Note: Descriptions are shown in the official language in which they were submitted.
B1?i7783-0e02
- 1 -
Title: METHOD AND APPARATUS FOR DETECTING MERCURY
FIELD OF THE INVEN'PION
This invention relates to the detection of
mercury. Tr;is invention ~r~ore particularly relates to the
detection of mercury in air, and to a method and apparatus
capable of providing a low detection Limit.
BACKGROUND OF THE INVENTION
There are many known methods of mercury
analysis, including: colorimetric; atomic absorption
coupled to vapour generation; atomic absorption following
gold trapping from vapour generation, and atomic
fluorescence coupled to vapour generator.
Atomic fluorescence is comn.only achieved in a
Cold Vapour Atomic Fluorescence Spectrophotometer (CVAFS).
CVAFS is preferred, as compared to atomic absorption,
since the phenomena is linear over a much wider range and
is not subject to positive interferences. Rather, it can
be subject to negative interference modes, with certain
molecular species causing quenching. The present
invention provides techniques for improving the
performance of such a device and for overcoming negative
interference.
It is known that gold is an excellent adsorber
& of mercury, which forms a gold amalgam with mercury.
However, many conventional instruments suffer from a
"memory" effect. This arises due to the use of gold
having substantial. thickness .in a detector cell, and
mercury migrating from the surface of the gold to the
interior, or at least below the surface. Consequently,
when the ma~rcury is flushed from a detector cell by
heating in an inert gas flow, in known manner, not all the
mercury is immediately re=leased. In subsequent cycles,
mercury from below the gold surface can migrate to the
surface and be flushed out, giving a false mercury
reading. While it has beern proposed to use such materials
as gold-coated sand, with the intention of providing a
BF'17783-0002
- 2 -
gold film so thin as to prevent this problem, such
technique has its own drawbacks. The gold-coated sand is
not always cr~mpletely stable, and the gold may not remain
plated or adrered to the sand particles, particularly when
subjected to hundreds and thousands of heating and cooling
cycles.
Pu:_-e gold is very selective and does not adsorb
most contaminants that can give false readings, which may
contaminate the flow path of the instrument. However,
problems have often been reported with the adsorption of
competing compounds. Thus, the activity of the gold can
be taken up by other compounds, and once used, the gold in
the cartridge will not be able to capture mercury. This
will give a false, low reading. During d<~sorption, the
competing compounds may be released. They may then
register a false positive reading, attenuate the actual
mercury signal, or contaminate the flow path. Organic:
compounds and water vapour are common examples of
contaminants that can be unintentionally entrairied.
In conventional inst=ruments, the flows of
different: gases, such as air, carrier gas, are not
controlled so as to give optimum performance. No
consideration has been given to optimizing the flows of
such gases.
In many conventional instruments, ambient air
and other contaminants can be passed through the detector
cell. To maintain the purity of the detector cell, and
prevent contamination, it is desirable that only a carrier
gas, containing mercury when present, be passed through
the detector cell.
Common types of detector cells have the interior
optical path filled with air. The ultraviolet radiation
produced by the lamp of t:he detector produce compounds in
air which absorb the ultraviolet light. The most
important. reaction is the break down of oxygen and the
creation of free radicals that recombine to produce ozone.
These UV adsorbent compounds decrease sensitivity of the
BF?t 7783-0002
~~~~.~.~.a
- 3 -
detector and cause significant baseline shift.
To calibrate known instruments, i.t is necessary
to provide manual injections using gas tight syringes.
This is cumbersome and awkward, and necessarily prevents
any automation of the device.
Ac~:ordingly, vt; is desirable to provide a
mercury detector, based on Cold Vapour Atomic Fluorescence
Spectrophotornetry which provides a much higher degree of
sensitivity. It is desirable that such a detector not
suffer from any memory e:Efects of the gold, and not be
susceptible to contaminants entering the detector
cartridges. It is desirable that the gas flows be
controlled to optimise, or. at least improve, usage of the
gases and performance of the apparatus as a whole.
It is further desirable that the apparatus be
capable of automatic operation, and include means for
automatic: rec:alibration.
It is also desirable that the detector itself
not be susceptible to 'the generation o~f ultraviolet
absorbent compounds which would decrease sensitivity.
