Note: Descriptions are shown in the official language in which they were submitted.
PCT/EP 92/01161
T~~A~L~LATi~~
H-19097/Pl
PCT
1 fN Halo 3 cyridylmethyl)1-N-methylamino-1-alkylamino-2-nitroethvlene
derivatives
for controllingLfleas in domestic animals
The present invention relates to 1-[N-(halo-3-pyridylmethyl)]-N-methylamino-1-
alkyl-
amino-2-nitroethylene derivatives of the following formula I for use in a
method of
controlling fleas in domestic animals, especially in dogs and cats, wherein
systemic
administration is preferred. The invention relates also to a method of
inhibiting the
infestation of dogs and cats by fleas which comprises the systemic
administration of the
said compound, in an amount effective against fleas, to the said domestic
animal, for
example the dog or the cat, by way of the digestive tract or the blood of the
host animal.
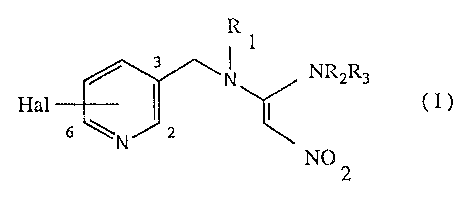
The 1-[N-(halo-3-pyridylmethyl)]-N-methylamino-1-alkylamino-2-nitroethylene
derivatives mentioned in the introduction have the following chemical swcture
of
formula I:
R
1
3 N ~2Rs
Hal I ~ ( I )
6 ~ J2
NO
2
wherein
Hal is halogen, such as fluorine, chlorine, bromine or iodine;
Rt is hydrogen, C~-Cbalkyl or C3-C~cycloalkyl;
R2 is hydrogen, Cr-C6alkyl or C3-C~cycloalkyl, and
R3 is hydrogen or C1-C~allcyl.
Owing to their pronounced activity against fleas, preference is given within
the scope of
211~~~J~~
-2-
formula I to the following subgroups:
Group a: Compounds of formula I wherein Hal is in the 6-position. Special
preference is
given to such compounds wherein Hal is fluorine, chlorine or bromine,
especially
chlorine.
Group b: Compounds of formula I wherein R1 is hydrogen, Ci-C3alkyl or C3-
Cbcyclo-
alkyl, preferably hydrogen, methyl or ethyl or cyclopropyl, especially ethyl.
Grou .c: Compounds of formula I wherein Rz is C1-C3alkyl or cyclopropyl,
especially
methyl.
Gro- uQd: Compounds of formula I wherein Hal is halogen, such as fluorine,
chlorine,
bromine or iodine; Rt is hydrogen, methyl, C3-Cbalkyl or C3-C~cycloalkyl and
R2 is
hydrogen or Ct-C6alkyl.
Within groups (a) to (d) preference is given above all to those compounds
wherein R3 is
hydrogen.
Owing to its pronounced systemic activity against fleas, special preference is
given to the
following compound:
1-[N-(6-chloro-3-pyridylmethyl)]-N-ethylamino-1-methylamino-2-nitroethylene,
and also
to its immediate homologues.
Within the scope of the present invention, depending on the number of carbon
atoms
indicated, the term "alkyl" is to be understood as meaning, for example, the
following
straight-chained and branched groups: methyl, ethyl, n-propyl, isopropyl, n-
butyl,
sec-butyl, tent-butyl, isobutyl, etc.. Here and hereinafter, Hal is to be
understood as being
fluorine, chlorine, bromine or iodine, preferably fluorine, chlorine or
bromine, but
especially chlorine. Depending on the number of carbon atoms indicated,
cycloalkyl by
itself or as part of a substituent is cyclopropyl, cyclobutyl, cyclopentyl,
cyclohexyl, etc.,
cyclopropyl being especially preferred.
Compounds of the structural type falling within the scope of formula I,
including
processes for their preparation, are described in EP-0 302 389. The compounds
of
formula I can be prepared in accordance with the methods disclosed in EP-0 302
389 or
2~~,;~t3~?
-3-
analogously to the compounds described therein. That publication discloses a
broad class
of compounds of which the above representatives constitute only a selected
subgroup,
although they do not appear therein as a group. The class of compounds
disclosed in
EP-0 302 389 is described as insecticidal and acaricidal. Plant-deswctive
insects and
mites are given as the preferred area of application. There is no reference to
activity
against fleas.
EF-0 302 833 likewise discloses a group of compounds which are structurally
similar to
those of the above formula I or which to some extent overlap structurally
therewith. The
essential structural difference between the compounds specifically disclosed
and,those of
formula I is that the former have an unsubstituted pyridyl group, whereas the
compounds
of formula I are mono-halogenated at the pyridyl radical. The compounds
disclosed in
EP-0 302 833 are said to be valuable active ingredients in pest control, while
being well
tolerated by warm-blooded animals and plants, and to be suitable for
controlling pests in
and on animals and plants. Among the animal-injurious insects, specific
reference is made
to the order Siphonaptera (fleas).
It has now been found, surprisingly, that in the case of the compounds of the
above
formula I, the introduction of a halogen atom into the pyridyl radical brings
about quite
unexpected and significantly increased activity against fleas. That is very
clear from a
comparison of the principal compound according to the invention and the
structurally most
similar compound from the prior art. The relevant comparison data are given
below in, the
Biological Examples.
