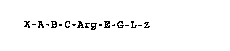Note: Descriptions are shown in the official language in which they were submitted.
~ ~ ` 21203~2
, ~ Merck Patent Gesellschaft
.,~
_ mit be~chrankter Haftung
,~ 6100 D a r m ~ t a d t
. .
., Linear adhe~i~o~ inhibitor~
The invention relate~ to novel linear peptides o~
- the formula I
X-A-B C-Arg-E-G-L-æ I,
- in which
: X i8 ~, acyl havi~g 1-10 C ato~m~, H-Asn, H-Val-A~n,
- 10 ~-A~p-Val-Asn, Fmoc-Gly-Gly, H-Lys-Gly-Gly,
~-Ly~-Pro, H-~yr-Gly-Gly, ~-Cy~-Gly-Gly,
I H-Cys(Trt)-Gly-Gly, H-Cys-Gly-Gly-Thr-Asp-Val-A~n
., or ~-Thr-Asp-Val-A~n,
A, B
" 15 and C ln each case independently of o~e a~other are
~` ab~ent or ar~ an amino acid radical, selected
.
from a group consiating of Ala, Arg, Asn, Asp,
~ A~p(OR), Cy~, Gln, Glu, Gly, Hi~, Ile, Leu, Ly~,
.1 Lys(Ac), Ly~AcNH~ y~(AcSH), Met, Orn, Ph2,
:, 20 4-Hal-Phe, Pro, Ser, Thr, Trp, Tyr or Val, where
', the amino acid radicals m~ntioned can al~o be
derivatizPd,
E i~ Gly, ~i~ or Leu-~
G i~ absent or i~ A~p or A~n,
~ 25 ~ i8 absent or i8 Gly, Ile, ~eu or ~eu-Leu,
'`. Z i~ ~2 0~ OE~
Hal iR F, Cl, Br or I ~nd
l Ac i~ alka~oyl having 1-10 C atoms,
-1l and their phy~iologically acceptable ~alt~.
Similar compounds are ~own, ~or example, from
1 European Patent Application EP 0 406 428.
.:l The obj eGt of the inve~tion wa~ to find no~l
:~ compounds ha~ing u~eful properties, i~ particular thos~
-, which can be u~ed fGr the productio~ of medicament~.
-~ 35 It ha~ bee~ found that the compounds of the
,~ formula I and th~ir salt2 have very u~e~ul propertie~. In
~l~ particular, they act aa integrin inh~bitor~, in which
ca~e they particularly lnhibit the in~eraction~ of ~3-
i~tegrin receptors with ligand~. This acti~n can be
- 2 _ 2 ~ 2 0 3 Q 2
demon~trat~d, ~or example, by the method which i~
de~cribed by J. W. Smith et al. in ~. Biol. Chem. 265,
12267-12271 (1990). In add.ttion, there ar~ a~ti-
jiinfli~mmiatory ef~ects. This action can al~o be
5 demonstrated with the aid of method~ which are ~nown from
the literature.
The compounds can be employed aa pharmaceutical
active compoundn in human and vetler~nary medicine, i~
particular for the prophylaxie aIld the treatment of
di~orders of the circulation, in thrombo~is, cardiac
infarct, arterioscleso~iA, inflammation~, apoplexy,
angina pectoris, tumours, onteolytic di~orders, in
particular osteoporosi~, angiogene~i~ and re~teno~i~
after angiopla~ty. They cian also be u ed i~ a ~upportive
role in wound-heali~g proce~se~.
The compounds are also suitable as antimicrobial agents
which avoid infections as they were caused for example
by bacteria, ~ungi or yeasts. The substances are useful
~as accompanying antimicrobial agents in cases where
¦20 operations are effected in order to insert non-corporal
materials, for example such as biomaterials, implants,
catheters or heart-pacemakers. They act as antiseptics.
j , .:
Z
':1
.!
212~302
. .
~ 2a -
''
.,
The abbre~iation~ o~ amino acid radicals shown
above and below stand ~or the radical~ of the followi~g
amino acid~:
,
Ala alanine
Arg arginine
A3n a~paragi~e
- Asp a~partic acid
- Asp(OR) a~partic acid ~~e~ter~
.~ Cit citrullin~
. .
., 10 Cy~ cy~tei~e
. D~b 2,4-diaminobutyric acid
Gln glutami~e
Glu glutamis acid
l ~ly glycine
`, 15 Hi~ hi~tidine
Ile isoleucine
Leu leucine
:,
~, Ly~ lysine
Ly~(Ac1 N'-acylly~i~e ~:
I~y8 (~cNH2) N'~ oaayllyBine
Ly~ (AcSH) N'-mercaptoacylly~i~e
-, Met methionine
,i Or~ orn~ thiIle
Phe pl-erlylala~ e
.
''
ii
- ` 2~2~3~2
: - 3 -
.:;
4-Hal-Phe 4-halophenylalanine
Pro proline
Ser ssrine
Thr threonine
5 Trp tryptophan
Tyr tyrosine
Val. valine.
In addition, the following have the meanings below:
BOC tert-butoxycarbonyl
:. 10 CBZ benzyloxycarbonyl
~, DCCI dicyclohexylcarbcdiimide
., DMF dimethylformamide
EDCI N-ethyl-N'- (3-dimethylaminopropyl)-
~: carbodiimide hydrvchloride
15 Et ethyl
j FMOC 9-fluore~ylmetho~ycarbo~yl
.. ¦ HOBt 1-hydroxybenzotriazole
~e me~hyl
~3~A 4-methylbenzhyd~ylamine
Mtr 4-methoxy-2,3,6-trimethylphenyl~ulfonyl
OBut tert-butyl e~ter
~Me methyl e~ter
"'............... OEt 0thyl e~ter
,1 POA ph~noxyac0tyl
25 TFA trifluoroaaetic aaid
,~ Trt trityl (triphenylmethyl).
If the amino acida me~tio~ed above can occur in
~everal enantio~eric fOrmB, the~ all the~e ~orm~ a~d al80
their mixtures (e.g. the DL-form~) are i~cluded above and
¦ 30 below, ~.g. as con~tituent~ of the compou~d~ of the
formula I. The amino acid~ a~d/or the amino acid radicals
~, can al~o be derivatized i~ a ~orm k~own per ~e.
The invention further relates to a process ~or
the preparation of a compound of the formula I according
~' 35 to Claim 1 or of one of it~ ~alt~, characterized in that
it i~ liberated ~rom one o~ it~ functional derivatives by
treating with ~ ~olvoly~ing o~ hydrogenolysing agent,
! or in that a peptide of the ~ormula II
.~
.
:;~
212~3~2
M-OH II
in which
: M ia (a) X, but not hydrogen, ~hnsc-Gly, ~_LYB_G1Y,
H-Ly~ Tyr-Gly, H-A~p Val, H-Tyr, H-Val,
H-Asp, ~-Cy~-Gly-Gly-Thr-A~p-Val, ~ CYR G1Y
Gly-Thr-A~p, H-Cys-Gly-Gly-Thr, ~-Cys-Gly-Gly,
-~ H-Cys-Gly, H-Cy~, ~-Cys(l'rt)-Gly, H-Cya(Trt~,
H-Thr-Asp-Val, ~-Thr-Asp or ~-Thr,
-i (b) X-A,
' (c) X-A-B,
~d) X-A-B-C,
.. (e) X-A-B-C-Arg,
(f) X-A-B-C-Arg-E or
(g) X-A-B-C-Arg-E-G
,
.
10 iB reacted with a~ ami~o co~pound of the form~la III
.,
`. ~-Q-Z III
~'
in which Z ha~ the mea~i~g indicat~d and
Q i8 (a) A-B-C-Ar~-E-G-L, Asn-A-B-C-Arg-E-G-L
Val-Asn-A-B-C-Arg-E-G-L, Pro-A-B-C-Arg-E-G-L,
Gly-A-B-C-Arg-E-G-L, Gly-Gly-A-B-C-Arg-E-G-L,
Asn-A-B-C-Arg-E-G-L, Val-Asn-A-B-C-Arg-E-G-L
Asp-Val-Asn-A-B-C-Arg-E-G-L,
~ Thr-Asp-Val-Asn-A-B-C-Arg-E-G-L, Gly-Thr-Asp-Val-
Asn-A-B-C-Arg-E-G-L or Gly-Gly Thr-Asp-Val-Asn-
A-B-C-ALg-E-G-L,
(
~.. . . -.. .. .. .. .. ..... . ... ..
