Note: Descriptions are shown in the official language in which they were submitted.
WO 93/0207~ 2 1 1 3 7 9 ~ PCI/US92/06003
- 1 -
TITI E
THIN FlLM DEPOLYMERIZATION
TO DIMERIC CYCLIC ESTERS
FIELD OF THE INVENTION
This invention relates to a reduced pressure reactive distillation
process for the preparation of dimeric cyclic esters. The process of the invention
comprises depolymerizing thin films of an oligomer of a hydroxycarboxylic acid
10 to form a dimeric cyclic ester and recovering the cyclic ester. The thin filmprocess of the invention affords rapid and substantially complete conversion to ~ -
dimeric cyclic ester, low hold-up times, little or no decomposition to undesirable
by-products, and bigh quality product in high yields.
BACKGROUND OF THE INVENTION
I)imeric cyclic esters of hydroxycarboxylic acids such as glycolide
(1,~dioxane-2,5~ione) and lactide (1,4-dioxane-3,5-dimethyl-2,5-dione), are
intermediates to bigh molecular weight polyhydroxycarboxylic acids which may
be useful in biomedical and other applications because of their ability to be
degraded biologically and hydrolytically to form physiologically and
enviromnentally acceptable by-products.
A process for producing dimeric cyclic esters may be found in
Aigner et al., European Patent Application Publicadon No. 264926. Aigner et
al., discloses conducting a thermolysis depolymerization reaction continuously in
2s a forced feed flow tube reactor (double screw extruder) under reduced pressure,
while maintaining an increasing temperature gradient along its length. The
dirneric cyclic ester product distills from the reaction mass through vapor ports
located at the downstream end of the reactor, while the higher-boiling residue is
extruded under the force-feed extrusion conditions.
3 0 lt is an object of this invention to provide an improved process for
converting low molecular weight oligomers or polymers of alpha-
hydroxycarboxylis composi~ions to cyclic esters in short residence times and at
high production rates.
It is another object of this invention to provide a process wherein
3s the polymer of an alpha-hydroxycarboxylic composition is substantially
completely converted to the cyclic ester, with substantially reduced racemization
and other undesirable decomposition reactions.
SUBSTITUTE SHEET
Wo 93/0207s Pcr/uS92/o6oo3
9~ 2
It is a further object of this invention to provide a process which
achieves rapid conversion to the dimeric cyclic ester while minimizing hold-up
time in the reactor and exposure of the reaction mass and product to thermal
stress.
SUMMARY OF THE INVENTIQN ;
This invention is based on the discovery that conversion of an
oligomer of an alpha-hydroxycar~oxylic acid moiety (e.g., an oligomer of lactic
acid), to a cyclic ester ~e.g., lactide), proceeds more rapidly than heretofore
believed when the reaction mass comprising the oligomer is spread as a thin
liquid or molten film onto a surface which has been heated to depolymerization
temperatures. Disposing the oligomer on the heated surface as a thin film,
preferably one having a relatively large surface area to film thickness ratio,
enables (1) heat to be transferred rapidly from the heating surface to the
1~ oligomer composition and, (2) reaction products (e.g., lactide) to be transferred
rapidly through the oligomer film and vaporized rapidly from the oligomer
surface (i.e., the reaction products are more volatile than the oligomer). This
makes feasible a short residence time, continuous process providing high
conversions and yields of the cyclic ester while reducing racemization and
decomposition or other undesirable side reactions occurring.
The invention is directed to an improved process for
depolymerizing (thermolyzing) a depolymerizable oligomer of an alpha-
hydroxycarboxylic acid composition to a dimeric gtclic ester under thin film
distillation conditions by heating to effective depolymerization and distillation
2s temperatures, whereby conversion of the oligomer to a vaporized ~yclic ester is
effected at short residence times.
The invention relates to a process for converting an oligomer of an
alpha hydro~ycarbosylic acid, an alkyl ester or salt thereof, comprising (1)
disposing a thin liquid film of a preformed oligomer o~ a heated surface in a
3 o reaction zone, (2) maintaining the surface at a temperature su~lcieIlt to heat the
film to a depolymerization temperature and convert the oligomer to dimeric
cyclic ester while m~intaining the reaction zone at a reduced pressure sufficiently
low to form a vapor product stream containing the dimeric cyclic ester, and (3)
recovering the product stream.
3s The process of the invention is particularly applicable to preparing
lactide, including L~lactide, in high yield and at a high conversion rate from an
oligomer of lactic acid or an ester or a nitrogen base salt thereof.
