Note: Descriptions are shown in the official language in which they were submitted.
~115~77
'"w O.Z. 0050/42593
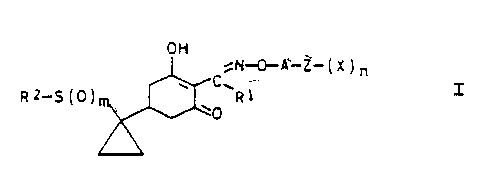
Cyclohexenoae oxime ethers
The present invention relates to novel cyclo-
hexenone oxime ethers of the general formula I
OH
~N-0-A-Z-(X)~
R1_S(Dy w ~~R1
in which the variables have the following meaning:
n is from 0 to 5;
m is from 0 to 2;
R1 is Cl-C6-alkyl;
R' is Cl-C,,-alkyl or benzyl;
A is Cl-C6-alkylene, C,-C6-alkenyleae or C3-C6-alkyaylene,
where these groups may carry from one to three Cl-C3-alkyl
groups and/or halogen atoms,
or a C3-C6-alkylene or C,-C6-alkenyleae group which, if
desired, is substituted by from one to three Cl-C,-alkyl
groups and is which one methyleae group is replaced with
an oxygen or sulfur atom, a sulfoxyl or sulfoayl group or
a group -N(Ra)-, where
R' is hydrogen, C1-C,,-alkyl, C,-C6-alkenyl or C,-C6-alkyayl;
Z is phenyl, a 5-membered heteroaromatic radical having
from one to three nitrogen atoms and/or one oxygen or
sulfur atom or a 6-mambered heteroaromatic radical having
from one to four nitrogen atoms, and
X is aitro, cyaao, halogen, Cl-C,-alkyl, partially or
completely halogenated C1-C,-alkyl, C1-C,-alkoxy, C1-C,
alkylthio, partially ~or completely halogeaated C
alkoxy, carboxyl, C1-C,-alkoxycarbonyl, benzyloxycarbonyl,
phenyl or pyridyl, where the aromatic radicals may be
unsubstituted or carry from one to three of the following
substituents: nitro, cyaao, halogen, C1-C,-alkyl,
partially or completely halogenated C1-C,-alkyl, C1-C,-
alkoxy, Cl-C,-alkylthio, partially or completely
halogeaated C1-C,-alkoxy, carboxyl, Cl-C,-alkoxycarboayl,
benzyloxycarbonyl or an amino group -NRbR', where
_~11~27~
- 2 - O.Z. 0050/42593
Rb is hydrogen, C1-C,-alkyl, C3-C6-alkenyl or C,-C6-alkynyl
and
R' is hydrogen, C1-C,-alkyl. C3-C6-alkenyl, C3-C6-alkynyl,
Cl-Cg-acyl or benzoyl which may be uasubstituted or in its
turn carry from one to three of the following radicals:
vitro, cyano, halogen, C1-C,-alkyl, C1-C,-alkoxy, C1-C,-
alkylthio, Cl-C,-haloalkyl, Cl-C,-haloalkoxy and/or Cl
alkoxycarbonyl,
and their agriculturally useful salts and esters of
C1-Cio-carboxylic acids and inorganic acids.
The present invention furthermore relates to
herbicides which contain these compounds as active
ingredients and a method for controlling undesirable
plant growth.
The literature has already disclosed herbicidal
cyclohexanediones of the general formula I'
OH
E~N~-0 I .
-~'~('~~(R
0
where E and D have, inter alia, the following meanings:
U.S. Patent 4,440,566 (D = benzyl; E = 2-ethylthio-
propyl);
EP-A 238 021 and EP-A 125 094 (D = benzyl or but-2-enyl;
E = a substituted 5-meanbered hetaryl radical):
EP-A 80 301 (D = benzyl or but-2-eayl; E = substituted
phenyl);
EP-A 177 913 (D = 2-thenyl or 3-thenyl; E = a substituted
5-membered to 7-membered heterocyclic structure)
U.S. Patent 4,432,786 (D = 5-chloro-2-thenyl: E = sub-
stituted phenyl)
EP-A 243 313 (D = (E)-3-chloroprop-2-eayls E = 1-methyl-
thiocycloprop-1-yl).
Furthermore, DE-A 38 38 309 describes in general
terms cyclohexenone oxime ethers of the type comprising
the compounds I, which ethers have herbicidal activity
zm~?77
- 3 - O.Z. 0050/42593
and in which, inter alia, E is a substituted 5-membered
to 7-membered heterocyclic structure and D ie a
substituted 4-phenylbutylene or 4-phenylbutenylene
radical.
It was an object of the present invention to
synthesize cyclohexenone oxime ethers which. compared
with the known members of this class of substances, have
greater selectivity in the control of weeds in gramineous
crops, such as rice and corn.
Accordingly this object is achieved by the
cyclohexenoae oxime ethers I defined at the outset, which
have a good herbicidal action preferably against species
from the grass family (Gramineae). They are tolerated
and hence selective in broad-leaved crops and in moao-
cotyledon plants which are not members of the Gramineae.
They include compounds which are also selective in
gramineous crops and at the same time control undesirable
grasses.
The cyclohexeaone oxime ethers of the formula I
are obtainable by various methods, preferably in a
conventional manner from already known derivatives of the
formula II (EP-A 243 313) and the corresponding hydroxyl
amines of the formula III (EP-A 169 521).
OH OH
,N-0-A-Z-(X)~
R2_S(0) ~ ~R1 + HZN-O-A-Z-(X)~ --~ R=-S(0) ~ \C~R1
II III I
The reaction is advantageously carried out in the
heterogeneous phase in a solvent at an adequate tempera-
ture below about 80°C in the presence of a base, the
hydroxylamine III preferably being used in the form of
the ammnonium salt.
Examples of suitable bases are carbonates,
bicarbonates, acetates, alcoholates or oxides of alkali
~11~~7'~
-~ - 4 - O.Z. 0050/42593
or alkaline earth metals, in particular sodium hydroxide,
potassium hydroxide, magnesium oxide and calcium oxide,
as well as organic bases, such as pyridine and tertiary
amines, eg. triethylamine. The base is added, for
example, in an amount of from 0.5 to 2 mol equivalents,
based on the ammoaium compouad.
Examples of suitable solvents are dimethyl
sulfoxidet alcohols, such as methaaol, ethanol and
isopropanol; aromatic hydrocarbons, such as benzene and
toluene: chlorohydrocarbons, such as chloroform and 1,2
dichloroethane; aliphatic hydrocarbons, such as hexane
and cyclohexane; esters, such as ethyl acetate, and
ethers. such as diethyl ether, dioxane and tetrahydro
furan. The reaction is preferably carried out in methan
0l using sodium bicarbonate as the base.
The reactioa is complete after a few hours. The
product can be isolated, for example, by evaporating down
the mixture, partitioning the residue between methylene
chloride and water and distilling off the solvent under
reduced pressure.
However. it is also possible to use the free
hydroxylamine base directly, for example in the form of
an aqueous solution. for this reactioa; a siagle-phase or
two-phase reaction mixture is obtained, depending on the
solvent used for the compound II.
Examples of suitable solvents for this variant
are alcohols, such as methanol, ethanol, isopropanol and
cyclohexanol; aliphatic and aromatic hydrocarbons and
chlorohydrocarbons, such as hexane, cyclohexane,
methylene chloride, toluene and dichloroethane; esters,
such as ethyl acetates nitriles, such as acetonitrile,
and cyclic ethers, such as dioxane and tetrahydrofuran.
