Note: Descriptions are shown in the official language in which they were submitted.
2 3
W~93/20696 PCT/US92/03076
REGULATION OF THE IMMUNE SYSTEM
Field of the Invention:
The present invention provides an improved means for
regulating the immune response, for ameliorating effects
of stress, and for avoiding untoward effects of chemother-
apy or exposure to irradiation by administration of andro-
stenediol (AED) and androstenetriol (AET~. The improved
means of regulating immune response can be utilized in
treating infectious diseases and immune diseases such as
diabetes and chronic fatigue syndrome, both diseases now
considered to be immune response related syndrome~.
Backaround of the ~nvention:
In vertebrates the development of host protection
against pathogens reguires a selective host immune re-
sponse that involves the mobilization of the humoral
and/or cellular mediated immune responses. Several fac-
- tors adversely affect the body's protective response
capability by causing prolonged immuno-suppression or
"down-regulation" of the immune system~ It is, in reali-
ty, more appropriate to speak of "mal-regulation" or
"deregulation" of the immune system than of "down-regula-
tion" since the result is a failure to protect the body
from assault. Immuno-suppression provides an opportunity
for pathogens to grow in the host. It does not matter
what causes the primary insult to immunity. The resulting
inability to muster the appropriate immune response has
the same effect. Among the many different causes of
immuno-suppression are viral, bacterial, fungal, yeast and
parasitic infections, chemotherapy, irradiation, severe
stress, chronic fatigue syndrome, diabetes mellitus and
some forms of steroid therapy.
It has long been known that patients receiving ste-
roid hormones of adrenocortical origin at pharmacological-
W093/20~96 ~ 3 PCT/US~2/03076
ly appropriate doses show increased incidence of infec-
tious disease. A.S. Fauci, immunolo.rev. 65, 133-155
~1982); and J.E. Parillo and A.S. Fauci, Annual Review of
Pharmacoloay and ~oxicoloqy 19, 179-201 (1979). Dehydro-
epiandrosterone, also known as 3-B-hydroxyandrost-5-en-17-
one or dehydroiso-androsterone (referred to hereinafter as
DHEA), is a 17-ketosteroid which is quantitatively one of
the major adrenocortical steroid hormones found in mam-
mals. M.E. Windholz, The Merck Index, Ninth Edition
(1976); K. Diem and C. Lentner, Geiay Scientific Tables
(1975). (Although DHEA appears to serve as an intermedi-
ary in gonadal steroid synthesis, the primary physiologi-
cal function of DHEA has not been fully understood. It has
~ been known, however, that levels of this hormone begin to
decline in the second decade of life, reaching 5~ of the
original level in the elderly.)
Clinically, DHEA has been used systemically and/or
topically for treating patients suffering from psoriasis,
gout, hyperlipemia, and in has been administered to post-
coronary patients~ W. Regelson et al., New York Academy of
Sciences 518, 260-273 (1988). In mammals DHEA has been
shown to have weight optimizing and anticarcinogenic
effects.
DHEA has been used clinically in Europe in conjunc-
tion with estrogen as an agent to reverse menopausal
symptoms and also has been used in the treatment of manic
depression, schizophrenia, and Alzheimer's disease. DHEA
has also been used clinically at 40 mg/kg/day in the
treatment of advanced cancer and multiple sclerosis.
(Regelson, supra) Mild androgenic effects, hirsutism,
and increased libido were the side effects observed. These
side effects can be overcome by monitoring the dose and/or
by using analogues.
U.S. Patent 5,077,284 entitled "Use of Dehydroepian-
drosterone to Improve Immune Response" describes the
subcutaneous or oral administration of DHEA to improve the
host's response to infections. U.S. Patent 4,978,~32
W093/20696 ~ 2 3 PCT/US92/03076
describes use of patch technology to deliver DHEA.
It is now disclosed that DHEA is a precursor in a
metabolic pathway which ultimately leads to more powerful
agents that increase immune response in mammals. That is,
DHEA acts as a biphasic compound: it acts as an immuno-
modulator when converted to androstenediol (5-androstene,
3B,17B diol hereinafter referred to as BAED) or andro~
stenetriol (5 androstene 3B,7B,17~ triol hereinafter
referred to as BAET), but n vitro has a certain lympho-
toxic and suppressive effect on cell proliferation prior
to its conversion to BAED and/or BAET. It is, therefore,
now understood that administration of DHEA shows superior
immunity enhancing properties as a result of its partial
~ conversion to a more active metabolite.
An agent that would advance the protective regulation
of the immune system without giving rise to undesirable
side effects seen with DHEA administration would provide
particularly advantageous improvement of host resistance
against infection. Protective regulation of the immune
system could then be effected using lower doses of the
chemotherapeutic agent, and would provide more immediate `
response with a wider range of protection.
Description of the Invention:
The present invention provides compounds and composi-
tions useful for enhancing the protective response of the
immune system against infections. The medicinal composi-
tions of the invention are also useful for treating other
complications often accompanying immune suppression. The
enhancement of the protective immune response may als~ be
referred to herein as up-regulation or regulation of
immune response.
The present invention provides means of synthesi2ing
both ~ and ~ sterio isomers of AET. Both isomers are
present in the body. However, since the activity of the
isomers varies, it is important that means of making the
separate isomers be provided. It is now known that the
W093/206~6 ~ PCT/US92/03076
BAET is the active agent in regulation of immune response.
It is, however, probable that the ~AET, when in excess, is
in equilibrium with BAET. However, because it is not
known at this time what the response delay interval is,
the BAET is the composition that is the isomer used thera-
peutically.
Using BAED and BAET and protected analogues avoids
certain side effects resulting from use of DHEA. The use
of BAED and BAET avoids many of the androgenic side ef-
fects that occur when the precursor DHEA is administered.
