Note: Descriptions are shown in the official language in which they were submitted.
2~~. ~~~~
2
The invention relates to a process for the preparation of
membrane-coated fertilizer granules for demand-related plant
feeding.
Nowadays, modern, environmentally acceptable and demand-related
plant feeding is based to an increasing extent on long-acting
fertilizers which are characterised by chemical modification of
,the nutrients or by coating soluble, fertilizer granules.
Coated long-acting fertilizers are described in numerous patents.
Urea formaldehyde resins, PE,'PP, alkyd resins, epoxy resins and
recently polyurethane resins, amongst others, are used as coating
agents (e.g., DE 3 544 451, US 3 264 088, GB 1 O11 463, EP 0 276
179, DE 2 834 513, US 3 223 518, NL patent 129 279). With the
systems mentioned, it has proved more or less possible to modify
the nutrient releases over time so that release periods can be
achieved for fairly short o.r fairly long growing times.
A disadvantage of the well known methods is that, until now,
parameters for demand-related plant feeding, e.g., homogeneity
of the individual particle coating, physical resilience, frost
resistance, could be solved only incompletely, if at all. In
particular, it has not been possible hitherto to control the
release of individual nutrients from special salt mixtures in
such as way that e.g., the release of potassium is delayed to a
greater extent compared with nitrogen.
The aim of the invention is to overcome the well known
disadvantages. An economic, industrial production process has
now been found which, with sufficient homogeneity of the
individual particle coating, makes it possible to produce
physically resilient, frost-resistant granulated material From
which the controlled, delayed release of nutrients takes place
as defined by Fick~s laws of diffusion.
An important prerequisite for obtaining high mechanical
resilience is the selection of a highly resilient coating
J
3
material which, when it is applied to the fertilizer granules,
shows its mechanical properties to their best advantage.
A solvent-free two-component polyurethane resin system -that can
be processed at room temperature and which can be cured by amine
catalysis within very short periods (compare DE 3544 451) has
proved to be suitable for coating according to the invention.
The coating process according to the invention is carried out in
a drum which is provided with special attachments. on the one
hand, specially shaped mixing blades.ensure the homogeneity of
the mixture, and on the other hand air channels lead into the bed
of granulated material so ws to be able to aerate said bed
intensively. The reaction takes place in a closed system.
The granules for said coating process must be largely spherical,
low-dust, attrition-resistant, break-proof and the nutrients
contained therein must be largely water-soluble. A narrow
particle size distribution of the granules is advantageous, the
factor 3 between the smallest and the largest particles of
granulated material being sufficient.
A coating process is described in DE 35 44 451 in which, with a
planned granulated material coating of 15% by wt. coating, based
on the end product, the coating compound composed of poly-
isocyanate and polyol is deposited 3 times in 5% portions with
simultaneous permanent catalyst gassing with amine-saturated
nitrogen gas at 2f °C. The outcome of such a coating leads to
an initial release of up to 18% after 24 hours (example 1).
The release of electrolytes from plastics-coated fertilizers in
an aqueous solution is determined by measuring the conductivity.
To this end, 10.0 g of the sample to be tested are added to 800
ml of water with a conductivity of less than 0.5 us/cm.~ The
water is stirred for t days at a constant rate of rotation of 300
rpm at 25 °C. The change in the salt content of the solution
~1~. i~~~
4
after t days is determined by conductimetry.
In--order to draw up a calibration curve, the~~c~ondudtivity is
measured .. in solutions with 0. 2; 0. 4; 0. 6; 0. 8; 1. 0; 2 . 0; 4 . 0;
6.0; 8.0 and 10.0 g of dissolved sample in 800 ml of water, the
conductivity being measured in mS/cm.
The rate of release R is then determined on the basis of the
calibration curve, the mean rate of release per day Rn, being
determined by R,~ - Rt,/t2 - t1 where Rte = rate of release after t1
days and R,2 = rate of release after t2 days.
Although the basic flow characteristic curve reveals a delay in
nutrient release, it does not give all the indications of the
presence of a true membrane. The following are to be regarded
as indices. of membrane-controlled diffusion (membrane function):
a) a measurable membrane activation time and
b) the applicability of Fick's law of diffusion according to the
following differential equation:
2~3.~~~~
dmi - - X x - 1 x .~ci (T) x F x T
dt n(T) f (ri) D
mi molar concentration of the substance i
t time
K constant: R/(6 ~r N) R: gas constant
N: Avogadro's constant
n(T) Viscosity membrane/solution (substance property of the
coating if well executed)
~ci(T) ' Concentration gradient of the substance i (substance
property of the care composition)
f(ri) Velocity factor of the particle i with the radius r
(substance property of the core composition)
F Membrane surface area (particle size distribution and surface
structure of the granules)
D Membrane thickness (effective thickness of coating deposited)
T Temperature in °Kelvin
~~.i ~a
6
The release of the nutrients in the time unit is dependent not
only on the total membrane surface, the membrane thickness and
' "the "'tem~ierature but ~ ahso ~ essentially 'on the concentration
difference between the individual dissolved substances inside the
coated granules and the solution surrounding the granules.
