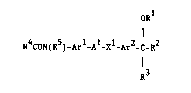Note: Descriptions are shown in the official language in which they were submitted.
2113913
- ,
ACETANILIDe DERIVATIVES
This invention concerns acetanilide derivatives and more
particularly acetanilide derivatives which are inhibitors of the enzyme
5-lipoxygenase (hereinafter referred to as 5-LO). The invention also
concerns processes for the manufacture of said acetanilide derivatives
and novel pharmaceutical compositions containing them. Also included --
in the invention is the use of said acetanilide derivatives in the
treatment of various inflammatory and/or allergic diseases in which the ~-
direct or indirect products of 5-L0 catalysed oxidation of arachidonic
acid are involved, and the production of new medicaments for such use.
As stated above the acetanilide derivatives described ~ 5~
hereinafter are inhibitors of 5-LO, which enzyme is known to be ~ ~ -
involved in catalysing the oxidation of arachidonic acid to give rise
via a cascade process to the physiologically active leukotrienes such
as leukotriene B4 (LTB4) and the peptido-lipid leukotrienes such as
leukotriene C4 (LTC4) and leukotriene D4 (LTD4) and various
metabolites. ~ ;
The biosynthetic relationship and physiological properties of
the leukotrienes are summarised by G.W. Taylor and S.R. Clarke in
Trends in Pharmacolo~ical Sciences, 1986, 7, 100-103. The leukotrienes
and their metabolites have been implicated in the production and
development of various inflammatory and allergic diseases such as
inflammation of the ~oints (especially rheumatoid arthritis,
osteoarthritis and gout), inflammation of the gastrointestinal tract
(especially inflammatory bowel disease, ulcerative colitis and --
gastritis), skin disease (especially psoriasis, eczema and dermatitis)
and respiratory disease ~especially asthma, bronchitis and allergic
rhinitis), and in the production and development of various
cardiovascular and cerebrovascular disorders such as myocardial
infarction, angina and peripheral vascular disease. In addition the
leukotrienes are mediators of inflammatory diseases by virtué of their
ability to modulate lymphocyte and leukocyte function. Other
physiologically active metabolites of arachidonic acid, such as the
prostaglandins and thromboxanes, arise via the action of the enzyme
cyclooxygenase on arachidonic acid.
- 2 - 21~3~13
It is disclosed in European Patent Application Nos. 0375457
A2 and 0385679 A2 that certain heterocyclic derivatives possess
inhibitory properties against S-L0. Furthermore European Patent
Applications Nos. 0409412, 0409413 and 0462812 are also concerned with
heterocyclic derivatives which possess inhibitory properties against
5-L0. We have now discovered that certain acetanilide derivatives
which possess some structural features which are similar to those of
the compounds disclosed in the above-mentioned applications but which
possess other structural features, in particular acetanilide groups,
which were not envisaged in those earlier applications are effective
inhibitors of the enzyme 5-L0 and thus of leukotriene biosyntheses.
Thus such compounds are of value as therapeutic agents in the treatment
of, for example, allergic conditions, psoriasis, asthma, cardiovascular
and cerebrovascular disorders, and/or inflammatory and arthritic
conditions, mediated alone or in part by one or more leukotrienes.
According to the invention there is provided an acetanilide
derivative of the formula I
ORl
,
R4coN(R5)-Arl-Al-xl-Ar2-c-R2
R3
.
wherein R4 is (1-4C)alkyl;
R5 is hydrogen or (1-4C)alkyl;
Arl is phenylene, pyridinediyl or pyrimidinediyl which may optionally
bear one or two substituents selected from halogeno, trifluoromethyl,
hydroxy, (1-4C)alkyl and (1-4C)alkoxy;
Al is a direct link to X1 or A1 is (1-4C)alkylene;
xl is oxy, thio, sulphinyl or sulphonyl;
Ar2 is thiophenediyl, furandiyl, thiazolediyl, oxazolediyl,
thiadiazolediyl or oxadiazolediyl which may optionally bear one or two
substituents selected from halogeno, trifluoromethyl, (1-4C)alkyl and
(1-4C)alkoxy; :
R1 and R2 together form a group of the formula -A2-X2-A3- which
'~ 9 ~ ~
together with the oxygen atom to which A2 is attached and with the
carbon atom to which A3 is attached define a ring having 5 or 6 ring
atoms, wherein A2 and A3, which may be the same or different, each is
(1-3C)alkylene and x2 is oxy, thio, sulphinyl or sulphonyl and which
ring may bear one, two or three (1-4C)alkyl substituents; and
R3 is (1-4C)alkyl;
or a pharmaceutically-acceptable salt thereof.
In this specification the generic term "alkyl" includes both
straight-chain and branched-chain alkyl groups. However references to
individual alkyl groups such as "propyl" are specific for the
straight-chain version only and references to individual branched-chain
alkyl groups such as "isopropyl" are specific for the branched-chain
version only. An analogous convention applies to other generic terms.
It is to be understood that, insofar as certain oi the
compounds of formula I defined above may exist in optically active or
racemic form by virtue of one or more asymmetric carbon atoms, the -
invention includes in its definition any such optically active or
racemic form which possesses the property of inhibiting 5-LO. The
synthesis of optically active forms may be carried out by standard
techniques of organic chemistry well known in the art, for example by
synthesis from optically active starting materials or by resolution of
a racemic form.
Suitable values for the generic terms referred to above
include those set out below.
A suitable value for R4 or R5 when it is (1-4C)alkyl is, for
example, methyl, ethyl, propyl, isopropyl or butyl.
Suitable values for substituents on Ar1 or Ar2 include, for
example:-
for halogeno: fluoro, chloro and bromo;
for (1-4C)alkyl: methyl, ethyl, propyl and
isopropyl;
for (1-4C)alkoxy: methoxy, ethoxy, propoxy and
isopropoxy.
_ 4 2113913
A suitable value for Ar1 when it is phenylene is, for
example, 1,3- or 1,4-phenylene.
A suitable value for Ar1 when i~ is pyridinediyl or
pyrimidinediyl is, for example, 2,4-, 2,5- or 3,5-pyridinediyl, or 2,5-
or 4,6-pyrimidinediyl.
A suitable value for A1 when it is (1-4C)alkylene is, for
example, methylene, ethylene or trimethylene.
A suitable value for Ar2 uhen it is thiophenediyl, furandiyl,
thiazolediyl, oxazolediyl, thiadiazolediyl or oxadiazolediyl is, for
example, 2,4- or 2,5-thiophenediyl, 2,4- or 2,5-furandiyl, 2,4- or
2,5-thiazolediyl, 2,4- or 2,5-oxazolediyl, 2,5-thiadiazoled:iyl or
2,5-oxadiazolediyl.
Uhen R1 and R2 together form a group of the formula
-A2-X2-A3- which together with the oxygen atom to which A2 is attached
and with the carbon atom to which A3 is attached define a ring having 5
or 6 ring atoms then a suitable value for A2 and A3, which may be the
same or dif$erent, when each is (1-3C)alkylene is, for example,
methylene, ethylene or trimethylene. Suitable values for the
(1-4C)ilkyl substituents which may be present on said 5- or 6-membered
ring include, for example, methyl, ethyl, propyl, isopropyl and butyl.
