Note: Descriptions are shown in the official language in which they were submitted.
)93/06086 2 1 1 9 ~ 7 I Pcr/US92~0648s ~ `
--1--
RETINOIDS AND THEIR USE IN TREATING SKIN DISEASES AND LEUKEMIA
Technical Field
The present invention relates to novel retinoids,
methods for their preparation, pharmaceutical compositions
comprising such compounds and the use of the compounds in0 treating the skin and in treating leukemia.
Background Art
Caucasians who have had a good deal of sun exposure in
childhood will show the following gross cutaneous
alterations in adult life: wrinkling, leatheriness,
yellowing, looseness, roughness, dryness, mottling
(hyperpigmentation) and various premalignant growths (often
subclinical). These changes are most prominent in light
skinned persons who burn easily and tan poorly. The baleful
e~fects of sunlight are cumulative, increasing with time.
Although the anatomic degradation of the skin is most
advanced in the elderly, the destructive effects of
excessive ~un exposure are already evident by the second
decade. Serious microscopic alterations of the epidermis
and dermis occur decades before these become clinically
visible. Wrinkling, yellowing, leatheriness, and loss of
elasticity are very late changes.
It is known to use vitamin A acid for the treatment of
acne as set forth in U.S. Patent No. 3,729,568. Other known
uses of vitamin A acid which were reviewed by Thomas and
Doyle in the Journal of American Academy of Dermatoloay, 4,
505-513 (1981), include, in addition to acne treatment,
treatment of senile comedones, nervus comedonicus, linear
verrucous nevus, plantar warts, pseudofolliculitis,
keratoacanthoma, solar keratosis of extremities,
callosities, keratosis palmaris et plantaris, Darier's
disease, ichthyosis, psoriasis, acanthosis nigricians,
lichen planus, molluscum contagiosum, reactive perforating
collagenosis, melasma, corneal epithelial healing,
geographic tongue, Fox-Fordyce disease, cutaneous metastatic
melanoma and keloids or hypertrophic scars. Vitamin A acid
derivatives (retinoids) are known to have prophylactic and
--2--
therapeutic effects on a great variety of tumors and are
being increasingly used as anti-tumor drugs. The use of
oral retinoic acid in treating leukemia has been reported by
R. P. Warrell, Jr. et al., New England Journal of Medicine,
324, 1385-1393 (1991). Additionally, R.C. E~fland et al.,
in Published European Patent Application No. 043522A2 refer
to the use of certain aminopyridinylmethanols and
aminomethylpyridinamines as topical anti-inflammatory agents
for the treatment of various dermatoses, while R. J. Yu et
al. in U.S. Patent No. 4,141,977 discloses the use of
several 6-nicotinamides and/or 2-substituted pyrizinamides
as topical agents for the treatment of psoriasis.
Disclosure of the Invention
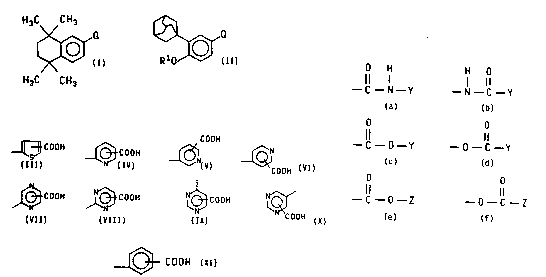
The present invention relates to a compound of the
formula:
r ~1
or a pharmaceutically acceptable salt or prodrug thereof,
wherein Rl is Cl to C8 alkyl and Q is selected from .
O H H O
Il I . 1 11
- 2 5 --C--N--Y --N--C--Y
,,_ O O
--C--O--Y --O--C--Y
O O
--C--O--Z and --O--C--Z
wherein Y is selected from
;
SUBSTITUTE SHEET
7 1
~ , .. . .... . .
COOH
~ C O O H ~ C O O H ~ ~;~
5 ~(~3 C O O H ~ C O O H N~ C O O H N~
and Z is selected from groups of the formula:
~ COOH
with the pharmaceutically acceptable salt and prodrug of the
formula I or II compound being derived from the aforesaid Y
and Z acid groups. These compounds are hereinafter also
referred to as the active compounds of the present
invention.