No special conditions with regard to the pressure
are required in general, the reaction is therefore
carried out at atmospheric pressure.
The novel cyclohexenone oxime ethers I are
evidently acidic, ie. they can form salts of alkali metal
z11~27'~
- 5 - O.Z. 0050/42593
or alkaline earth metal compounds and eaol esters.
Alkali metal salts of the compounds I can be
obtained by treating the 3-hydroxy compounds with sodium
hydroxide, potassium hydroxide or sodium or potassium
alcoholate in aqueous solution or in an organic solvent,
such as methanol, ethanol, acetone and toluene.
Other metal salts, such as manganese, copper,
zinc, iron, calcium. magnesium and barium salts, can be
prepared from the sodium salts in a conventional manner,
as can ammonium and phosphonium salts by means of
ammonia, phosphoaium, sulfonium or sulfoxonium
hydroxides.
The hydroxylamines III are generally obtained by
a number of knows process steps, starting from known
intermediates:
0 0
~ HO-A-Z-(x)~
fY
0 N~hl D N-O-A-1-t X I ~ N IN-CH ICH ~-Ohi
or
~ L AVZ Ix)~ ~ III
YI YII
L is a leaving group, for example halogen, such as
chlorine, bromine and iodine, or CH3SOz-O-.
The alkylatiag agents required for the synthesis
of the novel hydroxylamines of the formula III can be
prepared by conventional methods [cf. Rearrangement of
cyclopropylhetarylcarbinols: J. Heterocycl. Chem. 1~
(1976), 525, JP 55 051 004, JP 55 047 601; Houben-Weyl:
Methoden der Organischen Chemie, Volume 4/3, page 424 et
seq.; Coupling of metallized heterocycles with 1,m-
dibromoalkaaes: DE-A 28 21 409 and Chem. Ber. 114
(1981), 3667 and 3674; Heteroatom-interrupted alkylating
agents: DE-A 34 37 919; Tetrahedron Lett. 28 (1979),
2639, Org. Synth. Coll. Vol. 1_ (1944), 436;
DE-A 26 54 646; DE-A 2 714 561; J. Org. Chem. 52 (1987),
3587; DE-A 94 88 71; DE-A 94 88 72: J. Med. Chem. 26
(1983), 1570; Synthesis (1983), 675 and J. Org. Chem. 48
~115?'~7
- 6 - O.Z. 0050/42593
(1983) , 4970] .
If desired, the alkylating agents V can be
obtained [cf. Houben-Weyl, Methoden der Organischen
Chemie, Volume 5/3, page 862 et seq. and page 899 et seq.
and ibid., Volume 5/4, page 361 et seq.] in a convention-
al meaner from the carbinols IV [Coupling of aryl and
hetaryl iodides and bromides with l,w-alkenols or l,w-
alkyaols in the preseace of palladium catalysts:
Tetrahedron Lett. 24 (1975), 4467; Tetrahedron 35 (1979),
3291 Chem. Lett. (1977), 423; Houben-Weyl: Methoden der
Organischen Chemie, Volume 13/9B, page 964 et seq.].
The alkylating agent V and, if desired, also the
carbiaol IV are preferably coupled by the Mitsunobu
method [Syathesis (1981), 1; J. Med. Chem. 3_~ (1990),
187] with a cyclic hydroxyimide VI, and the resulting
protected hydroxylamine derivative VII is cleaved to give
the free hydroxylamine III, for example with 2-amino-
ethanol.
In the cyclic hydroxyimides VI, D is, for
example, C,- or C,-alkyleae, C,-alkenylene or a 5-membered
or 6-membered ring which contains not more than 3 double
bonds and may contain one nitrogen atom, for example
phenylene, pyridinylene, cyclopentylene and cyclohexylene
or cyclohexenylene. Examples of suitable substances are
the following:
0 0 0
( ~ \N-OH I \N-4H ( ~N-OH
N
0 - 0 0
0 0 0
I ~N~H N-OH I N-OH
0 0 0
0
~N-0H
0
~1152'~'~
-- - 7 - O.Z. 0050/42593
The reaction of the compounds V with the hydroxy-
imides VI is advantageously carried out in the presence
of a base. All bases which are capable of deprotonating
the hydroxyimides VI without attacking the amide system
are in principle suitable. These are in particular the
so called nonnucleophilic bases.
Examples are mineral bases, such as alkali metal
and alkaline earth metal carbonates, and alkali metal and
alkalise earth metal bicarbonates, and organic bases,
such as aliphatic, cycloaliphatic and aromatic tertiary
amines. Mixtures of these bases may also be used.
Examples of individual compounds are the follow-
ing bases: sodium carbonate, potassium carbonate,
magnesium carbonate, calcium carbonate, barium carbonate,
bicarbonates of these metals, tri.methylamine, triethyl
amine, tributylamine, ethyldiisopropylamine,
N,N-dimethylaniline, 4-N,N-dimethylaminopyridine, diaza-
bicyclooctane, diazabicyclouadecane, N-methylpiperidine,
1,4-dimethylpiperazine, pyridine, quiaoline, bipyridine
and phenanthroline. The economical bases sodium carbon-
ate and potassium carbonate are preferred.
The base is added in general in equivalent
amounts up to an excess of 5 equivalents, based on the
hydroxyimide. A larger excess is possible but has no
additional advantages. It is also possible to use a
smaller amount of base. However, from 1 to 3. in par-
ticular from 1 to 2, equivalents, based on the hydroxy-
imide VI, of the base are preferably used.
The use of nualeophilic bases, such as alkali
metal and alkaline earth metal hydroxides, in particular
sodium hydroxide and potassium hydroxide, is also pos
sible. In this case, it is advantageous to use the base
in equivalent amounts, based on the hydroxyimide VI, in
order to prevent a nucleophilic attack by the hydroxyl
ions on the carbonyl function of the amide group.
The starting compounds V are advantageously
reacted with the hydroxyimides VI in a solvent which is
2~i~~77
-~- - 8 - O.Z. 0050/42593
inert under the reaction conditions. Examples of advan
tageous solvents are polar aprotic solvents, such as
dimethylformamide, N-methylpyrrolidone, dimethyl sulfox
ide, aulfolane and cyclic ureas. The amount of solvent
is in general not critical.
The reaction of the starting compounds V with the
hydroxyimides VI may also be carried out using phase
transfer catalysis. In this case, solvents which form
two phases with water, preferably chlorohydrocarbons, are
used. Suitable phase transfer catalysts are the guater-
nary ammnoaium sad phosphonium salts, polyethylene gly-
cols, polyethylene glycol ethers and crown ethers, which
are conventionally used for such purposes and are des-
cribed in, for example, Dehmlow et al., Phase Transfer
Catalysis, pages 37-45 sad 86-93, Verlag Chamie, Weinheim
1980.
The phase transfer catalysts are advantageously
used in amounts of from 1 to 10, preferably from 3 to 5,
% by volume, based on the volume of the reaction mixture.
The reaction of the starting compounds V with the
hydroxyimidea VI is carried out in general at from 0 to
140°C, preferably from 2 to 100°C, in particular from 40
to 80°C. In an advantageous procedure, the hydroxyimide
VI is initially taken together with the base is the
solvent and the starting material V is metered into this
solution. It may prove advantageous to add the hydroxy-
imide at a lower temperature, for example at from 0 to
50°C, and to heat the reaction mixture to the actual
reaction temperature only after this addition.