A suitable value for R3 when it is (1-4C)alkyl is, for
example, methyl, ethyl, propyl or butyl.
A suitable pharmaceutically-acceptable salt of a compound of
the invention is, for example, an acid-addition salt of a compound of
the invention which is sufficiently basic, for example, an ;
acid-addition salt with, for example, an inorganic or organic acid, for
example hydrochloric, hydrobromic, sulphuric, phosphoric,
trifluoroacetic, citric or maleic acid. In addition a suitable
pharmaceutically-acceptable salt of a compound of the invention which
is sufficiently acidic is an alkali metal salt, for example a sodium or
potassium salt, an alkaline earth metal salt, for example a calcium or
magnesium salt, an ammonium salt or a salt with an organic base which
affords a physiologically-acceptable cation, for example a salt with
methylamine, dimethylamine, trimethylamine, piperidine, morpholine or
tris-(2-hydroxyethyl)amine. ~`
211~913
-- 5 --
Particular compounds of the invention include, for example,
acetanilide derivatives of the formula I, or
pharmaceutically-acceptable salts thereof, wherein:-
~a) Ar1 is phenylene which may optionally bear one or two
substituents selected from halogeno, hydroxy, (1-4C)alkyl and
(1-4C)alkoxy; and R4, R5, A1, X1, Ar2, R1, R2 and R3 have any of the
meanings defined hereinbefore or in this section defining particular
compounds;
(b) Ar1 is 1,4-phenylene which may optionally bear a substituent
selected from halogeno, hydroxy, (1-4C)alkyl and (1-4C)alkoay; and R4, :
R5, A1, X1, Ar2, R1, R2 and R3 have any of the meanings defined
hereinbefore or in this section defining particular compounds;
~c) Ar1 is 2,5-pyridinediyl; and R4, R5, A , X , Ar , R , R and
R3 have any of the meanings defined hereinbefore or in this section
defining particular compounds;
(d) A1 is a direct link to X1; and R4, R5, Ar1, 21, Ar2, R1, R2
and R3 have-any of the meanings defined hereinbefore or in this section
defining particular compounds;
(e) A1 is (1-4C)alkylene and X1 is oxy; and R4, R5, Ar1, Ar2, R1,
R2 and R3 have any of the meanings defined hereinbefore or in this "
section defining particular compounds;
(f) A1 is a direct link to X1 and X1 is thio; and R4, R5, Ar1,
Ar2, R1, R2 and R3 have any of the meanings defined hereinbefore or in
this section defining particular compounds;
(g) Ar2 is thiophenediyl; and R4, R5, Ar1, A1, X1, R1, R2 and R3
have any of the meanings defined hereinbefore or in this section
defining particular compounds;
(h) Ar2 is thiazolediyl; and R , R , Ar , A , X , R , R and R
have any of the meanings defined hereinbefore or in this section
defining particular compounds; or
(i) R1 and R2 together form a group of the formula -A2-X2-A3-
which together with the oxygen atom to which A is attached and with
the carbon atom to which A3 is attached define a ring having 5 ring
atoms, wherein each of A2 and A3 is methylene and x2 is oxy, and which
ring may optionally bear one or two substituents selected from methyl
and ethyl, and R3 is methyl or ethyl; and R4, R5, Ar1, A1, X1 and Ar2
21~3913
-- 6 --
have any of the meanings defined hereinbefore or in this section
defining particular compounds.
A preferred compound of the invention comprises an
acetanilide derivative of the formula I
wherein R4 is hydrogen, methyl, ethyl, propyl or isopropyl;
R5 is hydrogen, methyl, ethyl, propyl or isopropyl;
Ar1 is 1,4-phenylene which may optionally bear one substituent selected
from fluoro, chloro, hydroxy, methyl and methoxy;
A1 is a direct link to X1 and X1 is thio or sulphonyl, or A1 is
methylene and X1 is oxy;
Ar2 is 2,4- or 2,5-thiophenediyl or 2,4- or 2,5-thiazolediyl;
R1 and R2 together form a group of the formula -A2-X2-A3- which
together with the oxygen atom to which A2 is attached and the carbon
atom to which A is attached define a ring having 5 ring ato,ns wherein
each of A2 and A3 is methylene and x2 is oxy, and which ring may
optionally bear one or two substituents selected from methyl and ethyl,
and R3 is methyl or ethyl;
or a pharmaceutically-acceptable salt thereof. --
A further preferred compound of the invention comprises an -acetanilide derivative of the formula I ~
wherein R4 is methyl or ethyl; - -
R5 is hydrogen, methyl or ethyl;
Ar1 is 1,4-phenylene; --
A1 is a direct link to X1 and X1 is thio;
Ar2 is 2,4-thiophenediyl (with the X1 group in the 2-position);
R1 and R2 together form a group of the formula -A2-X2-A3- which ;~
together with the oxygen atom to which A2 is attached and with the
carbon atom to which A3 is attached define a ring having S ring atoms,
wherein A is -~(He)2-, A is methylene and X is oxy, and R3 is
methyl;
or a pharmaceutically-acceptable salt thereof.
A further preferred compound of the invention comprises an
acetanilide derivative of the formula I
wherein R4 is methyl or ethyl;
R5 is hydrogen, methyl or ethyl;
Ar1 is 1,4-phenylene;
'~ . . : . , ' .:
, . '
', ,-~., ': ' , ` '
'~ :', ' ............ ': . '
a'
Y:, ~ - ',
211391 3
-- 7 --
A1 is a direct link to X1 and X1 is thio;
Ar2 is 2,4-thiazolediyl (with the X1 group in the 2-position) or
2,5-thiazolediyl (with the X1 group in the 2-position);
R1 and R2 together form a group of the formula -A2-X2-A3- which
together with the oxygen atom to which A2 is attached and with the
carbon atom to which A3 is attached define a ring having S ring atoms,
~herein A2 is -C(Me)2-, A3 is methylene and X is oxy, and R3 is
methyl;
or a pharmaceutically-acceptable salt thereof.
A further preferred compound of the invention comprises an
acetanilide derivative of the formula I
wherein R4 is methyl;
R5 is methyl;
Ar1 is 1,4-phenylene or 2,5-pyridinediyl (with the -A1-X1- group in the
2-position);
A1 is a direct link to X1 and X1 is thio or sulphonyl;
Ar2 is 2,4-thiophenediyl (with the X1 group in the 2-position); :
R1 and R2 together form a group of the formula -A2-X2-A3- which - -
together with the oxygen atom to which A2 is attached and with the
carbon atom to which A3 is attached define a ring having 5 ring atoms, ` : ~
wherein A2 is -C(he)2-, A3 is methylene and x2 is oxy; and ;-
R3 is methyl; ::
or a pharmaceutically-acceptable salt thereof.
A specific especially preferred compound of the invention is, -
for example, the following acetanilide derivative of the formula I, or
a pharmaceutically-acceptable salt thereof~
N-methyl-4'-{4-l(4S)-2,2,4-trimethyl-1,3-dioxolan-4-yllthien-2-ylthio}-
acetanilide or
N-methyl-4'-{5-1(4R)-2,2,4-trimethyl-1,3-dioxolan-4-yl]thiazol-2-
ylthio}acetanilide.