With BAED and BAET it is possible to obtain rapid, con-
trolled enhancement of immune response. Furthermore, as
shown b~ the information below in Table 1, the BAED is
~ unexpectedly superior to DHEA for purposes of protecting
against viral assault since the protection offered differs
both quantitatively and qualitatively from protection
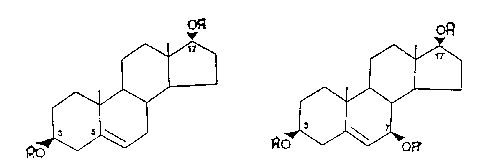
effected by DHEAo The structures of BAED and the ~ and B
isomers of AET are given below:
o~
~,ID~ ~/0~ ~
~ 4~ ~ ~ 4~ r ~ d~
In addition to the medicinal compositions, the inven-
tion encompasses analogues and derivatives of BAED and
~AET. As indicated later in the exemplification of syn-
thetic techniques, some of the substituted compounds are
useful as starting materials or intermediates in making
BAED and ~AET. BAED is available from commercial sources
and has been used as a raw material for production of
other steroids. (See U.S. Patent 2,521,586.) The product
used in testing was obtained from Sigma. BAED and BAET
may also be substituted with protective groups which, on
hydrolysis yield BAED or ~AET~ Hence, acylated and alky-
lated derivatives are useful as precursors to the BAED and
BAET~ Compounds such as those of the formula:
W093/20696 .~ ~ :'L ~ 1~ 2 ~ PCT/US92/03076
~oiJ~
wherein Rl may be H, alkenyl of 2-8 carbons, alkyl of 1-8
carbons, phenylalkyl of 1-4 carbons, phenyl or CO~" where-
in ~ is H: alkyl of 1-8 carbons , alkenyl of 2-8 c:arbons,
phenylalkyl whereîn the alkyl has 1-4 carbons (including
, benzyl) or phenyl~ Any phenyl moiety may have up to three
substituents chosen from among hydroxy, car~oxy of 1-4
carbons, halo, alkoxy of 1-4 carbons, alkyl of 1-4 car-
bons, or alkenyl of 2-4 carbons and wherein any alkyl may
be a straight chain, branched chain, or the alkyl may be
wholly or partially cyclized,
The acute ~irus infection used as a model in the
examples was chosen because of its widespread effects and
the severity of the model to show protection. The Cox-
sackievirus B4 is lethal to mice. In man, Coxsac~ie-
virùses cause such varied pathologies as upper respiratory
infection, pharyngitis, hemorrhagic conjunctivitis, menin-
gitis, exanthem, encephalitis, hepatitis, infantile diar-
rhea, paralysis, pericarditis, and myocarditis. It is now
believed that ~iruses of this ~roup also have a role in
the onset of ju~enile diabetes~
The use of BAED and ~AET and protected analogues as
taught herein provides high levels of protection to verte-
brates, including humans, against morbidity arising from
infections or exposure to immune suppressive influences.
In clinical medicine, treatment with BAED and BAET can
lower morbidity in patients exposed to pathogenic organ-
isms. These agents can be effectively used prophylacti-
c~lly in patients known to be particularly susceptible to
W093/20696 ~ 2 3 PCT/US92/0307~
infection. Patients undergoing surgery or chemotherapy or
patients suffering from burns, hypoplastic or aplastic
anemias, or diabetes are such susceptible patients who
would benefit from prophylactic administration of BAED
and/or BAET. Also among the causes of immuno-suppression
are viral, bacterial, fungal, yeast and parasitic infec-
tions, chemotherapy, irradiation, severe stress, chronic
fatigue syndrome and some forms of steroid therapy. The
compositions of the invention are particularly useful for
lo treating patients suffering from infections caused by
viruses that destroy the body's immune response, such as
human immunodeficiency virus (HIV~ and hepatitis.
Both BAED and ~AET are most efficiently formulated
~ using lipophilic carriers such as DMSO. For subcutaneous
administration to animals used in the examples, the active
agents were dissolved 1:1 DMSO/ethanol, then diluted for
subcutaneous administration to the animals. When the
compositions were administered by mouth, they were added
to chow to provide a composition containing .4~ AED. For
application to the skin, the AED may, for example, be
dissolved in carrier material containing DMSO and alcohol,
then applied to a patch. In such instances, the AED in
solution may be added to another carrier such as glycerol
before application of the composition to the support
material of the patch. For vaginal or rectal administra-
tion, the AED may be administered by suppository, enema,
or by application of creams, etc. Compositions of the
invention may be administered by any method that will
result in contact of the active agent with tissue of
ectodermal origin. Such methods include subcutaneous or
intradermal injection or topical application. One means
of topical application is the use of skin patches impreg-
nated with the active agent. This means of delivery is
advantageous since it is non-invasive and easily adminis-
tered by relatively unskilled care providers. Composi-
tions of the invention can also be used in veterinary
medicine to prevent morbidity that occurs during stress of
WOg3/20696 ~ O~ ~ PC~/US92/~3076
shipping. Administration of BAED and BAET can be effec-
tive as a means to prevent spread of infectious disease
and introduction of infectious organisms into the foods
for human consumption. BAED and BAET can be administered
by subcutaneous injection, in food or drink, by patches
applied to the skin, or by inhalation. A particular
health concern is the spread of infection through eggs.
Eggs are frequently infected during development in the
hen. Compositions containing active agents of the inven-
tion may be added to the feed or water to prevent bacteri-
al infection in the eggs.
When patches are used on animals or birds the skin
should be exposed directly to the patch. When a patch is
~ used, it may be necessary to pluck or shave the bird or
animal to expose the skin.
Othçr preferred methods of administration include
buccal, sublingual, nasal or endotracheal routes. Sprays
may be useful for this purpose. For nasal administration,
the active agent may be delivered as a powder that is
snorted. Inclusion complexes such as cyclodextrin inclu-
sion complexes are appropriate compositions for adminis-
tration to the oral-pharyngeal and nasal mucosa.
~AED and BAET may also be given with ~accines to en-
hance immune response. These agents may be administered
?5 either in a composition containing the vaccine or may be
given in a composition separate from the vaccine.
The compounds of the invention may also be admin-
istered to the intestinal mucosa by oral or rectal routes.
Suppositories, solutions for use as retention enemas, and
creams or jellies are appropriate carriers for use in
rectal administration.
Compounds of the invention may be applied to the
vaginal mucosa using creams, jellies, suppositories, or
douching solutions. In order to enhance immune re-
sponse at the site of exposure to infectious organisms,
the compounds may be added to prophylactic vaginal prepa-
rations or may be used as lubricants on condoms.
W093/20596 ~ O ~ 3 PCT/US92/03076
Administration of compositions of the invention has
proven to be highly effective as a means of protecting
against encephalitis and meningitis. The compositions of
the invention may be administered intrathecally either at
the spinal level or into the cisterna magna.
