Note: Descriptions are shown in the official language in which they were submitted.
' `'93~02~1 2 112 ~ 7 8 PCT/EP92/01626
INDOL~S
This invention relates to indole derivatives which
have steroid 5o~reductase inhibitory activity.
More particularly this invention relates to indoles,
their preparation and their use as testosterone-So~
reductase inhibitors.
The androgen class of steroi~al hormones, which
includes testosterone, is responsible for the difference
in the physical characteristics of males and females. of
all the organs that produce androgens, the testes produce'
these hormones in the greatest amounts. Over-production
of these hormones in the body results in many undesirable
physical manifestations and disease states, e.~. acne
! vulgaris t alopecia, seborrhoea, female hirsutism, benign
prostatic hypertrophy and male pattern baldness.
The principal androgen secreted by the testes is
testosterone and it is the primary androgen present in
male plasma. The principal mediator of androgenic
activity in certain organs such as the prostate and
sebaceous gland are the 5o~reduced androgens.
Testosterone is therefore the prohormone of 50~
dihydrotestosterone which is formed locally in the above
organs by the action of testosterone-So~reductase. The
presence of elevated levels of dihydrotestosterone in
many disease states has therefore focussed attention on
the synthesis of testosterone 5o~reductase inhibitors.
~ Testosterone So~reductase inhibitors may also be
r,~ useful in the treatment of human prostate
adenocarcinomas.
EP-A-0458207 discloses certain indole derivatives
which have testosterone 50~reductase inhibitory activity.
W~93/02051 PCT/EP92~0162fi
~ 6~ -2-
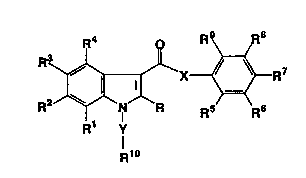
The present invention provides compounds of the
formula:-
X ~ -R7
Rl y
119
and pharmaceutically acceptable salts thereof,
wherein X is O, NH, N(C1-C4 alkyl), Cl-C4 alkylene, C2-C4
alkenylene or C2-C4 alkynylene, said alkylene,
alkenylene and alkynyléne groups being
optionally substituted by Cl-C4 alkyl or aryl;
Y is Cl-C6 alkylene optionally substituted by
Cl-C6 alkyl;
R is H, OH, halo, Cl-C4 alkyl or Cl-C4 alkoxy;
Rl, R2~ R3 and R4 are each independently selected
from H, Cl-C4 alkyl, Cl-C4 alkoxy, OH, halo and
CF3;
one of R6, R7 and RB-is Cl-C1s alkyl or ~ group of
the formula -Z(Cl-Cls alkyl), -Z(aryl) or
-ZtC3-C7 cycloalkyl), said alkyl group being
optionally interrupted by O, S(O)q~ NH or N~C1-
C6 alkyl), and said alkyl group and the alkyl
group of said -Z(C1-CI5 alkyl) group being
optionally su~stituted by Cl-Cl0 alkoxy, aryl,-
C3-C7 cycloalkyl or a group of the formula
-Z(aryl), and the remainder of R6, R7 and R8 and
Rs and R9 are each independently selected from
H, C1-C4 alkyl, C~-C4 alkoxy, halo and halo~Cl-C4
alkyl);
~ 93/02~1 PC~/EP92/01626
21~267~
R10 is COOH, COOR11 or CoNRl2R~3i
R1l is a biolabile ester-forming group;
R12 and R13 are each independently selected from
H and Cl-C4 alkyl;
Z is O, S(O]q~ NH or N(C1-C6 alkyl);
q is O, l or 2;
and "aryl", used in the definitions of X, R6, R7
and R~, means phenyl optionally substituted by
C1-C6 alkyl, C1-C6 alkoxy, C2-C6 al~.enyl, OH.,
halo, CF3, halo(C1-C6 alkyl), nitro, amino, c2-C6
alkanamido, C2-c6 alkanoyl or phenyl.
Alkyl, haloalkyl, alkenyl and alkoxy groups
containing three or more carbon atoms and alkanamido and
alkanoyl groups containing four or more carbon atoms may
be straight- or branched-chain.
The term "halo" means fluoro, chloro, bromo or iodo.
: The term 9'biolabile ester-forming group" is well
. understood in medicinal chemistry as meaning a group
~`si which forms an ester which can be readily cleaved in vivo
i~ to liberate the corresponding acid of the formula (I~
wherein R10 is COOH. A number of such ester groups are
well-known, for example in the penicillin area or in the
case of the angiotensin-converting enzyme (ACE) inhibitor
~ntihypertensive agents.
,~
, . .
~.
~,
.~
r
'S.~
. ~
:~,
~ 4_ PCT/EP92/0162
Esters of the formula (I) wherein Rl is -CO2(C~-C6
alkyl) are steroid S~reductase inhibitors per se but, in
general, where Rl is COORll such compounds are useful as
pro-drugs to provide compounds of the formula (I) wherein
Rl is COOH in vivo following oral administration. Such
esters are also useful as intermediates for the
preparation of compounds of the formula (I) wherein Rl is
COOH.
The suitability of any particular ester-forming
group for this purpose can be assessed by conventional in
vitro or in vivo enzyme hydrolysis studies. t
Examples of suitable biolabile ester-forming groups
are alkyl (e.g. C~-C6 alkyl), alkanoyloxyalkyl(including
alkyl, cycloalkyl or aryl substituted derivatives
thereof), arylcarbonyloxyalkyl (including aryl
substituted d~rivatives thereof), aryl, arylalkyl,
indanyl and haloalkyl: wherein alkanoyl groups have from
2 to 8 carbon atoms and alkyl groups have from 1 to ~
carbon atoms, all of which may be straight- or branched-
chain, and aryl means phenyl or naphthyl, both of which
may be optionally substituted by Cl-C4 alkyl, Cl-C4 alkoxy
or halo.
In addition to Cl-C6 alkyl, specific examples of
other biolabile ester-forming groups are benzyl, 1-~2,2-
diethylbutyryloxy)ethyl, 2-ethylpropionyloxymethyl, 1-(2-
ethylpropionyloxy)ethyl, 1-(2,4-dimethylbenzoyloxy)ethyl,
o~benzoyloxybenzyl, l-(benzoyloxy)ethyl, 2-methyl-1-
propionyloxy-l-propyl, 2,4,6-trimethylbenzoyloxymethyl,
1-(2,4,6-trimethylbenzoyloxy)ethyl, pivaloyloxymethyl,
phenethyl, phenpropyl, 2,2,2-trifluoroethyl, 1- or 2-
naphthyl, 2,4-dimethylphenyl, 4-t-butylphenyl and 5-
indanyl.
The pharmaceutically acceptable salts of the
~ compounds of the formula (I~ are the acid addition and
3 the base salts thereof.
-
2112~78
~93/02051 PCT/EP92/01626
Suitable acid addition salts are formed from acidswhich form non-toxic salts and examples are the
hydrochloride, hydrobromide, hydroiodide, sulphate,
bisulphate, phosphate, hydrogen phosphate, acetate,
maleate, fumarate, lactate, tartrate, citrate, gluconate,
benzoate, methanesulphonate, benzenesulphonate and ~-
toluenesulphonate salts.
Suitable base salts are formed from bases which form
non-toxic salts and examples are the aluminium, calci~lm,
lithium, magnesium, potassium, sodium, zinc and
diethanolamine salts.
For a review on suitable salts 5ee Berge et al, J.
Pharm. Sci., 66, l-19 (l97~).
In the above definitions relating to the present
invention:
Preferably X is O, NH, C1-C4 alkylene or C2-C4
~ alkenylene.
¦ More preferably X is O, NH, methylene, ethylene or
ethenylene.
Most preferably X is methylene.
Preferably Y is C1-C6 alkylene.
Most preferably Y is propylene.
:J ~ ~ ,
Preferably R is H or Cl-C4 alkyl.
Most preferably R is H.
Preferably R1, R2, R3 and R4 are each H.
~,
,
.~ _, . ..
WO93/02~1 ~ ~ PCT/EPg2/016
Preferably one of R6, R7 and RB is -O(C1-C15 alkyl),
the alkyl of said -O(C1-C1s alkyl) group being optionally
substituted by aryl, and the remainder of R6, R7 and R8
and R5 and R9 are each H.
More preferably one of R6, R7 and R9 is -O5H2(aryl) or
: -OCH(C1-C~ alkyl)(aryl) and the remainder of R6, R7 and X~
and Rs and R9 are each H.
:- Most preferably R7 is -OCH(CH3)(aryl) and R5, R6, R~
and R9 are each H.
Preferably R10 is COOH or COOR11.
~. Most preferably R10 is COOH.
',~
Preferably R11 is C1-C6 alkyl.
Most preferably R11 is ethyl.
Preferably Z is O.
Preferably "ary~" means phenyl optionally
substituted by from l to 3 substituents, more preferably
means phenyl optionally substituted by l or 2
substituents and most preferably means phenyl optionally
~ substituted by one substituent.
9 In a preferred aspect of the present invention
"aryl" means phenyl optionally substituted by C1-C6 alkyl,
C1-C6 alkoxy, halo, CF3, nitro or phenyl and more
preferably means phenyl optionally substituted by n-
~ propyl, isobutyl, methoxy, chloro, CF3, nitro or phenyl.
.~`-; Yet more preferably "aryl" means phenyl, 4-(n-
propyl)phenyl, 4-isobutylphenyl, 4-methoxyphenyl, 2,4-
dichlorophenyl, 3,4-dichlorophenyl, 4-
tri~luoromethylphenyl, 4-nitrophenyl or 4-phenylphenyl
and most preferably means 4-isobutylphenyl.
, . . .
s:~
~93no~ PCT/EP92/01626
_7_ 2 1 ~ 8
A compound of the formula (I) may contain one or
~ore asymmetric carbon atoms and/or one or more alkenyl
groups and may therefore exist in two or more
stereoisomeric forms. The present invention includes
both the individual stereoisomers of the compounds of the
formula (I) together with mixtures thereof. Separation
of diastereoisomers or cis and trans isomers may be
achieved by conventional techniques, e.g. by fractional
crystallisation, chromatography or H.P.L.C. of a
stereoisomeric mixture of a compound of the formula (I)
or a suitable salt or derivative thereof. An individual
enantiomer of a compound of the formula (I) may also be
prepared from a corresponding optically pure intermediate
or by resolution, such as by H.P.L.C. of a racemate using
a suitable chiral support or by fractional
crystallisation of the diastereoisomeric ~alts formed by
I reaction of a racemate with a suitable optically active
I acid or base.
¦ Particularly preferred embodiments of the compounds
of the formula (I) are
~ (R,S)-4-(-3-t4-(l-t4-(2-methylpropyl)phenyl~ethoxy)-
¦ phenylethanoyl]indol-l-yl)butanoic acid and
I (S)-4-(3-~4-(l-[4-(2-methylpropyl)phenyl~ethoxy)-
i phenylethanoyl]indol-l-yl)butanoic acid:
~ and the pharmaceutically acceptable salts thereof.