Preferred prodrugs of the present invention are
compounds wherein Z is selected from groups of the formula:
~ COOR2
and Y is selected from groups of the formula:
~ CO~R2 ~ ~ COOR
~ C O O R 2 ~ C O O R z $~C O O R Z a n d
wherein R2 is Cl to C8 alkyl, phenyl-CI to C8 alkyl or
substituted phenyl-CI to C8 alkyl wherein the substituted
phenyl may be substituted with one or two substituents
selected from Cl to C4 alkyl and halogen (i.e. fluoro,
chloro, bromo or iodo). Other prodrugs include compounds
SUB~;TITUTE SHEET
1 1 3 ~; ~ !I r
r r r
;
r
--4--
O O
wherein R2 is -CH-o-C-R4 or -CH2-NHC-oR4 wherein R3 is H, CH3,
l3
or C2H5 and R4 is Cl to C4 alkyl. Esters of alpha-amino
penicillins having such R2 groups are disclosed in United
States Patent 3,873,521.
The present invention also relates to the use of an
amount of a compound of the formula I or II, including a
pharmaceutically acceptable salt or prodrug thereof,
effective in moderating or retarding the effects of aging of
the skin and generally improving the quality of the skin or
in treating conditions of the skin selected from the group
consisting of psoriasis, acne and related disorders of
keratinization and epithelial differentiation, and skin
tumors.
The present invention also relates to a pharmaceutical
composition comprising a pharmaceutically acceptable carrier
and an amount of a compound .of the formula I or II,
including a pharmaceutically acceptable salt or prodrug
thereof, effective in moderating or retarding the effects of
aging of the skin and generally improving the quality of the
skin or in treating conditions of the skin selected from the
group consisting of psoriasis, acne and related disorders of
keratinization.and epithelial differentiation, and skin
tumors.
The present invention also relates to a pharmaceutical
com~osition useful for treating leukemia comprising a
pharmaceutically acceptable carrier and an amount of a
compound of the formula- I or II, including a
pharmaceutically acceptable salt or prodrug thereof,
effective in halting or ameliorating the symptoms of
leukemia. The composition is preferably administered as an
oral dosage form.
The present in~ention also relates to a method for
treating leukemia, which comprises administering to a
patient in need of such treatment an amount of a compound of
S~1BST~TU~: S~iE~
2 11~3 5 7 1
r
r r r
r
the formula I or II, including a pharmaceutically acceptable
salt or prodrug thereof, effective in halting or
ameliorating the symptoms of leukemia.
The following are preferred compounds of the present
5 invention:
4 - ( 5 , 6 , 7 , 8 - t e t r a h y d r o - 5 , 5 , 8 , 8 - t e t r a m e t h y l - 2 -
naphthoyloxy)-benzoic acid;
4- (5, 6, 7, 8-tetrahydro-5, 5, 8, 8-tetramethyl-2-
naphthyloxy-carbonyl)-benzoic acid;
6- (5, 6, 7, 8-tetrahydro-5, 5, 8, 8, -tetramethyl-2-
naphthoylamino)-nicotinic acid; and
6- [ 3- ( 1-adamantyl) -4-methoxybenzoylamino ] -nicotinic
acid .
Other specif ic compounds of the present invention are
the following:
3- (5, 6, 7, 8-tetrahydro-5, 5, 8, 8-tetramethyl-2-
naphthoyloxy)-benzoic acid;
2- ( 5, 6, 7, 8-tetrahydro-5, 5, 8, 8-tetramethyl-2 -
- naphthoyloxy)-benzoic acid;
6- (5, 6, 7, 8-tetrahydro-5, 5, 8, 8-tetramethyl-2-
naphthoyloxy)-nicotinic acid;
3- (5, 6, 7, 8-tetrahydro-5, 5, 8, 8-tetramethyl-2-
naphthyloxy-carbonyl)-benzoic acid;
2- (5, 6, 7, 8-tetrahydro-5, 5, 8, 8-tetramethyl-2-
naphthyloxy-carbonyl)-benzoic acid;
6- (5, 6, 7, 8-tetrahydro-5, 5, 8, 8-tetramethyl-2-
naphthyloxy-carbonyl ) -nicotinic acid;
5- ( S, 6, 7, 8-tetrahydro-5, 5, 8, 8 -tetramethyl-2 -
naphthoylamino)-thiophene-2-carboxylic acid;
5- (5, 6, 7, 8-tetrahydro-5, 5, 8, 8-tetramethyl-2-
naphthoylamino)-nicotinic acid;
6- (5, 6, 7, 8-tetrahydro-5, 5, 8, 8-tetramethyl-2-
naphthoylamino)-pyrazine-3-carboxylic acid;
2- (5, 6, 7, 8-tetrahydro-5, 5, 8, 8-tetramethyl-2-
naphthoylamino~-pyrimidine-5-carboxylic acid;
5-t3-(1-adamantyl)-4-methoxybenzoylamino~-thiophene-2-
carboxylic acid;
SU8STITUTE SHEET
S 7 ~
~ r ~
~ . r ~ ~ r r ~ . r , .
