Note: Descriptions are shown in the official language in which they were submitted.
W0~2/22579 1? ~ 81 PCI/US~210~972
MIMI~ PEPTIDES OF qPl~0
BackarQund of ~he InventiQn
Human immunodeficiency virus (HIV) is the cause
of acquired immunodeficiency syndrome and the HIV
05 external envelope glycoprotein~ gpl20, is associated
with v1ral infectivity and cytopathology including
cell fusion. gpl20 binds the cellular receptor of the
virus, CD4, and cells e~cpressing the envelope :fuse
with CD4-positive cells in culture. Fusion of
infected and noninfected cells and infection by
cell-free virions are important routes of HIY
infection.
It has been reported by Robey et al. ~çs~
~`~ Acad. S~i~ USA ~:7023-7027 (1986~) that gpl20
15 purified from ~irus-infected cells elicits antibodies
that neutralize the infectivity of HIV. Recombinant~
gpl20 or gpl60 expressed in a~variety of cell types
have also been shown to elicit~neutralizing antibodies
(see e.g., Lasky et al.~ ~S~ 2~3:209-233 :(1986~; :
20 Rusche et al., Proc. ~a1~_A~ad S~i. USA~84:1-5
(1987);~and Steimer et al., Vaccines 87:236-241
(1987)). The amino a~id sequence:of the envelope is
~known to vary between different HIV~isolates and ~the -~
;. neutrali~ing antibod~ies: elicited by the envelope of:
25 one HIV isolate are usually:eff:ective against only a
: sub~et~of he~erologous isolates. This observation
suggests that such neutralizing antibodies are :
: directed to a variable region of the envelope. This
idea is supported by the f act that observation
suggests that such neutralizing antibodies are
directed to a variable:region of the envelope;. This~
:
WO9~t/~2579 ~ 6~ ~ P~T/US92/04972
-2- ..
idea is supported by the fact that neutralizing
antibodies are elicited b~ an E. oli-produced
recombinant fragment;from the carboxyl-terminal region
of gpl20, PBl, thattcontains 37% of gpl20 and is from :~
05 a variable region of the envelope. This fragment :~.
- contains the dominant neutralizing epitopes (epitope
being the basic element or smallest unit of ~
recognition by a receptor such as an antibody), and a ~:
more complete understanding of the number, the
10 location, and the potential role of antibodies to ;
these epîtopes in preventing viral infection may ;
facilitate development of a subunit vaccine able to ~:
induce immunity to diverse HIV isolates. : ;
Matsushita et al. (J~_YirQl~ ~2107 2114 (1988))
15 reported the identlfication of a monoclonal antib~dy ~.
which is characterized by the ability to neutralize
infection by cell-free virus, and the ability to
prevent fusion of virus-infected cells. The~ antibody
binding site was mapped to a 25-amino acid segment of
20 gpl20, referred to herein as the V3~100p. This region
~: is known to contain major neutra~lizing epitopes.
Peptides which stimulate the production of antibodies .
which~are reactive with these immunodominant epitopes
yet which are not found in the~primary sequence o~ the
: 25 gpl20 protein would be useful~for immunization, for :
~: e~ample. Such peptides are referred to herein as
mimic peptides.
W092/~2s79 -3- 2 1 ~ 1 6 ~ 1 PCT/~Sg2/U4972
Summary of th~ InventiQn
This invention relates to peptides having a
primary amino acid sequence which is unrelated to the
primary gpl20 amino acid sequence and are, by
05 themselves, or conjugated to an immuno~enic carrier
molecule, able to stimulate the production of
anti-gpl20 antibodies. The anti-gpl20 antibodies
produced in response to peptides of the pres~nt
invention are directed toward a region of the gpl20 ~:~
lO molecule known as the V3-loop, which is known to
contain important epitopes.
The peptides can be form~lated in a : - :
physiologically acceptable carrier for use aæ a
vaccine composition. This composition can optionally
15 include an adjuvant. In addition, the peptide can be
conjugated to an im~unogenic carrier mol~cule to
increase immunogenicity. In this conte~t:the peptide
serve as an immunogen.