SU~STITUTE SHEET
wo g3/020~s 2 1 1 3 7 9 9 PCr/USs2~06003
- 3 -
ln one embodiment of the invention, the vapor product stream is
cooled, condensed and collected as a liquid. In a key aspect of this embodiment,the oligomer film is formed and depolymerized continuously, and the vaporized
product is collected continuously. In another aspect of the continuous process
s embodiment, the feed rate of the oligomer to the heated surface, the thickness of
the oligomer film, the temperature and the pressure are coordinated and
controlled such that the depolymerization proceeds substantially completely to ~ - `
form a vaporized product. Minor quantities, if any, of a residue (e.g., so-called
heel) may also be formed, but the presence of a residue does not affect overall
10 performance of the process. The residue can be removed periodically and
recycled or hydrolyzed as hereinafter described. For best results, thin film
depolymerization is conducted, according to the invention, within a wiped-film
evaporator.
The invention is a reactive distillation process comprising a series
15 of steps which include thermolysis/depolymerization of an open-chain poly
(hydroxycarbo~ylic) acid composition to a more volatile cyclic reaction product,followed by vaporization of the cyclic product from the thermolyzing mass and
condensation to recover the ~yclic ester product.
20BRIEF DESCRIPIlON OF THE DRAWINGS
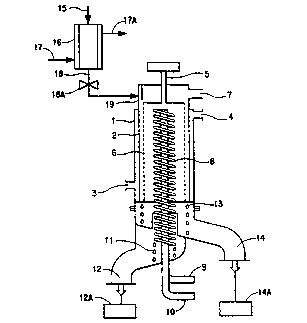
Fig. 1 is a sectional view of a wiped film evaporator with an
internal condenser.
Fig. 2 is a sectional view of the wiped film evaporator of Fig. 1
which is coupled with an external condenser.
25Fig. 3 is a schematic block diagram of the invention.
SU~STITlJTE SHEET
WO 93/0207~s PCI/US92/06003
31~
DETAILED DESCRIP~lON OF THE INVENTION :
The invention is directed to a process for preparing a cyclic ester ;
having the formula: -~
R2
Rl - ci C~
~C C--R
0 R2
wherein R1 and R2 are independently hydrogen or an aliphatic hydrocarbyl
radical having 1 to 6 carbon atoms. According to a key aspect of the process, anoligomer of an alpha-hydroxycarbo~ylic acid, an ester or a r~itrogen base salt
thereof, is introduced as a thin molten film into a reaction surface in a reaction
15 zone which is maintained at a reduced pressure and an elevated temperature.
The pressure and temperature within the reaction zone are maintained such that
the oligomer is depolymerized and a therrnolysis product, or products, are
vaporized to form a product stream containing the cyclic ester. The product
stream may be recovered by any suita~le method which does not adversely affect
20 the cyclic ester. ln some aspects of the invention, the product stream may becondensed which may be followed by one or more of redistillation, extraction
and/or crystallization from a sclvent to recover the desired cyclic ester product.
The oligomeric feed material may comprise an oligomer of an
oligomerizable alpha-hydroxycarbo%~lic acid, ester and/or a nitrogen base salt
25 thereof, which has the fo~nula:
rRl I
HO----C- C----OX
R2 O n
wherein n is an integer of 2 to 50: X is independently H, R3 or a cationic groupHA: Rl, R2, and R3 are independently H or a Cl-C6 hydrocarbyl radical: and
"A" is a nitrogen base. Preferably, Rl, R2 and R3, when other than H in the
above formula, is an alkyl group. More preferably, Rl and R2 are H or methyl,
35 as in glycolic acid (Rl=R2=H) and lactic acid (Rl=H, R2=methyl). The
cationic group HA is preferably derived from a nitrogen base, such as ammonia
or an alkyl amine, and preferably is ammonia or a tertia~ amine, such as
SUBSTITUTE SHEET
wo g3/02075 2 1 1 3 7 !~ 9 PCI`/US92/06003
:~
trimethylamine, triethylamine, methyldiethylamine, tripropylamine,
tributylamine, etc.
The degree of acceptable polymerization (i.e., the value of (n)) and
the resultant molecular weight can vary widely so long as the oligomer may be
5 rendered molten and depolymerized at the operating temperature. For best
results, the value of n is in the range of from about S to about 30 (e.g., not more ~ -
than about 25) and norrnally between about 10 and 15. The value of n tends to
increase during the course of the depolymerization reaction so that any beel (i.e., ~`
polymeric residue) remaining upon completion of the reaction usually has a
0 greater degree of polymerization than the starting oligomer. The heel may be
recycled to the reactor in accordance with the invention so long as the oligomer,
may be rendered molten and is depolymerizable. Alternatively, the heel can be
hydrolyzed to lower molecular weight alpha-hydroxy carboxylic acids, including
the monomeric acid, which can be re-polymerized to oligomers of the desired
degree of polymerization for reuse in the process.