S-[3-(1-adamantyl)-4-methoxybenzoylamino]-nicotinic
acid; -
6-[3-(1-adamantyl)-4-methoxybenzoylamino]-pyrazine-3-
carboxylic acid; and
2-[3-(1-adamantyl)-4-methoxybenzoylamino]-pyrimidine-S-
carboxylic acid.
The present invention also relates to a compound of the
formula:
HzNJ~COOR2
wherein R2 is Cl to C8 alkyl, phenyl-CI to C8 alkyl or
substituted phenyl-CI to C8 alkyl wherein the substituted
phenyl may be substituted with one or two substituents
o
selected from C~ to C4 alkyl and halogen, -CH-o-C-R4 or
R3
-CH2-NHC-oR4 wherein R3 is H, CH3, or C2H5 and R4 is Cl to C4
alkyl, which is useful in preparing compounds of the
formulae I and II wherein Q is -~c--~ ~ coOQ2 wherein R2
is as defined above. Preferably R2 is C~ to C6 alkyl.
Detailed Description
The active compounds of the present invention may be
prepared as follows:
Amides are prepared by converting a dicarboxylic acid
monoester derivative of the formula H02C-K-C02R2 wherein K is
heteroarylene and R2 is as defined above to its acid chloride
and reacting the acid chloride with an amine of the formula:
SU8STITUTE SHET
7 1
r r
r ~ r ~ ~ ~ 1 ~ r
~ . r , ~ ~ .
~ r ~ ~ r
r ~ ~ I
~NHZ ~l"NHz
wherein R1 is C~ to C8 alkyl, or by reacting an amino acid
ester of the formula H2N-K-C02R2 wherein K is heteroarylene
and R2 is as defined above with an acid chloride of the
formula:
COCI ~COCI
wherein Rl is C~ to C8 alkyl, in an inert solvent such as
tetrahydrofuran and in the presence of a tertiary amine
base, such as triethylamine, at a ~emperature of from about
-20C to reflux, preferably at about 0C to room
temperature, to yield compounds of the formulae-
~NH-C--Y.--CO R2 [~l"NH-C--K--C02R2
~,IC--NH-K--COzR~ C--NH-K--CO2RZ
Suitable solvents include tetrahydrofuran,
diethylether, dimethylformamide or toluene. The preferred
sol~ent is tetrahydrofuran. These intermediate compounds
may then be saponified to the corresponding carboxylic acids
by treatment with a base such as aqueous sodium hydroxide in
a solvent such as tetrahydrofuran or ethanol to
5~J~35TlTUT 5H~El
, ~ r ~ i r ~ -
.. r
--8--
give am des of the formulae I and II wherein Q is -C-NH-Y
or -NH-B-Y wherein Y is defined as above.
Esters of the formulae I or II are prepared by
converting a dicarboxylic acid mono-benzylester of the
formula HO2C-M-CO2CH2-phenyl wherein M is phenylene or
heteroarylene to its acid chloride and reacting it with a
hydroxy compound of the formula:
~OH ~"OH
or by reacting a hydroxy-acid benzyl ester of the formula
HO-M-CO2CH2-phenyl wherein M is phenylene or heteroarylene
with an acid chloride of the formula:
~ or ~ COCI
in either case in an inert solvent in the presence of a
tertiary amine base, such as triethylamine, at a temperature
between about -20C to reflux, preferably at room
temperature, to yield intermediates of the formulae:
SUBSTITUTE SHEET
7 ~ ~ r ~ ~ ~ ~
. ~ ~ ~ ~ r ~ r r
g
~0~l--C02CH2--pheny I,
[~,0~--n~OzCH2~heny l,
~-0~1~02CH2--pheny I and
~-O-t1~02CH2--pheny I
Suitable solvents include tetrahydrofuran, diethylether,
dimethylformamide, methylene chloride or toluene. The
preferred solvent is tetrahydrofuran.
The intermediate compounds (which also can be
considered prodrugs) can then be hydrogenated, .using a
suitable catalyst such as palladium on carbon in an inert
solvent, such as ethyl acetate, at temperatures between
about 0 and about 50C and at a pressure between about 14
and about 50 psi to provide the final esters of formulae I
,_ O O , O
11 11 11
and II wherein Q is -COOY, -OC-Y, -C-O-Z or -O-C-Z, where
Y and Z are as defined above.~ Other prodrugs wherein R2 is
O O
-CH-o-C-R4 or -CH2-NHC-oR4 may be prepared from compounds of
R3
formulae I and II as outlined in U. S. Patent No. 3,873,521.
