Note: Descriptions are shown in the official language in which they were submitted.
2118534
-I-
Background of the Invention
Field of the Invention
The present invention relates to reducing the non-volatile
inorganic contaminant content of refinery waste water discharged to
the environment. More particularly it relates to a process whereby
selenium is removed from the waste water and concentrated and the
selenium concentrate is disposed of in an environmentally responsible
manner.
Description of the Prior Art
Selenium in waste water streams is recognized as a
biological and health hazard and limits have been imposed on the
amount of selenium which may be discharged into receiving bodies of
water.
Because selenium is a component of refinery waste water,
being a natural constituent of the crude oil refined, the restriction
on the amount of selenium which may be discharged into receiving
bodies of water necessarily acts as a restriction on how such refinery
waste water streams can be handled and where and how they can be
disposed and can influence the entire refining process.
Various techniques have been considered as a means for
removing selenium from waste water streams.
Selenium can be removed from waste water streams, such as
those associated with power plants, by iron coprecipitation. In that
process a ferric iron salt is added to the waste water and a precipi-
tate of ferric hydroxide is produced through the adjustment of the pH.
The ferric hydroxide comes together as a flock like precipitate which
adsorbs contaminants in the waste water on its surface. By careful
control of the pH the selenium can be removed from the waste water.
~118~3~
-Z-
The treated waste water is sent to a settler and separated into clean
water and a sludge containing the contaminants. Due to its high
contaminants content (selenium and other metals etc.) the sludge is a
hazardous waste and its disposal is regulated. Further, a very large
volume of sludge is produced for the amount of contaminants removed.
Selenium can also be removed from waste water by contacting
with elemental iron at low pH which converts the selenium compounds in
the waste water into elemental selenium which is retained in the
elemental iron. See USP 4,405,464 and USP 4,940,549. This process
also produces a selenium containing sludge which poses disposal
problems.
While evaporation is commonly used to concentrate inorganics
in water streams, an issue exists as to what to do with the inorganics
concentrate. Because refinery waste water streams contain many varied
inorganic materials, evaporation practiced to concentrate the selenium
would produce a concentrate stream containing large amounts of
co-concentrated inorganics which would, again, constitute a disposal
and handling problem.
It would be an advantage if selenium and other harmful,
environmentally objectionable non-volatile inorganic materials could
be removed from refinery waste water streams, so as to minimize sludge
and contaminant concentrate handling problems and be disposed of in an
environmentally responsible way.
~I18534
-3-
Description of the Invention
Waste water streams preferably refinery waste water streams
contaminated with environmentally objectionable concentrations of
selenium compounds and other non-volatile inorganic compounds are
treated so as to reduce the concentration of selenium and other
inorganic compounds therein by a process comprising subjecting the
contaminated waste water stream to a non-chemical process whereby the
contaminants are concentrated in a concentrated stream and clean water
is produced as a product stream, passing the concentrate stream to a
coke quencher wherein the concentrate stream is used as coker quench,
the contaminants in the concentrate stream being deposited on the
quenched coke.
The non-chemical concentration process can be reverse
osmosis wherein the waste water stream is contacted with one face of a
selective separation membrane under elevated pressure sufficient to
overcome the osmotic pressure to thereby produce a permeate of clean
water of reduced selenium and other inorganic compound concentration
and a retentate of elevated selenium and other inorganic compound
concentration as a contaminants concentrate. The membrane separation
reverse osmosis process practiced will be the same as that practiced
for water desalination. Thus, membranes useful for water desalination
such as regenerated cellulose, cellulose esters, cellulose ethers and
mixtures thereof, polyamid as well as any other of the membranes found
useful for water desalination can be used. Reverse osmosis is
practiced by applying a pressure along the feed side of the membrane
sufficient to overcome the osmotic pressure. Pressure in the range of
100 to 800 psi can be used, but usually the pressure applied is in the
100 to 600 psi range. The membranes are used in elements of either
the spiral wound or hollow fiber configuration.