~ The invention also pertains to peptides which
-: ~ 20 serve as antiviral agen s and a method for interfering ::
:~ with viral propagation în an individual. The method ::~
: involves administering an effectiv~ amount of a
therapeutic composition~comp;rising a peptide in a
physiological carrier. The peptide has a primary
:: 25 amino acid sequence which is unrelated to the primary
amino acid sequence of an essential protein of a virus
Se.g., gpl20) and the pepti~e has the ability to:~
- i~terfere with a component involved in the function of ~:~
: the virus t In this conte~t, the peptide serves as an
30 antiviral agent.
In another aspec~, the invention pertains to a
method for ~creening an epitope librar~ with a
non-isolate specific antibody. Such antibodies
typically do not bind antigen with higA affinity.
W092/22579 PCTlUSg2/04972
~ 6~ -4-
Brief Descri~tion of the Drawinqs
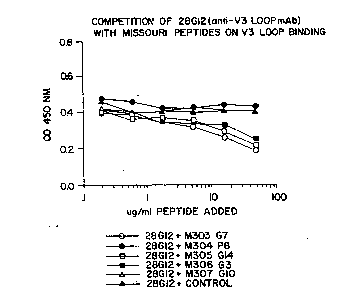
Fi~ure 1 is a schematic representation of results
from a competition assay for V3-loop binding.
Figure 2 is a schematic representation of rgsults
05 from a competition assay fbr recombinant gpl20
binding. ~`
Figure 3 is a schematic repr~sentation of results -;
from a direct binding assay with serum from mice
immun~zed with M306 G3.
~igure 4 is a ~chematic repr~sentation of results
from a direct binding assay with serum from mice
i~munized with M306 G3 conjugated to an immunogenic
carrier molecule. ~ :
Figure 5 is a schematic representation of results
15 from a competition esperiment in which Y3-loop was
attached to an ELISA plate and reacted with serum from j~
mic~ immunized with ~306 G3 conjugated ~o an
immunogenic carrier molecule~mi~ed with~variou~
: peptides. ;
Figure 6 is a schematic represent.ation of res~lts
from a comp~tition e~periment in which M306 ~3
conjugated to bovine serum al~umin was fised to an
ELISA plate and:reacted with se~um from mice immunized:
with M306 G3 and conjugated to an immunogenic ~arrier
25 molecule~mi~ed with various peptides.
Figure 7 is a schematic reprssentation of results
from a competition experiment in which V3-loop was
fixed to an ELISA plate and the antibody was an
affinity purified rat monsclonal known to b~ HIV ~ ;
30 neutralizing. ~ :
. ~
W092/2~79 -5- 2 1 ~ 1 6 8 1 P~T/US92~972
Figure 8 is a schematic representation of results
from a competition experiment in which recombinant ::
gpl20 was fixed to an ELISA plate and the antibody was
an affinity purified rat monoclonal known to be HIV
05 neutralizing.
..
Detailed De~criPtion of ~he ~n~ntiQ~ - .
One embodiment of subject invention is~based on
Appl~cants':discovery of pepti~des~, unrelated in
: primary amino acid sequence to the primary amino acid
10 sequence of gpl20, which stimulate the prodùction of
anti-gpl20 antibodies when administered ia:vivo to a ~ :
mammal. The primary amino acid sequence:refers to the
linear order of amino acid residues in a peptide or
protein. - ~-
: 15 Peptides of the invention~were identifie~
initially by screening an epitope library with a rat :~
monoclo~al a~tibody known to be HIV neutra~l:izi:ng in an
n:vitro assay. Such a method is des ribed, for
example, by Scott and smith (Sçi~nÇ~ 249:3~86-390
20 (~ggo)). Briefly, an epitope~;~library is a vast misture
Qf filamentous phage clones,:each displaying one
:peptide sequence on the v~irion surface.~ The survey is ~ ~ -
:~ accomplished using a:binding protein (e.g., a
monoclonal antibody) to affinity-purify~phage that ~ :
25 display tightly-binding peptides~ and~propagating:the:
purified pha~e in~E~ ~Qli. The~amino acid ~equence of:
: the peptides displayed on the phage are then
determined by sequencing the corresponding coding
region in the viral DNAs. Peptides identified in this
W092/~2S79 PCT/US92/04972
6~ -6-
manner that are also capable of competing for antibody
binding with the natural epitope are referred to
herein as mimic peptides because they mimic the
natural epitope, but in most instanc~s are unrelated
05 in primary sequence to;the natural epitope.
