Note: Descriptions are shown in the official language in which they were submitted.
2118~61
4- 19482/A
Acvloxvhexanoic Acid Derivatives
The invention relates tO novel chemical compounds that are derivatives of non-
hydrolysable analogues of peptides that are cleavable by aspartate proteases, that is to say
acylated 5-arnino-4-hydroxy-hexanoic acid derivatives and novel precursors thereof, to
processes for the preparation of those compounds, to pharmaceutical compositionscomprising those peptide analogues, and to the use thereof as medicarnents or in the
preparation of phannaceutical compositions for combating diseases caused by retro-
viruses.
Since the disease first appeared at the beginning of the 1980s, AIDS (Acquired Immuno-
deficiency Disease Syndrome) and its devastating consequences have been the subject of
numerous publications of a scientific or other nature. With the exception of minor
opinions to the contrary, the disease is thought to be caused by the HIV virus ~Human
Immunodeficiency Virus) of which two forms (HIV-l and HIV-2) have been described in
detail hitherto. At present, the treatment of the disease involves principally the use of
inhibitors of reverse transcriptase (a virus-specific enzyme), for example 3'-azid~
thymidine (AZT), although they have severe toxic side-effects. However, attempts have
also been made to introduce into the body, for example in the form of a recombinant
molecule or molecule fragme:nt, the T4-cell receptor which is present in certain cells of the
defence system of the human body and which is responsible for the anchoring and intro-
duction of infectious virus particles into those cells and thus for their infection. The
desired result is that binding sites on the virus particles would be titrated out and the
virions would therefore no longer be able to bind to the cells. Although treatment with
CD4 and derivatives appears to have the advantage of low toxicity, there is as yet no data
relating to its therapeutic effectiveness.
HIV has a genome organisation comparable to that of other retroviruses: the genome is
organised into the regions gag/pol/env. It has been shown that in HIV and other retr~
viruses the proteolydc maturation of the gag and gaglpol-fusion proteins is brought about
by a protease, HIV-protease, which is itself encoded by the pol-region of the viral
genome. Without that proteolytic cleavage, it is not possible for infectious virus particles
- 211866~
.
- 2-
to be formed, because, for example, the so-called "core proteins", that is to say structural
proteins of the v*us core, cannot be freed from their precursors.
According to WHO estimates, about ten million people are infected by HIV at present.
The disease is fatal in virtually all cases.
HIV- 1 and HIV-2 have in their genome regions that code for the HlV-protease which is
formed from a precursor protein that is thought to be cleaved autoproteolytically.
~he protease has an aspartate residue in its catalytic centre and is homologous with other
aspartate proteases. In the case of HIV it consists of 99 amino acids. The HIV-protease,
which has since been obtained also as a recombinant and as a chemically synthesised
molecule, has been treated with vaIious inhibitors, it having been shown that it too
functions as an aspartate protease. X-ray structural analysis of the HIV-protease and a
related enzyme from Rous' sarcoma virus supports the view that the en7yme, which is in
the form of a dimer in the active form, acts as an aspaltate protease. ;`
:
Owing to the central role of the HIV-protease in the processing of the said core proteins, it
is assumed that effective inhibition of that enzyme in vivs) will suppress the assembly of
mature virions, so that corresponding inhibitors can be used therapeutically. ~ - `
Prerequisites of therapeutic activity in vivo are the achievement of good inhibition of virus
replication in cell experiments and good bioavailability, for example a high level in the
blood that is sufficient to achieve adequately high concentrations in infected cells in the -
body.
The aim of the present invention is to provide novel compounds that exhibit advantageous
pharmacological propenies. :
I
Surprisingly it has now been found that the compounds according to the invention are
capable of achieving that aim.
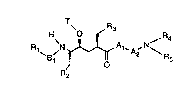
The compounds according to the invention are especially compounds of formula I'
':
. ~ ,
: '
-^ 2 ~
B, ~A,~, ~,N~ (I )
wherein T is an acyl radical of fonnula Z
RZ :~
o c C (~)
\
wherein :
R~ is unsubstituted or substituted hydrocarbyl wherein at least one car~on atom has been
replaced by a hetero atom with the proviso that a hetero atom is not bonded dinectly to the
carbonyl to which the radical RZ is bonded, aLkyl having two or more carbon atoms, lower
aLkenyl, lower alkynyl, aryl or unsubsdtuted or substituted amino, and wherein
Rl is hydrogen, lower alkoxycarbonyl, heterocyclylcarbonyl, benzyloxycarbonyl that is
unsubstituted or substituted by up to three radicals selected independendy of one another
from fluorine, halo-lower al~l, lower aLkanoyl, sul~o, lower aLkylsulfonyl and cyano,
heterocyclyloxycarbonyl wherein heterocyclyl is bonded via a carbon atom, one of the
mentioned carbonyl radicals wherein the bonding carbonyl group has been replaced by a
thiocarbonyl group, heterocyclylsulfonyl, lower aLkylsulfonyl or N-(heterocyclyl-lower
alkyl)-N-lower aLIcyl-aminocarbonyl,
Bl is a bond or a bivalent residue of an a-amino acid bonded N-terminally to Rl and C-
terminally to the amino group at the carbon atom carrying R2-CH2-,
R2 and R3 are each independently of the other phenyl or cyclohexyl, those radicals being
unsubstituted or substituted by from one to three radicals selected independen~y of one
another from hydroxy, lower alkoxy, halogen, halo-lower aLkyl, sulfo, lower ~lkylsulfonyl, . :
cyano and nitro,
Al is a bond between -C~) and A2 or is a bivalent residue of an a-amino acid bonded
N-terminally to the group -C=O and C-tenninally to A2,
A2 is a bivalent residue of an oc-amino acid bonded N-terminally to Al and C-terminally
-`` 2118~1
to the group NR4Rs, or
Al and A2 together form a bivalent residue of a dipeptide the central amide bond of
which has been reduced and which is bonded N-tcrminally to the group -C=O and C-terminally to the group NR4Rs, and
R4 and Rs together with the bonding nitrogen atom form unsubstituted or substituted
thiomorpholino or morpholino, ;or salts of those compounds where salt-forming groups are present.
In the description of the present invention, unless expressly deT~ned to the contrary, the
term "lower" used in the definition of groups or radicals, for example lower alkyl, lower
alkoxycarbonyl etc., means that the groups or radicals so defined contain up to and
including 7 and preferably up to and including 4 carbon atorns.
AsymmetTic carbon atoms which may be present in the substituents T, R1, Bl, R2, R3, A
and/or A2 and also in substituted thiomo~pholino or morpholino formed by R4 and Rs
together with the bonding nitrogen atom, can be in the (R)-, (S)- or (R,S)-configuration,
preferably in the (R)- or (S)-configuration. Accordingly the present compounds can be in
the form of mixtures of isomers or in the form of pure isomers, especially in the form of
diastereoisomeric mixtures, pairs of enantiomers or, preferably, pure enantiomers.
The general tenns and names used in the description of this invention preferably have the
following definidons; in the various categories of definition it is possible to use instead of
the general definitions any combinations of or individual radicals from the radicals
mentioned hereinabove and hereinbelow:
Unsubstituted or substituted hydrocarbyl RZ wherein at least one carbon atom has been
r~placed by a hetero atom with the proviso that a hetero atom is not bonded directly to the
carbonyl to which the radical RZ is bonded, is a saturated, partially saturated or unsatura- `
ted hydrocarbon radical having a maximum of 30 carbon atoms, preferably having up to
22 carbon atoms, and contains in place of at least one carbon atom, preferably in place of
each of from one to four carbon atoms, a hetero atom selected from nitrogen, oxygen and
sulfur and is unsubstituted or substituted by one or more radicals, preferably by up to three
substituents, especially by hydroxy, by lower alkoxy, such as methoxy, by lower alkoxy-
lower alkoxy, such as 2-methoxyethoxy, by lower alkoxy-lower alkoxy-lower alkoxy,
such as 2-(2-methoxyethoxy)ethoxy, by phenyl- or naphthyl-lower alkoxy-lower aLkoxy
(wherein phenyl or naphthyl is unsubstituted or substituted by one or more, preferably one,
- 211~
of the radicals halogen, such as fl~lorir~e, chlorine or bromine, lower alkyl, such as methyl,
halo-lower alkyl, such as trifluoromethyl, hydroxy, lower alkoxy, such as methoxy, lower
alkanoyloxy, carboxy, lower alkoxycarbonyl, phenyl-lower alkoxycarbonyl, cyano, carba-
moyl, mono- or di-lower alkylcarbamoyl, mono- or di-hydroxy-lower alkyl-carbamoyl,
heterocyclyl-lower alkyl wherein heterocyclyl is a saturated, partially saturated or un-
saturated single ring containing from 3 to 7, preferably from 5 to 7, ring atoms and up to
two hetero atoms selected from nitrogen, sulfur, oxygen and lower alkyl-, benzyl-, di-
phenylmethyl-, triphenylmethyl- or lower alkanoyl-substituted nitrogen, for example
piperidinomethyl, piperazin-l-ylmethyl, 4-lower alkyl-piperazin-1-ylmethyl, such as
4-methyl- or 4-ethyl-piperazin-1-ylmethyl, morpholinomethyl or thiomorpholinomethyl,
and nitro, which may be present independently of one another), by phenyl-lower alkanoyl-
oxy, such as benzyloxy, by halogen, such as fluorine, chlorine or bromine, by halo-lower
alkyl, such as trifluoromethyl or chloromethyl, by carboxy, by lower alkoxycarbonyl, by
phenyl-lower alkoxycarbonyl, such as benzyloxycarbonyl, by carbamoyl, by lower alkyl-
carbamoyl, by hydroxy-lower alkylcarbamoyl, by di-lower alkylcarbamoyl, by bis-
(hydroxy-lower alkyl)carbamoyl, by cyano, by oxo, by C3-C8cycloalkyl, such as cyclo-
butyl, cyclopentyl or cyclohexyl, or by aryl, preferably C6-CI4aryl, such as phenyl,
naphthyl, such as 1- or 2-naphthyl, or fluorenyl, such as fluoren-9-yl, those substituents
being independent of one another when several are present; a hetero atom preferably being
directly adjacent to the carbon atom in the radical hydrocarbyl RZ that is bonded to the
carbonyl group that bonds RZ in formula I' (that is to say being in the 3-position star~ing
from the RZ-bonding carbonyl group included in the count); and is especially ~ .
- heterocyclyl, bonded via a ring carbon atom, which is preferably a saturated, partially
saturated or unsaturated ring containing from 3 to 7, especially from 5 to 7, ring atoms and
containing one or more, especially up to a maximum of four, more especially up to two,
hetero atoms selected independently of one another from nitrogen, sulfur and oxygen; the
ring being present either as such or being once or twice, especially once, benzo-fused,
cyclopenta-, cyclollexa- or cyclohepta-fused; the ring system being substituted by up to
three radicals selected independently of one another from lower alkyl, phenyl-lower aIkyl,
diphenyl-lower alkyl, triphenyl-lower alkyl, such as triphenylmethyl, lower alkanoyl, .:
hydroxy, lower alkoxy, phenyl-lower alkoxy, such as benzyloxy, diphenylmethoxy or tri~
phenylmethoxy, hydroxy-lower alkyl, such as hydroxymethyl, halogen, such as fluorine, .
chlorine or bromine, cyano, lower alkoxycarbonyl, such as methoxy- or tert-butoxy-
carbonyl, phenyl-lower alkoxycarbonyl~ such as benzyloxycarbonyl, and halo-lower aLkyl,
such as chloromethyl or trifluoromethyl, or preferably being unsubstituted; selected espe-
~ 21186fil
- 6 -
cially from pyrrolyl, 2,5-dihydropyrrolyl, indolyl, indoli~inyl, isoindolyl, pyrrolidinyl,
such as pyrrolidin-3-yl or especially pyrrolidin-2-yl (in the (R,S)- or preferably the (R)- or
(S)-configuration), hydroxypyrrolidinyl, such as 3- or especially 4-hydroxypyrrolidinyl, : :
furyl, such as furan-3-yl or especially furan-2-yl, tetrahydrofuryl, thienyl, cyclohepta[b]- ..
pyrrolyl, imidazolyl, such as imidazol-2-yl, imidazol-3-yl or especially imidazol-5-yl,
N-triphenyl-lower alkyl-imidazolyl, such as N-triphenylmethyl-imidazolyl, pyrazolyl,
especially pyrazol-3-yl, oxazolyl, isoxazolyl, such as isoxazol-3-yl or -5-yl, thiazolyl, iso-
thiazolyl, such as isothiazol-3-yl or-S-yl, triazolyl, such as 1,2,3-triazol-4- or -5-yl or
1,2,4-tIiazol-5-yl, tetrazolyl, pyridyl, such as pyridin-4-yl or -3-yl or especially pyridin-
2-yl, quinolyl, such as quinolin-2-yl, isoquinolyl, especially isoquinolin- 1-yl or -3-yl,
piperidyl, especially piperidin-2-yl, ~-pyranyl, 4,5-dihydropyranyl, 4H-chromenyl,
chromanyl, ~-thiopyranyl, pyridazinyl, cinnolyl, phthalazinyl, quinazolinyl, quinoxalinyl,
pyrimidinyl, pyrazinyl, phenazinyl, phenoxazinyl, phenoth}azinyl, morpholinyl and
thiazinyl, each of which is bonded via a ring carbon atom; very special preference is given
to those of the mentioned radicals which contain a ring hetero atom directly adjacent to
the bonding ring carbon atom. Strong preference is given to the radicals furan-2-yl, (S)- or
(R)-pyrrolidin-2-yl and imidazol-4-yl, pyridin-2-yl, -3-yl or -4-yl, or isoquinolin-3-yl; and
also tetrahydropyranyl, such as 4-tetrahydropyranyl;
- lower alkyl (especially methyl, ethyl, n-propyl or n-butyl) which is substituted by at least
one radical selected from etherified or esterified hydroxy or (unoxidised or oxidised by 1
or 2 oxo groups) mercapto, unsubstituted or substituted amino and heterocyclyl, especially
by one of the mentioned radicals, and which may carry as a further substituent aryl, which
is as defined below at the end of this section, wherein
etherified hydroxy is especially lower alkoxy, such as methoxy, ethoxy or n-butoxy, or ~ :
lower alkoxy, such as methoxy, ethoxy or n-butoxy, substituted by one or two substi-
tuents, especially by aryl, more especially phenyl or naphthyl, by lower alkoxy, such as
ethoxy or methoxy, by lower alkylthio, such as methylthio or ethylthio, by loweralkoxy-lower alkoxy, such as 2-methoxyethoxy, by lower alkylthio-lower alkoxy, such
as 2-methylthioethoxy, by aryloxy or arylthio wherein aryl is as defined below, espe- :
cially phenyl or o-, m- or p-chlorophenyl, for example p-chlorophenyloxy, by amino,
N-lower alkylamino or N,N-di-lower alkylamino, such as 2-amino, 2-(N-lower alkyl)-
amino or 2-(N,N-di-lower alkyl)amino, for example 2-dimethylamino, by heterocyclyl as
deflned above for heterocyclyl RZ bonded via a ring carbon atom, especially 2-, 3- or 4-
pyridyl, or (additionally or preferably) by lower alkoxycarbonyl, such as methoxy-
t,.' ' ~:
~i' ,`~ .
2118661
- 7 -
carbonyl, by lower alkanoyloxy-lower alkoxycarbonyl, such as pivaloyloxymethoxy- :
carbonyl or acetyloxymethoxycarbonyl, by carboxy, by phenyl-lower alkoxy-
carbonylamino-lower alkoxy, such as 2-(benzyloxycarbonylamino)-ethoxy, by arnino-
lower alkoxy, such as 2-aminoethoxy, by di-lower alkylamino-lower alkoxy, such as
2-(dimethylamino)-ethoxy, or by N,N-di-lower alkylamino-lower alkoxy, such as
2-(dimethylamino)-ethoxy, or is aryloxy, especially phenyloxy, or (additionally or espe-
cially) tetrahydropyranyloxy, such as 4-tetrahydropyranyloxy;
esterified hydroxy is especially lower alkanoyloxy, such as acetyloxy, benzoylo~cy or
phenyl-lower alkanoyloxy, such as phenylacetyloxy;
etherified mercapto is especially lower alkylthio, such as methylthio, ethylthio or
n-butylthio, or lower alkylthio, such as methylthio, ethylthio or n-butylthio, substituted ~-
by one or two substituents, especially by aryl, more especially phenyl or naphthyl, by
lower alkoxy, such as ethoxy or methoxy, by lower alkylthio, such as methylthio or
ethylthio, by lower alkoxy-lower alkoxy, such as 2-methoxyethoxy, by lower alkylthio-
lower alkoxy, such as 2-methylthioethoxy, by aryloxy or arylthio wherein aryl is as
defimed below, especially phenyl or o-, m- or p-chlorophenyl, for example p-chloro-
phenyloxy, by amino, N-lower alkylamino or N,N-di-lower alkylamino, such as 2-amino,
2-(N-lower alkyl)amino or 2-(N,N-di-lower alkyl)amino, for example 2-dimethylamino,
by heterocyclyl as defined above for heterocyclyl RZ bonded via a ring carbon atom,
especially 2-, 3- or 4-pyridyl, or (additionally or especially) by lower aLkoxycarbonyl,
such as methoxycarbonyl, or is arylthio, such as phenylthio; it being possible for the
mercapto sulfur atom additionally or especially to be oxidised by one or preferably two
oxo groups, especially in lower alkoxycarbonyl-lower alkylsulfo, such as methoxy-
carbonyl-methylsulfo;
esteri~led mercapto is especially lower alkanoylthio, benzoylthio or phenyl-lower
alkanoylthio, such as phenacetylthio;
unsubstituted or substituted amino is especially amino or amino substituted by one or :::
two radicals selected from lower alkyl, such as methyl, heterocyclyl-lower alkyl wherein
heterocyclyl is as defined for heterocyclyl R~ bonded via a ring carbon atom, especially
heterocyclylmethyl, such as imidazolylmethyl, for example 4-imidazolylmethyl, orpyridylmethyl, for example 2-, 3- or 4-pyridylmethyl, each bonded via a ring carbon
atom, a}yl-lower alkyl, such as phenyl- or naphthyl-lower alkyl, for example phenyl- or
2 1 ~
- 8- ~ ~ -
naphthyl-methyl, lower alkanoyl, such as acetyl, lower alkoxycarbonyl, such as tert- -
butoxycarbonyl, and aryl-lower alkoxycarbonyl, such as phenyl-lower alkoxycarbonyl,
for example benzyloxycarbonyl; very especially one of the substituents of amino is lower
alkyl, especially methyl, and the other is hydrogen or one of the radicals mentioned
above as substituents of amino; and
heterocyclyl is especially as defmed above for heterocyclyl RZ bonded via a ring carbon
atom and is preferably a saturated, partially saturated or unsaturated ring and may also be
fused or substituted as above, especially as pyridin-2-yl, -3-yl or -4-yl;
or
- heterocyclyl-lower alkyl wherein lower alkyl is preferably methyl, 1- or 2-ethyl or
3-propyl and wherein heterocyclyl is as defined above for heterocyclyl RZ bonded via a
ring carbon atom, which is preferably a saturated, partially saturated or unsaturated ring
and may also be fused or substituted as above but may also be bonded via a ring nitrogen
atom, especially imidazol-l-yl, imidazol-2-yl, imidazol-S-yl or more especially imidazol~
4-yl, N-triphenyl-lower alkylimidazolyl, such as N-tTiphenylmethyl-imidazol-S-yl or espe-
cially -4-yl, or pyrazolyl, such as pyrazol- l-yl, -3-yl, -4-yl or -5-yl.
Alkyl RZ having two or more carbon atoms is especially ethyl, n-propyl, isopropyl, n-butyl
or l,l-dimethylethyl, or more especially C7-C20alkyl, so that T is, for example, propionyl,
butyryl, methylpropionyl, valeroyl or pivaloyl, or especially octanoyl, decanoyl or
palmitoyl.
Lower alkenyl RZ iS especially C2-C7alkenyl, more especially C2-C3alkenyl, wherein the
double bond is preferably in the l-position, so that T is, for example, acryloyl, crotonoyl,
isocrotonoyl or methacryloyl.
I
Lower alkynyl RZ is especially C2-C7aL~cynyl, more especially C2-C3alkynyl, wherein the
triple bond is preferably in the l-position, so that T is, for example, propynoyl. ~ ;
Aryl RZ by itself or aryl as a substituent in the above-mentioned radicals RZ with the
exception of aryl itself is especially C6-CI4aryl, more especially phenyl, naphthyl, such as
1- or 2-naphthyl, or fluorenyl, such as 9-fluorenyl, and is unsubstituted or substieuted by
up to three radicals selected independently of one another frorn lower aL~cyl, lower
t "': - ~ ` ` ~ ' ' . . .
~' . ' `' ' . ':
`'' ' , ` ' ` '
~ '' ' ' : '
- `` 21 ~86~1
alkanoyl, hydroxy, lower alkoxy, phenyl-lower alkoxy, such as benzyloxy, diphenyl-
rnethoxy or triphenylmethoxy, hydroxy-lower alkyl, such as hydroxymethyl, halogen, such
as fluorine, chlorine or bromine, cyano, lower alkoxycarbonyl, such as methoxy- or tert-
butoxy-carbonyl, phenyl-lower alkoxycarbonyl, such as benzyloxycarbonyl, halo-lower
alkyl, such as chloromethyl or trifluoromethyl, heterocyclyl-lower alkyl wherein hetero-
cyclyl is a saturated, partially saturated or unsaturated single ring containing from 3 to 7,
preferably from 5 to 7, ring atoms and up to two hetero atoms selected from nitrogen,
sulfur, oxygen and lower alkyl-, benzyl-, diphenylmethyl-, triphenylmethyl- or lower
alkanoyl-substituted nitrogen, for example piperidinomethyl, piperazin-l-ylmethyl,
4-lower alkyl-piperazin- 1 -yl-methyl, such as 4-methyl- or 4-ethyl-piperazin- l-ylmethyl,
morpholinomethyl or thiomorpholinomethyl, and nitro, which may be present indepen-
dently of one another, especially correspondingly substituted phenyl. Special preference is
given to o-, m- or p-chlorophenyl, chloro-lower alkylphenyl, such as p-chloromethyl~
phenyl, p-(morpholino-lower alkyl)phenyl, such as p-morpholinomethyl-phenyl, or
p-(thiomorpholino-lower alkyl)phenyl, such as p-thiomorpholinomethyl-phenyl, or also
phenyl. ~ .
Unsubsdtuted or substituted amino RZ carries at the nitrogen atom 1 or 2 substituents ~:
selected independently of one another from unsubstituted or substituted lower alkyl ~:
wherein the substituents of lower alkyl are preferably selected from hydroxy, lower . ~ :-
aL~coxy, lower aLtcanoyloxy, phenyl-lower alkanoyloxy, such as benzoyloxy or phenyl-
acetyloxy, halogen, such as fluorine, chlorine, bromine or iodine, especially fluorine or ~ :
chlorine, carboxy, lower alkoxycarbonyl, phenyl-lower alkoxycarbonyl, such as benzyl-
oxycarbonyl, cyano, oxo and phenyl or naphthyl each of which is unsubstituted or mono- :
or poly-substituted, preferably mono-substituted, for example by lower alkyl, for example
methyl, halo-lower alkyl, such as chloro- or bromo-methyl, halogen, for example fluorine
or chlorine, hydroxy, lower alkoxy, such as methoxy, lower alkanoyloxy, carboxy, lower ~ ~
alkyloxycarbonyl, phenyl-lower alkoxycarbonyl, halo-lower alkyl, such as trifluoromethyl, . ~ .
cyano and/or by nitro, especially phenyl substituted in the p-position by one of the ~ ~ :
mentioned radicals; especially selected from unsubstituted lower alkyl, such as methyl or
ethyl; and aryl that preferably has from 6 to 14 carbon atoms and is unsubstituted or ::
mono- or poly-substituted, preferably mono-substituted, for example by lower alkyl, for
example methyl, halo-lower alkyl, such as chloro- or bromo-methyl, halogen, for example
fluorine or chlorine, hydroxy, lower alkoxy, such as methoxy, lower alkanoyloxy, --
carboxy, lower alkyloxycarbonyl, phenyl-lower alkoxycarbonyl, halo-lower alkyl, such as
trifluoromethyl, cyano and/or by nitro, the nitrogen atom of the resulting N-substituted
.~
~ J . . ~
211~
1() :
carbamoyl group T carrying not more than one aryl radical; amino RZ being especially
amino, mono- or di-lower alkylamino, such as N-methyl-, N-ethyl-, N,N-dimethyl- or
N,N-diethyl-amino, or phenyl-lower aLkylamino wherein phenyl is unsubstituted or substi-
tuted by lower alkyl, for example methyl, halo-lower alkyl, such as chloro- or bromo-
methyl or trifluoromethyl, halogen, for example fluoIine or chlorine, hydroxy, lower
alkoxy, such as methoxy, carboxy and/or by cyano, pref~rably by up to three of those
substituents selected independently of one another, especially by one of those substituents,
for example in the p-position, such as in N-benzyl-, N-(4-fluorobenzyl)-, N-(4-chloro-
benzyl)-, N-(4-trifluoromethylbenzyl)- or N-(4-cyanobenzyl)-amino; special preference is
given to amino substituted at the nitrogen atom by only one radical, for example N-lower
alkylamino, such as N-methyl- or N-ethyl-amino, or phenyl-lower alkylamino wherein
phenyl is unsubstituted or substituted by lower alkyl, such as methyl, halo-lower alkyl,
such as chloro- or bromo-methyl or trifluoromethyl, halogen, such as fluorine or chlorine,
hydroxy, lower alkoxy, such as methoxy, carboxy and/or by cyano, preferably by up to
three of those substituents selected independendy of one another, especially by one of
those substituents, for example in the p-position, such as in N-benzyl-, N-(4-fluoro-
benzyl)-, N-(4-chlorobenzyl)-, N-(4-trifluoromethylbenzyl)- or N-(4-cyanobenzyl)-amino.
The definitions falling under the definition of unsubstituted or substituted amino R~ and ` ~:
the radical aminocarbonyloxy Rs may preferably be omitted from any of the definitions of
compounds of forsnula I' mentioned hereinabove and hereinbelow.
Especially preferred for RZ are the mentioned definitions with the exception of alkyl
having m~re than 2 carbon atoms, lower alkenyl and lower alkynyl; and also with the
exception of aryl. ~ -
In all definitions preference is given especially to those radicals RZ that contain in the
2-position or also in a higher position, such as the 3- or 4-position, a hetero atom selected
from nitrogen, oxygen and sulfur, especially nitrogen (especially valuable properties, for
example especially easy removal of T, are obtained in that case).
T is especially pyrrolidin-2-ylcarbonyl or -3-ylcarbonyl, such as (R)- or (S)-pyrrolidin-
2-ylcarbonyl ((D)- or (L)-prolyl), furan-3- or especially furan-2-ylcarbonyl, pyridyl-4-,
pyridyl-3- or especially pyridyl-2-ylcarbonyl, isoquinolin-1- or especially isoquinolin-
3-ylcarbonyl, pyrazin-2-ylcarbonyl, lower alkoxy-lower alkylcarbonyl, such as methoxy-
acetyl, n-butoxyacetyl or 3-methoxypropionyl, phenyl- or naphthyl-lower alkoxy-lower
alkylcarbonyl, such as benzyloxyacetyl, a-lower alkoxy-a-phenyl-lower alkylcarbonyl,
.'. . .............................. . .
'.,. ~ ~ ' '~ ' ;' , " ' ' ''' : ,..... ....
- 211~61
such as (R)- or (S)-a-methoxy-oc-pllenylacetyl, lower alkoxy-lower alkoxy-lower alkyl-
carbonyl, such as 2-(methoxyethoxy)acetyl, lower alkoxy-lower aL~oxy-lower aLIcoxy-
lower alkylcarbonyl, such as 2-(2-(methoxyethoxy)ethoxy)acetyl, o-, m- or p-chloro-
phenyloxy-lower alkoxy-lower aLkylcarbonyl, N,N-di-lower alkylamino-lower alkoxy-
lower alkylcarbonyl, such as 2-(N,N-dimethylamino)ethyloxy-acetyl or -3-propionyl, 2-,
3- or 4-pyridyl-lower alkyloxy-lower alkylcarbonyl, such as pysidin-2-ylmethoxyacetyl,
phenyloxy-lower alkylcarbonyl, such as phenoxyacetyl, lower alkylthio-lower aLkyl-
carbonyl, such as methylthioacetyl, phenyl-lower alkylthio-lower aLkylcarbonyl, such as
benzylthioacetyl, N,N-di-lower alkylamino-lower alkyicarbonyl, such as N,N-dimethyl-
amino-acetyl, -3-propionyl or -4-butyryl, N-lower alkylamino-lower alkylcarbonyl, such
as N-methylamino-acetyl or -3-propionyl, N-imidazol(-2-, -4- or -5-)ylmethyl-N-lower
alkylamino-lower alkylcarbonyl, such as N-(imidazol-4-ylmethyl)-N-methylaminoacetyl,
N-pyridin(-2-, -3- or -4-~ylmethyl-N-lower alkylamino-lower alkylcarbonyl, such as
N-pyridin-2-ylmethyl-N-methylaminoacetyl, N-phenyl-lower alkoxycarbonyl-N-lower
alkylamino-lower alkylcarbonyl, such as N-benzyloxycarbonyl-N-methylaminoacetyl,imidazol(-1-, -2-, -4- or -5-)yl-lower alkylcarbonyl, such as 3-(imidazol-4-yl)propionyl, or
N-triphenyl-lower alkylimidazol(-4- or -5-)yl-lower alkylcarbonyl, such as 3-(N-triphenyl- ~: :
methylimidazol-4-yl)propionyl, or pyrazol(- 1-, -3-, -4- or -5-)yl-lower aL~cylcarbonyl, such ::
as pyrazol- 1-ylacetyl, or also halo-lower alkylbenzoyl, such as p-chloromethylbenzoyl, p- :
(morpholino- or thiomorpholino-methyl)benzoyl or benzoyl, and (additionally or espe-
cially) lower alkanoyloxy-lower alkylcarbonyl, such as acetyloxyacetyl, lower alkoxy-
carbonyl-lower alkoxy-lower alkylcarbonyl, such as methoxycarbonylmethoxy-acetyl,
lower alkoxycarbonyl-lower alkylthio-lower alkylcarbonyl, such as methoxycarbonyl- :
methylthio-acetyl, lower alkoxycarbonyl-lower alkylsulfo-lower aL~cylcarbonyl, such as
methoxycarbonylmethylsulfo-acetyl, lower alkanoyloxy-lower alkoxycarbonyl-lower
alkoxy-lower alkylcarbonyl, such as pivaloyloxymethoxycarbonyl-methoxyacetyl or
acetyloxymethoxycarbonylmethoxy-acetyl, carboxy-lower alkoxy-lower alkylcarbonyl,
such as carboxymethoxyacetyl, N-phenyl-lower alkoxycarbonylamino-lower alkylcar-bonyl, such as 3-benzyloxycarbonylarnino-propionyl, amino-lower aL~cylcarbonyl, such as
3-aminopropionyl, di-lower alkylamino-lower alkoxy-lower alkoxy-lower alkylcarbonyl,
such as [2-(2-dimethylarninoethoxy)-ethoxy]-acetyl, N-phenyl-lower alkoxycarbonyl-
amino-lower alkoxy-lower alkoxy-lower alkylcarbonyl, such as 2-(2-N-benzyloxy-
carbonylaminoethoxy3ethoxy-acetyl, amino-lower alkoxy-lower alkoxy-lower aLIcyl-carbonyl, such as 2-(2-aminoethoxy)ethoxy-acetyl, or tetrahydrofuranyloxy-lower alkyl-
carbonyl, such as 2-(4-tetrahydrofuranyloxy)-acetyl or 2(R)-, 2(S)- or also 2(R,S)-(4-tetra-
hydrofuranyloxy)-propionyl.
- ,
2 1 1 ~
- 12-
Lower alkoxycarbonyl Rl preferably contains a branched lower alkyl radical, especially a
sec- or tert-lGwer alkyl radical, and is, for example, butoxycarbonyl, such as tert-butoxy-
carbonyl or isobutoxycarbonyl or (additionally or especially) ethoxycarbonyl. Tert- -
butoxycarbonyl is especially preferred.
Heterocyclylcarbonyl Rl contains especially a 5- or 6-membered heterocycle that contains
from 1 to 3 hetero atoms selected independently of one another from S, O and N, is un- ~ :
saturated or fully or part~ally saturated and is once or up to three times benzo-fused,
cyclopenta-, cyclohexa- or cyclohepta-fused, it being possible for the mentioned fused
rings to contain a further nitrogen atom as hetero atom, for example a heterocyclyl radical
selected from pyrrolyl, furanyl, thienyl, imidazolyl, pyrazolyl, oxazolyl, thiazolyl, pyridyl,
pyrazinyl, pyrimidinyl, indolyl, quinolyl, isoquinolyl, quinoxalinyl, ~-carbolinyl and a
benzo-fused, cyclopenta-, cyclohexa- or cyclohepta-fused derivative of those radicals,
which may also be fully or partially saturated, but are preferably partially saturated, or is
selected from pyridylcarbonyl, for example pyridyl-3-carbonyl, morpholinylcarbonyl, for
example morpholinocarbonyl, and benzofuranoyl, for example 3-benzofuranoyl, and also
alternatively or in addition thereto tetrahydroisoquinolylcarbonyl, for example tetrahydro-
isoquinolyl-3-carbonyl, preferably tetrahydroisoquinolyl-3(S)-carbonyl.
Benzyloxycarbonyl Rl is unsubstituted or substituted by up to three radicals selected
independently of one another from fluorine, halo-lower aL~cyl, for example trifluoromethyl
or pentafluoroethyl, lower alkanoyl, such as acetyl, propanoyl, butyryl or pivaloyl, sulfo,
lower alkylsulfonyl, for example methylsulfonyl, ethylsulfonyl, n-propylsulfonyl or
isopropylsulfonyl, and cyano. Preference is given to benzyloxycarbonyl that is unsubsti-
tuted or o-, m- or p-substituted, especially p-substituted, in the phenyl ring by a radical
selected from fluorine, trifluoromethyl, sulfo, methylsulfonyl, ethylsulfonyl and cyano, for
example benzyloxycarbonyl, fluorophenylmethoxycarbonyl, such as p-fluorophenyl-
methoxycarbonyl, trifluoromethylphenylmethoxycarbonyl, such as p-trifluoromethyl-
phenylmethoxycarbonyl, methylsulfonylphenylmethoxycarbonyl, such as p-methyl-
sulfonylphenylmethoxycarbonyl, or cyanophenylmethoxycarbonyl, such as p-cyano-
phenylmethoxycarbonyl.
Heterocyclyloxycarbonyl Rl contains as heterocyclyl especially a 5- or 6-membered
heterocycle that contains from 1 to 3 hetero atoms selected independently of one another
from S, O and N, is unsaturated or fully or partially saturated and is once or up to three
2 ~ 6 1
- 13-
times benzo-fused, cyclopenta-, cyclohexa- or cyclohepta-fused, it being possible for the
mentioned fused rings to contain a further nitrogen atom as hetero atom, for example a
radical selected from pyrrolyl, thienyl, imidazolyl, pyrazolyl, oxazolyl, thiazolyl,
pyrazinyl, pyrimidinyl, indolyl, quinolyl, isoquinolyl, quinoxalinyl, ~B-carbolinyl, or
additionally or especially ~-pyranyl or furanyl, and a benzo-fused, cyclopenta-, cyclohexa-
or cyclohepta-fused derivative of those radicals, which may also be fully or partially
saturated, the heterocyclyl radicals being bonded via a ring carbon atom to the oxygen of
the associated oxycarbonyl radical, preferably selected from pyrrolyl, thienyl, imidazolyl,
pyrazolyl, oxazolyl, thiazolyl, pyrazinyl, pyrimidinyl, indolyl, quinolyl, isoquinolyl, quin-
oxalinyl, ~-carbolinyl and a fully or partially saturated derivative of those radicals, for
example a partially saturated derivative of those radicals or indol-3-yl-oxycarbonyl,
benzothiazol-6-yl-oxycarbonyl or quinol-8-yl-oxycarbonyl, additionally or especially
tetrahydropyranyloxycarbonyl, such as 4-tetrahydropyranyloxycarbonyl, or tetrahydro-
furanyloxycarbonyl, such as 3(R)-, 3(S)- or also 3(R,S)-tetrahydrofuranyloxycarbonyl. In
a very especially preferred variant of the definition of Rl, the radicals falling under the
definition of the substituents heterocyclyloxycarbonyl are not included in any categories
of definition.
In the radicals mentioned it is also possible for the bonding carbonyl group to have been ~ -
replaced by a thiocarbonyl group. A carbonyl group is preferred. ~:
Lower aL~sylsulfonyl (= lower aLkyl-SO2-) Rl is preferably methylsulfonyl, ethylsulfonyl,
n-propylsulfonyl or isopropylsulfonyl. The compounds of formula I' wherein Rl is lower
alkylsulfonyl and the remaining radicals are as defined may preferably be omitted from
the definition of the compounds of formula I', or they are especially preferred.
Heterocyclylsulfonyl preferably contains as heterocyclyl one of the heterocyclesmentioned for heterocyclylcarbonyl ~I that is unsubstituted or substituted by lower alkyl, -
such,as methyl or ethyl, preference being given to heterocycles containing at least one
nitrogen atom that is bonded to the sulfur atom of the sulfonyl group, and is especially
piperidinosulfonyl, or piperazin-l-yl~sulfonyl, pyIrolidin-l-yl-sulfonyl, imidazol- ~ ~
idin-l-yl-sulfonyl, pyrimidin-l-yl-sulfonyl, quinolin-l-ylsulfonyl, morpholinosulfonyl or ~ -
thiomorpholinosulfonyl, especially thiomorpholinosulfonyl or morpholinosulfonyl, that is
unsubstituted or substituted by lower alkyl, such as methyl, at the nitrogen atom not
bonded to the sulfonyl sulfur atom. The compounds of formula I' wherein Rl is hetero-
cyclylsulfonyl and the remaining radicals are as de~med may preferably be omitted from
2 ~
- ]4 -
the definition of the compounds of formula 1', or they are especially preferred.
N-(heterocyclyl-lower alkyl)-N-lower alkyl-aminocarbonyl Rl preferably contains as
heterocyclyl one of the heterocycles mentioned for heterocyclylcarbonyl Kl, especially
pyridyl, such as 2-, 3- or 4-pyridyl, pyraz;nyl, pyrimidinyl, morpholinyl, such as morpho-
lino, thiomorpholinyl, such as thiomorpholino, or quinolyl, such as 2- or 3-quinolyl, and is
especially N-(heterocyclylmethyl)-N-methyl-aminocarbonyl, ~or example N-(pyridyl-
methyl)-N-methyl-aminocarbonyl, such as N-(2-pyridylmethyl)-N-methyl-aminocarbonyl.
The compounds of formula I' wherein Rl is N-(heterocyclyl-lower alkyl)-N-lower aL~cyl-
aminocarbonyl and the remaining radicals are as defimed may perferably be omitted from
the definition of compounds of formula I', or they are especially preferred.
A bivalent residue Bl of an a-amino acid bonded N-tenninally to Rl and C-terrninally to
the amino group at the carbon atom carrying R2-CH2- is preferably selected from glycine
(H-Gly-OH), alanine (H-Ala-OH), valine (H-Val-OH), norvaline (a-aminovaleric acid),
leucine (H-Leu-OH), isoleucine (H-Ile-OH), norleucine (a-aminohexanoic acid,
H-Nle-OH), serine (H-Ser-OH), homoserine (a-amino-~-hydroxybutyric acid), threonine
(H-Thr-OH), methionine (H-Met-OH), cysteine aI-Cys-OH~, proline (H-P~OH),
trans-3- and trans-4-hydroxyproline, phenylalanine (H-Phe-OH), p-fluorophenylalanine
(H-(p-F-Phe)-OH), tyrosine ~H-Tyr-OH), p-lower alkoxy-phenylalanine wherein lower
aLkoxy may be unbranched or branched, such as p-methoxy-phenylalanine
(H-(p-CH30-Phe)-OH), or also 4-isobutyloxyphenylalanine (H-(4-isobutyloxy-Phe)-OH)
or (especially) 4-(n-butyloxy)-phenylalanine (H (4-n-butyloxy-Phe)-OH), p-phenyl-lower
aLlcoxy-phenylalanine, such as p-benzyloxyphenylalanine (H-(p-BzlOPhe)-OH), 4-amino-
phenylalanine, 4-chlorophenylalanine, 4-carboxyphenylalanine, ~-phenylserine
(,B-hydroxyphenylalanine), phenylglycine, a-naphthylalanine (H-Nal-OH), cyclohexyl-
alanine (H-Cha-OH,~, cyclohexylglycine, tryptophan (H-Trp-OH), indoline-2-carboxylic
acid, 1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid, aminomalonic acid, aminomalonic
acid monoamide, aspar~ic acid (H-Asp-OH), asparagine (H-Asn-OH), glutamic acid
(H-Glu-OH), glutamine (H-Gln-OH), histidine (H-His-OH), arginine (H-Arg-OH~, lysine
(H-Lys-OH), ~-hydroxylysine, orni~hine (a,~-diaminovaleric acid), a,~-diaminobutyric
acid and a,~B-diaminopropionic acid, or alternatively and in addi~ion thereto 4-cyan~
phenylalanine (H-(p-CN-Phe)-OH), especially preferably the radical of a hydrophobic
amino acid, for example proline, phenylalanine, p-fluorophenylalanine, p-methoxy-
phenylalanine, p-benzyloxy-phenylalanine, tyrosine, phenylglycine, a-naphthylalanine,
cyclohexylalanine, cyclohexylglycine, or also 4-isobutyloxy- or especially 4-n-butyloxy-
2 ~ ~ 8 ~
- 15-
phenylalanine, or an aliphatic a-amino acid selected from glycine, valine, norvaline,
alanine, leucine, norleucine and isoleucine, especially valine, each of the mentioned
a-amino acids being in the D-, L- or (D,L)-form, preferably in the L-forrn, and being
linked especially to radicals Rl selected from lower alkoxycarbonyl, for example tert-
butoxycarbonyl, and heterocyclylcarbonyl, for example morpholinocarbonyl.
When Bl is a bond, Rl is bonded directly to the amino nitrogen atom that is bonded to the
carbon atom carrying the radical R2-CH2- in formula 1'.
Phenyl or cyclohexyl R2 or R3 is unsubstituted or substituted by up to three radicals :
selected independently of one another from hydroxy, lower alkoxy which may be un- ~:
branched or branched, such as methoxy or ethoxy or (addi~ionally or especially) isobutyl-
oxy or n-butyloxy (preferred), phenyl-lower aL~coxy, such as benzyloxy, halogen, for
example fluorine, halo-lower alkyl, for example trifluoromethyl, sulfo, lower aL~cyl-
sulfonyl, for example methyl- or ethyl-sulfonyl, cyano and nitro, preferably by one or two
of those radicals, especially selected from hydroxy, methoxy, benzyloxy, fluorine,
trifluoromethyl, sulfo, lower alkylsulfonyl, for example methyl- or ethyl-sulfonyl, and
cyano, and in the case of phenyl selected very especially from fluorine and cyano, and in
the case of cyclohexyl very especially from fluorine, trifluoromethyl, sulfo and lower ~ -
alkylsulfonyl, more especially fluorine; the mentioned substituents are bonded in the 2
3- or 4-position of the phenyl or cyclohexyl ring, especially in the 4-position, such as in
phenyl, cyclohexyl, 2-, 3- or 4-fluoro- or 2-, 3- or 4-cyano-phenyl or 4-fluorocyclohexyl,
especially in phenyl, cyclohexyl, 4-cyanophenyl or 4-fluorophenyl.
Especially preferably R2 is selected from phenyl, 4-hydroxyphenyl, 4-methoxyphenyl,
4-benzyloxyphenyl, 4-fluorophenyl, cyclohexyl and 4-trifluoromethylphenyl, while R3 is
selected from phenyl, 4-hydroxyphenyl, 4-methoxyphenyl, cyclohexyl, 2-, 3- or 4-fluoro-
phenyl, 4-trifluoromethylphenyl and 2-, 3- or 4-cyanophenyl.
More especially R2 is selected from phenyl, 4-fluorophenyl and cyclohexyl, while R3 is
selected from phenyl, cyclohexyl, 4-fluorophenyl and 4-cyanophenyl.
Greatest preference is given to the combinations: R2 phenyl and R3 phenyl; R2 cyclohexyl
and R3 4-cyanophenyl; R2 cyclohexyl and R3 4-fluorophenyl; and R2 and R3 each cyclo-
hexyl. Alternatively or in addition thereto, greatest preference is ~iven also to the
combinations R2 phenyl and R3 4-fluorophenyl; R2 phenyl and R3 4-cyanophenyl; R
2~186~1
- 16-
4-fluorophenyl and R3 4-fluorophenyl; R2 4-fluorophenyl and R3 4-trifluoromethylphenyl;
R2 4-trifluoromethylphenyl and R3 phenyl; R2 4-trifluoromethylphenyl and R3 4-fluoro-
phenyl; R2 4-trifluoromethylphenyl and R3 4-trifluoromethylphenyl; R2 hydroxyphenyl
and R3 phenyl; R2 phenyl and R3 hydroxyphenyl; R2 phenyl and R3 p-trifluoromethyl-
phenyl; R2 cyclohexyl and R3 p-methoxyphenyl; R2 cyclohexyl and R3 p-trifluoromethyl-
phenyl; R2 phenyl and R3 p-ben~yloxyphenyl; R2 p-methoxyphenyl and R3 p-benzyloxy-
phenyl; R2 p-ben~yloxyphenyl and R3 phenyl; R2 p-benzyloxyphenyl and R3 p-benzyloxy-
phenyl; R2 phenyl and R3 o-fluorophenyl; R2 phenyl and R3 m-fluorophenyl; R2 phenyl
and R3 p-methoxyphenyl; R2 p-methoxyphenyl and R3 p-hydroxyphenyl; R2 p-methoxy-phenyl and R3 phenyl; R2 phenyl and R3 o-methoxyphenyl; R2 phenyl and R3 m-methoxy-
phenyl; R2 p-methoxyphenyl and R3 p-methoxyphenyl; R2 p-methoxyphenyl and R3
m-methoxyphenyl; R2 p-methoxyphenyl and R3 o-rnethoxyphenyl; R2 phenyl and R3
m-cyanophenyl; R2 phenyl and R3 o-cyanophenyl; or R2 4-hydroxyphenyl and R3
4-hydroxyphenyl; and (additionally or especially) R2 = phenyl and R3= 2,4-difluoro-
phenyl; R2 = phenyl and ~3 = 4-isobutyloxyphenyl; R2 = cyclohexyl and R3= 4-benzyl-
oxyphenyl; R2 = cyclohexyl and R3= 4-hydroxyphenyl; R2 = phenyl and R3= 3,4-di-
methoxyphenyl; R2 = phenyl and R3= 3,4,5-trimethoxyphenyl; or R2 = phenyl aad R3=
2,3 ,4-trimethoxyphenyl.
Strong preference is given to the compounds wherein one of the radicals E~2 and R3isp-
methoxyphenyl and the other has one of the meanings mentioned, especially p-methoxy-
phenyl, p-fluorophenyl, phenyl or p-trifluoromethylphenyl.
A bivalent residue of an a-amino acid Al bonded N-tenninally to the group -~=0 and
C-terminally to A2 is, for example, one of the a-amino acids mentioned above for Bl, it
being possible for those amino acids to be in the (D)-, (L)- or (D,L)-form, pre~erably in the
(D)- or (L)-form, especially in the (L)-form. Preference is given to the hydrophobic a-
amino acids, especially the aliphatic hydrophobic a-amino acids, mentioned under B1, for
example glycine, valine or isoleucine, or additionally or especially leucine or phenyl-
glycine. In the mentioned a-amino acids the carboxy group bonded to A2 is not reduced or
is reduced, especially to a methylene group, for example in the mentioned hydrophobic
a-amino acids, such as in the reduced amino acid residues Gly(red), Val(red) or Ile(red),
especially in Val(red), the suffix (red) indicating the reduction of the carbonyl group of the
corresponding amino acid residue to the methylene group.
When Al is a bond, A2 is bonded directly to the carbonyl group at the carbon atom
' t
.,.:, . . . ~ , `,
2118661 -
- 17-
carrying the radical R3-CH2-.
A bivalent residue of an a-amino acid A2 bonded N-terminally to Al and C-terminally to
the group NR4Rs is, for example, one of the a-amino acids mentioned above for Bl, it :
being possible for those arnino acids to be in the (D)-, (L)- or (D,L)-form, preferably in the
(D)- or (L)-forrn, especially in the (L)-forrn. Preference is given to the hydrophobic a-
amino acids mentioned for Bl, for example glyeine, alanine, valine, leucine, isoleucine,
phenylalanine, p-fluorophenylalanine, tyrosine, p-methoxy-phenylalanine, p-benzyloxy-
phenylalanine, phenylglycine, a-naphthylalanine, cyclohexylalanine or cyclohexylglycine, .
preferably glycine, alanine, valine, leucine, phenylalanine, p-fluo}ophenylalanine, p-
methoxy-phenylalanine, p-benzyloxyphenylalanine or cyclohexylalanine, or also 4-iso-
butyloxyphenylalanine or (especially) 4-n-butyloxyphenylalanine, the mentioned residues : - -
being in the (D)- or (L)-form, but preferably in the (L)-form with the exception of phenyl-
alanine which is in the (L)- or the (D)-form. ~ :
A bivalent residue of a dipeptide, formed by Al and A2, the central peptide bond of which
has been reduced and which is bonded N-terrninally to the group -C=O and C-terminally
to the group NR4R5 preferably consists of two of the above-mentioned hydrophobic : .
a-amino acids, especially an N-terrninal amino acid residue selected from Gly(red),
Val(red) and Ile(red) and a C-terminal amino acid selected from glycine, phenylalanine,
tyrosine, p-methoxyphenylalanine, p-benzyloxyphenylalanine, cyclohexylalanine and
p-fluorophenylalanine, and also 4-isobutyloxyphenylalanine and (especially) 4-n-butyl-
oxyphenylalanine.
Especially preferably Al and A2 together forrn a bivalent residue of a dipeptide of the . '
formula Val-Phe, Ile-Phe, Val-Cha, Ile-Cha, Val-Gly, Val-(p-F-Phe), Val-(p-CH30-Phe),
Gly-(p-F-Phe); and alternatively or additionally the residue of a dipeptide of the forrnula
Val-Tyr, Ile-Tyr, Gly-Tyr, Ile-Gly, Val-(p-BzlOPhe), Val-Ile, Val-Ala, Val-Leu or
Val-Val, or (additionally or especially) phenylglycyl-(p-CH30-Phe), Val-(4-isobutyloxy-
Phe) or Val-(4-n-butyloxy-Phe); wherein the amino acids are in the (D~- or (L)-form, espe-
cially in the (L)-form, with the exception of (L)-Val-Phe in which Phe is in the (L)- or . . .
(D)-form, or a derivative thereof having a reduced central amide bond, for exarnple of the
forrnula Val(red)-Phe, bonded N-tenninally to the gToup -C=O and C-terrninally to the
group NR4Rs-
A preferred form of the invention relates either to compounds of formula I' wherein Bl is
- 2~18661
- 18 -
one of the mentioned bivalent residues of an a-amino acid and one of the radicals Al and
A2 is a bond and the other is one of the mentioned a-amino acids, or to compounds of
formula I' wherein Bl is a bond and Al and A2 are each one of the mentioned bivalent
residues of an a-amino acid or together forrn onc of the mentioned bivalent residues of a
dipeptide having a reduced central amide bond.
Thiomorpholino or morpholino formed by R4 and Rs together with the bonding nitrogen
atom is unsubstituted or substituted at one or more of the carbon atoms, preferably at one
or two carbon atoms, for e~ample one carbon atom, by lower aLtcyl, such as ethyl, propyl,
butyl, isobutyl, tert-butyl or especially methyl, by phenyl- or naphthyl-lower alkyl, such as
benzyl, 1- or 2-naphthylmethyl or phenyl-1- or phenyl-2-ethyl, especially phenyl-1- or
phenyl-2-ethyl, by hydroxy, by lower alkoxy, such as methoxy, ethoxy or tert-butoxy, by
amino, by lower alkylarnino, such as methyl- or ethyl-amino, or by di-lower alkylamino,
such as dimethylamino or diethylamino, by lower alkanoyl, such as acetyl or propionyl, by
phenyl- or naphthyl-lower aL~canoyl, such as phenylacetyl or l- or 2-naphthylacetyl, by
carboxy, by lower alkoxycarbonyl, such as isopropoxycarbonyl or tert-butoxycarbonyl, by
phenyl-, naphthyl- or fiuorenyl-lower alkoxycarbonyl, such as benzyloxycarbonyl, l- or
2-naphthylmethoxycarbonyl or 9-fluorenylmethoxycarbonyl, by carbamoyl, by mono- or
di-lower alkylcarbamoyl, such as dimethylcarbamoyl, by mono- or di-hydroxy-loweralkyl-carbarnoyl, such as di-hydroxymethyl-carbamoyl, by sulfo, by lower aLkylsulfonyl,
such as methylsulfonyl or ethylsulfonyl, by phenyl- or naphthyl-sulfonyl, wherein phenyl
may be substituted by lower alkyl, for example methyl or ethyl~ for example phenyl-
sulfonyl or toluenesulfonyl, by sulfamoyl, by halogen, for example fluorine or chlorine, by
cyano, by nitro and/or by oxo.
Very preferably R4 and R5 together with the bonding nitrogen atom forrn unsubstituted
thiomorpholino or morpholino, especially unsubstituted morpholino.
.
Salts lof compounds of formula I' that contain salt-forming groups, that is to say one or
more salt-forming groups, are especially acid addition salts, salts with bases or, where
several salt-forming groups are present, optionally also mixed salts or internal salts.
Salts are especially the pharmal~eutically acceptable salts of compounds of formula I' that
are non-toxic when used in the correct dose.
Such salts are formed, for example, from compounds of formula I' having one or more
' -. "-
-- 21~8661
,9 ~ :~
acidic groups, for example a carboxy group, and are, for example, the salts thereof with
suitable bases, such as non-toxic metal salts derived from metals of groups Ia, Ib, IIa and
IIb of the Periodic Table of Elements, especially suitable alkali metal salts, for example
lithium, sodium or potassium salts, or alkaline earth metal salts, for example magnesium
or calcium salts, and also zinc salts or ammonium salts, as well as those salts which are
formed with organic amines, such as unsubs~ituted or hydroxy-substituted mono-, di- or
tri-aL~ylamines, especially mono-, di- or tri-lower alkylamines, or with quaternary
ammonium compounds, for example with N-methyl-N-ethylamine, diethylamine, triethyl-
amine, mono-, bis- or tris-(2-hydroxy-lower alkyl)-amines, such as mono-, bis- or tris-
(2-hydroxyethyl)-amine, 2-hydroxy-tert-butylamine or tris(hydroxymethyl)methylamine,
N,N-di-lower aL~cyl-N-(hydroxy-lower alkyl)-amines, such as N,N-dimethyl-N-(2-
hydroxyethyl)-amine or tris(2-hydroxyethyl)amine, N-methyl-D-glucamine or quaternary
ammonium salts, such as tetrabutylammonium salts. The compounds of formula I' having
one or more basic groups, for example an amino or imino group, may form acid addition
salts, for example with inorganic acids, for example a hydrohalic acid, such as hyd~-
chloric acid, sulfuric acid or phosphoric acid, or with organic carboxylic, sulfonic9 sulfo or
phospho acids or N-substituted sulfamic acids, for example acetic acid, propionic acid,
glycolic acid, succinic acid, maleic acid, hydroxymaleic acid, methylmaleic acid, fumaric
acid, malic acid, tartaric acid, gluconic acid, glucaric acid, glucuronic acid, citric acid,
benzoic acid, cinnamic acid, mandelic acid, salicylic acid, 4-aminosalicylic acid,
2-phenoxybenzoic acid, 2-acetoxybenzoic acid, embonic acid, nicotinic acid or isonico-
tinic acid, and also with amino acids, for example glutamic acid or aspartic acid, and with
methanesulfonic acid, ethanesulfonic acid, 2-hydroxyethanesulfonic acid, ethane-1,2-
disulfonic acid, benzenesulfonic acid, ~-methylbenzenesulfonic acid, naphthalene-
2-sulfonic acid, 2- or 3-phosphoglycerate, glucose-6-phosphate, N-cyclohexylsulfamic
acid (with the formation of cyclamates) or with other acidic organic compounds, such as
ascorbic acid. Compounds of formula I' having acidic and basic groups may also form
internal salts.
For the purposes of isolation and purification it is also possible to use pharmaceutically
unacceptable salts.
The compounds of formula I' have valuable pharmacological properties. In particular,
they are active against retroviral diseases, especially against AIDS which is caused by
HIV, especially HIV- 1 or HIV-2. For example, they act as metabolic precursors of
compounds of formula I
2118661
- 20 -
R~ ~N~A~`A'N\ Il)
which contain a hydrogen atom in place of the radical T in compounds of formula I'1
while the remaining definitions are as defined for compounds of formula I'; and which
have anti-retroviral activity and are especially suitable in the treatment of AIDS as
inhibitors of the aspartate proteases of HIV-1 andlor HIV-2 (and possibly other re~o-
viruses that produce AIDS-analogous symptoms). The compounds of formula I' can there-
fore be used therapeutically against retroviral diseases, especially AIDS.
Compounds of formula I are mentioned and described in the European Patent Application
having the publication number EP 0 S32 466 (published on 17.03.1993).
The compounds of formula I are released from the compounds of formula I' in the body of
the animal to be treated, especially a warrn-blooded animal, including a human being.
Using the compounds of formula I' it is possible, for example, especially in the case of
enteral, preferably oral, administration of the compounds, to obtain advantageous
pharrnacokinetics different to those obtained on administration of the compounds o
formula I themselves.
The pharrnacodynamic prope.rties of the compounds of formula I' can be demonstrated, for
example, as follows:
The compounds of forrnula I' to be investigated or (for example as control) the coIres-
ponding compounds of forrnula I are dissolved in an organic solvent, such as dimethyl
sulfoxide (DMSO), in a concentration of 240 mg/ml. The resulting solutions are diluted
with 20% (w/v) hydroxypropyl-,3-cyclodex~in (HP,BCD) in order to obtain a concentration
of test compound of 12 mgfml. That solution is administered to mice orally by arti~lcial
special feeding in a dose of 120 mg/kg. 30, 60, 90 and 120 minutes after administration the
animals are sacrificed and blood is removed. Three or four animals are investigated per
time point. The blood is heparinised and prepared for analysis using one of the following
.~ ~
- 21~ 8661
- 21 -
two methods: in accordance with the first method whole blood is deproteinised by mixing
one part by volume of blood with one part by volume of acetonitrile; after centrifugation
(10 000 g, 5 minutes) the supernatant is analysed by reversed-phase HPLC. In accordance ;
with the second method 2 ,ul of a 1 mM solution of an internal standard (such as a different
compound of formula I) are added to 05 ml of heparinised blood. The blood is centrifuged
~10 000 g, 5 minutes) and the plasma is mixed with the same volume of acetonitrile. The
precipitated protein is removed by centrifugation (10 000 g, 5 minutes) and the super-
na~ant is concentrated by evaporation in vacuo. The residue is taken up in 0.1 ml of
phthalate buffer (O.OSM, pH 3) and 20 1ll of 3M NaCl. The mixture is extracted with 1 ml
and then again with 0.2 ml of diisopropyl ether (=DIPE; Merck, Darmstadt). The DIPE
fractions are combined and concentrated by evaporation in vacuo. The residue is
dissolved in 50% water (Baker) and 50% acetonitrile (v/v) prior to analysis by reversed-
phase HPLC.
The analysis by reversed-phase HPLC is carried out using a 125 x 4.6 mm Nucleosil(~
Cl8-column (reversed-phase material supplied by Macherey-Nagel, Duren, Fecleral
Republic of Germany, based on silica gel derivatised with hydrocarbon raclicals having
18 carbon atoms) equilibrated with a mobile phase of acetonitrile in water/0.1% trifluorc~
acetic acid. Depending upon the compound, the proportion of acetonitrile is advanta-
geously, for example, from 35 to 60 % by volume. The flow rate is 1 mVminute. Detection
is effected at 215 nm. Stanclards for the compounds in blood are worked up analogously to
the blood sarnples and used to establish standard curves on the basis of which the in vivo
concentrations are determinecl.
For the compounds of forrnula I' or of formula I, the concentration of the component of
formula I in the blood of mice 60 minutes after oral administration is preferably up to
104M, preferably from ~ x 10-8M to 5 x 10-5M.
The compounds of formula I exhibit inhibitory action on retroviral aspartate proteases,
especially HIV-protease-inhibiting actions. In the tests described below they inhibit espe-
cially the action of the HIV-protease of HIV-l in concentrations of from 10-6 to 10-9M, for
example with an ICso of from 10 to 1000 nM, especially from 10 to 100 nM. They, and
thus also the prodrugs of fonnula I', are therefore suitable agents against diseases caused ~ ;~
by that or related retroviruses (retroviral diseases), such as against AIDS or against the
preliminary stages thereof.
.,: . , ~ . . , ., . . ~
211g~1
The ability of the compounds of formula I to inhibit the proteolytic activity of, for
example, HIV- l-protease can be demonstrated, for example, in accordance with the
method described by A. D. Richards et al., J. Biol. Chem. 265(14),7733-7736 (1990). In
that method there is used as substrate for a recombinant HIV-l-protease (prepared in
accordance with Billich, S., et al., J. Biol. Chem. 263(34), 17905 - 17908 (1990)) a
synthetic chromophoric peptide (for exarnple HKARVL[NO2]FEANleS (Bachem,
Switzerland; see M. W. Pennington et al., Peptides 1990, ed.: E. Girault and D. Andrew
(1991), ESCOM Sci. Publ. B.V., pp. 787-789) or an icosapeptide such as
RRSNQVSQNYPIVQNIQGRR (prepared by peptide synthesis in accordance with known
methods: J. Schneider et al., Cell 54, 363-368 (1988)) that coIresponds to one of the
cleavage sites of the gag-precursor protein. That substrate and cleavage products thereof
can be analysed by high pressure liquid chromatography (HPLC).
For that purpose, an inhibitor of formula I to be tested is dissolved in dimethyl sulfoxide;
the enzyme test is carried out by adding suitable dilueions of the inhibitor in 20 mM
~-morpholinoethanesulfonic acid (MES) buffer pH 6.0 to the assay mix cornprising the
above-mentioned chromophoric peptide (67.2 ',IM) in 0.3M sodium acetate, O.lM NaCl
pH 7.4, or the above-mentioned icosapeptide (122 ',IM) in 20 mM MES buffer pH 6Ø
The size of each batch is 100 111. The reaction is started by the addition of in the first case
2 ~1 and in the second case 10 111 of HIV-l-protease and is stopped in the first case after
15 minutes by the addition of 100 111 of 0.3M HCI04, and in the second case after one - :
hour's incubation at 37C by the addition of 10 ~,11 of 0.3M HCI04. After centrifugadon of
the sample for S minutes at 10 000 x g in 100 ~1 (batch with chromophoric peptide) or
20 ~,ll (icosapeptide batch) of the rcsulting supernatant and after application to a
125 x 4.6 mm Nucleosil(9 C18-5,u-HPLC column (Macherey & Nagel, Duren) and elution,
the reaction products are quantified with reference to the peak height of the cleavage
product at 280 nm (batch with chromophoric peptide) or at 215 nm (batch with icosa-
peptide), gradient: 100 % el.l -> 50 % el.l/50 % el.2 (el.l: 75 % acetonitrile, 90 % H2O,
0.1 % trifluoroacetic acid (TFA); el.2: 75 % acetonitrile, 25 % H20, 0.08 % TFA) in the
course of 15 minutes; throughflow rate 1 ml/minute (el. = eluant3.
In that procedure there are determined for compounds of formula I, for exarnple, IC50
values (IC5Q = concentration that reduces the activity of the HIV l-protease by 50 % in
comparison with a control without inhibitor) of approximately 5û x 10-6 to 10-9M, espe-
cially 10-7 to 10-9M.
21~61 ~
- 23 -
In a further test it can be shown that the compounds of the present invention protect cells
that are normally infected by HIV from such an infection or at least retard such an
infection. In the test, the human T-cell leukaemia cell line MT-2 (Science 229, 563
(1985)), which is sensitive to the cytopathogenic effect of HIV, is incubated with HIV-1
alone or with HIV- 1 in the presence of one of tbe compounds according to the invention
and after several days the viability of the cells thus treated is assessed. For ehis purpose
the MT-2 cells are kept in RPMI 1640 medium (Gibco, Switzerland; RPMI 1640
comprises an amino acid mixture without L-Gln) that has been supplemented with 10 %
heat-inactivated foetal calf serum, L-glutamine, Hepes (2-[4-(2-hydroxyethyl)-1-piper-
azino]-ethanesulfonic acid) and standard antibiotics, at 37C in humidified air containing
5% C02. 50 ~LI of the respective Rst compound in culture medium and 100 ~,11 of HIV-1 in
culture medium (800 TCID50/ml) (TClD50 = Tissue Culture Infectious Dose 50 = dose
that infects 50 % of the MT-2 cells) are added to 4x103 exponentially growing MT-2 cells
in 50 ~,~1 of culture medium per well in 96-well microtitre plates. Parallel batches on a
further microtitre plate with cells and test compound receive 100 111 of culture medium
without the virus. After incubation for 4 days, the reverse transcriptase (RT) activity in ~ -
10 111 of the cell supernatant is determined. The RT activity is deterrnined in 50 mM Tris
(a,a,a-tris(hydroxymethyl)methylarnine, Ultra pur, Merck, Federal Republic of Gennany)
pH 7.8; 75 mM KCI, 2 mM dithiothreitol, 5 mM MgCl2; 0.05% Nonidet P-40 (detergent;
Sigma, Switzerland); 50 llg/ml of polyadenylic acid (Pharrnacia, Sweden); 1.6 llg/ml of
dT(12-18) (Sigma, Switzerland). The mixture is filtered through a 0.45 11 Acrodisc f11ter
(Gelman Sciences Inc., Ann Arbor) and stored at -20C. 0.1% (v/v) [alpha-32P]dlTP is
added to aliquots of that solution in order to obtain a final radioactive activity of
10 ~LCilml. 10 111 of the culture supernatant are transferred to a fresh 96-well microtitre
plate and 30 111 of the said RT cocktail are added thereto. After mixing, the plate is
incubated for 1.5 to 3 hours at 37C. 5 ~1 of that reaction mixture are transferred to
DE8 1-paper (Whatman). The dried filters are washed three times for 5 minutes with
300 mM NaCI/25 mM trisodium citrate and once with 95 % ethanol and again dried in the
air. Evaluation is made in a Matrix Packard 96-well counter (Packard, Zurich, Switær-
land). The ED90 values are calculated and are defimed as the lowest concentration of the
respective test compound that reduces the RT activity by 90 % in comparison with cell
batches not treated with the test compound. The RT activity is a measure of the repli-
cation of HlV- 1.
In that test the compounds of forrnula I exhibit, for example, an ED90 of from 10-~ to
10-sM, especially from 10-6 to 10-7M.
2118~61
- 24 -
The novel compounds of formula I of the present invention also exhibit advantageous
pharrnacological properties, which allow the assumption that they will exhibit the said
inhibitory actions in vivo. For example, in the case of intravenous or intraperitoneal
administration of 20 mg/kg of one of those compounds to mice, the level in the blood
1 hour after administration is preferably approximately the same as or higher than the
ED90 in the cell assay.
In the case of peroral administration of 120 mg/kg of one of those compounds, 30 minu~es
after administration, for example, the concentra~ions found in the blood of the mice are
above the ED90 in the cell assay and preferably constitute approximately 10 times the
ED90 in the cell assay.
For example, with Boc-Phe[C](p-CH3O)Phe-(L)-Val-(L)-Phe-morpholin-4-ylamide (seeExamples) after 30 minutes a concentration of 10.75 ~,lM can be obtained with an 13D90 of
0. 1 ~lM.
The level in the blood can be determined as above for the compounds of formula I in
mice.
In the definitions of compounds of formula I' mentioned hereinbelow, it is advantageously -
possible, ~or example in order to replac~ more general definitions by more specific defini-
tions, to use definitions of radicals from the above-mentioned general de~mitions or also to
omit individual defmitions.
