Note: Descriptions are shown in the official language in which they were submitted.
~2 1 1 4927
5 - This invention relates to the design of internal artificial tissue
replacement devices and particularly to the placement of an X-ray
identifiable array on the inner surface of the encapsulating shell of an
internal artificial tissue replacement device.
The need for an individual to introduce an artificial device into one's
10 body can arise from the desire to improve one's appearance, or from
circumstances beyond one's control. Accidental injury is a common source
of circumstance which may require artificial tissue replacement. In the past
decade the treatment of cancerous tissue has become a large source for the
need of tissue replacement operations.
Cancer treatment can include the removal of tissue which contain
cancer cells. The loss of tissue due to cancer treatment can be functionally
and aesthetically displeasing. To overcome the loss of tissue, artificial
devices may be placed internally to offset the effects of tissue loss. Once in
place however, it is difficult to monitor the position within the body or the
20 structural integrity of the device.
For example in cases of breast cancer, a mastectomy causes a
noticeable loss of tissue. When it is desired to undergo reconstructive
mammaplasty to replace to tissue lost from the mastectomy operation, it is
I~A21 14927
common to use an artificial device to replace the tissue below the skin.
Silicone gel filled implants are often used for this purpose. Saline filled and
multi-chamber implants are examples of other devices also used in
reconstructive mammaplasty.
The artificial devices in reconstructive mammaplasty are often filled
with silicone gel, as silicone is generally thought to be biocompatible inside
the human body. Once the implant is inside a person, it is difficult to monitor
the integrity of the outer cross-linked silicone shell which encapsulates the
implant. Rupture of this cross-linked silicone outer shell can enable the
silicone gel contents of the implant to move freely through the body. The
specific side effects of free silicone gel inside the body are unknown, and
are still under study, but never the less this situation must be avoided if
possible.
Currently the most effective way to monitor the integrity of the implant
is with Magnetic Nuclear Resonance Imaging (MRI). MRI is a reliable
method for diagnosing the intactness of the mammary prosthesis. MRI
requires on average an hour to perform a complete examination. Purchase
of MRI equipment for institutions is expensive, and highly trained staff are
required to operate the machines. Currently there are over one million
~A21 1~27
american women with an internal mammary prosthesis, and this figure is
increasing due to the higher rate of female breast cancer. MRI is not a
practical method to continuously monitor all of the women who have a
mammary prosthesis. An intermediate system for diagnosing the status of
5 an mammary prosthesis is required.
One of the reasons that MRI is successful in monitoring the implant is
that this method is able to isolate the silicone gel and display data relating
to the presence of only silicone. A more conventional method would be to
have a more identifiable material placed in an precise pattern on the surface
10 of the encapsulating shell.
If X-ray diffracting, absorbing, deflecting, diffracting, or blocking masses
were to be placed in a precise pattern within the encapsulating shell of the
implant, the implant would be viewable with a standard chest X-ray machine.
These chest X-ray machines are accessible in health care institutions in
15 metropolitan and rural locations. Chest X-ray machines and technicians are
widely available, less expensive, and take less time to obtain data on a
patient with implants.
With the assistance of computer imaging, the data from the X-ray can
be used to construct a three dimensional representation of the implant, with
~A21 14927
~ossible ranges of the current shape of the outer cross linked shell. If the
computer image is compared to data recorded from the initial installation of
the implant the accuracy of a diagnoses can be enhanced.
There are some restrictions based on the condition of the patient and
5 the shape and size of the artificial tissue replacement device which will have
an effect of the accuracy of the information obtained on an individual. MRI
provides a proven ability to diagnose silicone gel mammary prosthesis. The
level of success obtainable by the array is unknown at this time, but
information provided by the array is useful in that it can provide timely
10 information about the structure of the implant.