The outstanding activity against fleas exhibited by the compounds of formula 1
is of such
significance because the infestation by fleas of domestic animals and pets,
especially of
dogs and cats, is a problem for vets and animal owners alike to which there is
still no
adequate solution.
Owing to the complicated life cycle of the flea, none of the known methods of
controlling
these extremely troublesome parasites, which not only transmit diseases but
are also the
cause of unpleasant allergies, is totally satisfactory, especially since most
of the known
methods of control are based on the application of the active compound to the
habitat of
the various flea stages. As a result of the complex life cycle of the flea,
however, that
procedure is very laborious and cannot, in practice, cover all of the
habitats, and is
accordingly unreliable.
For example, control aimed at treating the adult fleas in the fur, which is
generally
effected by applying an anti-flea composition to the fur of the host animal,
takes no
account at all of the various juvenile stages of the fleas, which live not
only in the fur of
the animal, but also mainly on the floor, on carpets, on the animal's sleeping
place, on
chairs, in the garden and in all the other places with which the infested
animal comes into
contact. Adult cat and dog fleas (Ctenocephalides felis and C. cams) normally
live in the
fur of the host cat or host dog. They live on the blood of the host animal and
lay their eggs
in its fur. Since those eggs are not self-adhering, however, they generally
soon fall off and
can be found on the floor, on carpets, in the dog's or cat's basket, on the
chairs used by the
animal, in the garden, in the yard, etc..
That means that the whole of the pets' living area is contaminated with flea
eggs, from
v~hich the larvae develop within two days. The larvae have three distinct
stages of
development, each of which lasts three days. In the final stage the larva
spins its cocoon
and becomes a pupa. Under favourable conditions, i.e. at 33°C and a
relative humidity of
65 %, the metamorphosis from egg to pupa takes place in about 8 to 10 days.
After about
another 8 days the young, fully-foamed fleas develop in the cocoons that are
still lying on
the floor, the carpets, the sleeping places, the chairs, etc.. The young adult
fleas remain
there until they. sense the presence of an acceptable host animal, then they
hatch from their
cocoons and attempt to jump onto the host animal. Thus it takes at least three
weeks for
an egg to develop into a young adult flea that is capable of reinfesting the
host animal.
The young flea may, however, remain in its cocoon for months, possibly for up
to a year.
On the other hand, under less favourable conditions the development from egg
to young
adult flea may take from 4 to 5 months. To reach sexual maturity, fleas rewire
blood as
food in order to be able to reproduce, and that blood must be from the
appropriate host
animal. It is normally obtained from the excreta of the adult fleas living on
the host
animal. Those excreta contain large amounts of undigested blood.
That long life cycle, which proceeds separately from the host animal, has a
significant
influence on the successful control of fleas on the host animal.
Only when the fleas in the fur of the host animal can be successfully
controlled very
quickly, i.e. when all the adult fleas are killed within a very short time
with a suitable
active ingredient, is the cat or the dog protected against the risk of
reinfestation by newly
-5-
hatched young fleas from its living space.
Flea infestation of dogs and cats has unpleasant consequences not only fox the
animal to
be treated but also for the animal's owner. Such drawbacks lead, for example,
to local
irntation or troublesome itching and often result in intense scratching. A
large number of
the animals become allergic to the excreta of the fleas, which leads to very
itchy and
crusty skin changes around the sites of the bites on the animal's body. Those
skin changes
normally have a diameter of approximately 3 mm or more and often make the
animal
prone to biting and cause it to scratch, leading to loss of fur in places.
Furthermore, flea-infested animals are constantly exposed to the risk of
infestation by
dipylidium, a species of tape worm, which is transmitted by fleas.
Flea infestation is not only extremely troublesome for the affected animal,
but also has
unpleasant consequences for the animal's owner, since it will eventually
become evident
to him from the unusual behaviour of his pet that it is ill and suffering and
that he must
help it. What is more, it cai~ become unpleasant for the animal's owner if he
gives up
keeping his infested animal, or if it dies or is removed temporarily frorn its
usual
environment, since in the event of the prolonged absence of a suitable host
animal, the
newly hatched fleas on the floor will be forced to infest the human, although
they are
unable to live on human blood as their only source of food. Even when the dog
or the cat
is present, the animal's owner can still be bitten by the fleas.
In addition, dog and cat fleas, or their excreta, can lead to allergy-like
skin disorders in
some people, which in many cases means that the pet must be given up. The
desire for
effective control of fleas in dogs and cats has therefore existed for
centuries.
A number of conventional methods of control are known, but they have various
disadvant-
ages. If, fox example, flea combs surface-coded with an insecticide are used,
the animal's
owner has no alternative but to comb the animal intensively and often which,
depending
on the size of the animal, may take from a few minutes to an hour and will not
be accepted
patiently by every animal. However, not every animal owner is prepared to
devote the
time to this. The use of corresponding anti-flea shampoos is often
unsuccessful, since
most cats, and also many dogs, can be bathed, if at all, only by force, with
the result that
water and active ingredient are spilt and have to be cleared up. In addition,
the effect of
such a bath treatment lasts about a week at most, and the laborious procedure
has to be
2~~.3~0~>
-6-
repeated. The same or very similar problems can be expected with the use of
dips or
rinses. The use of dusting powders is generally also not accepted by the
animal without
resistance, since it takes several minutes to treat the whole surface of the
fur uniformly,
and some of the dust will inevitably get into the mouth, nose and eyes of the
animal. Even
with careful application, it cannot be ensured that the animal and the human
will not
inhale any powder. It is virtually inevitable that the human will also come
into contact
with the composition to a greater or lesser extent.