Active agents of the invention may be administered
via ocular route using compositions in the form of drops,
creams, or gel solutions or suspensions adapted for ocular
application.
BAED and BAET inhibit the adherence properties of
body cells. For purposes of effecting the anti-adherence
properties of the active agents of the invention may be
delivered directly to the epithelial tissue during sur-
, gery. An example of such use would involve the applica-
15 ' tion of compositions containing the active agents of the
invention to the omentum in conditions such as infection,
endometritis,and malignancies of the bowel and ovary
wherein adherence of foreign cells or particles to normal
cells of the peritoneal lining is a problem. Composi-
tions of the invention could, for example, be administered
as mists or sprays.
It has now been shown in,v~vo that DHEA is converted
to the BAED and both isomers of AET. The BAED and BAET
then act as regulators of the immune response. In the
skin, the conversion of DHEA to ~ED and subsequently to
AET appears to be one of the me-abolic pathways of DHEA.
The human skin has the enzymatic machinery to form 7B-OH
DHEA and to cause 7B hydroxylation of AED to yield BAET,
while the human adrenal cortex and liver can form only 7~-
hydroxylation of DHEA, but not 7B-hydroxylation. The
following table indicates the metabolic pathways of DHEA.
Trivial or common name systematic n~m~
(a) Dehydroepian~roSterone ~DHEA) 3B-Hydroxy-5-androsten-
17-one
(b) Dehydroepiandrosterone (DHEAS) 3B-Hydroxy 5
sulfate androsten-17-one-sulfate
W093/20696 ~ 2 3 PCT/US92/03076
(c) 7 B-OH Dehydroepiandrosterone-- -------
d) Androstenediol (AED) 5-androstene3~,17B,diol
e) Androstenetriol (AET) 5-androstene-3~,7B,17~,triol
tf) --- 7 keto-5- androstene-3B,17B, diol
(g) ~ -androstene-3B,7~,17B,triol
(h) --- 5-androstene-3B, 17~l diol-3-sulfate
(i~ Testosterone 17B-Hydroxy-4-androstene-3One
(j) Androstenedione 4-androstene-3,17 dione
(k) Dihydrotestoserone 17B-Hydroxy-5 B androstane-3-one
(1) Androsterone 3~-Hydroxy-5B androstan-17-one
DHEA (a) is known to have immune enhancing activity.
However,~dehydroepiandrosterone sulphate, "(b)", has been
found to have no enhancing effect on the immune system.
Both 5 androstene-3B, llB, 17B,-triol and 5 androstene~
16B, 17B,-triol have immunosuppressive effects. Testoster-
one, "(i)", is known to have an effect on the host immune
response, but this effect does not result in protection
from ~ethal infections. The qualitative and ~uantitative
effects of testosterone and the other sex hormones on the
immune response are both of a different nature and scope.
xam~le 1:
Use of androstenediol (5 androstene-3B, 17B, diol,
3S BAED) results in marked and significant resistance against
viral and bacterial infection. ~ose curve experiments were
conducted in the following manner: BAED and DHEA were
administered as a single depo dose of 8.3 mgs AED or 25
mgs. DHEA to SWR\J and C57BL\6J inbred mice. The mice
were then challenged with varying amounts of Coxsackie-
virus B4 (CB4) to determine protective value of the active
agents. BAED provided 50~ protective dose against cox-
sackievirus B4, which was as much as 100 times greater
than protection provided by DHEA. In addition, to the
0 2 3
W093/20~96 PCT/US92/03076
difference in ED50, the degree of protection against
virus mortality achieved with androstenediol was also
greater than the one obtained with DHEA injection. The
increased protection effected by BAED against virus in-
duced mortality was statistically significantly different
that the protection obtained by DHEA (P < 0.05).
TABLE 1
DHEA AND AED IN SWR/J MICE MORTALITY PER GROUP*
CB4 1 o5 VIRUS ALONE 1/ 6 DHEA o/ 6 AED O/ 6
CB4 106 VIRUS ALONE 3/6 DHEA 0/6 AED 0/6
CB4 1 o7 VIRUS ALONE 5/ 6 DHEA 1/6 AED 0/ 5
CB4 108 VIRUS ALONE 4/6 DHEA 1/6 AED 0/6
~ *No deaths occurred in control groups.
AED versus control p-value = 0.0001.
DHEA versus control p-value = 0.0017.
DHEA versus AED p-value = 0~0588.
As seen from these results, BAED i~ markedly more effi-
cient than the precursor DHEA in preventing mortality since
an effective dose of BAED which is 1/3 or less the dose
necessary to obtain an effect with DHEA is effective in
achieving protection from mortality. A similar protection
from virus mediated mortality was also observed in the
inbred C57BL/6J(b) strain. The two inbred mouse strains, the
SWR/J and the C57BL/6J, differ in their major hi~tocompati-
bility haplotypes, which are q and b respectively. These
results show that up-regulation of the immune response
achieved with BAED in str~ins of different histocompatibili-
ty may be independent of the major histocompatibility genes
on chromosome 17.
Recent reports show that the skin may have unique
immune functions. J.W. Streilein and R.E. Tigelar, Photo-
immunolooY, Parrish et al. eds. (Plenum Publishing, New
York, 1983) pp. 95-130. Indeed the skin is known to contain
a population of cutaneous immune cells, which include the
epidermal Langerhans cells and keratinocytes that produce an
epidermal thymocyte-activating factor, similar to IL-l, in
wo 93,206g6 ~ U ~ 3 Pcr/usg2/o3076
the murine system's Thy-l+ dendritic epidermal cell.
Exam~le 2:
A composition containing 8 mg BAED was administered
S subcutaneously to outbred ICR mice who were then challenged
with a lethal dose of Streptococcus faecalis strain
XlS15.OGlRF. Animals given BAED showed marked resistance to
morbidity as evident from reduction in mortality from 57% in
animals challenged with bacteria only, to 0% mortality in
animals infec~ed and treated with a single subcutaneous (SC)
dose of BAED. Moreover, BAED above a certain threshold dose
mediates a considerable proliferation of lymphocyte cells in
the spleen and thymus, but only in infected animals.
~ Administration of 8 mg/animal BAED without exposure to the
lS virus did not cause proliferation. Histopathological
examination of the organs of in~red SWR/J mice infected with
virus only and animals treated with BAED only, or BAED
treated and virus infected revealed that ~AED is effective
in protecting from virus-induced myocardiopathy, a~d
pancreopathy. Data presented in Table 2 below, shows that
~AED protec~s the host ICR inbred strain (Inst. of Cancer
Research strain now supplied by Holland Sprague Dowley
Company) from S. faecalis, but at a dose which is 1/3 the
dose of DHEA required for the same effect.
