Note: Descriptions are shown in the official language in which they were submitted.
21~03~1 :
W O 94/05652 PCT/EP93/02232
4,5-DICYANOIMIDAZOLE DERIVATIVES AND PESTICIDAL COMPOSITIONS CONTAINING THEM
The present invention relates to novel 4,5-dicyanoimidazolyl derivatives of formula I
below, which have anthelmintic, acaricidal and insecticidal activity. The invention relates
also to phannaceutical composi~ons and pesicidal compositions based on the compounds -~
of formula I. It relates also to the preparation of the active ingredients and compositions
and to the use thereof in the contr~l of helminths, especially nematodes, cestodes and
trematodes in warm-blooded animals, especially in domestic animals and productive live-
stock, and ~o the use thereof in the control of pests of the order Arthropoda, especially in
the control of insects and representatives of the order Acarina. The present invention
~elates lilcewise to valuable intermediates which can be used in the preparation of active ;~
ingredients, especially those of formula I below. -
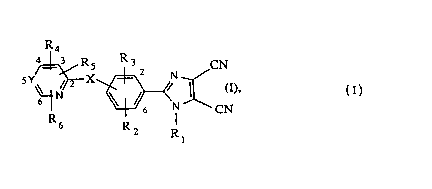
The novel 4,5-dicyanoimidazolyl derivatives have the following formula 1
R4
~N ~3~CN
wherein -
X is oxygen, sulfur, SO or S02; ~ -
Y is -CH= or -N=;
R1 is hydrogen, C1-C6alkyl, Cl-C6haloalkyl, Cl-C6hydroxyalkyl, Cl-C6cyanoaLkyl, or a - -
Cl-C6aL~cylene substituted by Cl-C6alkoxy, Cl-C6haloalkoxy, Cl-C6alkyl~hio, Cl-C6-
haloaLkylthio, C1-C6alkylsulfinyl, C1-C6haloalkylsulfinyl, C1-C6alkylsulfonyl, C1-C6- ~ -
haloaL~cylsulfonyl, Cl-C6hydToxyalkyl, Cl-C6alkyloxycarbonyl, Cl-C6alkylcarbonyl,
C1-C6aLkylcarbonyloxy or by COOH;
R2 is hydrogen, halogen, CN, C1-C6alkyl, C1-C6haloalkyl, C1-C6cyanoalkyl, Cl-C6-hydroxyaLkyl, Cl-C6alkoxy, Cl-C6haloalkoxy, Cl-C6alkylthio, Cl-C6haloalkylthio or
C3-C7cycloalkyl; --
R3 is hydrogen, halogen, Cl-C6alkyl, Cl-C6haloalkyl, cyano or nitro;
WO 94/056~2 2 1 2 ~ 3 4 1 PCI/EP93/02232
R4 is hydrogen, halogen, cyano, Cl-C6aLkyl, C1-C2haloaLkyl, Cl-C2aL~cylthio or c3-q- ~-
cycloaL~yl;
Rs is hydrogen9 halogen, nitro, Cl-C6aL~cyl, Cl-C2haloaLtcyl, Cl-C2aLIcylthio, Cl-C3-
alkoxy, C1-C3haloaLkoxy or C3-C7cycloalkyl; and
R6 is hydrogen or halogen; -
including the physiologically tolerable addition compounds.
In the definition of formula I according to the invention, the individual generic terms are - - ~
to be understood as having the following meanings: -
Within the scope of the present invention, the term aLtcyl by itself or as part of another
substituent is to be understood as meaning, for example, the following straight-chained
and branched groups, depending on the number of carbon atoms indicated: methyl, ethyl,
n-propyl, isopropyl, n-butyl, sec-butyl, tert-butyl, isobutyl, etc.. HaloaLlcyl by itself or as
part of haloalkoxy, haloallcylthio, haloalkylsulfinyl or haloalkylsulfonyl is a mono- to
per-halogenated aL~cyl substituent, for example CH2Cl, CHC12, CC13, CH2F, CH~;2, CF3, ~ -
CH2Br, CHBr2, CBr3, CH2I, CI3, CHCIF, CHBrCl, CFBrCl, C2Fs~ CH2cH2cL
CHClCH3, C2Cls, CHFCHC12, etc., but preferably CF3. Here and in the following,
halogen is to be understood as meaning fluonne, chlorine, bromine or iodine, preferably
fluorine, chlorine or bromine, but especially chlorine. Alkylthio is alkyl-S-; aL~cylsulfinyl
is aLkyl-S(O)-; aL~cylsulfonyl is alkyl-S(0)2-; alkylcarbonyl is aL~yl-C(O)-; aLlcylcarbonyl-
oxy is aLkyl-C(O)-O-; alkyloxycarbonyl is alkyl-O-C(O~ cylene is a saturated aliphatic
bridge member that is unbranched or branched, for example -CH2-; -CH2CH2-;
-CH2CH(CH3~; -CH2CH(CH3)CH2-; -CH2C(CH3)2-; etc..
Cycloalkyl by itself or as part of a substituent is, depending on the number of carbon
atoms indicated, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, etc.. Cyanoalkyl is an
alkyl group in which from one to three hydrogen atoms have been substituted by CN,
preferably an alkyl group in which a CN group is located at the terminal carbon atom.
Accordingly, hydroxyalkyl is an alkyl group in which from one to three hydrogen atoms
have been subsdtuted by OH, preferably an alkyl group in which an OH group is located at
the terminal carbon atom.
The expression "physiologically tolerable addition compounds" is to be understood as
meaning complexes or salts of a compound of formula I with an inorganic or organic base,
which are formed by the addition of an equivalent amount of a salt-forming base to the
WO 94/05652 2 12 0 3 4 1 PCr/EP93/02232
- 3 -
base molecule of forrnula I, or with solvents such as dimethylformarnide (DMF) or ~ -
dimethylacetamide (DMA). Examples of suitable inorganic bases are oxides, hydroxides,
carbonates and hydrogen carbonates of aLkali metals and alkaline earth metals (e.g. CaO,
BaO, NaOH, KOH, Ca(OH)2, ~lCO3, NaHCO3, K2C03 or Na2C03). Typical ~- -
representatives of suitable organic bases are telrabutylammonium hydroxide; trialkyl-
amines. such as triethylarnine, or dialkylarnines, such as diethyl- or dipropyl-amine.