More specifically, the steps invol~ed in the
screening of an epitope 1ibrary include providing an
epitope library containing bacterial cells infected
with fusion phage. The fusion phage are then
10 contacted with a biotinylated antibody under
condition~ appropriate ~or the binding of the antibody
to peptides displayed on the surface of the bacteria1
cells infected by the fusion pha~e. The mi~ture is
then contacted with a streptavidin-coated ~ubstrate
15 under conditi~ns appropriate for the binding of biotin
to streptavidin. ~on-~pecifical1y bound phage are
removed with a wash step. Boun~ phage are eluted and :
used to infect E ~oli cells and peptide s~quences
binding to the rat monoc1Ona1 antibody were
20 identified. Although these peptid~ sequences are
~: fourteen amino acid residues in 1ength~ it is be1ieved
that some or all of the he~apeptide amino acid
residu~s 5 through 10 are essential for the binding of
the peptides to the rat monoclonal antibody since ;~
25 amino acid residues 1 through 4 and 11 through 14 were
: identical for all the peptides in the epitope
1ibrary. However, it is possib1e that-some or a11 of
the amino acid residues 1 through 4 and 11 through 14
ar~ critical since amino acid residues 1 throuqh 4 and
30 I1 through 14 were part of the peptide that actually
demonstrated function in the E~emplification.
WO9~/22579 2 1 1 1 ~ 1 PcT/US92/04972 ;
Peptides having the same sequences as the
peptides identified by screening the epitope library, ~`~
e~cept for the addition of amino acid 1~ (alanine)
were synthesized and assayed for the ability to
05 stimulate an anti-gpl20 or anti-V3-loop response when
administered to mice n v vo, as described in the
E~emplification below. Murine antisera was tested
for reactivity in a direct binding assay as described
in detail in the E~emplification section. Three
10 peptides were identified which stimulate a response
which is cross-reactive with the natuxal HIV ~.
epitope. The three peptides are 15 amino acid
residues in length and can be represented generically
as follows~
15 Ala Asp Gly Ala AAl Gln AA2 Asn Arg AA3 Gly Ala Ala Gly Ala.
~.
In the generic formula above the symbols AAl, ~A2
and AA3 represent amino acid residues which are not
i
~:: perfectly conserv~d in the three mimic peptides
described herein. Thu~, the peptides of this
20 invention include peptides~of the generic formula set
forth above wherein AAI, AA2:and AA3 represent any
amino acid residue. In addition, the peptides~of the
invention~include fragments of the 15 amino acid
sequence which have the desired properties discussed
25 hereinO More preferably, AAl and AA3 are selected
from the ~roup of amino acids consisting:of serine,
threonine, methionine and cysteine and AA2 is
selected from the group consisting of lysine,
arginine, histidine, serine and threonine. Due t~
30 the critical nature of the he~apeptide, the peptides
of this invention can also be represented generically
including the following sequence:
W092/22579 PCT/US921~4972 `;
~ 6~ -8-
;;,.
AAl ~ln AA2 Asn Arg AA3
where symbols AAl, AA2 and ~A3 are the same as above
described~ the preferred peptides including the .~
following sequences being as follows: ;
05 Ser Gln Thr Asn Arg Met; ~;~
Met Gln Ser Asn Arg Ser; and
Ser Gln Arg Asn Arg Ser.
Peptides of the generic formula set forth above
can be routinely synthesized by standard chemi~al
.10 techni~ues or by recombinant DNA methodology. Such ~
techni~ues are well known in the art. Peptidé ~:
synthesizers are available commercially and standard
techniques have been described in numerous
publications including, for example,~Merrifield;(~
15 ~hem._~Q~. 35:~2149-2154 (1963~9 and Hun~apill:ar et
al. :(Na~ure~lQ:105~ 1984)). :It iæ well known to
one skilled in the art that the immunogenicity of ~:
small non-, or weakly-immu~ogenic molecules (haptens)
can be increased by c:onjugating th~ weakly- :
20 immuno~enic molecule to a carrier protein. Commonly
used carrier:proteins include~ keyhol~ limpet
: hemacyanin a~d bovine serum albumin. The peptides of
his invsntion can be conjugated to a carrier
mole~u~e, usin~ conventional metho~s, to enhance their
25 immunogenic:properties.
