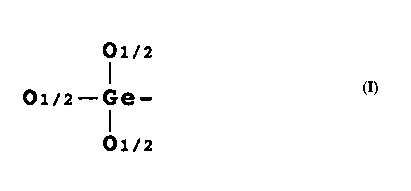Note: Descriptions are shown in the official language in which they were submitted.
WO 94/14826 PCT/JP93/01896
CA2 i i 7472
DESCRIPTION
Process for Isomerization of Compound of Aldose
Structure into Compound of Ketose Structure, and
Isomerization Agent or Accelerator Used Therein
Technical Field
The present invention relates to a process for
isomerizing a compound having an aldose structure into a
compound having a ketose structure, as well as to an
isomerization agent or accelerator used in said process.
Background Art
Carbohydrates are organic compounds which are
very important to living things as their energy sources,
etc. and which are present most abundantly on the earth.
They are composed mainly of monosaccharides. These
monosaccharides have typical structures in which 3 to 8
carbon atoms are bonded in a ring and the structures are
largely classified into two types.
That is, said structures are classified into al-
dose (an aldehyde-containing monosaccharide) and ketose
(a ketone-containing monosaccharide). Both aldose and
ketose are further classified into respective trioses,
tetroses, pentoses and hexoses depending upon the carbon
atom number of aldose or ketose.
Various reactions using monosaccharides are
known. As such a reaction which is used industrially,
1
WO 94/14816 PCT/JP93/01896
CA2ii7472
there is a reaction which comprises isomerizing glucose
(grape sugar) (an aldohexose) into fructose (fruit
sugar) (a corresponding ketohexose) to produce a high-
fructose syrup.
Said high-fructose syrup is a mixture of glucose
and fructose, obtained by isomerizing glucose partially.
Owing to the partial isomerization of glucose (having
low sweetness) into fructose (having high sweetness),
the high-fructose syrup has sweetness similar to that of
sucrose.
About 70~ of the high-fructose syrup is used in
cooling drinks and other drinks because fructose con-
tained therein has higher sweetness at lower tempera-
tures, and other portion is used in general foodstuffs
as a sweetener. The yearly production of high-fructose
syrup in the world is estimated about 8,000,000 kg.
Both glucose and fructose are hexoses similar in
structure. Chemical and enzymatic processes have hith-
erto been proposed for isomerization of glucose into
fructose, and it is currently conducted in industry to
isomerize glucose into fructose using an isomerization
enzyme, namely, glucose isomerase to produce a high-
fructose syrup.
That is, starch, for example, corn starch is
liquefied; the resulting liquid is subjected to sacchar-
ification using glucoamylase to obtain a starch syrup;
and passing the starch syrup continuously through an im-
mobilized enzyme obtained by immobilizing, using one of
2
WO 94/14826 PCT/JP93/01896
CAZi
various methods, a glucose isomerase produced by, a mi-
croorganism of, for example, Streptomyces genus, to iso-
merize the glucose contained in said solution into fruc-
tose.
The above isomerization reaction is an equilib-
rium reaction whose equilibrium point is 1 or there-
abouts. At the equilibrium point, about 50% of glucose
can be isomerized into fructose at a reaction tempera-
ture of about 60°C. In order to allow the isomerization
to proceed to such a level, however, a considerable
length of time is required, the reaction mixture is col-
ored owing to the heating for such a long time, and a
high cost is incurred for the purification and condensa-
tion steps required for product marketing. Hence, the
reaction is terminated when the isomerization has pro-
ceeded to a fructose content of about 42%.
As described above, the high-fructose syrup is
produced in order to allow glucose of mass production
and low cost to have sweetness similar to that of su-
crose. However, when the sweetness of sucrose is arbi-
trarily taken as 100, the above-mentioned high-fructose
syrup containing about 42% of fructose (this fructose
syrup is hereinafter referred to as 42%-fructose syrup,
in some cases) has a sweetness of 95-100 which is
slightly insufficient. Therefore, in the above isomer-
~ ization reaction alone, it is impossible to directly ob-
tain a high-fructose syrup having the same sweetness as
sucrose.
3
WO 94/14826 PCT/JP93/01896
CA2ii7472
Hence, there is currently produced in industry a
55%-fructose syrup having a sweetness of 100-110 by in-
creasing the fructose content in the 42%-fructose syrup
to 55%.
In order to produce a 55%-fructose syrup from
the 42%-fructose syrup, however, a large apparatus such
as a reactor packed with a cation exchange resin is re-
quired; moreover, a complicated operation must be con-
ducted, that is, continuous sugar separation is con-
ducted using said reactor to obtain a fructose syrup
containing about 95% of fructose and then this fructose
is mixed with the 42%-fructose syrup.