When using sprays, many people may be unpleasantly surprised to find that most
animals,
especially cats, run away or react aggressively at the mere sound of the
spray. In addition,
sprays also have all the disadvantages listed for dusting powders, added to
which they
become even more finely dispersed in the atmosphere and are therefore inhaled
by human
and animal. Fleas are frequently controlled by means of so-called flea
collars, which
ensure good effectiveness temporarily. This treatment has a certain weakness,
owing
especially to its locally very limited area of application. Although the
killing action in the
region of the neck and chest is generally 100 %, more remote parts of the body
are
scarcely affected. In addition, those collars are active for a limited time.
Furthermore,
many of the collars are unattractive and may annoy the animal. It is also
possible
nowadays to buy medallions, which can be hung from conventional collars and
are
supposed to be effective. Although they are attractive in appearance, the
action of those
medallions is unsatisfactory, since they have inadequate cont~nct with the
fur. Some anti-
flea organophosphorus compounds are also available as spot-on formulations and
are thus
applied to a locally limited area of the fur. They generally have good short-
term activity
against adult fleas, but the compositions used often have toxic properties
that present
problems: Some organophosphoms compounds have also been administered orally,
but
they are subject to strict safety restrictions and must on no account be
administered
simultaneously with other organophosphorus compounds.
Overall it may be said that the conventional methods, which seek to kill the
adult flea,
produce such unsatisfactory results chiefly because they depend on the
patience and the
skill of the user when handling the infested host animal. The success of the
current
compositions stands and falls with the frequency and thoroughness with which
the user,
normally the animal's owner, applies the active ingredient to the host animal
and the
thoroughness with which he disinfects the environment in which the host animal
lives.
The conventional methods are relatively expensive, time-consuming and not
especially
successful in the long term. In the short term, relief can certainly be
obtained with the
conventional compositions.
What has hitherto not been adequately taken into account in the case of the
conventional
methods and compositions is the fact that, owing to the particular life cycle
of the flea,
dogs and cats are repeatedly reinfested by new fleas, partly because contact
with the flea
eggs, flea larvae and young adult fleas on the floor and/or in the immediate
vicinity of the
animal is unavoidable, and partly because many pet animals constantly come
into contact
with infested members of their own species.
Constantly recurring reinfestation is inadequately prevented by conventional
compositions
or can be prevented only by the application of large amounts of disinfectant,
etc..
It has now been found, surprisingly, that using certain systemic methods of
administration
and using compounds of formula I as active ingredients, it is possible to
eliminate the
adult fleas very rapidly and completely and thus to intervene in the complex
development
cycle of the flea by blocking that cycle. Since those compounds exhibit all of
their
outstanding anti-flea activity also when they are administered to the host
animal
systemically, i.e. orally, parenterally, subcutanecusly, intramuscularly or
intravenously, it
is possible, by means of their specific periodic administration, in a simple
manner to break
the vicious circle of constantly recurring reinfestation described above until
all the
juvenile stages in the living area of the host animal have died. The fleas are
killed and
prevented from reproducing, the juvenile stages are prevented from maturing
and are no
longer able to infest the host animal, so that the living area of dogs and
cats can be kept
free of fleas for a prolonged period. The only unavoidable source of
reinfestation is
contact with infested members of the same species and that aspect can of
course be
excluded one hundred percent only by means of long-term txeatment. 'That
residual risk is,
however, of minor importance.
It has now been found that by oral administration, parenteral administration
or adminis-
tration by means of an implant, of compounds of formula I in an amount
effective against
fleas, it is possible for infestation by fleas of domestic animals, such as
cats and dogs, to
be drastically reduced or completely prevented.
What is astonishing in connection with the present invention, however, is that
full activity
can be achieved even when an active ingredient is administered to the host
animal in
relatively low concentrations and reaches its target of the adult flea only by
the circuitous
_g_
route via the gastro-intestinal tract and thus via the blood sucked up by the
flea. Since
systemic administration of the active ingredient brings about the total
mortality of the
adult fleas, it is now possible to eradicate the fleas. A combination of this
systemic
administration of the active ingredient with accompanying measures, for
example
disinfection of the living area of the host animal, makes it possible to
eliminate the flea
problem even more rapidly, but even without those accompanying measures the
flea
population is reduced completely or to an acceptable minimum within a few
weeks or at
the most months.
The compounds of formula I exhibit activity against juvenile flea stages
insofar as flea
laivae that hatch from the flea eggs are substantially dependent on the
excreta of the adult
fleas, since they live on that excrement. However, flea excreta comprise large
amounts of
undigested blood from the host animal and serve as a source of protein for the
developing
fleas. Since, however, the compounds of formula I kill the adult fleas very
rapidly, the
necessary excreta are not available and the juvenile stages are deprived of
their nutrient
base and therefore die before they reach the adult stage. This is also a
decisive
contributing factor in the interruption of the complex life cycle of the flea
and prevents the
host animals from being constantly reinfected in their preferred living area
by the eggs,
and the larvae hatching therefrom, that are scattered all over it.
The present invention thus has two objects, on the one hand the afore-
described method of
preventing the reinfestation of domestic animals by fleas, and simultaneously
of course the
inhibition of the reproduction of fleas.