SUMMARY OF THE PRESENT INVENTION
In accordance which the present invention, there
is provided a mercury detection apparatus comprising: a
main carrier gas inlet; a sample gas inlet; inlet valve
means connected to the carrier gas and sample gas inlets;
a cartridge including gold, for accumulatincJ mercury as an
amalgam, connected to the inlet valve means; an outlet
valve means connected to the cartridge; a sample gas flow
path extending through from the sample gas inlet through
the outlet and outlet valve means and through the
cartridge; a pump for pumping sample air through the
cartridge in the sample gas path; a vent connected to the
outlet valve means; and a mercury detector connected to
the outlet valve means; and a control unit: connected to
the inlet and outlet valve means to control thereof,
wherein with the sample gas inlet and the pump connected
to the cartridge by the inlet and outlet valve means, the
119113
2
-4-
pump draws sample a_i.r through the cartridge, and with the
carrier gas ir:let connected. by the inlet:. valve means to the
cartridge, the outlet valvf= means selectively connects the
cartridge to c::ne of the ven.i=, for venting re:~idual air, and
to the detector for detect.i.~:m of any meo~cury. The apparatus
includes a vF~nt three-way valve connected to the outlet
valve means, and to both the detector and the vent.
'!'he present invention also provides, a related
method, the method comprising the steps of:
a) passing sample gas containing mercury
through a cartridge containing go~_d, on which the mercury is
an adsorbed tc: form an ama Lc~am;
b) after a ltnown cxuantity of gas has passed
through the cartridge, t:ermin~itin.g the gas flow, and
flushing the c:artriage with an inert carrier gas, to flue>h
out residual air;
c) afters flushing of residual air,
connecting tr.e cartridge to a detector and heating the
cartridge to cause desorpt:i.on of mercur~~r and entraining the
mercury vapour in t:he carrier gas flaw; and passing the
carrier gas flow and entrained mercury to a detector for the
detection of mercury.
''he known <xuantit:y of gas is preferably
determined by sampling for a preset time, and integrating
the flow rate over that time, to obtain th.e total volurne
sampled. It may alternati.v~=_ly be obtained by passing a set
sample volume through the ~~~~rtridc~e .
t~nother aspect of the present invention
provides an apparatus for detecting mercury, the apparatus
comprising: a main carrier gas inlet; a sample gas inlet=;
two cartridges, each including gold, fo~~ accumulating
mercury as an amalgam; inl.~~t valve means connected to the
carrier gas and sample gas. inlets and comprising a fir:~t
inlet three-way valve connected between the sample gas inlet
and inlets of the two cartridges and a second inlet three-
way valve connected between the carrier gay; inlet and the
211911
-5-
inlets of the two cartridges; an outlet valve means
connected to the cartridges and comprising a first outlet
three-way valve connected between outlets of the two
cartridges and a second outlet three-way valve connected
between outle ~s of the two cartridges ; a sample gas flc>w
path exter~dinc~ from the sample gas inlet through the first
inlet and fir~;t outlet valves and through the cartridges; a
pump connected to first outlet valve for pumping sample air
through the cartridge in t;he sample gas path; a vent; a
mercury detector; means for heating the cartridges to desorb
mercury there'~n; a vent three-way valve connected between
the second outlet valve anal both the mercury detector and
the vent; and a control unit connected to the inlet and
outlet va:Lve means, to the= heating me<:~ns and to the vent
three-way valve for control thereof, wherein with the
sample gas inlet and the pump connected to one cartridge by
the first inlet valve and first outlet valve, the pump draws
sample air through the cartridge) and with the carrier gas
inlet connected by the second in-'yet valve to one cartridge
and through tc the vent three-way valve by the second outlet
valve, the vent three-way valve selec~~ively connects said
one cartridge to one of the vent, for venting residual aii~,
and the detector for detection of any mercury.
~he related method comprises the steps of:
(a) passing a sample gas stream through a
cartridge containing gold,. whereby an,;r mercury vapour is
adsorbed on the gold to form an amalgam, whale maintaining
the gold at .gin elevated t=emperature between ambient and
about 100'C;
(b) after a known valurne of: gas has been
passed through the chamber, terminat=ing the gas flow,
heating the cartridge to a higher temperature to cause the
mercury to revapourize, aru3 passing a carrier gas through
the cartridge to entrain the desorbed mercury vapour;
(c) passin.c~ the carrier gas with the
entrained mercury vapour to a detector, for detection of
,~,.~ ..
r 4j
3-0
__ 5a _
mercury.