Meanwhile, as the isomerization of other com-
pound having an aldose structure into a compound having
a ketose structure, there can be mentioned, for example,
isomerization of lactose (a dissacharide) into lactu-
lose. In this isomerization, however, unlike the above
isomerization of glucose into fructose, no enzyme effec-
tive for isomerization of lactose into lactulose has not
yet been found; therefore, the isomerization is cur-
rently conducted by adding, to lactose, sodium hydroxide
of a concentration not exceeding a given level and then
heating the mixture at 70°C or higher to isomerize lac-
tose into lactulose (Japanese Patent Publication No.
2984/1977). This process, however, gives a low isomer-
ization ratio, i.e. a low lactulose yield of 20% or less
(this is lower than the fructose yield). In order to
4
WO 94!14826 PCT/JP93/01896
CA2ii7472
obtain a high-lactulose syrup, the process has a problem
that the lactulose syrup obtained must be condensed.
An object of the present invention is to provide
a process which is free from the above-mentioned prob-
lems of prior art and which can isomerize a compound
having an aldose structure into a compound having a ke-
tose structure at a high isomerization ratio.
Another object of the present invention is to
provide a process which can isomerize a compound having
an aldose structure into a compound having a ketose
structure without requiring any special apparatus or any
complicated operation.
Still another object of the present invention is
to provide a process which can isomerize a compound hav-
ing an aldose structure into a compound having a ketose
structure, using an isomerization enzyme or without us-
ing any isomerization enzyme.
Still another object of the present invention is
to provide a process which can isomerize a compound hav-
ing an aldose structure into a compound having a ketose
structure without employing the condition of heating in
alkalinity (this condition is sometimes disadvantageous
for isomerization ratio) even when there has been found
no enzyme effective for said isomerization.
Still another object of the present invention is
to provide an isomerization agent or accelerator effec-
tive in the above process.
WO 94!14826 PCT/JP93/01896
CA2ii7472
Disclosure of the Invention
According to the present invention there is pro-
vided a process which comprises isomerizing a compound
having an aldose structure into a compound having a ke-
tose structure, by the use of or in the presence of an
organogermanium compound having a structural portion
represented by the following formula (I).
O1/2
O1/z-Ge- ( I )
O1/z
According to the present invention there is fur-
ther provided an isomerization agent or accelerator ef-
fective for the isomerization of a compound having an
aldose structure into a compound having a ketose struc-
ture, which agent or accelerator comprises, as an active
component, an organogermanium compound having a struc-
tural portion represented by the following formula (I).
O1/2
O1/z-Ge- ( I )
O1/z
Brief Description of the Drawing
6
WO 94/14826 PCT/JP93/01896
CA2ii7472
Fig. 1 is a graph showing a relation between re-
action time and isomerization ratio.
A case where an organogermanium compound
(23) was used as the present isomerization agent.
A case where an organogermanium compound
(18) was used as the present isomerization agent.
A case where an organogermanium compound
(1) was used as the present isomerization agent.
A blank
Best Mode for Carrying Out the Invention
The present invention is hereinafter described
in detail.
In the present invention, the isomerization of a
compound having an aldose structure into a compound hav-
ing a ketose structure is conducted by the use of or in
the presence of an organogermanium compound having a
structural portion represented by the above-mentioned
formula (I) with the remaining structure being a chain
or cyclic hydrocarbon, a substitution product or deriva-
tive thereof, or other organic group. Hence, descrip-
tion is made first on the organogermanium compound hav-
ing such a structure.
The organogermanium compound can be exemplified
by a compound represented by formula (II)
7
WO 94/14826 PCT/JP93/01896
CA2ii7472
Oi/z R1 R3
O~/2-Ge- ( C ) n-CH-COX1 (
Ol/z R2
[Rl, R2 and R3, which may be the same or different, in-
dependently represent a hydrogen atom, a lower alkyl
group, a substituted or unsubstituted phenyl group, a
carboxyl group, a carboxyalkyl group or an amino group
which is unsubstituted or substituted with appropriate
group(s); X1 represents a hydroxyl group, an O-lower
alkyl group, an amino group or a salt represented by OY1
(Y1 represents a metal or a basic group-containing com-
pound); and n represents an integer of 1 or more), which
contains, as a basic skeleton, a germylcarboxylic acid
derivative formed by bonding between a germanium atom
and a carboxylic acid derivative having three sub-
stituents R1, RZ and R3 and an oxygen-containing func-
tional group OX1, with the germanium atom in the basic
skeleton bonding to oxygen atoms at an atomic ratio of 2
(germanium) : 3 (oxygen).