TABLE 2
DOSE
B~CTE~UM AGENT mg/ANIM~ 24 hr. MD~n~TY
S. faecalis . none O 4/7 57%
S. faecalis DHEA 25 o/7 o
S. faecalis AED 8.3 o/~ o
Protection was accomplished in an extremely acute
infection where deaths occurred within 24 hours (4/7 deaths
in non-treated groups ~ersus 0/7 in the treated). Mice were
challenged with a lethal dose of plasmids containing
bacterium S. faecalis isolate X1515.OGlRF.
W093/20696 ~ ~ 3 - PCT/US92/03076
Example 3:
Wells containing monolayered urinary bladder tumor
cells were covered with a 5~ ~molar solution of either BAED
or DHEA overnight before being used in the assay. The
resu~ts are shown in Ta~le 3 below.
TABLE 3
X1515.OGlRF
WEL~ D08E NO. ADHERED% ADHERED
Media 1.78 x 106 1.77 x 106 99.4
DMSO 1.78 x 106 1.98 x Io6111.2
DHEA 1.78 x 106 1.01 x 10~ 56.7
AED 1. 78 X 106 1 . 05 X 10659 . O
As can be seen from Table 3, both DHEA and BAED inhibit
the adherence of the bacteria to human urinary bladder tumor
cells. It is believed that BAED and AET have an effect on
either membrane fluidity, or on a component which influences
adhesion and/or penetration into the ceil. Urinary Bladder
Tumor cells (EJ6) were analyzed by flow cytometry using the
fluorescent probe 1-4, trimethylammonio-phenyl-6 1,3,5 hexa-
triene tTMA DPH) as a membrane probe to determine the
effects of AED on membrane fluidity.
Result
A. Samples 1 and 2 represent CONTROL cells grown in
standard tissue cultùre media.
B. Samples 3 and 4 represent cells grown in the above
control media with 50 ~M AED added.
Sample A 1. 0.121256
Sample A 2. 0.120548
CONTROL AVERAGE 0.120902
AED 50 ~M 3. 0.135151
AED 50 ~M 4. 0.142453
TEST AVERAGE 0.138802
The difference of 0.017899 between the control and
BAED-treated cells is significant and demonstrates that BAED
caused a significant change in cell membrane adhesion. It
may be that an increase in cell membrane rigidity (decrease
fluidity) influences adherence.
~llf~23
WO93/206g6 PCT/US92/03076
13
ExamDl~ 4:
Cell culture media was inoculated with 4 x 107 Hela
cells. DHEA was added to a concentration 50 ~M and BAED
was added to a concentration of 50 ~M. The cell culture
exposed to DHEA showed a two-fold decrease in cell number.
The cell culture ~xposed to the ~AED, the control, and the
culture exposed to the carrier alone showed a two fold
increase or no change in the number of viable cells. These
observations indicate BAED lacks deleterious effects of DHEA
lo during the initial phase of cell response. Neither DHEA
nor BAED had an effect on the nu~ber of virus infectious
particles in vitro.
, Ex~mplo 5:
Mice were injected with 8 mg BAED or 25mg DHEA subcuta-
neously.~ The mice were then infected with Coxsackievirus
B4, 105 particles. The mice were sacrificed after 7 days.
The spleen lymphocytes were removed and stimulated with
Concavalin A, 5 ~g/ml in vitro. Stimulation was measured by
3H-thymidine incorporation method. The BAED had a profound
effect on the proliferation of spleen lymphocytes with as
much as 6.6 fold greater proliferation evidenced. The data
also indicates that, during the initial phase following
administration of the steroid DHEA results in some suppres-
sion of the i D une system.
The results described above indicate clearly the
advantage of using BAED or BAET rather than DHEA to increase
immune response to infections, since the initial immune
suppression or toxicity that precedes the immune up-regula-
tion that is seen in treatment with DHEA is avoided. Hence,
therapy with BAED and BAET, rather than DHEA is preferred to
improve immune response to infectious organisms.
The only change in structure between DHEA and AED is a
reduction of the keto group at the 17 position to a B
hydroxyl group. The AET which has the hydrogen at the 7
position replaced with a B hydroxyl group is produced by a
metabolic pathway in the skin. In other words, ~AET is a
o 2 3
W093/20696 PCT/US92/03076
14
down-stream product of metabolic pathway from DHEA in the
skin. The ~AET appears to be the most effective compound
for effecting immune up-regulation and protection from
untoward effects of stress, chemotherapy, and irradiation.
Preparatory methcd #l
An important aspect of the invention is the prepar~tion
of sterio-specific BAET for use as a medicinal. The
synthesis was accomplished using the 7-oxo-3~, 17~ acetoxy-
androst-5-ene as a starting material.
SYNTHESIS OF 3B,7~, 17B-TRIHYDROXYANDROST-5-ENE(I)
and 3B, 7~, 17~-TRIHYDROXYANDROST-5-ENE(II3:
~ Chromic oxide oxidation of 3B,17~-diacetoxyandrost-5-
lS ene in glacial acetic acid gave 3B,17~-diacetoxyandrost-5en-
7-one, (III), the intermediate to (I) or (II).
Aluminum isopropoxide reduction of (III) in isopropanol
gave ~I). li~hium tri ~sec-butyl) borohydride reduction of
(III) in tetrahydrofuran yielded (II~.
PREPARATION OF 3B,17B-DIACETOXYANDROST-5-EN-7-ONE(III)
37.4 g(.l mol) 3B,17B-diacetoxyandrost-5-ene (Steral-
oids A7850) in 400 ml glacial acetic acid was reacted with
30.06 g ( .3 mol) chromium(VI) oxide ~Aldrich 23,265-3)
dissolved in 20 ml H20 and 20 ml glacial acetic acid. The
CrO3 501ution was added drop-wise to the 3B,17B-diacetoxy-
androst-5-ene solution while maintaining the temperature at
55C for 4 hours. In order to decompose any unreacted CrO3,
methanol was added to the reaction mixture followed by
aqueous salt solution and ether. Evaporation of the ether
yielded 7.8 g(20% yield) of crude III,(details are given in
lab book #1, pp. 10-16). Crystallization from 95%`EtOH
yielded (III) m.p. 214-215-C, DSC peak 191-224 C, max at
220C Lit.,l m.p. 218-219-C, Lit. ,2 m.p. 224-225C (from
methanol).