Surprisingly, it has now been found that if the process is
' carried out in a particular way it is possible to produce
granules which, in terms of their release characteristics,
exhibit X11 the typical features of membrane-controlled
diffusian. With the process accarding to the invention, coated
granules were obtained which have a measurable characteristic
membrane activation time. The subsequent nutrient release
likewise confirms the active separation of the reserve of active
substances from the surrounding solution.
In arder to produce this complete membrane as a diffusion
regulator, the coating material to be applied~consisting of
polyisocyanate and polyol is metered in such a way that layer
thicknesses of 10 to 30 um, preferably 15 to 25 ~m are not
exceeded. In the case of a granulated material in the particle
size range of 2 to 4 mm and a median value of 3.0 mm, this means
a partial quantity of approx. 1 to 3% to be deposited,
particularly less than 2% by mass, based on the mass of granules
to be coated.
After a defined distribution and spreading time, this proportion.
of coating compound is caused to react spontaneously by means of
a highly concentrated amine mist as catalyst. The highly
concentrated amine mist is produced directly from undiluted amine
under airless conditions with a pressure of 2 to 10 bar,
preferably 3 to 5 bar, with a volume flow of 10 to 30_ml/s.
zn contrast to gassing with only a dilute amine-air mixture, the
highly concentrated amine mist makes it possible to' create, on
all the reaction sites simultaneously, such a high catalyst
potential on the entire coating compound deposited that
~1.~ ~~~~
simultaneous spontaneous curing takes place on each individual
particle-:, As a result, the extremely sensitive gel stage which
.represents the transition.between-free-flowi~ng~ res-i:n mixture and
tack-free coating surface will pass through extremely rapidly
without destruction of the resin coating that is just in the
process of forming.
. After the tack-free state has been reached, the amine is removed
to such an extent by aeration and degassing prior to the next
application of coating compound that premature initiation of the
reaction before the next distribution and spreading stage is
prevented. This separation of the individual reaction stages
according to the invention is important for the dense structure
of the individual particle membrane. Tmtermediate bonding of
individual particles to each other would lead to cracking when
the particles separate and hence damage to the surface formed
thus far, and would reduce substantially the quality of the
coating.
The temperature in the reaction bed affects both the distribution
and spreading of the coating and the reaction time. It was found
that in a temperature range of 25 to 50 °C, preferably in the
region of 30 to 40 °C, spreading and distribution are accelerated
sufficiently by a lowering of the viscosity without the reaction
time being reduced to the same extent. The amount of catalyst
is adjusted i.e. reduced, to the increased temperature whilst
maintaining the catalyst potential. Temperature control is
achieved by varying the inflow air temperature depending on the
process heat released.
After introduction of the amine mist and an appropriate reaction
time, aeration is carried out preferably by introducing air
directly into the inside of the bed of grar:ulated material. As
a result of the rapid gaseous exchange thereby made possible,
amine is removed from the bed of granulated material which is
thus prepared in an optimum time for the subsequent application
of coating compound.
2~.:~"~~n~
In order to develop the desired membrane thickness, the coating
process, described is repeated several times. It has become
.. ..apgar.en..t_in_; so doing tna~ .a .-a. g-ranulated~ -materiarl
temperature
of.30 °C at the beginning of the first coating cycle, e.g. by
. heating with air to an inflow air temperature of about 80 °C,
after the third coating application the temperature can be kept
in the optimum temperature range of 30 to 40 °C by cooling with
inflow air at ambient temperature. ,At the same time, the amount
of amine is reduced in stages. In this way, 2 - 5% by mass based
on the coating compound applied, is deposited with dimethyliso-
propylamine in the first coating process. This can then be
reduced to 0.5% by mass in the further coating stages.
A reproducible production process is ensured in a suitable manner
by process control and regulation by SPC (stored program
control).
Example 1
Preparation of membrane-coated fertilizer granules with a
nutrient release'time of 8 manths.