'This a;~pect of the present invention ensures
that the gold is maintained at a temperature above ambient,
so as to p-~event the adsorption of water, organic
contaminants ~;.nd the like.
Again i.t is preferred, although not essential,
to determine the sample gas volume by integrating the flow
over time.
'The present invention further provides a
permeatiori source, for use with ma_rcury detection apparatus,
the permeatioru source comprising a permeation chamber having
a chamber inlet and a chamber outlet; a:~ mer~~ury permeation
source withir; the ~>ermeation chamber; a heating unit for
maim=aining the permeation chamber at a substantially
constant temperature; valve means having an inlet for
carrier gas and an inlet connected to the permeation chamber
outlet, a permeation source output arid a permeation source
vent; and a ~econda.ry carrvier gas inlet r_onnected to the
permeation chamber and the inlet of the valve means.
s~:,,
BPi778:3~-0002
..
- 6 -
The provision of a permeation source enables the
device t:o operate automatically, and enables automatic,
unattended recalibration as required.
Preferably, inert gas is conserved, by setting
different glow rates for different purposes, more
preferably, there are 3 separate flow rates. The
cartridges are f7.ushed with carrier gas at a first
relatively high flow rage; during entrainment of mercury
vapour, carrier gas is set at a second, relatively low
flow rate; and, during idle or inactive periods, the
carrier gas flow is maintained at a third flow rate, below
the first and second flow rates, sufficient to maintain
the apparatus purged of air. This serves too conserve and
make optimal use of an inert gas supply.
DESCRIPTION_OF DRAWING FIGURES
Far a better understanding of the present
invention and to show more clearly how it may be carried
into effect, reference will now be made, by way of example
to the accompanying drawings, whicl-:~ show a preferred
embodiment of the present invention and in which:
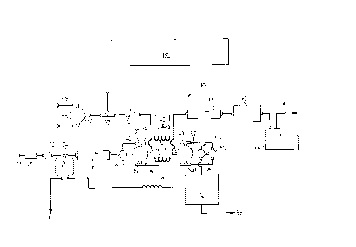
Figure 1 is a schematic flow diagram of an
apparatus in accordance with the present invention;
Figure 2 is a schematic diagram of a permeation
source of the apparatus of Figure I;
Figure 3 is a sc:hemati.c diagram of a detector of
the apparatus of Figure 1;
Figure 4 is a sectional view through a sampling
cartridge; and
Figure 5 is a graph showing a recovery
characteristic of the apparatus.
DESCRIPTION OF PREFERRED EMBODIMENT
The apparatus as a whole is indicated by the
reference 1(). The apparatus 7.0 includes a detector 12
first and second adsorbent cartridges 14, 15, and a mass
flow controller 16.
The structure of each cartridge is shown in
detail i_n Figure 4. Each cartridge 14, 15 comprises a
BP~7783-0:e02
_ 7 _
quartz glass tube 140 having a 6mm outside diameter and a
4mm inside diameter. A pure gold screen disk assembly is
indicated at 142. The tube 140 provides a retention
groove at 144 and defines a flow sealing orifice 146,
which also serves to ret:ain the gold di.=~k assembly in
position. Tne gold disk assembly comprises 100 disks, and
is approxima~ely 8mm long with a 4mm diameter. Total gold
surface area is approximately 1'7.7cm~.
A carrier. gas inlet 18 is connected through an
inlet filter 20, located at an inlet bulkhead, and then
through a stainless steel cutoff valve 22.
Th is valve 22 <~utomat:ically turns the carrier
gas supply on and off a.s power is supplied, to prevent
waste of carrier gas during a power failure and when the
instrument is turned off.
At 24, a T connector splits the flow into two
streams. One stream is d<~livered to a precision pressure
regulator 26. The regulator 26 in turn has an outlet,
connected by the T connector to a flow restrictor 28 for
a purge flow for the detector and to a permeation source
30, shown in detail. in Figure 2.