After the end of the reaction, water is advan
tageously added to the cooled reaction mixture, the
resulting hydroxylamine derivatives VII separating out as
crystalline solids or as oils. The hydroxylamine deriva
tives obtained in this manner can. if desired, be further
purified by recrystallization or by extraction.
The hydroxylamine derivatives VII may be
temporarily stored or converted immediately into the
z115~7'~
- 9 - O.Z. 0050/42593
hydroxylamines III (having free amino groups).
This conversion can be carried out by
conventional methods. as described in, for example,
DE-A 36 15 973 and the publications cited therein. The
process according to DE-A 36 15 973 is preferably used,
in which the hydroxylamines III are liberated by means of
ethanolamine. The liberation of the hydroxylamines III
with the aid of other bases, such as aqueous mineral
bases. amines. hydrazines. hydroxylamines or aqueous
acids, is also possible.
The hydroxylamines III can be isolated from the
reaction mixtures obtained is these processes by
conventional working-up methods, for example by extrac-
tion or by crystallization. To increase the tendency of
these hydroxylamiaes to crystallize, it may often be
advantageous to convert them into their salts with
mineral acids or organic acids. For this purpose, dilute
solutions of these acids are generally reacted With the
hydroxylamines, advantageously in approximately
equivalent amounts. As in the case of the hydroxylamines
III having a free amino group, the resulting
hydroxylammonium salts can be further processed directly
to the herbicides of the formula I or, if desired,
stored.
The cyclohexenone oxime ethers I may be obtained
in the preparation as isomer mixtures, both E/Z isomer
mixtures and enantiomer or diastereoisomer mixtures being
possible. The isomer mixtures can. if desired, be
separated by methods conventional therefor, for example
by chromatography or by crystallization.
The cyclohexenone oxime ethers I can be represen-
ted in a plurality of tautomeric forms, and the present
invention relates to all of them.
With regard to the biological activity, preferred
cyclohexenone oxime ethers of the formula I are those in
which the substituents have the following meanings:
n is from 0 to 5, in particular from 0, 1 or 2 or, where
~11~2'~'~
°'4 - 10 - O.Z. 0050/42593
Z is phenyl and X is in each case halogen, from 0 to 5~
when Z is a heterocyclic structure, the maximum possible
value of n corresponds to the number of substitutable
ring members; in the case of a plurality of radicals X,
the subtituents may be identical or different;
m is from 0 to 2, preferably 0;
R1 is C1-C6-alkyl, such as methyl, ethyl, propyl, n-butyl,
in particular ethyl and propyl;
R' is C1-C,,-alkyl, such as methyl, ethyl, propyl, n-butyl,
in particular methyl
A is C1-C6-alkylene. C,-C6-alkeaylene or C3-C6-alkynylene,
such as methylene, ethylene, propylene, butylene, pent-
ylene or hexylene~ propenyleae, prop-2-eaylene, butea-
yleae, but-2-enylene, but-3-eayleae. peatenylene, pent-2-
enylene, peat-3-eaylene, peat-4-enylene, hexenylene, hex-
2-enylene, hex-3-enylene, hex-4-enylene or hex-5-enylene~
prop-2-ynyleae, but-2-yaylene. but-3-ynylene, peat-2-
yaylene. pant-3-yayleae, pent-4-yayleae, hex-2-ynylene,
hex-3-ynylene, hex-4-ynylene or hex-5-yayleae and may be
substituted by from one to three methyl or ethyl radicals
and/or fluorine or chlorine atoms: is the case of the
unsaturated chains, both the cis sad the trana form may
occur; propylene. butylsne, prop-2-eayleae, but-2
enylene, but-3-enyleae and but-3-ynyleae are particularly
preferred
C,-C6-alkylene or C,-C6-alkeaylene in which a methylene
group is replaced with an oxygen or sulfur atom, a
sulfoxyl or sulfoayl group or -N(R')-, such as 3-oxa-
propylene, 3-azapropylene, 3-thiapropylene, 3-oxo-3-
thiapropyleae, 3,3-dioxo-3-thiapropylene. 3-oxabutylene,
3-azabutylene, 4-thiabutylene, 4-oxo-4-thiabutylene, 4,4-
dioxo-4-thiabutylene, 4-oxabut-2-enylene, 4-azabut-2-
enylene, 4-thiabut-2-enylene, 3-oxapentylene, 3-azapent-
ylene, 3-thiapentylene, 3-oxo-3-thiapentylene. 3,3-dioxo-
3-thiapentylene, 4-oxapentylene, 4-azapentylene, 4-thia-
pentylene, 4-oxo-4-thiapeatylene, 4,4-dioxo-4-thiapent-
ylene, 5-oxapeatylene, 5-azapentylene, 5-thiapentylene,
211527'
- 11 - O.Z. 0050/42593
5-oxo-5-thiapentyleae, 5,5-dioxo-5-thiapentylene,
5-oxapent-3-enylene. 5-azapent-3-enylene, 5-thiapeat-3-
enylene. 3-oxahexylene, 3-azahexylene, 3-thiahexylene,
3-oxo-3-thiahexylene,3,3-dioxo-3-thiahexylene,4-oxahex-
ylene, 4-azahexylene, 4-thiahexylene, 4-oxo-4-thiahex-
ylene, 4,4-dioxo-4-thiahexylene, 5-oxahexylene, 5-azahex-
ylene, 5-thiahexylene, 5-oxo-5-thiahexylene, 5,5-dioxo-5-
thiahexylene. 6-oxahexylene, 6-azahexylene, 6-thiahex-
ylene, 6-oxo-6-thiahexylene, 6,6-dioxo-6-thiahexylene, 6-
oxahex-4-enylene, 6-azahex-4-enylene or 6-thiahex-4
enyleae, which may be substituted by from one to three
methyl or ethyl radicals in the case of the unsaturated
chains, both the cis aad the traps form may occur; 3
oxapropyleae, 2-methyl-3-oxapropyleae, 3-oxsbutylene and
4-oxabutylene are particularly preferred;
Ra i s hydrogen
Cl-C~-alkyl, such as methyl, ethyl, n-propyl, 1-
methylethyl, n-butyl, 1-methylpropyl, 2-methylpropyl or
l,l-dimethylethyl, in particular methyl or ethyl:
C,-C6-alkenyl, such as 2-propeayl, 2-butenyl, 3-butenyl,
1-methyl-2-propenyl, 2-methyl-2-propeayl, 2-pentenyl, 3-
pentenyl, 4-peateayl, 1-methyl-2-butenyl, 2-methyl-2-
butenyl, 3-methyl-2-butenyl, 1-methyl-3-butenyl, 2-