Nature and Terms;
Since the invention can undertake an amorphous shape, in order to
illustrate the invention the drawings will describe an pear shaped implant
15 placed on the chest of a patient outside the ribcage and below the skin
tissue layer. The small end of the implant will be place closest to the head
of the patent and will be labelled as the "top" of the implant. The wide end
of the implant will be placed closest to the feet of the patient and be labelled
"bottom" for the orientation of the implant. The length of the pear will be
~ A 2 1 1 ~927
3riented from top to bottom with the "front" being the surface farthest away
from the ribcage of the patient, and the "back" being the surface closest to
the rib cage of the patient.
In the drawings which illustrate the embodiments of the invention,
5 Figure 1 is the "front" view of an implant, Figure 2 is a"side" view of the
implant across the "bottom" of an implant, Figure 3 is a "side" view from
"top" to "bottom", and Figure 4 is a cross sectional view along line 1,1 in
Figure 1. Figures 5, 6, and 7 illustrate a different embodiment of the array.
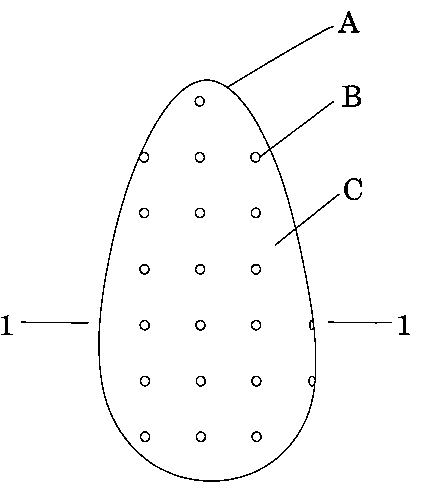
The breast implant illustrated consists of a cross-linked silicone outer
10 shell A which encapsulates the silicone gel contents C of the implant. The
cross-linked silicone shell A can also be composed of a different compound,
but should have the properties to keep the silicone gel from leaking out, be
biocompatible and have some flexibility to move with the body of the patient.
Calcium spheres B are embedded in the cross-linked silicone outer shell A,
15 and are placed over the entire cross-linked silicone shell A in the grid like
array as shown in figures 1, 2, and 3. The calcium spheres B can be
composed of other materials which block, diffract, or absorb X-rays.
The purpose of the calcium spheres B are that they, when bombarded
by X-ray radiation, will individually show up on an X-ray detecting or
~A21 14927
-sensitive medium. The fact that these calcium spheres B will be displayed
on the X-ray detecting medium will allow the outer shell of the breast implant
to be evaluated for integrity. The resulting display of the calcium spheres B
gives a physician more information about the integrity of the implant. When
5 the implant is first put in place a chest X-ray shortly afterwards will result in
a reference X-ray of the intact implant which can be used as a reference
point to compare future X-ray examinations. If for instance it is apparent that
there is a calcium sphere B missing, or that there is more space between
two adjacent calcium spheres B than there should be, than the physician can
10 call for further tests to verify the presence of a rupture in the outer shell A
of the implant.
The calcium spheres B will show up white on an X-ray. The bones of
the patient are also composed of calcium, but they tend to appear grey on
an X-ray. Due to the different contrast between the bones of the patient an
15 the calcium spheres B, the array can be distinctly identified with some
interference from the bones of the patient.
In figures 2 and 3 it can be shown that the "front" and "back" halves
of the breast implant have the grid array of calcium spheres B offset by half
of the distance between the calcium spheres B. In figure 2 line 2,2
~A21 14927
-represents the line dividing the top and bottom sections of the implant. As
it can be seen in figure 2 the array of calcium spheres B in the top section
are offset from the array of calcium spheres B in the bottom section.
Similarly in Figure 3 line 3,3 represents the division of the top and bottom
5 sections along the side of the implant.