The pressure regulator 26 provides a fixed
pressure that is applied to the flow restri.ctor 28, so as
to give a fixed, lower rate of flow. Here, it is
approximately 10 ml/min., to the o~>tical path of the
detector 12 , shown in Figure 3 and described in greater
detail below.
The other stream from the T connector 24 is
connected to the mass flow controller 16, as is explained
in greater detail below, the mass flow controller 16 is
capable of setting different flow levels, so as to give
greatly r_educ;ed carrier gas usage and shorter cycle times .
The following levels could be set:
during an initial flush phase of a detector
cycle, the controller is aet to allow a large carrier gas
flow, to allow a rapid flushing of air out of the
cartridge and surrounding fittings, thereby allowing
BP~7783-0002
_ g _
quicker c:ycl.ing;
during baseline and peak acquisition, the
carrier flow is set so .3s to produce optimally shaped
peaks; and
during idle periods, the flow is set for a low
value, whicr~ is just sufficient to peep the lines and
detector itself flushed and stable.
The=_ outlet of -the mass flow controller 16 is
connected to a first, inlet valve 34, a three-way solenoid
valve 34, which in turn is connected by T connectors 36
and 37, to the first and second cartridges 14 and 15.
Each cartridge is provided with a gold absorbent in a form
of a fine wire screen. The quantity of gold used and the
configuration of the wire screen is as such to ensure that
the active surface area is sufficiently large. Each
cartridge is also provided with a respective heater 40, 41
and a common cooling fan indicated schematically at 42.
The function of these components is detailed below.
Pure gold is used, instead of sand or glass
beads cc>ated with gold, since it is able to endure
hundreds of thousands of heating cycles without breaking
down.
The T connectors 36, :37 are also connected to a
second inlet three-way valve 46.
The outlets of the cartridges 14, 15 are
connected through outlet T connectors 48, 49 to a first
outlet three-way valve 50. The T connectors 48, 49 are
also connected to a second, outlet three-way valve 52.
The first and second inlet and outlet valves 34,
46, 50 and 52 are a11 three-way solenc>id valves.
The first outlet valve 50 is further connected
to a vent three-way valve 54, which has one outlet
connected to a vent indicated at 56 and on other outlet
connected to the detector 12. The detector in turn has a
detector vent 58.
The second inlet and outlet valves 46 and 52, as
indicated schematically, are larger than thE~ valves 34 and
B1?!~ 7783-0002
..
g _
50, so as to be capable of handling a greater flow rate.
The second outlet va:Lve 52 is connected to a
precision mass flow meter 60, which is used to measure
mass flow through each cartridge. The mass flow meter 60
is connected through a buffer tank 62 and a sample pump 64
to a pump exhaust fib.
A source selection valve 68 has an inlet 69 for
zero air and an inlet 70 Eor sample air. It is connected
through first and second inlet T connectors 72, 74. The
first inlet T connector 72 is connected to an outlet of a
permeation source detailed below, while the second inlet
74 provides an injection port.
Referring to Figure c:, this shows, in greater
detail, the permeation source 30, which provides a stable,
repeatable alternative to calibration x>y manual injection.
An aluminium block 80 is provided with a block heater 82.
The block 80 is intended to provide signiFicant thermal
inertia, so that the interior of the block is maintained
close to desired temperature set: point, within 0.05~C.
Within the block 80, there is a permeation
chamber 84. Within the chamber 84, a permeation tube 86,
which can be conventional., and which is retained by a
spring.
A permeation inlet 88 is connected through flow
restrictors 89 and 90 to the permeation chamber 84 and a
valve assembly indicated at 92.
The valve assembly 92 includes a permeation
shut-off valve 94 connected to a first permeation control
valve 96. The valve 9Ei is a specialized fast flush
solenoid valve, which is connected to a second permeation
control valve 98 and a permeation outlet 100, which is
connected to the T connector 72. The valve 96 has a
straight through gas path connected between valve 94 and
the source output 100. T:he connection to valve 98 is an
injection input which can be closed or opened to inject
mercury to the through gas path.