The oligomer should generally be free of dissolved gases, solvents
or other low boiling components to avoid or minimize the possibility of film
deterioration resulting from bubbling and flashing of the film when subjected toa reduced operating pressure. Also, the oligomer is preferably preheated at or
20 close to the operating temperature before it is fed to the reaction zone of the
evaporator.
The depolymerization process is advantageously carried out in a
- continuous manner using a thin film evaporator. The thin film evaporator may
comprise a heated cylindrical or tapered tubular reactor which includes means
25 for distributing the oligomer over the heated inner surface of the reactor. In one
aspect of the invention, the tubular reactor includes a series of rotable wiper
elements that maintain a close clearance from the wall or ride upon a film of
liquid on the waL~ In operation of such a reactor, the oligomer is continuously
fed to and disposed onto the heated surface of the reactor wall as a thin liquid30 film. For best results, the thin film is disposed onto the heated surface in a
manner sufficient to provide a relatively large surface area and a uniform
thickness. For example, the film thickness should be as thin as can be practicably
attained, and may range from about 0.05 to at least about 1.0mm, and normally
about 0.2 through O.Smm thick. For best results, the thickness of the thin film
35 will range from about Q3 to 1mm. Apparatuses which are acceptable for
creating continuous depolymerization conditions are exemplified by:
SUBSTITUTE SHEET ~:
W0 93/02075 PCI'/US92/06003 :
3~
- 6 -
A) A falling film evaporator, wherein the molten/liquified
oligomer flows downward along heated walls of the evaporator. The quality of
the film that forms depends primarily on the force of gravity, the viscosity of the
oligomer and its flow rate along the heated surface. For best results, a falling5 film evaporator of the wiped film type is employed and equipped ~,vith means for
spreading the incoming oligomer horizontally and vertically on the heat surface ; -
so as to create a thin film having a substantially uniform thickness and a
relatively large surface area. The ratio of surface area to film thickness is not
critical. However, in certain aspects of the invention it is desirable to create a
lo high surface area to film thickness ratio since for a given thickness a greater ratio
should increase the beat transfer from the heating means to a greater quantity of .
the liquid oligomer film, enhance tbe mass transfer of the depolymerization
products through and out of the film thereby into the reduced pressure space
above the film as vapor, and result in a greater quantity of product being formed
in a given time period.
B) A desirable wiped-film evaporator for carrying out the process
of the invention is equipped with rotating wiper elements that can be operated at
various speeds of rotation and adjusted to provide different spacings between the
heated evaporator wall and the wiper blades. The wiper blade spacing
determines film thickness, while wiper rotation rate and oligomer feed rate
determine the rate of film formation. Suitable wiper spacing (e.g., for
appropriate film thickness) and speed of rotation are readily determined by trial
for a~y particular oligom¢r composition, oligomer viscosity and other process
conditions (e.g., temperature, pressure, etc.)
2s The vapor product stream produced on depolymerizing the
oligomer to volatile products in the evaporator, is preferably contacted ~Anth acondeDsing surface, maintained at a temperature such that the product stream
condenses as a liquid, which is allowed to drain into a receiver. The condensing- surface may comprise an internal or an external condenser or a combination of
the two which is discussed below in more detail in connection with Figs. 1 and 2.
For example, the wiped film evaporator may include a heated surface which
surrounds and is spaced apart from an internal condenser. The wiped-film
evaporator may also be connected to an external condenser which functions as a . ~;
substitute for or as a supplement to the internal condenser. Tbe surface area ofthe condensing surface may be modified to control the manner in which the
product stream is condensed. For example, the surface area of a coiled tube
condenser may be increased by decreasing the diameter of the tube and ~-
SUBS~lTU~E SH~ET
Wo 93/0207s 2 1 1 3 7 9 9 PCI`/US92/061)~3
increasing the number of coils. The vapor product stream normally may
comprise the dimeric cyclic ester and other volatiles, including open-chain
hydroxycarboxylic acids (e.g., lactic acid, lactoyllactic acid, etc). The condensed
vapor product is readily separated into its constituents using methods such as
s distillation, extraction, crystallization, etc. ~;
The process of this invention is generally conducted in the
presence of a catalyst, which may be included in the oligomeric reactant before it
is fed to the evaporator. The catalyst can be any catalyst which is suitable forpromoting the thermolysis of the oligomers to cyclic esters. Suitable catalysts are
generally metals or compounds of metals of groups IV, V and VIII of the
Periodic Table. Preferred are metals of groups IV, notably Sn as the metal
(powdered), oxide, halogenide or carboxylate, or V, notably Sb, usually as the
oxide Sb203. Preferred herein are Sn (Il) carboxylates, especially those that are
soluble in the molten oligomer and exemplified by stannous bis(2-
ethylhexanoate), comanonly referred to as stannous octoate.