It is essential to the invention that the compounds of formula I are
administered in such a
manner that they can be taken up in sufficient amounts by the adult, sucking
flea with the
blood of the host animal and kill the adult flea rapidly before it can provide
sufficient
uncontaminated food for the flea larvae by way of its excreta. This is
achieved with the
compounds of the invention using various forms of administration, for example
by
administering the formulated active ingredient orally. "lFormulated" in this
case means,
for example, in the form of a powder, a tablet, granules, a capsule, an
emulsion or a foam,
in microencapsulated form, etc.; the animal need not necessarily be given the
composition
directly, rather it is advantageously mixed with its food, since that form of
administration
presents the fewest problems both to the host animal and to the person
administering the
composition. In addition to customary formulation ingredients, any composition
that is to
be administered orally may of course comprise further adjuvants that encourage
the host
_9_
animal to take the composition voluntarily, for example suitable odorants and
flavourings.
Oral administration, being easy to carry out, is preferred according to the
invention. A
further form of administration is parenteral administration, for example by
subcutaneous
injection or intravenous injection, or with a long-term composition (depot
form) in the
form of an implant.
Oral administration includes, for example, the administration of dog and cat
food ready
mixed with the active ingredient, for example in the form of biscuits or
treats, chewable
tablets, water-soluble capsules or tablets, in a water=soluble form that can
be added to the
food. in the form of drops or in other forms that can be mixed with the animal
food. The
implants also include any means that can be introduced into the body of.the
animal in
order to release active ingredient.
Percutaneous forms of administration include, for example, subcutaneous,
dermal,
intramuscular and even intravenous administration of injectable forms. In
addition to the
customary syringes with needles, needle-less high-pressure syringe devices, as
well as
pour-on and spot-on formulations, may be expedient.
By selection of a suitable formulation, it is possible to promote the ability
of the active
ingredient to penetrate through the living tissue of the animal, and/or to
maintain its
availability. That is important when, for example, a very sparingly soluble
active
ingredient is used, the low solubility of which requires means for enhancing
solubility,
since the animal's body fluid is capable of dissolving only small amounts of
active
ingredient at a time.
The active ingredient may also be present in a matrix formulation which
physically
prevents the active ingredient from decomposing and maintains the constant
availability of
active ingredient. The matrix formulation is injected into the body and
remains there as a
form of depot from which active ingredient is released continuously. Such
matrix
formulations are known to a person skilled in the art. They are generally wax-
like, semi-
solid substances, for example vegetable waxes and polyethylene glycols having
a high
molecular weight.
A high degree of availability of the active ingredient is also obtained by the
inti~oduction
of an implant of the active ingredient into the animal. Such implants are
widely used in
veterinary medicine and often consist of silicone-containing rubber. The
active ingredient
~1~.~~0
- to -
is dispersed in the solid rubber or is located inside a hollow rubber body.
Care must be
taken that the active ingredient selected is soluble in the rubber implant,
since it is first
dissolved in the rubber and then seeps continuously out of the rubber material
and into the
body fluid of the animal to be treated.
The rate of release of the active ingredient from the implant, and thus the
length of time
during which the implant exhibits activity, is generally determined by the
accuracy of the
calibration of the implant (amount of active ingredient in the implant), the
environment of
the implant and the polymer formulation from which the implant has been
produced.
Administration of the active ingredient by means of an implant is a further
preferred
component of the present invention. Such administration is extremely
economical and
effective, because a correctly dimensioned implant ensures that the
concentration of active
ingredient in the tissue of the host animal is constant. It is possible
nowadays for implants
to be so made and implanted in a simple manner that they are capable of
supplying the
active ingredient over a period of several months. Once the implant has been
made, the
animal is not disturbed further, and there is no further need to be concerned
about the
dose.
The administration of veterinary medicinal additives to animal food is well
known in the
field of animal health. It is usual first to prepare a so-called premix in
which the active
ingredient is dispersed in a liquid or is in finely divided form in solid
carriers. That
premix can normally comprise about 1 to 800 g of compound per kg of premix,
depending
on the desired final concentration in the food.
It is also known that active ingredients may be hydrolysed or weakened by the
constituents
of the food. Such active ingredients are routinely formulated in a protective
matrix, for
example in gelatin, before being added to the premix.
The present invention therefore relates to the object of eliminating the adult
fleas on the
domestic animal, as well as to preventing the further development of the flea
larvae by
depriving them of food, which is eduivalent to systemic prevention of the
reinfection of
domestic animals, especially of domestic animals by fleas. This is achieved by
adminis-
tering* to the said host animal orally, parenterally or by means of an implant
at least one
compound of formula I in an amount effective against fleas.
*Translator's note: "zusetzt" presumably in error for "verabreicht"
~~.1~~~~
-Il-
The present invention thus also relates to the object of preventing the
reproduction of
fleas, which comprises making available to fleas as food, by means of the
systemic
administration of the active ingredient to the host animal, contaminated blood
that
comprises at least one compound of formula I in an amount effective against
fleas. This is
most easily achieved by administering to the host animal, in the form of a
food additive, a
compound of formula I in an amount effective against fleas, and in that way
allowing it to
reach the fleas living on the host animal.
The compounds of formula I are advantageously administered in a dose of from
0.01 to
800, preferably from 0.1 to 200, especially from 0.5 to 30, mg/!cg of body
weight of the
host animal, oral administration being preferred.