The second permeation control valve 98 is a
BP~778:3--G~G2
- to -
three-way valve, having an inlet, connected to an outlet of
the permeation chamber 84, and a further outlet connected
to a permeation vent indicated at 102,
The permeation chamber 84 can be provided with
different sizes of permeation tubes. These could range,
for exampler in length from 1-.3 cm. The temperature in
the permeation chamber can be varied between 45-100~C,
with 50~C being a normal setting.
As shown in Figure 1, the permeation inlet 88 is
connected to the carrier gas inlet 18, for an inert gas,
which in this embodiment, is argon. Inert gas is used to
provide a ccjntinuous purge through the permeation source
30, so as to prevent contamination and eliminate any
possibility of oxidation within the permeation tube.
Referring to Figure 3, the detector 12 has a
housing 110. Within the housing 110, there are the
various elements o.f the detector 12, which can be largely
conventional.
Thus, there is a photomultiplier tube 112 and a
sample cuvette 1l4. An interference filter 116 is placed
between them. An ultraviolet lamp 118 provides the source
of W radiation.
The components 112-1l8 are enclosed, at least
partially, by an inner housing :120. This defines optical
paths from t:he lamp 118 to the cuvett~e 114, and in turn
from the cuvette 114 to the photomultiplier 112.
An optical path purge inlet 122 is provided at
one end, and an optical path outlet 124 is provided at the
outlet. As shown in Figure 1, the flow restrictor 28 is
connected to the inlet 122. This ensures that the optical
path is continuously purged with a flow of inert gas.
This avoid= quenching by certain molecular species
generated by the UT~ radiation.
The entire sample path is 1 /4 " OD Teflon, except
for the cartridges 14, 15 which are quartz glass . This
size is required due to t:he high sample flow rates. The
carrier gas components are 1/8" OD, thick walled Teflon
Bf',~7783_0002
- 11 -
and small bore miniature Teflon solenoid valves in order
to minimize dead volume, especially between the cartridges
14, 15 and tie detector 1:2.
The detector uses Colci Vapour Atomic
Fluorescence Spectrophotometry (CVAFS), t:o detect the
mercury. 'Mercury that passes into cuvette 114 is
illuminated by the ultraviolet lamp 118, which is a low
pressure mercury vapour lamp. Radiation at 253.7 nm
excites any mercury atoms present, which fluoresce and re
radiate at the same frequency. The photomultiplier tube
112 is at a right angle to the incident light from the
lamp 118, and receives some of this re-radiated light, but
not the direct .radiation :from the lamp 118.
As indicated schematically at 130, a control
circuit i.s connected to various elements of the apparatus,
namely the valves 22, 34, 46, 50, 52, 54, 68, 94, 96 and
9 8 , heaters 4 0 and 41 and f an 4 2 , the detector 12 , the
mass flow controller 16, the mass flow meter 60, and the
sample pump f>4. The control circuit includes an embedded
microprocessor, which can allow a wide range of features.
It can be provided with a suitable interface, including,
for example, a keyboard and display. 'rhe control circuit
130 can be provided with two or more analog outputs,
programmable for selective instrument readings, a serial
RS232 port, and digital outputs. Other inputs can be
provided as desired.
The microprocessor and the control circuit can
include a memory that allows the analyzer to retain
information, even in the absence of= power, including
information or data on instrument settings from parameters
from a recerva result, and two or more set measurement
cycles.
The precision mass flow meter E>0 is used to
measure sample flow rate through one cartridge 14, 15.
The microprocessor integrates flow rate over time, to
determine total volume of air or other gas passing through
the cartridge.
BPf'778_4-0002
- 12 -
The speed of the sample pump 64 is controlled by
a closed loop feed back controller, to ensure that the
flow rate is set. to give a desired aampling rate.
Controlling the pump speed results in quieter and more
energy effi~:ient operation, as well as F~rolonging pump
life.
Fc~r the carrier gas, t:he mass flow controller 1.6
is controlled to provide optimal flows of carrier gas, to
give the desired performance, during various steps of the
cycle. This can improve sensitivity and repeatability,
and conserve carrier gas, to give increased life time far
a cylinder of argon or at:her inert gas.
Each cartridge 14, 1.5 is subjected to cycles
which are short, giving small cartridge loadings, and are
alternated with cleaning cycles, which automatically are
performed before a calibration. Cycle times are
maintained short by having a high sample gas flow rate,
for example 21/min for 5,, 10 or. 15 minutes. It has been
found that this virtually eliminates the "memory effect"
previously associated with pure gold, cartridges.