Preference is given to compounds of formula I' wherein T is an acyl radical of the above-
mentioned formula :Z; wherein
.::
RZ isIheterocyclyl bonded via a ring carbon atom and selected from pyrrolyl, 2,5-dihydro-
pyrrolyl, indolyl, indolizinyl, isoindolyl, pyrrolidinyl, such as pyrrolidin-3-yl or especially
pyrrolidin-2-yl (in the (R,S)- or preferably (R)- or (S)-configuration), hydroxypyrrolidinyl,
such as 3- or especially 4-hydroxypyrrolidinyl, furyl, such as furan-3-yl or especially
furan-2-yl, tetrahydrofuryl, thienyl, cyclohepta[b]pyrrolyl, imidazolyl, such as imidazol-
2-yl, imidazol-3-yl or especially imidazol-5-yl, N-triphenyl-lower aLkyl-imidazolyl, such
as N-triphenylmethyl-imidazolyl, pyrazolyl, especially pyrazol-3-yl, oxazolyl, isoxazolyl,
such as isoxazol-3-yl or -5-yl, thiazolyl, isothiazolyl, such as isothiazol-3-yl or -5-yl,
~"., " ~ "" ,~'2', ' ' ~ "~ " ~ "
2 ~
- 25 -
triazolyl, such as 1,2,3-triazol-4- or -5-yl or 1,2,4-triazol-5-yl, tetrazolyl, pyridyl, such as
pyridin-4-yl or -3-yl or especially pyridin-2-yl, quinolyl, such as quinolin-2-yl, isoquin-
olyl, especially isoquinolin- 1-yl or -3-yl, piperidyl, especially piperidin-2-yl, ~y-pyranyl,
4,5-dihydropyranyl, 4H-chromenyl, chromanyl, ~-thiopyranyl, pyrida~inyl, cinnolyl,
phthalazinyl, quinazolinyl, quinoxalinyl, pyrimidinyl, pyrazinyl, phenazinyl, phen-
oxazinyl, phenothiazinyl, morpholinyl and thiazinyl, each of which is substituted by up to
three radicals selected independently of one another from lower aLkyl, phenyl-lower alkyl,
diphenyl-lower alkyl, triphenyl-lower alkyl, such as triphenylmethyl, lower alkanoyl,
hydroxy, lower alkoxy, phenyl-lower alkoxy, such as benzyloxy, diphenylmethoxy or tri-
phenylmethoxy, hydroxy-lower alkyl, such as hydroxymethyl, halogen, such as fluorine,
chlorine or bromine, cyano, lower alkoxycarbonyl, such as methoxy- or tert-butoxy- ~:
carbonyl, phenyl-lower alkoxycarbonyl, such as benzyloxycarbonyl and halo-lower aLkyl,
such as chloromethyl or trifluoromethyl, or preferably is unsubstituted, and each of which :
is bonded via a ring carbon atom; very special preference being given to those of the
mentioned radicals which contain a ring hetero atom directly adjacent to the bonding ring
carbon atom, more especially the radicals furan-2-yl, (S)- or (R)-pyrrolidin-2-yl and imid-
azol-4-yl, pyridin-2-yl, -3-yl or -4-yl, or isoquinolin-3-yl; or additionally or especially
tetrahydropyranyl; or
RZ iS lower alkyl (especially methyl, ethyl, n-propyl or n-butyl) which is substituted by at
least one radical selected from: -
lower alkoxy, such as methoxy, ethoxy or n-butoxy; lower aLkoxy, such as methoxy,
ethoxy or n-butoxy, substituted by one or two substituents, especially aryl, moIe espe-
cially phenyl, naphthyl, lower alkoxy, such as ethoxy or methoxy, lower aLkylthio, such . ~ :
as methylthio or ethylthio, lower alkoxy-lower alkoxy, such as 2-methoxyethoxy, lower
alkylthio-lower alkoxy, such as 2-methylthioethoxy, aryloxy or arylthio, especially
phenyloxy or o-, m- or p-chlorophenyloxy, for example p-chlorophenyloxy, amino,
N-lower alkylamino or N,N-di-lower alkylamino, such as 2-amino, 2-(N-lower alkyl)-
amino or 2-(N,N-di-lower alkyl)amino, for example 2-dimethylamino, and/or hetero-
cyclyl as last defined for heterocyclyl RZ bonded via a ring carbon atom, especially 2-, 3-
or 4-pyridyl; aryloxy, especially phenyloxy; lower alkylthio, such as methylthio,
ethylthio or n-butylthio; lower alkylthio, such as methylthio, ethylthio or n-butylthio,
substituted by one or two substituents, especially aryl, more especially phenyl or
naphthyl; arylthio, such as phenylthio; amino or amino substituted by one or two radicals
selected from lower alkyl, such as methyl, heterocyclyl-lower alkyl wherein heterocyclyl
` 2118~1
- 26 -
':
is as defined for heterocyclyl RZ bonded via a ring carbon atom, especially heterocyclyl-
methyl, such as imidazolylmethyl, for example 4-imidazolylmethyl, or pyridylmethyl,
for example 2-, 3- or 4-pyridylmethyl, each bonded via a ring carbon atom, aryl-lower
alkyl, such as phenyl- or naphthyl-lower alkyl, for example phenyl- s)r naphthyl-methyl,
lower alkanoyl, such as acetyl, lower alkoxycarbonyl, such as tert-butoxycarbonyl, and
aryl-lower alkoxycarbonyl, such as phenyl-lower alkoxycarbonyl, for example
benzyloxycarbonyl, with especially one of the substituents of amino being lower aLkyl,
especially methyl, and the other being hydrogen or one of the radicals mentioned above
as substituents of amino; heterocyclyl as defined above for heterocyclyl RZ bonded via a : .
ring carbon atom, especially as pyridin-2-yl, -3-yl or -4-yl; aml heterocyclyl-lower aLtcyl
wherein lower alkyl is preferably methyl, 1- or 2-ethyl or 3-propyl and wherein
heterocyclyl is as defined above for heterocyclyl RZ bonded via a ring carbon atom but
may also be bonded via a ring nitrogen atom, especially imidazol- 1-yl, imidazol-2-yl,
imidazol-5-yl or more especially imidazol-4-yl, N-triphenyl-lower alkylimidazolyl, such
as N-triphenylmethyl-imidazol-S-yl or especially -4-yl, or pyrazolyl, such as ~ ~
pyrazol-l-yl, -3-yl, -4-yl or -5-yl; and ~additionally or preferably) lower alkoxycarbonyl, : ~:
such as methoxycarbonyl; lower alkanoyloxy-lower alkoxycarbonyl, such as pivaloyl- ~:
oxymethoxycarbonyl or acetyloxymethoxycarbonyl; carboxy; phenyl-lower aL~coxy- ~ :
carbonylamino-loweralkoxy, suchas 2-(benzyloxycarbonylamino)-ethoxy; amino-loweralkoxy, sueh as 2-aminoethoxy; di-lower alkylamino-lower alkoxy, such as 2-(dimethyl-
arnino)-ethoxy; N,N-di-lower alkylamino-lower alkoxy, such as ~:
2-(dimethylamino)-ethoxy; lower alkoxycarbonyl-lower alkylsulfo, such as methoxy-
earbonyl-methylsulfo; lower alkanoyloxy, such as acetyloxy; and tetrahydropyranyloxy,
such as 4-tetrahydropyranyloxy; -
and which may carry as a further substituent aryl, which is as defined below; or
RZ iS aryl, especially chlorophenyl, chloro-lower alkylphenyl, such as o-, m- or p-chloro-
methylphenyl, p-morpholinomethyl-phenyl, p-thiomorpholinomethyl-phenyl, or also
phenyl;
aryl in the said definitions being especially phenyl, naphthyl, such as 1- or 2-naphthyl, or
fluorenyl, such as 9-fluorenyl, that is unsubstituted or substituted by up to three radicals
selected independently of one another from lower alkyl, lower alkanoyl, hydroxy, lower
aLl~oxy, phenyl-lower alkoxy, such as benzyloxy, diphenylmethoxy or triphenylmethoxy, -
hydroxy-lower alkyl, such as hydroxymethyl, halogen, such as fluorine, chlorine or
'~ ' :.
:
-27- 21l86
bromine, cyano, lower alkoxycarbonyl, such as methoxy- or tert-butoxy-carbonyl,
phenyl-lower alkoxycarbonyl, such as benzyloxycarbonyl, halo-lower alkyl, such as
chloromethyl or trifluoromethyl, piperidinomethyl, piperazin-l-ylmethyl, 4-lower aLIcyl-
piperazin-l-yl-methyl, such as 4-methyl- or 4-ethyl-piperazin-1-ylmethyl, morpholino-
methyl or thiomorpholinomethyl, and nitro, which may be present independently of one
another, and being especially correspondingly substituted phenyl;
Rl is hydrogen, tert-butoxycarbonyl, isobutyloxycarbonyl, pyridine-3-carbonyl, morpho-
linocarbonyl, 3-benzofuranoyl, 1,2,3,4-tetrahydro-isoquinoline-3-carbonyl, benzyloxy-
carbonyl substituted by up to three radicals selected independently of one another from
fluorine, halo-lower alkyl, lower alkanoyl, sulfo, lower alkylsulfonyl and cyano, or hetero-
cyclyloxycarbonyl wherein heterocyclyl is bonded via a carbon atom and is selected from
pyrrolyl, thienyl, imidazolyl, pyrazolyl, oxazolyl, thiazolyl, pyrazinyl, pyrimidinyl,
indolyl, quinolyl, isoquinolyl, quinoxalinyl, ~-carbolinyl, and additionally or especially
~-pyranyl and furanyl; and a fully or partially saturated derivative of those radicals, or
wherein the definition heterocyclyloxycarbonyl for Rl is absent, or is additionally or espe-
cially ethoxycarbonyl;
Bl is a bond (preferred) or a bivalent residue of an a-amino acid bonded N-terminally to
Rl and C-terrninally to the amino group at the carbon atom carrying R2-C~12-, preferably : -
the residue of a hydrophobic amino acid, for example proline, phenylalanine, p-fluoro-
phenylalanine, phenylglycine, a-naphthylalanine, cyclohexylalanine, cyclohexylglycine or ~ ; :
an aliphatic a-amino acid selected from glycine, alanine, valine, norvaline, leucine,
norleucine and isoleucine, especially valine, each of the mentioned a-amino acids prefer-
ably being in the D-, L- or (D,L)-forrn, especially in the L-form, and each of the
mentioned amino acids preferably being substituted by one of the radicals mentioned
under Rl selected from hydrogen, N-tert-butoxycarbonyl and morpholinocarbonyl,
R t and 1~3 are each independently of the other phenyl or cyclohexyl, those radicals being
unsubstituted or substituted by from one to three, preferably one or two, radicals selected
indepèndently of one another from hydroxy, methoxy, benzyloxy, fluorine, sulfo, lower
alkylsulfonyl, trifluoromethyl and cyano, additionally or especially from isobutyloxy and
n-butyloxy, as indicated above in the general de~mitions,
Al is a bivalent residue of a hydrophobic a-amino acid, as indicated above under the
general definitions, bonded N-terrninally to the group -C=O and C-terrninally to A2,
A2 is a bivalent residue of a hydrophobic a-amino acid, preferably as defined above under
the general definitions, bonded N-terrninally to Al and C-terminally to the radical NR4Rs,
the said amino acid residues being in the (D)- or (L)-forrn, preferably in the (L)-form, with
,;";
; ,, ., : : , .
., ~
;~ ,, , ~ ~ .. . . .
211~
- 28 -
the exception of phenylalanine which is in the (L)- or the (D)-forrn, ~:
especially Al and A2 form a bivalent residue of a dipeptide of the formula Yal-Phe,
Ile-Phe, Val-Cha, Ile-Cha, Ile-Gly, Val-Val, Val-Gly, Val-(p-F-Phe), Val-Tyr,
Val-(p-CH30-Phe), Val-(p-B~lOPhe), Val-Ile, Val-Ala, Val-Leu or Gly-(p-F-Phe), or
(additionally or especially) phenylglycyl-(p-CH30-Phe), Val-(4-isobutyloxy-Phe) or
Val-(4 n-butyloxy-Phe); wherein the amino acids are in the (D)- or (L)-forrn, especially in
the (L)-form, with the exception of (L)-Val-Phe in which Phe is in the ~L)- or (D)-form; or ~
Al and A2 together form a bivalent residue of a dipeptide consisting of two hydrophobic . ~:
o~-amino acids, prefeMbly two of those mentioned above under the general definitions, the -~ .
central arnide bond of which has been reduced and which is bonded N-terminally to the
group -C=O and C-terminally to the group NR4Rs, as indicated in the general definitions, ~ . .
for exarnple having the formula Val(red)-Phe, and
R4 and Rs together with the bonding nitrogen atom fonn thiomorpholino or morpholino,
especially morpholino, or additionally or especially 2,6-dimethylmorpholino;
and alternadvely or in addition thereto the compounds of formula I wherein Rl ismorpholinosulfonyl or N-(2-pyridylmethyl)-N-methyl-arninocarbonyl and the remaining :
radicals are as defined;
and the pharmaceutically acceptable salts of those compounds where salt-forming groups .
are present.
Greater preference is given to compounds of formula I' wherein :
T is pyrrolidin-2-ylcarbonyl or -3-ylcarbonyl, such as (R)- or (S)-pyrrolidin-2-ylcarbonyl
((D)- or a~)-prolyl), furan-3- or especially fuMn-2-ylcarbonyl, pyridyl-4-, pyridyl-3- or -
especially pyridyl-2-ylcarbonyl, isoquinolin-l- or especially isoquinolin-3-ylcarbonyl,
pyrazin-2-ylcarbonyl, lower alkoxy-lowe~. alkylcarbonyl, such as methoxyacetyl,
n-butoxyacetyl or 3-methoxypropionyl, phenyl- or naphthyl-lower alkoxy-lower alkyl-
carbonyl, such as benzyloxyacetyl, a-lower alkoxy-a-phenyl-lower alkylcarbonyl, such as . - . -:
(R)- or (S)-a-methoxy-a-phenylacetyl, lower alkoxy-lower alkoxy-lower alkylcarbonyl, ~ ::
such as 2-(methoxythoxy)acetyl, lower alkoxylower alkoxy-lower alkoxy-lower alkyl- .
carbonyl, such as 2-(2-(methoxyethoxy)ethoxy)acetyl, o-, m- or p-chlorophenyloxy-lower ..
alkoxy-lower alkylcarbonyl, N,N-di-lower alkylamino-lower alkoxy-lower alkylcarbonyl,
such as 2-(N,N-dimethylamino)ethyloxyacetyl, 2-, 3- or 4-pyridyl-lower alkyloxy-lower . .:
alkylcarbonyl, such as pyridin-2-ylmethoxyacetyl, phenyloxy-lower alkylcarbonyl, such as .
phenoxyacetyl, lower alkylthio-lower alkylcarbonyl, such as methylthioacetyl, phenyl-
lower alkylthio-lower alkylcarbonyl, such as benzylthioacetyl, N,N-di-lower aLkyl- : :.
amino-lower alkylcarbonyl, such as N,N-dimethylamino-acetyl, -3-propionyl or
.,,
~:
211~61
-29-
-4-butyryl, N-lower alkylamino-lower alkylcarbonyl, such as N-methylamino-acetyl or
-3-propiollyl, N-imidazol(-2-, ~4- or -5-)ylmethyl-N-lower alkylamino-lower alkyl-
carbonyl, such as N-(imidazol-4-ylmethyl)-N-methylaminoacetyl, N-pyridin(-2-, -3- or
-4-)ylmethyl-N-lower alkylamino-lower alkylcarbonyl, such as N-pyridin-2-ylmethyl-
N-methylaminoacetyl, N-phenyl-lower alkoxycarbonyl-N-lower alkylamino-lower alkyl-
carbonyl, such as N-benzyloxycarbonyl-N-methylaminoacetyl, imidazol(-l-, -2-, -4- or
-5-)yl-lower aL~cylcarbonyl, such as 3-(imidazol-4-yl~propionyl, N-triphenyl-lower aL~cyl-
imidazol(-4- or -5-)yl-lower alkylcarbonyl, such as 3-(N-triphenylmethylimidazol-4-yl)-
propionyl, or pyrazol(-l-, -3-, -4- or -5-)yl-lower alkylcarbonyl, such as pyrazol-l-yl-
acetyl, or also halo-lower alkylbenzoyl, such as p-chloromethylbenzoyl, p-(morpholino- or ~:
thiomorpholino-methyl)benzoyl or benzoyl, or (especially) lower alkanoyloxy-lower
alkylcarbonyl, such as acetyloxyacetyl, lower alkoxycarbonyl-lower aLlcoxy-lower aLkyl-
carbonyl, such as methoxycarbonylmethoxy-acetyl, lower aLtcoxycarbonyl-lower aLkyl-
thio-lower alkylcarbonyl, such as methoxycarbonylmethylthio-acetyl, lower aLkoxy-
carbonyl-lower alkylsulfo-lower alkylcarbonyl, such as methoxycarbonylmethylsulfo-
acetyl, lower alkanoyloxy-lower alkoxycarbonyl-lower aLkoxy-lower aLlcylcarbonyl, such
as pivaloyloxymethoxycarbonyl-methoxyacetyl or acetyloxymethoxycarbonylmethoxy-
acetyl, carboxy-lower alkoxy-lower alkylcarbonyl, such as carboxymethoxyacetyl,
N-phenyl-lower alkoxycarbonylamino-lower alkylcarbonyl, such as 3-benzyloxycarbonyl-
arnino-propionyl, amino-lower alkylcarbonyl, such as 3-aminopropionyl, di-lower alkyl-
arnino-lower alkoxy-lower alkoxy-lower aL~cylcarbonyl, such as 12-(2-dimethylarnino-
ethoxy)-ethoxy]-acetyl, N-phenyl-lower aL~coxycarbonyl-amino-lower alkoxy-lower
alkoxy-lower alkylearbonyl, such as 2-(2-N-benzyloxycarbonylaminoethoxy)ethoxy-
acetyl, amino-lower alkoxy-lower alkoxy-lower alkylcarbonyl, such as 2-(2-amino-ethoxy)ethoxy-acetyl, or tetrahydropyranyloxy-lower alkylcarbonyl, such as 2-(4-tetra-
hydropyranyloxy)-acetyl or 2(R)-, 2(S)- or also 2(R,S)-(4-tetrahydropyranyloxy)-propionyl;
Rl is hydrogen, tert-butoxycarbonyl, isobutyloxycarbonyl, pyridine-3-carbonyL mo~pho-
linocarbonyl, 3-benzofuranoyl or 1,2,3,4-tetrahydro-isoquinoline-3-carbonyl, or especially
ethoxycarbonyl, 4-~etrahydropyranyloxycarbonyl or te~ahydrofuran-2(R or S)-yloxy-
carbonyl; or alternatively and in addition thereto morpholinosulfonyl, N-(2-pyridyl-
methyl)-N-methyl-aminocarbonyl;
Bl is a bond (preferred) or a bivalent residue of the a-amino acid valine bondedN-terminally to Rl and C-terminally to the amino group at the carbon atom carrying
R2-CH2-, in the latter case Rl preferably being hydrogen, tert-butoxycarbonyl or morpho-
linocarbonyl, or alternatively or in addition thereto morpholinosulfonyl or N-(2-pyridyl-
~- 21~8661
-30-
methyl)-N-methyl-aminocarbonyl,
R2 and R3 are each independently of the other phenyl or cyclohexyl, those radicals being
unsubstituted or substituted by from one to three, preferably one or two, radicals selected
independently of one another from hydroxy, methoxy, benzyloxy, fluorine, sulfo, lower
alky}sulfonyl, cyano and trifluoromethyl, and especially from isobutyloxy and n-butyloxy,
Al is a bivalent residue of one of the a-amino acids glycine, valine, isoleucine and espe-
cially leucine and phenylglycine bonded N-terrninally to the group -C=O and C-terminally
to A2,
A2 is a bivalent residue of one of the a-amino acids glycine, alanine, valine, leucine,
isoleucine, phenylalanine, tyrosine, cyclohexylalanine, p-methoxy-phenylalanine,p-benzyloxyphenylalanine, p-fluorophenylalanine and especially p-(isobutyloxy)- or p-(n-
butyloxy)-phenylalanine bonded N-terminally to Al and C-terrninally to the group NR4Rs,
or
Al and A2 together form a bivalent residue of a dipeptide having a reduced central peptide
bond that consists of an N-terminal amino acid residue selected from Gly(red), Valtred)
and Ile(red) and a C-terminal amino acid residue selected from glycine, phenylalanine,
cyclohexylalanine, tyrosine, p-methoxy-phenylalanine and p-fluorophenylalanine, and ~at ~ -
is bonded N-terminally to the group -C=O and C-terminally to the group NR4Rs, as - ~:.
defined above for Al and A2, and . .
R4 and Rs together with the bonding nitrogen atom form thiomorpholino, morpholino or
preferably 2,6-dimethylmorpholino, especially mo~pholino,
and the pharmaceutically acceptable salts of those compounds where salt-forming groups
are present.
Special preference is given to compounds of formula I' wherein
T is pyrrolidin-2-ylcarbonyl or -3-ylcarbonyl, such as (R)- or (S)-pyrrolidin-2-ylcarbonyl
((D)- or (L)-prolyl), furan-3- or especially furan-2-ylcarbonyl, pyridyl-4-, pyridyl-3- or ~ ~
especially pylidyl-2-ylcarbonyl, isoquinolin- 1- or especially isoquinolin-3-ylcarbonyl, ~ ~ :
pyrazin-2-ylcarbonyl, lower alkoxy-lower alkylcarbonyl, such as methoxyacetyl, n-
butoxyacetyl or 3-methoxypropionyl, phenyl- or naphthyl-lower alkoxy-lower alkyl-
carbonyl, such as benzyloxyacetyl, a-lower alkoxy-a-phenyl-lower alkylcarbonyl, such as
(R)- or (S)-a-methoxy-a-phenylacetyl, lower alkoxy-lower alkoxy-lower alkylcarbonyl,
such as 2-(methoxyethoxy)acetyl, lower alkoxy-lower alkoxy-lower alkoxy-lower alkyl-
carbonyl, such as 2-(2-(methoxyethoxy)ethoxy)acetyl, o-, m- or p-chlorophenyloxy-lower ~ .
alkoxy-lower alkylcarbonyl, N,N-di-lower alkylamino-lower alkoxy-lower alkylcarbonyl, ~:
such as 2-(N,N-dimethylamino)ethyloxyacetyl, 2-, 3- or 4-pyridyl-lower a~cyloxy-lower
`` 21~8661
- 3 1 -
alkylcarbonyl, such as pyridin-2-ylmethoxyacetyl, phenyloxy-lower alkylcarbonyl, such as
phenoxyacetyl, lower alkylthio-lower alkylcarbonyl, such as methylthioacetyl, phenyl-
lower aL~cylthio-lower aLlcylcarbonyl, such as benzylthioacetyl, N,N-di-lower alkylamino-
lower alkylcarbonyl, such as N,N-dimethylamino-acetyl, -3-propionyl or -4-butyryl,
N-lower alkylamino-lower alkylcarbonyl, such as N-methylamino-acetyl or -3-propionyl,
N-imidazol(-2-, -4- or -5-)ylmethyl-N-lower allcylamino-lower alkylcarbonyl, such as N-
(imidazol-4-ylmethyl)-N-methyl~ninoacetyl, N-pyridin(-2-, -3- or-4-)ylmethyl-N-lower
aL~ylamino-lower alkylcarbonyl, such as N-pyridin-2-ylmethyl-N-methylaminoacetyl,
N-phenyl-lower alkoxycarbonyl-N-lower alkylamino-lower alkylcarbonyl, such as
N-benzyloxycarbonyl-N-methylaminoacetyl, imidazol(-l-, -2-, -4- or -S-)yl-lower aL~cyl-
carbonyl, such as 3-(imidazol-4-yl)propionyl, N-triphenyl-lower aLkylimidazol(-4- or
-S-)yl-lower aL~cylcarbonyl, such as 3-(N-~iphenylmethylimidazol-4-yl~propionyl, or
pyrazol(-l-, -3-, -4- or -5-)yl-lower aL~cylcarbonyl, such as pyrazol-l-ylacetyl, or also
halo-lower alkylbenzoyl, such as p-chloromethylbenzoyl, p-(morpholino- or thiomorpho-
lino-methyl)benzoyl or benzoyl,
Rl is hydrogen, tert-butoxycarbonyl, isobutyloxycarbonyl, pyridine-3-carbonyl, morpho-
linocarbonyl, 3-benzofuranoyl or 1,2,3,4-tetrahydro-isoquinoline-3-carbonyl; or alterna-
tively and in addition thereto morpholinosulfonyl or N-(2-pyridylmethyl)-N-methyl-
aminocarbonyl,
Bl is a bond or a bivalent residue of ~he a-amino acid valine bonded N-terrninally to R
and C-terrninally to the amino group at the carbon atom carrying R2-CH2-, in the latter
case Rl preferably being hydrogen, tert-butoxycarbonyl or morpholinocarbonyl, oralternatively or in addition thereto morpholinosulfonyl or N-(2-pyridylmethyl)-N-methyl-
aminocarbonyl,
R2 and R3 are each independently of the other phenyl or cyclohexyl, those radicals being
unsubstituted or substituted by one or two radicals selected independently of one another
from hydroxy, methoxy, benzyloxy, fluorine, sulfo, lower alkylsulfonyl, cyano and tri-
fluoromethyl,
Al is a bivalent residue of one of the a-amino acids glycine, valine and isoleucine bonded
N-terminally to the group -C=O and C-terminally to A2,
A2 is a bivalent residue of one of the a-amino acids glycine, alanine, valine, leucine,
isoleucine, phenylalanine, tyrosine, cyclohexylalanine, p-methoxy-phenylalanine,p-benzyloxyphenylalanine and p-fluorophenylalanine bonded N-terminally to Al andC-terminally to the group NR4Rs, or
Al and A2 together forrn a bivalent residue of a dipeptide having a reduced central peptide
bond that consists of an N-terminal amino acid residue selected from Gly(red), Val(red)
~ 2118~61
- 32 - . ::
and lle(red) and a C-terminal amino acid residue selected from glycine, phenylalanine,
cyclohexylalanine, tyrosine, p-methoxy-phenylalanine and p-fluorophenylalanine, and that
is bonded N-terminally to the group -C=O and C-terminally to the group NR~Rs, asdefined above for Al and A2, and
R4 and Rs together with the bonding nitrogen atom form thiomorpholino or morpholino,
especially morpholino,
and the pharmaceutically acceptable salts of those compounds where salt-forming groups
are present.
Very special preference is given to a compound of formula I' wherein
T is pyTrolidin-2-ylcarbonyl or -3-ylcarbonyl, such as (R)- or ~S)-pyrrolidin-2-ylcarbonyl
((D)- or (L)-prolyl), furan-3- or especially furan-2-ylcarbonyl, pyridyl-4-, pyridyl-3- or
especially pyridyl-2-ylcarbonyl, isoquinolin-l- or especially isoquinolin-3-ylcarbonyl,
pyrazin-2-ylcarbonyl, lower alkoxy-lower alkylcarbonyl, such as methoxyacetyl,
n-butoxy-acetyl or 3-methoxypropionyl, phenyl- or naphthyl-lower alkoxy-lower aLlcyl- -:
carbonyl, such as benzyloxyacetyl, a-lower alkoxy-a-phenyl-lower aLkylcarbonyl, such as
(R)- or (S)-a-methoxy-a-phenylacetyl, lower alkoxy-lower aL~oxy-lower alkylcarbonyl, .
such as 2-(methoxyethoxy)acetyl, lower alkoxy-lower alkoxy-lower alkoxy-lower aLtcyl-
carbonyl, such as 2-(2-(methoxyethoxy)ethoxy)acetyl, o-, m- or p-chlorophenyloxy-lower
alkoxy-lower alkylcarbonyl, M,N-di-lower alkylamino-lower alkoxy-lower alkylcarbonyl,
such as 2-(N,N-dimethylamino)ethyloxyacetyl, 2-, 3- or 4-pyridyl-lower alkyloxy-lower
alkylcarbonyl, such as pyridin-2-ylmethoxyacetyl, phenyloxy lower aL~cylcarbonyl, such as :
phenoxyacetyl, lower alkylthio-lower alkylcarbonyl, such as methylthioacetyl, phenyl-
lower alkylthio-lower alkylcarbonyl, such as benzylthioacetyl, N,N-di-lower alkylamino-
lower aLkylcarbonyl, such as N,N-dimethylamino-acetyl, -3-propionyl of-4-butyTyl,
N-lower alkylamino-lower alkylcarbonyl, such as N-methylamino-acetyl or -3-propionyl,
N-imidazol(-2-, -4- or -S-)ylmethyl-N-lower alkylamino-lower alkylcarbonyl, such as ~ :
N-(imidazol-4-ylmethyl)-N-methylaminoacetyl, N-pyTidin(-2-, -3- or-4-)ylmethyl-
N-lower alkylamino-lower alkylcarbonyl, such as N-pyridin-2-ylmethyl-N-methylamino-
acetyl, N-phenyl-lower alkoxycarbonyl-N-lower alkylamino-lower alkylcarbonyl, such as
N-benzyloxycarbonyl-N-methylaminoacetyl, imidazol(-l-, -2-, -4- or -5-)yl-lower alkyl-
carbonyl, such as 3-(imidazol-4-yl)propionyl, N-triphenyl-lower alkylimidazol(-4- or -5-)-
yl-lower alkylcarbonyl, such as 3-(N-triphenylmethylimidazol-4-yl)propionyl, or pyrazol-
(-1-, -3-, -4- or -5^)yl-lower alkylcarbonyl, such as pyrazol-l-ylacetyl, or also halo-lower ~ :
alkylbenzoyl, such as p-chloromethylbenzoyl, p-(morpholino- or thiomorpholino-methyl)- . .
benzoyl or benzoyl,
.:. ' '~
211 ~661
- 33 -
Rl is tert-butoxycarbonyl,
R2 is phenyl, cyclohexyl, p-hydroxyphenyl, o-, m- or p-methoxyphenyl, p-benzyloxy-
phenyl, o-, m- or p-fluorophenyl, p-trifluoromethylphenyl or o-, m- or p-cyanophenyl,
especially phenyl, p-hydroxyphenyl, p-methoxyphenyl, p-benzyloxyphenyl, p-fluoro-
phenyl or p-trifluoromethylphenyl,
R3 independently of R2 has one of the definitions given for R2,
Al is a bivalent residue of one of the a-amino acids glycine, (L)-valine and (L)-isoleucine
bonded N-terminally to the group -C=O and C-terminally to A2,
A2 is a bivalent residue of one of the ol-amino acids glycine, alanine, valine, leucine,
isoleucine, phenylalanine, tyrosine, cyclohexylalanine, p-methoxy-phenylalanine, p-
benzyloxyphenylalanine or p-fluorophenylalanine bonded N-terminally to A1 and
C-terminally to the group NR4Rs, and
the radical -NRsR6 is morpholino,
and salts thereof where salt-forming groups are present,
or (additionally or especially) the compounds of forrnula I', or salts thereof, wherein T is
lower alkanoyloxy-lower alkylcarbonyl, such as acetyloxyacetyl, lower alkoxycarbonyl-
lower alkoxy-lower alkylcarbonyl, such as methoxycarbonylmethoxy-acetyl, lower
alkoxycarbonyl-lower alkylthio-lower alkylcarbonyl, such as methoxycarbonylmethyl-
thio-acetyl, lower alkoxycarbonyl-lower alkylsulfo-lower alkylcarbonyl, such as methoxy-
carbonylmethylsulfo-acetyl, lower aLkanoyloxy-lower alkoxycarbonyl-lower alkoxy-lower
alkylcarbonyl, such as pivaloyloxymethoxycarbonyl-methoxyacetyl or acetyloxymethoxy-
carbonylmethoxy-acetyl, carboxy-lower aLkoxy-lower alkylcarbonyl, such as carboxy-
methoxyacetyl, N-phenyl-lower alkoxycarbonylamino-lower aLIcylcarbonyl, such as -
3-benzyloxycarbonylamino-propionyl, amino-lower aLkylcarbonyl, such as 3-amino-
propionyl, di-lower alkylamino-lower alkoxy-lower alkoxy-lower alkylcarbonyl, such as
[2-(2-dimethylaminoethoxy)-ethoxy]-acetyl, N-phenyl-lower alkoxycarbonyl-amino-lower
alkoxy-lower alkoxy-lower alkylcarbonyl, such as 2-(2-N-benzyloxycarbonylamino-
ethoxy)ethoxy-acetyl, amino-lower alkoxy-lower alkoxy-lower alkylcarbonyl, such as
2-(2-aminoethoxy)ethoxy-acetyl, or tetrahydropyranyloxy-lower alkylcarbonyl, such as
2-(4-tetrahydropyranyloxy)-acetyl or 2(R)-, 2(S)- or also 2(R,S)-(4-tetrahydropyranyl-
oxy)-propionyl and the remaining radicals are as defined immediately above.
Great preference is given to a compound of formula I' wherein T is an acyl radical of
formula Z, as indicated above, wherein ~ .
RZ is hydrocarbyl wherein at least one carbon atom has been replaced by a hçtero atom
21186~1 ~
- 3 4 -
. -
with the proviso that a hetero atom is not bonded directly to the carbonyl to which th~ .
radical R~ is bonded, alkyl having two or more carbon atoms, lower alkenyl, lower aLcynyl
or aryl, preferably as defined above, especially wherein
RZ is heterocyclyl bonded via a ring carbon atom and selected from pyrrolyl, 2,5-dihydro-
pyrrolyl, indolyl, indolizinyl, isoindolyl, pyrrolidinyl, such as pyrrolidin-3-yl or especially
pyrrolidin-2-yl (in the (R,S)- or preferably the (R)- or (S)-configuration), hydroxypyrrol- . ~:
idinyl, such as 3- or especially 4-hydroxypyrrolidinyl, furyl, such as furan-3-yl or espe- ~ ~
cially furan-2-yl, tetrahydrofuryl, thienyl, cyclohepta[b~pyrrolyl, imidazolyl, such as imid- .
azol-2-yl, imidazol-3-yl or especially imidazol-S-yl, N-triphenyl-lower aLkyl-imidazolyl,
such as N-triphenylmethyl-imidazolyl, pyrazolyl, especially pyrazol-3-yl, oxazolyl,
isoxazoiyl, such as isoxazol-3-yl or -S-yl, thiazolyl, isothiazolyl, such as isothiazol-3-yl or
-S-yl, triazolyl, such as 1,2,3-triazol-4- or -S-yl or 1,2,4-triazol-5-yl, tetrazolyl, pyridyl,
such as pyridin-4-yl or -3-yl or especially pyridin-2-yl, quinolyl, such as quinolin-2-yl,
isoquinolyl, especially isoquinolin-1-yl or -3-yl, piperidyl, especially piperidin-2-yl, - ~ .
~-pyranyl, 4,5-dihydropyranyl, 4H-chromenyl, chromanyl, ~-thiopyranyl, pyridazinyl, :
cinnolyl, phthalazinyl, quinazolinyl, quinoxalinyl, pyrimidinyl, pyrazinyl, phenazinyl, - :
phenoxazinyl, phenothiazinyl, morpholinyl and thiazinyl, each of which is substituted by i~
up to three radicals selected independently of one another from lower alkyl, phenyl-lower
alkyl, diphenyl-lower alkyl, triphenyl-lower alkyl, such as triphenylmethyl, lower
alkanoyl, hydroxy, lower alkoxy, phenyl-lower alkoxy, such as benzyloxy, diphenyl-
methoxy or triphenylmethoxy, hydroxy-lower alkyl, such as hydroxymethyl, halogen, such ~ i~
as fluorine, chlorine or bromine, cyano, lower alkoxycarbonyl, such as methoxy- or tert-
butoxy-carbonyl, phenyl-lower alkoxycarbonyl, such as benzyloxycarbonyl and halo- :
lower alkyl, such as chloromethyl or trifluoromethyl, or pre~erably is unsubstituted, and ~:
each of which is bonded via a ring carbon atom; very special preference being given to
those of the mentioned radicals which contain a ring hetero atom directly adjacent to the
bonding ring carbon atom, more especially the radicals furan-2-yl, (S)- or (R)-pyrrolidin- . .
2-yl and imidazol-4 yl, pyridin-2-yl, -3-yl or -4-yl, or isoquinolin-3-yl; or additionally or
especially tetrahydropyranyl; or
RZ is lower alkyl (especially methyl, ethyl, n-propyl or n-butyl) which is substituted by at
least one radical selected from
.
lower alkoxy, such as methoxy, ethoxy or n-butoxy; lower alkoxy, such as methoxy,
ethoxy or n-butoxy, substituted by one or two substituents, especially aryl, more espe-
211g ~ 61
- 35 -
cially phenyl, naphthyl, lower alkoxy, such as ethoxy or methoxy, lower alkylthio, such
as methylthio or ethylthio, lower alkoxy-lower alkoxy, such as 2-methoxyethoxy, lower
alkylthio-lower alkoxy, such as 2-methylthioethoxy, aryloxy or arylthio, especially
phenyloxy or o-, m- or p-chlorophenyloxy, for example p-chlorophenyloxy, amino,
N-lower alkylamino or N,N-di-lower alkylamino, such as 2-amino, 2-(N-lower
alkyl)amino or 2-(N,N-di-lower alkyl)amino, for example 2-dimethylamino, and/or
heterocyclyl as last de~lned for heterocyclyl RZ bonded via a ring carbon atom, especially
2-, 3- or 4-pyridyl; aryloxy, especially phenyloxy; lower alkylthio, such as methylthio,
ethylthio or n-butylthio; lower alkylthio, such as methylthio, ethylthio or n-butylthio,
substituted by one or two substituents, especially aryl, more especially phenyl or
naphthyl; arylthio, such as phenylthio; amino or amino substituted by one or two radicals
selected from lower alkyl, such as methyl, heterocyclyl-lower alkyl wherein heterocyclyl
is as defined for heterocyclyl RZ bonded via a ring carbon atom, especially heterocyclyl-
methyl, such as imidazolylmethyl, for example 4-imidazolylmethyl, or pyridylmethyl,
for example 2-, 3- or 4-pyridylmethyl, each bonded via a ring carbon atom, aryl-lower
alkyl, such as phenyl- or naphthyl-lower alkyl~ for example phenyl- or naphthyl-methyl,
lower alkanoyl, such as acetyl, lower alkoxycarbonyl, such as tert-butoxycarbonyl, and
aryl-lower alkoxycarbonyl, such as phenyl-lower alkoxycarbonyl, for example
benzyloxycarbonyl, with especially one of the substituents of amino being lower aLkyl,
especially methyl, and the other being hydrogen or one of the radicals mendoned above
as substituents of amino; heterocyclyl as defined above for heterocyclyl RZ bonded via a
ring carbon atom, especially as pyridin-2-yl, -3-yl or -4-yl; and heterocyclyl-lower alkyl -
whereiin lower alkyl is preferably methyl, 1- or 2-ethyl or 3-propyl and whereinheterocyclyl is as defined above for heterocyclyl RZ bonded via a ring carbon atom but
may also be bonded via a ring nitrogen atom, especially imidazol-1-yl, imidazol-2-yl,
imidazol-5-yl or more especially imidazol-4-yl, N-triphenyl-lower alkylimidazolyl, such
as N-tTiphenylmethyl-imidazol-5-yl or especially -4-yl, or pyrazolyl, such as
pyrazol- 1-yl, -3-yl, -4-yl or -5-yl; or (additionally or preferably) lower alkoxycarbonyl,
such as methoxycarbonyl; lower alkanoyloxy-lower alkoxycarbonyl, such as pivaloyl-
oxymethoxycarbonyl or acetyloxymethoxycarbonyl; carboxy; phenyl-lower
alkoxycarbonylamino-lower alkoxy, such as 2-(benzyloxycarbonylamino)-ethoxy;
amino-lower alkoxy, such as 2-aminoethoxy; di-lower alkylamino-lower alkoxy, such as
2-(dimethylamino)-ethoxy; or N,N-di-lower alkylamino-lower alkoxy, such as 2-(di-
methylarnino~-ethoxy; lower alkoxycarbonyl-lower alkylsulfo, such as methoxy-
carbonyl-methylsulfo; lower alkanoyloxy, such as acetyloxy; and tetrahydropyranyloxy,
such as 4-tetrahydropyranyloxy~ : .
.... ~ ~
2118~1
- 36- ::
and which may also carry as a further substituent aryl, which is as defined below; or
RZ is aryl, especially chlorophenyl, chloro-lower aLlcylphenyl, such as o-, m- or p-chloro-
methylphenyl, p-morpholinomethyl-phenyl, p-thiomorpholinomethyl-phenyl, or also
phenyl;
aryl in the said defimitions being especially phenyl, naphthyl, such as 1- or 2-naphthyl, or
fluorenyl, such as 9-fluorenyl, tha~ is unsubstituted or substituted by up to three radicals
selected independently of one another from lower alkyl, lower alkanoyl, hydroxy, lower
alkoxy, phenyl-lower alkoxy, such as benzyloxy, diphenylmethoxy or triphenylmethoxy, .
hydroxy-lower alkyl, such as hydroxymethyl, halogen, such as fluorine, chlorine or :
bromine, cyano, lower alkoxycarbonyl, such as methoxy- or tert-butoxy-carbonyl, .
phenyl-lower alkoxycarbonyl, such as benzyloxycarbonyl, halo-lower alkyl, such as .
chloromethyl or trifluoromethyl, piperidinomethyl, piperazin-l-ylmethyl, 4-lower aLkyl- ~ . ~
piperazin- l-yl-methyl, such as 4-methyl- or 4-ethyl-piperazin-1-ylmethyl, morpholino- :
methyl or thiomorpholinomethyl, and nitro, which may be present independently of one
another, and being especially correspondingly substituted phenyl;
T in the most preferred form being in the form of pyrrolidin-2-ylcarbonyl or -3-ylcarbonyl,
such as (R)- or (S)-pyrrolidin-2-ylcarbonyl ((D)- or (L)-prolyl), furan-3- or especially
furan-2-ylcarbonyl, pyridyl-4-, pyridyl-3- or especially pyridyl-2-ylcarbonyl, isoquin-
olin-l- or especially isoquinolin-3-ylcarbonyl, pyrazin-2-ylcarbonyl, lower aLkoxy-lower
alkylcarbonyl, such as methoxyacetyl, n-butoxyacetyl or 3-methoxypropionyl, phenyl- or :
naphthyl-lower alkoxy-lower alkylcarbonyl, such as benzyloxyacetyl, a-lower alkoxy-a-
phenyl-lower alkylcarbonyl, such as (R)- or (S)-a-methoxy-or.-phenylacetyl, lower
alkoxy-lower alkoxy-lower alkylcarbonyl, such as 2-(methoxyethoxy)acetyl, lower
alkoxy-lower alkoxy-lower alkoxy-lower alkylcarbonyl, such as 2-(2-(methoxyethoxy)- ~ .
ethoxy)acetyl, o-, m- or p-chlorophenyloxy-lower alkoxy-lower alkylcarbonyl, N,N-di-
lower alkylamino-lower alkoxy-lower alkylcarbonyl, such as 2-(N,N-dimethylamino)-
ethyloxyacetyl, 2-, 3- or 4-pyridyl-lower alkyloxy-lower alkylcarbonyl, such as pyridin- :
2-ylmethoxyacetyl, phenyloxy-lower alkylcarbonyl, such as phenoxyacetyl, lower alkyl-
thio-lower alkylcarbonyl, such as methylthioacetyl, phenyl-lower alkylthio-lower alkyl-
carbonyl, such as benzylthioacetyl, N,N-di-lower alkylamino-lower alkylcarbonyl, such as
N,N-dimethylamino-acetyl, -3-propionyl or -4-butyryl, N-lower alkylamino-lower alkyl-
carbonyl, such as N-methylamino-acetyl or -3-propionyl, N-imidazol(-2-, -4- or -5-)yl-
.. , " " I~_ ?'
211~661
- 37 -
methyl-N-!ower alkylamino-lower alkylcarbonyl, such as N-(imidazol-4-ylmethyl)-
N-methylaminoacetyl, N-pyridin(-2-, -3- or -4-)ylmethyl-N-lower alkylamino-lower alkyl-
carbonyl, such as N-pyridin-2-ylmethyl-N-methylaminoacetyl, N-phenyl-lower aLkoxy-
carbonyl-N-lower alkylamino-lower alkylcarbonyl, such as N-benzyloxycarbonyl-
N-methylaminoacetyl, imidazol(-l-, -2-, -4- or-5-)yl-lower alkylcarbonyl, such as 3-
(imidazol-4-yl)propionyl, or N-triphenyl-lower alkylimidazol(-4- or -5-)yl-lower alkyl-
carbonyl, such as 3-(N-triphenylmethylimidazol-4-yl)propionyl, or pyrazol(-l-, -3-, -4- or
-5-)yl-lower aLIcylcarbonyl, such as pyrazol- l-ylacetyl, or also in the forrn of halo-lower
alkylbenzoyl, such as p-chloromethylbenzoyl, p-(morpholino- or thiomorpholino-methyl)-
benzoyl or benzoyl, especially in the fonn of methoxyacetyl or in the form of 2-pyridyl-
carbonyl; or (additionally or especially) lower aLlcanoyloxy-lower alkylcarbonyl, such as
acetyloxyacetyl, lower alkoxycarbonyl-lower aL~coxy-lower aL~cylcarbonyl, such as
methoxycarbonylmethoxy-acetyl, lower alkoxycarbonyl-lower alkylthio-lower a~yl-
carbonyl, such as methoxycarbonylmethylthio-acetyl, lower alkoxycarbonyl-lower aL~cyl-
sulfo-lower alkylcarbonyl, such as methoxycarbonylmethylsulfo-acetyl, lower aL~anoyl-
oxy-lower aLIcoxycarbonyl-lower alkoxy-lower alkylcarbonyl, such as pivaloyloxy-methoxycarbonyl-methoxyacetyl or acetyloxymethoxycarbonylmethoxy-acetyl, carboxy-
lower alkoxy-lower alkylcarbonyl, such as carboxymethoxyacetyl, N-phenyl-lower
alkoxycarbonylamino-lower aLIcylcarbonyl, such as 3-benzyloxycarbonylamino-propionyl,
amino-lower aL~ylcarbonyl, such as 3-aminopropionyl, di-lower alkylamino-lower
alkoxy-lower alkoxy-lower alkylcarbonyl, such as [2-(2-dimethylaminoethoxy)-ethoxy]- -
acetyl, N-phenyl-lower alkoxycarbonyl-aminolower alkoxy-lower aLkoxy-lower aL1cyl-
carbonyl, such as 2-(2-N-benzyloxycarbonylaminoethoxy)ethoxy-acetyl, amino-loweralkoxy-lower alkoxy-lower aL~cylcarbonyl, such as 2-(2-aminoethoxy)ethoxy-acetyl, or
tetrahydropyranyloxy-lower alkylcarbonyl, such as 2-(4-tetrahydropyranyloxy)-acetyl or
2(R)-, 2(S)- or also 2(R,S)-(4-tetrahydropyranyloxy)-propionyl;
.
and wherein
either (especially preferred) Rl is tert-butoxycarbonyl; Bl is a bond; R2 is phenyl; R3 is
phenyl; Al is the bivalent residue of (L)-valine (-(L)-Val-) bonded N-terminally to the
group -C=O and C-terminally to A2; A2 is the bivalent residue of phenylalanine
(-(L)-Phe-) bonded N-terrninally to Al and C-terrninally to the group -NR4Rs; and R4 and
Rs together with the bonding ni~rogen atom fo~n morpholino;
or Rl is tert-butoxycarbonyl; Bl is a bond; R2 is phenyl; R3 is p-methoxyphenyl; Al is the
- -- 2118661
- 38 -
bivalent residue of (L)-valine (-(L)-Val-) bonded N-terminally to the group -C=O and
C-terminally to A2; A2 is the bivalent residue of p-methoxy-phenylalanine
(-(L)-(p-CH30-Phe)-) bonded N-terminally to Al and C-terminally to the group -NR4Rs; ~ :
and R4 and Rs together with the bonding nitrogen atom form morpholino;
or Rl is tert-butoxycarbonyl; Bl is a bond; R2is phenyl; R3is p-fluorophenyl; Al is the
bivalent residue of (L)-valine bonded N-terminally to the group -C=O and C-terminally to
A2; A2is the bivalent residue of phenylalanine bonded N-terminally to Al and
C-terminally to the group -NR4R5; and R4 and Rs together with the bonding nitrogen atom : - ;
form morpholino; :
or Rl is teirt-butoxycarbonyl; Bl is a bond; R2 is phenyl; R3is p-fluorophenyl; Al is the : -
bivalent reisidue of (L)-valine bonded N-terminally to the group -C=O and C-terminally to . : .
A2; A2is the bivalent residue of p-methoxy-phenylalanine bonded N-terminally to Al and . .
C-terminally to the group -NR4R5; and R4 and Rs together with the bonding nitrogen atom
form morpholino;
or (especially preferred) Rl is tert-butoxycarbonyl; Bl is a bond; R2 is phenyl; R3is
p-methoxyphenyl; Al is the bivalent residue of (L)-valine (~(L)-Val-) bonded N-terminally
to the group -C=O and C-terminally to A2; A2 is the bivalent residue of phenylalanine . .
(-(L)-Phe-) bonded N-terrninally to Al and C-terminally to the group -NR4R5; and R4 and ~ ~
R5 together with the bonding nitrogen atom form morpholino; ~ :
and pharmaceutically acceptable salts thereof where salt-forming groups are present.
Very special preference is given to a compound of formula I' ~herein T is 4(S)-
(2-furanylcarboxy); 4(S)-[4-(dimethyl-amino)-butyryloxy]; 4(S)-(N-Z N-methyl-amino-
acetyloxy); 4(S)-(methylamino-acetyloxy); 4(S)-[N-(imidazole-4-methyl)-N-methyl-aminoacetyloxy]; 4(S)-[3-(1-triphenylmethyl-imidazol-4-yl)-propionyloxy]; 4(S)-[3-(4-
imidazolyl)-propionyloxy]; 4(S)-(methoxy-acetyloxy); 4(S)-(2-picolinoyl); 4(S)-(benzyl-
oxy-acetyloxy); 4(S)-[(S)-a-methoxy-a-phenyl-acetyloxy]; 4(S)-[(R)-oc-methoxy-
a.-phenyl-acetyloxy]; 4(S)-(l-pyrazolylacetyloxy); 4(S)-(isoquinoline-3-carbonyloxy); :
4(S)-(pyrazinecarbonyloxy); 4(S)-(4-oc-chloromethyl-benzoyloxy); 4(S)-[4-(4-morpho-
lino)methyl-benzoyloxy]; 4(S)^(isonicotinoyloxy); 4(S)-(nicotinoyloxy); 4(S)-(3-methoxy-
propanoyloxy); 4(S)-[(4-chlorophenoxy)methoxyacetyloxy]; 4(S)-[2-(2-methoxyethoxy)-
acetyloxy]; 4(S)-(butyloxyacetyloxy); 4(S)-[2-[2-(2-methoxyethoxy)ethoxy]acetyloxy)]; ~ -
. ~'.
2118661
- 39 -
4(S)-(methoxyacetyloxy); 4(S)-(phenoxyacetyloxy); 4(S)-[(S)-a~-methoxy-a-phenylacetyl-
oxy); 4(S)-[(R)-a-methoxy-a-phenylacetyloxyl; (N,N-dimethyl-aminoacetyloxy); 4(S)-
[N-(pyridine-2-methyl)-N-methyl-aminoacetyloxy]; 4(S)-[3-(dimethyl-amino)-propionyl-
oxy]; 4(S)-[3-(N-ZN-methyl-amino)-propionyloxy]; 4(S)-(3-methyl-amino-yropionyl-oxy); 4(S)-[(dimethylaminoethoxy)-acetyloxy]; 4(S)-[(2-pyridylmethoxy)-acetyloxy];
4(S)-(methoxy-acetyloxy); 4(S)-(pyridine-2-carboxy); 4(S)-(methylthioacetyloxy); 4(S)- ~ ~:
(benzylthioacetyloxy); 4(S)-((L)-prolyloxy); 4(S)-((D)-prolyloxy); 4(S)-((L)-(N~ prolyl)-
oxy); 4(S)-((~)-(N-Z-prolyl)oxy); 3-(N-Z-amino)-propionyloxy; 3-amino-propionyloxy;
(3-dimethylamino-propoxy)-acetyloxy; 2-(2-dimethylamino-ethoxy)-ethoxy-acetyloxy;
(4-dimethylamino-butoxy)-acetyloxy; (2-benzyloxy)acetyloxy; (2-acetyloxy)acetyloxy;
2-(4-tetrahydropyranyloxy)acetyloxy; 2(R)-(4-tetrahydropyranyloxy)-propionyloxy; 2-(2-
amino-ethoxy)ethoxy-acetyloxy); 2-(2-benzyloxycarbonylamino-ethoxy)ethoxy-acetyl-
oxy; (methoxycarbonyl-methoxy)-acetyloxy; (methoxycarbonyl-methylthio)-acetyloxy;
(methoxycarbonyl-methylsulfo)-acetyloxy; (pivaloyloxymethoxycarbonyl)-methoxy-ace-
tyl; (carboxymethoxy)-acetyloxy; or (acetoxymethoxycarbonyl-methoxy)-acetyloxy; and
Rl is tert-butoxycarbonyl; Bl is a bond; R2 is phenyl; R3 is phenyl; Al is the bivalent
residue of (L)-valine (-(L)-Val-) bonded N-terminally to the group -C=O and C-terminally
to A2; A2 is the bivalent residue of phenylalanine (-(L)-Phe-) bonded N-terminally to Al
and C-terminally to the group -NR4Rs; and R4 and Rs together with the bonding nitrogen
atom form morpholino;
or the compound of formula I' wherein T is as last defined, and has especially one of the
mentioned meanings, and
Rl is tert-butoxycarbonyl; Bl is a bond; R2 is phenyl; R3 is p-methoxyphenyl; Al is the
bivalent residue of (L)-valine (-(L)-Val-) bonded N-terminally to the group -C=O and
C-terminally to A2; A2 is the bivalent residue of phenylalanine (-(L)-Phe-) bonded
N-terminally to Al and C-terminally to the group -NR4Rs; and R4 and Rs together with the : ::
bonding nitrogen atom form morpholino;
or in each case a pharmaceutically acceptable salt thereof where salt-forrning groups are
present.
:
Greater preference is given to a compound of formula I' wherein T is methoxyacetyl or
pyridin-2-ylcarbonyl and
-- 211~61
- 40 - . -
either Rl is tert-butoxycarbonyl; Bl is a bond; R2 is phenyl; R3 is phenyl; Al is the
bivalent residue of (L)-valine bonded N-terminally to the group -C=O and C-terminally to
A2, A2 is the bivalent residue of phenylalanine bonded N-terminally to Al and
C-terrninally to the group -NR4Rs; and R4 and Rs together with the bonding nitrogen atom
form morpholino (especially preferred);
or Rl is tert-butoxycarbonyl; Bl is a bond; R2 is phenyl; R3 is p-methoxyphenyl; Al is the
bivalent residue of (L)-valine bonded N-terminally to the group -C=O and C-terminally to
A2; A2 is the bivalent residue of p-methoxy-phenylalanine bonded N-tenninally to Al and
C-terminally to the group -NR4R5; and R4 and Rs together with the bonding nitrogen atom
form morpholino;
or R1 is tert-butoxycarbonyl; Bl is a bond; R2 is phenyl; R3 is p-fluorophenyl; A1 is the :
bivalent residue of (L)-valine bonded N-terminally to the group -C=O and C-terminally to :.
A2; A~ is the bivalent residue of phenylalanine bonded N-terminally to A1 and
C-terrninally to the group -NR4Rs; and R4 and Rs together with the bonding nitrogen atom
form moIpholino;
':
or R1 is tert-butoxycarbonyl; B1 is a bond; R2 is phenyl; R3 is p-fluorophenyl; A1 is the
bivalent residue of (L)-valine bonded N-terminally to the group -C=O and C-terminally to -
A2; A2 is the bivalent residue of p-methoxy-phenylalanine bonded N-terminally to Al and
C-terminally to the group -NR4Rs; and R4 and Rs together with the bonding nitrogen atom :~
form morpholino;
or Rl is tert-butoxycarbonyl; Bl is a bond; R2 is phenyl; R3 is p-methoxyphenyl; A1 is the
bivalent residue of (L)-valine bonded N-teIminally to the group -C=O and C-terminally to
A2; A2 is the bivalent residue of phenylalanine bonded N-terminally to A1 and
C-terminally to the group -NR4Rs; and R4 and Rs together with the bonding nitrogen atom
forrn ~norpholino ~especially prefeIred);
and phannaceutically acceptable salts thereof where salt-forming groups are present.
Greatest preference is given to all compounds of formula I' or the individual compounds
of formula I' mentioned in the Examples and the pharrnaceutically acceptable salts thereof
where salt-forming groups are present.
.
,~. . , , . : . . .:
,. ~ , . . ~. .. .
.~ .~ ' ':,`~ ' ' :. . .'!,'
,.,'! . , ' , , ~,. : ' ' ' '
. , . ' ' "'.; , '
'-;i, ' ' , '
" ~ . ,
,,'.' ' , :` '' .~ ' ' . '
2 ~ 6 1
- 41 -
The compounds of formula I' and salts of those compounds having at least one salt-
forming group are obtained in accordance with processes known per se, for example as
follows:
a) for the preparation of a compound of fonnula
" .
R~ ~N~ 2 Rs (Ib ),
R2
wherein Rl' has the meanings given for Rl in compounds of formula I' with the exception
of hydrogen, the hydroxy group present at the carbon atom adjacent to the carbon atom
carrying the radical R2-CH2- is in free or protected form and the remaining radicals are as
defined for compounds of formula I', an acid of formula : -
Rl'-OH (II),
or a reactive acid derivative thereof, wherein Rl' has the meanings given for Rl in
compounds of formula I' with the exception of hydrogen, is condensed with an amino
compound of forrnula ~ ~:
T
N~A1~A~N\ (III'), ~ ;
P(2 : ~
I
or with a reactive derivative thereof, wherein the radicals are as defined for compounds of :
formula I', free functional groups in the starting mateIials of formulae lI and III', with the
exception of those participating in the reaction, being if necessary in protected form, and,
if desired, any protecting groups present are removed, or ~ -
b) for the preparatlon of a compound of formula
:` 21186~ ~
- 42 -
.
1~ B~ ~ A1` A ' N \ (Ic'),
wherein Bl' may be the same residues as Bl in compounds of formula I' but may not be a
bond, the hydroxy group present at the carbon atom adjacent to the carbon atom carrying
the radical R2-CH2- is in free or protected fonn and the remaining radicals are as defined
for compounds of formula I', a carboxylic acid of formula
Rl-Bl'-OH (IV), :
or a reactive acid derivative thereof, wherein Rl is as defined for compounds of forrnula I'
and Bl' is as last defined, is condensed with an amino compound of fonnula
T
H~ ~A1~ ,N~ (V ), ~ ~
R2 ~ ::
or with a reactive derivative thereof, wherein the radicals are as defined for compounds of
formula I', free functional groups in the star~ng materials of forrnulae IV and V', with the
excepdon of those par~cipating in the reaction, being if necessary in protected form, and,
if desired, any protecting groups present are removed, or :
c) a carboxylic acid of formula
T R3
B1 ~OH (VI~),
R2
or a reactive derivative thereof, wherein the radicals are as defined for compounds of
..... . . . ~ . . . . ~
~.,, .:
.~ .~ , . . ~ .; .,
--``` 2118~1
- 43 -
fonnula I', is condensed with an amino compound of formula
H/ `A'N\ (VII),
or with a reactive derivative thereof, wherein the radicals are as defined for compounds of
formula I', free functional g~oups in the starting materials of formulae VI' and VII, with
the exception of those participating in the reaction, being if necessary in protected forrn, :
and, if desired, any protecting g~oups present are removed, or
d) for the preparation of a compound of formula ~ :
.
Rl N~ f~A,~ ~ \ (Id'),
: :
.
wherein Al' and A2' are as de~fined for Al and A2 in compounds of f~rmula I', but Al' is
not a bond and the peptide bond between Al ' and A2' is not in reduced form, the hydroxy ~ ~
group present at the carbon atom adjacent to the carbon atom carrying the radical R2-CH2- : `
is in free or protected form and the remaining radicals are as defined for compounds of
formula I', a carboxylic acid of formula
T\ R3 ~:
H O ~
Rl~ ~N `~ ~ ` OH (VIII'),
R2 . '~
~`` 211~61
- 44 -
or a reactive derivative thereof, wherein the radicals are as last de~med, is condensed with : :
an amino compound of formula -
H--A2'--N ' 4 ::
Rs .
or with a reactive derivative thereof, wherein the radicals are as last defined, free ~ ~
functional groups in the starting materials of forrnulae VIII' and IX, with the exception of ~-
those participating in the reaction, being if necessary in protected form, and, if desired, :
any protecting groups present are removed, or
e) a carboxylic acid of formula
T R3 -
B1 ~A1-`A2 OH (X'),
O
R2
or a reactive derivative thereof, wherein the radicals are as def;ned for compounds of
formula I', is condensed with an amino compound OI formula
H ~ N ~ (XI),
R5
or with a reactive derivative thereof, wherein the radicals are as defined for compounds of
forrnula I', free functional groups in the starting materials of formulae X' and XI, with the
exception of those participating in the reiaction, being if necessary in protected form, and,
if desired, any protecting groups present are removed, or
'r.~ ,, . . " , ':
!'i.~ r~ ~r~ " ' "
211~61 ~:
- 45 -
f) in a compound of formula I' wherein the substituents are as defined above with the
proviso that in the compound of fonnula I' in question at least one functional group is
protected by protecting groups, the protecting groups are removed, or
g~ a compound of formula I
H OH ~ R4
`~A1~ ,N~ (I)
s
wherein the radicals are as defined for compou.nds of formula I', is reacted with a ~ :
carboxylic acid of formula XXV : :
T-OH (XXV),
wherein T is as defined for compounds of forrnula I', or with a reactive acid derivative
thereof, free functional groups in the starting materials of formulae XXV and I, with the
exception of those participating in the reaction, being if necessary in protected form, and,
if desired, any protecting groups present are removed, or
h) for the preparation of a compound of formula I' wherein T is a radical of formula Z
wherein R~ is lower alkyl substituted by etherified or esterified hydroxy or mercapto, by
unsubstituted or substituted amino or by heterocyclyl bonded via nitrogen, and the ~ ~ .
remaining radicals are as defined for compounds of formula I', a compound of formula
Wl
(C mH2m)
o - C R3
Rl~ ,N~Al.A N~
R2 ~
wherein Wl is a leaving group, -(CmH2m) is a lower alkylene radical (m is from 1 to 7) : :
2 ~
- 46 -
and the remaining radicals are as defined for compounds of formula I', is reacted with a
compoundoffonnula
L-H (XXVII),
wherein L is etherified or esterified hydroxy or mercapto, unsubstituted or substituted
amino, or heterocyclyl bonded via nitrogen, or with a reactive derivative thereof, free
functional groups in the starting materials of formulae XXVI and XXVII, with theexception of those participating in the reaction, being if necessary in protected form, and,
if desired, any protecting groups present are -removed,
and/or, if desired, a compound of formula I' obtainable in accordance with any one of the
above processes a) to h) having at least one salt-forming group is converted into its salt
and/or an obtainable salt is converted into the free compound or into a different salt and/or
isomeric mixtures of compounds of formula I' which may be obtainable are separated
andlor a compound of forrnula I' according to the invention is converted into a different
compound of formula I' according to the invention.
The above-defined processes are described in detail below:
Process a) (Formation of an amide bond)
In starting materials of formulae II and III', functional groups, with the exception of
groups that are intended to participate in the reaction or that do not react under the
reaction conditions, are protected independently of one another by protecdng groups.
Protecting groups for functional groups in starting materials the reaction of which is to be
avoided, especially carboxy, amino, hydroxy, mercapto and sulfo groups, include espe-
cially those protecting groups (conventional protecting groups) which are customarily
used in the synthesis of peptide compounds, and also in the synthesis of cephalosporins
and penicillins as ~vell as nucleic acid derivatives and sugars. Those protecting groups
may already be present in the precursors and are intended to protect the functional groups
in question against undesired secondary reactions, such as acylation, etherification, esteri-
fication, oxidation, solvolysis, etc.. In certain cases the protecting groups can additionally
---`` 2118661
- 47 -
cause the reactions to proceed selectively, for example stereoselectively. It is character-
iStiC of protecting group~ that they can be removed easily, i.e. without undesired secondary
reactions taking place, for example by solvolysis, reduction, photolysis, and also enzymat-
ically, for example also under physiological conditions. Protecting groups may also be
present in the end products, however. Compounds of formula I' having protected
functional groups may have grcater metabolic stability or pharmacodynamic properties - -
that are better in some other way than the corresponding compounds having free
functional groups.
The protection of functional groups by such protecting groups, the protecting groups them-
selves and the reactions for their removal are described, for example, in standard works ~ -
such as J. F. W. McOmie, "Protective Groups in Organic Chemistry", Plenum Press, -
London and New York 1973, in Th. W. Greene, "Protective Groups in Organic Synthesis",
Wiley, New York 1981, in "The Peptides", Volume 3 (E. Gross and J. Meienhofer, eds.),
Academic Press, London and New York 1981, in "Methoden der organischen Chemie",
Houben-Weyl, 4th edition, Volume 15/I, Georg Thieme Verlag, Stuttgart 1974, in H.-D.
Jakubke and H. Jescheit, "Aminosauren, Peptide, Proteine" ("Amino acids, peptides,
proteins"), Verlag Chemie, Weinheim, Deerfield Beach and Basle 1982, and in Jochen
Lehmann, "Chemie der Kohlenhydrate: MonosacchaTide und Derivate" ("The Chemistryof Carbohydrates: monosaccharides and derivatives"), Georg Thieme Verlag, Stuttgart
1974. -
A carboxy group is protected, for example, in the form of an ester group which can be
cleaved selectively under mild conditions. A carboxy group protected in esterified foIm is ~ -
esterified especially by a lower alkyl group that is preferably branched in the l-position of
the lower alkyl group or substituted in the 1- or 2-position of the lower alkyl group by suit-
able substituents.
A protected carboxy group esterified by a lower alkyl group is, for example, methoxy-
carbonyl or ethoxycarbonyl.
A protected carboxy group esterified by a lower alkyl group that is branched in the
l-position of the lower alkyl group is, for example, tert-lower alkoxycarbonyl, for example
tert-butoxycarbonyl. -
A protected carboxy group esterified by a lower alkyl group that is substituted in the 1- or
;, : .... .. ,, . . . .. - . . . . .
2 ~
- 48 -
2-position of the lower alkyl group by suitable substinlents is, for example, arylmethoxy-
carbonyl having one or two aryl radicals, wherein aryl is phenyl that is unsubstituted or
mono-, di- or tri-substituted, for example, by lower alkyl, for example tert-lower alkyl,
such as tert-butyl, lower alkoxy, for example methoxy, hydroxy, halogen, for example
chlorine, and/or by nitro, for example benzyloxycarbonyl, benzyloxycarbonyl substitute~ -
by the mentioned substituents, for example 4-nitrobenzyloxycarbonyl or 4-methoxy-
benzyloxycarbonyl, diphenylmethoxycarbonyl or diphenylmethoxycarbonyl substituted by
the mentioned substituents, for example di(4-methoxyphenyl)methoxycarbonyl, and also
carboxy esterified by a lower alkyl group, the lower alkyl group being substituted in the 1-
or 2-position by suitable substituents, such as l-lower aL~coxy-lower alkoxycarbonyl, for
example methoxymethoxycarbonyl, l-methoxyethoxycarbonyl or l-ethoxyethoxy-
carbonyl, l-lower alkylthio-lower alkoxycarbonyl, for example l-methylthiomethoxy-
carbonyl or l-ethylthioethoxycarbonyl, aroylmethoxycarbonyl wherein the aroyl group is
benzoyl that is unsubstituted or substituted, for example, by halogen, such as bromine, for
example phenacyloxycarbonyl, 2-halo-lower alkoxycarbonyl, for example 2,2,2-trichloro-
ethoxycarbonyl, 2-bromoethoxycarbonyl or 2-iodoethoxycarbonyl, as well as 2-(tri-substi-
tuted silyl)-lower alkoxycarbonyl wherein the substituents are each independently of the
others an aliphatic, araliphatic, cycloaliphatic or aromatic hydrocarbon radical that is
unsubstituted or substituted, for example, by lower alkyl, lower alkoxy, aryl, halogen
and/or by nitro, for example lower alkyl, phenyl-lower alkyl, cycloalkyl or phenyl each of
which is unsubstituted or substituted as above, for example 2-tri-lower alkylsilyl-lower
alkoxycarbonyl, such as 2-tri-lower alkylsilylethoxycarbonyl, for example 2-trimethyl-
silylethoxycarbonyl or 2-(di-n-butyl-methyl-silyl)-ethoxycarbonyl, or 2-triarylsilylethoxy-
carbonyl, such as triphenylsilylethoxycarbonyl.
A carboxy group may also be protected in the form of an organic silyloxycarbonyl group.
An organic silyloxycarbonyl group is, for example, a tri-lower alkylsilyloxycarbonyl
group, for example trimethylsilyloxycarbonyl. The silicon atom of the silyloxycarbonyl
group can also be substituted by two lower alkyl groups, for example methyl groups, and
by an amino group or carboxy group of a second molecule of formula I'. Compoundshaving such protecting groups can be prepared, for example, using dimethylchlorosilane
as silylating agent.
A carboxy group may also be protected in the form of an internal ester with a hydroxy
group present in the molecule suitably spaced from the carboxy group, for example in the
~-position with respect to the carboxy group, that is to say in the form of a lactone, prefer-
; . . . ~ ~
., ~ ~ ,~ , . . .
2118~
- 49 -
ably a ~-lactone.
A protected carboxy group is preferably tert-lower alkoxycarbonyl, for example tert-but- ~ - -
oxycarbonyl, benzyloxycarbonyl, 4-nitrobenzyloxycarbonyl, 9-fluorenylmethoxycarbonyl ~ -
or diphenylmethoxycarbonyl, or a carboxy group protected in the form of a lactone, espe- ~ ~ :
cially a ~-lactone.
A protected amino group may be protected by an amino-protecting group, for example in
the form of an acylamino, arylmethylamino, etherified mercaptoamino, 2-acyl-lower
aL~c-1-enylamino or silylamino group or in the form of an azido group.
In an acylamino group, acyl is, for example, the acyl radical of an organic carboxylic acid
having, for example, up to 18 carbon atoms, especially an unsubstituted or substituted, for
example halo- or aryl~substituted, lower alkanecarboxylic acid or an unsubsdtuted or
substituted, for example halo-, lower alkoxy- or nitro-substituted, benzoic acid, or, prefer-
ably, of a carbonic acid semiester. Such acyl groups are preferably lower aLkanoyl, such ;
as formyl, acetyl, propionyl or pivaloyl, halo-lower aLkarloyl, for example 2-haloacetyl,
such as 2-chloro-, 2-bromo-, 2-iodo-, 2,2,2-~ifluoro- or 2,2,2-trichloro-acetyl, unsubsti-
tuted or substituted, for example halo-, lower aLkoxy- or nitro-substituted, benzoyl, such as
benzoyl, 4-chlorobenzoyl, 4-methoxybenzoyl or 4-nitrobenzoyl, lower alkoxycarbonyl,
preferably lower alkoxycarbonyl that is branched in the 1-position of the lower alkyl
radical or suitably substituted in the 1- or 2-position, for example tert-lower aLkoxy-
carbonyl, such as tert-butoxycarbonyl, arylmethoxycarbonyl having one, two or three aryl
radicals which are phenyl that is unsubsdtuted or mono- or poly-substituted, for exarnple,
by lower alkyl, especially tert-lower alkyl, such as tert-butyl, lower alkoxy, such as
methoxy, hydroxy, halogen, such as chlorine, and/or by nitro, for example benzyloxy-
carbonyl, 4-nitrobenzyloxycarbonyl, diphenylmethoxycarbonyl, 9-fluorenylmethoxy-carbonyl or di(4-methoxyphenyl)methoxycarbonyl, aroylmethoxycarbonyl wherein thearoyllgroup is preferably benzoyl that is unsubstituted or substituted, for example, by
halogen, such as bromine, for example phenacyloxycarbonyl, 2-halo-lower alkoxy~
carbonyl, for example 2,2,2-trichloroethoxycarbonyl, 2-bromoethoxycarbonyl or 2-iodo~
ethoxycarbonyl, 2-(tri-substituted silyl)-lower alkoxycarbonyl, for example 2-tri-lower
alkylsilyl-lower alkoxycarbonyl, such as 2-trimethylsilyle~hoxycarbonyl or 2-(di-n-butyl-
methyl-silyl)-ethoxycarbonyl, or triarylsilyl-lower alkoxycarbonyl, for example 2-tri-
phenylsilylethoxycarbonyl.
- 211~
5()
In an arylmethylamino group, for example a mono-, di- or especially tri-arylmethylamino
group, the aryl radicals are especially unsubstituted or substituted phenyl radicals. Such
groups are, for example, benzyl-, diphenylmethyl- or especially trityl-amino.
In an etherified mercaptoamino group the mercapto group is especially in the form of
substituted arylthio or aryl-lower aLkylthio, wherein aryl is, for example, phenyl that is
unsubstituted or substituted, for éxample, by lower alkyl, such as methyl or tert-butyl,
lower alkoxy, such as methoxy, halogen, such as chlorine, and/or by nitro, for example
4-nitrophenylthio.
In a 2-acyl-lower alk-l-enyl radical that can be used as an amino-protecting group, acyl is,
for example, the corresponding radical of a lower alkanecarboxylic acid, of a benzoic acid
that is unsubstituted or substituted, for example, by lower alkyl, such as methyl or tert-
butyl, lower alkoxy, such as methoxy, halogen, such as chlorine, and/or by nitro, or espe-
cially of a carbonic acid semiester, such as a carbonic acid lower alkyl semiester. Corres-
ponding protecting groups are especially l-lower alkanoyl-lower alk-l-en-2-yl, for
example l-lower alkanoyl-prop-l-en-2-yl, such as l-acetyl-prop-l-en 2-yl, or lower
aLkoxycarbonyl-lower alk-l-en-2-yl, for example lower aLkoxycarbonyl-prop-l-en-2,-yl,
such as l-ethoxycarbonyl-prop-1-en-2-yl.
