carbon atoms, methyl and methoxy in turn being particularly
preferred, aryl having up to 10 ring carbon atoms, in particular phenyl, where the aryl
radicals may or may not have substituents in turn, for example Cl-C4aLkyl- or
Cl-C4alkyloxy substituents; or a~ylalkyl, in particular aryl-(cl-ca~)aLlcyl~ very particularly
arylmethyl, in particular bénzyl.
Pyrazoles of this type are cornmercially obtainable or can be obtained by the customary
rnethods, for example by reaction of appropriately selected i,i~2-diketones, for example a
1,3-diketone, with hydrazine with or without a solvent (for example toluene).
If mixtures of epoxy resins and polyisocyanates are employed, the epoxy resin preferably
. : . , - , .
, j i~ . . . . . .
~;s ~
- 8 -
makes up at least 50, or even better at least 70 per cent by weight of the total resin
component.
The compositions mentioned advantageously comprise the compound of the formula 1 in
an amount of 0.1 to 10 per cent by weight, based on the total aunount of epoxy resin and
dicyandiamide and any polyisocyanate present. Dicyandiamide is in general present in
amounts of 2-20 per cent by weight, and the epoxy resin or epoxy resin/polyisocyanate
mixture is present in amounts of 80-98 per cent by weight, in each case based on the total
amount of dicyandiamide, epoxy resin and any blocked or non-blocked polyisocyanate
present. The following preferred composition which comprises dicyandiamide in anamount of about 10 per cent by weight and 1,4-bis(N,N'-dimethylureido)benzene in an
amount of about S per cent by weight, based on the total amount of epoxy resiDs,dicyandiamide and 1,4-bistN,N'-dimethylureido~benzene, may serve as an example.
In addition to the constituents mentioned, the compositions according to the invention can
also contain other customary constituents in the customary amounts, for example viscosity
regulators, extenders, fillers, reinforcing agents, metal particles, pigments, dyes, organic
solvents, plasticizers, adhesion promoters, fungicides, antioxidants, flow control agents,
diluents, for example reactive diluents, and others.
The curable mixtures according to the invention can be employed quite generally for the
production of cured products and can be employed in a formulation appropriate for the
particular specific field of use, for exaInple as coating compositions, paints, pressing
compositions, irnmersion resins, casting resins, impregnaiing resins, lamina~ing resins or
matnuc resins.
They are suitable, ~or example, for production of prepregs, laminated materials or
composite materials, for the production of all types of shaped articles or for enclosing
electrical or electronic components. The compositions according to the invention are
particularly suitable as 1- or 2-component adhesives, for example for gluing on ICs. A
field of use specifically for the compositions based on mixtures of epoxy resins and
polyisocyanates is the production of cured products with modifying agents which ha~e
reacted in the product, such as plasticizers or flexibilizers, where, for example, the
polyisocyanate component is the modifying agent for the epoxy resin component
(interpenetrating polymer networks).
~, ,, , .
~. ' . , .
,i i .
,,-., i .,,
. . ~
~ ' , ' ~ ; ''; ' :
;
,,.j, . .
:,,
_ 9
Applications where it is impoltant to achieve a high adhesion of cured material according
to the invention are particularly preferred, i.e. the use of the compositions according to the
invention as coating agents, for the production of prepregs and, in paIticular, as a
therrnosetting, preferably one-component adhesive.
The mixtures according to the invention can rapidly be cured fully at relatively low
temperatures. Temperatures in the range from 20 to 200C, preferably from 60 to 1 80C,
in particular 80 to 120C, are in general used for full culing. Curing can be effected here
by supplying heat in any form. It can also be carried out, for example, with the aid of
microwaves or by induction heating, in which case the compositions must of course
comprise electrically conductive particles, for example metal particles, for the latter. Full
curing is as a rule effected with simultaneous shaping to shaped articles, impregnations,
coatings or gluings.
A particular advantage of the compositions according to the invention is, however, that
they can already be cured fully at particularly low temperatures in the range below 140C,
in particular between 120 and 140C, at a rate which is adequate in practice. The gelling
times of compositions according to the invention are in fact in general below 30 minutes
even at these temperatures.