The catalyst will be employed in catalytically-effective amounts,
whicb can vary widely depending upon the particular feed material employed and
the rcaction conditions. The optimum catalytically-effective amounts for any
particular sy~stem can readily be determined through trial runs. For example,
with stannous octoate as the catalyst, the quantity of catalyst will generally be
such that tbe rcaction mass contains from about 0.01 to about 5% by weight,
usual~r from about 03 to 3% and for bcst results, at lcast about 1~o. Higher
catalyst loadings are more desirable because oligomer residence time decreases
with increases in the initial catalyst concentration, thereby improving the dimeric
2s cyclic ester production rate.
~- In one aspect of the invention, it may be desirable to admix a
solvent of the cyclic ester with the oligomer before the oligomer is introduced
into the evaporator. A suitable solvent must be substantially inert under the
depolymerization process conditions and be generally equal to and/or less
3 o volatile than the cyclic ester. A solvent can improve the fluidity of the oligomer
and maintain the cleanliness of the heating wall within the wiped-film
evaporator. A suitable solvent having a volatility generally equivalent to the
cyclic ester may also serve to maintain the cleanliness of the condenser by
washing cyclic ester from the condenser surface.
Suitably effective temperatures in the evaporator can vary widely provided
the temperature is below the decomposition temperature of the dimeric cyclic
ester being formed. Normally, the temperature is in the range of from about
SUBSTITUTE SHEET
wO g3/02075 ~ ~3~ 9~` Pcr/US92/o6u~3
- 8 -
200C to 290C. The optimum temperature range for any particular oligomer-
to-cyclic ester conversion will vary with the composition of the oligomer. For
example, for the production of L-or D- lactide the temperature is usually in therange from about 250C through about 270C and, for glycolide from about
s 260C through 280C .
The surface of the evaporator's heating zone can be heated by any
expedient means. The heating zone is advantageously constructed of a thermally
conductive material so that heat can be supplied from an outside source through
the wall of the heating zone to its internal evaporation surface and thus heat the
0 oligomer film to depolymerization temperatures. Heat may be supplied, for
example, electrically by wrapping the outside of the heating zone with heating
tape or by jacketing the zone with an electrically-heated mantle. Alternatively,the heating zone may be jacketed such that hot oil may be circulated through it
and in this way bring the internal heating surface to the desired temperature. If
15 desired, thermocouples can be placed at the external surface of the evaporator's
heating zone to monitor and record the depolymerization temperature.
The depolymerization process is carried out under subatmospheric
pressures which are consistent with the vapor pressure of the cyclic ester beingrecovered at the operating (depolymerizing) temperature. The pressure can vary
2 o from below about lmm of Hg upwards and is normally below about 20mm of Hg.
Generally, the pressure is the range of about 1 to Smm of Hg.
An important aspect of the invention is that one or more of the
following process conditions, such as the oligomer composition, oligomer
viscosity, oligomer feed rate, speed of film formation (e.g., rotation rate of the
25 wiper blades), f~m thickness, temperature, pressure and oligomer catalyst
loading, can be coordinated such that substantially all the oligomer being fed is
converted to a vapor product stream rapidly and substantially completely with
little or no heel formation. Control of these process conditions makes feasible a
short residence time continuous process affording high conversion of oligomer ~;
3 o and high yields of the desired dimeric cyclic ester with little or no occurrence of
racemization, decomposition or other undesirable side reactions, such as
charring. Residence times can be extremely short, i.e., generally are a matter of
minutes, o&en between about 1 and 10 minutes. While it may be desirable to
achieve as high a conversion/ production rate as possible by eliminating heel
35 formation, it is generally advisable to maintain the depolymerization conditions
such that a small quantity of oligomer heel can be removed from the evaporator
as the reaction proceeds since the heel retains the catalyst, which might
~ SUBSTITUTE SHEET ~-
21~379~
WO 93/0207~ PCI`/US92~06003
otherwise leave deposits on the evaporator walls. The heel may be recycled
directly to the evaporator or it may first be hydrolyzed to lower molecular weight
alpha-hydroxy carboxylic acids which may be recycled along with the catalyst to
form additional oligomer which is then used for producing the cyclic ester.