A silylamino group is, for example, a tri-lower alkylsilylamino group, for example
trimethylsilylamino or tert-butyl-dimethylsilylamino. The silicon atom of the silylamino
group can also be substituted by only two lower alkyl groups, for example methyl groups,
and by the amino group or carboxy group of a second molecule of formula I'. Compounds
having such protecting groups can be prepared, for example, using the corresponding
chlorosilanes, such as dimethylchlorosilane, as silylating agents.
An amino group can also be protected by conversion into the protonated forrn; suitable
corresponding anions are especially those of strong inorganic acids, such as sulfuric acid,
phosphoric acid or hydrohalic acids, for example the chlorine or bromine anion, or of
organic sulfonic acids, such as p-toluenesulfonic acid.
Preferred a nino-protecting groups are lower alkoxycarbonyl, phenyl-lower alkoxycar-
bonyl, fluorenyl-lower alkoxycarbonyl, 2-lower alkanoyl-lower alk-1-en-2-yl and lower
alkoxycarbonyl-lower alk-l-en-2-yl, especially tert-butoxycarbonyl and benzyloxy-
carbonyl.
~ 2~18661 ~ -
- 51 -
A hydroxy group can be protected, for example, by an acyl group, for example lower
alkanoyl that is unsubstituted or substituted by halogen, such as chlorine, such as acetyl or
2,2-dichloroacetyl, or especially by an acyl radical of a carbonic acid semiester mentioned
for protected amino groups. A hydroxy group can also be protected by tri-lower alkylsilyl,
for example trimethylsilyl, triisopropylsilyl or tert-butyl-dimethylsilyl, a readily -
removable etherifying group, for example an alkyl group, such as tert-lower alkyl, for
example tert-butyl, an oxa- or a thia-aliphatic or -cycloaliphatic, especially 2-oxa- or
2-thia-aliphatic or -cy loaliphatic, hydrocarbon radical, for example 1-lower aL~toxy-lower
alkyl or l-lower alkylthio-lower alkyl, such as methoxymethyl, 1-methoxyethyl, 1-ethoxy-
ethyl, methylthiomethyl, 1-methylthioethyl or 1-ethylthioethyl, or 2-oxa- or 2-thia-cyclo-
aL~cyl having f.rom S to 7 ring atoms, such as 2-tetrahydrofuryl or 2-tetrahydropyranyl, or a ~-
corresponding thia analogue, and also by 1-phenyl-lower alkyl, such as benzyl, diphenyl-
methyl or trityl, wherein the phenyl radicals can be substituted, for example, by halogen,
for exarnple chlorine, lower alkoxy, for example methoxy, and/or by nitro. A preferred
hydroxy-protecting group is, for example, 2,2,2-trichloroethoxycarbonyl, 4-nitrobenzyl-
oxycarbonyl, diphenylmethoxycarbonyl, benzyl or trityl.
Two hydroxy groups, especially adjacent hydroxy groups, occurnng in a molecule, or a
hydroxy group and an amino group that are adjacent to one another, can be protected, for
example, by bivalent protecting groups, such as a methylene group that is preferably
substituted, for example, by one or two lower aLkyl radicals or by oxo, for example unsub- -
stituted or substituted alkylidene, for example lower alkylidene, such as isopropylidene,
cycloalkylidene, such as cyclohexylidene, a carbonyl group or benzylidene.
A hydroxy group adjacent to a carboxy group can be protected by the formation of an
internal ester (lactone), especially a ~-lactone.
Preferably a protected hydroxy group is protected by tri-lower alkylsilyl or in the forrn of a
lactone, especially by tert-butyl-dimethyl-silyl or in the form of a ~-lac~one.
A mercapto group, for example in cysteine, can be protected especially by S-aLkylation
with unsubstituted or substituted alkyl radicals, by silylation, by $hioacetal formation, by
S-acylation or by the forrnation of asymmetric disulfide groupings. Preferred mercapto-
protecting groups are, for example, benzyl that is unsubstituted or substituted in the phenyl
radical, for example by methoxy or by nitro, such as 4-methoxybenzyl, diphenylmethyl
- 52 - 2 ~ 6 ~
that is unsubstituted or substituted in the phenyl radical, for example by methoxy, such as
dil4-methoxyphenyl)methyl, triphenylmethyl, pyridyldiphenylmethyl, trimethylsilyl,
benzyl~hiomethyl, tetrahydropyranyl, acylaminomethyl, such as acetamidomethyl, iso-
butyrylacetamidomethyl or 2-chloroacetamidomethyl, benzoyl, benzyloxycarbonyl oralkyl-, especially lower alkyl-aminocarbonyl, such as ethylaminocarbonyl, and also lower
alkylthio, such as S-ethylthio or S-tert-butylthio, or S-sulfo.
A sulfo group can be protected, for example, by lower alkyl, for exarnple methyl or ethyl,
by phenyl or in the form of a sulfonamide, for example in the form of an imidazolide.
In the context of this Application, a protecting group, fo~ example a carboxy-protecting
group, is to be understood as being expressly also a polymeric carrier thae is bonded in a
readily removable manner to the functional group, for example the carboxy group, to be
protected, for example a carrier suitable for the Merrifield synthesis. Such a suitable
polymeric carrier is especially a polystyrene resin, weakly cross-linked by copolymerisa-
tion with divinylbenzene, that carries bridge members suitable for reversible bonding.
The acids of forrnula II are carboxylic acids or sulfonic acids.
The carboxylic acids of fonnula II either contain a free carboxy group or are in the form of
a reactive derivative, for example in the form of an activated ester derived from the free
carboxy compound, in the form of a reactive anhydride, or in the form of a reactive cyclic
amide. The reactive derivatives may also be fonned in situ.
Activated esters of compounds of forrnula II having a carboxy group are especially esters
unsaturated at the linking carbon atom of the esterifying radical, for example of the vinyl
ester type, such as vinyl esters (obtainable, for exarnple, by transesterification of a corres-
ponding ester with vinyl acetate; activated vinyl ester method), carbamoyl esters
(obtainable, for example, by treatment of the corresponding acid with an isoxazolium
reagent; 1,2-oxazolium or Woodward method), or 1-lower alkoxyvinyl esters (obtainable,
for example, by treatment of the corresponding acid with a lower alkoxyacetylene; ethoxy-
acetylene method), or esters of the amidino type, such as N,N'-disubstituted arnidino ~ -
esters (obtainable, for example, by treatment of the corresponding acid with a suitable
N,N'-disubstituted carbodiimide, for example N,N'-dicyclohexylcarbodiimide; carbo-
diimide method), or N,N-disubstituted amidino esters (obtainable, for example, by
treatment of the corresponding acid with an N,N-disubstituted cyanamide; cyanamide
:- 211g661 ~ ~.
- 53 -
method), suitable aryl esters, especially phenyl esters substituted by elec~on-attracting
substituents (obtainable, for example, by treatment of the corresponding acid with a
suitably substituted phenol, for example 4-nitrophenol, 4-methylsulfonylphenol, 2,4,5-tri- ~ :
chlorophenol, 2,3,4,5,6-pentachlorophenol or 4-phenyldiazophenol, in the presence of a
condensation agent, such as N,N'-dicyclohexylcarbodiimide; activated aryl estersmethod), cyanomethyl esters (obtainable, for example, by treatment of the corresponding
acid with chloroacetonitrile in the presence of a base; cyanomethyl esters method), thio-
esters, especially unsubstituted or substituted, for example nitro-substituted, phenylthio
esters (obtainable, for example, by treatment of the corresponding acid with unsubstituted
or substituted, for example nitro-substituted, thiophenols, inter alia by the anhydride or :
carbodiimide method; activated thiol esters method), or especially amino or amido esters
(obtainable, for example, by treatment of the corresponding acid with an N-hydroxyamino . ~ :
or N-hydroxyamido compound, for exarnple N-hydroxysuccinimide, N-hydroxypiperidine,
N-hydroxyphthalimide, N-hydroxy-S-norbornene-2,3-dicarboxylic acidimide, l-hydroxy~
benzotriazole or 3-hydroxy-3,4-dihydro-1,2,3-benzotriazin-4-one, for example by the :
anhydride or carbodiimide method; activated N-hydroxy esters method). Internal esters,
for example ~-lactones, can also be used.
Anhydrides of acids may be symmetric or preferably mixed anhydrides of those acids, for : .`
example anhydrides with inorganic acids, such as acid halides, especially acld chlorides :~
(obtainable, for example, by treatrnent of the corresponding acid with thionyl chloride,
phosphorus pentachloride or oxalyl chloride; acid chloride method), azides (obtainable, for
example, from a corresponding acid ester via the corresponding hydrazide and treatment
thereof with nitrous acid; azide method), anhydrides with carbonic acid semiesters, for
example carbonic acid lower alkyl semiesters (obtainable, for example, by treatment of the
corresponding acid with chloroformic acid lower alkyl esters or with a l-lower aL~oxy-
carbonyl-2-lower alkoxy-1,2-dihydroquinoline; mixed O-aLkylcarbonic acid anhydrides
method), or anhydrides with dihalogenated, especially dichlorinated, phosphoric acid
(obtainable, for example, by treatment of the corresponding acid with phosphorusoxychloride; phosphorus oxychloride method), anhydrides with other phosphoric acid
derivatives (for example those obtainable with phenyl-N-phenylphosphoramidochloridate
or by reaction of alkylphosphoric acid arnides in the presence of sulfonic a~id anhydrides
and/or racemisation-reducing additives, such as N-hydroxybenzotriazole, or in the
presence of cyanophosphonic acid diethyl ester) or with phosphorous acid derivatives, or
anhydrides with organic acids, such as rnixed anhydrides with organic carboxylic acids
(obtainable, for example, by treatment of the corresponding acid with an unsubstituted or
': I '
~-` 211~Sl
- 54 -
substituted lower alkane- or phenyl-lower alkane-ca~boxylic acid halide, ror example
phenylacetic acid chloride, pivalic acid chloride or trifhJoroacetic acid chloride; mixed
carboxylic acid anhydrides method) or with organic sulfonic acids (obtainable, for
example, by treatment of a salt, such as an alkali metal salt, of the corresponding acid with
a suitable organic sulfonic acid halide, such as a lower alkane- or aryl-, for example
methane- or p-toluene-sulfonic acid chloride; mixed sulfonic acid anhydrides method) and
symmetric anhydrides (obtainable, for example, by condensation of the corresponding acid
in the presence of a carbodiimide or l-diethylaminopropyne; symmetric anhydridesmethod).
Suitable cyclic arnides are especially amides having five-membered diazacycles of
aromatic character, such as amides with imidazoles, for example imidazole (obtainable,
for example, by treatment of the corresponding acid with N,N'-carbonyldiimidazole;
imidazole method), or pyrazole, for exarnple 3,5-dimethylpyrazole (obtainable, for
example, via the acid hydrazide by treatment with acetylacetone; pyrazolide method).
As mentioned, derivatives of carboxylic acids that are used as acylating agents may also
be forrned in situ. For example, N,N'-disubstituted amidino esters may be formed in situ
by reacting, for example in the presence of a suitable base, such as triethylamine, a
mixture of the starting material of formula lII' and the acid of formula II used as acylating
agent, in the presence of a suitable N,N'-disubstituted carbodiimide, for example
N,N'-cyclohexylcarbodiimide. In addition, amino or amido esters of the acids used as
acylating agents may be formed in the presence of the starting material of formula III' to
be acylated, by reacting a mixture of the corresponding acid and amino starting materials
in the presence of an N,N'-disubstituted carbodiimide, for example N,N'-dicyclohexyl-
carbodiimide, and of an N-hydroxyamine or N-hydroxyamide, for example N-hydroxy-succinimide, where appropriate in the presence of a suitable base, for example 4-dimethyl-
amino-pyridine. Moreover, activation in situ can be achieved by reaction with
N,N,N',N'-tetraalkyluronium compounds, such as O-benzotriazol-1-yl-N,N,N',N'-tetra-
methyluronium hexafluorophosphate~ Finally, phosph~ic acid anhydrides of the
carboxylic acids of formula II can be prepared in situ by reacting an alkylphosphoric acid
amide, such as hexamethylphosphoric acid triamide, in the presence of a sulfonic acid
anhydride, such as 4-toluenesulfonic acid anhydride, with a salt, such as a tetrafluoro- ~ -
borate, for example sodium tehafluoroborate, or with another derivative of hexamethyl- ~-
phosphoric acid triamide, such as benzotriazol-l-yl-oxy-tris(dimethylamino)phosphonium
hexafluoride, preferably in the presence of a racemisation-reducing additive, such as
--` 21186~1
N-hydroxybenzotriazole.
The amino group of compounds of forrnula III' that participates in the reaction preferably
carries at least one reactive hydrogen atom, especially when the carboxy group reacting
therewith is present in reactive form; it may, however, itself have been derivatised, for
example by reaction with a phosphite, such as diethylchlorophosphite, 1,2-phenylene-
chlorophosphite, ethyl dichlorophosphite, ethylenechlorophosphite or tetraethylpyro-
phosphite. A derivative of such a compound having an amino group is, for example, also
a carbamic acid halide, the amino group that participates in the reaction being substituted
by halocarbonyl, for example chlorocarbonyl.
Condensation to form an amide bond can be carried out in a manner known ~ se, for
example as described in standard works, such as Houben-Weyl, "Methoden der organ-
ischen Chemie", 4th edition, Volume 15/II (1974), Volume IX (1955), Volume E 11
(1985), Georg Thieme Verlag, Stuttgart, "The Peptides" (E. Gross and J. Meienhofer,
eds.), Volumes 1 and 2, Academic Press, London and New York, 1979/1980, or
M. Bodansky, "Principles of Peptide Synthesis", Springer-Verlag, Berlin 1984.
The condensation of a free carboxylic acid with the corresponding amine can be carried
out preferably in the presence of one of the customary condensation agents. Customary
condensation agents are, for example, carbodiimides, for example diethyl-, dipropyl-,
N-ethyl-N'-(3-dimethylaminopropyl)-carbodiimide or especially dicyclohexylcarbo-diimide, also suitable carbonyl compounds, for example carbonylimidazole, 1,2-
oxazolium compounds, for example 2-ethyl-5-phenyl-1,2-oxazolium 3'-sulfonate and2-tert-butyl-5-methylisoxazolium perchlorate, or a suitable acylamino compound, for
example 2-ethoxy 1-ethoxycarbonyl-1,2-dihydroquinoline, N,N,N',N'-tetraaL~cyluronium
compounds, such as O-benzotriazol-1-yl-N,N,N',N'-tetramethyluronium hexafluoro-
phosphate, also activated phosphoric acid derivatives, for example diphenylphosphoryl
azide,l diethylphosphoryl cyanide, phenyl-N-phenylphosphoroamidochloridate, bis(2-oxo-
3-oxazolidinyl)phosphinic acid chloride or 1-benzotriazolyloxy-tris(dimethylamino)-
phosphonium hexafluorophosphate.
In a manner analogous to the types of reaction mentioned for condensation for the
carboxylic acids of forrnula Il, in the condensation the sulfonic acids of forrnula II having
a terminal sulfonyl group may also be reacted with compounds of formula III' to form the
corresponding sulfonamides of formula Ib'.
,s - , . . ........... .
," ,~
2 ~
- 56-
For example, it is possible to use activated sulfonic acid esters, for example the corres-
ponding aryl esters, such as phenyl esters, especially those substituted by nitro groups, it
being possible for the amine component of forrnula Ib' also to be used in the form of an -
alkali metal amide, for example an alkali metal arylamide, such as sodium anilineamide.
or an alkali metal salt of nitrogen-containing heterocycles, for example potassium
pyrrolide.
It is also possible to use reactive anhydrides, such as the corresponding symmetric acid
anhydrides (which can be prepared, for example, by reaction of the alkylsulfonic acid
silver salts with alkylsulfonyl chlorides) or, preferably, the corresponding asymmetric acid
anhydrides, for exa nple anhydrides with inorganic acids, such as sulfonyl halides, espe-
cially sulfonyl chlorides (obtainable, for example, by reaction of the corresponding
sulfonic acids with inorganic acid chlorides, for example thionyl chloride, sulfuryl
chloride or phosphorus pentachloride), with organic carboxylic acids (obtainable, for
example, by treatment of a sulfonic acid halide with the salt of a carboxylic acid, such as
an alkali metal salt, analogously to the above-mentioned method for the preparation of
mixed acid anhydrides), or azides (obtainable, for example, from a corresponding sulfonic
acid chloride and sodium azide or via the corresponding hydrazide and treatment thereof
with nitrous acid analogously to the above-mentioned azide method).
If desired, an organic base may be added, for exarnple a tri-lower alkylamine having bulky
radicals, such as ethyl diisopropylamine, and/or a heterocyclic base, for example pyridine,
4-dimethylaminopyridineorpreferablyN-methylmorpholine. ~ -
The condensation of activated esters, reactive anhydrides or reactive cyclic amides with
the corresponding amines is customarily carried out in the presence of an organic base, for
example simple tri-lower alkylamines, for exarnple triethylamine or tributylamine, or one
of the above-mentioned organic bases. If desired, a condensation agent is additionally
used, for example as described for free carboxylic acids.
The condensation of acid anhydrides with amines can be effected, for exarnple, in the
presence of inorganic carbonates, for example ammonium or alkali metal carbonates or
hydrogen carbonates, such as sodium or potassium carbonate or hydrogen carbonate(usually together with a sulfate), while the reaction of sulfonic acid halides, such as
sulfonic acid chlorides, can be carried out in the presence of hydroxides, for example
211~6~1
-57-
alkali metal hydroxides, such as sodium hydl oxide or potassium hydroxide.
Carboxylic acid chlorides, for example the chlorocarbonic acid derivatives derived from
the acid of formula II, are condensed with the corresponding amines preferably in the
presence of an organic amine, for example the above-mentioned tri-lower alkylamines or
heterocyclic bases, where appropriate in the presence of a hydrogen sulfate.
The condensation is preferably carried out in an inert, aprotic, preferably anhydrous,
solvent or solvent mixture, for example in a carboxylic acid arnide, for exampleformamide or dimethylformamide, a halogenated hydrocarbon, for example methylenechloride, carbon tetrachloride or chlorobenzene, a ketone, for example acetone, a cyclic
ether, for example tetrahydrofuran, an ester, for example ethyl acetate, or a nitrile5 for
example acetonitrile, or in a mixture thereof, as appropriate at reduced or elevated
temperature, for example in a temperature range of from approximately -40C to approx-
imately +100C, preferably from approximately -10C to approximately +50C, and
without an inert gas or under an inert gas atmosphere, for example a nitrogen or argon ~ ~ -
atmosphere.
Aqueous, for example alcoholic, solvents, for example ethanol, or aromatic solvents, for
example benzene or toluene, may also be used. When alkali metal hydroxides are present
as bases` acetone can also be added where appropriate.
The condensation can also be carried out in accordance with the technique known as
solid-phase synthesis which originates from R. Merrifield and is described, for exarnple, in
Angew. Chem. 97, 801 - 812 (1985), Naturwissenschaften 71, 252 - 258 (1984) or in R. A.
Houghten, Proc. Natl. Acad. Sci. USA 82, 5131 - 5135 (1985).
The freeing of functional groups protected by protecting groups in the resultingcompounds of formula I' having protected functions is effected in accordance with one or
more of the methods mentioned under Process f).
. .
Process b) (Formation of an amide bond)
' '
In starting materials of formulae IV and V', functional groups, with the exception of the
groups that are intended to participate in the reaction or that do not react under the
reaction conditions, are protected independently of one another by protecting groups.
- ~ ' - ' . . ' :. : -
2118~61
- ~8 -
The protecting groups, the free carboxylic acids and the reactive derivatives thereof, the
free amines and the reactive derivatives thereof and the processes used for condensation
are entirely analogous to those described under Process a) for the fonnation of an amide
bond starting from compounds of formulae II and III' except that carboxylic acids of
formula IV are used instead of those of formula II and amino compounds of formula V'
are used instead of those of formula III'.
The freeing of functional groups protected by protecting groups in the resultingcompounds of formula I' having protected functions is effected in accordance with one or
more of the methods mentioned under Process f).
Process c) (Formation of an amide bond)
In starting materials of formulae VI' and VII, functional groups, with the exception of the
groups that are intended to participate in the reaction or that do not react under the
reaction conditions, are protected independently of one another by protecting groups.
The protecting groups, the free carboxylic acids and the reactive derivatives thereof, the ;~
free amines and the reactive derivatives thereof and the processes used for condensation
are entirely analogous to those described under Process a) for the formation of an amide
bond starting from compounds of forrnulae II and lII' except that carboxylic acids of
foTmula VI' are used instead of those of formula II and amino compounds of for nula VII -
are used instead of those of formula III'.
The freeing of functional groups protected by protecting groups in the resuitingcompounds of formula I' having protected functions is effected in accordance with one or
more of the methods mentioned under Process f).
I
Process d) (Formation of an amide bond)
In starting materials of formulae VIII' and IX, functional groups, with the exception of the
groups that are intended to participate in the reaction or that do not react under the
reaction conditions, are protected independently of one another by protecting groups.
The protecting groups, the free~carboxylic acids and the reactive derivatives thereof, the ~ -
-~ 211g6~1 .
- s9 -
free amines and the reactive derivatives thereof and the processes used for condensation
are entirely analogous to those described under Process a) for the formation of an amide
bond starting from compounds of formulae II and III' except that carboxylic acids of
formula VIII' are used instead of those of formula II and amino cornpounds of formula IX
are used instead of those of formula III'.
The freeing of functional groups protected by protecting groups in the resultingcompounds of formula I' having pr~tected functions is effected in accordance with one or
more of the methods mentioned under Process f).
Process e) (Forrnation of an amide bond)
In starting materials of formulae X' and XI, functional groups, with the exception of the
groups that are intended to participate in the reaction or that do not react under the
reacdon conditions, are protected independently of one another by protecting groups.
The protecting groups, the free carboxylic acids and the reactive derivatives thereof, the
free amines and the reactive derivatives thereof and the processes used for condensation
are entirely analogous tO those described under Process a) for the formation of an amide
bond starting from compounds of forrnulae II and III' except that carboxylic acids of
formula X' are used inst.ead of those of formula II and amino compounds of formula XI
are used instead of those of formula III'.
A reactive derivative of such a compound of formula XI having an amino group is, for
example, also an isocyanate in which the amino group participating in the reaction has
been modified in the form of an isocyanate group, in that case there being obtainable only
compounds of formula I' that carry a hydrogen atom at the nitrogen atom of the amide
group formed by the reacdon.
The freeing of functional groups protected by protecting groups in the resultingcompounds of formula I' having protected functions is effected in accordance with one or
more of the methods mentioned under Process f).
Process f) (Removal of protecting groups)
The removal of protecting groups that are not constituents of the desired end product of
~", , "` "' .", '' , `'
" ~ ' ' ' '` ' ' ', .
2118661
- 60-
formula I', for example the carboxy-, amino-, hydroxy-, mercapto- and/or sulfo-protecting
groups, is effected in a manner known per se, for example by means of solvolysis, espe-
cially hydrolysis, alcoholysis or acidolysis, or by means of reduction, especially hydro-
genolysis or by means of other reducing agents, as well as photolysis, as appropriate
stepwise o~ simultaneously, it being possible also to use enzymatic methods. The removal
of the protecting groups is described, for example, in the standard works mentioned
hereinabove in the section relating to protecting groups.
.
For example, protected carboxy, for example tert-lower alkoxycarbonyl, lower alkoxy-
carbonyl substituted in the 2-position by a ~isubstituted silyl group or in the 1-position by
lower alkoxy or by lower alkylthio, or unsubstituted or substituted diphenylmethoxy-
carbonyl can be converted into free carboxy by treatment with a suitable acid, such as
formic acid, hydrogen chloride or trifluoroacetic acid, where appropriate with the addition
of a nucleophilic compound, such as phenol or anisole. Unsubstituted or substituted -
benzyloxycarbonyl can be freed, for example, by means of hydrogenolysis, i.e. bytreatment with hydrogen in the presence of a metal hydrogenation catalyst, such as a
palladium catalyst. In addition, suitably substituted benzyloxycarbonyl, such as 4-nitro-
benzyloxycarbonyl, can be converted into free carboxy also by reduction, for example by - ~ -
treatment with an aLkali metal dithionite, such as sodium dithionite, or with a reducing
metal, for example zinc, or a reducing metal salt, such as a chromium(II) salt, for exarnple
chromium(II) chloride, customarily in the presence of a hydrogen-yielding agent that, ~ -
together with the metal, is capable of producing nascent hydrogen, such as an acid, espe-
cially a suitable carboxylic acid, such as an unsubstituted or substituted, for example
hydroxy-subsdtuted, lower alkanecarboxylic acid, for example acetic acid, formic acid,
glycolic acid, diphenylglycolic acid, lactic acid, mandelic acid, 4-chloromandelic acid or
tartaric acid, or in the presence of an alcohol or thiol, water preferably being added~ By
treatment with a reducing metal or metal salt, as described above, 2-halo-lower alkoxy-
carbonyl (where appropriate after conversion of a 2-bromo-lower alkoxycarbonyl group
into al corresponding 2-iodo-lower alkoxycarbonyl group) or aroylmethoxycarbonyl can
also be con~erted into free carboxy. Aroylmethoxycarbonyl can be cleaved also by treat- ~ - -
ment with a nucleophilic, preferably salt-forming, reagent, such as sodium thiophenolate
or sodium iodide. 2-(tri-substituted silyl)-lower alkoxycarbonyl, such as 2-tri-lower alkyl-
silyl-lower alkoxycarbonyl, can also be converted into free carboxy by treatment with a
salt of hydrofluoric acid that yields the fluoride anion, such as an alkali metal fluoride, for
example sodium or potassium fluoride, where appropriate in the presence of a macrocyclic
polyether ("crown ether"), or with a fluoride of an organic quaternary base, such as tetra-
211~fi6;~ :
- 61 -
lower alkylammonium fluoride or tn-lower alkylaryl-lower alkylammonium fluolide, for
example tetraethylammonium fluoride or tetrabutylammonium fluoride, in the presence of
an aprotic, polar solvent, such as dimethyl sulfoxide or N,N-dimethylacetamide. Carboxy
protected in the form of organic silyloxycarbonyl, such as tri-lower aLt~ylsilyloxycarbonyl,
for example trimethylsilyloxycarbonyl, ean be freed in cùstomary manner by solvolysis,
for example by treatment with water, an alcohol or an acid, or, furthermore, a fluoride, as
described above. Esterified carboxy can also be freed enzymatically, for example by
means of esterases or suitable peptidases, for example esterified arginine or lysine, such as
lysine methyl ester, using trypsin. Carboxy protected in the form of an internal ester, such
as in the form of ~-lactone, can be freed by hydrolysis in the presence of a hydroxide-
containing base, such as an alkaline earth metal hydroxide or, especially, an aL~cali metal
hydroxide, for example NaOH, KOH or LiOH, more especially LiOH, the correspondingly
protected hydroxy group being freed at the same time.
A protected amino group is freed in a manner known per se and, according to the nature of
the protecting groups, in various ways, preferably by solvolysis or reduction. Lower
alkoxycarbonylamino, such as tert-butoxycarbonylamino, can be cleaved in the presence
of acids, for example mineral acids, for example a hydrogen halide, such as hydrogen
chloride or hydrogen bromide, especially hydrogen bromide, or sulfuric or phosphoric
acid, preferably hydrogen chloride, in polar solvents, such as water or a carboxylic acid,
such as acetic acid, or ethers, preferably cyclic ethers, such as dioxane; 2-halo-lower
aLtcoxycarbonylamino ~where appropriate after conversion of a 2-bromo-lower alkoxy-
carbonylamino group into a 2-iodo-lower alkoxycarbonylamino group), aroylmethoxy-
carbonylamino or 4-nitrobenzyloxycarbonylamino can be cleaved, for example, by treat-
ment with a suitable reducing agent, such as zinc in the presence of a suitable carboxylic
acid, such as aqueous acetic acid. Aroylmethoxycarbonylamino can be cleaved also by
treatment with a nucleophilic, preferably salt-forming, reagent, such as sodium thio-
phenolate, and 4-nitrobenzyloxycarbonylamino also by treatment with an aLkali metal
dithionite, for example sodium dithionite. Unsubstituted or substituted diphenylmethoxy-
carbonylamino, tert-lower aLkoxycarbonylamino or 2-(tri-substituted silyl)-lower aL~coxy-
carbonylamino, such as 2-tri-lower alkylsilyl-lower alkoxycarbonylamino, can be cleaved
by treatment with a suitable acid, for example formic acid or trifluoroacetic acid, for
example in a halogenated hydrocarbon, such as methylene chloride or chlo~oforrn (espe-
cially when hydroxy protected by benzyl is not to be freed at the same time); unsubstituted
or substituted benzyloxycarbonylamino can be cleaved, for example, by means of
hydrogenolysis, i.e. by treatment with hydrogen in the presence of a suitable hydrogena-
211~
- 62 -
tion catalyst, such as a palladium catalyst, for example bonded to a carrier, such as carbon,
preferably in polar solvents, such as di-lower alkyl-lower alkanoylamides, for example
dimethylformamide, ethers, such as cyclic ethers, for example dioxane, esters, such as
lower alkanoic acid lower alkyl esters, for example ethyl acetate, or alcohols, such as
methanol, ethanol or propanol, with methanol being especially pre~erred, preferably
approximately at room temperature; unsubstituted or substituted triarylmethylamino or
formylamino can be cleaved, for example, by treatment with an acid, such as a mineral
acid, for example hydrochloric acid, or an organic acid, for example formic, acetic or tri-
fluoroacetic acid, where appropriate in the presence of water, and triphenylmethylamino ~-
can be cleaved especially by hydrogenolysis with a noble metal or noble metal oxide as
catalyst, such as platinum, palladium or, especially, palladium hydroxide, the catalyst - ~ ~
preferably being bonded to a carrier, such as carbon, silica gel or aluminium oxide, in inert ~ .
solvents, such as an ether, preferably a lower alkyl-lower alkanoate, such as ethyl acetate,
at temperatures of from 20 to 80C, especially from 50 to 70C, if necessa~y under
elevated pressure, for example approximately from 1 to 10 bar; and an amino group
protected in the form of silylamino can be freed, for example, by means of hydrolysis or
alcoholysis. An amino group protected by 2-haloacetyl, for example 2-chloroacetyl, can
be freed by treatment with thiourea in the presence of a base, or with a thiolate salt, such - -
as an alkali metal thiolate of thiourea, and subsequent solvolysis, such as alcoholysis or
hydrolysis, of the resulting substitution product. An amino group protected by 2-(tri- - :;
substituted silyl)-lower alkoxycarbonyl, such as 2-tri-lower alkylsilyl-lower aL~coxy-
carbonyl, can be converted into the free amino group also by treatment with a salt of
hydrofluoric acid that yields fluoride anions, as indicated above in connection with the
freeing of a correspondingly protected carboxy group. Likewise, silyl, such as trimethyl-
silyl or tert-butyl-dimethylsilyl, bonded directly to a hetero atom, such as nitrogen, can be
removed using fluoride ions, preferably with a fluoride of an organic quaternary nitrogen
base, such as tetra-lower alkylammonium fluoride or tri-lower alkylaryl-lower alkyl-
ammonium fluoride, for example tetraethylammonium fluoride or tetrabutylammoniumfluoride, in the presence of an aprotic, polar solvent, such as dimethyl sulfoxide or N,N-di-
methylacetamide, or especially an ether, such as tetrahydrofuran, at temperatures of from
0 to 50C, especially at about room temperature.
Amino protected in the form of an azido group is converted into free amino, for example,
by reduction, for example by catalytic hydrogenation with hydrogen in the presence of a
hydrogenation catalyst, such as platinum oxide, palladium or Raney nickel, by reduction
using mercapto compounds, such as dithiothreitol or mercaptoethanol, or by treatment
- 211~
- 63 -
with zinc in the presence of an acid, such as acetic acid. The catalytic hydrogenation is
preferably carried out in an inert solvent, such as a halogenated hydrocarbon, for example
methylene chloride, or in water or in a mixture of water and an organic solvent, such as an
alcohol or dioxane, at approximately from 20C to 25C, or with cooling or heating.
A hydroxy or mercapto group protected by a suitable acyl group, by a tri-lower alkylsilyl
group or by unsubstituted or substituted l-phenyl-lower alkyl is freed analogously to a
correspondingly protected amino group. A hydroxy or mercapto group protected by
2,2-dichloroacetyl is freed, for example, by basic hydrolysis, and a hydroxy or mercapto
group protected by tert-lower alkyl or by a 2-oxa- or 2-thia-aliphatic or -cycloaliphadc
hydrocarbon radical is freed by acidolysis, for example by treatment with a miner~l acid or
a strong carboxylic acid, for example trifluoroacetic acid. A hydroxy group protected by
benzyloxy is freed, for example, by hydrogenolysis, that is to say by treatment with
hydrogen in the presence of a suitable hydrogenation catalyst, such as a palladium
catalyst, for exarnple bonded to a caTrier, such as carbon, preferably in polar solvents, such
as di-lower alkyl-lower alkanoylamides, for example dimethylformamide, ethers, such as
cyclic ethers, for example dioxane, esters, such as lower alkylalkanoates, for example
ethyl acetate, or alcohols, such as methanol, ethanol or propanol, with methanol being
especially preferred, preferably at about room temperature. Mercapto protected by pyridyl-
diphenylmethyl can be freed, for example, using mercuryaI) salts at pH 2-6 or by zinc/-
acetic acid or by electrolytic reduction; acetamidomethyl and isobutyrylamidomethyl can
be freed, for example, by reaction with mercury(II) salts at pH 2-6; 2-chloroacetamido-
methyl can be freed, for example, using 1-piperidinothiocarboxamide; and S-ethylthio,
S-tert-butylthio and S-sulfo can be freed, for example, by thiolysis with thiophenol, thio-
glycolic acid, sodium thiophenolate or 1,4-dithiothreitol. l'wo hydroxy groups or an
adjacent amino and hydroxy group which are protected together by means of a bivalent
protecting group, preferably, for example, by a methylene group mono- or di-substituted
by lower alkyl, such as lower alkylidene, for example isopropylidene, cycloalkylidene, for
example cyclohexylidene, or benzylidene, can be freed by acid solvolysis, especially in
the presence of a mineral acid or a strong organic acid. A tri-lower alkylsilyl group is
likewise removed by acidolysis, for example by a mineral acid, preferably hydrofluoric
acid, or a strong carboxylic acid. 2-halo-lower alkoxyf.~arbonyl is removed using the
above-mentioned reducing agents, for example a reducing metal, such as zinc, reducing
metal salts, such as chromium(II) salts, or using sulfur compounds, for example sodium
dithionite or preferably sodium sulfide and carbon disulfide. Esterified hydroxy groups,
for example lower alkanoyloxy, such as acelyloxy, can also be freed by esterases, and
- 211~61
- 64 -
acylated amino can be freed, for example, by suitable peptidases.
A sulfo group protected in the form of a sulfonic acid ester or sulfonamide is freed, for
example, by acid hydrolysis, for example in the presence of a mineral acid, or preferably
by basic hydrolysis, for example with an alkali metal hydroxide or alkali metal carbonate,
for example sodium carbonate.
The temperatures for the freeing of the protected functional groups are preferably from
-80 to lOO~C, especially from -20 to 50C, for example from 10 to 35C, such as in the
region of room temperature.
:: .
When several protected functional groups are present, if desired the protecting groups can
be so selected that more than one such group can be removed simultaneously, for example
by acidolysis, such as by treatment with trifluoroacetic acid, or with hydrogen and a
hydrogenation catalyst, such as a palladium-on-carbon catalyst. Conversely, the groups ~ -
can also be so selected that they cannot all be removed simultaneously, but rather in a
desired sequence, the corresponding intermediates being obtained.
. ,
Process g) (Formation of a carboxylic acid ester)
~ '
The acylation of the hydroxy group is effected, for exarnple, in a manner known per se ~ ~ -
using an acid of formula XXV as defined above wherein T is as defined with the exception -
of unsubstituted or substituted aminocarbonyl, or a reactive derivative of a compound of
formula XXV wherein T is as defined for compounds of formula I. A suitable reactive
derivative is, for exarnple, a carboxylic acid of forrnula XXV'
T-ZI (XXV')
~ .
wherein T is one of the radicals defined above for compounds of formula I', preferably
one of the radicals meneioned with the exception of unsubstituted or substituted amino-
carbonyl, and wherein Zl is reactively activated hydroxy (the compound of forrnula XXV'
therefore contains, instead of a hydroxy function bonded to the carbonyl group, reactively
activated hydroxy, preferably as deFmed below). The free carboxylic acid of forrnula XXV
can be activated, especially also in si~u, for example, by serong acids, such as a hydrohalic,
sulfuric, sulfonic or carboxylic acid, or acidic ion exchangers, for exarnple by hydro-
chloric, hydrobromic or hydriodic acid, sulfuric acid, an unsubstitueed or substituted, for
-` 211~61
-- 65 -
example halo-substituted, alkanecarboxylic acid, or by an acid of formula XXV, prefer-
ably with an excess of the acid of formula XXV, if necessary with the binding of the
resulting water of reaction by water-binding agents, with removal of the water of reaction
by azeotropic distillation or with extractive esterification, by acid anhydrides, especially
inorganic or more especially organic acid anhydlides, for example carboxylic acid
anhydrides, such as lower alkanecarboxylic acid anhydrides (with the exception of formic
acid arlhydride), for example acetic anhydr~de, or by suitable activating or coupling
reagents of the type mentioned below. T-ZI may especially also be a carboxylic acid azide
(Zl = azido; obtainable, for example, by reaction of a corresponding acid ester via the
corresponding hydrazide and treatment thereof with nitrous acid); a carboxylic acid halide
(Zl = halogen, especially chlorine or bromine), especially an acid chloride or bromide,
obtainable, for exarnple, by reaction with organic acid halides, especially with oxalyl
dihalides, such as oxalyl dichloride, with inorganic acid halides, for example with acid
halides of phosphorus or sulfur, such as phosphorus trichloride, phosphorus tribromide,
phosphorus pentachloride, phosphorus pentabromide, phosphorus oxychloride, phosphorus
oxybromide, thionyl chloride or thionyl br.omide, or especially under mild condi~ions with
tetra-lower aLkyl-a-halo-enamines, for example tetramethyl-a-halo-enarnines, especially
l-chloro-N,N,2-trimethyl-1-propenamine (preferably by reaction under an inert gas, such
as nitrogen, in inert solvents, especially chlorinated hydrocarbons, such as methylene
chloride or chloroform, or ethers, such as diethyl ether, dioxane or tetrahydrofuran, or
mixtures thereof, at preferred temperatures of from -78 to 50C, especially from -60 to
30C, for example from -10C to room temperature (cf. I:)evos, A., et al., J. C. S. Chem.
Commun. 1979, 1180- l l X l, and Haveaux, B., et al., Org. Synth. 59, 26 ( 1980~), it being
possible for the resulting acid halide, for example the acid chloride of formula XXV'
wherein Zl is chlorine, also to be used further directly in si~u, for example by reaction with
the compound of formula I in the presence of tertiary nitrogen bases, such as pyridine or
4-dimethylaminopyridine (DMAP, which is preferably added in catalytic amounts) or both
of those bases, at preferred temperatures of from -20 to 50C, especially from 10C to
40C; an activated ester wherein Zl is the radical of an alcohol having electron-attracting
substituents, especially cyanomethoxy or aryloxy wherein aryl is preferably phenyl or
naphthyl that is mono- or poly-substituted by halogen, nitro and/or by cyano, for exarnple
nitrophenoxy, such as 4-nitrophenoxy or 2,4-dinitrophenoxy, or polyhalophenoxy, such as
pentachlorophenoxy; or a symmetrical or, preferably, asymmetrical acid anhydride which
can be obtained, for example, by the action of a salt, for example an alkali metal salt, of an
acid of formula XXV or its reaction partner, preferably a lower alkanecarboxylic acid,
such as acetic acid, such as the sodium or potassium salt, on a complementary acid halide,
^" 211866~
- 66-
especially, in the case of the reaction with a salt of a carboxylic acid of formula XXV, a :~
carboxylic acid halide, for example chloride, such as acetyl chloride, and, in the case of
the reaction of a carboxylic acid halide of formula XXV' wherein Zl is halogen, for
example chlorine or bromine, with a salt of a lower alkanecarboxylic acid, especially -
sodium or potassium acetate. There may be used as activating and coupling reagents for
activating carboxylic acids of formula XXV in situ also carbodiimides, for example
~d,N'-di-CI-C4alkyl- or N,N'-di-Cs-C7cycloalkyl-carbodiimide, such as diisopropylcarbo-
diimide or N,N'-dicyclohexylcarbodiimide, advantageously with the addition of an -
activating catalyst, such as N-hydroxysuccinimide or unsubstituted or substituted, for
example halo-, Cl-C7alkyl- or C~-C7alkoxy-substituted, N-hydroxy-benzotriazole or
N-hydroxy-S-norbornene-2,3-dicarboxamide, Cl-C4alkyl haloformate, for example
isobutyl chloroformate, suitable carbonyl compounds, for example N,N-carbonyldiimid- ;
azole, suitable 1,2-oxazolium compounds, for example 2-ethyl-S-phenyl-192-oxazolium - -
3'-sulfonate or 2-tert-butyl-5-methyl-isoxazolium perchlorate, suitable acylamino
compounds, for example 2-ethoxy-1-ethoxycarbonyl-1,2-dihydroquinoline, or suitable
phosphoryl cyanamides or azides, for example diethylphosphoryl cyanamide or diphenyl-
phosphoryl azide, also triphenylphosphine disulfide or 1-Cl-C4alkyl-2-halopyridinium
halides,forexample 1-methyl-2-chloropyridinium iodide.
If two free carboxy groups are present in the compound of formula XXV it is also possible
for an internal anhydride to be present as activated acid derivative.
Zl is preferably halogen, such as chlorine or bromine, and also acyloxy, for example lower ~ `
alkanoyloxy, such as acetyloxy.
The reaction with an acid halide, such as an acid chloride, of formula XXV' (Zl = Cl) is
carried out especially in an ether, such as dioxane, tetrahydrofuran, or a nitrile, such as
acetonitrile, or mixtures thereof, in the presence or absence of pyridine and in the absence
or, preferably, in the presence of tertiary nitrogen bases, such as 4-dimethylaminopyridine,
ethyl diisopropylamine, triethylamine or mixtures of two or more of those bases, with or
without a protective gas, such as argon, at temperatures of from 0 to 80C or the reflux
temperature, for example from room temperature to 50C or the reflux temperature if that
is lower than 50C.
For the specific case where RZ in formula I' is unsubstituted or substituted amino there are
suitable for the introduction of the corresponding radical T (unsubstituted or substituted
21~ ~6~1
- fi7 -
aminocarbonyl) in the reaction with compounds of formula I especially the compounds of
formula XXV' wherein Zl is halogen, such as chlorine, and wherein T is unsubstituted or
substituted aminocarbonyl, which can be prepared, for example, by reaction of the
complementary amines, for example unsubstituted or substituted alkylamines, aryl-lower
alkylamines or arylamines, as defined for unsubstituted or subs~ituted amino RZ, with
phosgene or analogues thereof that contain instead of chlorine other halogen atoms, espe-
cially bromine, preferably in the presence of tertiary nitrogen bases, such as pyridine or
triethylamine, and in inert solvents, for example chlorinated hydrocarbons, such as
methylene chloride or chloroform, ethers, such as diethyl ether, tetrahydrofuran or
dioxane, or carboxylic acid amides, such as dimethylformamide. Also suitable are corres-
ponding N-carbonylazolides of formula XXV' (Zl = N-containing heterocycle, such as
1-imidazolido) which are obtained, for example, by reaction with the corresponding
N,N'-carbonyldiazolides, such as N,N'-carbonyldiimidazole, under conditions as just
described for phosgene and analogues having other halogen atoms. The reaction ofcompounds of formula I with the corresponding compounds of formula XXV' is then
likewise earried out under those conditions (ef. Staab, H. A., Angew. Chemie 74, 407
(1962)).
For the specifie case of the introduction of aminocarbonyl T or an N-monosubstituted
aminocarbonyl group T there is suitable as activated acid derivative especially the corres-
ponding isocyanate of formula XXV"
Q-N=C=O (XXV")
wherein Q is an amino-protecting group, for example trihaloacetyl, such as trifluoro- or
triehloro-aeetyl, or one of the unsubstituted or substituted lower alkyl radieals or aryl
radieals mentioned above in the definition of unsubstituted or substituted amino RZ
wherein the amino group carries a substituent, it being possible, when Q is an amino-
protecting group, to obtain after the reaction with the compound of formula I the corres-
ponding eompound of formula I' wherein Rs is free aminocarbonyloxy by removal of the
proteeting group Q, as described for the freeing of amino protected by acyl under
Process f), especially by aeid hydrolysis, or, when Q is one of the mentioned substituted or
unsubstituted lower alkyl radicals or aryl radieals, a corresponding eompound of formula I
eontaining aminocarbonyl T monosubstituted at the nitrogen atom. Both aminocarbonyl
and N-monosubstituted aminocarbonyl T can be eonverted into N-disubstituted amino-
earbonyl T by alkylation with a further unsubstituted or substituted lower alkyl radical
. .
. .
- 21~g~1
- 68 -
using suitable starting materials and conditions analogous to those described below under
"Additional Process Steps".
The mentioned reactions can be carried out under reaction conditions known per se, at
customary temperatures, in the presence or, especially when lower alkanoyl anhydr;des
are used to activate the carboxylic acid of formula XXV, in the absence of inert solvents
or diluents, for example in acid amides, for example carboxylic acid amides, such as
dimethylformamide, dimethylacetamide or 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimi-
dinone (DMPU), or amides of inorganic acids, such as hexamethylphosphoric acid
triamide, ethers, for example cyclic ethers, such as tetrahydrofuran or dioxane, or acyclic
ethers, such as diethyl ether or ethylene glycol dimethyl ether, halogenated hydrocarbons,
such as halo-lower aLtcanes, for example methylene chloride or chloroform, ketones, such
as acetone, nitriles, such as acetonitrile, acid anhydrides, such as acetic anhydride, esters,
such as ethyl acetate, bisalkane sulfimes, such as dimethyl sulfoxide, nitrogen hetero-
cycles, such as pyridine, or mixtures of those solvents, especially in anhydrous solvents or
solvent mixtures, it being possible to select for the above-mentioned reactions the
particular solvents that are suitable in each case, there being used, as appropriate and
expedient, salts of the compounds used, especially metal salts of carboxylic acids that are
used, such as the aL~cali metal or aLI~aline earth metal salts, for exarnple sodium or
potassium salts, in the absence or the presence of catalysts, such as dimethylarnino-
pyridine, condensation agents or neutralising agents, such as tertiary nitrogen bases, for :
example pyridine, triethylamine, N-methylmorpholine, dimethylaminopyridine or ethyl
diisopropylamine, and, depending on the nature of the reaction and/or the reactants, under
atmospheric pressure or in a closed vessel, under normal pressure or under elevated
pressure, for example at the pressure produced in the reaction mixture under the reaction ~ :
conditions in a closed tube, and/or in an inert atmosphere, for example under an argon or
nitrogen atmosphere. Preference is given to reaction conditions that are mentioned
specifically in any particular case or, especially, that are analogous to those mentioned in
the Examples. The course of the reaction is advantageously monitored using customary
methods of analysis, especially using thin-layer chromatography. From those reaction
conditions it is possible to select the reaction conditions that are suitable for each of the
reactions described in this text, reaction conditions that are specifically mentioned being
especially preferred.
The reaction according to the invention is preferably carried out under mild conditions,
especially at temperatures of from -1t) to 60C, for example from 0 to room temperature
:,.. ~,~ ~ , .. .
211~
- 69 -
or at slightly elevated temperatures up to about 50C, for example approximately from 0
to 40C. Both in the case of the reaction with a carboxylic acid halide of formula XXV'
wherein Zl is halogen, such as chlorine or bromine, and in the case of the reaction with an
anhydride, especially a symmetrical anhydride (Zl = O-T), the corresponding compound
of formula XXV' (halide and T-O-T, respectively) is used especially in an approximately
equimolar amount in relation to the compound of formula I or in excess, for example from
0.95 to 10 times the molar amount, preferably from 1.05 to 5 times the molar amount.
In starting materials of forrnulae I, XXV, XXV' and XXV", functional groups, with the
exception of groups that a~e intended to participate in the reaction or that do not react
under the reaction conditions, are protected independently of one another by protecting
groups.
The protecting groups are entirely analogous to those described under Process a) for the
formation of an amide bond starting from compounds of formulae II and III'. The freeing
of functlonal groups protected by protecting groups in the resulting compounds of
forrnula I' having protected functions is effected in accordance with one or more of the
methods mentioned under Process f).
Process h) (Nucleophilic substitution)
In starting materials of for nulae XXVI and XXII, functional groups, with the exception of
groups that are intended to participate in the reaction or that do not react under the
reaction conditions, are protected independently of one another by protecting groups.
The protecting groups are entirely analogous to those described under Process a) for the
formation of an amide bond starting from compounds of formulae II and III'.
A leaving group Wl is especially a nucleofugal leaving group selected from hydroxy
esterified by a strong inorganic or organic acid, such as hydroxy esterified by a mineral
acid, for example a hydrohalic acid, such as hydrochloric acid, hydrobromic acid or
hydriodic acid, or by a strong organic sulfonic acid, such as a lower alkanesulfonic acid
that is unsubstituted or substituted, for example by halogen, such as fluorine, or an
aromatic sulfonic acid, for example a benzenesulfonic acid that is unsubstituted or substi-
tuted by lower alkyl, such as methyl, halogen, such as bromine, and/or by nitro, ~or
example a methanesulfonic acid, p-bromotoluenesulfonic acid or p-toluenesulfonic acid,
21186~
- 70 -
and hydroxy esterified by hydra~oic acid.
The reaction between a compound of formula XXVI and a compound of formula XXVII
wherein L is etherified or esterified hydroxy or mercapto is preferably carried out in the
presence of a base, for example a hydroxide-containing base, such as a metal hydroxide,
for example an alkali metal hydroxide, such as sodium or potassium hydroxide, or espe-
cially a metal carbonate or hydrogen carbonate, such as sodium or potassium carbonate or ~-
sodium or potassium hydrogen carbonate, there also being suitable in those cases, in
addition to the solvents mentioned hereinbelow, preferably ketones, such as lower
aLlcanones, or aqueous and protic solvents, or especially with the use of a metal alcoholate
ur thiolate as activated compound of forrnula XXVII or with the preparation thereof in situ
in the presence of a strong base, for example an alkali metal hydride, such as sodium
hydride, or in the presence of an aLkali metal, such as sodium, in the absence or presence
of a suitable solvent, especially an aprotic solvent, for example DMPU, an ether, such as
diethyl ether, dioxane or tetrahydrofuran, or a carboxylic acid amide, such as dimethyl-
formamide, at temperatures of from 0C to the reflux temperature, especially from 20C
to the reflux temperature, if necessary under a protective gas, such as nitrogen or argon.
In the case of the reaction of compounds of fonnula XXVII wherein L is unsubstituted or
substituted amino or is heterocyclyl bonded via nitrogen, the reaction is preferably carried
out in the absence of a base or in the presence of a sterically hindered nitrogen base, espe-
cially a tertiary nitrogen base, such as 4-dimethylaminopyr~dine, pyridine or triethylamine,
in an aqueous or non-aqueous solvent, such as an aqueous or non-aqueous alcohol, for
example ethanol or methanol, esters, such as diethyl esters, ethers, such as diethyl ether,
dioxane or tetrahydrofuran, carboxylic acid amides, such as dimethylforrnarnide, or aceto-
nitrile, at temperatures of from 0C to the reflux temperature, especially from 20 to
100C.
l:)epending on the reaction conditions, the substitution can take place in the form of a
first-order or second-order nucleophilic substitution.
The freeing of functional groups protected by protecting groups in the resultingcompounds of formula I' having prooected functions is effected in accordance with one or ~-~
more of ~e methods mentioned under Process f).
AdditionalProcess StePs
6 6 1
- 71 -
In the additional process steps, which are optional, functional groups of the starting
compounds that are not intended to take part in the reaction may be unprotected or may be
in protected form, for example they may be protected by one or more of the protecting
groups mentioned above under Process a). The protecting groups may be retained in the
end products or some or all of them may be removed in accordance with one of themethods mentioned under Process f).
Salts of compounds of formula I' having at least one salt-forming group may be prepared
in a manner known per se. For example, salts of compounds of formula I' having acid
groups may be formed, for example, by treatment with metal compounds, such a3 aLkali
metal salts of suitable organic carboxylic acids, for example the sodium salt of 2-ethyl-
hexanoic acid, with organic aLtcali metal or alkaline earth metal compounds, such as the
corresponding hydroxides, carbonates or hydrogen carbonates, such as sodium or
potassium hydroxide, carbonate or hydrogen carbonate, with corresponding calciumcompounds or with ammonia or a suitable organic amine, stoichiometric amounts or only
a small excess of the salt-forming agent preferably being used. Acid addition salts of
compounds of formula I' are obtained in customary manner, for example by treatment
with an acid or a suitable anion exchange reagent. Internal salts of compounds of
fonnula I' containing acid and basic salt-forming groups, for example a free carboxy
group and a free amino group, may be formed, for example, by the neu~alisation of salts,
such as acid addition salts, to the isoelectric point, for example with weak bases, or by
treatment with ion exchangers.
Salts can be converted in customary manner into the free compounds; metal and
ammonium salts can be converted, or example, by treatment with suitable acids or acidic
ion exchangers, and acid addition salts, for example, by treatment with a suitable basic
agent or basic ion exchangers.
Stereoisorneric mixtures of compounds of formula I', that is to say mixtures of diastereo-
isomers and/or enantiomers, such as, for example, racemic mixtures, can be separated into
the corresponding isomers in a manner known per se by suitable separating processes. For
example, mixtures of diastereoisomers can be separated into the individual diastereo-
isomers by fractional crystallisa~ion, chromatography, solvent partition etc. Racemates can
be separated from one another, after conversion of the optical antipodes into dias,ereo-
isomers for example by reaction with optically active compounds, for example optically
21~6Gl :~:
achve acids or bases, by chromatography on column mate}ials covered with optically
active compounds or by enzymatic methods, for example by selective reaction of only one
of the two enantiomers. This separation can be carried out either at the stage of one of the
starting materials or wi~h the compounds of formula 1' themselves.
In an obtainable compound of formula I' wherein T is a radical of formula Z wherein RZ iS
phenyl- or naphthyl-methoxycarbonyl-substituted amino or imino, for example in amino-
lower alkyl N-substit~ted by those radicals or amino-lower alkyl N,N-disubstituted by
phenyl- or naphthyl-methoxycarbonyl and by lower alkyl, in N-phenyl- or naphthyl-
methoxycarbonyl-pyrrolidin-2-yl or -amino-lower alkoxy-lower alkoxy-lower aL~cyl, the
amino or imino group in the radical T in question can be converted into the co7responding
amino- or N-lower alkylamino-lower alkylcarbonyl group by removal of phenyl- or
naphthyl-lower alkoxycarbonyl, for example by hydrogenolysis, that is to say by treatrnent ~ :
with hydrogen in the presence of a suitable hydrogenation catalyst, such as a palladium
catalyst, for example bonded to a carrier, such as carbon, preferably in polar solvents, such
as di-lower alkyl-lower alkanoylamides, for example dimethylformamide, ethers, such as
cyclic ethers, for example dioxane, esters, such as lower alkanoic acid lower alkyl esters,
for example ethyl acetate, or alcohols, such as methanol or ethanol, especially at room
temperature.
By reductive amination it is possi~le to convert amino-lower aL~cyl RZ mono-N-substituted
by heterocyclyl-lower alkyl, such as imidazolylmethyl or pyridylmethyl, each bonded via
a ring carbon atom, by aryl-lower alkyl, such as phenyl- or naphthyl-lower aL~cyl, or espe-
cially by lower alkyl, as a constituent of T in a compound of formula I' into lower alkyl RZ
N,N-disubstituted by an additional radical selected from heterocyclyl-lower alkyl, such as
imidazolylmethyl or pyridylmethyl, each bonded Yia a ring carbon atom, and aryl-lower
alkyl, such as phenyl- or naphthyl-lower aL~cyl. The reaction between a corresponding
mono-N-substituted compound of formula I' and a corresponding heterocyclyl- or aryl-
lower alkyl ketone or lower aL~canealdehyde is carried out with catalytic hydrogenation, for
example in the presence of a noble metal catalyst, such as platinum or especially
palladiumj bonded to a carrier, preferably carbon, or a heavy metal catalyst, such as Raney
nickel, at normal pressures or at pressures of from 1 to 100 bar, pre~erably in the presence
of a noble metal catalyst at approximately normal pressure, in organic solvents, for
example alcohols, such as ethanol or especially methanol, in ~he absence or, preferably, in
the presence of a carboxylic acid, such as a lower alkanoic acid, for example acetic acid,
at preferred temperatures of from 1) to 50C, for example at room temperature, or with
~: ~
~: ~
2118~1
- 73 -
reduction by means of a complex boron hydride, such as sodium cyanoborohydride.
In a compound of formula I~ wherein T is N-triphenylmethyl-imidazolyl-lower alkyl-
carbonyl, the triphenylmethyl radical can be removed, as described under Process f),
yielding the corresponding compound of formula I' wherein T is imidazol(-2-, -4- or
-5-)yl.
In a compound of formula I' wherein one or more of the radicals R2, R3 and A2 iS substi-
tuted by benzyloxy, the benzyloxy radical can be removed, as described under Process f),
yielding the corresponding compounds of formula I' containing hydroxy in the place of
benzyloxy.
In an obtainable compound of forrnula I', an amino or carboxamide group can be substi-
tuted, a carboxy group present in free or reactive forrn can be esterified or amidated or an
esterified or amidated carboxy group can be converted into a free carboxy group.
The substitution of a carboxamide group or of another primary or secondary amino group,
for example for the formation of the carbamoyl derivatives mono- or di-lower aL~cyl-
carbamoyl and mono- or di-hydroxy-lower aL~cylcarbamoyl mentioned above as a substi-
tuent of thiomorpholino or morpholino formed by R4 and Rs together with the bonding
nitrogen atom, or with the formation of the above-mentioned derivatives of the substituent
amino at thiomorpholino or morpholino formed by R4 and Rs together with the bonding
nitrogen atom, or for the formation of N,N-disubstituted amino RZ in compounds of
formula I' wherein the nitrogen of the amino groups to be reacted is bonded to hydrogen,
is effected, for example, by alkylation.
Suitable agents for alkylating a carboxamide group in a compound of formula I' are, for
example, diazo compounds, for example diazomethane. Diazomethane can be
decomposed in an inert solvent, the free methylene formed reacting with the carboxamide
group in the compound of formula I'. The decomposition of diazornethane is carried out
preferably catalytically, for example in the presence of a noble metal in finely divided
form, for example copper, or a noble metal salt, for example copper(I~ chloride or
copper(II) sulfate.
Alkylating agents are also mentioned in German Offenlegungsschrift 2 331 133, for
example aLkyl halides, sulfonic acid esters, Meerwein salts or l-subsdtuted 3-aryl-
` 211~6~
- 74 -
triazenes, which can be reacted under the conditions mentioned therein with a compound
of fonnula I' containing a carboxamide group.
Further alkylating agents are selected from corresponding alkyl compounds that carry a
substituent X wherein X is a leaving group. A leaving group is especially a nucleofugal
leaving group selected from hydroxy esterified by a strong inorganic or organic acid, such
as hydroxy esterified by a mineral acid, for example a hydrohalic acid, such as hydro-
chloric, hydrobromic or hydriodic acid, or by a strong organic sulfonic acid, such as an
unsubstituted or substituted, for example halo-substituted, such as fluoro-substituted,
lower alkanesulfonic acid, or an aromatic sulfonic acid, for example a benzenesulfonic
acid that is unsubstituted or substituted by lower alkyl, such as methyl, by halogen, such as
bromine, and/or by nitro, for example a methanesulfonic, trimethanesulfonic or p-toluene-
sulfonic acid, and hydroxy esterified by hydrazoic acid.
The reaction can be carried out under the conditions of a first-order or second-order
nucleophilic substitution.
For example, one of the compounds containing a substituent X wherein X is a leaving
group with high polalisability of the electron shell, for example bromine or iodine, can be
reacted in a polar aprotic solvent, for example acetone, acetonitrile, nitromethane,
dimethyl sulfoxide or dimethylformamide. The substitution reaction is carried out if
desired at reduced or elevated temperature, for example in a temperature range of from
approximately -40 to approximately lOOC, preferably from approximately -10 toapproximately 50C, and if desired under an inert gas, for example under a nitrogen or
argon atmosphere.
For the esterification or amidation of a carboxy group in a compound of formula I', for
example for the amidation of a free carboxy group of an amino acid, such as Glu or Asp,
with ammonia, or of a free carboxy group at thiomorpholino or morpholino formed by R4
and Rs together wi~h the bonding nitrogen atom, if desired the free acid can be used or the
free acid can be converted into one of the above-mentioned reactive derivatives and
reacted with an alcohol, with ammonia, or with a primary or secondary amine, or, in the
case of esterification, the free acid or a reactive salt, for example the caesium salt, can be
reacted with a reactive derivative of an alcohol. For example the caesium salt of a
carboxylic acid can be reacted with a halide or sulfonic acid ester corresponding to the
alcohol. The esterification of the carboxy group can also be carried out with other
i . . . ~ .
2~8~1
- 75 -
customary alkylating agents, for example with dia~omethane, alkyl halides, sulfonic acid
esters, Meerwein salts or 1-substituted 3-aryltriazenes, etc.
For the conversion of an esterified or amidated carboxy group into the free carboxy group
it is possible to use one of the methods described above for the removal of carboxy-
protecting groups or, if desired, alkaline hydrolysis under customary reaction conditions,
such as those mentioned in Organikum, 17th edition, VEB Deutscher Verlag der Wissen-
schaften, Berlin 1988.
:'
In a compound of formula I', an esterified carboxy group can be converted into an unsub-
sdtuted or substituted carboxamide group by aminolysis with ammonia or with a primary
or secondary amine. The aminolysis can be carried out in accordance with the customary
reaction conditions, such as those mentioned for such reactions in Organikum, 15th
edition, VEB Deutscher Verlag der Wissenschaften, Berlin (East) 1976.
A free amino group present in a compound of formula I' can be acylated, for example to
introduce one of the radicals mentioned for R1 other than hydrogen. The acylation is
carried out in accordance with the methods mentioned above under Process a) or one of ~ ~-
the methods mentioned for protecting groups or, for example, according to one of the
procesæs mentioned in Organikum, 17th edition, VEB Deutscher Verlag der Wissen-
schaften, Berlin (East) 1988.
In an obtainable compound of forrnula I' wherein the substituents are as defined and at
least one free hydroxy group is present and the remaining functional groups are in pro-
tected form, the free hydroxy group, for example the hydroxy group at thiomorpholino or
morpholino formed by R4 and Rs together with the bonding nitrogen atom, can be acylated
or etherified.
;: . ' .
The acylation can be carried out with acylating reagents according to one of the methods
mentioned under Processes a) to e) or g) or according to one of the methods mentioned for
protecting groups or according to one of the processes mentioned in Organikum, 17th
edition, VEB Deutscher Verlag der Wissenschaften, Berlin (East) 1988.
The etheri~lcation can be carried out with the above-mentioned alkylating agents and
under the same reaction conditions, for example with diazomethane, alkyl halides,
sulfonic acid esters, Meerwein salts, I-substituted 3-aryltria~enes, etc.. Preference is given
21~ 8~Ç;1
- 76 -
to the reaction with corresponding alkyl halides, such as lower alkyl iodides or bromides,
in the presence of caesium carbonate in suitable solvents or solvent mixtures, for example
in N,N-di-lower alkyl-lower alkanoylamides, such as dimethylformamide or dimethyl-
acetamide, or ethers, such as dioxane, or mixtures thereof, at temperatures of from 0 to
the reflux temperature, preferably from 3û to 60C, for example at about 50C.
In a compound of fonnula I', any groups that correspond to protecting groups, and also
suitable radicals Rl other than hydrogen can be removed in accordance with one of the
methods mentioned under Process f), especially by hydrolysis, for example in the presence
of bases, such as alkali metal or alkaline earth metal hydroxides, for example sodium
hydroxide, or acids, such as organic acids or mineral acids, for example a hydrogen halide,
such as hydrogen chloride. The hydrolysis is effected under the customary conditions, for
example in aqueous solution or in anhydrous solvents, especially in ethers, such as
dioxane, at temperatures of from -50C to the reflux temperature of the reacuon mixture in
question, for example from 0 to 50C, preferably in the presence of a protective gas, such
as argon or nitrogen, or by hydrogenolysis ~for example in the case of benzyloxycarbonyl
radicals~, preferably in polar solvents, such as alcohols, for example me~anol or ethanol,
or esters, such as lower aLtcyl-lower alkanoates, for example ethyl acetate, at the last- -
mentioned temperatures and in the presence of suitable hydrogena~ion catalysts, such as a
palladium catalyst, which is preferably bonded to a carrier, such as carbon.
In a compound of forrnula 1' wherein at least one of the radicals R2 and R3 is a phenyl
group and/or one or more of the radicals Bl, Al and A2 is phenylalanine, it being possible
for each of the phenyl radicals to be substituted, as described above, the corresponding
phenyl radical(s) can be reduced, for example hydrogenated, selectively to (a) corres-
ponding cyclohexyl radical(s). The hydrogenation is preferably carried out in the presence
of a catalyst that allows the selective hydrogenation of double bonds in the presence of
peptide bonds, especially a catalyst comprising heavy metal oxides, such as a
Rh(IIl)tPt(VI) oxide catalyst according to Nishimura (S. Nishimura, Bull. Chem. Soc.
Japan 33, 566 (1960)), in suitable solvents, especially water, alcohols, such as methanol or
ethanol, esters, such as ethyl acetate, or ethers, such as dioxane, for example in methanol,
at temperatures of from 0 to 150C, preferably from 10 to 50C, for example at room
temperature, and at hydrogen pressures of from 1 to 50 bar, for example at normal
pressure.
Pharmaceutical Compositions:
" .~.. . . . . ~ . . ... .
~ 2118~1
The invention relates also to pharmaceutical compositions comprising compounds of
formula 1' and to novel compounds of formula I.
The pharmacologically acceptable compounds of the present invention may be used, for
example, in the preparation of pharmaceuticaLI compositions that comprise an effective
amount of the active ingredient together or in admixture with a significant amount of
inorganic or organic, solid or liquid, pharmaceutically acceptable carriers.
:'~
The pharmaceutical compositions according to the invention are compositions for enteral,
such as nasal, buccal, rectal or oral, or parenteral, such as intramuscular or intravenous,
administration to warm-blooded animals (human beings and animals) that comprise an
effective dose of the pharmacological active ingredient alone or together with a significant
amount of a pharmaceutically acceptable carrier. The dose of the active ingredient
depends on the species of warm-blooded animal, body weight, age and individual
condition, individual pharmacokinetic data, the disease to be treated and the mode of
adrninistradon.
The invendon relates also to pharmaceutical composidons and a method for treating
diseases caused by retroviruses, for example AIDS or the preliminary stages thereof, espe-
cially when HIV-2 or more especially HIV-1 is the cause of the disease, preferably ;
wherein a compound of for nula I' according to the invention is present in an amount that ~ ~
is therapeutically effecdve against retroviral diseases, such as AIDS and the preliminary -
stages thereof, in a pharmaceueical composition that is suitable for administradon to a
warm-blooded animal, especially a human being, for the treatment of a retroviral disease,
such as AD~S, or wherein a therapeutically effective amount of a compound of formula I' `
according to the invention is administered in a treatment method to a warm-blooded ~ :
animal, for example a human being, who on account of one of the~entioned diseases,
especially AIDS, requires such treatment, in an amount that is therapeutically effective
against retroviral diseases, such as AIl:)S and the preliminary stages thereof. The dose to
be administered to warm-blooded animals, for example human beings of approximately
70 kg body weight, is from approximately 3 mg to approximately 10 g, preferably from
approximately 40 mg to approximately 4 g, for example approximately from 300 mg to
1.5 g per person per day, divided preferably into 1 to 3 single doses which may, for
example, be of the same size. Usually, children receive half of the adult dose. -
.
~ :'
2~1~5~1
- 78 -
The pharmaceutical compositions comprise from approximately 1 % to approximately ~ -
95 %, preferably from approximately 20 % to approximately 90 %, active ingredient.
Pharmaceutical compositions according to the invention may be, for example, in unit dose
form, such as in the form of ampoules, vials, suppositories, dragées, tablets or capsules.
The pharmaceutical compositions of the present invention are prepared in a manner known
per se, for exarnple by means of conventional dissolving, Iyophilising, mixing, granulating
or confectioning processes.
Solutions of the active ingredient, and also suspensions or dispersions, and especially
isotonic aqueous solutions, dispersions or suspensions, are preferably used, it being
possible, for example in the case of Iyophilised compositions that comprise the active
ingredient alone or together with a carrier, for exarnple mannitol, for such solutions,
dispersions or suspensions to be made up prior to use. The pharrnaceutical compositions
may be sterilised andlor may comprise excipients, for example preservatives, stabilisers,
wetting agents and/or emulsifiers, solubilisers, salts for regulating the osmotic pressure
and/or buffers, and are prepared in a manner known per se, for example by means of
conventional dissolving or Iyophilising processes. The said solutions or suspensions may
comprise viscosity-increasing substances, such as sodium carboxymethylcellulose,carboxymethylcellulose, dextran, polyvinylpyrrolidone or gelatin.
Suspensions in oil comprise as the oil component the vegetable, synthetic or semi-
synthetic oils customary for injection purposes. There may be mentioned as such espe-
cially liquid fatty acid esters that contain as the acid component a long-chained fatty acid
having from 8 to 22, especially from 12 to 22, carbon atoms, for example lauric acid,
tridecylic acid, myristic acid, pentadecylic acid, palmitic acid, margaric acid, stearic acid,
arachidic acid, behenic acid or corresponding unsaturated acids, for example oleic acid,
elaidic acid, erucic acid, brassidic acid or linoleic acid, if desired with the addition of anti-
oxidants, for example vitamin E, ~-carotene or 3,5-di-tert-butyl-4-hydToxytoluene. The
alcohol component of those fat~y acid esters has a maximum of 6 carbon atoms and is a
mono- or poly-hydric, for example a mono-, di- or tri-hydAc, alcohol, for example
methanol, ethanol, propanol, butanol or pentanol or the isomers thereof, but especially
glycol and glycerol. The following examples of fatty acid esters are therefore to be
mentioned: ethyl oleate, isopropyl myristate, isopropyl palmitate, "Labrafil M 2375"
(polyoxyethylene glycerol trioleate, Gattefossé, Paris), "Miglyol 812" (triglyceride of
saturated fatty acids with a chain length of C8 to Cl2, Huls AG, Germany), but especially
` 211~61
-79-
vegetable oils, such as cottonseed oil, almond oil, olive oil, castor oil, sesame oil, soybean
oil and more especially groundnut oil.
The injection compositions are prepared in customary manner under sterile conditions; the
same applies also to introducing the compositions into ampoules or vials and sealing the
containers. -
Pharmaceutical compositions for oral administration can be obtained by combining the
active ingredient with solid czrriers, if desired granulating a resulting mixture, and
processing the mixture, if desired or necessary, after the addition of appropriate excipients,
into tablets, dragée cores or capsules, or by preparing dispersions, preferably with
phospholipids, which are introduced into vials. It is also possible for the active ingredients
to be incorporated into plasdcs calriers that allow the active ingredients to diffuse or be
released in measured amounts.