The compounds of the formulae I and II are carboxylic
4~ acids and form salts with bases. Examples of these salts
SUB~ T iTUTE SHEET
` ` ~ r
r r ~ ;
--10--
are the salts with alkali metals such as sodium and
potassium; or alkaline earth metals such as calcium, and
magnesium; the salts with ammonia; and the salts with
organic bases such as methylamine, ethylamine, diethylamine,
trimethylamine, triethylamine, pyridine, picoline, arginine,
lysine, triethanolamine, and meglumine. The salts are
prepared by reacting a compound of the formulae I or II with
1 equivalent of base in a suitable inert solvent.
The active compounds of the present invention are
useful in moderating and retarding the effects of aging of
the skin and generally improving the quality of the skin,
particularly huma~n facial skin, resulting in a more youthful
appearance. The compounds may be administered by topical
application beginning in middle age when aging changes first
become evident clinically. Certain of the anatomic
alterations may be corrected and at least partially
reversed, accompanied by improvement in the appearance of
the skin. More specffically, the compounds of the present
invention have the following advantages inter alia:
Clinical
Effacement of fine wrinkles
Smoother surface
Lighten pigmente~ blotches
Skin has more turgor
Large pores less noticeable
Skin feels livelier and tighter
Hi~oloaic
Thicker epidermis
Normalizes atypia and pre-malignant changes
Atrophy and dysplasia corrected
S~imulate blood flow; new vessels formed
Stimulate fibroblasts with collagen formation
Increase ground substance
Melanin with keratinocytes is decreased
The active compounds of the present invention suppress
the hyperkeratinization of human tissue cells and are useful
SUBSIITl~J~ SH~:ET
9 S ~7 1
r r ~ ~ r r
in the systemic and topical treatment of dermatological
ailments linked to a keratinization disorder
(differentiation proliferation) or epithelial
differentiation. They are also useful in treating
S dermatologic ailments with inflammatory and/or immuno-
allergic components (e.g., psoriasis and acne) and also have
anti-tumoral activity. These compounds can also be employed
in the treatment of cutaneous atrophy. These compounds are
also usefully employed in the field of ophthalmology and
principally in the treatment of corneopathy. Unless
otherwise indicated, treatment can be systemic or topical.
Treatment of leukemia is generally systemic.
The effectiveness of the active sompounds of the
present invention in retarding the effects of aging of the
skin and generally improving the quality cf the skin may be
evaluated in the rhino mouse model described below.
Three groups of mice, each having five mice are used.
One group receives test agent, one group receives vehicle,
and the third group is untreated. The mice used are female
rhino (age about 7 weeks). The dorsal surface of each
animal is treated with 100 ~1 of topical agents, once a day,
5 times a week for 4 weeks. - At the end of the treatment
period, animals are clinically assessed for effacement of
skin folds and wrinkles. Representative animals are
photographed with untreated controls.
For histologic evaluation, dorsal skin biopsies (strips
about 1 cm long) are processed for light microscopy.
Sections are evaluated on an ordinal scale for normalization
of the epidermis and utriculi (abnormal hair follicles~.
For the split skin assessment, a 2 cm2 portion of dorsal
skin is excised. Epidermis is separated from the dermis
with 0.5% acetic acid and processed for light microscopy and
microphotography. Utriculi are measured from the
photographs for numerical data (mean + S.D.).
The active compounds of this invention may be tested
according to the following procedùre which shows the
differentiation of malignant cells, whereby the
SUBSTITUTE SHEET
r ~ r ~ r
r ~ r
r ~ ~` ~
r
-12-
differentiation of human acute promyelocytic leukemia cells
(HL-60) and their conversion to mature granulocytes
(myelocytes) can be assayed by an observation of the
morphological changes of nuclei and further by the
measurement of the degree of reduction of nitro-blue
tetrazolium (NBT) which is induced by a test compound tProc.
Natl. Acad. Sci. U.S.A., 77, 2936-2940 (1980)~.