methyl-3-butenyl, 3-methyl-3-butenyl, l,l-dimethyl-2-
propenyl, 1,2-dimethyl-2-propenyl, 1-ethyl-2-propenyl, 2-
hexenyl, 3-hexenyl, 4-hexenyl, 5-hexenyl, 1-methyl-2-
peatenyl, 2-methyl-2-pentenyl, 3-methyl-2-peateayl, 4-
methyl-2-pentenyl, 1-methyl-3-pentenyl, 2-methyl-3-
penteayl, 3-methyl-3-peatenyl, 4-methyl-3-penteayl, 1-
methyl-4-penteayl, 2-methyl-4-pentenyl, 3-methyl-4-
pentenyl, 4-methyl-4-pentenyl, 1,1-dimethyl-2-butenyl,
1,1-dimethyl-3-butenyl, 1,2-dimethyl-2-butenyl, 1,2-
dimethyl-3-butenyl,l,3-dimethyl-2-butenyl,l,3-dimethyl-
3-butenyl, 2,2-dimethyl-3-butenyl. 2,3-dimethyl-2-
buteayl, 2,2-dimethyl-3-buteayl, 1-ethyl-2-butenyl. 1-
ethyl-3-buteayl, 2-ethyl-2-buteayl, 2-ethyl-3-buteayl,
1,1.2-trimethyl-2-propenyl, 1-ethyl-1-methyl-2-propeayl
~11~?77
- 12 - O.Z. 0050/42593
and 1-ethyl-2-methyl-2-propenyl, in particular 2-propenyl
and 2-butenyl;
C3-C6-alkyayl, such as 2-propyayl, 2-butynyl, 2-peatynyl,
3-pentynyl, 4-pentynyl, 1-methyl-3-butynyl, 2-methyl-3
butynyl, 1-methyl-2-butynyl, l,l-dimethyl-2-propynyl, 1
ethyl-2-propynyl, 2-hexynyl, 3-hexynyl, 4-hexynyl, 5-
hexynyl, 1-methyl-2-pentynyl, 1-methyl-3-pentynyl, 1-
methyl-4-pentyayl, 2-methyl-3-peatynyl, 2-methyl-4-
pentynyl, 3-methyl-4-pentynyl, 3-methyl-2-pentyayl, 1,1-
dimethyl-2-butynyl,l,l-dimethyl-3-butynyl,l,2-dimethyl-
3-butyayl, 2,2-dimethyl-3-butynyl, 1-ethyl-2-butynyl, 1-
ethyl-3-butynyl, 2-ethyl-3-butynyl or 1-ethyl-1-methyl-2-
propynyl, in particular 2-propynyl aad 2-butynyl;
Z is phenyl,
5-membered hetsryl, such as furanyl, pyrrolyl, thienyl,
imidazolyl, pyrazolyl, isoxazolyl, oxazolyl, isothiazol-
yl, thiazolyl, oxadiazolyl, thiadiazolyl or triazolyl, in
particular furanyl and thienyl, or
6-membered hetaryl, such as pyridyl, pyridazyl, pyrimid
y1, pyrazyl, triazyl, tetrazyl, in particular pyridyl and
pyrimidyl;
X is vitro, cyano,
halogen, such as fluorine, chlorine, bromine and iodine,
is particular fluorine and chlorine
Cl-C,-alkyl, such as methyl, ethyl, n-propyl, 1-
methylethyl, n-butyl, 1-methylpropyl, 2-methylpropyl and
l,l-dimethylethyl, in particular methyl and 1,1-
dimethylethyl,
Cl-C,-alkoxy, such as methoxy, ethoxy, n-propoxy, 1
methylethoxy, n-butoxy, 1-methylpropoxy, 2-methylpropoxy
or 1,1-dimethylethoxy, in particular methoxy, ethoxy, 1
methylethoxy and 1,1-dimethylethoxy,
Cl-C~-alkylthio, such as methylthio, ethylthio, propyl
thio, 1-methylethylthio, butylthio, 1-methylpropylthio,
2-methylpropylthio and 1,1-dimethylethylthio, in par
ticular methylthio and ethylthio,
C1-C,-haloalkyl, such as fluoromethyl, difluoromethyl,
X1152?'~
- 13 - O.Z. 0050/42593
trifluoromethyl, chlorodifluoromethyl, dichlorofluoro-
methyl, trichloromethyl, 1-fluoroethyl, 2-fluoroethyl,
2,2-difluoroethyl, 2,2.2-trifluoroethyl, 2-chloro-2,2-
difluoroethyl, 2,2-dichloro-2-fluoroethyl, 2,2,2-
trichloroethyl, peatafluoroethyl and 3-chloropropyl, in
particular difluoromethyl, trifluoromethyl. 2,2,2-
trifluoroethyl and pentafluoroethyl,
C1-C,-haloalkoxy, such as difluoromethoxy. trifluoro
methoxy, chlorodifluoromethoxy, dichlorofluoromethoxy, 1
fluoroethoxy, 2-fluoroethoxy, 2,2-difluoroethoxy,
1,1,2,2-tetrafluoroethoxy, 2,2,2-trifluoroethoxy, 2-
chloro-1,1,2-trifluoroethoxy and pentafluoroethoxy, in
particular trifluoromethoxy,
carboxyl,
Cl-C,-alkoxycarbonyl, such as methoxycarboayl, ethoxy-
carbonyl, a-propoxycarboayl, 1-methylethoxycarbonyl, n-
butoxycarboayl and l.l-dimethylethoxycsrbonyl, in par-
ticular methoxycarbonyl, ethoxycarboayl and 1,1-dimethyl-
ethoxycarbonyl. especially methoxycarbonyl, as well as
benzyloxycarbonyl, phenyl and pyridyl, where the aromatic
radicals are in each case unsubstituted or in turn may
carry from one to three of the following radicals:
vitro, cyano, carboxyl, beazyloxycarbonyl, halogen as
stated above in general and in particulars C1-C~-alkyl as
stated above, in particular methyl, ethyl and 1-methyl-
ethyl ~ C1-C,,-alkoxy as stated above, in particular methoxy
and ethoxy~ Cl-C,-alkylthio as stated above, in particular
methylthiot C1-C,-haloalkyl as stated above, in particular
trifluoromethyl; Cl-C,.-haloalkoxy as stated above, in
particular difluoromethoxy and trifluoromethoxy, and/or
C1-C,-alkoxycarboayl as stated above, in particular
methoxycarbonyl and ethoxycarbonyl, or
an amino group -NRbR~, where
Rb is hydrogen, Cl-C,-alkyl, C3-C6-alkeayl and C,-C6-alkynyl
as stated in general and in particular for R' and
R° is hydrogen, C1-C,-alkyl, C3-Cg-alkenyl or C3-C6-alkyayl
as stated in general and is particular for R'
211~2'~'~
- 14 - O.Z. 0050/42593
C1-C6-acyl, such as acetyl, propionyl, butyryl,
2-methylpropionyl, peatanoyl, 2-methylbutyryl,
3-methylbutyryl, 2,2-dimethylpropionyl, hexanoyl, 3-
methylpentanoyl, 3-methylpentanoyl, 4-methylpentanoyl,
2,2-dimethylbutyryl, 2,3-dimethylbutyryl, 3,3-dimethyl-
butyryl and 2-ethylbutyryl, in particular acetyl and
propionyl,
or benzoyl, where the aromatic radical may be
unsubstitued or furthermore carry from one to three
radicals selected from a group consisting of vitro,
cyano, carboxyl, benzyloxycarbonyl, halogen as stated
above in general and in particular, Cl-C,-alkyl as stated
above, in particular methyl, C1-C~-alkoxy as stated above,
in particular methoxy or ethoxy, Cl-C,-alkylthio as stated
above, in particular methylthio, Cl-C,-haloalkyl as stated
above, in particular trifluoromethyl, Cl-C,-haloslkoxy as
stated above, in particular difluoromethoxy and
trifluoromethoxy, and C,-C,-alkoxycarbonyl as stated
above, in particular methoxycarbonyl and ethoxycarbonyl.