A good dose that can be administered to the host animal regularly is from 0.5
to
100 mg/kg of body weight. Administration is advantageously effected daily or
weekly.
The total dose may vary for the same active ingredient from one species of
animal to
another as well as within a species of animal, since it depends inter alia on
the weight and
the constitution of the animal.
When used according to the invention, the active ingredient is not normally
administered
in pure form, but preferably in the form of a composition which comprises in
addition to
the active ingredient constituents that assist administration, suitable
constituents being
those that are tolerated by the host animal. It is of course possible, as well
as controlling
the adult fleas in accordance with the invention, additionally to use
conventional methods
that control the juvenile flea stages, although the latter is not absolutely
essential.
Such compositions to be administered in accordance with the invention
generally
comprise from 0.1 to 99 % by weight, especially from 0.1 to 95 % by weight, of
a
compound of formula I and from 99.9 to 1 % by weight, especially from 99.9 to
5 % by
weight, of a solid or liquid, non-toxic adjuvant, including from 0 to 25 % by
weight,
especially from 0.1 to 25 % by weight, of a non-toxic surfactant.
Whereas commercial products will preferably be formulated as concentrates, the
end user
will normally employ dilute formulations.
The compositions may also contain further auxiliaries such as stabilisers,
antifoams,
::~,~1
- 12-
viscosity regulators, binders, tackifiers as well as other active ingredients
for obtaining
special effects.
The materials known from veterinary medicinal practice for oral and parenteral
adminis-
tration and for implants can be used as formulation excipienis. Some examples
are given
below.
Suitable excipients are especially fillers, such as sugars, for example
lactose, saccharose,
mannitol or sorbitol, cellulose preparations and/or calcium phosphates, for
example tri-
calcium phosphate or calcium hydrogen phosphate, and binders, such as starch
pastes
using, for example, corn, wheat, rice or potato starch, gelatin, tragacanth,
methylcellulose
and/or, if desired, disintegrators, such as the above-mentioned starches, also
carboxy-
methyl starch, cross-linked polyvinylpyrrolidone, agar, alginic acid or a salt
thereof, such
as sodium alginate. Adjuvants are especially flow conditioners and lubricants,
for
example silicic acid, talc, stearic acid or salts thereof, such as magnesium
or calcium
stearate, and/or polyethylene glycol. Drag~e cores can be provided with
suitable,
optionally enteric, coatings, there being used inter alia concentrated sugar
solutions which
may comprise gum arabic, talc, polyvinylpyrrolidone, polyethylene glycol
and/or titanium
dioxide, or coating solutions in suitable organic solvents or solvent
mixtures, or, for the
preparation of enteric coatings, solutions of suitable cellulose preparations,
such as acetyl-
cellulose phthalate or hydroxypropylmethylcellulose phthalate. Dyes,
flavourings or
pigments may be added to the tablets or drag~e coatings, for example for
identification
purposes or to indicate different doses of active ingredient.
Other orally administxable compositions are dry-filled capsules comprising
gelatin, and
also soft sealed capsules comprising gelatin and a plasticiser, such as
glycerol or sorbitol.
The dry-filled capsules may comprise the active ingredient in the form of
granules, for
example in admixture with fillers, such as lactose, binders, such as starches,
and/or
glidants, such as talc or magnesium stearate, and, if desired, stabilisers. In
soft capsules,
the active ingredient is preferably dissolved or suspended in suitable
liquids, such as fatty
oils, paraffin oil or liquid polyethylene glycols, to which stabilisers may
also have been
added. Preference is given inter alia to capsules that are easily bitten
through or
swallowed without being chewed.
Suitable for parenteral administration are especially aqueous solutions of an
active
ingredient in water-soluble form, for example in the form of a water-soluble
salt, and also
CA 02113908 2002-04-05
21489-8808
-13-
suspensions of the active ingredient, such as corresponding
oily injection suspensions, there being used suitable
lipophilic solvents or vehicles, such as fatty oils, for
example sesame oil, or synthetic fatty acid esters, for
example ethyl oleate, or triglycerides, or aqueous injection
suspensions that comprise viscosity-increasing substances,
for example sodium carboxymethylcellulose, sorbitol and/or
dextran, and, optionally, stabilisers.
The compositions of the present invention can be
prepared in a manner known per se, for example by means of
conventional mixing, granulating, confectioning, dissolving
or lyophylising processes. For example, pharmaceutical
compositions for oral administration can be obtained by
combining the active ingredient with solid carriers,
optionally granulating a resulting mixture, and processing
the mixture or granules, if desired or necessary after the
addition of suitable excipients, to form tablets or dragee
cores.
According to one aspect of the present invention,
there is provided a use of a compound of formula (I):
R1
3
NRZR3
Hal
6 \N Z N02
wherein: Hal is halogen; Rl is hydrogen, C1-Csalkyl or C3-
C~cycloalkyl; R2 is hydrogen, C1-C6alkyl or C3-C~cycloalkyl,
and R3 is hydrogen or C1-C6alkyl, in the preparation of a
composition for the systemic control of adult fleas on a
domestic animal.
CA 02113908 2002-04-05
21489-8808
-13a-
According to another aspect of the present
invention, there is provided a use of a compound of
formula (I)
R1
3
NR2R3
Hal
~ Jz ~ (I) .