The characteristics of the heel which is formed, determines the
degree to which it may be recycled. The practicality of recycling the heel
typically is related to the degree to which it is polymerized. For example, heels
which have a relatively high degree of polymerization (as shown by a high
molecular weight) are more difficult to recycle due in part to the reduced
o quantity of hydroxyl groups on the end of the polymer chain. Any heel which
may be produced in the process will have had a shon residence time in the
wiped-film evaporator and, therefore, possess relatively low molecular weights
which typically permits these heels to be directly recycled or readily transformed
into a recyclable form.
The flow of oligomer to the heated wall of the wiped-film
evaporator can vary widely depending on tbe oligomer, its viscosity and its
temperature (viscosity decreasing with increasing temperature); also on the
dcpolymerization temperature and configuration of the evaporator itself. The
flow of oligomer into the wiped film evaporator should be sufficient for the
oligomer to form a film under gravitational flow onto the surface of the heated
wall, but it should not be so fast as to flood the evaporator. The oligomer flowrate should be coordinated witb the depolymcrization temperature so tbat a tbin
film is established and maintained throughout the run and depolymerization
proceeds sucb that most, if not substantially all the oligomer, is depolymerized to
2s the desired ~yclic ester product. Further, the flow rate of the oligomer should
also be coordinated witb tbe rotation rate of the wiper blades (i.e., the wiper
blades cause the oligomer to form a film along the heated surface of the wiped-
film evaporator). For example, a relatively high oligomer flow rate should be
accompanied with a rapid v~riper blade rotation rate to ensure effective
depolymerization of the oligomer without excessive charring or beel formation.
However, in some cases, it is advantageous to leave a minor proportion of
oligomer unconverted so that flowable "heel" can be removed and in this way
maintain a clean heating wall (i.e., a heating wall substantially free of
decomposition products and deposits).
The invention may be better understood with reference to the
drawings.
SUBSTITUTE SHEET
wO 93/02075 3~g~ PCr/USg2/06003
- 10 -
The apparatus of Fig. 1 consists essentially of a jacketed cylindrical
evaporator 1 evacuatable to reduced pressures through linel by a vacuum pump
not shown. Evaporator 1 contains internal condenser.~ spaced apart from inside
wall ~ of evaporator 1 and a rotatable cylindrically shaped wiper blade
s mechanism S disposed between wall 2 and condenser ~. Wiper blade mechanism
S has a plurality of wiper blades ~ adapted to spread liquid oligomer evenly anduniformly over wall 2, the wiper blade mechanism ~ also has a plurality of
openings~ between the blades and along the circumference of the cylindrical
sbape formed by the blades, whereby wall ~ communicates with condenser 8
0 through the combined 6A openings. The spacing between wall ~ and blades 6
can be fixed or adjusted to provide a variety of wall-to-wiper blade spacings.
Wiper blade mechanism 5 is rotatable at various controlled speeds by a motor
drive not shown at SA.
Evaporator wall 2 is heated from an external surface to operating
temperatures by circulating appropriately hot oil through the jacket, the oil
entering at 3 and exiting at 4.
Thermocouples placed at the jacket of 1 provides a measure of the
temperature at wall ~. The depolymerization temperature developed at wall 2 is
a function of the temperature of the heating fluid, which temperature is readilydetermined by trial for any particular oligomer.
The temperature of condenser ~ is controllable by circulating
coolant through it via entering and leaving lines ~ and 10.
Evaporator 1 is adapted to receive molten oligomer from jacketed ;
feed vessel 16. The oligomer, fed to 16 through line.L~, is maintained molten by -~
cira~lating sufficiently hot oil through the jacket, the oil entering at 17 and
exiting at~ The temperature of the molten oligomer should be below but
reaso~ably close to the depolymerization temperature. Valve 18A controls the
feed rate of the molten oligomer to evaporator 1. Normally, valve 18A is -
adjusted such that about SOOcc of oligomer will pass into the jacketed cylinderical
evaporator in from about 45 rninutes through about 2 hours. Deflector plate 19
is positioned to direct molten oligomer fed via line 18 to wall 2 of the evaporator
so that it can flow under gravity down the wall and can be spread by wiper blades
vertically and horizontally to a substantially uniform thickness over the entireinside wall of the evaporator.