Particularly preferred cyclohexenone oxime ethers
of the formula I are shown in Table 1 below.
~11~~77
""~' - 15 - 0. Z . 0050/42593
. .. .. ..
' ~ _ .. .. ~
_ _ ...
~
.r .' ~. .... _ Z = S
'r ~ =
N cV N
N N
E c c E E
~ E
cw n ~ ' W
n O w
. ~ ~ s ~ o
~
. s o vo vo
- N
' ~o ~o 'o ~ - z
"
' N N fW W T
t0 r, Y
p, .. .' .... .. .' E .' .' .'
~ _ ~ .' , .'
.'
V = = Z = ~ w ~ ~ ~
~ ~ ~
r W ~- ~ Z ~ ~ ~ r
1 r Z ~ Z
Z
' 'N . " N O N NN N
N N N N N
N
v ' ~ v E E E E
E E E ~ ~
E
..r ~.r w v r v ~r v
v w w
I
=
e7 O O O ~1 O O O O
O O O O vl1 O W
u1
!r1 N N O~ CO Ot N N
...~ h O N N
~_ O~
p ~p tD t0 tp t0 tC ,t ~
r~ n s v .t rw r~
.~ ~D
.~ '
"
E
...' ...~ "" ~ ...~ ...' "
...' ... .
..'
..~
., = _
~ ~ ~'1 (""f ~1 l f'1 P'1 !'~1
N N ? ~ P'1 ~1 f~"1 N N
N O N
W --1 '
.r
N Vf N VI N V1 N VI N
E E N H N W Vf ~
E ' E
w '.... w r v w ~ ....
~.. .v w w rr w r a
~. 1~
I
~ ~ ~ ~ ~ A
~ ~ ~ e'f
Q ~ vl'1
V , O ~ .- e' C
?~ O cv D
~
11 ~ N N N N N N N N N
~ W n N N ;.ID N tO
f~ 1G
E
O
Y1 V
N
1 ~.r
~'I ~'1 ~'1 r~'1 ~'f ~'1'1 ~'1 ~'I '1
a a ''1 ~'1 a c
a a
c a
a ~ G
a, a '~ "~I ~ '~w~ a a a a
~ a ~ a a ~
a ~
a s ~
'
, ~ w w v ~ w w w v v
w w w i i
~ i ~
~ ~ ~
V N ~ ~ ~ V' ~ ~ er ar
/ d' ~' a' ~f' eh ~'
Z /
O
1 I I I
V V V V
11 II a II I I
V = Z Z Z 1 1 O O
I 1
H r n H ~ O I 1
= O
y
Z Z Z V ~y n ~1
V I N
H H I Z
H O
' O
'
N H H Z
_
H N v
N H
V V V (, H Z Z
N Z
U V rv N
y1 N Vf Uf V H ,y
V "r H
N
f~I
z ~ v~ v v
'
L L. L L U u 1 I 1
V I 1
,y +~ r.~ ,r I I
I
.~ ~ '~ T T
T T ?~ ~ p~ d
a O. a O O ~ O
O ~ O ~' T T T
O L L
L T L T
L a a
t
a s a s y y ..1 I
a. ,
,r ~"~ 1 a~ 1 V W r
Gi C C ( UJ IrJ
4J C C
C
W 4J
N f"7 C ~l7 1~ O ~ N
GG OD O
O O O O O O O
O O
<i e1 e1 ei a-1 c1 e-f e-1 e1
a1 e-; ri
zti~277
.~- - 16 - O.Z. 0050/42593
o .. .. .. .. ._ ,. ._ .. _ .., .. .. .. ..
'-'= _ ~=z = z z = =__'_"== ~_
.V h N N N N N S
N N N N N N N
E E E E ~ V ' ~ ~ ' E ' E ~ E E E
u~
.w O O vc'~ en O O u1 ~ O ~ ~ ... coat ~ ~ ~ s Os O O
CO m C~ GO wt1 ,~1
= 1~ 1~
v0 10 10 IC1 1C1 .? ~T .T ~T d ? S ~ ? ~ .J .J
W .. .. .. ., ., .. .. .. .. ~ . . ...~ .. .. .. .. ._
~.. ~ ~ w w w w ~ .~ ~ ... .... ~ w Z ~ S ~ ~r ... w
S : S Z S Z ~ S Z Z = Z ~ Z ~ S t Z Z S
N ~ N ~- N N !'r1 P'1 N1 !~1 R1 i!'1 ~ ~ N N N N N N N N
. . i~'1 . e~1 v v
8 6 E E v _ ~ In H N v1 In v 6 v E ~ E ~ E E
.~ v v ~ ..,r a .",r v /~ ~ ~ .~ ~. ~. ~ .r v r ~ r
x
,r o In o w o w In In In In In In ui o In o m o In o In In In
... .r w.mr ~ P1 P1 !'~'1 ~ N'1 w .r .r .w .~ ~ ~ ~ N N N N N ~ N .~
.? f~ d Pmf1 K1 N N N .? N ? N f'~ N P. .r f'~ ? I~ .t tW1 !w .y e"~ s N1
._ .. .. .. .. .. .. .. .. E .. E .. .. .. .. ~~ ~. .. .. .. .. .. ..
~ w w w ~ w w w w w w ~ ..~ .r .~ w w ,.." w w w w .~ w w ..~
~._ _ _ =r. _= i T. _ _ __ _= T.= _ _ =a
,."~ e~1 ... erg .~. trf trt e~f ... th .. e~"1 O ~"1 O e~'1 ... e!1 ... e~f
... ~'1 .~ e~1 N ~f N t= O e~1 O
_ N1 cr'1 . ~ . . . O . O
H ~ t/1 ~ H V1 III H IR N N H VI H H III H v VI v t/f v 1/1 v 1I1 N
~~ .r v .~ r.. ..r r. .~ rr w w v n, .... ~ v .r ~ ~ .~w ~,.. w .a v ~. v ~r v
f~. v 11
I 1 1 I
en ill ill ill er1 111 Il1 it7 ill u1 !~1 Il1 P1 iCf p Il1 Q ~ 41 1l1 It1 1!1
i'1 Il1 P1 Il1 !~1 O f'1 O
=r~'1 ~. Q1 .~ 01 wr w.. ,r .r ..r wr ~.. Q ... 0 C1 Il'1 01 IC1 ~ m ... ~ .~.
~ .-. (',~ .-. Ice. ~ f~
QI N tap N i0 N N N 1O N 10 N f'~ N f~ .-. tQ ,r ~ N t0 N 1p N Ip N ip N t0 N
iD
C ' ( C C C G
U ~ ~i O O ~-I .-i
-- ;~ s m m ~. ~1 ~.1 ~, n, >, ~, cii a
C G ~ d ~ H
a GW
07 O O s~ r-I .C~ r-1 .C .C O ~ -"~ "U'~ O 0 Q G
H H ~ ~ ~ O ~ O ~ ~ ~ U a a' U ~ ~ a~
N NN~~r'110~1'1~1''lcrf~~NN~'d'NN
Z
V V
eV N
Z = N ;" y = 1 I I
h H = _ ~ r O 0
~. =
~
Q O ~ ~ ~ ~ Z r
H N .
i ~ = T.