~ ` 2~2~302
~` - 5 -
(b) B-C-Arq-E-G-L,
. (c) C-Arg-E-G--L,
(d) Arg-E-G-L,
. (e) E-G-L,
(f~ G-L or
~:. (g) L
... .
j3 and/or in that a free amino group i~ optionally acylated
and/or a compound of the for~ula I :is converted into one
of it~ ~alt8 by treating with an acid or a base.
The radical~ A, B, C, E, ~, L, M, Q, X and Z
above and below have the meanings indicated in the
formulae I, II and III, if not expressly stated
~ otherwise.
.', I~ the abo~e formulae, alkyl i~ preferably
methyl, ethyl, isopropyl or tert-butyl.
X is preferably hydrogen, ~-Asn, Fmoc-Gly-Gly or
Cy~(~rt)-Gly-Gly, but particularly preferably ~ CYB
~l Gly-Gly. In addition, X can al~o be acyl having 1-10
C atom~, acyl preferably being al~anoyl having 1-8, in
particular 1, 2, 3 or 4 C atom~, specifically preferably
~¦ 15 formyl, acetyl, propio~yl, butyryl, i30butyryl, pentznoyl
~, or hex~noyl, but al80 aryl-CO, ~uch a~ e.g. benzoyl, o-,
m- or p-toluyl, o-, m- or p-methoxyben~oyl and al~o 2-
naphthoyl or else aryl-CnHl~-CO ~n = 1-4), such aa e.g.
-I Ph-C~2-Co, Ph(C~3)2-co, Ph-(C~2)3Co or Ph-(C~)~-CO
: 20 (Ph = phe~yl), where Ph ca~ also be sub~tituted by an
OCX3 or C~3 group.
The group A is pr~erably Ala, Leu or Ser, in
particular Gly, or else i8 not present. B i8 preferably
A~n or A~p. C i~ pre~rab1y Ala, al~o D-Ala, Hi~ and in
~, 25 particular Gly. ~ i~ preferably Gly or ~is. G i~
preferably Asp, while L i~ preferably ~eu. Z is N~2, but
particularly preferably OH.
~ Accordingly, the invention in particular relates
i to thoae co~pound~ o the formula I in which at least one
of the said radicals ha~ one of the preferred meanings
indicated above.
A preferred group o~ ~iompo~nda ~an be expres~ed
'I
.,
:~
2~2~302
-- 6
by the formula Ia, which corresponds to the formula I and
in which ~, C, E, X and Z have the meaning~ indicated
, there and
A i~ Gly,
~, 5 G i~ A~p and
t, L is Leu.
A ~urther group of preferred compound~ can be
expres~ed by the part ~ormulae Iaa to Iad, which
otherwise corre~pond to the formula I or Ia, but in which
in Iaa: X i8 hydrogen or acety.l
A is Gly,
B i~ Asn or A~p,
C i~ ab~ent, i~ Ala or Gly,
E is Gly or Hi8,
G is A8p,
~,
L is Leu and
Z i~ NH2 or OH,
. .
in Iab: X ia hydrogen or acyl,
. A is absent or i~ Gly,
`~ 20 B i~ absent or i8 A~n,
~ C is Gly,
., E i~ Gly or
G is A8p,
L is Leu and
Z i~ N~2 or OH,
~, i~ Iac: X i8 hydrogen,
A i8 ab~ent or i~ Gly,
B i~ absent or i~ A~n,
C i8 absent or i~ Gly,
~ i~ Gly or ~i 8,
G i~ A~p,
L is Leu and
Z i~ OH or NH2
'
2~203~2
: - 7 -
in Iad X is H-Asn, H-Val-Asn, H-Asp-Val-Asn, F~oc-
Gly-Gly, H-Lys-Gly-Gly, H-Tyr-Gly-Gly,
- ~I-Cys(Trt~-Gly-Gly, H-Cys-Gly-Gly, H-Cys-
Gly-Gly-Thx-Asp-Val-Asn or H-Thr-Asp-
; Val-Asn,
. .
A is Gly,
; B i~ Asn,
tJ C ia Gly,
E i~ Gly or ~i 8,
~, G i~ Asp,
'~ L is Leu and
'-' Z iR N~2 or O~.
Particularly suitable compounds are tho~e which
corre~pond to th~ formula I and in which A, B, C, X and
Z have the aboveme~tioned preferred meaning~, but in
~1 which the central amino acid radical i8 L-Arg, not D-Arg,
`'' E i~ Gly and al~o ~ia, not D-Ri~, ~ i8 A~p and L i~ Leu.
l The compound~ of the formula X and also the
~, 15 ~tarting material~ for their preparatio~ are otherwi~e
prepared by know~ methods, as are de~cribed in the
literature (e.g. i~ th~ standard wor~s such as
Houben-Weyl, Methoden der organischen Ch~mie, (Me~hodR of
Organic Chemistry) ~eorg-Thieme-Verlag, Stuttgart), in
particular under reaGti.o~ condition~ which are known and
~uitable for the ~aid reaction~. I~ thin context, u3e ca~
~, also be made of known variant~ which are not me~tioned in
i more detail her~.
The starti~g substances ca~ al~o be formed in
aitu, if desired, ~uch that they are not i~olat~d ~rom
.the reaction mixture, but immediately react~d ~urther ~o
give the compound~ o~ the form~la I.
~i The compound~ o~ ths ~o~mula I can be obtained by
liberati~g t~em rom their functional deri~ative~ by
'~ 30 801voly8i~ in particular h~drolyais, or by
hydrogenoly~ia.
Pre~erred starting material~ for the 801~01y8i~
, or hydrogenoly~is ara tho~e which contain appropriate
protected amino and/or ~ydro~yl group~ instead of ona or
~'
~'''; . : . . `
2~203~2
. - - 8 -
more free ami~o and/or hydroxyl groups, preferably tho~e
which carry an amino protecting group in tead o~ an H
:. atom which i~ bonded to an N atom, e.g. those which
correspond to the ormula I, but contain an NHR' group
(in which R' is an amino protecting group, e.g. BOC or
CBZ) in6tead of an N~a group.
In addition, starting mat~rials are preferred
~ which carry a hydroxyl protecting group in~tead o~ the
~:~ H atom of a hydroxyl group, e.g. thos~ which corre,spond
to the formula I, but contain a~ R''O-phe~yl group (in
. which R'' i~ a hydroxyl protecting group~ inst~ad of a
hydroxyphenyl group.
Several - identical or different - protected
amino and/or hydroxyl group,~ can ba pre,sent i~ the
molecule of the starting material. If the protsctive
groupR pre~e~t are different from one another, in many
ca,~es they can be rQmoved electively.
The expre,~6ion "amino protecting group" i~
`: generally known and relate,s to group,s which are suitable
for protecti~g (for blocki~g) an amino group from chemi-
cal reactions, but which are easily removable, after th~
de6ired chemical reactio~ has been carried out at other
po,sitions in the molecule. ~ypical groups of t~is type
are, in particular, un,Yubstituted or ,sub,~tituted acyl,
aryl, aralkoxymethyl or aralkyl groups. ~ the ami~o
, protecting group,s are r~moved a ter the de,sired reaction
, (or reaction sequencej, their nature and 8iZP i8
`' otherwise not critical; but tho,~e ha~ing 1-20, in
'! particular 1-8, C atom~ are pref erred . The expre,~sion
"acyl groupn is to be ta~en in it8 wide~t sen~e in
. .,
connection with the preuent process and the preRent
-i compound6. It includes acyl group~, derived from
aliphatic, araliphat~c, aromatic or heterocyclic
~ carboxylic acid~ or sulfonic acids and i~ particular
.-1 35 alkoxycarbonyl, aryloxycarbonyl a~d, in particular,
;¦ aralkoxycarbonyl group~. ~xample~ of acyl group~ of this
~r~ type are alkanoyl such as acetyl, propio~yl or butyryl;
~-l aralkanoyl ~uch a~ phe~ylac~tyl; aroyl ~uch aB b~nzoyl or
toluyl; aryloxyalkanoyl ~uch a~ POA; alkoxycarbonyl ~uch
21203~
g
as msthoxycarbonyl, ethoxycarbonyl, 2,2,2-trichloro-
ethoxycarbonyl, BOC, 2-iodoethoxycarbonyl; aralkyloxy-
carbonyl ~uch a~ Q Z (ncarbobenzoxyn), 4-met~o~ybenzyl-
oxycarbonyl, FMOC, and aryl~ul~onyl ~uch a~ Mtr.