The following sub-groups within the scope of fonnula I, inter alia, are preferred on
account of their pronounced activity: -
Gruup tA): Compounds of formula I wherein
X is oxygen or sulfur;
Y is -CH= or -N=;
Rl is hydrogen, Cl-C3alkyl, C1-C3haloaLkyl, C1-C3hydroxyaLkyl, or a Cl-C4alkylene
substituted by Cl-C3aL~coxy, Cl-C3haloaL~coxy, Cl-C3aLkylthio, Cl-C3haloaL~cylthio,
Cl-C3allcylsulfinyl, Cl-C3haloalkylsulfinyl, Cl-C3alkylsulfonyl, Cl-C3haloaLkyl-sulfonyl, C1-C3hydroxyaLkyl, Cl-C3alkyloxycarbonyl, Cl-C3alkylcarbonyl or by COOH;
R2 is hydrogen, halogen, CN, Cl-C3alkyl, Cl-C3haloalkyl, Cl-C3cyanoalkyl, C1-C3-aLkoxy, C1-C3haloaL~coxy, Cl-C3aL~ylthio, Cl-C3haloalkylthio or C3-C6cycloalkyl;R3 is hydrogen, halogen, Cl-C3alkyl, Cl-C3haloalkyl, cyano or nitro;
R4 is hydrogen, halogen, cyano. C1-C3aLkyl, halomethyl, methylthio or C3-C6cycloaL~cyl;
Rs is hydrogen, halogen, nitro, C1-C3aLkyl, halomethyl, methylthio, C1-C2aL~coxy,
Cl-C2haloalkoxy or C3-C6cycloalkyl; and
R6 is hydrogen, fluorine, chlor,ine or bromine;
including the physiologically tolerable addition compounds. ~
Group (B): Compounds of formula I wherein -
X is oxygen or sulfur,
Y is -CH= or -N=;
Rl is hydrogen, Cl-C3alkyl, Cl-C3haloallcyl, Cl-C3hydroxyalkyl, or a Cl-C4alkylene
substituted by Cl-C3alkoxy, Cl-C3haloalkoxy, Cl-C3alkylthio, Cl-C3haloalkylthio, - -
Cl-C3aL~cylsulfinyl, Cl-C3haloalkylsulfinyl, Cl-C3alkylsulfonyl, Cl-C3haloaL~cyl- . ``
sulfonyl, Cl-C3hydroxyalkyl, Cl-C3aLtyloxycarbonyl, Cl-C3alkylcarbonyl or by COOH;
R2 is hydrogen, halogen, C1-C2haloaLkyl or C1-C2aLkoxy;
R3 is hydrogen, halogen, Cl-C4alkyl, Cl-C3haloaL~yl, cyano or nitro;
R4 is hydrogen, halogen, Cl-C~alkyl or methylthio;
WO 94/05652 2 1 2 0 3 ~ 1 PCr/EP93/02232
- 4 -
:
Rs is hydrogen, halogen, nitro, Cl-C2aL`cyl, OCF3, C3-C6cycloalkyl or CF3; and
R6 is hydrogen, fluorine or chlorine;
including the physiologically tolerable addition compounds.
Group (C~: Compounds of fonnula I wherein
X is oxygen or sulfur,
Y is -CH= or -N=;
Rl is hydrogen;
R2 is hydrogen, fluorine, chlorine, bromine, CN, Cl-C2haloalkyl or Cl-C2aL~oxy; -
R3 is hydrogen, halogen, Cl-C3allcyl, Cl-C3haloalkyl, cyano or nitro;
R4 is hydrogen, fluorine, chlorine, bromine, Cl-C2alkyl or methylthio;
Rs is hydrogen, fluorine, chlorine, bromine, nitro, Cl-C2aL~cyl, C3-C6cycloalkyl, OCF3 or
CF3; and
R6 is hydrogen, fluorine or chlorine;
including the physiologically tolerable addition compounds.
Group (D): Compounds of formula I wherein
X is oxygen or sulfur,
Y is -CH= or -N=;
Rl is Cl-C2alkyl, preferably methyl; -
R2 is hydrogen, halogen, Cl-C2aLkyl. Cl-C2haloaLkyl or Cl-C2aL~oxy;
R3 is hydrogen, halogen, C1-C3alkyl, Cl-C3haloalkyl, cyano or nitro;
R4 is hydrogen, fluorine, chlorine, bromine or Cl-C2alkyl;
Rs is hydrogen, fluorine, chlorine, bromine, nitro, Cl-C2alkyl, C3-C6cycloaLkyl, OCF3 or
CP3;and :
R6 is hydrogen, fluorine or chlorine;
including the physiologically tolerable addition compounds.
Group (E): Compounds of formula I wherein
X is oxygen;
Y is -N=;
Rl is hydrogen, Cl-C3alkyl, Cl-C3haloalkyl, or a Cl-C4alkylene substituted by Cl-C3-
aLIcoxy, Cl-C3haloalkoxy, Cl-C3alkylthio, Cl-C3haloaL~cylthio, Cl-C3aL~cylsulfinyl,
Cl-C3haloalkylsulfinyl. Cl-C3alkylsulfonyl, Cl-C3haloalkylsulfonyl, Cl-C3hydroxy-
aLkyl, C1-C3aLIcyloxycarbonyl, Cl-C3alkylcarbonyl or by COOH;
R2 is hydrogen, halogen, Cl-C2haloalkyl, Cl-C2haloalkoxy or Cl-C2alkoxy;
WO 94/056~2 2 1 2 0 3 ~1 PCI/EP93/02232
R3 is hydrogen, halogen, Cl-C3alkyl, Cl-C3haloaLkyl, cyano or nitro;
R4 is hydrogen, halogen, Cl-C2alkyl or CF3;
Rs is hydrogen, halogen, nitro, C1-C2aL~cyl, OCF3 or CF3; and
R6 is hydrogen or chlorine;
including the physiologically tolerable addition compounds.
Group (F): Compounds of formula I wherein
X is oxygen or sulfur,
Y is^CH=;
Rl is hydrogen, Cl-C3aLkyl or Cl-C2hydroxyalkyl;
R2 is hydrogen, fluorine, chlorine, CF3, CN, C1-C2alkyl or C1-C2alkoxy;
R3 is hydrogen, fluorine or chlorine;
R4 is hydrogen; - -
Rs is hydrogen, methyl, fluorine, chlorine, nitro, C1-C2aLkoxy, OCF3 or CF3; andR6 is hydrogen or chlorine;
including the physiologically tolerable addition compounds.
Group (G): Compounds of formula l wherein
X is oxygen or sulfur;
Y is -CH= or -N=;
Rl is C1-C3haloaLIcyl, or a Cl-C4alkylene substituted by Cl-C3alkoxy, C1-C3haloaL~coxy.
Cl-C3hydroxyalkyl or by COOH;
R2 is hydrogen, halogen, CN, Cl-C3alkyl, Cl-C3haloaL~cyl, Cl-C3cyanoalkyl, Cl-C3-
aLIcoxy, Cl-C3haloalkoxy, Cl-C3alkylthio, Cl-C3haloalkylthio or C3-C6cycloalkyl;R3 is hydrogen, halogen, Cl-C3alkyl, Cl-C3haloalkyl, cyano or nitro;
R4 is hydrogen, halogen, Cl-C6aL~cyl, Cl-C2haloalkyl or C3-C7cycloalkyl;
Rs is hydrogen, halogen, Cl-C6alkyl, Cl-C2haloalkyl, Cl-C2haloalkoxy or C3-qcyclo-
aLkyl; and - -
R6 is hydrogen;
including the physiologically tolerable addition compounds.
~ .':..
Group (H): Compounds of forrnula I wherein
X is oxygen or sulfur;
Y is -CH- or -N=;
Rl is Cl-C3haloalkyl, or a Cl-C4alkylene substituted by Cl-C3alkoxy;
R2 is hydrogen, halogen, C1-C3alkyl, Cl-C2haloalkyl or Cl-C )alkoxy;
wo 94/056s2 212 0 3 ~1 PCr/EPs3/02232
-6-
R3 is hydrogen, halogen, Cl-C3haloaL~cyl, cyano or nitro;
R4 is hydrogen, halogen, Cl-C2haloalkyl or C3-qcycloaL~cyl;
Rs is hydrogen, halogen or Cl-C2haloalkyl; and
R6 is hydrogen;
including the physiologically tolerable addition compounds.