The substituents R1, RZ and R3, which may be the
same or different, independently represent a hydrogen
atom; a lower alkyl group such as methyl, ethyl, propyl,
butyl or the like; a substituted or unsubstituted phenyl
group; a carboxyl group; a carboxyalkyl group; or an
amino group which is unprotected or protected with a
protective group such as acetyl or the like. The sub-
8
WO 94!14826 PCT/JP93/01896
CA2ii7472
stituent X1 represents a hydroxyl group, an O-lower
alkyl group, an amino group or a salt represented by OY1
(Y1 represents a metal such as sodium, potassium or the
like (the metal need not be monovalent), or a basic com-
pound typified by lysozyme or a basic amino acid such as
lysine].
The substituents R1 and R2 bond to each carbon
of the carbon chain represented by (C)n (n is an integer
of 1 or more) present at the a-position of the germanium
atom. Accordingly, when n is 1, 2, ...n, R1 becomes
R11. R12. -~~Rln. and R2n becomes R21, R22. ...R2n. The
substituent R3 bonds to the methylene group present be-
tween said carbon chain and the oxygen-containing func-
tional group.
The organogermanium compound used in the present
invention can therefore be exemplified by those shown in
the following Tables 1-5.
9
WO 94/14826 PCT/JP93/01896
CA2ii7472
Table 1
R1
Compound I
(C) n R3 X 1
No. I
R2
1 CH2 H OH
CH3
2 I H OH
CH
3 CH2 CH3 OH
CH3
4 I CH3 OH
CH
CH3
I
C H OH
I
CH3
C6H5
6 I H OH
CH
C6H5
7 I CH3 OH
CH
8 CH2 CH2COOH OH
C6H5
I CH2COOH OH
CH
CH2 H ONa
WO 94/14826 PCT/JP93/01896
CA2ii7472
Table 2
RI
Compound I
(C) n R3 X I
No. I
R2
11 CH2 H NH2
CH3
12 I H NH2
CH
13 CH2 CH3 NH2
CH3
14 I CH3 NH2
CH
CH3
I
15 C H NH2
I
CH3
C6H5
16 I H NH2
CH
C6H5
17 I CH3 NH2
CH
11
WO 94/14826 PCT/JP93/01896
CA2ii7472
Table 3
R1
Compound
(C) n R3 X1
No. I
R2
18 CH2 NH2 OH
CH3
19 I NH2 OH
CH
CH3
I
20 C NH2 OH
I
CH3
CH3
21 I NH2 OCH3
CH
CH3
I
22 C NH2 OCH3
I
CH3
C6H5
23 I NH2 OH
CH
C6H5
24 I NH2 OCH3
CH
25 CH2 NH2 OCH3
26 CH2 NH2 ONa
12
WO 94/14826 PCT/JP93/01896
CA2ii7472
Table 4
R1
Compound I
(C) n R3 X1
No. I
R2
27 CH2 NHCOCH3 OH
CH3
2$ I NHCOCH3 OH
CH
CH3
I
29 CH2 NHCOCH3 OH
I
CH3
CH3
30 I NHCOCH3 OCH3
CH
CH3
I
31 C NHCOCH3 OCH3
I
CH3
C6H5
32 I NHCOCH3 OH
CH
C6H5
33 I NHCOCH3 OCH3
CH
34 CH2 NHCOCH3 OCH3
35 ~ CH2 NHCOCH3 ONa
13
WO 94/14826 PCT/JP93/01896
CA2ii7472
Table 5
R,
Compound I
( C ) n R 3 X ,
No. I
Rz
36 CHZCHZ H OH
CH3
37 I H OH
CHCH2
CH3
38 I H OH
CH2CH
39 CH2CH2 CH3 OH
CBHS
40 I H OH
CHCHZ
4 1 CHZCH2 NH2 OH
42 CHZCHZ H NH2
43 CHZCHz NHCOCH3 OH
44 CH2CH2CH2 H OH
CH3
45 I H OH
CHCHZCH2
CH3
46 I H OH
CHZCHCHz
47 CH2CH2CH2 CH3 OH
CBHS
48 I H OH
CHCHZCHZ
49 CHZ (CH2) ZCHZ H OH
CH3
50 I H OH
CH (CHZ) ZCH2
1 CHZ (C H2) 3CH2 H OH
14
WO 94/14826 PCT/JP93I01896
CA2ii7472
Of the compounds shown in Tables 1-5, those
shown in Tables 1-4 represented by the following formula
(III) are preferable from the availability standpoint:
O1/2R4R6
Oi~a-Ge-C-CH-COR2 ( III )
O1/zR5
wherein R4, R5 and R6, which may be the same or differ-
ent, independently represent, similarly to R1, R2 and
R3, a hydrogen atom, a lower alkyl group, a substituted
or unsubstituted phenyl group, a carboxyl group, a car-
boxyalkyl group or an amino group which is unsubstituted
or substituted with appropriate group(s); and X2 repre-
sents, similarly to X1, a hydroxyl group, an O-lower
alkyl group, an amino group or a salt represented by OY2
(Y2 represents a metal or a basic group-containing com-
pound).