Depolymerization products vaporized from the oligomer heated to
depolymerization temperatures at wall 2 pass through openings.~, condense at
least in part on condenser 8 and are collected as condensateL, via lineL2, in
SUBSTITUTE SHEET -
WO 93/0207~ PCI'/USg2/06003
21137!J9
- 11 -
receiver 12A. Molten material not converted to vapor collects as heel 13, via line
14, in receiver 14A.
Condensate 11 can be further processed by any means known to
the art for recovering and further refining the cyclic ester product, if desired.
Similarly, the recovered "heel" may be recycled to the reactor for
conversion to additional quantities of cyclic ester. If its degree of polymerization
is higher than desired, it may be further processed to convert it to an oligomerhaving a more optimum degree of polymerization.
Fig. 2 incorporates the features of Fig. 1 and includes as well
external condenser 20 cornrnunicating with evaporator 1 through insulated line 7(insulation not shown). Condenser 20 is cooled by a coolant (e.g., ethylene
glycol, water, mL~tures thereof, etc.) circulating through it via lines 21 and 22, and
is so sized and placed as to condense substantially all vaporized product not
condensed by condenser 8, condensate 23 passing through line 24 to receiver
24~ Uncondensed product vapor, if any, leaving conderlser 20 through line 7A
passes through cold trap 26 maintained at a low temperature, for example that
provided by a solid CO2 - alcohol mixture 27. Sensor 28 measures the reduced
pressure established by the vacuum pump.
It should be noted that in the above representative thin film
evaporators the distance between the wall at which the oligomer is
depolymerized and the condensing surface can vary widely. For example, in an
evaporator of the internal condenser ~pe it may be very small, for example, on
the order of the mean free path of the dimeric cyclic ester depending on the
operating pressure or it may be, as in evaporators with external condensers, quite
large, for exarnple, many times the mean free path of the cyclic ester. For the
present invention, it has been found that the distance bet veen heating surface
and condensing surface is not critical provided the pressure is sufficiently low and
the depolyrnerization temperature is sufficiently high to ensure an adequate
cyclic ester production rate. Optimum ~emperatures and pressures for a given
3 0 oligomer in a given evaporator are readily determined by trial. Evaporators with
external condensers, which imply relatively large heating surface to condensing
surface distances, are preferred herein for the more complete recoveries of the
vapor products they provide.
Now referring to Fig. 3 which is a schematic block diagram of the
3 5 operation for producing the cyclic ester, concentrated aqueous lactic acid,
preferably containing about 8~90~o by weight lactic acid (e.g., 88% acid as is
available comrnercially), is fed through line 31 to converter 32 where it is further
SUBSrITUTE SHEET
WO 93/0207~ PCI`/US92/06003
~9~
1, -
concentrated by distillation and polymerized to po1ylactic acid (PLA) with
further removal of water-of-reaction by heating gradually to about 160C, then to
175C usually under reduced pressure or with a N2 sweep. The aqueous
distillate removed during this concentration and polymerization stage is passed
through 33 to concentrator ~4, where it is dehydrated to concentrated lactic acid
for recycle. Polylactic acid produced in 32 is sent through ~6 to a thin film
evaporator 37, such as illustrated in Figs. 2 and 3, where it is converted to the
corresponding dimeric cyclic ester(e.g., lactide), as described above. The
condensed product produced in evaporator 37 containing lactide, minor
o proportions of lactic acid and still minor proportions of volatilized water-soluble
oligomers generally having 2 to 3 lactic acid units, can be further
processed/purified by solvent treatments. For example, the condensed product
exits the thin film evaporator through 38 and enters an extractor 39, where it is
extracted with a suitable solvent, for example acetone, to form a solution of ~-
lactide and its lactic acid value impurities.
With a water-miscible solvent such as acetone, the solution is
concentrated to start precipitation of lactide, and diluted with water, preferably
water cooled to ~5C, in an amount sufficient to precipitate the lactide `
substantially completely, leaving the lactic acid values in the aqueous solution. -
Lactide, substantially free of its impurities, is separated, as by filtration orcentrifugation, removed through line 41 and purified further if desired, by
washing, drying and recrystallizadon from non-reactive solvents (e.g., toluene). ~
When a water-immiscible solvent is employed (e.g., methyl isobutyl ketone), the `;
organic solution produced in~, is thoroughly extracted with water in an amount
sufficient to remove substantially all the lactic acid values from the solution, and
the resulting aqueous phase separated from the organic. The lactide-containing
organic phase is removed via line 40 and lactide recovered from the solution by
any means known to the an (e.g., solvent evaporation), crystallization and
recrystallization, as necessary or desired.