N NOz
wherein: Hal is halogen; R1 is hydrogen, C1-C6alkyl or C3-
C~cycloalkyl; R2 is hydrogen, C1-C6alkyl or C3-C~cycloalkyl,
and R3 is hydrogen or Cl-Csalkyl, for the systemic control of
adult fleas on a domestic animal.
According to a further aspect of the present
invention, there is provided a use of a compound of formula
(I)
R1
3
NRZR3
Hal
N NOz
wherein: Hal is halogen; R1 is hydrogen, C1-C6alkyl or C3-
C~cycloalkyl; R2 is hydrogen, C1-Csalkyl or C3-C-,cycloalkyl,
and R3 is hydrogen or Cl-C6alkyl, in the preparation of a
composition for systemic administration to blood of a
domestic animal for preventing fleas from reproducing on the
said domestic animal.
According to one further aspect of the present
invention, there is provided a use of a compound of formula
(I)
CA 02113908 2002-04-05
21489-8808
-13b-
R1
3
NRZR3
Hal
),
N NOZ
wherein: Hal is halogen; R1 is hydrogen, C1-C6alkyl or C3-
C-,cycloalkyl; R2 is hydrogen, Cl-C6alkyl or C3-C~cycloalkyl,
and R3 is hydrogen or Cl-C6alkyl, for systemic administration
to blood of a domestic animal for preventing fleas from
reproducing on the said domestic animal.
According to one other aspect of the present
invention, there is provided a systemically active
composition for preventing flea infestation of a domestic
animal comprising in an amount effective against fleas, a
compound of formula (I):
R1
3
NRZR3
1s Hal 6 \ Jz ~ ( I ) ,
N NOZ
wherein: Hal is halogen; R1 is hydrogen, C1-C6alkyl or C3-
C~cycloalkyl; R2 is hydrogen, C1-C6alkyl or C3-C~cycloalkyl,
and R3 is hydrogen or Cl-Csalkyl, together with an excipient
that is physiologically tolerable for the animal.
According to still a further aspect of the present
invention, there is provided a food additive for the
systemic control of fleas in a domestic animal, which
comprises as active ingredient, in an amount effective
against fleas, as a compound of formula (I):
CA 02113908 2002-04-05
21489-8808
-13c-
R1
3
NR2R3
Hal
N NOZ
wherein: Hal is halogen R1 is hydrogen, C1-C6alkyl or C3-
C~cycloalkyl; RZ is hydrogen, C1-Csalkyl or C3-C~cycloalkyl,
and R3 is hydrogen or Cl-C6alkyl.
According to yet a further aspect of the present
invention, there is provided a commercial pack containing
individual doses of a compound of formula (I):
Ri
3
NRzR3
Hal ~ (I) ,
6 \N Z NOZ
wherein: Hal is halogen; R1 is hydrogen, C1-C6alkyl or C3
C~cycloalkyl; R2 is hydrogen, C1-C6alkyl or C3-C~cycloalkyl,
and R3 is hydrogen or C1-C6alkyl, in formulated form for oral
administration against fleas in a domestic animal, wherein
the compound is present in individual ready-for-use doses of
from 0.01 mg/kg of body weight of the animal to
approximately 800 mg/kg of body weight of the animal,
together with instructions for the use thereof in treating
or preventing flea infestation in the animal.
The following Examples illustrate the invention
described above, but do not limit its scope in any way.
Temperatures are given in degrees Celsius.
~113~(~C~
- 14-
Table: Examples of compounds of formula I that can be used in accordance with
the
invention
R
1
N ~2R3
Hal ~ I (I)
6 ~N 2
N02
Comp. Hal Rt R2 R3 melting point
No. in °C
1.1 6-Cl CH3 CH3 H 87-90
1.2 6-CI C2~I5 CH3 H yellow
oil
1.3 6-Cl CH3 C2H5 H 112-114
1.4 6-Cl CH3 C3H~-i H 133-135
1.5 6-Cl CH3 C4-H9-n H
1.6 6-CI CH3 C4-H~-t H
1.7 6-Cl CH3 C4-H9-s H
1.8 6-Cl CH3 CS-H~-n H
1.9 6-Cl CH5 C6-Ht3-n H
1.10 6-F Cl-I3 CH3 H 100-100.5
1.11 5-Br CI-I3 CI-I3 I-I 116-117
1.12 2-Cl CH3 CH3 H 106-113
1.13 6-CI C3I-h-iS0 CI-I3 I-I
1.14 6-Cl Ca-I-h-n CI-I3 H
1.15 6-CI C4-H~-tert CH3 H
1.16 6-Cl C4-Eh-sec CI-I3 H
1.17 6-Cl C~-Ht3-n CH3 H
1.18 6-Cl cyclopropylCEI3 II 104-105
1.19 6-CI cyclobutyl CI-I3 H
1.20 6-CI cyclopentylCH3 H
1.21 6-Cl cyclohexyl CH3 H
1.22 6-CI cycloheptylCH3 H
1.23 6-Cl H CI-I3 H 158-161
2113~0~~
- IS -
Comp. Hal Rr RZ R3 melting
point
No. in C
1.24 6-Cl C2H5 H H 159-161
1.25 6-Cl CH3 H H 202 decomp.