The organogermanium compound having the above
structure can be produced by various methods (for exam-
ple, Japanese Patent Publication No. 40159/1984,
Japanese Patent Kokai (Laid-open) No. 86890/1991 and
Japanese Patent Kokai (Laid-open) No. 62885/1990).
Description is made on the production of organogermanium
compounds represented by formula (III).
An organogermanium compound of formula (III)
wherein X2 is a hydroxyl group, can be produced by, for
WO 94/14826 PCT/JP93/01896
CA2ii7472
example, hydrolyzing a trihalogermylpropionic acid
(e. g., trichlorogermylpropionic acid) having sub-
stituents R4 to R6, as shown in the following formula.
R4 R6
I I H20
cisGe-c-cH-coox ~ (zl=)
I
RS
An organogermanium compound of formula (III)
wherein XZ is an O-lower alkyl group, can be produced
by, for example, reacting the above trichlorogermylpro-
pionic acid with thionyl chloride or the like to convert
said acid into a corresponding acid halide, reacting
said halide with an alcohol corresponding to said lower
alkyl group, and hydrolyzing the reaction product. An
organogermanium compound of formula (III) wherein X2 is
an amino group, can be produced by, for example, react-
ing said acid halide with ammonia and then hydrolyzing
the reaction product.
An organogermanium compound of formula (iii)
wherein XZ is a salt represented by OYZ and YZ is a
metal, can be produced by reacting a compound of formula
(III) wherein XZ is a hydroxyl group, with a hydroxide
of Y2. An organogermanium compound of formula (III)
wherein XZ is a salt represented by OYZ and YZ is a ba-
sic group-containing compound, can be synthesized by a
known acid-base reaction.
16
WO 94/14826 PCT/JP93/01896
CA2ii7472
Organogermanium compounds of formula (III)
wherein n is larger than 1, can be produced basically in
accordance with the above-mentioned methods.
That the thus produced organogermanium compound
is represented by the above-shown general formula (II)
can be well supported by the results of instrumental
analyses (e. g., NMR absorption spectrum, IR absorption
spectrum) obtained for said compound.
The formulas (II) and (III) representing the
organogermanium compound of the present invention, each
represents said compound in its crystal state. It is
known that the present compound, for example compound
(II), takes a structure represented by the following
formula (II'), in water.
OH R1 R3
OH-Ge- (C) n-CH-COX1
OH R2
The organogermanium compounds (II) and (IZI) can
be represented also by other structural formulas. For
example, the compound (II) is the same as a compound
represented by the following structural formula (II" ).
R1 R3
Ge-(C)n-CH-COX1
R2 2O3
17
WO 94/14816 PCTIJP93/01896
CA2ii7472
In the present invention, the organogermanium
compound which is represented by at least one of above
formulas cari be used, regardless of their crystal struc-
tures.
The organogermanium compound used in the present
invention has very low toxicity. For example, a com-
pound (II) wherein n=1, R1=RZ=R3=A and X1=OH [compound
No. 1, this compound is hereinafter referred to as
organogermanium compound (1) in some cases], shows a
LDSp of 6 g/kg or more when orally administered to mice
and 10 g/kg or more when orally administered to rats.
In the present invention, as described previ-
ously, a compound having an aldose structure is isomer-
ized into a compound having a ketose structure by the
use of or in the presence of an organogermanium compound
having a structural portion represented by the above
formula (I). The compound to be isomerized may be any
compound which has, in the molecule, the following al-
dose structure represented by Fischer's projection for-
mula
O
I I
C-H
I
(OH)H-C-OH(H)
I
(OH)H-C-OH(H)
la
WO 94/14826 PCT/JP93/O1S96
CA2ii7472
and which can be isomerized into a compound having the
following ketose structure represented by Fischer's pro-
jection formula
CH2-OH
I
C=O
I
(OH)H-C-OH(H)
via an interim stage of formation of a cis-ene-diol
structure as shown below.