The aqueous solution containing substantially all the lactic acid
values and residual organic solvent is sent through line 41 to concentrator 42
where any organic solvent present is stripped therefrom, and the aqueous
solution concentrated for recycle (e.g., to 88% lactic acid), the organic solvent
and excess water being removed via line 43, the recycle stream through 47. The
3 5 polylactic acid residue remaining in the thin-film evaporator 37 is removed
through 44. If necessary or desired, the residue (or heel) can be hydrolyzed back
to monomeric (e.g., lactic acid), by passing it to hydrolyzer 45 where it is heated
SUBSrlTUTE SHEET
Wo 93/0207~ Pcr/US92/o6oo3
2113799
- 13 -
with water at the boil until the residue is converted substantially completely to an
aqueous solution.
The hydrolysate is filtered and concentrated, if necessary, to, for
example, 88% lactic acid for recycle. Preferably, the proportion of water
5 employed for hydrolysis will be sufficient to provide an hydrolysate having the
desired lactic acid concentration. Also, one could use dilute aqueous lactic acid
for hydrolysis. Lactic acid hydrolysate for recycle leaves hydrolyzer 45 through47, is combined with the lactic acid recycle stream 48 (from 43) and 35 (from 34)
to form combined stream 49, which is recycled to line 31, then to converter 2
lo along with make up concentrated lactic acid.
The overall operation, representing a single illustrative recycle
stage, can be repeated to achieve a lined-out recycle process wherein the amountof recycle material will equal the amount of lactic acid material generated in
each pass. Recycle of recovered lactic acid enables the quantity of fresh lacticacid feed to the converter to be reduced and the overall yield of lactide thereby `~
increased.
The following examples are provided to illustrate, not limit the
scope of the appended claims. Unless specified otherwise, commercially
available materials were used to practice the following examples.
EXAMPLES
A UIC Industries (Joliet, Illinois, USA) Model KDL4 wiped film
evaporator was used in each of the following examples. The evaporator included
a vertically arranged cylindrical housing which possessed about 0.043 square
25 meters of evaporator surface. The evaporator was modified to include an
external condenser downstream from its internal condenser which is illustrated in
Figure 2. The evaporator was jacketed for heating its evaporator surface with
circulating hot oil and was also equipped with (a) a jacketed feed funnel, also
heatable with circulating hot oil, ~b) a feed means for metering molten oligomer30 into the evaporator at control1ed rates, the feed being provided at the top and
allowed to flow down the interior cylindrical wall of the evaporator, (c) glass-reinforced Teflon~ polytetra-fluoroethylene rollers serving as rotating wiper
blades for mechanically spreading the oligomer as a thin film both horizontally
and vertically over the heated wall. The wiper blades were rotated by a Janke-
3s Kunkel model no. RW20 drive mechanism which had a setting of 2.5. The wiperblade spacing was set to provide a film thickness of about O.5mm. The unit
SUBSTITUTE SHEET
WO g3/02075 PCr/USg2/06003
3~9~ ~
included means for evacuating and maintaining the evaporator under reduced
pressure.
EXAMPLE 1
~ An oligomer of lactic acid was prepared by gradually heating a
mixture of about 1650 grams 88% L,lactic acid containing less than about 1% D-
lactic acid and about 185 grams stannous octoate up to a temperature of about
180C while a stream of N2 gas was passed through the mass to facilitate
removal of water. The process was continued until the oligomeric produced
0 show an average chain length of 10, determined by titration in methanol with
methanolic sodium methoxide using phenolphthalein as the indicator. The
resultant oligomer contained about 0.69% by weight of the stannous octoate
catalyst.
B. The oligomer prepared in step (A) above, was depolymerized
under the following conditions:
The wiper roller of the wiped film evaporator was set at agitation
setting of about 2.5; about 260C oil was circulated through the evaporator
jacket and about 150C oil through the feed funnel jacket. Hot water was
circulated through the condensers to a temperature of about 90C and the
evaporator was evacuated and maintained at about 1 mm of Hg pressure.
Preheated (150C) molten oligomer was fed to the evaporator at a rate of about
273 grams/hour. Disdllate from tbe evaporator surface was collected as
drainage from the condensers at the rate of abut 234.5 grams/hour. A heel was
drained from the heated evaporator wall, was collected at the rate of about 35.5grams/hour. The wiped-film evaporator was operated for about 85 minutes.
