Note: Descriptions are shown in the official language in which they were submitted.
` ~ 1;L5t3~1 :
The pre~ent invention relateR to novel derivatives of
quinolonecarboxylic acid and napthyridonecarboxylic acid,
processes ~or their preparation and their use a~ medica-
ments, in particular as antiviral agent~.
The antibacterial e~fect of certain quinoloneaarboxylic
acids ha~ been known for a relatively long time. Little
is known about the antiviral e~ficacy of such ~ubstances
[c~. JP 022 64 724 ~2, US 4 959 363; EP 431 991 Al].
Quinolonss ~or the treatment of AIDS-infected cells are
describ~d in: AIDS 4(12~, 1283 (1990); Cell Struc. funct.
15(5), 295 (1990); EP 394 553 A2; WO 90 135 42 Al; in
these cases, a protecti~e ef~ct i8 evident for certain
cell lines without the ~irua being de~troyed. The des-
cribed substance~ al~o h~ve an antibacterial e~ect.
The pre~ent a~vantion now relats~ to no~el derivatiYes o~
guinolonecarboxylic ac~d and ~apthyridonecarboxylic acid
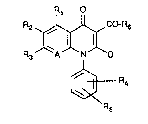
o~ the ge~ral formula (I)
R1
R2~,~C-R6
R3 1A~N~D (I) ,,
~XR :
:: ~
' .'
~ - ~
Le A 29 518
~ :
... , .. , .. .. ...... , ., . . .. ~ .. . ..... . . . ... .......... .. ........ . .. . ... .... . . . . .
in which
Rl represents h~drogen, hydroxyl, mercapto, halogen,
straight-chain or branched alkyl having up to 3
carbon atoms, pexfluoroalkyl having up to 3 carbon
atom~, stralght-chain or branched alkoxy or alkyl-
thio having in each case up to 6 carbon atom~,
arylthio having 6 to 10 carbon atoms, or
represents the group o~ the form~la -NR7R~,
in which
R7 and R8 are id~ntical or di~erent and denote
hydrogen or ~traight-chain or branched alkyl
ha~ing up to 6 carbon atoms,
R2 repre~ents h~drogen, nitro or halogen,
: R3 repreaents a reeidus of th~ fo~mula ~ N
R10
~. ''~ ,,
: -: - -
:~ 15 i~ which :~
;~ R9 a~d Rl are identical or di~ferent a~d denote -
hydrogen, straight-chain or branched alkyl
having up to 6 carbon atoms, or a re~idue of -~
the formula
~e A 29 518 - 2 -
,.. ,.. ~...... .... .... , .. ,.. ,.. ,.. ,... , .......... , . ~
2 1
R12 ~ N~
R13 N
\~ or
N N
in which
R1l, R12 and R13 are identiaal or different and
denote hydrogen, halogen, nitro,
~, 5 hydroxyl, cyano, mercapto, arylthio ha~- ~
: ing 6 to 10 carbon atom~, straight-chain ~ :
~ .
or branched alkyl, alkoxy, acyl or alkyl-
thio ha~ing in each ca~e up to 6 carbon
atom~, or per~luoroalkyl having up to 4
; carbon atoms,
~ A repr-s-3ts a nitrogen atom or represe~ts the group - -~
R~ of th~ormula -CR
: in whi~h
Rl4 denotes hydro~en, ~ haloge~, mothyl,
5~ hydroxyl, mathoxy, vinyl or a}kinyl,
R :~r~pr~e~ts a residue of the formNla -(CH~a_N
. ~ :
~ ,
:
: : :
~: Le A 29 518 - 3 -
~ .. ~.. .... .. .... . .. . . . . ..... . . . . . . . . . . . ..
l3 7 ~
in which
a de~otes a number 1, 2, 3 or 4,
Rls and Rl6, together with the ~itrogen atom, ~orm an
imidazolyl, pyrrolyl, 1,2,3-triazol-1-yl,
1,2,3-triazol-2-yl, 1,2,4-triazol-1-yl,
1,2,4-triazol-4-yl or tetrazolyl ring,
Rs represents hydrogen, hydroxyl, halogen, or repre~
~e~t~ ~traight-chain or bra~ched alkyl or alkoxy
ha~ing in each ca~e up to 6 aarbon atom~
R6 repre~ents hydroxyl, benzyloxy, morpholino, or
B ~raight-chai~ or branched alkoxy having up to 6
carbon atom~, whera the latter can be substituted
idantically or dif~erently up to 3 time~ by halog n,
hydroxyl, or by a heterocyclic re~i~ue of the
r CH3 ~::
:formula LOXCH ~ r
-
.
r-pr~s~t~ a group of t~- formula _NRl7R~
in which --
. -
Rl7 a~d Rl8 are ide~tical or dif~erent a~d denote -~:
~ -
;~ '
~ Le A 29 518 - 4 -
'
,.......... .. . ..... . . ........... .... ... . . . .
0 2 1
hydrog~n or 8 traight-chain or branched alkyl
having up to 8 carbon atoms, which i~ option-
ally aubstituted identically or differently up
to 3 times by hydroxyl or by straight-chain or
branched alkoxy having up to 6 carbon atoms,
or
R6 represent~ a re~idue of the ~ormula - N N-R19
, in which :~
"'`'
R~9 denotes hydrogan or stra~ght-chain or branched
alkyl having up to 4 carbon atom~,
D repre~ents hydroge~, a~ino, phenyl, cyano, hydroxyl,
or reprQs~nta straight-chain or ~ranched alkenyl,
alkylthio or alkoxycarbonyl ha~i~g in each ca~e up i~
,
~: : : to 6 carbon atoms, or : ~:
1:5~ : ~repre3~nts straight-chain or b~anch~d al~yl haYing
up:to 6 car~on atoms, whioh i8 optionally eu~titu- -:-~
e-d~ by :stralght-chain or branched alko~ycarbonyl
ha~ing up to 4 carbon ato~s,
,
: ~d hydrates a~d salts thereof, optio~ally in an i~o~eric
~form.
P~ysiologically ha~mless 8alt8 of tho compounds ac~ording .-
~; '
e A 29 518 - 5 -
:
. ',, ,.. ,.:... .. ............... ................... ....
0 ~ :1
to the invention can be ~alts of the compounds according
to the invention with mineral acids, carboxylic acid~ or
sulphonie acids. Those which are particularly preferred
are, or example, salt~ with hydrochloric acid,
S ~ydrobromic acid, sulphuric acid, phosphorie aeid,
methanesulphonic acid, ethanesulphonia aeid, toluene-
sulphonie aeid, benzenesulphonie aeid, naphthalene-
disulphonie aeid, aeetic aeid, propionie aeid, laetie
aeid, tartarie aaid, eitrie aeid, fumarie aeid, maleie
aeid or benzoie aeid.
Physiologieally harmle~s ~alts ean also be alkali me~al,
alkaline earth metal, silver and guanidinium salts of the
eompounds aeeording to the invention.
Compound~ of the general formula (I) are preferred
in whieh
Rl represent~ hydrogen, ~ydroxyl, ~ereapto, fluorine,
ehlorine, bromi~e, ~traight-ehain or branehed alkyl
having up to 3 earbo~ atom~, tri~l~oromethyl,
straight-ehain or branehed alkoxy or alkylthio
having in eaeh ease up to 4 earbon atoms, phe~yl-
thio, or repre~e~ts the group of the formula -NR7R8,
--::?. .
,
in whieh
R7 and Ra are identieal or dif~erent and denote
hydrogen or straight-ehain or bra~ehed alkyl
Le A 29 518 - 6 -
. ......... .
l) 2 1
having up to 4 carbon atoms,
R2 represent~ hydrogen, nitro, fluorine, chlorine or
bromine,
Rg-N N -
R3 represent~ a residue of the formula ~
R10
in which
R9 and R10 are identical or dif~erent and denote
hydrogen, straight-chain or branched alkyl-
ha~ing up to 4 carbon atoms, or
denote a ro~idue of the ~ormula ~-
Rl1 ~
R.2 ~ ~ N~
10 ~ \~ or
N N
.
~ :in w~ich
: : Le A 29 518 - 7 -
.. i....... .... .. ......... .... .... ........... ....... . ... .
~ ~S021
R11 and R~2 and R1~ are identical or dif~erent
a~d denote hydrogen, fluorine, chlorine,
bromine, nitro, hydroxyl, cyano, phenyl-
thio, straight-chain or branched alkyl,
alkoxy, acyl or alkylthio having in each
ca~e up to 4 carbon atoms, or trifluoro-
methyl,
A represent~ a ~itrogen ~tom or represent~ the group
of the formula -CR
.
in which
:
R~i denote~ hydrogen, fluorine, chlorine,
~ i
bromine, methyl, hydro~yl, methoxy, vinyl :~
or alkinyl,
R15 .,
R~ repreaent~ a re~idue o the for~ula -(CH2)a - N
\R16
~ .
:~ 15 in which
: a de~ot-e a number 1, 2 or 3,
Rl~ and R~6, together with the n~trogen ato~, form an
: i~idazolyl, pyrrolyl, 1,2,3-triazol-1-yl,
: 1,2,3-triazol-2-yl, 1,2,4-trlazol-1-yl,
~ .
Le A 29 518 - 8 -
... ,,,,.. ,.. ,.. ,.,.,.,.. ,:,............. ....... .... .... ......... . .
1,2,4-triazol-4-yl or tetra~olyl ring,
Rs repre~ents hydrogen, hydroxyl, fluorine, chlorine,
bromine, or represents ~traight-chain or branched
alkyl or alkoxy having in each case up to 4 carbon
atoms,
R6 represents hydroxyl, benzyloxy, morpholino, or
straight-chain or branched alkoxy having up to 5
carbon atom~, whare the latter can be sub~titu~ed
identically or differently up to 2 times by halogen,
hydroxyl, or by a heterocyclic re~idue of the ~ .
... ~, -
:.,..:.,
~ r CH3
formula ~ O X CH3
:
~ repre~ent~ a group of the formula -NRl7Rla,
:~ :
:~ in whlch
:~ Rl7 and ~le ara ide~tical or dif~erent and de~ote
, 15 : hydroge~ or straight-chain or branched alkyl
ha~ing up o 6 carbon atoms, which i~ option-
ally substituted identically or differently up
: to 2 times by hydroxyl or by straight-chain or
branohed alkoxy ha~ing up to 6 carbon atom~
Le A 29 518 - 9 -
:~ .
21 L~O~l
or
R6 repre~ents a residue o~ the formula - N N-R19
in which
R19 denote~ hydrogen or straight-chain or branched
alkyl having up to 4 carbon atom~,
and
D repre~nts methyl, Yi~yl, phenyl or hydrogen,
and hydrat~s and ~alt~ thereo, optionally in zn isomeric
form.
;~ 10 Compound~ of the general fonmula (I) ar~ particularly
; pref-rred~
i~ ~hich
. Rl represa~ts hydrog~n, fluori~e, chlorine, bromine or
m~thyl,
R2 reprasent~ hydroge~, 1uori~e or chlorina, ~ ~
: ~,
~ .
~e A 29 518 - 10 -
L 1~021
Rg-N N--
R' represents a residua of the formula ~
R10
in which
R9 and Rl are identiaal or different a~d denote
hydrogen, straight-chain or branched alkyl
having up to 4 carbon atoms, or denote a resi-
due of the ~ormula
R
R~ 2 ~ N~
13
N N
~ ~ or
: ~ N N
; ~; '
which
: ~ Rll, R~3 and Rl3 are identical or di ~er~t and
denote hydrog~n, fluorine, chlorine, bro-
mi~e, nitro, hydroxyl, cyano, phenylthio,
: : straight-chain or branch~d al~yl, alkoxy, - -~
acyl or alkylt~io ha~ing i~ each ca~e up
to 3 carbo~ atoms, or trifluoromethyl,
: ~ .
~ Le A 29 518 - 11 -
.
.
~:
A represent~ a nitrogen atom or repr~sents the group
of the formula -CRl4,
i~ whiah
Rl4 denote~ hydrogen, fluoxine or chlorine,
R1s
R~ represe~ts a residue of the formula -CH~ N
R16
i~ which
Rl5 a~d Rls, togethar with the nitrogen atom, for~ an
imidazolyl, pyrrolyl, 1,2,3-triazol-1-yl, .
1,2,3-triazol-2-yl, 1,2,4-triazol-1-yl or
; lO 1,2,4-triazol-4-yl r~ng,
Rs repre8-nt3 hydroge~,
~: R6 repre8ent8 hydroxyl, benzyloxy, morpholi~o or,
straight-chai~ or branched alkoxy ha~ing up to 4
:~
arbosl ato~ns, where the latter can optio~ally be
15: 8ub8tituted by fluori~e, chlorine, bromino, ~-
, ~
' ~ :
-"-
Le A 29 518 - 12 - :-
~:
.. : ...... . ........................... ... .. ... ...... . ... . ... . ... .
r CH3
hydroxyl, or by a re~idue of the formula ¦ XCH
r 3
or represe~ts a group o~ the formula -NR~7R~8,
in which
R17 and R18 are identical or diferent and denote -~
hydrogen, or 3traight-chain or branched alkyl
having up to 4 carbon atoms, which i~ option-
ally substituted i~entically or dif~erently up
to 2 times ~y hydroxyl or by straight-chain or
branched alko~y having up to 6 carbon atoms,
or
RC repre~ent~ a residue of the formula - N N-R19
in which
Rl9 denotes hydkoge~ or ~traight-chain or branched
~: alkyl having up to 3 carbon atom~, -
~ 15 and
:i' D represents hydrogen,
~ .
-:
Le A 29 518 - 13 - i~
-- ~llàO~l
and hydrates and ~alt~ thereof, optionally i~ an isomeric
form.
In addition, a proces~ for preparing the compound~ of the
general formula (I) according to the invention has been
found which i8 characterized in that
compound of the general ~ormula (II)
Rl O
R2 ~,,CO-R6
E A N D (II)
~- ~ R4
~X '
R5
in ~hich
Rl, Rl, R~, Rs, R6, A a~d D ha~e the abovementioned me~ning
and
..,,~ .
E repr~se~ts haloge~, pre~erably chlorine and
fluorine,
are reacted with com~ounds of ths ge~eral ~ormula (III)
R3-~ (III)
i~ which
'~ ~
Le A 29 518 - 14 -
..................... ...................... .. .. ... . ...... . ... . .
l L ~ 0 21
R3 has the abovementioned mea~ing,
in inert sol~ents, optionally in the presence of acid-
capturing agents,
and, in the case of the acids, the esters are hydrolyzed,
and, in the casQ of the eater~, the acids are reacted
with the corresponding alcohols according to cu~tomary
methods,
and, in the case o~ the amide~, the acids are amidated,
~ optionally after prior activation and in the pre~ence o
a ba~e a~d/or auxiliary 8ub8ta~ce.
The proce~s according to the in~ention may be clarified,
by way o~ ex~ple, by the following formula ~cheme:
F ~ Co2H A
~: ~ + C6Hs-H N-H 9'
N - N~
': :
,. ';
Le A 29 518 - 15 -
.. . ............ ... . . . .. .... ... . ... . . . . ... . . .. . . .. . .... . . . .. . .. .
:, ";:;,,, " ~, ,;"",, ~,,,4".~,"~, ' .,., . ~ - . . ~.~. - . ,
0 ,~ 1
F CO2H F ~ CO2-CzH5
~N ~ ~ C2HsOH ~ ~
N ~ N~`N N - N `
~ .
O O
¦ O F~C-NH(CHz)z-CH3 : :
1 ) C~OC2H5 / N(C2HS)3 N~
2) H2N~ H2)2-CHa ~ [~ ~
N,N~ : -
,
;~: : :: :: .
.: ~ o
:: F~3~co24H~ ~
F N ~ . ~ ~
~ ~ ~ ~, + C~Hs~ N-H a ~ - ~
:~ .
; , ~ .'
Le A 29 518 - 16
:
.
: ~.. ,. ,.,., ~.. .... ,.. ,.......... ~.. .......... ...... . .
<IMG>
The customary inert solvents, which are not altered under
the reaction conditions, are suitable for use as solvents
for all the process steps. These solvents preferably
include organic solvents, such as ethers, for example
diethyl ether, dioxane or tetrahydrofuran, or hydro-
carbons, such as benzene, toluene, xylene, cyclohexane,
or petroleum fractions, or halogeno hydrocarbons, such as
methylene chloride, chloroform, carbon tetrachloride, or
dimethyl sulphoxide, N,N-dimethylformamide, hexamethyl-
phosphoric triamide, sulpholane, ethyl acetate, pyridine,
0 ~
triethylamine, N-methylpyrrolidone, anisole or picoline.
It is al~o po~ible to use mixture~ of the said ~olvents.
Dim~thyl sulphoxide, N,N-dimethyl~ormamide and N-methyl-
pyrrolidone are pre~erred.
The austomary basic compounda are suitable for u~e as
basa~ for individual reaction steps. These compounds
include, for example, alkali metal or alkaline earth
metal hydroxides, ~yridine, triethylamine, dii~opropyl-
ethylamine or N-methylpiperidi~e, or bicyclic amidine~,
such as 1,4-diazabicyclot2.2.2]octane (DABC0), 1,5-
d~azabicyclo[4.3.0]non-5-ene ~DBN) or 1,5-diazabicyclo-
5.4.0]undec-5-ene (DBU). Diisopropylethylamine and DABC0
~ .;
are pr~ferred.
The ba es are in general employed in a quantity of 1 to
3 mol, pre~erably o~ 1 to 1.5 mol~ based on 1 mol of the
corresponding carboxylic acid.
The proce~s i8 generally carried out in a temperature
ra~ge of +0C to ~160~, pr~erably o~ ~0C to l140C.
In general, atmoepheric pressure i8 amployed. HoweYer, it
20 i8 al80 po~sible to carry out the p~oces~ under negative
pres~ure or under exce~ pr~ssure (e.g. i~ a range from -
O.S to 5 bar).
Suitable colvents to u~e ~or the hydrolysie are wafer or
water i~ combination with a CuBtomary organic BolVent~
Th- lattar pre~erably includ~ alcohol~, such as methanol,
.