450kg of a spherical granulated material NPK 16-l0-20 are placed
in a sealable drum and heated to a granulated material
temperature of 30,°C by passing through a stream of air preheated
to 80 °C. Whilst the drum is rotating, 8.8 kg of a polyol-
polyisocyanate mixture are applied dropwise to the granules an
the first stage and mixed for a total of 2 mins.
Subsequently, without any further inflow of air and the drum
being closed, dimethylisopropylamine is applied under airless
conditions via two wide-jet nozzles, diameter 0.4 mm, with a
pressure of about 4 bar and a volume flow of 18.7 ml/s. Af°ter
a reaction time of 1 minute, the amine concentration in the bed
of granulated material is reduced to below 250 ppm within a
further 4 minutes by connecting up inflow air and off-gas in a
controlled manner.
~~:~"''°~'s~
'J ~ 'U V
9
The sequence of stages resin application, mixing time, amine
application, reaction time and aeration is passed through six
..>.times;-~:.as.can be -seen ~~rom-=the-~tabTe below':- " ~- ~ w
Table
Stage Resin Catalyst Temperature
(C) of
inflow air ranules
1. 8.8 kg 0.24 kg 80 30
2. 8.8 kg 0.20 kg 80 33
3. 8.8 kg o.18 kg 80 35
4. 8,8 kg 0.16 kg 80 38
5. 8.8 kg 0.14 kg 20 38
6. 8.8 kg 0.14 kg 20 36
Total 52.8 kg 1..06 kg
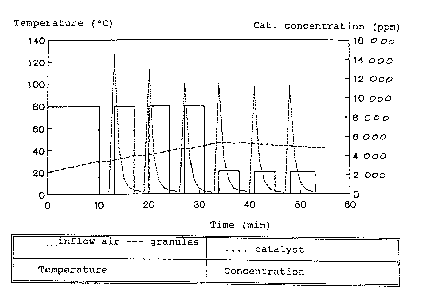
The coating process with the parameters inflow air and granulated
material 'temperature and catalyst concentration is shown again'
by way of a graph in figure 1.
The resin membrane produced in this way fulfils the conditions
of the Fick diffusion equation given above.
Above a11, a measurable membrane activation can be observed which
becomes particularly noticeable at low temperatures by a marked
delay in the onset of nutrient release, as can be seen from
figure 2. The temperature dependence of cumulative nutrient
release from the granulated material prepared according to
example 1 to be expected from Fick's law of diffusion is shown
in this ffigure.
Figure 3 shows the rate of diffusion as a function of the
thickness of the coating surrounding the granules. The granules
measured in this figure were prepared with different quantities
of resin, as in example 1.
Figure 4 shows the characteristic dissolution behaviour of an NPK
16-10-20 fertilizer on the basis of the individual nutrient
:;-~snlubil.it.ies in, a closed, ,system with simulation~~of water ingress
_arid nutrient release, as would be expected in the ideally coated
fertilizer core.
Figure 5 shows the actual ratios of nutrient release of the
fertilizer granules prepared according to the invention according
to example 1.
The change in the NPK ratios during the period of nutrient
release of the fertilizer granules coated according to example
1 (figure 5) corresponds to the theoretical expectation (Figure
4). This analytical finding shows that the saturated solution
present in the interior of the individual coated particle leads
to the same nutrient ratios after membrane penetration into the
outside solution.
Figure s shows the high degree of homogeneity of the product from
the production process according to the invention (similar to
example 1 with 4 coating stages) in a comparison between the
individual particle measurements of the granulated material A
according to the invention and a comparative product B
(OsmocoteR, Sierra, Heerlen Nb).
In the a~,~plication of coated long-acting fertilizers which axe
to be used for supplying substrates, mechanical mixing, storage
stability of substrates already supplied in general but also
unforeseeable frost effects are important critical factors under
practical conditions.
In figure 7 it is shown how the granulated material. prepared
according to the invention behaves in comparison with other
coated products (OsmocoteR, Sierra, Heerlen NZ) during storage
with and without intermittent frost action. Whilst the
comparative product has completely lost its property of delayed
nutrient release after frost action, this property is only
i~~~~~~~~~
slightly impaired with the granulated material prepared according
to the invention.
.Lt .is shown in figure 8 how the granulated material prepared
according to the invention behaves in comparison with other
coated products (comparative product as above) in terms of their
mechanical resilience during substrate preparation. The nutrient
release behaviour of granules produced according to the invention
is increased only slightly even with vigorous i.e., repeated
mechanical mixing, whereas the comparative product exhibits an
unacceptable direct salt release from destroyed granules.