Another embodiment of the subject invention is
based on Applicants' discovery of peptides, unrelated
in primary amino acid seguence to the primary amino
acid sequence of an essential protein of a virus
(e.g., HIV gpl20), which have the ability to
wo 92,2257g 2 1 1 1 6 ~ 1 P~TIUS92/04972 `~
_9 _
interfere with a component involved in the function
of the virus. In a pre$erred embodiment, these
peptides have the ability by themselves, or
conjugated to an immunogeni~ carrier molecule, to
05 stimulate the production of antibodies specifically
reactive with the essential viral protein when :
administered to a mammal such as the peptides set
forth above which are mimic peptides for the V3 loop.
One skilled in the art wilI recogniz~ that the
10 amino acid seguences of th~ p2ptides set forth above
represent only the essential portion required for
.
immunogenic or anti-viral a~tivity. ~ peptide~which
is useful as an immunogen or anti-vi~ral aqent is not~
necessarily limited to a mintmal sequence shown
15 above, but mu:st have at least a portion of an amino
acid sequence shown above~which provides immunogenic
or anti-viral activity. In addition, peptides which
include modifiea amino acids~a~re included~within the
scope of the invention provided that the
~ modifications (e.g., conservative substitutions) do
not adversely affect the functional characteristics
~`~ of the psptides.
Uses ~Qr the m~m~ ~eptides
t is w~ll known in~the art:~that antibodies
25 directed to the V3-loop can prevent viral infection
- by both ~ree and cell-associated virus. The mimic
peptides can be used, for example, as immunogens to
stimulate an anti-gpl20 immune response in an
individual. Such a response could be protective in
30 the sense that the individual to whom the peptides
are administered may be immune to infection by the
HIV virus.
''
WO92/2257g PCTiUS92/04972 .:
6~ o- ~
To be used as a vaccine, the mimic peptides are
formulated in a composition and administered
according to conventional protocols. Such a
comp~sition includes at least one mimic peptide, by
05 itself or conjugated to~n immunogenic carrier
molecule, in an amount sufficient to stimulate an `:
immune response in a host. The mimic peptide is :
contained in a physiologically acceptable carrier. ;:
Such carrier~ include, ~or example, fillers,
10 non-tosic buffers, physiological saline solution,
etc~ The composition can also include adjuvants,
protease inhibitors, lymphokines, or compatible drugs-
. where such a combination is:seen as d~sirabl:e oradvantageous. -
Although the role of the V3-loop has not been
elucidated, it appears that the V3-loop has a viral:
function which leads to viral propa~ation. ~ Other
patho~enic viruses contain proteins which have a
viral function which lead to viral propagation~
Peptides which are unrelated in primary amino
acid sequence to the primary amino acid seque~ce of
an essential protein of a virus, preferably mimic
peptides, can therefore be used-in a therapeutic
composition to interfers with viral repl~ication in an
25 individual. This can occur, for e~ample, by
interference of the binding of a viral product with a
cellular or viral component. The mimic peptides~ can
be used as decoy molecules to inhibit the natural
function of an essential viral protein (e.g.,~ the HIV
30 gpl20 protein). By introducing an effective amount
of the mimic peptides to an ir~fected or non-in~ected
individual, it is reasonable to predict that their
W092/22579 2 11 1~ 1 PCT/US92/~4g72
presence will interfere with viral replication
thereby slowing or preventing the spread of the
infection. `-
EXEMP~IFICATION
05 Direct bindin~_~saY
Direct bindin~ between test peptides and test
antibody (affinity purified or:in serum~ was
determined by~ELISA assay. Costar~ELA/RIA plates
w~r~ coated by overnight incubation with 50 ~1 of a
10 solution containing the test peptides or controls.
Following the overnight incubation, the p:lates were ~
washed 3 times with 1 X Phosphate Buffered Saline ~`
(PBS). The plates were then blocked with 1% Bovine :~-
Serum Albumin (BSA) essentia~lly globulin free (Sigma~
15 ~at. #7638~ at room temperature for absut l~hour or
more.
: The blocker was removed and about 100:~1 of
affinity purified 1 antibody ~or test serum~ was
; added`in~a dilution series. The dilutions~ were made
20 :in PBS-T~FCS :(l X PBS, 0.05% Tween-20, 2:.5% Fetal
Calf~Serum (FCS).~ The serum~was incubated~on the
plate for~:about 1 hour~at room:temperature.
~:~: Following~this incubation:, the plates:were washed 3
times wit~ PBS-T/FCS. ~ ~ :
2 antibody was then added (at about 100 ~1 per
well) and incubated at room temperature for:about 1
hour. The 2 antibody i~ typically an anti-species~
antibody which is biotin labeled. This incubation is
followed by 3 PBS-T/FCS washes.