O
II H \C/OH CHz_pH
I H ~ ~C~ ~ C=O
H-C-0H OH I
I
As the compound having the above aldose struc-
ture, monosaccharides and their derivatives such as
shown below at the left side can be mentioned. They are
isomerized into compounds shown below at the right side
glyceraldehyde ~ dihydroxyacetone
erythrose, threose -~ erythrulose
ribose, arabinose -~ ribulose
xylose, lyxose ~ xylulose
allose, altrose -~ psicose
glucose, mannose ~ fructose
19
WO 94/14826 PCT1JP93/01896
~~~i7472
gulose, idose -> sorbose
galactose, talose -~ tagatose
As the compound having the aldose structure, re-
ducing disaccharides and their derivatives such as shown
below at the left side can also be mentioned. They are
isomerized into compounds shown below at the right side.
maltose -~ maltulose
lactose --> lactulose
Trisaccharides and higher, and also polysaccha-
rides and their derivatives can be isomerized. In that
case, they must have an aldose structure at the molecu-
lar end. Incidentally, for some (e. g. maltose and lac-
tose) of the above compounds which can be isomerized,
there has been found no enzyme capable of isomerizing
them into corresponding compounds each having a ketose
structure.
Of the compounds of ketose structure, lactulose
is clinically used for the improvement of psychoneurosis
associated with hyperammonemia, tremors of hands and
fingers, etc.
In the isomerization of a compound having an al-
dose structure according to the present invention, an
isomerization enzyme may or may not be used. when no
isomerization enzyme ,is used, the isomerization may be
conducted under the same conditions as employed in the
conventional isomerization of glucose into fructose us-
ing an isomerization enzyme, for example, at room tem-
perature to 60-90°C in the presence of an alkali such as
WO 94/14826 PCT/JP93/01896
CA2ii7472
sodium hydroxide, calcium hydroxide or the like. In the
isomerization using no enzyme, it is also possible to
use the alkaline portion of the electrolytic water ob-
tained by the polarization of water using a particular
apparatus therefor.
The concentration of the organogermanium com-
pound used in the isomerization are not particularly re-
stricted because it is determined depending upon isomer-
ization time, desired isomerization ratio, etc.
However, as an example, 1~ by weight or more of the
organogermanium compound is added to a 10~ by weight to
solution of the compound having an aldose structure.
In the isomerization of the present process, the
isomerization ratio increases generally with an increase
in the reaction time. Therefore, the isomerization ra-
tio is controlled by controlling the reaction time,
whereby a desired isomerization ratio is obtained.
In the isomerization process according to the
present invention, an isomerization enzyme may be used
as in the conventional isomerization of glucose into
fructose using an isomerization enzyme.
Description is made on a case of isomerization
of glucose into fructose using an isomerization enzyme.
First, starch (e.g. corn starch) is liquefied using a-
amylase produced by, for example, Bacillus genus; the
resulting liquid is subjected to saccharification using
glucoamylase produced by, for example, Aspergillus
niger, to obtain a starch syrup. Incidentally, this
21
WO 94/14826 PCT/JP93/01896
CA~ii7-472
starch syrup contains about 93-95% of glucose. In the
saccharification, there may be used, in combination,
pullulanase which is an enzyme for cleavage of a-1,6-
glycoside linkage of starch; in this case, the glucose
content in the resulting starch syrup is about 96%.
The starch syrup is purified and condensed as
necessary; then, there is added, as necessary, a metal
ion of magnesium, manganese or cobalt required by glu-
cose isomerase used in the subsequent isomerization
step. From the standpoint of food safety, magnesium ion
is preferred as the metal ion.
The resulting starch syrup is subjected to an
isomerization step. Glucose isomerase used in this step
maybe any as long as it can isomerize glucose into fruc-
tose. Examples of glucose isomerase are those enzymes
produced by microorganisms belonging to Streptomyces
genus, Bacillus genus, Arthrobacter genus,
Microbacterium genus, etc. Specific examples of the en-
zyme are as follows.
Lactobacillus brevis
Bacillus coagulans
Brevibacterium pentosoaminoacidium
Arthrobactor sp.
Actinoplanes missouriensis
Streptomyces phaeochromogenus
Streptomyces rubiginosus
Streptomyces albus NRRL-5778
Streptomyces griseofuscus
22
WO 94/14826 PCT/JP93/01896
CA2ii7472
The above-mentioned glucose isomerase is allowed
to act on the above-mentioned starch syrup in the pres-
ence of the above-mentioned organogermanium compound to
isomerize glucose in the syrup into fructose. This step
may be conducted in a mixture of the starch syrup, the
organogermanium compound and the glucose isomerase; how-
ever, it is also possible that the glucose isomerase be
immobilized according to one of conventional methods to
prepare an immobilized enzyme and the starch syrup con-
taining the organogermanium compound be continuously
passed through the immobilized enzyme. Incidentally, in
the present invention, a microbial cell preparation
whose proteins other than glucose isomerase have been
inactivated, may be used in place of the isomerization
enzyme.