Within groups A to H, those compounds of fo~nula I wherein the pyridinyl-X- group or
the pynmidinyl-X~ group is bonded in the para-position are especially preferred.
Within groups A to H, special preference is given also to those compounds of formula I ~
wherein X is oxygen. The pyridinyl representatives are also of interest. -
Especially preferred individual representatives of the compounds of formula I include:
2-[4-(3-chloro-S-triauoromethylpyridin-2-yl-oxy)]phenyl-4,5-dicyanoimidazole;
l -methyl-2-[4-(3-chloro-5-trifluoromethylpyridin-2-yl-oxy)lphenyl-4,5-dicyanoimidazole;
2-[2-fluoro-4-(3-chloro-5-trifluoromethylpyridin-2-yl-oxy)]phenyl-4,5-dicyanoimidazole;
2-14-(4-trifluoromethyl-~cyclopropyl-pyrimidin-2-yl-oxy)]phenyl-4.5-dicyanoimidazolc; ~ '
and
2-[2-(chloro)-4-(4-trifluoromethyl-6-cyclopropyl-pyrimidin-2-yl-oxy)]phenyl-4,5-dicyano-
imidazole.
Other interesting representatives from groups G and H are, for exarnple:
' -
2-L~(3-chloro-S-trifluoromethylpyridin-2-yl-oxy)]-1-ethoxy nethyl-4,5-dicyanoimidazole;
2-12-chloro-4-(4-trifluoromethyl-~cyclopropyl-pyrimidin-2-yl-oxy)]phenyl-1-ethoxy-
methyl-4,5 -dicyanoimidazole;
2-[4-(3-chloro-5-trifluoromethylpyridin-2-yl-oxy)]- 1 -carboxy-
methyl-4,5-dicyanoimidazole; and
2-13-chloro-4-(4-trifluoromethyl-~cyclopropyl-pyrimidin-2-yl-oxy)]phenyl- 1-(3,3,3-tri-
fluoropropyl)-4 ,5~icyanoimidazole.
The compounds of formula I are prepared either
(a) by cyclising a compound of forrnula Il
WO 94/05652 2 1 2 0 3 4 1 PCI/EP93/02232
~4
H~
6 R 2 H- CN
wherein the substituents Rl, R2, R3, R4, R~s, R6, X and Y are as defined under formula I,
with a suitable cycling agent and, in those cases in which Rl in the compound offoqmula II is hydrogen, in~oducing an alkyl radical mentioned under Rl in formula I by
subsequent aLkylation, ~
or -: -
(b) by reacting a compound of formula III ;
H-X~<N 3~ CN ` '
R2 Rl ~ ~
wherein the substituents R1s R2, R3 and X are as defined under forrnula I, with a ~--
compound of forrnula IV -
R -:
~Rs
y~ /~ Z (IV), :
R
wherein R4, Rs, R6 and Y are as defined under formula I and Z is a customary leaving
group.
The cyclisation of variant (a) may be ca~ied out using cyclising agents known ~r se.
WO 94/05652 2 1 2 ~ 3 4 1 PCI /EP93/02232
Examples of suitable cyclising agents are: ~
Lead tetraacetate in glacial acetic acid or a mixture of glacial acetic acid and an aromatic ~ -
inert hydrocarbon, for example benzene, toluene or xylene ~cf. F.P. Stephens e~t aL, J.
Chem. Soc., 2971 (1949) and 1722 (1950)~. -
lodine in a polar organic solvent, for example N-methyl-2-pyrrolidone, ~vith the addition
of an inorganic base, such as sodium acetate, and at elevated temperature [cf.
EP-269 238].
Organic oxidising agents, such as 2,3-dichloro-5,6-dicyano- 1 .4-benzoquinone (DDQ), in a -
polar inert solvent, such as acetonitrile, and at elevated temperature lcf. EP-283 173].
Aqueous organic oxidising agents, such as sodium hypochlorite [cf. Jpn. Kokai TOKKYO
KO~O 79, 112, 8611 or N-chlorosuccinimide in a polar organic solvent. such as N,N-
dimethylformamide (DM~;), vith the addition of a soluble organic base, such as nicotin-
arnide ~c Synthesis 1058 (1984)~.
In the cyclisation, the reaction temperatures are custo.narily from +20 to +100C,
preferably from +40 ~o ~90C.
In variant (a), a compound of formula lI wherein R1 is hydrogen is preferably used as
starting material and one of the aLkyl substituents mentioned under Rl from the group
Cl-C6aLkyl, Cl-C6haloalkyl, Cl-C6hydroxyalkyl, Cl-C6cyanoalkyl, and a Cl-C6-
aL~cylene substituted by Cl-C6alkoxy, Cl-C6haloalkoxy, Cl-C6alkylthio, Cl-C6halo-
aLkylthio, Cl-C6alkylsulfinyl, Cl-C6haloalkylsulfinyl, Cl-C6alkylsulfonyl, Cl-C6halo-
alkylsulfonyl, Cl-C6hydroxyalkyl, Cl-C6alkyloxycarbonyl, Cl-C6alkylcarbonyl, Cl-C6-
aL~cylcarbonyloxy or by COOH, is subsequently introduced by means of one of the
customary aL~ylating methods.
Variant (b) lreaction of III with IV~ is preferably calried out in the presence of an inert
polar organic solvent, such as dimethyl sulfoxide (DMSO), N,N-dimethylacetamide
(DMA) or DM~. the reaction advantageously being calried out in the presence of an
inorganic base at temperatures of from 0 to +180C, preferably from +20 to +160C.
Examples of suitable inorganic bases are oxides, hydroxides, carbonates and hydrogen
- carbonates of alkali metals and alkaline earth metals (e.g. CaO, BaO, NaOH, KOH,
WO 94/05652 2 1 2 0 3 ~1 PCT/EP93/02232
Ca(OH)2, KHCO3, NaHCO3, K2C03 and Na2C03), and also acetates, for example ;
CH3COONa and CH3COOK. Also suitable as bases are aL~ali metal alcoholates, for
example sodium ethanolate, sodium propanolate, potassium tert-butanolate and sodium ~--
methanolate. The base is advantageously added in an amount of from 100 to 200 % of the -
equimolar amount, based on the reactants. The water of reaction formed in this reaction
may optionally be removed from the reaction mixture by means of a customary entrainer,
for example methylene chloride, benzene or toluene.
Customary leaving groups in connection with reaction (b) are to be understood as being,
for cxample, halogen, especially chlorine, bromine or iodine, preferably chlorine, or
sulfonyl radicals, such as benzoylsulfonyl, paratosyl or lower alkylsulfonyl, especially
mesyl.
The compounds of formula IV are known or can be prepared analogously to known -~
representatives. - -
-
The compounds of formula Il are novel and, on account of their structure, are csp~cially
suitable for the preparation of the active end products of formula I or of other active
substances having that partial structure. They therefore form an important part of the -
present inven~ion. ; -~
The compounds of formula III wherein R l =H are prepared by cyclising a compound of
forrnula V
. .
R3 -
H-X~ ~ (V),
R2 H- I CN
R
,
wherein R2, R3 and X are as defined under forrnula I and Rl is H, as described under
reacion (a) in the case of formula lI and, in those cases in which Rl in the compound of
forrnula V is hydrogen, introducing an alkyl radical mentioned under Rl in formula I by
subsequent alkylation.