I The compounds of formula (I) provided by the
, . , . .................. ~,....... .
invention;may be prepared by the following methods:-
l) The compounds of the formula (I) wherein R10 is COOHand X, Y, R and R1 to R9 are as previously defined
for a compound of the formula (I) may be prepared by
cleavage of an ester of the formula:-
:~ '
.
WO93~U~1 PCT/EP92/0162~
.ol~
R2 r~ R ....
R1 y
COOR
wherein R14 is a suitable ester-forming group and X,
Y, R and R1 to R9 are as previously defined for a
compound of the formula (I).
;:
A plethora of suitable ester-forming groups that may
be cleaved ~o provide the corresponding carboxylic
acid are known to the skilled man, see, e.g., T.W.
Greene and P.G. Wuts, "Protective Groups in Organic
Synthesis", Wiley-Interscience (2nd edition, lg9l).
Where R14 is an ester-form.ing group that may be
removed by hydrolysis, e.g. Cl-C6 alkyl or an
alternative biolabile ester-forming group as
previously defined (i.e. a compound of the fonmula
(Ij wherein R10 is COOR1l), the hydrolysis may be
carried out under acidic or basic conditions, e.g.
using an aqueous solution of sither a suitable
mineral acid or a suitable inorganic base.
Preferably the hydrolysis is carried out under basic
conditions.
,; .
In a typical procedure an ester of the formula (II)
~' is treated with an aqueous solution of a suitable
base, e.g. sodium or potassium hydroxide, and in the
presence of a suitable organic co-solvent, e.g.
;
r,`'
: `
~93/O~K1 2 1 1 2 S 7 ~ PCT/EP92/01626
_9_
tetrahydrofuran or a C1-C4 alkanol such as methanol.
The hydrolysis is typically carried out at from room
temperature to the reflux temperatur~ and preferably
is carried out at room temperature. The product is
obtained as a base salt which may be-converted to
thie carboxylic acid by acidif ication in the work-up
procedure.
Where R14 is an ester-forming group that may be
removed by reduction, e.g. benzyl, the reduction may
be carried out by catalytic hydrogenation using,
e.g., palladium-on-charcoal, as the catalyst.
2) The compounds of the formula (I) wherein R10 is COOH
and X, Y, R and R1 to R9 are as previously defined
for a compound of the formula (I3 may be prepared by
~ hydrolysis of a compound of the formula (I) wherein
- R10 is CoN~12R13 and X, Y, R, R1 to R9, R12 and R13 are
as previously defined for a compound of the formula
The hydrolysis may be carried out under acidic or
basic conditions, e.g. using an aqueous solution of
~o either a suitable mineral acid, e.g. hydrochloric or
3 sulphuric acid, or a suitable inorganic ~ase, e.g.
sodium or potassium hydroxide, at from room
temperature to the reflux tempPratur~. When basic
hydrolysis conditions are used the product is
obtained as a base salt which may be converted to
the carboxylic acid by acidification in the work-up
procedure.
3) The compounds of the formula (I) wherein R10 is COO~
and X, Y, R and R1 to R9 are as previously defined
for a compound of the formula (I) may be prepared by
hydrolysis of a compound of the formula:-
~6
WO93/02~1 PCT/EP92/016~
q 6~ ~ -10-
R4 0 R9 ,12
R~ X ~R7
R2~ R R5 R~
Rl l
CONHNHR 1B
wherein X, Y, R and R1 to R9 are as previously
defined for a compound cf ~he formula (I) and Rls is
H or C1-C~ alkyl.
The hydrolysis may be carried out under acidic or
basic co~ditions, e.g. using an aqueous solution of
either a suitable acid, e.g. hydrochloric or acetic
I acid, or a suitable inorganic base, e.g. sodium or
! potassium hydroxide, at from room temperature to the
- reflux temperature. When basio hydrolysis
, conditions are used the product is obtained as a
I base salt which may be converted to the carboxylic
acid by acidi~ication in the work-up procedure.
.
~'9 93/02~1 2 ~ ~ 2 ~ ~ 8 PCT/EP92/01626
4) The compounds of the formula (I) wherein R10 is COOH
and X, Y, R and Rl to R9 are as previously de~ined
for a compound of the formula (I) may be prepared by
hydrolysis of a compound of the formula:-
R3 x.~R ,.. ,(IV)
R2~r R R5 R6
R1 y
CN
.
wherein X, Y, R and Rl to R9 are as previously
~, defined for a compound of the formula (I).
.
The hydrolysis may he carried out under acidic orbasic conditions, e.g. using an aqueous ~olution of
either a suitable acid, e.g. hydrochloric or
sulphuric acid, or a suitable inorganic base, ~.g.
sodium or potassium hydroxide, at from room
temperature to the reflux temperature. When basic
conditions are used hydrogen peroxide may optionally
be present and also the product is obtained ~s a
base salt which may be converted to the carboxylic
acid by acidification in the work-up procedure.
~.
; ~
, ~,
,,~
.,
W093/0205~ ~ ~?,6~ PCT/EP92/0162
. -12-
S) The compo~nds of the formula (I) wherein R10 is COOH
and X, Y, R and R1 to R9 are as previously defined
for a co~pound of the formula (I) may be prepared by
acidic hydrolysis of a compound of the formula:-
R~ R7
R1 y
r
R16 R17
wherein X, Y, R and R1 to R9 are as previouslydefined for a compound of the formula (I) and Rl~ and
R17 taken together represent ethylene, said ethylene
group being optionally substituted by phenyl or C1-C4
alkyl ~preferably methyl). Preferably R16 and R17
ta~èn together represent -CH2C(CH3) 2- .
The hydrolysis may be carried out using an aqueous
solution of a suitable acid such as hydrochloric
acid at from room temperature to the reflux
temperature.
., .
93/02051 21 ~ 2 6 7 8 PcT/Ep92lol626
The compounds of the formula (I) wherein R10 is CONH2
and X, Y, R and R1 to R9 are as previously defined
for a compound of the formula (I) may be prepared by
partial hydrolysis of a compound of the formula (IV~
wherein X, Y, R and R1 to R9 are as previously
defined for a compound of the formula (I). The
hydrolysis may be carried out using concentrated
sulphuric acid at from 0OC to room temperature.
The compounds of the formula (I) wherein Rl is COOR
and X, Y, R, R1 to R9 and R1l are as previously
defined for a compound of the formula (I) may be
prepared by esterification of a compound of the
formula (I) wherein Rl is COOH and X, Y, R and Rl to
R9 are as previously defined for a compound of the
formula (I) with an alcohol of the formula R1lOH
wherein R1l is as previously defined for this method.
The reaction may be carried out under classical
esterification conditions such as by using an excess
of the alcohol and with acid catalysis, e.g~ by
sulphuric acid or p-toluenesulphonic acid, at from
room temperature to the reflux temperature. The
water generated during the reaction may be removed
by azeotropic distillation or by the use of a
dehydrating agent or a molecular sieve.
~he esterification may also be carried out ~y
reacting the acid with the alcohol in the presence
of a dehydrating agent, e.g.
dicyclohexylcarbodiimide or diethylazodicarboxylate/
triphenylphosphine (see O. Mitsunobu, Synthesis,
19~1, 1).
g
WO93/U~ PCT/EP92/01626
-14-
- Alternatively the esterification may be carried out
by first forming an activated ester or imidazolide
~ derivative of the carboxylic acid, followed by
:: reaction of the activated ester or imidazolide n
situ with the alcohol of the formula RllOH. An
`~ activated ester may be formed by reacting the
carboxylic acid with l-hydroxybenzotriazoie in the
presence of a suitable dehydrating agent, e.g. l-(3-
N,N-dimethylaminopropyl)-3-ethylcarbodiimide, and in
a suitable solvent, e.g. dichloromethane, at room
temperature. An imidazolide may be formed by
~ reacting the carboxylic a~id with l,l'-
; carbonyldiimidazole in a suitable solvent, e.g.
- dichloromethane, at room temperature.
8) The compounds of the formula tI) wherein R10 is COOR~
wherein X, Y, R, R1 to R9 and R~1 are as previously
defined for a compound of the formula (I) may be
prepared by reaction of a compound of the formula:-
,~
"s
~j
X~R
. . R1 y
~r~ coz1
S ~ .
." ~
j.~
r '
~''~93/02051 2 1 ~ 2 v 7 8 PCT/EP52/016~
-15-
wherein X, Y, R and R1 to R9 are as previously
defined for a compound of the formula (I) and Z1 is a
suitable leaving group, e.g. chloro or bromo, with
an alcohol of the formula R11OH wherein R11 is as
previously defined for this method.
....
The reaction may be carried out in the presence of
an acid acceptor, e.g. pyridine, and in a suitable
solvent, e.g. dichloromethane, at from OC to room
temperature.
.
9) The compounds of the formula (I) wherein R10 is COOR
1 wherein X, Y, R, R1 to R9 and R11 are as previously
! defined for a compound of the formula ~I) may be
, prepared by reaction of a base salt of a compound of
! the formula (I~ wherein R10 is COOH and X, Y, R and
to R9 are as previously defined for a compound of
the formula (I) (i.e. a car~oxylate base salt) with
a compound of the formula R11Z2 wherein R11 is as
previously defined for a compound of the formula (I)
and Z2 i5 a suitable leaving group, e.g. halo,
preferably bromo or iodo, or p-toluenesulphonyloxy.
Preferred base salts of the compounds of the formula
(I) for use in this method are the sodium and
potassium salts. The reaction ~ay be carried out in
a~suitable solvent, e.g. dimethylformamide or
tetrahydrofuran, at from room temperature to the
reflux temperature.
:
10) The compounds of the.formula (I) wherein Rl~ is
CoNR12R13 and X, Y, R, R1 to R9, Rl2 and R13 are as
previously defined for a compound of the formula (I)
may be prepared by reaction of a compound of the
~-~ formula tI) wherein R10 15 COOH and X, Y, R and R1 to
,
~, .
6~ ~
; WO9~1 PCT/EP92/01626
-16-
R9 are as previously defined for a compou~d of the
formula (I), with an amine of the formula Rl2R13NH
wherein R12 and R13 are as previously defined for this
method, in the presence of a dehydrating agent, e.g.
dicyclohexylcarbodiimide. The reaction may be
carried out in a suitable organic solvent, e.g.
dichloromethane, at from room temperature to the
reflux temperature.
Alternatively the reaction may be carried out by
first forming an activated ester or imidazolide
derivative of the carboxylic acid, followed by
reaction of the activated ester or imidazolide in
~ij situ with the amine of the formula R12R13NH. Suitable
procedures for the formation of an activated ester
or imidazolide are described in method (7).
11) The compounds of the formula (I) wherein Rl is
CoNR12R13 and X, Y, R, R1 to R9, Rl2 and Rl3 are as
previously defined for a compound of the formula (I)
may be prepared by reaction of a compound of the
formula (VI) wherein X, Y, R, R1 to R9 and Z~ are as
previously defined for a compound of the formula
(VI), with an amine of the formula R12R13NH wherein Rl2
and R13 are as previously defined for this method.