Normal phase tlc: EtOAC-cyclohexane-EtOH (45:45:10),
Rf=0.86. IR bands (cm -1: 1737,1666 (Lit. ,2 1728,1668).
W093/20696 ~ U 2 3 PCT/US92/03076
H NMR (CDC13),(d), ppm: 0.81(s,3H), 1.25 (s,3H), 2.02(s,6H),
2.25(m,H at C-4), 2.5(m,H at C-8),4.6(t,H at C-17),4.7(m,H
at C-3), 5.72(s,1H); (Lit. ,2 0.80(s,3H), 1.20(s,3H), 2.03
(s,6H), 4.62(m,lH), 5.71(g,lH).) Reverse phase lc/ms(fast
atom bombardment detection) detected m/z 389(M+HJ' ion in
the major lc peak,(Lit.,2 m/z 388(M ). Tentative C-13 nmr,
(d), ppm assignments (CDC13) are:
Carbon 1 2 3 4 5 6 7 8 9
ppm 38.35 3S.74 72.04 43.03 164.22 126.44 201.18 65.82 49.69
Carbon 10 11 12 13 14 15 16 17 18
ppm 37.76 27.26 35.95 44.72 44.95 25.83 27.55 81.90 12.05
Carbon 19 20 21 22 23
ppm 20.67 171.14 21.24 170.23 21.17
PREPARATION OF 3B,7B,17B-TRIHYDROXYANDROST-5-ENE~I)
3~,~17B-diacetoxyandrost-5-en-7-one was subjected to
reduction by aluminum isopropoxide in isopropanol to give
3B,7~,7B-trihydroxyandrost-5-ene.
PREPARATION OF 3~,7~,17B-TRIHYDROXYANDROST-5-ENE~II)
5.1 ml(5.1 ~mol) lithium tri(sec-butyl)borohydride
(Aldrich L-Selectride) in tetrahydrofuran was rapidly added
to 499 mg(l.28 mmol) of 3B,17~-diacetoxyandrost-5-en-7-one
in 15 ml of freshly distilled tetrahydrofuran under nitrogen
while stirring for 1.5 hours at ice-bath temperature. 0~9 g
KOH in 15 ml methanol was added, reaction mixture refluxed
for 0.5 hours, and then added 37.5 ml of 10~ NaCl solution.
After cooling in freezer(-20~, crystals formed which were
filtered to yield 123.6 mg (19%) (II), m.p. 239-45C.
Crystallization from methanol yielded (II), m~p. 249.5-
253-C. 1 H nmr (CD(OD)3), (d),ppm: 0.75(s,3H),1.01(s,3H),
3.1(m,1H), 3.6(t,1H),3.7(d,1H),5.50(d,1H).
Tentative C-13 nmr, (d),ppm assignments (CD(OD)3) are:
Carbon 1 2 3 4 5 6 7 8
ppm 37.49 32.12 72.01 42.91 146.75 124.88 65.46 39.09
Carbon 9 10 11 12 13 14 15
ppm 43.58 38.55 21.49 30.65 93.65 45.35 38.13
W093/20696 PCT/US92/03~76
hl~ ~02:~
16
Carbon 16 17 18 19
ppm 42.57 82.30 11.5719.53
DISCUSSION
Stereochemistry was assigned to 3~,7B,17B-trihydroxy-
androst-5ene(I) and 3B,7~,17B-trihydroxyandrost-5-ene(II)
by comparison with cholest 5-ene-3B,7B-diol and cholest-5-
ene-3B,7~-diol proton nmr(Lit.3). L-Selectride reduction
of (III) to produce (II) was carried out using the same
reaction conditions given by Morisaki for the preparation
of 7~-hydroxycholesterol4. Carbon-13 resonances for stere~-
isomers (I) and (II) are shown. (Multiplicities (ppm))
, ISOMER(II) ISOMER(I)
1 37.19 T37.77
2 ~ 32.12 T32.30
3 72.01 D72.12
4 42.91 T41.24
14~.75 *144.10
6 121.88 ~127.43
7 65.46 D74~00
8 39.09 D38.29
9 43.58 D50.08
38.55 *37.73
11 21.49 T21.90
12 30.65 T30.86
13 43.65 *44.27
14 45O35 D52.31
38.31 T2~.60
16 42.57 T42.57
17 82.30 D82.30
18 11.57 Q11.57
19 19.53 Q19.53
Q=quartet, T=triplet, D=doublet and *= quaternary C
(ISOMER II, 7-OH axial)(ISOMER I 7-OH equatorial)
W O 93/20696 PC~r/US92/03076
FE ~ NCES
1. Adolf Butenandt et al. Ber. 71B, 1316-22(1938).
2. Anthony J. Pearson et al., J. Chem. Soc. Perkin Trans.
I, 267-273(1985).
3. Leland L. Smith et al., J.Org.Chem.,Vol.38, No.l, 119-
123 (1973).
4. Masuo Morisaki et al., Chem. Pharm. Bull. 35(5)1847-
i852, (1987)
1o PreP~ratory method #2
A second method of preparing the ~ and B isomers of
AET has been developed. The process uses, as a starting
material, 3B, 17B diacetoxyandrost-5-ene, a commercially
, available reagent (Stereloids A7850). This compound is
- 15 oxidized with chromium hexacarbonyl (Cr(Co) 6) to form 3B.
17B-dîacçtoxyandrost-5-en-7-one as described by Pearson
(Pearson, et al., J. Chem Soc. Perkin. Trans. 1, 1985,
267.) The enone formed was then reduced with triisobutyl-
aluminum (TIBA) to give the acetylated 7~-hydroxy (3a) or
7B-hydroxy (3b) product depending on the solvent used.
.
PREPARATION OF 3B, 7~. 17~-TRIHYDROXYAN~OST-5-ENE.
