Note: Descriptions are shown in the official language in which they were submitted.
rr: '
21186~8
"I~PROVED SPENDABLE ANODE FOR ANTICORROSION PROTECTION
OF OFFSHORE STRUCTURES, ~ND PROCESS FOR HANUF~CTURING
IT".
The present invention relates to a novel
spendabLe anode for the anticorrosion protection of
offshore structures, vhich anode, thanks to its
particular composite-structure con~iguration, besides
making it possible the necessary amount of anodic
material to be decreased ~ith self-explanatory
economic advantages, also improves the protection
state of the surfaces of the offshore structure, ~ith
protective compact deposits being formed in a larger
amount.
The present invention also relates to the process
for acconplishing such a spendable anode.
According to the prior art, the anticorrosion
protection of offshore structures by means of
spendable anodes represents today one of the most used
techniques in this field, and its developme~.t.s.m~d~.it
possible optimal anodic material from the vie~points
of efficiency and ~eight and cost savings to be found
out, such as aluminum, zinc and magnesium alloys.
According to such a technique, the spendable
anode, made of an anodic material having a more
negative electrochemical potential than of the
material ~hich constitutes the offshore structure to
be protected, generates a bias ~hich in its turn
causes a protection current to be established, ~hich
causes protective calcareous deposits to be formed on
the same strucuture.
Such a bias clearly is the h;gher, the more
negative the electrochemical potentiaL of the anodic
material used.
On the other hand, the above said necessary
protection current for the adequate protection of the
offshore structure depends, besides such marine
conditions as temperature, salinity, oxygen content,
and so forth, also, and above all, on the surface
condit;ons of the offshore structure to be protected,
i.e., ~hether calcareous deposits are present or less.
From the above it results hence that during the early
steps of the life time of the offshore structure to be
protected, during which the protective calcareous
deposits have not been adequately formed yet, the need
exists for having the maximal protection current,
which may then decrease during the subsequent life
steps of the structure, as a function of the quality
of the calcareous deposit formed during said initial
step.
from the above, it derives that an essential
element in the design of any spendable anode is the
ability of the latter to supply the high demand of
starting protection current during the initial step of
the life of the offshore structure, besides being
capable of preserving the protection state of said
structure throughout the subsequent steps of the
useful operating life of the latter. Inasmuch as,
however, the starting current ~hich a spendable anode
is capable of supplying depends, bes~des the marine
conditions and its electrochemical potential, also on
:, - :- :.- -:.:: ,,, - .. : : : :-, . .. ::. - . .. ..
2~
its surface and, consequently, its geometry, the above
said condition practical-y results in that the
geometric dimensions and the ~e;ght of the spendable
anode have to be determined as a function of the
S starting current required by the offshore structure to
be protected, and, respectively, of the protection
current ~hich must be generated during the subsequent
operating steps of the same structure, after the
formation of the protective calcareous deposit.
No~, as magnesium and its alloys display the
highest negative eLectrochemical potential, one might
think that the spendable anode made of magnesium aLloy
is the optimal solution for the anticorrosion
protection of offshore structures because it, by
generating a high bias and, consequently, a high
initial anodic current, is capable of suppLying a very
effective protection of the above said structure, by
causing compact calcareous deposits to be formed,
~hich reduce the magnitude of the required protection
current during the subsequent steps of the useful
operating life of said structure. Actually,
ho~ever, such an anode of magnesium alloy, by
displaying a lo~ anodic efficiency, is not capable of
covering the ~hole useful operating life of the
offshore structure to be protected, unless it is used
in such large amounts as to render it economically
- unecceptable, o~ing to its large ~eight and high cost.
In fact, the presently most largely used
spendable anodes for the protection of offshore
structures are aluminum or zinc alloys uhich secure,
2~$~
even uhen small ueight amounts thereof are used, the
fulL protection of the structure to be protected
throughout the who~e life span thereof, ~ith the
demand for a high initial current being satisfied by
S suitabLy increasing the geometric size of said anodes.
The purpose of the present invention precisely is
of obviating the above said draubacks and hence
supply;ng a spendable anode for the anticorrosion
protection of offshore structures uhich is capable of
1û supplying the high starting current uhich is initially
demanded in order to create an effective protective
layer of calcareous deposit on the offshore structure
to be protected, although its geometrical dimens;ons
are kept small, as uell as of securing the
anticorrosion protection throughout the operating life
span of the structure, ~ith its weight being anyuay
kept small.