The reaction may~-be carried out in the prèsence of
an acid acceptor,~e.g. pyridine, and in a suitable
solvent, e.g. dichloromethane, at from 0C to room
temperature.
12) The compounds of the formula (I) wherein R10 is
CoNR12R13 and X, Y, R, R1 to R9, Rl2 and Rl3 are as
previously defined for a compound of the formula (I)
may be prepared by reaction of a compound of the
W.093/02051 2 I ~ ~ ~ 7 ~ PCT/EP92/01626
-17-
formula (II) wherein R14 is a suitable ester-forming
group, e~g. C1-C6 alkyl or an alternative biolabile
ester-forming group as previously defined (i.e. a
compound of the formula (I) wherein Rl is COOR11), or
p-nitrophenyl, and X, Y, R and R1 to R9 are as
previously defined for a compound of the formula
(I), with an amine of the formula R12R13NH wherein R12
and R13 are as previously defined for this method.
The reaction may be carried out in a suitable
solvent, e.g. a C1-C4 alkanol, at from room
temperature to the reflux temperature. The reaction
is usually carried using an excess of the amine~ and
in a sealed reaction vessel.
.
13) The compounds of the formula (I) wherein R10 is COOH
or CoNR12R13, X is C1-C4 alkylene, C2-C4 alkenylene or
C2-C4 alkynylene, said alkylene, alkenylene and
' alkynylene ~roup~ being optionally substituted by C1-
~ C4 alkyl or aryl, and Y, R, R1 to R9, R12 and R13 are
Ji as previously defined for a compound of the formula
, may be prepared by acidic hydrolysis of a
compound of the formula:-
R7 ....
R
. COR20
.,
.,
,
W093/02~ PCT/EP92/01626
- -18-
wherein X, Y, R and Rl to R9 are as previously
defined for this method, Rl8 and Rl9 are either each
C~-C4 alkyl or when taken together represent c2-c3
alkylene, said alkylene group being optionally
substituted by C1-C4 alkyl, and R20 is OH, OR21 wherein
R21 is a suitable ester-forming group that may be
removed by hydrolysis, e.g. Cl-.C6 alkyl or an
alternative biolabile ester-forming group as
previously defined, or NR12R13 wherein R~2 and ~13 are
as previously defined for this method. The
hydrolysis may be carried out using a suitable acid, .
e.g. hydrochloric acid or p-toluenesulphonic acid,
in the presence of water.
..
A compound of the formula (VII) may be prepared by
first forming the corresponding ketal of a compound
of the formula (VIII) wherein X, R and R1 to Rg are
as pre~iously defined for this method ~y reacting
with the corresponding alcohol under acidic
conditions, e.g. see T.W. Greene, "Protective Groups
in Organic Synthesis", Wiley-Interscience (1981),
followed by N-alkylation of the ketal by a similar
procedure to that described in method (14) for
alkylation of compounds of the formula (VIII).
,
~ 14) All the compounds of the formula (I) wherein X, Y, R
j and Rl to Rl are as previously defined for a
compound of the formula (I) may be prepared by
alkylation of a base salt (i.e. the N-deprotonated
form) of a compound of the ~ormula:-
~S
'
~93/02051 PCT/EP92/01626
2 ~ ~ 8
R4 o R~ ,R8
R3~``X ~R7
R2~q~ R Rs R6
R1 H
., ~
wherein X, R and R1 to R9 are as previously defined
for a compound of the formula (I), with a compound
of the formula Z3-Y-cooR22, Z3-y CoN~12~13 or a b
salt of a compound of the formula Z3-Y-CooH, as
appropriate, wherein Y. R1~ and R~3 are as previously
defined for a com~ound of the formula (I), Z3 iS a
leaving group, e.g. halo, preferably chloro, bromo
or iodo, methanesulphonyloxy or p-
toluenesulphonyloxy, and R22 is a biolabile ester-
forming group as previously defined for Rll. The
preferred ~ase salts of the compounds of the formula
Z3-Y-CooH are the alkali metal and alkaline earth
metal salts, e.g. the sodium and potassium salts.
Thé preferred base salts of the compounds of the
,
formula (VIII) are the alkali metal salts, e.g. the
,~ sodium and potassium salts.
, . . .
~,
~,
" , . . ..
W093/02~1 PCT/EP92/01626
~ 6 ~ -20-
The reaction may be performed by initial
deprotonation of the compound of the formula (VIII)
with a suitable base, e.g. sodium hydride, Eollowed
by reaction of the resulting anion with the compound
f th formula Z3-Y-cooR22~ Z3-Y-coNRI2R13 or a base
salt of the compound of the formula Z3-Y-CooH, as
required. The reaction may be carried o~t in a
suitable solvent, e.g. N,N-dimethylformamide or
tetrahydrofuran, at from 0C to the reflux
temperature and preferably at about room
temperature. The reaction may also be carried out
using potassium carbonate as the base and in 2-
butanone as the solven~ at about the reflux
temperature of the solvent.
Alternatively the reaction ~ay be carried out under
phase transfer conditions where a suitable ba~e is
sodium or potassium hydroxide.
Where a compound of the formula (I) wherein Rl is
COOH is required the product is obtained as a base
salt which may be converted to the carboxylio acid
by acidification in the work-up procedure.
-
15) The compounds of the formula (I) wherein X is C1-C4
alkylene, C2-C4 alkenylene or C2-C4 alkynylene, said
alkylene, alkenylene and alkynylene groups being
optionally substituted by C1-C~ alkyl or aryl, and Y,
R and R1 to R10 are as previously defined for a
compound of the formula (I), may be prepared by
acylation of an indole of the formula:-
r~ , ~, ~ . . .
~ 93/02~1 21 ~ 2 ~ 78 PCT/EP92/01626
-21- -
R4
R3~
R2~r R
R1 y
~oR~3
or, where R is OH, a base salt thereof,
or of a base salt of an indole of the formula:-
R3 ~4
R2~1 ~ R
R~ Y
CO2H
wherein Y, R and R~ to R4 are as previously definedfor a compo~nd of the for~ula (I) and R23 is either
oR24 wherein R24 iS a biolabile ester-forming group as
previously dèfined for R11, or is NR12R13 wherein R12
and R13 are as previously defined for a compound of
the formula (I), with a compound of the formula:-
R9 8
O
Il ~ ~
Z4~1\ X~ R7 ~ (XI)
>=<
~5 ~6
22- PCT/EP92/01626
wherein X and Rs to R9 are as previously defined for
this method and Z4 is a leaving group, e.g. halo,
preferably c~loro, and in the presence of a Lewis
acid where R is not OH and optionally in the
presence of a Lewis acid where R is OH. Suitable
Lewis acids include aluminium chloride and
diethylaluminium chloride.
The reaction may be carried out in a suitable
solvent, e.g. toluene, at from room temperature to
the reflux temperature.
The preferred base salts of the indoles of the
formula (X) are the alkali metal and alkaline earth
metal salts, e.g. the sodium and potassium sa.lts.
"
Where a compound of the formula (I) wherein Rl is
COOH is required the product is obtained as a base
salt which may be converted to the carboxylic acid
by acidification in the work-up procedure.
Where a compound of the formula (I) wherein R is OH
is required the compounds of the formulae (IX) and
(X) must be in the form of an enolate salt.
Accordingly'an indole of the formula (IX~ where R is
OH or a base salt of an indole of the formula (X)
where R is OH should first be treated with one
equivalent of a suitable biase, e.g. calcium
hydroxide, to form an enolate salt which may then be
acylated with a compound of the formula ~XI),
optionally in the presence of a Lewis acid.
Incorporation of an acidification step in the work-
up procedure affords the compound of the formula (I)
wherein R is OH.
~
.
,Y,~
, !
,. . .
',,j
~'~93/02051 21~ 2 ~ 7 & PCT/EP92/01626
-23-
16) The compounds of the formula (I) wherein Rl is COOH,
X is 0, NH, N(CI-C,~ alkyl) or C1-C~ alkylene, said
alkylene group being optionally substituted by C1-C~
alkyl or aryl, and Y, R and R1 to R9 are as
previously defined for a compound of the formula
(I), may be prepared by oxidative cleavage of a
compound of the formula:-
R ~ ~ X ~ R7
R2 ~1 r R R5 R6
wherein Zs is -CH=CH2, -CH=CH(Cl-C4 alkyl), -CH=C(Cl-C4
alkyl) 2 or -CECH and X, Y, R and Rl to R9 are as
previously defined for this method.
The reaction may be carried out by ozonolysis or by
treatment with aqueous potassium permanganate
solution.
i, 17) The compounds of the formula (I) wherein X is 0, R
is either COOR11 or CoNR~2Rl3 and Y, R, R1 to R9, R11,
R12 and R13 are as previously defined for a compou~d
of the formula (I~, may be prepared by
esterification of a compound of the formula:-
~,
li
.,,
~'
,i
~,
W093/~2~1 ~16~ PCT/EP92/01626
-24-
R~co2H
R1 y
Co(oRll or NR12R13~
wherein Y, R, R1 to R4, R11 R12 and R13
previously defined for this method, with a phenol of
the formula:-
t
. ` .
~9 ~R8
HO~27 ~..(XIV)
, R5 R~
!.~wherein Rs to R9 are as previously defined for
,...
~d this method. A similar esterification
proced~re to any one of those described in
method (7) may be used.
., ~
,~
,
i, .
~,,
~,;
~93/02~1 ~112 6 7 8 PCT/EP92/01626
-25-
18) The compounds of the ~ormula (I) wherein X, Y, R and
Rl to Rl are as defined for a compound of the
formula (I~ in method (17) may be prepared by
reaction of a compound of the formula:-
R3~CoZ6
R1
CO(ORl1 or NRl2Rl3)
wherein Y, R, R1 to R4, R11 Rl2 and R13previously defined for this method and Z6 iS a
leaving group, e.g. chloro or bromo, with a phenol
of the formula (XIV) wherein Rs to R9 are as
. ~ pre~iously defined for this method.
The reaction may be carried out in the presence of
an acid acceptor, e.g. pyridine, and in a suitable
solvent, e.g. dichloromethane, at from OC to room
temperature.
19) Th~ compounds of the formula (I) wherein X is NH or
N(Cl-C4 alkyl), R10 is either COORll or CoNRl2Rl3 and Y,
R, R1 to R9, R11, R12 and R13 are as previously defined
for a compound of the formula (I), may be prepared
by reaction of a compound of the formula (XIII) or
- an activated ester or imidazolide thereof, wherein
R Rl to R4 R11 R12 and Rl3 are as previou51Y
defined for this method, with an amine of the
formula:-
''
.
WO93/02~1 ~ PCT/EP92/016
26-
R9 ~8
~R7 .... (X
)=<~
i R5 R6
wherein R24 is H or C1-C4 alkyl and R5 to R9 are as
previously defined for this method.
The reaction may be carried out in the presence of a
suitable dehydrating agent, e.g.
dicyclohexylcarbodiimide, and in a suitable organic
; solvent, e.g. dichloromethane, at from room
temperature to the reflux temperature.