Preferred amino protecting groups arle BOC and Mtr, and in
.addition CBZ, FMOC, benzyl and ~cety~l.
. Th0 expre~sion "hydroxy protecting group" i8 al~o
generally known and relate~ to group~ whiah are suitable
for protecting a hydroxyl group from ch~mical reactions,
but which are ea~ily remov~ble, after tha de~ired chemi-
.~cal reaction has bean carried out at other po~itionB in
- the molecule. Typical group~ of th~ type are the above-
mentio~ed unsubstituted or ~ub~tituted aryl, aralkyl or
acyl group~, and in additio~ alao alkyl group~. The
nature and ~ize of the hydroxy protecting groups i~ not
critical, a~ they are removed again after the desired
chemical reaction or reaction sequence; pre~erred groups
are those having 1-20, in particular 1-10 C atoms.
Example~ of hydroxyl protectin~ group~ are, inter alia,
~ 20 benzyl, p-nitrobenzoyl, p-toluene~ulfonyl and acetyl,
- benzyl and acetyl being particularly preferred. The COO~
. group~ in aspartic acid and glutamic acid are preferably
protected in the form of their tert-butyl e8te28 (e.g.
A~p (OBut)).
The functional derivative~ of the c~mpounds of
the formula I to be used as 3tarti~g materials can be
, prepared by cu~tomary method~ o~ ami~o acid a~d peptide
i synthe~ia, ~uch as are deDcribed e.g. in the ~aid
standard works and patent applicatio~, a~d e.g. also by
the Merrifield ~olid pha~e method (B.~. Gy~in a~d R.8.
. Merrifield, J. Am. Chem. Soc. 94, 3~02 et seq. (1972)).
The liberation o f the compounds of the ~ormula I
from their functional derivatiYes i~ carried out
. depending on the protecti~g group used - e.g. with stro~g
35 acid~, preferably with TFA or perchloric acid, but alao
with other atrong inorganic acids auch a~ hydrochloric
acid or ~ul~uric acid, or ~trong organic carboxylic acida
auch as trichloroacetic acld or ~ul~onic acids such a~
ben~ene- or p-tolueneaulfo~ic acld. The presence o~ an
.;` ~ ,~: . : '. ' , . ' `
`:. ` ' . :
` 21203~2
- 10 -
.
addit~onal inert solvent i~ possible, but not alway~
necesRary. Suitable inert 601vent~ are preferably
organic, for example carboxylic acid~ ~uch as ac~tic
acid, ethers ~uch a~ tetrahydrofuran or dioxane, amides
such as DMF, halogenated hydrocarbonn ~uch a~ dichloro-
methane, and in addit~o~ al~o alcohol~ auch a~ methanol,
ethanol or isopropanol a~d al~o watar. ~n addition,
mixtures o~ the abovementioned solvent~ are ~uitable. TFA
is preferably used in an exce~ w.ithout addition o~ a
-I 10 further solv~nt, perchloric acid in the for~ of a mixture
of acetic acid and 70 % perchloric acid i~ ~he ratio 9:1.
The reaction temperature~ for ths cleavage are
expediently between about O and about 50, preferably
between 15 and 30 (room t~perature).
The group~ BOC, OBut a~d Mtr ca~ be remo~ed e.g.
preferably u~ing TFA i~ dichloromethane or uqing about 3
to 5 N ~Cl in dioxane at 15-30, the FMOC group using an
about 5- to 50 % 801ution of dimethylami~e, diethylamine
or piperidine in DMF at 15-30.
Protecti~g groups which can be removed by hydro-
genolysi~ (e.g. CBZ or be~zyl) can be removed, e.g. by
treating with hydrogen i~ the presence of a catalyQt
(e.g. a noble metal cataly~t ~uch a~ palladium, preferab-
ly on a carrier such a~ carbon). Suitable sol~entR in
thi~ ca~e are tho~e i~dicated above, in particular e.g.
~, alcohol ~uch a3 methanol or ethanol or amide~ ~uch a~
DMF. The hydrogenoly~i~ i carried out, a8 a rule, at
i temperatures betwee~ about O and 1~0 and pressures
¦ between about 1 and 200 bar, preferably at 20-30 and 1-
10 bar. Hydrogenoly~i~ of the CBZ group is ea~ily carried
I out e.g. vn 5 to 10 % Pd-C i~ methanol or u~i~g ammonium
¦ formate ~instead Of ~2) on Pd-C in methanol/DMF at
20-30.
~, Compounds of the formula I can also be obtained
;j 35 by reaction of a compound of the formula II under
conde~sing condi'cions knowIl per ~e for peptide syr~these~,
i, a~ are de~cribed e . g . i~ :E~ouben-~eyl, loc cit . volume
15/II, pages 1 to 806 (1974).
The reactio~ 3 pre~erably carried out irl the
i
2~ 20~02
- 11
presence of a dehydrating agent, e.g. a carbodiimide ~uch
~ as DCCI or EDCI, and in addition propanepho~phonic
'~ anhydride (compare A~gew. Ch~m. 92, 129 (1980)), diphen-
ylphosphoryl azide or 2-athoxy-N-ethoxycarbonyl-1,2-di-
hydroquinoline, in an inert ~olvent, 2 . g . a haloge~ated
hydrocarbon Ruch as dichloromethane, a~ ether such aa
- tetrahydrofuran or dioxane, an ~nide such a~ DMF or
- dimet~ylacetamlde, a nitrile such ac acetonitrile, or i~
mixtures of these ~olvents, at temperature~ between about
-10 and 40, preferably between O and 30.
Instead of II, ~uitable reactive derivatives of
these sub~tances can also be employed in the reaation,
e.g. tho~e in which reactive groups are intermediately
blocked by protecti~g groups. The anino acid deri~-
ativ~ II can be u3ed e.g. in the form of their activated
ester~ which are expediently formed in situ, e.g. by
s addition o~ HOBt or N-hydroxy~uccinimide.
I The starting materials of the formula II are, a~
a rule, novel. They can be prepared by known methods,
e.g. the abo~ementioned method~ of peptide synthe~i~ and
of removal of protective groups.
a rule, protected peptide esters of the
formula R'-M'-OR'' are i~itially ~ynthesized, where M'
,
~or~e~pond~ to the radical ~ reduced at the N-ter~inal
i25 end by an ~ atom, e.g. BOC-M'-OMe or ~moc-M'-OMe. These
`¦are hydrolysed to a~ids of the fo~mula R'-M'-O~, e.g.
BOC-M'-O~ or Fmoc-N'-O~ and then condensed with a
compound of the formula III, which i8 optionally likewi~e
provided with corre~ponding protective groups at
po~itions which should ~ot be acces~ible to the reaction.
In the case of compoundR of the formula III,
~peptide e~ters of the formula R'-Q-~'-R'', such a~ e.g.
-,BOC-Q-Z'-OMe or Fmoc-Q-Z'-OMe~ are li~ewi~e ~ynthesized,
wh0re Z' i~ -NH- or -O- and then, before the condensation
3~ for the preparation of compound~ of the formula I is
carxied out, the protecti~e group R~ i8 cleaved in a
known manner, e.g. Fmoc by treatment with a
piperidine/DMF ~olution.
Particularly advantageou~ly, the m3re recent
. . .
: -
:,., : :
,.. .: ~ : , , , ~ ;
2l2n3~2
12 -
methods of peptide ~ynthesis according to modifi~d
~errifield technique6 and u~ing peptide ~ynthe~i~
apparatus a~ i8 described e.g. in Peptide~, Proc. 8th Am.
Pept. Symp., ~da. V. Hruby and D.H. Rich, ~ierce Comp.
III, pp. 73-77 (1983) by A. Jonczyk and ~. Meienho~er
(Fmoc ~trategy) or the techniqu88 presented in Angew.
Chem. 104, 375-391 (1992) can be u~ed. Method3 of thiQ
type are known per se and their description at thi~ poi~t
i8 therefore unnecessary.