2II8534
-4-
The non-chemical concentration process can also take the
form of evaporation or steam generation. The waste water stream is
heated in a dedicated boiler or in a heat exchanger utilizing waste
heat (such as a steam evaporator). The hot waste water is flashed
wherein clean steam is vented as overheads and used elsewhere in the
refinery where clean steam is required, or cooled for disposal. The
bottoms from the flash vessel are recovered as a contaminants concen-
trate.
This contaminants concentrate, whether it is from the
evaporator or steam generator or the retentate from the reverse
osmosis unit is sent to the coker as coker quench. The selenium
compounds in the contaminants concentrate will remain in the coke.
Waste water streams which contain selenium and other
inorganic compounds are generated at various locations in the
refinery; they are all eventually gathered at the sour water stripper
where the volatile forms of selenium as well as ammonia and H2S etc.
are removed. Selenium contamination of water streams may occur any
time an aqueous stream contacts a selenium containing oil stream.
Selenium is most often present in high concentrations in steam
condensate streams, where the steam was in contact with the oil and
"stripped" the selenium out of the oil stream. The selenium then
remains in the condensed water stream. As previously stated in
refinery operations the selenium containing streams are gathered at
the sour water stripper. Actual stripping of the water before being
treated by the present invention is not necessary, but is preferred.
When contaminant concentration takes the form of steam generation or
reverse osmosis, the waste water stream can preferably be subjected to
various filtering, neutralizing and softening procedures known in the
art prior to the practice of the steam generation or reverse osmosis
concentration step. Such pretreatment removes constituents in the
waste water known to be harmful to steam generator equipment or
reverse osmosis membrane. If the stream contains a significant
quantity of H2S and volatile forms of selenium, a stripping step is
recommended to prevent these volatile materials from escaping into the
atmosphere when evaporation is practiced as the concentration step.
.211853
-5-
It has been discovered that an evaporation process can be
employed in treating the waste water stream because the selenium in
sour water stripper waste water is primarily in the non-volatile form.
Most of the volatile forms of selenium have been removed during the
stripping process.
Evaporation permits concentration of the non-volatile
selenium compounds in the bottom fraction from the evaporator. Any
degree of evaporation and concentration can be practiced, the object
being to reduce the overall volume of contaminated waste water through
the production of a separate clean water stream. It has been found
that over 97% of the selenium compounds in the waste water are concen-
trated in the liquid concentrate stream when the waste water is
concentrated at a 7:1 concentration ratio. Thus, a concentration
ratio in the range of about 2:1 to 15:1 or more preferably about 2:1
to 50:1 or more, most preferably about 2:1 to 100:1 or more will be
typical, depending of course on the volume of waste water, the concen-
tration of contaminants on the waste water, and the coker quench water
requirements. It would, of course, be desirable to produce as concen-
trated a stream as possible of the lowest volume so as to maximize
clean steam/water production.
The selenium concentrate stream is used as coke quench and
can replace all or part of the currently used coke quench and thus,
may have the added benefit of replacing a contaminated stream
currently used for that service, such as a sour water stream. The
selenium in the contaminants concentrate used as coke quench becomes
attached to the coke and will not volatize when the contaminants
concentrate contacts the hot coke from the coker. Coke from any type
of coker source can be quenched by this process. The concentration
step is preferably adjusted to result in the production of a contami-
nants concentrate stream of just sufficient volume to satisfy the coke
quench requirements of the coke plant. Replacement of a sour water
coke quench stream by a selenium contaminant stream has the added
benefit of reducing the total SOx released to the main refinery stack.
Thus, the present invention acts to reduce two environmentally objec-
tionable contaminants.
X118534
-6-
Brief Description of Drawings
Figure 1 presents a schematic of the present invention
wherein the method of concentrating the contaminated waste water
stream is evaporation.