Alternatively the reaction may be carried out
by first forming an activated ester or
imidazslide derivative of the carboxylic acid,
followed by reaction of the activated ester or
imidazolide in_situ with the amine. Suitable
procedures for the formation of an actiYated
ester or imidazolide are described in method
(7).
.~
,,
,~
~093/02~1 2 1 ~ 2 6 7 8 PCT/EP92/016~
-27-
20) The compounds of the formula (I) wherein X, Y, R and
Rl to R10 are as defined for a compound of the
formula (I) in method (19) may be prepared by
reaction of a compound of the formula (XV) wherein
Y, R, Rl to R4, R11, R12 and R1~ are as previously
defined for this method and Z6 iS as previously
defined for a compound of the formula (XV), with an
amine of the formula (XVI) wherein R5 to R9 and R24
are as previously defined for an amine of the
formula (XVI). The reaction may be carried out in
the presence of an acid acceptor, e.g. pyridine, and~
in a suitable solvent, e.g. dichloromethane, at from
0C to room temperature.
21) The compounds of the formula (I) wherein X is NH or
N(C1-C4 alkyl), R10 is COOH or CoNR~2R13 and Y, R, R1 to
; R9, R12 and R13 are as defined for a compound of the
formula (I), may be prepared by reaction of a
compound of the formula:-
,~
~ R3~COOR2s
,, 1 11 1 1 .... (XVII)
7! R2~r~R
" R1 y
,. I
~ CO~ )
, .
~.
~ or a base salt thereof,
~.
.,
WO93/020~ q~ PCT/EP92/01
-28-
wherein Y, R, R1 to R4, R12 and R13 are as previously
defined for this ~ethod and R2s is a suitable ester-
forming group, e.g. C~-C4 alkyl or p-nitrophenyl,
with an amine of the formula (XVI) wherein Rs to R9
are as previously defined for this method and R24 iS
as previously defined for a compound of the formula
: (XVI~.
~,
The reaction may be carried out in a suitable
solvent, e.g. a C1-C4 alkanol, at from room
temperature to the reflux temperature.
22) The compounds of the formula (I) wherein R10 i.s COOH
and X, Y, R and R1 to R9 are as previously defined
for a compound of the formula ~I), may be prepared
J by oxidation of a compound of the formula:--
~!
,
~ R4 O R~ ~8
r~ x~ Q~V~
CH20H
;'
wherein X, Y, R and R1 to R~ are as previously
defined for a compound of the formula (I). A
suitable oxidising agent for this purpose is
chromium trioxide in pyridine.
~'.
~g3n~51 PCT/EP92/01626
211267~
. -29-
23~ The compounds of the formula (I) wherein X is C2-C4
alkylene optionally substituted by C1-C~ alkyl or
aryl, and Y, R and R1 to R10 are as previously
defined for a compound of the formula (I), may be
prepared by reduction of a compound of the formula
(I) wherein X is C2-C4 alkenylene or C2-C~ alkynylene,
said al~enylene or alkynylene group being optionally
substituted by Cl-C4 alkyl or aryl, and Y, R and
to R10 are as previously defined for a compound of
the formula (I).
The reduction may be carried out using hydrogen in
the presence of a suitable catalyst, e.g. palladium-
on-charcoal, and in a suitable solvent, e.g. ethanol
or ethyl acetate, at from room temperature to the
reflux temperature and at a pressure of from one to
five atmospheres (l.Ol x 105 to 5. 07 X 105 Pa).
,, .
The compounds of the formula (I) wherein one of
R6, R7 and RB is a group of the formula -Z(C1-Cl5
alkyl) or -Z (C3-C7 cycloalkyl), the alkyl of
said -Z(C1-C15 alkyl) group being optionally
substituted by C1-C1o alkoxy, aryl, C3-C7
cycloalkyl or a group of the formula ~Z(aryl),
Z i5 0, S, NH or N(Cl-C6 alkyl) and the
remainder of R6~ R7 and R8,-R5, R9, X, Y, R, R1 to
R~, R10 and "aryln~are as previously defined for
a compound of the formula (I), may be prepared
by reaction of a compound of the formula:-
.
~,.
~,
,
,,
J
W093/0~ PCT/EPg2/016
R4 0
~ X ~ R~ ....
R2 ~ ~ R R26 R27
! Rl Y
or a base salt thereof,
wherein one of RZ7, R28 and ~29 iS a group of theformula -Z7-H wherein Z7 iS 0, S, NH or N(Cl-C6
alkyl) and the remainder of R27, R28 and R29 are
as previously defined for this method for the
remainder of R6, R7 and R8, R2~ and R30 are as
pre~iously defined for this method for R5 and R9
and X, Y, R, Rl to R4 and Rl are as previously
defined for this method, with a compound of the
formula R3lZ8 wherein R3l is C~ s alkyl or C3-C7
cycloalkyl, as appropriate, said alkyl group
being optionally substituted by Cl-C10 alkoxy,
aryl, C3-C7 cycloalkyl or a group of the formula
-Z(aryl), wherein "aryl" and Z are as
previously defined for this method, and Z8 iS a
suitable leaving group, e.~. halo, preferably
chloro, ~romo or iodo, methanesulphonyloxy or
p-toluenesulphonyloxy.
j:
,. ~.
~93/02051 2 ~ 1 2 6 7 8 PCT/EP92/01626
-31-
The preferred base salts of the compounds of the
formula (XIX) are the sodium and potassium salts.
Where Z7 is O or S the reaction is preferably carried
out usi~g a base salt (i.e. a phenoxide or
thiophenoxide base salt) of a compound of the
formula (XIX) which may be generated in situ from
the corresponding phenol or thiophenol of the
formula (XIX) using a suitable base, e.g. sodium or
potassium hydroxide or sodium hydride, a~d in a
suitable solvent, e.g. ethanol or N, N~
dimethylformamide, at from 0C to the reflux
; temperature.
Where Z7 is NH or N(C1-C6 alkyl) a compound of` the
formula (XIX) may be reacted with a compound of the
formula R31Z8 in the presence of an additional acid
' acceptor, e.g. pyridine, and in a suitable organic
solvent, e.g. dichloromethane.
25) The compounds of the formula (I) wherein R10 i5 COOR
or CoNR12R13, one of R6~ R7 and R~ is a group of the
~ formula -O(Cl-Cls alkyl), -O(aryl) or -O(C3-C7
i cycloalkyl), the alkyl of said -O(C1-C~5 alkyl) group
~ being optionally substituted by C1-C10 alkoxy, aryl,
~,.
C3-C7 cycloalkyl or a group of the formula ~(a~yl~,
. and the remainder of R6, R7 and R'3, R5, R9, X~ Y, Z,
- R, ~1 to R4, R11, R12, R13 and "~ryl" are as previously
,r" defined for a csmpound of the formula (I), may be
~ prepared by reaction of a compound of the formula
-. (XIX) wherein one of R27, R28 and R29 is OH and the
remainder of R27l R23 and R29 and R26 and R30
, .,
. previously defined for a compound of the formula .
'J (XIX) in method (24) and X, Y, R, R1 to R4 and R10 are
as previously defined for this method, with a
compound of the formula R32oH wherein R32 is C1-C15
alkyl, aryl or C3-C7 cycloalkyl, as appropriate,
, .
WO 93/0205~ Pcr/Ep92/ol6~2
--32--
said alkyl group being optionally ~:ubstituted by Cl-
C10 alkoxy, aryl, C3-C7 cycloalkyl or a group of the
formula -Z(aryl), wherein "aryl" and Z are as
previously defined for this method, in the presence
of a suitable dehydrating agent, e.g.
diethylazodicarboxylate/triphenylphosphine. The
reaction may be carried out in a suitable solvent,
e.g. tetrahydrofuran, at from room temperature to
the reflux temperature.
26) The compounds of the formula (I~ wherein X is CH(Cl- '
C lkyl) Rl is coo~1' or CoNR12R13 and Y, R, R to R ,
~ ~1l, R12 and Rl3 are as previously defined for a
- compound of the formula (I), may be prepared by
alkylation of a base salt of a compound of the
~i formula (I~ wherein X is CH2 and Y, R and Rl to R13
are as previously defined for this method, with a
compound of the formula (Cl-C4 alkyl)Z9 wherein Z9 is
a suitable leaving group, e.g. halo, preferably
chloro, bromo or iodo, methanesulphonyloxy or
p-toluenesulphonyloxy.
The preferred base salts of the csmpounds of the
formula (I) for use in this method are the sodium
and potassium salts.
The reaction may be carried out by first reacting a
compound of the formula (Ij wherein X is CH2 with a
suitable base, e.g. sodium hydride, and in a
suitable solvent, e.g. N,N-dimethylformamide, at
~, , ,
~ from 0C to room temperature, followed by in situ
¦ alkylation of the base salt formed with a compound
of the formula (C~-C4 alkyl)Z9.
~,-
~ 93/02051 21 1~ ~ 7 8 PCT/EP92/01626
-33-
All of the above reactions and the preparations of
novel starting mater~als used in the preceding
methods are conventional and appropriate reagents
and reaction conditions for their performance or
preparation as well as procedures for isolating the
desired products will be well known to those skilled
in the art with reference to literatu~e precedents
and the Examples and Preparations hereto.
A pharmaceutically acceptable salt of a compound of
the formula ~I) may be readily prepared by mixing
together solutions of a compound of the for~ula (I)
and the desired acid or base, as appropriate. The
salt may precipitate from solution and ~e collected
by filtration or is recovered by evaporation of the
solvent.
~,
The compounds of the ~ormula (I) are steroid 5o~
reductase inhibitors and they are therefore useful
in the curative or prophylartic treatment of
diseases or conditions such as acne vulgaris,
alopecia, seborrhoea, female hirsutism, benign
prostatic hypertrophy and male pattern baldness.
Certain compounds of the formula (I) are alco
useful in the treatment of human prostate
adenocarcinomas. ~ -
The compounds of the formula (I) may be tested invitro for steroid So~reductase inhibitory activity
,,
using prostate tissue from rats or humans.
~,
The compounds of the formula (I) may be tested for
potency in inhibiting rat steroid 5c~reductase using
ventral prostate tissue from male rats. In
determining inhibitory potency against rat prostatic
50~reductase the following procedure was employed:-
~.,
,~
i~
.:
W093/0205~ & PCT/EP92/01626
-34-
Rat prostates were minced into small pieces. The
tissue was homogenised in Buffer A (20mM sodium
phosphate, pH 6.5, buffer containing 0.32M sucrose
and lmM dithiothreitol) with a Brinkman Polytron
(Kinematica, Luzern, GmBH), and then homogenised
with a motor driven (lOOOrpm) Potter Elvehjem
(teflon-to-glass) homogeniser. Prostate particles
were obtained by centrifugation at 105,000G for 60
minutes. The pellet was washed in 4 volumes of
Buffer A and recentrifuged at 105,000G. The
resulting pellet was dispersed in Buffer A (lml per
g of prostate tissue originally used) with a motor
driven Potter Elvehjem homogeniser as described
above. The particulate suspension was stored as lml
samples at -70C.