1.26 5-Cl CH3 CI-i3 H
1.27 4-Ci CH3 CH3 H
1.28 6-Ci cyclopropylC2H5 H 144-146
1.29 6-F C2H5 CH3 H oil
1.30 6-Br C2H5 CH3 H 79-80
1.31 6-Ci H CH3 CH3 97-98
1.32 6-Br CH3 CH3 H 130-131
1.33 6-Ci CH3 CH3 CH3 110-112
1.34 6-Cl H Calls CH3 87-88
1.35 6-Ci CaH7-iso H ~ H resin
1.36 6-Cl H H H 188-190
1.37 6-Cl C3I-h-n H H 185-186
1.38 6-CI C3H~-n H CH3 102-103
1.39 6-Cl C3H~-iso H CH3 119-120
i.40 6-Cl cyclopropylH cyclo- 113-115
propyl
In the Formulation Examples that follow, the term active ingredient stands for
1-(N-
(6-chioro-3-pyridylmethyl)]-N-ethylamino-1-methylamino-2-nitroethylene.
Example 1: Tablets comprising 25 mg of active ingredient can be prepared as
follows:
Constituents (for 1000 tablets)
active ingredient 25.0 g
lactose 100.7 g
wheat starch 7.5 g
polyethylene glycol 6000 5.0 g
talcum 5.0 g
magnesium stearate 1.8 g
~~,. ~.~'~~3~
- 16-
demineralised water q.s.
Preparation: All the solid ingredients are first forced through a sieve having
a mesh sire of
0.6 mm. Then the active ingredient, the lactose, the talcum and half the
starch are mixed
together. 'The other half of the starch is suspended in 40 ml of water and the
suspension is
added to a boiling solution of the polyethylene glycol in 100 ml of water. The
resulting
starch paste is added to the main batch and the mixture is granulated, if
necessary with the
addition of water. The granules are dried overnight at 35°, forced
through a sieve having a
mesh size of 1.2 mm, mixed with the magnesium stearate and campressed to form
tablets
having a diameter of about 6 mm which are concave on both sides.
Example 2: Tablets comprising 0.02 g of active ingredient are prepared as
follows:
Composition (fox 10 000 tablets)
active ingredient 200.00 g
lactose 290.80 g
potato starch 274.70 g
stearic acid 10.00 g
talcum 200.00 g
magnesium stearate2.50 g
colloidal silica 32.00 g
ethanol q.s.
A mixture of the active ingredient, the lactose and 194.70 g of potato starch
is moistened
with an ethanolic solution of the stearic acid and granulated through a sieve.
After drying,
the remaining potato starch, the talcum, the magnesium stearate and the
colloidal silica are
mixed in and the mixture is compressed to form tablets each weighing 0.1 g,
which may, if
desired, be provided with dividing notches for finer adaptation of the dose.
Example 3: Capsules comprising 0.025 g of active ingredient can be prepared as
follows:
Composition (for 1000 capsules)
active ingredient 25.00 g
lactose 249.80 g
gelatin 2.00 g
corn starch 10.00 g
-17-
talcum 15.00 g
water q.s.
The active ingredient is mixed with the lactose, the mixture is moistened
uniformly with
an aqueous solution of the gelatin and granulated through a sieve having a
mesh size of
1.2-1.5 mm. The granules are mixed with the dried corn starch and the talcum
and
introduced in 300 mg portaons into hard gelatin capsules (size 1).
Example 4: Premix (food additive)
0.25 part by ~.veight of active ingredient and .
4.75 parts by weight of secondary calcium phosphate, alumina, Aerosil,
carbonate or
challc are mixed until homogeneous with
95 parts by weight of an animal food.
Exam~e 5: Premix (food additive
0.40 part by weight of active ingredient and
5.00 parts by weight of Aerosil/chalk (1:1) are mixed until homogeneous with
94.6 parts by weight of a commercial dry food.
Example 6' Emulsifiable concentrate
20 parts by weight of active ingredient are mixed with
20 parts by weight of the emulsifier, e.g. a mixture of alkylarylpolyglycol
ether with
alkylarylpolysulfonates, and with
60 parts by weight of a solvent, until the solution has been completely
homogenised.
Emulsions of the desired concentration are obtained by dilution with water.
Exampe 7: Solutions (e. . for use as a drink additive)
15 percent by weight active ingredient in 2,2-dimethyl-4-hydroxymethyl-1,3-
dioxolane,
percent by weight active ingredient in diethylene glycol monoethyl ether,
10 percent by weight in polyethylene glycol 300, and
5 percent by weight in glycerol.
Example 8: Soluble powder
25 parts by weight of active ingredient
1 part by weight of sodium lauryl sulfate,
3 parts by weight of colloidal silica gel, and
-18-
71 parts by weight of urea.
The ingredients are mixed together and ground with one another until
homogeneous.
Other biologically active compounds or adjuvants that are neutral towards the
active
ingredients and that have no adverse effect on the host animal to be treated,
as well as
mineral salts or vitamins, can be added to the compositions described.
Biological Examples
Example 9' Coinnarison test of action against Ctenocephalides felis (cat flea)
In accordance with the following protocol, both the active ingredient
according to the
invention, 1-[N-(b-chloro-3-pyridylmethyl)]-N-ethylamino-1-methylamino-2-nitro-
ethylene having the chemical structure:
'CH 3
Compound (A) of / ~ IN,~ NHCH 3
the invention I
CI \N N02
and the structurally most similar compound mentioned in EP-0 302 833, of the
formula:
CH 3
Compound (B) ~ NHICH
of the prior art ~ ~ \ N 3
I
N NO 2
are tested for comparison purposes against an untreated control group of
fleas.