Sample time, for example, flow hates and other
steps in the adsorption. cycle are programmable. The
control circuit 130 can be set for recording two, or
possibly more, complete sets of cycle parameters.
The instrument can be calibrated either by
manual injection through the injection port provided at
the T inlet connector 74, or alternatively, it can be
automatically calibrated from the permeation source 30,
allowing long term unattended operat:eon. The source 30
can also be used at any time for manual recalibration.
While in use, the source selection valve 68 i.s
normally sw:i_tched to the sample ambient air of inlet 70.
It is switched to the inlet 69 for zero air, for the
following operations: cartridge cleaning operation; zero
phase calibration; span phase calibration; or when the
control circuit 130 otherwise switches the valve 68.
In use, with the valve 68 connected to the
BP~'778:3~-0u02
- 13
sample air inlet 70, air is alternately and sequentially
passed through the cartridges 14, 15. The air is drawn
through the cartridges by the sample pump 64.
A~. indicated, t=he lines for the air flow are
relatively 7.arge, as are the valves 46 and 52, to enable
a relatively volume of air to be fussed through each
cartridge. Air i~. drawn through the cartridge 14, until
the desired volume is detected by the mass flow meter 60.
Ai_r flow is switched t:o the r_artridge 14, at the
end of the adsorption cycle for the cartridge 15. The
pump 64 draws air through the cartridge 14, with the
cartridge 15 being subjected t:o a flush and desorption
cycle, detailed below. At the end of the cycle, cartridge
and associated dead paces in connections to valves
15 etc. will contain air.
After the valves 46, 52 have been switched to
connect the air flow to the cartridge 14, the valves 34
and 50 are simultaneously switched tc> connect the
cartridge 15 to the carrier gas inlet 18 and to the
detector' 12.
Before any gas i_s passed to the detector 12, the
vent three-way valve 54 is switched to the vent 56.
The mass flow controller 16 is then set to a
relatively high flow for a "flush" phase. This flushes
out the dead volume associated with the cartridge 15 of
any residual air and other impurities, typically for 20-60
seconds.
The flow is then reduced to a second, lower flow
rate, which is used for the remainder of the desorption
cycle. To give the flow time to stabili;ae, there is a
measurement delay time of 1-10 seconds. This delay allows
stale, poss~.bly contaminated air to be flushed out of the
cartridge before a baseline measurement is taken.
A further baseline acquisition delay, of again
1-10 seconds is then allowed, i~o a further stabilization
of the carrier flow and i=lushing of the cartridge.
The second, lower flaw rate is set by the mass
13P#7783-0002
~.~l.~~l~~
- 14 --
flow controller 16, and i.s such as to ensure well-defined
narrow peaks in the detector 12.
At. the end of the flush phase, the vent three
way valve 54 is switched to connect the cartridge 15 to
the detector 12.
Ttie next step in the desorption cycle is for the
instrument to make a baseline acquisition measurement.
This measures the level and noise of the baseline, and can
provide warning ~of a :number of potential instrument
problems. Amongst these, there are: excessive baseline
shift, potentially caused by cell contamination, photo
multiplier tube ageing or lamp ageing; high baseline
noise, which is usually a sign of an ageing lamp. The
recommended range for baseline ac:~quis_ition is 5-:30
seconds.
The cartridge 15 is then heated by the
respective heater 41. At the start of heating, there is
an integration delay, usually set in the range 10-20
seconds. This is the number of seconds between the start
of heating and the activation of the peak integrator. The
value should be set large enough to avoid integrating
baseline noise, but small enough to ensure that the
baseline immediately preceding the start of the peak :is
captured.
The heater on duration can be set independently
for each cartridge 14, :15, in case the heaters heat at
different rates. Excessive heating time rnay shorten the
life of, or damage, the cartridges. The heating time is
set to be long enough to ensure that all mercury is
desorbed during each burn. As a general. rule, heating
should start when detector voltage stops :Falling after a
peak, and the recommended range is 20-40 seconds.