The following components, dissolved in Buffer B
(40mM sodium phosphate buffer, pH 6.5~, were added
to a test tube: 500~1 of ~3H]-testosterone (l~Ci,
lnmol; Du Pont, NEN Research Products, Stevenage,
U.K.), 100~1 of 0.5mM NADPH, a compound of the
formula (I) dissolved in 5~1 of dimethyl sulphoxide,
and Buffer B to give a final reaction volume of lml.
The mixture was warmed to 37C and the reaction
started by addition of an aliquot of prostate
particulate suspension. The reaction mixture was
incubated at 37C for 30 minutes and then quenched
by addition with vigorous ~ixing of 2ml of ethyl
acetate containing 20~g each of testosterone and 5c~
dihydrotestosterone as carriers. The aqueous and
organic layers were separated by centrifugation at
2000G for 10 minutes. The organic layer was
transferred to a second test tube and evaporated to
dryness under nitrogen. The residue was dissolved
in 50-80~1 of absolute ethanol and spotted onto a
silica gel 60 F254 TLC plate (E. Merck, Darmstadt,
Germany) and developed in chloroform:acetone
~18~:15).
~}
~'~93/02051 21 ~ ~ 6 7 8 PCT/EP92/01626
-35-
The radiochemical content in the bands of the
substrate (testosterone) and the product (5o~
dihydrotestosterone) was determined with a RITA
Radio TLC Analyser (Raytest Instruments Ltd.,
Sheffield, U.K.). The percent of recovered
radiolabel converted to 5o~dihydrotestosterone was
calculated and used to determine enzyme activity.
All incubations were conducted so that no more than
15% of substrate (testosterone) was converted to
product .
The experimentally obtained data for a range of
inhibitor concentrations was computer fitted to a
sigmoidal dose-response curve and concentrations of
compound giving 50% inhibition of 50~reductase
activity (IC50's) were calculated using a SIGFIT
p~ogram (De Lean, A., Munson, P.J~ and Rodbard, D.,
American Journal of Physiology~ 235, E97 (1978)).
J
The compounds of the formula ~I) may be tested for
potency in inhi~iting human steroid 5~reductase
using tissue-from hyperplastic human prostates. In
determining inhibitory potency against human
prostatic 5o~reductase the following procedure was
employed:-
,, ; . .
.. . .
~ Frozen human prostate tissue was pulverised in
7~j liquid nitrogen using a steel mortar and pestle.
. The powdered tissue was homogenised in 4 volumes of
Gi Buffer A (20mM sodium phosphate, pH 6.5, containing
O.32M sucrose, lmM dithiothreitol and 50~M NADPH~
with an Ultra-Turrax (Janke and Kunkel GmBH & Co.,
~' Staufen i.BR., Germany). The homogenate was
': centrifuged at 500G for 5 minutes, to remove large
particles of tissue, and the supernatant was then
centrifuged at lOO,OOOG for l hour. The resulting
. i
WO93/020~ 36- PCT/EPg2/01
pellet was dispersed in Buffer A (lml per g of
prostate tissue originally used) with the Ultra-
Turrax homogeniser. This particulate preparation
was then filtered through 2 layers of cheesecloth
and the filtrate was stored as lml samples at -70C.
.,
The following components, dissolved in Buffer B
(20mM citrate phosphate buffer, pH 5.2), were added
to a test tube: SOO~l of r3H] testosterone (l~Ci,
lnmol; Du Pont, NEN Research Products, Stevenage,
U.K.), lOO~l of NADPH regeneration system (5mM
NADPH, 50mM glucose 6-phosphate, 5 units/ml glucose
6-phosphate dehydrogenase), a compound of the
formula (I) dissolved in 5~1 of dimethyl sulphoxide,
and Buffer B to give a final reaction volume o lml.
~1 The mixture was warmed to 37OC and the reaction
started by addition of an aliquot of prostate
particulate suspension. The reaction mixture was
incubated at 37C for 30 minutes and then quenched
by addition with vigorous mixing of 2ml of ethyl
acetate containing 20~g each of testosterone and 5c~
dihydrotestosterone as carriers. The aqueous and
organic layers were separated by centrifugation at
2000G for lO minutes~ The organic layer was
transferred to a second test tube and evaporated to
dryness under nitrogen. The residue was dissolved
in 50-80~l of absolute ethanol and spotted onto a
silica gel 60 F254 TLC plate (E. Merck, Darmstadt,
Germany) and developed in chloroform:acetone
(185:15).
~",
7i?
" .
':
~093tO2~1 21.~ 2 ~ 7 ~ PCT/EP92/01626
-37-
The radiochemical content in the bands of the
substrate (testosterone) and the product (5c~
dihydrotestosterone) was determined with a RITA
Radio TLC Analyser (Raytest Instruments Ltd.,
Sheffield, U.K.). The percent of recovered
radiolabel converted to 5o~dihydrotestosterone was
calculated and used to determine enzyme activity.
All incu~ations were co~ducted so that no more than
lS% of substrate (testosterone~ was converted o
product.
The experimentally obtained data for a range of
inhibitor concentrations was computer fitted to a
sigmoidal dose-response curve and concentrations of
compound giving 50% inhibition of 5~reductase
activity (ICso's) were calculated using a SI~FIT
prsgram (De Lean, A., Munson, P.~. and Rodbard, D.,
Ameri~an Journal of Physiology, 235, E97 tl97&)).
The compounds of the formula (I) may be tested for
potency in inhibiting steroid 5~reductase activity
in human prostate adenocarcinomas using cell lines
DUl45 and HPC36M. In determining inhibitory potency
against 5~reductase the following procedure was
employed:-
- .. . . .
Human prostate adenocarcinoma cell lines were grown
in Dulbecco's Modified Eagles medium (DMEM)
containing 5~ serum. The cells were recovered from
the medium by centrifugation, washed in serum free
~MEM and suspended at 5-lO x 106 cells/ml. in serum
f ree medium .
~,
ç
WOg3/0205l ~ PCT/EPY2/016~6
-38-
The following components were added to a test tube:
10~1 of ~3H]-testosterone (l~Ci, 20 pmol) dissolved
in ethanol (Du Pont, NEN ~esearch Products,
Stevenage, U.K.) and 5~1 of an ethanol solution of a
compound of the formula (I). The ethanol was
evaporated under nitrogen and the testosterone and
the compound redissolved in 0.25ml of serum free
medium containing 0.25~mol NADPH. The mixture was
warmed to 37C and the reaction started by addition
of 0.25ml of cell suspension (1.2-2.5 x 106 cells).
The reaction mixture was incubated at 37C for 2
hours and then quenched by addition w~th vigorous
mixing of 1.5ml of ethyl acetate containing 20~g
each of testosterone and 5o~dihydrotestosterone as
carriers. The aqueous and organic layers were
separated by centrifugation at 2000G for 10 minutes.
The organic layer, containing testosterone and its
metabolites, was transferred to a ~econd test tube
and evaporated to dryness under nitrogen. The
residue was dissolved in 50-80~1 of absolute
ethanol, spotted onto a silica gel 60 F254 TLC plate
(E. Merck, Darmstadt, Germany) and developed in
dichloromethane:acetone (185:15)~
.
The radiochemical content in the bands of the
substrate (testosterone) and the product (5o~
dihydrotestosterone) was determined wi~h a RITA
Radio TLC Analyser (Raytest Instruments Ltd.,
Sheffield, U.K.). The percentage of recovered
radiolabel converted to S~dihydrotestosterone was
calculated and used to determine enzyme activity~
All incubations were conducted so that no more than
15% of substrate (testosterone~ was converted to
product.
~.
~93/02~1 21 1 2 6 7 8 PCT/EP92/01626
-39-
The experimentally obtained data for a range of
inhi~itor concentrations was computer fitted to a
sigmoidal dose-response curve and concentrations of
compound giving 50% inhibition of 5o~reductase
activity (IC50's) were calculated using a SIGFIT
program (De Lean, A., Munson, P.J. and Rodbard D.,
American Journal of Physiology, 235, E97 (1978)).
For human use, the compounds of the formula (I) can
be administered alone but will generally be
administered in admixture with a pharmaceutical
carrier selected with regard to the intended route
of administration and standard pharmaceutical
practice. For example, they can be administered
orally in the form of tablets containing such
. excipients as starch or lactose, or in capsules or
. ovules either alone or in admixture with excipients,
~ or in the form of elixirs, solutions or suspensions
r~ containing flavouring or colouring agents. They can
be injected parenterally, for example,
1 intravenously, intramuscularly or subcutaneously.
For parenteral administration, they are best used in
the form of a sterile aqueous solution which may
contain other substances, for example, enough salts
or gluc~sP to make the solution isotonic with blood.
~7 For oral and parenteral administration to human
patients the daily dosage level of the compounds of
the formula (I) will be from O.Ol to 2Q mg/kg (in
single or divided doses3 and preferably will be from
O.l to lOmg/kg except for the treatment of human
prostate adenocarcinomas where doses of up to
20mg/kg may be used. Thus tablets or capsules of
the compounds will contain from lmg to 0.5g of
active compound for administration singly or two or
more at a time, as appropriate. The physician in
,,i~,
PCT/EP92/01626
-40-
any event will determine the actual dosage which
will be most suitable for an individual patient and
it will vary with the age, weight and respons~ of
the particular patient. The above dosages are
exemplary of the average case; ~here can, of course,
be individual instances where higher or lower dosage
ranges are merited and such are within the scope of
this invention.
Alternatively, the compounds of the formula (I) can
be administered in the form of a suppository or
pessary, or they may be applied topically in the
form of a lotion, solution, cream, ointment or
dusting powder. For example, they can be
incorporated into a cream consisting of an aqueous
emulsion of polyethylene glycols or liquid paraffin;
or they can be incorporated, at a concentration
between l and 10%, into an ointment consisting of a
- white wax or white soft paraffin base together with
: such stabilizers and preservatives as may be
required.
The compounds of the formula (I) may also be
administered together with an ~antagonist (e.g.
prazosin or doxazosin), an antiandrogen (e.g.
flutamide) or an aromatase inhibitor (e.g.
atamestane), particularly for the curative or
prophylactic treatment of benign prostatic
hypertrophy.
Thus the invention further provides:-
i) a pharmaceutical composition comprisin~ a
compound of the formula (I), or a
~J pharmaceutically acceptable salt thereof,
`' together with a pharmaceutically acceptable
diluent or carrier;
., .
