Note: Descriptions are shown in the official language in which they were submitted.
211~72~
- 1 -
PH/5- 19450/A
Phenyliminothiadiazabicyc}ononanone salts havin~ herbicidal properties
The present invention relates to novel phenyliminothiadiazabicyclononanone salts and to
the use thereof as herbicides.
Herbicidally active phenyliminothiadiazabicyclononanone derivatives are disclosed, inter
alia, in EP-A-0 238 711~ EP-A-0 273 417, EP-A-0 312 064 and JP Patent Kokai Hei
1-186894. These derivatives carry different substituents at the phenyl ring.
Salts of this class of compounds having good herbicidal properties have now been found.
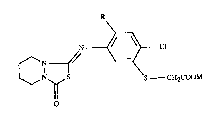
Accordingly, the invention relates to the compounds of formula I
N ~ Cl
--N // \=~
~N~S S CH2COOM (I),
wherein
R is hydrogen or fluoro; and
M is an alkali metal ion or an alkaline earth metal ion or an ammonium cation.
Preferred alkali metal and alkaline earth metal hydroxides as salt formers are the
hydroxides of lithium, sodium, potassium, magnesium or calcium, more particularly those
of sodium or potassium.
Illustrative examples of amines suitable for salt-forrnation are primary, secondary and
tertiary aliphatic and aromatic amines, typically methylamine, ethylamine, n-propylamine,
isopropylamine, the four isomeric butylamines, n-amylamine, isoamylamine, hexylamine,
heptylamine, octylamine, nonylamine, decylamine, pentadecylamine, hexadecylamine,
211~72~i
heptadecylamine, octadecylamine, methyl ethylamine, methyl isopropylamine, methyl
hexylamine, methyl nonylamine, methyl pentadecylamine, methyl octadecylamine, ethyl
butylamine, ethyl heptylamine, ethyl octylamine, hexyl heptylamine, hexyl octylamine,
dimethylamine, diethylamine, di-n-propylamine, diisopropylamine, di-n-butylamine,
di-n-amylamine, diisoarnylamine, dihexylamine, diheptylamine, dioctylamine,
ethanolamine, n-propanolamine, isopropanolamine, N,N-diethylethanolamine,
N-ethylpropanolamine, N-butylethanolamine, allylamine, n-buten-2-ylamine,
n-penten-2-ylamine, 2,3-dimethylbuten-2-ylamine, dibuten-2-ylamine, n-hexen-2-ylamine,
propylenediamine, diethanolamine, trimethylamine, triethylamine, tri-n-propylamine,
triisopropylamine, tri-n-butylamine, triisobutylamine, tri-sec-butylamine, tri-n-amylamine;
heterocyclic amines such as pyridine, quinoline, isoquinoline, morpholine,
thiomorpholine, N-methylmorpholine, N-methylthiomorpholine, piperidine, pyrrolidine,
indoline, quinuclidine and azepine; primary arylamines such as anilines, methoxyanilines,
ethoxyanilines, o-, m- and p-toluidines, phenylendiamines, benzidines, naphthylamines
and o-, m- and p-chloroanilines.
Typical examples of quaternary ammonium bases are the cations of haloammonium salts,
including the tetramethylammonium cation, the trimethylbenzylammonium cation, the
triethylbenzylammonium cation, the tetraethylammonium cation, the trimethylethylammo-
nium cation, and also the ammonium cation.
The invention further relates to herbicidal compositions that contain the novel compounds
as active ingredients, to the preparation of said compounds, and to the use of said
compounds and compositions for selective weed control.
The process of this invention for the preparation of the compounds of formula I is carried
out in general accordance with known processes, inter alia as described in EP-A-O 312 064
and ~.P-A-O 468 924, and comprises thiophosgenating a compound of formula ll
R
H2N cl (Il)
~ .. ,
S--CH2COOM
under standard conditions, to the isothiocyanate of forrnula III
: '
.