The above purpose is substantially achieved by
using a composite-structure spendable anode in uhich
the anodic material, generally consisting of zinc or
aluminum alloys, uhich constitutes an external coating
applied onto the carrier uhich supports the same
anode, is externally coated, in its turn, by a second
anodic material, generally magnesium alloys, having an
electrochemical potential still more negative than of
the above said zinc or aluminum alloyed-based anodic
material.
The advantages displayed by such a composite-
structure spendable anode over to the traditional
anodes knoun from the prior art substantially derive
21186~8
from the actions of initial bias;ng and of anodic
efficiency ~hich a spendable anode should perform
being caused no~ to be carried out by two different
anodic materia~s, so as to take the maximal advantage
from their intrinsic properties.
In fact, in that ~ay, the magnesium aLloy ~h;ch
constitutes the outermost coating, ~ith lo~ anodic
capacity and high negative electrochemical potential,
will operate dur;ng the first step of the useful
operating life of the offshore structure to be
protected, and hence makes it possible the geometric
dimension of the same anode to be reduced, whilst the
internal anodic material, constituted by aluminum or
zinc alloys, ~hich starts acting only after that all
tS of said outermost magnesium coating has been
consumed, will operate on surfaces which are already
biased and coated by compact calcareous deposits,
which hence require low protection current values,
with the weight amount of said internaL anodic
material, necessary in order to protect the structure
during the residual portion of the useful operating
life of the latter, being consequently decreased.
Experimental tests demostrated that the use of
composite-structure spendable anodes according to the
present invention makes it possible a considerably
~arge economing saving, of the order of 20~., to be
obtained, as compared to the use of traditional
spendable anodes kno~n from the prior art.
Summing-up, the spendable anode for the
anticorrosion protection of an offshore structure,
21 186~8
comprising a carrier means provided with an external
coating made of an anodic material having a more
negative electrochemical potential than of the
materiaL which constitutes said offshore strurture to
be protected, is characterized, according to the
present invention, in that said anodic material is
provided, in its turn, with an external coating of a
second anodic material with a still more negative
electrochemical potential than of the above said
anodic material.
Then, according to a preferred feature of the
present invention, the process for manufacturing such
a spendable anode, suitable for the anti-corrosion
protection of offshore structures, comprises the steps
of casting said anodic material which constitutes the
external coating of the carrier means which supports
the same anode into a suitable ingot mould or chill or
mould and then casting, into another suitable ingot
mould or chill or mould, said second anod;c material
around the anode formed during the preceding step.
The invention is better explained now by
referring to the accompanying drawing, which displays
a preferred embodiment supplied for merely
exemplifying, non-limitative purposes, because
technical, structural or technological changes may be
always supplied without departing from the scope of
the present invention. So, for example, rather than by
means of sequential steps of casting into ingot moulds
or chills or moulds the two anodic materials which
coat, in sandwich fashion, the anode carrier means,
7.
2I18658
the composite-structure anode can also be manufactured
by spraying said second anodic material onto said
support already coated with the above said anodic
materiaL ~ith lower negative electrochemical
potential, or coating the Latter with said second
anodic material by means of a plating process.
On the other hand, it is evident that all of the
above said processes make it possible composite anodes
with any cross-section and length features to be
manufactured.
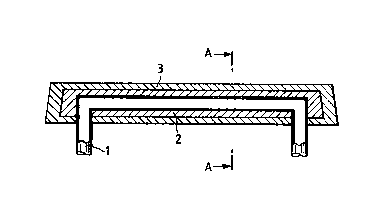
In said drawing:
figure 1 shows a perspective view of a spendable
anode for the anticorrosion protection of offshore
structures, manufactured according to the invention;
Figure 2 displays a longitudinal sectional view
made along the middle of the anode of Figure 1;
Figure 3 displays a front sectional view made
along section line AA of figure 2.
Referring to the above Figures, w;th the
reference numeral 1 the carrier means is indicated of
the anode to be manufactured, which carrier means,
charged to a suitable ingot mould or chill or mould,
not shown in Figure, is provided with an external
coating by casting an anodic material 2 with a more
negative electrochemical potential than of the
material which constitutes the structure to be
protected.
Said coated carrier means is then charged to
another suitable ingot mould or chill or mould, also
not displayed in Figure, and, in its turn, is provided
21186~
~ith an externa~ coating by casting an anodic material
3 ~ith a more negative e~ectrochemical potential than
of the anodic materiaL 2.