Suitable carriers are especially fillers, such as sugars, for example lactose, saccharose,
mannitol or sorbitol, cellulose preparations and/or calcium phosphates, for exarnple tri- :
calcium phosphate or calcium hydrogen phosphate, and also binders, such as starch pastes
using, for example, corn, wheat, rice or potato starch, gelatin, tragacanth, methylcellulose,
hydroxypropylmethylcellulose, sodium carboxymethylcellulose and/or polyvinyl-
pyrrolidone, and/or, if desired, disintegrators, such as the above-mentioned starches, also
carboxymethyl starch, crosslinked polyvinylpyrrolidone, agar, alginic acid or a salt
thereof, such as sodium alginate. Excipients are especially flow conditioners and
lubricants, for example silicic acid, talc, stearic acid or salts thereof, such as magnesium or
calcium stearate, andlor polyethylene glycol. Dragée cores are provided with suitable,
optionally enteric, coatings, there being used, inter alia, concentrated sugar solutions
which may comprise gum arabic, talc, polyvinylpyrrolidone, polyethylene glycol andlor
titanium dioxide, or coating solutions in suitable organic solvents, or, for the preparation
of enteric coatings, solutions of suitable cellulose preparations, such as ethylcellulose
phthalate or hydroxypropylmethylcellulose phthalate. Capsules are dry-filled capsules
made of gelatin and also soft, sealed capsules made of gelatin and a plasticiser, such as
glycerol or sorbitol. The dry-filled capsules may comprise the active ingredient in the
form of granules, for example with fillers, such as lactose, binders, such as starches,
and/or glidants, such as talc or magnesium stearate, and if desired with stabilisers. In soft
capsules the active ingredient is preferably dissolved or suspended in suitable oily
excipients, such as fatty oils, paraffin oil, fatty acid esters or lower alkylene glycols, such
211~61
- 80-
as 1,2-propylene glycol monolaurate (mixture of the two constitutional isomers;
Gattefossé S.A., Saint Priest, France), (E~)Gelucire (glycerides and partial polyglycerides of
fatty acid; Gattefossé S.A., Saint Priest, France) or liquid polyethylene glycols, such as
PEG 300, it likewise being possible for stabilisers and/or antibacterial agents to be added.
Dyes or pigments may be added to the tablets or dragée coatings or to the capsule casings,
for example for identification purposes or to indicate different doses of active ingredient.
Especially preferred as pharmaceutical compositions are phospholipid-stabilised
dispersions of the active ingredient, preferably for oral administration, comprising
a) a phospholipid or several phospholipids of the formula
1CH2--O--R
R --O--CH O
A I ll ~ Ra (A),
CH2--O--P--O--(CnH2n)--N~ Rb
o~3 RC
wherein RA iS C10-20aCYI. RB is hydrogen or Cl0 20acyl, Ra~ Rb and RC are hydrogen or
Cl4alkyl and n is an integer from two to four, if desired
b) a further phospholipid or several further phospholipids
c) the active ingredient and
d) a pharmaceutically acceptable carrier liquid and, if desired, fu~her excipients and/or
preservatives.
The pr~cess for the preparation of those dispersions is as follows: a solution or suspension
of components a) and c) or a), b) and c), but preferably of a) and b) in a ratio by weight of
from 20: 1 to 1: S, especially from 5: 1 to 1: 1, is converted into a dispersion by dilution
with water and the organic solvent is then removed, for example by centrifugation, gel
filtration, ultrafiltration or especially by dialysis, for exarnple tangential dialysis, prefer-
ably against water, and then, preferably after the addition of excipients or preservatives
and if necessary with the establishment of an acceptable pH value by the addition of
pharmaceutically acceptable buffers, such as phosphate salts or organic acids (pure or
dissolved in water), such as acetic acid or citric acid, preferably from pH 3 to 6, for
example pH 4 - 5, the dispersion obtained is concentrated (unless it already has the correct
active ingredient concentration) preferably to an active ingredient concentration of from 2
211866~ :
- 81 -
to 30 mg/ml, especially from 10 to 20 mg/ml, concentration preferably being effected in
accordance with the methods last mentioned for the removal of an organic solvent, espe-
cially by ultrafiltration, for example using an apparatus for carrying out tangential dialysis
and ultrafiltration.
The phospholipid-stabilised dispersion that can be prepared in accordance with that
process is stable ~or at least several hours at room temperature, is reproducible as regards
the proportions of the components and is toxicologically acceptable and is therefore espe-
cially suitable for oral administration to human beings.
The size of the particles obtained in the dispersion is variable and is preferably from
approximately 1.0 x 10-X to approximately 1.0 x 10~5rn, especially from approximately
10-7 to approximately 2 x 104m.
The nomenclature for the phospholipids of formula I and the numbering of the carbon ~ `
atoms are in accordance with the recommendations of the IUPAC-IUB Commission on
Biochemical Nomenclature (CBN) (sn-nomenclature, stereospecific numbering) given in
the Eur. J. of Biochem. 79, 11-21 (1977) "Nomenclature of Lipids".
In a phospholipid of formula A, RA and RB having the definitions Cl0 20acyl are prefer-
ably straight-chained Cl0 20alkanoyl having an even number of carbon atoms and
straight-chained Cl0 20alkenoyl having a double bond and an even number of carbon
atoms.
Straight-chained Cl0 20alkanoyl RA and RB having an even number of carbon atoms are,
for example, n-dodecanoyl, n-tetradecanoyl, n-hexadecanoyl or n-octadecanoyl.
Straight-chained Cl0 20alkenoyl RA and R8 having a double bond and an even number of
carbon atoms are, for example, 6-cis-, 6-trans-, 9-cis- or 9-trans-dodecenoyl, -tetra-
decenoyl, -hexadecenoyl, -octadecenoyl or-icosenoyl, especially 9-cis-octadecenoyl
(oleoyl). ;~
In a phospholipid of formula A, n is an integer from two to four, preferably two. The
group of the formula -(CnH2n)- is unbMnched or branched alkylene, for example 1,1-
ethylene, 1,1-, 1,2- or 1,3-propylene or 1,2-, 1,3- or 1,4-butylene. 1,2-Ethylene (n=2) is -
preferred.
.: .
` 21~6~1
- 82 -
Phospholipids of formula A are, for example, naturally occurring cephalins wherein Ra~ Rb
and Rc are hydrogen, or naturally occurring lecithins wherein Ra~ Rb and Rc are methyl,
for example cephalin or lecithin from soybeans, bovine brain, bovine liver or hen's eggs
having different or identical acyl g~oups RA and RB or mixtures thereof.
Synthetic, substantially pure phospholipids of formula A having different or identical acyl
groups RA and RB are preferred.
The term "synthetic" phospholipid of forrnula A defines phospholipids that have a uniform
composition as regards RA and RB. Such synthetic phospholipids are preferably the
lecithins and cephalins defimed below, the acyl groups RA and RB Of which have a de~med
structure and are derived from a defined fatty acid having a degree of purity higher than
approximately 95 %. RA and RB may be identical or different and may be unsaturated or
saturated. RA is preferably saturated, for example n-hexadecanoyl, and RB is preferably
unsaturated, for example 9-cis-octadecenoyl (= oleoyl).
The term "naturally occurAng" phospholipids of formula A defines phospholipids that do
not have a uniform composition as regards RA and RB. Such natural phospholipids are
likewise lecithins and cephalins the acyl groups RA and RB of which are structurally
undefinable and are derived from naturally occurring fatty acid mixtures. - -
The term "substantially pure" phospholipid defines a degree of purity of more than 70 %
(by weight) of the phospholipid of formula A, which can be established by suitable deter-
mination methods, for example by paper chromatography.
Special preference is given to synthetic, substantially pure phospholipids of formula A
wherein RA is straight-chained Cl0 20alkanoyl having an even number of carbon atoms and
RB islstraight-chained Cl0 20alkenoyl having a double bond and an even nurnber of carbon
atoms. Ra~ Rb and Rc are methyl and n is two.
In an especially preferred phospholipid of formula A, RA is n-dodecanoyl, n-tetra-
decanoyl, n-hexadecanoyl or n-octadecanoyl and RB is 9-cis-dodecenoyl, 9-cis-tetra- ~ -
decenoyl, 9-cis-hexadecenoyl, 9-cis-octadecenoyl or 9-cis-icosenoyl. Ra~ Rb and Rc are
methyl and n is two.
-- ~1186~1
: ,,
- 83 -
A vcry especially preferred phospholipid of formula A is syn~hetic 1-n-hexadecanoyl-
2-(9-cis-octadecenoyl)-3-sn-phosphatidyl choline having a purity of more than 95 %.
Preferred natural, substantially pure phospholipids of formula A are especially lecithin
(L-a-phosphatidyl choline) from soybeans or hcn's eggs.
The names given in brackets are also customarily used for the acyl radicals in the
phospholipids of forrnula A: 9-cis-dodecenoyl (lauroleoyl), 9-cis-tetradçcenoyl (myrist-
oleoyl), 9-cis-hexadecenoyl (palmitoleoyl), 6-cis-octadecenoyl (petroseloyl), 6-trans-octa-
decenoyl (petroselaidoyl), 9-cis-octadecenoyl (oleoyl), 9-trans-octadecenoyl (elaidoyl),
1 l-cis-octadecenoyl (vaccenoyl~, 9-cis-icosenoyl (gadoleoyl), n-dodecanoyl (lauroyl),
n-tetradecanoyl (myristoyl), n-hexadecanoyl (palmitoyl), n-octadecanoyl (stealoyl),
n-icosanoyl (arachidoyl).
Other phospholipids are preferably esters of phosphatidic acid (3-sn-phosphatidic acid)
with the mentioned acyl radicals, such as phosphatidyl serine and phosphatidyl ethanol-
amme.
Sparingly soluble active ingredients may also be present in the form of water-soluble, .
pharmaceutically acceptable salts, as defined ~bove.
The carrier liquid d) comprises the components a), b) and c) or a) and c) as liposomes in ~ -
such a manner that for a period of from several days up to several weeks no solids or solid
aggregates, such as micelles, re-form and the liquid comprising the said components is
administrable, preferably orally, if necessary after filtration.
The carrier liquid d) may comprise pharmaceutically acceptable, non-toxic excipients, for
example water-soluble excipients that are suitable for producing isotonic conditions, for :
example ionic additives, such as sodium chloride, or non-ionic additives (structure
formers), such as sorbitol, mannitol or glucose, or water-soluble stabilisers for the
liposome dispersion, such as lactose, fructose or sucrose.
In addition to the water-soluble excipients, the carrier liquid may also comprise emulsi-
fiers, wetting agents or surfactants that can be used for liquid pharmaceutical formula-
tions~ especially emulsifiers, such as oleic acid, non-ionic surfactants of the fatty acid
polyhydroxy alcohol ester type, such as sorbitan monolaurate, monooleate, monostearate
2~18~1
- 84-
or monopalmitate, sorbitan tristearate or ~ioleate, polyoxyethylene adducts of fatty acid
polyhydroxy alcohol esters, such as polyoxyethylene sorbitan monolaurate, monooleate,
monostearate, monopalmitate, tristearate or trioleate, polyethylene glycol fatty acid esters,
such as polyoxyethyl stearate, polyethylene glycol-400-stearate, polyethylene glycol-
2000-stearate, especially ethylene oxide/propylene oxide block polymers of the Pluronic(~)
type (Wyandotte Chem. Corp.) or the Synperonic(3' type (ICI).
Preferred preservatives are, for example, antioxidants, such as ascorbic acid, or micr~
bicides, such as sorbic acid or benzoic acid.
Startin~ materials:
The present invention relates also to novel starting materials and/or intermediates and to
processes for their preparation. The starting materials used and the reaction conditions
selected are preferably those which result in the compounds described as being preferred.
All starting materials can preferably be prepared analogously to the processes merltioned
in the Examples and Reference Examples.
In the preparation of all star~ng materials, free functional groups that are not intended to
participate in the reaction in question, may be unprotected or may be in protected form, for
example they may be protected by the protecting groups mentioned above under
Process a). If necessary, the functional groups that are not intended to participate in the
reaction are always in protected for n (cf. for example the protection of compounds of
formula XXI in the reaction, for example, to form compounds of formula XXII', see
below, at the carboxy group that is not to be reacted, in order to avoid lactonisation~.
Those protecting groups can be removed at suitable times by the reactions described under
Process f). The compounds having salt-forming groups can also be used in the form of
salts, and at any stage salts can be formed or converted into the free compounds again.
In the formulae, unless the stereochemistry of asymmetric carbon atoms is defined direcdy
by the choice of colresponding bond symbols~ the configuration of asymmetric carbon
atoms is indicated by the configuration symbol which is selected from (S), (R) and (S,R).
The carboxylic or sulfonic acids of folmula II, or reactive derivatives thereof, are known
and are commercially available or can be prepared in accordance with processes known
- `~, .:, '.'' ! ~.. . . ..
- -``` 21~8~61 ::
~ ~5 -
per se.
The compounds of formula III' are known or can be prepared in accordance with
processes known per se. They can be obtained, for example, from compounds of formula
~ COOH
HN (XII),
ia
wherein R2 is as defined for compounds of formula I' and Pa is an amino-prolecting . . :
group, especially lower alkoxycarbonyl, such as tert-butoxycarbonyl, or phenyl-lower
alkoxycarbonyl, such as benzyloxycarbonyl, (or analogues thereof containing hydrogen in
place of Pa, which can then be protected subsequently), by reduction to a compound of
formula ~ ~ i
.. .
R CHO .'
HN (XIII) -
Pa
(or the analogue having hydrogen in place of Pa) wherein the radicals are as last defined.
The reduction of amino acid ~erivatives of formula XII to the corresponding aldehydes
XIII is effected, for example, by reduction to the corresponding alcohols and subsequent
oxidation to the aldehydes of formula XIII.
I
The reducdon to the alcohols is effected especially by hydrogenation of the corresponding ~ -
acid halides or other activated carboxylic acid derivatives mentioned under Process a), or
by reaction of activated carboxylic acid derivatives of compounds of formula XII, espe-
cially anhydrides with organic carboxylic acids, preferably those of haloformic acid esters,
such as chloroformic acid isobutyl ester, (which are preferably obtained by reaction of
compounds of formula XII in the presence of basic amines, for example tri-lower alkyl-
amines, such as triethylamine, in organic solvents, such as cyclic ethers, for example ~ :
- 211~
- 86-
dioxane, at temperatures of from -50 to 80C, preferably from 0 to 50C) with complex
hydrides, such as alkali metal borohydrides, for example sodium borohydride, in aqueous
solution in the presence or absence of the organic solvents last used, at temperatures of
from -50 to 80C, preferably from 0 to 50C. The subsequent oxidation of the resulting
alcohols is preferably effected with those oxidising agents which selectively convert the
hydroxy group into an aldehyde group, for example chromic acid or derivatives thereof,
such as pyridinium chromate or tert-butyl chromate, dichromate/sulfuric acid, sulfur
trioxide in the presence of heterocyclic bases, such as pyridine/SO3 (preferably dissolved
in di-lower alkyl sulfoxides, such as dimethyl sulfoxide, aromatic solvents, such as
toluene, or mixtures of those solvents), also nitric acid, pyrolusite or selenium dioxide, in
water, aqueous or organic solvents, such as halogenated solvents, for example methylene
chloride, carboxylic acid amides, such as dimethylformamide, and/or cyclic ethers, such as
tetrahydrofuran, in the presence or absence of basic amines, for example tri-lower aL~cyl-
amines, such as triethylamine, at temperatures of from -70 to 100C, preferably from -70
to -50C or from -10 to 50C, for example as described in European Patent Application
EP-A-0 236 734. .:
Direct reduction of the compounds of fo~mula XII to the aldehydes is also possible, for
example by hydrogenation in the presence of a partially poisoned palladium catalyst or by
reduction of the corresponding amino acid esters, for exarnple the lower alkyl esters, such
as ethyl esters, with complex hydrides, for example boron hydrides, such as sodium boro-
hydride, or preferably aluminium hydIides, for example lithium aluminium hydride,
lithium tri(tert-butoxy)aluminium hydride or especially diisobutylaluminium hydride, in
non-polar solvents, for example in hydrocarbons or aromatic solvents, such as toluene, at .
from -100 to 0C, preferably from -70 to -30C, and subsequent reaction to form the
corresponding semicarbazones, for example with the corresponding acid salts of semi-
carbazones, such as semicarbazide hydrochloride, in aqueous solvent systems, such as
alcohol/water, for example ethanol/water, at temperatures of from -20 to 60C, preferably
from l lO to 30C, and reaction of the resulting semicarbazone with a reactive aldehyde, for
example formaldehyde, in an inert solvent, for example a polar organic solvent, for
example a carboxylic acid amide, such as dimethylformamide, at temperatures of from -30
to 60C, preferably from 0 to 30C, and then with an acid, for example a strong mineral
acid, such as a hydrogen halide, in aqueous solution, if desired in the presence of the
solvent previously used, at temperatures of from -40 to 50C, preferably from -10 to
30C. The corresponding esters are obtained by reaction of the amino acids with the
corresponding alcohols, for example ethanol, analogously to the conditions used in the
:: 21186~
- 87 -
condensation under Process b), for example by reaction with inorganic acid halides, such
as thionyl chloride, in organic solvent mixtures, such as mixtures of aromatic and
alcoholic solvents, fnr example toluene and ethanol, at temperatures of from -50 to 50C,
preferably from -10 to 20C.
The preparation of compounds of formula XIII is carried out in an especially preferred
manner under conditions analogous to the reaction conditions mentioned in J. Org. Chem.
47, 3016 (1982), J. Org. Chem.43, 3624 (1978) or J. Org. Chem. Sl,392l (1986).
For the synthesis of a compound of formula III', a compound of fo~mula XIII is then
reacted with a reactive tetraaLlcylsilane, preferably a halomethyl-tri-lower aL~cylsilane, such
as chloromethyltrimethylsilane, in an inert solvent, for example an ether, such as diethyl ~;
ether, a cyclic ether, such as dioxane, or an ester, such as ethyl acetate, at temperatures of
from -100 to 50C, preferably from -65 to 4ûC, there being obtained compounds of
formula
OH R
R2~Si~RR87 (XIV)
HN .
Pa
:,
wherein R6, R7 and R8 are lower alkyl, for example methyl, and the remaining radicals are
as last defined; the resulting compounds are converted in the presence of a Lewis acid,
such as boron trifluoride ethyl etherate, in an inert solvent, especially a halogenated hydro-
carbon, such as methylene chloride or chloroform, with subsequent aftertreatment with an
aqueous base, for example sodium hydroxide solution, at temperatures of from -30 to
80C, especially from 0 to 50C, with elimination and protecting group removal, into an
olefinic compound of formula
R2~ (XV)
H2N
:-` 2118~6~
- 88 -
wherein R2 is as defined for compounds of formula I'; an amino-protecting group Pa is
re-introduced into the corresponding olefin, as described under Process a) for the intro-
duction of amino-protecting grollps, especially with the aid of an acid anhydride in a
chlorinated hydrocarbon, such as methylene chloride or chloroforrn, at temperatures of
from -50 to 80C, especially from 0 to 35C, there being obtained a protected amino-
olefin of formula
R2 1 (S) ~
HN (XVI)
Pa
in which the radicals are as last defined; the double bond is converted into an oxirane,
preferably stereoselecdvely using peroxides, especially peroxycarboxylic acids, for
example haloperbenzoic acid, such as m-chloroperbenzoic acid, in an inert organic
solvent, preferably a halogenated hydrocarbon, such as methylene chloride or chloroform, ~ -
at temperatures of from -50 to 60C, especially from -10 to 25C, and, if necessary,
diastereoisomers are separated, there being obtained an epoxide of foImula
(R)_ o
R2~ (XVII)
HN
Pa ~ :
in which the radicals are as last defined; a suitable malonic acid diester, for example
malonic acid dimethyl ester or malonic acid diethyl ester, is added to the olefims in
question, for example by activation of the methylene group of the malonic acid diester by
means of an aLt~ali metal, for example sodium, in a polar anhydrous solvent, such as an
alcohol, for example methanol or ethanol, at temperatures of from -50 ~o 80C, especially
from 0 to 35C, and the solution is treated with an acid, such as a carboxylic acid, for
example citric acid, there bçing obtained a lactone of formula .
211~
- 89-
(S)~ Rg
R2~0 (XVIII)
HN
Pa
wherein Rg is lower aLkoxy, for example methoxy or ethoxy, and the remaining radicals
are as last defined; if desired, in those compounds in which R2 is phenyl that is unsu~sti-
tuted or substituted as described for compounds of formula I', that radical is reduced to ~ :
cyclohexyl, especially by hydrogenation, preferably in the presence of catalysts, such as
noble metal oxides, for example mixtures of Rh(III)/Pt(VI) oxides (in accordance with
Nishimura), preferably in polar solvents, such as alcohols, for example methanol, at
normal pressure or at up to S bar, preferably at norrnal pressure, at temperatures of from
-20 to 50C, preferably from 10 to 35C; a compound of forrnula XVIII obtained directly
or after hydrogenation is reacted with a reagent that introduces the radical R3-CHr, for
example of the formula R3-CH2-W wherein R3 is as de~med for compounds of formula I'
and W is a nucleofugal leaving group selected from hydroxy esterified by a strong
inorganic or organic acid, such as hydroxy esterified by a mineral acid, for example a
hydrohalic acid, such as hydrochloric, hydrobromic or hydriodic acid, or by a strong
organic sulfonic acid, such as an unsubstituted or substituted, for example halo-substi-
tuted, such as fluoro-substituted, lower aLlcanesulfonic acid or an aroma~ic sulfonic acid,
for example benzenesulfonic acid that is unsubstituted or substituted by lower aLkyl, such
as methyl, halogen, such as bromine, and/or by nitro, for example a methanesulfonic, ~
methanesulfonic or p-toluenesulfonic acid, and hydroxy esterified by hydrazoic acid, espe-
cially bromide, in an anhydrous polar solvent, for example an alcohol, such as ethanol, in
the presence of an alkali metal, for example sodium, at temperatures of from -50 to 80C,
preferab1y from 0 to 35C, yielding a compound of formula
R3 o
(S) ~ Rg
R2~\o (XIX)
HN
Pa
`:, ' ' ': :
2118~1
- so -
wherein the radicals are as last defined; the compound of formula XIX is hydrolysed and
decarboxylated, for example by hydrolysis by means of a base, such as an aLkali metal
hydroxide, for example lithium hydroxide or sodium hydroxide, at temperatures of from
-50 to 80C, preferably approximately from 0 to 35C, in an organic solvent, for
example an ether, such as dimethoxyethane, or an alcohol, such as ethanol, and subsequent
decarboxylation by heating in an inert solvent, preferably a hydrocarbon, for example an
aromatic hydrocarbon, such as toluene, at ~emperatures of from 40 to 120C, preferably
from 70 to 120C, there being obtained a compound of formula
R2~0 (XX)
HN
Pa
wherein the radicals are as last defined; the resulting (R,S,S)- and (S,S,S)-isomers are
separated by column chromatography, and the (R,S,S)-isomer is used further and, for the
purpose of opening the lactone ring, is reacted with a base, such as an aLIcali metal
hydroxide, for example lithium hydroxide or sodium hydroxide, in an inert solvent, such
as an ether, for example dimethoxyethane, or an alcohol, such as ethanol, yielding a
compound of formula :
OH R3 :
R2,_~3~(R) oH (~XI)
HN
Pa
wherein the radicals are as last defined; there is introduced into the resulting compound a
hydroxy-protecting group Py, for example one of the hydroxy-protecting groups
mendoned under Process a) under the conditions mentioned therein, especially a tri-lower
aL~cylsilyl group with the aid of the corresponding halo-tri-lower aLIcylsilane~ for example
tert-butyldimethylchlorosilane, in a polar solvent, such as a di-lower aLlcyl-lower aLIcanoyl-
amide, such as dimethylformamide, in the presence of a sterically hindered amino
2113~61
- 91 - . .
compound, such as a cyclic amine, for example imidazole, at temperatures of from -50 to
80C, preferably from 0 to 35C, yielding a compound of formula
Py
O ~R3
(~IJ~(R) (XXII)
2 r (s~ COOH
HN
Pa
wherein the radicals are as last defimed; or is acylated directly with a compound of
formula XXV or with a reactive derivative thereof, as defined above, with the introduction
of the radical T, as described above under Process g), there being obtained the corres~
ponding compound of formula
:
T
b ~R3 .:
2 r (s, COOH
HN
Pa :
~,
which contains the radieal T in place of Py and in which the remaining radicals are as
defined; and a compound of formula III' having the radicals indicated under Process a) is
prepared from a compound of formula XXII or XXlI', for example by condensation with a ~ `
compound of formula VII wherein the radlcals are as defined under Process c), under the
conditions indicated for Process a), especially by ~n situ reaction in the presence of a
condensation agent, such as benzotriazol-l-yl-oxy-tris(di-methylamino~phosphonium
hexafluorophosphate or O-benzotriazol-l-yl-N,N,N',N'-tetramethyluronium hexafluoro- .
phosphate, a sterically hindered amine, such as N-methylmorpholine, and a compound
hindering racemisation, such as 1-hydroxybenzotriazole, in a polar solvent, preferably an
acid amide, for example a di-lower alkylamino-lower alkanoylamide, such as dimethyl-
formamide, at temperatures of from -50 to 80C, especially from 0 to 35C, and by
subsequent protecting group removal of Pa, as described under Process f), provided Pa is
not a radical corresponding to the residue H-BI- as defined above for compounds of
formula I' with the exception of a bond, condensation with a compound of the formula
, . :., - :, ~, ,.,, , :
2~1~661
- 92 -
H-BI'-OH wherein Bl' is as defined under Process b), under the last-mentioned condensa-
tion conditions and finally removal of Py (only in the case of a compound of
rormula XXII) and/or further protecting groups, as described under Process f), and if
nececssary (in the case of a compound having a free hydroxy group prepared from a
compound of formula XXII) the introduction of T, as described under Process g). For the
preparation of a compound of formula III' there is also possible successive condensation
of a compound of formula XXII or XXII' with compounds that introduce the residues
-Bl-, -A~ Ar~ -Al-A2-. A2-NR4Rs andlor -NR4Rs of the compolmd of formula VII,
under conditions analogous to those mentioned for Process a), and when a compound of
formula XXII is used the protecting group Py is removed according to one of the methods
described under Process f) and the radical T must then be introduced using a compound of ~ :
formula XXV or a reactive derivative thereof under the reaction conditions men~ioned for
Process g).
The route from an above-mentioned compound of formula XVIII to a compound of : -
formula XX may also be as follows~
Hydrolysis of a racemic compound of formula XVIII (which can be prepared from the
racemate of a compound of formula XVI via the corresponding racemate of a compound : -
of formula XVII) and decarboxylation under conditions analogous to those employed for
the hydrolysis and decarboxyladon of compounds of formula XIX result in a compound : . -:
which is analogous to the compound of formula XIX but in which the radicals R3-CH2-
and R9-(C=O)- are absent and which is in the form of a racemate; that compound is then
reacted with a compound of the formula R3-CH2-W, as defimed below of forrnula XVIII,
wherein W is one of the nucleofugal leaving groups mentioned there, especially halogen, ~:
such as bromine or chlorine, by deprotonation in the presence of a strong base, such as an
alkali metal bis(tri-lower aLkylsilyl)amide, for example lithium bis(trimethylsilyl)amide,
followed by alkylation with the compound of the ~ormula R3-CH2-W (preferably yielding
the 1 '(S),3(R)-(R3-CH2-),S(S)- and the 1 '(R),3(S~-(R3-CH2-),5(R)-compound of
formula XX, that is to say a racemate).
The afore-mentioned compounds of formula XV can also be in the (R,S)-configuration at :
the carbon atom carrying the radical -NH2 instead of in the (S)-configuration shown, and
the compounds of formulae XII, XIII, XIV and especially those of formulae XVI, XVII,
XVIII, XIX, XX, XXI and/or XXII can also be in the (R,S)-configuration at the carbon
atom carrying the radical Pa-NH- instead of in the (S)-configuration. The afore-mentioned
211~661
- 93 -
compounds of formulae XVI, XVII and XVIII ca l also be in the form of racemates, that is
to say the optical antipodes of the formulae shown are also possible. It is possible to
obtain from those racemates, for example, corresponding compounds of formula VI' (for
example racemates if Rl and Tl do not contain centres of asymmetry) so that in this
manner a compound of fonnula I' can be obtained wherein either the carbon atom carrying
R2-CH2- is in the (S)-configuration, the carbon atom carrying T-O- is in the (S)-configura-
tion and the carbon atom carrying R3-CH2- is in the (R)-configuration (2R,4S,SS), or the
mentioned carbon atoms have the opposite configuration (2S,4R,5R); or mixtures of
compounds of formula VI' or I' having those two configuradons may also be obtained.
Corresponding racemic mixtures or mixtures of diastereoisomers can (preferably) be
separated into the individual isomers at any stage.
A compound of formula XX wherein the radicals are as defined is also prepared from a
compound of formula XIII wherein the radicals are as defined, by reacting an aldehyde of
formula XIII with a 2-halopropionic acid ester, especially a 2-iodopropionic acid ester,
such as 2-iodopropionic acid ethyl ester, there being obtained a compound of formula
(RIS)
(S)A
R2~o~ (xxm)
HN
Pa
wherein the radicals are as defined and wherein the carbon atom carrying the radical
Pa-NH- may alternadvely also be in the (R,S)-configuration.
The reaction is carried out first with the formation of the homoenolate of the 2-halo-
propionic acid ester in the presence of a mixture of Zn/Cu in a di-lower aL~cyl-lower
aLkanoylamide, such as dimethylacetamide, at temperatures of from 0 to 100C, espe-
cially from 2G to 80C. In a further batch, preferably under protective gas, such as
nitrogen or argon, a titanium tetrahalide, such as titanium tetrachloride, is added to a
tetra-lower alkyl orthodtanate, such as tet;aisopropyl orthodtanate, in an aromatic solvent,
such as toluene or xylene, in the presence of a halogenated hydrocarbon, such asmethylene chloride, and the mixture is stirred at from 0 to 50C, especially from 20 to
30C, there being fonned the corresponding dihalodtanium di-lower alkanolate or prefer-
:; : .
,:........ ,., .:.. .. : :, :: - , ~ . . .:
i:: ~ ,.. ... : ....... ~ .
~ 2118~1
- 94 -
ably the trihalotitanium lower alkanolate, especially trichlorotitanium diisopropanolate.
The zinc homo-enolate solution is added dropwise thereto at temperatures of from -50 to
0C, especially from -40 to -25C, and then the aldehyde of formula XIII in a halogenated
hydrocarbon, for example m.ethylene chloride, is added dropwise, the reaction taking place
at from -50 to 30C, preferably approximately from -20 to 5C, with the formation of an -
ester, especially an ethyl ester, of the compound of folmula XXIII. That ester is then
hydrolysed to forrn the compound of formula XIII, as defined above, preferably in an
organic solvent, such as an aromatic compound, for example in toluene or xylene, in the
presence of an acid, such as a carboxylic acid, for example acetic acid, at temperatures of
from 20C to the boiling point of the reaction mixture, especially from 70 to 100C. lf
necessary, diastereoisomers are separated, for example by chromatography, for example
on silica gel with an organic solvent mixture, such as a mixture of aLkane and ester, such
as lower alkane and lower alkyl-lower alkanoyl ester, such as hexane/ethyl acetate.
From the compound of formula XXIII, the corresponding compound of formula XX is
then obtained by deprotonation with a strong base, yielding the carbanion which is forrned
at the or.-carbon atom adjacent to the oxo group of the lactone, and by subsequent nucleo-
philic substitution of the radical W of a compound of the fonnula R3-CH2-W wherein R3
and W are as defined above for the preparation of compounds of formula XIX (W is espe-
cially bromo), the reaction preferably resulting stereoselectively in the (R)-configuration -~
at the carbon atom ca~rying the radical R3-CH2- in the compound of formula XX. The
reaction with the strong base, especially with an alkali metal organosilicon amide
compound, for exarnple an alkali metal bis(tri-lower alkylsilyl)amide, such as lithium bis-
(trimethylsilyl)amide, or with an alkali metal di-lower alkylamide, such as lithium diiso- ~ :
propylamide, is preferably calllied out in an inert organic solvent, especially an ether, for
example a cyclic ether, such as tetrahydrofuran, or 1,3-dimethyl-3,4,5,6-tetrahydro-
2(1H)-pyrimidinone (DMPU), or mixturçs of those solvents, at temperatures of from -100
to 0C, preferably from -78 to -50C, and the nucleophilic substitution is effected in situ . ~ .
by the addition of the compound of the formula R3-CH2-W, in the samç solvent at
temperatures of from -100 to 0C, preferably from -60 to -40C. ~
A compound of formula XV wherein the radicals are as defined and wherein preferably ~ : -
the carbon atom carrying the group -NH2 is in the (R,S~-configuration can also be obtained
by converting a formic acid ester, for example a formic acid lower alkyl ester, such as
formic acid ethyl ester, by reaction with allylamine at temperatures of from 20 to 70C,
especially from 50 to 60C, into formic acid allylamide. That amide is ~hen dehydrated
: '
` 2~1~6~1
under protective gas, such as nitrogen or argon, preferably with an acid halide, such as
phosphorus oxychloride, phosgene or especially an organic sulfonic acid halide, for
example an arylsulfonic acid chloride, such as toluenesulfonic acid chloride, in the
presence of a base, for example a tri-lower alkylamine, such as triethylamine, or especially
a mono- or bi-cyclic amine, such as pyridine or quinoline, at temperatures of from 50 to
100C, especially from approximately 80 to approximately 100C. An allyl isocyanide is
formed which is converted by reaction with an organolithium salt, for example lower
aL~cyllithium, such as n-butyllithium, into the corresponding lithium salt, the reaction
preferably being carried out in an inert organic solvent, especially an ether, such as
dioxane or diethyl ether, or an aLlcane, for example hexane, or a mixture of those solvents,
at temperatures of from -120 to -50C, especially approximately from -100 to -90C. The
lithium salt formed is then reacted in situ with a compound of the formula R2-CH2-W
wherein R2 is as defined for compounds of formula I and W is as defined above for
compounds of the formula R3-CH2-W, especiially bromine, preferably by the dropwise
addition of R2-CH2-W in an organic solvent, for example an ether, such as tetrahydro-
furan, at the temperatures last mentioned and with subsequent heating at from 0 to 50C,
preferably from 20 to 30C, yielding an isocyanide of formula
R2
(XXIV)
C-N
wherein the radicals are as defined. The compound of formula XXIV is then hydrolysed,
preferably in an aqueous solution to which an acid has been added, for example in an
aqueous hydrohalic acid, such as hydrochloric acid, especially in concentrated hydro-
chloric acid, at temperatures of from -20 to 30C, especially approximately from 0 to
10C, yielding the compound of formula XV wherein the radicals are as last defined and
wherein the carbon atom carrying the group -NH2 is preferably in the (R,S)-configuration.
Compounds of formula IV are known or can be prepared in accordance with processes
known per se, for example by condensation of carboxylic or sulfonic acids of formula II,
or reactive derivatives thereof, with amino compounds of the formula H-BI'-OH wherein
Bl' is as defined for compounds of forrnula IV, the condensation being carried out as last
--:`; 2118~61 :
- 96 -
descnbed, or in the case of compounds of formllla II wherein Rl' is N-(heterocyclyl-
lower alkyl)-N-lower alkylaminocarbonyl, such as N-(2-pyridylmethyl)-N-methyl-amino-
carbonyl, analogously to EP 0 402 646 of 19.12.1990, Example 218.
A compound of formula V' is prepared, for example, from a compound of formula XXII ~ ~ -
or XXII' by condensation with a compound of formula VII or successive condensation
with compounds (for example H-Al'-OH, H-A2'-OH, H-AI-A2-OH or the compound of
~ormula XI, wherein the residues are in each case as defined above) that correspond to
fragments of the compound of formula VII. The condensation conditions are analogous to
those described for the preparation of the compounds of formula III'; when a compound of
formula XXII is used, T is then introduced by reaction with a compound of forrnula XXV
or with a reactive derivative thereof, as defined under Process g), under the conditions
mentioned for Process g).
A compound of formula VI' is prepared, for example, from an amino compound of
fonnu1a XXII or XXII', for example by the introduction of a carboxy-protecting group, as
described under Process a), and removal of the protecting group Pa, as described under
Process f), by condensaffon with a carboxylic acid of the formula Rl-Bl-OH wherein the
radicals are as defined for compounds of formula I'. When a compound of forrnula XXII is
used, T is then introduced by reaction with a compound of forrnula XXV or with areactive derivative thereof, as defined under Process g), under the conditions mentioned
for Process g).
A compound of formula VII is prepared, for example, from the corresponding amino acid
H-Al'-OH or H-A2'-OH or the peptide H-Al-A2-OH and the arnine components of
formula XI, wherein the radicals are in each case as defined above, by condensation
analogously to the process described under Process a). For the preparation of a compound
having a reduced peptide bond between Al and A2, the peptide bond between Al and A2
can be reduced, preferably at the dipeptide stage, for example with hydrogen in the
presence of heavy metal or noble metal catalysts, such as platinum or palladium,optionally on carriers, such as activated carbon, or by means of complex hydrides, prefer-
ably complex hydrides, for example lithiurn aluminium hydride or diisoarnyl borane in
polar solvents, such as alcohols, for example ethanol, or ethers, such as cyclic ethers, for
example tetrahydrofuran, at temperatures of from 0 to 150C, preferably from 20C to the
boiling point of the reaction mixture in question. The amine of formula XI is known or is
prepared in accordance with methods known per se.
2~1$~
- 97 -
A sompound of formula VIII' can be prepared, for example, from a compound of
formula VI' by condensation with an amino acid that introduces the residue Al'. The
reaction is callied out analogously to the conditions described under Process a).
A compound of forrnula IX is prepared, for example, from an amino acid H-A2'-OH,wherein A2' is as defined under Process d), and an amine of for nula XI wherein the
radieals are as defined for compo-mds of formula I, by condensation.
A compound of formula X' is prepared, for example, fiom a compound of formula VI'
and from the corresponding amino acid H-AI '-OH or H-A2'-OH or thei peptide
H-AI-A2-OH wherein the residues are in each case as defined above, by condensadon
analogously to the process described under Process a). For the preparation of a compound
having a reduced peptide bond between Al and A2, the peptide bond between Al and A2 is
reduced, preferably at the dipeptide stage, for example with hydrogen in the presence of
heavy metal or noble metal catalysts, such as platinum or palladium, optionally on
carriers, such as activated carbon, or by means of complex hydIides, preferably cornplex
hydrides, such as lithium aluminium hydride or diisoamyl borane in polar solvents, such
as alcohols, for example ethanol, or ethers, such as cyclic ethers, for example tetrahydro-
furan, at temperatures of from 0 to 150C, preferably from 20C to the boiling point of
the reaction mixture.
The amine of formula XI is known Md is commercially available or is prepared in
accordance with methods known per se.
Compounds of forrnula XXV are known or can be prepared in accordance with processes
known per se, or they are commercially available.
There may be mentioned by way of example the preparation of a compound of
formula XXV wherein T is arylcarbonyl substituted by heterocyclyl-lower alkyl wherein
heterocyclyl is bonded vfa a ring nitrogen atom, which is preferably effected by reaction
of a halo-lower alkyl-substituted, such as chloro- or bromo-methyl-substituted, aryl-
carboxylic acid, such as chloromethylbenzoic acid or bromomethylbenzoic acid, wi~h a
corresponding heterocyclic nitrogen base, such as piperidine, piperazine, 1-lower alkyl-
piperazine or especially morpholine or thiomolpholine, with nucleophilic substitution of
thehalogen atorn.
2118~
- g8 -
Compounds of formula I can be prepared in accordance with the processes given in the
European Patent Application having the publication number EP O 532 466 (published on
17th March 1993; an English language equivalent has been applied for, for example, in ~ -
South Africa). That European Patent Application is therefore included in this text by
reference.
A compound of fonnula I can preferably be prepared analogously to a compound of
formula I' in accordaince with one of Processes a) up to and including f), by using in
Process a), instead of a compound of formula III', a compound of formula III - -
\o ~R3 ~ R4
H B~ ~ A2 ~ R5 (III),
R2 : :
,.:
wherein the radicals are as defined for compounds of formula III', yielding the compound
of formula Ib which contains a hydrogen atom in place of T in a compound of formula Ib'
and in which the remaining radicals are as deSned for compounds of formula Ib', (which
can be prepared analogously to a compound of formula III' from a compound of
for nula XXII but omitting the acylation with a compound of formula XXY), or by using
in Process b)"nstead of a compound of formula V', a compound of formula V
H ~ :
H C) ~ ~ R4
~N `~ A2 ~ (V),
H - R5 ~ :
R2 :
wherein the radicals are as defined ~or compounds of formula V', yielding a compound of
formula Ic which contains a hydrogen atom in place of T in a compound of formula Ic'
and in which the remaining radicals are as defined for compounds of formula Ic', (which
can be prepared analogously to a compound of formula V' from a compound of ~ -
formula XXII but omitting the acylation with a compound of formula XXV), or by using :
in Process c), instead of a compound of formula VI', a compound of forrnula VI
6 ~
99
R, N~30H (Vl),
R2
wherein the radicals are as defined for compounds of forrnula VI', (which can be prepared
analogously to a compound of formula VI' from a compound of formula XXII but
omifflng the acylation with a compound of for nula XXV), or by using in Process d) for
the preparation of a compound of formula Id which contains a hydrogen atom in place
of T in a compound of formula Id' and in which the remaining radicals are as deflned for
compounds of formula Id', inst~ad of a compound of formula VIII', a compound of
foImula VIII
\~ R3
\ B / ~ Al '` OH (VIII),
O
R2 , ~, .
wherein the radicals are as defined for compounds of formula VIII', (which can be ~:
prepared analogously to a compound of formula VIII' from a compound of formula VI), or
by using in Process e), instead of a compound of formula X', a compound of formula X :
.
H~ R3
B1 ~ 1~A2~
R2 ~:
wherein the radicals are as defined for compounds of formula X', (which can be prepared
analogously to a cornpound of formula X' from a compound of formula VI), or by us;ng in . ~:
Process f), instead of a compound of forrnula I' wherein at least one functional group is in
protected form, a compound of formula I having at least one protected functional group, ~ .
211866~ ~
- 100-
with removal of protecting groups.
The present invention relates also to the intermediates of formula I and their salts where
salt-forming groups are present, if they are novel. They likewise have pharmaceutical
activity, as described above for the compounds of formula I that can be freed from the
compounds of formula I', and can be present in pharmaceutical compositions instead of
compounds of formula I'; the corresponding pharmaceutical compositions are obtained by
using compounds of formula I instead of compounds of formula I' above in the description
of the pharmaceutical compositions. The compounds can therefore be used in the
treatment of diseases and, lL~ce the compounds of formula I', can be incorporated into
pharmaceutical compositions and used for the treatment of retroviral diseases, such as
AIDS, especially by inhibition of HIV-I- or HIV-II-protease.
They are preferably compounds of forrnula I
R1~ 13 ~ ~ A1` A ~ N ~ (I)
wherein
Rl is hydrogen, lower alkoxycarbonyl, heterocyclylcarbonyl, benzyloxycarbonyl that is
unsubsdtuted or substituted by up to three radicals selected independendy of one another
from fluorine, halo-lower alkyl, lower aLkanoyl, sulfo, lower alkylsulfonyl and cyano,
heterocyclyloxycarbonyl wherein heterocyclyl is bonded via a carbon atom, one of the
mentioned carbonyl radicals wherein the bonding carbonyl group has been replaced by a
thiocarbonyl group, heterocyclylsulfonyl, lower allcylsulfonyl or N-(heterocyclyl-lower
alkyl)-N-lower alkylaminocarbonyl, as defined for compounds of formula I', especially
tert-butoxycarbonyl;
R2 is phenyl, cyclohexyl, lower alkoxyphenyl, especially o-, m- or p-methoxyphenyl,
benzyloxyphenyl, especially p-benzyloxyphenyl, p-fluorophenyl, p-triiluoromethylphenyl `
or p-hy&roxyphenyl,
R3 is phenyl, lower alkoxyphenyl, especially o-, m- or p-methoxyphenyl, p-trifluor~
methylphenyl, o-, m- or p-cyanophenyl, benzyloxyphenyl, especially p-benzyloxyphenyl,
o-, m- or p-fluorophenyl or hydroxyphenyl,
-=~ 2118~1
- 101 -
A~ is the bivalent reisidue of the amino acid (L)-valine, (L)-iss)leucine or glycine bonded
N-terminally to the group -C=O and C-terminally to A2,
A2 is the bivalent residue of the amino acid glycine, alanine, valine, leucine, isoleucine,
phenylalanine, tyrosine, cyclohexylalanine, p-lower alkoxyphenylalanine, such asp-methoxy-phenylalanine, p-benzyloxyphenylalanine or p-fluorophenylalanine bonded
N-terminally to Al and C-terrninally to the group NR4Rs, and
R4 and R5 together with the bonding nitrogen atom form unsubstituted or substituted
thiomorpholino or rnorpholino, especially molpholino,
with the proviso that at least either one of the radicals R2 and R3 is benzyloxyphenyl or A2
is the bivalent residue of p-benzyloxyphenylalanine, while the remaining radicals are as
defined, when either R3 is other than o- or m-fluorophenyl, o- or m-cyanophenyl or o- or
m-methoxyphenyl, or when A2 is other than alanine or leucine;
or salts of those compounds where salt-forming groups are present.
Of those compounds preference is given to the compounds of formula I wherein Rl is
tert-butoxycarbonyl, R2 is phenyl, p-benzyloxyphenyl or o-, m- or p-methoxyphenyl, R3 is
phenyl, p-benzyloxyphenyl, o-, m- or p-methoxyphenyl, o-, m- or p-fluorophenyl or
hydroxyphenyl, Al is the bivalent residue of the amino acid (L)-valine, (L)-isoleucine or
glycine, especially of (L)-valine, bonded N-terminally to the group -C=O and C-terminally
to A2, A2 is the bivalent residue of the amino acid glycine, alanine, valine, leucine, iso-
leucine, phenylalanine, tyrosinç, cyclohexylalanine, p-lower alkoxyphenylalanine, such as
p-methoxy-phenylalanine, p-benzyloxyphenylalanine or p-fluorophenylalanine, especially
of phenylalanine, tyrosine, p-methoxy-phenylalanine, p-benzyloxyphenylalanine orp-fluorophenylalanine, bonded N-tertninally to Al and C-terminally to the group NR4Rs,
and R4 and Rs together with the bonding nitrogen atom form morpholino,
with the proviso that at least either one of the radicals R2 and R3 is benzyloxyphenyl or A2
is the bivalent residue of p-benzyloxyphenylalanine, while the remaining radicals are as
defined.
.
Special preference is given also to the compounds of ~ormula I wherein Rl is tert-butoxy-
carbonyl, R2 is phenyl, R3 is o- or m-fluorophenyl, o- or m-cyanophenyl or o- or m-lower
alkoxyphenyl, such as o- or m-methoxyphenyl, Al is the bivalent residue of the amino
acid (L)-valine, (L)-isoleucine or glycine, especially of (L)-valine, bonded N-terminally to
21~8~61
- 102-
the group -C=O and C-terminally to A2, A2 is the l~ivalent residue of the amino acid
glycine, alanine, valine, leucine, isoleucine, phenylalanine, tyrosine, cyclohexylalanine,
p-lower alkoxyphenylalanine, such as p-methoxy-phenylalanine, p-benzyloxyphenyl-alanine or p-fluorophenylalanine, especially of phenylalanine, tyrosine, p-lower aL~oxy-
phenylalanine, such as p-methoxy-phenylalanine, p-benzyloxyphenylalanine or p-fluoro-
phenylalanine, bonded N-terminally to A1 and C-terrninally to the group NR4Rs, and R4
and Rs together with the bonding nitrogen atom form morpholino. `
Special preference is given to a compound of formula I having the name: ~ ~
Boc-Phe[C](o-CN)Phe-(L)-Val-(L)-Phe-morpholin-4-ylamide, ~ ::
Boc-Phe[C](m-CN)Phe-(L)-Val-(L)-Phe-morpholin-4-ylamide, .
Boc-Phe[C](p-BzlO)Phe-(L)-Val-(L)-Phe-moIpholin-4-ylamide,
Boc-Phe[C](p-BzlO)Phe-(L)-Val-(L)-(p-BzlOPhe)-morpholin-4-ylamide,
Boc-(p-BzlO)Phe~C]Phe-(L)-Val-(L)-Phe-morpholin-4-ylamide,
Boc-(p-BzlO)Phe[C]Phe-(L)-Val-(L)-(p-BzlOPhe)-morpholin-4-ylamide,
Boc-(p-13zlO)Phe[C](p-BzlO)Phe-(L)-Val-(L)-Phe-morpholin-4-ylarnide,
Boc-(p-BzlO)Phe[C](p-BzlO)Phe-(L)-Val-(L)-(p-BzlOPhe)-morpholin-4-ylamide,
Boc-Phe[C](o-F)Phe-(L)-Val-(L)-Phe-molpholin-4-ylamide, :
Boc-Phe~C](o-F)Phe-(L)-Val-(L)-(p-CH30-Phe)-morpholin-4-ylamide,
Boc-Phe[C](m-F)Phe-(L)-Val-tL)-Phe-morpholin-4-ylamide, '
Boc-Phe[C](m-F)Phe-(L)-Val-~L)-(p-CH30-Phe)-morpholin-4-ylamide,
Boc-Phe[C]Phe-(L)-Val-(L)-Leu-morpholin-4-ylamide,
Boc-Phe[C]Phe-(L)-Val-(L)-Ala-morpholin-4-ylamide,
Boc-(p-CH30)Phe[C](p-BzlO)Phe-(L)-Val-(L)-Phe-morpholin-4-ylamide,
Boc-(p-CH30)Phe[C](p-BzlO)Phe-(L)-Val-(L)-(p-CH30-Phe)-morpholin-4-ylamide,
Boc-(p-CH30)Phe[C](p-BzlO)Phe-(L)-Val-(L)-Tyr-morpholin-4-ylamide, ~ :
Boc-(p-CH30)Phe[C](3-CH30)Phe-(L)-Val-(L)-Phe-morpholin-4-ylamide,
Boc-(p-CH30)Phe~C](~-CH30)Phe-(L)-Val-(L)-Phe-morpholin-4-ylamide,
Boc-Phe~C](3-CH30)Phe-(L)-Val-(L)-Phe-morpholin-4-ylamide,
Boc-Phe[C](2-CH30)Phe-(L)-Val-(L)-Phe-morpholin-4-ylamide,
Boc-Phe[C](3-CH30)Phe-(L)-Val-(L)-(p-CH30-Phe)-morpholin-4-ylamide,
Boc-Phe[C]t2-CH30)Phe-(L)-Val-(L)-(p-CH30-Phe)-morpholin-4-ylamide or
Boc-Phe[C](p-BzlO)Phe-(L)-Val-(L)-(p-CH30-Phe)-morpholin-4-ylamide
or alternatively a compound having the name:
. " . ' ' : : , . . ~ . . ,, ~, . , '
:
^`~ 21~ 8~1
- 103-
Boc-(p-CH30)Phe[C]Phe-(L)-Val-(L)-Phe-morpholin-4-ylamide (very especially
preferred),
Boc-(p-CH30)Phe[C](p-CH30)Phe-(L)-Val-(L)-Phe-morpholin-4-ylarnide (very
especially preferred),
Boc-(p-CH30)Phe[C]Tyr-(L)-Val-(L)-Phe-morpholin-4-ylamide,
Boc-(p-CH30)PhelC]Phe-(L)-Val-(L)-(p-CH30-Phe)-murpholin-4-ylamide,
Boc-(p-CH30)Phe[C]Phe-(L)-Val-(L)-Tyr-morpholin-4-ylamide,
Boc-(p-CH30)Phe[C](p-CH30)Phe-(L)-Val-(L)-~p-CH30-Phe)-morpholin-4-ylarnide,
Boc-(p-CH30)Phe[C](p-CH30)Phe-(L)-Val-(L)-Tyr-morpholin-4-ylamide,
Boc-(p-CH30)Phe[C]Tyr-(L)-Val-(L)-(p-CH30-Phe)-morpholin-4-ylamide or
Boc-(p-CH30)Phe[C]Tyr-(L)-Val-(L)-Tyr-molpholin-4-ylamide.
Finally, the compound of formula I having the name:
Boc-Phe[c](p-CH30)Phe-(L)-Val-(L)-Phe-morpholin-4-ylamide
(- 5(S)-(tert-butoxycarbonylamino)-4(S)-hydroxy-2(R)-(p-methoxy-phenylmethyl~-
6-phenyl-hexanoyl-(L)-valyl-(L)-phenyl-alanyl-morpholin4-ylamide) is very especially ~ :
preferred.
A compound of forrnula XXVI can be prepared, for example, from a compound of
formula I by reaction with a carboxylic acid of forrnula
Wl-(CmH2m)COOH (XXVII)
wherein the radicals are as de~med for compounds of formula XXVI, or wit`h a reactive
acid derivative thereof that contains in place of the carboxy group a radical -(C=0)-Zl
whe~ein Zl is as defined for compounds of formula XXV', under conditions analogous to
those mentioned for Process g).
The isocyanates of formula XXV" can be prepared, for exarnple, from the corresponding
arnine precursors by conversion of the amino group into the isocyanato group, for example
by reaction with phosgene at elevated temperature, for example under reflux conditions, or
by the dropwise addition of the primary, secondary or tertiary amine, which is liquid or
dissolved in a solvent, to an excess of phosgene in a suitable solvent (toluene, xylene, ~ --
ligroin, chlorobenzene, a-chloronaphthalene etc.) with cooling (for example to -50 to
0C), there being formed intermediately a mixture of carbamoyl chloride and amine
~:` 211~
- 104-
hydrochloride, which is then phosgenated further at elevated temperature (for example at ;
from 50C to the reflux temperature) until completely dissolved, with HCI being
eliminated.
The remaining starting compounds are known, are prepared according to processes known
per se and/or are commercially available. ``
The following applies generally to all the processes mentioned hereinabove and
hereinbelow: ` -
As a result of the close relationship between the compounds of formula I' and their salts
and starting materials (starting compounds and intermediates) in free form and in the form
of their salts, hereinabove and hereinbelow any reference to the free compounds or their
salts should be understood as including also the corresponding salts or free compounds,
respectively, as appropriate and expedient.
All the above-mentioned process steps can be carried out under reaction conditions known
per se, preferably the reaction conditions specifically mentioned, in the absence or,
usually, in the presence of solvents or diluents, preferably those solvents or diluents which
are inert towards the reagents used and are solvents therefor, in the absence or presence of `
catalysts, condensadon agents or neutralising agents, for example ion exchangers, such as
cation exchangers, for example in the H ' form, and depending upon the nature of the
reaction andJor the reactants, at reduced, normal or elevated temperature, for example in a
temperature range of from approximately -100C to approximately 190C, preferably from
approximately -80C to approximately 150C, for example at from -80 to -60C, at room
temperature, from -20 to 40C or at reflux temperature, under atmospheric pressure, or in a
closed vessel, optionally under pressure and/or in an inert atmosphere, for example under
an argon or nitrogen atmosphere.
I
At all stages of the reaction it is possible for any isomeric mixtures which may occur to be
separated into the individual isomers, for example diastereoisomers or enantiomers, or into
desired mixtures of isomers, for example racemates or diastereoisomeric mixtures, for
example analogously to the methods described under "Additional Process Steps".
In certain cases, for example in the case of hydrogenation, it is possible to obtain stereo-
selective reactions, so that, for example, individual isomers can be obtained more easily.
i'` .' ' ' ' ~'`''~ ' . ' ' '
21~ ~661
- 105-
The solvents from which the solvents suitable for any particular reaction can be selected
include, for example, water, esters, such as lower alkyl-lower alkanoates, for example
ethyl acelate, ethers, such as aliphatic ethers, for example diethyl ether, or cyclic ethers,
for example tetrahydrofuran, liquid aromatic hydrocarbons, such as benzene or toluene,
alcohols, such as methanol, ethanol or 1- or 2-propanol, nitriles, such as acetonitrile,
halogenated hydrocarbons, such as methylene chloride, acid amides, such as dimethyl-
formamide, bases, such as heterocyclic nitrogen bases, for example pyridine, carboxylic
acid anhydrides, such as lower alkanoic acid anhydrides, for example acetic anhydride,
cyclic, linear or branched hydrocarbons, such as cyclohexane, hexane or isopentane, or
mixtures of those solvents, for example aqueous solutions, unless indicated to the contrarv
in the description of the processes. Such solvent mixtures can also be used in working-up, ~ -
for example by chromatography or partition.
The compounds, including their salts, may also be obtained in the forrn of hydrates, or
their crystals may include, for example, the solvent used for crystallisation.
The invention relates also to those fonns of the process in which a compound obtainable
as intermediate at any stage of the process is used as starting material and the remaining
process steps are carried out, or in which a starting material is formed under the reaction
conditions or is used in the form of a derivative, for example in protected form or in the
form of a salt, or a compound obtainable in accordance with the process of the invention is
produced under the process conditions and is processed further in situ. In the process of
the present invention it is preferable to use those starting materials which result in the :~ ;
compounds described at the beginning as being especially valuable. Reaction conditions :
analogous to those mentioned in the Examples are especially preferred.
Where necessary, protected starting compounds can be used at any stage of the process
ænd the protecting groups can be removed at suitable stages of the reaction. - ~ -
Protecting groups and the manner in which they are introduced and removed are as ~ ~
described under Processes a) and f). ~ ~ -
The following Examples are intended to illustrate the invention but do not limit the scope
thereof in any way. The Reference Examples listed at the beginning are starting
compounds (of formula I) for the Examples (of forrnula I') listed thereafter.
~O,', .. ,.,, ' . . , ,: ' : ' . ' ,'. . - . , ' ' ' ' '
211~61
- 106- .
Any reference to "Reference Examples~ relates either to the ~Reference Examples~ specif-
ically described hereinafter or to the Examples mentioned in European Patent Application
EP 0 532 466 (published 17th March 1993, incorporated in the present text by reference)
(the numbers of those Examples are thus identical to the "Reference Example" numbers
given below).
:
Temperatures are given in degrees Celsius (C). Where no temperatu~e is specified, the
reaction takes place at room temperature. The Rf values, which indicate the ratio of the
seepage propagation of the substance in question to the seepage propagation of the eluant
front, are determined on thin-layer silica gel plates by thin-layer chromatography (TLC) in
the following solvent systems:
TLC eluant systems:
A hexane/ethyl acetate 1:1
B ethyl acetate - ;
C hexane/ethyl acetate 4:1
D hexanetethyl acetate 2:1
E hexane/ethyl acetate 3:1
F methylene chloride/methanol 9:1
G chloroform/methanoUwater/glacial : ~:
acetic acid 85:13:1.5:0.5
H ethyl acetate/methanol 9:1
hexane/ethyl acetate 1:2
J chloroform/methanoUacetic acid/water 75:27:5:0.5
K ethyl acetate/acetic acid 19:1
L methylene chloride/methanol 7:3 :~
, M methylene chloride/ether 49:1
N methylene chloride/ether 3:1
O ethyl acetaterI~IF 9:1
P hexane/ethyl acetate 8:1
Q methylene chloride/hexane/ether 10:10:1
R methanol
S ethyl acetate/hexane 3:1
T ethyl acetate/ethanol 97:3
" ' ' ' '
'"~ /: ,., ~ .. i
~,~. , ,
- ` 2 ~
- 107-
U THF/ethyl acetate 3:1
V methylene chloride/l~lF 2:1
W methylene chloride/'propanol/- ;
methanoUtriethylamine 8:3:3: 1
X acetonitrile/ethyl acetate 1~
Y ethyl acetate/ethanol 9S:S
Z methylene chloride/methanol 12:1
A' methylene chloride/diethyl ether 1:1
B' methylene chloride/tetrahydrofuran 4:1
C' methylene chloride/tetrahydrofuran 1:1
The above-mentioned letter codes for TLC syst~ms are also used in some cases to indicate
the eluants in column chromatography.
The abbreviation "Rf(A)", for example, indicates that the Rf value was determined in -
solvent system A. The quantitative ratio of solvents to one another is always given in paris
by volume (v/v). The quantitative ratio of the solvents used in the definition of the eluant
systems for column chromatography are also given in parts by volume (v/v).
The other shortened names and abbreviations used have the following meanings:
abs. absolute
atm physicalatmospheres - ~ ~
Anal. elemental analysis ~-
Boc tert-butoxycarbonyl
BOP benzotriazol-l-yloxy-tris(dimethyl-
amino)phosponium hexafluorophosphate
brine saturated sodium chloride solution
calc. calculated
(unit of pressure) - 1 atm corresponds to 1.013 bar
DCC dicyclohexyl~arbodiimide
DEPC pyrocarboxylic acid diethyl ester
DIPE diisopropyl ether
DMAP 4-dimethylaminopyridine
DMF dimethylformamide
DMPU 1,3-dimethyl-3,4,5,6-tetrahydro- 2(1H)-pyrimidinone
.. ,, .. .. .... . ::
- `~ 2 1 ~
- 108-
DMSO dimethyl sulfoxide
ether diethyl ether
FAB-MS Fast-Atom-Bombardment mass spectroscopy
h hour(s)
HBTU O-benzo~riazol-l-yl-N,N,N',N'-
tetramethyluronium hexafluorophosphate
HOBt l-hydroxybenzotriazole
HV highvacuum ;
IR in~rared spectroscopy
min minute(s)
m.p. melting point
NMM N-methylmorpholine
org. orgamc
Pd/C palladium on activated carbon (catalyst)
propanol isopropanol
Rl~ rotary evaporator
RT room temperature
sat. saturated
TBAF tetrabutylammonium fluoride (~ihydrate)
TFA irifluoroacetic acid
1~ tetrahydrofuran
TLC thin-layer chromatography
Z benzyloxycarbonyl
.~ .
Mass spectroscopic data are obtained according to the "Fast-Atom-Bombardment"
(FAB-MS) method. The mass data relate to the protonated molecule ion (M+H)+.
The values for the IR spectra are given in cm-l, with the solvent in question indicated in
round brackets.
The abbreviations customary in peptide chemistry are used to denote divalent residues of
natural a-amino acids. The configuration at the a-carbon atom is indicated by the prefix
(L.)- or (D)-. -Cha- denotes cyclohexylalanyl, -(p-F-Phe)- denotes phenylalanyl substituted
in the p-position of the phenyl ring by fluorineg -(p-CH3O-Phe)- denotes phenylalanyl
substituted in dle p-position of the phenyl ring by a methoxy group, -(p-BzlOPhe)- denotes
211~6~1
- 109-
phenylalanyl substituted in the p-position by a benzyloxy group, -(p-CN-Phe)- denotes
phenylalanyl substituted in the p-position of the phenyl ring by a cyano group, -(4-n-
butyloxy-Phe)- denotes phenylalanyl substituted in the p-position of the phenyl Iing by
n-butoxy and -(4-isobutyloxy-Phe)- denotes phenylalanyl substituted in the p-position of
the phenyl ring by isobutoxy. ~:
HPLC-gradients: -
20 % ~ 100 % a) in b) for 20 min.
II 20 % ~ 100 % a) in b) for 35 min.
I~ 20 % ~ 100 % a) in b) for 30 min.
IV 20 % ~ 100 % a) in b) for 20 min and then
100 % a) for 8 min. ~
Eluant a): acetonitrile + 0.05 % TFA; eluant b): water ~ 0.05 % TFA. Column (250 x ~ -
4.6 mm) filled with "Reversed-Phase" material Clg-Nucleosil(E~) (5 ~lm mean particle size,
silica gel covalently derivatised with octadecylsilanes, Macherey & Nagel, Duren, Federal
Republic of Germany). Detection by UV absorpdon at 215 nm. The re~ention times (tRe~)
are given in minutes. Flow rate 1 mVmin.
':
The following abbreviations for residues are defined by the corresponding fonnulae and
names:
The residue with the abbreviation -Phe[C]Phe- denotes the divalent residue of 5(S)-
amino-2(R)-benzyl-4(S)-hydroxy-6-phenylhexanoic acid and has dle formula
,
211~
- 1 10 - '
H OH ~0
N ~
~ O
The residue with the abbreviation -Cha[C](p-CN~Phe- denotes the divalent residue of ;
~(S)-amino-2(R)-(p-cyanophenylmethyl)-6-cyclohexyl-4(S)-hydroxyhexarloic acid and
has the fonnula
j~N
H OH ~ ;
~N~
O'~
The residue with the abbreviation -Cha[C]Cha- denotes ~e divalent residue of ~(S)-
amino-6-cyclohexyl-2(R)-cyclohexylmethyl-4(S)-hydroxyhe~canoic acid and has the
fo~mula
H\
N
0"
18661 ~
- 111 - ~ .
The residue with the abbreviation -Cha[C](p-F)Phe- denotes the divalent residue of 5(S)-
amino-6-cyclohexyl-2(R3-(p-fluorophenylmethyl)-4(S) hydroxyhexanoic acid and has the
formula :
~, F
H OH
~N~ , ~;~
O
~ : ~.'~ '
The residue with the abbIeviadon -(p-F)Phe[C]Phe- denotes the divalent residue of 5(S)-
amino-2(R)-benzyl-6-(p-fluorophenyl)-4(S)-hydroxyhexanoic acid and has the fo~nula .
~ .- " ' : '"~;
H OH ~J
N ~ ::
O '',
F :
The further formulae of ceintral building bloc~cs shown below correspond to the following
abbreviations, which are used in the Exarnples which follow.
~'; "
` 211~6~1
-
- 1 1 2 - ~
H~ ~ ~ ~ U~ j~ f~
X~ ~
X y y
-Phe[C](p-F)Phe- H F
-Phe[C](p-CN)Phe- H CN -Cha[C](p-CH30)Phe~ CH30
-Phe[C](p-CH30)Phe- H CH30 -cha[c](p-cF3)phe- CF3
-Phe[C](p-CF3)Phe- H CF3 -ChatC](p-Bzlo)Phe-
-(p-F)Phe[C](p-F)Phe- F F C6H5-CH2-O
-(p-F)Phe[C](p-CN)Phe- F CN -Cha[C]Tyr-OH
-Tyr[C]Tyr- OH OH
-Tyr[ClPhe- OH H
-Phe[C]Tyr- H OH
-(p-BzlO)Phe[C](p-BzlO)Phe-
C6H5CH20 C6H5CH2
-(p-BzlO)Phe[C]Phe- C6H5CH2o H
-Phe[C](BzlO)Phe- H C6H5CH20
-(p-CH30)Phe[C~(p-CH30)Phe
CH3-O CH3-O
-(p-CH30)Phe[C]Phe- CH3 0 H
-(p-CH30)Phe[C](p-BzlO)Phe-
CH3-O CcHscH20
-(p-CH30)Phe[C]Tyr- CH3-O OH
Accordingly, -Phe[C](p-F)Phe- colTesponds to the divalent residue of S(S)-amino-2(E~)-(p-fluorophenylmethyl)-4(S)-hydroxy-6-phenylhexanoic acid; -Phe[C](p-CN)Phe-
corresponds to the divalent residue of 5(S)-amino-2(R)-(p-cyanophenylmethyl)-4(S)-
211~6~1
- 113-
hydroxy-6-phenylhexanoic acid; -Phe[C](p-CH30)Phe- corresponds to the divalent
residue of 5(S)-amino-4(S)-hydroxy-2(R)-(p-methoxyphenylmethyl)-6-phenylhexanoicacid; -Phe[C](p-CF3)Phe- corresponds to the divalent residue of 5(S)-amino-4(S)-hydroxy-6-phenyl-2(R)-(p-~ifluoromethylphenylmethyl)-hexanoic acid;
-(p-F)Phe[C](p-F)Phe- corresponds to the divalent residue of 5(S)-amino-6-(p-fluoro-
phenyl)-2(R)-(p-fluorophenylmethyl)-4(S)-hydroxyhexanoic acid;
-(p-F)Phe[C](p-CN)Phe- corresponds to the divalent residue of 5(S)-amino-2(R)-(p-cyano-
phenylmethyl)-6-(p-fluorophenyl)-4(S)-hydroxyhexanoic acid; -Cha[C](p-CH30~Phe-
corresponds to the divalent residue of 5(S)-amino-2(R)-(p-methoxyphenylmethyl)-6-
cyclohexyl-4(S)-hydroxyhexanoic acid; -Cha[C](p-CF3)Phe- corresponds to the divalent
residue of 5(S)-amino-6-cyclohexyl-4(S)-hydroxy-2(R)-(p-tnfluoromethylphenylmethyl)- -
hexanoic acid; -Tyr[C]Tyr- corresponds to the divalent residue of 5(S)-amino-4(S)-
hydroxy-6-(p-hydroxyphenyl)-2(R)-(p-hydroxyphenylmethyl)-hexanoic acid; -Phe[C]Tyr-
corresponds to the divalent residue of 5(S)-amino-4(S)-hydroxy-2(R)-(p-hydroxyphenyl-
methyl)-6-phenylhexanoic acid; -Tyr[C]Phe- corresponds to the divalent residue of 5(S)-
amino-4(S)-hydr~xy-6-(p-hydroxyphenyl)-2(R)-benzylhexanoic acid;
-(p-BzlO)Phe[C](p-BzlO)Phe- corresponds to the divalent residue of 5(S)-amino-6-(p-
benzyloxyphenyl)-2(R)-(p-benzyloxyphenylmethyl)-4(S)-hydroxyhexanoic acid;
-(p-BzlO)Phe[C]Phe- corresponds to the divalent residue of 5(S)-amino-6-(p-benzyloxy-
phenyl)-4(S)-hydroxy-2(R)-benzylhexanoic acid; -Phe[C](p-BzlO)Phe- corresponds to the
divalent residue of 5(S)-amino-2(R)-(p-benzyloxyphenylmethyl)-4(S)-hydroxy-6-phenyl-
hexanoic acid; -(p-CH30)Phe[C](p-CH30)Phe- corresponds to the divalent residue of
5(S)-arnino-4(S)-hydroxy-6-~p-methoxyphenyl)-2(R)-(p-methoxyphenylmethyl)-hexanoic
acid; -(p-CH30)Phe[C]Phe- corresponds to the divalent residue of 5(S)-amino-4(S)-
hydroxy-6-(p-methoxyphenyl)-2~R)-phenylmethylhexanoic acid;
-(p-CH30-)Phe[C](p-BzlO)Phe- corresponds to the divalent residue of S(S)-amino-2(R)-
(p-benzyloxyphenylmethyl)-4(S~-hydroxy-6-(p-methoxyphenyl)-hexanoic acid; and
-(p-CH30)Phe[C]Tyr- corresponds to the divalent residue of 5(S)amino-4(S)-hydroxy-
2(R)-(p-hydroxyphenylmethyl)-6-(p-methoxyphenyl)-hexanoic acid.
-Cha[C](p-BzlO)Phe- denotes the divalent residue of 5(S)-amino-2(R)-(p-benzyloxy-
benzyl)-6-cyclohexyl-2(R)-4(S)-hydroxyhexanoic acid; -Cha[C]Tyr- accordingly denotes
the divalent residue of 5(S)-amino-6-cyclohexyl-4(S)-hydroxy-2(R)-(4-hydroxybenzyl)-
hexanoic acid.