:
~` 2~1~72~
SC~ C~
S--CH2COOM
and subsequently reacting said isothiocyanate with the hexahydropyridazine of formula V
~NH
~NH ~(V)
to the compound of formula IV
S R
C ~<CI (IV)
S--CH2C.OOM
in which compounds of formulae II, m and IV R is hydrogen or fluoro; and ~ is an aLkali
metal ion or an aL~aline earth metal ion or an ammonium cation, and sub~equently reacting
the compound of formula IV with phosgene, diphosgene (t~ichloromethyl chlorofo~mate)
or triphosgene (bis(trichlorome~yl)carbonate) to give the c~mpound of fc~rmula L
This process is illustrated in more detail in the follow~ng reaction scheme.
Reaction scheme:
R R .::
H2N~- Cl Cl-CtS)-CI ~ ~ S=C=N~ Cl : .:
Cl-CH2CH2~1 /H20 \~
CaC03
S----CH2COOM S--CH2C~OOM
II ll[I
2~15725
- 4 -
~NH S R
C ~ c~ Cl-C(O~CI
C2H50H NH CI~F12CH2-CI
S--C}12COOM
IV
N~CI
~1 ~ ~<
I~N ~ S S--CH2COOM
I
The isothiocyanate of formula III is obtained in per se known manner by thiophosgenating
the appropriate amine of formula II. Such processes are desc;ribed, inter alia, in
EP-A-0 304 920, pages 53 and 55; EP-A-0 312 064, page 11, last paragraph, and page 12,
lines 12-18; and EP-A-0 468 924, page 21, lines 29-47, page 22, Example H2, and
page 27, Example H17.
The compound of folmula IV is obtained in per se known m~mner by reacting the
isothiocyanate of formula III with hexahydropyridazine of formula V. 5Ud~l reactions are
described, inter alia, in l~P-A-0 312 064, page 11, last paragr~ph, page 12, lines 19-24;
EP-A-0 468 924, page 16, last paragraph, and page 17, first paragraph, page 19,
lines 42-46 and page 22, Example H3.
The cyclisation of the hexahy~ pyridazinylthiocarbonylamino derivative of fonnula IV
may conveniently be carried out with phosgene, diphosgene (trichloromethyl
chloro~ormate) or tdphosgene (bis(trichloromethyl)carbonate) in an inert solvent at low
temperature, preferably in the range from 0 to 50C, most pn~ferably from 0 to 15C Such
cyclisadon reactions are described, inter alia, in EP-A-0 312 064, page 11, lines 1-40 and
13xamples 1 to 5 on pages 13 and 14; and in EP-A-0 468 924., page 19, lines 47-51,
page 23, Exarnple H4 and page 28, Example H19. ~ ~
The intermediates of formulae III and IV are novel and have been specially developed for ~ -
the synthesis of the compounds of fonnula I. They lilcewise constitute an object of the
211~725
invention.
The preparation of the starting compound of formula II is described in ~P-A-0 126 419.
The hexahydropyridazine of formula V is known or can be !prepared by processes known
from the literature, inter alia in Bull. Soc. Chim. France 1957, 704; EP-A-0 304 920,
pages 9-11 (schemes 2-4); and l~P-A-0 468 924, page 19, last paragraph, page 20, first
paragraph and page 28, ~xample H18.
The fina~ product of formula I can be isolated in conventional manner by cautious
concentration and/or evapora~on of the solvent under vacuum, and by recysta~lising or
trituralting the solid residue in a solvent in which it is not readily soluble, typically an
ether, an alromatic hydrocarbon or a chlorinated hydrocarbon.
The compounds of formula I or compositions containing them may be applied in thepractice of this invention by all standard methods of application used in agIiculture,
typica~ly preemergence application, postermergence applica~tion and seed dressing, as well
as using different methods and techniques, for example controlled release. ~or controlled
release, a solution of the herbicide is applied to a mineral glanular ca~ier or to a
polymerised granulate (urea/formaldehyde) and the dried. A coating can then be
additionally applied (coated granules) that allows the herbicide to be released at a
controlled rate over a specific period of time.