','
,,
~93/02051 21 ~ 2 6 7 8 PCT/EP92/01626
-41-
ii) a compound of the formula (I), or a
pharmaceutically acceptable salt or composition
thereof, for use as a medicament;
iii) the use of a compound of the formula (I), or of
a pharmaceutically acceptable salt or
composition thereof, for the manufacture of a
medicament for inhibiting a steroid So~
reductase;
iv) the use of a compound of the formula (I), or of
a pharmaceutically acceptable salt or
composition thereof, for the manufacture of a
medicament for the curative or prophylactic
treatment of acne vulgaris, alopecia,
seborrhoea, female hirsutism, benign prostatic
hypertrophy, male pattern baldness or a human
prostate adenocarcinoma;
iv) a ~ethod of treatment of a human to inhibit a
-steroid 5~reductase which comprises ~reating
said human with an ef~ective amount of a
compound of the f ormula (I) or with a
pharmaceutically acceptable salt or composition
~,~
thereof;
.-~vi) a metho~ of treatment of a human to cure or
prevent acne vulgaris, alope~ia, seborrhoea,
female hirsutism, benign prostatic hypertrophy,
male pattern baldness or a human prostate
adenocarcinoma which comprises treating said
~-.human with an effective amount of a compound of
the formula (I) or with a pharmaceutically
acceptable salt or composition thereof; and
Vii) novel intermediates of the formulae (II) (with
the proviso that R14 is not as defined for R11),
(IV), (VIII) or a base salt thereof and (XIX)
or a base salt thereof.
;:i
,~
~,,
}~ 1
,. ..
W093/02051 ~ PCT/EP92/016
- -42-
The following Examples illustrate the preparation of
the compounds of the formula (I):-
ILLUSTRATIVE METHOD 1
(R . S ~ -4 - ( 3- r 4- ( 1- r 4-(2-Methvlpro~Yl)phen~llethoxy)-
benzovllindol-l-yl~butanoic acid
A solution of (R,S)-4-(3-[4-(l-t4-(2-
methylpropyl)phenyl]ethoxy)benzoyl]indol-l-yl)butanoic
acid ethyl ester (3.8g) in tetrahydrofuran(THF) ~35ml)
and methanol (35ml) was treated with 2N sodium hydroxide
solution ~35ml). After stirring at room temperature for '
2 hours the mixture was cautiously concentrated in vacuo
to a volume of about 50ml then cooled in an ice-bath and
.acidified with 2N hydrochloric acid solution. The acid
phase was extracted with ethyl acetate (lOOml3, the
organic extract dried (sodium sulphate) and concentrated
in vacuo to provide the title compound as a white foam,
I (3.27g)-
i EXAMPLES 1 to 18
The following compounds of the general formula:-
:!
. O
~X~ R7
~' (CH2)3cooH
, .
~, or base salts thereof, were prepared by hydrolysis of the
corresponding ethyl esters (see Examples l9 to 36) by
similar methods to that used in illustrative Method 1.
v
?
~) 93/020~;1 2 1 ~ 2 68 PCI`/EP92/01626
~, ,~, _ ," ," . N I ~ I
c~l, ~ ~ t--I I I I I - ~ o
D ~ ~ ~ ~ N C~l ~ ~ ~D O I ^ ~'
S_ E~ o ~ ~ OZ,~
o ~ ~ ~ 11 t ) a) z ~ Q
- !
rT = ~ o
I ~ , ~ ~
~ _ ~ ~
WO93/02051 ?~ PCr/EW2NI6
--44--
r O N ~ I I N l
N ~ N ~ a~ I N ~ E I o I ¦
~ u~ ' ~--u~ ~ ~) ~ I U) l
~ ~ .30 ~N . E ~ ~ = Y ~ E N ~ E ~
~ O C~ Z I E ~ O C~ , N ~
Ir ~ ~
11
~ I ~ l~o
~ O C~
1~
L~
~Y!D93/02051 2 11 2 G 7 8 PCI/EP92/01626
--45--
_ . I
N ~ ~~ I ~ N C) N ~ I
I ~ I ~' t`i ~ ~ N a:)
'7i¦ t~GII~ ~J Q ¦ ~ I ~ ~ I O I
¦ C~~ I ~--Uq C I <D Z E N N C
l . _~ ~ ~ IL ~.) (~ _ _~ ~ OD
I_ .~
¦ N I_ + ~ ~
E_ _ _
I
1~
~ - _ I
~ . _ .
W093~02 1 ?,6 (3 PCl`/EP92/016~
q~
--46--
. . _ . - . _
I
~ o I ~ , E ~ ~'~ o I ~
~ ~ ~ ~ O ^O N ~ N C~l In ^
Z co ~ Z ~ C~i ~n ~ ~
~ ~ I I co ~n c~ . I g
u~ l ~ ~ l o)
a ~ ~ C"e :~ s~ ~ N ~ ~0 "o "~ ~ G
~: ~ I ~ z u~ ' ~ ~ I ~Z N 11) 0 0
O ~u~ I E N ~ C Q r~ N ~t .
r ~ 0
E _ .
t
X O ,
.
F
Ul Z _
~0 93/02051 21 i 2 1~ 7 8 PCI`/EP92/01626
--47--
r ~ ~ I ~ _
l c~ o c~ ~i ..... ~ I o O
~ ~ .
I
~ ~n o
WO 93/020~ PCI/EP92/016~ ~
,6 _48
_ _
z ~ '`'--~ Q Z ~, ~ ~ 0 c~
un o ~ C~l I I I ~ o '`' I o I
G ~ Z ~ ~ ~ E ~ Z o ~ ~ ~ a
I .
. __ _
~ U~ ~
I . _ .
_ _
X O
~ , . .
~ ~ _ ~ . ~ -
~ 93/02051 2 1 1 2 ~ 7 8 PCI`/EP92/01626
--49-- .
~ _ -- .
Z ~ o E I ~ c~ o
N o ,~ ,_ ~ ~ ~j ~' o ~o:
u~ ~ O Z ~ I I " ~'Z ~ I I I: I
C I a:~ ~ I O ~O ~Oru~ O XU) I ~ C~ ~ 0 a
O I ~ N U~ ~ t~ O~ I N ~ r~
1~
~Z ~ ~
L~ _, _
6 ' PCr/EPg2/016~
~ - ^ I I I . _~ I ~
~ _
~ 93/02051 21 1 2 6 7 8 PCI~/EP92/01626
--51--
r . _ .
I -
o I ~ ~ e~
Z a, ~." a, ~ E Z ~ Q
, ;~ o ~ Q _ a) ~ Co _ ,
N ~ N ~ ~ _ N N
c ~ ",a) ~ E _,- C7 ~ ~ ",~ ~ ~ E E E c~ E E
6 C I ~ Z c ~ ~ , I ~ Z o o ~n o ~ ~ o
' a~ O ~ 1 ~ o
o ~ () O ~ ~_~
~ -- -.
X O C~
~ -
Q
U~Z .- oo
W0 93/02051 ~ PCI'/EP92~016;~
ILLUSTRATIVE METHOD 2
~ ,S) -4-(3-r4-~l-r4-(2-Methvlprot~vl)~henvll-
ethoxY)benzovl~indol-l-yl)butanoic acid ethyl_ester
A suspension of sodium hydride (60% dispersion in
oil, 716mg) in dry dimethylformamide(DMF) (15ml) at ooc
and under a nitrogen atmosphere was treated dropwise with
a solution of 4-(3-t4-hydroxybenzoyl]indol-1-yl)butanoic
acid ethyl ester (5.24g) in dimethylformamide (30ml).
After stirring for one hour at room temperature a
solution of o~methyl-4-(2-methylpropyl)benzyl bromide
(3.95g) in dimethylformamide (5ml) was added to the
mixture at 0C. The resultant mixture was stirred
overnight at room temperature. The reaction was
partitioned between lN hydrochloric acid solution (lOOml)
and ethyl acetate (200ml). The separated organic layer
was washed successively with lN sodium hydroxide solution
(lOOml), saturated aqueous brine (lOOml) and then water
(lOOml). The organic layer was dried (MgSO4) and :-
concentrated in vacuo to provide a yellow oil. Column
chromatography (silica, 4.1 hexane/ethyl acetate)
provided, after e~aporation of the appropriate fractions,
the title compound, (3.8g).
~-~93tO2~1 21 12 6 7 8 PCT/EP92/01626
-53-
EXAMPLES 19 to 30
The following compounds of the general formula:-
~" ~ R7
r
(CH2)3CO2CH2CH3
were prepared by alkylation of the corresponding phenol
derivatives (see Preparation 4 and Example 37~ with the
corresponding alkyl bromides (see, e.g., Preparations 11
to 13~ by similar methods to that used in illustrative
Method 2.
WO 93/020~ ?~6~ PCI~/EP92/01626
r - - ~ - --
I N I N ~ - I N
~ U~ O I C l ~
o0~ z ~
a ~ S ~ O ~ z ~
I _ ~
~ 0
E c~l ~ ~n ~
IQ~
I ~- ~ , .
~ ~' ~
~O 93/020~;1 2 1 ~ 2 ~ 7 ~ PCI /EP92/01626
--55--
_ , _ _ _ _
I N I N N ~
C~ _ I U~ In I
I I I I ~--I o E
~ ~ .
Z ~ ~ Q ~
a7 I--I I I ~ I I ~I I ~ -~-
E ~ ~ E E ~ E c ~n E ~ I
~:Z u~ ~7 o ~ ~n Z o o u~ , .
CD . _
~C ~ ~ +
.
E _ . .
I _ . _ .
. _ .~
E :
~Z ~ ~
-
WO 93/~ PCr/EP92/016
- --5 6--
_
- I U~ - I C~
N V 0 ' I I I I ~ I I I I
~ 11 C~ ~
Z I o Z --~ ~' ~ E (~ N ~. CD ~ G
.~n ~ ~I I ~--I _ (~) I I I I I
~ C~ o N I I ~ I ~ E ~n 'n E E
~ C I C~ Z o o ~ ~ ~ Z ~ ~
3 ~- ~ NOO~X~ IC~100~ .
IL ( ) o I ~ ~ ~i ~ ~
. _ ~
+ ~+
~:~
- . - -
Q~ . l
. . _ _ _ _
_ _ _
X O O
_ _ T _ _
I ~
Ll~Z ~ __ ~ _ .
'~ 93/02051 _57- 21 1 2 6 7 8 PCI~EP92/01626
~ S ~ ~ ~ S ~
=~
WO 93/02051 ~Q~ PCI`/EP92/016
58-
~_ ' _ - . .,
~D I
_ _ U~
0 I 0 N cn _ I E
11 0 ~ . Q . ~ ~ E ~, -~,
Z C) ~ N ~ ~ E I 2~O ~ N --~ ~ E
_I I I ~ ~ Z~ I ~ I I I I I I
C ~ _ E ~--I c "a~ ~ _ CJ ~
~: Z u~ o o u~ ~ I Z u~ o o
' C~ ` 00 ~ O ~ - o ~ c~ -
I ~ ~ . <o m LL (.) I I ~ ~ c~
~ .
._
t~ _ ~T~
O O
. /
~iVO 93/02051 21 12 B 7 ~ PCI`/EP92/01626
--59--
. -
I I I I I I I
I I _~ ~
_ _ ~ I I I ~--
~ ~ E v ~'` U~ . . ~
S ~ I ~` > 0 ~ E
~o --I I I o I --I I I o I
C~ ~ C`~ ~ ~ C~
c ~ ~ ~ s ~ E
z o o o u~ Z u~ o ~ o~ o
I tD ) C~l ~ Q I ~) C~ ~
~ . ~
t ~
. ... .