A solution of 3~,17~-diacetoxyandrost-5-en-7-one
(0.499~ g., 1.285 mmol) and tetrahydrofuran (20 mL, dried
over MgSO4) were mixed under a nitrogen atmosphere. TIBA~
(2 mL, 2mmol, in lM toluene) was added dropwise by syringe
The solution was stirred at room temperature for about 7
hours. The reaction was terminated by the addition of
ethyl acetate (1.4mL), the methanol ~lOmL), and finally,
by addition of 10 mL of 50% acetic acid. This solution
was added to 100 mL water and extracted with ethyl ace-
tate (3 times with 50 mL.) The organic layers were com-
bined and then washed with saturated sodium bicarbonate
solution tSO mL), saturated sodium chloride solution (2
times with 50 mL) and water ~50 mL). The organic layer
was then dried over magnesium sulfate and the solvent
removed ~y rotary evaporation to yield >~6% crude product.
W093/20696 ~ 2 3 PCT/US92/03076
18
~H NMR indicated that the crude product contained 3B,17~-
diacetoxyandrost-5-en-7-one (starting material), 65% and
3B,17B-diacetoxy-7~-hydroxyandrost-5-ene, 35%.
PREPARATION OF 3B,7B,17B-TRIHYDROXYANDROST-5-ENE
A solution of 3B,17~ diacetoxyandrost-5-en-7-one
(0.9581 g, 2.466 mmol) and pentane (30 mL), dried over
MgSO4) were mixed under a nitrogen atmosphere. TIBA (9.5
mL, 9.5 mmol, lM in toluene) was then added dropwise by
syringe. The solution was stirred at room temperature for
about 1 hour. The reaction was terminated by the addition
of diluted hydrochloric acid (approximately 5mL). This
solution was added to water ~lOO mL) and extracted with
, ethyl acetate (3 times with 50 mL). The organic layers
were combined and then washed several times with saturat-
ed sodium bicarbonate solution (50 ml), saturated sodium
chloride solution (two times with 50 mL,) and water ~SO
mL). The organic layer was dried over magne-ium sulfate
and the solv~nt removed by rotary evaporation to yield 86%
crude product. tH NMR indicated that the crude product
contained 3B,17B,-diacetoxy-7-B-hydroxyandrost-5-en-7-one
(~6%) and 3B,17B diacetoxy-7-~-hydroxyandrost-5-ene.
In both of the above instances, the final products
were reduced in accord with preparatory method #1~
The compositions of the invention containing BAED
and BAET, are useful in treatment any condition wherein a
condition related to immune response is present including
diabetes mellitus, chronic fatigue syndrome, stre~s,
chemotherapy, and exposure to irradiation. One of the
more depressing results of many of these conditions that
involve aberrations of the immune response includes alope-
cia. The compounds of the învention are effective for
treating the alopecia resulting from such aberrations of
immune response, including effec~s of chemotherapy and
irradiation. In alopecia, the ~AET should be of consider-
able benefit for use in treàtment. The compositions of
both BAED and BAET are useful in overcoming other untoward `~
W O 93/20696 ~ PC~r/US92/03076
19
effects of immune suppression arising in these conditions.
Example 6-.
SWR/J inbred mice were injected with single depo
doses of 0.5, 2.0, 4.0 and 8.0 mg/animal of BAED, then
challenged with 107 PFU Coxsackievirus B4. The results are
shown below:
AED dose, mg/animal ~ cumulative sur~ival
0 17
~.5 83
2 100
4 100
8 100
Based on these results, the theoretical dose (extrap-
olated) Qf ~AED necessary to achieve 50~ protection from
infection wi~h 107 PFU of CB4 is 0.25 mg/animal.
The example demonstrates that ~AED is 50 to 100 times
more potent than DHEA for protection from Coxsackievirus
B4.
Example 7:
In an attempt to evaluate effect of BAED on patholo-
gies of the heart and pancreas in infected mammals, three
groups of inbred SWR/J mice were compared. The groups
studied included animals infected with virus only, animals
treated with BAED only, and animals infected with virus
and protected with BAED. The comparison revealed the
following:
Heart Pancreas
AED unremarkable unremarkable
VIRUS focal areas of multiple severe necrosis
necrosis with substantial (Pancreopathy)
myocardial calcification.
AED PLUS no evidence of no evidence of `
virus induced myocardiopathy virus induced
pancreopathy
W093/20696 ~ 0 2 3 - PCT/US92/03076
These results demonstrate that BAED given as a single
depo dose at 8.0 mg/mouse protected the heart tissue from
virus induced myocardiopathy, and also protected the
pancreas from virus induced necrosis of this organ. These
results show that BAED can be used effectively in the
protection from virus-induced cardiovascular and pancre-
atic disease, in particular myocardiopathies and pancreo-
pathies. Previously there were no effective drugs to
protect these organs from ~irus induced damage.
Exam~le 8:
Effectiveness of BAED, ~AET, and BAET were compared
by challenging mice with Coxsackievirus B4, 5.0 x 107
~ pfu/animal. The foliowing data represents total umulative
- 15 mortality (n/6) at indicated days post infection with
virus.
Cumulative mortality
Treatment Group (DaYs Post Infection)
2 4 6 8 _ 10 12 14 16
Virus alone 0 1 6 * * * * *
BAED .5 mg/virus 0 0 1 1 6 * * *
~AET .5 mg/virus o 1 4 4 4 4 4 4
~AET .5 mg/virus 0 0 1 1 2 2 4 4
While the ~AET appeared to protect two animals from
the virus, the înitial mortality would indicate that at
least in early stages, there is little or no protection
from the ~AET. Other studies indicate that there is far
less protection with ~AET than with either ~AE~ or BAET.
Clearly, the BAET is the most effective agent for both
early and longer term protection. It appears that there
is a limited protection attributable to ~AET. Therefore,
this isomer would not be as beneficial as ~AET for effect-
ing enhancement of immune response.
The use of ~AED or BAET as active agents to provide
regulation of the immune system makes it possible to ef-
wo 93/206g6 ~ Dr~3 PCT/US92/03076
21
fectively regulate the host immune response against viral,
bacterial and other infections~ In the case of virus-
induced heart or pancreatic infection where no other anti-
viral chemotherapeutic modality exists BAED and BAET have
value as prophylactic prote tive agents. The protective
value of ~AED and BAET is particularly important to pa-
tients undergoing surgical procedures or suffering inju-
ries where resistant strains of organisms such as pseudo-
monas present a serious threat. Examples of such patients
are those undergoing bowel surgery or suffering from gun-
shot wounds of the abdomen. Patients with history of con-
ditions such as rheumatic fever would also benefit from
prophylactic use of BAED and ~ET. The mode of adminis-
, tration in a particular case will depend on the infectious
agent against which protection is sought and the target
tissue o~f the organism. While the administration subcuta-
neously as a depo is effective against systemic infections
as shown by the data presented above, when the composi-
tions are given to assist the body in meeting an infection
in a particular tissue, it may be advantageous to admin-
ister the active agents to the tissues most affected.