A compound of the invention comprising an acetanilide ~ :
derivative of the formula I, or a pharmaceutically-accèptable salt
thereof, may be prepared by any process known to be applicable to the
preparation of structurally-related compounds. Such procedures are
provided as a further feature of the invention and are illustrated by
the following representative examples in which, unless otherwise
21 13913
d R4 R5 Arl Al X1 Ar2, R1, R2 and R3 have any of the
meanings defined hereinbefore, provided that when there is a hydroxy
group in Ar1 then any such group may optionally be protected by a
conventional protecting group which may be removed when so desired by
conventional means.
(a) The coupling, conveniently in the presence of a suitable
base, of a compound of the formula II
R4CoN(R5)-Arl-Al-z II
wherein Z is a displaceable group with a compound of the formula III
oRl
Hx1_Ar2_c_R2 III
'
R3
A suitable base for the reaction is, for example, an alkali ~ ~;
or alkaline earth metal carbonate, (1-4C)alkoxide, (1-4C)alkanoate, "
hydroxide or hydride, for example sodium carbonate, potassium
carbonate, barium carbonate, sodium ethoxide, potassium butoxide,
sodium acetate, sodium hydroxide, potassium hydroxide, sodium hydride
or potassium hydride. Alternatively a suitable base for the reaction
is, for example, an organic amine base such as pyridine, 2,6-lutidine,
collidine, 4-dimethylaminopyridine, triethylamine, N-methylmorpholine
or diazabicyclol5.4.0lundec-7-ene.
A suitable displaceable group Z is, for example, a halogeno
or sulphonyloxy group, for example a chloro, bromo, iodo,
methanesulphonyloxy or toluene-4-sulphonyloxy group.
The reaction is conveniently performed in a suitable inert
solvent or diluent, for example, one or more of water, a (1-4C)alcohol
such as methanol, ethanol and propanol, pyridine, 1,2-dimethoxyethane,
tetrahydrofuran, 1,4-dioxan or a dipolar aprotic solvent such as
N,N-dimethylformamide and dimethylsulphoxide. The reaction is
conveniently performed at a temperature in the range, for example, 10
, ~ ~ . . ............. . . .
",
~........ ., .: . ::~
~ , : ;'` .,,; - ~-.;
2113913
g
to 150C, conveniently at or near 130C.
The starting materials of the formulae II and III may be
obtained by standard procedures of organic chemistry. The preparation
of such starting materials is described within the accompanying
non-limiting Examples. Alternatively necessary starting materials are
obtainable by analogous procedures to those illustrated which are
within the ordinary skill of an organic chemist. The disclosures of
European Patent Applications Nos. 0375457, 0385679, 0409412, 0409413
and 0462812 are particularly relevant to the preparation of suitable
starting materials. -
(b) The cyclisation, conveniently in the presence of a suitable
acid, of a compound of the formula IV
OH -- -
,.
R4coN(Rs)-Arl-Al-xl-Ar2-c-A3-x2-H IV
R3
with an appropriate aldehyde or ketone, or with the corresponding
hemiacetal or acetal derivative thereof.
A suitable acid for the cyclisation reaction is, for example,
an inorganic acid such as hydrochloric, sulphuric or phosphoric acid,
or, for example? an organic acid such as 4-toluenesulphonic acid or
trifluoroacetic acid. The cyclisation reaction is conveniently
performed in a suitable inert solvent or diluent, for example 1,2-
dimethoxyethane or tetrahydrofuran. Preferably the reaction is
performed using the appropriate aldehyde or ketone as both a reactant
and diluent. The cyclisation is effected at a temperature in the
range, for example, 20 to 150C, conveniently at or near the boiling
point of the diluent or solvent.
The tertiary alcohol starting material of the formula IV may
be obtained by standard procedures of organic chemistry. The
preparation of examples of such starting materials is described within
the accompanying non-limiting Examples which are provided for the
purpose of illustration only.
: 2113913
- 10 -
(c) The coupling of a compound of the formula V
R CON(R5)-Arl-A1-xl_z V
wherein Z is a displaceable group as defined hereinbefore, or --
alternatively, when X is a thio group, Z may be a group of the formula
R4coN(R5) _Arl_Al xl
with an organometallic reagent of the formula VI
OR
:~ , ,
2 2
M-Ar -C-R VI
R3
wherein H is an alkali metal or alkaline earth metal such as lithium or
calcium or N represents the magnesium halide portion of a conventional
Grignard reagent.
The coupling reaction is conveniently performed in a suitable
inert solvent or diluent as defined hereinbefore and at a temperature
in the range, for example, -80 to +50C, conveniently in the range
-80C to ambient temperature.
The preparation of the starting materials of the formulae V
and VI may be obtained by standard procedures of organic chemistry.
(d) For the production of those compounds of the formula I
wherein Xl is a sulphinyl or sulphonyl group, or wherein R1 and R2
together form a group of the formula -A2-X2-A3- and x2 is a sulphinyl
or sulphonyl group, the oxidation of a compound of the formula I
wherein Xl is a thio group or wherein Rl and R2 together form a group
of the formula -A2-X--A3- and x2 is a thio group.
A suitable oxidising group is, for example, any agent known
in the art for the oxidation of thio to sulphinyl and/or sulphonyl, for
- ~
~113913
- 11
example, hydrogen peroxide, a peracid (such as 3-chloroperoxybenzoic or
peroxyacetic acid), an alkali metal peroxysulphate (such as potassium
peroxymonosulphate~, chromium trioxide or gaseous oxygen in the
presence of platinum. The oxidation is generally carried out under as
mild conditions as possible and with the required stoichiometric amount
of oxidising agent in order to reduce the risk of over oxidation and
damage to other functional groups. In general the reaction is carried
out in a suitable solvent or diluent such as methylene chloride, ..
chloroform, acetone, tetrahydrofuran or tert-butyl methyl ether and at
a temperature, for example, at or near ambient temperature, that is in
the range 15 to 35C. Uhen a compound carrying a sulphinyl group is
required a milder oxidising agent may also be used, for example sodium
or potassium metaperiodate, conveniently in a polar solvent such as
acetic acid or ethanol. It will be appreciated that when a coDpound of ~- --
the formula I containing a sulphonyl group is required, it may be
obtained by oxidation of the corresponding sulphinyl compound as well
as of the corresponding thio compound.
Conventional protecting groups for a hydroxy group which may
be present in Ar1 are set out hereinafter.
A suitable protecting group for a hydroxy group is, for
example, an acyl group, for example a (2-4C)alkanoyl group (especially ~ -
acetyl), an aroyl group (especially benzoyl) or an arylmethyl group
(especially benzyl). The deprotection conditions for the above
protecting groups will necessarily vary with the choice of protecting
group. Thus, for example, an acyl group such as an alkanoyl or an
aroyl group may be removed, for example, by hydrolysis ~ith a suitable
base such as an alkali metal hydroxide, for example lithium or sodium
hydroxide. Alternatively an arylmethyl group such as a benzyl group
may be removed, for example, by hydrogenation over a catalyst such as
palladium-on-charcoal.