UIZ C~l .
WO93~02051 ~ ~ PCT/EP92/01626
60-
EXAMPLE 31
4-~3- r 2-(4- r 1-(4- r 2-Methvl~ro ~ll~h~nYl)ethoxyl-
PhenYl~ro~anoyllindol-l-yl)butanoic acid ethyl ester
(racemate)
CH3
~0~ CH3
~"~ CH3
r
(C~2)3co2cH2cH3
lî ~u CH3
~t~3J~ ~' CH3
(CH2)3CO2CH2CH3
A solution of (R,S)-4-[3-(4-~ 4-[2-
methylpropyl]phényl)ethoxy]phenylethanoyl)indol-l-
yl~butanoic acid ethyl ester (see Example 19) (522mg) in
DMF (5ml) was treated with sodium hydride (60% dispersion
in oil, 43mg) and stirred at room temperature for 10
minutes. Methyl iodide (62~1) was added and stirring was
continued for 16 hours at room temperature. The mixture
was diluted with ethyl acetate (30ml) and washed with lN
hydrochloric acid solution (30ml) and water (30ml). The
organic layer was dried (MgS04) and evaporated to give a
yellow oil which was purified by flash chromatography
~silica, 3:1 hexane/ethyl acetate) to give, after
evaporation of the appropriate fractions, the title
compound as a yellow oil (247mg).
W093/02~1 PCT/EP92/01626
21~2678
-61-
H-NMR (CDCl3): ~ = O.90(d,6H), 1.30(t,3H), 1.50(d,2H),
1.55(d,3H), 1.80(m,1H), 2.15(m,2H), 2.24~m,2H),
2.40(m,2H), 4.10(m,4H), 4.38(q,lH), 5.20(q,lH),
6.78(d,2H), 7.05(d,2H), '7.10-7.40(m,7H), 7.62~d,1H),
8.40(m,1H) ppm.
EXAMPLE 32
(R.S)-4-(3- r N-(4- r 1- r4-~-MethvlproP~l~ehenyl)-
ethoxylPhenvl~carbamovl1indol-1-yl)butanoic acid ethyl
ester
NH ~ OH
--co2cH2cH3
1) NaH,DMF
2~ S
Br~cH3
O Ç~3
~NH ~ }0 ~CH3
CO2CH2CH3
W~93/020~1 PCT/EP92/016
62-
A solution of 4-(3-~N-(4-hydroxyphenyl)carbamoyl]-
indol-1-yl)~utanoic acid ethyl ester (see Preparation 2)
(220mg) in ~MF (5ml) was treated with sodium hydride (60%
dispersion in oil, 26mg). After stirring for 30 minutes
a solution of o~methyl-4-(2-methylpropyl)benzyl bromide
(see Preparation 12) (174mg) in DMF (2ml) was added and
stirring continued for 4S minutes. The mixture was
diluted with ethyl acetate (30ml) and washed ~uccessively
with 2N hydrochlo~ic acid solution (SOml), water (S x
3Oml) and saturated brine (2 x 3Oml).
The organic layer was dried (MgSO~) and evaporated to give
a yellow oil (265mg). Flash chromatography (silica, 3:1
hexane/ethyl acetate) gave, after evaporation of the
appropriate fractions, the title compound as a gum which
crystallised from diethyl ether, (193mg), m.p. 101-103C.
Pound C,7S.S6; H,6.97; N,S-39; C33H38N204 requires:
C,75.30; H,7.28; N,5.32%.
H-NMR (CDCl3): ~ = O.90(d,6H), 1.25(t,3H), 1.60(d,3H),
1.81(m,1H), 2.15(m,2H), 2.30(t,2H), 2.45(d,2H),
4.10(q,2H), 4.20(t,2H), 5.25(q,1H), 6.85(d,2H),
7.10(d,2H), 7.25-7.50(m,7H), 7.55(s,1H), 7.75(s,~H),
8.00(m,1H) ppm.
93/020~ 2 S 7 ~ PCI/EP92/01626
--63--
EXAMPLE 3 3
,4-(3-rN-(4-Benzyloxyphenvl)car-bamo~ ind
vl) butanoic acid ethvl ester
C~NH ~oCH2~3
NaH,DMF
~, 2) E~r~~CO2c H2cH3
~NH ~OCH2 ~3
cs)2cH2~H3
W093/02~1 ~ PCT/EP92/016
-64-
A solution of 3-(N-[4-benzyloxyphenyl]carbamoyl)-lH-
indole (see Preparation 3) (4.0g) in DMF (60ml) was added
to a suspension of sodium hydride t60% dispersion in oil,
515mg) in DMF (20ml~ After stirring for 30 minutes at
20C a solution of ethyl 4-bromobutyrate (2.52g) in DMF
(20ml) was added. After stirring for 30 minutes at 20C
the DMF was removed in vacuo. The resultant off-white
solid was triturated with ethyl acetate and filtered.
The filtrate was absorbed onto silica gel and
chromatographed (silica, 1:1 ethyl acetate/hexane) to
give, after evaporation of the appropriate fractions, the,
desired product, (850mg), m.p. 124-126C. Found:
C,73.91; H,6.38; N,6.14; C28H28N204 requires: C,73.66;
H,6.18; N,6.13%.
..
lH-NM~ (CDCl3): ~ = 1.20(t,3H), 2.15(m,2H), 2.25(t,2~),
4.10(q,2H), 4.20~t,2H), 5.04(s,2H3, 6.95ld,2H), 7.25-
7.50(m,8H), 7.55(d,2H), 7.65(s,1H), 7.75(s,1H),
8.0~(m,lH) ppm.
EXAMPLES 34 to 36
The following compounds of the general formula:-
e~x~ -}ocH2{~3
(cH2)3co2cH2cH3
were prepared by similar methods to that used in Example
33 using the corresponding lH-indoles (see Preparations 4
to 6) and ethyl 4-bromobutyrate as the starting
materials~
.
~093/02~1 2 ~ 7 `~PCT/EP92/01626
-65-
, ~ .-
Example m.p.
No. X (C) m/z Analysis/NMR
I . _ _ _
34 0 96- _ Found: C,73.07; H,5.87;
97 N,3.03;
C29H27N05 requires: C,73.34;
H,5.94; N,3.05%.
H-NM~ (CDCl~ = 1.30
(t,3H), 2.25(m,2H), 2.40
(t,2H), 4.20~q,2H), 4.35
(t,2H), 5.10(s,2H), 7.05
(d,2H), 7.20(d,2H), 7.30-
7.50(m,8H), 8.00(s,1H),
8.30(m,1H) ppm.
, _ _ . ,
CH=CH 83- 468 Found: C,77.00; H,6.11;
ttrans) 84 (M+l)+ N,3.01;
C2~H2sN04 requires: C,77.06;
H,6.25; N,3.00%.
H-NMR (CDCl3): ~ - 1.28
(t,3H), 2.30 (m,2H~,
2.40(m,2H), 4O20 (m,2H),
4.35(m,2H), 5.15 (s,2H),
7.02~d,2H~, 7.28-7.55
(m,9H), 7~65~d,2H), 7.84
(d,lH~, 7.94 (s,lH),
8.55(m,1H~ ppm.
__ _ _ _
WO93/02051 PCT/EP92/016~
~b ``
7 &
66-
, _ -
Example m.p.
No. X (C) m/z Analysis/NMR
, . _
361 CH2 _ _ Found: C,76.22; H,6.29;
N,2.98;
C~gH29NOI requ~res: C, 76. 46;
H,6.42; N,3.07%.
H-NMR (CDC13): ~ - 1c30
(t,3H), 2.20(m,2H), 2.35
(t,2H~, 4.10(s,2H), 4.15
(q,2H), 4.25(t,2H), 5.05 ,
(s,2~), 6.95(d,2H), 7.25-
7.45(m,10H), 7.80(s,lH),
8.45(m,1H) ppm.
1, . .
The reaction mixture work-up was to add lN
hydrochloric acid solution and extract the mixture
with ethyl acetate. The organic layer was washed
with }:~rine and then water, dried and concentrated in
vacuo to provide the required product. The
chromatographic work-up used in Example 33 was hence
unnecessary.
.~,
~IU )93/02051 21 i 2 6 Y PCI/EP92/01626
- --67--
EXAMPLE 3 7
4- ( 3- r 3- ( 4-BenzYloxyphenYl ) propano~l 1 indol-1-
Yl ) butanoic acid ethyl ester
r~ 0 3
co2cH2cH3
H2, Pd/C,
'1, CH3CO2C2H5
~ 1 1 o~3
I CO2CH2~H3
co2~H2cH3
WO93/02051~?~ PCT/EP92/016~
-68- -
A solution of (E)-4-(3-~3-(4-benzyloxyphenyl)-
propenoyl~indol-l-yl)butanoic acid ethyl ester (see
Example 35) (lg) in ethyl acetate (25ml) wa~ hydrogenated
in the presence of 10% palladium-on-charcoal (250mg) at
4.15 x 105 Pa for 4.5 hours. The reaction was filtered
through a cellulose-based filter aid and the filtrate
concentrated in vacuo. The residual oil was
chromatographed (silica, 40% ethyl acetate/hexane) to
first provide, after combination and evaporation of the
appropriate fractions, the title compound, (580mg), m.p.
123-5C, m/z-= 442 (M+l)~. Found: C,75.89; H,6.20;
N,3.17; Cz8H2~NO4 requires: C,76.17; H,6.16; N,3.17%.
lH-MMR (d6-DMSO): ~ = 2.00 (quintet,2H), 2.25(t,2H),
2.90(t,2H), 3.12(t,2H), 4.2S(t,2H), 5.05(s,2H),
6.90(d,2H), 7.20-7.48(m,9H), 7.60(d,1H), 8.20(d,1H),
8.40(s,1H), 12.25(s,br,1H) ppm.
Further elution provided, after combination and
evaporation of the appropriate fractions, 4-(3-t3-(4-
hydroxyphenyl)propanoyl]indol-1-yl)butanoic acid ethyl
ester, (24Omg).
lH-NMR (CDCl3): ~ = 1.25(t,3H~, 2.14(quintet,2H),
2.28(t,2H), 3.02(m,2H), 3.12(m,2H), 4.02-4.26(m,4H),
5.62(s,lH), 6.75(d,2H), 7.10(d,2H), 7.22-7.40(m,3H),
7.54(s,1H), 8.40(m,1H) ppm.
!~093/02~1 2 1 1 ~ 6 7 ~ PCT/EP92/01626
~69-
The following Preparations illustrate the
preparation of certain starting materials used in the
previous Examples:-
ILLUSTRATIVE METHOD 34-(3~4-Hvdroxvbenzovllindol-l-yl)butanoic acid ethYl
ester
A solution of 4-(3-[4-benzyloxybenzoyl3indol-l-
yl)butanoic acid ethyl ester (13.4g) in ethyl acetate
(300ml) was hydrogenated at 4.15 x lOs Pa in the presence
of 10% palladium-on-charcoal (3g3 at room temperature for
4 hours. The catalyst was removed by fi~tration of the
reaction through a cellulose-based filter aid and the
filtrate was concentrated in vacuo to a pale pink solid.