The compounds of formula I may be used in unmodified folm, i.e. as obtained in the
synthesis, but preferably they are processed in conventional manner with the assistants
custornarily employed in formulation technology to emulsifiable concenlrates, directly
sprayable or dilutable solutions, dilute emulsions, wettable powders, soluble powders,
dusts, granulates or microcapsules. Just as with the type of compositions, the methods of
application such as spraying, atomising, dusting, scattering ~M pouring, are chosen in
accordance with the intended objectives and the prevailing c ircumstances.
The formulations, i.e. the compositions containing the compound of formula I and usually
one or more than one formulation assistant, are prepared in lknown manner, e.g. by
homogeneously mixing and/or grinding the herbicide with extenders, typically solvents,
solid carriers or surface-active compounds (surfactants).
21~72~
- 6-
Suitable solvents are typically: aromatic hydrocarbons, preferably the fractions containing
8 to 12 carbon atoms such as of alkylbenzenes, typically xylene mixtures or alkylated
naphthalenes; aliphatic and cycloaliphatic hydrocarbons such as paraffins, cyclohexane or
tetryhydronaphthalene; alcohols such as ethanol, propanol or butanol; glycols and their
ethers and esters such as propylene glycol or dipropylene glycol ether, ketones such as
cyclohexanone, isophorone or diacetone alcohol; strongly polar solvents such as
N-methyl-2-pyrrolidone, dimethyl sulfoxide or water; vegetable oils and their esters such
as rapeseed oil, castor oil or soybean oil; and also silicone oils.
The solid carriers typically used for dusts and dispersible powders are usually natural
mineral fillers such as calcite, talcum, kaolin, montmorillonite or attapulgite. To improve
the physical properties it is also possible to add highly dispersed silicic acid or highly
dispersed absorbent polymers. Suitable granulated adsorptive carriers are porous types,
including pumice, broken brick, sepiolite or bentonite; and suitable nonsorbent carriers are
materials such as calcite or sand. In addition, innumerable pregranulated materials of
inorganic or organic origin may be used, especially dolomite or pulverised plant residues.
Depending on the ~ype of compound of formula I to be formulated, suitable surface-active
compounds are nonionic, cationic andlor anionic surfactants having good emulsifying,
dispersing and wetting properties. Surfactants will also be understood as comprising
mixtures of surfactants.
Suitable anionic surfactants may be water-soluble soaps as well as water-soluble synthetic
surface-active compounds.
Suitable soaps are the alkali metal salts, alkaline earth metal salts, ammonium salts or
substituted ammonium salts of higher fatty acids (C10-C22), e.g. the sodium or potassium
salts of oleic or stearic acid, or of natural fatty acid mixtures which can be obtained, inter
alia from coconut oil or tallow oil. Further suitable soaps are also the fatty acid methyl
taurin salts.
.
More often, however, so-called synthetic surfactants are used, especially fatty sulfonates,
fatty sulfates, sulfonated benzimidazole derivatives or alkylarylsulfonates.
The fatty alcohol sulfonates or sulfates are usually in the form of alkali metal salts,
alkaline earth metal salts, ammonium salts or substituted ammonium salts, and they
,~f~
2~1572~
contain a C8-C2~aL~cyl radical which also includes the alkyl moiety of acyl radicals, e.g. the
sodium or calcium salt of ligninsulfonic acid, of dodecylsulfate, or of a mixtur~ of fatty
alcohol sulfates obtained from natural fatty acids. These compounds also comprise the
salts of sulfated and sulfonated polyadducts of fatty alcohols and ethylene oxide. The
sulfonated benzimidazole derivatives preferably contain 2 sulfonic acid groups and one
fatty acid radical containing 8 to 22 carbon atoms. Exarnplary aLkylarylsulfonates are the
sodium, calcium or triethanolamine salts of dodecylbenzenesulfonic acid, dibutylnaphtha-
lenesulfonic acid, or of a condensate of naphthalenesulfonic acid and formaldehyde.