2118661
- 114-
H~N ~CF3 ~N
F ~ -
-(p-F)Phe[C](p-CF3)Phe- Y
-(CF3)Phe[ClPhe- H
-(CF3)Phe[C](p-F)Phe- F
~(CF3)Phe[C](p-CF3)Phe- ~F3
The residue -(p-F)Phe[C](p-CF3)Phe- accordingly corresponds to the divalent residue of
5(S)-amino-4(S)-hydroxy-6-(p-fluorophenyl)-2~R)-(p-trifluoromethylphenylme~yl)-
hexanoic acid.
The symbol l ~ is intended to indicate that the residues -(CF3)Phe[C]Phe-,
-(CF3)Phe[C](p-F)Phe- and -(CF3)Phe[C](p-CF3)Phe-, which correspond to the divalent
residues of 5-amino-2-phenyl-4-hydroxy-6-(p-trifluoromethylphenylmethyl)-hexanoic
acid, 5-amino-2-(p-fluorophenylmethyl)-4-hydroxy-6-(p-trifluoromethylphenyl)-hexanoic
acid and 5-amino-2-(p-trifluorome~ylphenylmethyl)-4-hydroxy-6-(p-trifluoromethyl-
phenyl)-hexanoic acid, may be present in the corresponding Examples in the form of a
mixture of the 2(R),4(S),S(S)-isomer with the 2(S),4(R),5(R)-isomer.
Further meanings of corresponding divalent residues are as follows:
-Phe[C](m-CN)Phe- denotes the divalent residue of
5(S)-amino-2(R)-(m-cyanophenylmethyl)-4(S)-hydroxy-6-phenylhexanoic acid;
-Phe[C](o-CN)Phe- denotes the divalent residue
of 5(S)-amino-2(R)-(m~cyanophenylmethyl)-4(S)-hydroxy-6-phenylhexanoic acid; : -Phe[C](o-F)Phe- denotes the divalent residue of
5(S)-amino-2(R)-(o-fluorophenylmethyl)-4(S)-hydroxy-6-phenylhexanoic acid;
-PheCC](m-F~Phe- denotes the divalent residue of
3`~ . .
~ ` . . `: . .
2118~61
- 115-
5(S)-amino-2(R)-(m-fluorophenylmethyl)-4(S)-hydroxy-6-phenylhexanoic acid;
-(p-CH30)Phe[C](3-CH30)Phe- denotes the divalent residue of :.
5(S)-amino-2(R)-(m-methoxyphenylmethyl)-4(S)-hydroxy-6-(p-methoxyphenyl)-hexanoic
acid;
-(p-CH30)Phe[C](2-CH30)Phe- denotes the divalent residue of
5(S)-amino-2(R)-(o-methoxyphenylmethyl)-4(S)-hydroxy-6-(p-methoxyphenyl)-hexanoic
acid; . .
-Phe[C](3-CH30)Phe- denotes the divalent residue of
5(S)-amino-2(R)-(m-methoxyphenylmethyl)-4(S)-hydroxy-6-phenylhexanoic acid; and
-Phe[C](2-CH30)Phe- denotes the divalent residue of
S(S)-amino-2(R)-(o-methoxyphenylmethyl)-4(S)-hydroxy-6-phenylhexanoic acid.
Finally,
-Phe[C](p-isobutyloxy)Phe- corresponds to the divalent residue of
5(S)-amino-4(S)-hydroxy-2(R)-(p-isobutyloxybenzyl)-6-phenyl-hexanoic acid;
-Phe[C~(3,4-dimethoxy)Phe- corresponds to the divalent residue of
5(S)-amino-2(R)-(3,4-dimethoxybenzyl)-4(S)-hydroxy-6-phenyl-hexanoic acid;
-Phe[C](3,4,5-trimethoxy)Phe- corresponds to the divalent residue of
5(S)-amino-4(S)-hydroxy-6-phenyl-2(R)-(3,4,5-trimethoxybenzyl)-hexanoic acid; and
-Phe[C](2,3,4-trimethoxy)Phe- corresponds to the divalent lesidue of
5(S)-amino-4(S)-hydroxy-~phenyl-2(R)-(2,3,4-trimethoxybenzyl)-hexanoic acid.
Reference ExamPles (mentioned in the follow~ng with a description of the p~eparation
only if an additional synthesis is described)~
Reference Examples 1 to 41 and a description of the preparation thereof (which may
involve a novel synthesis) are to be found in the above-mentioned EP 0 532 466. They are
asfollows: .~
!
ReferenceExample 1: Boc-Cha~Cl(~-F)Phe~L)-Val-(L~-P.he-morpholin-4-~,rlamide~ -
Reference Example 2: Boc-Phe~ClPhe-~L)-Val~ -Phe-mor~holin-4-vlamide (Note: the
5(S)-[l(S)-(Boc-amino)-2-phenylethyl]-3(R)-phenylmethyl-dihydrofuran-2-(3H)-one
mentioned as first compound in Reference Example 2b) is prepared preferably as follows
(method of preparation that is suitable also for larger amounts):
211~
i) S(S)-I l(S)-(Boc-amino)-2-phenylethyll-dihydrofuran-2-(3H)-one
(A.E. DeCamp, A.T. Kawaguchi, R.P. Volante, and I. Shinkai, Tetrahedron Lett.32, 1867
(1991)). Under a nitrogen atmosphere, 173 g of Zn/Cu (preparation: R.D. Smith, H.E.
Simmons, W.E. Parham, M.D. Bhavsar, Org. Synth., Coll. Vol 5, 855 (1973)~ and 280 ml
of dimethylacetamide are added to a solution of 375 g (1.65 mol) of 3-iodopropionic acid
ethyl ester [Reference Example 21 D)l)a)] in 1700 ml toluene and the mixture is stirred
vigorously for 1 h at RT and then for 4 h at 80C ( ~ zinc homoenolate solution). In a
second apparatus (nitrogen atmosphere), 127 ml (1.14 mol) of titanium tetrachloride are
added to a solution of 122 ml (0.40 mol) of tetraisopropyl orthotitanate in 350 ml of
toluene and 1900 ml of methylene chloride with slight cooling at an internal temperature
of from 15 to 25C, and the mixture is stirred for 15 min at RT ( ~ yellow solution) and
cooled to -40C ( ~ partial crystallisation of the trichlorotitanium isopropanolate). The
zinc homoenolate solution, cooled to RT, is filtered under an argon atmosphere through a
G3 glass frit and added dropwise to the trichlorotitanium isopropanolate, the temperature
being maintained at from -30C to -25C ( ~ deep red soludon), and the mixture is stirred
for S min at -25C and cooled to -40C. Subsequently a solution of 233 g (0.85 mol) of
N-Boc-phenylalaninal (preparation: D.J. Kempf, J. Org. Chem. 51, 3921 (1986), then
crystallisation from hexane (0C, approximately 18 h), washing with cold hexane, drying)
in 1500 ml of methylene chloride is added dropwise and the mixture is stirred for 15 h at
from -22 to -18C and finally for 1 h at 0C. The reaction mixture is taken up in 10 1 of
ice-water and 12 1 of tert-butyl methyl ether and stirred vigorously for from 7 to 10 min.
The aqueous phase is removed and extracted twice with 10 1 of ether each time; the
organic phases are washed with 8 l of water, 8 1 of sat. sodium hydrogen carbonate
solution, 8 l of water and 5 l of bnne, dried with sodium sulfate and concentrated by
evaporation (~ crystalline S(S)-(Boc-arnino)-4(S)-hydroxy-6-phenylhexanoic acid ethyl
ester).
The above intermediate is heated for 2.5 h at 100C in 6500 ml of toluene and 230 ml of
acetic acid under an argon atmosphere. The cooled reaction mixture is poured, with
stirring, onto 6 l of ice-water, the aqueous phase is rémoved and extracted twice with
2000 ml of toluene each time, and the organic phases are washed with 5 1 of sat. sodium
hydrogen carbonate solution,5 l of 40 % sodium hydrogen sulfite solution, 4 l of water
and 4 l of brine and dried with sodium sulfate. Concentration of the organic phases by
evaporation to a residue of approximately 300 g and the addition of 800 ml of hexane
(stilred for several hours to complete the reaction) yields crystalline lactone, which
according to HPLC contains approximately 10 % of the (SR) epimer (TLC Rf(E)=0.08;
tRet(II)=18.8 min). That material is used in the next step. The pure title compound may be
~` 211g661
- 117 - ~ -
obtained by column chromatography (SiO2, hexane/ethyl acetate 2:1): TLC Rf(E)=0.14;
tRe,~II)=19.2 min; [c~]D=17.7 (c=l; ethanol).
ii) 5(S)-~l(S)-(Boc-amino)-2-phenylethyll-3(R)-phenvlmethYldih-/drofuran-2-(3H)-one
(A.K. Ghosh, S.P. McKee, and W.J. Thompson, J. Org. Chem.56, 6500 (1991)). Under a
nitrogen atmosphere, a solution of 1943 g (6.32 mol) of 5(S)-[l(S)-(Boc-amino)-2-phenyl-
ethyl]-dihydrofuran-2-(3H)-one in 12.0 1 of THF and 1.9 1 of 1,3-dimethyl-3,4,5,6-tetra-
hydro-2(1H)-pyrimidinone is cooled to -75C, at an internal temperature of below -70C
14000 ml of lithium bis(trimethylsilyl)amide (lM) in THF (Aldrich) are added, and the
mixture is then stirred for 20 min at -75C. Over a period of 1 h, 835 ml (7.00 mol) of
benzyl bromide are added thereto, the internal temperature not being allowed to exceed
-70C, and the mixture is stirred for 30 min at -75C to complete the reaction. There are
then added to the clear solution at from -75 to -70C 2320 ml of propionic acid (90 min)
followed by 2320 ml of water (1 h), the temperature being allowed to rise to -10C. The ;
reaction mixture is poured onto 30 1 of ethyl acetate and 35 1 of 10 % citric acid solution,
and the aqueous phase is removed and re-extracted twice with 10 1 of ethyl acetate each
time. The organic phases are washed three times with 12 1 of sat. sodium bicarbonate
solution each time, with 20 1 of brine and twice with 20 1 of water each time, and concen-
trated, and the oily residue is taken up in 10 1 of toluene and concentrated by evaporation
to a residual volume of approximately 5 l. Filtration of the evaporation residue through 4
kg of Merck silica gel (0.063-0.200 mm), subsequent washing with toluene and
crystallisation of the crude product from hexane (4 1 of hexane/kg of crude product) yields
the title compound: TLC Rf(D)=0.54; FAB-MS (M+H)+=414.]).
Reference Example 3: Boc-Cha[C](p-CN)Phe-(L)-Val-(L)-Phe-morpholin-4-ylamide,
Reference Example 4: H-PheLC]Phe-(L)-Val-(L)-Phe-morpholin-4-ylamide,
Reference Example S: 3-Benzofuranoyl-Phe[C]Phe-(L)-Val-(L)-Phe-rnorpholin-4-yl- ~ :
amide,
Reference Example 6: Nicotinoyl-Phe[C]Phe-(L)-Val-(L)-Phe-morpholin-4-ylamide,
Reference Example 7: Morpholinocarbonyl-Val-Phe[C]Phe-(L)-Val-(L)-Phe-rnorpholin-
4-ylamide,
Reference Example 8: Boc-Cha[C]Cha-(L)-Val-(L)-Cha-morpholin-4-ylamide,
Reference Example 9: Boc-Cha[C](p-F)Phe-(L)-Val-(L)-(p-F-Phe)-morpholin-4-ylamide,
Reference Example 10: Boc-Cha[C](p-F)Phe-(L)-Val-(L)-(p-CH3O-Phe)-morpholin-4-
ylamide,
Reference Example 11: Boc-Cha[C]~p-F3Phe-(L)-Val-(L)-Cha-morpholin-4-ylamide,
Reference Example 12: 1,2,3,4-Tetrahydroisoquinoline-3(S)-carbonyl-Val-Phe[C]Phe-
2~1~661
118 -
(L)-Val-(L)-Phe-morpholin-4-ylamide,
Reference Example 13: Boc-Phe[C~Phe-(L~-Val-(L)-(p-F-Phe)-morpholin-4-ylamide,
Reference Example 14: Boc-Phe[C]Phe-(L)-Val-(L)-(p-CH30-Phe)-morpholin-4-ylamide,
Reference Example 15: Boc-Phe[C]Phe-(L)-Val-(L)-Cha-morpholin-4-ylamide,
Reference Example 16: Boc-Phe[C]Phe-(L)-Ile-(L)-Phe-morpholin-4-ylamide,
Reference Example 17: Boc-Phe[C]Phe-(L)-Val-Gly-morpholin-4-ylamide,
Reference Example 18: Boc-Phe[C]Phe-(L)-Ile-Gly-morpholin-4-ylamide~
Reference Example 19: Boc-Phe[CJPhe-(L)-Val-~L)-Val-morpholin4-ylamide,
Reference Example 20: Boc-Phe[C]Phe-(L)-Val-(L)-Phe-thiomorpholin-4-ylamide,
Reference Example 21:
A) 1) one of the compounds mentioned under B) to J) below in which the
radical -thiomorpholin-4-ylamide stands in place of
-morpholin-4-ylamide;
B) 1) Boc-(p-F)Phe[C](p-F)Phe-(L)-Val-(L)-Phe-morpholin-4-ylamide,
2) Boc-(p-F)Phe[C](p-F)Phe-(L)-Val-(L)-(p-F-Phe)-morpholin4-ylamide,
3) Boc-(p-F)Phe[C](p-F)Phe-(L)-Val-(L)-(p-CH30-Phe)-morpholin-4-
ylamide,
4) Boc-(p-F)Phe[C](p-F)Phe-(L)-Val-(L)-Cha-morpholin-4-ylamide,
5) Boc-(p-F)Phe[C](p-F)Phe-(L)-Ile-(L)-Phe-morpholin-4-ylamide,
C) 1) Boc-(p-F)Phe~C](p-CN)Phe-(L)-Val-(L)-Phe-morpholin-4-ylamide,2) Boc-(p-F)Phe[C](p-CN)Phe-(L)-Yal-(L)-(p-F-Phe)-morpholin-4-ylamide,
3) Boc-(p-F)Phe[C](p-CN)Phe-(L)-Val-(L)-(p-CH30-Phe)-morpholin-
4-ylamide,
4) Boc-~p-F)Phe[C](p-CN)Phe-(L)-Val-(L)-Cha-morpholin-4-ylamide,
5) Boc-(p-F)Phe[C](p-CN)Phe-(L)-Ile-(L)-Phe-morpholin-4-ylamide,
and
D) 1) Boc-Phe[C](p-F)Phe-(L)-Val-(L)-Phe-morpholin-4-ylarnide.
2) Boc-PhelCl(p-F)Phe-~L)-Val-(L)-(p-F-Phe)-morpholin-4-Ylamide
Analogously to Reference Example 1), 350 mg (0.395 mmol) of S(S)-(Boc-
amino)-4(S)-(tert-butyldimethylsilyloxy)-6-pheslyl-2(R)-(p-fluorophenyl-
211~
- 119- '
methyl)-hexanoyl-(L)-Val-(L)-(p-F-Phe)-morpholin-4-ylamide are
deprotected with 374 ~ng (1.19 mmol) of TBAF in 3 ml of DMF to yield th~
title compound: tRC,(II)=23.3 min; FAB-MS (M+H)+=765.
The starting material is prepared as follows:
2)a) 5(S)-(Boc-amino)-4(S~-(tert-butvldimethylsilvloxy)-6-phenyl-2(R)- ~ ;~
(p-fluorophenylmethyl)-hexanoYI-(L)-Val-(L)-(p-F-Phe)-morpholin-4- ~ :
ylamide
Analogously to Reference Example 9f), 265 mg (0.485 mmol) of 5(S)-(Boc-
amino)-4(S)-(tert-butyldimethylsilyloxy)-6-phenyl-2(R)-(p-fluorophenyl- ;~
methyl)-hexanoic acid [Reference Example 21 D) l)e)] and 188 mg
(0.53 mmol) of H-(L)-Val-(L)-(p-F-Phe)-morpholin-4-ylamide (Reference :
Example 9e) in 4.6 ml of NMM/CH3CN 0.25M are reacted with 202.6 mg
(0.53 mmol) of HBTU: TLC Rf(D)=0.28; tRet(II)=33.4 min.
3) Boc-Phe~Cl(p-F)Phe-(L)-Val-(L~-(p-CH~O-Phe~morpholin~-vlamide
Analogously to Reference Example 1), 280 mg (0.312 mmol) of 5(S)-(Boc-
amino)-4(S)-(tert-butyldimethylsilyloxy)-6-phenyl-2(R)-(p-fluorophenyl-
methyl)-hexanoyl-(L)-Val-(L)-(p-CH3O-Phe)-morpholin-4-ylamide are
deprotected with 295 mg (0.94 mmol) of TBAF in 3 ml of DMF to yield the
title compound. Column chromatography (siO2, ethyl acetate/hexane 4
ethyl acetate) yields the pure title compound: TLC Rf(B)=0.56;
tRet(II)=23. I min; FA13-MS (M+H)+--777.
The starting material is prepared as follows:
3) a) 5(S)-(Boc-amino)-4(S)-(tert-butyldimethYlsilyloxy)-6-phenyl-2(R)-
(p-fluorophenylmethyl)-hexanoyl-(L)-Val-(L)-(p-CH~O-Phe)-morpholin-4-
ylamide
Analogously to Reference Example 9f), 200 mg (0.366 mmol) of
5(S)-(Boc-amino)-4(S)-(tert-butyldimethylsilyloxy)-6-phenyl-2(R)-(p-fluoro-
phenylmethyl)-hexanoic acid [Reference Example 21 D) l)e)] and 146 mg
(0.402 mmol) of H-(L)-Val-(L)-(p-CH30-Phe)-morpholin-4-ylamide
(Reference Example lOe) in 3.6 ml of NMM/CH3CN 0.25M are reacted with ~ -
153 mg (0.402 mmol~ of HBTU: TLC Rf(D)=0.22; tRet(II)=33.1 min.
. ~
': ~
~; " ' ~ ' ' ~ '; ' ' ' . ' ! . ` '
211~6~1
- 120-
4) Boc-PherCl(p-F)Phe-(L)-Val-(L)-Cha-morpholin-4-vlamide
(described in EP 0 532 466)
S) Boc-Phe~CI(p-F)Phe-(L)-Ile-(L)-Phe-morpholin-4 vlamide
Analogously to Reference Example 1), 220 mg (0.251 mmol) of 5(S)-(Boc-
arnino)-4(S)-(tert-butyldimethylsilyloxy)-6-phenyl-2(R)-(p-flus)rophenyl-
methyl)-hexanoyl-(L)-Ile-(L)-Phe-morpholin-4-ylamide are deprotected with
240 mg (0.75 mmol) of TBAF in 3 ml of DMF to yield ~he ti~e compound.
Column chromatography (SiO2, ethyl acetate/THF 9:1) yields the pure title
compound: TLC Rf(0)=0.3; tRet(II)=23.9 min; FAB-MS (M+H)+=761.
The starting material is prepared as follows:
5) a) S(S~-(Boc-amino)-4(S~-(tert-butyldimethYlsilvloxv~-6-phen~/1-2(R)-
(p-fluorophenylmethvl)-hexanovl-(L)-Ile-(L)-Phe-morpholin-4-vlamide
Analogously to Reference Example 9f),200 mg (0.366 mmol) of 5(S)-(Boc-
amino)-4(S)-~tert butyldimethylsilyloxy)-6-phenyl-2(R)-(p-fluorophenyl-
methyl)-hexanoic acid [Reference Example 21 D) l)e)] and 140 mg
(0.403 mmol) of H-(L)-Ile-(L)-Phe-morpholin-4-ylamide (Reference
Example 16b) in 3.5 ml of NMM/CH3(:N 0.25M are reacted with 153 mg
(0.40 mmol) of HBTU: TLC Rf(D)=0.16; tRe~(II)=34.4 min. -
E) 1) Boc-Phe~Cl(p-CN)Phe-(L2~Val-(L)-Phe-mor~pholin-4-ylamide
(described in EP 0 532 466)
2) Boc-Phe[C](p-CN)Phe-(L)-Val-~L)-(p-F-Phe)-morpholin-4-ylamide
(described in EP 0 532 466)
3) Boc-Phe[C](p-CN)Phe-(L)-Val-(L)-(p-CH30-Phe)-morpholin-4-ylamide
4) Boc-Phe[C](p-CN)Phe-(L)-Val-(L)-Cha-morpholin-4- ylamide
5) Boc-Phe~Cl(p-CN)Phe-(L)-Ile-(L~-Phe-morpholin-4-ylamide
(described in EP 0 532 466)
F) 1) Boc-Phe~Cl(p-CH~O)Phe-(L)-Val-(L~-Phe-morpholin-4-ylamide
Analogously eo Reference Example 1), 417 mg (0.48 mmol) of 5(S)-(Boc-
amino)-4(S)-(tert-butyldimethylsilyloxy)-6-phenyl-2(R)-(p-methoxyphenyl-
methyl)-hexanoyl-(L)-Val-(L)-Phe-morpholin-4-ylamide are deprotected
with 301 mg (0.95 mmol) of TBAF in 5 ml of DMF to yield the title
.'~"
2 1 1 ~ 1~ 6 1 ~ ~
- 121 -
: .
compound: TLC Rf(F)=0.4; tRet(I)=15.8 min; FAB-MS (M+H)+=759. ;
: '" ', '
The Startillg material is prepared as follows:
1) a) p-MethoxvbenzYl iodide
A solution of 1.7 ml (12.8 mmol) of 4-methoxybenzyl chloride (Fluka;
Buchs/Switzerland) in 25 ml of acetone is stined at RT with 9.4 g ;
(62.6 mmol) of sodium iodide. A gas chromatogram of the reaction mixture
after 90 min indicates complete reaction, and the reaction mixture is there-
fore poured onto ether and washed with 10 % sodium thiosulfate solution and -
brine. The organic phase is dried with Na2S04 and concentrated by evapora-
tion to yield the title compound: lH-NMR (200 MHz, CD30D: 3.78 (s, 3 H),
4.54 (s, 2 H), 6.8-6.95 and 7.2-7.4 (2m, each 2 H).
1) b) 5(S)-rl(S)-(Boc-amino)-2-PhenYleth~-3(R)-(P-methoxy~-
methvl)-dihydrofuran-2-(3H)-one
Analogously to Reference Ex~mple 21 D) l)c), 2.98 g (9.74 mmol) of S(S)-
[l(S)-(Boc-amino)-2-phenylethyl]-dihydrofuran-2-(3H)-one [Reference
Example 21 D) I)b)] dissolved in 40 ml of THF are deprotonated at -75C
with 19~5 ml of 1ithium bis(tnmethylsilyl)arnide lM in l~IF and alkylated
with 2.9 g (11.7 mmol) of p-methoxybenzyl iodide in 20 ml of THF
(45 min). Column chromatography(SiO2, hexane/ethyl acetate 2:1) and
digestion from DIPE yields the pure title compound: TLC Rf(D)=0.32;
tRet(I)=16.7 min.
. , .
1) c) 5(S)-(Boc-amino)-4(S)-hYdroxy-6-phenvl-2(R)-(p-methoxYPhen
methY1)-hexanoic acid ~;
Analogously to Reference Example li), 1.7 g (3.99 mmol) of 5(S)-[l(S)-
(Boc-amino)-2-phenylethyl] -3(R)-(p-methoxyphenylmethyl)-dihydr~
furan-2-(3H)-one in 43 ml of dimethoxyethane and 11 ml of water are --
hydrolysed with 16 ml of lM lithium hydroxide solution. Stirring in ether
yields the pure title compound: TLC Rf~F)=0.53; tRett~I)=14.2 min; FAB-MS
(M+Na)+=466.
;
211~6~
- 122-
1) d) 5(S)-(Boc-amino)-4(S)-(tert-butyldimethylsilvloxy)-6-phenyl-2(R)-
(p-methoxvphenylmethvl)-hexanoic acid
Analogously to Reference Example lj), 0.93 g (2.10 mmol) of 5(S)-(Boc-
amino)-4(S)-hydroxy-6-phenyl-2(R)-(p-methoxyphenylmethyl)-hexanoic
acid in 20 ml of DMF are silylated with 1.4 g (9.64 mmol) of tert-butyl-
dimethylchlorosilane and 1.17 g (17.2 mmol) of imidazole. The silyl ester
function is hydrolysed with 1.7 g of potassium carbonate in methanol
(23 rnl)/ I~F (7 rnl)/water ~7 ml) and the crude product is stirred in hexane toyield the title compound: tRet(l)=20.6 min; FAB-MS (M+H)+=558.
.
1) e) 5(S)-(Boc-amino)-4(S)-(tert-butvldimethvlsilYloxv)-6-PhenY1-2(R~-
(p-methoxvphenvlmethyl)-hexanoyl-(L)-Val-(L)-Phe-morpholin-4-ylamide
Analogously to Reference Example 9f), 300 mg (0.537 mmol) of 5(S)-(Boc-
amino)-4(S)-(tert-butyldimethylsilyloxy)-6-phenyl-2(R)-(p-rnethoxyphenyl-
methyl)-hexanoic acid and 197 mg (0.59 mmol) of H-(L)-Val-(L)-Phe-
morpholin-4-ylamide (Reference Example lo) in 5.2 ml of NMM/CH3CN
0.25M are reacted with 224 mg (0.59 mmol) of HBTU: tRet(I)=22.1 min;
FAB-MS (M+H)+=873.
2) Boc-Phe[C](p-CH30)Phe-(L)-Val-(L)-(p-F-Phe)-morpholin-4-ylamide
3) Boc-Phe~Cl~p-CH~O)Phe-(L)-Val-(L)-(p-CH~0-Phe)-morpholin-4-vl-
amide
Analogously to Reference Example 1), 200 mg (0.22 mmol) of 5~S)-(Boc-
amino)-4(S)-(tert-butyldimethylsilyloxy)-6-phenyl-2(R)-(p-methoxyphenyl- .
methyl)-hexanoyl-(L)-Val-(L)-(p-CH30-Phe)-morpholin-4-ylarnide are
deprotected with 210 mg (0.66 mmol) of TBAF in 3 ml of DMF to yield the
title compound. Column chromatography (SiO2, methylene chlolide/-
methanol 19:1) yields the pure title compound: ILC Rf(F)=0.66; ~-
tRe,(II~=22.5 min; FAB-MS (M+H)+=789.
The starting matelial is prepared as follows:
3) a) 5(S)-(Boc-amino)-4(S)-(tert-butyldimethylsilvloxy)-6-phenyl-2(R)-
(p-methoxyphenYlmethyl)-hexanoyl-(L)-Val-(L)-(p-CH~0-Phe)-morpholin-
4-ylamide
Analogously to Reference Example 9f), 200 mg ~0.358 mmol) of 5(S)-(Boc- -
2118~61
- 123-
amino)-4(S)-(tert-butyldimethylsilyloxy)-6-phenyl-2(R)-(p-methoxyphenyl-
methyl)-hexanoic acid and 143 mg (0.39 mmol) of H-(L)-Val-(L)-(p-CH30-
Phe)-morpholin-4-ylamide (Reference Example lOe) in 3.6 ml of
NMM/CH3CN 0.25M are reacted with 149 mg (0.39 mmol) of HB~:
tRe,(II)=33.2 min.
4) Boc-Phe[C](p-CH30)Phe-(L)-Val-(L)-Cha-morpholin-4-ylamide
5) Boc-Phe[C](p-CH30)Phe-(L)-Ile-(L)-Phe-morpholin-4-ylamide
G) 1) Boc-PhelCl(p-CF~)Phe-(L)-Val-(L)-Phe-morpholin-4-ylamide
Analogously to Reference Example 1), 120 mg (0.13 mmol) of 5(S)-(Boc-
amino)-4(S)-(tert-butyldimethylsilyloxy)-6-phenyl-2{R)-(p-trifluoromethyl-
phenylmethyl)-hexanoyl-(L)-Val-(L)-Phe-morpholin-4-ylamide are depro-
tected with 124 mg (0.39 mmol) of TBAF in 3 ml of DMF to yield the title -
compound. Precipitation with DIPE from a concentrated solution in DMF
yields the pure title compound: tRet(II)=24.7 min; FAB-MS (M+H)+=797.
The starting material is prepared as follows:
1) a) S(S)-rl(S)-(Boc-arnino)-2-phenyl-ethvll-3(R)-~p-trifluoromethYIPhenvl-
methvl)-dihydrofuran-2-(3H)-one
Analogously to Reference Example 21 D) l)c), 1.0 g (3.26 mmol~ of 5(S)-
[l(S)-~Boc-amino)-2-phenylethyl]-dihydrofuran-2-(3H)-one [Reference
Example 21 D) l)b)] dissolved in 20 ml of THF are deprotonated at -75C
with 6.5 ml of lithium bis(trimethylsilyl)amide lM in THF and aLkylated
stalting at -75C with 0.93 g (3.91 mmol) of p-trifluoromethylbenzyl
bromide (Fluka; Buchs/Switzerland) (warrning up during 45 min up to
-60C). Column chromatography (SiO2, hexane/ethyl acetate 2:1) yields the
pure title compound: TLC Rf(D)=0.4; tRet(II)=27.0 min; FAB-MS
(M+H-butene)+=408.
1) b) S(S)-~Boc-amino)-4(S)-hvdroxv-6-phenvl-2(R)-(p-trifluoromethYl-
phenYlmethyl)-hexanoic acid
Analogously to Reference Example li), 4.3 g (9.3 mmol) of 5(S)-[I(S)-
(Boc-arnino)-2-phenylethyl]-3(R)-(p-trifluoromethylphenylmethyl)-dihydro-
furan-2-(3H)-one in 100 ml of dimethoxyethane und 25 ml of water are
.~.. , . .~ , .
,-
~s,
6 1
- 124-
hydrolysed with 37 ml of lM lithium hydroxide solution to yield the title
compound: TLC Rf(H)=0.68; tRC,(II)=22.5 min.
1) c) 5(S)-(Boc-aminoL4(S)-(tert-butyldimethylsilyloxy)-6-phenyl-2(R)-
(p-trifluoromethylphenylmethvl)-hexanoic acid
Analogously to Reference Example lj), 3.2 g (6.65 mmol) of 5(S~-(Boc-
amino)-4(S)-hydroxy-6-phenyl-2(R)-(p-trifluoromethylphenylmethyl)-
hexanoic acid in 25 ml of DMF are silylated with 4.6 g (30.6 mmol) of tert-
butyldimethylchlorosilane and 3.7 g (54.5 mmol) of imidazole. Hydrolysis of
the silyl ester function with 5.5 g of potassium carbonate in methanol
(75 ml)/~ (22 ml)/water (12 ml) yields the title compound~
tRe,(II)=32.7 min.
I) d) 5(S)-(Boc-amino)-4(S)-(tert-butYldimethYlsilvloxY)-6-phenYl-2(R)-
(p-trifluoromethvlDhenvlmethvl)-hexanovl-(L)-Val-(L)-Phe-morpholin-4-
ylamide
Analogously to Reference Example 9f), 200 mg (0.335 mmol) of 5(S)-(Boc-
amino)-4(S)-(tert-butyldimethylsilyloxy)-6-phenyl-2(R)-(p-trifluoromethyl-
phenylmethyl)-hexanoic and 123 mg (0.369 mmol) of H-(L)-Val-(L)-Phe-
morpholin-4-ylamide (Reference E~arnple lo) in 3.2 ml of NMM/CH3CN
0.25M ar~ reacted with 140 mg (0.37 mmol) of HBTU: tRe,(II~=34.8 min.
2) Boc-Phe~C](p-CF3)Phe-(L)-Val-(L)-(p-F-Phe)-morpholin-4-ylamide
3) Boc-Phe[C~(p-CF3)Phe-(L)-Val-(L)-(p-CH30-Phe)-morpholin-4-ylamide
4) Boc-Phe[C]lp-CF3)Phe-(L)-Val-(L)-Cha-morpholin-4-ylamide
S) Boc-Phe[C](p-CF3)Phe-(L)-Ile-(L)-Phe-morpholin-4-ylamide
H) 1) Boc-Cha[C](p-CN)Phe-(L)-Val-(L)-(p-F-Phe)-morpholin-4-ylamide -~ i
2) Boc-Cha[C3(p-CN)Phe-(L)-Val-(L)-(p-CH30-Phe)-morpholin-4-ylamide
3) Boc-Cha[C](p-CN)Phe-(L)-Val-(L)-Cha-morpholin-4-ylamide
4) Boc-Cha[(:](p-CN)Phe-(L)-Ile-(L)-Phe-morpholin-4-ylamide
I) l) Boc-Cha[C](p-CH30)Phe-(L)-Val-(L)-Phe-morpholin-4-ylarnide
2) Boc-Cha[C](p-CH3O)Phe-(L)-Val-(L)-(p-F-Phe)-morpholin-4-ylamide
3) Boc-Cha~C](p-CH3O)Phe-(L)-Val-(L)-(p-CH3O-Phe)-morpholin-4-
ylamide
2~1g~
- 125-
4) Boc-Cha[C] (p-CH30)Phe-(L)-Val-(L)-Cha-morpholin-4-ylamide
S) Boc-Cha[C](p-CH30)Phe-(L)-Ile-(L)-Phe-morpholin-4-ylamide
J) 1) Boc-Cha[C](p-CF3)Phe-(L)-Val-(L)-Phe-morpholin-4-ylamide
2) Boc-Cha[C](p-CF3)Phe-(L)-Val-(L)-(p-F-Phe)-molpholin-~ylamide
3) Boc-Cha[C](p-CF3)Phe-(L)-Val-(L)-(p-CH30-Phe)-molpholin-4-ylamide
4)Boc-Cha[C](p-CF3)Phe-(L3-Val-(L)-(~ha-morpholin-4-ylamide ::
5) Boc-Cha[C](p-CF3)Phe-(L)-Ile-(L)-Phe-morpholin-4-ylamide
Reference Example 22: Analogously to one of the above-ment~oned Reference Examples,
or in the manner indicated in detail in the case in question, the following are prepared by ~ -
selection of the corresponding starting compounds:
A) Boc-(L)-Val-Phe[C]Phe-(L)-Val-(L)-Phe-morpholin-4-ylamide; :
B) H-(L)-Val-Phe[C]Phe-(L)-Val-(L)-Phe-morpholin-4-ylamide;
C) Boc-Cha~Cl(p-F~Phe-(L)-Ile-(L~-Phe-morpholin-4-vlamide :
(described in EP 0 532 466)
D) Boc-Cha[C](p-F)Phe-(L)-Val-(L)-Cha-morpholin-4-ylamide;
E) Bor-Phe[C]Phe-(L)-Val-(D)-Phe-morpholin-4-ylamide;
F~ Boc-Phe[C]Phe-(L)-Val(red)-(L)-Phe-morpholin-4-ylamide;
G) Isobutyloxycarbonyl-Phe[C]Phe-(L)-Val-(L)-Phe-morpholin-4-ylamide;
H) Boc-Cha[C](p-CN)Yhe-(L)-Val-(L)-Phe-thiomorpholin-4-ylamide;
I) Boc-Cha~Cl(p-F)Phe-(L)-Val-(L)-Phe-thiornorpholin-4-Ylamide
(described in EP 0 532 466)
J) The compounds from Reference Example 22 A) to 22 G), in which the radical
-thiomorpholin-4-ylamide stands in place of -morpholin-4-ylamide.
2~i~6~1
- 126-
Note: Reference Examples 23 to 32 do not relate to compounds of fonnula 1. ~:
Reference Example 33: Boc-(p-F)PherCl(P-CF~)Phe-lL)-Yal-(L)-PheAmorDholin-4-
vlamide
(descnbed in EP O 532 466) ~ :
.~ . .
Reference Example 34: Boc-(p-F)PherCl(p-CF~)Phe-(L)-Val-(L)-(l)-F-Phe)-morpholin-4-
Ylamide ,
(described in EP O 532 466)
Refereince Example 35: Boc-(p-F)PherCl(~-CF~Phe-(L)-Val-(L)-(P-C~I~O-Phe)-morph~lin-4-ylamide
(described in EP O 532 466)
:' :';:
Reference Example 36: MorDholinosulfonYl-(L~-Val-Phe~ClPhe-(L)-Val-(~)-Phe-morDho-
lin-4-Y1amide
(described in EP O 532 466)
Reference ~3xampl~ 37: MorPholinosulfonyl-PherClLPhe-(L)-Val-(L)-Phe-morPhol n-~yl :nide :
(described in EP O 532 466)
. ~
Reference Example 38: N-(N-(2-PYridYlmethYl~-N-methYl-aminocarbonvl)-(L)-va~
PherClPhe-(L)-Val-(L)-Phe-morDholin-4-ylamide ',
(desclibed in EP O 532 466)
Reference Example 39: 5(S)-(Boc-amino)-4(S)-(acetoxY)-6-Phenvl-2(R)-~henYlmethY
hexanoyl-(L)-Val-(L)-Phe-morpholin-4-Ylamide
(descTibed in EP O 532 466)
Reference Example 40:
The following compounds are obtained in a manner analogous to that described in one of
the above-mentioned Reference Examples: -~ .
A) Boc-(p-CF3)Phe[C]Phe-(L)-Val-(L)-Phe-morpholin-4-ylamide
B) Boc-(p-CF3)Phe[C]Phe-(L)-Val-(L)-(p-F)Phe-morpholin-4-ylamide
-- 21~86~1
- 127-
C) Boc-(p-CF3)Phe[C]Phe-(L)-Val-(L)-(p-CH30)Phe-morpholin-4-ylamide
D) Boc-~p-CF3)Phe[C]Phe-(L)-Val-(L)-Cha morpholin-4-ylamide
E) Boc-(p-CF3)Phe[C]Phe-(L)-Ile-(L)-Phe-morpholin-4-ylamide
F) Boc-(p-CF~)PherCl(p-F~Phe-(L)-Val-(Ll-Phe-morDholin-4-ylamide
Analogously to Reference Example 1), 136 mg (0.146 mmol) of a mixture of 5(S)-(Boc-
amino)-4(S)-(tert-butyldimethylsilyloxy)-6-(p-trifluoromethylphenyl)-2(R)-(p- fluoro-
phenylmethyl)-hexanoyl-(L)-Val-(L)-Phe-morpholin-4-ylamide and 5(R)-(Boc-amino)-4(R)-(tert-butyldimethylsilyloxy)-6-(p-trifluoromethylphenyl)-2(S)-(p-fluorophenyl-
methyl)-hexanoyl-(L)-Val-(L)-Phe-morpholin-4-ylamide are deprotected with 92 mg
(0.293 mmol) of TBAF in 1.5 ml of DMF to yield the title compound: tRet(II)=26.0 min;
FAB-MS (M+H)+=815.
The starting material is prepared as follows:
a) rac. N-Boc-1-(P-trifluoromethvlphenyl)-3-buten-2-amine
Analogously to Reference Example ld), 10.5 g (48.8 mmol) of rac. l-(p-trifluoromethyl-
phenyl)-3-buten-2-amine (Reference Example 40 P) c)) and 13.8 g (63.4 mmol) of Boc
anhydride are reacted in 100 ml of methylene chloride. The reaction mixture is washed
with O.lN HCl and 2 portions of brine, and the aqueous phases are extracted with 2
portions of methylene chloride. The organic phases are dried with Na2SO4, concentrated
by evaporation and precipitated with hexane from a concentrated solution in methylene
chloride to yield the title compound: TLC Rf(P)=0.27; tRet(II)=25.5 min; Anal.: calc. C
60.94%,H6.39%,N4.44%,F18.07%;foundC61.15%,H6.43%,N4.27%,F
18.09%.
b: 2(R)-r 1 (S)-(Boc-amino)-2-(P-trifluoromethvlphenvl)-ethYll-oxirane and
2(S)-~l(R)-(Boc-amino)-2-(p-trifluoromethylphenyl)-ethvll-oxirane
Analogously to Reference Example ld), 13.5 g (42.8 mmol) of rac. N-Boc-1-(p-trifluoro-
methylphenyl)-3-buten-2-amine and 36.B g (214 mmol) of m-chloroperbenzoic acid are
reacted in 284 ml of chloroform. The reaction mixture is partitioned between 3 portions of
methylene chloride, 10 % Na2SO3 solution, sat. Na2CO3 solution, water and brine, and
column chromatography (SiO2, hexane/ethyl acetate 4:1) of the crude product yields the
racemate of the title compounds: TLC E~f(C~=0.15; tRe,(II)--22.9 min; Anal.: calc. C
58.00 %, H 6.08 %, N 4.23 %, F 17.20 %; found C 58.03 %, H 6.33 %, N 4.45 %, F
17.02 %.
-~-" 21186~1
- 128 -
c: 5(S)-~l(S)-(Boc-amino)-2-(p-tritluoromethYlphenyl)-ethvll-3(R~s)-carbethoxydihydr
furan-2-(3H)-one and 5(R)-I 1 (R)-(Boc-amino)-2-(p-trill methylphenYI)-ethvll-
3(S~R)-carbethoxvdihydrofuran-2-(3H)-one
Analogously to Reference Example le), 9.6 g (29.0 mmol) of a mixture of 2(R)-[l(S~
(Boc-amino)-2-(p-trifluoromethylphenyl)-ethyl]-oxirane and 2(S)-[ l (R)-(Boc-amino)-2-
(p-trifluoromethylphenyl)-ethyl]-oxirane in 48 ml of ethanol and 5 ml of THF are reacted
with sodium diethylmalonate (prepared from 153 ml of ethanol, 2 g (87 mmol) of sodium
und 15.4 ml (101 mmol) of malonic acid diethyl ester). Crystallisation by the addition of
hexane to a concentrated solution in ethyl acetate yields a mixture of the tide compounds:
TLC Rf(D)=0.40; tRe,(II~=24.1 min and 24.6 min; FAB-MS (M+Na)+=468.
d: 5(S)-~ 1 (S)-(Boc-amino)-2-(p-trifluoromethYlphenvl)-ethyll-dihvdrofuran-2-(3H)-one
and S(R)-rl(R)-(Boc-amino)-2-(1~-trifluoromethvlphenvl)-ethvll-dihvdrofuran-2-(3H)-one
Analogously to Reference Example lh),9.0 g (20.2 mmol) of a mixture of 5(S)-[l(S)-
(Boc-amino)-2-(p-trifluoromethylphenyl)-ethyl]-3(R,S)-carbethoxydihydrofuran-2-(3H)-
one and S(R)-[l(R)-(Boc-amino)-2-(p-trifluoromethylphenyl)-ethyl]-3(S,R)-carbethoxy-
dihydrofuran-2-(3H)-one in 166 ml of dimethoxyethane are hydrolysed with 86.9 ml of lN
aqueous LiOH solution. Decarboxylation of the resulting carboxylic acids in 350 ml of ~
toluene (9 h 120C) and crystallisation of the crude product by the addition of hexane to a ~ -
concentrated solution in ethyl acetate yields the title compound in the form of a racemate:
tRet(II)=23.2 min; Anal.: calc. C 57.90 %, H 5.94 %, N 3.75 %, F 15.26 %; found C
57.70%,HS.78%,N3.82%,F15.42%.
e: 5(S)-rl(S)-(Boc-amino)-2-(p-trifluoromethYlPhenYl)-ethvll-3(R)-~p-fluorophenvl- -
methYl)-dihvdrofuran-2-(3H)-one and S(R)-~l(R)-(Boc-arnino~-2-(p-trifluoromethYl-
PhenYl)-ethvll-3(S)-(p-fluorophenvlmethyl)-dihvdrofuran-2-(3H)-one
Analogously to Reference Example 21 D) l)c),700 mg (1.88 mmol) of a mixture of 5(S)- -
[ l (S)-(Boc-amino)-2-(p-trifluoromethylphenyl)-ethyl~-dihydrofuran-2-(3H)-one and
5(R)-[l(R)-(Boc-amino)-2-(p-~ifluoromethylphenyl)-ethyl]-dihydrofuran-2-(3H)-onedissolved in 3.4 ml of THF and 0.38 ml of 1,3-dimethyl-3,4,5,6-te~ahydro-2(1H)-pyIimid-
inone are deprotonated at -75C with 3.67 ml of lithium bis(trimethylsilyl)amide lM in
THF, and alkylated at -75C (40 min) with 0.242 ml (1.88 mmol) of 4-fluorobenzyl brom-
ide (Fluka; Buchs/Switzerland). Column chromatography (SiO2, hexane/ethyl acetate 2:1)
yields the tide compound: TLC Rf(D)=0.59; tRe,(ll)=26.6 min; FAB-MS (M+H)+=482.
-- 211~
- 129-
f) S(S)-(Boc-amino)-4(S)-hydroxy-6-(p-trifluoromethylphenvl)-2(R)-(p-fluorophen
methyl)-hexanoic acid and 5(R)-(Boc-amino)-4(R)-hydroxv-6-(p-tlifluoromethYlphenvl)-
2(S)-(p-fluorophenylmethyl)-hexanoic acid
Analogously to Reference Example li), 1.1 g (2.28 mmol) of a mixture of 5(S)-[l(S)-
(Boc-amino)-2-(p-~ifluoromethylphenyl)-ethyl]-3(R)-(p-fluorophenylmethyl)-dihydro-
furan-2-(3H)-one and 5(R)-[l(R)-(Boc-amino)-2-(p-trifluoromethylphenyl)-ethyl]-3(S)-
(p-fluorophenylmethyl)-dihydrofuran-2-(3H)-one in 37 ml of dimethoxyethane and 19 ml
of water are hydrolysed with 9.1 ml of lM lithium hydroxide solution. The reaction
mixture, par~ally concentrated by evaporation, is poured onto a mixture of ice, 112 ml of
sat. NH4Cl solution, 9.4 ml of 10 % citric acid solution and 46 ml of methylene chloride,
and methanol is added until the precipitated solid has dissolved to give a clear solution in
the 2 phases. The aqueous phase is extracted with 2 portions of methylene chloride/-
methanol approximately 9:1, and the organic phases are washed with brine, dried with
Na2SO4 and concentrated by evaporation: TLC Rf(D)=0.15; tRe,(II)=22.7 min.
g) 5(S)-(Boc-amino)-4(S)-(tert-butvldimethYlsilyloxv)-6-(p-trifluoromethylphenvl)-2(R)
(p-fluorophenvlmethyl)-hexanoic acid and 5(R)-(Boc-amino)-4(R)-(tert-butvldimethvl-
silyloxy)-6-(p-trifluoromethylphenvl)-2(S)-(p-fluorophenvlmethYl)-hexanoic acid
Analogously to Reference Example lj), 1.1 g (2.20 mmol) of a mixture of 5(S)-(Boc-
amino)-4(S)-hydroxy-6-(p-trifluoromethylphenyl)-2(R)-(p-fluorophenylmethyl)-hexanoic
acid and 5(R)-(Boc-amino)-4(R)-hydroxy-6-(p-trifluoromethylphenyl)-2(S)-(p-fluoro-
phenylmethyl)-hexanoic acid in 2.4 ml of DMF are silylated with 1.52 g (10.1 mmol~ of
tert-butyldimethylchlorosilane and 1.2 g (18.0 mmol) of imidazole. The silyl ester
function is hydrolysed with 1.8 g of potassium carbonate in 50 rnl of methanolllHF/water
3:1 1 and, after extraction and column chromatography (siO2, hexane/ethyl acetate 2:1),
yields the title compound: TLC Rf(D)=0.16; tRCt(II)=32.7 min; FAB-MS (M+H)+=614.
h) S(S)-(Boc-amino~-4(S)-(tert-butYldimethvlsilyloxv)-6-(P-trifluoromethvlphenYl)-2(R)-
(P-fluorophenYlmethvl)-hexanoyl-(L)-Val-(L)-Phe-morpholin-4-ylamide and 5(R)-(Boc-
amino)-4(R)-(tert-butYldimethvlsilvloxy)-6-(p-~ifluoromethvlphenYl)-2(S)-(p-fluoro-
PhenvlmethYl)-hexanovl-(L)-Val-(L)-Phe morpholin-4-Ylamide
Analogously to Reference Example 9f), 200 mg (0.326 mmol) of a mixture of 5(S)-(Boc-
amino)-4(S)-(tert-butyldimethylsilyloxy)-6-(p-trifluoromethylphenyl)-2(R)-(p-fluoro-
phenylmethyl)-hexanoic acid and 5(R)-(Boc-amino)-4(R)-(tert-butyldimethylsilyloxy)-6-
(p-trifluoromethylphenyl)-2(S)-(p-fluorophenylmethyl)-hexanoic acid and 119 mg
(0.358 mmol) of H-(L)-Val-(L)-Phe-morpholin-4-ylamide (Reference Example lo) in
211~6~1
-130-
3.1 ml of NMM/CH3CN 0.25M are reacted with 136 mg (0.36 mmol) of HBTU to yield
the title compound: tRe,(II)=34.5 min; FAB-MS (M+H)+=929.
G) Boc-(p-CF3)Phe[C](p-F)Phe-(L)-Val-(L)-(p-F)Phe-morpholin-4-ylamide
H) Boc-(p-CF3)Phe[C](p-F)Phe-(L)-Val-(L)-Cha-morpholin-4-ylamide
I) Boc-(p-CF3)Phe[C](p-F)Phe-(L)-Val-(L)-(p-CH30)Phe-morpholin-4-ylamide
J) Boc-(p-CF3)Phe[C](p-F)Phe-(L)-Ile-(L)-Phe-morpholin-4-ylamide
K) Boc~ CF~)PherCl(p-CF~)Phe-(L)-Val-(L)-Phe-morPholin-4-vlamide
Analogously to Reference Example 1), 110 mg ~0.112 mmol) of a mixture of 5(S)-(Boc-
amino)-4(S)-(tert-butyldimethylsilyloxy)-6-(p-trifluoromethylphenyl)-2(R)-(p-trifluoro-
methylphenylmethyl)-hexanoyl-(L)-Val-(L)-Phe-morpholin-~ylamide and 5(1?)-(Boc-
amino)-4(R)-(tert-butyldimethylsilyloxy)-6-(p-trifluoromethylphenyl)-2(S)-(p-trifluoro-
methylphenylmethyl)-hexanoyl-(L)-Val-(L)-Phe-morpholin-4-ylamide are d~protectedwith 71 mg (0.225 mmol) of TBAF in 1.1 ml of DMF to yield the title compound:
tRe,(I)=18.0 and 18.6 min (12%); FAB-MS (M~H)t=865.
The starting material is prepared as follows:
a: 5~S~-r1(S)-(Boc-amino)-2-(p-trifluoromethylphenvl)-ethyll-3(R)-(p-trifluoromethyl-
Dhenvlmethyl)-dihvdrofuran-2-(3H)-one and 5(R)-~l(R)-(Boc-amino)-2-(p-trifluoro-methYlphenvl)-ethyll-3(S)-~p-trifluoromethylphenylmethyl)-dihydrofuran-2-(3-H)-one
Analogously to Reference Example 21 D) l~c), 1.5 g (4.02 mmol) of a mixture of S(S~-
[ 1 (S)-(Boc-amino)-2-(p-trifluoromethylphenyl)-ethyl]-dihydrofuran-2-(3H)-one and
5(R)-[l(E~)-(Boc-amino)-2-(p-~ifluoromethylphenyl)-ethyl]-dihydrofuran-2-(3H)-one
dissolved in 7.3 ml of THF and 0.81 ml of 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimid-
inone are deprotonated at -75C with 7.86 ml of lithium bis(trimethylsilyl)amide lM in
THF and alkylated at -75C (40 min) with 1.01 g (4.02 mmol) of 4-trifluoromethylbenzyl
bromide (Fluka; Buchs/Switzerland). Column chromatography (SiO2, methylene
chloride/hexane/ether 10:10:1) yields the title compound: TLC Rf(Q)=0.23;
tRe,(II)=27.7 min; FAB-MS (M+H-Boc)+=432.
b) 5(S)-(Boc-amino)-4(S)-hydroxY-6-(p-trifluoromethylphenyl)-2(R)-(p-trifluoromethyl-
phenvlmethyl)-hexanoic acid and 5~R)-(Boc-amino)-4(R)-hYdroxv-6-(p-trifluoromethyl-
phenyl)-2(S)-(p-trifluoromethvlphenylmethyl)-hexanoic acid
Analogously to Reference Example li), 1.43 g (2.69 mmol) of a mixture of 5(S)-[I(S)-
2 ~
- 131 -
(Boc-amino)-2-(p-trifluoromethylphenyl)-ethyl]-3(R)-(p-trifluoromethylphenylmethyl)-
dihydrofuran-2-(3H)-one and 5(R)-[l(R)-(Boc-amino)-2-(p-trifluoromethylphenyl)-
ethyl]-3(S)-(p-trifluoromethylphenylmethyl)-dihydrofuran-2-(3H)-one in 43 ml of
dimethoxyethane and 22 ml of water are hydrolysed with 10.7 ml of lM lithium hydroxide
solution. The reaction mixture, partially concentrated by evaporation, is poured onto a
mixture of ice, 132 ml of sat. NH4Cl solution, 11 ml of 10 % citric acid solution and 54 ml
of methylene chloride, and methanol is added until the precipitated solid has dissolved.
The aqueous phase is extracted with 2 ps)rtions of methylene chloride/methanol approx-
imately 4:1, and the organic phases are washed with brine, dried with Na2SO4 and concen-
trated by evaporation: tRe~(lI)=24.2 min; FAB-MS (M+H)+=550.
c) S(S)-(Boc-amino)-4(S~-(tert-butvldimethylsilyloxv)-6-(p-trifluoromethvlphenvl~-2(R)-
(p-trifluoromethvlPhenvlmethvl~-hexanoic acid and 5(R)-(Boc-amino)-4(R)-(tert-butYl-
dimethvlsilvloxY)-6-(p-trifluoromethylphenyl)-2(s)-(p-trifluoromethylphenvlmethyl)
hexanoic acid
Analogously to Reference Example lj), 1.38 g (2.51 mmol) of a mixture of 5(S)-(Boc-
amino)-4(S)-hydroxy-6-(p-trifluoromethylphenyl)-2(R)-(p-trifluoromethylphenylmethyl)-
hexanoic acid and 5(R)-(Boc-amino)-4(R)-hydroxy-6-(p-trifluoromethylphenyl)-2(S)-
(p-trifluoromethylphenylmethyl)-hexanoic acid in 5.7 ml of DMF are silylated with 1.74 g
(11.6 mmol) of tert-butyldimethylchlorosilane and 1.4 g (20.6 mmol) of imidazole.
Hydrolysis of the silyl ester function with 2.1 g of potassium carbonate in 55 ml of
methanol/l~/water 3:1:1 yields the title compound: TLC Rf(A)=0.25; tRet(l)=21.8 min.
d) 5(S)-(Boc-amino)-4(S)-(tert-butvldimethYlsilYloxY)-6-(p-trifluoromethvlPhenyl)-2(R)
(p-trifluoromethvlphenYlmethyl)-hexanoYl-(L)-Val-(L)-Phe-morpholin-4-YIamide and
S(R)-(Boc-amino)-4(R)-(tert-butyldimethylsilvloxy~-6-(p-trifluoromethYlphenYI)-2(S)-
(p-trifluoromethvlPhenvlmethyl)-hexanovl-(L)-Val-(L)-Phe-morpholin-4-vlamide
Analogously to Reference Exarnple 9f), 200 mg (0.301 mmol) of a mixture of 5(S)-(Boc-
amind)-4(S)-(tert-butyldimethylsilyloxy)-6-(p-trifluoromethylphenyl)-2(R)-(p-trifluoro-
methylphenylmethyl)-hexanoic acid and 5(E~)-(Boc-arnino)-4(R)-(tert-butyldimethylsilyl-
oxy)-6-(p-trifluoromethylphenyl)-2(S)-(p-trifluoromethylphenylmethyl)-hexanoic acid and
110 mg (0.331 mmol) of H-(L)-Val-(L)-Phe-morpholin-4-ylamide (Reference Example
lo) in 2.8 ml of NMMlCH3CN 0.25M are reacted with 126 mg (0.33 mmol) of HBTU. Inthe course of the reaction, one of the diastereoisomers precipitates from the reaction
mixture and can be fil~ered off ( ~ 5(R),4(R)~2(S)). By means of extraction (analogously
to Reference Example 9f) of tlle mother liquor and column chromatography (SiO2,
~'' :' : . : '
~` 2 1 1 ~
- 132-
hexane/ethyl acetate 1:1), the other diastereoisomer (~ 5(S),4(S),2(R)) can be isolated in
a diastereoisomenc punty of approximately 90 % (according to lH-NMR spectroscopy):
TLC Rf~A)=0.15; tRe~ 23.0 min (diastereoisomer not separated); lH-NMR (300 MHz,CD30D, after cristallisation (~ 5(R),4(R),2(S))): inter alia 0.60 (dd, (H3C)2CH),
lH-NMR (300 MHz, CD30D, a~ter chromatography ( ~ 5(S),4(S),2(R))): inter alia 0.91
(dd, (H3C)2CH).
L) Boc-(p-CF3)Phe[C](p-CF3)Phe-(L)-Val-(L)-(p-F)Phe-morpholin-4-ylamide
M) Boc-(p-CF3)Phe[C](p-CF3)Phe-(L)-Ile-(L)-Phe-morpholin-4-ylamide
N) E~oc-(p-CF3)Phe[C](p-CF3)Phe-(L)-Val-(L)-(p-CH3O)Phe-morpholin-4-ylamide
O) Boc-(p-CF3)Phe[C](p-CF3)Phe-(L)-Val-(L)-Cha-morpholin-4-ylamide
P) The compounds according to the above Reference Exarnples A) to O), in which the
radical -morpholin-4-ylamide has been replaced by the radical -thiomoIpholin-4-ylamide.
The starting material is prepared as follows:
a) N-Allvlformamlde
A solution of 300 ml of al1ylamine in 1288 ml of ethyl formate is heated for 8 h at 60C.
The reaction mixture is concentrated in a rotar~y evaporator and the residue is distilled over
a Vigreux column (77C; 1 mbar): lH-NMR (200 MHz, CDCl3): 8.2-7.95 (m,1 H),
6.5-5.8 (sb,1 H),5.9-5.7 (m, 1 H),5.3-5.05 (m,2 H),3.95-3.75 (m,2 H).
- ~ ~
b) A11Y1 isocyanide
(U. ScholL~copf, R. Jentsch, K. Madawinata and R. Harms, Liebigs Ann. Chem., (1976)
2105). Under a nitrogen atmosphere,517 g of quinoline and 286 g of p-toluenesulfonic
acid chloride are placed in a vessel at 90C. A vacuum of 2-4 mbar is applied and 85 g of
N-allylformamide are added dropwise, the resulting isocyanide being distilled off
continuously at an internal temperature of 85-95C over a Vigreux column into a
cond~nsation trap (acetone/dry ice). When the reaction is complete, the distillate is
immediately distilled once more over a Vigreux column (nitrogen atmosphere, nor nal
pressure; 100C): IH-NMR (200 MHz, CDCl3): 5.9-5.7 (m, 1 H), 5.45 (d, 16 Hz, 1 H),
5.32 (d, 10 Hz, 1 H), 4.05 (m, 2 H); IR (CH2Cl2): 2150, 1650.
:
c) rac. l-(p-TrifluoromethYlphenyl)-3-buterl-2-amine
Under a nitrogen atmosphere, 4.5 g of allyl isocyanide are dissolved in 100 ml of
THF/ether/pentane abs. 4:1:1 and cooled to -100C. At from -100 to -90C, 42 ml of
... ~: .::, . .;. .. .. ; : ~ ....... :
2 ~
- 133-
n-butyllithium (1.6M in hexane) are added dropwise, whereupon first vf all a yellow
colour forms and, shortly before the end of the addition, a solid is precipitated. The
reaction mixture is allowed slowly to warm to -70C and is then cooled again to -100C.
At ~rom -100 to -85C, a solution of 16 g of p-trifluoromethylbenzyl bromide (Fluka;
Buchs/Switzerland) in 10 ml of THF is added dropwise, and the mixture is slowly warmed
to RT. The reaction mixture is concentrated by evaporation using a rotary evaporator
(80 mbar; 30C), and the residue is poured onto 150 ml of ice-water and extracted 3 times
with ether. The ethereal phases are concentrated by evaporation, 85 ml of methanol and
17 ml of concentrated hydrochloric acid are added to the brown reisidue at 0C, and the
mixture is left overnight in a refrigerator. The mixture is concentrated by evaporation
using a rotary evaporator and the residue is partititioned between 2 x 150 ml of 2M hydro-
chloric acid and 2 x 200 ml of ether. The combined aqueous phases are rendered alkaline
with solid sodium hydroxide, with cooling, and extracted with 3 portions of ethyl acetate.
The organic phases are washed with brine, dried with sodium sulfate, concentrated by
evaporation and distilled in a bulb tube (0.1 mbar; 170C) to yield the pure title
compound: lH-NMR (200 MHz, CDCl3): 7.56 and 7.32 (2d, 8 Hz, each 2 H), 5.96-5.78(m, 1 H), 5.19-5.02 (m, 2 H), 3.68-3.55 (m, 1 H), 2.87 and 2.71 (AB x d, Jab= 13 Hz, J1=6
Hz, J2= 8 Hz, 2 H), 1.4 (sb, 2 H).
Further reaction analogously to Reference Examples 1 d) to 1 k) and 1), 9 f) and 9, 10 f)
and 10, 15 a) and 15 or 16 c) and 16 results in the compounds mentioned above under a) to
o).
ReferenceExample 41:
The following are prepared analogously to one of the a~ove Reference Examples:
A) Boc-Phe~ClPhe-(L~-Val-(L)-Tvr-morpholin-4-vlamide
Anal'ogously to Reference Example 1), 360 mg (0.418 mmol) of 5(S)-(Boc-amino)-4(S)-
(tert-butyldimethylsilyloxy)-6-phenyl-2(R)-phenylmethylhexanoyl-(L)-Val-(L)-Tyr-morpholin-4-ylamide are deprotected with 263 mg ~0.84 mmol) of TBAF in 7 ml of DMF
to yield the title compound: TLC Rf(B)=0.28; tRe,(II)=20.1 min; FAB-MS (M+H)+=745.
The starting material is prepared as follows:
211~fi61
- 134-
a: Z-(L)-Tvr-morpholin-4-Ylamide
9.08 g (44 mmol) of DCC are added to an ice-cooled suspension of 14.04 g (40 mmol) s)f
Z-(L)-Tyr-OH in 750 ml of methylene chloride and the mixture is stirred for 20 min.
Subsequently, 10.81 g (80 mmol) of HOBT and a solution of 5.23 g (60 mmol) of
morpholine in 50 ml of methylene chloride are added. The mixture is stirred for 18 h at RT
and then ~lltered. The filtrate is washed with sat. NaHCO3 solution, water and brine and
the aqueous phases are extracted with 2 portions of methylene chloride. The organic
phases are dried with Na2SO4, concentrated by evaporation and subjected to column
chroma~ography (SiO2, ethyl acetate) to yield the title compound: TLC Rf(B)=0.39.
b: H-(L)-Tvr-morPholin-4-ylamide
A solution of 2.05 g (5.3 mmol) of Z-(L)-Tyr-morpholin-4-ylamide in 91 ml of methanol
is hydrogenated for 1.5 h at RT in the presence of 0.5 g of 10 % PdlC. Filtration through
(~Celite (filter aid of diatomaceous earth, John-Manville Corp.) and concentration of the ~ `
filtrate by evaporation yields the title compound: TLC Rf(R)=0.34. -
c: Z-(L~-Val-(L)-Tyr-morpholin-4-ylamide
At 0C, 5.18 g (25 mmol) of DCC and 3.73 g (27.S mmol) of HOBT are added to a
solution of 6.3 g (25 mmol) of Z(L)-Val-OH in 400 ml of methylene chloride and the
mixture is then stirred for 20 min. A solution of 6.27 g (25 mmol) of H-(L)-Tyr-morpholin-4-ylamide in 600 ml of methylene chloride is subsequently added. After the
mixture has been stirred for 18 h at RT, working up is carried out as described in
Reference Example 41 A) a): TLC Rf(B)=0.50.
d: H-(L)-Val-(L)-TYr-morPholin-4-Ylamide
Hydrogenation in the presence of 1 g of 10 % Pd/C for 1.5 h at RT of a solution of 4.83 g
(10 mmol) of Z-(L)-Val-(L)-Tyr-morpholin-4-ylamide in 182 ml of methanol, followed by
filtration through (E~Celite, concentration of the filtrate by evaporation and column
chromatography (SiO2, methylene chloride/methanol 9:1), yields the title compound: TLC
Rf(F)=0.30; FAB-MS (M+H)+=350.
e: 5(S)-(Boc-amino)-4(S)-(tert-butyldimethylsilyloxy)-6-phenyl-2(R)-phenvlmethyl-
hexanovl-(L)-Val-(L)-TYr-morpholin-4-ylam_de
Analogously to Reference Example lk), 300 mg (0.569 mmol) of 5(S)-(Boc-amino)-4(S)-
(tert-butyldimethylsilyloxy)-6-phenyl-2(R)-phenylmethylhexanoic acid (Reference
Example 2d)) in 5 ml of DMF are activated with 277 mg (0.626 mmol) of BOP, 84.5 mg
, . . - ~ . ~ : . . . .
-` 211~6~1
- 135-
(0.625 mmol) of HOBT and 157 1ll (1.42 mmol) of NMM, and reacted with 198.6 mg
(0.568 mmol) of H-(L)-Val-(L)-Tyr-morpholin-4-ylamide in 2 ml of DMF (2 h RT).
Column chromatography (SiO2, ethyl acetate/hexane 2:1) yields the title compound: TLC
Rf(I)=0.26; tRet(II)=32.2 min; FAB-MS (M~H)+=859.
B) Boc-TYr~ClPhe-(L)-Val-(L)-Phe-morpholin-4-vlamide
Hydrogenation in the p~esence of 82 mg of 10 % Pd/C at RT of 165 mg (0.197 rnmol) of
Boc-(p-BzlO)Phe[C]Phe-(L)-Val-(L)-Phe-morpholin-4-ylamide (Reference Example 46)dissolved in 12 ml of methanol, followed by filtration through ~Celite and concentradon
of the filtrate by evaporation, yields the title compound: tRet(I)=13.9 min; FAB-MS
(M+H)+-745.
C) Boc-TyrrClPhe-(L)-Val-(L)-Tyr-morpholin-4-vlarnide
Hydrogenation in the presence of 120 mg of 10 % Pd/C at RT of 235 mg (0.25 mmol) of
Boc-(p-BzlQ)Phe[C]Phe-(L)-Val-(L)-(p-BzlOPhe)-morpholin-4-ylamide (Reference
Example 47) dissolved in 30 ml of methanol, followed by ~lltration through (~)Celite,
concentration of the filtrate by evaporation and crystallisation from ethyl acetate/hexane,
yields the title compound: TLC Rf(B)=0.43; tRet(I)=12.1 min; FAB-MS (M+H)+=761.
D) Boc-PherClTyr-(L)-Val-(L)-Phe-mor~olin-4-Ylamide
~lydrogenation in the presence of 70 mg of 10 % Pd/C at RT of 140 mg (0.168 mmol) of
Boc-Phe[C](p-BzlO)Phe-(L)-Val-(L)-Phe-molpholin-4-ylamide (Reference Example 44)dissolved in 10 ml of methanol, followed by filtration through ~)Celite and concentration
of the filtrate by evaporation, yields the title compound: tRet(II)=19.9 min; FAB-MS
(M+H)+=745. ;
E) Boc-PherC1Tyr-(L)-Val-(L)-TYr-morpholin-4-ylamide
Hydrogenation in the presence of 75 mg of 10 % Pd/C at RT of 140 mg (0.159 mmol) of
Boc-Phe[C](p-BzlO)Phe-(L)-Val-(L)-(p-BzlOPhe)-moIpholin-4-ylarnide (Reference
Example 45) dissolved in 10 ml of methanol, followed by fil~ration through (3~Celite and
concentration of the filtrate by evaporation, yields the title compound: tRet(II)=17.2 min;
FAB-MS (M+H)+-761.
F) Boc-TYrrClTvr-(L)-Val-(L)-Phe-moIpholin-4-~lamide
Hydrogenation in the presence of 85 mg of 10 % Pd/C at E~T of 188 mg (0.178 mmol) of
Boc-(p-BzlO)Phe[C](BzlO)Phe-(L)-Val-(L)-Phe-morpholin-4-ylarnide (Reference
~" 2118~
- 136-
Example 48) dissolved in 20 ml of methanol, followed by filhration through (3)Celite and
ConCentrahon of the filtrate by evaporation, yields the title compound: TLC Rf(B)=0.50;
tRe,(I)=12.1 min; FAB-MS (M+H)+-761.
: .
G) Boc-Tyr~ClTvr-(L)-Val-(L)-Tyr-morpholin-4-ylamide
Hydrogenation in the presence of 85 mg of 10% Pd/C at RT of 209 mg (0.20 mmol) of
Boc-(p-BzlO)Phe[C3(p-BzlO)Phe-(L)-Val-(L)-(p-BzlOPhe)-morpholin-4-ylamide
(Reference Example 49) dissolved in 20 ml of methanol, followed by ~lltration and
concentration of the filtrate by evaporation, yields the title compound: TLC Rf(B)=0.15;
tRe,(I)=10.6 min; FAB-MS (M+H)+=777.
H) The compounds according to the above Reference Examples A) to G), in which the
radical -morpholin-4-ylamide has been replaced by the radical -thiomorpholin-4-ylamide.
The Reference Examples mentioned in the following are not explicitly disclosed in EP 0
532 466.
Reference Example 42: Boc-PherCl(o-CN)Phe-(L)-Val-(L)-Phe-morpholin-4-Ylamide
Analogously to Reference Example 1), 145 mg (0.167 mmol) of 5(S)-(Boc-amino)-4(S)-
(tert-butyldimethylsilyloxy~-6-phenyl-2(R)-(o-cyanophenylmethyl)-hexanoyl-(O-Val-
(L)-Phe-morpholin-4-ylamide are deprotected with 105 mg (0.334 mmol) of TBAF in 4 ml
of DMF. P~ecipitation with DIPE from a concentrated solution in DMF yields the pure
ti~le compound: tRet(I)=15.7 min; FAB-MS (M+H)+=754.
The starting material is prepared as follows:
a) 5(S)-~l(S)-(Boc-amino)-2-phenYlethvll-3(R)-(o-cYanophenYImethYl)-dihydrofuran-2
(3H)-one
Analogously to Reference Example 21 D) l)c), 2.0 g (6.55 mmol) of S(S)-[l(S)-(Boc-
amino)-2-phenylethyl]-dihydrofuran-2-(3H)-one [Reference Example 21 D~ l)b)]
dissolved in 40 ml of THF are deprotonated with 13.1 ml of lithium bis(trimethylsilyl)-
amide lM in THF and alkylated (75 min) with 1.4 g (7.2 mmol) of 2-bromomethylbenzo-
nitrile (Fluka; Buchs/Switærland). Column chromatography (SiO2, hexane/ethyl acetate
2:1) and stirring in hexane yields the pure title compound: TLC Rf(D)=0.45;
tRet(I)=16.2 min.