The conditions employed for the isomerization of
glucose into fructose according to the present inven-
tion, can be the same as used in the conventional known
isomerization processes. That is, the isomerization may
be conducted, for example, in neutrality to weak alka-
linity at 60-90°C.
In the isomerization of glucose into fructose
according to the present invention, the isomerization
ratio increases with the lapse of the reaction time, as
shown in Examples given later. Therefore, it is possi-
ble to control the reaction time to control the isomer-
ization ratio and thereby obtain a desired isomerization
23
WO 94/14826 PCT/JP93/01896
CA2'~ ~ 772
ratio, for example, a ratio of isomerization to fructose
of about 55~ or more.
In the present invention, the amount of the
organogermanium compound used can be determined depend-
ing upon the intended isomerization ratio, etc. The
organogermanium compound can be used in a concentration
range of, for example, 1/100 M or more.
The present invention is hereinafter described
in more detail with reference to Examples.
Example 1
(1) Synthesis of organogermanium compounds
Trichlorogermane (Cl3GeH) was added to acrylic
acid (CH2CHCOOH) to obtain trichlorogermylpropionic acid
(Cl3GeCH2CH2COOH). It was hydrolyzed to synthesize an
organogermanium compound (1). In the same manner were
synthesized organogermanium compounds (2) to (51).
(2) Preparation of substrate solutions
A solution containing 40~ glucose and 1.2 M
organogermanium compound was prepared according to the
following procedure. 0.8 g of anhydrous glucose was
dissolved in 0.8 ml of deionized water. To the solution
was added, in small portions, 0.407 g of the organoger-
uranium compound (1) as an isomerization accelerator of
the present invention [a compound represented by formula
(II) wherein n=1, R1=R2=R3 and X1=OH] while the pH of
the solution was maintained at very weak alkalinity, to
completely dissolve the compound in the solution.
24
WO 94/14826 PCT/JP93/01896
CA2ii7472
Thereto was added 4.9 mg of magnesium sulfate, and the
pH of the resulting mixture was adjusted to 8Ø Then,
deionized water was added to make the total volume 2.0
ml, whereby a substrate solution was prepared.
Two other substrate solutions containing the
organogermanium compounds (18) [a compound represented
by formula (II) wherein n=1, R1=R2=H, R3=NH2 and X1=OH]
and (23) [a compound represented by formula (II) wherein
n=1, R1=H, R2=C6H5, R3=NH2 and X1=OH], respectively,
were prepared in the same manner as above except that
the organogermanium compounds (18) and (23) were used in
amounts of 0.443 g and 0.638 g, respectively (these
amounts corresponded to 1.2 M of germanium).
(3) Preparation of enzyme
An isomerization enzyme (glucose isomerase) ex-
tracted from the cells of Streptomyces griseofuscus S-41
was purified according to a known method using an ion
exchange column, a gel filtration column or the like,
until a single band was obtained electrophoretically.
The resulting purified enzyme was used as a standard en-
zyme.
(4) Enzymatic isomerization reaction
In a small test tube were placed 0.7 ml of the
above substrate solution, 0.1 ml of a 200 mM MOPS buffer
solution (pH 8.0) and 0.2 ml of a solution containing
the above-prepared standard enzyme (5.69 mg/ml). The
test tube was placed in a water bath of 60°C and the
mixture in the test tube was subjected to a reaction.
WO 94/14826 PCT/JP93/01896
CA2ii7472
Each 50 ul of the reaction mixture was taken and added,
at regular intervals, to 50 pl of 0.5 N perchloric acid
placed in a microvial, to terminate the reaction. The
amount of formed fructose in the microvial was deter-
mined by high-performance liquid chromatography using a
column [LC7A, SCR-101 (N) manufactured by Shimadzu
Corp.] to examine the change with time, of ratio of iso-
merization of glucose into fructose.
(5) Results
As shown in Fig. 1, in the blank using no
organogermanium compound, the reaction reached an equi-
librium in about 6 hours and the isomerization ratio was
as low as 50~. When the organogermanium compound of the
present invention was added as an isomerization acceler-
ator, both the initial reaction rate and the isomeriza-
tion ratio in equilibrium were superior to those of the
blank. That is, the initial reaction rate was 40-50~
higher than that of the blank, in all cases and there
was substantially no difference in initial reaction rate
between different organogermanium compounds. Meanwhile,
the isomerization ratio in equilibrium varied depending
upon the kinds of organogermanium compounds used; and
the compound (23) gave an isomerization ratio of 99~,
the compound (18) gave an isomerization ratio of 80$,
and the compound (1) gave an isomerization ratio of 75~.