- Le A ~9 518 - 18 -
, . ,
;.' . '. ' .' ' i .,. `, ., .' .: '. ' ' -' .`"''.'-" ;- ' i - ' ' '' ' ' " '
- 2 ~
ethanol, propanol, i~opropanol or butanol, or ethers,
such a8 tetrahydrofuran or dioxane, or dimethyl~ormamide
or dimethyl ~ulphoxide. The use of alcohol~, ~uch as
methanol, ethanol, propa~ol or isopropanol, i~ parti-
cularly pre~erred.
The hydrolysis i~ e~fected using bases, for example
alkali metal or alkaline earth matal hydroxide~, prefer-
ably lithium hydroxide or sodium hydroxide, in one of the
above listed solve~ts, preferably dimethyl~ormamide,
tetrah~drofuran or ethanol in combinatiQn with water.
The hydroly~is can also be ef~ected using acids, ~uch as,
for example, acetia acid, hydrochloric acid, hydrobromic
acid, metha~esulphonic acid, sulphuric acid or perchloric
acid.
The hydrolysis is generally carried out in a temperature
range of 0C to +130~, preferably of +20C to +110C.
I~ ge~eral, the hydrolysi~ i8 carried out u~der atmo~-
pheric pressure. ~owe~er, it is also po~sible to carry it
; out under negative pressure or u~der exce~ pre~ure
(e.g. from 0.5 to 5 bar).
, '
I~ carryi~g out the hydroly~is, the acid i~ generally
employed i~ a quantity o~ 1 to 3 mol, pre~erably of 1 to
1.5 mol, ba~ed on 1 mol of the ester. The use o~ molar
qua~titia~ of the react2~ts is particularly preferred.
Le A 29 518 - 19 -
.. . . . .
a 2 ~.
The hydroly~i~ of tert-butyl esters i~ generally effected
u~ing acid~, ~uch as, for example, hydrochloric acid or
trifluoroacatic acid, in the pre~ence of one of the
abov~mentioned solvents and/or water or their mixture~,
preferably ui~ing dioxane or tetrahydrofuran.
The esteri$ication of the acids i8 effected
a) according to a customary method by reacting the
acids with the corresponding alcohols optionally in
o~e of tha above listed eiolve~ts in the presie~ce of
a catalyst. In this case it i8 preferred that the
corresponding alcohol i8 also employed as the 801
vent~
b) Alternativ~ly, the acid is activated by methods of
organic chOEmistry which are al80 well known and
co~verted, for example using Cl-COlC2~s, into the
mixed anhydride, which i8i co~verted into the e3ter
in ~ccordance with a). . ~ :~
Inorga~ic acid~, such a~0 for example, sulphuric acid, or
inorganic acid chlorides, ~uch asi, ~or example, thio~yl
chloride, ~ay be employed as cataly~t for a). In the ca~e
: of b~, one o~ the above liat~d base~, which i8 inert
under the reaction conditions, such a~, for exa~ple,
triethylamine, pyridine or bicyclic amidinea, can option-
ally be added.
In gs~eral, 0.01 to 1, preferably 0.05 to 0.5, mol of
cataly~t are employed in the case o~ a), and 0.5 - 2,
pxef~rably 1 - l,S, mol of catalyst i~ th~ case of b3, in
i~ .
Le A 29 518 - 20 -
.. . . . . . . . .
0 ~ ~
each case based on 1 mol of r~action partner.
The amidation i8 in general effected in inert aolvents
and optionally in the presence o~ one of the above li0ted
baaes and o~ a dehydrating agent.
In this context, inert organic ~olvents which are not
al~ered under the reaction conditions are suitable for
u~e as sol~ents. These include halogeno hydrocarbon~,
such as dichloro~ethane, trichloromethane, tetrachloro-
methane, 1,2-dichloroethanQ, trichloroethane, tetra-
chloroethane or trichloroethylene, hydrocarbons, such a~benzene, xylene, toluene, hexane, cyclohexane or petro-
leum fraction~, nitromethane, d~athylformamide, ace~o-
nitrile or hexamethylphosphoric triamide. It i8 also
po~sible to employ mixtures o~ the ~olven~s. Dichloro-
15 methane i3 particularly preferred.
The amidation i~ i~ general carried out in a temperaturerange from 0C to 150~, preferably at 25C to 40C.
. .
The amidation i8 in general carxied ou~ under at~ospheric
pressure. However, it i~ also pos~ible to carry out the
proce~s under negative pressure or under excess pre~sure
(e.g. in a range ~rom 0.5 to 5 bar~.
;: ' .
Iu ~arrying out the amidation, the ba~e i8 in general
employed in a quantity of 1 to 3 mol, pre~erably of 1 ~o
1.5 ool, based on 1 ool o~ the carboxylic aoid.
Le A 29 518 - 21 -
. ~'.
1) 2 1
. .
The compounds of the general ~ormula (II) are novel and
can be prepared by fir~t conv~rting compound~ of the
general formula (IV)
R~ 0
R2 ~C02-R6,
E A L R16 :
in which ::~
S ~ Rl, Rl, A and E have ths abovementioned meaning,
L repre8ents halogen, preferably chlorine or fluorine,
~, .,.^
R6' represents C1-C~-al~yl,
and
Rl6 repre~ent8 Cl-C4-alkoxy or C1-C~-dial~ylamino,
; 10 by reaetion with amin~s o~ the ge~eral formula (V)
NH2
:~ ~ Rs
:~ : R4
in which
~:: R4 and Rs have the abov~mention~d mea~i~g,
.
~ in one o the abo~e li~ted so1v~nt8, preferably ethanol,
~ .
~ Le A 29 518 - 22 -
~;' 211~02~
into the compound~ of the general formula (VI)
Rl O
RZ ~ CO-R6'
1 11 11 .
E ~ A ~ L VhNH (VI)
R ::~ 4
Rs - ;
in which
Rl, R~, R~, Rs, A, E, L and R6 have the aboveme~tioned
mea~ing, :~
, ~.
and, in a ~inal step, ayclizing the latter compounds in
one o~ the above li~ted 801~ent8 and one of the above
li8ted ~a888~ preerab1y DMF and K2CO3/RF,
and, i~t~e ca~e where D ~ ~, varying these ~ub~titue~t~
according to cu~tomary m~thods.
. 10 ~:: Th~:proce~ e~eral carried out in a temperature
rang~ rom +0C to ~150C, pre~rably o~ +0C to ~120C. ~:
In general, at~o8pheric ~r~8ure is employed. However, it
i8~ al~o poo~ible to carry out:the pro~e~ under negati~e
pr2ssure or under exces~ pre8~ure (e.g. i~ a range from
~ 0.5 to 5 bar).
: Thé compounds o~ ths ge~eral formulae ~rV) a~d (V) are
- . ~
: , -- -::
.,
Le A 29 518 - 23 ~
~: .,: : .
~: .
,
-` ~11~0~1
known per ~e or can be prepared in aacordance with
publi~hed ~ethods.
The compou~d~ of the general formula (VI) are noval and :
can~ ~or example, be prepared aB de~cribed above~
Surpri~ingly, the compound~ according to the invention
were found to have an effect in lentivirus-infected cell
aultureR. It was possible to demonstrate this using ~IV
~iru8 ai~ all exiample.
HIV in~ection_in cell culture
L~'i'
: 10 The ~IV test was carried out according to the method of
Pauwel~ et al ~cf. ~ournal of Virological Method~ 20,
(1988), 309-321~ with minor modi~ications.
Normal human blood lymphocyte~ (P~L'~) wera e~riched on
Ficoll-~ypague and ~timulated with phytohaemagglutinin
(90 ~g/ml) i~d i~terleukin-2 (40 ~/ml) in RPMI 1640
containi~g 20% foetal cal~ 8erum. For the i~4~ction with
infec~ious ~IV, the PBL'a were p~lleted and the cell
pellet wa8 3ubs~gue~tly su~pended in 1 ml o~ HIV viru~
ad~orptiou solution ia~d the BUspen~io~ wa8 l~cubated at
37C for 1 hour.
;"','~':'
Th~ ~iru8 adsorption solutio~ was cent~i~uged and the
in~ected cell pellet wa8 take~ up in growth medium ~uch
that a concentration of 1 x 105 cells per ml was
obtained. The cell~ which had been infected in thi~ way
:
Le A 29 518 - 24 - . ~
.
~1 :l 5021
wer~ then pipett~d into the well~ o~ 96-w~ll microtiter
plates in th~ ilmount of 1 x 104 cells/well.
The first vertical row of the microtiter plate contained
only growth medium and cells which had not been infected
but whiah had otherwi~e been treated exactly as described
above (cell control). The ~econd vertical row of the
microtiter plate aontained only ~IV-infected cells (virus
control) in growth medium. The remaining wells contained
the compounda according to the in~ention in differing
concentratio~s, starting from the well~ of the third
vertical row of the microtiter plate, from which the
~f~ substances under te~t were diluted up to 21 times in
doubling dilution 8tep8.
The te6t samples were incubated at 37C until the
syn~tiu~ ~orm2tio~ which i8 typical for HIV appeared i~
the untreated viru~ control (between day 3 and day 6
following infection), at which time the syneytium
formatio~ wa8 evaluated micro~copically. Under the~e te~t
conditions, approxim3tely 20 syncytia arose in the
untreat~d virus co~rol, whlle no ~yncytia w~re eviden}
. ....... :. in the untreated cell control.
Each ICso ~alue was determln~d as the ~oncentration of the
:- ~ compou~d according to the i~ventio~ at which 50% (iabout
10 8y~cytia) of the ~irus-induced sy~cytia were sup-
prossed by treatment with th~ compound according to the
invention.
, :
~ ' ~
~ .
Le A 29 518 - 25 -
.. ,.. ,.. :.. , .. , .. ,.. ,.. ,.. , .. . .. , :
It Wa~ fOUnd that the COmPOUnd~ aCCOrding tO the inVen-
tiOn PrOteCt HIV-infeCted Ce11~ frOm VirU8-indUCed Ce11
de~trUCtiOn.
Tab1e A:
EX. NO. ICSO (~m)
8 3
14 0.7
0.2
23 0.4 .
31 0.7
39 0.25
0 2
~5 6~ 0.7
92 0.6
113 2
146 0.7
The COmPOUnd8 aCCOrding tO the in~e~tiOn rePreBent
Va1Ua~1e aCtiVe CO~POUnd~ ~Or thQ treatmQnt a~d PrOPh~1-
axi3 Of di8ea~e~ 1n hUman a~d VeterinarY madiCine which
are CaU8ed bY retrOVi~U~e8.
hO8e area8 Of indiCatiOn i~ hUman mediCina WhiCh m~y be
m2~tiOUed bY WaY Of example are:
1.) Th~ trea~me~t a~d PrOPhY1aXi8 Of hUman retrOVira1
nfeCtiOn~.
: 2.) FOr the treabme~t Or PrOP~Y1aXi~ Of di8ea8e~ (AIDS)
CaU8ed bY HIV I (hUman i~mUnOde4iCienCY VirU~
PreViOn~1Y termed HTLV III/LAV) a~d HIV II, and the
: ~ :
.:
~ .
~' : --:: -:..... :
LQ A 29 518 - 26 -
............... :
5~1
stages as~ociated therewith, ~uch as ARC (AIDS-
related complex) and LAS (lymphadenopathy syndrome),
a~ well as of the immune weakness and encephalopathy
cau~ed by thi~ virus.
3.) For the treatment or prophylaxi~ of an HTLV-I or
HTLV-II in~ection.
4.) For the treatment or prophylaxi~ of the AIDS-carrier
condition.
Those indicatio~ in veterinary medicine which may be
li~ted by way of exam~le are:
In~ection~ with
a) Maedi~ na (in sheep and goat~).
b) progressive pneumonia viru~ (PPV~ ~in 3heep and
goat~) ~
c) caprin~ arthriti~-e~cephalitia virus (in ~heep and
goats~ -
d~ zwoeger~iekte viru8 (in aheep)
: e) equ~e infectiou~ anaemia viru8 (0~ the horse)
) infections caused by feline leukaem~a ~i~u~
g) infection~ caused by ~ellne i~munode~cie~cy virus
~ :: (FIV~
:~: h) infection~ cau~ed by ~i~ian immNnodeficie~y viru~
(SIV) :
The above li~ted poi~ts 2, 3 and 4 fro~ the areas of
indication in huma~ mediain~ are pre~erred.
,, .
: The pre~ent i~vention includes pharmaceutical .-
: ~
. - :
Le A 29 518 - 27 -
::
...... .............. .... ... . ... . . .. . . .
2 1 1 ~ 0 h ~l
23189-759
preparations which, in addition to non-toxic, inert and
pharmaceutically appropriate excipients, contain one or more
compounds of the formula (I), or which consist of one or more
active compounds of the formula (I), as well as processes for
producing these preparations.
The active compounds of the formula (I) are preferably
intended to be present in ~he above listed pharmaceutical
preparatlons at a concentration of about 0.1 to 99.5, preferably
of about 0.5 to 95 ~ by weight of the total mixture.
The invention also extends to a commercial package
containing a compound of the invention, together with instructions
for its use as an antiviral agent.
In addition to the compounds of the formula (I), the
above listed pharmaceutical preparations may also contain further
pharmaceutical active compounds.
The above listed pharmaceutical preparations are
produced in a customary manner in accordance with known methods,
for example by mixing the active compound(s) with the
excipient(s). ~
In general, it has proved to be advantageous both in ~ ~-
human and veterinary medicine, in order to obtain the desired
results, to administer the active compound(s) according to the -~-
invention in total quantities of about 0.5 to about 500,
preferably 1 to 100, mg~kg of body weight every 24 hours,
optionally in the form of several individual doses. An individual
dose preferably contains the active compound(s) in quantities of
about 1 to about 80, in particular 1 to 30, mg~kg of body weight. -
It may, ~
2 8
~ 211~02~
however, be nece~ary to deviate ~rom the said do~age~,
nam~ly in dep~ndence on the nature and the body weight of
the subject to be treated, on the nature and ~everity of
the disea~e, on the nature of the preparation and the
mode o~ adminiatration of the medicament, and on the
period or interval within which adminl~tration take~ :
place.
Starti~a comPound~
Example I
2-(4-~mi~obenzyl)-2~-1,2,3-triazole
NH2
N '~ :
N ~ ~
: : ~
a~ 2-(4-Nitrobe~zyl)-2H-1,2,3-triazole
15.6 g (0.072 mol) of 4-~itrobenzyl bromide are heated to ~ :
roflux for 6 hour~ in 70 ml of acetone together with
5.0 g (0.072 mol) o~ 1,2,3-triazole and 20.2 g (0.15 mol) ~ :
o potas~ium carbonat~. Af ter cooli~g, the mixture ia
:; f iltered and the sol~ent i~ r~moved in ~acuo. The crude -
~: product is purified by colum~ chromatograp~y (~llica gel,
methyle~e chloride/~eth~ol 98/2).
.
Le A 29 518 - 29 -
2;ll~0~1
Yiald: 3.87 g (26% of theory)
Melting point: 96-100C
b. 2-(4-Aminobenzyl)-2-H-1,2,3-triazole
3.5 g (0.017 mol~ of 2-(4-nitrobenzyl)-2H-1,2,3-triazole
are hydrogenated under atmo~pheric pressure in 50 ml of
ethyl acetate and in the presence of 1 g of Pd/C (10%).
The catalyst is filtered off and sub~equently washad
thoroughly with dimethyl~ormamide. The solvents are
xemoved in vacuo.
Yield: 2.1 g (72% o~ theory)
H-NMR (d6-DMSO): 8 5.4 (2H); d 6.5 (2H~; d 7.0
(2H); 8 7.7 (2H).
: ~ '
. -
Le A 29 518 - 30 -
~,.... ....................... ... ..... . .. ..... . . . ....... . ......
2 l
Example II and ExamPle III
1-(3-AmiD,obenzyl)-1~-1,2,3-tria~ole (II) an,d
2-(3-Ani~ob~nzyl)-2U-1,2,3-triazole ~III)
N~2 NH2
l 11 l 11
`N N~N`N
~ N \~
.~ a. 1-(3-Nitrobenzyl)-lH-1,2,3-triazole and
2-(3-nitrobenzyl)-2H-1,2,3-triazole ~-
12.3 g (0.072 mol) of 3-nitrobenzyl chloride and 5.0 g of
1,2,3-triazole ar~ heated to reflux for 6 hours in 70 ml
o~ acetonQ together with 29.2 g ~,0.15 mol) o~ pota~8ium : :
carbon,ata. The cooled mixture i8 ~iltered and the solvent ~ ~:
: 10 i~ removed i~ vacuo. The crude product i8 fractionated by
:: column, chrom~tography (silica gel, methylene chloride/
metha~,ol 9~/4).
: Yield: 4.1 g of 1-(3-~itrob~D,zyl)-1~-1,2,3-triazole ~,28%
: o~ theory) :
Melting point: 96-97C
: 4.6 g of ~-(3-nitrobenzyl)-2H-1,2,3-triazole (31~ of ~ :~
theory) . --
, , . ':
' ~
:~:
-
Le A 29 518 - 31 -
....................... .. . . . .. ... . ..... .. ............. .......... ... . .. ... .. ......... . .. ..................... . .. .
~lla~i
Melting point: 91-93C
4.1 g (0.02 mol) of 1-~3-nitrobenzyl)--lH-1,2,3-triazole
are hydrogenated under atmo~pheric pres~ure in 100 ml of
ethyl acetate together with ~ g of Pd/C (10%). The
catalyst i~ filtered off and then washed thoroughly with
dimethyl~ormamide. The ~ol~ent~ are remo~ed in ~acuo.
Yield: 3.2 g (94% of theory) (Example II)
H-NMR (CDCl3): ~ 5.4 (2H); m 6.5-6.7
(3H); t 7.15 ~lH); 8 7.50 (lH); 8 7.70 ~lH).
4.6 g (0.023 mol) o~ 2-(3-nitrobenzyl)-2~-1,2,3-triazole
. are hydrogenated in an analogous manner to Example II.