WO 94/05652 ~12 (~ 3 ~ I pcr/Ep93/o2232
- 10-
In ~at reachon it is especially advantageous to use iodine/N-methylpyrrolidone as
cyclising agent.
In the cyclisation of compounds of fonnula V, a compound of formula V wherein Rl is
hydrogen is preferably used as starting material and one of the alkyl substituents
men~oned under Rl from the group Cl-C6alkyl, Cl-C6haloalkyl, Cl-C6hydroxyalkyl,
Cl-C6cyanoalkyl, and a Cl-C6alkylene substituted by Cl-C6alkoxy, Cl-C6haloalkoxy,
Cl-C6aL~cylthio, Cl-C6haloalkylthio, Cl-C6alkylsulfinyl~ Cl-C6haloalkylsulfinyl, Cl-C6-
alkylsulfonyl, C1-C6haloaLkylsulfonyl, Cl-C6hydroxyaLIcyl, Cl-C6alkyloxycarbonyl,
Cl-C6aL~ylcarbonyl, Cl-C6alkylcarbonyloxy or by COOH, is subsequently introduced by
means of one of the customary alkylating methods.
The compounds of formula III may also be prepared from compounds of formula VI
CH jX~N I~ CN
wherein Rl, R2, R3 and X may have all the meanings given under formula I, by
demethylation at the CH3-X- group. There may be used as demethylating agent, forexample, aluminium chloride in chlorobenzene, it being possible to proceed analogously
to J.W. ApSimon et aL, Can. J. Chem. 60~ 308 (1982). The compounds of for nula VI may
in turn be prepared according to methods known in the literature.
The compounds of formula Il are prepared by reacting compounds of formula VII
~ ~6 (Vll),
wherein R2, R3, Rs, R6, R7, X and Y are as defined under forrnula 1, with 2,3-diamino-
'''`
WO g4/05652 2 1 2 (~ 3 4 1 PCI /EP93/02232
maleic acid dini~ile (DAMN) of formula IX
H2N~/CN
11~ '~) ~--,.
H-N~ CN
wherein Rl is as defined under formula I, and, in those cases in which Rl in thecompound ~f fonnula lX is hydr~gen, introducing an alkyl radical mentioned under Rl in
fomlula I by subsequent alkylation.
.~ - -
The compounds of fonnula V are prepared in a completely analogous manner, namely by
reacting a compound of formula VI~ -
, ~
R 3 H -
H-X ,~
R 2
wherein R2, R3 and X are as defined under formula 1, u~th DAM~ (of forrnula IX).
The reac~ns with DAMN may be carried out analogously to the reactions described in ~ -~
the literature, for example analogously to DE 3 726 044 or R.W. Begland et al., J. Org.
Chem. 39, 2341 (1974).
,~
In the reactions wieh a compound of formula IX, a compound of formula lX wherein Rl is
hyd~ogen is preferably used as starting material and one of the alkyl substituents
mentioned under Rl from the group Cl-C6alkyl, Cl-C6haloalkyl, Cl-C6hydroxyaLlcyl, -~ `
Cl-C6cyanoalkyl, and a Cl-C6alkylene substituted by Cl-C6alkoxy, Cl-C6haloalkoxy,
Cl-C6alkyldlio, Cl-C6haloalkylthio. Cl-C6alkylsulfinyl, Cl-C6haloalkylsulfinyl, Cl-C6- '
alkylsulfonyl, Cl-C6haloalkylsulfonyl, Cl-C6hydroxyalkyl, Cl-C6alkyloxycarbonyl,Cl-C6alkylcarbonyl, Cl-C6alkylcarbonyloxy or by COOH, is subsequently introduced by
means of one of the customaJy alkyladng methods.
wo 94/05652 ~ 3 ~; PCI/EP93/02232
The compounds of formula VIII are known or can be prepared analogously to the known
representatives, for examp]e as described in J. Chem. Eng. Data 28, 39 (1983);
R.N. Young et aL, Tetrahedron Letters 25, 1753 (1984) or L.C. Raiford et aL, J. Amer.
Chem. Soc. 52, 4576 (1930).
The compounds of formula VII are novel, and the present invention relates also to them.
They are valuable precursors in the preparation of the active substances of formula I.
There is already a partial s~ucture in them that is important for the activity of the end
products. The compounds of formula VII are prepared by reacting compounds of
formula VIII with compounds of for nula IV, the reaction preferably being carried out in
an inert polar solvent, such as DMSO or DMF, and advantageously in thc presence of an
inorganic base at temperatures of from 0 to +180C, preferably from +20 to +160C.
Examples of suitable inorganic bases are oxides, hydroxide~s, carbonates and hydrogen
carbonates of alkali metals and aL~caline earth metals (e.g. CaO, BaO, NaOH, KOH,
Ca(OH)2, KHCO3, NaHCO3, K2CO3 and Na2CO3), and also acetates, for example
CH3COONa and CH3COOK. Also suitable as bases are alkali metal alcoholates, for
example sodium ethanolate, sodium propanolate, potassium tert-butanolate and sodium
methanolate. The base is advantageously added in an amount of from 100 to 200 % of the
equimolar amount, based on the reactants. The water of reaction formed in this reaction
may optionally be removed from the reaction mixture by means of a customary entrainer,
for example methylene chloride, benæne or toluene.
It has now been found that the compounds of formula I according to the invention are
valuable active ingredients in pest control while being well tolerated by warm-blooded
animals, fish and plants. Thus, they not only exhibit a broad activity spectrum against
helminths, such as nematodes, cestodes and trematodes, that parasitise the animal
organism. especially mammals, their action being directed especially against nematodes
(roundworms), but they may also be used equally successfully against phytopathogenic
insects and arachnids which occur on useful plants and ornarnentals in agriculture,
especially in cotton, vegetable and fruit crops, in forestry, in the protection of stored goods
and material stocks, and in the hygiene sector, especially on domestic animals and produc- ;
tive livestock. They are effective against all or individual development stages of normally
sensitive and also resistant species of insects and arachnids.
As a particular feature of the compounds of formula l attention is drawn to the surprisingly
high degree to which they are tolerated by wann-blooded animals, which makes them
WO 94/05652 212 0 3 41 PCI/EP93/02232
- 13- ~-
superior to many other anthelmintics. The practical handling thereof in the treatment of
worm-infested animals is extraordinarily facilitated by the fact that they are tolerated by -~ -
the treated anirnals without symptoms even at relatively high doses.
As anthelmintics, the novel compounds of formula I according to the invention are
suitable, for example, for controlling parasitic nematodes of the orders (according to - ~
K.I. Sl~rajabin) ~ -
Rhabditida
Ascaridida
Spirurida ;
Trichocephalida
or for controlling cestodes of the orders (according to Wardle & McLeod)
Cyclophyllidae
Pseudophyllidae
or for controlling trematodes of the order
Digenea
in domestic animals and productive livestock, such as cattle, sheep, goats, horses, pigs, red
deer, cats, dogs and fowl. They may be administered to the animals either as a single dose
or repeatedly, the single doses preferably being from 1 to 50 mg per kg of body weight,
depending on the species of animal. In many cases, protracted administration may result
in an improved action or may permit the use of smaller total doses.