Figure 4 is a cross section through line 1,1 of figure 1. The cross
section illustrated by figure 4 shows that the calcium spheres B are
contained within the cross-linked encapsulating shell A of the implant and do
not come in direct contact with the silicone gel C of the implant. Thus the
10 loss of a calcium sphere B could be caused by exposure by a rupture on the
outer surface of the shell surrounding the calcium sphere B, but not deep
enough to continue through to the silicone gel C of the implant. If this
situation occurs it is possible that the cross-linked encapsulating shell A
could potentially rupture at this point at a later time and the implant may
15 need to be replaced before a rupture occurs as a preventative action. There
are no calcium spheres B on the bottom section of the implant in figure 4 as
they are offset and not in the plane of line 1,1 in figure 1.
Since figures 1, 2 ,3, and 4 illustrate a pear shaped implant, the
implant should not flip from top to bottom once inside the patient. The pear
~A 2 I 14927
shaped implant generally is also designed with a flat or shallow convex back
side which will keep the implant in a consistent front, back orientation once
inside the patient. This consistent orientation of the pear shape implant will
have the same surface as the front at all times and the same surface as the
5 back at all times. By offsetting the array of calcium spheres B between the
front and back grids it will make the resulting X-ray picture more valuable as
the front and back arrays of calcium spheres B will be offset and not on top
of each other. With implants which have a rounder shape it may not be
feasible to offset the front and back array of calcium spheres B as the
10 orientation once inside can not be predicted, and the implant may move
around once inside due to the shape of the implant.
Figures 5, 6, and 7 illustrate how the array could be placed on a
pancake shaped implant. Figure 5 represents the "front" surface of the
implant with D representing oval rather than round masses in a horizontal
15 orientation. Figure 6 represents the "back" surface of the implant with E
representing the oval masses in a vertical orientation. Figure 7 represents
the view taken of both arrays superimposed-on one another. As it is
represented in Figure 7 the "front" and "back" arrays are individually visible
due to the orientation and shape of each array. This can add more
~A21 14927
Information as rotation of oval masses D or E can be used as a factor to
indicate possible rupture of the encapsulating envelope of the implant. The
use of oval masses D or E can have the disadvantage of causing a rupture
by poking through the surface of the implant where a fold or sharp curve in
5 the encapsulating shell occurs.
As noted earlier the X-ray blocking, diffracting, deflecting, or absorbing
spheroid masses are depicted as being the calcium spheres B. Calcium is
a good example of a material to be used in the array as it has all of the
properties required of a material for this purpose. The properties of a
10 material for use in the array are as follows:
Biocompatible
Biodegradable
Diffract, block, deflect, or absorb X-rays
Non reactive to silicone gel or cross-linked shell
Non reactive during cross linking silicone process
Can be produced in specific size and shape
Show up on an X-ray differently than the bones of the patient
~A21 1 4~27
- Heavy metals can be put into compounds which meet the above
requirements, but may not be practical in the day to day operations of the
patient. Day to day examples such as airport metal detectors may
discourage the use of heavy metals.
The disadvantages of the array in general are that if the patient has
silicosis, or another lung condition which causes the lungs to show up as a
white cloud, the array will not show through the cloud around the lung area.
In situations where a rupture causes the shell's surface to contract rather
than expand, the calcium spheres B will move closer together, and it will be
difficult to tell if the closeness of spheres is caused by a fold or a rupture. In
some circumstances the calcium spheres B will interfere with other objects
which need to be viewed by a chest X-ray.
In current use are multiple chamber tissue replacement devices. These
multi chamber devices are in use to better control the shape and size of an
implant. The array may be placed within the chambers of the implant to
monitor the position or integrity of these inner chambers. As time continues
and technology advances the complexities of the construction of internal
artificial tissue replacement devices will increase. An attempt to include the
unknown direction of the implant construction will be addressed in the claims
~ A21 1 4927
,D~llowing the conclusion of the description of the invention.
In summery, the above representation of the invention is attempting by
means of words to describe an array of masses which will block, diffract, or
absorb X-rays to be placed within the encapsulating layer of a tissue
5 replacement device in such a manner as to be recorded on an X-ray
recording media and that the resulting picture or data recorded will provide
a medical doctor with information which can assist in the diagnosis of the
intactness of an the internal tissue replacement device.