211~
- 137-
b) 5(S)-(Boc-amino)-4(S)-hvdroxy-6-phenyl-2(R)-(o-cYanophenylmethYl)-hexanoic acid
Analogously to Reference Example li), 1.67 g (3.97 mmol) of 5(S)-[l(S)-(Boc-amino)-
2-phenylethyl]-3(R)-(o-cyanophenylmethyl)-dihydrofuran-2-(3H)-one in 37 ml of
dimethoxyethane and 20 ml of water are hydrolysed with 16 ml of lM lithium hydroxide
solution to yield the title compound: tRe~(I)=13.8 min.
c) S(S)-(Boc-amino)-4(S)-(tert-butyldimethylsilyloxy)-6-phenvl-2(R)-(o-cyanophenyl-
methvl)-hexanoic acid
Analogously to Reference Example lj), 0.85 g (1.93 mmol) of S(S)-(Boc-amino)-4(S)-
hydroxy-6-phenyl-2(R)-(o-cyanophenylmethyl)-hexanoic acid in 10 ml of DMF are
silylated with 1.34 g (8.9 mmol) of tert-butyldimethylchlorosilane and 1.08 g (1~.9 mmol)
of imidazole. Hydrolysis of the silyl ester function with 1.6 g of potassium carbonate in
40 ml of methanoUIHF/water 5:1:2, followed by column chromatography (SiO2, ethylacetate/hexane 1: 1), yields the title compound: ILC Rf(A)=0.4; tRe,(I)=20.0 min.
d) 5(S)-(Boc-amino)-4(S)-(tert-butyldimethvlsilyloxv)-6-phenyl-2(10-(o-cyanophenYl-
methyl)-hexanovl-(L)-Val-~L)-Phe-morpholin-4-ylamide "
At 5C, 41 mg (0.20 mmol) of DCC are added to 100 mg (0.18 mmol) of S(S)^(Boc-
amino)-4(S)-(tert-butyldimethylsilyloxy)-6-phenyl-2(R)-(o-cyanophenylmethyl)-hexanoic
acid in 3 ml of THF. After 10 min, 66 mg (0.20 mmol) of H-(L)-Val-(L)-Phe-morpholin-
4-ylamide (Reference Example lo) and 27 mg (0.20 mmol) of HOBT are added and themixture is stirred for 17 h at RT. The reaction mixture is filtered and the filtrate is parti-
tioned between 3 portions of ethyl acetate, 10 % citric acid solution, water, sat. NaHCO3
solution and brine. The organic phase is dried with Na2SO4, concentrated by evaporation
and digested in DIPE to yiekl the title compound: tRet(I)=21.8 min; FAB-MS
(M+H)+=868.
Reference Example 43: Boc-PherCl(m-CNlPhe-(L~-Val-(L)-Phe-morPholin4-ylamide
Analogously to Reference Example 1), 131 mg (0.151 mmol) of 5(S)-(Boc-amino)-4(S)-
(tert-butyldimethylsilyloxy)-6-phenyl-2(R)-(m-cyanophenylmethyl)-hexanoyl-(L)-Val-
(L)-Phe-morpholin-4-ylamide are deprotected with 95 mg (0.301 mmol) of TBAF in 4 ml
of DMF. Precipitation with DIPE from a concentrated solution in DMF yields the pure
title compound: tRet(I)=15.7 min; FAB-MS (M+H)+=754.
The starting material is prepared as follows:
211~6~1
- 138-
a) S(S)-r 1 (S)-(Boc-amino)-2-phenylet}lvll-3(R~-(m-cyanophenylmethyl)-dihvdrofaran-2-
(3H~-one
Analogously to Reference Example 21 D) l)c), 2.0 g (6.55 mmol) of 5(S)-[l(S)-(Boc-
amino)-2-phenylethyl]-dihydrofuran-2-(3H)-one [Reference Example 21 D) l)b)]
dissolved in 40 ml of THF are deprotonated at -75C with 13.1 ml of lithium bis(~
methylsilyl)amide lM in THF and alkylated (60 min -60C) with 1.4 g (7.2 mmol) of
3-bromomethylbenzonitrile (Fluka; BuchstSwitzerland). Column chromatography (SiO2,
hexane/ethyl acetate 2:1) and stilring in hexane yields the pure title compound: TLC
Rf(D)=0.41; tRet(I)=16.1 min.
b) 5(S)-(Boc-amino)-4(S)-hvdroxY-6-phenYl-2(R)-(m-cYanoPhenylmethyl)-hexanoic acid
Analogously to Reference Example li), 1.6 g (3.8 mmol) of 5(S)-[l(S)-(Boc-amino)-
2-phenylethyl]-3(R)-(m-cyanophenylmethyl)-dihydrofuran-2-(3H)-one in 37 ml of
dimethoxyethane and 20 ml of water are hydrolysed with 15.2 ml of lM lithium hydroxide
solution to yield the title compound: tRet(I)=13.8 min.
c) S(S)-(Boc-amino)-4(S)-(tert-butYldimethvlsilYloxy~-6-phenY1-2(R)-(m-cvanoPhenYl-
methyl)-hexanoic acid
Analogously to Reference Example lj), 1.4 g (3.2 mmol) of 5(S)-(Boc-amino)-4(S)-
hydroxy-6-phenyl-2(R)-(m-cyanophenylmethyl)-hexanoic acid in 20 ml of DMF are -
silylated with 2.2 g (14.6 mmol) of tert-butyldimethylchlorosilane and 1.8 g (26 mmol) of
imidazole. Hydrolysis of the silyl ester function with 2.6 g of potassium carbonate in
55 ml of methanol/l~lF/water 8:1:2, followed by column chromatography (siO2, ethyl
acetate/hexane 1:1), yields the title compound: TLC Rf~A)=0.39; tRet(I)=19.8 min.
d) 5(S)-(Boc-amino)-4(S)-(tert-butvldimethylsilyloxy)-6-phenvl-2(R)-(m-cvanophenyl-
methYI)-hexanoYl-(L)-Val-(L2-Phe-morpholin-4-ylamide
Analogously to Reference Example 42 d), 100 mg (0.18 mmol) of 5(S)-(Boc-amino)-
4(S)-(tert-butyldimethylsilyloxy)-6-phenyl-2(R)-(m-cyanophenylmethyl)-hexanoic acid in
3 ml of THF are activated with 41 mg (0.20 mmol) of DCC and reacted with 66 mg
(0.20 mmol) of H-(L)-Val-(L)-Phe-morpholin-4-ylamide (Reference Example lo) and
27 mg (0.20 mmol) of HOBT to yield the title compound: tRe,(I)=21.5 min; FAB-MS
(M+H)+=868.
E~eference Example 44: Boc-Phe~Cl(p-BzlO)Phe-(L)-Val-(L)-Phe-morpholin-4-~lamideAnalogously to Reference Example 1), 560 mg (0.59 mmol) of S(S)-(Boc-amino)-4(S)-
r~, . . ~ ;~ ` ' '. .' ', . :
'`': : .: :~, ' `
2 ~
- 139-
(tert-butyldimethylsilyloxy)-6-phenyl-2(R)-(p-benzyloxyphenylmethyl)-hexanoyl-(L)-
Val-(L)-Phe-mo}pholin-4-ylamide are deprotected with 372 mg (1.18 mmol) of TBAF in
8.4 ml of DMF to yield the title compound: tRe,(II)=26.1 min; FAB-MS (M+H)+=835.The starting material is prepared as follows~
a) p-BenzvloxYbenzvl iodide
A solution of 1.0 g (4.3 mmol) of 4-benzyloxybenzyl chloride (Fluka; Buchs/Switzerland)
in ~ ml of ace~one is stirred at RT with 3.13 g (20.9 mmol) of sodium iodide. According to
a gas chromatrogram, the reaction is complete after 90 min. Working up as described in
Reference Example 21 F) 1) a) yields the title compound: IH-NMR (200 MH~, CDCl3:4.48 (s, 2 H),5.06 (s, 2 H),6.85-6.95 (m, 2 H),7.25-7.48 (m,7 H).
b) S(S)-rl(S)-(Boc-amino)-2-phenvlethyll-3(R)-(p-benzvloxvphenylmethyl)-dihydro-furan-2-(3H)-one
Analogously to Reference Example 21 D) l)c), 1.13 g (3.70 mmol) of 5(S)-[l(S)-(Boc-
amino)-2-phenylethyl]-dihydrofuran-2-(3H)-one [Reference Example 21 D) l)b)]
dissolved in 4.8 ml of THF and 0.75 ml of 1,3-climethyl-3,4,5,6-tetrahydro-2(1H)-pyrimid-
inone are deprotonated at -75C with 7.25 ml of lithium bis(trimethylsilyl)amide lM in
THP, and alkylated (15 min) with 1.2 g (3.7 mmol) of p-benzyloxybenzyl iodide in 2 ml of
THF. Column chromatography (SiO2, hexane/ethyl acetate 2:1) yields the pure tidecompound: TLCRf(D)=0.30; tRet(II)-28.2min;FAB-MS (M+H)+=502.
c) 5(S)-(Boc-amino)-4(S)-hYckoxY-6-Phenyl-2(R)-(p-benzYloxy-uhenvlmethyl)-hexanoic
acid
Analogously to Reference Example li), 1.4 g (2.79 mmol) of 5(S)-[l(S)-(Boc-amino)-
2-phenylethyl]-3(R)-(p-benzyloxyphenylmethyl)-dihydrofuran-2-(3H)-one in 45 ml of
dimethoxyethane and 23 ml of water are hydrolysed with 11 rnl of lM lithium hydroxide
solution. The reaction mixture, pa~tially concentrated by evaporation, is poured onto a
mixture of ice, 137 ml of sat. NH4CI solution,11 ml of 10% citric acid solution and 56 ml
of methylene chloride, and methanol is added until the precipitated solid has dissolved.
The aqueous phase is extracted with 2 portions of methylene chloride/methanol
approximately 10:1, and the organic phases are washed with brine, dried with Na2SO4 and
concentrated by evaporation: tRet(II)=24.0 min; FAB-MS (M+H)+=520.
211~6~1
- 14() -
d) S(S~-(Boc-amino)-4(S)-(tert-butvldimethvlsilyloxv)-6-phen~ -2(R)-(p-benzvloxy-
phenvlmethvl)-hexanoic acid
Analogously to Reference Example lj), 1.4 g (2.69 mmol) of 5(S)-(Boc-amino)-4(S)-
hydroxy-6-phenyl-2(R)-(p-benzyloxyphenylmethyl)-hexanoic acid in 2.9 ml of DMF are
silylated with 1.87 g (12.4 mmol) of tert-butyldimethylchlorosilane and 1.5 g (22 mmol)
of imidazole. Hydrolysis of the silyl ester function with 2.2 g of potassium carbonate in
63 ml of methanol/'I~F/water 3: 1: 1 and column chromatography (siO2, hexane/ethyl
acetate 2:1) of the crude product yields the title compound: TLC R~D)=0.17;
tRet(II)=33.7 min; FAB-MS (M+H)+=634.
: .
e) 5(S)-(Boc-amino)-4(S)-(ter~-butvldimethvlsilYloxy)-6-phenYl-2(R)-(P-benzvloxv-
~henylmethyl)-hexanoyl-(L)-Val-(L)-Phe-morpholin-4-vlamide
Analogously to Reference Example 9f), 400 mg (0.631 mmol) of 5(S)-(Boc-amino)-4(S)-
(tert-butyldimethylsilyloxy)-6-phenyl-2(R)-(p-benzyloxyphenylmethyl)-hexanoic acid and
231 mg (0.69 mmol) of H-(L)-Val-(L)-Phe-morpholin-4-ylamide (Reference Example lo)
in 5.7 ml of NMMlCH3CN 0.25M are reacted with 263 mg (0.69 mmol) of HBTU: TLC ~ -
Rf(A)=0.3; tRet(II)=35.8 min; FAB-MS (M+H)+=949.
Reference Example 45: Boc-Phe~Cl(p-BzlO)Phe-(L)-Val-(L)-(p-BzlC)Phe)-morpholin-4-
,rlamide
Analogously to Reference Example 1), 665 mg (0.63 mmol) of 5(S)-(Boc-arnino)-4(S)-
(tert-butyldimethylsilyloxy)-6-phenyl-2(R)-(p-benzyloxyphenylmethyl)-hexanoyl-(L)-
Val-(L)-(p-BzlOPhe)-morpholin-4-ylamide are deprotected with 398 mg (1.26 mmol) of
TBAF in 9 ml of DMF. Precipitation with DIPE from a concentrated solution in
methylene chloride yields the pure title compound: tRet(II)=28.7 min; FAB-MS
(M+H)+=941.
The starting material is prepared as follows:
a: Boc-(L)-(p-BzlOPhe)-morpholin-4-vlamide
9.08 g (44 mmol) of DCC are added to an ice-cooled suspension of 14.84 g (40 mmol) of
Boc-(L)-Tyr(Bzl)-OH (Bachem; Bubendorf/Switzerland) in 650 ml of methylene chloride
and the mixture is stirred for 20 min. Subsequently, 10.81 g (80 mmol) of HOBT and a
solution of 5.23 g (60 mmol) of morpholine in 50 ml of methylene chloride are added.
After having been stirred for 18 h at RT the mixture is filtered. The filtrate is washed with
sat. NaHCO3 solution, water and brine and the aqueous phases are extracted with 2
~; :. . .
-~-` 211~6~1 -
- 141 -
po}tions of methylene chlonde. The organic phases are concentrated by evaporation and
again dissolved in a small amount of methylene chloride, the residual undissolved dicyclo-
hexylurea is filtered off and the filtratc is concentrated by evaporation to yield the title
compound: TLC Rf(B)=0.69.
b: H-(L)-~P-BzlOPhe~-morpholin-4-vlamide
At 0C, 30 ml of TFA are added to a solution of 1.0 g (2.69 mmol) of Boc-(L)-
(p-BzlOPhe)-morpholin-4-ylamide in 30 ml of methylene chloride. After 45 min, the
solvent is evaporated off, and the residue is dissolved in ethyl acetate and washed with sat.
NaHC03 solution, 2x water and brine. The aqueous phases are extracted with 2 portions of
ethyl acetate. The organic phases are dried with Na2SO4 and concentrated by evaporation
to yield the title compound: TLC Rf(F)=0.42.
c: Boc-(L)-Val-(L)-(p-BzlOPhe)-morpholin-4-ylamide
At 0C, 2.68 g (13 mmol) of DCC and 1.93 g (14.3 mmol) of HOBT are added to a
solution of 2.8 g (13 mmol) of Boc-(L)-Val-OH in 350 ml of methylene chloride and the ~ -
mixture is then sdrred for 20 min. A solution of 4.41 g (13 mmol) of H-(L)-(p-BzlOPhe)-
morpholin-4-ylamide and 1.8 ml (13 mmol) of triethylamine in 250 ml of methylenechloride is then added. After stirring for 18 h at RT, working up is caIried out as described
in Reference Example 41 A) a). This yields the pure title compound after column
chromatography (SiO2, ethyl acetate/hexane 3:1): TLC Rf(S)=0.50.
~,
d: H-(L)-Val-L~-(P-BzlOPhe)-morpholin-4-ylamide - --
Analogously ~o Reference Example 45 b), 5.7 g (10.6 mmol) of Boc-(L)-Val-(L)-
(p-BzlOPhe)-morpholin-4-ylamide in 120 ml of methylene chloride are cleaved with120 ml of TFA to yield the title compound: TLC Rf(F)=0.42.
e) 5(S)-(Boc-amino)-4(Sl-(tert-butYldimethYlsilyloxy)-6-phenYl-2(R)-(p-ben
phenvlmethYl)-hexanoyl-(L)-Val-tL)-(p-BzlOPhe)-morpholin-4-ylamide
Analogously to Reference Example 9f), 400 mg (0.631 mmol) of S(S)-(Boc-amino)-4(S)-
(tert-butyldimethylsilyloxy)-6-phenyl-2(R)-(p-benzyloxyphenylmethyl)-hexanoic acid
(Reference Example 44 d)) and 305 mg (0.69 mmol) of H-(L)-Val-(L)-(p-BzlOPhe)- ~ - -
morpholin-4-ylamide in 5.7 ml of NMM/CH3CN 0.25M are reacted with 263 mg
(0.69 mmol) of HBTU: TLC Rf(A)=0.31; t~et(II)=37.1 min; FAB-MS (M+H)+=1055.
,. ~.. , .. ~. .. . .
- `
~ 211~6~
- 142-
Reference Example 46: Boc-(p-B~IO)Phe~ClPhe-(L)-Val-(L)-Phe-morpholin-4-ylamide
Analogously to Reference Example 1), 664 mg (0.70 mmol) of 5(S)-(Boc-amino)-4(S)-
(tert-butyldimethylsilyloxy)-6-(p-benzyloxyphenyl)-2(R)-(phenylmethyl)-hexanoyl-(L)-
Val-(L)-Phe-morpholin-4-ylamide are deprotected with 440 mg (1.40 mmol) of TBAF in
7 ml of DMF. Crystallisation from ipropanoVhexane yields the pure title compound: TLC
Rf(D)=0.32; tRet(I)=17.8 min; FAB-MS (M+H)+=83S.
The starting material is prepared as follows:
a: N-Boc-(p-benzyloxyphenylalaninol)
Analogously to Reference Example 21 B) l)b),37.1 g (100 mmol) of Boc-(L)-
(p-BzlOPhe)-OH (Bachem; Bubendorf/Switzerland) in 116 ml of THF are activated atfrom -5C to -10C with 15.33 ml (110 mmol) of triethylamine and 14.36 ml (110 mmol)
of chloroformic acid isobutyl ester in 70 ml of THF. The filtered reaction mixture is
introduced dropwise into 7.57 g (200 mmol) of sodium borohydride, treatment with water
and digestion in hexane yield the title compound: TLC Rf(A)=0.50; FAB-MS
(M+H)+=358.
b: N-Boc-(P-benzYloxYPhenylalaninal)
Analogously to Reference Example 21 B) l)c), a solution of 3.5 ml of (49 mmol) of
DMSO in 60 ml of methylene chloride is added at -60C to 4.76 g (37.5 mmol) of oxalyl
chloride in 33.6 ml of methylene chloride. The addition of 8.94 g (25 mmol) of N-Boc-(p-
benzyloxyphenylalaninol) in 150 ml of methylene chloride, 14 ml (100 mmol) of triethyl-
amine in 30 ml of methylene chloride and aqueous working up (extracdon of the aqueous
phases with ethyl acetate) yields the crystalline dtle compound: TLC Rf(A)=0.71;IH-NMR (200 MHz, CDCI3): 1.44 (s, 9 H), 3.06 (d, J=6 Hz, 2 H), 4.39 (m, 1 H), 6.86-6.98
and 7.03-7.15 (2m, each 2 H),7.30-7.48 (m,5 H),9.62 (s, 1 H).
c: S(S)-~l(S)-(Boc-amino)-2-(P-benzy~oxvphenvl)ethyll-dihvdrofuran-2-(3H)~one
Analogously to Reference Example 21 D) l)b), the zinc homoenolate is formed from7.7 ml (57.1 mmol) of 2-iodopropionic acid ethyl ester in 100 ml of toluene, 6.0 g
(91.8 mmol) of Zn/Cu and 9.69 ml of dimethylacetamide. The zinc homoenolate is
transferred by means of cannula to trichlorotitanium isopropanolate (prepared from
4.17 ml (14.2 mmol) of tetraisopropyl orthotitanate and 4.41 ml (40.2 mmol) of titanium
tetrachloride in 12 ml of toluene and 69 ml of methylene chloride) which has been cooled
to from -40C to -25C. The mixture is heated for 5 min at -25C and cooled again to
'; Yr ~
~` 211~6~1
- 143-
-40C. A solution of 9.7 g (27 mmol) of N-Boc-(p-benzyloxyphenylalaninal) in 24.5 ml of
methylene chloride is then added dropwise and the mixture is stirred for 15 h atapproximately -20C and then for 1 h at 0C. The reaction mixture is poured onto 0.4 lcg
of ice-water and 0.5 1 of ether, and stirred vigorously fs r 10 min. The aqueous phase is -~
removed and extracted with 2 portions of ether. The organic phases are washed with - -
water, sat. sodium hydrogen carbonate solution, water and brine, dried with sodium sulfate
and concentrated by evaporation ( ~ crystalline 5(S)-(Boc-amino)-4(S)-hydroxy-6-(p-
benzyloxyphenyl)-hexanoic acid ethyl ester).
The above intermediate is heated for 2.5 h at 100C in 220 ml of toluene and 6.73 ml of
acetic acid. 0.5 1 of water is added to the cooled reaction mixture, the aqueous phase is
removed and extracted with 2 portions of ether, and the organic phases are washed with
sat. sodium hydrogen carbonate solution, water and brine, dried with sodium sulfate and
concentrated by evaporation. Crystallisation of the residue from ether/hexane yields the
pure title compound: l'LC Rf(E)=0.28; tRe,(II)=23.5 min; lH-NMR (200 MHz, CDCI3): ;
1.40 (s, 9 H), 2.03-2.2 and 2.44-2.64 and 2.73-2.98 (3m, each 2 H), 3.95 and 4.48 (2m,
each 1 H), 4.62 (d, J=9 Hz, 1 H), 6.87-6.97 and 7.09-7.21 (2m, each 2 H), 7.27-7.48 (m, 5
H)- `
d: 5(S)-rl(S)-(Boc-amino)-2-(p-benzyloxyphenvl)-ethyll-3(R)-(phenvlmethYl)-dihydro-
furan-2-(3H!-one . .
Analogously to Refere!-ce Example 21 D) l)c), 2.47 g (6.0 mmol) of 5(S)-[l(S)-(Boc-
amino~-2-(p-benzyloxyphenyl)ethyl]-dihydrofuran-2-(3H)-one dissolved in 12 ml of THF
and 1.2 ml of 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone are deprotonated at
-70C with 11.73 ml of lithium bis(trimethylsilyl)amide lM in THF and alkylated
(75 min) with 0.713 ml (6.0 mmol) of benzyl bromide in 3 ml of THF. Column chromato-
graphy (SiO2, hexane/ethyl acetate 4:1 ~ 2:1) and crystallisation from ether/hexane yields
the pure title compound: TLC Rf~D)=0.36.
e) 5(S)-(Boc-amino~-4($)-hYdroxv-6-(p-benzvloxYphenvl)-2(R)-(phenvlmethYl)-hexanoic
. .
acid
Analogously to Reference Example li), 0.502 g (1.00 mmol) of 5(S)-[l(S)-(Boc-amino.)-
2-(p-benzyloxyphenyl)-ethyl]-3(R)-(phenylmethyl)-dihydrofuran-2-(3H)-one in 16 ml of -
dimethoxyethane and 8.6 ml of water are hydrolysed with 4 ml of lM lithium hydroxide
solution. The reaction mixture, partially concentrated by evapora~on, is poured onto a
mixture of ice, 49 ml of sat. NH4CI solution, 4.1 ml of 10 % citric acid solution and
methylene chloride, and ethanol is added until the precipitated solid has dissolved. The
211~
- 144-
:
aqueous phase is ex~racted with 2 portions of methylene chloride/ethanol approximately
9: 1, and the organic phases are washed with brine, d~ied with Na2SO4 and concentrated by
evaporation: TLC Rf(A)=0.22.
f) 5(S)-(Boc-amino)-4(S)-(tert-bu~vldimethvlsilvloxy)-6-(p-benzvloxv~h~nvl)-2(R)-
(phenYlmethyl)-hexanoic acid
Analogously to Reference Example lj), 1.04 g (2.00 mmol) of 5(S)-(Boc-amino)-4(S)-
hydroxy-~(p-benzyloxyphenyl)-2(R)-(phenylmethyl)-hexanoic acid in 7 ml of DMF are
silylated with 1.39 g (9.0 mmol) of tert-butyldimethylchlorosilane and 1.12 g (16.4 mmol)
of imidazole. Hydrolysis of the silyl ester function with 1.6 g of potassium carbonate in
46 ml of methanoVl~IF/water 3:1:1 and column chromatography (SiO2, hexane/ethyl
acetate 4: 1 ~ 2:1 ~ 1:2) of the crude product yields the title compound: TLC
Rf(A)=0.69.
g) S(S)-(Boc-amino~-4(S)-(tert-butyldimethYlsilyloxv)-6-(p-benzYloxvphenyl)-2(R)-
(phellvlmethyl)-hexanoyl-(L)-Val-(L)-Phe-morpholin-4-vlamide
Analogously to Reference Example 9f), 126.7 mg (0.200 mmol) of S(S)-(Boc-amino)-4(S)-(tert-butyldimethylsilyloxy)-6-(p-benzyloxyphenyl)-2(R)-(phenylmethyl)-hexanoic
acid and 73.3 mg (0.22 mmol) of H-(L)-Val-(L)-Phe-morpholin-4-ylarnide (Reference
Example lo) in 1.88 ml of NMM/CH3CN 0.25M are reacted wi~h 83.4 mg ~0.22 mmol) of
BTU: TLC R~(A)=0.33; FAB-MS (M+H)+=949.
Reference Example 47: Boc-(p-BzlO)Phe~ClPhe-(L)-Val-(Ll-(p-BzlOPhe)-mor~holin-~
Ylamide
Analogously to Reference Example 1),788 mg (0.85 mmol) of 5(S)-(Boc-amino)-4(S)-(tert-butyldimethylsilyloxy)-6-(p-benzyloxyphenyl)-2(R)-(phenylmethyl)-hexanoyl-(L)-
Val-(L)-(p-BzlOPhe)-morpholin-4-ylamide are deprotected with 534 mg (1.70 mmol) of
TBAF in 8.5 ml of DMF. Crystallisation from ipropanol/hexane yields the pure title
compound: TLC Rf~B)=0.51; tRet(I)=l9.0 min; FAB-MS ~M+H)+=941.
The starting material is prepared as follows:
a) 5(S)-(Boc-amino)-4(S)-(tert-butYldimethvlsilyloxv)-6-(p-benzyloxvphenyl)-2(R)-
(phenvlmethyl)-hexanoyl-(L)-Val-(L)-(P-BzlOPhe)-morpholin-4-ylamide
Analogously to Reference Example 9f),560 mg (0.88 mmol) of 5(S)-(Boc-amino)-4(S)-
(tert-butyldimethylsilyloxy)-6-('oenzyloxyphenyl)-2(R)-(phenylmethyl)-hexanoic acid and
2118~61
- 145-
425 mg (0.968 mmol) of H-(L)-Val-(L)-(p-BzlOPhe)-morpholin-4-ylamide (Reference
Example 45 d)) in 8.27 ml of NMM/CH3CN 0.25M are ~eacted with 366.9 mg
(0.968 mmol) of HBTU: TLC Rf(A)=0.33; FAB-MS (M~H)+=1055. ;
'~"
Reference Example 48: Boc-(p-BzlO)Phe~Cl(p-BzlO)Phe-(L)-Val-(L)-Phe-morDholin-4-
Ylamide ,
Analogously to Reference E~xample 1), 1.10 g (1.0 mmol) of 5(S)-(Boc-amino~-4(S)-(tert-
butyldimethylsilyloxy)-6-(p-benzyloxyphenyl)-2(R)-(p-benzyloxyphenylmethyl)-
hexanoyl-(L)-Val-(L)-Phe-morpholin-4-ylamide are deprotected with 628 mg (2.0 mmol)
of TBAF in 10 ml of DMF. Crystallisation from hot ipropanol yields the pure title -
compound: TLC Rf(A)=0.61; tRet(I)=l9.1 min; PAB-MS (M~H)+=941.
The starting material is prepared as follows:
a: 5(S)-rl(S)-(Boc-amino)-2-(P-benzYloxYphenyl)-ethvll-3(R)-(p-benzyloxyphen
methyl)-dihvdrofuran-2-(3H)-one
Analogously to Reference Example 21 D) l)c), 2.47 g (6.0 mmol) of 5(S)-[l(S)-(Boc-
amino)-2-(p-benzyloxyphenyl)ethyl]-dihyd~furan-2-(3H)-one (Reference Exarnple 46 c)) ~ -
dissolved in 12 ml of ~HF and 1.2 ml of 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrirnid-
inone are deprotonated at -70C with 11.73 ml of lithium bis(trimethylsilyl)amide lM in
TE~ and aLIcylated (60 min) with 1.946 g (6.0 mmol) of p-benzyloxybenzyl iodide
(Reference Example 44a)) in 3 ml of THF. Column chromatography (SiO2, hexane/ethyl
acetate 4: 1) and crystallisation from ethyl acetate~exane yields the pure title compound~
TLC Rf(D)=0.45; tRet(I)=19.9 min; FAB-MS (M+H)~=608.
b) 5(S)-(Boc-amino)-4(S)-hYdroxv-6-(p-benzyloxYphenYl)-2(R)-(p-benzylox~,rPhen
methYl)-kexanoic acid
Analogously to Reference Example li), 2.7 g (4.43 mmol) of S(S)-[l(S)-(Boc-amino)-2-
(p-benzyloxyphenyl)-ethyl]-3(R)-(p-benzyloxyphenylmethyl)-dihydrofuran-2-(3H)-one in
59 ml of dimethoxyethane and 31.8 ml of water are hydrolysed with 14.8 ml of lM
lithium hydroxide soludon. I'he reaction mixture, partially concentrated by evaporadon, is
poured onto a mixture of ice, 181 ml of sat. NH4CI soludon, 16.2 ml of 10 % citric acid
solution and 400 ml of ethyl acetate, and THF is added until the precipitated solid has
dissolved. The aqueous phase is extracted with 2 portions of ethyl acetate, and ~e organic
phases are washed with brine, dried with Na2SO4, concentrated by evaporation anddigested in hexane: TLC Rf(A)=0.07.
~\
2~136~1
- 146-
c~ 5(S)-(Boc-amino)-4(S)-(tert-butvldimethylsilvloxy)-6-(p-benzvloxvphenvl)-2(R)-
(p-benzvloxyphenvlmethvl)-hexanoic acid
Analogously to Reference Example lj~, 2.44 g (3.90 mmol) of 5(S)-(Boc-amino)-4(S)-
hydroxy-6-(p-benzyloxyphenyl)-2(R)-(p-benzyloxyphenylmethyl)-hexanoic acid in 14 ml
of DMF are silylated with 2.70 g (17.6 mmol) of tert-butyldimethylchlorosilane and 2.18 g
(32 mmol) of imidazole. Hydrolysis of the silyl ester function with 3.2 g of potassium
carbonate in 90 ml of methanolJIHF/water 3:1:1 and column chromatography (SiO2,
hexane/ethyl acetate 2: 1 ~ 1: 1) of the crude product yields the title compound: TLC
Rf(A)=0.53; FAB-MS (M+H)+=740.
d) 5(S)-(Boc-amino)-4(S)-(tert-butyldîmethvlsilvloxY)-6-(p-benzvlox~henYI)-2(R~-
(p-benzyloxyphenvlmethvl)-hexanoyl-(L)-Val-(L)-Phe-morpholin-4-Ylamide
Analogously to Reference Example 9f),740 mg (1.00 mmol) of S(S)-(Boc-amino)-4(S)-
(tert-butyldimethylsilyloxy)-6-(p-benzyloxyphenyl)-2(R)-(p-benzyloxyphenylmethyl)-
hexanoic acid and 367 mg (1.10 mmol) of H-(L)-Val-(L)-Phe-morpholin-4-ylamide
(Reference Example lo) in 9.39 ml of NM~VCH3CN 0.25M are reacted with 417 mg
(1.10 mmol) of HBTU: lLC Rf(A)=0.27; FAB-MS (M+H)+=1055.
Reference Example 49: Boc-(p-BzlO)PherC~(p-BzlO)Phe-(L)-Val-(L)-(~BzlOPhe)-
morpholin-4-ylamide
Analogously to Reference Example 1), 0.85 g (0.73 mmol) of 5(S)-(Boc-amino)-4(S)-
(tert-butyldimethylsilyloxy)-6-(p-benzyloxyphenyl)-2(R)-(p-benzyloxyphenylmethyl)-
hexanoyl-(L)-Val-(L)-(p-BzlOPhe)-morpholin-4-ylamide are deprotected with 460 mg(1.46 mmol) of TBAF in 7.3 ml of DMF. Digestion in ethyl acetate/hexane yields the pure
title compound: TLC Rf(B)=0.66; tRet(I)=20.2 min; FAB-MS (M+H)+=1047.
The starting material is prepared as follows:
.
a: 5(S~-(Boc-amino~-4(S)-(tert-butvldimethYlsilYloxv)-6-(p-benzvloxyphenyl)-2(R)-
tp-benzvloxYPhenYImethvl)-hexanoYl-(L)-val-(L)-(p-Bzlophe)-morpholin-4-ylamide
Analogously to Reference Example 9f),740 mg (1.00 mmol) of 5(S)-(Boc-amino)-4(S)-
(tert-butyldirnethylsilyloxy)-6-(p-benzyloxyphenyl)-2(R)-(p-benzyloxyphenylmethyl)- -
hexanoic acid (Reference Example 48) and 483 mg (1.10 mmol) of H-(L)-Val-(L)-
(p-BzlOPhe)-morpholin-4-ylamide (Reference Example 45) in 9.39 ml of NMM/CH3CN
0.25M are reacted with 417 mg (1.10 mmol) of HBTU: TLC Rf(A)=0.19.
'?~,:` ;~. . ,
-" 2113~61
- 147-
Reference Example 50: Boc-PhelCl(o-F)Phe-(L)-Val-(L)-Phe-morpholin-4-vlamide
Analogously to Reference Example 1), 190 mg (0.219 mmol) of 5(S)-(Boc-amino)-4(S)-
(tert-butyldimethylsilyloxy)-6-phenyl-2(R)-(o-fluorophenylmethyl)-hexanoyl-(L)-Val-
(L)-Phe-morpholin-4-ylamide are deprotected with 138 mg (0.438 mmol) of TBAF in 3 ml
of DMF to yield the title compound: TLC Rf(A)=0.23; tRet(I)=16.0 min; FAB-MS
(M+H)+= 747.
The starting material is prepared as follows:
a) 5(S)-rl(S)-(Boc-amino)-2-phenvl-ethvll-3(R)-(o-fluorophenvlmethvl)-dihydrofuran-2-
(3H)-one
Analogously to Reference Example 21 D) l)c), 5.0 g (16.37 mmol) of 5(S)-[l(S)-(Boc-
amino)-2-phenylethyl]-dihydrofuran-2-(3H)-one [Reference Example 21 D) l)b)]
dissolved in 75 ml of THF are deprotonated at -75C with 32.7 ml of lithium bis(tri-
methylsilyl)amide lM in THF and aLIcylated starting at -75C with 2.1 ml (18.0 mmol) of
o-fluorobenzyl bromide (Fluka; Buchs/Switzerland) (waTming up du~ing 60 min up to
max. -60C). Column chromatography (SiO2, hexane/ethyl acetate 3:1) yields the tide
compound: TLC Rf(D)=0.61.
b) 5(S)-(Boc-amino)-4(S)-hvdroxY-6-Phenvl-2(R)-(o-fluoroPhenvlmethvl)-hexanoic acid ~ ;
Analogously to Reference Example li), 4.5 g (10.8 mmol) of 5(S)-[l(S)-(Boc-arnino~
2-phenylethyl]-3(~)-(o-fluoro~henylmethyl)-dihydrofuran-2-(3H)-one in 170 ml of
dimethoxyethane are hydrolysed with 43.5 ml of lM lithium hydroxide solution. The
evaporation residue of the reaction mixture is poured onto a mixture of ice, 120 ml of sat.
ammonium chloride solution and 240 ml of 10 % citric acid solution, and extracted with 3
portions of methylene chloride. The organic phases are washed with water and brine, dried
over Na2SO4 and concentrated by evapora~ion: tRet(I)=14.5 min.
,,
c) 5(S)-(Boc-amino)-4(S)-(tert-butvldimethYlsilYloxY)-6-phenvl-2(R)-(o-fluoroPhen~
methyl)-hexanoicacid
Analogously to Reference Example lj), 1.5 g (3.47 mmol) of 5(S)-(Boc-amino)-4(S)-
hydroxy-6-phenyl-2(R)-(o-fluorophenylmethyl)-hexanoic acid in 15 ml of DMF are
silylated with 2.4 g (16 mmol) of tert-butyldimethylchlorosilane and 1.95 g (28.5 mmol)
of imidazole. Hydrolysis of the silyl ester function with 2.8 g of potassium carbonate in
50 ml of methanol/I~F/water 4: 1: 1 yields the title compound after column chromato-
~.
-- 2118~1
- 148-
graphy (SiO2, hexane/ethyl acetate 2:1): TLC Rf(D)=0.33; tRet(I)=20.7 min.
d) 5(S)-~Boc-amino)-4(~)-(tert-butyldimethylsilvloxy)-6-phenyl-2(R)-(o-fluorophen
methyl)-hexanoyl-(L)-Val-(L)-Phe-morpholin-4-ylamide
Analogously to Reference F.xample 9f), 150 mg (0.27 mmol) of 5(S)-(Boc-amino)-4(S)-
(tert-butyldimethylsilyloxy)-6-phenyl-2(R)-(o-fluorophenylmethyl)-hexanoic acid and
101 mg (0.30 mmol) of H-(L)-Val-(L)-Phe-morpholin-4-ylamide (Reference Example lo)
in 2.6 ml of NMM/CH3CN 0.25M are reacted with 115 mg (0.30 mmol) of BTU:
tRet(I)=22.3 min.
Reference Example 51: Boc-PherCl(o-F)Phe-(L)-Va ~-(p-CH30-Phe)-morpholin-4
amide
Analogously to Reference Example 1), 332 mg (0.37 mmol) of 5(S)-(Boc-amino)-4(S)-
(tert-butyldimethylsilyloxy)-6-phenyl-2(R)-(o-fluorophenylrnethyl)-hexanoyl-(L)-Val-
(L)-(p-C~30-Phe)-morpholin-4-ylarnide are deprotected with 350 mg (1.11 mmol) ofTBAF in 3 ml of DMF. Digestion from DIPE and ether yields the title compound: TLC
Rf(B)=0.50; tRoL(I)=16.0 min; FAB-MS (M+H)+=777.
l'he starting material is prepared as follows:
a) S(S)-(Boc-amino)-4(S)-(tert-butYldimethvlsil~loxY)-6-PhenYl-2(R)-(o-fluoroPhenyl-
methvl)-hexanovl-(L)-Val-(L)-(p-CH~O-Phe)-morpholin-4-ylarnide
Analogously to Reference Example 9f~, 205 mg (û.375 mmol) of 5(S)-(Boc-amino)-4(S)- ~-
(tert-butyldimethylsilyloxy)-6-phenyl-2(R)-(o-fluorophenylmethyl)-hexanoic acid
(Reference Exarnple 50c) and 150 mg (0.412 mmol) of H-(L)-Val-(L)-(p-CH30-Phe)-
morpholin-4-ylamide (Reference Example lOe) in 3.6 ml of NMM/CEI3CN 0.25M are
reacted with 156 mg (0.412 mmol) of BTU. The crude product (foam) is stirred to yield
the tide compound: TLC Rf(A)=0.16; tRet(I)=22.6 min.
I
Reference Exarnple 52: Boc-PherCl(m-F)Phe-(L)-'Val-(L)-Phe-morpholin-4-Ylamide
Analogously to Reference Example 1), 181 mg (0.24 mmol) of S(S)-(Boc-amino)-4(S)-
(tert-butyldimethylsilyloxy)-6-phenyl-2(R)-(m-fluorophenylmethyl)-hexanoyl-(L)-Val-
(L)-Phe-morpholin-4-ylamide are deprotected with 151 mg ~0.478 mmol) of TBAF in 3 ml
of DMF. Digestion in hexane yields the title compound: TLC Rf(A)=0.54;
tRe,(I)=16.2 min; FAB-MS (M+H)+=747.
~ 2 ~
- 149-
.,:
The starting material is prepared as follows:
a) 5(S)-rl(S)-(Boc-amino)-2-phenylethyll-3(R)-(m-fluorophenYlmethvl)-dihvdrofuran-2-
(3H)-one
Analogously to Reference Example 21 D) l)c), 5.0 g (16.37 mmol) of 5(S)-[l(S)-(Boc-
amino)-2-phenylethyl]-dihydrofuran-2-(3H)-one [Reference Example 21 D) l)b)]
dissolved in 75 ml of THF are deprotonated at -75C with 32.7 ml of lithium bis(~i-
methylsilyl)amide lM in THF and aLlcylated starting at -75C with 3.4 g (18.0 mmol) of
3-fluorobenzyl bromide (Fluka; Buchs/Switzerland) (warming up during 60 min up to ~-~
max. -50C). Column chromatography (SiO2, hexane/ethyl acetate 3:1) yields the tide
compound: TLC Rf(D)=0.6; tRet(I)=17.2 min.
b) 5(S)-(Boc-amino)-4(S)-hydroxy-6-phenvl-2(R)-(m-fluorophenylmethvl)-hexanoic acid
Analogously to Reference Example li), 3.7 g (8.95 mmol) of 5(S)-[l(S)-(Boc-amino)-
2-phenylethyl]-3(R)-(m-fluorophenylmethyl)-dihydrofuran-2-(3H)-one in 140 ml of
dimethoxyethane are hydrolysed with 35.8 ml of lM lithium hydroxide solution.
Extraction of the evaporatlon residue of the reaction mixture from a mixture of ice, 120 ml
of sat. ammonium chloride solution and 240 ml of 10 % citric acid solution with a large
amount of methylene chlolide (solubility!) yields the title compound: tRe,(I)=14.6 min.
c) 5(S~-(Boc-amino)-4(S)-(tert-butvldimethvlsilyloxv)-6-~henyl-2(R)-(m-fluorophen~rl- ~ -
methvl)-hexanoic acid
Analogously to Reference Example lj), 2.7 g (6.25 mmol) of 5(S)-(Boc-amino)-4(S)-
hydroxy-6-phenyl-2(R)-(m-fluorophenylmethyl)-hexanoic acid in 30 ml of DMF are
silylated with 4.33 g (28.8 mmol) of tert-butyldimethylchlorosilane and 3.51 g
(51.3 mmol) of imidazole. Hydrolysis of the silyl ester function with 5.1 g of potassium
carbonate in 100 ml of methanol~I~/water 4:1:1 yields the title compound aftercolumn
chromatography (SiO2, hexane/ethyl acetate 2:1): tRe,(I)--20.8 min.
!
d) 5(S)-(Boc-amino)-4~S~-(tert-butvldimethylsilyloxy2-6-phenvl-2(R)-(m-fluorophen
methvl)-hexanoYl-(L)-Val-(L)-Phe-morPholin-4-vlamide
Analogously to Reference Example 9f), 150 mg (0.27 mmol) of 5(S)-(Boc-amino)-4(S)-
(tert-butyldimethylsilyloxy~-6-phenyl-2(R)-(m-fluorophenylmethyl)-hexanoic acid and
101 mg (0.30 mmol) of H-~L)-Val-(L)-Phe-morpholin-4-ylamide (Reference Example lo)
in 2.6 ml of NMM/CH3CN 0.25M are reacted with 115 mg (0.30 mmol) of HBTU.
Column chromatography ~SiO2, hexane/ethyl acetate 1:1) yields the title compnund: TLC
~ ;.. ,,, . .. ~
. ., . , - :: . .
211~6~1
- 150-
Rf(A)=0.28; tRe,(I)=23.0 min.
Reference Example 53: Boc-Phe~Cl(m-F)Phe-(L)-Val-(L)-(p-CH~O-Phe)-morpholin-4-
vlamide
Analogously to Reference Example 1), 336 mg (0.37 mmol) of 5(S)-(Boc-amino)-4(S)-
(tert-butyldimethylsilyloxy)-6-phenyl-2(R)-(m-fluorophenylmethyl)-hexanoyl-(L)-Val-
(L)-(p-CH30-Phe)-morpholin-4-ylamide are deprotected with 350 mg (1.12 mmol) of
TBAF in 3 ml of DMF. Diges~ion from l:)IPE and ether yields the ti~le compound: TLC
Rf~B)=0.48; tRet(I)=16.1 min; FAB-MS (M+H)+=777.
The starting material is prepared as follows:
a) 5(S)-(Boc-amino)-4(S)-(tert-butvldimethvlsilYloxv)-6-phenvl-2(0-(m-fluoroPhenYl-
methyl)-hexanovl-(L)-Val-(L)-(p-CH30-Phe)-morpholin-4-vlamide :,
Analogously to Reference E~xample 9f), 205 mg (0.375 mmol) of 5~S)-(Boc-amino)-4(S)-
(tert-butyldimethylsilyloxy)-6-phenyl-2(R)-(m-fluorophenylmethyl)-hexanoic acid (Ref-
erence Example 52c) and 150 mg (0.412 mmol) of H-(L)-Val-tL)-~p-CH3O-Phe)-morph~lin-4-ylamide (Reference Example 10e) in 3.6 ml of NMMJCH3CN (0.25M3 are reactedwith 156 mg (0.412 mmol) of HBTU. The crude product is sdrred in an ultra-sound bath
in hexane to yield the title compound: TLC Rf(A)=0.16; tRe,(I)=22.5 min.
: :.
Reference Example 54: Boc-PherClPhe-(L)-Val-(L)-Leu-morpholin-4-vlamide -
Analogously to Reference Example 1, 900 mg (1.11 mmol) of S(S)-Boc-amino4(S)-(tert-
butyldime~ylsilyloxy)-6-phenyl-2(R)-phenylmethylhexanoyl-(L)-Val-(L)-Leu-morpho-lin~4-ylamide aTe deprotected with 608 mg (1.93 mmol) of TBAF in 12 ml of DMF toyield the tide compound: tRe, (I) = 16 min; FAB-MS (M+H+)= 695.
The starting material is prepared as follows:
.
a) Z~(Sl-Val-(L)-Leu-morpholin-4-Ylamide
Analogously ~o Reference Example 41 A) a)l 3.98 g (10.9 mmol) of Z-(L)-Val-(L)-
Leu-OH (Bachem, Switzerland) are converted into the title compound with 0.87 ml -
(10 mmol) of morpholine. TLC Rf (methylene chloride/methanol 9:1) = 0.6.
b) H-(S)-Val-(L)-Leu-morpholin-l-Ylamide
Analogously to Reference Example 41 A) b), 4.7 g (10.9 mmol) of Z-(L)-Val-(L)-Leu-
211~G~l
- 15 1 -
molpholin-4-ylamide are converted into the title compound by hydrogenation in the
presence of I g of 10 % Pd~C. TLC Rf (methylene chloride/methanol 9: 1) = 0.3: FAB-MS
(M+H+)= 300.
c) 5~S)-(Boc-amino)-4(S)-(tert-butyldimethylsilvloxv)-6-phenv!-2(R)-phenylmeth
hexanoyl-(L)-Val-(L)-Leu-morpholin-4-ylamide
Analogously to Reference Example 1 k),750 mg (1.4~ mmol) of S(S)-(Boc-amino-4(S)-
(tert-butyldimethyl-silyloxy)-6-phenyl-2(R)-phenylmethylhexanoicacidand508mg
(1.696 mmol) of H-(L)-Val-(L)-Leu-morpholin-4-ylamide are reacted in DMF: tRet(I) = ~;
22.4 min; FAB-MS (M+H+)= 809.
Reference Example 55: Boc-Phe~ClPhe-(L)-Val-(L)-Ala-morPholin-4-vlamideAnalogously to Reference Example 1, 623 mg (0.81 mmol) of 5(S)-(Boc-arnino)-4(S)-
(tert-butyldimethylsilyloxy)-6-phenyl-2(R)-phenylmethylhexanoyl-(L)-Val-(L)-Ala-morpholin-4-ylamide are deprotected with 456.5 mg (1.447 mmol) of TBAF in 11 ml of
DMF to yield the title compound: tRet(II)= 19.6 min; FAB-MS (M+H+)= 653.
The starting material is prepared as follows:
~ -
a) ZtL)-Val-(L)-Ala-morPholin-1-vlamide
Analogously to Reference Example 41 A) a), 2 g (6.21 mmol) of Z-(L)-Val-(L)-Ala-OH
(Bachem, Switzerland) are converted into the title compound with 0.54 ml (6.21 mmol) of
morpholine. TLC Rf(methylene chloride/methanol: 9/1)= 0.61.
b) H-(L)-Val-(L)-Ala-mor~h~ in-l-Ylamide
Analogously to Reference Example 41 A) b), 2.4 g (6.2 mmol) of Z-(L)-Val-(L)-Ala-
morpholin-1-ylamide are converted into the title compound by hydrogenation in the
presence of 0.4 g of 10% PdJC. TLC Rf(methylene chloride/methanol: 9/1)= 0.53;
FAB-IMS (M+H+)- 258.
c) 5(Sl-(Boc-amino)-4(S)-~tert-butyldimethYlsilvloxv)-6-phenvl-2(R)-phenylmethY
hexanovl-(L)-Val-(L)-Ala-morpholin- 1 -vlamide
Analogously to Reference Example lk, 500 mg (0.947 mmol) of S(S)-(Boc-amino)-4(S)-
(tert-butyldimethylsilyloxy)-6-phenyl-2(R)-phenylmethylhexanoicacidand292.4mg
(1.136 mmol) of H-(L)-Val-(L)-Ala-morpholin-4-ylamide are reacted in DMF: tRet(II)=
32.4 min; FAB-MS (M+H+)= 767.
-; 211~661
- 152-
Reference Example 56: The following compounds are prepared analogously to one of the
above Reference Examples:
A) oc-(p-CH~O~Phe~ClPhe-(L)-Val-(L)-Phe-morpholin-4-Ylamide
B) Boc-(~-CH~O)Phe~Cl(p-CH~O~Phe-(Ll-Val-(L)-Phe-morpholin-4-vlamide
C) Boc-(p-CH30)PherCl(p-BzlO)Phe-(L)-Val-tL)-Phe-m,orpholin-4-ylamide
D) Boc-(p-CH~O)Phe~ClTyr-(L)-Val-(L)-Phe-morpholin-4-vlamide
E) Boc-(p-CH~O)PherClPhe-(L~-Val-(L~-(p-CH~O-Phe)-morpholin-4-vlamide
F) Boc-(p-CH~O)PherClPhe-(L)-Val-(L)-Tvr-morpholin-4-vlamide
G) Boc-(p-CH~,O)PherCl(p-CH~O)Phe-(L)-Val-(L)-(p-CH30-Phe)-morpholin-~ylamide
H) Boc-(p-CH~O~PherCl(p-CH~O)Phe-(L)-Val-(L)-Tvr-morpholin-4-vlamide
I) Boc-(p-CH~O)PherCl(p-BzlO)Phe-(L)-Val-(L)-(p-CH3O-Phe)-morPholin-4-vlamide
J) ~ ~ 30)PherCl(p-BzlO)Phe-(I~-Val-~L)-Tyr-morpholin-4-vlamide
K) Boc-(p-CH~O)PherClTyr-~L)-Val-(L)-(p-CH30-Phe)-morpholin-4-vlamide
L) Boc~ CH~O)PherClTvr-(L)-Val-(L)-Tyr-morDholin-4-Ylamide
M) Boc-(p-c~l3o)phercl(3-cH3o)phe-(L)-val-(L)-phe-morpholin-4-ylamide
N) Boc-(~-CH~O)PherCl(2-CH30)Phe-(L)-Val-(L)-Phe-morpholin-4-ylamide
O) Boc-PherCl(2-CH~O)Phe-(L)-Val-(L)-Phe-morpholin-4-vlamide
101.6 g of TBAF are added to a solution of 140.3 mg of S(S)-(Boc-amino)-4(S)-(tert-
butyldimethylsilyloxy)-6-phenyl-2(R)-(2-methoxyphenylmethyl)-hexanoyl-(L)-Val-(L)-
Phe-morpholin-4-ylamide in 3 ml of DMF, and the reaction mixture is stirred at RT for ~ :
211~6~
- 153-
21 h. The faintly yellowish solution is diluted with approximately 30 ml of ethyl acetate,
washed in succession once with water, once with saturated sodium bicarbonate solution
and twice with brine until neutral, and dried over sodium sulfate. After removal of the
solvent, the residue is purified on silica gel (eluant B) to yield the title compound. TLC
Rf(B)=0.26. FAB-MS (M+H)+=759. ~ -
The starting material is prepared as follows: ~
56 O) a) 2-Methoxvbenzyl chloride -
16.8 ml of thionyl chloride are added dropwise over a penod of approximately 30 minutes
to 10 ml of 2-methoxybenzyl alcohol (Fluka, Buchs, Switærland) and 53.76 g of diiso-
propylaminomethylpolystyrene (Polyhunig base; Fluka, Buchs, Switærland; copolymer of
98 % styrene and 2 % divinylbenzene, diisopropylaminomethylated) in 200 ml of abs.
ether. After having been stirred for a further 1.5 h at 0C, the mixture is filtered with
suction and the filtrate is concentrated in a rotary evaporator and under a high vacuum.
The residue is purified by chromatography on silica gel (eluant: hexane/ethyl acetate 6:1).
TLC Rf(C)=O.5. lH-NMR (200 MHz, CDC13): 7.42-7.24 (m, 2H); 7.0-6.84 (m, 2H); 4.68 -
(s,2H); 3.9 (s,3H).
56 O) b) 2-Methoxvbenzyl iodide
9.3 g of sodium iodide are added to 2 g of 2-methoxybenzyl chloride in 22 rnl of abs.
acetone and the mixture is stirred overnight at RT. The reaction mixture is diluted with
250 ml of ether and washed with 10% sodium thiosulfate solution and brine. The title
compound is obtained by drying over sodium sulfate and removing the solvent and is
further processed without being purified. TLC Rf(C)=0.46.1H-NMR (200 MHz, CDCl3):
7.36-7.2 (m, 2H); 6.92-6.8 (m, 2H); 4.48 (s, 2H); 3.91 (s, 3H).
56 O) c) 5(S)-rl(S)-(Boc-amino)-2-phenvlethyll-3(R~-(2-methoxyphenylmethvl)-di-
hydrotfuran-2-(3Hl-one
Under a nitrogen atmosphere, a solution of 1 g of 5(S)-[l(S)-(Boc amino)-2-phenylethyl~-
dihydrofuran-2-(3H)-one [for preparation see under Reference Example 2 i)] in 4 ml of
abs. THF and 0.66 ml of DMPU is cooled to -75C, 6.42 ml of lithium bis(trimethylsilyl)-
amide (lM) in THF (AldTich, Steinheim, Federal Republic of Germany) are added at an
internal temperature of below -70C, and the mixture is then stirred for 20 min at -75C.
812 mg of 2-methoxybenzyl iodide in 2 ml of abs. THF are added dropwise to the reaction
solution over a period of 10 min using a syringe, during which the internal temperature
~ ` 2 ~
- 154-
must not exceed -70C, and the mixture is stirred for 1 h at -75C to complete the reaction.
1.22 ml of propionic acid followed by 1.22 ml of water are then added to the clear solution
at from -75C to -70C using a syringe, the temperat-lre rising to -30C. The reaction
mixture is subsequently diluted with 50 ml of ethyl acetate and stirred cold (ice/water
cooling) for 5 min with 20 ml of 10% citric acid solution. The aqueous phase is removed,
and the organic phase is washed in succession with brine, sat. sodium bicarbonate solution
and again with brine. The combined aqueous phases are re-extracted twice with ethyl
acetate. The combined organic phases are dried over sodium sulfate and concentrated. The
title compound is obtained in the form of a brownish oil. Purification is carried out by
chromatography on silica gel. Chromatography on silica gel (eluant E) yields the pure title
compound. TLC Rf(hexane/ethyl acetate 2.5:1)=0.54. MS M+=425.
56 O) d) 5(S)-(Boc-amino)-4(S)-hvdroxy-6-phenyl-2(R)-(2-methoxvphenylmethvl)-
hexanoic acid
4.45 ml of a lM lithium hydroxide soludon are added dropwise at RT to a soludon of
474 mg of 5(S)-[l(S)-(Boc-amino)-2-phenylethyl]-3(R)-(2-methoxyphenylmethyl)-
dihydrofuran-2-(3H)-one in 18 ml of dimethoxyethane and 9.07 ml of water. The reacdon
mixture is then stirred for 3 h at RT, diluted with ethyl acetate and THF and washed in a
separadng funnel with a mixture of 54.78 ml of sat. ammonium chloride soludon and
4.58 ml of 10% citric acid solution, followed by bnne and water, until neutral. The tdtle
compound is obtained after drying over sodium sulfate and removing the solvent and is
further processed without being further purified. TLC Rf(hexane/ethyl acetate 2.5:1)=0.15
56 O) e) 5(S~-(Boc-amino)-4(S)-(tert-butvldimethYlsilyloxv)-6-phenvl-2(R)-(2-methoxy-
~enYlmethYl)-hexanoic acid
614 mg of imidazole and 796 mg of tert-butyldimethylchlorosilane are added, withstirring, to a soludon of 500 mg of S(S)-(Boc-amino)-4(S)-hydroxy-6-phenyl-2(R)-(2-
methoxyphenylmethyl)-hexanoic acid in S ml of DMF. After having been stirred for 20 h
at I~T~ the reaction solution is poured onto ice-water and extracted with ethyl acetate. The
organic phase is washed with 10% citric acid solution and brine and dried over sodium
sulfate~ The solvent is then concentrated by evaporation and the residue is chromato-
graphed on silica gel (eluants E and A) to yield the title compound. Rf(hexane/ethyl
acetate 2.5:1)=0.12. FAB-MS (M+H)+=558.
56 O) f) 5(S)-(Boc-amino)-4(S)-ltert-butYldimethylsilvloxy)-6-phenyl-2(R)-(2-
methoxyphenvlmethyl)-hexanoyl-(L)-Val-(L)-Phe-morpholin-4-vlamide
- 2 ~
- 155- .
A mixture of 100 mg of 5(S)-(Boc-amino)-4(S)-(tert-butyldimethylsilyloxy)-6-phenyl-
2(R)-(2-methoxyphenylmethyl)-hexanoic acid,74.71 mg of HBTU and 65.68 mg of H-
(L)-Val-(L)-Phe-morpholin-4-ylamide [for preparation see under Reference Example 1 o)]
in 1.68 ml of a 0.25M solution of NMM in acetonitrile is stirred for 15 h at RT under
argon. The solution is concentrated to dryness, and the residue is taken up in ethyl acetate
and washed in succession with 10% citric acid, water, sat. sodium bicarbonate soludon
and brine. The title compound is obtained after drying over sodium sulfate and removing
the solvent and is further processed without being purified. TLC Rf(A)=0.20. FAB-MS
(M+H)+=873.
P) Boc-PheLC1~3-CH~O)Phe-(L)-Val-(L)-Phe-morpholin-4-ylamide
Analogously to E~eference Example 56 O), 356 mg of 5(S)-(Boc-amino)-4(S)-(tert-butyl-
dimethylsilyloxy)-6-phenyl-2(R)-(3-methoxyphenylmethyl)-hexanoyl-(L)-Val-(L)-Phe-
morpholin-4-ylamide in 6.09 ml of DMF are desilylated with 206 mg of TBAF to yield the -
title compound: Purification is carried out by chromatography on silica gel (eluant B).
TLC Rf(B)=0.37. FAB-MS (M+H)+=759.
The starting material is prepared as follows: ;
56 P) a) 3-MethoxybenzYI iodide
Analogously to Reference Example 56 O) b), the title compound is obtained from 2 ml of
3-methoxybenzyl chloride (Fluka, Buchs, Switzerland) and g.72 g of sodium iodide in
23 ml of abs. acetone. TLC Rf(hexane/ethyl acetate 2.5:1)=0.71.1H-NMR (200 MHz,
CDCl3): 7.20 (m, lH); 7.0-6.87 (m, 2H); 6.78 (dxd, lH); 4.42 (s, 2H); 3.8 (s,3H).
56 P) b) 5(S)-~l(S~-5Boc-amino)-2-phenylethvll-3(R~methoxyphenylmethYI)-dihydro-furan-2-(3H)-one
Analogously to Reference Example 56 O) c), 1.5 g of 5(S)-[l(S)-(Boc-amino)-2-phenyl-
ethyl]-dihydrofuran-2-(3H)-one [prepared according to Reference Example 2i)] in 3 ml of
abs. THF are deprotonated (-75C) with 9.62 ml of lithium bis(trimethylsilyl)amide (lM
in THF) and with the addition of 0.998 ml of DMPU, and alkylated with 1.22 g of
3-methoxybenzyl iodide. Chromatography on silica gel (eluant E~ yields the pure t;tle
compound. TLC Rf (hexane/ethyl acetate 2.5~ -0.32. FAB-MS (M+H)+=426.
56P) c)5(S)-(Boc amin ~-4(S)-hydroxy-6-phenvl-2(R)-(3-methoxyphenylmethyl)-
hexanoic acid
k:
. . . :. , ~ ~ . : :: ,` .`:
.;:.. -`: ` : : .~: .
i . .: , .;
--` 2 ~ 6 1
- 156-
Analogously to Reference Example 56 O) d), 1.315 g of 5(S)-[l(S)-(Boc-amino)-2- -
phenylethyl]-3(R)-(3-methoxyphenylmethyl)-dihydrofuran-2-(3H)-one in 49.9 mlof
dimethoxyethane and 25.16 ml of water are hydrolysed with 12.36 ml of lithium
hydroxide solution lM to yield the title compound which is directly further processed.
TLC Rf(A)=0.09. FAB-MS (M+H)+ =444.
56 P) d) 5(S)-(Boc-amino)-4(S)-(tert-butvldimethylsilyloxv)-6-PhenYI-2(R)-(3-methoxY-
phenylmethyl)-hexanoic acid
Analogously to Reference Example 56 O) e), 1.3 g of 5(S)-(Boc-amino)-4(S)-hydroxy-6-
phenyl-2(R)-(3-methoxyphenylmethyl)-hexanoic acid in 13 ml of DMF a~e silylated with
1.987 g of tert-butyldimethylchlorosilane and 1.646 g of imidazole. Chromatography on
silica gel (eluants E, D and A) yields the pure title compound. TLC Rf(D)~).06. FAB-MS
(M+H)+=558.
56 P) e) 5(S)-(Boc-aminol-4(S~-(tert-butvldimethylsilyloxy)-6-phenyl-2(R)-(3-methoxy-
phenvlmethyl)-hexanovl-(L)-Val-(L)-Phe-morpholin-4-ylamide
Analogously to Reference Example 56 O) f), 192.2 mg of 5(S)-(Boc-amino)-4(S)-(tert-
butyldimethylsilyloxy)-6-phenyl-2(R)-(3-methoxyphenylmethyl)-hexanoic acid and
126.4 mg of H-(L)-Val-(L)-Phe-morpholin-4-ylamide [prepared according to Reference
Example lo)] in 3.23 ml of NMM/CH3CN 0.25M a~ reacted with 143.7 mg of HBTU to
yield the title compound. TLC Rf(A)=0.25. FAB-MS (M+H)+=873.
Q) Boc-PherCl(3-CH3O)Phe-fL~-Val-(L~-[p-CH3O-Phe~-morpholin-4-Ylamide
R) Boc-Pher(:~l(2-CHso)phe-(L2-val-(L~-(~H3o-phe2-morpholin-4-ylamide
S) Boc-PherCl(p-BzlO~Phe-(L~-Val-(L~-(p-CH~O-Phe~-morpho i_-4-ylamide
Analogously to Reference Example 1), 345 mg (0.352 mmol) of 5(S)-(Boc-amino)-4(S)-
(tert-butyldimethylsilyloxy)-6-phenyl-2(R)-[(p-benzyloxyphenyl)methyl]-hexanoyl-(L)- -
Val-(L)-(p-CH30-Phe)-morpholin-4-ylamide are deprotected with 222 mg (0.704 mmol)
of TBAF in 5 ml of DMF. Digestion from DIPE in an ultrasound bath yields the title
compound: TLC E~f(B)=0.28; tRe~(I)=17.6 min; FAB-MS (M+H)+=865. -
The starting material is prepared as follows:
211~
- 157-
a) S(S)-(Boc-amino)-4(S)-(tert-butyldimethylsilyloxy)-6-phenyl-2(R)-~(p-benzvloxY-
phenyl)methyllhexanoyl-(L)-val-(L)-(p-cH3o-phe)-morpholin-4-ylarnide
Analogously to Reference Example 9f), 263 mg (0.415 mmol) of 5(S)-(Boc-amino)-4(S)-
(tert-butyldimethylsilyloxy)-6-phenyl-2(R)-[(p-benzyloxyphenyl)methyl]-hexanoic acid
(Reference Example 44d) and 137 mg (0.377 mmol) of H-(L)-Val-(L)-(p-CH30-Phe)-
morpholin-4-ylamide (Reference Example 10e) in 3.6 ml of NMM/CH3CN 0.2~M are
reacted with 157 mg (0.415 mmol) of HBTU to yield the title compound: tRet(I)=23.5 min.
Reference Example 57: Boc-Phe~ClTyr-(L)-Val-(L)-(p-CH3O-Phe)-morpholin-4-vlamideHydrogenation of 70 mg (0.081 mmol) of Boc-Phe[C](p-BzlO)Phe-(L)-Val-(L)-
(p-CH30-Phe)-morpholin-4-ylamide (Reference Example 56S)) in 8 ml of methanol in the
presence of 35 mg of 10% Pd/C yields the title compound after filtration through Celite
and concentration by evaporation: TLC Rf(B)=0.17; tRe,(I)=14.1 min; FAB-MS
(M+H)+=775.
Reference Example 58: Boc-PherCl(p-CN)Phe-(L)-Val-(L)-(p-CH3O-Phe)-morpholin-4-
vlamide
Analogously to Reference Example 1), 322 mg (0.36 mmol) of S(S)-(Boc-amino)-4(S)- -~
(tert-butyldimethylsilyloxy)-6-phenyl-2(R)-(p-cyanophenylmethyl)-hexanoyl-(L)-Val-
(L)-(p-CH30-Phe)-morpholin-4-ylamide are deprotected with 340 mg (1.08 mmol) of
TBAF in 3 ml of DMF over a period of 19 h. Digestion of the crude product from DIPE in
an ultrasound bath yields the title compound: TLC Rf(Y)=0.41; tRet(I)=15.4 min; FAB-MS
(M+H)+=784.
The starting material is prepared as follows:
a) 5(S)-(Boc-amino)-4(S)-(tert-butvldimethylsilyloxy)-6-phenYl-2(R)-(p-cYanoPhenvl-
methvl)-hexanoyl-(L)-Val-(L)-(p-CH~O-Phe)-morpholin-4-vlamide
Under a nitrogen atmosphere, 200 mg (0.362 mrnol) of 5(S)-(Boc-amino)-4(S)-(tert-butyl-
dimethylsilyloxy)-6-phenyl-2(R~-(p-cyanophenylmethyl)-hexanoic acid [Reference
Example 21 E)l)c)] are dissolved in 6 ml of abs. THF and, at 5C, 82 mg ~0.398 mmol) of
DCC are added. After 10 min 145 mg (0.398 mmol) OI H-(L)-Val-(L)-(p-CH30-Phe)-
morpholin-4-ylamide (Reference Example 10e) and 54 mg (0.398 mmol) of HOBT are
added and the mixture is s~irred for 17 h at RT. The reaction mixture is filtered, and the
filtrate is taken up in ethyl acetate and washed with 10 % citric acid solution, water, sat.
NaHCO3 solution and brine. The aqueous phases are extracted twice with ethyl acetate,
.. - , , ,
,. ., -
- 158-
and the organic phases are dried with Na2SO4, concentrated by evaporation and digested
in DIPE to yield the title compound: tRet(I)=21.8 min; FAB-MS (M+H)~=898.
Reference Example 59: 5(S)-(Boc-amino)-4(S)-hYdroxY-6-phenY1-2(R~-l(2.4-difluoro-
phenyl)-methyll -hexanoYl-(L)-Val-(L)-Phe-mor~holin-4-vlamide
Analogously to Reference Example 1), 264 mg (0.30 mmol) of 5(S)-(Boc-amino)-4(S)-
(tert-butyldimethylsilyloxy)-6-phenyl-2(R)-[(2,4-difluorophenyl)-methyl]-hexanoyl-
(L)-Val-(L)-Phe-morpholin-4-ylamide are deprotected with 190 mg (0.60 mmol) of TBAF
in S ml of DMF for 17 h. Digestion of the crude product from a small amount of
methylene chloride and DIPE/hexane 3:1 in an ultrasound bath yields the title compound:
TLC Rf(B)=0.71; tRCt(I)=16.1 min; FAB-MS (M+H)+=765.
~ .
The starting material is prepared as follows:
a) S(S)-rl(S)-(Boc-amino)-2-phenYlethY11-3(R)-r(2.4-difluorophenYl)methYll-dihYdro~
furan-2-(3H)-one
Analogously to Reference Example 21 D) l)c),5.0 g (16.37 mmol) of 5(S)-[l(S)-(Boc- ~ ~ -
amino)-2-phenylethyl]-dihydrofuran-2-(3H)-one [Reference Example 21 D) l)b~]
di~solved in 100 ml of THF are deprotonated at -75C with 32.7 ml of lithium bis(tri-
methylsilyl)amide lM in THF, and alkylated starting at -75C with 2.51 ml (19.6 mmol)
of 2,~difluorobenzyl bromide (Aldrich; Milwaukee/USA) (warming up during 2 h up to
max.-60C). Column chromatography (SiO2, hexane/ethyl acetate 2:1) yields the tide
compound: TLC Rf(D)=0.5; tRe,(I)=17.2 min.
b) 5(S)-(Boc-amino)-4(S~-hYdroxv-~Phenvl-2(R)-~(2.4-difluorophenYl)methvll-hexanoic
acid
Analogously to Reference Example li), 3.1 g (7.18 mmol) of 5(S)-[l(S)-(Boc-amino)-2-
phenylethyl]-3(R)-[(2,4-difluorophenyl)methyl]-dihydrofuran-2-(3H)-onein77mlof
dimethoxyethane and 19 ml of water are hydrolysed with 28.7 ml of lM lithium hydroxide
solution (19 h RT): tRet(I)=14.7 min.
c) 5(S)-(Boc-amino)-4(S)-(tert-butvldimethylsilvloxy)-6-phenvl-2(R)-~(2.4-difluoro-
phenyl)methvll-hexanoic acid
Analogously to Reference Example 1;),3.2 g (7.12 mmol) of 5(S)-(Boc-amino)-4(S)-hydroxy-6-phenyl-2(R)-[(2,4-difluorophenyl)methyl]-hexanoic acid in 67 ml of l:)MF are
silylated with 4.93 g (32.7 mmol) of tert-butyldimethylchlorosilane and 3.97 g
211g6~1
- 159- .
(58.4 mmol) of imidazol~. Hyd}olysis of the silyl ester function with 5.9 g of potassium
carbonate in 77 ml of methanol, 20 ml of THF and 20 ml of water yields the titlecompound after column chromatography (SiO2, hexane/ethyl acetate 2:1): TLC
Rf(D)=0.22; tRe,(I)=20.8 min.
d) 5(S)-(Boc-amino)-4(S)-(tert-butyldimethvlsilyloxy)-6-phenvl-2(R)-r(2~4-difluoro-
phenvl)-methyll -hexanoyl-(L)-Val-(L)-Phe-morpholin-4-vlamide
Analogously to Reference Example 9f), 200 mg (0.35 mmol) of 5(S)-(Boc-amino)-4(S)-
(tert-butyldimethylsilyloxy)-6-phenyl-2(R)-[(2,4-difluorophenyl)methyl]-hexanoic acid -
and 130 mg (0.39 mmol) of H-(L)-Val-(L)-Phe-morpholin-4-ylamide (Reference Exarnple
lo) in 3.4 ml of NMMICH3CN 0.25M are reacted with 148 mg (0.39 mmol) of HBTU to
yield the title compound: tRet(I)=22.4 min. ~ -
Reference Example 60: Boc-Phe~ClPhe-(L)-Val-(L)-Phe-trans-(2~6)-dimethvlmorPholin
4-ylamide:
Analogously to Reference Example 1,770 Ing (0.89 mmol) of 5(S)-(Boc-amino)-4(S)-(tert-butyldimethylsilyloxy)-6-phenyl-2(R)-(phenylmethyl)-hexanoyl-(L)-Val-(L)-Phe-
trans-(2,6)-dimethylmorpholin-4-ylamide are deprotected with 280 mg (0.89 mrnol) of
TBAF in 10 ml of DMF. Digestion from DIPE and ether yields the title compound: TLC
Rf(D')=0.26; tRe~(I)=17.1 min.; FAB-MS(M+H)+=757.