The carrier used in a given instance will depend on
the mode of administration. Both AED and AET are lipo-
philic compounds. They are more soluble than DHEA in
water. Solvents for lipophilic steroids are known in the
art and would be used as carriers for these compounds.
Examples of such carriers are ~lycols such as polypropyl-
ene glycol, polyethyl ne glycol. and cyclodextrins, espe-
cially the intrinsically amorphous cyclodextrins. Other
vehicles that should be considered include fatty acid
esters of polyoxyethylene sorbatan (Tweens) or sorbitan
(Spans) to prepare oil-in-water emulsions.
ExamPle 9:
Capsules of a formulation of AED for oral adminis- -
tration is prepared by containing 15 mg. BAED, 150 mg.
starch, and 5 mg. magnesium stearate. The capsules are
W093/20696 ~ 0 2 3 PCT/US92/03076
administered daily or twice a day to achieve a daily dos-
age of 15 mg. per day.
In the laboratory, BAED was added to the chow of the
animals at a rate of .4% of the diet. When the animals
who had been fed the diet containing BAED were exposed to
Coxsackievirus B4 by injection in accord with the teach-
ings of Example 1, the animals who had been fed the BAED
survived, while ~ontrol animals all died.
Exam~le 10:
A preparation for application to the skin or mucosa
may be prepared in the following manner:
Ingredient %w/w
, AED 0.5~
glyceryl monostearate 3.0%
propylene glycol 13.0~
Petrolatum 83.5%
When BAED or BAET or their analogues are administered
orally, the active agents may be utilized more efficiently
îf t}.e active agents are protected from destruction and
absorption in the upper gastro-intestinal tract. The ac-
tive agents are most effective when the period of exposure
to the mucosa of the intestinal tract is increased. Hence
use of capsules containin~ the active agents in formula-
tions that effect slow release in the intestine are ap~ro-
priate for treatment of intestinal disorders such as
Crohn's disease and colitis. Use of retention enemas for
treatment of inflammation of the large bowel is also
appropriate.
A formulation for administration as a retention enema may
be formulated in the following manner:
Exam~le 11:
Ingredient w/w
BAET 4%
Propylene glycol 96%
WO93/206g6 ~ PCT/US92/03~76
23
When the active agent is administered to the mucosa
of the oral cavity, it may be administered as a ~uccal
tablet or spray for use in the oral-pharyngeal cavity and
the nasal cavities.
The compositions could also be administered to the
bronchial tree via inhalation. This means of administra-
tion would be particularly useful in treating patients
with lung infections or in treating other lung conditions
such as black lung disease or emphysema that often are
complicated by opportunistic infections. The compositions
could be given by aerosol into the trachea or administered
in mist along with other agents used in respiration thera-
PY- .
~ The administration of the BAED and BAET to the skin
can be accomplished using patches wherein a support is
impregna~ed with the active agent or using implants that
provide slow release of the active agents.
A patch for the administration of AET or AED can be
formulated as adhesive patches containing the drug. For
example, the patch may be a discoid in which a pressure-
sensitive silicone adhesive matrix containing the active
agent may be covered with a non-permeable backing. The
discoid may either contain the active agent in the adhe-
sive or may have attached thereto a suppor~ made of mate-
rial such as polyurethane foam or gauze that will hold the
active agent. Before use, the material containing the
active agent would be covered to protect the patch.
Example 12:
.
A patch composed of trilaminate of an adhesive matrix
sandwiched between a non-permeable backing and a protec-
tive covering layer is prepared in the following manner:
To a pressure-sensitive silicone adhesive composition
BIOPSATM Q7-292Q (Dow Corning Corp., Midland, Michigan,
U.S.A.) in cyclohexane (50% w/v) is added sufficient BAED
to provide a .5% BAED composition. The adhesive is ap-
plied to a polyester film to provide in successive layers -
W~93/20696 PCT/US92/03076
~1~o~23
24
to provide about 2 mg of active agent per cm2. The film
containing the adhesive is then made into patches of 10
cm2. For patches would be covered with a protective layer
to be removed before application of the patch. Patches
may be prepared containing permeation enhancers such as
cyclodextrin, butylated hydroxyanisole, or butylated
hydroxytoluene. However, it should be remembered that the
active agants of this invention are effective on applica-
tion to the epidermal tissue. When the patches are to be
applied to thin or abraded skin, there is little need to
add a permeation enhancer.
Compositions of the invention can be administered as
a prophylactic during radiation therapy or chemotherapy or
, after exposure to irradiation whether the exposure occurs
as a result of environmental accident or therapy. Other
instances when use of these immune up-regulators would be
appropriate is in treatment of ~urns, hypoplastic and ap-
lastic anemias, diabetes, and in the elderly during epi-
demics. Their use is also beneficial in preventing or
mitigating ef~ects of exposure to dangerous infectious
organisms, as was demonstrated by the data related to car-
diopathies and pancreopathies. Such use is particularly
indicated in populations exposed to organisms that target
the immune system, such as HIV infections. In certain
instances the compositions ta~ght herein can also be used
as immune modulators in the production of blocking anti-
bodies to counteract hypersensitivity reactions.
As indicated previously, patients scheduled to under-
go bowel surgery or other "dirty" surgical procedures
could receive a dose of BAED or BAET prophylactically.
Use of the compositions as taught herein before invasive
dental procedures or oral surgery should be considered.
As indicated in the tables and previous discussion,
the compositions of the invention can be usad to prevent
adhesion of bacteria to the tissues. The prevention of
cell to cell adhesion resulting from administration of the
compositions also has applications for prevention of
- W093/20696 h ~ i ~ O ~ 3 PCT/US92/03076
thrombosis. Compositions of the invention can be adminis-
tered as a slow drip into blood vessels when prevention of
formation of a thrombus is necessary. An example of such
use would be a drip into an artery following thrombo-
lectomy or for prevention of cerebral throbosis. Instil-
lation into the bladder could also be beneficial for
prevention or treatment of urinary infection. The admin-
istration of the BAED or BAET can be employed as a means
of preventing formation of infectious foci.