When a pharmaceutically-acceptable salt of a compound of the
formula I is required, it may be obtained, for example, by reaction of
sald compound with a suitable acid or base using a conventional ~ .
procedure. ~hen an optically active form of a compound of the formula
I is required, it may be obtained by carrying out one of the aforesaid
procedures using an optically active starting material, or by
2113913
- 12 -
resolution of a racemic form of said compound using a conventional
procedure.
As stated previously, the compounds of the formula I are
inhibitors of the enzyme 5-LO. The effects of this inhibition may be -
demonstrated using one or more of the standard procedures set out
below:- -
a) An in vitro assay system involving incubating a test -
comp~und with heparinised human blood, prior to challenge with the
calcium ionophore A23187 and then indirectly measuring the inhibitory
effects on 5-LO by assaying the amount of LTB4 using specific
radioimmunoassays described by Carey and Forder (Prosta~landins,
Leukotrienes Ned., 1986, 22, 57; Prosta~landins~ 1984, 28, 666; Brit.
J. Pharmacol., 1985, 84, 34P) which involves the use of a protein-LTB4
conjugate produced using the procedure of Young et alia
(Prostaglandins, 1983, 26(4), 605-613). The effects of a test compound
on the enzyme cyclooxygenase (which is involved in the alternative
metabolic pathway for arachidonic acid and gives rise to
prostaglandins, thromboxanes and related metabolites) may be measured
at the same time using the specific radioimmunoassay for thromboxane
B2(TxB2) described by Carey and Forder (see above). This test provides
an indication of the effects of a test compound against 5-L0 and also ~-~
cyclooxygenase in the presence of blood cells and proteins. It permits
the selectivity of the inhibitory effect on 5-L0 or cyclooxygenase to
be assessed.
b) An ex vivo assay system, which is a variation of test a)
above, involving administration to a group of rats of a test compound
(usually orally as the suspension produced when a solution of the test
compound in dimethylsulphoxide is added to carboxymethylcellulose),
blood collection, heparinisation, challenge with A23187 and
radioimmunoassay of LTB4 and TxB2. This test provides an indication of
the bioavailability of a test compound as an inhibitor of 5-L0 or
cyclooxygenase.
c) An in vivo system involving measuring the effects of a
test compound administered orally against the liberation of LTB4
induced by zymosan within an air pouch generated within the
subcutaneous tissue of the back of male rats. The rats are
- ~ - - . ---. . - - - . - . . . - .. .. . . . . . . . . . .
2113~13
anaesthetised and air pouches are formed by the injection of sterile
air (20ml). A further injection of air (lOml) is similarly given after
3 days. At 6 days after the initial air injection the test compound is
administered (usually orally as the suspension produced when a solution
of the test compound in dimethylsulphoxide is added to ~ ~
hydroxypropylmethylcellulose), followed by the intrapouch injection of ~ -
zymosan (lml of a 1% suspension in physiological saline). After 3 -
hours the rats are killed, the air pouches are lavaged with ~ ;
physiological saline, and the specific radioimmunoassay described above
is used to assay LTB4 in the washings. This test provides an
indication of inhibitory effects against S-LO in an inflammatory
milieu.
Although the pharmacological properties of the compounds of
the formula I vary with structural changes as expected, in general
compounds of the formula I possess 5-LO inhibitory effects at the
following concentrations or doses in one or more of the above tests
a)-c)~
Test a): IC50 (LTB4) in the range, for example, 0.01-40~H
IC50 (TxB2) in the range, for example, 40-200~M; -~
Test b): oral ED50(LTB4) in the range, for example,
O.1-lOOmg/kg;
Test c): oral ED50(LTB4) in the range, for example,
0.1-50mg/kg.
No overt toxicity or other untoward effects are present in
tests b) and/or c) when compounds of the formula I are administered at
several multiples of their minimum inhibitory dose or concentration. ~ ;~
Thus, by way of example, the compound N-methyl-4'-{4-l(4S)-
2,2,4-trimethyl-1,3-dioxolan-4-yl]thien-2-ylthio}acetanilide has an
IC50 of <0.04~M against LTB4 in test a) and an oral ED50 f
approximately 1.5 mg/kg versus LTB4 in test c); and the compound
N-methyl-4'-{5-[(4R)-2,2,4-trimethyl-1,3-dioxolan-4-yl]thiazol-2-
ylthio}acetanilide has an IC50 of 0.07~M against LTB4 in test a).
In general those compounds of the formula I which are particularly
preferred have an IC50 of <l~H against LTB4 in test a) and an oral ED50
of <10 mg/kg against LTB4 in tests b) and/or c).
, .,
,, . . - ... .
21~3913
- 14 -
These compounds are examples of compounds of the invention
uhich show selective inhibitory properties for 5-LO as opposed to
cyclooxygenase, which selective properties are expected to impart
improved therapeutic properties, for example, a reduction in or freedom
from the gastrointestinal side-effects frequently associated with
cyclooxygenase inhibitors such as indomethacin.
According to a further feature of the invention there is
provided a pharmaceutical composition which comprises an acetanilide
derivative of the formula I, or a pharmaceutically-
acceptable salt thereof, in association with a pharmaceutically-
acceptable diluent or carrier.
The composition may be in a form suitable for oral use, for
example a tablet, capsule, aqueous or oily solution, suspension or
emulsion; for topical use, for example a cream, ointment, gel or
aqueous or oily solution or suspension; for nasal use, for example a
snuff, nasal spray or nasal drops; for vaginal or rectal use, for
example a suppository; for administration by inhalation, for example as
a finely divided powder such as a dry powder, a microcrystalline form -
or a liquid aerosol; for sub-lingual or buccal use, for example a
tablet or capsule; or for parenteral use (including intravenous,
subcutaneous, intramuscular, intravascular or infusion), for example a
sterile aqueous or oily solution or suspension.
In general the above compositions may be prepared in a conventional
manner using conventional excipients.
The amount of active ingredient (that is an acetanilide
derivative of the formula I, or a pharmaceutically-acceptable salt
thereof) that is combined with one or more excipients to produce a
single dosage form will necessarily vary depending upon the host
treated and the particular route of administration. For example, a
formulation intended for oral administration to humans will generally
contain, for example, from 0.5 mg to 2 g of active agent compounded
with an appropriate and convenient amount of excipients which may vary
from about 5 to about 98 percent by weight of the total composition.
Dosage unit forms will generally contain about 1 mg to about 500 mg of
an active ingredient.
2113913
- 15 -
According to a further feature of the invention there is
provided an acetanilide derivative of the formula I, or a
pharmaceutically-acceptable salt thereof, for use in a method of
treatment of the human or animal body by therapy.
The invention also includes a method of treating a disease or
medical condition mediated alone or in part by one or more leukotrienes
which comprises administering to a warm-blooded animal requiring such
treatment an effective amount of an active ingredient as defined above.
The invention also provides the use of such an active ingredient in the
production of a new medicament for use in a leukotriene mediated
disease or medical condition.
The size of the dose for therapeutic or prophylactic purposes
of a compound of the formula I will naturally vary according to the
nature and severity of the conditions, the age and sex of the animal or
patient and the-route of administration, according to well known
principles of medicine. As mentioned above, compounds of the formula I -
are useful in treating those allergic and inflammatory conditions which
are due alone or in part to the effects of the metabolites of ~ ~ -
arachidonic acid arising by the linear (5-LO catalysed) pathway and in
particular the leukotrienes, the production of which is mediated by "-
5-LO. As previously mentioned, such conditions include, for example,
asthmatic conditions, allergic reactions, allergic rhinitis, allergic
shock, psoriasis, atopic dermatitis, cardiovascular and cerebrovascular
disorders of an inflammatory nature, arthritic and inflammatory joint
disease, and inflam~atory bowel diseases.