The HL-60 cells are cultured in plastic flasks in RPMI-
1640 medium supplemented with 5% heat inactivated fetal calf
seru~ and antibiotics (penicillin G and streptomycin). The
cells (3xlO4/ml) are then cultured with a compound of the
present invention for 4 days. Growth inhibition of the
cells by the test compounds is then determined by counting
the number of cells by microscope and relative ratio was
e:camined by taking the number of cells by control (without
test compoundj as 100%. The cells are fixed and stained
with Wright-Giemsa stain to examine the morphological
changes of the nuclei;
The cells treated with the present compounds are
differentiated -to mature granulocytes (myelocytes,
metamyelocytes and neutrophils), just as the cells treated
with retinoic acid. The biochemical activity of cells
treated with the compound is measured as follows:
The cells after 5 days incubation are centrifuged and
diluted with RPMI-1640 medium supplemented with 5% fetal
calf serum, to provide a definite number of the cells. To
the diluted cell suspension are then added 200 mg/ml of 12-
O-tetradodecanoylphorbol-13-acetate (TPA) and the resulting
culture medium is then incubated for 20 minutes at 370C in
the presence of 0.1% of NBT. Thus, the mature
differentiated cells containing blue-black formazan are
counted by microscopy, so that the ratio of the cells having
~he ability to reduce N8T, to total cells, can be
calculated.
The active compounds of the present invention can be
used for determining the type of leukemia a patient may have
by incubating a sample of a patient's blood in vitro in the
S~JBS I ITIITF C~
3 lj 7
r ~ r ~ ~ ~ ~ r
r ~ ~ ~ r
r
r
-13-
presence of a compound of the present invention in ananalogous manner as described in the morphological assay for
the HL-60 cells: Only promyelocytic leukemia cells, but no
lymphocytic leukemia cells, differentiate to mature
granulocytes, which can be clearly determined by
observation with a microscope [Sabo in Cells, 14, 533
~1982)~. When the incubation is performed in a soft agar,
promyelocytic leukemia cells do not form a colony, since the
differentiated cells do not proliferate further. Thus,
these compounds are very useful in the determination of
promyelocytic leukemia, which enables one to select the
appropriate therapeutic method.
The antileukemic activity of the active compounds of
the present invention may be demonstrated by testing for
their ability to treat HL-60 (human derived leukemia cells)
nude mice by the following procedure:
A test compound is suspended in 10% (v/v) Tween 80
(trademark) in a concentration of 10 mg/ml. Cells (sxlo7) of
HL-60 are transplanted subcutaneously to a nude mouse ~;
(BALB/c, nu/nu female Nihon Clea). At the end of days 9, 14
and 17 after the transplantation, 0.1 ml of the suspension
per 10 g of body weight of mouse are administered pQr os two
time5 at intervals of 7 hours (200 mg/kg/day). Tumor
volumes are measured after 4, 6, 8 and 11 days after the
first administration. Compounds effective against HL-60
cells are also expected to be effective against
neuroblastoma, squamous cells carcinoma, and melanoma.
The pharmaceutical compositions containing the active
compounds of the present invention as the main component are
formulated in a conventional manner, using conventional
carriers for formulation, including ~excipients. The
medicaments may be administered orally as tablets, pills,
capsules, granules, etc., or may be administered
parenterally as injections such as intravenous injections,
intramuscular injections, etc., in the form of ointments,
creams and the like for external application in particular
for the treatment of dermatological disorders. They may be
S~5~S J ~TUT~ SHE:;T
" ~ ' ,, r, ~ r
,. ~ r ~ ~ r
r ~ ~ r ~
r ~ r ~ .
1 4
used as aerosols, suppositories, etc. The doses of the
medicaments are properly determined according to each case
on considering the symptom, the age of patient, sex, etc.,
but are usually about 1 to about 300 mg per day for an adult
in case of oral administration and about 1 to about 100 mg
per day for an adult in case of parenteral administration,
the daily amount usually being administered in 2 or 3
separate dosages.
The active compounds of the present invention retard
the effects of normal aging of the skin due to impairment of
the differentiation of epidermal epithelial cells and due to
loss of collagen fibers, abnormal changes in the elastic
fibers and deterioration of small blood vessels of the
dermis of the skin. The compounds are applied topically to
the epidermis of the skin in a program of maintenance
therapy, whereby epithelial growths are substantially
reduced and prevented and the skin substantially regains and
maintains its firmness, turgor and elasticity during the
the~apy. Generally, the maintenance therapy is begun in
middle age when epithelial growths and other aging changes
begin to appear clinically. For topical application, the
active compounds of the present invention may be applied to
the skin in any suitable non-toxic, dermatologically
acceptable vehicle (preferably a non-volatile, emollient or
lub~icating vehicle, such as an oleaginous substance, which
helps hydrate the skin) in an amount and at a frequency
which are insufficient to cause excessive irritation of the
. _ .
skin. Generally, concentrations in the range of about
0.0005 to about 0.05% and pr ferably, from about 0.005~ to
about 0.025% by weight of the vehicle are preferred.