In the case of the animal pests from the group of the insects and Acarina, the activity of -
the compounds of fonnula I according to the invention may manifest itself in the death of
the pests immediately or only at a later date, for example at moulting, or in reduced `
oviposition and/or a reduced hatching rate. The above-menioned animal pests include:
of the order Lepidoptera for example
Acleris spp., Adoxophyes spp., Aegeria spp., Agrotis spp., Alabama argillaceae, Amylois
spp., Anticarsia gemmatalis, Archips spp., Argyrotaenia spp., Autographa spp., Busseola
fusca, Cadra c~utella, C~posina nipponensis, Chilo spp., Choristoneura spp., Clysia
ambiguella, Cnaphalocrocis spp., Cnephasia spp., Cochylis spp., Coleophora spp.,
Crocidolomia binotalis, ~yptophlebia leucotreta, Cydia spp., Diatraea spp., Diparopsis
castanea, Earias spp., Ephestia spp., Eucosma spp., Eupoecilia ambiguella, Eupr~ctis spp.,
Euxoa spp., Grapholita spp., Hedya nubiferana, Heliothis spp., Hellula undalis, Hyphantria
cunea, Keiferia lycopersicella, Leucoptera scitella, Lithocollethis spp., Lobesia botrana,
Lymantria spp., Lyonetia spp., Malacosoma spp., Mamestra brassicae, Manduca sexta,
WO 94/05652 2~ ~ 2 Q ~ 41 PCI~/EP93/02232
- 14-
Operophtera spp., Ostrinia nubilalis, Pammene spp., Pandemis spp., Panolis flammea,
Pectinophora gossypiella, Phthorimaea operculella, Pieris rapae, Pieris spp., Plutella ~ -
xylostella, Prays spp., Scirpophaga spp., Sesamia spp., Sparganothis spp., Spodoptera spp.,
Synanthedon spp., Thaumetopoea spp., Tortnx spp., Trichoplusia ni and Yponomeuta
spp.;
of the order Coleoptera for example
Agriotes spp., Anthonomus spp., Atomaria linearis, Chaetocnema tibialis, Cosmopolites
spp., Curculio spp., Dermestes spp., Diabrotica spp., Epilachna spp., Eremnus spp.,
Leptinotarsa decemlineata, Lissorhoptrus spp., Melolontha spp., Orycaephilus spp.,
Otiorhynchus spp., Phlyctinus spp., Popillia spp., Psylliodes spp., Rhizopertha spp.,
Scarabeidae, Sitophilus spp., Sitotroga spp., Tenebrio spp., Tribolium spp. and
Trogoderma spp.; ~ -
of the order Orthoptera for example
Blatta spp., Blattella spp., Gryllotalpa spp., Leucophaea maderae. Locusta spp.,Periplaneta spp. and Schistocerca spp.;
of the order Isoptera for example
Reticulitermes spp.;
of the order Psocoptera for example
Liposcelis spp.;
of the order Anoplura for example
Haematopinus spp., Linognathus spp., Pediculus spp., Pemphigus spp. and Phylloxera ~;
spp.; '- :.
of the order Mallophaga for example
Damalinea spp. and Trichodectes spp.; :
of the order Thysanoptera for exarnple
Frankliniella spp., Hercinothrips spp., Taeniothrips spp., Thrips palmi, Thrips tabaci and
Scirtothrips aurantii;
of the order Heteroptera for example
Cimex spp., Distantiella theobroma, Dysdercus spp., Euchistus spp., Eurygaster spp.,
Leptocorisa spp., Nezara spp., Piesma spp., Rhodnius spp., Sahlbergella singularis, ~:
Scotinophara spp. and Triatoma spp.;
of the order Homoptera for example
Aleurothrixus floccosus, Aleyrodes brassicae, Aonidiella spp., Aphididae, Aphis spp., ~
Aspidiotus spp., Bemisia tabaci, Ceroplaster spp., Chrysomphalus aonidium,
Chrysomphalus dictyospermi, Coccus hesperidum, Empoasca spp., Eriosoma larigerum,
Erythroneura spp., Gascardia spp.. Laodelphax spp., Lecanium corni, Lepidosaphes spp.,
WO 94/05652 212 0 3 41 PCI/EP93/02232 -;
- 15-
Macrosiphus spp., Myzus spp., Nephotettix spp., Nilaparvata spp., Paratoria spp., ~ -
Pemphigus spp., Planococcus spp., Pseudaulacaspis spp., Pseudococcus spp., Psylla spp.,
Puhrinaria aethiopica, Quadraspidiotus spp., Rhopalosiphum spp., Saissctia spp.,Scaphoideus spp., Schizaphis spp., Sitobion spp., Trialeurodes vaporariorum, Trioza -
erytreae and Unaspis citri;
of the order Hymenoptera for example - -
Acromyrmex, Atta spp., Cephus spp., Diprion spp., Diprionidae, Gilpinia polytoma,
Hoplocampa spp., Lasius spp., Monomorium pharaonis, Neodiprion spp., Solenopsis spp.
and Vespa spp.;
of the order Diptera for example
Aedes spp., Antherigona soccata, Bibio hortulanus, Calliphora erythrocephala, Ceratitis ~-
spp., Chrysomyia spp., Culex spp., Cuterebra spp., Dacus spp., Drosophila melanogaster,
Fannia spp., Gastrophilus spp., Glossina spp., Hypoderma spp., Hyppobosca spp.,
Liriomyza spp., Lucilia spp., Melanagromyza spp., Musca spp., Oestrus spp., Orseolia
spp., Oscinella frit, Pegomyia hyoscyami, Phorbia spp., Rhagoletis pomonellL Sciara spp., ~-
Stomoxys spp., Tabanus spp., Tannia spp. and Tipula spp.; ~ ;
of the order Siphonaptera for example
Ceratophyllus spp. and Xenopsylla cheopis,
of the order Acarina for example
Acarus siro, Aceria sheldoni, Aculus schlechtendali, Amblyomma spp., Argas spp.,Boophilus spp., Brevipalpus spp., Bryobia praetiosa, Calipitrimerus spp., Chorioptes spp., ~ -
Dermanyssus gallinae, Eotetranychus carpini, Eriophyes spp., Hyalomma spp., lxodes - -
spp., Olygonychus pratensis, Ornithodoros spp., Panonychus spp., Phyllocoptruta oleivora, ~
Polyphagotarsonemus latus, Psoroptes spp., Rhipicephalus spp., Rhizoglyphus spp., ~ ~-
Sarcoptes spp., Tarsonemus spp. and Tetranychus spp.; and
of the order Thysanura for example ~-~
Lepisma sacchanna. - --
The compounds of formula I according to the invention are therefore highly suitable inter
alia for controlling pests from the group of the insects and arachnids in cotton, fruit, ~ - -
maize, soybean, citrus and vegetable crops. In particular, they control plant-eating insects,
such as Anthonomus grandis, plant-eating insect larvae, such as the larvae of Spodoptera
littoralis or Heliothis virescens, sucking insects, such as Aphis craccivora or Bemisia
tabaci, and soil insects, such as Diabrotica balteata.
.
The compounds of formula l may also be used as dressing agents for pro~ecting seeds
WO 94/056~2 2 t 2 0 :3 41 PCI'lEP93~02232
- 16-
(fruit, tubers, grains) and plant cuttings against noxious insects as well as against phyto-
pathogenic noxious insects that occur in the soil. The invention relates also to composi-
tions comprising compounds of formula I as active ingredient, especially crop-protecting
compositions, and ~o their use in the agricultural sector, including farming, horticulture
and forestrv, or related fields.