In using a compound of the formula I for therapeutic or
prophylactic purposes it will generally be administered so that a daily
dose in the range, for example, 0.5 mg to 75 mg per kg body weight is
received, given if required in divided doses. In general lower doses
will be administered when a parenteral route is employed. Thus, for
example, for intravenous administration, a dose in the range, for
example, 0.5 mg to 30 mg per kg body weight will generally be used.
Similarly, for administration by inhalation, a dose in the range, for
example, 0.5 mg to 25 mg per kg body weight will be used.
2113913
- 16 -
Although the compounds of the formula I are primarily of
value as therapeutic agents for use in warm-blooded animals (including
man), they are also useful whenever it is required to inhibit the
enzyme 5-LO. Thus, they are useful as pharmacological standards for
use in the development of new biological tests and in the search for
new pharmacological agents.
By virtue of their effects on leukotriene production, the
compounds of the formula I have certain cytoprotective effects, for
example they are useful in reducing or suppressing certain of the
adverse gastrointestinal effects of the cyclooxygenase inhibitory non-
steroidal anti-inflammatory agents (NSAIA), such as indomethacin,
acetylsalicylic acid, ibuprofen, sulindac, tolmetin and piroxicam.
Furthermore, co-administration of a 5-LO inhibitor of the formula I
with a NSAIA can result in a reduction in the quantity of the latter
agent needed to produce a therapeutic effect, thereby reducing the
likelihood of adverse side-effects. According to a further feature of
the invention there is provided a pharmaceutical composition which
comprises an acetanilide derivative of the formula I, or a
pharmaceutically-acceptable salt thereof as defined hereinbefore, in
conjunction or admixture with a cyclooxygenase inhibitory non-steroidal `-
anti-inflammatory agent (such as those mentioned above), and a
pharmaceutically-acceptable diluent or carrier.
The cytoprotective effects of the compounds of the formula I
may be demonstrated, for example in a standard laboratory model which
assesses protection against indomethacin-induced or ethanol-induced
ulceration in the gastrointestinal tract of rats.
The compositions of the invention may in addition contain one
or more therapeutic or prophylactic agents known to be of value for the
disease under treatment. Thus, for example a known platelet ~-
aggregation inhibitor, hypolipidemic agent, anti-hypertensive agent,
beta-adrenergic blocker or a vasodilator may usefully also be present
in a pharmaceutical composition of the invention for use in treating a
heart or vascular disease or condition. Similarly, by way of example,
an anti-histamine, steroid (such as beclomethasone dipropionate),
sodium cromoglycate, phosphodiesterase inhibitor or a beta-adrenergic ~ -
stimulant may usefully also be present in a pharmaceutical composition
:~:
-
21~3913
- 17 -
of the invention for use in treating a pulmonary disease or condition.
The invention will now be illustrated in the following
non-limiting Examples in which, unless otherwise stated~
(i) evaporations were carried out by rotary evaporation in
vacuo and work-up procedures were carried out after removal of residual
solids by filtration;
(ii) operations were carried out at ambient temperature,
that is in the range 18-25C and under an atmosphere of an inert gas
such as argon;
(iii) column chromatography (by the flash procedure) and
medium pressure liquid chromatography (MPLC) were performed on Nerck
Rieselgel silica (Art. 9385) or Herck Lichroprep RP-18 (Art. 9303)
reversed-phase silica obtained from E. Nerck, Darmstadt, Germany;
(iv) yields are given for illustration only and are not
necessarily the maximum attainable;
(v) the structures of the end-products of the formula I were
confirmed by NHR and mass spectral techniques; unless otherwise stated,
CDCl3 solutions of the end-products of the formula I were used for the
determination of the NMR spectral data, chemical shift values were
measured on the delta scale and the following abbreviations are used~
s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet;
(vi) intermediates were not generally fully characterised
and purity was assessed by thin layer chromatographic, infra-red (IR)
or ~MR analysis, ~;
(vii) melting points are uncorrected and were determined
using a Nettler SP62 automatic melting point apparatus or an oil-bath
apparatus; melting points for the end-products of the formula I were -~
determined after recrystallisation from a conventional organic solvent
such as ethanol, methanol, acetone, ether or hexane, alone or in -
admixture; `
(viii) the following abbreviations have been used:-
-, ::
THF tetrahydrofuran; -
DHF N,N-dimethylformamide. ~ -~
2113913
- 18 -
Fxamp~e 1
A mixture of N-methyl-4'-iodoacetanilide (0.52 g),
2-mercapto-4-1(4S)-2,2,4-trimethyl-1,3-dioxolan-4-yllthiophene
(0.48 g), potassium carbonate (0.4 g), cuprous chloride (0.055 g) and
DMF (5 ml) was stirred and heated at 130C for 2 hours. The mixture
was cooled to ambient temperature and partitioned between ethyl acetate
and water. The ~rganic phase was washed with water and with brine,
dried (MgS04) and evaporated. The residue was purified by column
chromatography using a 5:1 mixture of methylene chloride and ethyl
acetate as eluent. There was thus obtained N-methyl-4'-{4-[(4S)-2,2,4-
trimethyl-1,3-dioxolan-4-yllthien-2-ylthio}acetanilide (0.68 g, 90%) as
an oil;
NMR Spectrum: (CD3SOCD3) 1.33 (s, 3H), 1.4 (s, 3H), 1.53 (s, 3H),
1.7-1.9 (s, 3H); 3.3 (s, 3H), 3.95-4.06 (q, 2H), 7.2-7.35 (m, 4H), 7.4
(d, lH), 7.65 (d, lH);
Elemental Anal~sis: Found C, 60.1; H, 6.1; N, 3.4;
C19H23N03S2 requires C, 60.4; H, 6.1; N, 3.7%.
The N-methyl-4'-iodoacetanilide used as a starting material
was obtained as follows:-
Acetyl chloride (1.84 g) was added dropwise to a stirredmixture of 4-iodoaniline (5 g), triethylamine (3.5 ml) and methylene
chloride (50 ml) which had been cooled to 10C in an ice-bath. The ;~
mixture was stirred at 10C for 3 hours. The mixture was partitioned
between ethyl acetate and water. The organic phase was washed with
water and with brine, dried (HgS04) and evaporated. There was thus
obtained 4'-iodoacetanilide (5.52 g), m.p. 179-181C.
Sodium hydride (60% dispersion in mineral oil, 0.92 g) was
added portionwise to a stirred solution of 4'-iodoacetanilide (5.5 g)
in DMF (35 ml) which had been cooled to 10C in an ice-bath. The
mixture was stirred and allowed to warm to ambient temperature during 2
hours. Hethyl iodide (1.6 ml) was added and the mixture was stirred at
ambient temperature for 66 hours. The mixture was poured onto a
mixture of dilute aqueous hydrochloric acid and ice. The precipitate
was isolated, washed in turn with water and petroleum ether and dried.