Corresponding phosphates, typically salts of the phosphated polyadduct of p-nonylphenol
with 4 to 14 mol of ethylene oxide, or phospholipids, are also suitable.
Nonionic surfactants are preferably polyglycol ether derivatives of aliphatic orcycloaliphatic alcohols, or saturated or unsaturated fatty acids and aLIcylphenols, said
derivatives containing 3 to 30 glycol ether groups and 8 to 20 carbon atoms in the
(aliphatic) hydrocarbon moiety and 6 to 18 carbon atoms in the aLtcyl moiety of the
alkylphenols.
Further suitable nonionic surfactants are the water-soluble polyadducts of polyethylene
oxide with polypropylene glycol, ethylenediaminopolypropylene glycol and
ylpolypropylene glycol containing 1 to 10 carbon atoms in the aL~cyl chain, which
polyadducts contain 20 to 250 ethylene glycol et~ er groups and 10 to 100 propylene glycol
ether groups. These compounds usually contain 1 to 5 ethylene glycol units per propylene
g1ycol unit.
Illustrative examples of nonionic surfactants are nonylphenol polyethoxyethanols,
polyethoxylated castor oil, polyadducts of polypropylene and polyethylene oxide, tributyl-
phenoxy polyethoxyethanol, polyethylene glycol and octylphenoxy polyethoxyethanol.
Fatty acid esters of polyoxyethylene sorbitan are also suitable nonionic surfactants,
typically polyoxyethylene sorbitan trioleate.
Cationic surfactants are pre~erably quaternary amrnonium salts carrying, as N-substituent,
at least one C8-C22alkyl radical and, as further substituents, unsubstituted or halogenated
lower aLl~yl, benzyl or hydroxy-lower aLlcyl radicals. The salts are preferably in the form of
halides, methyl sulfates or ethyl sulfates, for example stearyl trimethylammonium chloride
211~72~
or benzyl bis~2-chloroethyl)ethylammonium bromide.
The surfactants customarily employed in the art of formulation are described, inter alia, in
"Mc Cutcheon's Detergents and Emulsi~lers Annual", Mc Publishing Corp.,
Glen Rock,New Jersey, 1988, H. Stache, "Tensid-Taschenbuch" (Handbook of
Surfactants), Carl Hanser Verlag, Munich/Vienna 1981, and M. and J. Ash, "Encyclopedia
of Surfactants", Vol I-III, Chemical Publishing Co., New York, 1980-81.
The herbicidal compositions will usually contain from 0.1 to 99 % by weight, preferably
from 0.1 to 95 % by weight, of a compound of formula I, from 1 to 99.9 % by weight of a
solid or liquid adjuvant, and from 0 to 25 % by weight, preferably from 0.1 to 25 % by
weight, of a surfactant.
Whereas it is preferred to formulate commercial products as concentrates, the end user
will norrnally use dilute formulations.
The compositions may also contain further ingredients such as stabilisers, vegetable oils or
epoxidised vegetable oils, (epoxidised coconut oil, rapeseed oil or soybean oil), antifoams,
typically silicone oil, preservatives, viscosity regulators, binders, tackifiers, as well as
fertilisers or other chemical agents for obtaining special effects.
In particular, preferred formulations are made up as follows (throughout, percentages are
by weight):
Emulsifiable concentrates: ~ -
herbicide: 1 to 90 %, preferably 5 to 50 %
surfactant: 5 to 30 %, preferably 10 to 20 %
liquid carrier: 15 to 94 %, preferably 7() to 85 %
Dusts:
herbicide: 0.1 to 50 %, preferably 0.1 to 1 %
solid calTier: 99.9 to 90 %, preferably 99.9 to 99 %
- 21~572~
Suspension concentrate:
herbicide: 5 to 75 %, preferably 10 to 50 %
water 94 to 24 %, preferably 88 to 30 ~/o
surfactant: 1 to 40 %, preferably 2 to 30 %
Wettable powder:
herbicide: 0.5 to 90 %, pref~rably 1 to 80 %
surfactant: 0.5 to 20 %, preferably 1 to 15 %
solid carrier: S to 95 %, preferably 15 to 90 %
Granulate:
herbicide 0.5 to 30 %, preferably 3 to 15 %
solid carrier: 99.5 to 70 %, preferably 97 to 85 %
The compounds of formula I are usually applied with success in concentrations of 0.001 to
2 kg/ha, preferably 0.005 to 1 kg/ha. The concentration required to achieve the desired
action can be determined by experimentation. It will dependl on the type of action, the
development stage of the cultivated plant and of the weed, as well as on the application
(locus, time, method) and, based on these parameters, can vary over a wide range.