H n V H Uev
V V 1 1 I ~ N V V n1
I
H H H n H
H f Z Z = _ '
Z Z i Z Z S o ~ V V V V V V
Z
V V V V V I~ .. v 1 1 t
V
I 1 I I 1 v
1
.. ~ ,~.
r
, ' d T Z 2
T T a .
a y ~ o ... o
.. O ~ ~ G 'r O "~ L _ L y,i L T
O ~'1
a, L r T ' " a t
L . a c a ~ a
a r - a ~ I .r
a 1 r I
~ aJ rr I ~ I ..r C 4J C
1 C 41 C W
~I:1y - 4J 4J C t,u C J
C
f~ C If'7 tw m 07 O e-1 N c~9 Q tf: CO rw OD
GC N
r1 e1 N N N C'! N N N N
ei ei <l e1 e~1 e~ r: e~ e! e~l e1 r1 e-1 e-1 e-1
e-~
~11~277
- 17 - O.Z. 0050/42593
U .. .. .. ., . , _
_ ..
:..
x ~ = - _
_
_
N N .~ .r - eV
N ~
N
B E E E W.i
~ V
O O 5l1 u'1 ~1 K1
e'1 e~
er1 ert IC1 ~ ? .?
n , t~
? s ? s ? ~ ?
d ~
1 .. . .. .. .. ..
~r , _ ~" . ~
.~ ... . S
. .-. ..
Z
.
_ _ _ _
N
N N N N N N
N
O
.r B E E E ' E
V '''
0 0 ~ ~ ~ an o
w ~
... ... ... N N ~. N
....... . O
Z . Z -Z
=
s ~ ,s a ~ s .~ r~
en a a n r:
a
.. .. .. .. .. .,
g ~ E ~ ~ , ...
~ .~ ...~,.,
. .r ... ...
-
.
. ... .~ .
.~. . ..
.
~
=
l~1 e~"1~ ~"'~e'1 w e1 N
O O O O ... w
b O , O ~O
i O i 6
V v v vi v~ ~ V 9
' v in
.a ~ I f~ ~ ~.. V
I~ ~ .r
I
P1 c~1 ~'1 Il1 Ir ~ t11 O
O O O O ~7 ~i1
... ~., ... .-. ~ m ... CD
1~ h m 4~ CO
N ~O N N N N N ~p t0
O t0 1D lp N
C
fy
~Oi ~ ~r ~ ~G~ ?,
E O C O ~ ~ O
-.
Q O
w w
~ ~
N N N ~' sf' N N d'
I I
I I O O I
p O 1 I O
I
I I ~ ~ I t
H w n n O O S
t .r S -
U v' ~ v n
H = S
V y - V
V V V V V
fV N N N N N 'r
_. = r_
V V V V V V V
I I I I I I I
a a a a
T ~ 7s ~ ~ L
li a ~ W C t~s7a c
Q) O e-1 N fry<t Il7
N t"7 C'9 fh M f'7 l'7
e1 ei r1 e1 e1 e~l a~
211527'7
- 18 - O.Z. 0050/42593
.. .. ..
z i _ ~ ~
_ N ~ _
N N N ~ - fJN
E ~ . E
_-
~,
s ~' .a .: s s s .~o
....," ' '
.. .' ..
N N N N r N N
N e
~ ~ E .r i E v,i
N ~. ... E ...r~.. J.. .,. _ .-v
. ~ ..
N O ~ ~
N ~ O O O O O O
N
N ....... N ...N . ...
... ~.'
V ~ S n ~ ~ s ~ ~
~ ~ ~
S s S s s
' .' s
.
.'
n ' .. .' .'~ .' ..E .. ....
- ......IL ~ E
~ .... ~
""
_ _ ,"~' .~ ...--....
W y N ' ' S S S S
r S S
N
E -1 ~ . ...
r O o .-.O o ...o -...
O V E D ~ O E O o O
~ o
.. ... ",~ .....~ E ' E E ~ ' E
.~". ~ s n ~ r
~..
I ... r ..r.. .
rs
00 p o 0 0 0
O
~ ~ ~ ~ 0 0
o O ~
p ; ~
..., ~ ." ~ ,.; ~, ' '
~o ~D .......~D_: ~:~p....r....
~p ~p
C
ac
C C ~ C
t _ ~ d '~ .-a
.-~I
C C a ~ flr
O ' O O
S
O H w w w w s~ p N
D D
C t ~ 0 0 ~ ~
, r
r
U Cj a~ ep ~ ~ W W U
N 1 ~ ~ ~ ~ ( (
T
N d "~ N N N N d' er
S
V
n
Z
V
I I 1 I
O O 1 1 O O
I I O O I I
1 I
a v v
~ ~ ~ v
N N N N _ _
V S S S S Z = i
I
N N
N
N N N N N N V S
S Z S S S = = S
V
a v a v v a v a V
I I I I I I I I ~
I
'- '~ '"I T
a ' a
_ a a a.
o -. o ~ o
'' ~' ~ ~
a r a a .
y a
~
i
I wr I .v I r ( 1
C 4J G lu C 4J ~ W
=
O O O O O O O O O ~
N N N N N N N N N N
211577
~'~ - 19 - O.Z. 0050/42593
a .. ..
~ ~ ~ ..
''' x z x = x = '-
N N N N N
E L E E ~ v E
.. In ~nO p w
~O ~G~?' .s N N
d W t y~ s ~ tG
~
N .. .... .. . .,
= x x = ~- _
V N N N N N N N N N
E V L ~ E E y y vi
V ~/r.. ~ r v
~~ O 1~ O ~ K1 t~1 O O O
V N N N N N N ~ .r
...
II ~ .? n .~fWt ~ ? ~ crf
~ f~'1f'1 u"1
. E ..
N N N N N N N N O
O r-1 O O O N
rr O O O
o ~ E E E
s ~
r ~
tl1 O Il1O IC1 ~ tC~ IC1
O O O O
r~ 0D I~~ n ~ f~ t~
f~ t~ n t~
O t0 O ~D p O O t0
O ~O t0 O
O
C
at
1
N ~
~1 ~l -1
C C C C
O CC ~ ~ a) ~ ri
C ~ C, G O O O
H
o ~ ~ ~ ~ a
0 0 0 0 0 0 0
N ~ iC .C ~: 1 1 I 1 .C
.... .a~ ~ a~ ~ U
.
v ~ ~ ~ ~ 1
x N e!~ ~ N N N N ~1
V
Z
V
n
Z
V
I I
O
~
I O O
I I
a = i o 0
_V _V I 1 V V
N
x x
1
N N H N ~I _N
V V V V V V tj
1 1 I I t i I
.~ r~
a a
a -. o ~. a 0
o 0
'' a s a as'.
t~. W '''rI rr I I
C 4J C 1y C
a-1 N f'7 C' 1l~ CD fw
O O O O O O O
('~ M N C'~ l'7 C'7 M
~11527'~
- 20 - O.Z. 0050/42593
The cyclohexenone oxime ethers I are suitable as
herbicides, in particular for controlling plant species
from the Gramineae family (grasses). In general, they
are tolerated and are thus selective in broad-leaved
crops and in monocotyledon plants which do not belong to
the Gramineae, in particular in corn and rice. Some
derivatives of the compounds I may, however, also exhibit
selectivity in Gramiaeae, so that undesirable grasses can
be selectively controlled.