The residence dme in the reactorwas about 6.0 minutes. ~-
The ratdo of the distillate collection rate to the oligomer feed rate
was about 85.9% 1(234.5/273) X 100]. The ratio of the sum of the distillate and
heel collection rates to the feed rate was 98.9% [(270/273) X 100] which
3 o indicated substantially complete recovery of the thermolysis products ~distillate).
The distillate contained about 69.5% L-lactide, 6.5~o meso-lactide
and 0.04%, D-lactide, which was determined by high pressure liquid
chromatography. The remainder of the distillate consisted essentially of lactic
acid and volatilized water-soluble lactic acid oligomers amounting to about 11303 5 milliequivalents of acidity per kilogram of distillate. The above lactide content
on a 100~ basis, corresponds to about 91.2% L-lactide, 8.3% meso-lactide and
0.5% D-lactide.
SUBSTIJUTE SHEET
WO g3/02075 PCI`/US92/0~003
- 1s211~7g9
Although the data a~ove indicate that some racemization had
occurrcd during the depolymerization step, the proportion of meso-isomer was
acceptable.
s EXAMPLE 2
The procedure of Example 1 was repeated except that ~a) the
lactic acid was oligomerized to an average chain length of 18 and the oligomer
contained about 0.S9% by weight of the stannous octoate, (b) the oligomer feed
rate was about 25S g/hr, (c) the distillate recovery rate was about 244 g/hr ando (d) the heel recovely rate was nil. Thus, the ratio of distillate rate to feed rate
was about 95.7%.
The distillate contained abut 77.6% L,lactide, 6.1% meso-lactide
and 0.2% D-lactide. The remaining 16.1% of distillate consisted of lactic acid ~;
and volatilized lactic acid oligomers corresponding to abut 6S0 rnilliequivalents
of acidity/kg of distillate. On a 100% basis, the lactide content correspond to ~-
about 92.S% L-, 7.3% meso and 0.24% D^lactide.
EXAMPLE 3
Example 1 was repeated except that (a) the oligomer contained
2.9% by weight of stannous octoate, (b) 170C oil was circulated through the
feed funnel jacket and 270C oil through the evaporator jacket, (c) the feed rate
of oligomer to the evaporator was 941 grams/hour.
Distillate was collected at the rate of 731 grams/hour, and
unconverted material (heel) at 197 grams/hour. The duration of the run was 0.6
hours. The residence time in the reactorwas about 4 minutes.
The ratio of the distillate collection rate to the oligomer feed rate
was M.7% [731/941 X 100]; the ratio of the sum of the distillate and heel
collection rates to the feed rate was 98.6% [928/941 X 100].
The distillate was found to contain 64.4% L,lactide, 5.92%
mesolactide and 0.62% D-lactide, by HPI~, and 1334 me/kg of acidity. The
lactide content on a 100% basis corresponded to 9Q8~o L-, 8.3% meso- and 0.8~o
D-lactide.
EXAMPLE 4
This example was carried out in a 4" wiped film still made by Pope
Scientific Co., Menomonee Falls, WI. The still contained a jacketed vertical
SUBSTlTllTE SHEE~
wo 93/0207~ Pcr/US92/o6oo3
16 -
evaporator section equipped with a variable speed drive film wiper mechanism
and coupled to an external condenser.
A lactic acid oligomer was prepared by gradually heating 2000
grams of 88% L-lactic acid and 20 grams of stannous octoate to 180C and ;
5 holding at that temperature for 1 hour. The pressure was reduced and heating at
180C was continued to remove water until the resulting oligomer showed an
average chain length of 11.8. :
The evaporator was evacuated and heated by circulating 245C oil
through its jacket. 1344 grams of the oligomer was fed to the evaporator at the ~;
rate of about 1209 grams/hour over a period of about 50 minutes. During this
time, the pressure in the evaporator ranged between 2.5 and 10mm of Hg. 197
grams of distillate was condensed by the external condenser; 1156 grams of heel
remained in the still. The distillate contained 52.1% ~lactide, 3.64% meso-
lactide, Q93% D-lactide and 2700 me/kg of acidity. On a 100% basis, the lactide
15 portion contained 91.9% L-, 6.4% meso- and 1.64% D-lactide.
Although a few exemplary embodiments of the present invention
have been described above in detail, those skilled in the art will readily
appreciate that the present invention embraces many combinations and
variations other than those exemplified.
,:
SUBSTITUTE SHEET