The compounds of forrnula I have excellent herbicidal properties, which make them
preeminently suitable for application in crops of cultivated plants, especially in cereals,
rice, maize and soybeans.
Crops will be understood as meaning also those that have been made tolerant to herbicides
or classes of herbicides by conventional breeding or genetic engineering methods.
The invention is illustrated by the following non-limitative Examples.
211~725
- 10-
, . .
1. Preparation of the novel compounds of formula I
ExampleP1: 1-(2-Chloro-4-fluoro-5-isothiocYanatophenYlthio)aceticacid
S=C N~ Cl (III)
S--CH2COOH
A solution of 11.8 g of 1-(5-amino-2-chloro-4-fluorophenylthio)acetic acid in 100 ml of
ethylene chloride is added dropwise, with stirring, at room temperature to ia mixture of
7.5 g of calcium carbonate, 5 ml of thiophosgene in 100 ml of of ethylene chloride and
100 ml of water. After stirring for 6 hours at room temperature, the solid constituents of
the reaction mixture are isolated by filtration and taken up in a mixture of ethyl
acetate/water. A solution of 2N hydrochloric acid is stirred ,lnto the resultant suspension
and stirring is continued for 1/2 hour. The organic phase is separated, dried over sodium
sulfate and then concentrated by evaporation, giving 11.5 g of 1-(2-chloro 4-fluoro-5-iso
cyanatophenylthio)acetic acid with a melting point of 11 8C.
Example P2: 1-1 2-Chloro-4-fluoro-5-( 1 -hexahYdropYridazinvlthiocarbonYlamino)
~henYlthiolacetic acid
S
CN_C_HN~CI (IV)
S--CH2COOH
A solution of 11.5 g of 1-(2-chloro-4-fluoro-5-isothiocyana~tophenylthio)acetic acid in
100 ml of ethylene chloride is added dropwise, with stirring;, at room temperature to a
solution of 5.0 g of hexahydropyridazine in 100 ml of ethanol. After stining for 3 hours at
room temperature, the reaction mixture is consentrated by evaporation under vacuum,
giving 14.5 g of 1-[2-chloro-4-fluoro-5-~1-hexahydropyridaLzinylthiocarbonylamino)-phen-
ylthio]acetic acid with a melting point of 110-118C.
-^ 211~72~
~ixample P3: 9-1 4-Chloro-2-fluoro-5-( 1 -oxycarbonylmethvlthio)phenvliminol-8-thia-
1 .6-diazabicYclo~4.3.01nonan-7-one
N Cl
CN~S S_CH~COO~I (la)
A solution of 14.5 g of 1-[2-chloro-4-fluoro-5-(1-hexahydropyridazinylthiocarbonylami-
no)phenylthio]acetic acid in 100 ml of ethylene chloride is added dropwise, with stirring,
at 0-5C to 40 ml of a 20 % solution of phosgene in toluene. The reaction mixture is
stirred at room ternperature for 6 hours and then poured into ice-water. The organic phase
is separated, dried over sodium sulfate and concentrated by evaporation under vacuum,
giving 15.0 g of 9-[4-chloro-2-fluoro-5-(1-oxycarbonylmethylthio)phenyliminoJ-8-thia-
1,6-diazabicyclo-[4.3.0]nonan-7-one with a melting point of 170-172C.