Various non-toxic, dermatologically acceptable vehicles
or carriers will be evident to those of ordinary skill in
- the art. Volatile vehicles which dry or otherwise harm the
skin, such as alcohol and acetone, should be avoided. An
ointment base (without water) is preferred in the winter and
in subjects with very dry skin. Examples of suitable
ointment bases are petrolatum, petrolatum plus volatile
SUBSTIT~JTE SHEET
7~
~ ,. . . . . . . . .
r
--15--
silicones, and lanolin. In warm weather and often for
younger persons, emulsion (cream) bases, which are mixtures
of oils and water are preferred. Examples of suitable cream
bases are Eucerin (trademark, Beiersdorf), cold cream (USP),
S Purpose Cream (trademark, Johnson ~ Johnson) and hydrophilic
ointment (USP).
The treatment according to the present invention
relating to aging of the skin is intended to continue
indefinitely; otherwise, the effects of aging may reappear
after treatment is terminated. That is, the treatment of
the present invention may be considered to be intervention
therapy in decelerating the aging process. If the
intervention is stopped, there may be regression to the
original state.
lS Usuall~, there is little point in beginning the
treatments of the present invention relating to aging until
middle age when the effects of aging begin to appear. The
particular program of maintenance therapy according to the
present invention will vary depending upon the individual
being treated. Generally, depending upon the age and state
of the skin when treatments begin, once a day applications
for up to 6 months may be necessary to reduce and control
the effects of aging which have already occurred. Once a
stabilized sXin control has been obtained, the frequency of
25- application of an active compound of the present invention
may be reduced, for example, to two or three times a week,
and in some cases only once a week for the rest of the
person's life. That is, once the aging process has been
controlled, a maintenance dose on the order of two
applications per week may be sufficient to maintain that
state.
SUB~ T IT~l E SHEE:T
7 ~
.. ... . . . .. . . . .
~' ~ ' ~ r r . ..
~ . ~ ~ ~ r
~ ~ r . I
-16-
EXAMPLE 1
4-(5,6 7,~-Tetrahydrc-5 5,8.8-tetramethyl-2-naphthoyl-
oxy)-benzoic acid
A 500 ml three-neck round-bottom flask equipped with a
stirrer and a condenser was charged with 5.0 g (0.0217 mole)
of 2-acetyl-5,6,7,8-tetrahydro-5,5,8,8,-tetramethyl-
naphthalene and 261.1 ml of a 3~ NaOCl solution ~149.1 ml of
5.25% NaOCl (Clorox) (trademark)], and combined with 112 ml
water and 4.7 ml of a 45% aqueous KOH solution. The mixture
was heated with an oil bath to reflux under stirring for 2
hours, allowed to cool to room temperature and extracted
with ethyl acetate. The aqueous layer was acidified with lN
HCl and the precipitated product was extracted with ethyl
acetate. This organic layer was then washed with water and
with saturated sodium chloride solution, dried over
anhydrous sodium sulfate and evaporated in vacuo to give,
after trituration with petroleum ether, 4.0 g (79%) of
5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthoic acid, mp
174-6C; MS: m/e, 232; NMR (CDCl3) consistent with product.
Anal.: Calcd. for C15H20O2: C, 75.55; H, 8.68; Found: C, 76.01;
H, 8.40.
A 50 ml three-neck round-bottom flas~ equipped with a
stirrer, a condenser and a nitrogen inlet tube was charged
with 2.15 g (0.00927 mole) of 5,6,7,8-tetrahydro-5,5,8,8-
tetramethyl-2-naphthoic acid (prepared as described above),
5 ml (8 g; 0.067 mole) of thionyl chloride and a catalytic
amount of pyridine. The resulting orange solution was
hea~ed at reflux for 2.5 hours, cvoled and evaporated in
vacuo to give the acid chloride as an orange-brown oil. A
250 ml three-neck round-bottom flask equipped with a
stirrer, condenser and a nitrogen inlet tube was charged
with a solution of 2.11 g (0.00925 mole) of benzyl 4-
hydroxybenzoate in 50 ml of dry tetrahydrofuran, cooled to
0C in an ice-bath, at which point 1.3 ml (0.934 g; 0.00g25
mole) of triethylamine and then the acid chloride prepared
above (slurried in 50 ml of tetrahydrofuran) were added.