There was thus obtained N-methyl-4'-iodoacetanilide (5.1 g), m.p.
140-142C;
' :: : ' .: . :
:' :
: ' , : : ~ . : . , :
2113913
- 19 -
NMB Spectrum: (CD3SOCD3) 1-8 (s, 3H), 3.16 (s, 3H), 7-15 (d, 2H), 7-85
(d, 2H).
The 2-mercapto-4-1(4S)-2,2,4-trimethyl-1,3-dioxolan-4-yl]-
thiophene used as a starting material was obtained as follows:-
n-Butyl-lithium (1.55H in hexane, 37 ml) was added to a
stirred mixture of methyltriphenylphosphonium bromide (21.4 g) and THF
(100 ml~ which had been cooled to 10C. The mixture was stirred at
ambient temperature for 90 minutes. The mixture was recooled in an
ice-bath and a solution of 4-acetyl-2-bromothiophene (Synth. Comm.,
1981, 29-34; 11 g) in THF (60 ml) was added. The mixture was stirred
at 0C for 1 hour and at ambient temperature for 2 hours. The mixture
was partitioned between diethyl ether and a saturated aqueous ammonium
chloride solution. The organic phase was washed with water and with
brine, dried (HgS04) and evaporated. The residue was purified by
column chromatography using hexane as eluent. There was thus obtained
2-bromo-4-isopropenylthiophene (9 g, 85%).
n-Butyl-lithium (1.6M in THF, 6.1 ml) was added to a stirred
solution of a portion (1.85 g) of the product so obtained in diethyl ~ -
ether (20 ml) which had been cooled to -80C. The mixture was stirred
at -80C for 1 hour. A solution of dimethyl disulphide (0.942 g) in
diethyl ether (5 ml) was added. The mixture was stirred and allowed to
warm to -40C during 2 hours. The mixture was poured onto a mixture of
a saturated solution of ammonium chloride and ice and extracted with
diethyl ether. The organic phase was washed with water and with brine,
dried (HgS04) and evaporated. The residue was purified by column
chromatography using hexane as eluent. There was thus obtained
4-isopropenyl-2-methylthiothiophene (1.35 g, 87X);
NMR Spectrum: 2.08 (s, 3H), 2.5 (s, 3H), 5.0 (m, lH), 5.3 (m, lH), 7.13
(d, lH), 7.25 (d, lH).
After repetition of the previous reaction, the compound so
obtained (3.1 g) was added to a vigorously stirred mixture o~ an osmium
dihydroquinine complex known as AD-mix-~ (25.2 g; J. Or~. Chem., 1992,
57, 2768-2771), tert-butanol (90 ml) and water (90 ml) which had been
cooled to 0C. The mixture was stirred at 0C for 6 hours and stored
at -5C for 16 hours. Sodium sulphite (10 g) was added and the mixture
was stirred and allowed to warm to ambient temperature. The mixture
~1~3gl3
- 20 -
was partitioned between ethyl acetate and water. The organic phase was
dried (HgSO4) and evaporated. The residue was purified by column
chromatography using a 5:3 mixture of hexane and ethyl acetate as
eluent. There was thus obtained 4-[(2S)-1,2-dihydroxyprop-2-yl]-2-
methylthiothiophene (3.41 g, 92%), m.p. 71-73C, lalpha]25 = + 14.8
(conc. = 0.5 g per 100 ml of methylene chloride).
A mixture of the product so obtained, acetone dimethyl acetal
(2.6 g), 4-toluenesulphonic acid (0.04 g) and acetone (50 ml) was
stirred and heated to reflux for 1 hour. The mixture was cooled to
ambient temperature and neutralised by the addition of dilute aqueous
potassium carbonate solution. The mixture was partitioned between
ethyl acetate and water. The organic phase was washed with water,
dried (MgSO4) and evaporated. The residue was purified by column
chromatography using a 19:1 mixture of hexane and ethyl aceta~e as
eluent. There was thus obtained 2-methylthio-4-1(4S)-2,2,4-trimethyl-
1,3-dioxolan-4-yllthiophene in 87% yield as an oil (3.57 g);
NHR SPectrum: (CD3SOCD3) 1.3 (s, 3H), 1.4 (s, 3H), 1.5 (s, 3H), 2.5
(s, 3H), 3.9-4.0 (q, 2H), 7.1 (d, lH), 7.3 (d, lH). -~
A mixture of a portion (0.5 g) of the product so obtained,
sodium methanethiolate (0.5 g) and DMF (5 ml) was stirred and heated to ~ -~
130C for 35 minutes. The mixture was cooled to ambient temperature
and partitioned between ethyl acetate and water. The mixture was
acidified by the addition of lM aqueous citric acid solution. The ~ -~
organic layer was washed with water and with brine, dried (MgSO4) and
evaporated. There was thus obtained 2-mercapto-4-l(4S)-2,2,4-tri- `~
methyl-1,3-dioxolan-4-yllthiophene (0.48 g).
~xample 2
Using an analogous procedure to that described in Example 1,
N-methyl-4'-iodoacetanilide was reacted with 2-mercapto-5-[(4R)-2,2,4-
trimethyl-1,3-dioxolan-4-yl]thiazole to give N-methyl-4'-{5-[(4R)-
2,2,4-trimethyl-1,3-dioxolan-4-yllthiazol-2-ylthio)acetanilide in 72
y~eld, m.p. 65-70C;
NhR Spectrum: (CD3SOCD3) 1.3 (s, 3H), 1.35 (s, 3H), 1.6 (s, 3H),
1.85-1.90 (s, 3H), 3.3 (s, 3H), 4.0 (m, 2H), 7.45 (d, 2H), 7.65 (d,
2H), 7.7 (s, lH).
;- ....... ., - , .
:: : : . ~
..... . . .
. - - ~ ::
-: . . .
2113913
- 21 -
The 2-mercapto-5-1(4~)-2,2,4-trimethyl-1,3-dioxolan-4-yl]-
thiazole used as a starting material was obtained as follows:-
A mixture of 2-bromothiazole (32.8 g), potassium iodide
(2 g), sodium methanethiolate (14.7 g) and methanol (200 ml) was
stirred and heated to reflux for 8 hours. The bulk of the solvent was
evaporated and the residue was partitioned between diethyl ether and
water. The organic phase was washed with water and with brine, dried
(HgS04) and evaporated. The residue was distilled to give
2-methylthiothiazole (18.2 g) as an oil (b.p. 58-60C at 10 mm Hg).
n-Butyl-lithium (1.55M in hexane, 30 ml) was added to a
stirred solution of 2-methylthiothiazole (5.4 g) in diethyl ether which
had been cooled to -10C. The mixture was stirred at -10C for
20 minutes. A solution of acetone (9.2 ml) in diethyl ether (10 ml)
was added. The mixture was s~irred at -5C for 4 hours. The mixture
was partitioned between diethyl ether and a saturated aqueous ammonium
chloride solution. The organic layer was washed with brine, dried ~ -~
(MgSO4) and evaporated. The residue was purified by column
chromatography using a 3:2 mixture of hexane and ethyl acetate as
eluent. There was thus obtained 5-(2-hydroxyprop-2-yl)-2-methyl-
thiothiazole (6.2 g, 80%) as an oil. "
A mixture of the product so obtained, triethyl orthoformate
(5.8 ml), 4-toluenesulphonic acid (0.065 g) and ethanol (40 ml) was
stirred and heated to reflux for 1 hour. The bulk of the ethanol was
evaporated and the residue was partitioned between diethyl ether and
water. The organic phase was washed with brine, dried (HgSO4) and
evaporated. The residue was purified by column chromatography using a
10:1 mixture of toluene and ethyl acetate as eluent. There was thus
obtained 5-(2-ethoxyprop-2-yl)-2-methylthiothiazole (5.2 g, 65%) as an
oil.