At the end of heating, thE~re is a peak delay
time, to allow for the peak to finish eluting. During
this time, the integrator is kept active. Immediately
upon completion of this period, the area of the largest
peak found is reported for the desorption. If the
BI?i'7783-0002
~~~.~:1.:~3
- 15 -
current cycle is a calibration, t=he appropriate correction
factor is stored, while if it is an ambient run, the
existing calibration correction factors are used to
convert t:he area into a c~~ncentration.
There is then a cartridge cool down time, which
is the numbr;r of second=s after_ expia~ation of the peak
delay time that is required for the cartridge to cool
sufficiently, and to once again adsorb mercury. Carrier
gas flow rate is set at the third idle level at the start
of this period. This time delays thf~ end of the
desorption cycle enough to ensure that a cartridge is
always cool enough to begin sampling. During this period,
the fan 42 is operated.
At the end of the cool down period, an idle time
commences, during which t:he cartridge 15 lies idle with
the third idle flow rate of carrier gas being supplied.
The cartridge 15 awaits the cartridge 14 to end its
sampling period. The sampling period for each cartridge
cannot be set: shorter thar.~ the total time required for the
desorption cycle so that usually the cartridge undergoing
desorption will have a certain idle period. The sampling
period is recommended to be set in the range of 300-3600
seconds.
At the end of the peak delay time, the cooling
fan 42 is turned on to cool the cartridge 15. Whereas fan
4 cools both cartridges 14, 15, this will not have a
significant effect on the temperature of the cartridge 14,
undergoing adsorption, since its temperaturE~ above ambient
is small, compared to the desorption temperature.
At a11 times, t:he heaters 40, 41 are operated to
maintain the cartridges at the desired minimum
temperature, between ambient and about 100~C, preferably
in the range 45-75~C, with 50~C being a normal setting.
At the end of a preset time, the cartridge 14 is
fully loaded and total volume sampled is determined by
integration from the flow rate served by the MFM 60. The
valves 4E anti 52, as well. as the valves 34 and 50 are then
8F'1'7783-002
- 16 -
switched. The sample air flow is then switched to the
cartridge 15 , and the car. Tier gas is switched to the f first
cartridge 14.
The cartridge 14 is then subjected to the same
cycle sequen::e as outlined above.
Th~~ mass flow controller is also controlled by
the control circuit 130, to maintain a third, different
flow rate, during idle periods. During such periods,
where no collectian or detection is taking place, the
carrier gas flow rate is set to a very low value to
conserve carrier gas. The rate is maintained just
sufficiently high enough to maintain the various lines
purged, so as to prevent contamination and infiltration of
air or other contaminants.
During detection, the regulator 26 provides a
desired flow to the detector 12 and the permeation source
30. As described above, the flow through the detector 12,
shown in Figure 2, maintains this flushed of air, so as to
give improved performance.
For the permeation source 30, this ensures that
carrier gas at the desired flow rate is always available.
When the permeation source 30 is not in use, the
flow restrictor 90 is connected by the valves 94, 96 to
the outlet 100 and simultaneously the permeation source
itself is connected throv~gh to t:he permeation vent 102.
Ca librati.on of t: he device or apparatus 10 can be
achieved by manual operation, or can be set to occur
automatically at predetermined times, by the control
circuit 130. During calibration, the valve 94 is
activated to close off the flow to it, and the valves 96
and 98 are operated to connect the permeation chamber 84
through to the permeation outlet 1()0. This injects
mercury at a known level or concentration into the line
connected to the .inlet valve 46. Simultaneously, the
valve 68 will be switched to connect to a zero air inlet,
so that the exact mercury concentration will be known.
The detector 12 can be calibrated accordingly. As a
BF'#7783-0002
- 17 -
second calibration point, a reading can be taken with the
zero air, with no mercury injected.
To turn off the permeation source, for an idle
state, the second F>ermeat:ion control valve 98 is switched
back to the position connecting the permeation source to
the permeation vent: 102. Simultaneously, the valve 94 is
opened, and the first permeation control valve 96 is
closed to cut. off the connection from valve 98. The valve
94 is maintained opened for a set time to flush out the
line connecting source 30 to the T connector 72. Then the
valve 94 is closed, so that no :mercury contamination can
occur from the source 30.