Yield: 3.9 g ~97% of theory) (Example III)
Exam~le IV and ~xamp19 V
1-(2 ~;~obenzyl)-~-1,2,3-triazole ~IV) and ~ :
2-(2-aminobe~yl)-2~-1,2,3-tria~ole (V)
NH2 NH2
[~6~N
a. l-(2-Nitrobe~zyl)~ 1,2,3-triazole and 2-(2-nitro-
be~zyl)-2~-1,2,3-triazole
~ 15.5 g (0.072 mol) o~ 2-nitrobenzyl bromide and 5.0 g
:
Le A 29 518 - 32 -
-~ 2 ~ i3 ~ ~ 1
(0.072 mol) of 1,2,3-triazole are heated to reflux for
4 houris in 70 ml of acetone together with 20.2 g
(O.15 mol) of pota~ium carbonate. The cooled mixture i8
filtered and the sol~ent 18 r~mo~ed in vacuo. The crude
product is ~ractionat~ed by aolumn chromatography (silica
gel, methylene chloride/methanol 96/4).
Yield: 3.0 g of 2-(2-nitrobenzyl)-2H-1,2,3-trlazole
(20% of theory)
Melting point: 77-80C
and
6.8 g of 1-(2-nitrobenzyl)-lX-1,2,3-triazole
(46% of theor~
~elting point: 110-115C
b. 1-(2-Aminobenzyl)-lH-1,2,3-triazole
6.8 g (0.033 mol) of 1-(2-nitrobenzyl)-lX-1,2,3-triazole -~
are hydroge~ated undar atmospheric pre~sure in 100 ml of ~ ~ ~
ethyl acetate and in he pr~si~ncc o~ 1 g o~ Pd/~ (10%). ~:
Th~ catalyst is filtered off and the~ wa~hed thoroughly ~:-
with dimethylformamide. The ~ol~enSs are remo~ed ~n
va~uo.
Y~eld: 5.6 g (97~ of theory~
l~-NMR (CDCl3): 8 5.45 (2~); m 6.65-6.85
: (2~); m 7.10-7.25 (2~); a 7.50 (1~3; 8 7.70 (1~
3.0 g (0.015 mol) of 2-(2-nitrobenzyl)-2~-1,2,3-triazole
are hydrog~nated in a~ a~alogou~ m~nner to Example b. ~ -~
Yield: 2.4 g :(91% o~ theory)
l~-NMR (CDc13) ~ 5.50 (2~); m 6.60-6.85 .-
;; ~'
.
:
Le A 29 518 - 33 -
^ ~:1 L.~21
(2H); m 7.05-7.35 (2H); 8 7.55 (2H)
Example VI
6,7-Di1uoro-1,4-d~hydro-4-o~o~ 4~ -1,2~3-~riazol-1-
yl-methyl)-phenyl]-3-qui~oli~ecarbo~ylic acid
O O
F ~-~`OH
ll l
F~N~ : ~
. . .
",. ~
. :
~N~N~N
~/
a. Ethyl 2-(2,4,5-trifluorobenzoyl)-3-t4-(1~-1,2,3-
: triazol-l-yl-methyl)-phenylamino] acrylate
11.5 g (0.038 ~ol) of ethyl 3-ethoxy-2-(2,4,5-trifIuoro-
benzoyl)-acrylate are initially introduced into 75 ml of :~-~
ethanol and 6.7 g (0.038 mol) of 1-(4-aminobenzy~
1,2,3-triazole are added dropwi~. The mixture is sub- :
,~ ~ sequ~tly stirr~d at room temperaure for 12 hours and th~
~olvent is the~ remoYed in vacuo.
:~ :Cruds yield: 1~,8 g
b. ~thyl 6,7-Di~luoro-1,4-dihydro-4-oxs-1-~4~ -1,2,3-
~ 15 ~riazol-1-yl-methyl)-ph~yl]-3-quinoline~arboxylate
; ` :
Le A 29 518 - 34 -
:~ :
V ~ l
15 g (0.035 mol) of the produet obtained under a. are
h~ated at 100C for 5 hours in 70 ml of dimethylformamide
together with 5.7 g (0.041 mol) o potassium carbonate.
The ~olvent i8 r~moved in vacuo and the re~idue 18
stirred up with water. Drying then take~ place at about
100C.
Yield: 13.2 g (92% of theory)
Melting point: 183-185C
g (24 mmol) of the ester obtained under b. are
stirred, together with 0.6 g (24 mmol) o~ lithium
hydroxide, at room temparature for three day~ in a
mixture consisting of 100 ml of tetra ffl drofura~, 5 ml of
dimethylformamide and 5 ml of water. Subsequently,
filtration with ~uction take~ plac~ and the re~idue i8-
stirred up thoroughly with iso-propanol ~d the~ dried at
about 100~.
Yield: 8.2 g (89% of thaory~
Melting point: ~280C
~-:
, .
`.~
:
' -
Le A 29 SI8 - 35 -
........ . ....... . .. . .. . . .
1150~1
Example VII
~thyl 7-chloro-6-fluoro-1,4-~ihydro-4-Qxo-l-t4-~lE-1,2,3-
triazol-l-yl-methyl)-phe~yl]-1,8-naphthyridino-3-
car~ylate
O O
Cl N~
.:`i.J ,N~
~/ : .
5 a.Ethyl 2-(2,5-diahloro-4-fluoro-nicotinoyl)-3-t4-(lH- ~
1,2,3-triazol-1-yl-methyl)-phenylamino~-acrylate - .
2.9 g (8.6 m~ol~ of ethyl 3-ethoxy-2-(2,5 dichloro-4-
fluoro-nicotinoyl)-acrylate and 1.5 g (8.6 mmol) o~ 1~(4-
amlnobenzyl)-l~-1,2,3-triazola are ~tirred at room
10 . temperature for 12 hours in 20 ml o~ ethauol. The 801v~nt
i~ then remo~ed in vacuo.
Yi~ld: 3.65 g (91% o~ theory)
Melti~g point: 153-158C
::
3.5 g (7.~ mmol) o~ ~he product obtained u~der a. are
heated at 120C ~or 5 hour~ in 20 ml o~ dimethylformamide
together with 1.3 g l9~4 mmol) of potassium carbonate. --
Le A 29 518 - 36 -
!
......... _ .. . ..
2 1
The cooled mixture i8 added to ice water and the precipi-
tated product i8 i~olated~ It i8 dried at about 100C.
Yield: 3.05 g (94% of theory)
Melting point: 216-220C
Exam~le VIII
~thyl 6~7-di~luoro-1,4-dihydro-4-o~o-1-t4-(2~-1,2,3-
triazol-2-yl-methyl)-phenyl]-3-quinolin~carbo~ylate
O o .
F ~`OE~
N'
N :
a. Ethyl 2-~2,4,5-trifl~orobenzoyl)-3-~4-(2~-1,2,3-
triazol-2-yl-methyl)-phenylamino]-acrylate
: ::
3.6 g (0.012 mol) o~ ethyl 3-ethoxy-2-(2,4,5-trifluoro-
benzoyl)-acrylate a~d 2.1 g 10.038 mol) o~ 2-(4-ami~o-
;' benzyl)-2~-1,2,3-triazole are ~tirred at room temperature
ov~rnight in 25 ml of ethanol. All the volatile co~-
ponents are then re~oved in vacuo.
: 15 Yield: 5.0 g (97~ o~ theory)
Melting point: 108-110C
~e A 29 518 - 37 - ~
... . ...... . .. . .. .. . . . . . . . .. . .. .. ... .. . .. . . . . . . . ... .. . . . . . .
. . . . . . ............ . .. . .. . . . .. ......
`~ 2.1 1.~21
5.0 g (0.012 mol) of the product obtained under a. are
heated at 100C for S hours in 25 ml of dimethylformamide
together with 1.9 g (0.014 mol) o~ pota88ium carbonate.
The cooled mixture i8 then added to ice water and the
precipitated product i8 i~olated. It i~ dried at about
100~
Yield: 4.0 g (85% o~ theory)
Melting point: 203-206C
,~
', ! ' -, ' .~
I
,
~e A 29 518 - 38 -
..... ~................. . . .... .
-~ 2 l1~21
Example IX
Ethyl 6,~-difluoro-1,4-dih~dro-4-oxo-1-t4-(1~-1,2,4-
triazol-l-yl-me~hyl)-phanyl]-3-quinolinecarboxylate
O O
F ~ OEt
F N
~ . ~
, N
N
a. ~thyl 2-~2,4,5-trifluorob~nzoyl)-3-t4-(1~-1,2,4-
: 5 triazol-l-yl-methyl)-phenylamino]-acrylate
17.2 g (0.052 ~ol) o~ ethyl 3-etho~y-2-(2,4,5-tri~luoro-
benzoyl~-acrylate and 10,0 g (0.057 mol) o~ 1-(4-amlno-
:~ ` be~zyl)~ 1,2,4-triazole are 3tirred at room t2mperature
~:~ : overnig~t in 100 ~1 o~ etha~ol. The pracipitatsd product
: 10 ia iaolat~d a~d wa~hod with ethanol.
; Yield: 20.2 g (82~ o~ theory)
~:~ ` Melting p~t: 137-139~
4.5 g (0.011 mol) o~ the product obtai~ed under a. are
heated: at 100C for ~even houx~ i~ 25 ml o~ dimethyl-
15 : formamide togather with 1.9 g (0.014 mol) of potaa~ium ::
car~onat~. Th0 cooled ~ixture i8 added to ice water a~d
'
Le A 29 518 - 39 -
~ '
21~0~1
the pre~ipi~ated product i8 isolated. It i8 dried at
about 100C.
Yield: 2.94 y (68% of theory)
Melting point: 226-228C
Example X
~thyl 7-cbloro-6-~luoro-1,4-dihy~ro-4-oxo-1-t4~ -1,2,4-
triazol-l-yl-methyl)-phenyl]-1,8-naphthyridine-3-
carboxylate
O O ::
~;-.i F ~
., 1
Cl' ~N~ ~ N '
N,N
~ N
a. Ethyl 2-(2,5-dichloro 4-~luoro-nicotinoyl)-3-[4-(lH-
1,2,4-triazol-1-yl-met~yl~-phenylamino]-acrylate
9.7 g (0.029 mol) of et~yl 3-ethoxy-2-(2,5-dichloro-4-
;J fluoro-ni~otinoyl)-acrylats and 5.0 g (0.029 mol) of 1-(4-~mi~ob~nzyl)~ 1,2,4-triazole are stirred at room
temperaturQ for thre~ hour~ in 60 ml of ethanol. The
15 : ~olvent is removed in vacuo.
Yield: 13.5 g o~ crude product
Le A 29 518 - 40 -
.. . ... . ..... .. .. . . .. .. . . .
2~150~1
13.3 g (28 mmol~ of the product obtain~d under a. areheated at 100C for four hours in 70 ml of dimethylform-
amide together with 5.0 g (36 mmol~ of potas~ium carbon-
ate. The aooled mixture i~ added to ice water and the
preaipitated product i~ isolated. It is dxied at about
100C.
Yield: 10.8 g (85% of theory)
Melting point: 225-228C
Example XI
6,7-Difluoro-1,4-dih~dro-1-[4-(N-i~idazolyl-methyl)-
:~ phenyl]-4-oxo-3-quinolinecarbo~ylic aaid
O O
F ~OH
F N
~3
N ~ ~ :
~ N
a. ~thyl 2-(2,4,5-trifluorobenzoyl)-3-~4-(N-ilidazolyl-
methyl)-phe~ylami~ol-acrylat~
17.2 g (0.057 mol) o~ ethyl 3-ethoxy-2-(2,4,5-trifluoro-
be~zoyl)-acrylate and 10.0 g (0.057 mol) of N-(4-amlno-
be~zyl)-i~ida~ole ar- ~tirred at room te=por~tur~
.-
::
Le A 29 518 - 41 - ~
2115~
overnight in 100 ml o ethanol. All the volatile
component~ are removed in vacuo.
Yield: 24.0 g of crude product
b. Ethyl 6,7-Di~luoro-1,4-dihydro-1-~-(N-imidazolyl-
S methyl~-phenyl]-4-oxo-3-~uinolinecarboxylate
24.0 g of the product obtained under a. are heated at
100C ~or 8iX hour~ in 200 ml o dimethylformamide
together with 14.3 g (0.1 mol) of pota~sium carbonate.
All the volatile component~ are removed in ~acuo and the
re~idue i8 puri~ied by column chromatography (silica g~l,
.` methyle~e chlorid~/methanol 96/4).
Yield: 15.3 g (65~ o~ theory over two ~teps~
Melting point: 251-254C
200 mg (0.49 mmol) o~ the e~ter obtained under b. are
heat~d at 80C ~or two hours in a mixture consisting of
1 ml o~ ac~tic acid, 1 ml o~ water and 0.1 ml of co~cen-
trated sulphuric acid~ After cooliug, the mixtur~ i~
: added to a little ~ce wat~r and th~ product i8 isolat~d.
Yield: 180 mg (96% o~ theo~y)
~elting poi~t: ~280C
~xample XII
~: ~thyl 6,7-difluoro-1,4-dibydro-4-oxo-1-C3-(1~-1,2,3-
triazol-l-yl-~ethyl)-phe~yl]-3-quicoli~ecarbo~ylate
.: :
~ '
: Le A 29 518 - 42 -
.. . . ~ i -
0 2 :L
o o
F ~ OEt
~ N ~
a. Ethyl 2-(2,4,5-trifluorobenzoyl)-3-~3-(lH-1,2,3-
triazol-1-yl~methyl)-phenylamino]-acrylate
: 5.9 g (0.019 mol) o~ ethyl 3-ethoxy-2-(2,4,5-trifluoro-
.~ benzoyl)-acrylate and 3.4 g ~0.019 mol) of 1-(3-amino-
: S benzyl)~ 1,2,3-triazola are ~tirred o~ernight in 40 ml
of athanol. All the ~olatile compo~ents ar~ then remo~ed
in vacuo.
Yield: 7.8 g (92~ o~ theory) ~:~
- Melting point: 126-128C .
: ~13 7.7 g (0.018 mol) of tho product obtai~ed under a. are
. heat-d at 100C for ~our hours in 45 ml of d~methylform-
amlde:~ogether with 3.1 g (0.022 mol3 o pota88ium
carbonate. The cooled mixture i~ added to 180 ml of ice
:wat~r and~the precipitated product i8 ~olated. It i8
lS ~ dried at a about 100C.
YiQld: 6.a ~ (85% of theory)
M-lein~ pOi~t: 187-189C
xample XIII
Le A 29 518 - 43 -
.
~."
.. .. ..... ... . . ... . . . . .
~` 2~ 1~0~1
~thyl 6,7-difluoro-1,4-dihydro-1-~3-(2~-1,2,3-triazol-2-
yl-~thyl)-phQ~yl]-4-oxo-3-guinoli~ecar~oxylate
O O ' '
F ~l` OEt
~ .
. ~ N~
a. Ethyl 2-(2,4,5-trifluorobenzoyl)-3-~3-2H-1,2,3-
triazol-2-yl-methyl~-phenylamino]-acrylate
:` 5 4.0 g (0.013 molj of ethyl 3-ethoxy-2-(2,4,5-trifluoro- : ~
benzoyl)-acrylate and 2.3 g (0.013 mol) of 2-(3-amino-- ~ -
benzyl)-2~-1,2,3-triazole are ~tirr~d overnight in 25 ml
of ethanol. All the volatile component3 are then removed ~ :
in vacuo.
Yield: 5.5 g of orude product
5.5 g (0~013 ~ol) o~ the product obtai~ed under a. are
haat~d at 100C ~or four hour~ i~ 30 ml of dim~t.hylform-
amide togethsr with 2.3 g (0.017 mol) o$ pota~ium
carbonate. The coolad mixture is added to 180 ml o$ ice
: 15 water~and the precipitated product is i~olated. It i~ ~
dried at about 100C. :;
Y~ield: 4.5 g (86~ of theory)
~eltlng point: 144-146C
Examvle XIV
: :~
~ ~e A 29 518 - 44 -
.
~: -
O ~ ~
~thyl 6,7-difluo~o-1,4-dihydro-1-t3-(1$-1,2,4 tria~ol-l-
yl-methyl)-phenyl]-4-oxo-3-quinoli~ecarbo~ylate
O O
F ~ OEt
~ N~\
a. ~thyl 2-~2,4,5-trifluorobenzoyl)-3-~3-(lH-1,2,g-
triazo-l-yl-methyl)-phenylamino]-acrylate
-: .
8.7 g (0.029 mol) o~ eth~l 3-ethoxy-2-(2,4,5-trifluoro-
benzoyl)-acrylate and 5.0 g (0.029 mol) of 1-(3-i~mino-
benzyl)-1~-1,2,4etriazole are stirred o~er~ight in 60 ml
:~ o~ e~hanol. All th~ volatile components are then removed
in vacuo.
Yield: la.4 g of crud~ product
12.4 g (0.013 mol) of the product obtained u~der a. are
:~ heaked at 100C for two hour~ in 80 ml of dimethylfor~-
mide togethor wlth 5.4 g ~0.039 mol) of po~assium
ciarbonate. The cooled mlxture iB added to i~a wat~r, and
then this mixture i~ extracted with ms~hylene chloride
a~d the ~olvent removod o~ a rota ~ evaporator. Dryi~g
: ` ta~e~ place at about 100C.
:~ Yield: 10.0 g (84% of theory~
~ : Melting point: 175-180C
::
Le A 29 518 - 45 -
, ,
,
-
0 2 ~
Example XV
~thyl 7-chloro-6-fluoro-1,4-dihydro-4-oxo-1-t3-(L~-1,2,4-
triazol-l-yl-methyl)-phe~yl]-1,8-naphthyridin~-3-
carboxylate
O O
C~X~OEt
N ~=\
N ~
~
a. Ethyl 2-(2,5-dichloro-4-fluoro-nicotinoyl)-3-[3-(lH-
~ 1,2,4-tria~ol-1-yl-methyl)-phenylamino]-acrylate
;~ ~ 5.7 g (0.017 mol) o~ ethyl 3-ethoxy-2-(2,5-dichloro-4-
fluoro-~icotinoyl)-acrylate and 3~0 g (0.017 mol) o~
1-(3-~minobenzyl)-lH-1,2,4-triazole a~e ~tirred at roo~
temperature ov2rnight in 5 ml of ethanol. The ~olvent i8
then r~oved in vacuo.