A mixture of a portion (4.37 g) of the material so obtained,
boron trifluoride ether complex (6 ml) and methylene chloride (10 ml)
was stirred at ambient temperature for 3 hours. The mixture was
evaporated and the residue was partitioned between diethyl ether and a
saturated aqueous potassium carbonate solution. The organic phase was
washed with brine, dried (MgS04) and evaporated. The residue was
purified by column chromatography using a 50:3 mixture of hexane and
- '~113913
- 22 -
ethyl acetate as eluent. There was thus obtained 5-isopropenyl-2-
methylthiothiazole (2.55 g, 74X) as an oil.
NHR SPectrum: 2.1 (s, 3H), 2.7 (s, 3H), 5.0-5.2 (m, 2H), 7.5 (s, lH).
Using analogous procedures to those described in the third to
fifth paragraphs of the portion of Example 1 which is concerned with
the preparation of starting materials, the product so obtained was
converted in turn into:-
5-[(2R)-1,2-dihydroxyprop-2-yll-2-methylthiothiazole in 83% yield, m.p.
107-109C;
2-~ethylthio-5-1(4R)-2,2,4-trimethyl-1,3-dioxolan-4-yl]thiazole in 88%
yield as an oil,
NMR Spectrum: (CD3SOCD3) 1.3 (s, 3H), 1.4 (s, 3H), 1.6 (s, 3H), 2.65
(s, 3H), 4.0-4.1 (q, 2H), 7.55 (s, lH); and
2-mercapto-5-l(4R)-2,2,4-trimethyl-1,3-dioxolan-4-yllthiazole in
quantitative yield.
~xample 3
The procedure described in Example 1 was repeated except that ;~
racemic 2-mercapto-4-(2,2,4-trimethyl-1,3-dioxolan-4-yl)thiophene was ~-
used in place of 2-mercapto-4-[(2S)-2,2,4-trimethyl-1,3-dioxolan-4-yl]-
thiophene. There was thus obtained N-methyl-4'-[4-(2,2,4-trimethyl-
1,3-dioxolan-4-yl)thien-2-ylthio)acetanilide in 69% yield;
NHR Spectrum: (CD3SOCD3) 1.33 (s, 3H), 1.4 (s, 3H), 1.53 (s, 3H),
1.7-1.9 (s, 3H)j 3.3 (s, 3H), 3.95-4.06 (q, 2H), 7.2-7.35 (m, 4H), 7.4
(d, lH), 7.65 (d, lH).
The racemic 2-mercapto-4-(2,2,4-trimethyl-1,3-dioxolan-4-yl)-
thiophene used as a starting material was obtained as follows:-
n-Butyl-lithium (1.6N in THF, 28.5 ml) was added to a stirred
mixture of 2,4-dibromothiophene (J. Org. Chem., 1988, 53, 417; 10 g)
and diethyl ether (150 ml) which had been cooled to -70C. After 1
hour, a solution of dimethyl disulphide (3 ml) in diethyl ether (10 ml)
was added and the mixture was stirred and allowed to warm to -20C
during 1 hour. The mixture was poured into water. The organic phase
was washed with brine, dried (HgS04) and evaporated. The residue was
purified by column chromatography using hexane as eluent. There was
thus obtained 4-bromo-2-methylthiothiophene (4.76 g7 90%).
. ,.-, . - -. ., - . . - ,. . - - . . . ~ . . . .
r, ,r, ~
-- 2113913
- 23 -
n-Butyl-lithium (1.5M in hexane, 16.6 ml) was added to a
stirred solution of 4-bromo-2-methylthiothiophene (5.2 g) in diethyl
ether (150 ml) which had been cooled to -78C. The mixture was stirred
at -70C for 1 hour. A solution of 1-tetrahydropyran-2-
yloxypropan-2-one (4.3 g) in diethyl ether (5 ml~ was added. The
mixture was stirred at -70C for 1 hour and then allowed to warm to
-30C. The mixture was poured onto a mixture of ice and a saturated
aqueous solution of ammonium chloride. The mixture was extracted with
diethyl ether. The organic layer was washed with brine, dried (MgS04)
and evaporated. The residue was purified by column chromatograpny
using a 10:3 mixture of hexane and ethyl acetate as eluent. There was
thus obtained 4-(2-hydroxy-l-tetrahydropyran-2-yloxyprop-2-yl)-2-
methylthiophene (5.35 g, 75X) as an oil.
A mixture of the product so obtained, 2N aqueous hydrochloric
acid (3 ml) and methanol (20 ml) was stirred at ambient temperature for
1.5 hours. The bulk of the methanol was evaporated and the residue was
partitioned between ethyl acetate and a dilute aqueous potassium
carbonate solution. The organic phase was washed with brine, dried
(NgS04) and evaporated. The residue was purified by column
chromatography using a 2:1 mixture of hexane and ethyl acetate as
eluent. There was thus obtained 4-(1,2-dihydroxyprop-2-yl)-2-
methylthiothiophene (3.08 g, 82%), m.p. 40-42C.
A mixture of a portion (1 g) of the compound so obtained,
acetone dimethyl acetal (1 ml), 4-toluenesulphonic acid (0.01 g) and
acetone (12 ml) was stirred and heated to reflux for 45 minutes. The
mixture was cooled to ambient temperature and neutralised by the
addition of a dilute aqueous potassium carbonate solution. The mixture
was extracted with ethyl acetate. The organic phase was washed with
brine, dried (NgS04) and evaporated. The residue was purified by
column chromatography using a 20:1 mixture of hexane and ethyl acetate
as eluent. There was thus obtained
4-(2,2,4-trimethyl-1,3-dioxolan-4-yl)-2-methylthiothiophene (0.97 g,
81X) as an oil.
A mixture of a portion (0.347 g) of the material so obtained,
sodium methanethiolate (0.35 g) and DNF (4 ml) was stirred and heated
to 130C for 30 minutes. The mixture was cooled to ambient temperature
2113913
- 24 -
and partitioned between diethyl ether and a dilute aqueous citric acid
solution. The organic phase was washed with brine, dried (HgSO4) and
evaporated. There was thus obtained 2-mercapto-4-(2,2,4-trimethyl-
1,3-dioxolan-4-yl)thiophene as an oil.