The cyclohexenone oxime ethers I or the herbi-
cides containing them can be applied, for example, is the
form of directly sprayable solutions, powders, suspen-
sions, including concentrated aqueous, oily and other
suspensions sad dispersions, emulsions, oil dispersions,
pastes, dusting agents, broadcasting agents or granules,
by spraying, nebulizing, dusting, broadcasting or pour-
ing. The application forms depend on the intended uses;
they should in any case ensure a very fine distribution
of the novel active ingredients.
The compounds I are suitable is general for the
preparation of directly sprayable solutions, emulsions,
pastes or oil dispersions. Suitable inert additives are
mineral oil fractions having a medium to high boiling
point. such as kerosene or diesel oil, as well as coal
tar oils and oils of vegetable or animal origin, ali-
phatic, cyclic sad aromatic hydrocarbons, eg. toluene,
xylene. paraffin, tetrahydronaphthalene, alkylated
naphthalenes or derivatives thereof, methanol, ethanol,
propanol, butanol, cyclohexanol, cyclohexanone, chloro-
benzene or isophorone, or strongly polar solvents, such
as N,N-dimethylformamide, dimethyl sulfoxide, N-methyl-
pyrrolidone or water.
Aqueous application forms can be prepared from
emulsion concentrates. dispersions, pastes. wettable
powders or water-dispersible granules by adding water.
For the preparation of emulsions, pastes or oil disper-
sions, the substrates, as such or dissolved in an oil or
~11~277
'" - 21 - O.Z. 0050/42593
solvent, can be homogenized in water by means of wetting
agents, adherents. diapersanta or emulsifiers. However,
concentrates which consist of active iagredieat, wetting
agents, adherents, dispersants or emulsifiers and possib-
1y solvents or oil and which are suitable for dilution
with water can also be prepared.
Suitable surfactants are alkali metal, alkaline
earth metal and ammonium salts of aromatic sulfonic
acids, for example lignin-, phenol-, naphthalene- and
dibutylnaphthaleneaulfonic acid, and of fatty acids,
alkyl- sad alkylarylsulfonatee, alkyl sulfates, lauryl
ether sulfates and fatty alcohol sulfates, and salts of
sulfated hexa-, hepta- sad octadecanols, and of fatty
alcohol glycol ethers, condenaates of aulfonated naph-
thalene and its derivatives with formaldehyde, conden-
satea of naphthalene or of naphthaleaesulfonic acids with
phenol and formaldehyde. polyoxyethyleae octylphenol
ethers, ethoxylated isooctyl-, octyl- or nonylphenol,
alkylphenol polyglycol ethers, tributylphenyl polyglycol
ethers. alkylaryl polyether alcohols, iaotridecyl al
cohol, fatty alcohol/ethylene oxide condensates, ethox
ylated castor oil, polyoxyethylene alkyl ethers or
polyoxypropylene, lauryl alcohol polyglycol ether
acetate, sorbitol esters. ligainsulfite waste liquors or
methylcelluloae.
Powders, broadcasting agents and dusting agents
can be prepared by mixing or milling the active
ingredients together with a solid carrier.
Granules, for example coated, impregnated and
homogeneous granules. can be prepared by binding the
active ingredients to solid carriers. Solid carriers are
minerals such as silica gel, silicas, silicates, talc,
kaolin, limestone, lime, chalk, bole, loess, clay,
dolomite, kieselguhr, calcium sulfate, magnesium sulfate
and magnesium oxide, milled plastics, fertilizers, such
as ammonium sulfate, ammonium phosphate, ammonium nitrate
and ureas, and vegetable products, such as grain flour,
~11a27'~
~""' - 22 - O.Z. 0050/42593
bark meal, wood meal and nutshell meal, cellulosic
powders or other solid carriers.
The formulations contain in general from 0.01 to
95, preferably from 0.5 to 90, % by weight of active
ingredient. The active ingredients are used in a purity
of from 90 to 100%. preferably from 95 to 100% (according
to the Nl~t spectrum) .
Examples of such formulations are:
I. A solution of 20 parts by weight of compound No.
1.03 in a mixture consisting of 80 parts by
weight of xylene. 10 parts by weight of the
adduct of from 8 to 10 mol of ethylene oxide with
1 mol of N-monoethanololeamide, 5 parts by weight
of the calcium salt of dodecylbenzenesulfoaic
acid and 5 parts by weight of the adduct of 40
mol of ethylene oxide with 1 mol.of castor oil.
By finely distributing the mixture in 100,000
parts by weight of water, a dispersion which
contains 0.02% by weight of the active ingredient
is obtained.
II. A dispersion of 20 parts by weight of compound
No. 1.05 in a mixture consisting of 40 parts by
weight of cyclohexanone, 30 parts by weight of
isobutanol and 20 parts by weight of the adduct
of 70 mol of ethylene oxide with 1 mol of
isoocytlphenol and 10 parts by weight of the
adduct of 40 mol of ethylene oxide with 1 mol of
castor oil. A mixture of this dispersion with
100,000 parts by weight of water contains 0.02%
by weight of the active ingredient.
III. A dispersion of 20 parts by weight of compound
No. 1.07 in a mixture consisting of 25 parts by
weight of cyclohexanone, 65 parts by weight of a
mineral oil fraction boiling within a range from
210 to 280°C and 10 parts by weight of the adduct
of 40 mol of ethylene oxide with 1 mol of castor
oil. A mixture of this dispersion with 100,000
211577
- 23 - O.Z. 0050/42593
parts by weight of water contains 0.02% by weight
of the active ingredient.
IV. A mixture, milled in a hammer mill, of 20 parts
by weight of compound No. 1.09, 3 parts by weight
of the sodium salt of diisobutylnaphthalene-a
sulfonic acid, 17 parts by weight of the sodium
salt of a ligninsulfonic acid obtained.from a
sulfite waste liquor and 60 parts by weight of
silica gel powder. By finely distributing the
mixture in 20,000 parts by weight of water, a
spray liquor which contains 0.1% by weight of the
active ingredient is obtained.
V. An intimate mixture of 3 parts by weight of
compound No. I.11 sad 97 parts by weight of
finely divided kaolin. Thin dusting agent
contains 3% by weight of active ingredient.
VI. A stable oily dispersion of 20 parts by weight of
compound No. 1.01, 2 parts by weight of the
calcium salt of dodecylbenzeaesulfoaic acid, 8
parts by weight of fatty alcohol polyglycol
ether, 2 parts by weight of the sodium salt of a
phenolsulfoaic acid/urea/formaldehyde coadeasate
and 68 parts by weight of a paraffinic mineral
oil.
The herbicides or the active ingredients can be
applied by the preemergence or postemargsace method. If
the active ingredients are less well tolerated by certain
crops, it is possible to use application methods in which
the herbicides are sprayed with the aid of the sprayers
in such a way that the leaves of the sensitive crops are
as far as possible not touched, whereas the active
ingredients reach the leaves of undesirable plants
growing underneath or the uncovered soil surface (post-
directed, lay-by).
The application rates of active ingredient are
from 0.001 to 3.0, preferably from 0.01 to 1, kg/ha of
active ingredient (a.3.), depending on the aim of
~11527'~
'"' - 24 - O.Z. 0050/42593
control, the season, the target plants and the stage of
growth.