Further nore, the present invennon relates also to the preparation of those compositions,
which comprises homogeneously mixing and/or grinding the active ingredient with one or
more substances or groups of substances described herein. The formulation stages can be
supplemented by kneading, granulating (in the case of granules) and, optionally, compres-
sing (in the case of pellets). Also included is a prophylactic and/or curative method of
controlling plant pests andlor helminths in mammals, which comprises applying the
compounds of formula I according to the invention, or the compositions according to the
invention, to the locus of the pest. -
Target subjects in the case of use as anthelmintics are all warm-blooded animals which
may be infested with helminths, especially mammals and fowl, but most especially ~-
domestic animals, productive livestock and pet animals, such as cows, horses, donkeys, ~ `
sheep, goats, llamas, camels, red deer, pigs, dogs, cats, rabbits, chickens, turkeys, ducks,
geese, pheasants, pal~idges, etc., as well as all fur-producing breeding animals. Of
course, infested zoo animals may also be treated successfully.
It is generally known that of the endoparasites occurring in warm-blooded animals it is
particularly the helminths that cause great damage to the animals they infest. Where there ~-
is cbronic and especially epidemic occurrence of worm-related disorders in herds of
animals, such damage caused by helminthiases can assume serious economic proportions. - -
In the animals affected, the damage manifests itself inter alia as losses in productivity,
reduced resistance to other diseases, and increased mortality. Especially dangerous
worm-related disorders are caused by helminths parasitising the gastrointestinal tract and
other organs and, despite numerous prophylactic measures, still occur relatively frequently
in ruminants, such as cattle, sheep and goats, as well as in horses, pigs, fowl, red deer,
dogs and cats.
In this description the term "helminths" is to be understood as meaning especially parasitic
worms that belong to the Platyhelminthes (cestodes, trematodes) and Nemathelminthes
(nematodes and related species), that is to say tapeworms, sucker worrns and roundworms
WO 94/05652 2 12 0 3 41 pcr/Ep93/o2232
of the gastrointestinal ~act and other organs (e.g. the liver, lungs, kidneys, lymph vessels,
blood, etc.).
There is therefore an urgent need to provide therapeutic compositions suitable for control- -
ling helminths in all their development stages and for guarding against attack by such
parasites.
Although a number of substances having anthelmintic activity are known that have been
proposed for controlling the various species of helminth, these have not proved completely - ~ -
satisfactory, either because at a tolerable dose it is not possible to make full use of their
activity spectrum, or because at therapeutically effective doses they exhibit undesired
side-effects or properties. ln this respect the resistance tO certain classes of substances,
which is occurring more and more nowadays, is also becoming increasingly significant.
"Albendazol". which is described, for example, in the literature (British Pat. No.
1 464 326; Am. J. Vet. Res. 38, 1425-1426 (1977); Am. J. Vet. Res. 37, 1515-1516 (1976);
Am. J. Vet. Res. 38, 807-808 (1977); Am. J. Vet. Res. 38, 1247-1248 (1977, has only a ~ ~
limited anthelmintic activity spectrum in ruminants. Its action against benzimidazole- ~ -
resistant n~matodes and adult liver flukes is completely inadequate, and in particular the
pathogenically important immature migrating forms of the latter are not affected at doses
that the host animal can tolerate.
It has surprisingly been found that the novel compounds of formula I have a broad activity
spectrum against helminths, such as nematodes, cestodes and trematodes, that parasitise
the animal organism, especially warm-blooded animals, their action being directed
cspecially against nematodes (roundwo~ns).
Target crops to be protected against phytopathogenic pests within the scope of the present -
invention comprise e.g. the following species of plants: cereals (wheat, barley, rye, oats,
rice, maize, sorghum and related crops), beet (sugar beet and fodder beet), pomes, stone
fruit and soft rruît ~apples, pears, plums, peaches, almonds, cherries, strawbe~ries,
raspber,ries and blackberries), leguminous plants (beans, lentils, peas, soybeans), oil plants
(rape, mustard, poppy, olives, sunflowers, coconut, castor oil plants, cocoa beans, ground-
nuts), cucumber plants (cucumber, marrows, melons), fibre plants (cotton, flax, hcmp,
jute), citrus fruit (oranges, lemons, grapefruit, mandarins), vegetables (spinach, lettuce~
asparagusl cabbages, carrots, onions, tomatoes, potatoes, paprika), Iauraceae (avocados,
cinnamon, camphor), or plants such as tobacco, nuts, coffee, aubergines, sugar cane, tea,
wo 94~05652 212 Q 3 41 pcr/Eps3/o2232
- 18-
pepper, vines~ hops, bananas and natural rubber plants, as well as ornamentals.
The compounds of formula I are normally applied in the form of compositions and can be
applied to the crop area or plant to be treated, simultaneously or in succession, with further
compounds. These further compounds can be fertilisers or micronutricnt donors or other
preparations that influence plant growth. They can also be selective herbicides as well as
insecticides, fungicides, bactericides, nematicides, molluscicides or mixtures of several of
these preparations, if desired together with further carriers, surfactants or other ~ ~
applicadon-promodng adjuvants customarily employed in formulation technology. ~ -
The good pesticidal activity of the compounds of formula I according to the invention
corresponds to a mortality of at least 50-60 % of the mentioned pests. ~ -
. ~
The activity of the compounds of the invention and of the compositions comprising them
against animal pests can be substantially broadened and adapted to prevailing circum- - -
stances by the addition of other insecticides andlor acaricides. Examples of suitable --
additives include representatives of the following classes of active ingredients: organo-
phosphorus compounds, nitrophenols and derivatives thereof, formamidines, ureas,carbamates, pyrethroids, chlorinated hydrocarbons, and Bacillus thuringiensis
preparations.
The compounds of formula 1 are used in unmodified form or, preferably, together with the
adjuvants conventionally employed in formulation technology, and can therefore be
formulated in known manner e.g. into emulsifiable concentrates, directly sprayable or
dilutable solutions, dilute emulsions, wettable powders, soluble powders, dusts, granules,
and also encapsulations in polymer substances. As with the nature of the compositions, ~ -
the methods of application, such as spraying, atomising, dusting, scattering or pouring, are
chosen in accordance with the intended objectives and the prevailing circumstances.
A preferred method of applying a compound of formula 1, or an agrochemical composition
which comprises at least one of said compounds, is foliar application. The frequency of
application and the rate of application depend on the risk of infestation by thecorresponding pathogen. However, the compounds of formula I can also pcnetrate the
plant through the roots via the soil (systemic action) if the locus of the plant is
impregnated with a liquid formulation, or if the compounds are applied in solid form to the
soil, e.g. in g~anular form (soil application). In paddy rice crops, such granules may be
'~ '
WO ~4/05652 2 1 2 0 3 4 1 pC~/E;P93/02232
- 19-
:'
applied in metered amounts to the flooded nce ~leld. The compounds of formula 1 may,
however, also be applied to seeds (coating), either by impregna~ing the seeds wi~h a liquid
formulation comprising the active ingredient, or by coating them with a solid formulation.
The formulations, i.e. the compositions, preparations or mixtures comprising ~hecompound (active ingredient) of formula I, or combinations of those compounds with
other agTochemical active ingredients, and, where appropriate, a solid or liquid adjuvant, ;
are prepared in known manner, e.g. by homogeneously mixing and~or grinding the active
ingredients with extenders, e.g. solvents, solid carriers and, where appropriate, surface~
active compounds (surfactants)~ - -
Suitable solvents are: aromatic hydrocarbons, preferably the fractions containing 8 to 12
carbon atoms of alkylbenzenes, e.g. xylene mixtures or aLkylated naphthalenes, alipha~c
or cycloaliphatic hydrocarbons, such as cyclohexane, paraffins or tetrahydronaphthalene,
alcohols such as ethanol, propanol and butanol, and glycols and their ethers and esters,
such as propylene glycol, dipropylene glycol ether, ethylene glycol, ethylene glycol
monumethyl or monoethyl ether, ketones, such as cyclohexanone, isophorone or
diacetonol alcohol, strongly polar solvents, such as N-methyl-2-pyrrolidone, dimethyl
sulfoxide or dimethylformamide, or water, vegetable oils, such as rape oil, castor oil,
coconut oil or soybean oil; and, where appropriate, also silicone oils.
The solid carriers used, e.g. for dusts and dispersible powders, are norrnally natural
mine~al fillers such as calcite, talcum, kaolin, montmorillonite or attapulgite. In order to
improve the physical properties it is also possible to add highly dispersed silicic acids or
highly dispersed absorbent polymers. Suitable granulated adsorptive caIriers are porous
types, for example pumice, ~roken brick, sepiolite or bentonite; and suitable nonsorbent
carriers are calcite or sand. In addition, a great number of granulated materials of
inorganic or organic nature can be used, e.g. especially dolomite or pulverised plant
residues.
Depending on the nature of the compound of formula I to be formulated, or of the -
combinations of those compounds with other insecticides or acaricides, suitable
surface-active compounds are non-ionic, cadonic and/or anionic surfactants having good ;~
emulsifying, dispersing and wetting properties. The term "surfactants" will also be
understood as comprising mixtures of surfactants.
21203~1 -
WO 94/056~2 PCr/EP93/02232
- 20 -
Both so-called water-soluble soaps and also water-soluble synthetic surface-active
compounds are suitable anionic surfactants.
Suitable soaps are the alkali metal salts, alkaline earth metal s~lts or unsubstituted or ~:
substituted ammonium salts of higher fatty acids (Clo-c22)~ e.g. the sodium or potassium
salts of oleic or stearic acid, or of natural fatty acid mixtures which can be obtained e.g. ; --
from coconut oil or tall oil. Fatty acid methyltaurin salts may also be mentioned as
surfactants.
More f~equently, however, s~called synthetic surfactants are used, especially fatty
sulfonates, fatty sulfates, sulfonated benzimidazole derivatives or alkylarylsulfonates.
The fatty sulfonates or sulfates are usually in the form of alkali metal salts, aLkaline earth
metal salts or unsubstituted or substituted ammonium salts and generally contain a
Cg-Cæalkyl radical, which also includes the alkyl moiety of acyl radicals, e.g. the sodium
or calcium salt of lignosulfonic acid, of dodecyl sulfate or of a mixture of fatty alcohol
sulfates obtained from natwal fatty ~cids. These compounds also comprise the salts of
sulfated and sulfonated fatty alcohoVethylene oxide adducts. The sulfonated benz- -
imidazole derivatives preferably contain 2 sulfonic acid groups and one fatty acid radical
containing approximately 8 to n carbon atoms. Examples of alkylarylsulfonates are the
sodiurn, calcium or triethanolamine salts of dodecylbenzenesulfonic acid, dibutyl-
naphthalenesulfonic acid, or of a condensate of naphthalenesulfonic acid and fonnal-
dehyde. Also suitable are corresponding phosphates, e.g. salts of the phosphoric acid ester
of an adduct of p-nonylphenol vith 4 to 14 mol of ethylene o~ide, or phospholipids.
Non-ionic surfactants are preferably polyglycol ether derivatives of aliphadc orcycloaliphadc alcohols, or saturated or unsaturated fatty acids and alkylphenols, said
derivadves containing 3 to 30 glycol ether groups and 8 to 20 carbon atoms in the
(aliphatic) hydrocarbon moiety and 6 to 18 carbon atoms in the alkyl moiety of the
aL~ylphenols. Purther suitable non-ionic surfactants are the water-soluble adducts of
polyethylene oxide with polypropylene glycol, ethylenediaminopolyprowlene glycol and -
aLkylpolypropylene glycol containing 1 to 10 carbon atoms in the alkyl chain, which
adducts contain 20 to 250 ethylene glycol ether groups and 10 to 100 propylene giycol
ether groups. These compounds usually contain 1 to 5 ethylene glycol units per propylene
glycol unit.
WO94/05652 212 0 341 PCr/EP93/02232
- 2 1 -
,
Representative examples of non-ionic surfactants are nonylphenolpolyethoxyethanols,
castor oil polyglycol ethers, polypropylene/polyethylene oxide adducts, tributylphenoxy-
polyethoxyethanol, polyethylene glycol and octylphenoxypolyethoxyethanol. ~atty acid
esters of polyoxyethylene sorbitan, e.g. polyoxyethylene sorbitan trioleate, are also
suitable non-ionic surfactants.
~, -,
Cationic surfactants are preferably quaternar,v ammonium salts which contain, asN-substituent, at least one Cg-C22aLkyl radical and, as further substituents, unsubstituted
or halogenated lower alkyl, benzyl or hydroxy-lower alkyl radicals. The salts are
preferably in the f~rm of halides, methyl sulfates or ethyl sulfates, e.g. stearyltrimethyl- -
ammonium chloride or benzyldi(2-chloroethyl)ethylammonium bromide.
The surfactants customarily employed in formulation technology are described, for
example, in the following publications:
"McCutcheon's Detergents and Emulsifiers Annual", MC Publishing Corp., Glen Rock,
NJ, USA, 1988,
H. Stache, '~ensid-Tæchenbuch". 2nd edition, C. Hanser Verlag, Munich, Vienna, 1981,
M. and J. Ash, "Encyclopedia of Surfactants", Vol. I-III, Chemical Publishing Co., New
York, 1980-1981.
The pesticidal compositions for crop protection usually comprise 0.1 to 9~ %, preferably ~
0.1 to 95 %, of a compound of formula I or of combinations of that compound vith other -
insecdcides or acaricides, 1 to 99.9 % of a solid or liquid adjuvant, and 0 to 25 %,
preferably 0.1 to 25 %, of a surfactant. Whereas commercial products will preferably be
formulaW as concentrates, the end user will normally employ dilute formulations, which
have considerably lower concentrations of active ingredient. Typical applicationconcent~ations are from 0.1 to 1000 ppm, preferably from 0.1 to 500 ppm. The ~ates of
application per hectare are generally from 1 to 2000 g of active ingredient per hectare, ~ .
preferably from 10 to 1000 g/ha, especially from 20 to 600 g/ha.
Preferred forms of administration for use in warm-blooded animals in the control of
helminths include solutions, emulsions, suspensions (drenches), feed additives, powders,
tablets, including effervescent tablets, boli, capsules and microencapsulations. Care must
21203~1 ~
WO 94/05652 PCr/EP93/02232
be taken to ensure that the formulation adjuvants are physiologically tolerable.