Example 2
26
CA 02117472 2005-02-18
72057-29
(1) Preparation of weakly alkaline electrolytic water
by electrolysis
Water was passed through an apparatus for elec-
trolysis [e.g. Microcluster* manufactured by
Asahi Glass Co., Ltd.]. The alkaline portion of the re-
sulting electrolytic water was taken to use as a weakly
alkaline electrolytic water.
(2) Preparation of glucose solutions
14 g or 28 g of anhydrous glucose was dissolved
in about 80 ml of the weakly alkaline electrolytic water
prepared above. The same electrolytic water was further
added to make the total volume 100 ml, whereby a 14 %
glucose solution and a 28% glucose solution were pre-
pared. The 14% glucose solution had pH 9.l and the 28%
glucose solution had pH 8.61, right after the prepara-
tion.
(3) Preparation of organogermanium compound solutions
1.847 g of the organogermanium compound (18) was
weighed and added to about 2 ml of deionized water. The
mixture was made weakly alkaline (pH 8.00 or 8.53) with
a small amount of sodium hydroxide. The same deionized
water was further added to make the total volume of 3
ml. The final concentration of the compound (18) in the
solution was 1.67 M.
(4) Isomerization
200 N1 of the 14% or 28% glucose solution and
200~r1 of the organogermanium compound solution (pH 8.00
or 8.53) were placed in a small test tube. Also, 200 ul
*Trade-mark
27
WO 94/14826 PCT/JP93/01896
C-A2 i 17472
of the 14% or 28% glucose solution and 200 N1 of the
weakly alkaline electrolytic water were placed in a
small test tube. Each test tube was thoroughly shaken
and then placed in a water bath of 80°C to give rise to
a reaction. 1-3 hours later, 50 ul of the reaction mix-
ture was added to 50 ul of 0.5 N HC104 to terminate the
reaction. Thereafter, the mixture was diluted 100-fold
with deionized water to determine the amount of formed
fructose and the amount of residual glucose by high-per-
formance liquid chromatography using 7A (a column) manu-
factured by Shimadzu Corp.
The results are shown in Table 6.
Table 6
Run Glucose pH of reaction Isomerization
No. mixture ratio (%1
concentration
I%1
1 (Ge)14 7.17 48.0
2 14 8.75 2.1
3 (Ge)7 7.52 73:1
4 7 9.03 3.1
(Ge)14 7.81 65.0
6 14 8.74 2.0
7 (Ge)7 8.15 94.7
8 7 9.03 3.5
9 (Na,Ge) 14 8.61 98.9
14 10.62 32.3
(Na)
28
WO 94/14826 PCT/JP93/01896
CA2ii7472
As is clear from Table 6, the isomerization ra-
tios of glucose were 2.0 to 3.5% when a glucose solution
was dissolved in a weakly alkaline electrolytic water
alone. Meanwhile, when an organogermanium compound so-
lution was further added, the isomerization ratios of
glucose were 48.0 to 94.7%. Further, the isomerization
ratio of glucose was 32.3% when sodium hydroxide was
added to a glucose solution, while the isomerization ra-
do of glucose was 98.9% when an organogermanium com-
pound solution was further added.
Incidentally, in Table 6, (Ge) shows cases in
which an organogermanium compound solution was added;
(Na) shows a case using sodium hydroxide and deionized
water; in other cases, isomerization was conducted using
weakly alkaline electrolytic water alone and without us-
ing any of (Ge) and (rla).
Example 3
Other compounds represented by formula (I) were
subjected to 3-hour isomerization in the same manner as
in Example 2. The results are shown in Table 7.
Incidentally, compounds other than those shown in Table
7 showed substantially the same isomerization ratios.