Example P4: Sodium salt of 9-~4-chloro-2-fluoro-5-(1-oxvcarbonylmethv;lthio)-
phenvlirninol-8-thia-1 ,6-diazabicvclor4.3.0lnonan-7-one
F
~N ~ Cl
CN~S S_CH~COON.
0.28 g of sodium methylate is added at room ternperature to a suspension of 1.94 g
(0.005 mol) of 9-[4-chloro-2-fluoro-5-(1-oxycarbonylmethylthio)phenylimino]-8-thia-
1,6-diazabicyclo[4.3.0]nonan-7-one in 75 ml of methanol, wherupon the suspension goes
into solution. Stirring is continued for 15 minutes at room temperature and the solvent is
then cautiously rernoved by evaporation under vacuum. The residual glassy product is
digested in diethyl ether, giving 1.7 g (83 % yield) of the desired sodiurn salt of 9-[4-chlo-
- 211~725
ro-2-fluoro-5-(1-oxycarbonylmethylthio)phenylimino]-8-thia- 1 ,6-diazabicycloL4.3.0~no-
nan-7-one as a powder; m.p. 203C.
Example P5: Sodium salt of 9-~4-chloro-5-(1-oxYcarbonvlrnethvlthio)PhènYlimino1-8-thia-1 ~6-diazabicvclof4.3.01nonan-7-one
N~CI
~N~
I~N~S S--C}12COC)Na (1.002)
O
is obtained in general accordance with the procedure described in Example P4: :
m.p. 208-215C.
The compounds of forrnula I listed in Table 1 can be prepared in analogous manner.
211572~
- 13-
Table 1: Compounds of formula I
R
\~_
N ~/ \\~--Cl
--`N~
s----cH2coo M
Cmpd. R M Physical data
1.001 F Na m.p. 203C
1.002 H Na m.p. 208-215C
1.003 F H2N o m.p. 120C
1.004 H H2N o
1.005 F Li
1.006 H Li
1.007 F K
1.008 H K
1.009 F Mg
1.010 H Mg
1.011 F Ca
1.012 H Ca
1.013 F NH4
1.014 H NH4
1.015 F OEI3NH3
1.016 H OEI3NH3
1.017 F (CH3)2N~2
1.018 H (CH3)2NH2
1.019 F (CH3)3N~
1.020 H (C~3)
1.021 F C2HsNH3
1.022 F (c2Hs)2N~2
r~ 2115725
- 14-
Cmpd. R M Physical data
1.023 F (C2Hs)3NnH
1.024 p C4HgNH3
1.025 F (C4~s)4N
1.026 F HOCH2CH2~n~3
1.027 H HOCH2CH2NH3
1.028 F ~HOOEI2OEI2)2NH2
1.029 H ~HocH2cH2)2NnH2
1.030 F (HOCH2CH2)3NH
1.031 H (HOCH2CH2)3NH
1.032 F C6Hs-CH2NH3
1.033 H C6Hs-CH2NH3
1.034 F C6Hs-NH3
1.035 H C6Hs-NH3
1.036 F HsC2OCO-CH2NH3
1.037 NH2
1.038 H ~l2
1.039 F H2N
1.040 H H2N
1.041 F ~1
1.042 F HN~
1.043 H HN~
1.044 F H2C=CH-C~H2NnH3
1.045 H H2C=CH-C~H2NnH3
1.046 F ~C=~-CH2N~l3
1.047 H Hc~c-cH2NH3
-~ 211572~ :
2. Formulation Examples for compounds of forrnula I (throu~hout. percenta~es are by
wei~ht)
, .~ .
F1. Wettable powder a) b) c)
compound of formula I 25 % 50 % 75 %
sodium ligninsulfonate 5 % 5 %
sodium laurylsulfate 3 % - 5 %
sodium diisobutylnaphthalene-
sulfonate - 6 % 10 %
octylphenoxy polyethoxyethanol
(7-8 mol EO) - 2 %
highly dispersed silica 5 % 10 % 10 %
kaolin 62 % 27 %
The active ingredient is thoroughly mixed with the adjuvants and the mixture is
thoroughly ground in a suitable mill to give wettable powders which can be diluted with
water to give suspensions of any desired concentration.