Figure 2 presents a schematic of the present invention
wherein the method of concentrating the contaminated waste water
stream is steam generation.
Figure 3 presents a schematic of the present invention
wherein the method for concentrating the contaminated waste stream is
membrane separation.
Detailed Description of Drawings
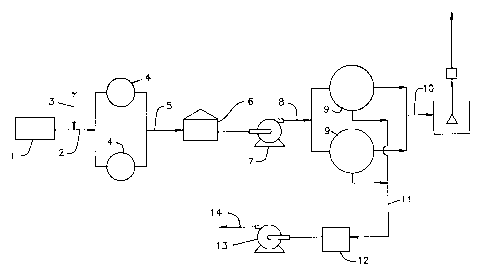
Figure 1 presents one embodiment of the process wherein
waste water from the sour water stripper (1) is fed via line (2) to
filters (4) charged with any appropriate filtering media for solids/-
oil removal such as walnut media or anthracite/garnet. The pH of the
sour water in line (2) is adjusted to about 7-8 by the addition of
acid (e. g., sulfuric acid) via line (3). The filtrate from filters
(4) is fed via line 5 to a surge holding tank either under N2
atmosphere or pressurized wherein the water is also permitted to stand
and any insoluble particulate matter settles out. Filtered water from
tank (6) is fed via pump (7) and line (8) to brine concentrators (9)
wherein any convenient technique such as vapor compressors, steam,
etc. can be used to cause the evaporation. The vapor exchanges heat
with incoming waste water and condenses to form product water which is
recovered via line 10 and sent to clean water sump 10A and then via
line lOb to consumers of clean water in the refinery or to discharge.
Concentrated brine from the concentrators (9) is sent via line 11 to a
storage tank (12) and then via pump (13) and line (14) to the coker as
quench water.
Figure 2 presents another embodiment wherein elements (1)
through 7 are as described in Figure 1. In Figure 2 the filter water
is sent via pump (7) to water softeners (8) wherein the sour water
~11$~~
_7_
contacts e.g., a sodium zeolite softener. The softened water is sent
via line 9 to a holding tank 10 which is either under a N2 atmosphere
or pressurized and then via line (11) to pump 12. Part of the
softened sour water can be sent via line (13A) to the water softener
regeneration facility (13B). Softened water is sent via line 13 to
preheat exchanger 14 shown as powered by steam via lines 18 and 15
from the steam drum (17). Low pressure steam from heat exchanger 14
is fed via line 16 to steam drum 17. Steam drum 17 is powered by
employing a side stream (19) from the steam drum 17 sent to heat
exchanger 20 wherein the stream 19 is indirectly heated using high
pressure steam (e.g., 600#) via line 24. This heated stream 19 is
returned to steam drum 17 and provides the heat needed to generate the
steam in steam drum 17. A sour steam overhead is recovered from steam
drum 17 via line 18. The high pressure steam from exchanger 20 is
sent via line (24) to flash drum (25) wherein low pressure steam is
recovered via line (26) as overhead and condensate is recovered via
line (27) and sent to a deaerator. Concentrated bottoms are recovered
from steam drum (17) via line (21) cooled in exchanger (22) and sent
via line 23 to the coker as coke quench water.
Figure 3 presents yet another embodiment of the present
invention wherein again elements (1) through (7) are as described in
Figure 2. In Figure 3 the softened water is sent via line (9) to
cartridge filters (10) (e. g., 5 micron cartridge filter) wherein fine
particulate matter is removed. The filtered water from 10 is sent via
line 11, pump 12 and line 13 to the reverse osmosis membrane unit
which produces a concentrate and high quality permeate. The concen-
trate is sent via line 15, hold up drum (16) pump (17) and line (18)
to the coker for use as coke quench water. Clean water is recovered
as permeate via line (18) and sent to water storage tank (20). Clean
water is sent via line (21), pump (22) and line (24) to the consumers
of clean water in the refinery or to discharge. A side stream via
line 23 can be used in the softener regeneration facility (25).