A ba~e of the formula I can be co~verted into the
appropriate acid addition salt u~ing an acid. Suitable
acid~ for thi~ reaction are in particular tho~e which
yield phy~iologically acceptable ~alt~. Inorganic acids
can thu~ be used, e.g. ~ulfuria acid, nitric acid,
15 hydrohalic acid~ such as hydrochloric acid or hydrobromic
acid, phosphoric acids such as orthophosphoric acid and
~ul~amic acid, a~d in addition organic acid~, in par-
ticular aliphatic, alicyclic, araliphatic, aromatic or
heterocyclic mono- or polyba~ic carboxylic, ~ul~o~ic or
20 ~ulfuric acida, e.g. formic acid, acetic acid, propionic
acid, pivalic acid, diethylacetic acid, malonic acid,
succinic acid, pimelic acid, fumaric acid, maleic acid,
j lactic acid, tartaric acid, malic acid, benzoic acid,
-i salicyl c acid, 2- or 3-phe~ylpropio~ic acid, citric
25 acid, gluconic a~id, a~corbic acid, ni~otinic acid,
¦ i~onicotinic acid, methane- or ethane3ul0nic acid,
' ethanedisulfonic acid, 2-hydroxyethane~ulfonic acid,
i benze~esulfonic acid, p-toluenesulfonic acid, naphthal-
ene-mono- and -disulfonic acids, and lauryl~ul~uric acid.
30 Sal 8 with phyaiologically unacceptable acids, e.g.
, , picrates, can be u~ed for the isolation and/or purifica-
i tion of the compound6 o the fo~mula I.
~ On the other hand, an acid of the formula I can
`¦ be converted into one of it~ physiologically acceptable
~ 35 metal or ammonium salt~ by reaction with a ba~e. Suitable
i salts here are in partiaular the ~odium, potassium,
I mag~esium, calcium and a~monium ~alts, and al~o
~ub~tituted ammonium ~alt~, e.g. the dimathyl-, diethyl-
or dii~opropylammonium 8alt8, monoethanol-, dietha~ol- or
`!
..;~ . .. .. . . . . . . . .. . . .
~ 21203~2
~ ~ - 13 -
,, .
triethanolammonium salt~, cyclohexyl- or dicyclohexyl-
ammonium ~al~s, dibenzylethylenediammonium ~alt~, and
; furth0rmore e.g. salt~ with N-methyl-D-glucamine or ~ith
arginine or lysine.
: .
5In addition, the novel compound~ o~ the formula
I can be used as integrin ligands for the preparation of
'. column8 for affinity chromatography for the preparation
o~ integrins in pure form.
The ligand, i.e. a peptidle derivative o~ the
formula I, i~ in thi~ case covalently coupled to a
polymeric ~upport via anchor ~unctions.
, Suitable pol~meric support materials are the
;' polymeric ~olid pha~e~ known per ~e ~n peptide chemistry,
having preferably hydrophilic propertiea, for example
cro~linked poly~ugar~, ~uch a~ cellulose, Sepharose or
Sephadex , acryla~ides, polymer~ baaed on polyethyle~e
glycol or Tentakel polym2rs .
Suitable a~chor f~ction~ which are linked to the
polymeric supports are preferably linear alkylene chains
~¦20 having 2-12 C atoms, which are bonded directly to the
polymer at one end and have a functional group, auch a~
e.g. hydroxyl, amino, ~ercapto, maleimido or -COOE at the
'~other end and are suitable to be linked to the C- or N-
terminal ~ectio~ of the respective peptide.
l 25It is po~ le i~ this ca~e that the peptid~ be
¦ bonded directly or likewi~e via a ~econd anchor functio~
~ to the a~chor o~ the polymer. It i8 also po~sible that
i peptide~ which contain amino acid rad~cals with
functionalized ~ide chain~ are bo~ded to the a~chor
¦3 0 fu~ction of the polymer via the~e.
;Moreover, certain amino acid radicals which are
a con~tituent of the peptides o~ the formula I can b~
modi~ied in their ide chain~ in ~uch a way that they are
available for anchorage via e.g. SH~ 0~, NH2 or C00
,35 groups with the anchor o~ the polymer.
jIn khi~ ~on~ection, unusual amino acids are
po~ible, ~uch as ~.g. phenylalani~e derivative~ which
carry a mercapto, hydroxyl, amino or aarboxyalkyl chain
in position 4 o~ the phenyl ring, the ~unctio~al group
.~
-1
.
~ . 2120302
:. '
- 14 -
. being located at the end o~ the chain.
Example~ of amino acid radical~ whose side chaln
can be used directly as an anchor function are e.g. Lys,
Orn, Arg, Dab, A~p, A~, Glu, ~ln, Ser, Thr, Cys, Cit ox
Tyr.
Example~ of N-terminal an~hor~ are radicals, such
as e.g. -co-cnH2~-NH~ -CO-CnHln-OH, -CO-C~H2~-SH or
-CO-C~H~-COOH, where n = 2-12, the le~gth of the alkylene
chain not being critical and it optionally al~o being
po~sible to replace this partially or completely e.g. by
appropriate aryl or alkylaryl radicals.
C-termi~al anchors can be, for example, -O-C~H~n-
S~l ~ CD~2n - Ox ~ - O - Cn}~SI - N~2 ~ - - C~2~, - COO~I ~ -NH-Cn~I2n-SH,
; -NH-CnH2n-O~, -NH-C~2~ or -N~-C~a2~-COO~, w~at has
already been ~aid in the preceding ~ection applying to n
and also to the alkylene chain.
'~ The N- and C-terminal anchors can al80 be uRed as
anchor componen~s for an already functionalized side
chain of an amino acid radical. Suitable amino acid
radicals here, for example, ara those Ruch as Lys(CO-
. CsHlo-N~z)~ Asp(N~-C3~6-COO~ or Cy8 (C3H6-NH2), the anchor
;, always being bonded to the ~u~ctio~al group of the side
~' chain.
~he preparation of the material~ for affinity
, 25 chromatography i~ carried out u~der condition~ such as
i are customary Por the condensation of ami~o acids and are
knowm per se and have already been outlined in the
~ ~ection for the preparation of the compou~d~ of the
-. formula I, or are described i~ Pierce, Immunotechnology
Catalog & Handbook (1990)).
The no~el compound3 of the formula I and their
physiologically acceptable alts can be u~ed ~or the
production o pharmaceutical pre~aration~ by bringing
them into a suit~ble dosage form together with at least
one excipient or auxiliary a~d, if desired, together with
one or more other active compound (8) . The preparations
~ thus obtained c~n be employed a0 medicament~ in human or
:, ~eterina~y medici~e. Suitable exclpient ~u~tance~ are
-, organic or inorganic ~ubsta~ce~ which are ~uit:able or
. ,
212~302
- 15 -
enteral (e.g. oral or rectal), parenteral ~e.g.
intravenous injsction) or local (e.g. topiaal, derma7,
ophthalmic or naeal) adminiatration or ~or adminiutratisn
in the ~orm of an inhalant spray and which do not react
with the novel compound~, ~or example water or aqueou~
isotonic ~aline solution, lower alcohol~, vegetable oils~
benzyl alcohol~, polyethylene glycols, glycerol
triacetate and other fatty acid glyceride~, gelatin, soya
lecithin, carb~hydrates such as lactose or starch,
~O m~gneaium stearate, talc, cellulose and petroleum jelly.
Tablets, coated tablets, capsules, ~yrups, ~uice~ or
drops, in particular, are u~ed for ~ral administration;
film tablets and capsule~ having enteric coating~ or
capsule hells are especially of interest. Suppo~itorie~
are used for rectal admini~tration, and solution~,
preferably oily or aqueou~ ~olutions, and in addition
suspen~ion~, emulsiona or implant~, ~re u~ed for
parenteral administration. Solutions, e.g., which can be
used in tha form o~ eye drops, and in addition, e.g.
~20 suspeu~ion~, emul~ions, creams, oin~ment~ or compresse~
iare suitable for topical application. Spray~ can be u~ed
which co~tai~ the active compound either di~sol~ed or
su~pended in a propellant ga~ or propellant gas mixture
(e.g. CO2 or chloro~luorohydrocarbons) for admini~tration
a~ inhala~ spray~. The acti~e compou~d hera i~
expediently used in micronized form, it b~ing possi~l~
for one or more additional phy~iologically tolsrable
801~ent to be present, eng. ethanol. Inhalant solutions
ca~ be administered with the aid of cu~to~ary inhalers.