F2. Emulsifiable Concentrate
compound of formula I 10%
calcium dodecylbenzenesulfonate 3 %
octylphenoxy polyethoxyethanol
(4-5 Mol EO) 3 %
polyethoxylated castor oil
(36 mol EO) 4 %
cyclohexanone 30 %
xylene mixture 50 %
Emulsions of any desired concentration can be prepared from such concentrates bydilution with water.
F3. Dusts a) b)
compound of formula I 5 % 8 %
talcum 95 %
kaolin - 92 %
-~ 211~7~5
- 16-
.
Ready for use dusts are obtained by intimately mixing the carriers with the active
ingredient.
.:, :
F~. Extruder ranulate ~;
compound of formula I 11) %
sodium ligninsulfonate 2 %
carboxymethyl cellulose 1 %
kaolin 87 ~/o
The active ingredient i9 mixed with the adjuvants, the mixture is ground and moistened
with water. Tnis mixture is extruded and subsequently dried in a strearm of air.
F5. Coated ~ranulate
compound of formula I 3 %
polyethylene glycol 200 3 %
kaolin 94 %
The finely ground active ingredient is uniformly applied to the kaolin moistened with
polyethylene glycol in a mixer to give a non-dusting coated granulate.
F6. Suspension concentrate
compound of formula I 40%
propylene glycol 10 %
nonylphenoxy polyethoxyethanol
(15 mol EO) 6 %
sodium ligninsulfonate 10 %
carboxymethyl cellulose 1 %
silicone oil in the form of a 75 %
aqueous emulsion 1 %
water 32 %
The finely ground active ingredient is intimately mixed with the adjuvants to give a
suspension concentrate from which suspensions of any desired concentration can be
prepared by dilution with water.
:~ 2 1 1 5 7 2 ~ ~
., .
- 17 -
3. Biolo~ical Examples
Example B 1: Pre-emer~ence herbicidal action
Dicot test plants are sown in plastic pots in standard soil. Immediately after sowing, an
aqueous suspension of the test compound prepared from a 25 % wettable powder
(l~xample F1, a)) is sprayed on to the plants in a concentration of 2 kg a.i./ha (5001 of
water/ha). The test plants are then cultivated in a greenhouse under optimum conditions.
The test is evaluated after 3 weeks on a rating scale from 1 to 9 (1 = total damage, 9 = no
action). Ratings from 1 to 4 (especially from 1 to 3) denote good to very good herbicidal
action.
Table B1: Preemer~ence Action:
Cornpound of Test plant:
formula Sinapis Solanum Ipomoea Seta- Cyperus
ria
1.001 1 1 4 2 3
1.0û2 1 1 4 2 3
1.003 1 1 1 4 2
The same results are obtained by formulating the compounds of formula I according to
Examples F2 to F6.
Example B2: Post-emer~ence herbicidal action (contact herbicide)
Dicot plants are cultivated in a greenhouse in plastic pots with standard soil and sprayed in
the 4- to 6-leaf stage with an aqueous suspension prepared from a 25 % wettable powder
(Example F1, a)) of the test compound in a concentration of 2 kg a.i./ha (50û 1 of
water/ha). The test plants are then cultivated in a greenhouse under optimum conditions.
The test is evaluated after about 18 days on a rating scale from 1 to 9 (1 = total damage,
9 = no action). Ratings from 1 to 4 (especially from 1 to 3) deonote good to very good
herbicidal action.
21t572~
-18-
Table B2: Post-emergence action
Compound of Test plan~:
fonnula Sina- Sola- Ipo- Seta- Stel- Cyperus
pis num moea ria laria
1.001 1 1 1 3 2 3
1.002 1 1 1 3 2 3
1.003 1 1 1 1 3 4
The same results are obtained by formulating the compounds of formula I according to
E~xtttttples F'.t to 1~6.