BAED and ~AET are both effective in overcoming ef-
fects of ultraviolet depravation. Hence, administration
of the compositions to overcome the effects of depression
related to light deprivation (usually called seasonal
~ adaptive disorder) is appropriate.
~AED and ~AET may be used as adjuncts in vaccination
to incr~ase response to an immunogen. Not only will these
agents increase response to the vaccine, they will also
incrase ability to protect against disease befor2 the body
has responded with increase in specific antibodies. Such
use is particularly appropriate in instances where inhi-
bition of immune response can be a complicating factor as
is the case in patients suffering from, for example,
malignancies, AIDS, or environmental factors such as ex- -
posure to pesticides. It is, of course, understood that
use as adjunct to vaccination would be appropriate in
vertebrates other than man, including vaccination of pets,
dairy animals, meat-producing animals, fish, and chickens.
Chickens are particularly prone to develop infectious
diseases when living in confined conditions. CoccidiosisJ
Salmonella infections, viral infections, including those
giving rise to malignancies such as leukemia and sarcoma
(caused by a retrovirus) are particularly common among ~-
chickens grown under modern commercial conditions. The
active agents of the invention may be given by any means
that results in contact of the agent with tissue of ecto~
dermal origin. `
The effect of Coxsackie~irus on humans has been noted ~`-
W093/2069~ PCT/US92/03076
0 2 3
26
previously. The value of avoiding such effects, espe-
cially in children, is clear. Effects of other viruses
such as chickenpox-herpes zoster is considered an impor-
tant cause of debilitating illness in the elderly. Fur-
thermor,e, chickenpox in susceptible adults often causes
severe illness. In children, chicken pox can cause death
when the child is subjected to immuno suppressive therapy
or is genetically i~mune deficient. BAED and BAET a use-
ful for prophylatic treatment of susceptible persons who
have been exposed to infection. Finally, the protection
of the fetus and newborn from HSV infection is a very im-
portant application of the invention. BAED and ~AET can
be administered during the third trimester to HSV-infected
, women as a means of protecting the newborn.
In vitro, these compounds can be used in commercial
setting ~o induce lymphocyte proliferation. The use of
BAED and BAET would increase yield of products of such
proliferation in tissue culture. In the clinical setting,
BAED and BAET can be given to e~fectively enhance pa-
tients' ability to combat infections. Patient lymphocytes
may be withdrawn, reproduced in vitro in media containing
BAED or BAET to increase proliferation of lymphocytes, and
the lymphocy~es so primed for response could then be rein-
troduced i`nt`o the patient~ In cases such as malignancy or
other cellular disease, the malfunctioning cells can be
inactivated by known means before proliferation in growth
media.
The compositions of the invention may also be used
prophylactically to protect animals from the consequences
of infection by pathogenic organi5ms. It is known that
under the stress of shipment to market animals often
become susceptible to infections that are not ordinarily
serious, but that can cause the animals to loss much
weight en route to the packing house. Such loss may be
avoided by administration BAED and BAET and analogues
disclosed herein. The active agents can be given by
patch, injection, or in feed. Because the active agents
W093/2069~ 2 3 PCT/US92/03076
are most effective when the period of exposure to the tis-
sue of ectodermal origin is extended, when the active
agents are administered through the GI tract, compositions
should be modified to extend the period of exposure of the
active agent to the intestinal mucosa and to protect the
agents from destruction in the upper GI tract. Hence, use
of capsules that effect slow release in the intestine is
appropriate. The capsules may be placed in baits for
administration to animals. To treat infections of the
large bowel, the active agents may be given by retention
enema.
~AED and BAET may be administered to the mucosa of
oral, pharyngeal, and nasal cavity by tablet, a lozenge,
, by administration as a spray for use in the oral-pharyn-
geal cavity, or as a nasal spray.
Administration to the skin may be accomplished using
patches wherein a support to be applied to the skin is
impregnated with the active agent. If the host is a
mammal or bird, it may be necessary to shave or pluck the
region to which the patch is applied. -
A preferred method of administration is by subcutane-
ous injection as a depo. The method is particularly
appropriate for administration of the active agents to
mammals, since subcutaneous injection is easily performed
and the effect is relatively long lasting.
BAED and BAET are already present in the body as
natural components. They do not pose a serious toxic
problem at levels known to be safe; they appear to be are
chemically quite stable.
The dosages used will depend on the size and condi-
tion of the host. Test data indicated in this application
was obtained in small animals. In larger adult mammals
daily dosage of .2 to 30 mg/da. of AED a preferred dosage.
For AET the preferred dosage is usually in the range of
.001 to 20 mg/da, with .001 to 1 mg/da. being the more
preferred dosage. However, the dosage will vary depend-
ing on the route of administration. Subcutaneous, inha-
W093/206~6 ~ O~ ~ PCT/US92/03076
28
lation and intrathecal administration are to be methods
that would require lower dosages of the active agents.
It is, of course, understood that analogues of ~AED
and BAET having protective groups can be administered to
the host as a means of delivering BAED or BAET to target
tissues. Acylation is a preferred method af protecting
the compounds. Acylated compounds wherein R1 is COR2 are
also appropriate compounds for use as starting material
from which to make BAED and BAET.
The active agents, ~AED and ~AET, can be given in
conjunction with other active agents which may be given
simultaneously or may be incorporated in compositions
containing BAED or BAET. BAED and BAET can be given with
, anti-infective agents such as antibiotics, antiviral
agents, antifungals, antiparasitic agent to potentiate the
activity~ of these drugs by up-regulating protective immune
response. Antiviral agents include, for example, Di-
deoxyinosine, AZT, acyclovir, etc. Other a~tive agents
that may be combined with the AED and AET include antial- -
lergic medications such as epinephrine.
Finally, medicinal compositions containing BAED and
BAET are particularly valuable for use in combating viral
infections in patients who have suffered from infections
exacerbated by immuno-suppressive therapy. One of the
major complications in patients with tissue transplants is
the opportunistic infection with viruses that ordinarily
do not cause serious disease symptoms. Use of the compo-
sitions of the invention, which result in rapid protective
regulation of the immune response, allows the medical team
to place the patient on ~Isee-saw~ therapy to avoid trans-
plant rejection while regulating the immune response to
avoid overwhelming infection.