The novel compound~ ca~ al~o be lyophilized and the
lyophilizates obtained used e.g. ~or the productio~ of
injection preparation~0 The injections can be
administered a~ a bolus or as a continuous infusio~ (e.g.
intra~enous, intramuscular, subcutaneou~ or intxathecal).
The preparations indicated can be sterilized and/or ca~
contain auxiliaries ~uch as pre~ervatives, stabilizers
and/or wetting agentB, emul~i~iers, ~alts for in~luencing
o~motic pressure, buffer ~ubstance~, colorantQ and/or
flavouring~. If de~ired, they can al~o contain one or
. . .
- 2~20302
- 16 -
more other active compound~, e.g. one or more vitamins.
The ~ub~tance~ ac~ording to th~ inv~ntion aan A~
a rule be administ~red i~ analogy to other kn~wn
; commercially available peptidas, but i~ particular i~
analogy to the compound~ descxibed in US-A 4,472,305,
preferably in doaages between about 0.05 and 500 mg, in
particular between 0.5 and 100 mg, per do~ag~ u~i~. The
daily dose i~ preferably between ~out 0.~1 and 2 mg/kg
of body weight. The ~pecific do~e ~or each intended
patient depends, howe~er, on ma~y different factors, ~or
example the activity of the ~pecific co~pound employ~d,
: the age, body weight, general nta~e o~ health, ~ex, the
diet, the time and route of admi~istration, and the rate
o~ excretion, pharmaceutical combi~ation and Beverity of
-15 the particular disorder to which the thsrapy applie~.
Parenteral ad~iniatration is preferred.
~11 temperature~ above and below are stated in
C. In the following example~, ~cu3tomary working up"
mean3: water i~ added, if nece~ary, the ~ixture i8
neutralized a~d extract~d with ether or dichloromethane,
.the orga~ic pha~e i~ separated off, dried over 30dium
~ulfat2, ~iltered and evaporated and the ra~idue i~
purified by chromatography on silica g~l and/or crystal-
lizatio~. RT = ret~ntion tim~ (mi~ute~3 for ~PLC o~ a
Lichrosorb RP 3elect B (250-4.7 ~m) column, eluent:
0.3 % TFA in water; i~oprop~nol gradient from 0-80 % by
vol. in 50 min at 1 ~l/~i~. Flow and d~tection at 215 nm.
M~ = molecular pea~ in the ma8~ ~pectrum, obtained by the
fast atom bombard~ent method (FAB~, a~ a rul2 i M~
i.e. the ma~ of the particular c~mpound increased by
1 ma~ u~it.
2.2 g o~ BOC-Asp-~ly-OH are di~aolved in a
mixture o~ 150 ml of di~hloromethane and 20 ml of DMF,
- 35 cooled to 0 and the~ treated with 0.5 y o~ DCCI, 0.3 g
of ~OBt, 0.23 ml of N-methylmorpholine a~d 1 equivalent
of ~-Arg-~i~-A~p Leu-OMe lboth peptides ara obtainable
according to modified Merrifield tecbnique method~]. Th~
-............... .. ~ .,,
` 2~20302
~ - 17 -
reaction mixt~re 1~ etirred for 20 hour,~ at 0 a~d ~or
6 hours at room temperature. It i~ conce~trate,d, treated
with a mixed bed ion exchanger and added to an aqueous
- NaHCO3 solution. The product which deposit~ ~ filtered
off with suction and wa~he,d with water. Washing with
ethyl acetate~petroleum ether giveE, BOC-Asp-Gly-Arg-~is-
Ae,p -Leu-OMe.
! The ~ollowing ar~ obtai~ed analogou~ly by
conde~,ation of H-Arg-~is-A~p-Leu-OMe
'! 10 with BOC-Gly-OH:
BOC-Gly-Arg-His-Asp-Leu-OMe;
with BOC-Asn-Gly-Asp-Gly-OH:
~OC-Asn-Gly-Asp-Gly-Arg-His-Asp-Leu-OMe,
:.
with BOC-Val-Asn-Gly-Asp-Gly-OH:
BOC-Val-Asn-Gly-Asp-Gly-Arg-His-Asp-Leu-OMe;
with BOC-Asp-Val-Asn-Gly-Asp-Gly-OH:
BOC-Asp-Val-Asn-Gly-Asp-Gly-Arg-His-Asp-Leu-OMe
with BOC Lys-Gly-Gly-Gly-Asp-Gly-OH:
BOC-Lys-Gly-Gly-Gly-Asp-Gly Ar~-His-Asp-Leu-OMe;
15 with BOC-Tyr-Gly-Gly-Gly-Asp-Gly-OH:
BOC-Tyr-Gly-Gly-Gly-Asp-Gly-Arg-His-Asp-Leu-OMe;
with BOC-Ala-Asp-Gly-OH:
BOC-Ala-Asp-Gly-Arg-His-Asp-Leu-OMe;
with BOC-D-Ala-Asp-Gly-OH:
BOC-D-Ala-Asp-Gly-Arg-His-Asp-~eu-OMe;
with BOC-Gly-Asp-~la-OH:
BOC-Gly-Asp-Al2-Arg-His-Asp-Leu-OMei
.,~: . : ~ ... :: .
... . :
, ,. . : - : : ~
.:; . . : :: .- -
- . 2~20302
with BOC-Gly-D-Asp-Gly-OH:
BOC-Gly-D-Asp-Gly-Arg-His-Asp-Leu-OMe;
with BOC-Gly-Asp-D-Ala-OH:
BOC-Gly-Asp-D-Ala-Arg-His-Asp-Leu-OMe;
with BOC-Gly-Asp-OH:
~ BOC-Gly-Asp-Arg-His-Asp-Leu-OMe;
'I with BOC-Cys~Trt)-Gly-Gly-Gly-As~-O:~:
BOC-Cys(Trt)-Gly-Gly-Gly-As~ s-.. s~-~eu-OMe;
,
with BOC-Cys-Gly-Gly-Gly-Asp-OH:
BOC-Cys-Gly-Gly-Gly-Asp-Arg-His-Asp Leu-OMe;
.,
with BOC-Cys(Trt)-Gly-Gly-Gly-Asp-Gly-OH:
BOC-Cys(Trt)-Gly-Gly-Gly-Asp-Gly-Arg-His-Asp-Leu-
' OMe;
~ith BOC-Cys-Gly-Gly-Gly-Asp-Gly-OH:
BOC-Cys-Gly-Gly-Gly-Asp-Gly-Arg-His-Asp-Leu-OMe;
with BOC-Cys-Gly-Gly-Thr-Asp-Val-Asn-Gly-Asp-Gly-OH:
BOC-Cys-Gly-Gly-Thr-Asp-Val-Asn-Gly-Asp-Gly-Arg-His-
Asp-Leu OMe;
,~
with BOC-Thr-Asp-Val-Asn-Gly-Asp-Gly-O~:
~ BOC-Thr-Asp-Val-Asn-Gly-Asp-Gly-Ars-His-Asp-1eu-
`~ OMe.
.~
19 ~xa~ 2
¦ The followi~g are o~tained analogou~ly to
:i,
.~
` ~ 2120302
., 1 g
~ Example 1 by reaction of BOC-Gly-A~p-Gly-O~
',~
with H-Axg-His-Asp-OMe:
BOC-Gly-Asp-Gly-Arg-His-Asp-OMe;
with H-Arg-His-OMe:
BOC-5~-Asp-Gl~-A s-.~is-OM_
with H-Arg-His-Asn-Leu-OMe:
BOC-Gly-Asp-Gly-Arg-His-Asn-Leu-OMe;
~,
~, 5 with H-Arg-Gly-Asp-Leu-OMe:
:1 BOC-Gly-Asp-Gly-Arq-Gly-Asp-Leu-OMe;
:
with H-Arg-His-D-Asp-Leu-OMe:
. BOC-Gly-Asp-Gly-Arg-His-D-Asp-Leu-OMe;
with H-Arg-D-His-Asp-Le-u-OMe:
, BOC-Gly-Asp-Gly-Arg-D-His-Asp-Leu-OMe;
'";1
-i with H-D-Arg-His-Asp-Leu-OMe:
~ BOC-Gly-Asp-Gly-D-Arg-His-Asp-Leu-OMe.