A Streptavidin-HRP (horseradish peroxidase)
conjugate (Southern Biotechnology Associates,
Birmingham, AL) was added at:an appropriate dilution
: ~
.
W O 92/22579 P ~ /U~;92/04972
~typically 1/10,000) in PBS-T/FCS and incubated at ~-
room temperature for about 1 hour. ThiS ~tep was ;:.
ollowed by 3 washes with PBS-T/FCS. The assay was
developed using the TMB Microwell Substrate System
05 (Kirkegaard & Pexry ~abs, Gaithersburg, :r!lD). The
optical density (OD) was read on an ELISA reader at
450 nanom~ters. ` '
C:ompetit on çxp~iments
Competition e~cperiments:wexe carried out by ~'~
10 mising each test peptide and co~trol peptide in ~,
solution with a test antibody a~ determininq the
~, effect (if any) of the peptide in solution on bindin~ ~'
of t~e antibody to a peptide attached to the plate in
an ELISA assay. The protocol is essentially as
15 described above in connection with the ELI5A assay
except that peptides (or peptide c:onjugates) to be
tested or ability to compete with;the fixed peptides ~
are mi:~ed with the antibody and incubated for about ~;
: ~ 30 minutes prior to the addition of the antibody to
20 the ELISA plate.
Idçn~ification of antibodie ~reactive With V3-loop
Four rat monoclonal antibodies which react:
specifi~ally with HIV-IIIB were tested for V3-loop
binding. Each of these antibodies e~chibits
25 anti-syncytia activity in an n vitro assay. The
antibodies were designated 24D3, 20G3, lG7 and 28G12.
In a direct binding a~say, of the type de~cribed
above, pol~styrene plates were coated with cyclized
V3-loop (50 ,ug/ml), non-cyclized V3 loop (50 ~giml)
30 and recombinant gpl20 (2 llg/ml). Each of the 4 rat
monoclonal antibodies was tested in the assay. In
W092t22S7~ PC~`/US92/04972
2111~1
addition a murine control which was known to bind
V3-loop was included as a positive control. The
results from this bindin~ assay clearly showed that
all four rat monoclonal antibodies react specifically
05 with the cyclized and non-cyclized form of V3-loop.
Antibody lG7 demonstrated the strongest reactivity.
Two of the antibodies were biotinylated (lG7 and
28Gl~) by conventional methods. Specifically, in
separate 1.5 ml polypropylene tubes, 167 ~1 (500 ~g)
10 of 28G12 and 250 ~1 of lG7 (500 ~g) wer~ added. To
each tube was added 12 ~1 o~ a biotin solution and
the tubes were incubated at room temperature, with -~
agitation, for 2 houræ. The biotin solution was
prepared by dissolving 1-3 mgs NHS-LC Biotin (Pierce
15 Cat. #21335; MW 556.59 daltons) in 456.6 ~1 distilled
water~mg of reagen~s. The final concentration waæ
3.93 mM. The solution was made fresh each ~ime the
e~perime~t was performed. : ~:
To prevent biotinylation of the carrier
20 ovalbumin, 500 ~1 of lM ethanalamine was added (pH 9
a-nd the mi~ture was incubated at room temperature for
2 hours. 20 ~1 of carrier ovalbumin (10 ~g/ml was
also added) and the biotinylated antibodies were
diluted against 1 X TBS overnight. The concentration
: 25 of each was determined to be about 400-500 ~g/ml.
The material was concentrated in a CentriconR
30-kilodalton ultrafilter (Amicon~j~ and washed three ~-
times with 2ml TBS to re~ove unconjugated biotin.
300 ~1 of TBSJ0.02~ NaN3 and 40 ~1 (7.44 X 1012
30 virions) W kil led blocking phage was added directly
to the CentriconR. The mi~ture was vortexed to
thoroughly mi~ the blocking phage with the antibody
solution, then reconcentrated. The blockin~ phage
WOg2/22579 PCT/US92/04972
-14- .
6~ ~ `
are meant to block anti~odies that cross-react with
the filamentous phage in general~ apart from the
foreign peptide they display. The retentate was
collected in the conical cup by back-centrifugation,
05 as described in the Centricon~ instructîons, and
stored at 4C.