Test protocol:
20 adult fleas of the species Ctenocephalides felis are introduced into a flat
round cage
closed off at both ends with gauze. A vessel sealed at the bottom with a
parafilm
membrane is then placed on the cage. The vessel contains blood comprising 1.0
ppm of
- 19-
active ingredient and is heated to a constant temperature of 37°C. The
fleas take up the
blood through the membrane. Evaluation is effected 24 and 48 hours after the
start of the
test. The percentage reduction in population (% activity) is determined from a
comparison
of the number of dead fleas given treated blood with those given untreated
blood (control
group). 24 hours after treatmene the blood is replaced with fresh blood that
has likewise
been treated and the test is continued with the surviving fleas. The untreated
blood for the
control group is also replaced after 24 hours.
Results: All three groups of fleas [(Control group/untreated blood); (Group
A/blood
treated with compound A of the invention) and (Group B/blood treated with
compound B
from the prior art)] begin to take up blood as soon as they are placed in the
test apparatus.
The behaviour of the control group and of Group B (prior art) remains
virtually unchanged
over the duration of the test. In contrast, only half an hour after the start
of the test the first
toxic signs appear in Group A (treatment according to the invention). After 24
hours the
following individual results are observed using 2 x 20 fleas in each case (in
% mortality):
Compound ppm a.i. TeSt 1 Test 2 Test 3 Test 4
B 1.0 0% 5% 0% 0%
A 1.0 100 % 95 % 95 % 100
Control 0.0 0 % 0 % 0 % 0%
Example 10: In vivo comyarison test of the action against Ctenocephalides
felis (cat flea)
In this comparative test, as in Example 9, compound A of the invention is
again tested on
cats and compared with the.structurally most similar compound B of the prior
art.
When other compounds from the Table are tested with the compounds of formula I
given
by way of example, entirely comparable results are obtained.
Testprotocol:
6 female domestic cats 1-2 years old and having a body weight of from 3.0 to
4.0 kg are
divided into three groups of two animals. One group is already infested with
fleas, but
remains untreated and serves as control group. One of the other groups
receives test
compound A and the other receives test compound B, in each case in a dose of
10 mg/kg
-20-
of body weight, by means of a gelatin capsule directly into the back of the
throat. Each of
the test compounds has been mixed beforehand with lactose 1:1. Immediately
after
administration of the test compounds the cats are infested with 20 fleas each
(16 female
and 4 male fleas) in the region of the ridge between the shoulderbones.
Further infestation
with a further 20 fleas is effected in the case of compound A on days +2, +4
and +6. In
the case of the cats treated with compound B and in the case of the comparison
group,
further infestation with fleas is unnecessary, since all the fleas from the
initial infestation
have survived.
The flea eggs are collected daily and counted. The number of dead fleas found
is also
determined daily. The results for the 2 treated groups are compared both with
each other
and with those for the control group.
Results: The number of dead fleas found and the egg production are shown in
the
following Tables 1 and 2:
Table 1: Number of fleas killed
Compound Cat no . dead fleas on day . . .
0 +1 -r2 +3 +4 +5 +6 +? +8 +9
325 12* 1 0* 11 5* 0 2* 0 0 0
A
B
Control
343 9* 1 0* 8 2* 0 2* 0 0 0
351 0* ~. 0 0 0 0 0 0 0 0
266 0* 0 0 0 0 0 0 0 0 0
339 0* 0 0 0 0 0 0 0 0 0
599 0* 0 0 0 0 0 0 0 0 0
* Infestation with 20 fleas
~1.~..~)~Jc~
-21 -
In the case of compound A, only 6 hours after infestation with the first 20
fleas there is
60 % mortality. Even on the second. day after fresh infestation with a further
20 fleas, a
further 55 % of the fleas are killed. Not until day 6 does the activity of
compound A fall
to approximately 10 %. In contrast, no significant difference is found between
the control
group and the group of cats treated with compound B. Compound B proves
ineffective
and unusable for oral administration against fleas at the low test
concentrations.
Table 2: Egg production
Compound Cat no . egg production on day...
+2 +3 +4 +5 +6 +7 +8 +9
A
B
Control
325 0* 0 0* 0 37* 233 250 370
343 0* 0 0* 60 0* 186 240 283
351 240 306 403 423 350 333 343 290
266 106 266 386 313 286 290 326 303
339 60 133 186 300 310 313 290 233
599 96 110 156 146 166 116 200 170
* Infestation with 20 fleas
In the group treated with compound A, no flea eggs are found after the first
and second
infestations with fleas. In the group of cats treated with compound B and in
the control
group, the female fleas produce the usual number of eggs; no significant
differences are
found.
The comparison test shows that compound A has good systemic activity against
adult fleas
for a period of about 3-4 days. Compound B, on the other hand, can be classed
as
completely ineffective at the test close. It could be neither predicted nor
expected that the
mono-halogenation of the pyridyl group would bring about such a significant
increase in
activity as regards the systemic action against fleas.
~ ~'~ ~:. ~ C'j
2,1.a.~a ~~
-22-
The compounds for which physical data are given in the Table of compounds that
may be
used in accordance with the invention exhibit activity against fleas that is
comparable with
that of compound A of the invention. Especially striking is the outstanding
activity of
compounds 1.1 to 1.4, 1.10 to 1.12, 1.18, 1.28, 1.29 and 1.36 to 1.40.