'^I
'.' The following are obtained analog~usly to
li 10 Example 1 by raaction oE 30C-Gly-Asn-Gly-O~
i with H-Arg-His-Asn-Leu-OMe:
' BOC-Gly-Asn-Gly-Arg-His-Asn-Leu-OMe;
with H-Arg-His-Asp-Leu-OMe:
:j BOC-Gly-Asn-Gly-Arg-His-Asp-Leu-OMe.
i
' ~ J,
2~20302
- 2~ -
. Rxam~l~ 3
; The following are obtai~ed analo~ou~ly to
~xample 1 by co~den#ation of:
BOC-Leu-Asp-His-Arg-OH with H-Gly-Asp-Gly-O~t:
BOC-Leu-Asp-His-ALg-Gly-Asp-Gly-OEt;
.,
'
BOC-Lys-Gly-Gly-Gly-Asp-Arg-Leu-OH with H-His-Asp-Gly-OEt:
BOC-Lys-Gly-Gly-Gly-Asp-Arg-Leu-His-Asp-Gly-OE~;
BOC-Lys-Pro-Ser-Asp-OH with H-Gly-Arg-Gly-OEt:
BOC-Lys-Pro-Ser-Asp-Gly-Arg-Gly-OEt;
BOC-Arg-His-OH ~nth H-Asp-Leu-OMe:
: BOC-Arg-His-Asp-Leu-OMe;
BOC-D-Lys-D-Pro-D-Ser-D-Asp D-Gly-OH with H-D-Arg-D-Gly-OMe:
BOC-D-Lys-D-Pro-D-Ser-D-Asp-D-Gly-D-Arg-D-Gly-OMe;
.
..~ BOC-Leu-Asp-His-OH ~nth ~-Arg-Gly-Asp-OMe:
BOC-Leu-Asp-His-Arg-Gly-Asp-OMe;
~I BOC Leu-Asp-His-OH with H-Arg-Gly-OMe:
BOC-Leu-Asp-His-Arg-Gly-OMe;
., .
-~ BOC-Gly-Arg-His-OH with H-Asp-Leù-Leu-OMe:
Jl BOC-Gly-Arg-His-Asp-Leu-Leu-OMe;
BOC-Arg-Gly-OH with H-Asp-Leu-OMe:
BOC-Arg-Gly-Asp-Leu-OMe;
:Z
212~3~2
- 21 -
BOC-Cys-Gly-Gly-Gly-Asp-Arg-OH with H-Leu His-Gly-OMe:
BOC-Cys-Gly-Gly-Gly-Asp-Arg-Leu-His-Gly-OMei
~'1
':1
BOC-Cys-Gly-Gly-Gly-Asp-Gly-Arg-OH with H-His-Asp-Ile-OMe:
"J,BOC-Cys-Gly-Gly-Gly-Asp-Gly-Arg-His-Asp-Ile-OMe.
"~
...
~ample ~
1.3 g of BOC-A~p-Gly-Arg~ -Asp-~eu-OMe are
dissolved in 60 ml of methanol, treated with 1.5 ml of
2 N NaO~ nolution and stirred for 4 hours at ~5. After
j5 remo~al o~ the ~olvent, the re~idue i~ ta~e~ up in water,
;the p~ i8 adjusted to 3 by the addition of dilute ~Cl and
the mixture i8 extracted ~lth ethyl acetate. The extract
'i8 dried over Na2SO4. Removal of the solvent gi~es BOC-
r;~iA~p-Gly-Arg-Ein-A~p-Leu-O~, which i~ take~ up in 20 ml of
?lo 2 N HCl in dioxane a~d atirred ~or 2 hours at room
temperature. The rea~tion mix ure iB concentrated to
.1~dry~e~ and the re~idu~ purified by RPLC. ~-A~p-~ly-Arg-
Hi~-Asp-Leu-OH is obtained; ~T = 9.5; M~ 712.
,The ~ollowing ar~ obtained analogoualy by removal
,~15 of the protective groups, starting from the compound~
.~
from Example 1:
H-Gly-Arg-His-Asp-Leu-OH; RT = 9.2; M+ 597;
::j
H-Asn-Gly-Asp-Gly-Arg-His-Asp-Leu-OH; RT = 11.3; M~ 883;
~1H-Val-Asn-Gly-Asp-Gly-Arg-His-Asp-Leu-OH; RT = 12 9; M+ 982;
1
:1,
~ 2120302
- 22 -
H-Asp-Val-Asn-Gly-Asp-Gly-Arg-His-Asp-Leu-OH; RT = 13,4;
M+ 1097;
H-Lys-Gly-Gly-Gly-Asp-Gly-Arg-His-Asp-Leu-OH; RT = 11.5;
M+ 1011;
H-Tyr-Gly-Gly-Gly-Asp-Gly-Arg-His-Asp-Leu-OH; RT = 13,8;
M+ 1046;
H-Ala-Asp-Gly-Arg-His-Asp-Leu-OH; RT = 10,7i M+ 783;
H-D-Ala-Asp-Gly-Arg-His-Asp-Leu-OH; RT = 11,1; M+ 783;
.
H-Gly-Asp-Ala-Arg-His-Asp-Leu-OHi RT = 11,0; M+ 78~;
~'',
' H-Gly-D-Asp-Gly-Arg-His-Asp-Leu-OH; RT = 10,5; M+ 770;
.
! H-Gly-Asp-D-Ala-Arg-His-Asp--Leu-OH; RT = 11,9; M+ 78q;
, H-Gly-Asp-Arg-His Asp-Leu-OH; RT = 8,1; M+ 712;
; H-Cys~Trt)-Gly-Gly-Gly-Asp-Arg-His-~sp-Le~-OH; RT = 27,7;
M+ 1171
1~
;
~ , . . . .
21~3~2
- 23 -
H-Cys-Gly-Gly-Gly-Asp-Arg-His-A~?-Leu-OH; RT = 11.7;
M+ 929;
.
H-Cys(Trt)-Gly-Gly-Gly-Asp-Gly-Arg-:lis--Asp-~eu-OH; R~ = 27
M+ 1228;
.
H-Cys-Gly-Gly-Gly-Asp-Gly-Arg-His~ p-Leu-OH; RT = 11,7;
M+ 987;
. H-Cys-Gly-Gly-Thr-Asp-Val-Asn-Gly-.rsp-Gly-Arg-His-Asp-Leu-OH;
RT = 12.7; M+ 1415;
H-Thr-Asp-Val-Asn-Gly-Asp-Gly-Arg-.Jis-Asp-Leu-OH.
B~a~pl~ 5
The following are obtained analogouRly to
Example 4 by hydrolysi~ and removal of the BOC protective
group~, starting fro~ the compou~d~ ~rom ~xampl3 2:
H-Gly-Asp-Gly-Arg-His-Asp-OH; RT = 3.5; M+ 656;
H-Gly-Asp-Gly-Arg-His-OH; RT = 3.5; Mi 541;
H Gly-Asp-Gly-Arg-His-Asn-Leu-OHi RT = 11.2; M+ 769i
H-Gly-Asp-Gly-Arg-Gly-Asp-Leu-OH; RT = 11,4; M+ 689;
H-Gly-Asp-Gly-Arg-His-3-Asp-Leu-OH; RT = 11.7; M+ 769;
H-Gly-Asp-Gly-Arg-D-His-Asp-Leu~OHi RT = 11 3; M~ 769;
~ 212~3~2
- 24 -
H-Gly-Asp-Gly-D-Arg-His-Asp-Leu-OH; RT = 10,9; M+ 769.
. H-Gly-Asn-Gly-Arg~His-Asn-Leu-OHi RT = 10, 8i M+ 767;
H-Gly-Asn-Gly-Arg-His-Asp-Leu-OHi RT - ll. 6i M+ 768 .
., .
. .~;
-~ Ea~gple 6
. The following are obtai~led analogou~ly to
.'. Example 4 star~ing from the co~pound~ from ~xample 3 by
hyd~olysis and removal of the BOC protective groups:
H-Leu-Asp-His-Arg-Gly-Asp-Gly-OH; RT = ll.1; M+ 769;
., .