Yleld: 7.7 g o$ crude product
7.7 g (16 mmol) of the product ob~ained under a. are
heated at 100C ~or two hours in 50 ml o~ dimethylform-
amid~ together with 3.4 g (24 mmol) of potas~ium carbon-
ats. T~e ~ooled mixture i8 added to ice water and th~ 8
mixturQ i~ then extracted with methylene chloride and the -
org ~ ic pha~e i8 then dried ov~r sodium sulphate a~d
evaporated off on a rotary evaporator. Drying takes place --
~: .
~:: .
':
. ::
Le A 29 518 - 46 -
~ .
~:
~-~ 2:L 15021
at about 100C.
Yield: 4.9 g (67% o~ theory over two steps)
Melting point: 186-189C
Exam~le XVI
6,7-Di1uoro-1,4-dihydro-1-l3-(N-imidazolyl-methyl)-
phe~yl]-4-o~o-3-quin~lLnecarbo~ylic acid
O O
F ~ OH
~,,
~ ~ N
: a. Ethyl 2-(2,4,5-trifluorobenzoyl)-3-~3-(N-imldazolyl~
methyl)-phe~ylamino]-a~rylate ~ ;~
: 8.7 g (0.029 mol) of ethyl 3-ethoxy-2-(2,4,5-trifluoro- ~-`
: 10 be~zoyl~-acrylate and 5.0 g (0.029 mol) of N-(3-amino- -:~
benzyl)-imidazol are stirred at room temperature
over~ight in 60 ml of ethanol. All the volatile com- ::
: ponent~ ar~ then removed i~ vacuo.
~ield: 12.3 of crude product
.-._~
;~ }5 b. Ethyl 6,7-difluoro-1,4-dihydro-1-t3-(N-imidazolyl- m~thyl)-phen~1]-4-oxo-3-quinolinecarboxylate
, .
12 . 3 g of ~he product obtained under a. are heated at --
', :.,
.
~e A 29 518 - 47 -
~S~.,:., """ ~ 'A,.: `,'. tr ' ~
100C for two hours in 70 ml of dimethylformamide
together with S g (0.036 mol) of potas~ium carbonate. The
cooled mixture i8 added to ice water and thi~ mixturs i~
the~ extracted with methylene chloride and the solvent i8
then removed in a rotary evaporator. The crude product i8
puri~isd by column chromatograph~ (~ilica gel, methylene
chloride/methanol 96/4).
Yield: 3.3 g (28~ of theory over two step~
Melting poin~: 193-194C
200 mg (0.49 mmol) o~ the e~ter obtained under b. are
heated at 80C for two hours in a mixture consisting of
1 ml of acetic acid, 1 ml of water and 0.1 ml of con-
centrated sulphuric acid. After cooling, the mixture i~
add~d to a little ic~ water and the product i~ isolated.
Yield: 130 mg (68~ o~ theory)
Melting point: ~280C
~xample XVII
~thyl 6,7-difluoro~ -dihy~ro-4-o~o-1-t2-(1~-1,2,3- :
; tria~ol-l-yl-m~th~ phQnyl]-3-quinolinscarbo~ylate
O O -'
F ~ OEt
F N
~f N ' N~N
. .
~' ' ' "'.
Le A 29 518 - 48 - -
~. ,,,,.~ ,~
211.i~21
a. Ethyl 2-(2,4,5-trifluorobenzoyl)-3-[2~ -1,2,3-
triazol-1-yl-methyl)-phenylamino]-acrylate
5.0 g (0.016 mol) of ethyl 3-ethoxy-2-(2,4,5-trifluoro-
benzoyl)-acrylate and 2.8 g (0.016 mol) of 1-(2-amino-
S banzyl)-1~-1,2,3-triazole are ~tirred overnight in 30 ml
o~ ethanol. A11 the volatile compon~nt~ are then removed
in vacuo.
Yield: 6.4 g of crude product
Melting point: 124-126C -
6.0 g (0.014 mol) of the product obtained under a. are
heated at 100C for ~our hours in 35 ml of dimethylform-
amide together with 2.4 g (0.017 mol) of pota~sium
carbonate. The cooled mixture i~ added to 180 ml of ice
water and the precipitated product is i~olated. It i8 .
dried at about 100C. -~
Yield: 3.9 g (68% of theory)
Melti~g point: 224-226C
~thyl 6,7-difluoro-1,4-dihydro-4-o~o-1-t2-(2~-1~2,3-
triazol-2-yl-~ethy})-phenyl3-3-gQinoli~ecarbQ~ylat~
: .
Le A 29 518 - 49 -
. . . . .. . . .. . .
o o
F ~ OEt
~ N~
: N
a. Ethyl2-(2,4,5-trifluorQb~zoyl)-3-~2-(2H,-1,2,3-
triazol-2-yl-mcthyl)-phenylamino]-acrylate
: 3.9 g (0.013 mol) o~ ethyl 3-ethoxy-2-(2,4,5-trifluoro-
benzoyl)-acrylate and 2.3 g (0.013 mol) of 2-(3-amino-
S benzyl)-2~-1,2,3-triazole are stirred over~ight in 25 ml
o~ ethanol. All the volatile components are then removed
in ~acuo.
Yield:~4.9 g (89% o~ theory)
:: Melting point: 98-99C
~: :
4.5 g (0.011 mol) o~ the produc~ obtained under a. are
heated at 100C for four hours i~ 25 ml of di~ethylform-
amide: together wit~ 1.7 g (0.012 ~ol~ oE potaasium
arbonate. The ~ooled mixture i~ added to 180 ml o~ ice
w~ter and the precipitated product ie isola~ed. It i~
:: :
~: 15~ : ~ dried at about 100C.~ - .
Yield::3.7 g (8B% o~ theory~ -
lSing poi~t: 144-145C
,: .
. .
: Le A_29 518 - 50 -
- .~.
~ 2 1
Example XIX
~thyl 6,7-difluoro-1,4-dihydro-4-o~o-1-~2-~1~-1,2,4-
triazol-1-yl-methyl)-phenyl]-3-quinoli~carbo~ylate
O O
F ~3~1~OEI
N'
a. Ethyl 2-(2,4,5-trifluorobenzoyl)-3-12-(lH,-1,2,4-
triazol-1-yl-methyl~-ph~nylamino~-acrylate
8~7 g (0.029 mol) o~ ethyl 3-ethoxy-2-(2,4,5-tri~luoro-
benzoyl)-acrylate and S.0 g ~0.029 molj o~ 1-(3-amino-
benzyl)~ 1,2,4-triazole are stirred overnight in 60 ml
o ethanol. ~ll the volatile ~omponent~ are then r~moved
in va~uo.
Yield: 14.3 g o crude product
12.4: g o~ the produ~P o~tai~ed unde~ a. are heated at
100C for 3.5 hour~ in 80 ml of d~mathyl~ormamide
together w~th 5.7 g (0.04 mol) of potassium car~onato.
The cooled mixture is add~d to ice wa r and thi~ mixture
i8 the~ ~xtracted with methylene chloride and the solvent
the~ remo~sd on a rotary e~aporator. The crude product i~
purified by colum~ chromatography (~ilica gel~ methyle~e
chlo~idej~ethanol 96/4).
:~ .
~ : :
~ Le A 29 518 - 51 -
~ ,.,.'~
Yield: 6.5 g (54% of theory)
Melting point: 214-216C
ExamE~le XX
6,7-Difluoro-1,4-dihydro-1-12-(N-imidazolyl-m~thyl)-
phenyl]-4-oso-3-quinoline~ar~o~ylic a~id
O O
F ~ OH
N
N
_ Ethyl 2-(2,4,5-trifluorobenzoyl)-3-t2-N-imidazolyl-
mathyl)-phe~ylamino~-acrylate
7.85 g (0.026 mol) o$ et~yl 3-ethoxy-2-(2,4,5-trifluoro-
benzoyl)-acrylate a~d 4.5 g (0.026 mol) o~ N-(2-amino-
benzyl)-imidazola are stirred at room temperature
over~ght in 50 ml of ethanol. All the volatile com-
ponents are remo~d in ~acuo and the re~idue i~ puri~ied
by chromatography.
Yield: 7.87 g (70~ of theory)
-" :-
b. Ethyl 6,7-difluoro-1,4-dihydro-1-t2-~N-imidazolyl-
methyl~-phe~yl]-4-oxo-3-qui~olinecarboxylate
. .
7.8 g ~0.018 mol) of the product obtained under a. are .-
Le A 29 518 - 52 -
heated at 100C ~or 3.5 hours in 45 ml of dimethyl~orm-
amide to~ether with 3.1 g (0.022 mol) of potassium
carbonate. The cooled mixture i~ added to ice water and
this mixture i8 then extracted with methylene chlorlde
and~the solvent removed on a rotary evaporator. The crude
product is purified by column chromatography (silica gel,
meth~le~e chloride/methanol 96/4).
Yield: 4.7 g (64% o~ theory)
Melting point: 225-227C
200 mg (0.49 mmol) o~ the e~ter obtained under b. are
heated at 80C for two hours in a mixture con~i~ting of
1 ml of acetic acid, 1 ml of water and 0.1 ml of co~cen-
trated sulphuric acid. After cooling, the mixture i8 ..
neutralized with dilute sodium hydroxide solution and-
then extraated with methylene chloride a~d the solvent
removed on a rotary evaporator.
Yield: 67 mg (37% of theory)
Melting poi~t: 210-214C (with decompo~ition)
Example_XXI
6,7,8-Tri1uoro-1~4-dibydr~-4-~xo-1-~4~ -1,2,3-triazol-
l-yl-s~ rl)-phenyll-3-q!linolinecarboxylic acid
- .,...,~
'`.:`~
Le A 29 518 - 53 - - -
`` ` 2 ~ 2 :1
o o
F
F ~3
N
: a. Ethyl 2-(2,3,4,5-tetrafluorobQnzoyl)-3~4-(lH-1,2,3
~ triazol-l-yl-methyl)-phenylamino]-acrylate
: 12 g ~0.069 mol) of 1-(4-aminob~zyl)-lH-1~2,3-triazole
are initially i~troduced into 120 ml of ethanol and
5~ 22.0 g (0.069 mol) of et~yl 3-ethoxy-2-(2,3,4,5-tatra-
luoroben~oyl)-acrylate i~ 30 ml o~ methylene chlorlda
::
~: are added at room t~mperature. The ~ixture ia 8ub8e-
::~ : que~tly stirred ~t room temper~ture for 12 hours and the
solvent is then removed i~ vacuo~
Crude yiald: 30.8 g
b. Ethyl 6,7,8-Trifluoro-l,~-dihydro-4-oxo-1-t4-(1~-
: 1,2,3-triazol-1-y}-methyl)-pha~yll-3-qui~olinecarboxylate
30.8 g (0.069 mol) of th~ produet obtained under a. are
heated at 90C for three hours ~n 130 ml of dim~thylform-
5 ~ amide together with 4.4 g (0.076 mol) of potassiu~
fluoride. The ~olvent i8 then removed in vacuo and the
_
~' : '
2 ~
residue i8 taken up in methylene chloride/water. Afterback extraction, the combined organic pha~es are wa~hed
with water until neutral and then driad over sodium
sulphate. After removing the solvent in vacuo, the
residue i8 stirred up w~th ethyl acetate/i~ooctane 1:1.
Yield: 23.2 g (79~ o~ theory)
Melting point: 170-172C
23.2 g (54.2 mmol) of the ester obtained under b. are
stirred at room temiperature overnight together with 1.3 g
(54.2 mmol) of lithium hydroxide in a mixture con~isting
of 240 ml of tetrahydrofuran, 100 ml of dimethylformamlde
`.~- and 12 ml o~ water. After rOEmoving the solvents in vacuo,
the crudo product is puri~ied by column chrom~tograph~
( 8il iaa gel, methylene chloride/methanol 95/5).
Yield: 10.7 g (89% of theory)
Melting point: 170-172C
,
,~:
,`
LQ A 29 518 - 55 -
Example XXII
8-Chloro-6,7-difluoro-l,4-dihydro-4-oxo-l-14-~l~-l,2,3-
triazol-l-yl-methyl)-p~en~ll-3-quinolinecarbo~ylic acid
O O
F ~ OH
F--~/~N :
Cl f~ .
: ~ N - N~N
a. Ethyl 2-(3-chloro-2,4,5-trifluorobenzoyl)-3-~4-(lH-
~; : 5 1,2,3-triazol-l-yl-methyl)-phenylzmino]-acrylate
8 g (0.046 mol~ o~ l-(4-aminobenzyl)-lH-1,2,3-triazole
ar~ initially i~troducsd into 80 ml o ethanol a~d 14.7 g
~; (0.046 mol) of ethyl 3-ethoxy-2-~3-c~loro-2,4,5-tri-
luorobenzolyl~-acrylate in 4 ml of methylene chloride :~
10~ are th~n added at room temp~rature. The mixture i~
ub~eque~tly stirred ~t room t~mp~rature ~or l~ hours and m~ ~
the ~ol~e3t i8 then removed in vacuo. ~ ;
Crude yield: 2I.3 g ~.
b. ~thyl 8-chloro-6,7-di~luoro-1,4-dihydro-4-oxo-l-t4-
; :15~ 1,2,3-triazol-1-yl-methyl)-phenyl]-3-gui~oline-
carboxylate
~,
Le A 29 518 - 56 - ~-
., ,
2 l
21.3 g (0.069 mol) o~ the product obtained under a. are
heated at 90C for three hours in 90 ml of dimethylform-
amide together with 4.9 g (0.050 mol) of potassium
fluoride. The ~olvent i~ ramoved in vacuo and the residue
i8 the~ tak~n up in m~thylene chloride/water. A~tar back
extraction, the comb~ned organic phase~ are wa~hed with
water until neutral and then dried over ~odium sulphate.
The ~olvent is removed in vacuo.
Yield: 12.2 g (60% o theory)
Melting point: 120-122C
12.0 g (27 mmol) of the ester obtained under b. Pre
-~ stirred at room temperature for three days together with
700 mg (27 mmol) of lithium hydroxide in a mixture con-
sisti~g o~ 120 ml o~ tetrahydro~uran, 50 ml of dimethyl--
formamide and 6 ml o~ water. After removing the solvent~
in vacuo, the crude product is purified by column chro-
matography (silica gel, me~hylano chloride/methaQol/
acatic aaid 10/1/0.5).
Yi~ld: 9.4 g (84% of theory~
Melting point: 201-203C
::
~ A 29 518 - 57 -
. .
.,
0 2 1
Example XXIII
8-~hloro-6,7-di~luoro-1,4-dihydro-4-oxo-1-[4-(2~-1,2,3-
triazol-2-yl-methyl)-phenyll-3-qui~oline~arbo~ylic acid
O O
F~ ~J~OH
11 11
F ~ N ~
: ~ N
: N ~:
The title compound was obtained in analogy with Example
XXII.
Melting point: ~250C
::,
:,
~: :: : :,. :
'
- .: -
Le A 29 518 - 58 - ~
S ~ 2 ~
... .
'~ Example XXIV
, ~
.~ 6,7,8-Trifluoro-1,4-dihydro-~-oxo-1-~4-(1~-1,2,4-triazol-
~, l-y~ ~hyl)-phenyll-3-quinoiineaarboxyli~ acid
,:,, O O
~JI~OH
F ,~1~
~J ,
? N,N :
:~ a. Ethyl 2-(2,3,4,5-tetrafluorobenzoyl)-3~t~-(lH-1,2,~-
: 5 triazol-l-yl-me hyl)-phQnylami~o]-acrylat~
3.9 g (0.022 mol) of 1-(4-aminob~nzyl)-1~-1,2,4-triazole
are initially introdllced into 40 ml of ethanol and 7 . 2 g
(0.022 mol) of ethyl 3-sthoxy-2-(2,3,4,5-tetrafluoro- .~
m` ~ b~zoyl)-acrylate in 2 ml o$ methylQ~e chlorid~ are th~n ~:
: ~10 ~ a:dded at room tamperature. The mixturQ i~ ~ub~e~uently -~
~ stirred a~ room ~amp~ratur2 for 12 ~ours and the solYent
: ~ ~` i8 :than remo~ed in ~acuo.
Crude yield:~ 10.0 g
b. ~th~l 6,7,8-trifluoro 1,4-dihydro-4-oxo-1-t4~
: 15 ~1,2,4-triazol-1-yl-methyl)-phenyl]-3-guinol~necarboxrlate
:
~,
Le A 29 518 - 59 -
~ .
~.
-~ 2115~27
:; `
i 10.0 g (0.022 mol) of the product obtained under a. are
,i heated at 90C for three hours in 50 ml of dimethyl~orm-
amide togethar with 1.4 g (0.025 mol) of potas~ium
~luoride. The solvent i~ r~moved in vacuo and the residue
5 ii~ then taken up in methylene chloride/water. After back
extraction, the combined organic phase~ are wa~hed with
water until neutral and then dried o~er isodium ~ulphate.
The crude product i~i puri~ied by column chromatography
(i8ilica g~l, methylene chloride/methanol 95:5).
Yield: 8.9 g (94% of theory)
Melting point: 160-162C ~-
8.9 g (20.8 mmol~ of the ester obtained under b. are
stirred at room temperature overnight together with
500 mg (20.8 mmol) of lithium hydroxide in a mi~ture
co~sisting of 90 ml of tetrahydrofuran, 4.5 ml of di- -
methylformi~mide and 4.5 ml o~ water. After removing the
~olvent in vacuo, the arude product i8 purified by column
chromatography (~ilica gel, methylene chlorids/methanol
ss/~
Yield: 6.2 g (75% of theory)
M~lting point: 256-258C
, ~ :
.... ,i : ~
:,
-
Le A 29 518 - 60 -
3 .~
. 2~1.5~2:1
~' '
pj Example XXV
8-C~loro-6,7-di~luoro-1,4-dihydro-4-o~o-1-[4-~1~-1,2,4-
1 triazol-1-yl-methyl)-ph~nyl]-3-quinolineaarbo~ylic acid
O O
: ~ :
:~ ~ N
~ N
The title ao~pou~d waa obtained in analogy with Example .
XXIV.
Melting point: 232-234C
i .
. .
.
,
;, ~
:' ~ , - :
Le A 29 518 - 61 -
.
2 1
Example XXVI
,
N-Isopropyl-l6,~-difl~oro-1,4-dihydro-4-o~o-1-[4-(lH-
1~2,3-triazcl-1-yl-methyl)-phe~yl]-3-quinolinecarbox-
~1; ~m;de]
~ O O
F~H ~
,,- ~
` " .
~ N - N ~ : ~
2.67 g (7.0 mmol) of 6,7-difluoro-1,4-dihydro-4-oxo-1-t4-
(1~-1,2,3-triazol-1-yl-methylJ-phenyl]-3-~ui~oline-
carboxylic acid are sus~ended in 25 ml of dry tolue~e a~d
the ~u~p~n~io~ i8 boiled for drying on a water separator.
Subseguently, 0.55 ml (7.5 mmol~ of thionyl chloride is
added to the cooled sUspension, which is then boiled
until gas e~olut~o~ ceas~s; 1.3 ml (15 m~ol~ of iso~ro-
pylamine are then add~d at room temperature and boili~g
i8 ~ub~equently continued for a ~urther two hours. For
-:' the working upj the cooled solution i8 stirred i~to ~:~
: 15 lN hydrochloric acid and the~ extracted with methylene
chloride. The combined organic p~a~as are washed with
saturated ~odium hydroge~ carbonate ~olu~icn a~d satura-
ted sodium chloride 801utio~. Following drying over
Le A 29 518 - 62 -
.~
i!
~. sodium sulphate, the solution i~ evaporated on a rotary
evaporator and ~urification i~ then carried out using
column chromatography (siliaa gel, methylene chloride/
! methanol 9/1).
Yield: 0.7 g (24~ of theory)
Melting point: 223-225C
Example XXVII
N~N-Dii~opropyl-[6,7-di~luoro-1,4-dihydro-4-oxo-1-14-(I~-
1,2,3-triazol-1-yl-~thyl)-ph~nyl]-3-quinolinecarbox-
amide]
~ .
;. o o
F~
- N~N
0.28 ml (2.7 mmol) of triethylamine i8 added at 0~ to
O.9S g (2.5 mmol~ of 6,7-difluoro-1,4-di~ydro-4-oxo-1-14-
'-3 ~ (1~-1,2,3-triazol 1-yl-~sthyl~-phe~yl]-3-quinoli~-
carboxylic acid in 10 ml of acetone. Sub~eguently,
0.38 ~1 (2,7 mmol) of ethyl chloroformate is added drop-
Wi8~ at this temperature within the space of 30 minutes.
After adding 0.38 ml (2.7 mmol) o diisopropylamine, the
: .,
Le A 29 518 - 63 -
2 1
. . .
mixture i8 boiled under reflux ~or one hour. For the
working up, the ~ixture i8 added to saturated sodium
hydrogen carbonate solution and this mixture is then
.. extracted with ethyl aaetate. The combined organic phases
S are wa~hed with saturated ~odium chloride solution and
, dried over sodium sulphate.
Yield: 1.1 g (95% of theory)
Melting point: 85-87C
The compound~ listed in Table I are obtained in analogy
with Example XXVII: :
` Table I:
o o
X~R6 ~.
~3N~ ~N
:
Ex. No. R6 ~.p.tC]
XXVIII -NHC(CH~0~)2C~3 171-173
;~ XXIX -N~C~2~ (0~) OE3 75_77
' lS XXX -N~CH2C~20~ 90-92
XXXI -N~CH2CH~OC~3 156-158
-
~,
Le A 29 518 - 64 -
. J
2 ~
... .
Example XXXII
~.
Ethyl 7-chloro-1,4-dihydro-4-oxo~ 4-(lH-1,2,4-triazol-
l-yl-m~thyl)phenyl]-1,8-naphthyridine-3-aarboxylate
O
~COOCzHs
,N ;~:
N
a. Diethyl (~,6-dichloronicotinoyl)malonate
,~ .
~: 5 7.2I~g (0.075 mol3 o~ m~gns~iu~ chloride are initially
introduced at 0C into 75 ml of absolute acstonitrile and
: 12.12 g (0.075 mol) o~ di~thyl malonate are added drop-
wise~while eooling in ac ice bath~ Subsequently, 15.34 g
(0.150 mol) of triethylami~e are addad dropwisa at 0C,
; : ~ 10 as are, a~ter subsequantly stirr~ng ~or 50 mlnu~es,
17.0 g~ (0.075 mol) of 2,6-dichloronicotinoyl chloride
(Hsl~etica Chimica Acta 59, 222 (1976)); the mlxture is
' the~ stirred o~ernight while being hQa ed to roo~ tem-
perature. 80 ml o~ 18% ~tr~n~th hydrochlori~ acid are
~;~ :: : 15 subaequently added and the mlxture i~ extracted with
met~y}~tert-butyl ether, followed by drying over sodium
sulphate and concentratio~ in vacuo. --
Le A 29 518 - 65 -
r~ ~ 1 ;1 5 ~
~,
b. Ethyl ~2,6-dichloronicotinoyl)acetate
,
The crude diethyl (2,6-dichloronicotinoyl)-malonate i~
heated to reflux for 2 hours in 45 ml o water containlng
90 mg o~ p-toluene~ulphonic acid. The mixture i~ extrac-
ted with methylene chloride, followed by washing withwater, drying over 30dium sulphate and concentration in
vacuo. The crude product is purified on silica gel
(eluent, dichloromethane).
Yield: 14.2 g (72% of theory over two steps) .
c. ~thyl 2-(2,6-dichloronicotinoyl)-3-ethoxyacrylate ~
43 g (0.162 mol) of the product ~rom b. are heated at ~.
150-160C for two hours in 38.1 g (0.26 mol) of ethyl
orthoformate and 42.4 g (0.42 mol) of ace~ic aDhydride. - ~ :
All the readlly ~olatile constituent~ are distilled o~f -~
under ~igh vacuum at a bath temperature of up to 100C
and the crude product i~ used directly ~or ~urther
reaction.
Crude yield: 50.~ g -
d. Ethyl 2-(2,5-dichloronicotinoyl)-3-[4~ -1,2,4-
triazol-1-yl-methyl)ph~nylamino]-acrylate
, ,~
- 7 g (0.022 mol) of the product obtained under c. and ~.
3.8 g (0.022 mol) of 1-(4-aminobenzyl) lH-1,2,g-triazole
are stirred at room tem~erature o~arnight in 40 ml of ~ :~
etha~ol. The 801Yent i8 removed in ~acuo.
Yi~ld: 9.7 g of ~rude product .-
'~
Le A 29 518 - 56 - -
~ 21 1 ~0~1
, . .
, .,~.
26.0 g (O.OS8 mol) of the product obtained in d. are
heated at 80C for four hours in 140 ml of dimethylform-
amide together with 9.5 g ~0.066 mol) of potasnium
aarbonate. The cooled mixture i~ added to ice water and
the precipltated product is isolat~d and dried at about
100C.
Yield: 18.7 g (78~o of theory)
M.p.: 253-256C
,. ..
: .
,
"
.
~ .
'
i~
Le A 29 51B - 67 -
2 ~
, .
''I
., Pr~parati~
Example l:
! ~ .
'`i
hyl 6-fluoro-7-14-(4-~luorophff~yl)-piperazLn-1-yl]-1,4-
i~ dihydro-4-oxo-1-~4-~1~-1,2,3-triazol-1-yl-methyl)-
; 5 phenyl]-3-guinolinecarbo~ylate
i o
F~ ~,COOEt
FJ3~ J
, N
~=/ .
490 mg (1.2 mmol) of ethyl 6,7-di~luoro-1,4-dihydro-4-
oxo-1-14-(1~-1,2,3-triazol-1-yl-methyl)-phsnyl]-3-quino-
linecarboxylate ar3 heated at 1~0C for eight hours in
12 ml o~ N-methylpyrrolidona together with 650 mg
(3.6 m~ol) o~ N-(4-fluorophe~yl)-piperazina. All the
~olatile aompone~ta ar~ removed u~d~r high vacuum and the
re idue i~ stirred up with iso-propanol a~d dried at
about 100C.
Yield: 580 mg (84% of theory)
M-lti~g poi~t: 128-130C
'
Le A 29 518 - 68 ~
1 5 ~ 2 1
;~ Example 2:
.~
~th~l 6- luoro-1,4-di~ydro-4-~xo-7-(3-phanylpiperazin-
l-yl3-1-[4-(1~-1,2,3-triazQl-l-yl-methyl)-phenyl]-3-
1 qui~oli~eaarbo~ylata
F~COOEt
S The title compound i8 obtained in a~alogy with Example
~ 1 by reaating with 3-phanylpipera~ine after 3tirring up
: i~ acetonitrile.
Nelting point: 219-221C
: .-r~
Le A 29 518 - 69 -
- ~ ' . :,
'
~c ;; 2 1 1 ~ 0 2 1
.
ExamPle 3:
6-Fluoro-1,4~dihydro-~-o~o-7-(4-phenylpiperazin-1-yl)-
l-t4-(1~-1,2,3-triazol-1-yl-~ethyl)-phenyl]-3-
quinolinecarboxylic acid
o
F ~,COOH
~ N ~J ¢~
;'~
286 mg (0.75 mmol) o~ 6,7-difluoro-1,4-dihydro-4-oxo-1-
l4-(1~-1,2,3-triazol-1-yl-methyl)-phenyl]-3-quinoline-
~arboxylic acid and 364 mg (2.25 mmol) of N-phen~l-
piperazine are heated at 120C for one hour in 7.5 ml
of N-methylpyrrolidone. All the volatile constituent~
are remo~ed u~der high vacuum a~d the ra~idue i3
stirred up with aceton~trile and dri0d at about 100C.
Yield: 150 m!~ (38% o~ theory)
~el~ing point: ~290C
t'.l,` '~,
'- Ths compounds li~ted in Table 1 are prepared in analogy
;; 15 with the dlr~ctions in Example 3:
,
" -
' -"
Le A 29 518 - 70 -
;~
~ ` ~ 211~J21
:`.
~, Table 1:
:.~ o
i' F~COOH
R , N ~J
Ex. No. Reaction cond. I~:olated from M.p. ~C] R8
~ F ~
4 1 h/ 140 C Is~prop 22S - 227
: ~ 5 1 hl120-C Is~prop >280 ~OEt
6 16h lRT Acetonitrile ~ 280 <~
::: ~ ~< -'
7 1 h /100 C Acetonitrile 138-140
8 1 h/100-C Acetonitrile >280
~ ~ MeO ~ =~
9 ;: 3 h l60-C DMF/wate~ 236 - 238 '~ . ~
:, ; ~ -
~: ~N
1 h 1120'C Isc~p~op 217-219 <
. ! , : ~ .
'~
Le A 29 518 - 71 - ~
.
-
2:l~5~2~
ExamE~ e 11
6-Fluoro-l,~-dihy~ro-4-oxo-7-(3-ph~nylpipsrazin-1-yl)-
1-[4-(1~-1,2,3-triazol-l-yl-~athyl)-phsnyl]-3-
;~, qui~oli~eaarbo~ylic acid
; ! O
~CC OH
) ~1N~
286 mg (0.75 mmol) o~ 6,7-difluoro-1,4-dihydro-4-oxo-1-
4-(1~-1,2,3-triazol-l-yl-methyl)-phenyl]-3-quinoli~e-
carboxylic acid a~d 365 mg ~2.25 ~mol) o~ 3-phenyl- -
pip~razi~ are h~ated at 120C for o~e hour i~ 7.5 ml
o~ N-methylpyrrolidone. All th~ volatile compo~ents are
removed in va~uo and the residue i8 ~tirr~d u~ with
: iso-propanol and dr~ed at about 100C.
~ ~ Yield: 210 m~ ~53% of thaory) :-
- ~ M~lt1ng po~nt: 163-165C -
Le A 29 518 - 72 -
: .'
' . '
' `~.
~: .
"
~ Example 12
~;i 6-Fluoro-1,4-dihydro-4-~xo-7-[4-(4-hydro~yphe~yl3-
piperazin-1-yl]-1-~4-(1~-1,2,3-triazol-1-yl-methyl)-
phen~l]-3-qui~olina~arbo~ylic acid
o
~ COOH
HOJ~ ¢~
:`: N'' ~N
~=/ .
286 ms (0.75 m~ol) o~ 6,7-d~fluoro-1,4-dihydro-4-oxo-1-
~4-(1~-1,2,3-triazol-1-yl-methyl)-phe~yl]-3-quinoline-
carboxyli~ acid ~re ~eated at 120C for two hours in
7.5 ml o~ N-methylpyrrolidons together ~ith 290 mg
(O.86 mmol) o~ N-(4-hydroxyphe~yl)-piperazlne and 385
mg (3.4 mmol) of dlazabicyclo~2.2.2]octana. All the
:: volatile components are rQmovad u~der high vacuum and
th~ residue i8 stirred up w~t watqr and driad at about ~ :
100C. :
Yield: 390 m~ (96% o~ theory) ~:
1,.... ,.-~
: 15 Melting point: 269-271C
~: The compou~ds listed i~ Table 2 are prepared in analogy ~ :~
with th~ direction~ in ~xample 12:
Le A 29 518 - 73 -
",.~ a 2 l
.i Table 2:
., o
,1 F~3,COOH
R~
N' ~`N
Ex. No. Isolated from M.p. CC] R8
13 Is~prop 248-250 SC8Hs
14 Water 133-135 ~
c~ . ~:
Water 25~256 c
Le A 29 518 - 74 -
.
21
..;i
;;~ Exam~le 16
i ~thyl 6-~luoro-1,4-dihydro-4-o~o-7-(4-phenylpiperazin-
yl)-1-[4-(1~-1,2,3-triazol-1-yl-~ethyl)-phQnyl~-1,8-
naphthyridi~e-3-carb~xylate
F ~COOEt
~N~J
, N'
~=/
. .
~ 5 0.5 g (1.2 mmol) of ethyl 7-~hloro-6-fluoro-1,4-
: dihydro-4-oxo-1-t4-(lH-1,2,3-triazol-1-yl-msthyl)-
phe~yll-1,8-naphthyridine-3-carboxylate are hsated at
120~ for our hours in 12 ml of dimethyl sulphoxide
together wit~ 190 ~g (1.2 mmol) o~ N-phenylplp~razine
~; 10 and 460 mg (4.1 ~mol) of diazabicyclot2.2.2]octa~e. All
the ~olatile componsnts are r~mo~ed u~der high Ya~Uum
a~d the residue i~ ~tirr~d up with water and dried at
about 100~
Y~eld: 430 mg (66% of theory)
Melting point: 201-203C
The compound~ listed in Table 3 aro prepared in analogy
: w1th the directions iD Example 16:
: he A 29 518 - 75 -
. . . .. . ... . ..... . .. . . . . . ..
0 2 1
.,,~ ' .
~ ' .
~ Tabl e 3:
~'' O
i~ ~;~COOEt
I ~ N~N
Ex. No.M.p. ~C] Ra ~:
17 180-183 F ~
8 115-117 C~ :
9 86-89 - ~
Cl ~
-
:
i
~e A 29 518 - 76 -
, : ,
~ 2 ~ 0 ~ 1
Y
Example 20
i ~-Fluoro-1,4-dihydro-4-o~o-7-(4-phenylpiperazin-1-yl)-
-1,2,3-triazol-1-yl-~eth~l)-phenyl]-1,8-
naphthyridine-3-carbo~ylic acid
o
F X~3~COOH
~N N
[3,1~, ~
N
300 mg (0.5 mmol~ o~ the ester ~rom Example 16 are
heated at 50~C ~or 1~5 hours together with 1 ml of 1 N ::
sodium hydroxide solution in a mixture consi~ting o~ 5
:~ ml of athanol and S ml o~ water. Thi~ mixture i~ then
nautralized with dilut~ ~ydrochloric acid and the
solve~ts are ramoved in vacuo. The ros~due i~ purif~ed ~-
by colum~ chroma~ography (silica gel, methyle~e .
chloride/methanol 9/1).
Yiold: 240 mg (85% of theory)
, Mal~ing pol~t: 279-281~ (with decompo~ition)
~The compounds listed in Tabl~ 4 are prepared in analogy
~; ~ with the dir~ctions i~ Ex2mple 20:
: :
Le A 29 51a - 77 -
~:
2~
.j Table 4:
O
F~COOH
R"N~J ~1
~N
~ -~
¦: Ex. No. M.p. ~C] Ra
21 225-227(decomp.) F~
22 238-240 (decomp.) c
~; 23 200-204(decomp.)
cl
,~s`: : ~
:
~ ?~
,~ : , .
-
:~
Le A 29 518 - 78 -
' ':
~ 21~S~21
Example 24
~thyl 6-fluoro-1,4-di~ydro-4-o~o-7-(4-phe~ylpiperazin-
1-yl)-1-l4-(2~-1,2,3-triazol-2-yl-methyl)-phenyl]-3-
guinoli~e-carbo~ylate
~ o
F ~3~COOEt
;
N
0.5 g (1.2 ~mol) of ethyl 6,7-difluoro-1,4-dihydro-4- :
oxo-l-~4-(2H-1,2,3-triazol-2-yl-methyl)-phenyl]-3-
guinolinecarboxylate are heated at 120C ~or three
hours in 12 ml o~ di~ethyl sulphoxide together with 190
~g (1.2 mmol) o~ N-phenylpipera~ine and 460 mg (4.1
mmol~ of diazabicylco~2.2.2]octa~e. All the ~olatil~
~omponents are removed under high vacuum a~d the
r2~idue i~ stirrad up with water and dried at about
~: 100C.
;~ Yield: 580 mg (80% of theory)
-; 15 Melting point: 230-232C .:
The compound~ ted in Table 5 are prepared in analogy
~: w1th the directions in E~ample 24:
:
:
Le A 29 518 - 79 -
:' "
~ `~
~11S~21
;:i Table 5:
.~ o
F~3~CoO~t
~ R, ' J ¢~
~ ~ NN'3
Ex. No. M.p. LC~ RB
~; ~5 æ7-229 F
~: ~ 26 187-189 a~
~7 189-190
. .
^.. J ~ :
:~ :
`:
Le A 29 518 - 80
~ -
-
: 211~i~21
Example 28
6-Fluoro-1,4-dihydro-4-oxo-7-(4-phe~ylpiperazin-1-yl)-
1-[4-(2~-1,2~3-triazol-2 yl-methyl)-phQnyll-3-
quinoline-carboxylic acid
o
F ~,COOII
¢~
: '~
: ~: N
0.5 g (0.9 mmol~ o~ the estar obtained in Example 20
are heated at 50C for l.S hours together with 1.8 ml
of 1 N sodium hydroxide solution in a mixture
con~i~ting o~ 9 ml of ethanol and 9 ml of water. This
mixture is then neutralized with dilute hydroohloria
aci:d and the 801vent~ are r~moved in vacuo. The resid~e
i8 purified by column chromatography (silica gel,
methylene chloride/~ethanol 9/1).
: : Yield: 0.39 g ~82% o~ theory)
M-lting polnt: 208-210C
15 The cQmpounds li~ted in Table 6 are prepared in analogy
: : with the dire~tions in Example 28
'
~. .
,
Le A 29 518 - 81 -
~:s
c~
2~1S~
,'. Table 6:
~' o
F~3,COOH
N '=~
N
Ex. No. M.p. ~C] R~
29 247-249 F
246-248 Cl~
;~ ~ 31 197-201 .
Cl
.
; ~ .
" ~
,~ ~ :
Le A 29 518 - 82 - -
~ d
21~21
Exampl e 3 2
~hyl 6-fluoro-1,4-dihydro-4-o~o-7-(4-phenylpiperazi~-
l-yl)-1-14-~lE-1,2,4-triazol-1-yl-~ethyl)-phenyl]-3-
quinoline-carbo~ylate
i, o
F~,COOEI
~N ~J l?
N
N
O.S g (lt2 mmol~ of ethyl 6,7-difluoro-1,4-dihydro-4-
oxo~ 4-(1~-1,2,4-triazol-1-yl-methyl)-phenyl~-3-
quinoli~-carboxylate are ~eated at 120C for three
: ~ hour~ i~ 12 ml o~ dimethyl sulphoxide together with
190 mg (1.2 mmol) o~ N-p~auylpipera~ine and 460 ~g ~4.1
mmol) of dlazabiayclo[2.2.2]octane. All the volatile
compo~ents are then r~moved undsr high vacuum a~d the
~: rasidue i8 ~tirred Up with water and dried at about
100C.
Yield: 600 mg (89% of theory)
Melting poi~t: 217-218C
The compound~ ted in Table 7 are prepared i~ analogy -
with the directions in Example 32: ~ ~
, . .
Le A 29 518 - 83 - ~
!:. ~' ' `' ~
2 1
. . .
Table 7:
F~3,COOEt
' R" ' J ~q
'.: y;~
.. j ,
'' ~N' ~>
I= N
]
13x. No. M.p. [C] R8
:
33 72-74 F~
34 98-100 Cl~
110-112
36 128-1 30
e ~ F,C
\
37 1 26-1 28 Cl ~\
F3C
38 Z21-225 ~ .
~ ~ ~J ~ ~
.
- `:
Le A 29 518 - 84 ~
2 ~ 0 2 1
Exam~le 39
.i:
6-Fluoro-1,4-dihydro-4-oxo-7-(4-ph~nylpiperazi~-1-yl)-
l-t4-(1~-1,2,4-triazol-1-yl-methyl)ph~nyl] 3-qui~oline-
, carbogyli~ acid
,~ O
F~13~COOH
~N N
~N~J
~ N' ~>
~=N
0.5 g (0.9 mmol) of the ester obtained in Example 32
are heat~d at 50C ~or 1.5 hour~ togather with 1.8 ml
of 1 N sodium hydroxide 801ution in a mlxture
consisting o~ 9 ~1 o~ ethanol and 9 ml of wa~er. Thi8 :
mixture i~ than ~eutralized with dilute hydrochloric ::
acid a~d th~ 801ve~t8 are removed i~ va~uo. Th~ resid~e -~
i8 purifi~d by ~olum~ c~romatography (~ a gel,
: methyle~a ~hloride/~etha~ol 9/1)~
Yiald: 0.46 g (82~ of theory)
: ~elti~g poi~t: 234-236C
: 15 The ~ompou~ds listed in Table 8 are prepared i~ a~alogy
with th~ directions in Example 39:
~ . -
Le A 29 518 - 85 - :
; ~
211~021
Tabl e 8:
.~, o
~i F ~COOH
R~ ' J
N ~ N~
Ex. ~o. M.p. [Cl R8
246-248 F
; 41 244 246 cl~
42 179-181 c~
~: :
43 264-268 ~=~
F,C
44 23g-241 Cl~'
F3C~
198-202
'~
I~-- A29 518 86 - ~
f ~
0 2 1
i`;
i~ Examp l e 4 6
: :~
~thyl 6-fluoro-1,~-dihydro-4-o~o-7-(4-phenylpiperazin-
l-yl)-1-[4-(1~-1~2,4-triazol-1-yl-methyl)ph~nyl3-1,8-
i ~aphthyridi~e-3-carbo~ylate
o
F~ ~,,COOEI
;
~` N~
: ~ ~ N ~:~
0.5 g (1.2 m~ol) o~ ethyl 7-chloro-6-fluoro-1,4-
dihydro-4-oxo-1-t4-(lH-1,2,4-triazol-1-yl-methyl)-
phenyl]-1,8-naphthyridine-3-carboxylate ar~ heated at :~
120C for 8ix hour~ in 12 nl of d~m~thyl sulphoxide
` ~ together with 190 mg (1.2 mmol) of N-phe~ylpiperazine~::
}O and 460 mg (4.1 mmol) of diazabicyclo[2.2.2]octa~e. All
the volatile compon~nts are r~mo~ed u~der high vacuum.
~ ; The residue i3 ~tirred up with water a~d purified by
::~ colu~n chro~atography (silica gel, methyle~e
chlor~de/methanol 96/4).
Yield: 500 m~ (78% of theory)
Malt~ng point: 179-180C
ThQ com2ounds listed in Table 9 are prepared in analogy ~ ;
with the directions in Example 46~
~-
.
.
Le A 29 518 - 87 -
: :
2 1
., ,i ,
i, Table 9~
r ~N ~
~>
: ::
Ex. No. ~.p. [C] R8 ~:
,~
201-203 F
48 183-185 Cl~
49 170-172
a i--
I
: ~; : i ~ :
~ I.e A 29 :518 - 88 -
:~ :
: :
.
:: : :
2 1
Example 50
6-Fluo~o-1,4-dihydro-4-oxo-7-(4-phe~ylpiperazi~-1-yl)-
1-[4-(1~-1,2,4-triazol-1-yl-~ethyl)phenyl]-1,8-
nap~th~ridi~a-3-carboxylic acid
.~ o
F~COOH
,;1 ~` N ~ N N : :.
0.45 g (0.8 mmol) of the ester from ~xample 46 i8
heated at 50C ~or 1.5 hours togathe~ wit~ 1.6 ml of
1 N sodium hydroxid~ 801ution in a mixture consisting
o~ 8 ml of ethanol and 8 ml of water. This mixture i8
t~n neutralized with dilute hydrochloric acid and the ::
solvents are remov~d in vacuo. Tha residue i8 puri~ied -~.
by aolumn chromatography (~ilica gel, methylene
chloride/meth~nol 9jl). ~
Yield: 0.39 mg ~90% o~ theory) ~ ;
Melt~g point: 234-238C (with decomposition)
The compounds li~ted in Table 10 are prepared in
analogy with the direction~ in Example S0:
'' .
:; ~
:: :
Le A 29 51B - 89 -
.. . . . . . . .
~ 2 ~ 2 1
Table 10
~Nl~
N>
EX. No. M.p. ~C~ R8 ~ ~
51 252-25~; F ~ ~.
52 212-214 Cl~ ..
S~ 217-220
Ls ~A 29 518 - 90 -
~ :
`
~1 ~ 5021
.~, .
~1 Example 54
i.,i
`A,~
~thyl 6-fluoro-1,4-dihydro-1-~4-(N-imida~olyl-
met~yl)p~e~l]-4-oxo-7-(4-phe~ylpiperazin-1-yl)-3-
guinoline-carbo~ylate
!~i F ~ COOE~
', N~
~=N
`':i - - -:
0.5 g (1.2 mmol) of ethyl 6,7-difluoro-1,4-dihydro-1-
t4-(N-imidazolyl-methyl)-phenyl]-4-oxo-3-
:~ quinolinecarboxylate i8 heated t 120C for fi~e hour~
in 12 ml of dimethyl sulphoxide together with 190 mg
(1.2 mmol) of N-phenylpiperazine and 460 ~g (4.1 mmol)
oE diazabicyclo~2.2.2]octan~. All thQ ~olatila
-~ compo~ent~ are removed und~r high vacuum and the
re~idua is stirred up wîth water aud dried at about
100C.
Yield: 580 mg (86~ of theory) ~
Melting point: 130-133C :~-
Th~ aom~ound~ listed in Table 11 are prepared in
analogy with the directio~s in Example 54~
-:
~e A 29 518 - 91 -
0 2 1
.;~ Table 11:
F ~3,COOEt
~N N
: Ex. No. M.p. [C] R8
55 :180-184 F~ .:~
56 187-188 cl~
57 155-tS9
::: ~: :: : ~ :
Le A 29 518 - 92 - ~
:: :
:
: ~ :
?; '
2 ~
;i!~ Example 5~
.i '.
6-Fluoro-1,4-dihydro-1-l4-(N-imidazolyl-~e~hyl)-
phanyll-4-o~o-7-(4-ph~nylpiperazin-1-yl)-3-quinoline-
carboxylic acid
o
FX~,C0011 ~:
13~N~J ~ ~
.~.~x~ N~,
S n.s g (0.9 m~ol) of the ester obtained in Example 55 i8
heatad at 50C for 1.5 hours together with 1.8 ml o~ :
: 1 N sodiu~ hydroxide solution in a mixture co~i~ting
~f 9 ml o~ etha~ol and 9 ml of water. Thi~ mixture i8
the~ neutralized with dilute hydrochloric acid and the
sol~ents are removed i~ vacuo. The residue is purified
: ~ by coIum~ ch~o~atography (silica gel, methylene
chloride/met~a~ol 9/1). :~
; Yield: 0.42 g (89% of theory)
Melting point: 248-251~
:: :
The compou~d~ ted in Table 12 arQ prepared in
a~alogy with the directio~s in Exæmple 58: -
Le A 29 518 - 93 - ~.
.... .~ , ... . .
2 1 1 ~ ~ 21
Table 12:
o
F ~3~COOH
F~a ,N~J
N~
Ex. No. M.p. tC] R8
59 20Z-205 (decomp.) F~
242-246 (decomp.) Cl~--
61 22~227 (decomp.)
:~ - c
: :` ~` : :
:
'
Le A 29 518 - 94 -
.
s ~ 2 1
Example 62
~thyl 6-fluoro-1,4-dihydro-4-oxo-7-(4-phenylpiperazin-
.1 1-yl)-1-13-(1~-1,2,3-triazol-1-yl-m~thyl1phe~yl~-3-
~i quinoline-carboxylate
i
,~COOEt
3~ N ~ ~ N
. 5 0.5 g (1.2 mmol) of ethyl 6,7-difluoro-1,4-dihydro-4-
oxo-1-~3-(1~-1,2,3-triazol-1-yl-methyl)-phenyl]-3- :-
quinolinecarboxylata i8 heated at 120e ~or three hours
in 12 ml o~ dim~thyl sulphoxide tog~ther with 190 mg
~: ~ (1.2 mmol) of N-phenylpiperazine and 460 mg (4.1 mmol)
o~ diazabiayclo~2.2.2]octane. All the volat~le
compone~t~ are ramoYed under high vacuum and the
residue i8 stirred up with wat~r a~d dried at about
100C.
Yield: 650 mg (97~ of theory) `~
Melting point: 170-172~
; The compounds li3ted in Table 13 are preparQd in
analogy with the directions in Exampla 62:
LQ A 29 518 - 95 -
-
G
2 ~ 1~021
~,N~3~ Tabl e 1 3:
F~,COOEI
.~J ~,S:~
Ex. No. ~-p- 1C] R8
63 117-119 F~
~, 64 128-130 Cl~
:~ 65 174-175
,~ ;
:: :
; ~ :
~ : '
:, :
~ -
-- .
Le A 29 518 - 96 -
. .,,, ::
. A ' . ', ,, ,. ~
0 2 1
;~i ExamRle 66
'~
6-Fluoro-1,4-dihydro-4-oxo-7-(4-ph~nylpiperazin-1-yl)
1-l3-(1~-1,2,3-triazol-1-yl-meth~l)phe~yl]-3-quinoli~e-
carbo~ylic acid
o
F ~COOH
13~N ~J ¢~ N~
,,, :
0.55 g (0.9 mmol) of the estsr obtained in ~xample 62
i~ heated at 50~C for 1.5 hour~ together with 1.8 ml of
: 1 N ~odium hydroxida solution in a mixture con~i~ting
o~ 9 ~1 o~ ~thanol and 9 ml of water. Thi~ mixture i~ -
the~ neutralized with dilute hydrochloric acid and the
solYents are remo~ed in vacuo. The residue i~ puri~ied
by columA chro~ato~raphy (silica gel, met~ylene
. . chloride/methanol 9/1).
Yisld: 0.31 g (6~% of theory)
Neltlng point: 243-245C -:
:~' 15 The compou~d~ listQd in Table 14 are prepared in
~~ analogy with the diractions in ~xample 66:
~':
: ~ ~
..
''..' ~- :~
Le A 29 518 - 97 -
~ ` -- 2 ~ 2 ~
~j Table 14 ~
~,.t O
F~13,COOH
, R"N~JN ~N~>
Ex. No. M.p. [C~ Rt
67 233-234 F ~
.,~ .,~, j:
68 Z50-253 Cl~
63 238-24
' ~; Cl
~: .
Le A 29 518 - 98 - ~
- ,,;
~ ~ ~71 2 ~
Exa~ple 7 0
~thyl 6-fluoro-1,4-dihydro-4-oxo-7-(4-phenylpiperazin-
l-yl)-1-C3-(2~-1,2,3-tria~ol~2-yl-methyl)phenyl]-3-
gui~oline-carboxylate
.~ ~ o
~ ~ ~ ,COOEt
[3~N J ~ N
~~ 5 0.5 g (1.2 mmol) of ethyl 6,7-difluoro-1,4-dihydro-4-
~ oxo-1-~3-(2~-1,2,3-triazol-2-yl-meth~l)phenyl]-3-
I qui~olinecarboxylate i8 heated at 120C for three hour~
in 12 ml of dimethyl sulphoxide together with 190 mg
(1.2 mmol) o~ N-pheAylpipera2ine and 460 my (4.1 mmol3
of diazabicyclo~2.2.~]octane. All the volatile
components are removed under high ~acuum a~d the
residue i8 3tirred up with water and dried at about
~: 100C.
Yield: 59G mg (89% o~ theory)
. 15 Mel~ing point: 112-115~ ~:
The compounds listed in Table 15 are prepared in
analogy with the direc~ion~ in Example 70:
: ..
Le A 29 518 - 99 -
;:` ~
~` 2 t 1~021
.i!,;~
Table 15:
,~,.,~. o
b ~N~
Ex. No. M.p. lC~ R9
71 139-142
,~ 72 108-110 c
73 110-112
1~; cl ,
s
~ '
:
.
Le A 29 518 - 100 -
.
,?,
-'1
:li Example 74
,:.
;, 6-Fluoro-1,4-dihydro-4-o~o-7-(4-phenyl~iperazin-1-yl)-
3-(2~-1,2,3-triazol-2-yl-methyl)phenyl]-3-quinoline-
car~o~ylic acid
:: o
F~,COOH
3~N~J [~ N
~ 5 0.5 g (0.9 mmol) of the e~ter obtained in Example 70 i8
: heated at 50C for 1.5 hours togethar with 1.8 ml of
1 N sodium hydroxide solution in a mixture consi~ting
of 9 ml of et~anol and 9 ml of water. This mixture i8
then neutralized with dilute hydrochlo~ic acid a~d the
solvents are removed in ~acuo. The re~idue i~ purified
.: by colum~ chromatography (sili~a gel, methylene
chloride/metha~ol 9/1).
Yield: 0.2 g (42* of theory)
~: Melting point: 233-235C
The compou~ds listed in Table 16 are prepared in
_~ analogy with the direction~ in Exzmple 74:
.,."".~
.
: ~ ' ',
Le A 29 518 - lO1 -
''~Sl~
0 2 1
Table 16:
?j F~3,COOH
, N J ~ N ~
~! Ex. No. M.p. [C] R8
~` 75 208-210 F~
76 2~;9-263 Cl~
'::
~ 77 202-208 ~
~ ,, Cl
:~ :
~:, , ' , '
: : .
::
Le A 29 518 - 102 -
~ 113021
' Example 78
., .
~thyl 6-fluoro-1,4-dihydro-4-o~o-7-(4-phe~ylpiperazin-
yl)-1-[3-(1~-1,2,4-triazol-1-yl methyl)phenyl]-3-
. qui~oli~e-car~o~ylate
o
N ~
N
,. 5 0.5 g (1.2 mmol) of ethyl 6,7-difluoro-1,4-dihydro-4-
:~ OXG-1- [3~ ,1, 2,4-triazol-1-yl-methyl)-phenyl]-3-
quinolinecarboxylate i~ heated at 120C for 8iX hour~
in 12 ml of dimethyl sulphoxide together with 190 mg
; (1.2 mmol) o~ N-phe~ylpiperazina and 460 mg (4.1 mmol) :~
of diazabicyclo~2.2.2]oata~e. All the volatil~
components are re~oved und~r high vacuum a~d the
residue i~ ~tirred up with water a~d dried at about
100C.
Yield: 600 mg (89% of thaory)
~eltlng point: 160-162C
~ The compounds list~d in Tab1Q 17 are prepared i~
: ~ analogy with the directions in Ex ~ le 78:
~: :
.
~, .
Le A 29 518 - 103 -
. . . .. .. .
~`i ` ---` ` ~1 ~ 5 ~21
,
,~. Table 17:
o
F~3,COOEt
.~ ~8 ' ~J [~ N'`~N
:::
:
Ex. No. M.p. [C] R8
79 160-162 F~
158-160 c
81 1 341
cl . '
- ::
:: :: : :
. : ~:
: :
~ ~:
:: '
Le ~ 29 518 - 104 -
`.
2 ~ ) 2 -I
,, .
$ Example a2
, 6-Fluoro-1,4-dihydro-4-oxo-7- (4-p~e~ylpiperazi~-1-yl)-
:~, 1-C3-(1~-1,2,4-triazol-1-yl-~ethyl~phenyl~-3-qui~oline-
carboxylic acid
o
F X~COOH
' ~ [~ N ~J 1$~ N ~\ ~
:!5 0.5 g (0.9 mmol) of the ester obtained in Example 78 is
-~ heated at 50C for 1.5 houxs together with 1.8 ml of
1 N sodium hydroxide solution in a mixture consisting :
of 9 ml of ethanol a~d 9 ml of water. Thi~ ~ixture i8
the~ nautralized with dilute hydrochlori~ acid and the
~olve~ts are removed in vacuo. The residue i8 purified : -~
~ by column ¢hromatography (sili~a g~l, methylene
: chloride/methanol 9/1).
Yield: 0.44 g (93% of theory)
:~: Melti~g point: 256-260~C
Tha compounds listed in Table 18 are prepared in
analogy with tke direction~ in Example 82:
'~ ,. '~'
:
~e A 29 51B - 105 -
., ~
`~` ~
;2~
Ta~ble 18:
F ~3,COOH
R8 ' J ~,NN ~N
; ~x. No. M.p. lC] R8
83 202-206 F
84 264-268 cl
i~
,.".~
~ ~ 85 221-225 c
:
'
;~ ~ ,
~: ' ~ "'''. ~ . .
~ ` -:
:
~ ' ~
L~ A 29 518 - 106 - -:
0 2 1
~ Example 86
.,.~.
~thyl 6-fluoro-1,4-dihydro-4-oxo-7-(4-phe~ylpip~ra~in~
yl)-1-[3-(1~-1,2,4-triazol-1-yl-methyl)phenyl]-1,8-
naphthyridi~e-3-carbaxylate
o -:
F~COOE:
0.5 g (1.2 mmol) of ethyl 7-chloro-6-fluoro-1,4-
dihydro-4-oxo~ 3-(1~-1,2,4-triazol-1-yl-methyl)-
~:~ phenyl]-1,8-naphthyridine-3-aarboxylate i8 heated at
120C ~or 4 hours in 12 ml of dimethyl sulphoxida
together with 190 mg ~1.2 mmol) of N-phenylpiperazine
and 460 mg (4.1 nmol) of diazabicy¢lot2.2.2~octa~e. All
the ~olatile ~o~po~e~ts are r~mo~ed u~dar high vacuum.
. ~he residue i8 ~tirr~d up with water and puri~ied by
;~: col~mn chrnmatography (~ilica gal, methylene
chloride/~ethanol 9/1).
Yield: 280 mg (43% of theory~
-~ Melting point: 109-111C
,i,
The compounds listed in Table 19 are prepared in
: analogy with the directions in ~xample 86:
~::
:
he A 29 518 - 107 -
`--
Table 19:
o
: ~N ~l~COOEt
; R~ ~N
:~:
: :~
Ex. No. M.p. [C] R~ .
:; 87 205-207 F
88 136-138 U~
89 t 33-135
:~ : C~
:
" ::
- 108 - ~'
;
;
i' Exiample 9O
il
J 6-Fluoro-1,4-dihydro-4-oxo-7-(4-phenylpiperaZin-1-yl)-
[3-(1~-1,2,4-tria201-1-yl-m~thyl)phenyl]-1,8-
~aphthyridine-3-carbo~ylic acid
F~, COOH
3, N J ~ N ~\
',.~,`
0.25 g (0.4 mmol) of the ester from Exi~ple 45 is
heated at 50C ~or 1.5 hours together with 0.8 ml of
1 N ~odiu~ hydroxid~ 801ution in a mixture con~i~ting
of ~ ml of ~thianol and 4 ml of water. T~ia mixture i~
t~en neturaliz~d with dilute hydrochloric acid and the
sol~ent~ are r~oved in vacuo. The re~idue i9 purified
by ~olum~ chromatography (sil~ca gel, methylene
chloride/mothanol 9/1).
Yield: 0.21 g (90% of theory)
~elting point: 264-268C (with deco~po~ition)
,~
. 15 Ths compoundd li~ted in Table 20 are prspared in
' a=alogy ~ith the dir-ctio=~ x~mple 90:
;~
Le A 29 518 - 109 -
~:15~21
Table 20:
F ~,COOH
~N N
,NJ ~ ~N
Ex. No. M.p. tC] R8
91 213-218 F
92 200'202 Cl~
93 246-250
'
~, ,. j ~
! ' : `. .
~`~' ' ''' ~
~ Le A 29 518 - 110 -
'- ~
2 ~ 2 1
Example 94
~thyl 6-~luoro-1,4-dihydro-1-~3-(N~ dazolyl-~eth~
ph~nyl]-4-o~o-7-~4-phQnylpip~razin-l-yl)-3-guinoline-
carbo~ylate
o
F~,COOEt
,~ N N
:~:5 0.5 g (1.~ mmol~ o~ ethyl 6,7-difluoro-1,4-dihydro-1-
~3-(N-imidazolyl-methyl)-phenyl]-4-oxo-3-~uinoline-
carboxylate i~ heated at 120C ~or four hour~ in 12 ml
o~ dimethyl sulphoxide together with 190 mg (1.2 ~mol)
of N-ph~ylpiperazi~e and 460 mg (4.1 mmol) o$
diazabicyclo~2.2.2]octana. All the volatile components
are removed under high vacuu~ and the residue i~
: ~tirred up with water and dried at about 100C.
: Yield: 590 mg (88% of theory)
~; Mslting po~t: 187-190C
The compounds listed i~ Table 21 are prepared in
analogy with t~e directions in ~xample 94:
`:
.
;~ ~ .' :.''.
'~ ~
Le A 29 518 - 111 -
6$~
Table_21:
F ~COOEt
~NJ
Ex. No. M.p. [Cl R8
/=\ '
168-170 F
.~ 96226-229 Cl~
97190-193
~ ~ Cl
~ .
. , .::
''`t
~-'
Le A 29518 - 112 - :
:
Example 98
6-Fluoro-1,4-dihydro-1-[3-(N-~m;dazolyl-~ethyl)phenyl]-
4-o~o-7-(4-phe~ylpiperazin-1-yl)-3-quinoli~e-aarboxylic
acid
o
F X~COOH
0.5 g (0.9 mmol) o~ the ester obtained in Example 95 i~
heated at 50C for 1.5 hours together with 1.8 ml of
1 N sodium hydroxide solution in a mixture consisting
of 9 ml o~ ethanol and 9 ml of water. This mixture i~
the~ neutralized with dilute hydrochloric acid and the
~olvents are remo~ed i~ ~acuo. The residue i8 purifi~d
:~ by colu~n chro~atography (sàlica gel, m~thylena
chloride/methanol 9/1).
: ~ Yi~ld: 0.4~ g (959c o~ theory) ~ ~ -
~ Meltlng point: 207-210C
: ,~ 15 The compou~d~ listed in Table 22 are prepared in `~-
~ ~ : analogy with ~h~ directions in Example 98~
~ - .
.
Le A 29 518 - 113 -
'2~
Table 22:
F ~3,COOH
~N N
R, ' J ~ N
~3x. No. M.p. lC] R8
99 190-192 F~
00 ~ 300
101 260-Z63 ~
Cl - -
.:
~: .
Le A 29 518 - 114 - -
"'~ ',
'.
ExamPle 102
~thyl 6-~luoro-1,4-dihydro-4-oxo-7-(4-ph~nylpiperazin-
l-yl)-l-12-(1~-1,2,3-triazol-1-yl-methyl)pheuyl]-3-
qui~oline-aarbo~ylate
o
F~,CC)OEt
~N N
¢~N~,J ~--N' ~N
: S 0.5 g (1.2 mmol) of ethyl 6,7-di~luoro-1,4-dihydro-4-
oxo-l-[2-~lH-1,2,3-triazol-1-yl-methyl)-phenyl]-3-
quinolinecarboxylate is heated at 120C for thr~e hour~ :
in 12 ml of dimethyl ~ulphoxidc together with 190 ~g
(1.2 mmol) of N-phenylpiperazine and 460 mg (4.1 mmol)
of diazabicylaot2.2.2]octane. All the volatile
components are r~mo~ed under high vacuum and the
~ : - residue i8 ~t~rr~d up with water and dried at about "~
:~ ~ lOQC.
~: Yield: 580 my (86% o~ theory) --~-
` ~elting point: 138-141C ;~
~3 : ~':-
:~ The compounds listed in Table 23 are prepared in
~ analogy with ~he direction3 in Example 102~
-
Le A 29 518 - 115 - ~
2 1
Table 23:
F~l~COOE~
--N~N~
~,NJ~--N'N`
~ ~ .
EX. No.M.p. ~C~ Ra
103119-121F~
104128-131c~
~: 105125-128 ~ ~`~
:: c~
' :. ~ '
:~ ` :
: :: . ::~ .
- :-
' , .
L8 A 29518 - 116 -
~... `
21:l5021
Example 106
6-Fluoro-1,4-dihydro-4-oxo-7-(4-ph~nylpiperazin-1-yl)-
1-l2~ 1,2,3-triazol-1-yl-m~thyl)phQnyl~-3-quinoli~e-
carbo y li~ aaid
o
F~,COOH
~N N
N~J [~N~ \N
.` 5 0.5 g (0.9 mmol) of the ester obtained in Example 102 ~
i8 heated at 50C for 1.5 hour~ together with 1.8 ml of - .
1 N sodium hydroxide solution in a mixture consisting
of 9 ml of ethanol and 9 ml of water. Th~s mixture i8
:~ then neturalized with dilute hydrochloric acid and the
0 801vent8 ~re removed in vacuo. The residu~ i8 Furified
by colu~n ~hromatography (~ilica gel, methylen-
chlorids~methanol 9/1).
Yield: 83 ~g (18% of t~eory) ~ :
: ~ ~ Melting point: 235 238~ ~with deaomposition)
. - .,:
The~compound~ listed in Table 24 are prepared in
analogy wit~ the directi~ns in Example 106
:::
: -
-
, ::
:
:
.
Le A 29 518 - 117 -
2 ~ n 2 l
Table 24:
o
F~COOH
Ex. No. M.p. ~Cl R
07 209-211 F~ ..
108 208-211 Cl~
109 228-231 ~ -
: Cl . .~:
.
:: . .::
: :~ :
:~,::: : : :
:`:
:
~ ~ .
~ .
Le A 29 518 - 118 -
.
.... . ,, ,, ' .
Example 110
Ethyl 6-fluoro-1,4-dihy~ro-4-o~o-7-(4-phenylpiperazin-
l-yl)-1-1~-~2~-1,2,3-triazol-2-yl-me~hyl~phanyl]-3-
quinoli~e-carbo~ylate
F~"COOEI
N=~
0.5 g 11.2 mmol) of ethyl 6,7-difluoro-1,4-dihydro-4-
oxo-l-t2-(2H-1,2,3-triazol-2-yl-methyl)-phenyl]-3-
quinolinecarboxylate i8 heated at 120C ~or three hours
in 12 ml o~ dimethyl sulphoxide together with 190 mg
(1.2 mmol) o~ N-phe~ylpiperazine and 460 mg (4.1 mmol) ~ :
of diazabicylc~[2.2O2]octa~e. All the volatil~
~omponents are removed u~der high vacuum a~d the
residue i8 stirr~d up with water and dried at about
100C.
Yield: 590 mg (80~ o~ theory~
15: Melti~g point: 198-200C
~`:
: ~ The compou~d~ listed in Table 25 are prepared in
v a~alogy with ~ho directions i~ Example 110:
1: ~
, .
he A 29 518 - 119 -
2 ~
Table 25:
F ~3~COOE~
Ex. ~o. M.p. ~Cl R8
; 111217-719 F ~ ~ ;
~ ~ 112208-210 Cl~
; ~ 113 167-168
~: Cl
::: : : :
"
.
,:
:
:`
: .
'
Le A 29 518 - 120 -
...... .. ...... . . . . . .. .. .
2:~ `Lag~
Example 114
6-Fluoro-1,4-dihydro-4-oxo-7-(4-phQnylpiperazin-l-yl)-
1-[3-(2~-1,2,3-triazol-2-yl-methyl)~h~uyl]-3-guinoline-
carboxylia ac~d
F~,C0011
N--~> `:`
0.5 g (0.9 mmol) of the ester obtained in Example 110
is heated at 50C for 1.5 hours together with 1.8 ml of :~
1 N aodium hydroxide solution in a Lixture con8i8ting : :~
of 9 ml of ethanol and 9 ml of water. Thi~ mixture is
then ncturalized with dilute hydrochloric acid a~d the
~ 10 solvent~ are removed in vacuo. The re~i~ue i~ puri~ied
:~~ by colum~ chromatography ~silica gel, methyle~e
chlor~de/metha~ol 9/1).
Yield: 28b mg (60% o~ theory)
Melting poi~t: 270-273C
The compounds li~ted i~ Table 26 are prepared in
r~ analogy w~th the directions in Example 114
,, .",.................................................................. ..
.
. .
La A 29 518 - 121 -
` -
2 ~ 2 1
Table 26-
o
F ~3,COC1H
~ Ra J ~--N :~
EX. No. M~p. ~C] R8
115 271-272 F
116 291 295 a
117 273-276 ~
.:
: ~
;,:~: ~ :
: ::
..~
Le A 29 SI8 - 122 - ~:
-
. ~.... ..... ... . .. ..
2 ~
Exam~le 118
~thyl 6-fluoro-1,4-di~ydro-4-o~o-7-(4-phanylpipera2in-
1-yl)-1-12-(1~=1,2,4-triazol-1-yl-~ethyl)ph~nyl]-3-
quinolin~carbo~ylate
F~COOEt
~N~J [~f N~
'.~:"'~'''"
0.5 g (1.2 mmol) of ethyl 6,7-difluoro-1,4-dihydro-4-
oxo~ 2-(1~-1,2,4-triazol-1-yl-methyl)-phenyl]-3-
quinoline~arboxylate is heated at 120C ~or ~our hour~ -
in 12 ml af di~ethyl sulphoxide together with 190 mg
(1.2 mmol) of N-phenylpiperazine a~d 460 mg (4.1 mmol)
of diazabicyclo~2.2.2]octane. All the volatile
:~ component~ are removed under high vaouum and the
re~idue ia stirred up with water and dried at about
~ 100C. ~ :
:~: Yield: 420 mg ~62% o~ theory)
~ 15 Melting ~oint: 214-217~
.. ~
~' Tho compound~ listed in Table 27 are prepared in
ana1ogy w1th th- dlrection~ x~p1e 118:
: '
Le A 29 518 - 123 -
....... . . . . .. .
2 ~
Tabl~ 27:
o
F ~3~COOE~
R" ~J ~f N ~>
Ex. No. M.p. [C] Ra
; 119229-232 F
"
12022~!-225 Cl~
121213-216
Cl
~ '
: :' '
~``''
,.
:
Le A 29 518 - 124 -
'`'.-~
Exam~le 122
6-Fluo~o-1,4-dihydro-4-oxo-7-~4-phenylpiperazin-1-yl)-
1-l2-(1~-1,2,4-triazol-1-yl-methyl)phenyl]-3-quinoline-
carbo~ylia acid
F~17,COOH
`N
[~--N ~ N~?
0.35 g (0.6 mmol) of the e8t9r obtained in Example 118
i8 heated at 50C for two hours together with 1.2 ml of
1 N sodium hydroxide ~olution in a ~ixture con~i~ting
of 6 ml of etha~ol and 6 ml of water. Thi~ mi~ture i~
the~ neturali~ed with dilute hydrochloric acid and the
~olve~ts are ramo~ed i~ vacuo. Th~ residue i~ purified
by colum~ chroMatography (silica gel, methylene
chloride/metha~ol 9/1).
Yield: 280 ~g (B9% o~ theory~
Melting poi~t: 270-274C
Th~ compou~ds li~ted in Table 28 are prepared in
analogy with the dire~t~o~s in ~xample 122
~, ;;)
Le A 29 518 - 125 -
Table 2 8:
F ~3,COOH
R8 ' `J ~ N~N`>
Ex. No. ~.p. ~C] R8
123227-229 F
24257-258 Cl~
~ ~ 125258-261
.~: Cl
'~
~ . , :
Le A 29 518 - 126 - ~ ~ ~
. . ,~,
Example 126
~thyl 6-fluoro~ dihydro-1-12-~N-imidazolyl-methyl)-
phenyll-4-o~o-7-~4-p~en~lpiperàzi~-1-yl)-3-quinolina-
carbQxylate
F ~COOEt
~N N
[~N~J ~--N~
: ; 5 O.S g (1.2 mmol) of ethyl 6,7-di~luoro-1,4-dihydro-1-
[2-(N-imidazolyl-methyl)-ph0nyl]-4-oxo-3-
guinoli~ecarboxylate i8 heated at 120C for two hours
in 12 ml o~ dimethyl ~ulphoxide together with 190 mg
(1.2 mmol) of N-ph2nylpiperazine and 460 ~g (4.1 mmol)
of diazabicyclo~2.2.2]oc~ane. All the volatile
aomponent~ are removed u~der high vacuum and ~he
re~idue i~ stirr~d up with water ~nd dried at about
100C.