Because of the wide range of application methods,
the cyclohexeaone oxime ethers I or agents containing
them can be used in a further number
of crops for elimin-
ating undesirable plants. Examples of suitable crops
are
the following:
Botanical name Common name
Allium cepa onions
Ananas comosus pineapples
Arachis hypogaea peanuts (groundnuts)
Asparagus officinalis asparagus
Beta vulgaris spp. altissima sugarbeets
Beta vulgaris app. rapa fodder beets
Brassica napus var. napus rapeseed
Brassica napua var. nspobraesica swedes
Hrassica rapa var. silvestris beets
Camellia sinensis tea plants
Carthamus tinctorius safflower
Carya illinoinensis pecan trees
Citrus limon lemons
Citrus sinensis orange trees
Coffea arabica (Coffea canephora,
Coffea liberica) coffee plants
Cucumis sativua cucumbers
Cynodon dactylon Bermuda grass
Daucus carota carrots
Elaeis guineansis oil palms
Fragaria vesca strawberries
Glyciae max soybeans
Gossypium hirsutum
(Gossypium arboreum, cotton
Gossypium herbaceum,
Gossypium vitifolium)
Helianthus aanuus sunflowers
Hevea brasiliensis rubber plants
Hordeum vulgare barley
zlm~7~
°° - 25 - O.Z. 0050/42593
_Hotanical same Common name
Humulus lupulus hops
Ipomoea batatas sweet potatoes
Juglans regia walnut trees
Lens culinaris lentils
Linum usitatissimum flax
Lycopersicon lycopersicum tomatoes
Malus spp. apple trees
Manihot esculenta cassava
Medicago sativa alfalfa (lucerne)
Muses app. banana plants
Nicotiana tabacum tobacco
(N. rustica)
Olea europaea olive trees
Oryza sativa rice
Phaseolus lunatua limabeans
Phaseolus vulgaris snapb eans~ green
beans dry beans
Picea abies Norway spruce
Pinus app. pine trees
Pisum sativum English peas
Prunus avium cherry trees
Prunus persica peach trees
Pyrus communis pear trees
Ribes sylvestre redcurrants
Riciaus communis castor-oil plants
Saccharum officinarum sugar cane
Secale cereals rye
Solanum tuberosum . Irish potatoes
Sorghum bicolor (s. vulgare) sorghum
Theobroma cacao cacao plants
Trifolium pratense red clover
Triticum aestivum wheat
Triticum durum durum wheat
Vicia faba tick beans
Vitis vinifera grapes
z1m277
- 26 - O.Z. 0050/42593
Botanical name Common name
Zea mat's Indian corn, sweet
corn, maize
To extend the action spectrum and to achieve
synergistic effects, the cyclohexenone oxime ethers I can
be mixed and applied together with a large number of
members of other groups of herbicidal or growth
regulating active ingredients. Examples of components
for the mixture are diazines. 4H-3,1-benzoxazine deriva
tives, benzothiadiaziaones, 2,6-dinitroanilines, N-
pheaylcarbamates, thiolcarbamates, halocarboxylic acids,
triazines, amides, ureas, diphanyl ethers, triazinones,
uracils. benzofuran derivatives, cyclohsxane-1,3-dione
derivatives which carry, for example. carboxyl or
carbimino is the 2-position, quinoliascarboxylic acid
derivatives, imidazolinoaes. sulfonamides, sulfoaylureas,
aryloxy- and hetaryloxypheaoxypropioaic acids and their
salts, eaters and amides and others.
It may also be useful if the compounds I, alone
or in combination with other herbicides, are also applied
as a mixture with further crop protection agents, for
example with agents for controlling pests, phytopatho
genic fungi or bacteria. The miscibility with mineral
salt solutions which are used for eliminating nutrient
and trace element deficiencies is also of interest. It
is also possible to add noaphytotoxic oils and oil
concentrates.
Preparation Examples
2- (1- (4-trees- (4-Fluorophenyl) -but-3-enyloximino]
propyl]-3-hydroxy-5-(1-methylthiocyclopropyl)-2-cyclo
hexen-1-one (compound 1.1)
A mixture of 2.0 g (7.9 mmol) of 3-hydroxy-5-(1-
methylthiocyclopropyl)-2-propionyl-2-cyclohexea-1-one,
1.6 g (9.0 mmol) of O-(4-tracts-(4-fluorophenyl)-but-3-
enyl]-hydroxylamine and 100 ml of methanol was stirred
for 24 hours and then evaporated dower under reduced
pressure. Yield: 100%
zim277
'"'' - 27 - O.Z. 0050/42593
1H-Nl~t (200 I~iz, in CDC13) : b [ppm] = 0.77 (m, 2H) ; 0.97
(m, 2H); 1.10 (t, 3H); 1.60 (m, 1H); 2.13 (s. 3H);
2.40-2.80 (m, 6H); 2.90 (q, 2H); 4.17 (t, 2H); 6.10 (dt,
1H); 6.43 (d, 1H); 7.00 (m, 2H); 7.30 (m, 2H): 14.80
(s. 1H) .
2- [1- (4-traps- (4-Chlorophenyl) -but-3-eayloximino] -
propyl]-3-hydroxy-5-(1-methylthiocyclopropyl)-2-cyclo-
hexen-1-one (compound 1.3)
A mixture of 2.0 g (7.9 mmol) of 3-hydroxy-5-(1
methylthiocyclopropyl)-2-propionyl-2-cyclohexen-1-one,
1.8 g (9.0 nmaol) of O- [4-tracts- (4-chlorophenyl) -but-3
enyl]-hydroxylamine and 100 ml of methanol was stirred
for 24 hours and than evaporated down uader reduced
pressure. Yield: 100%
1H-Nl~t (200 l~z. in CDC1,) : 6 [ppm] = 0.77 (m, 2H) ; 0 . 97
(m, 2H); 1.10 (t, 3H); 1.60 (m, 1H); 2.13 (s, 3H);
2.40-2.80 (m, 6H): 2.90 (q, 2H); 4.20 (t, 2H); 6.20 (dt,
1H); 6.43 (d, 1H); 7.27 (s, 4H); 14.70 (s, 1H).
Use Examples
The herbicidal action of the cyclohexenone oxime
ethers of the formula I was demonstrated by greenhouse
experiments:
The vessels used were plastic flowerpots
containing loamy sand with about 3.0% of humus as a
substrate. The seeds of the test plants were sown
separately according to species.
Ia preemergence treatment, the active ingredients
suspended or emulsified in water were applied directly
after sowing, by means of finely distributing nozzles.
The vessels were lightly watered is order to promote
germination and growth and were then covered with trans
parent plastic covers until the plants had begun to grow.
This covering ensures uniform germination of the test
plants, unless germination had been adversely affected by
the active ingredients.
For the postamergence treatment, the test plants
- 28 - O.Z. 0050/42593
were initially grown in the test vessels or were planted
in the test vessels a few days beforehand. The active
ingredients suspended or emulsified in water were not
applied until a height of growth of from 3 to 15 cm had
been reached, depending on the form of growth. The
application rate for the postemergence treatment was 0.25
kg/ha of a.i.
The plants were kept at 10-25°C or 20-35°C,
according to species. The test period extended over 2 to
4 weeks . During this time. the plants were tended and
their response to the individual treatments was
evaluated.
Evaluation was based on a scale of from 0 to 100.
100 means no emergence of the plants or complete destruc
tion of at least the above-ground parts and 0 means no
damage or normal growth.
The plants used in the greenhouse experiments
consisted of the following species:
Botanical name Coam~on name
Echinochloa crus-galli barnyard grass
Setaria faberii Giant foxtail
Setaria viridis Green foxtail
The result showed that gramineous plants can be
very readily controlled postemergence with compound No.
1.05.