Trituration with cold diethyl ether gave a white powder,
(8.24g).
PREPARATIONS_1 an~d 2
The following compounds of the general formula:-
~X~3OH
(CH2)3GO2cH2cH3
were prepared by hydrogenation of the correspondingbenzyl ethers (see Examples 33 and 36) by similar methods
to that used in illustrative Method 3.
WO93/02~1 PCT/EP92/016
70-
Prep. m.p.
No. X (~C) m/z Analysis/NMR
_
1 CH2 _ 366 lH-NMR (CDCl3): ~ =
(M+l)+ 1.25(t,3H), 2.20(m,2H),
2.35(t,2H), 4.10(s,2H),
4.15(q,2H), 4.25(t,2H~,
6.80(d,2H), 7.20(d,2H~,
7.25-7.45(m,3H),
7.75(s,1H), 8.40(m,lH)
ppm~
21 NH 193- _ Found: C,68.88; H,5.98;
196 N,7.40;
~2lHz2N204 requires
C,68.83; H,6.05; N,7064%.
H-NMR (d6-DMS0~: ~ =
l-O5(t,3H), 2.00(m,2H) r
2.15(t,2H), 3.90(g,2H),
4.05(t,2H), 6.60(d,2H),
7.10(m,2H), 7.25(d,lH),
7.30(d,2H), 7.75(s,1H),
8.05(d,lH), 8.45(s,lH),
8.55(s,lH) ppm.
~ ~ I _~ ~
1 Hydrogenation carried out at 40C.
`-~ 93/02051 21 2 6 7 PCT/FP92/01626
-71-
PREPARATION 3
3-(N- r 4-Benz~loxvPhenvllca~bamoyl)-lH-indole
~,COOH ~N~O
H H
A stirred solution of lH-indole-3-carboxylic acid
(6.0g) in dichloromethane (lOOml) was treated with 1-
hydroxybenzotriazole hydrate (5.Og) and 1-(3-N,N-
dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride
(14.2g) followed by triethylamine (21ml) and
4-benzyloxyaniline hydrochloride (9.65g). The mixture
was stirred at room temperature for 2 hours, diluted with
di~hloromethane and washed successively with water (2 x
l90ml), 2N hydrochloric acid (4 x lOOml3 and saturated
aqueous brine t~ x 50ml). The organic layer was dried
(Na2SO4), filtered and evaporated. During the evaporation
the desired product crystallised as a white solid and was
collected by filtration (5.89g). The mother liquors were
evaporated ~o a pale brown oil and chromatographed
(silica, 1:1 ethyl acetate/hexane3 to give a further crop
of the desired product, ~995mg), m.p. 211-214C.
Found: C,77.55; H,5.51; N,8.26; C22HlBN202 requires:
C,77.18; H,5.30; ~,8.18%.
IH-NMR (d6-DMSO): ~ = 5.05(s,2H), 6.95(d,2H), 7.10(m,2H),
7.25-7.45(m,6H), 7.60(d,2H), 8.10(s,1H~, 8.15(d,1H),
9.60(s,1H), 11.70(s,br,1H) ppm.
WO93~02~1 PCT/EP92/0162
72-
PREPARATION 4
lH-Indole-3-carbo*ylic acid 4-ben~yloxvphenvl est~
COOH
N
H
1) (cocl)2~cH2cl2~DMF
~3CH2o ~;30H,pyridine,C~CI2
~3OCH2 ~3
A suspension of lH-indole-3-carboxylic acid (l0g) in
dichloromethane (250ml) was cooled to OC and treated
wit~ oxalyl chloride (8.9ml) and dimethylformamide (DMF)
~5 drops). After stirring for one hour the clear
solution was evaporated and azeotroped three times with
dichloromethane to give the acid chloride as a brown
crystalline solid.
~ 93~02051 PCT/EP92/01626
2112~78
-73-
To a solution of 4-benzyloxyphenol (12.40g) in
dichloromethane (200ml) was added pyridine (7.Sml)
followed by a solution of the acid chloride prepared
above in dichloromethane (200ml). After stirring
overnight the mixture was evaporated and partitioned
between ethyl acetate (lOOml) and 2N hydrochloric acid
(50ml). The separated organic layer was washed with 2N
hydrochloric acid (2 x 50ml) and then saturated brine (2
x 50ml). The organic layer was dried (MgSO4), evaporated
and the residue crystallised from dichloromethane to give
the title compound as a white solid, (19.57g), m.p. 188- '
189C.
Found: C,77.07; H,4.77; N,4.05; C22HI7No3 requires:
C,76.95; H,4.99; N,4.08%.
lH-NMR (CDCl3): ~ = 4.90(s,2H), 6.85(d,2H), 7.00(d,2H),
7.05-7.40(m,8H), 7.90(s,lH), 8.00(m,lH), ll.OO(s,br,lH)
ppm.
ILLUSTRATIVE METHOD 4
3-(4-Benzvloxvbenzovl)-lH-indole
A mechanically stirred solution of indole (30.0g) in
sodium dried diethyl ether (450ml) was treated dropwise -
with methylmagnesium iodide (8~ml of 3.OM solution in
diethyl ether). After stirring for one hour at 20C
4-benzyloxybenzoyl ch~oride (67.3g) was added. Stirring
was continued for two hours at 20C and then lN
hydrochloric acid (250ml) added to the mixture and the
reaction was allowed to stand overnight. The resulting
precipitate was filtered off and triturated with hot
ethyl acetate (3 x lOOml) to give the desired compound as
a pale pink solid, ~40.9g).
WO 93/02051 PCIIEP92~016s,~
PREPARATIO~S _ 5 and 6
The following indoles of the general formula:-
¢~X~OCH
N
H
were prepared from lH-indole and the corresponding acid
chlorides tsee Preparations 7 and 8) using similar
methods to that used in illustrative Method 4.
~ ` ') 93/0205l PCI /EP92/01626
2112678
--75--
l _ _ . _ I :
Prep . X ( PC j m/ Z Analysis/NMR
No._ . __.
CH2 - 341 lH-N~ (d6-DMS0) i5 =
5.00(s,2~I~,
6 . 90 (d, 2EI), 7 .10-
7 . 40 (m, lOH),
8.10(d,1H),
8.45(s,1H~,
12.00(s,br,1H) ppm.
_ . _ _ _ I
Ç CH--CH ~ (M541), 5 15 (s 2H), 7 . lo-
8 . 3 0 (d , 1H) ,
8 . 65 (d, lH) ppm.
___ . - . ~
W093/02051 PCT/EP92/01626
76- -
ILLUSTRATIVE METHOD 5
4-Benzvloxv-2.3-dimethylbenzovl chloride
4-Benzyloxy-2,3-dimethylbenzoic acid (2.0g) was
suspended in dichloromethane (lOml) and treated with
oxalyl chloride (l.3ml) and dimethylformamide (DMF) (2
drops). After stirring overnight the homogeneous
solution was evaporated to give a white solid which was
azeotroped three times with toluene to give the title
compound as a white powder (2.24g).
PREPARATION 7
4-Benzyloxy~henacvl chloride
The title compound was prepared using a similar
method to that described in illustrative Method 5 except
using 4-benzyloxyphenylacetic acid as the starting
material. The material obtained was used immediately.
PREPARATION 8
(E)-3-(4-BenzYloxy~henYl)Propenovl chloride
The title compound was prepared using a similar
method to that described in illustrative Method 5 except
using (E)-3-(4-benzyloxyphenyl)propenoic acid as the
starting material. The material obtained was used
immediately.
PREPARATION 9
l-(4-n-Pro~vl~henvl~butan-l-ol
~ solution of 4-n-propylbenzaldehyde (7.4g) in
diethyl ether (60ml) was cooled to 0C and treated with a
2.OM solution of n-propylmagnesium chloride in diethyl
ether (27.5ml). The react~on was stirred overnight,
diluted with diethyl ether and quenched with saturated
aqueous ammonium chloride solution. The organic layer
was separated, washed with saturated aqueous ammonium
chloride solution and dried (MgSO4). The organic layer
was filtered and evaporated to give a colourless oil
which was purified by chromatography (silica, 4:l
- hexane/ethyl acetate) to provide, after evaporation of
the appropriate fractions, the desired product, (4.06~),
m/z = 192(M')-
Wo93/02051 2112 ~ 7 8 PCT/EP92/01626
-77-
~H-NMR (CDC13): ~ = l.OO(m,6H), 1.20-1.40(m,2H),
1.70(q,2H), 1.75-l.90(m,3H), 2.60(t,2H), 4.60(m,lH),
7.10(d,2H), 7.30(d,2H) ppm.
PREPARATION 10
(R,S)-1-(4- r 2-Methvl~ropy~ henYl)ethanol
A solution of 4-isobutyrylacetophenone (lO.og) in
methanol (50ml) was cooled to 0C and treated portionwise
with sodium borohydride (3.23g). After stirring
overnight at room temperature the reaction was quenched
with lN hydrochloric acid (SOml) and ethyl acetate
(lOOml) added. The organic layer was separated, dried
(MgSO4) and evaporated to give the title compound as a
clear oil, (10.02g), m/z = 178(M~). Found: C,79.69;
H,9.90; C12H180.1/7 H20 requires: C,79.68; H,10.~9%.
1H-NMR (cDel3): ~ = O . 9o (d,6H), 1. 50 (d, 3H~, 1. 85 (m ~lH),
2.50(d,2H), 4.85(q,lH), 7.15(d,2H), 7.30(d,2H) ppm.
PREPAR~TIONS 11 to 13
The following alkyl bromides were prepared by
dissolving the corresponding alcohol (see, e.gO,
Preparations 9 and 10) in dichloromethane and cooling the
solution in an ice-bath whilst saturating with dry
hydrogen bromide. After stirring the mixture for a ~hort
period the reaction was evaporated in vacuo to provide
the desired alkyl bromlde which was used directly without
characterisation.
._ _ ~ I
Preparation . Alkyl bromide
No. . .
11- 1-Bromo-1-~4-n-propylphenyl)butane.
. . _ . . I
12 ~Methyl-4-t2-methylpropyl)benzyl
bromide.
131 c~(4-n-Propylphenyl~-4-n-propylbenzyl
bromide.
I _ _ ,
1 For starting material see EP-A-291245.
WO93/02~1 PCT/EP92/016
-78-
Pharmacoloqical activitY
A selection of compounds of the formula (I) was
tested in vitro for steroid 5o~reductase inhibitory
activity using ventral prostate tissue from male rats
according to the procedure outlined on pages 33 to 35 of
the specification. The results obtained are`presented in
Table l.
Table l
~ ~ -- .
Example No. ~ IC ; n~)
lO00
7 138
12 ~
In addition, the compound of Example 36 was tested n
vitro for steroid 5o~reductase inhibitory activity using
tissue from hyperplastic human prostates by the procedure
outlined on pages 35 to 37 of the specification. An IC50
value of 89.7nM was obtained for this compound.