The invention is demonstrated by reference to the following
non-limiting examples.
2118534
_$_
Example 1
Waste water samples from two sour water strippers and the
coker liquid product fractionator overhead condensing drum were
distilled to determine the selenium concentration levels in the
distillation contaminants concentrate bottoms and the amount of
selenium carried overhead in the steam. The results of the distilla-
tion of 200 and 300 ml samples are reported in Tables IA and B.
Example 2
The various water streams of Example 1 containing varying
amounts of selenium were employed as coke quench elutriant. The data
in Table 2 indicates that coke retains substantially all of the
selenium present in the selenium concentrate stream. The concentrated
sour water stripper bottoms was produced by distillation to a 7:1
concentration ratio.
211853
_g_
c a c a
o ~n ~n an ~n an ~n ~n o ~ o
~n ~n v~
a r ~ N a r
O O O O O O O O O l0 ~ +~
O t~! V V V V V V V V V ~
tn
~ i t i
i +~
O C d C
~ ~
> O N N N N N N N N N N N > GJ N
N O
O U O U N
C O O O O O O O O O O O C O
O
O O V V V V V V V V V V V O O V
V
U (~ U N
E E
O .. O ..
N N
r N d0 Iw 00 t0 Ln O C1 M 01 r tn Q1
E ~ M ~ ~ lD
C N CO 00 p1 rW0 1~ D 00 00 C N 01
t~ O W 00 t0 rr
O t0 .~ ~ ~ N M ~ ~ ~ ~ ~ -r G7 t~ M
i .~ i ~
r ~ r f
O O O O
N N
N N
N N
H
d O
C +~ C +~
O t6 O t~
r ~ O r
+~ r E O O O O O O O O O O O +~ r E O
O tf7
t~ X O O tn O tn O tt7 O O tn O td X O O
J ~l7 tn J ~'
r O r N r~ r~ N rm--i N r~ r-~ r O r M
~ E
r L O r i O
r Q7 r Q~
+~ Q i.~ C.
H a N a
Q r m r
r~D .-~~
r O r- O
r ~ r
t0C t~ C
O
r C r C
G7 O N O
r (/~ ~ r
O ~ O
4. r of C1 N O IW N tl7 I~ Ln N 1~ 4- r f~ O
~ ~ N ~ O
O C i M 00 t0 00 01 N ~ l0 00 O C i M
~ 00 D1 ~f7
G7 C1 .~ Iw 1n C1 N Op r~ O +~ M
i~ N 01 t0 00 N
N r- N r C ~
C C1
+~ G7 r~ .~ M ~ N et .-~ ~ +~ O O
O t0 N
r N U r N U
O C O C
N O N O
G7 U d U
DC
a a
+~ +~ a~
a~
C r- C r
G7 N r
r
U r O 1~ O 117 O 1n O O Ll7 U r O
tn O Ln 00
i +~ N ll~ fw N tn tw N 1~ 1~ i +~ O
O N N Vl
a r 2 r
D G
i i
G7 N O
+~ i i a +.> i
E E
t~! O N O N ~ ~ (C O
~C N
3 a~ o_ E G. E a~ 3 a~ a.