Screeninq of the:~it~Pe librar~ ~:
Biotinylated antibodies, prepared as described
above, were u~ed to screen an ~pitope library (Scott
10 and Smith, Scien~e 249:386-390 ~1990)). The epitope
library was screened essentially as descri~ed by
. Scott and Smith (E~æ~). In this way, peptides were:
discovered which specifically bind the HIV :~
neutralizing rat monoclonal anti~odies described ~:
15 a~ove. Four 15 amino acid~ length synthetic peptides
were produced which contain the 14 amino acid peptide
identified by 6creening the~epitope library. These
: p~ptides are:referred to herein as M303 G7,:~M305 G14,
M306 G3 and M307 G10 and the sequences~of these
: 20 peptides are as follows:
M303 G7: Ala Asp Gly Ala Ser Gln Thr Asn Ar~
Met ~Gly Ala ~Ia Gly Ala
: M305 G14: Ala Asp Gly Ala Met Gln~Ser Asn Arg
, Ser Gly Ala Ala~ Gly Ala
M306 G3: Ala Asp Gly Ala Ser Gln Arg Asn Arg
Ser Gly Ala Ala Gly Ala
M307 ~10: Ala Asp Gly Ala Ser Gln Asn Asn Gly
Ser Gly Ala Ala Gly Ala
'.
W092/22579 PCT/US92/0497
-15-
2111~1
For purposes of reference, the V3-loop sequence is: ;
~ly Cys Thr ~rg Pro Asn Asn Asn Thr Arg Lys Ser Ile
Arg Ile Gln Arg Gly Pro Gly Arg Ala Phe Val Thr Ile
Gly Lys Ile Gly Asn Met Arg Gln Ala His Cys Gly. The
05 cyclized ~orm of the V3-loop is generated by joining
the amino terminus and the carboxy terminu~ of the
linear molecule. ~:-
Reactiv ~y_of ~0~ ~7.~ ql4 and ~30~ G3 wi~h rat ~:
monQçl~nal 2~I2
The M303 G7, M305 G14, M306 G3 and M307 G10 :~
peptides were syn~hesized by conve~tional methods and
tested for direct binding with xat monoclonal
antibody 28G12 by the ELISA assay described above.
Each of the four peptides was attached to separate
15 wells of an ELISA plate. Appropriate controls were
includ~d such a~:an V3-loop positive control in an
ELISA well and a BS~ negative control in a separate ~
well. ~-
In th~ direct binding assa~, khe antibody showed
20 strong binding to the positive controls (V3-loop and
recombinant gpl20) but showed binding to ~303 G7,
M305 G14, M306 G3 and M307 G10 at near background
levels or sli~htly eIe~ated. This may be an artifact
.
of the small size ~f t~ese peptides (15 amino:acids
25 as compared to 38 for ~3-loop)~
Surprising results were obserYed when the test
peptides were tested i~ the competition assay
described above. Specifically, when three of the
f our test peptid~s were incubated with the 28G12
30 ~ntibody in solution prior to incubation with plates
coated with ~3-loop or recombinant gpl20 a
significant decrease in antibody binding, as
W092/22579~ PCT/VS92/04972
evidenced by a decrease on the OD4so determination,
was observed. When tested in this manner, the
peptide M307 G10 was the only test peptide that did
not result in a significant decrease in antibody
05 binding. This M307 G10 peptide had glycine as the
- ninth amino acid residue, whereas the other three
test peptides, M303 G7, M305 G14 and M306 G3 all had
arginine as the ninth amino acid residue. In the
competition e~periment, the control peptides us~d
10 were lambda repressor amino acid residues 12-26, said
peptide having the sequence Leu Glu Asp ~la Arg Arg
Leu Lys Ala Ile Tyr Glu Lys Lys Lys (CO~T~OL in :
Figure~ 1-7) and a synthesized peptide, M304 P8,
containing the sequence of a 14 amino acid peptide
15 that did not bind biotinylated antibody during the
screening of the epitope library, said M304 P8
peptide having the sequence Ala Asp Gl~ Ala Ile Ser
Asn Leu Ile Ser Gly Ala Ala Gly Ala. The resul'rs of
this e~periment are shown in Figures 1 a~d 2. -
20 Immunization Qf mice with ~m c ~Ptid~
BALB~C mice were immunized u~ing conventional
methods with each of the three mimic peptid~s. The
mice were primed on Day 0 with 50 ~9 per mouse of
either M306 G3 in complete Freund's adjuvant ~CFA) or
25 M306 G3 conjugated to keyhole limpet hemacyanin (RLH~
în CFA. At 21 days post-immunization, thc mice were
tail bled for a 1 response and the serum was
collected. The mice were then boosted with M306 G3
or M306 G3 KLH in incomplete Freund's adjuvant
3~ (IFA)o Two weeks after the boost the mice were bled
for a 2 response. Two weeks after the 2 bleedin~
the mice were bled for a late 2 response. The mice
W092/22579 PCT~US92~04972
21115~1
were again boosted and bled 2 weeks later for a 3~ ~.