Example 4 -~
Sufficient sodium acetate was added to a solution of
potassium peroxymonosulphate (0.3 g) in water (2 ml) in order to bring
the acidity of the solution to pH5-6. The resultant solution-was added
dropwise to a stirred solution of N-methyl-4'-[4-(2,2,4-trimethyl-1,3-
dioxolan-4-yl)thien-2-ylthio]acetanilide (0.13 g) in methanol ~4 ml)
which had been cooled to 0C. The mixture was allowed to warm to
ambient temperature and was stirred for 4 hours. The mixture was ~ -
partitioned between ethyl acetate and water. The organic phase was
washed with brine, dried (HgSO4) and evaporated. The residue was - ~-
purified by column chromatography using increasingly polar mixtures of
toluene and ethyl acetate as eluent. There was thus obtained
N-methyl-4'-14-(2,2,4-trimethyl-1,3-dioxolan-4-yl)thien-2-ylsulphonyl]- .
acetanilide (0.114 g, 80%);
NhR Spectrum: (CD3SOCD3) 1.3 (s, 3H), 1.4 (s, 3H), 1.5 (s, 3N), 1.95-
(s, 3H), 3.2 (s, 3H), 3.9-4.1 (m, 2H), 7.6 ~m, 2H), 7.85 (d, lH), 7.9
(d, lH), 7.95-8.05 (m, 2H).
~:
Exa~ple 5
Using an analogous procedure to that described in Example 1,
2-chloro-5-(N-methylacetamido)pyridine was reacted with 2-mercapto-4-
(2,2,4-trimethyl-1,3-dioxolan-4-yl)thiophene to give
5-(N-methylacetamido)-2-[4-(2,2,4-trimethyl-1,3-dioxolan-4-yl)thien-2-
ylthiolpyridine in 15% yield;
NMR S~ectrum: (CD3SOCD3 + CD3C02D) 1.38 (s, 3H), 1.45 (s, 3H), 1.6 (s,
3H), 1.85 (s, 3H), 3.15 (s, 3H), 4.0-4.1 (m, 2H), 7.0 (d, lH), 7.4 (d,
lH), 7.7 (m, 2H), 8.4 (d, lH).
The 2-chloro-5-(N-methylacetamido)pyridine used as a starting
material was obtained as follows:-
A solution of acetyl chloride (3 ml) in methylene chloride(10 ml) was added dropwise to a stirred mixture of
- 2 1 1 3 g l 3
- 25 -
5-amino-2-chloropyridine (5 g)~ triethylamine (6 ml) and methylene
chloride (60 ml) which had been cooled to 0C. The ~ixture was allowed
to warm to ambient temperature and was stirred for 2 hours. The
mixture was partitioned between dilute aqueous sodium hydroxide
solution and methylene chloride. The organic phase was washed with
water, dried (NgS04) and evaporated. There was thus obtained
5-acetamido-2-chloropyridine (6.2 g, 93%), m.p. 145-148C. ~ -
Sodium hydride (60X dispersion in mineral oil, 0092 g) was
added portionwise to a stirred solution of 5-acetamido-2-chloropyridine
(4 g) in DHF (40 ml) which was cooled to 5C. The mixture was stirred -
at 5C for 1 hour. The mixture was cooled to 0C and methyl iodide
(1.43 ml) was added. The mixture was allowed to warm to ambient
temperature and was stirred for 3 hours. The mixture was partitioned
between ethyl acetate and water. The organic phase was washed with
water, dried (HgS04) and evaporated. The residue was purified by
column chromatography using ethyl acetate as eluent. There was thus
obtained 2-chloro-5-(N-methylacetamido)pyridine (1.8 g, 42X), m.p.
42-45C.
~xa~ple 6 "
The following illustrate representative pharmaceutical dosage
forms containing the compound of formula I, or a
pharmaceutically-acceptable salt thereof (hereafter compound X), for
therapeutic or prophylactic use in humans:
(a) Tablet I m~/tablet
Compound X.................................... 100
Lactose Ph.Eur................................ 182.75
Croscarmellose sodium......................... .12.0
Haize starch paste (5X w/v paste)............. ..2.25
Hagnesium stearate............................ ..3.0
2113913 ~-
- 26 - ~ -
(b) Tablet II m~/tablet
Compound X............................................... 50
Lactose Ph.Eur......................................... 223.75 -
Croscarmellose sodium................................... 6.0
Naize starch............................................ 15.0
Polyvinylpyrrolidone (5X w/v paste)..................... 2.25
Magnesium stearate...................................... 3.0
(c) Tablet III mg/tablet ~- i
Compound X.............................................. 1.0
Lactose Ph.Eur......................................... 93.25
Croscarmellose sodium................................... 4.0
Maize starch paste (5X w/v paste)....................... 0.75
Magnesium stearate...................................... 1.0
(d) Capsule m~/capsule
Compound X.................................... 10 ~ ~`
Lactose Ph.Eur ............................... 488.5
Magnesium stearate ........................... 1.5
(e) Iniection I (50 m~/ml)
Compound X ................................... 5.0% w/v
1M Sodium hydroxide solution ................. 15.0% v/v
0.1M Nydrochloric acid
(to adjust pH to 7.6)
Polyethylene glycol 400....................... 4.5% w/v
Water for injection to lOOX
(f) Injection II (lO m~/ml)
Compound X ................................... 1.0% w/v
Sodium phosphate BP .......................... 3.6X w/v
O.lM Sodium hydroxide solution ............... 15.0% v/v
Water for in~ection to 100%
;~ 2113913
. ,.
- 27 -
(g) Injection III (lmg/ml,buffered to pH6) :
Compound X .................................. O.lX w/v
Sodium phosphate BP ......................... 2.26% w/v
Citric acid ................................. 0.38X w/v
Polyethylene glycol 400 ..................... 3.5% w/v ~ -
~ater for injection to 100%
(h) Aerosol I mg/ml
Compound X .................................. 10.0
Sorbitan trioleate .......................... 13.5
Trichlorofluoromethane ...................... 910.0
Dichlorodifluoromethane ..................... 490.0 -
(i) Aerosol II mg/ml
Compound X .................................. 0.2
Sorbitan trioleate .......................... 0.27
Trichlorofluoromethane ...................... 70.0
Dichlorodifluoromethane ...................... 280.0
Dichlorotetrafluoroethane ................... 1094.0
.. `
(;) Aerosol III mg/ml
Compound X ............................................................ 2.5
Sorbitan trioleate .......................... 3.38
Trichlorofluoromethane ............................................... 67.5
Dichlorodifluoromethane ............................................. 1086.0 ~:
Dichlorotetrafluoroethane ............................................ 191.6
(k) Aerosol IV mg/ml
Compound X ............................ ~................................ 2.5
Soya lecithin ............................... ........................... 2.7 ~.
Trichlorofluoromethane ...................... .......................... 67.5
Dichlorodifluoromethane ..................... ........................ 1086.0
Dichlorotetrafluoroethane ................... ......................... 191.6
~1~3913
- 28 -
, -:
Note
The above formulations may be obtained by conventional
procedures well known in the pharmaceutical art. The tablets (a)-(c)
may be enteric coated by conventional means, for example to provide a
coating of cellulose acetate phthalate. The aerosol formulations ~ -
(h)-(k) may be used in conjunction with standard, metered dose aerosol ~-
dispensers, and the suspending agents sorbitan trioleate and soya
lecithin may be replaced by an alternative suspending agent such as ~ -
sorbitan monooleate, sorbitan sesquioleate, polysorbate 80,
polyglycerol oleate or oleic acid.
TS37415
04JAN94
BST/MB
.~ .- .
- '
, :- .: