Note: Descriptions are shown in the official language in which they were submitted.
- 2~909
. .
ELECTROLYTIC CELL WITH GAS ELECTRODES AND - . .
METHOD FOR ELECTROLYSIS BY THE SAME
FIELD OF THE INVENTION ~`
The present invention relates to an electrolytic
cell and a method of electrolysis by the same for the ~:
production of an alkali and an inorganic acid (or
organic acid) from a salt of an inorganic acid (or
organic acid). More particularly, the present
invention relates to an electxolytic cell and a method
lo of electrolysis by the same for the production of
caustic soda and hydrochloric acid from sodium
chloride, the production of an alkali and sulfuric
acid from sodium sulfate, and the production of an
alkali and amino acid from a salt of an amino acid.
BACK~ROUND OF_THE INVENTION
A branch of the old basic chemical industry is
electrolysis for the production of sodium hydroxide
and chlorine from an aqueous solution of alkali ~ --
, .
chloride, especially brine. Electro~ysis in the early
stage, which employed mercury as the cathode, yielded
alkall hydroxide and chlorine of extremely hiqh
purity. However, the use of this conventional method
is now restricted because of large energy consumption ~-
(approximately 3000 kWh/ton-alkali hydroxide) and
environmental pollution due to mercury. The
";
--1-- :
' :~:`.
, ,
. .
r~ , .
~ - 21i~9~9
, ~, .:.~::
conventional method has been replaced by a new one
which employs an asbestos diaphragm. However, it has ;
- the disadvantage of yielding low-purity alkali
hydroxide which needs subsequent separation from
alkali chloride and also yielding chlorine containing
a large amount of oxygen. In addition, its energy
consumption including that for product purification is
equal to or larger than that of the mercury method,
although it requires a small amount of energy for
, . -, ~ .
lo electrolysis itself. Moreover, it poses a problem
associated with the production of cancer by asbestos.
At present, the method for electrolysis of alkali
chloride has been shifted to the ion exchange membrane
method.
The ion exchange membrane consists of feeding a
purified aqueous solution of alkali chloride
(especially brine) to the anode compartment of the
electrolytic cell (which is divided into the anode and
cathode compartments by a cation exchange membrane)
, ~
~ 2a and pure water (if necessary) to the cathode
,
compartment, thereby producing chlorine in the anode
; compartment and alkali hydroxide (30-50%) in the
cathode compartment. This method consumes 20-30% less
energy than the conventional method (2200-2500
kWh/ton-alkali hydroxide). This method is used for
the production of more than 80% of alkali hydroxide in
,.: ::: ~, .
~ ., :
-2~
'~ , .' ,:.,
~ ` 21~0~
. .~. .
Japan. In addition, this method is also used for
electrolysis of other alkali compounds such as sodium
sulfate.
The present inventors have proposed a new method
for performing electrolysis using a gas electrode as
the anode, without evolution of gases (such as
chlorine and ox~gen) from the anode, which uses less
energy than the conventional ion exchange membrane
method as disclosed in EP 0 522 382 Al. This new
lo method employs an electrolytic cell constructed as
shown in Fig. 1. The electrolytic cell proper 100 is
divided into an anode compartment 110, an intermediate
compartment 120, and a cathode compartment 130 by two
ion exchange membranes (that is, an anion exchange
membrane 115 and a cation exchange membrane 125). The
anode compartment 110 is further divided into a gas
compartment 111 and a solution compartment 112 by a
gas electrode 114 provided with a current collector
~ 113.
; ~ zo This electrolytic cell for the ion exchange
membrane method, however, has the following
disadvantages because the electrodes, current
collector, and ion exchange membranes are arranged
vertically. First, the gas electrode 114 and current
collector 113 in the anode compartment 110 are in
contact with each other under a pressure which differs
-3-
~` 2~909 ` ~
gradually from one place to another in the vertical
direction. This leads to an uneven current
- distribution. Second, the cathode compartment 130
gives off gas bubbles, which causes an uneven current
distribution in the vertical direction. Third,
condensed water accumulates on the bottom of gas
compartment lll. This prevents complete gas diffusion
and results in an uneven gas distribution. A gas
distributor is necessary to avoid this problem.
lo Fourth, the apparatus requires an external circulating
line 140 and a gas-liquid separating column 150 (as
shown in Fig. l) to separate gas effectively from the
cathode. The third and fourth disadvantages may be
eliminated by the aid of auxiliary equipment, but
tRere are no means to eliminate the first two
., ~ ~....
disadvantages (uneven current distribution). "
:'-'~'.''`''.'
SUMMARY OF THE INVENTION ;~
The present invention was completed to eliminate ~
the above-mentioned disadvantages (such as uneven ;`~ -
~20 current distribution) involved in the conventional ion
exchange membrane method and the conventional
electroly~ic cell therefor, without resorting to the ; `
~; use of any auxiliary equipment. Accordingly, it is an ~ -
:: ' f: : ~
~; i object of the present invention to provide an ;~
~25 electrolytic cell with a gas electrode and a method
:~,
-4- ~
~': :';'~'',''
,; ''' ` '-",
.,
2~909 :
for electrolysis by said electrolytic cell. The
electrolytic cell and method are intended to
- efficiently produce an alkali and an inorganic acid
(or organic acid) from a salt o:E an inorganic acid (or ~
organic acid). ~;
The present invention is embodied in an
electrolytic cell characterized in that the
electrolytic cell proper is divided horizontally into
an anode compartment and a cathode compartment having
lo a cathode by an approximately horizontal ion exchange
membrane, said anode compartment being divided into a
solution compartment and a gas compartment by an
approximately horizontal hydrogen gas electrode
equipped with a current collector. The electrolytic
cell may be modified such that said ion exchange
; membranes have an intermediate compartment formed
between them. ;~
The present invention is also embodied in a
method for electrolysis by means of an electrolytic
cell which is divided horizontally into an anode
compartment and a cathode compartment having a cathode
by an approximately horizontal ion exchange membrane,
said anode compartment being divided into a solution
compartment and a gas compartment by an approximately
horizontal hydrogen gas electrode equipped with a
current collector, said method comprising supplying
~ 2~909
said solution compartment with an aqueous solution of
alkali salt, thereby electrolytically producing an
- acid in said solution compartment and an alkali
hydroxide in said cathode compartment. The method may
s be modified such that said ion exchange membranes have
an intermediate compartment formed between them which
is fed with an aqueous solution of alkali salt.
sRIEF DESCRIPTION OF THE DRAWINGS ~ -~
Fig. 1 is a schematic vertical sectional view
0 showing a conventional vertical electrolytic cell.
Fig. 2 is a schematic vertical sectional view
showing an embodiment of the electrolytic cell
provided with a gas electrode pertaining to the
present invention. ~ ~
DETAILED DESCRIPTION OF THE INVENTION `~ ~ :
The invention will be described in more detail in ~;
the following.
A feature of the present invention resides in
: ~ .
positioning the electrolytic cell proper horizontally, -~
thereby also positioning the gas electrode ~as the
anode), ion exchange membrane, and cathode ;
. ,:
horizontally. This structure makes uniform the
pressure under which the gas electrode and the current
collector are in contact with each other, thereby
-6-
''. ' `'' . '
''. ,; '~'~
~` 211~909
: :
eliminating the uneven current distribution.
Moreover, this arrangement permits the condensed water
originating from the gas electrode to escape from the
system without coming into contact with the gas
electrode, thereby eliminating the uneven current
distribution for certain. The removal of the
condensed water improves the gas distribution,
obviating the necessity of adding auxiliary equipment
such as a distributor. With the electrolytic cell
lo positioned horizontally, it is possible to reduce the
thickness of the cathode compartment in the vertical
direction. This alleviates the adverse effect of
bubbling due to gas evolution. The overall effect is
the reduction in electrolytic potential.
lS The electrolytic cell pertaining to the present
invention may be constructed of substantially the same
constituents as in the conventional vertical
electrolytic cell. For example, the gas electrode may
be the conventional one having a hydrophobic part and
;~ 20 a hydrophilic part. It may be formed by coating one
side of the substrate carrying a catalyst metal with
polytetrafluoroethylene (to render that side
hydrophobic). The cathode may be the same in material
and shape as the conventional one. A preferred
example of the cathode is a perforated nickel plate
coated with a noble metal oxide. The hydrogen for
-7-
f-, 2~1~gO9 -~' .;~,
depolarization in the anode compartment may be
supplied from a separate source or by recycling the
hydrogen evolved in the cathode compartment.
The electrolytic cell of the present invention
has its inside divided into the cathode compartment
and anode compartment by one ion exchange membrane or
into the cathode compartment, intermediate
compartment, and anode compartment by two ion exchange
membranes. The anode compartment is divided into the
lo solution compartment and the gas compartment by the
gas electrode. Alternatively, the gas electrode may
be provided with an ion exchange membrane on its side
facing the solution compartment so as to ensure the
~ gas-liquid separation.
; 15 The electrolytic cell of the present invention
permits the same electrolytic reaction as the
conventional one used for inorganic salts. Therefore,
it may be used for the production of caustic soda and
hydrochloric acid from sodium chloride by
electrolysis, the production of caustic soda and
~; sulfuric acid from sodium sulfate by electrolysis, and
the production of alkali and amino acid from a salt of
an amino acid by electrolysis. ;
The intermediate compartment or solution
compartment of the electrolytic cell should be fed
-8-
:~ 211~09
with an electrolyte which is properly selected
according to the intended electrolytic reaction.
- The invention will be described with reference to
the accompanying drawings.
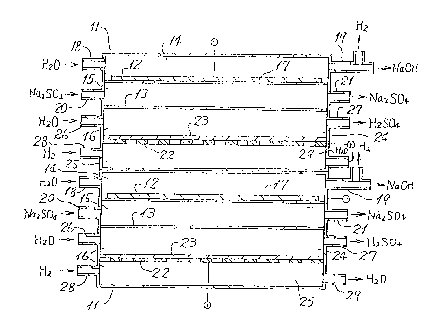
Fig. 2 is a schematic vertical sectional view
showing an embodiment of the electrolytic cell
pertaining to the present invention. It is to be
noted that two units of the electrolytic cell are
placed one on top of the other, each made up of a
o cathode compartment, an intermediate compartment, and
- an anode compartment.
Each unit of the electrolytic cell is a box-type
electrolytic cell proper 11. The inside of the
electrolytic cell proper 11 is divided into a cathode
compartment 14, intermediate compartment 15~ and anode
compartment 16 by a cation exchange membrane 12 and
; ~ anion exchange membrane 13 both positioned
horizontally. In the cathode compartment 14 is a
cathode 17 of perforated plate which is in contact
with the upper side of the cation exchange membrane
: 12. The cathode compartment 14 has on its both sides` ~ an inlet 18 for water or a dilute aqueous solution of
sodium hydroxide and an outlet 19 for hydrogen and
caustic soda. The intermediate compartment 15 has on
its both sides an inlet 20 for an aqueous solution of
, ;'.
_g_ : : ~
r'~~
9 0 ~
sodium sulfate and an outlet 21 for an aqueous
solution of sodium sulfate.
- A gas electrode 23, provided with a current
collector 22, is placed horizontally in an anode -
compartment 16. The anode compartment 16 is divided
into a solution compartment 24 (upper) and a gas ~ ;
compartment 25 (lower) by the gas electrode 23. The - ~;
solution compartment 24 has on one side an inlet 26
for water or dilute sulfuric acid and on the other ~ `~
lo side an outlet 27 for sulfuric acid. The gas
compartment 25 has on one side an inlet 28 for ~
hydrogen and on the other side an outlet 29 for ~ -
condensed water.
;~ The electrolytic cell constructed as mentioned
above permits the uniform current supply and hence the ~-~
: - .
.: .
~ smooth electrolytic reaction on the gas electrode,
`~ because the gas electrode 23 and current collector 22
in the anode compartment 16 are in uniform contact ~ -
with each other. The fact that the outlet l9 of the
- :
cathode compartment is only slightly above the cathode ~ ;
17 permits the hydrogen evolved by the cathode 17 to ;
be discharged easily from the system. Moreover, the -~
above-men,tioned arrangement protects the gas electrode
from deterioration because the condensed water evolved
by the gas compartment 25 of the anode compartment
does not accumulate on the bottom of the gas
,:
-1O- "
", ~.:'.'
21~909
compartment. This protects the gas electrode 23 and
current collector 22 from coming into contact with the
condensed water.
The invention will be described with reference to
S the following examples which demonstrate the
electrolysis of an aqueous solution of alkali
chloride. The examples are not intended to restrict
the scope of the invention.
Example 1
Electrolysis of sodium sulfate was carried out
using a single unit of the electrolytic cell (sho~n in
Fig. 2) which is specified as follows:
The electrolytic cell proper measures 1274 mm
deep, 366 mm wide, and gO mm high. It is horizontally
divided into a cathode compartment (22 mm thick), an
intermediate compartment (3 mm thick~, and an anode
compartment (25 mm thick) having a solution
compartment (3 mm thick), by a cation exchange
membrane ("Nafion #324" made by DuPont) in the cathode
~20 compartment and an anion exchange membrane ("Celemion
#AAV" made by Asahi Glass Co., Ltd.) in the anode
' compartment, respectively. A gas electrode (made by ~;
E-TEX Co., Ltd.) is horizontally positioned in the
anode compartment and separated from the anolyte by a
cation exchange membrane ("Nafion #117"), so that the
' ~
~' ~'''''"'``;','
r
2 1 ~ ~1 9 0 9 ~
anode compartment is divided into the solution
compartment and the gas compartment. The gas
electrode is provided with a current collector of
platinum-plated perforated titanium. The upper side
s of the "Nafion #324" is in contact with an activated
cathode (having an electrode effective area of 35 dm2)
which is a perforated nickel plate with nickel plating
in which ruthenium oxide powder is dispersed.
The cathode compartment was fed with pure water
;~ 10 to produce a 19~ aqueous solution of caustic soda.
The intermediate compartment was fed with a 20%
aqueous solution of sodium sulfate which was recycled
at a flow rate of 3 liters/minute from a 5-meter high
head tank. The anode compartment was fed with 17%
sulfuric acid which was recycled at a flow rate of 3
liters/minute. The hydrogen gas evolved in the
cathode compartment was immediately discharged from
the cathode compartment, washed with water to remove
caustic soda mist, and recycled to the hermetically
~o sealed gas compartment. To the hydrogen stream was
added moistened hydrogen ~at a flow rate of 0.05 m3~h)
from a commerciaI hydrogen bomb. The pressure in the
gas compartment was 200 mmAq.
Using the electrolytic cell constructed as
~25 described above, electrolysis was carried out at about
60C with an electric current of 700 A so as to
-12-
ri 211~909
produce caustic soda in the cathode compartment and
sulfuric acid in the anode compartment. It was found
that the electrolytic potential was 3.10 V, which
remained almost unchanged for one month. The current
s efficiency for caustic soda and sulfuric acid was 90%
and 80%, respectively. After continued electrolysis
for one month, the electrolytic cell was disassembled
for inspection. Nothing anomalous was found in the
constituent elements.
lo Comparative Example 1
An electrolytic cell of the conventional vertical
type as shown in Fig. 1 was constructed from the same
constituent elements as in Example 1. The catholy~e
was circulated spontaneously from an externally
installed gas-liquid separating column. The hydrogen
separated by the gas-liquid separating column was
introduced into the gas compartment adjacent to the
anode compartment in which it was used as depolarizing
agent as in Example 1. The electrolytic cell was fed
~20 with an aqueous solution of sodium sulfate and
sulfuric acid in the same manner as in Example 1.
Electrolysis was carried out at 60C with a current of
700 A. The initial electrolytic potential was 3.25 V,
which began to increase in the fourth week and
-13~
:':
` 2~1~909 i ~;
. .
eventually reached 3.5 V after one month.
Electrolysis was then discontinued.
The electrolytic cell was disassembled for
inspection. Whitish discoloration was found in the
cation exchange membrane which separates the gas
electrode from the anolyte. SEM observations revealed
that the discoloration is due to anomalous wear and
local deterioration. Discoloration was also found in
the current collector and gas electrode at the bottom
lo of the electrolytic cell. The results of analysis
revealed that this discoloration was caused by the
peeling (anomalous wear) of platinum from the current
collector. It is considered that the local corrosion
was accelerated by the condensed water which
accumulated on the bottom of the gas compartment,
exerting a higher pressure on the bottom than to other
parts.
The present invention provides an electrolytic
, ., .~ :. . .
cell characterized in that the electrolytic cell
proper is divided horizontally into an anode
compartment and a cathode compartment having a cathode
by an approximately horizontal ion exchange membrane,
said anode compartment being divided into a solution
compartment and a gas compartment by an approximately
2s horizontal hydrogen gas electrode equipped with a
current collector. The electrolytic cell may have two
-14-
9 ~ ~ ~
ion exchange membranes which form an intermediate
compartment between them.
The electrolytic cell of the present invention
offers the following advantages. The gas electrode
and the current collector are in contact with each
other under a uniform pressuxe. This allows for
uniform current supply and the smooth electrolytic ~;
reaction on the gas electrode. The cathode
compartment permits hydrogen evolved therein to be
lo discharged easily from the system. The anode ;
compartment does not permit the accumulation of
condensed water This protects the current collector ~ ;~
and gas electrode from deterioration by condensed
water. The overall effect is the stable electrolytic
potential which is lower by about 0.2 V than that of
the electrolytic cell of the conventional vertical ~
type. ~ -
The electrolytic cell of khe present invention
allows for an efficient, economical operation for ;
electrolysis.
While the invenkion has been described in detail
and with reference ko specific embodiments thereof, it
; will be apparent to one skilled in the art that
various changes and modifications can be made therein
without departing from the spirit and scope thereof. ~
,:.` . ;.,...:
-15- ;