The mixture was heated under stirring to 60C for 5 hours
5'~ 1 lT'~3~ S~
~ 9 ~ 7 ~
~, . ~, , .
.
--17--
and then kept at room temperature overnight. After
evaporation of the solvent in vacuo, the residue was taken
up in ethyl acetate/water, the organic layer was washed with
water, dried over anhydrous sodium sulfate and then
S evaporated in vacuo to a yellow solid. This solid was
dissolved in 30 ml of tetrahydrofuran, 10% Pd/C was added
and the mixture was hydrogenated at 10 psi on a Parr shaker
for 2.5 hours, and then hydrogenated with fresh catalyst for
another hour when TLC (thin layer chromatography) analysis
(ethyl acetate) indicated the reaction to be complete. The
catalyst was removed by filtration over diatomaceous earth
tCelite(trademark)] and washed with ethyl acetate, the
filtrate was evaporated in vacuo and the residual white
solid (2.6 g) recrystallized from absolute ethanol to give
1.39 g (43%) of 4-(5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-
naphthoyloxy)-benzoic acid as white crystals, mp > 230C;
MS: m/e, 352; NMR (DMS0-d6) consistent with product. Anal.
Calcd. for C22H24O4:;C, 74.98; H, 6.86. Found: C, 75.14; H,
6.69.
EXAMPLE 2
4-(5 6 7 8-TetrahYdro-5r5 8 8-tetramethyl-2-naphthyl-
ox~y-carbonvl)-benzoic acid
A 50 ml three-neck round bottom flask equipped with a
stirrer, a condenser and a nitrogen inlet tube was charged
with 0.4 g (O.OOlS6 mole) of terephthalic acid mono-benzyl
ester tprepared according to G. J. Atwell and B. F. Cain, in
J. Med. Chem., 10, 706 (1967)], l.lS ml (1.86 g; 0.0156
mole) of thionyl chloride and a catalytic amount of
pyridine. The mixture was heated at reflux for 2 hours.
The solution was then kept at 650C overnight, cooled and
evaporated in vacuo to give the acid chloride as a yellow
semi-solid. A 50 ml three-neck round-bottom flask equipped
with a stirrer, condenser and a nitrogen inlet tube was
charged with a slurry of 0.305g (O.OOlS mole) of S,6,7,8-
tetrahydro-5,5,8,8-tetramethyl-2-naphthol ~prepared
according to H.A. Bruson and J.W. Kroeger, J. Am. Chem.
Soc~., 62, 36 (1940)] in 25 ml of dry tetrahydrofuran, 0.207
5~ 1T~JTE SHEET
3 7 i~
r r
r
-18-
ml (0.15 g; 0.0015 mole) of triethylamine and 0.410 g
(0.0015 mole) of the acid chloride prepared above. The
mixture was heated under stirring to reflux for 2 hours and
then kept at 50OC overnight, at which point TLC analysis
(hexane/ethyl acetate 1:1) indicated that the reaction was
complete. After evaporation of the solvent in vacuo, the
residue was taken up in ethyl acetate/water, the organic
layer was washed with water, dried over anhydrous sodium
sulfate and evaporated in vacuo to give, after trituration
with hexane, 230 mg (35%) of a white solid; MS: m/e, 442;
NMR (DMSO-d6) consistent with product. This solid was
dissolved in 30 ml of ethyl acetate, 10~ Pd/C was added and
the mixture was hydrogenated at 40 psi on a Parr shaker for
4 hours when TLC analysis (hexane/ethyl acetate 1:1)
indicated that the reaction was complete. The catalyst was
removed by filtration over diatomaceous earth ~Celite
(trademark)] and washed with ethyl acetate, the filtrate was
evaporated in vacuo and the residual white solid triturated
with hexane to give 85 mg t46%) of the title compound as
white crystals, mp > 2-20C; MS: m/e, 3S2; NMR (DMS0-~)
consistent with product; Anal. Calcd. for CnH24O2: C, 74.98;
H, 6.86. Found: C, 74.50; ~, 6.59.