response. Two weeks after the 3 bleeding, the mice
were bled for a late 3 response. The boost schedule
was repeated until the late 5 r~sponse was
05 determined. All blood was collected in
Becton-Dickinson serum collection tubes and the serum
separated out and used in direct and competition
binding experiments. ~:
The results of direct binding e~periments using
10 mouse immune serum are presented in Figures 3 and 4.
Figure 3 represents an avera~e value of assays
performed after each bleed from 4 mice immunized with
M306 G3. The figure legend lists the antigen which
was bound to the plate. The control was bovine serum
15 albumin (BSA)-. As can be seen clearly in Figure 3,
immunization with M306 G3 mimic peptide led to the
production of murine antibodies specifically reactive
with both V3-loop and the M306 G3 peptide. The
immunized mice had never been esposed to KLH and ~`
20 the~refore the reactivity seen in Figure 3, middle
bar, is due to specific reactivity with the mimic
peptide. As can be seen~in the later bleeds,
particularly the 4 bleed,~immunization with the
mimic peptide results in the production of murine
25 antibodies which are specificaIly reactive with the
V3-loop peptide.
Figure 4 shows the results of a similar
experiment in which M306 G3 conjugated to KLH was ~;
used to immunize 4 mice. The data presented in
30 Figure 4 represents an average value for the 4 mice.
The antigen which was fixed to the plate is shown in
the legend. KLH was not present on the plate and
therefore the middle bar represents reactivi~y toward
O ~/2257g PCI/US92/04972
~ 18-
the mimic peptide specif ically. Again the antiserawas reactive with V3-loop although a greater
disparity was observed between ~he relative
affinities of the antisera for V3-loop versus M306 G3
05 than was observed in the e~periment described in the
preceding paragraph.
Competition experiments were carried out using
the mimic peptides and the mouse immune serurn. As -
shown in Figu~e 5, in a f irst set o escperiments,
10 serum from a mouse immunized wi~h Mi306 G3 KLH was ;-:
mi~ed with peptides prior to incubation:with V3-loop
f i~ed to an ELISA plate. A significant dscrease in ~ ;`
the OD reading was o~served when t~e serum was
~ ~.
preincubated with either V3-loop or M306 G3 as
15 compared with the OD reading when the seru~ was
preincubated~with PBS. This result indicates that
mice immunizeid with the M306 G3 K~H conjugate produce ~::
antibodie& which cross-react with both M306 G3 and `~
~3-loop.
:Figure:6 shows the results of competition
e2periments in which M306 G3 BSA was fixed~ to the
ELISA plate and the serum was from a mouse immunized
with M306 G3 KLH. As ca~ be seen in the figure~ the
M306 G3 peptide was able to compete effecti~ely at
25 an~igen concentrations exceeding l0 ~g/ml. ::~
Competition ~periments were also carried out
using the affinity purified 28G12 monoclonal
antibody. Figure 7 shows the results~of a
competition experiment with this antibody using ELISA
30 plates to whieh V3-loop had been attached. Again,
M306 G3 mimic peptide showed:competitive behavior at
concentrations e~ceeding 10 ~g/ml.
W092/22579 -19 2 1 ~ 1 6 8 1 PCT/US92/04972
. ~
Figure 8 shows the results of a 28G12 competition :~
experiment in which recombinant gpl20 was fixed to
the ELISA plate. In this e~periment, the inclusion
of the M306 G3 peptide resulted in a substantial
05 reduction in the OD determination indicating
effective competition~
EquivalentS
Those skilled in the art will know, or be able to
ascertain using no more than routine experimentation,
10 many equivalents to the specific embodiments of the
invention described herein. These and all other
equivàlents are intended to be encompassed by the
~ following claims.
~ ~;