.j H-Lys-Gly-Gly-Gly-Asp-Arg-Leu-His-Asp-Gly-OHi RT = 9 8i
' M+ 1011;
., .
~ H-Lys-Pro-Ser-Asp-Gly-Arg-Gly-OHi RT = 3.5i M+ 71 6i
: 1 :
.' H-Arg-His-Asp-Leu-OH; RT = 8,0; M+ 590i
,.j, :
,l H-D-Lys-D-Pro-D-Ser-D-Asp-D-Gly-D-Arq-D-Gly-OH; RT = 24. 7;
M+ 938i
~ 1 .
i~ H Leu-Asp-His-Arg-Gly-Asp-OHi RT = 11. 5; M+ 712;
l' H-Leu-Asp-His-Arg-Gly-OH; RT = 3.9; M+ 597;
. '
-.1 H-Gly-Arg-His-Asp-Leu-Leu-OH; RT = 15.3; M+ 710i
,':lj
:, H-Arg-Gly-Asp-Leu-OHi RT = 7.0; M+ 460i
i: -
i ;!
, ................................ .
`:~
2~2~3~2
~ 25 -
; H-Cys-Gly-Gly-Gly-Asp-Arg-Leu-His-Gly-OH; RT = 7.2; M+ ~71;
H-Cys-Gly-Gly-Gly-Asp-Gly-Arq-His-Asp-Ile-OH; RT = 15.1;
M+ 986.
Lxample 7
0.9 g of ~-Arg-~is-A~p-Leu-OR i~ dissolved in
200 ml of aqueou~ DMF and treated dropwi~e with stirring
with 0.5 g o~ acetyl chloride, di~o}ved i~ 10 ml of
- 5 dichloromethane. The reaction mixture i8 ~tirrsd for
15 minute~ and ~trongly concentrat0d. The produ¢t which
deposits i~ separated off. ~3C-CO-Axg-~i~-Aap-Leu-OR i~
obtaineds RT = 12.4; k~ 582.
The followi~g are obtained by acetylation
of H-Gly-Asp-Gly-Arg-His-OH:
H3C-CO-Gly-Asp-Gly-Arg-His-OH; ~ :
::
of
H-Arg-Gly-Asp-Leu-OH:
H3C-CO-Arg-Gly-Asp-Leu-O~; RT = 13.0; M+ 502;
. of H-Lys-Pro-Ser-Asp-Gly-Arg-Gly-OH:
H3C-CO-Lys-Pro-Ser-Asp-Gly-Arg-Gly-OH
~ H-Arg-His-Asp-Leu-OH:
I H3C-CO-Arg-His-Asp-Leu-OH;
i of H-D-Lys-D-Pro-D-Ser-D~Asp-D-Gly-D-Arg-D~Gly-OH:
H3C-CO-D-Lys-D-Pro-D-Ser-D-Asp-D-Gly-D-Arg-D-Gly-OH;
- . . :: . ~ : . :: . : : :
` 2~203~2
- 26 -
,,
of H-Leu-Asp-~is-Arg-Gly-Asp-OH:
H3C-CO-Leu-Asp-His-Arg-Gly-Asp-O.H;
. .
of H-Leu-Asp-His-Arg-Gly-OH:
H3C-CO-Leu-Asp-His-Arg-Gly-OH.
.
~x~ple 8
Analogously to Example 1, condensation o~ E3C-CO-
j 5 Gly-A~p-Gly-Arg-Hi.F-OH with H-A~p-Leu-OMe and sub~et~ui~t
,~1 hydrolysii~ giYes H3C-CO-Gly-A~-~p-Gly-Arg-~is-Asp-hteu~
~ RT = 1401; ~ 811.
. . ~
ample 9
2.0 g of BOC-Gly-Arg-HiR-Asp-Leu-OMi3 are stirred
, 10 for two hours in 25 ml of 4 N hydrochloric acid in
'i dioxane. The reaction mixture is the~ conce~trated, the
residue i& dissolved in 100 ml of DMF and the solutio~ i8
j cooled to 0. 1 equivalent of Fmoc-Gly-Gly-Gly-A~p~OH,
1.3 g of TBT~ and 1.0 ml of triethylami~e are th~ added
succe~sively. The solution i~ ~tirr~d for 2 hour~ at 0
~, and for 12 hours at room temperatur~. After fre~h
.~, concentration, the concentrate i8 poured into an Na~CO3
3i solutio~. The product which depo~its dur~ng the cour~e o~
this i~ ~iltered o~f and dissolved in 50 ml o~ m~thanol,
and the solution i6 treated with 1.5 ml of 2 N NaO~
~olution, stirred for 4 hours at 25 a~d wor~ed up i~ th~
I cu~tomary manner. F~oc-Gly-Gly-Gly-AQp-Gly-Arg-Ei~-Asp-
;~ leu-O~ iFi obtained;
RT = 28.0; Mr 1105.
The following are obtained analogoueily by
co~di-n~iation of H-Arg-Hi~-A6p-Leu-O~e
, wit~
s Fmoc-Gly-OH:
Fmoc-Gly-Arg-His-Asp-Leu-OH;
,A
212~3~2
- 27 -
with Fmoc-Asn-Gly-Asp-Gly-oH:
~` Fmoc-Asn-Gly-Asp-Gly-Arg-His-Asp-Leu-OH;
with Fmoc-val~Asn-Gly-Asp-Gly-oH:
Fmoc-Val-Asn-Gly-Asp-Gly-Arg-His--Asp-Leu-OH;
with Fmoc-Asp-~al-Asn-Gly-Asp-Gly-OH:
' Fmoc-Asp-Val-Asn-Gly-Asp-Gly-Arg--His-Asp-Leu-OH
with Fmoc-Lys-Gly-Gly-Gly-Asp-Gly-OH:
- Fmoc-Lys-Gly-Gly-Gly-Asp-Gly-Arg-His-Asp-Leu-OH;
with Fmoc-Tyr-Gly-Gly-Gly-Asp-Gly-OH:
Fmoc-Tyr-Gly-Gly-Gly-Asp-Gly-Arg-His-Asp-Leu-OH;
With Fmoc-Ala-Asp-Gly-oH
; Fmoc-Ala-Asp-Gly-Arg-His-Asp-Leu-OH;
with Fmoc-D-Ala-Asp-Gly OH:
Fmoc-D-Ala-Asp-Gly-Arg-His-Asp-Leu-OH;
Fmoc-Gly-Asp-Ala-OH:
Fmoc-Gly-Asp-Ala-Arg-His-Asp-Leu-OH;
;~ with Fmoc-Gly-D-Asp-Gly-oH
Fmoc-Gly-D-Asp-Gly-Arg-His-Asp-Leu-OH;
with Fmoc-~ly-Asp-D Ala O
Fmoc-Gly-Asp-D-Ala-Arg-His-Asp-Leu-OH;
' Fmoc-Gly-Asp-OH:
' Fmoc-Gly-Asp-Arg-His-Asp-Leu-OH;
:,
2120302
~, - 28 -
.
with
.J Fmoc-Cys~Trt)-Gly-Gly-Gly-Asp-OH:
~ Fmoc-Cys(Trt)-Gly-Gly-Gly-Asp-Arg-His-Asp-Leu-OH;
.~ .
Fmoc-Cys-Gly-Gly-Gly-Asp-OH:
~ Fmoc-Cys-Gly-Gly-Gly-Asp-Arg-~: s-Asp-Leu-OH;
.'i
~1 Fmoc-Cys~Trt)-Gly-Gly-Gly-Asp-Gly-OH:
,¦ Fmoc-Cys(Trt)-Gly-Gly-Gly-Asp-Gly-AIg-His-Asp-Leu-OH;
3 with Fmoc-Cys-Gly-Gly-Gly-Asp-Gly-OH:
.,~ Fmoc-Cys-Gly-Gly-Gly-Asp-Gly-Arg-His-Asp-Leu-OH;
-, 5 with Fmoc-Cys-Gly-Gly-Thr-Asp-Val-Asn-Gly-Asp-Gly-OH:
3 Fmoc-Cys-Gly-Gly-Thr-Asp-Val-Asn-Gly-Asp-Gly-Arg-His-Asp-
Leu-OH;
.~ with Fmoc-Thr-Asp-Val-Asn-Gly-Asp-Gly-OH:
Fmoc-Thr-Asp-Val-Asn-Gly-Asp-Gly-Arg-His-Asp-Leu-OH.