The starting cornpound is prepared as follows:
a) 5(S)-(Boc-amino)-4(S~-(tert-butyldimeth~rlsilYloxv)-6-phenvl-2(R)-(phenylmedhYl)-
hexanoYl-(L~-Val-(L)-Phe-trans-(2~6)-dimethylmorpholin-4-Ylamide:
Analogously to Reference Example 9f),420 mg (0.97 mmoi) of 5(S)-(Boc-amino)-4(S)-
(tert-butyldimethylsilyloxy)-6-phenyl- 2(R)-(phenylmethyl)-hexanoic acid (Reference
Example 50c) and 512 mg (0.97 mmol) of H-(L)-Val-(L)-Phe-trans-(2,6)-dimethyl-
morpholin-4-ylarnide in 10 ml of NMM/CH3CN 0.25M are reacted with 405 mg df
HBTU. Chromatography on silica gel with ethyl acetat~exane (1:1) yields the tidecompound: tRe,(I)=22.9 min.
b) H-(L)-Val-(L)-Phe-trans-(2.6)-dimethvlmorpholin-4-ylamide:
402 mg (1.1 mmol) of HBTU are added to a solution of 400 mg (1 mmol) of Z-(L)-Phe-
(L)-Val-OH and 120 mg (1 mmol) of trans-(2,6)-dimethylmorpholine in 10 ml of
NMM/CH3CN 0.25M and the mixture is stirred for 96 h at RT. The reaetion mixture is
211~
- 160-
diluted with ether and washed in succession with water, 10 % ci~c acid, sat. sodium
bicarbonate solution, water and sat. sodium chloride solution. The organic phases are dried
over sodium sulfate and concentrated by evaporation. The residue is hydrogenated as
described in Reference Example 1 m) with Pd/C in methanol and yields the title
compound in the form of an amorphous solid.
Reference Example 61: Boc-Phe~Cl(p-isobutYloxY)Phe-(L)-Val-(L)-Phe-morPholin-4-Yl-
amide:
233 mg (0.7 mmol) of caesium carbonate are added to a solution of 155 mg (0.2 mmol) of - :~
Boc-Phe[C~Tyr-(L3-Val-(L)-Phe-morpholin-4-ylamide from Reference Exarnple 41D in18 ml of dioxane and the mixture is stirred for 12 h. 1.1 ml (9.4 mmol) of isobutyl iodide
are added to the milky suspension and the reaction mixture is stirred for 2 h at RT and then
heated for 6 h at 80 C. The reaction mixture is cooled and diluted with methylene
chloride, solids are removed by filtration and the ffltrate is concentrated by evaporation.
The title compound is obtained after purification by chromatography on silica gel with
ethyl acetate/hexane (95:5): TLC Rf(C')=0.56; tRet(I)=18.1 min.; FAB-MS (M+H)+=801.
Reference Example 62: Boc-Phe~Cl(p-isobutyloxy)Phe-(L)-Val-(L)-(n-isobutvloxv-Phe)-
mor~holin-4-vlamide:
Analogously to Reference Example 61, the dtle compound is obtained from 114 mg
(0.14 mmol) of 5(S)-(Boc-amino)-4(S)-hydroxy-6-phenyl-2(R)-(4-hydrox,Yphenyl-
methyl)-hexanoyl-(L~-Val-(L)-(p-isobutyloxy-Phe)-morpholin-4-ylamide, 163 mg
(0.5 mmol) of caesium carbonate and 0.4 ml (3.4 mmol) of isobutyl iodide after purifica-
tion by chromatography on silica gel with ethyl acetate: TLC Rf(C')=0.55 -tRet(I)=20.1.;FAB-MS (M+H)+=873.
The starting material is prepared as follows:
a) S(S)-(Boc-amino)-4(S)-hvdroxy-6-phenvl-2(R)-(4-hvdroxYphenvlmethvl)-hexanoYI-(L)-Val-(L)-(p-isobutvloxY-Phe)-morpholin-4-ylamide:
A solution of 408 mg (0.4 mmol) of 5(S)-(Boc-amino)-4(S)-(tert-butyldimethylsilyloxy)-
6-phenyl-2(R)-(4-benzyloxyphenylmethyl)-hexanoyl-(L)-Val-(L)-(p-isobutyloxy-Phe)-
morpholin-4-ylamide in 6 ml of methanol is hydrogenated for 5 h with 76 mg of Pd/C 5 %
in the presence of I atm hydrogen pressure. The catalyst is removed by fil~ation, the
filtrate is concentrated by evaporation, and the residue is chromatographed on silica gel -
with ethyl acetate/methanol (19:1). In addition to 5(S)-(Boc-amino)-4(S)-(tert-butyl-
' --` 211~6gl
- 161 - --
dimethylsilyloxy)-6-phenyl-2~R)-(4-hydroxyphenylmethyl)-hexanoyl-(L)-Val-(L)-(p-isobutyloxy-Phe)-morpholin-4-ylamide obtained as a by-product, the title compound is
obtained: TLC Rf(C')=0.37.
b) 5(S)-(Boc-amino)-4(S)-(tert-butyldimethylsilyloxv)-6-phenyl-2-(R~-(4-benzYloxy-
phenylmethyl)-hexanoyl-(L)-Val-(L)-(p-isobutyloxy-Phe)-molpholin-4-ylamide:
Analogously to Reference Example 9f, 291 mg (0.45 mmol) of 5(S)-(Boc-amino)-4(S)-
(tert-butyldimethylsilyloxy)-6-phenyl-2(R)-(4-benzyloxyphenylmethyl)-hexanoic acid
(Reference Example 44d~ and 203 mg (0.5 mmol) of H-(L)-Val-(L)-(p-isobutyloxy-Phe)-
morpholin-4-ylamide (Reference Example 70b) in 4.6 ml of NMM/CH3CN 0.25M are
reacted with 193 mg of HBTU: TLC Rf(E')=0.39; FAB-MS (M+H)+=1021.
Reference Example 63: S(S)-~4-(TetlahVdropyranyl~oxvcarbonylaminol-4(s)-hvdroxv-6
phenvl-2(R)-benzylhexanovl-(L)-Val-(L)-Phe-morpholin-4-vlamide
0.331 ml (2.38 mmol) of triethylamine are added to 500 mg (0.795 mmol) of S(S)-
(amino)-4(S)-hydroxy-6-phenyl-2(R)-benzylhexanoyl-(L)-Val-(L)-Phe-morpholin-4-
ylamide and reacted, with cooling to 5C, widh 262 mg (1.59 mmol) of chloroformic acid
4-tetrahydropyranyl ester (Chemical Abstracts Registry No. 89641-80-5). After having
been stirred for a further hour at RT, the reacdon mixture is poured onto water and
extracted 3 times with ethyl acetate. The combined organic phases are washed widh water,
saturated sodium bicarbonate solution and brine, dried over sodium sulfate, and then
concentrated under reduced pressure. The tide compound is purified by column chromato-
graphy (SiO2, methylene chloride/methanol); TLC Rf (B)= 0.3; tRet(I) = 14.28 min;
FAB-MS (M+H~)= 757.
Reference Example 64: 5(S)-r3(S)-(TetrahYdrofuranvl~oxvcarbonvlaTninol-4(S)-hvdroxv-
6-phenvl-2(R)-benzvlhexanovl-(L)-Val-(L)-Phe-morPholin-4-Ylamide
Analogously to Reference Example 63,500 mg (0.795 mmol) of 5(S)-(amino)-4(S)-
hydroxy-6-phenyl-2(R)-benzylhexanoyl-(L)-Val-(L)-Phe-morpholin-4-ylamide are reacted
with 363 mg (2.41 mmol) of chloroformic acid 3(S)-tetrahydrofuranyl ester. The title
compound is purified by precipitation with ether; TLC R~ (B)= 0.25; tRet(I)= 13.86 min;
FAB-MS (M+H+)= 743.
The starting matelial is prepared as follows:
-`` 21186~
- 162 - -
a) Chloroformic acid 3(S)-tetrahydrofuranyl ester
14.1 ml (27.24 mmol) of a 20 % solu~ion of phosgene in toluene are added dropwise to
14 ml of toluene. After the mixture has been cooled in an ice-bath, a solution of 2 g
(22.7 mmol) of (S)-(+)-3-hydroxy-tetrahydrofuran (JPS CHIMIE, Bevaix, Switzerland) in
a small amount of toluene is added, the mixture is then stirred for 1 h at RT, and the
excess phosgene is çxpelled with argon. After concentration in a rotary evaporator at
reduced pressure, distillation is carried out for the purpose of purification. Boiling point at
12 torr: 130C.
' ' .
Refer~nce Example 65: 5(S)-~3(R)-(TetrahvdrofuranYl)oxYcarbonYlaminol-4(S)-hYdr
6-phenyl-2(R)-benzylhexanovl-(L)-Val-(L)-Phe-morpholin-4-ylamide
Analogously to Reference Example 63, 500 mg (0.795 mmol) of 5(S)-(amino)-4(S)-
hydroxy-6-phenyl-2(R)-benzylhexanoyl-(L)-Val-(L)-Phe-morpholin-4-ylamide are reacted
with 363 mg (2.41 mmol) of chloroformic acid 3(R)-tetrahydrofuranyl ester. The title
compound is purified by column chromatography (SiO2, ethyl acetate to ethyl acetate/-
acetone: 9/1 ); TLC Rf(ethyl acetate/acetone: 4/1)= 0.62; tRet(IV)= 14.06 min; FAB-MS
(M+H= 743.
The starting material is prepared as follows:
a) Chloroformic acid 3(R)-tetrahYdrofuranyl ester
12.5 ml (24.15 mmol) of a 20 % solution of phosgene in toluene are cooled in an ice-bath,
a solution of 1.06 g (12.03 mmol) of (R)-(+)-3-hydroxytetrahydrofuran (JPS CHIME,
Bevaix, Switzerland) in a small amount of toluene is then added dropwise ~nd, after the
mixture has been stirred at RT for 2 h, the excess phosgene is expelled with argon. After
concentration by evaporation in a rotary evaporator under reduced pressure, the crude title
compound is further processed without being further purified.
Reference Example 66: 5(S)-Ethoxvcarbonylamino-4(S)-hydroxy-6-phenyl-2(R)-benzyl-
hexanoyl-(L)-Val-(L)-Phe-morpholin-4-ylamide
Analogously to Reference Example 63, 500 mg (0.795 mmol) of 5(S)-(amino)-4(S)-
hydroxy-6-phenyl-2(R)-benzylhexanoyl-(L)-Val-(L)-Phe-morpholin-4-ylamide are reacted
with 0.151 ml (1.585 mmol) of chloroformic acid ethyl ester (Fluka, Buchs, Switzerland).
The title compound is purified by crystallisation (ethyl acetate/ether); TLC Rf(B)= Q.4;
tRet(IV)= 14.51 min; FAB-MS (M+H+)= 701.
; .,, - : . . ,, ~ ~ . . ,
~1186~1
- 163-
Reference Example 67: 5(s)-(Boc-amino)-4(s)-hydroxy-6-cyclohexyl-2(R)-(4-ben
phenylmethvl)-hexanoyl-(L)-Val-(L)-Phe-morpholin-4-vlamide
Analogously to Reference Example 1, 1.45 g (1.517 mmol) of S(S)-(Boc-amino)-4(S)-
(tert-butyldimethylsilyloxy)-6-cyclohexyl-2(R)-(4-benzyloxybenzyl)-hexanoyl-(L)-Val-
(L)-Phe-morpholin-4-ylamide in 12 ml of DMF are reacted with 0.958 g (3.03 mmol) of
TBAF trihydrate to yield the title compound. The title compound is purified by crystallisa-
tion (hexane). TLC Rf (B)= 0.5; tRet(IV)= 18.99 min; FAB-MS (M+H+)= 841.
The starting compounds are prepared as follows:
a) 5(S)-~ 1 (S)-(Boc-amino)-2-cYclohexYlethyll -dihYdrofuran-2-(3H)-one
A solution of 5 g (16.37 mmol) of 5(S~-~l(S)-(Boc-amino)-2-phenylethyl]-dihydrofuran-
2-(3H)-one in 50 ml of methanol is hydrogenated for 2 h at RT under normal pressure in
the presence of 0.5 g of Nishimura catalysi. The catalyst is removed by filtration and the
filtrate is then concentrated in a rotary evaporator and dried under a high vacuum. TLC Rf
(D)= 0.5; FAB-MS (M+H+)= 312.
b) 5(S)-r 1 (S)-(Boc-amino)-2-cYclohexvlethvll-3~R)-(4-benzyloxYbenzvl)-dihvdrofuran-2-
(3H)-one
Analogously to Reference Example 21 D) 1) c), 30.9 g (99.26 mmol) of 5(S)-[l(S)-(Boc-
arnino~-2-cyclohexylethyl]-dihydrofuran-2-(3H)-one are reacted with 200 ml (200 mmol)
of lidhium bis(trimethylsilyl)amide lM in THF and 34 g (104.8 mmol) of 4-benzyloxy-
benzyl iodide to yield the title compound. The title compound is purified by column
chromatography (SiO2, h~xane/ethyl acetate: 4/1 to 1/1) and crystallisation (hexane/ethyl
acetate); TLC Rf (C)= 0.33; tRet(IV)= 20.41 min; FAB-MS (M+H= 508.
c) 5(S)-(Boc-amino)-4(S)-hvdroxv-6-cYclohexyl-2(R)-(4-benzYloxvbenzYl)-hexanoic acid
Analogously to Reference Example 1 i), 2.4 g (4.728 mmol) of 5(S)-[l(S)-(Boc-amino)-
2-cyclohexylethyl]-3(R3-(4-benzyloxybenzyl)-dihydrofuran-2-(3H)-one in 10 ml of 1,2-di-
methoxyethane are reacted with 9.45 ml of lM LiOH solution to yield the tide compound,
which is purified by crystallisation from hexane. TLC Rf (E) = 0.33; tRet(IV)= 18 min;
FAB-MS (M+H+)= 526.
d) 5(S)-(Boc-amino)-4(S)-(tert-butYldimethvl)silYloxY-6-cyclohexvl-2(R)-~4-benzyloxy-
benzyl)-hexanoic acid -
Analogously to Reference Example l j), 28.8 g (54.8 mmol) of 5(S)-(Boc-amino)-4(S)-
.
il . '~; ' ~ , ,
2 1 1 8 ~
- 164-
hydroxy-6-cyclohexyl-2tR)-(4-benzyloxybenzyl)-hexanoic acid in 288 ml of DMF areconverted into the title compound with 35.8 g (237.6 mmol) of tert-butyldimethylchloro-
silane and 30 g (237.6 mmol) of imidazole. The title compound is purified by column
chromatography (SiO2, hexane/ethyl acetate: 4/1 to 1/1); TLC Rf (E)= 0.33; tRCt(IV)=
23.72 min;
' ~' .
e) ~(S)-(Boc-amino)-4(S)-(tert-butvldimethvl)silYloxv-6-cvclohexvl-2(R)-(4-hvdroxy-
phenylmethvl)-hexanoyl-(L)-Val-(L)-Phe-morpholin-4-ylamide
3 g (4.69 mmol) of 5(S)-(Boc-amino)-4(S)-(tert-butyldimethylsilyloxy)-6-cyclohexyl-
2(R)-(4-benzyloxybenzyl)-hexanoic acid in 40 ml of DMF are cooled in an ice-bath to 5C :
with 1.91 g (5.16 mmol) of H-(L)-Val-(L)-Phe-morpholin-4-ylamide, and 0.783 ml
(5.16 mrnol) of DEPC and 2.3 ml (16.41 mmol) of triethylamine are added. After the
mixture has been stirred for 1.5 h at RT it is poured onto water and extracted three times
with ethyl acetate. The combined organic phases are washed with water, saturated sodium
bicarbonate solution (twice) and brine, dried over sodium sulfate and then concentrated
under reduced pressure. The title compound is purified by column chromatography (SiO2,
hexane/ethyl acetate: 1/1); TLC Rf (A)= 0.3; tRet(IV) = 25.3 min; FAB-MS (M+H+)= 955.
Reference Example 68: 5(S)-(Boc-amino)-4(S)-hYdroxv-6-cYclohexY1-2(R)-(4-hYdr
phenvlmethyl)-hexanoyl-(L)-Val-L)-Phe-morpholin-~ylamide
Analogously to Reference Example 1, 0.69 g (0.797 mmol) of 5(S)-(Boc-amino)-4(S)-
(tert-butyldimethylsilyloxy)-6-cyclohexyl-2(R)-(4-hydroxyphenylmethyl)-hexanoyl-(L)-
Val-(L)-Phe-morpholin-4-ylamide in 5 ml of DMF are reacted with O.S g (1.59 mmol) of
TBAF trihydrate to yield the title compound. The title compound is purified by crystallisa~
tion (ether). tRet(IV)= 15.52 min; FAB-MS (M+H+)= 751.
The starting compound is prepared as follows:
a) S(S)-(Boc-amino)-4(S)-(tert-butYldimethYl)silyloxy-6-cyclohexyl-2(R~-(4-hydr
phenylmethyl)-hexanovl-(L)-Yal-(L)-Phe-morpholin-4-vlamide
Analogously to Reference Example 45, 19.5 g (20.41 mmol) of S(S)-(Boc-amino)-4(S)-
hydroxy-6-cyclohexyl-2(R)-(4-benzyloxyphenylmethyl)-hexanoyl-(L)-Val-(L)-Phe-
mo~pholin-4-ylamide (Reference Example 67 e) in 400 ml of methanol are hydIogenated
in the presence of 4 g of 10% Pd/C. The title compound obtained after working up is
further reacted without additional purification; TLC Rf (A) 0.28; tRe,(IV) = 21.99 min;
FAB-MS (M+H+)= 866.
21~8~1
- 165-
Reference Example 69: 5(S)-(Boc-amino)-4(S)-hydroxv-6-cyclohexYI-2(R)-(4-methoxY-
phenylmethyl)-hexanoyl-(L)-Val-(L)-Phe-morpholin-4-ylamide
Analogously to Reference Example 1, 3.93 g (4.469 mmol) of 5(S)-(Boc-amino)-4(S)-
(tert-butyldimethylsilyloxy)-6-cyclohexyl-2(R)-(4-methoxyphenylmethyl)hexanoyl-(L)-
V~l-(L)-Phe-morpholin-4-ylamide in 15 ml of DMF are reacted with 2.82 g (8.94 mmol)
of TBAF trihydrate to yield the title compound. The title compound is purified by precip-
itation (hexane). TLC Rf (B)= 0.64; tRe,(IV)= 17.34 min; FAB-MS (M+H+)= 765.
The starting compound is prepared as follows:
a) S(S)-(Boc-amino)-4(S)-(tert-butYldimethvl)silyloxY-6-cyclohexvl-2(R)-(4-methoxY-
phenylmethyl)-hexanoyl-(L)-Val-(Ll-Phe-morpholin-4-ylamide
Analogously to Reference Example 70 a), a solution of 4 g (4.623 mmol) of 5(S)-(Boc-
amino)-4(S)-(tert-butyldimethyl)-silyloxy-6-cyclohexyl-2(R)-(4-hydroxyphenylmethyl)-
hexanoyl-(L)-Val-(L)-Phe-morpholin-4-ylamide in 70 ml of dioxane is reacted with 6.02 g
(18.49 mmol) of caesium carbonate and 9.1 ml (92.46 mmol) of methyl iodide. The title
compound obtained after working up is further processed without being further puri~led.
TLC Rf (I)= 0.36; tRet(IV)= 24 min; FAB-MS (M+H+)= 880.
Reference Example 70: S(S)-(Boc-amino)-4(S)-hvdroxY-6-PhenY1-2(R)-benzvlhexanovl-
(L)-Val-(L)-(4-isobutvloxy-Phe)-morDholin-4-ylamide
Analogously to E~eference Example 1, 201 mg (0.22 mmol) of 5(S)-(Boc-amino)-4(S)-
(tert-butyldimethylsilyloxy)-6-phenyl-2(R)-benzylhexanoyl-(L)-Val-(L)-(4-isobutyloxy-
Phe)-morpholin-4-ylamide are deprotected with 139 mg (0.44 mmol) of TBAF in 5 ml of
DMF over a period of 18.5 h. Column chromatography (SiO2, ethyl acetate/hexane: 4/1)
yields the tide compound: TLC R~(B)= 0.38; tRet(I)= 18.3 min; FAB-MS (M+H+)= 801.
The starting compounds are prepared as follows:
a) N-(BenzvloxYcarbonY!~-(L)-Val-(L)-(4-isobutYloxy-Phe)-morpholin-4-ylamide
A solution of 1.93 g (4 mmol) of N-(benzyloxycarbonyl)-(L)-Val-(L)-Tyr-morpholin-4-yl-
amide (Reference Example 41 A) c)) in 8 ml of 1/1 DMF/dioxane is treated with 2.6 g
(8 mmol) of caesium carbonate and 2.31 ml (20 mmol) of isobutyl iodide and then heated
to 50C. After 1.25 h, 2.6 g (8 mmol) of caesium carbonate and 2.31 ml (20 mmol) of
isobutyl iodide are again added, followed by a further 2.31 ml (20 mmol) of isobutyl
.,
.. .... ~
2~i8~1
- 166-
iodide after 2.15 h and after 4 h. After having been stirred for a total of 5.75 h at 50C, the
reaction mixture is poured onto ice/water and extracted three times with methylene
chloride. Drying over sodium sulfate is followed by concentration in a rotary evaporator.
Column chromatography ~SiO2, ethyl acetate/hexane 4/1) yields the title compound: TLC
Rf (B)= 0.43.
b) H-(L~-Val-(L~-(4-isobusvloxy-Phe~-morpholin-4-ylamide
Analogously to Reference Example 10 e), 1.5 g (2.78 mmol) of N-(benzyloxycarbonyl)-
(L)-Val-(L)(4-isobutyloxy-Phe)-morpholin-4-ylamide in 40 ml of methanol are hydrogen-
ated in the presence of 0.2 g of 10% Pd/C. The title compound is further used without
being further purified: TLC Rf (F)= 0.44.
c) 5(S~-(Boc-amino~-4(S)-(tert-butvldimethvlsilyloxy~-6-phenyl-2(R)-benzvlhexanoYl- .
(L~-Val-(L)-(4-isobutYloxy-Phe)-morpholin-4-ylamide
Analogously to Reference Example 10 f), 118 mg ~0.22 mmol) of 5(S)-(Boc-amino)-4(S)-
(tert-butyldimethylsilyloxy)-6-phenyl-2(R)-benzylhexanoic acid and 100 mg (0.25 mmol)
of H-(Lj-Val-(L)-(4-isobutyloxy-Phe)-morpholin-4-ylamide in 2.19 ml of NMMJCH3CN0.25M are reacted with 94.8 mg (0.24 mmol) of HBTU ~o give the title compound. -
Column chromatography (SiO2, ethyl acetate/hexane: 4/1) yields the title compound: TLC
Rf (B)= 0.54.
Reference Example 71: 5(5)-(Boc-amino)-4~S)-hydroxv-~phenyl-2lR)-bonzvlhexanoYl-
(L)-Leu-(L)-(p-CH~,O-Phe)-morPholin-~ylamide '.
Analogously to Reference Example 1, 189 mg (0.22 mmol) of 5(S)-(Boc-amino)-4(S)-(tert-butyldimethylsilyloxy)-6-phenyl-2(R)-benzylhexanoyl-L)-Leu-(L)-(p-CH30^Phe)-
morpholin-~ylamide are deprotected with 139 mg (0.44 mmol) of TBAF in S ml of DMF
over a period of 18 h. Column chromatography (SiO2, ethyl acetate/hexane: 4/1) yields the
title compound: TLC Rf(B)= 0.37; tRe,(I)= 16.6 min; FAB-MS (M+H~)= 773.
.
The starting compounds are prepared as follows:
a) N-(BenzyloxYcarbonvl)-(L~-Leu-L)-(p-CH~O-Phe)-morpholinvlamide
0.45 g (1.68 mmol) of (L)-N-benzyloxycarbonyl-leucine (Fluka, Buchs, Switzerland) and -~
0.444 g (1.68 mmol) of H-(L)-(p-CH30-Phe)-morpholinylamide in 70 ml of methylenechloride are reacted with 0.347 g (1.68 mmol) of DCC and 0.25 g of HOBT to give the
title compound. Column chromatogaphy (SiO2, ethyl acetate/hexane: 4/1) yields the tide
j . . . . ~, . . ..
2~661
- 167-
compound: TLC Rf (B)= 0.28.
b) H-(L)-Leu-(L~-(p-CH~O-Phe~-morpholin-4-Ylamide
Analogously to Reference Example 10 e), 0.73 g (1.43 mmol) of N-(benzyloxycarbonyl)-
(L)-Leu-(L)-(p-CH3Q-Phe)-morpholin-4-ylamide in 20 ml of methanol is hydrogenated in
the presence of 0.1 g of 10% Pd/C. The title compound is further used without being
fu}ther purified: TLC R~ (F)= 0.47.
c) 5(S)-(Boc-amino~-4(S!-(tert-butyldimethylsilyloxv~-6-phenyl-2~R)-benzvlhexanoyl-
(L)-Leu-(L)-(p-CH~O-Phe~-morpholin-4-ylamide
Analogously to Reference Example 10 f),118 mg (0.22 mmol) of S(S)-(Boc-amino)-4(S)-
(tert-butyldimethylsilyloxy)-6-phenyl-2(R)-benzylhexanoic acid and 91 mg (0.25 mmol)
of H-(L)-Leu-(L)-(p-CH30-Phe)-morpholin-4-ylamide in 2.19 ml of NMM/CH3CN 0.25M
are reacted with 94.8 mg (0.24 mmol) of HBTU to give the title compound. Column
chromatography (SiO2, ethyl acetate/hexane: 4/1) yields the title compound: TLC Rf (B)=
0.57.
d) ~1~
Analogously to Reference Example 70 a), a solution of 2 g (5.2 mmol) of Z-(L)-Tyr-
molpholin-4-ylamide (Reference Example 70 e)) in 100 ml of 1:1 DMF/dioxane is reacted
with 3.38 g (10.4 mmol) of caesium carbonate and 0.324 ml (5.2 mmol) of methyl iodide.
Column chromatography (SiO2, ethyl acetate/hexane: 4/1) yields the title compound: TLC
Rf (ethyl acetate/hexane: 4/1)= 0.34.
e) H-(L)-(p-CH~O-Phe)-morpholin-4-ylamide.
Analogously to Example 70 b), a solution of 3.9 g (9.8 mmol) of Z-(L)-(p-CH3O-Phe)-
morpholin-4-ylamide in 150 ml of methanol is hydrogenated in the presence of 1.2 g of
10% Pd/C. After working up, the title compound is further used without additional
purification. TLC Rf (F)= 0.32.
Reference Example 72: 5(S)-(Boc-amino)-4(S)-hydroxY-6-phenyl-2(R~-benzYlhexanovl-
(L)-Val-(L)-(4-n-butyloxy-Phe)-morpholin-4-Ylamide
Analogously to Reference Example 1,201 mg (0.22 mmol) of 5(S)-(Boc-amino)-4(S)-
(tert-butyldimethylsilyloxy)-6-phenyl-2(R)-benzylhexanoyl-(L)-Val-(L)-(4-n-butyloxy-
Phe)-morpholin-4-ylamide are deprotected with 139 mg (0.44 mmol) of TBAF in S ml of
DMF over a period of 16.5 h. Column chromatography (SiO2, ethyl acetate/hexane: 4/1)
~ . .
~: 2118661
- 168-
yields the title compound: TLC Rf(B)= 0.39; tRC~(I)= 18.2 min; FAB-MS (M~H+)= 801.
The starting compounds are prepared as follows:
a) N-(BenzvloxYcarbonvl)-L~-Val-(0-(4-n-butyloxy-Phe)-morpholin-4-vlamide
Analogously to Reference Example 70 a), a solution of 0.48 g (0.1 mmol) of N-(benzyl-
oxycarbonyl)-(L)-Val-(L)-Tyr-morpholin-4-ylamide in 0.2 ml of 1/1 DME/dioxane istreated with 65 mg (0.2 mmol) of caesium carbonate and 11.9 111 (0.1 mmol) of n-butyl ; --
iodide. Column chromatography (SiO2, ethyl acetate/hexane 4/1) yields the title
compound: TLC Rf (B)= 0.47.
b) H-(L)-Val-(L)-(4-n-butyloxy-Phe)-morpholin-4-vlamide
Analogously to Reference Example 10 e), 1.38 g (2.6 mmol) of N-(benzyloxycarbonyl)-
(L)-Val-(L)-(4-n-butyloxy-Phe)-moIpholin-4-ylamide in 40 ml of methanol are hydrogen-
ated in the presence of 0.2 g of 10% PdlC. The title compound is further used wi~out
being further purified: TLC Rf (F)= 0.45.
c) StS)-(Boc-amino)-4(S)-(tert-butyldimethvlsilvloxv)-6-phenyl-2(R)-benzvlhexanovl-
(L)-Val-(L)-(4-n-butvloxy-Phe)-morpholin-4-vlamide
Analogously to Reference Example 10 f), 118 mg (0.2~ mmol) of 5(S)-(Boc-amino)-4tS)-
(tert-butyldimethylsilyloxy-6-phenyl-2(R)-benzylhexanoic acid and 98 mg (0.25 mmol) of
H-(L)-Val-(L)-(4-n-butyloxy-Phe)-morpholin-4-ylamide in 2.19 ml of NMM/CH3CN
0.25M are reacted with 94.8 mg (0.24 mmol) of HBTU to yield the title compound. The
title compound, TLC Rf (B)= 0.57, is further used without additional purification.
Reference Example 73: 5(S~-{Boc-amino)-4(S)-hYdroxv-6-PhenY1-2(R~-benzYlhexan
~O-Phe)-morpholin-4-ylamide
Analogously to Reference Example 1, 380 mg (0.41 mmol) of 5(S)-(Boc-amino)-4(S)-(tert-butyldimethylsilyloxy)-6-phenyl-2(R)-benzylhexanoyl-(L)-phenylglycyl-(L)-
(p-CH30-Phe)-morpholin-4-ylamide are deprotected with 265 mg (0.82 mmol) of TBAFin 10 ml of DMF over a period of 17 h. Column chromatography (SiO2, ethyl acetate/- -
hexane: 2/1 to 4/1) yields the title compound: TLC Rf(B)= 0.47; tRet(I)= 16.4 min;
FAB-MS (M+H+)= 793.
The starting cornpounds are prepaTed as follows:
2118~1
- 169-
a) N-(t-Butyloxvcarbonyl)-(L)-phenyl~lycyl-~L)-(p-CH3O-Phe)-morpholin-4-Ylamide
0.44 g (2 mmol) of N-tert-butyloxycarbonyl-(L)-phenylglycine and 0.53 g (2 mmol~ of
H-(L)-(p-CH30-Phe)-morpholinylamide in 80 ml of methylene chloride are reacted with
0.413 g (2 mmol) of DCC and 0.297 g of HOBT to give the title compound. Column
chromatography (SiO2, ethyl acetate/hexane: 4/1) yields the title compound: TLC R
(ethyl acetate/hexane: 4/1)= 0.31.
b) H-(L)-Phenyl~lYcvl-(L)-(p-CH~O-Phe)-morpholin-4-vlamide
A solution of 900 mg (1.81 mmol) of N-(t-butyloxycarbonyl)-(L)-phenylglycyl-(L)-(p-CH30-Phe)-moIpholin-4-ylamide in 19 ml of formic acid is stirred for 2 h and then
concentrated in a rotary evaporator and dissolved in ethyl acetate. The solution is washed
in succession 4 times with sodium bicarbonate, once with water and once with brine, dried
over Na2SO4 and then concentrated. The ti~le compound is further used without being
further purified. TLC Rf (B)= 0.12.
c) S(S)-(Boc-amino~-4(S)-(tert-butYldimethYlsilYloxv)-6-phenYI-2(R)-benzvlhexanovl~
(L)-phenvlglycyl-(L~-~p-CH30-Phe)-morpholin-4-ylamide
Analogously to Reference Example lû f), 118 mg (0.22 mmol) of 5(S)-(Boc-amino)-4(S)-
(tert-butyldimethylsilyloxy)-6-phenyl-2(R)-benzylhexanoic acid and 96 mg (0.25 mmol)
of H-(L)-phenylglycyl-(L)-~p-CH30-Phe)-morpholin~-ylamide in 2.19 ml of
NMMICH3CN 0.25M are reacted with 94.8 mg (0.24 mmol) of HBTU to give the title
compound. Column chromatography (SiO2, ethyl acetate/hexane: 4/1) yields the title
compound: TLC Rf (B)= 0.57.
Reference Example 74: Boc-PherCl(3~4-dimeithoxv)Phe-(L)-Val-(L)-Phe-morpholin-4-Yl-
_mide
Analogously to Reference Example 56 O), 141 mg of 5(S)-(Boc-amino)-4(S~-(tert-butyl-
dimethylsilyloxy)-6-phenyl-2(R)-(3,4-dimethoxyphenylmethyl)-hexanoyl-(L)-Val-(L)-
Phe-morpholin-4-ylamide in 3 ml of DMF are desilylated with 100.4 mg of TBAF to yield
the tide compound. Purification is carried out by chromatography twice on silica gel
(eluants ethyl acetate/hexane 4:1 and B). TLC Rf(B)i=0.21. FAB-MS (M+H)+=789.
The starting material is prepared as follows:
74 a) 3,4-Dimethoxybenzyl chloride
Analogously to Reference Example 56 O) a), the title compound is obtained from 10 g of
211~6~1
- 170 -
3,4-dimethoxybenzyl alcohol (Fluka, Buchs, Switzerland),46.2 g of diisopropylamino-
methylpolystyrene (poly-Hanig base) and 4.62 ml of thionyl chloride in 200 ml of abs.
ether. TLC Rf(C)=0.31.1H-NMR (200 MHz, CDC13): 7.0-6.87 (m, 2H); 6.82 (d, lH); 4.56
(s, 2H); 3.9 (s, 3H); 3.87 (s,3H).
74 b) 3~4-Dimethoxybenzyl iodide
Analogously to Reference Example 56 O) b), the title compound is obtained from 6.185 g
of 3,4-dimethoxybenzyl chloride and 24.19 g of sodium iodide in 62 ml of abs. acetone.
TLC Rf(C)=0.40.1H-NMR (200 MHz, CDM3): 6.95 (dxd, lH); 6.88 (d, lH); 6.75 (d, lH);
4.47 (s, 2H); 3.87 (s, 3H); 3.86 (s,3H).
74 c) S(S)-rl(S)-(Boc-amino)-2-phenylethyll-3(R)-(3~4-dimethoxv~henylmethyl)-dihydr
furan-2-(3H~-one
Analogously to Reference Example 56 O) c), 1 g of 5(S)-[l(S)-(Boc-amino~-2-phenyl-
ethyl]-dihydrofuran-2-(3H)-one [prepared according to Reference Example 2i)] in 4 ml of
abs. THF is deprotonated (-75C) with 6.42 ml of lithium bis(trimethylsilyl3amide (lM in
THF) and with the addition of 0.66 ml of DMPU, and aL~cylated with 911 mg of 3,4-di-
methoxybenzyl iodide. Chromatography on silica gel (eluants D, A and hexane/ethyl
acetate 1:2) yields the pure title compound. TLC Rf(A)=0.42. MS M+=455. -
74 d) 5(S)-(Boc-amino)-4(S)-hydroxy-6-phenyl-2(R)-(3,4-dimethoxvphenvlmethyl)-
hexanoic acid
Analogously to Reference l~xample 56 O) d),778 mg of 5(S)-[l(S)-(Boc-amino)-2-
phenylethyl]-3(R)-(3,4-dimethoxyphenylmethyl)-dihydrofuran-2-(3H)-one in 27.67 ml of
dimethoxyethane and 13.91 ml of water are hydrolysed with 6.83 ml of lithium hydroxide
solution lM to yield the title compound which is directly further processed. TLCRf(A)=0.07.
74 e)' 5(S)-(Boc-amino)-4(S)-(tert-butvldimethvlsilyloxv)-6-phenyl-2(R)-(3,4-dimethoxy-
phenylmethyl)-hexanoic acid
Analogously to Reference Example 56 O) e), 804 mg of 5(S)-(Boc-amino)-4(S)-hydroxy-
6-phenyl-2(R)-(3,4-dimethoxyphenylmethyl)-hexanoic acid in 5.94 ml of DMF are
silylated with 1.162 g of tert-butyldimethylchlorosilane and 946.6 mg of imidazole.
Purification of the title compound is carried out by chromatography twice on silica gel
(eluants D, A, ethyl acetate/hexane 2: 1 and B). TLC Rf(A)=0.27. MS M+=557.
211~
- 171 -
74 f) 5(S)-(Boc-amino)-4(S)-(tert-butyldimethvlsilyloxy~-6-phenvl-2 (R)-(3.4-dimethoxv-
phenvlmethyl~-hexanovl-(L)-Val-(L)-Phe-morpholin-4-ylamide
Analogously to Reference Example 56 O) f), 100 mg of 5(S)-(Boc-amino)-4(S)-(tert-
butyldimethylsilyloxy)-6-phenyl-2(R)-(3,4-dimethoxyphenylmethyl)-hexanoic acid and
62.3 mg of H-(L)-Val-~L)-Phe-morpholin-4-ylamide [prepared according to Reference
Example lo)] in 1.59 ml of NMM/CH3CN 0.25M arei reacted with 70.9 mg of HBTU to
yield the title compound. TLC Rf (ethyl acetate/hexane 2:1)=0.19. FAB-MS (M~H)+=903.
Reference Example 75: Boc-PhelCl(3,4~5-trimethoxy)Phe-(L)-Val-(L)-Phe-morpholin-4-
Ylamide
Analogously to Reference Example 56 O), Sl l mg of 5(S)-(Boc-amino)-4(S)-(tert-butyl-
dimethylsilyloxy)-6-phenyl-2(R)-(3,4,5-trimethoxyphenylmethyl)-hexanoyl-(L)-Val-(L)-
Phe-morpholin-4-ylamide in 9.36 ml of DMF are desilylated with 323.5 mg of TBAF to
yield the title compound. Purification is carried out by chromatography on silica gel
(eluants B and H). TLC Rf(B)=0.13. FAB-MS (M+H)+=819.
The starting material is prepared as follows:
75 a) 3,4~5-Trimethoxybenzyl iodide
Analogously to Reference Example 56 O) b), the title compound is obtained from S g of
3,4,5^trimethoxybenzyl chloride (Fluka, Buchs, Switzerland) and 16.89 g of sodium iodide
in 40 ml of abs. acetone. TLC Rf(C)=0.27.1H-NMR (360 MHz, CDCI3): 6.60 (s,2H~;
4.44 (s,2H); 3.86 (s, 6H); 3.83 (s,3H).
.
75 b) 5(S)-~l(S)-(Boc-amino)-2-~YlethY11-3(R)-(3~4,5-trimethoxvphenvlmethYl)- ~ :~
dihvdrofuran-2-(3H)-one
Analogously to Reference Example 56 O) c), 1 g of 5(S)-[l(S)-(Boc-amino)-2-phenyl-
ethyl]-dihydrofuran-2-(3H)-one [prepared according to Reference Example 2i)] in 4 ml of
abs. ~F is deprotonated (-75C) with 6.42 ml of lithium bis(trimethylsilyl)amide (lM in
THF) and with the addition of 0.66 ml of DMPU, and alkylated with 1.008 g of 3,4,5-tri-
me.thoxybenzyl iodide. Chromatography on silica gel (eluants hexane/acetone 3: 1) yields
the title compound. TLC Rf(hexane/acetone 3:1)=0.22. FAB-MS M+=485.
75 c)S(S)-(Boc-arnino)-4(S)-hYdroxv-6-Phenyl-2(R)-(3~4~5-trimethoxyphenylmeth
hexanoic acid
Analogously to Reference Example 56 O) d), 1.097 g of S(S)-[l(S)-(Boc-amino)-
-:` 2~661
- 172-
2-phenylethyl]-3~R)-(3,4,5-trimethoxyphenylmethyl)-dihydrofuran-2-(3H)-one in
36.48 ml of dimethoxyethane and 18.39 ml of water are hydrolysed with 9.03 ml oflithium hydroxide solution lM to yield the title compound, which is further processed
without being purified.
75 d) 5(S)-(Boc-amino)-~(S)-(tert-butvldimethylsilvloxY)-6-phenYI-2(R)-(3,4.5-tri-
methoxvphenvlmethyl)-hexanoic acid
Analogously to Reference Example 56 O) e),1.526 g of 5(S)-(Boc-amino)-4(S)-hydroxy-
6-phenyl-2(R)-(3,4,5-trimethoxyphenylmethyl)-hexanoic acid in 15.16 ml of DMF are
silylated with 2.11 g of tert-butyldimethylchlorosilane and 1.683 g of imidazole. Purifica-
tion of the title compound is carried out by chromatography twice on silica gel (solvents:
hexane, A, ethyl acetate/hexane 2:1). TLC Rf(B)=0.39. FAR-MS (M+H)+=618.
75 e) 5(S)-[Boc-amino)-4(S)-(tert-butyldimethvlsilyloxy)-6-Phenvl-2(R)-(3~4.5-tri-
methoxvphenvlmethyl)-hexanoYl-(L)-Val-(L)-Phe-morpholin-4-Ylamide '
Analogously to Reference Example 56 O) f),316.5 mg of 5(S)-(Boc-amino)-4(S)-(tert-
butyldimethylsilyloxy)-6-phenyl-2(R)-(3,4,5-trimethoxyphenylmethyl)-hexanoic acid and
171 mg of H-(L)-Val-(L)-Phe-morpholin-4-ylamide [prepared according to ReferenceExample lo)] in 4.82 ml of NMM/CH3CN 0.25M are reacted with 213.39 mg of HBTU toyield the title compound. TLC Rf(B)=0.45. FAB-MS (M+H)+=~33.
Reference Example 76: Boc-PherCl(2.3.4-trimethoxy)Phe-(L)-Val-(L)-Phe-morPholin-4-
vlamide
Analogously to Reference Example 56 O), 407 mg of 5(S)-(Boc-amino)-4(S)-(tert-butyldi-
methylsilyloxy)-6-phenyl-2(R)-(2,3,4-trimethoxyphenylmethyl)-hexanoyl-(L)-Val-(L)-
Phe-morpholin-4-ylamide in 7.41 ml of DMF are desilylated with 256 mg of TBAF toyield the title compound. Purification is carried out by chromatography on silica gel
(eluant: B). TLC R~(B)=0.12. FAB-MS (M+H)+=819.
The starting material is prepared as follows:
76 a) 2.3.4-TrimethoxYbenzYI chloride
Analogously to Reference Example 56 O) a), the title compound is obtained from 10.22 g
of 2,3,4-trimethoxybenzyl alcohol (Aldrich, Steinheim, Federal Republic of Germany),
33.75 g of diisopropylaminomethylpolystyrene (poly-Hunig base) and 10.6 ml of thionyl
chloride in 200 ml of abs. ether. Purification is calTied out by chromatography on silica gel
: .^~; - ,., . . ~ . .
- 211~6~1 ~
- 173-
(eluant: E). TLC Rf(E)=0.47. IH-NMR (360 MHz, CDC13): 7.05 (d, lH); 6.65 (d, lH);
4.61 (s, 2H); 3.98 (s, 3H); 3.86 (s, 3H); 3.85 (s, 3H).
76 b) 2,3~4-Trimethoxvbenzvl iodide
The title compound is obtained from 2.34 g of 2,3,4-trimethoxybenzyl chloride and 7.87 g
of sodium iodide in 18.6 ml of abs. acetone analogously to Reference Example 56 O) b),
and is further processed without being purified. TLC Rf(hexane/ethyl acetate 2.5~ 0.44.
lH-NMR (200 MHz, CDC13): 7.03 (d, lH); 6.60 (d, lH); 4.50 (s, 2H); 4.05 (s, 3H); 3.85 ~ -
(2xs, 6H).
76 c) 5(S)-1 l (S)-(Boc amino)-2-phenvlethyll-3(R)-(2,3~4-trimethoxyphenylmethYl)-
dihydrofuran-2-(3H)-one
Analogously to Reference Example 56 O) c), 1.5 g of 5(S)-[l(S)-(Boc-amino)-2-phenyl-
ethyl]-dihydrofuran-2-(3H)-one [prepared according to Reference Example 2i)] in 6 ml of
abs. THF are deprotonated (-75C) with 9.62 ml of lithium bis(trimethylsilyl)amide (lM
in THF) and with the addition of 0.998 ml of DMPU, and alkylated with 1.51 g of 2,3,4-
trimethoxybenzyl iodide. Chromatography on silica gel (eluants: hexane/ethyl acetate 1:2)
yields the pure title compound. TLC Rf(A)=0.53. FAB-MS M+=485.
76 d) 5(S)-lBoc-amino)-4(S~-hYdroxv-6-phenyl-2(R)-(2.3.4-trimethoxv~henYlmethYl)-
hexanoic acid -
Analogously to Reference Example 56 O) d), 1.354 g of 5(S)-[l(S)-(Boc-amino)-2-
phenylethyl]-3(R)-(2,3,4-triTnethoxyphenylmethyl)-dihydrofuran-2-(3H)-onein43.36ml
of dimethoY.yethane and 21.86 ml of water are hydrolysed with 10.74 ml of lithium
hydroxide solution lM to yield the title compound, which is further processed without -
being purified. TLC Rf(A)=0.03. MS M+-H20 = 485.
76 e) 5(S)-(Boc-amino)-4(S)-(tert-butyldimethYlsilvloxy)-6-phenYl-2(R)-(2.3,4-tri-
methox~,rDhenvlmethvl)-hexanoic acid
Analogously to Reference Example 56 03 e), 1.308 g of S(S)-(Boc-arnino)-4(S)-hydroxy-
6-phenyl-2(R)-(2,3,4-trimethoxyphenylmethyl)-hexanoic acid in 13 ml of DMF are
silylated with 1.816 g of tert-butyldimethylchlorosilane and 1.443 g of imidazole. Purifica- -
tion of the title compound is carried out by chromatography twice on silica gel (solvents:
A andethyl acetatefhexane 1.5:1). TLC Rf(ethyl acetate/hexane 2:1)=0.02. FAB-MS -
(M+H)+=618.
--` 21~86~1
- 174-
76 f) 5(S)-(Boc-amino)-4(S)-(tert-butyldimethylsilvloxy)-6-phenyl-2(R)-(2~3~4-tri- - :
methoxyphenylmethyl)-hexanovl-(L)-Val-(L)-Phe-morpholin-4-ylamide
Analogously to Reference Example 56 O) f), 250 mg of 5(S)-(Boc-amino)-4(S)-(tert-
butyldimethylsilyloxy)-6-phenyl-2(R)-(2,3,4-~imethoxyphenylmethyl)-hexanoic acid and
148.4 mg of H-(L)-Val-(L)-Phe-morpholin-4-ylamide [prepared according to Reference
Example lo)] in 3.807 ml of NMM/CH3CN 0.25M are reacted with 168.8 mg of HBTU toyield the title compound. TLC Rf(B)=0.64.
Examples of compounds of formula I':
Example 1: 5(S)-(Boc-amino)-4(S)-(2-furanvlcarboxY)-6-PhenY1-2(~-phenvlmeth~vl-
hexanoyl-(L)-Val-(L)-Phe-morpholin-4-vlamide
Under a nitrogen atmosphere, 74111 (0.75 mmol) of 2-furancarboxylic acid chloride
(Pluka; Buchs/Switzerland) are added ~o a solution of 365 mg (0.50 mmol) of
Boc-Phe[C]Phe-(L)-Val-(L)-Phe-morpholin-4-ylamide (Example 2) and 10 mg of DMAP
in 3 ml of dioxane and 0.6 ml of pyridine and the mixture is stiIred for 44 h at RT. Since,
according to HPLC, there is still some starting material present, a further 0.1 ml of
2-furanecarboxylic acid chloride and 1 ml of pyridine are added. After a further 2 days, the
reaction mixture is diluted with ethyl acetate and washed with sat. NaHCO3 soludon,
water and brine. The aqueous phases are extracted with 2 portions of ethyl acetate and the
combined organic phases are dried with Na2SO4 and concentrated by evaporation.
Precipita~ion with DIPE/hexane 1:2 from a concentrated solution in methylene chloride
yields the pure title compound: TLC Rf~I~=0.23; tRe~(I)=17.5 min; FAB-MS (M+H)+=823.
Example 2: 5(S)-(Boc-amino)-4(S)-(N~N-dimethvlaminoacetvloxv)-6-phenvl-2(R~-
phenvlmethvlhexanoYl-(L)-Val-(L)-Phe-morpholin-4-ylamide
Under a nitrogen atmosphere, 130 mg (0.82 mmol) of N,N-dimethylacetyl chloride (in the
form of the hydrochloride salt) [preparation: N.H. Kramer and H.F.G. Linde, Arch. Pharm.
(Weinheim) 324, 433 (1991)] are added to a solution of 300 mg (0.41 mmol) of
Boc-Phe[C]Phe-(L)-Val-(L)~Phe-morpholin-4-ylamide (Reference Example 2) and a small
amount of DMAP in 1 ml of pyridine, and the mixture is stirred for 9 h at 60C. Since,
according to HPLC, there is still some starting material present, a further 130 mg of
N,N-dimethylacetyl chloride hydrochloride is added. After a further 2 h at 60C, the
reaction mixture is diluted with ethyl acetate and washed with sat. NaHCO3 solution, 2x
water and brine. The aqueous phases are extracted with 2 portions of ethyl acetate, and the
.,, . ~,
. , ~. ...
21~6~
- 175-
combined organic phases are dried with Na2SO4 and concent~ated by evaporation. Column
chromatography (SiO2, ethyl acetate/ethanol 97:3 ~ 95:5) and digestion in DIPE in an
ultrasound bath yields the crystalline title compound: TLC Rf(T)=0. 11; tRe,(II)=19.5 min;
FAB-MS (M+H)-~=8 14.
Exarnple3: S(S)-(Boc-amino)-4(S)-~4-(dimethylamino)-butyr~rloxyl-6-phenvl-2(R)
phenvlmethvlhexanovl-(L)-Val-(L)-Phe-morpholin-4-vlamide
Analogously to Example 1), 200 mg (0.27 mmol) of Boc-Phe[C]Phe-(L)-Val-(L)-Phe- ~-~
morpholin-4-ylamide (Reference Example 2) and a small amount of DMAP in 5 ml of
dioxane/acetonitrile 4:1 and 372 111 (2.2 mmol) of N-ethyldiisopropylamine are reacted
with 161 mg (0.87 mmol) of 3-(dimethylamino)butyryl chloride (in the form of the hydro-
chloride salt). Digestion of the crude product from DIPE yields the title compound:
tRet(I)=13.8 min; FAB-MS (M+H)+=842. ~ ;
The starting material is prepared as follows:
a: 4-(DimethYlamino)butyrvl chloride hydrochloride
10 g (60 mmol) of 4-dimethylaminobutyric acid hydrochloride (Janssen; Bruggen/-
Gerrnany) are heated for approximately 3 h at 65C in 30 ml of SOCl2. SOCI2 is
evaporated off and the residue is stirred to yield the title compound: TLC of a sample
dissolved in methanol, R~(U~=0.67; TLC of 4-dimethylaminobutyric acid hydrochloride,
Rf(U)=0.50.
Example 4: S(S)-(Boc-amino)-4(S)-(N-Z-N-methYlaminoacetYloxv)-6-phenY1-2(R)- ~ -
phenvlmethvlhexanovl-(L!-Val-(L)-Phe-morpholin-4-vlamide
Under a nitrogen atmosphere, 339 ,ul (2.4 mmol) of 1-chloro-N,N,2-trimethyl-1-propen-
amine [B. Haveaux, A. Dekoker, M. Rens, A.R. Sidani, J. Toye, L. Ghosez, M. Murakami,
M. Yoshioka, and W. Nagata, Organic Syntheses 59, 26 (1980)] are added at 0C to446 mg (2.00 mmol) of Z-sarcosine (Bachem; Bubendorf/Switzerland) in 10 ml of
methylene chloride. After 2 h at 0C, the mixture is concentrated by evaporation under
HV. The residue is taken up in 2 ml of dioxane and, with ice-cooling, a solution of 1.093 g
(l.S mmol) of Boc-Phe[C]Phe-(L)-Val-(L)-Phe-morpholin-4-ylamide (Reference Example -
2) in 1.3 ml of pyridine and 10 ml of dioxane is added. Since, according to HPLC, there is
still some starting material present after 60 h at RT, 17 mg of DMAP and an additional
2.0 mmol of Zsarcosyl chloride ~preparation analogous to above) in 2 ml of dioxane are
added. After 18 h at RT, the mixture is concentrated by evaporation and chromatographed
- ' ~:" ` ; - ' :; ': ' ' ! . ' ~ c;
2 1 1 ~
- 176-
(SiO2, ethyl acetate/hexane 3:1). Digestion in hexane yields the p~lre title compound: TLC
Rf(B)=0.74; tRet(I)=18.6 min; FAB-MS (M+H)+=934; Anal: calc. C 68.15 %, H 7.23 %, N
7.50%;foundC68.15%,H7.54%,N7.50%.
Example 5: S(S)-(Boc-amino)-4(S)-(methylaminoacetyloxY)-6-phenyl-2(R)-PhenYI-
methylhexanovl-(L)-Val-(L)-Phe-morpholin-4-Ylamide '
200 mg (0.21 mmol) of 5(S)-(Boc-amino)-4(S)-(N-ZN-methylaminoacetyloxy)-6-
phenyl-2(R)-phenylmethylhexanoyl-(L)-Val-(L)-Phe-morpholin-4-ylamide (Example 4)dissolved in 10 ml of ethyl acetate are hydrogenated at RT in the presence of 0.20 g of
10 % Pd/C (approximately 3 d), the mixture is ~lltered through ~)Celite, the filtrate is
concentrated by evaporation and the oil obtained is crystallised from hexane (ultrasound
bath) to yield the title compound: TLC Rf(B)=0.45; tRe,(I)=13.3 min; FAB-MS
(M+H)+=800.
Example 6: S(S)-(Boc-amino)-4(S~-~N-limidazol-4-methvl)-N-methvlaminoacetYloxvl-6-
~henvl-2(R)-phenvlmethvlhexanoyl-(L)-Val-(L)-Phe-morpholin-4-ylamide
A solution of 160 mg (0.20 mmol) of 5(S)-(Boc-amino)-4(S)-(methylaminoacetyl-
oxy)-6-phenyl-2(R)-phenylmethylhexanoyl-(L)-Val-(L)-Phe-morpholin-4-ylamide
(Example S) and 23 mg (0.24 mmol) of imidazole-4-carbaldehyde (Ph.D. Stein and St.E.
Hall, US-Patent 4 977 174, 11th Dec. 1990) in 2 ml of methanol and 26 mg (0.44 mmol)
of acetic acid is hydrogenated in the presence of 20 mg of 5 % PdlC at RT for approxi-
mately 3 h. The mixture is filtered through (~Celite, and the filtrate is concentrated by
evaporation and paltitioned between 3 portions of ethyl acetate, sat. NaHCO3 solution,
water and brine, subjected to column chromatography (SiO2, ethyl acetate/l~ 3: 1I :3) auld crystallised from acetonitrile/DIPE/hexane to yield the title compound: TLC
Rf(U)=0.13; tRet(I)=12.2 min; FAB-MS ~M+H)+=880.
Example 7: 5(S)-(Boc-amino)-4(S)-rN-(PYIidine-2-methyl)-N-methylaminoacetyloxyl-6
phenYl-2(R)-phenvlmethyl-hexanovl-(L)-val-(L)-phe-morpholin-4-ylamide
A solution of 80 mg (0.10 mmol) of 5(S)-(Boc-amino)-4(S)-(methylaminoacetyloxy)-~phenyl-2(R)-phenylmethylhexanoyl-(L)-Val-(L)-Phe-morpholin-4-ylamide ( xample 5)
and 12.9 mg (0.12 mmol) of freshly distilled pyridine-2-carbaldehyde (Fluka; Buchs/-
Switzerland) in 1.5 ml of methanol and 13 mg (0.22 mmol) of acetic acid is hydrogenated
at RT in the presence of 15 mg of 5 % Pd/C. Filtration through (~)Celite and column
chromatography (SiO2, ethyl acetate ~ ethyl acetate/ethanol/triethylamine 90:10:1) yields
the title compound: TLC Rf(V)=0.3; tRet(I)=14.3 min; FAB-MS (M+H)+=891.
2118661
- 177-
Example 8: 5(s)-(Boc-amino)-4(s)-l3-(l-triphenylmethvlimidazol-4-yl~-propion
6-phen~11-2(R)-phenylmethvlhexanovl-(L)-Val-(L)-Phe-morpholin-4-ylamide
Analogously to Example 4), 2.0 g (5.2 mmol) of 3-(1-triphenylmethylimidazol-4-yl)-
propionic acid dissolved in 20 ml of methylene chloride are converted into the acid -
chloride with 1.1 ml (7.8 mmol) of 1-chloro-N,N,2-trimethyl-1-propenamine. The
evaporation residue is dissolved in 12 ml of dioxane and added to a solution of 1.23 g
( 1.7 mmol) of Boc-Phe[C]Phe-(L)-Val-(L)-Phe-morpholin-4-ylamide (Reference Example
2) in 4 ml of pyridine. According to HPtLC, there is still a large amount of starting material
present after 18 h at RT, and therefore 50 mg of DMAP and a further 3 mmol of 3-(1-tri-
phenylmethylimidazol-4-yl)-propionic acid chloride (preparation as described above~ are
added. After a further 24 h at RT, the reaction mixture is concentrated by evaporation, and
the residue is dissolved in ethyl acetate and washed with 3x water and brine. The aqueous
phases are extracted with 2 portions of ethyl acetate. Column chromatography (SiO2,
methylene chloride/l~IF 9:1 ~ methylene chloride/'I~/triethylamine 90:10:1) yields the :
title compound: tRet(I)=17.7 min; FAB-MS (M+H)+=1093.
The starting material is prepared as follows:
.
a: Imidazol-4-Ylpropionic acid (sodium salt)
1.38 g (10 mmol) of urocanic acid (Aldrich) are dissolved in 10 ml of lN aqueous NaOH
solution. The solution is diluted with 10 ml of methanol, 80 mg of 5 % Pd/C catalyst are
added and the mixture is hydrogenated for approximately 1 h at RT. Filtraeion through ~ -
(~9Celite and concentration by evaporation yields the title compound: lH-NMR (200 MHz,
C20): 2.40 and 2.73 (2t, J=7 Hz, each 2 H), 6.77 and 7.56 (2s, each 1 E~
b: 3-(1-TriphenvlmethYlimidazol-4-Yl)-propionic acid
With stirring, 3.4 g (12.2 mmol) of triphenylchloromethane are added in portions over a
period of 4 h to a 2-phase mixture of 10 mmol of imidazol-4-ylpropionic acid (in the form
of the sodium salt), 4 ml of water, 4.6 ml (33 mmol) of triethylamine and 8 ml of
ipropanol. After the reaction mixture has been stirred for a further 4 h, 10 g of silica gel
are added and the mixture is concentrated to dryness and applied in the form of a powder
to a silica gel column (methylene chloride). Elution with methylene chloride/lpropanoV-
methanoVtriethylamine 8:3:3:1 yields the title compound: TLC Rf(W)=0.77;
tRet(I)=l 1.8 min; FAB-MS (M+H)~=383.
2 ~
- 178-
Example 9: 5(S)-(Boc-amino)-4(S)-l 3-(4-imida~olyl)-propionyloxvl-6-phenyl-2(R)
phenylmethylhexanoyl-(L)-Val-(L)-Phe-morpholin-4-ylamide
0.50 mmol of S(S)-(Boc-amino)-4(S)-[3-(1-triphenylmethylimidazol-4-yl)-propionyl-
oxy] -6-phenyl-2(R)-phenylmethylhexanoyl-(L)-Val-(L)-Phe-molpholin-4-ylamide
(Example 8) dissolved in 20 ml of ethyl acetate are hydrogenated for 8 days at 60C and at
a hydrogen pressure of approximately 4 atrn (approximately 4.052 bar or 0.4052 MPa) in
the presence of 0.3 g of 20 % palladium hydroxide on carbon. Removal of the catalyst by
filtration, concentration of the filtrate by evaporation, and column chromatography (SiO2,
acetonitrile/ethyl acetate/triethylamine 50:50:1) yields the title compound: TLCRf(X)=0.09; tRe,(I)=13.3 min; FAB-MS (M+H)+=851.
Example 10: 5(S)-(Boc-amino)-4(S)-(methoxvacetvloxv)-6-(P-fluorophenYl)-2(13~-
(p-fluorophenylmethvl)-hexanovl-(L)-Val-(L)-Phe-morpholin-4-vlamide
Analogously to Exarnple 1), 100 mg (0.13 mmol) of Boc-(p-F)Phe[C](p-F)Phe-(Lj-
Val-(L)-Phe-morpholin-4-ylamide [Reference Example 21 B) 1)] and a small arnount of
DMAP in 1 ml of dioxane and 0.16 ml of pyridine are reacted with 36 ~,11 (0.39 mmol) of
methoxyacetic acid chloride (Fluka; Buchs/Switzerland). Digestion in DIPE yields the
title compound: TLC Rf(B)=0.38; tRet(I)=16.9 min; FAB-MS (M+H)+=837.
Example 11: S(S)-(Boc-amino)-4(S)-(2-picolinovl)-6-(p-fluorophenvl)-2(R)-(P-fluQr
Phenvlmethyl)-hexanoYl-(L)-Val-(L)-Pke-morpholin-4-ylamide
Under a nitrogen atrnosphere, 32.2 mg (0.26 mmol) of 2-picolinic acid (Fluka; Buchs/-
Switzerland) in 0.5 ml of methylene chloride are converted into the acid chloride at 0C
with 22 ~11 (3.157 mmol) of 1-chloro-N,N,2-trimethyl-1-propenamine [B. Haveaux, A.
Dekoker, M. Rens, A.R. Sidani, J. Toye, L. Ghose~, M. Murakami, M. Yoshioka, and W.
Nagata, Organic Syntheses 59, 26 (1980)]. After 45 min, 1 ml of dioxane, 0.26 ml of
pyridine, 100 mg (0.13 mmol) of Boc-(p-F)Phe[C](p-F)Phe-tL)-Val-(L)-Phe-morpholin-
4-ylamide [Reference Example 21 B) 1)] and 0.3 mg of DMAP are added. Since,
according to HPLCj the reaction is not complete after 18 h at RT, further acid chloride is
added until complete conversion has taken place. The dark reaction mixture is diluted with
methylene chloride and washed twice with sat. NaHCO3 solution, water and brine. The
aqueous phases are extracted with 2 portions of methylene chloride, and the organic
phases are dried with Na2SO4, concentrated by evaporation and chromatographed (SiO2,
ethyl acetate). Decolouration of the red-coloured solution of the product in ethyl acetate
using activated carbon yields the title compound: TLC Rf(B)=0.16; tRe,(l)=16.9 min;
FAB-MS (M+H)+=870.
~; ~::: : :~ :ji
2~8~1
- 179-
Example 12: 5(S)-(Boc-amino)-4(S)-~~d e-2-carboxvl)-6-Rhellvl-2(R)-(p-fluoro-
phenylmethvl)-hexanovl-(L)-Val-(L)-Phe-morpholin-4-vlamide
Analogously to Example 11), 49 mg (0.40 mmol) of 2-picolinic acid in 3 ml of methylene
chloride are converted into the acid chloride at RT with 62 ~ll (0.44 mmol) of l-chloro-
N,N,2-trimethyl-1-propenamine (17 h). A solution of 150 mg (0.20 mmol) of Boc-
Phe[C](p-F)Phe-(L)-Val-~)-Phe-morpholin-4-ylamide [Reference Example 21 D) 1)~ and
1.5 mg of DMAP in 2.3 ml of pyridine is added thereto. Since, according to HPLC, there
is still some starting material present after 17 h at RT, a further 0.6 mmol of 2-picolinic
acid chloride is added. After a further 17 h, the reaction mixture is poured onto sat.
NaHCO3 solution and extracted with 3 portions of methylene chloAde. The organic phases
are washed with water and brine, dried with Na2SO4 and concentrated by evaporation. The -
dark-red product is dissolved in ethyl ace~ate, treated with activated carbon, ~lltered, and
concentrated by evaporation again. Digestion in an ultrasound bath in DIPE yields the tide
compound: TLC Rf(B)=0.52; tRe,(I)=18.8 min; FAB-MS (M~H)+=852.
Example 13: 5(S)-(Boc-amino)-4(S)-(methoxyacetvloxy)-6-phenYl-2(R)-(P-fluorophenyl-
methvl)-hexanovl-(L)-Val-(L~-Phe-morpholin-4-ylamide
Analogously to Example 1), 150 mg (0.20 mmol) of Boc-Phe[C](p-F)Phe-(L)-Val-(L)-Phe-morpholin-4-ylamide [Reference Example 21 D) 1)] and 1.2 mg of DMAP in 1.5 ml
of dioxane and 0.24 ml of pyridine are reacted with 27 ~,11(0.30 mmol) of methoxyacedc
acid chloride (Flulca; Buchs/Switzerland). Extraction with ethyl acetate yields the dde
compound: TLC Rf(B)=0.7; tRet(I)=16.9 min; FAB-MS (M~H)t=819.
Example 14: 5(S)-(Boc-aminol-4(S)-(benzvloxvacetYloxy)-6-phenYI-2(R)-(p-fluoro-
phenvlmethvl)-hexanoyl-(L)-yal-(L)-Phe-morpholin-4-vlamide
Analogously to Example 1), 150 mg (0.20 mmol) of Boc-Phe[C](p-F)Phe-(L)-Val-(L)-Phe-morpholin-4-ylamide [Reference Example 21 D) 1)] and 1.2 mg of DMAP in 1.5 ml
of dioxane and 0.24 ml of pyridine are reacted with 2 portions each of 47.5 ~11 (0.30 mmol)
of benzyloxyacetyl chloride (Fluka; Buchs/Switzerland). Digesdon in hexane/DIPE 1:4 ~ -
yields the title compound in the forrn of a foam: TLC Rf(A)=0.24; tRet(l)=18.7 min;
PAB-MS (M+H)+=895.
Example 15: 5(S)-(Boc-amino~-4(S)-l(S~-a-methoxy-a-phenylacetvloxYl-6-Phenvl-2(R)-
(p-fluorophenylmethYl)-hexanovl-(L)-Val-(L)-Phe-morpholin-4-ylamide
Under a nitrogen atmosphere, 66.5 mg (0.40 mmol) of (S)-a-methoxy-a-phenylacetic acid
2~1~6~1
- 180-
(Fluka; Buchs/Switærland) in 3 ml of methylene chloride are converted at RT into the
acid chloride with 62 ~,ll (0.44 mmol) of 1-chloro-N,N,2-trimethyl-1-propenamine [B.
Haveaux, A. Dekoker, M. Rens, A.R. Sidani, J. Toye, L. Ghosez, M. Murakami, M.
Yoshioka, and W. Nagata, Organic Syntheses 59, 26 (1980)]. After 30 min, a solution of
150 mg (0.20 mmol) ~f Boc-Phe[C](p-F)Phe-(L)-Val-(L)-Phe-morpholin-4-ylamide
[Reference Example 21 D) 1)] and 1.5 mg of DMAP in 2.3 ml of pyridine is added and the
mixture is then stirred for 3 h. The reaction mixture is then poured onto NaHCO3 soludon
and extracted with 3 portions of methylene chloride. The organic phases are washed with
water and brine, dried with Na2SO4 and concentrated by evaporation. Digesdon in DIPl~
yields the dde compound in the fonn of a foam: TLC Rf(B)=0.73; tRe,(I)=18.6 min;FAB-MS (M+H)+=895.
Example 16: S(S)-(Boc-amino)-4(S)-r(R)-a-methoxv-a-phenvlacetvloxvl-6-phenyl-2(R)-
(P-fluorophenvlmethvl)-hexanovl-(L)-Val-(L)-Phe-morpholin-4-ylamide
Analogously to Example 15), 66.5 mg (0.40 mmol) of (R)-a-methoxy-a-phenylacetic acid
(Fluka; Buchs/Switzerland) in 3 ml of methylene chloride are converted into the acid
chloride wi~ 62,ul (0.44 mmol) of 1-chloro-N,N,2-trime~hyl-1-propenamine, and reacted
with a soludon of 150 mg (0.20 mmol) of Boc-Phe[C](p-F)Phe-(L)-Val-(L)-Phe-morpho-
lin-4-ylamide [Example 21 D) 1)] and 1.5 mg of DMAP in 2.3 ml of pyridine to yield the
dtle compound: TLC Rf(A)=0.30; tRet(l)=18.4 min; FAB-MS (M+H)+=895.
Example 17: 5(S)-~Boc-amino)-4(S)-(l-mrazolylacetYloxv)-6-phenyl-2(R)-phenylmethhexanovl-(L)-Val-~ Phe-mor~holin-4-vlamide
Analogously to Example 4,340 ~11 (2.4 mmol) of 1-chloro-N,N,2-trimethyl-1-propenarnine
[B. Haveaux, A. Dekoker, M. Rens, A.R. Sidani, J. Toye, L. Ghosez, M. Murakami, M.
Yoshioka and W. Nagata, Organic Syntheses 59,26, (1980)] are added at 0C, under a
nitrogen atmosphere, to 252 mg (2 mmol) of l-pyrazolylacetic acid (Jones et al., J. Org.
Chem. 19, 1428-32 (1954)) in 15 ml of methylene chloride. After the mixture has been
stirred for 30 minutes at room temperature, a solution of 729 mg (1 mmol) of Boc-
Phe[C]Phe-(L)-Val-(L)-Phe-morpholin-4-ylamide (Example 2) and 5 mg of 4-dimethyl-
aminopyridine in 5 ml of pyridine is added. After 155 minutes at room temperature, the
mixture is stirred for a further 2 hours at 40C. Since, according to TLC, there is still some
starting material present, an additional 2 mmol of l-pyrazolylacetic acid chloride (for
preparation see above) is added. After a further 1.5 hours, the reaction mixture is poured
onto 150 ml of a 2:1 mixture (v/v) of water/saturated aqueous sodium bicarbonate solution
and extracted three times with methylene chloride. The combined organic phases are
:-- 2118661 ; ~
- 181 -
washed in succession with water and brine, dried over sodium sulfate, and then concen-
trated. Drying under HV is followed by chromatography (SiO2, methylene chloride/-
methanol from 100:0 to 97:3). Digestion in diisopropyl ether and a brief treatment in an
ultrasound bath yields the pure title compound: TLC Rf(ethyl acetate)= 0.34. tRet(I)=
16.8min.