Exam~le 1: Preparation of N-C-HN~NHO' N' 3
112.09 g (0.7 mol) of 1,4-phenylene diisocyanate (from Akzo Chem. Co.; now obtainable,
for example, from AldIich or Fluka) and 1.6 litres of acetic acid ethyl ester (ethyl acetate)
are heated under reflux in a 2 litre round-bottomed flask with a reflux condenser and
ni~ogen in- and outlet. 63.1 g (1.4 mol) of dimethylamine are now passed in. The mixture
is allowed to cool to room temperature and the white suspension is ~lltered through a G4
suction filter. After washing with ethyl acetate, the product is dried under a bigh vacuum
at 110C for 14 hours. 174 g (99.3% of theory) of dle desired product are obtained.
Meltin~ point: > 300(~.
Elemental analvsis: calculated C 57.58% H 7.25% N 22.38%
found C57.50% H7.24% N22.03%
. ....... - - ~ , i , , ~ : .
' ' " ,' : ,
" ,'. ' :. ~ ' ' ' :
*',
r ~ ; ' . ,' ' ' . '
~' ' ~ .' '' '
~ .,'j ' . , .
- 10- :
IR (KBr): l)(C=O) at 1640 cm~l; no -NCO band ( ~) 2280 cm~
visible.
H-NMR (DMSO-d6~ 2.90 ppm, s, 12H; 7.27 ppm, s, 4H; 8.06 ppm, s, 2H.
H3C~ O o Cl-13
Example 2: Preparation of ,N C HN~ NHC - N
A solution of 64.88 g (0.6 mol) of 1,4-phenylenediarnine in 600 millilitres of pyridine is
initially introduced into a 1.5 litre sulfonadng flask with a reflux condenser, nitrogen in-
and outlet, ~hermometer and dropping funnel, and 141.95 g (1.32 mol) of
dimethylcarbarnoyl chloride are added dropwise. An exothermic reaction takes place. The
rnixture is allowed to react at 100C for 12 hours and then cooled, and is poured onto a
mixture of ice and lN hydrochloric acid. It is filtered through a G4 suction fîlter and the
product is washed v~ith water and then with ethanol and ethyl acetate and dried under a
high vacuurn at 50C for 14 hours. 116.8 g (77.8% of theory) of the desired product are
obtained in the form of a violet powder.
Melting point: > 300C.
Elemental analvsis: calcula~ed C 57.5~% H 7.25% N 22.38%
found C 57.56% H 7.23% N 22.~3%
lH-NMR (DMsO-d6~ 2.90 ppm, s, 12H; 7.28 ppm, s, 4H; 8.10 ppm, s, 2H.
Exam~ P~paration of .N - ~ - HN~ NH C - N, analogously to US-A-2,993,044.
136.08 g (1.26 mol) of 1,4-phenylenediamine, 161.28 g (5.04 mol) of sulfur and 315
millilitres of methanol are initially introduced into a 6.3 litre steel autoclave with
mechanical stirring (anchor stirrer). Thereafter, 340.2 g (7.56 mol) of dimethylamine are
forced in, followed by carbon monoxide, Imtil a pressure of 22 bar prevails in the
autoclave. During this operation, the temperature rises from initially 18C to 36C. The
pressure drops to 10 bar in the course of 75 minutes. The mixture is heated ~o 100C and
left at this temperature for 2 hours (pressure of 10 bar). Thereafter, it is cooled ~o room
, ~ , , , - ~ . . .
:. ~: , . .
.: :. . . . . ..
.. : ~. ~ . . .
, ,.: . . .
~ : ' . . .
11 "~
temperature, the au~oclave is washed out with methanol and the resulting black suspension
is heated at 60C for one hour and filtered hot. The residue is washed with hot methanol
until the wash liquid is colourless, and is dried at 110C under a high vacuum for 14 hours.
296 g (94% of theory) of the desired product are obtained.
Elemental analYsis: calculated C 57.58% H 7.25% N 22.38%
found C 57.42% H 7.28% N 22.38%
IR (KBr): ~(C=O) at 1640 cm~l.
IH-NMR (DMSO-d~2~ 2.99 ppm, s, 12H; 7.44 ppm, s, 4H; 8.23 ppm, s, 2H.
The products of Examples 1, 2 and 3 all have the same accelerating effect and the same
latency properlies in the compositions described in this specification.