EXAMPLE 3
6-(5 6 7 8-Tetrahydro-5 5 8 8-tetramethyl-2-na~hthoyl-
amino)-nicotinic acid
A 500 ml three-neck round-bottom flask equipped with a
stirrer and a condenser was charged with 11.81 g (0.085
mo~é) of 6-aminonicotinic acid and 60 m~ of thionyl
chloride; 5 ml of pyridine were then added and the mixture
was refluxed under stirring for l hour. After evaporation
in vacuo, the residual orange-brown oil was cooled to ooc
and carefully treated with 50 ml of methanol. After the
slight exotherm subsided, the yellow solution was evaporated
ia vacuo and the residue was taken up in ethyl
acetate/water. The organic layer was washed with aqueous
sodium bicarbonate solution and water, dried over anhydrous
sodium sulfate and evaporated in yacuo to give, after
~STIT~JTE S~.~EET
7 1
.. . . . ..
r r r
'
--19--
crystallization of the residue from chloroform/hexane, 8.71
g (65%) of methyl 6-aminonicotinate, mp 153-155C; MS: m/e,
152; NMR (CDCl3j consistent with product.
A 500 ml three-neck round-bottom flask equipped with a
stirrer and a condenser was charged with 7.67 g (0.049 mole)
of methyl 6-aminonicotinate (prepared as described above)
and 150 ml of tetrahydrofuran; 6.81 ml (4.96 g; 0.049 mole)
of triethylamine and 12.29 g (0.049 mole) of 5,6,7,8-
tetrahydro-5,5,8,8-tetramethyl-2-naphthoylchloride(prepared
as described in Example 1) were then added and the mixture
stirred at room temperature overnight. After evaporation in
vacuo, the residue was partitioned between water and ethyl
acetate, the organic layer was washed with water, dried over
anhydrous sodium sulfate and evaporated in vacuo. The
residue was purified by column chromatography on silica,
using hexane/ethyl acetate as the eluant. The major LP spot
wa~ collected to give a tan solid [NMR (CDCl3) consistent
with the methyl es~er of the desired product]. This
material was dissoived in 50 ml of tetrahydrofuran, 50 ml of
2N NaOH was added and the mixture was stirred at room
- temperature overnight. After partial evaporation ln vacuo
to remove the organic solvent, the aqueous solution was
acidified to pH 4 with 6N HCl to give a cream-colored
precipitate, which was filtered and recrystallized from
ethanol to afford 6.32 g (37%) of the title compound as
white crystals, mp > 240C; MS: m/e, 352; NMR (DMSO-d6)
consistent with product. Anal. Calcd. for ~H~N2O3: C,
71.56; H, 6.86; N, 7.95. Found: C, 71.24; H, 7.11; N, 7.87.
EXAMPLE 4
6-r3-(1-AdamantYl)-4-methoxv-benzoylamino1-nicotinic
acid
A 150 mL three-neck round-bottom flask equipped with a
stirrer and a condenser was charged with 250 mg (0.0016
mole) of methyl 6-aminonicotinate (prepared as described in
Example 3) and 15 mL of tetrahydrofuran; 0.23 mL (0.16 g;
0.0016 mole) of triethylamine and 0.5 g (0.0016 mole) of 3-
~1-adamantyl)-4-methoxybenzoyl c~loride (prepared according
5~33~1TlJIE S~?FT
,~
a, ~
~ ~ ~ . . .. . .
~ ~ r ~ ' ' '
-20-
to the method of B. Shroot et al., as described in U.S.
Patent No. 4,927,928) were then added and the mixture
stirred at room temperature overnight. After evaporation in
vacuo, the residue was partitioned between water and ethyl
acetate, the organic layer was washed with water and brine,
dried over anhydrous and evaporated in vacuo. The residue
was purified by column chromatography on silica, using
hexane/ethyl acetate as the eluant. The major and less
lipophilic spot was collected to give a tan solid tNMR
(CDCl3) consistent with methyl ester of the desired product].
This material was dissolved in 5 mL of tetrahydrofuran, 3 mL
of 2N aqueous NaOH were added and the mixture was stirred at
room temperature overnight. After partial evaporation n
vacuo to remove the organic solvent, the aqueous solution
was acidified to pH 4 with 6N HCl to give a white
precipitate, which was filtered and recrystallized from
ethanol to afford 203 mg (31%) of the title compound as
white crystals, mp >250C; MS: m/e, 406; NMR (DMSO-d6)
consistent with product. Anal. Calcd. for C24H26N2O4-H2O: C,
67.91; H, 6.65; N, 6.60. Found: C, 67.81; H, 6.82; N,
6.43.
EXAMPLE 5
The compounds of Examples 1-3 were tested in the rhino
mouse model hereinbefore described at a concentration of
2S 0.1%. All of the compounds improved the appearance of the
skin.
SUBSTITUTF SHEET