I
.~ Eb~a~l~ 10
007 g of BOC-Gly-Asp-Gly-Arg(BOC) -~18-A9p-L~u-O~
3 i~ dissolved in 100 ml o~ dichloromethane, treated with
:' 10 1.4 ~qui~al~nts of ~BHA r~in and stirred for 24 hours at
~, room tempera~ure. Removal of tha ~olve~t gi~e~ BOC-Gly-
A~p-Gly-Arg~BOC)-Hi~-A~p-~eu-MBEL~ re~in, whi~h i~ ta~e~
up in 20 ml of 2 M ~Cl in dioxane and stirred for 2 hours
at room temperature. Subsequent treatme~t ~ith TFA yields
15 H-Gly-A8p-Gly-Arg-Hi~-A8p-Leu-NH2; RT = 8 . 8; ~ 768 .
The following peptide amide~ are obtainad
analogou~ly by reaction with MBHA resi~:
i
ro~ BOC-Arg~BOC)-His-Asp-Leu-OH:
H-Arg-His-Asp-Leu-NH2; RT = 5.4; M~ 539;
2120302
29 -
from BOC-Arg~BOC)-Gly-Asp-Leu-OH:
,~
/H-Arg-Gly-As~-Le~-NH2; RT = 4,7; M+ 459;
, .
'.~
~ from BOC-Gly-Asp-Gly-Arg(BOC)-Gly-Asp-Leu-OH:
,,~H--Gly-Asp-Gly-Arg-Gly-As~-Leu-N~i2.
,.. .
,Analogously to Example 7, starti~g fro~ ~-Gly-
:.:
Asp-Gly-Arg-~i~-Asp-~eu-NH2, H3C-CO-Gly-Aap-Gly-Arg-~is-
Bp-heU-N~ iB obtained by a~etylatio~ of the peptide; RT
= 12 . 4; ~ 810 .
The following are obtained a~alogou ly-
from H-Arg-His-Asp-Leu-NH2
'1
,H3C-CO-Arg-His-Asp-Leu-NH2i RT = 11.1; M+ 581i
~.10 from H-Arg-Gly-Asp-Leu-NH2
''5H3C-CO-Arg-Gly-Asp-Leu-NH2i RT = 11,3; M~ 5Q1.
, .
mp12 12
80 mg of ~-Thr-Asp-Val-AQn-Gly-A~p-Gly-Arg-Ei~-
Asp-~eu-OH are dis~olved three to four tlme~ i~ O.01
m ~Cl and ~reeze-dried after ea~h dis~olving operatlon.
~'15 Subsequent purification by ~PLC give~ ~-Thr-A~p-Yal-A~n-
~,Gly-A~p-Gly-Arg-Hi~-A~p-Leu-O~ ~ HCl.
-,The ~ollowi~g i8 obtained analogously
~i$rom ~-Thr-A~p-Val-A~n-Gly-Asp-Gly-Arg-~ Asp-~eu-O~ by
treatment with TFA:
:`
, .
; ,, .
:.i
.. ,.. ,. . . ... ,.. ,, ; . . ~ ` .... .
,.... . ~ ..... . .. ~ .. ... . - .. . . ..
. . . ~ .
~l2n~n2
- 30 -
. ' .
:H-Thr-Asp-Val-Asn-Gly-Asp-~ly-Arg-His-Asp-Leu-~H x TFA;
lRT = 11.8i M+ 1198.
! ~ :
~amplo 13
To prepare af~inity pha~eR, 0.9 g o~ N-maleimido-
1, Cs~10-CO-NH-C3H6-polymer tobtainable~by conden~ation o~i N-
:~ 5 maleimido-C~ -COOH with ~2N-C3H6-polymer] i~ su~pended in
~i 10 ml of O . 1 M sodium phosphate buffer at p~ 7 and 1
3equivalent of H-CyF-Gly-Gly-Gly-Asp~Arg-His-A~p-Leu-O~ i~
added at 4. The mixture is ~tirred ~or 4 hour~ ~ith
simultaneou~ warming of the reaction ~ixture to room
10 temp~rature, and the solid residue i~ filtered off and
washed twice with 10 ml each of buf~er ~olution (p~ 7~
~7 and then three times with 13 ml eac~ of water. ~-Cys[3-
(N-maleimido-CsHlO-CO-NH-C3H6-polymer)}-Gly-Gly-Gly-A~p-
Arg-~i~-Asp-Leu-O~ i8 obtained.
15 k~mple 1~
~nalogously to Example 1, the ~ollowing polymeric
phase is obtained by condensation o$ polymer-0-~3~6-N~2
[~ommercially available] andHOOC-C4H8-CO-Gly-Asp-Gly-Arg-
,~is-Asp-Leu-OMe Eobtainable by conden~atio~ o adipic
i20 acid with H-Gly-A~p-Gly-Arg-His-Asp-Leu-OMe under the
.3, condition~ mentioned]:
'~ Polymer-O-C3H6-NH-CO-CqH3-CO-Gly-A~;p-Gly-Arg-~ -A~p-Leu-
OMe, From this, hydrolysis in meth~nol using 2 N NaO~
~olution a~cording to Ex.4 g.i~es polym~r-O-C3~6-N~-CO-~8-
,25 CO-~ly-~p-Gly-Arg-Hi -A~p-Leu OH.
~, The examples below relate to pharmaceutical
preparation~.
,.
k~ample A: Injectio~ ~ials
A solution of 100 g of an active compound of the
30 formula I and 5 g of disodi~un hydrogenpho~phate i~ 3 1 o~
doubly di~tilled water i~ adjusted to p~ 6.5 with 2
hydrochloric acid, æter~le filterad, filled i~to
,i~jection ~ials and lyophilized under sterile conditio~,
iand the Yials are ~ealed in a st0rile manner. ~ach
!
, .. , . ~ ; ` ,; . .
.~.. - .. . ~ ... ~' . . . . ; . ,
2l2~3n2
- 31 -
injection vial contains 5 mg of active compound.
~xample B: Suppositories
A mixture of 20 g of an active compound o~ the
for~sula I is fused with 100 g of ~oya lecithin a~d 1400 g
of cocoa butter, and the mixture i~ poured into mould~
and al}owed to cool. Each UppoQitox~ coutain~ 20 mg of
acti~o compound.
k~a~ple C: Solution
: A s~lution of 1 g of an active compound of the
formula I, 9.38 g of Na~2PO~ 2H20, 28.48 g of Na2HPO, 12H~O
- and 0.1 g of benzalkonium chloride i~ prepared i~ 940 ml
o~ dou~sly di~tilled water. The ~olution i8 adjust~d to
p~ 6.8, made up to 1 1 a~d sterilized by irradiation.
This solution can be u~ed in the form of cye drop~.
~xample D: Ointment
500 mg of an active compound of the fonmula I are
mixed with 99.5 g of petroleum jelly under aaeptic
co~dition~.
kx~mpSle E: Tablet~
A mixture of 1 kg of active c~mpound of the
formula I, 4 kg of lactose, 1.2 kg of potato starch,
' O.~ kg of talc and 0.1 kg of mayne3iu~ Btearate i~
`. pressed to gi~e tablets in a customary manner, such that
s each tablet contains 10 mg of active com~ound. . .
3xa~ple ~: Coated tablet~
' Tablets are pressed analogously to Ex~mple ~ a~d
; then coated in a customary manner wit~ a coating
~ 3ucrose, potato starch, talc, tragacanth and colorant.
j~,
~xam~Sle Gs ~ap~ules
Hard gelatin capsules a~e ~illed with 2 kg o~
acti~e compound of the formula I in the cu~tGmary manner,
~uch that each capsule contains 20 mg o~ activa c~mpound.
~, , 2l2n~0~
- 32 -
~xample ~: Ampoules
A ~olution of 1 kg of active compou~d of the
formula I in 60 1 of doubly di~tilled water i~ sterilo
.,filtered, filled into ampoule~, lyophilized under ~tsrile
,.,5 conditions and the ampoules are sealed in a ~ter~lo
émanner. Each ampoule contains 10 mg of active compound.
!
-
. .
-
i~
'
: '
'
i
,`
'': : : . ` ~ ` ` `
',:: . ~ : , ` ``