Calibration requires zero and span calibration
points. This is usually obtained first by passing zero
gas through valve 68 to the device. It then samples for
one full period on each cartridge (usually 2 x 5 - 10
minutes), The area for each desorption is recorded as the
expected response of the instrument when the input mercury
is nil. Practically, as is well known, there is usually
a small response due to rE~sidual mercury in the zero air,
or due to residual contamination.
The second or span point i.s provided by the
permeation source as indicated or by manually injecting
mercury. During this phase, valve 68 is still open to
feed zero air to the analyzer. Analyt_Lcally, it is
preferable to ensure that t:he zero and sampling conditions
are as alike as possible. Thus, the same flow through the
cartridges is maintained. Any residual mercury in the
zero gas would also be present during the span phase, thus
ensuring that any slope of a calibration line is
unaffected. Any residual. mercury in the zero gas would
manifest itself as an offset in the final readings not as
a sensitivity error. Further, the gas flow through the
permeation source requires only a small volume of
calibration gas. Zero gas is required to make up the
additional volume required by the device.
The detector 12 uses cold vapour atomic
Be#~~a_,-.o002
- 18 _
fluorescence spectrophotometry for detection of mercury,
since it is more sensitive and linear over a much wider
range, compared to other techniques. The flushing with
the carrier gas eliminates a major negative interference
mode, and effectively prevents the quenching that it can
cause. 'fhe iltravi_olet radiation produces compounds that
absorb ultraviolet light. The most important reaction is
the breakdown of oxygen and the creation of free-radicals
which combine with oxygen to produce ozone, which absorbs
ultraviolet light. These ultraviolet absorbing compounds
decrease sensitivity of the detector and cause significant
baseline drift. This absorption is eliminated by the
sealed detector with the purged optical path described
above.
The provision o:f the permeation source 30 which
can be automatically controlled enables the apparatus to
make unattended calibrations. Known techniques rely on
manual injection using gas-tight syringes,. to calibrate
mercury-detection instruments.
The arrangement of valve assembly 92 for the
permeation source 30 ensures that even with the permeation
source 30 turned off, there is no possibility of mercury
leaking into the system, to contaminate subsequent
samples. In the off configuration, the mercury from the
permeation source passes only through the valve 98, and
not through the main connection to the outlet 100, and is
vented.
By maintaining the cartridges heated, it has
been found that certain compounds that normally interfere
with florescence and mercury detection in. the detector
cell area eliminated. The temperature is such as to
prevent condensation of water and any organic compounds
that may be present: in thc= sample stream .
This has been confirmed by tests using common
urban pollutants. High, although unquantified,
concentrations of H2S and S02 gas were added to zero air
and sampled by the analyzer. Test runs were made to
BP~f778V-.GJ02
- 19 -
confirm that these compounds cause no false positive
readings. The sample air was then spiked by the injection
of mercury t:o establish that the compounds did not cause
suppression of normal readings.
The use of pure gold as an adsorption medium has
often been associated with a "memory' effect, where the
quantity of mercury desorbed depends upon t:he past history
of the mercury, as well as current exposure. In effect,
mercury previously adsorbed is assumed to have migrated to
the interior of the gold, so that it is only released at
some much later time, thereby leading to inaccuracies in
later readings. Thus, a single heating ~~ycle has been
assumed to be insufficient to remove all the mercury from
the gold.
Here, short cycle times are used, typically 10
minutes or Less, and more preferably 5 minutes or less.
This is believed to prevent the migration of the mercury
from the surface of the gold.
Tc test the recovery rate, the device was
exposed to large mercury concentrations (40-70ng/cubic
metre) for approximately 15 hours while monitoring at 10
minutes intervals. The source was provided by laboratory
air spiked with additional mercury. Although the source
was not accurately quantified, it was sufficiently large
to exceed the upper calibration point of the instrument.
The instrument. was than exposed to zero. The
analyzer reported an immediate drop to less than 1~ of the
former readings within the first cycle. Figure 4
summarizes this test.
The results demonstrate conclusively that the
memory effect does not occur to any significant degree at
the loadings and cycle times used.
It is bel ieved that this is due to the following
factors: short adsorptio:n/desorption cycles; low mercury
loadings of :Less than 900pg; and incorporation of cleaning
cycles to purge the cartridges before any significant
operations start.