Yield: 470 mg (71% of theory)
~alting point: 117-119C
Ihe compounds listed in Table 29 are prepared in
, ~ i,
analogy with the directions in Example 126:
,
~' ~
. . :
Le A 29 518 - 127 -
2 ~
Table 2 9:
F~3,COOEt
~N N
~8 J [~
~x. No. ~I.p. ~C] R~
127 122-124 F
128123-125 a~
; 129107-109
~' ~
~: :
::
:: ~ : ::
. . ~ : :
~.; ""
::
~::
: ~ ;
: ~
' -
Le A 29 518 - 128 -
,:
2 ~
Example 130
6-Fluoro-1,4-dihydro-1-[2~ dazolyl-~ethyl)-
ph~yl]-4-o~o-7-(4-ph~nylpiperazin-1-yl3-3-qui~oline-
carbo~ylic aaid
o
F ~COOH
N J ~ N~
`
0.4 g (0.7 mmol) of the ester obtained in ~xample 126
is heated at 50~ for 1.5 hours together with 1.4 ml o~
1 N sodium hydro~ide solution in a mixture con~isting
of 7 ml o~ ethanol a~d 7 ml o~ water. Thi~ mixture i~
then n~utraliz~d with dilute hydrochloric acid a~d the
801~ent8 are rem~v~d ~ vacuo- The residu~ i8 purified
;~ by column chromatography (silica gel, methyle~e
ahloride/methanol 9/1).
Yield: 0.29 mg (79% o~ th~ory)
;:~ ~elting point: 260-263C
v 15 The compou~ds li~ted in Tabls 30 are prepared in ~: :
a~logy with the directions in Example 130: ~
. . ~
',~,
:`:
. ~
..~ .
Le A 29 518 - 129 -
..
-
Tab1e 3 0:
o
F~,COOH
R8 ~NJ ~--N~
EX. NO. M.P. ~C] Ra
131258-262 F~ ::
132250-254 Cl~
133275-278
. .
. .
~: , : ::
, ~ : : .'~'.
:::~ : :
.
,
~ ; ' ,",
LQ A 29 518 - 130 -
.
. . . .
i, ~ ,
3 ~ 2 ~
Example_134
6,8-Difluoro-i,4-dihydro-~-oxo-7-~4-ph~nylpiperazi~-1-
yl~-1-l4-(1~-1,2,3-triazol-1-yl-methyl~phe~yll-3-
quinoline-carbo~ylic acid
F ~COOI-I
¢~N J F Ir
, N ~
300 mg (0.75 mmol) o 6,7,8-trifluoro-1,4-dihydro-4-
oxo-1-~4-(1~-1,2,3-triazol-1-yl-methyl)-phenyl]-3-
: guinolineaarboxylic acid are heated at 100C i~ 8 ml o~
DMSO together with 410 ~g (2.25 mmol~ of N-(4-fluoro-
phenyl)-piperazi~. All the volatile compo~e~t~ are
ramoved u~der hig~ ~acuu~ and the residue is stirred up
w~t~ iso-propanol and the re~lting ~olid i8 i~olated.
Wash~ng ~ repeatQd with i~o-propanol and diethyl ether
a~d dryi~g then takes place at about 100C.
~:,~ Yield: 387 ~g (92~ o~ theory) ~ --
~ ' 15 ~elt~ng point: 222-224C
:~: Tha compounds listed i~ Table 31 are prepared in
a~alosy with the directions in Example 134: ~;
~ ~ .
Le A 29 518 - 131 -
,j ~
2 ~ 2 ~
Table 31:
F ~, COOH
--NJ~N~J
N ~:
Ex. No. ~q-p. [C] RU
1 35200-202
:~ M~O
136194-196 f ~ ~:
R ` :: ~
~37~Z0-222 f=< -
`: .
a : ~
138225-2 7 /=< `
t39226-228 H
: ~ ~ 1402~8-250 Me
~,, ' " ~ `
: ~ ~ ' '"
.
Le A 29 518 - 132 -
: .
X l
Example 141
8-Chloro-6-fluoro-1,4-di~ydro-4-oso-7-[4-(4
~luoropheQ~l)-pipe~az~-l-yl]-l-l~-(lE-1,2,3-triazol-1-
yl-~e~hyl)-phenyl]-3-guinoli~e-~arbo~ylic a~id
F ~,~ COOH
~N~N~J
,13,NJ C' ¢~
' ' - ' N ~ N~
The titla compound i8 obtained in analo~y with ~xample
134 by r~acting 8-chloro-6,7-difluoro-1,4-dihydrs-4-
oxo~ 4-(lH-~,2,3-triazol-1-yl-met~yl)-phe~yl]-3-
quinolinecarhoxyl~c acid with N-(4-fluorophe~yl)- --
piperazine.
~Melti~g point: 1~8-130C
,
,:,,...
.
. .
Le A 29 518 - 133 -
` Exampla_142 2 ~1 ~ a 2 ~
8-Chloro-6-~luoro-1,4-dihydro-4-oxo-7-[4-(2-
l chloroph~nyl)-piperazi~-l-yl]-1-[4-~lE-1,2,3-triazol-1-
yl-m~thyl)p~enyl]-3-quinoli~e-aarbo~ylic acid
F ~COOH
l 11 11
~N ~ N
~CI
N ~ ~N
~/ '~"
The title compound is obtained i~ analogy with Example
134 by ~eacting 8-chloro-6,7-di$1uoro-1,4-dihydro-4-
oxo~ 4-(lH-1,2,3-triazol-1-yl-methyl)-phe~yl~-3-
quinoli~ecarboxyl~c acid with N-(2-chlorophenyl)-
piperazine .
Moiti~g polnt: 158-160C
~.
:
; ,
Le A 29 518 -- 134 -
Exam~le 143
8-~hloro-6-~luioro-1,4-dihydro-4-o~o-7-l4-(4-
~luoropheniyl)-piperazin-l-yl]-1-[4-(2~1,2,3-triazol-2-
yl-~ethiyl)phiQ~yl]-3-quiDiol~Die-carbo~ylic aa~d
~,COOH
~¢~NJ Cl
N
The title compounid ~8 obtain~d in analogy with ~xample
:~ 134 by reacting 8-chloro-6,7-di~luo~o-1,4-dihydro-4- -oxo-l-C4-(2~-1,2,3-triazol-2-yl-methyl)-phenyl]-3- :
quinolinecarboxylic acid with N-(4-~luorophe~
piperazine.
~elting poiDit: 213-215C
~' ~:
,
-
.. :
Le A 29 51~ - 135 -
:l `
¦ Exam~le 144
8-Chloro-6-fl~oro~ dihydro-4-oxo-7-t4-(2-
chlorophe~yl)-piperazin-1-yl]-l-t4-t2~-1,2,3-triazol-2-
yl-~e~hyl)ph~nyl~-3-quinoli~e-carbo~ylic acid
F~,COOH
¢~N~J Cl I î 1 N
N ~ ~bH
N =::/
The title compou~d i8 obtained in analogy with ~xampl~
134 by reacting 8-chloro-6~7-difluoro 1,4-dihydro-4-
oxo-1-[4-(2~-1,2,3-triazol-2-yl-methyl)-phenyl]-3-
quinolinecarboxylic acid with N-(2-chlorophe~yl)-
piperazi~e.
~ ~elting point: 228-230C (undar decomposition)
In a~alogy with the directions in Example 144,
6,7,8-trifluoro-1,4-dihyd~o-4-oxo-1-t4-(1~-1,2,4-
tria~ol-1-yl-methyl)-phenyl]-3-qu~olinecarboxylic acid
::: reacta to give the following products:
:
-
Le A 29 518 - 136 -
,
Table 3 2:
o
F~ 3~COOH
R ~NJ F ~
N~3
~ N : .:
Ex. No. M.p. ~C~ R8
145 Oil H
~: ~ ~ OEt . .
146 297 (decomp.) ~__
:~ ~ 147 . 278-280
a
148 217-219
149 222-224 -CôHs
~::: : :
~: :
: ;. ~
;
: :
.
~; :
.
Le A 29 518 - 137 - ~
2 ~ 2 1
Example 150
8-Chloro-6-1uoro-1,4-dihydro-4-o~o-7-t4-(4-
fluorophenyl)-pipera~in-l-yl]-1-~4-(1~-1,2,4-triazol-1-
yl-methyl~pha~yl]-3-quinoli~e-carbo~ylia a~d
F~l~,COOH
~NJ~N~
~NJ Cl ~
~` N' ~>
\-= N
The title compound i8 obtained in analcgy with Example
134 by roaa~ing 8-ahloro-6,7-dl luoro-1,4-dihydro-4-
oxo-1-~4-(lH-1,2,4-triazol-1-yl-me~yl~-phe~1]-3-
qulnolinecarboxylic acid with ~-(4-fluorophenyl)-
: piperazlne.
: :~ 10 ~elting poi~t: 248-250~C
~::;,6
'
La A 29 518 - 138 - ~-~
?
.
0 ~ ~
Example 151
8-Chloro-6-fluoro-1,4-dihydro~4-oxo-7-t4-(2-
chloropheni~l)-piperazin-l-yl]-1-[4~ -1,2,4-triazol-1
yl-~othyl)p~ienyl]-3-gui~oliDia-carboYylic acid
o
F ~ COOH
~N ~ N
N ~J Cl
~CI ~J
.~,............................ ~N~N~>
\-= N
. .
The titl~ compound i~ obtained in aDialogy with Bxaimple ~ -
134 by reacti~ig 8-chloro-6,7-difluoro-1,4-dihydro-4-
~:~ oxo-l-E4-(lH-l~2~4-triazol-l-yl-m~thyl)-phenyl]-3
,j, quinoli~ecarboxylic acid with N-(2-chlorophi~yl)-
piperazi~e.
~: ~10 Melti~g poi~t: 248-250C
` .
. ~. ,, :
;~ ,
., . - :
Le A 29 518 - 139 -
,
2 1
Example 152
.~
6-Fluoro 1,4-dihydro-4-oxo-7-l4-(4-~luorophenyl)-
piperazin-l-yl~ 4-(1~-1,2t3-~riazol-1-yl-
. methyl)ph~nyl]-3-g~inoli~ecarboxylic acid propyla~ide
o o
F ~ ~ N ~
~[3~ N ~J ~
f ~ N ~ ~N
0.46 ml ~3.3 mmol) of triethylamine is added at 0~ to
1.32 g (3 mmol) of 6-~luoro-1,4-dihydro-4-oxo-7-l4-(4-
fluorophe~y})-piperazin-l-yl]-1-[4-(lE-1,2,3-triazol-1-
yl-methyl3-phenyl3-3-quinolin2carboxylic acid in 10 ml
of acetone. Subsequently~ 0.34 ml of ethyl
chloro~onmate is addRd dropwi~e at this temperature
within the space of 30 minutes. After adding 0.30 ml
(3.6 ....~.ol) of propylamine, the mixture i8 boiled u~der
reflux for 1.5 hours.
For the working up, the mixture i8 added to saturated
:~';, ' 15 sodium hydroge~ carbonate solution, and then extraated
: with me~hylene chloride followed by drying over sodium
sulphate. The crude product obtai~ed by e~aporation i~ -
~: chromatographed on silica gel (methylene chloride/
methanol 96/4).
Le A 29 518 - 140 -
' .
:::
i'' . . ~ ' ~ ! ', `
Yield: 0.8 g (57~ of theory)
Melting point: 202-204C
2 . ~
The compounds ll~ted in Tabli~ 33 are prepa~ed in
. analogy with the~ directionn in Example 152:
Tablei2 33-
o o
~R"
fN N
i, ~N J ¢~
N~N~
~ .
.
EX. No. M.p. ~C] RC
153194-196 -N N~H3
154 152-154 -NH(;H2CH20CH3
155 110-112 -N O
.:': . :.
156 223-225 -NHCH2CH2OH
"~
.
Le A 29 518 - 141 -
15~21
,
Exam~le 157
Ba~zyl 6-fluoro-1,4~di~ydro-4 o~o-7-t4-(4-
~luorophenyl~-piperazin-l-yl]-1-[4-(1~-1,2,3-triazol-1-
yl-~ethyl)phenyl]-3-quinoli~eaarboxylate
o o
l~N~ ~\[3
,~ N ~J
:';=`` N
. . N ' ~N
.,
0.14 ml (1.2 mmol) of triet~ylamine i~ added at 0C to
:~ 0.44 g (1.0 mmol) of 6-~luoro-1,4-dihydro-4-oxo-7-[4-
(4-fluorophenyl)-piparazin-1-yl]-1 ~4-(lH-1,2,3-
triazol-1-yl-methyl)-phenyll-3-quinoli~ecarboxyli~ acid
in 10 ml o~ ac~tone. Subseque~tly, 0.14 ml (1.2 mmol)
of ethyl ~hloro~ormate i3 added dropwise at this
temperature within . ~ 30 ~inutes. After ~:
; ~ addi~g 0.30 ~1 (3.6 mmol) of b~nzyl alcohol, t~ :
mixturQ i~ boiled under reflux ~or 2 hours. For the
~t~ working up, th~ ~ixtur~ i8 added to saturated ~odium
hydrog~n carbo~ate solution and this mixture i8 then .~
: extra~ted with ethyl acetate. The ~ombin2d organic - ~-
: pha~s are washed wi~h saturated sodium chloride
solution a~d dried over sodiu~ sulphate. The crude
product obtained ~y evaporatlon i8 chrom~tographed on
.... ~ ~ .;
~ -
Le A 29 51B - 142 - ~:
silica gel 60 (eluent: methylene chlorid~methanol =
9/1) .
Yield: 0.35 g (55% o~ theory)
Melting point: 226-228C
S The aompou~ds listed in Table 34 are prepared in
analogy with the directions in Example 157:
Tabl~ 34:
o o
/~1 ~RC
~N
~ Ex. No. M.p.[C] R8 ;~ :
; 158 ~235 -OCH3 ~
.
159 198-200 -OCH2CH2CI ~
~l ~ .
160 ~235 X
~ ~ H3C CH3 "
CH3
161 163-165~ ~ ~ c~ ~ :
~:
'
. .
Le A 29 518 - 143 -
. ..... .. . . . .. . . .. .. . . . . ..
i
' Example 162
i
~thyl 1,4-dihy,dro-4-o~o~7-(4-phanylpiperazin-1-yl)-1-
[4-(1~-1,2,4-triazol-1-yl-methyl)pha~yl]-1,8-
naphthyridi~ecarbo~ylate
o
N J~COOC2Hs
13'``' ~
N ..
: 5 0.5 g (1.2 mmol) of ethyl 7-chloro-1,4-dihydro-4-oxo-1-
[4-~lH-1,2,4-triazol-1-yl-meth~l)-phenyl]-1,8-
~; naphthyridino-3-carboxylate i~ stirred at room
tempsratura o~ern~ght i~ 12 ml of d~methyl sulphoxide
together with 0.58 g (3.6 mmol) of N-methylpiperazinQ.
All the volatile compo~nts arQ the~ ramo~ed u~der high
: ~aauum. The residuQ i8 added to wa~er a~d the mixtur~ ::
is extracted with dichlor~methane. The crude product
obtained after rotary evaporatio~ is purified on ~ilica
g~l (eluent diahloromet~a~a/~ethanol 96/4).
Yiold: 475 mg (73% of theory)
M.p.: 170-171C
. ~
. .
LQ A 29 518 - 144 -
2 1
, Example 163
.
~thyl 7-[~-~4-fluorophQnyl)piper~zin-l-yl]-1,4-dihydro-
4-oxo-1-14-~ 1,2,4-triazol-1-yl-methyl~phanyl~ 8-
naphthyridi~e-3-~arbo~ylat~
,~3~CCOC2H,
~N~J ~ ~
~ N :
;-. N' ~ :
N
" :,
The title compou~d i8 obtain~d i~ analogy with Example :-
162 by reacting w~th 1-(4-fluorophenyl~piperazine. ~
M~p.: 176-178C ::
.:
- ~' .
.,;,. ~ ~
~ ~ :
.. _................ : -
.
La A 29 518 - 145 -
..... , .. ~ .. , .. , ....... ... ...... .. . .. . .... , . ,, . ~ .. ,, . ".. ............................... .... ......... . . .... . .. ..
.. .... ........ . ...... .
2 1 :L ~ ~ 2 ~
Example 164
1,4-Dihydro-4-oxo-7- (4-phenylpiperazil~L-l-yl) ~ 4- (l~I-
1, 2, 4-tria~ol-1-yl-~ethyl) phenyl] -1, 8~napl~thyridine-3 -
carbo~ acid
~3~ COOH
N N
N
0.45 g (0.84 mmol) of the e~ter obtai~ed in 13xample 162 .
i8 stirred at 50C for one hour together ~ith 1.68 ml
of 1 N ~odium hydroxide ~olution in a mixture ~ -:
consiltirLg o~ 8 ml o~. e~haDol and 8 ml of water. Thi~ :
m~xture i~ then neutralized wit~ dilute hydrochloric -~
acid a~d the 801VeIlti~ are.remo~d i~ va~uo. The residue
puri~ied on s~ a gel (sluent,
di~:hloromethane~methanol 9 6 /4 ) .
YiQld: 158 mg (37% o~ theory)
M.p.: 286-287C : :
, ' :
:: ~
Le A 29 518 - 146 - ~
, . " ,. ~ .. . . . . ... .
~ j
5 ~ 2 ~
3 Exampls 165
7-14-(4-Fluorophanyl~piperazin-1-yl]-1,4-dihydro-4-o~o-
4-(1~-1,2,4-triazol-1-yl-methyl)phenyll-1,8-naph-
thyridi~e-3-car~oxylic acid
~COOH
~N 1N~N~
,N~
: S In analogy with ~xample 164, tha ester from Example 163
reacts to giva the title compound.
M.p.: 284-2~5~
"
~;:
Le A 29 518 - 147 -
.......... .. .... . . . .... .