29
WO 94/14826 PCT/JP93/01896
CA2ii7472
U
~~ NC t~N V O .Hu7sY O O 07 Mtp
M! M t0 GOO t~t~N M ..nOf WIff
?!~ < M M N It7M 0p t~
M
x t~< t~N M
V ! Y Of 00 t~ N ~t0
A Ml' M 1p ~ pf M W tp M 1A M n~
z ~.~ ~ ~ .o~ M ~o~o ~ a .~ <M
M!~ Ost~ N M o0OIN O 00 n op
WM W t~ I'N .-~Oun .~ M O OCO
N~f N .~ t0 C' CO W N.~
x MM ~ C a0tp .~N a M N t~. Ot
N O00 ! v0 0pM p C~O t~ N ID Nt~
z nto n to ton inr n n r .c cv
~~ tDN t0O .rM C M T ID t~ tD TIp
OIt~ I~t~ b o0 10O N OI t~ u7 N.~
N Q
N
,xT'x MM tAM M N 47OIN 1~ p .r
b OO ! N O M N OI.r Op pf pp Nd.
z MN N N N M .~ M N M N
"~ <CO C M t0M t0M N n < !' t0M
MN t~O Op.~ M < N O pu Op .-n.~
a M.w .-r.r .~ n N O < N
a
~"'IF' x na0 O M t0.-nN M u7 tp t0 1t7 pp~
O
!~.r O tD t~O .w0 O t~ N 0p NOp
,Z <V M M M C ~p? C M M M N.~
IG.r N O < < Iwp M O I~ N NV
O O f~ M M !~OIN 10 N t0 IDID
_N .w ~i a M M N
'IF x ICM N M COV eJN tp M N M %O
b NO W y~ t~C' O M O OI ~ .r nN
'~."NN .~ N .~N N .-n N ..n
a
.-nC~ M ID t~a M t0N Oo N M NO
.-nOoO O W N O O .~ M N 0poD
D
.rN a < V V
N
iF ~x MtD M < ~ Op O ~ .H Ip M a7. Qn
N QN .~N O N OIC~e7.V M C~ OIp
,Z, NN N N N N .-~N N N N N
a
'_~,t~m N Ic M ao ..N a c M .. ma
VIIIt0t~ N M OWf700 V' OD N tpN
~ aa ooc~ I~m toa o. a~ m op mn
iF ~x QI07 M < < 00 .yO a0 7 1f7t0 NI~
N 00(~ tptp M sT I~I~t~ tp N N M01
z o,of a a a m m a o, o~ o, of mI
~ -.N M a I mo opo n N a ., inlc
N N M MM
U
z xx
~ ~ ~
A
C7 U t7 Ur V UYC~ V V p fJC7
WO 94/14826 PCT/JP93/01896
CA2ii7472
Example 4
The same isomerization test as in Example 2 was
conducted with slight modifications.
That is, 200 N1 of one of various saccharide so-
lutions and 200 ul of one of various organogermanium
compound solutions were placed in a small test tube.
The mixture was adjusted to pH 10 with an aqueous sodium
hydroxide solution, after which the tube was placed in a
water bath of 80°C for a reaction. 3 hours later, the
reaction was terminated and the amount of each saccha-
ride isomerized was determined by high-performance liq-
uid chromatography using 7A (a column) manufactured by
Shimadzu Corp.
The results are shown in Table 8.
Table 8
Organogermanium Isomerization (%)
compound Galactose Ribose MaltoseArabinose Xvlose Mannose
1 38.67 27.76 78.91 20.87 40.66 37.23
8 40.60 29.60 80.41 22.22 33.26 39.18
17 16.38 27.06 53.84 7.96 25.88 16.53
22 19.72 57.98 49.26 11.49 31.24 38.20
Blank 7.60 2.89 12.59 2.75 5.59 12.48
Industrial Applicability
31
WO 94/14826 PCT/JP93/01896
C~2~3~4~2
As is shown from the foregoing Examples, the
present isomerization process is free from the problems
of prior art and can isomerize a compound having an al-
dose structure into a compound having a ketose structure
without requiring any special apparatus or any compli-
cated operation.
This implies that an isomerized saccharose of
desired concentration can be supplied, in a desired
amount, to a processed food plant, for example, a plant
for production of cold drink using an isomerized saccha-
rose, by installing therein a small isomerization unit
utilizing the technique of the present invention.
Further, when said isomerization unit is incorporated
into a processed food production line, costs associated
with transportation, storage and feeding of raw materi-
als can be reduced significantly.
Further, the present process can isomerize a
compound having an aldose structure into a compound hav-
ing a ketose structure in the presence or absence of an
isomerization enzyme. Even when no effective isomeriza-
tion enzyme is found to exist for a particular compound
of aldose structure to be isomerized into a correspond-
ing ketose structure, the present process can isomerize
such a compound into a compound having a ketose struc-
ture by not resorting to the condition of heating under
alkalinity (this condition is sometimes disadvantageous
in isomerization ratio).
32
WO 94/14826 PCT/JP93/01896
CA2ii7472
Furthermore, the present isomerization agent or
accelerator effective for and used in carrying out the
present process is very safe and highly stable.
33