~xample 4: A mixture of in each case 20 g of epoxy resin based on bisphenol A (epoxide
equivalent 185-190 g/equivalent; viscosity (at 25C in accordance with DIN 53015):
10,000-12,000 mPa.s; Alaldit(~GY-250); 2 g of dicyandiamide and 1 g of accelerator from
Example 1 is passed over a triple roll mill twice for homogenization. This mixture has the
properties shown in Table 1 (mixture A). For comparison, the properties of a mixture
which is otherwise identical but comprises the same amount of chlorotoluron as the
accelerator instead of the curing accelerator according to the invention (mixture B) and the
properties of a corresponding mixture without any accelerator addition (rmxture C) are
also shown in the table.
Table 1:
~ ~ Exo- Tensile lap-sheat Gelling time in numltes at
Mix- thermicity ~H Stabiaty slrength 3 (Al/Al)
ture maximum [J/g] at 40-C with ISO 4587IOO'C 120-C 140'C 160C
_ r
A 132'C 400 >100 d 19AN/mm2>60 23 6 3
B 149'C 300 < 14 d 17.9Ntmm2 >60 11.5 5 1.25
C 202'C 443 >100 d 19.5N/mm2 >60 >60>60 31
1 determined by DSC at a heating rate of lO'C/rninute
2 d = days before doubling of the viscosily of the mixture occurs
3 after curing at 160-C for 60 minutes
When ~he data given for mixtures A and B are compared, the greatly increased stability of
~ . . . ~ , . ,
, . , , ~ ~ . .
., . ~ .: .
: ' '
:, :
fi ~ ~
- 12-
the composition according to the invention ~mixture A) compared with a composition
comprising chlorotoluron (mix~e B) can clearly be seen, although both compositions
have about the same reactivity at elevated temperature and accordingly gel and also cure
in practically ~he same time at these temperatures. A compaIison of the values for
mixtures A and C furthermore shows that the stability of the thermosetting composition A
acclerated according to the invention is even comparable to that of a non-accelerated but
otherwise identical composidon (mixture C).
Example 5- The following adhesive mixture (mixture D) is passed over a triple roll mill
twice for homogenization:
9.52 g of bisphenol A diglycidyl ether
2.36 g of 1,4-butanediol diglycidyl ether
0.34 g of crude non-purified bisphenol A diglycidyl ether (5.2-5.4 equivalents of
epoxide/lcilogram)
6.00 g of adduct of bisphenol A diglycidyl ether/carboxyl-terrninated
butadiene/acrylonitrile copolymer/cashew nut shell oil (3.0-3.5 equivalents of
epoxide~cilogram)
0.70 g of dicyandiamide
0.60 g of silicon dioxide
0.48 g of an accelerator according to Pxamples 1-3
For comparison, an identical mix~e which comprises, however, the identical amount of
chlorotoluron as an accelerator is homogenized in the same manner (mixture E). ~ ~.
Important properties of the two rnixtures are compared in Table 2.
.,,-. . ~, - :
,, , , ,
:. : ., -: ............. . .
,, - :
: . .
, . .
- 13-
Table 2:
-- E~o- ,, 2 Tensile lap-shear Gelling t;me in mu utes at
ML1~- thcmucity ~H Stablllty strength3 (Al/A1)
~re maximum [J/g] at 40'C with ISO 4587 lOO'C 120'C 140'C 160'C
. _ _ _
D 157-C252 >100 d 303N/mm2 >60 29 8.3 23
E 152'C 237 < 30 d 35.6N/mm2 >60 19 8.2 3.5
_ _
1 determined by DSC at a heating rate of lO'C/minute
2 d = days be.fore doubling of the vicosity of the mi~ture occurs
after curing at 160'C for 60 minutes
The inc~eased stability/reactivi~y ratio of composition D according to the invention can
again be clearly seen.
;. ~ , . . . ... . .
f.,,.,.;.,, ,, ' '' ' ::,
( ' . '' : "
5.: ::. : . . :
:: . . :
,, i . :
~'', :,:.. .. :: .
,,: ,: . : ~ ::
" ,'.¢:' ','''~ ' ' ' '' ~ :
. ~.,:. . , . :