E
O i a O a O s O i a
O
i-1 r +a r i-1 i i-~ r
~
N tn i +~ i +~ O N N i
+~
+~ O +~ O > ~ +~
O
3 cn ~ tn .a O 3 cn
.a
zns.~s~
I . i
-ep
I 1 O
V
I 1 O
N
E
1 ~ >> N
O I
1 N O t0 ~ t0 ~ l0 tQ
r- rt! O N O ~O O O
r I
1 N +~ C
r 'C
C I
1 ~ ~ r-~ O r1 .~ r~ l0 r
O \ I N N M M <l7 lG
Q
1 E iL
~r I
1 r- I E
o
1 I Q
O
N
I I r
~
I 1 C
I I N
~
I >> I r
Q
I ~ ~ O O O O O O O V
+~ I O O
E
I 3 awv o er o ~ 0 I N
v 1 I o 1 0 ~ o
n:
I O +~ t0 Ll7 l0 LL) t0 lp
O \ 1 l0 l0 LI)
G)
i I r- cb C~
L 1
i
d 1 4- i N N N N N N C
O r 1
+~
~.d 1 Y 1 r
i
~
e,~ I I i
2
3, 1 I Q
Q I 1 N
i I I
~
7 1 N 1 d
O
OU I E E E
I
N I O~I
>1 1 r i Q1 1~ O1 t0 01 ~I >>
I l0 1~ LL7 N IW
l0
i 1 C ~ r'
1
C I Q O ~ M 00 wt N ~' .~ C)
N 1 O 1~ l0 tff
M
r I r L ~ .-H r-1 .~ tD +~
C I N ~ N ~ Ln
M
>>r I Q V
I
r 1 (n r i
~ 1
aa~ 1 E 1 0
C.CC 1 1 V
Q 1 I V
O I I
Q~
~ 1 1
+~ r
N 1 I
O fd
i I Q N O
V 1
O 1 r N d 1~ ~ ~7 ~ 1
N N E I I ~ 1 ~ 1~
Q~
r I >Z N N N N N N N >>
+~ f0
+.~ I
(U I E ~
~ i I
e0 1 ~ E r
N N ~ I
3
I N 1 Q
Q
i I 1 V
f-
C I 1 N r
O
r I 1 E
O
N 1 1 O 4-
N I 1 +1 r
N 1 1 +~ "C
~
V 1 * U .~ ~ .-~ O W O
Q L I N N O O
C I G7 C N M 1 N r-~ L N t G7
d d 1 lp I O <f' N
e~ 1 tn O tp Iw tD ~ t0 M >> L
d a I 00 ~ N
r 1 (' I i
r
f0 1 I N .~ Q 01 N
i
1 I
tn 1 I i O
E I 1 NO N N r O O
N
Q I 1 -p~C N ~C E i ~L >Z
~ E
r 1 Q 1 r0O i E O O +~ O r
C O
C I r- t NV O O V +~ N U N O
e~ i~
G1 1 C. I L+~ .1~+~ 'C ~ N
i~
r I E 1 iO t~ +~ C1 O i p1 C >> Q
d C
O
O 1 r0 I NC 3 O O C L N C G7 O ~ Q i
C) +~
L
N I N I >r ~C L r ~C +~ r ~C r 'p
~ O
I I O+~ ~ +~ L ~C .i-~ C r a ~0
C O C O i O
L
1 I !t1 r0 L ~Sf 3 ~ r r i
r V d r a~ V
V N
1 1 ir N O r 1Z r r O 'p N
C
O.
I I OOtrr O >2r-rL O L O.C r
N
C.rr
1 I +~V i ~0 V r O V e,~e0
e0 e0 U ~
c0 r
I I cbi O +~ r L i O i +~ L N N C
C +~ C C N
C i
I I Cr > O L r ~ N r O O ~ O O
r O r O r E
+~
I I OU O I~ +> U N U H d C O
IL f- U LL
4. N
I I r N ~ Q L
I I ~ Q N O +~ t0
i
I I U i +~ +~ d r
i~
I m0 Q td i II i
N
I I i +~ i ~ II
I 1 LLr e6 r- +~ r- d ?~ >
i
I I rv 3 c~ C ec >, ea a~
1 1 ir r- r r Q r r- II Rf O
'O +~
I 1 Q+~ td i +~ t0 V +~ ed
1 I ~Lr C O r C C r C L \.a I""
~.
1 1 OC r O C r O C r dL r
I I UI--I t~ 1--I U 1--a LL >2r-
4. l.t_ Y