Example 18: S(S)-(Boc-amino)-4(S)-(isoquinoline-3-carbonvloxv)-6-PhenYl-2(R)-Phenvl-
methylhexanoyl-(L)-Val-(L)-Phe-morpholin-4-vlamide
Analogously to Example 4, the corresponding acid chloride is prepared from 173 mg
(1 mmol) of isoquinoline-3-carboxylic acid (Aldrich, Federal Republic of Germany) with
250 ~,11 of 1-chloro-N,N,2-trimethyl-1-propenamine. In the subsequent step, a solution of
365 mg (0.5 mmol) of Boc-Phe[C]Phe-(L)-Val-(L)-Phe-morpholin-4-ylamide (Reference
Example 2) and 5 mg of 4-dimethylaminopyridine in 3 ml of pyridine is added to the acid
chloride. After working up analogously to Example 17, the product is chromatographed
(SiO2, ethyl acetate/hexane from 4: 1 to 100:0). Digestion in diisopropyl ether yields the
pure title compound: TLC Rf(ethyl acetate)= 0.28; tRe,(I)= 17.2 min.
Exarnple 19: S(S)-(Boc-amino)-4(S)-(pyrazinecarbonyloxv)-6-phenyl-2(R~-phenvlmethy -
hexanovl-(L~-Val-(L)-Phe-morpholin-4-vlamide
Analogously to Example 4, the corresponding acid chloride is prepared from 248 mg
(2 mmol) of pyrazinecarboxylic acid (Fluka, Switzerland) w;th 340,ul of 1-chloro-N,N,2-
trimethyl-l-propenamine. In the subsequent step, a solution of 729 mg of Boc-
Phe[C]Phe-(L)-Val-(L)-Phe-morpholin-4-ylamide (Reference Example 2) and 5 mg -
4-dimethylaminopyridine in ~ ml of pyridine is added to the acid chloride. After working
up analogously to Example 17, the product is chromatographed (SiO2, ethyl acetate/- -
hexane from 4:1 to 100:0). Digestion in diisopropyl ether yields the pure title compound:
TLC Rf(ethyl acetate)= 0.31; tRe,(I)= 16.4 min.
Example 20: S(S)-(Boc-amino)-4(S)-(4-a-chloromethvlbenzoYloxY~-6-phenvl-2(RI-
phenylmethylhexanoyl-(L)-Val-(L)-Phe-morpholin-4-ylamide
Analogously to Example 4, the corresponding acid chloride is prepared from 680 mg
(4 mmol) of a-choro-p-toluic acid (Fluka, Switzerland) with 800 ~ul of 1-chloro-N,N,2-tri-
methyl-l-propenamine. In the subsequen~ step, a solution of 729 mg (1 mmol) of Boc-
Phe[C]Phe-~L)-Val-(L)-Phe-morpholin-4-ylamide (Reference Example 2) in 5 ml of
pyridine is added to the acid chloride. After working up analogously to Example 17, the
product is chromatographed (SiO2, ethyl acetate). Digestion in diisopropyl ether yields the ~ -
211~6~1
- 182-
pure title compound: TLC R~(ethyl acetate) 0.5; tRC,(II)= 28.2 min.
FJxample 21: 5(S)-~Boc-amino)-4(S)-~4-(4-morpholino)methYlbenzoYloxyl-6-phenY
2(E~)-phenylmethylhexanoy~ -val-(L)-phe-morpholin-4-ylamide
Analogously to Example 4, the corresponding acid chloride is prepared from 258 mg
(1 mmol) of a-(4-mo!pholino)-p-toluic acid (p-(morpholin-4-ylmethyl)benzoic acid;
preparation according to US-Patent 4 623 486 dated 18.11.1986 (Lombardino et al.)) with
350 111 of 1-chloro-N,N,2-trimethyl-1-propenamine. In the subsequent step, a solution of
365 mg (0.5 mmol) of Boc-Phe[C]Phe-~L)-~al-(L)-Phe-morpholin-4-ylamide (Reference
Example 2) in 2.5 ml of pyridine is added to the acid chlolide. After working upanalogously to Example 17, column chromatography (SiO2, ethyl acetate then methylene
chloridelmethanol 100:0 to 96:4) yields the pure title compound: TLC Rf (ethyl acetate)=
0.28; tRe,(lI)= 20.1 min;
Example 22: 5(S)-(Boc-amino)-4(S)-(isonicotinoYloxY)-6-phenY1-2(R)-Phenylmeth
hexanovl-(L)-Val-(L)-Phe-morpholin-4-ylamide
Analogously to Example 4, ehe corresponding acid chloride is prepared from 260 mg
(2 mmol) of isonicotinic acid (Fluka, Switzerland) with 340,ul of 1-chloro-N,N,2-tri-
methyl-l-propenamine~ A solution of 729 mg (1 mmol) of Boc-Phe[C]Phe-(L)-Val-(L)-
Phe-morpholin-4-ylamide (Reference Example 2) and 5 mg of 4-N,N-dimethylamino-
pyridine in 5 ml of pyridine is then added. After working up analogously to Example 17,
column chromatography (SiO2, ethyl acetate) yields the pure title compound: TLC R
(ethyl acetate)= 0.27; tRet(I)= 15.3 min; FAB-MS (M+H+)= 834.
Example 23: 5(S)-(Boc-amino)-4(S)-(nicotinoYloxv)-6-Phenvl-2(R)-phenylmeth
hexanoYl-(L)-Val-(L)-Phe-morPholin-4-Ylamide
Analogously to Example 4, the corresponding acid chloride is prepared from 740 mg
(6 mmol) of nicotinic acid (Fluka, Switzerland) with 1200 ~,11 of 1-chloro-N,N,2-tri-
methyl-l-propenamine. In the subsequent step, a solution of 1100 mg (1.5 mmol) of Boc-
Phe[C]Phe-(L)-Val-~)-Phe-morpholin-4-ylamide (Reference Example 2) and 10 mg of
4-N,N-dimethylaminopyridine in 5.5 ml of pyridine is added to the acid chloride. After
working up analogously to Example 17, column chromatography (SiO2, ethyl acetate)
yields the pure title compound: TLC Rf(ethyl acetate)= 0.33; tRe,(lI)= 22.4 min.
:-
- ~` 2 ~
- 183-
Example 24: 5(s~-~Boc-amino)-4(s~-(2-picolinovloxy)-6-phenyl-2(R)-phenylmeth
hexanoyl-(L)-Val-(L)-Phe-morpholin-4-ylamide
Analogously to Example 41, the corresponding acid chloride is prepared from 985 mg
(8 mmol) of 2-picolinic acid (Fluka, Switzerland) with 1600 ~LI of 1-chloro-N,N,2-tri-
methyl-1-propenamine. In the subsequent step, a solution of 1450 mg (2 mmol) of 13OC-
Phe[C]Phe-(L)-Val-(I,)-Phe-morpholin-4-ylamide (Reference Example 2) and 10 mg of
4-N,N-dimethylaminopyridine in 7.5 ml of pyridine is added to the acid chloride. After
working up analogously to Example 17, column chromatography (SiO21 hexane/ethyl
acetate 1/1 to ethyl acetate) yields the pure title compound: TLC Rf(ethyl acetate)= 0.23;
tRet(II)= 28.4 min.
Example 25: S(S)-(Boc-amino)-4(S)-(3-methoxY~roPanoyloxY)-6-phenvl-2(R)-phenYl-
methvlhexanovl-~L)-Val-(L)-Phe-morpholin-4-ylamide
Analogously to Example 4, the corresponding acid chloride is prepared from 387 ~1
(4 mmol) of 3-methoxypropionic acid (Fluka, Switzerland) with 680 111 of l-chloro-
N,N,2-trimethyl-1-propenamine. In the subsequent step, a solution of 1450 mg (2 mmol)
of Boc-Phe[C]Phe-(L)-Val-(L)-Phe-morpholin-4-ylamide (Reference Exarnple 2) and
10 mg of 4-N,N-dimethylaminopyridine in 10 ml of pylidine is added to the acid chloride.
After working up analogously to Example 17, column chromatography (SiO2, ethyl
acetate) yields the pure title compound: TLC Rf(ethyl acetate)= 0.37; tRet~I)= 17.3 min;
FAB-MS (M+H+)= 815.
Example 26: 5(S)-(Boc-amino)-4(S)-~(4-chlorophenoxY)methoxYacetyloxy)1-6-phenYl-2(R)-phenvlmethylhexanoyl-(L)-Val-(L)-Phe-morpholin-4-ylamide
Analogously to Example 4, the corresponding acid chloride is prepared from 217 mg
(1 mmol) of (4-chlorophenoxy)methoxyacetic acid (Cretin et al., Phytochemistry 22(12),
2661-64 (1983)) with 170 ,ul of 1-chloro-N,N,2-trimethyl-1-propenamine. In the
subsequent step, a solution of 365 mg (0.5 mmol) of Boc-Phe[C]Phe-(L)-Val-(L)-Phe-
morpholin-4-ylamide (Reference Example 2) and 5 mg of 4-N,N-dimethylaminopyridine
in 2.5 ml of pyridine is added to the acid chloride. After working up analogously to
Example 17, column chromatography (SiO2, ethyl acetate) yields the pure title
compound: TLC Rf(ethyl acetate)= 0.5; tRet(I)= 19.1 min; FAB-MS (M+H+)= 927.
Example 27: 5(S)-(Boc-amino)-4(S)-~2-(2-methoxvethoxY)-acetyloxY)1-6-phenY1-2(R)-
phenylmethylhexanovl-(L~-Val-~)-Phe-morpholin-4-Ylamide
Analogously to Example 4, the corresponding acid chloride is prepared from 240
211~
- 184-
(2 mmol) of 2-(2-methoxyethoxy)acetic acid (Fluka, Switzerland) with 340 ~,11 of1-chloro-N,N,2-trimethyl-1-propenamine. In the subsequent step a solution of 729 mg
(1 mmol) of Boc-Phe[C]Phe-(L)-Val-(L)-Phe-molpholin-4-ylamide (Reference
Example 2) and 5 mg of 4-N,N-dimethylaminopyridine in 5 ml of pyridine is added to the
acid chloride. After working up analogously to Example 17, column chromatography(SiO2, methylene chloride/methanol: 100/0 to 97/3) yields the pure title compound: TLC
Rf(ethyl acetate)= 0.33; tRe,(I)= 16.9 min; FAB-MS (M+H+)= 845.
Example 28: 5(S)^(Boc-amino)-4(S)-(butvloxYacetvloxy)-6-phenYI-2(R)-phenvlmethvl-
hexanoyl-(L)-Val-(L)-Phe-morpholin-4-ylamide
Analogously to Example 4, the corresponding acid chloride is prepared from 265 mg
(2 mmol) of 2-(n-butoxy)acetic acid (Janssen, Netherlands) with 340 ~11 of 1-~hloro-
N,N,2-trimethyl-1-propenamine. In the subsequent step, a solution of 729 mg (1 mmol) of
Boc-Phe[C]Phe-(L)-Val-(L)-Phe-morpholin-4-ylamide (Reference Example 2) and 5 mgof 4-N,N-dimethylaminopyridine in 5 ml of pyridine is added to the acid chloride. After
working up analogously to Example 17, column chromatography (SiO2, ethyl acetate)
yields the pure title compound: TLC Rf(ethyl acetate)= 0.44; tRet(I)= 18.7 min; FAB-MS
(M+H+)= 843.
Example 2~: 5(S1-(Boc-amirlo)-4(S)-[2-r2-(2-methoxyethoxv)-ethoxYlacetvloxY)1-6-phenvl-2(R)-~henvlmethylhexanoYl-(L)-Yal-L)-Phe-morDholin-4-vlamide
Analogously to Example 4, the corresponding acid chloride is prepared from 314 111
(2 mmol) of 2-[2-(2-methoxyethoxy)ethoxy]acetic acid (Fluka, Switzerland) with 340 ~
of 1-chloro-N,N,2-trimethyl-1-propenamine. In the subsequent step, a soludon of 729 mg
(1 mmol) of Boc-Phe[C]Phe-(L)-Val-(L)-Phe-morpholin-4-ylamide (Reference :
Example 2) and 5 mg of 4-N,N-dimethylaminopyridine in S ml of pyridine is added to the
acid chloride. After working up analogously to Example 17, column chromatography(SiO2, methylene chloride/methanol: 100/0 to 97/3) yields the pure title compound: TLC
RE(ethyl acetate)= 0.27; tRe,(I)= 16.7 min; FAB-MS (M+H+)= 889.
Example 30: S(S)-(Boc-amino)-4(S)-(methoxYacetYloxv)-6-PhenYl-2(R)-phenylmeth
hexanovl-(L)-Val-(L)-Phe-morpholin-4-vlamide
Analogously to Example 35, 729 mg (1 mmol) of Boc-Phe[C]Phe-(L)-Val-(L)-Phe-
morpholin-4-ylamide (Reference Example 2) are converted with 100 ~LI (1.1 mmol) of ;
methoxyacetic acid chloride (Fluka, Switzerland) into the title compound, which is
purified by column chromatography (SiO2, ethyl acetate/hexane: 4/1). TLC Rf(ethyl
` ` 211866~
- 18~-
acetate)= 0.43; tRc,(l)= 16.8 min; FAB-MS (M~-H+)= 801.
Example 31: 5(S)-(Boc-amino)-4(S)-(phenoxvacetyloxv)-6-phenYl-2(R)-phenvlmethYI-hexanovl-(L)-Val-(L)-Phe-morpholin-4-vlamide
Analogously to Example 35, 1450 mg (2 mmol) of Boc-Phe[C]Phe-SL)-Val-(L)-Phe-
morpholin-4-ylamide (Reference Example 2) are converted with 360 111(2.6 mmol) of
phenoxyacetyl chloride (Fluka, Switzerland) into the title compound, which is purified by
column chromatography (SiO2, ethyl acetate/hexane: 4/l). TLC Rf(ethyl acetatelhexane:
4/l)= 0.37; tRet(I)= 18.5 min; FAB-MS (M+H+)= 863.
Example32: 5(S)-(Boc-amino)-4(S)-~(S)-a-methoxv-oc-phenvlacetyloxy)-6-phenvl-2(R)-
phenvlmeth~lhexanoyl-(L)-Val-(L)-Phe-morpholin-4-ylamide
Analogously to Exarnple 4, the corresponding acid chloride is prepared from 332.4 mg
(2 mmol) of (S)-a-methoxy-a-phenylacetic acid (Fluka, Switzerland) with 340 ~11 of 1-
chloro-N,N,2-trimethyl-1-propenamine. In the subsequent step, a solution of 729 mg
(1 mmol) of Boc-Phe[C]Phe-(L)-Val-(L)-Phe-morpholin-4-ylamide (Re~erence
Example 2) and 5 mg of 4-N,N-dimethylaminopyridine in S ml of pyridine is added to the
acid chloride. After working up analogously to Example 17, column chromatography(SiO2, ethyl acetate) yields the pure title compound: TLC Rf(ethyl acetate)= 0.43; tRet(I)=
18.5 min; FAB-MS (M+H+)= 877. ~ :
Example 33: 5(S)-(Boc-amino)-4(S)-r(R)-a-methoxy-a-~henYlacetyloxy)l-6-phenyl-
2(R)-phenvlmethYlhexanovl-~L)-Val-(L~-Phe-morpholin-4-vlamide
Analogously to Example 4, the corresponding acid chloride is prepared from 333 mg
(2 mmol) of (S)-a-methoxy-a-phenylacetic acid (Fluka, Switzerland) with 340 ~,11 of 1-
chloro-N,N,2-trimethyl-1-propenamine. In the subsequent step, a solution of 729 mg
(1 mmol) of Boc-Phe[C]Phe-(L)-Val-(L)-Phe-morpholin-4-ylamide (Reference
Example 2) and 5 mg of 4-N,N-dimethylaminopyridine in 5 ml of pyridine is added to the
acid chloride. After working up analogously to Example 17, column chromatography(SiO2, ethyl acetate) yields the pure title compound: TLC Rf(ethyl acetate)= 0.5; tRet(I)=
18.3 min; FAB-MS (M+H+)= 877. ~ -~
Example 34: 5(S)-(Boc-amino)-4(S)-(valeroyloxY)-6-phenyl-2(R)-phenYlmethyl-
hexanoyl-(L)-Val-(L)-Phe-morpholin-4-ylamide
Analogously to Example 35, 729 mg (I mmol) of Boc-Phe[C]Phe-(L)-Val,-(L)-Phe-
morpholin-4-ylamide (Reference Example 2) are converted with 181 111(1.5 mmol~ of
2118661
186-
valeroyl chloride (Fluka, Switzerland) into the title compound, which is purified by
column chromatography (SiO2, ethyl acetate/hexane: 4/1). TLC Rf(ethyl acetate/hexane:
4/1)= 0.33; tRe,(I)= 18.9 min; FAB-MS (M+H+)= 813.
Example 35: 5(S)-(Boc-amino)-4(S)-(Pivaloyloxv)-6-phem/l-2(R)-phenvlmethvl- -
hexanovl-(L)-Val-(L)-Phe-morpholin-4-vlamide
Under an argon atmosphere, a solution of 130 111(1.05 mmol) of pivaloyl chloride in
0.4 ml of pyridine is added over a period of 10 minutes to a solution of 510 mg (0.7 mmol)
of Boc-Phe[C]Phe-(L)-Val-(L)-Phe-mo~pholin-4-ylamide (Reference Example 2) in 4 ml
of pyridine. After the further addition twice of 150 ~,11 of pivaloyl chloride and 5 mg of
4-N,N-dimethylaminopyridine, no more starting material can be detected by TLC after a
total reaction time of 3.75 hours at 50C. The reaction mixture is poured onto approxi-
mately 40 ml of saturated aqueous sodium bicarbonate solution and extracted three times
with methylene chloride. The combined extracts are washed with brine, dried over sodium
sulfate and concentrated. The subsequent column chromatography (SiO2, methylene
chloride/methanol: lOOtO to 98.5/1.5) yields the pure title compound. TLC Rf(methylene
chloride/methanol: 95/5)= 0.6; tRet(II)= 28.3 min; FAB-MS (M+H+)= 813.
Exarnple 36: 5(S)-(Boc-amino)-4(S~ almitovloxv)-~PhenYl-2(R)-Phenylmethhexanovl-(L)-Val-(L)-Phe-morpholin-4-ylamide
Analogously to Example 35, 729 mg (1 mmol) of Boc-Phe[C]Phe-(L)-Val-(L)-Phe-
morpholin-4-ylamide (Reference Example 2) are converted with 456 ~1(1.5 mmol) of `
palmitic acid chloride (Fluka, Switzerland) into the title compound, which is purified by
column chromatography (Si()2, ethyl acetate~exane: 1/1). TLC Rf~ethyl ace~ate/hexane:
A/1)= 0.43; tRet(100% acetonitrile, isocratic)= 13.8 min; FAB-MS (M+H+)= 967.
Example 37: 5(S)-(~oc-amino~-4(S)-(butvroYloxY)-6-phenY1-2(R)-Phenylmeth
hcxanoYl-(L)-val-(L)-phe-morpholin-4-ylamide
Analogously to Example 35, 729 mg (1 mmol) of Boc-Phe[C]Phe-(L)-Val-(L)-Phe- ~ i
morpholin-4-ylamide (Reference Example 2) are converted with 156 1l1(1.5 mmol) of
butyric acid chloride (Fluka, Switzerland) into the title compound, which is purified by
column chromatography (SiO2, ethyl acetate/hexane: 4/1). TLC Rf(ethyl acetate)= 0.43;
tRe,(I)= 18.3 min; FAB-MS (M+H+)= 799. ~ ~ -
211~fi~
- 187-
.:
Example 38: 5(Sl-(Boc-amino)-4(S)-(propanoyloxy)-6-phenyl-2(R)-phenylmeth
hexanoyl-(L)-Val-(L)-Phe-morpholin-4-ylamide
Analogously to Example 35, 729 mg (1 mmol) of Boc-Phe[C]Phe-(L)-Val-(L)-Phe-
morpholin-4-ylamide (Reference Example 2) are converted with 131 ,LI (1.5 mmol) of
propionyl chloride (Flulca, Switzerland~ into the title compound, which is purifled by
column chromatography (SiO2, ethyl acetate/hexane: 4/1). TLC Rf(ethyl acetate)= 0.43;
tRel(I)= 17.6 min; FAB-MS (M+H+)= 785.
Example 39: S(S)-(Boc-amino)-4(S)-(benzovloxY)-6-PhenYl-2(R)-phenylmethylhexanoyl- . :
(L)-Val-(L)-Phe-morpholin-4-ylamide
Analogously to Example 35, 365 mg (1 mmol) of Boc-Phe[C]Phe-(L)-Val-(L)-Phe-
morpholin-4-ylamide (Re~erence Example 2) are converted with 64 ~11(1.1 mmol) ofbenzoyl chloride (Aldrich, Federal Republic of Germany) into the title compound, which
is purified by column chromatography (SiO2, ethyl acetate). TLC Rf(ethyl acetate)= 0.5;
tRet(II)= 27.6 min; FAB-MS (M+H+)= 833.
Example 40: S(S)-(Boc-amino)-4(S)-(methvll~ropionvloxv)-6-Dhenvl-2(R)-PhenYlmethYl-
hexanovl-(Ll-Val-(L)-Phe-morpholin-4-ylamide
Analogously to Example 2), Boc-Phe[C]Phe-(L)-Val-(L)-Phe-morpholin-4-ylamide
(Reference Example 2) is reacted with an excess of isobutyric acid chloride (Fluka;
Buchs/Switzerland): tRe (I)=18.2 min; FAB-MS (M+H)~=799.
Example 41: The following compounds are prepared analogously to one of the aboveprocesses:
A) 5(S)-(Boc-amino)-4(S)-[3-(dimethylamino)-propionyloxy3-6-phenyl-2(R)-
phenylmethylhexanoyl-(L)-Val-(L)-Phe-morpholin-4-ylamide
B) 5(S)-(Boc-amino)-4(S)-[3-(N-Z-N-methylamino)-propionyloxy]-6-phenyl-
2(R)-phenylmethylhexanoyl-(L)-Val-(L)-Phe-morpholin-4-ylamide :::
C) 5(S)-(Boc-amino)-4(S)-(3-methylaminopropionyloxy)-6-phenyl-2(R)-
phenylmethylhexanoyl-(L)-Val-(L)-Phe-morpholin-4-ylamide
D) 5(S)-(Boc-amino)-4(S)-[(dimethylaminoethoxy)-acetyloxy]-6-phenyl-2(R)-
(p-fluorophenylmethyl)-hexanoyl-(L)-Val-(L)-Phe-morpholin-4-ylamide -E) 5(S)-(Boc-amino)-4(S)-[(2-pyridylmethoxy)-acetyloxy]-6-phenyl-2(R)-
(p-fluorophenylmethyl)-hexanoyl-(L)-Val-(L)-Phe-morpholin-4-ylamide
F) 5(S)-(Boc-amino)-4(S)-(methoxyacetyloxy)-6-phenyl-2(R)-(p-methoxy-
: , .
:.: : , .. : ,
-- 2l~6fll
- 188-
phenylmethyl)-hexanoyl-(L)-Val-(L)-(p-CH30-Phe)-morpholin-4-ylamide
C~) 5(S)-(Boc-amino)-4(S)-(pyridine-2-carboxyl)-6-phenyl-2(R)-(p-methoxy-
phenylmethyl)-hexanoyl-(L)-Val-(L)-(p-CH30-Phe)-morpholin-4-ylamide
H): 5(S)-(Boc-amino~-4(S)-(2-methylthio)acetyloxy-6-phenvl-2(R)-benzvlhexanoYl-(L)-
Val-(L)-Phe-morpholin-4-ylamide
Analogously to Example 4, a solution of 546 mg (0.75 mmol) of 5(S)-(Boc-amino)-4(S)-
hydroxy-6-phenyl-2(R)-benzylhexanoyl-(L)-Val-(L)-Phe-morpholin-4-ylamide and 5 mg
of DMAP in 5 ml of pyridine is added to a mixture of 0.174 ml (2 mmol) of 2-methylthio-
acetic acid (Fluka, Buchs, Switzerland) and 0.34 ml (2.4 mmol) of 1-chloro-N,N,2-tri-
methyl-l-propenamine in 15 ml of methylene chloride. After working up and columnchromatography (SiO2, ethyl acetate~exane: 4/1), the title compound is isolated: TLC
Rf(B)= 0.41; tRe,(I)= 17.9 min; FAB-MS (M+H+)= 817.
I) 5(S)-(Boc-amino)-4(S)-(benzylthioacetyloxy)-6-phenyl-2(R)-phenylmethylhexanoyl-
(L)-Val-(L)-Phe-morpholin-4-ylamide
J): 5(S)-(Boc-amino)-4(S)-r(L)-(prolvl)oxyl-6-phenyl-2(R~-phenylmethylhexanoyl-(L)
Val-(L)-Phe-morpholin-4-ylamide
1.12 g (1.166 mmol) of 5(S)-(Boc-amino)-4(S)-[(L)-(N-Z-prolyl~oxy]-6-phenyl-2(R)-
phenylmethylhexanoyl-(L)-Val-(L)-Phe-morpholin-4-ylamide (Example 41 L)) in 20 ml
of ethyl acetate are hydrogenated in the presence of 120 mg of lû % PdlC. Filtration,
concentration of the filtrate by evaporation, and digestion from DIPE yields the title
compound: tRe,(I)=13.8 min; FAB-MS (M~H)+=826.
K) 5(S)-(Boc-amino)-4(S)-~lD)-(prolYl)oxvl-6-uhenYI-2(R)-PhenYlmethvlhexanoyl-(L)
Val-(L)-Phe-morpholin-4-ylamide
950 mg (0.989 mmol) of 5(S)-(Boc-amino)-4(S)-[(D)-(N-Z-prolyl)oxy]-6-phenyl-2(R)-
phenylmethylhexanoyl-(L)-Val-(L)-Phe-morpholin-4-ylamide (Example 41 M) in 20 ml of
ethyllacetate are hyidrogenated in the presence of 0.20 g of 10 % Pd/C. Filtration, concen-
tration of the filtrate by evaporation, column chromatography (SiO2, THF/ether 1:1) and
digestion from DIPE yields the title compound: tRet(I)=13.5 min; FAB-MS (M+H)+=826.
-: , , - -
L) S(S)-(Boc-amino~-4(S~-r(L)-(N-Z-Prolvl)oxYl-6-Phenyl-2(R)-~-kenvlmethylhexanovl- ;
(L)-Val-L)-Phe-morpholin-4-Ylamide
Under a nitrogen atmosphere, 683 mg (2.74 mmol) of Z-(L)-proline in S ml of methylene
chloride are reacted at RT with 232 ,ul (1.644 mmol) of 1-chloro-N,N,2-trimethyl-1-
211~6~ ~
- 189-
propenamine [B. Haveaux, A. Dekoker, M. Rens, A.R. Sidani, J. Toye, L. Ghosez, M.
Murakami, M. Yoshioka, and W. Nagata, Organic Syntheses 59, 26 (1980)]. After 30 min,
2.7 ml of pyridine, 1.00 g (1.37 mmol) of Boc-Phe[C]Phe-(L)-Val-(L)-Phe-morpholin-4-
ylamide (Reference Example 2) and 3 mg of DMAP are added. Since, according to HPLC,
there is still some starting matenal present after 17 h at RT, a further 1.3 mmol of Z(L)-
proline is activated with 0.8 mmol of 1-chloro-N,N,2-trimethyl-1-propenamine and added.
After a further 17 h, the reaction mixture is poured onto sat. NaHC03 solution and
extracted with 3 portions of methylene chloride. The organic phases are washed with
water and brine, dried with Na2SO4 and concentrated by evaporation. Column
chromatography (SiO2, ethyl acetate~ yields the title compound: TLC Rf(B)=0.50,
tRe,(I)=20.0 min; FAB-MS (M+H)+=961.
M) S(S)-(Boc-amino)-4(S~-~(D)-(N-Z-prolvl)oxvl-6-phenYl-2(R)-phenvlmethylhexanoYl-
(L)-Val-(L)-Phe-morpholin-4-vlamide
Under a nitrogen atmosphere, 683 mg (2.74 mmol) of Z(D)-proline in S ml of methylene ~ -
chloride are reacted at RT with 232 ~11(1.644 mmol) of 1-chloro-N,N,2-trimethyl-1-
propenamine. After 30 min, 2.7 ml of pyridine, 1.00 g (1.37 mmol) of Boc-Phe[C~Phe-
(L)-Val-(L)-Phe-morpholin-4-ylamide (Reference Example 2) and 3 mg of DMAP are
added. Since, according to HPLC, there is still some starting material present after 17 h at ~;
RT, a further 683 mg of Z(D)-proline are activated with 232 ~1 of 1-chloro-N,N,2-~
methyl-l-propenarnine and added. After a further 17 h, working up is carried outanalogously to E3xample 41L~: TLC Rf(B)=0.47; tRet(I~=19.3 min; FAB-MS (M+H)+=960.
There is used as star~ng material for a number of compounds 5(S)-(Boc-amino)-4(S)-
(chloroacetyloxy)-6-phenyl-2(R)-(p-fluorophenylmethyl)-hexanoyl-(L)-Val-(L)-Phe-morpholin4-ylamide (which can be prepared by reacting chloroacetic acid with
5(S)-(Boc-amino)^4(S)-hydroxy-6-phenyl-2(R)-(p-fluorophenylmethyl)-hexanoyl-(L)-Val-(L)-Phe-morpholin-4-ylamide analogously to Example 4) in which the chlorine atom
is converted into a substituted N, S or O atom by nucleophilic substitution, for example in
the preparation of compounds D) with dimethylaminoethanol, and compounds E) with2-pyridylmethanol .
Example 42: Analogously to one of the processes described in Examples 1 to 41, unless
specifically described in the afore-mentioned Examples the compounds of formula I of
Refe~ence Examples 1 to 22 and 33 to 56 are converted, by replacing the 4(S)-hydroxy
group in the central non-hydrolysable peptide building block, into the corresponding
~,............ . . . .
, . .
~:. . ..
2 1 1 ~
- 1~0-
4(S)-acyloxy compounds in which one of the following radicals stands in place of the
4(S)-hydroxy group in each case:
A) 4(S)-(2-furanylcarboxy)~
B) 4(S)-[4-(dirnethylamino)-butyryloxy];
C) 4(S)-(N-Z-N-methylaminoacetyloxy);
D) 4(S)-(methylaminoacetyloxy);
E) 4(S)-[N-(imidazol-4-methyl)-N-methylaminoacetyloxy];
F) 4(S)-[3-(1-triphenylmethylimidazol-4-yl)-propionyloxy];
G) 4(S)-[3-(4-imidazolyl)-propionyloxy];
H) 4(S)-(methoxyacetyloxy); ~:I) 4(S)-~(2-picolinoyl); -
J) 4(S)-(benzyloxyacetyloxy); :
K) 4(S)-[(S)-a-methoxy-ol-phenylacetyloxy];
L) 4(S)-[(R)-a-methoxy-a-phenylacetyloxy]; : .
M) 4(S)-(l-pyrazolylacetyloxy);
N) 4(S)-(isoquinoline-3-carbonyloxy);
O) 4(S)-(pyrazinecarbonyloxy); -~ - ;
P) 4(S)-(4-a-chloromethylbenzoyloxy); ~ ~-
Q) 4(S)-[4-(4-morpholino)methylbenzoyloxy];
R) 4(S)-(isonicotinoyloxy); :::S) 4(S)-(nicotinoyloxy);
T) 4(S)-(3-methoxypropanoyloxy)
U) 4(S)-[(4-chlorophenoxy)methoxyacetyloxy)];
V) 4(S)-[2-(2-methoxyethoxy)acetyloxy)];
W) 4(S)-(butyloxyacetyloxy);
X) 4(S)-[2-[2-(2-methoxyethoxy)ethoxy]acetyloxy)];
Y) 4(S)-(methoxyacetyloxy);
Z) 4(S)-(phenoxyacetyloxy);
AA) 4(S)-[(S)-a-methoxy-a-phenylacetyloxy); ~ :
AB) 4(S)-[(R)-a-methoxy-a-phenylacetyloxy)];
AC) (N,N-dimethylaminoacetyloxy);
AD) 4(S)-[N-(pyridine-2-methyl)-N-methylaminoacetyloxy].
Example 43: Analogously to one of the processes described in Examples 1 to 41, unless
speci~lcally described in the afore-mentioned Examples the compounds of formula I of -~
2~1~6~1
- 191 -
Reference Examples 1 to 2,2 and 33 to 56 are converted, by replacing the 4(S)-hydroxy
group in the central non-hydrolysable peptide building block, into the corresponding
4(S)-acyloxy compounds in which one of the following radicals stands in place of the
4(S)-hydroxy group in each case:
A) 4(S)-[3-(dimethylamino)-propionyloxy];
B) 4(S)-[3-(N-Z-N-methylamino)-propionyloxy];
C) 4(S)-(3-methylaminopropionyloxy);
D) 4(S)-[(dimethylaminoethoxy)-acetyloxy]; ~ -
E) 4(S)-[(2-pyridylmethoxy)-acetyloxy];
F) 4(S)-(methoxyacetyloxy);
G) 4(S)-(pyridine-2-carboxyl);
H) 4(S)-(methylthioacetyloxy);
I) 4(S)-(benzylthioacetyloxy);
J) 4(S)-((L)-prolyloxy);
K) 4(S)-((D)-prolyloxy);
L) 4(S)-((L)-(N-Z-prolyl)oxy);
M) 4(S)-((D)-(N-Z-prolyl)oxy).
Example 44: S(S)-(Boc-amino)-4(S)-r3-(N-Z-amino)-propionvloxYl-6-phenvl-2(R)-
phenylmethYlhexanoyl-(L)-Val-(L)-Phe-morpholin-4-ylamide
Under a nitrogen atmosphere, 612 mg (2.74 mmol) of Z-~-alanine in S ml of methylene
chloride are reacted at RT with 232 111 (1.644 mmol) of 1-chloro-N,N,2-trlmethyl-1-
propenamine. After 40 min, 2.7 ml of pyridine, 1.00 g (1.37 mmol) of Boc-Phe[C]Phe-
(L)-Val-~L)-Phe-morpholin-4-ylamide (Reference Example 2) and 3 mg of DMAP are
added. Since, according to HPLC, there is still some starting material present after 17 h at
RT, a further 612 mg of Z-~-alanine are activated with 232 ,Ll l-chloro-N,N,2-trimethyl-
l-propenamine and added. After a further 17 h, working up is carried out analogously to
Example 41L): TLC Rf(B)=0.38; tRet(I)=18.3 min; FAB-MS (M+H)+=934.
Example 45 5(S)-(Boc-amino)-4(S)-(3-aminopropionyloxy)-6-phenYl-2(R)-Phenyl-
methylhexanovl-(L)-Val-(L)-Phe-morpholin-4-Ylamide
Hydrogenation of 1.00 g (1.07 mmol) of S(S)-(Boc-amino)-4(S)-[3-(N-Z-amino)-
propionyloxy]-6-phenyl-2(R)-phenylmethylhexanoyl (L)-Val-(L)-Phe-morpholin-4-yl-amide in 20 ml of ethyl acetate ;n the presence of 0.2 g of 10 % Pd/C, followed by ~lltra-
tion, concentration of the filtrate by evaporation and digestion from DIPE, yields the title
',', ' ': .'.': :,. : , ' , ,, ' , : , , ` ' ' , " '. :
- 211~6~1
- 192-
compound: tRet(I)=13.1 min; FAB-MS (M+H)+=800.
Example 46: 5(S~-(Boc-amino)-4(S)-~(3-dimethylaminopropoxY)-acetYloxvl-6-phenYl-2(R~-phenvlmethvlhexano~rl-(L)-Val-(L)-Phe-mo~pholin-4-ylamide
A solution of 0.124 mmol of 5(S)-(Boc-amino)-4(S)-(iodoacetyloxy)-6-phenyl-2(R)-phenylmethylhexanoyl-(L)-Val-(L)-Phe-morpholin-4-ylamide in 0.3 ml acetone is added
to 13 mg (0.124 mmol) of 3-dimethylaminopropanol (Fluka; Buchs, Switzerland), the
mixture is then washed twice with 0.3 ml of acetone each time and, after the addition of
15.6 mg (0.187 mmol) of NaHCO3, is stir ed for 17 h at RT tHPLC: fully reacted). The
solvent is carried off in a stream of nitrogen, and the residue is suspended in a small
amount of methylene chloride and poured onto a mixture of ice-water and methylene
chloride. The organic phase is removed and washed with water and brine, and the aqueous
phases are extracted twice with methylene chloride. The organic phases are dried with
Na2SO4, concentrated by evaporation, and precipitated with DIPE from a concentrated
solution in acetone to yield the title compound: tRet(I)=13.5 min; FAB-MS (M+H)+=872.
The starting material is prepared as follows:
a) 5(S)-(Boc-amino)-4(S)-(iodoacetvloxy)-6-phenyl-2(R)-phenvlmethylhexanoYl-(L)-Val-(L)-Phe-morPholin-4-Ylamide
90.5 mg (0.604 mmol) of sodium iodide are added to a solution of 100 mg (0.124 mmol) :
of 5(S)-(Boc-amino)-4(S)-(chloroacetyloxy)-6-phenyl-2(R)-phenylmethylhexanoyl-tL)-
Val-(L)-Phe-morpholin-4-ylamide in 0.3 ml of acetone, and the mixture is stirred for 1.5 h
at RT, during the course of which a precipitate separates out. Since HPLC after 1 h
already indicates complete reaction to the title compound, the supernatant solution is --
pipetted off and immediately used in the above step: tRe,(I)=18.2 min.
b) 5(S)-tBoc-amino)-4tS)-tchloroacetvloxY~-6-Phenyl-2tR)-phenylmethylhexanoYl-tL)-
Val-tL)-Phe-morpholin-4-Ylamide
. .,
Under a nitrogen atmosphere 2.00 g (2.74 mmol) of Boc-Phe[C]Phe-(L)-Val-(L)-Phe-morpholin-4-ylamide (Reference Example 2~ are introduced into 20 ml of acetonitrile and
12 ml of dioxane, and 1.3 ml of pyridine, a small amount of DMAP and f1nally 1.17 g
(6.85 mmol) of chloroacetic acid anhydride are added. After having been stirred for 24 h at
RT, the mixture is poured onto ice-water and extracted three times with ethyl acetate. The
organic phases are washed with 2 portions of sat. NaHCO3 solution, water and brine, dried
with Na2SO4 and concentrated by evaporation. Column chromatography (SiO2, methylene
.' . ' ' . ` : .'' . . . ' , .~ , ~ ; 1,,, ',. '. '' ' ' . I:.' ' ' ' '
g ~ ~
- 193-
chloride/ether 1:1) yields the title compound: TLC Rf(A')=0.16; tRc,(I)=17.7 min;
FAB-MS (M+H)+=805.
Example 47: S(S)-(Boc-amino)-4(S)-~2-(2-dimethylaminoethoxv)-ethoxYacetvloxYl-6-
~nyl-2(R)-phenylmethylhexanoyl-(L)-Val-(L)-Phe-morpholin-4-ylami(lei
A solution of 0.285 mmol of 5(S)-(Boc-amino~-4(S)-(iodoacetyloxy)-6-phenyl-2(R)-phenylmethylhexanoyl-(L)-Val-(L)-Phe-morpholin-4-ylamide in acetone (Example 46a) is
added to 37.9 mg (0.285 mmol) of 2-(2-dimethylaminoethoxy)-ethanol (BASF;
Ludwigshafen, Germany), the mixture is then washed twice with 0.7 ml of acetone each
time, 36 mg (0.43 mmol3 of NaHCO3 are added, and the mixture is stirred for 20 h at RT
to complete the reaction. The mixture is worked up analogously to Example 46 and the
crude product is s~irred with DIPE to yield the title compound: t,Re~(I)=13.5 min; FAB-MS
(M+H)+=902.
Example 48: S(S)-(Boc-amino)-4(S)-~(4-dimethvlaminobutoxY)-acetYloxY1-6-Phen
2(R)-phenvlmethvlhexanoyl-(L)-Val~ -Phe-morpholin-4-vlamide
A solution of 0.124 mmol of 5(S)-(Boc-amino)-4(S)-(iodoacetyloxy)-6-phenyl-2(R)-phenylmethylhexanoyl-(L)-Val-(L)-Phe-morpholin-4-ylamide in acetone (Example 46a) is
added to 14.5 mg (0.124 mmol) of 4-dimethylamino-1-butanol (BASF; Ludwigshafen,
Germany), and the mixture is then washed twice with 0.3 ml of acetone each time and,
after the addition of 26 mg (0.187 mmol) of K2CO3, stirred for 18 h at RT to complete the
reaction. Since, according to HPLC, there is still some iodoacetate present, a further 0.25
equivalents of 4-dimethylarnino-1-butanol is added. Working up after 17 h analogously to
Example 46 yields the tide compound: tRet(I)=13.5 min; FAB-MS (M+H)+=886.
Example 49: 5(S)-(Boc-amino)-4(S~-~2-(2-methoxYethoxy)-acetYloxYl-6-Phenyl-2(R)
(p-methoxvphenYl)methylhexanovl-(L)-Val-(L)-Phe-morpholin-4-ylamide
Under a nitrogen atmosphere, 0.25 ml of pyridine, l50 mg (0.198 mmol) of Boc-
Phe[C](p-CH3O)Phe-(L)-Val-(L)-Phe-morpholin-4-ylamide (Reference Example 21 F) 1))
and a small amount of DMAP are added to 75 mg (~ 60 % strength; 0.296 mmol) of 2-(2-
methoxyethoxy)-acetic acid chloride in 1.5 ml of dioxane. After 17 h at RT, the reaction
mixture is poured onto sat. NaHCO3 solution and extracted three times with methylene
chloride, and the organic phases are washed with sat. NaHCO3 solution, water and brine,
dried with Na2SO4 and concentrated by evaporation. Column chromatography (SiO2,
methylene chlorideJI~lF 4:1) and stirring with hexane yields the title compound: TLC
Rf(B')=0.37; tRe,(I)=16.7 min; FAB-MS (M+H)+=875.
~ 2118~
- 194-
The stiarting material is prepiared as follows: ;
a) 2-(2-Methoxyethoxv)-acetic acid chloride
Under a nitrogen atmosphere, 2.56 ml (29.8 mmol) of oxalyl chloride and 2 drops of DMF
are added to 1.69 ml (14.9 mmol) of 2-(2-methoxyethoxy)-acetic acid (Fluka; Buchs/-
Switzerland) in 75 ml of methylene chloride. After 17 h at RT, the reaction mixture is
carefully concentrated by evaporation in a RE with the exclusion of moisture. A IH-NMR
spectrum of the residue exhibits the signals of the title compound in addition to signals of
z40 % solvent (especially methylene chloride): lH-NMR (200 MHz, CDCI3): 3.37 (s,MeO), 3.58 and 3.77 (2d, J=5 Hz, CH2-CH2), 4.48 (s, CH2). That mixture is used above.
-: ' :. '
Exarnple 50: 5(S~-(Boc-amino)-4(S~-(2-picolinoYloxY)-6-PhenYl-2(R~-(p-methoxvohenvl)
methYlhexanoyl-(L)-Val-(L)-Phe-morDholin-4-Ylamide
Under a nitrogen atrnosphere, 48.6 mg (0.395 mmol) of 2-picolinic acid in 0.78 ml of
methylene chloride are reacted at 0C with 33.5 111 (0.237 mmol) of 1-chloro-N,N,2-tri-
methyl-l-propenamine. After 30 min, 0.39 ml of pyridine, 150 mg (0.198 mmol) of Boc-
Phe~Cl(p-C~I30)Phe-(L~-Val-(L)-Phe-morpholin-4-ylamide (Refer~nce Example 21 F) 1))
and 0.4 mg of DMAP are added thereto. Since, according to HPLC, there is still some
Boc-Phe[C](p-CH30)Phe-(L)-Val-(L)-Phe-morpholin-4-ylamide present after 17 h at RT,
portions each of 48.6 mg of 2-picolinic acid are each activated with 33.5 ~Ll of l-chlor~
N,N,2-trirnethyl-1-propenamine and added until, after a reaction time of 16 h in each case,
all star~ng material has ~acted. Working up analogously to Example 41L) iand column
chromatography (siO2, methylene chloride/I~IF 4:1) yields the tide compound: TLCRf(B')=0.30; tRet(I)=16.5 min; FAB-MS (M+H)+=865.
Example 51: 5(S)-(Boc-amino)-4(S)-f2-r2-(2:methoxvethoxY)ethoxYl-acetvloxvl-6-
phenvl-2(R~-(p-methoxvphenvl)methvlhexanovl-(L)-Val-(L)-(p-CH3~?-Phe)-morpholin-4-vlamide
Under a nitrogen atmosphere, 58 111(0.38 mmol) of 2-[2-(2-methoxyethoxy)ethoxy]-acetic
acid (Fluka; Buchs/Switzerland) in 0.7 ml of methylene chloride are reacted at 0C with
32 ,ul tO.228 mmol) of 1-cihloro-N,N,2-trimethyl-1-propenamine. After 30 min, 0.37 ml of
pyridine, 150 mg (0.190 mmol) of Boc-Phe[CJ(p-CH30)Phe-(L)-Val-(L)-(p-CH30-Phe)-morpholin~-ylarnide (Reference Example 21 F) 3)) and a small amount of DMAP are
added thereto. Since, according to HPLC, there is still some Boc-Phe[C](p-CH30)Phe-
(L)-Val-(L)-(p-C~30-Phe)-morpholin-4-ylamide present after 19 h at RT, a further 58
211~6~1
- 195-
of 2-[2-(2-methoxyethoxy)ethoxy]-acetic acid is activated with 32 ~11 of 1-chloro-N,N,2-
trimethyl-l-propenamine and added. Working up after 17 h analogously to Example 41L),
column ch~omatography (SiO2, methylene chloride/l~lF 4:1) and sti}ring in DIPE yields
the title compound: TLC Rf(B')=0.23; tRet(I)=16.5 min; FAB-MS (M+H)+=949.
Example 52: 5(S)-(Boc-amino)-4(S)-(2-picolinoYloxv)-6-Phenvl-2(R)-(P-methoxvPhenvl)
methylhexanoyl-(L)-Val-(L)-(p-CH~O-Phe)-morpholin-4-~vlamide
Under a nitrogen atmosphere,46.8 mg (0.380 mmol) of 2-picolinic acid in 0.75 ml of
methylene chloride are reacted at 0C with 32111 (0.228 mmol) of 1-chloro-N,N,2-tri-
methyl-l-propenamine. After 30 min, 150 mg (0.190 mmol) of Boc-Phe[C~(p-CH3O)Phe-
(L)-Val-(L)-(p-CH30-Phe)-morpholin-4-ylamide (Reference Exarnple 21 F) 3)) in 0.38 ml
of pyridine and 0.4 mg of DMAP are added thereto. Since, according to HPLC, there is
still some Boc-Phe[C](p-CH30)Phe-(L)-Val-(L)-(p-CH30-Phe)-morpholin-4-ylamide
present after 17 h at RT, a further 46.8 mg of 2-picolinic acid is activated with 32111 of
l-chloro-N,N,2-trimethyl-1-propenamine and added. Working up after 18 h analogously to
Example 41L), column chromatography (SiO2, methylene chlorideJI~3F 4:1) and stirring
with hexane yields the title compound: TLC Rf(B')=0.37; tRe~(I)=16.4 min; FAB-MS(M+H)+=894.
Exarnple 53: 5(S)-~Boc-amino)-4(S)-(2-benzvlo~Y)acetYloxv-6-Phenyl-2(R)-benzvl-
hexanoyl-(L)-Val-(L)-Phe-morpholin-4-ylamide
Analogously to Example 1,730 mg (1 mmol) of 5(S)-(Boc-amino)-4(S)-hydroxy-6-
phenyl-2(R)-benzylhexanoyl-(L)-Val-(L)-Phe-morpholin-4-ylamide are reacted with
0.237 mi (1.5 mmol) of benzyloxyacetyl chloride (Fluka, Buchs, Switzerland) and 5 mg of
DMAP in 5 ml of pyndine to yield the title compound. Column chromato~raphy (SiO2,
ethyl acetate) yields the title compound: TLC R~(B)= 0.5; tRet(I)= 19 min; FAB-MS
(M+H+)= X77.
Example 54: 5(S)-(Boc-amino)-4(S~-(2-acetvloxv)acetvloxv-~-Phenvl-2(R)-ben
hexanoyl-(L)-Val-(L)-Phe-morpholin-4-vlamide
Analogously to Example 1,730 mg (1 mmol) of 5(S)-(Boc-amino)-4(S)-hydroxy-6-
phenyl-2(R)-benzylhexanoyl-(L)-Val-(L)-Phe-morpholin-4-ylamide are reacted with
0.166 ml (1.5 mmol) of acetyloxyacetyl chloride (Aldrich, Steinheim, Federal Republic of
Germany) and 5 mg of DMAP in 5 ml of pyridine to give the title compounmd. Column
chromatography (SiO2, ethyl acetate) yields the title compound: TLC Rf(B~= 0.41; tRet(I)
= 17.3 min; FAB-MS (M+H~)= 829.
~113~
- 196
Example 55: 5(S)-(Boc-amino)-4(S)-I2-(4-tetrahydropvranyloxy)acetyloxv-6-phen
2(R)-benzylhexanoyl-~L)-'Val-(L)-Phe-morpholin-4-ylamide
Analogously to Example 4, a solution of 36S mg (0.5 mmol) of 5(S)-(Boc-amino)-4(S)-
hydroxy-6-phenyl-2(R)-benzylhexanoyl-(L)-Val-(L)-Phe-morpholin-4-ylamide and 5 mg
of DMAP in 4 ml of pyridine is added to a mixture of 160 mg (1 mmol) of 4-tetrahydro-
pyranylacetic acid (Chemical Abstracts-Registry No. 85064-61-S) and 0.17 ml (1.2 mmol)
of 1-chloro-N,N,2-~imethyl-1-propenarnine in 10 ml of methylene chloride. After
working up and column chromatography (SiO2, ethyl acetatelhexane: 4/1) the titlecompound is isolated: TLC Rf (methylene chloridelmethanol: 4/1)= 0.57; tRet(I) =16.9 min; FAB-MS (M+H+)= 871.
Example56: 5(S)-(Boc-amino)-4(S)-r2(R)-(4-tetrahYdropYranyloxY~l-propion
Phenyl-2(R)-benzvlhexanoyl-(L)-Val-(L)-Phe-morpholin-4-ylamide
Analogously to Example 4, a solution of 219 rng (0.3 mmol~ of 5(S)-(Boc-amino)-4(S)-
hydroxy-~phenyl-2(R)-benzylhexanoyl-(L)-Val-(L)-Phe-morpholin-4-ylamide and 5 mgof DMAP in 1.5 ml of pyridine is added to a mixture of 105 mg (0.6 mmol) of 2(R)-(4-
tetrahydropyranyl)-propionic acid (Beilstein E III/IV, Vol. 18, p 3851) and 0.11 ml
(0.72 mmol) of 1-chloro-N,N,2-trimethyl-1-propenamine in 5 ml of methylene chloIide. -
After working up and column chromatography (SiO2, ethyl acetatelhexane: 4/1) the title
compound is isolated: TLC Rf(B)- 0.37; tRet(I)= 17.3 min; FAB-MS (M+H+)= 885.
Exarnple 57: 5(Sl-(Boc-amino)-4(S)-r2-aaminoethoxy)ethoxyacetvloxv)l-6-phenYl-
2(R)-benzYlhexanoYl-(L)-Val-(L)-Phe-morpholin4-v!amide
Analogously to Exarnple 45, 0.83 g (0.82 mmol) of 5(S)-(Boc-amino)-4(S)-[2-(2-benzyl-
oxycarbonylaminoethoxy)ethoxyacetyloxy)]-6-phenyl-2(R)-benzylhexanoyl-(L)-Val-(L)-
Phe-morpholin-4-ylamide (Example 58) in 20 ml of methanol is hydrogenated in thepre~ence of 0.1 g OI 10% Pd/C. Column chromatography (SiO2, methylene chloride/-methanol: 95/5 to 80/20) yields the title compound: TLC Rf (methylene chloride/-
methanol: 9/1)= 0.15; tRet(I) = 13.1 min; FAB-MS (M+H+)= 874. ~ ~
Example58: S(S)-(Boc-amino)-4(S~-r2-(2-benzvloxYcarbonvlaminoethoxv)ethoxvacetvl- -~ ~ -
oxy~l-6-phenYI-2(R)-benzylhexanoYl-(L)-Val-(L)-Phei-morpholin-4-ylamide -:
Analogously to Example 4, a solution of 1.û9 g (1.5 mmol) of 5(S)-(Boc-amino)-4(S)-
hydroxy-~phenyl-2(R)-benzylhexanoyl-(L)-Val-(L)-Phe-morpholin-4-ylamide and 10 mg
of DMAP in 7.5 ml of pyridine is added tO a m;xture of 0.9 g (3 mmol) of 2-(2-benzyloxy-
2 ~
- 197-
carbonylamino)-ethoxy)-ethoxyacetic acid and 0.51 ml (3.6 mmol) of 1-chloro-N,N,2-
trimethyl- 1-propenamine in 20 ml of methylene chlonde. After working up and column
chromatog;raphy (SiO2, methylene chloride to methylene chloride/methanol: 98/2) the $itle
compound is isolated: TLC R~(B)= 0.41; tRe,(I)= 17.95 min; FAB-MS (M+H+)= 1008.
The starting compounds are prepared as follows:
a) 2-f2-(Phthalimidoethoxy)-ethoxvl-acetic acid ethvl ester
A solution of 10.S g (50 mmol) of 2-[2-(chloroethoxy)-ethoxy]-acetic acid ethyl ester
(Chemical Abstracts-Registry No. 82227-25-6) in 100 ml of DMF is reacted with 9.25 g
(50 mmol) of potassium phthalirnide (Fluka, Buchs, Switærland) and 50 mg of
18-crown-6 ether (1,4,7,10,13,16-hexaoxacyclooctadecane, Fluka, Buchs, Switzerland)
and the mixture is stirred for 2.5 h at 100C. The reaction mixture is cooled and then
concentrated under reduced pressure. The residue is taken up in ether and washed twice in
each case with water and brine. After drying and concentrating, the tide compound is
obtained by column chromatography (SiO2, ethyl acetatefhexane: 1/1); TLC Rf (ethyl
acetate/hexane: 4/1)= 0.45;
b) 2-r2-(Aminoethoxy~-ethoxyl-acetic acid (see also E.P. 0 410 280 A1)
A solution of 9.6 g (29.9 mmol) of 2-[2-(phthalimidoethoxy~-ethoxy]-acetic acid ethyl
ester in 195 ml of 20% aqueous HCl is stirred at reflux for 1.5 h. The mixture is cooled
and then concentrated in a rotary evaporator, and the residue obtained is taken up in 45 ml
of water. Insoluble material is removed by filtration and the ffltrate is extracted ti ree times
with ethyl acetate. The combined organic phases are washed with water and brine, dried
and concentrated. The white crystalline material obtained is siirred in 110 ml of 6N HCI
for 17.5 h, cooled and concentrated, and the residue is taken up in 20 ml of water. The
aqueous phase is washed three times with ethyl acetate and lyophilised under a high
vacuum. TLC Rf (methyl ethyl ketone/acetic acid/water: 311/1) = 0.45.
c) 2-,r2~(BenzYloxvcarbonYlaminoethoxv~-ethoxYl-acetic acid
A suspension oiE 6.7 g (12.5 mmol) of 2-[2-(aminoethoxy)ethoxy]-acetic acid in 30 ml of
THF and 10 ml of water is adjusted to pH 10 with 2N NaOE~, and reacted dropwise with
1.95 ml of chloroformic acid benzyl ester (Fluka, Buchs, Switærland). While the mixture
is being sdrred for 2.5 h, the pH is maintained between 9 and '9.5 by 2N NaOH. After
concentration of the reaction mixture under reduced pressure, the resuldng aqueous phase
is washed twice with ethyl acetate, adjusted to pH 1.8 with 2N sulfuric acid and extracted
21~61
.:
- 198-
3 times with ethyl acetate. The combined organic phases are then washed with water and
brine, dried and concentrated under reduced pressure. The title compound so obtained is
further used without being further purified. TLC Rf (ethyl acetate/acetic acid: 9/1) = 0.39.
Example 59: S(S)-(Boc-amino)-4(S)-~(methoxvcarbonYlmethoxv)-acetYloxvl-6-PhenY
2(R)-benzylhexanovl-(L)-Val-(L)-Phe-morpholin-4-Ylamide
Analogously to Example 4, a solution of 219 mg (0.3 mmol) of S(S)-(Boc-amino)-4(S)-
hydroxy-6-phenyl-2(R)-benzylhexanoyl-(L)-Val-(L)-Phe-morpholin-4-ylamide and 5 mg
of DMAP in 1.5 ml of pyridine is added to a mixture of 89 mg (0.6 mmol) of diglycolic
acid monomethyl ester (Chem. Ber. 55, 670 (1922) and 0.11 ml (0.72 mmol) of l-chloro-
N,N,2-trimethyl- l-propenamine in 5 ml of methylene chloride. After working up and
column chromatography (SiO2, ethyl acetate/hexane: 4/1) the title compound is isola~ed.
TLC Rf(B)= 0.35; tRe,(IV)= 16.84 min; FAB-MS (M+H+)= 859.
Example 60: 5(S)-(Boc-amino)-4(S~-r(methoxycarbonylmethYlthio)-acetyloxyl-6-phenvl-
2(R)-benzvlhexanoyl-(L)-Val-(L)-Phe-mor~holin-4-ylamide
Analogously to Example 4, a soludon of 219 mg (0.3 mmol) of 5(S)-(Boc-arnino)-4(S)-
hydroxy-6-phenyl-2(R)-benzylhexanoyl-(L)-Val-(L)-Phe-morpholin-4-ylamide and 5 mg -
of DMAP in 1.5 ml of pyridine is added to a mixturei of 98 mg ~0.6 mmol) of thiodi- ` ~ ~ `
glycolic acid monomethyl acetate (Indian J. Chem. 2SB, 880 (1986)) and 0.11 ml
(0.72 mmol) of 1-chloro-N,N,2-trimethyl-1-propenamine in S ml of methylene chloride.
Working up and column chromatography (SiO2, ethyl aceeate/hexane: 4/1) yield the title
compound.
Example 61: 5(S)-(Boc-amino)-4(S)-~(methox~carbonvlmethYlsulfo)-acetvlox~rl-6-
Phenvl-2~R)-benzvlhexanoYl (L)-Val-(L)-Phe-morpholin-4-ylamide
Analogously to Example 4, a solution of 219 mg (0.3 mmol) of 5(S)-(Boc-amino)-4(S)-
hydroxy^6-phenyl-2(R)-benzylhexanoyl-(L)-Val-(L)-Phe-morpholin-4-ylamide and 5 mg
of DMAP in 1.5 ml of pyridine is added to a mixture of 117 mg (0.6 mmol) of S,S-dioxo-
thiodiglycolic acid monomethyl ester and 0.11 ml (0.72 mmol) of 1-chloro-N,N,2-
trimethyl-l-propenamine in S ml of methylene chloride. After working up and column
chromatography (SiO2, ethyl acetatelhexane: 4/1,~ the title compound is isolated.
The starting compound is prepared as follows:
21186~1
,.
199 _
a) S~S-Dioxo-thiodi~lvcolic acid monomethvl ester
Thiodiglycolic acid monomethyl ether (Indian J. Chem. 25B, 880 (1986)) in a methanoV-
water mixture is oxidised with potassium peroxomonosulfate at approximately 25C to
yield the ~itle compound, which is further used.
Example 62: 5(S)-(Boc-amino)-4(S)-r~pivalovloxYmethoxYcarbonYlmethoxv)-acet
6-Rhenyl-2(R~-benzvlhexanoyl-(L)-Val-(L)-Phe-morpholin-4-vlamide
Iodomethyl pivalate (Chem. Abstr. Registry No. 53064-79-2) is added to S(S)-(Boc-
amino)-4(S)-[(carboxymethoxy)-acetyloxy]-6-phenyl-2~R)-benzylhexanoyl-~L)-Val-(L)-
Phe-morpholin-4-ylamide. The subsequent column chromatography yields the tide
compound.
Example 63: 5(S)-(Boc-amino)-4(S)-~(carboxymethoxv)-acetYloxvl-6-phenvl-2(R)-
benzvlhexanovl-(L)-Val-(L~-Phe-molpholin-4-vlamide
Diglycolic acid anhydride (Fluka, Buchs, Switzerland) is added to a solution of 219 mg
(0.3 mmol) of StS)-(Boc-amino)-4(S)-hydroxy-6-phenyl-2(R)-benzylhexanoyl-(L)-Val-
(L)-Phe-morpholin-4-ylamide in pyridine. After working up and column chromatography
(SiO2, ethyl acetate~ the title compound is isolated.
Example64: (S)-(Boc-amino)-4(S)-r(acetoxvmethoxycarbonYlmethoxv)-acetYloxYl-6
Phenvl-2(R)-benzY!hexanoyl-(L)-Val-(L)-Phe-morpholin-4-Ylamide
Analogously lo Example 62, 5(S)-(Boc-amino)-4(S)-[(carboxymethoxy)-acetyloxy]-6-phenyl-2(R)-benzylhexanoyl-(L)-Val-(L~-Phe-morpholin-4-ylarnide is reacted with
bromomethyl acetate (Aldrich, Steinheim, Federal Republic of Gerrnany). The subsequent
column chromatography yields the title compound.
Example 65:
Analogously to one of the processes described in Examples 1 to 64, unless explicitly
described in the afore-mentioned Exarnples the compounds of formula I of Reference
Examples 57 to 64 are ~onverted, by replacing the 4(S)-hydroxy group in the central non-
hydrolysable peptide building block, into the corresponding 4(S)-acyloxy compounds in : ;
which one of the following radicals stands in place of the 4(S)-hydroxy group:
A) 4(S)-(2-furanylcarboxy)-;
B) 4(S)-[4-(dimethylamino)^butyryloxy];
C) 4(S)-(N-ZN-methylaminoacetyloxy);
D) 4(S)-(methylaminoacetyloxy);
2il86~1
- 2()0-
E) 4(S~-[N-(imidazole-4-methyl)-N-methylaminoacetyloxy];
F) 4(S)-[3-(1-triphenylmethylimidazol-4-yl)-propionyloxy];
G) 4(S)-[3-(4-imidazolyl)-propionyloxy];
H) 4(S)-(methoxyacetyloxy);
I) 4(S)-((2-picolinoyl);
J) 4(S)-(benzyloxyacetyloxy);
K) 4(S)-[(S)-c~-methoxy-o~-phenylacetyloxy]; ~ :
L) 4(S)-[(R)-oc-methoxy-a-phenylacetyloxy];
M) 4(S)-(l-pyrazolylacetyloxy);
N) 4(S)-(isoquinoline-3-carbonyloxy); .
O) 4(S)-(pyrazinecarbonyloxy);
P) 4(S)-(4-oc-chloromethylbenzoyloxy);
Q) 4(S)-[4-(4-morpholino)-methylbenzoyloxy];
R) 4(S)-(isonicotinoyloxy); :.
S) 4(S)-(nicotinoyloxy);
T) 4(S)-(3-methoxypropanoyloxy) . -
U) 4(S)-[(4-chlorophenoxy)-methoxyacetyloxy)];
V) 4(S)-[2-(2-methoxyethoxy)-acetyloxy)];
W) 4(S)-(butyloxyacetyloxy);
X) 4(S)-[2-[2-(2-methoxyethoxy)-ethoxy]-acetyloxy)];
Y) 4(S)-(methoxyacetyloxy);
Z) 4(S)-(phenoxyacetyloxy); .
AA) 4(S)-[(S)-a-methoxy-a-phenylacetyloxy);
AB) 4(S)-[(R)-a-methoxy-oc-phenylacetyloxy)];
AC) (N,N-dimethylaminoacetyloxy);
AD) 4(S)-[N-(pyridine-2-methyl)-N-methylaminoacetyloxy];
AE)4(S)-[3-(dimethylamino)-propionyloxy];
AF) 4(S)-[3-(N-Z-N-methylamino)-propionyloxy];
AG) 4(S)-(3-methylaminopropionyloxy);
AH) 4(S)-[(dimethylaminoethoxy)-acetyloxy];
AI) 4(S)-[(2-pyridylmethoxy)-acetyloxy];
AJ) 4(S)-(methoxyacetyloxy);
AK) 4(S)-(pyridine-2-carboxyl);
AL) 4(S)-(methylthioacetyloxy);
AM) 4(S)-(benzylthioacetyloxy~;
AN) 4(S)-((L)-prolyloxy); :~
2~18~1
.
- 201 -
AO) 4(S)-((D)-prolyloxy);
AP) 4(S)-((L)-(N-Z-prolyl)oxy);
AQ) 4(S)-((D)-(N-Z-prolyl)oxy).
Example 66: Analogously to one of the processes described in Examples 1 to 64, unless
explicitly described in the afore-mendoned Examples the compounds of formula I of
Reference Examples I to 22 and 33 to 64 are converted, by replacing the 4(S)-hydroxy
group in the central non-hydrolysable peptide building block, into the corresponding
4(S)-acyloxy compounds in which one of the following radicals stands in place of the
4(S)-hydroxy group:
3-(N-Z-amino)-propionyloxy;
3-aminopropionyloxy;
(3-dimethylaminopropoxy)acetyloxy; :
2-(2-dimethylaminoethoxy)-ethoxyacetyloxy;
(4-dimethylaminobutoxy)acetyloxy;
(2-benzyloxy)acetyloxy;
2-acetyloxy)acetyloxy;
2-(4-tetrahydropyranyloxy)-acetyloxy;
2(R)-(4-tetrahydropyranyloxy)-propionyloxy;
2-(2-aminoethoxy)-ethoxyacetyloxy~; - . .
2-(2-benzyloxycarbonylaminoethoxy)-ethoxyacetyloxy);
(methoxycarbonylmethoxy)-acetyloxy; ~ `
(methoxycarbonylmethylthio)-acetyloxy; .
(methoxycarbonylmethylsulfo)-acetyloxy; .:
(pivaloyloxymethoxycarbonylmethoxy;
(carboxymethoxy)acetyloxy;
(acetoxymethoxycarbonylmethoxy)acetyloxy.
Example 67: Gelatin solution
A sterile-filtered aqueous solution of one of the compounds of formula I' mentioned in the
preceding Examples 1 to 43 and 44 to 66 or of formula I from the Reference Examples 42
to 76 that in addition comprises 20 % cyclodextrin, and a sterile gelatin solution preserved ~ ~ .
with phenol, are so mixed under aseptic conditions, with heating, that 1.0 ml of a solution -
of the following composition is obtained:
21186~
.~
.
- 202-
active ingredient 3 mg
gelatin 150.0 mg
phenol 4.7 mg
dist. water with 20 % cyclodex~ins 1.0 ml
Example 68: Sterile drv substance for in1ection
5 mg of one of the compounds of formula I' mentioned in the preceding Examples 1 to 43
and 44 to 66, or of formula I from the Reference Examples 42, to 76, are dissolved as
active ingredient in 1 ml of an aqueous solution with 20 mg of mam~itol and 20% cyclo~
dextrins as solubilisers. The solution is sterile-~lltered and introduced under aseptic
conditions into a 2 ml ampoule, deep-frozen and lyophilised. Before use, the Iyophilisate
is dissolved in 1 ml of distilled water or 1 ml of physiological saline. The solution is
administered intramuscularly or intravenously. The formulation can also be introduced
into double-chamber disposable syringes.
Example 69: Nasal sPray
500 mg of finely ground (<5.0 llm) powder of one of the compounds of formula I'
mentioned in the preceding Examples 1 to 43 and 44 to 66, or of f~rmula I from the
Reference Examples 42 to 76, are suspended as active ingredient in a mixture of 3.5 ml of
Myglyol 812(~) and 0.08 g of benzyl alcohol. The suspension is introduced into a container
fitted with a metering valve. 5.0 g of Freon 12(~ are introduced lmder pressure into the
container through the valve. The "Freon" is dissolved in the Myglyollbenzyl alcohol
mixture by shaking. The spray container contains approximately 100 single doses which
can be administered individually.
Example 70: Film-coaeed tablets
The following ingredients are processed to produce 10 000 tablets each comprising
100 mg of active ingredient:
active ingredient 1000 g
corn starch 680 g
colloidal silicic acid 200 g
magnesium stearate 20 g
stearic acid 50 g
sodiumcarboxymethyl starch 250g
water quantum satis ¦
,.::: , ~ . - ,
.. ~ . . .
211~6Gl
- 203 -
A mixture of one of the compounds of formula I' mentioned in the preceding Examples 1
to 43 and 44 to 66, or of forrnula I from the Reference Examples 42 to 76, as active
ingredient, 5Q g of corn starch and colloidal silicic acid is processed with starch paste
prepared from 250 g of corn starch and 2.2 kg of demineralised water to form a moist
mass which is forced through a sieve of 3 mm mesh size and dlied in a fluidised bed drier
at 45 for 30 min. The dry granules are pressed through a sieve of 1 mm mesh size, mixed
with a pre-sieved rnixture (1 mm sieve) of 330 g of corn starch, the magnesium stearate,
the stearic acid and the sodium carboxymethyl starch, and compressed into slightly
biconvex tablets.
Example 71: Orallv administrable dispersion 1
625 mg of one of the compounds of formula I' mentioned in the preceding Examples 1 to
43 and 44 to 66, or of formula I from the Reference Examples 42 to 76, as acdve
ingredient, and 625 mg of POPC (1-palmitoyl-2-oleoylphosphatidylcholine = 1-hexa-
decanoyl-2-(9-cis-octadecenoyl)-3-sn-phosphatidylcholine) are dissolved in 25 ml of
ethanol. The solution is diluted with ten times the amount of water. The ethanolic solution
is for that purpose added dropwise at room temperature, at a rate of 10 mVmin, to the
prescribed amount of water. The ethanol is removed from the mixture by tangential
dialysis ("Cross Flow Filtration") against 1750 ml of water (System: Minitantg), 700 cm2
polyethersulphone membrane with an exclusion limit of 100 kD, supplied by Millipore
(USA)). The mixture is concentrated to 15 mg of actdve ingredient by ultrafiltration using
the same system. After the addition of 1.24 mglml of citric acid and 1.24 mgtml of
disodium hydrogen phosphate.2 H2O to adjust the pH to 4.2 and the addition of 1 mgtml
of sorbic acid as andmicrobial preservatdve, the dispersion is concentrated to 15 mg/ml
again and introduced into vials, for example having a capacity of 20 ml. The dispersion
particles have a diameter of from 0.1 - 2 ~lm. They are stable for at least six months at
from +2 to 8C and are suitable for oral administration.
:'
Example 72: Orally administrable dispersion 2 -
The preparation is canied out analogously to Example 71, except that 25 mg of active
ingredient and 50 mg of POPC are used to prepare the ethanolic solution.
Example 73: Orally administrable dispersion 3
The preparation is carlied out analogously to Example 71, except that 25 mg of active
ingredient and 125 mg of POPC are used to prepare the ethanolic solution.
` 211~6~1
- 2()4 -
E~xamplc 74: Or ~lly a(Jm~i.s;r.lb!c d_~E~slon 4
'I'hc preparation is carricd out analogously to l~xamplc 71, except that 50 mg of active
ingrcdient and 50 mg of POPC arc used to prepare the ethanolic solution.
~xample 75: ~2~1~1~s~
Thc preparation is carried out analogously to any one of Examples 71 to 74, except that
activc ingrcdient and phssphatidyl choline from soybeans or phosphatidylcholine from
cg6 yolk (70 - 100 ~ pure) are used instead of POPC to preparc the ethanolic solution. If
dc~ircd, an antioxidant, such as ascorbic acid, is added in a concentration of 5 mg/ml.
~,
~ ' r
~1 ,