Suitable binders for tablets and boli are chemically modif~ed, water- or alcohol-soluble,
polymeric natural substances, such as starch, cellulose or protein derivatives (e.g. methyl-
cellulose, carboxyrnethylcellulose, ethylhydroxyethylcellulose, proteins such as æin,
gelatin and the like) as well as synthetic polymers, for exarnple polyvinyl alcohol, poly-
vinylpy~olidone, etc.. Tablets also comprise fillers (e.g. starches, microcrystalline
cellulose, sugars, lactose, etc.), lubricants and disintegrators.
If the anthelmintic compositions are in the form of feed concentrates, then there are used
as carriers, for example, high-performance feed, fodder grain or protein concentrates. In
addition to the active ingredients, such feed concentrates or compositions may comprise
additives, vitamins, antibiotics, chemotherapeutics, or other pesticides, especially
bacteriostats, fungistats, coccidiostats, or hormone preparations, substances having an
anabolic action, or substances that promote growth, affect the meat quality of animals for
slaughter, or that are otherwise beneficial to the organism. If the compositions or the ~ - -
compounds of formula I contained therein are added directly to the feed or to the animal's
drinking water, then the finished feed or the finished drinking water preferably comprises
thc acdve ingr~licnts in a concentration of approximately from 0.0005 to 0.02 ~ by
weight (5-200 ppm). - -
The compositions according to the invention may be administered to the animals to be ~ ~ -
treated perorally, parenterally or subcutaneously, the compositions being in the form of
solutions, emulsions, suspensions (drenches), powders, tablets, boli and capsules. --
The anthelmintic compositions according to the invention usually comprise 0.1 to 99 96 by -~
weight, preferably 0.1 to 95 % by weight, of a compound of formula I, Ia or mixtures
thereof, 99.9 to 1 % by weight, preferably 99.8 to 5 % by weight, of a solid or liquid
adjuvant, including 0 to 25 % by weight, preferably 0.1 to 25 % by weight, of a surfactant. - -
Whereas commercial products will preferably be formulated as concentrates, the end user
will normally employ dilute formulations.
Such compositions may also comprise further additives, such as stabilisers, antifoams, - ~-
viscosity regulators, binders, tackifiers, as well as other active ingredients for obtaining
specialeffects.
WO 94/056~2 212 0 3 41 PCl/EP93/02232
- 23 -
The present invention relates also to such anthelmintic compositions employed by the end
user.
In each of ~e methods of pest control according to the invention, or in each of the
pesticidal compositions according to the invention, the compounds of formula I may be
used in any of their spatial configurations, their mixtures, or in the form of their salts.
The invention relates also to a method for the prophylactic protection of warm-blooded -
animals, especially productive livestock, domestic animals and pet animals, against parasi-
tic helminths, which method comprises administering the compounds of formula 1, or the
active ingredient formulations prepared therefrom, to the animals in the form of an
additive to their feed or their drinking water, or in solid or liquid form orally, by injection
or parenterally. The invention relates also to the compounds of forrnula I according to the
invention for use in one of the mentioned methods.
The following Examples selve solely to illustrate the in~,-ention, without limiting it.
Preferred fonnulations are composed especially of the following constituents (throughout,
percentages are by weight):
Emulsifiable concentrates:
ac~vc ingredient:1 to 90 %, preferably 5 to 20 %
surface-active agent:1 to 30 %, preferably 10 to 20 %
liquid carrier:5 to 94 %, preferably 70 to 85 %
Dusts:
active ingredient:0.1 to 10 %, preferably 0.1 to 1 %
solid carrier99.9 to 90 %, preferably 99.9 to 99 %
Suspension concen~rates:
acdve ingredient:S to 75 %, preferably 10 to 50 %
water 94 to 24 %, preferably 88 to 30 %
surface-active agent:1 to 40 %, preferably 2 to 30 %
WO 94/0~652 2 1 7 ~ 3 ~ ~ pcr/Eps3/o2232
-24-
Wettable powders:
active ingredient:0.~ to 90 %, preferably 1 to 80 %
surface-active agent:0.5 to 20 %, preferably 1 to 15 % -
solid carrier:S to 95 %, preferably 15 to 90 %
Granules:
active ingredient:O.S to 30 %, preferably 3 to 15 % ~ - -
solid carrier99.5 to 70 %, preferably 97 to 85 %
. .,
The cornpositions may also comprise further additives, such as stabilisers, e.g. vegetable ; -
oils or epoxidised vegetable oils (epoxidised coconut oil, rape oil or soybean oil),
antifoarns, e.g. silicone oil, preservatives, viscosity regulators, binders, tackifiers, as well
as fertilisers or other active ingredients for obtaining special effects.
The following Exarnples serve to illustrate the invention, but they do not limit it.
The symbol "h" stands for hour, "min." stands for minute, and "N" means no~mality. --;
1. Preparation ExamPles
" ' '
1.1. PreParation of 2-chlor~r~trifluoromethvl-6-cvcloproPvl-pYrimidin-2-Y
benzaldehyde (precursor) ~ ~
A mixture of 44.5 g of 2-chloro-~trifluoromethyl-6-cyclopropylpyrimidine, 31.3 g of ~ -
2-chloro4-hydroxybenzaldehyde, 55.3 g of anhydrous K2CO3 and 350 ml of DMSO is
stirred for 18 h at ~60C under a nitrogen atmosphere. After cooling to room temperature, -~
the mixture is filtered, and the filtrate is poured onto ice-water and exsracted several times
with ethyl acetate. The combined organic phases are washed in succession with lNhydrochloric acid, lN sodium hydroxide solution and saturated sodium chloride solution,
dried over magnesium sulfate and concèntrated by evaporation. The crystalline crude
product is dissolved in ethyl acetate and the solution is purified with activated carbon and
filtered. The solvent is evaporated off, diethyl ether is added to the residue that remains,
the mixture is stiIIed, and the crystals that form are isolated by filtration. N-hexane is -
added to the mother liquor, and the resulting crystals are likewise isolated by filtration. A
total of 31.9 g of 2-chloro-4-14-trifluoromethyl-6-cyclopropyl-pyrimidin-2-yl-oxyl- -
benzaldehyde having a melting point of 92-95C is obtained.
WO 94/056~2 21 2 0 3 ~! 1 PCI/EP93/02232
- 25 -
1.2. Preparation of the Schiff's base of 2-chloro-4-r4-trifluoromethyl-6-cyclopropyl-
Pvrimidin-2-yl-oxvl-benzaldehvde (precursor)
A mixture of 20.6 g of 2-chloro-4-[4-trifluoromethyl-6-cyclopropyl-pyrimidin-2-yl-oxy]-
benzaldehyde, 6.5 g of DAMN and 150 ml of ethanol is heated under reflux for 25 h. The
reaction mixture is brought to room temperature and then concentrated completely by
evaporation under reduced pressure. The residue is taken up in ethyl acetate, and
activated carbon is added. After filtration, the ester solution is concentrated completely,
and the residue is chromatographed on silica gel with n-hexane/ethyl acetate (2/1 to 1/1).
The crystalline product obtained after concentration is isolated by filtration, washed with a
small amount of diethyl ether and dried, yielding 12.1 g of the Schiff's base of 2-chloro-
4-[4-tlifluoromethyl-6-cyclopropyl-pyrimidin-2-yl-oxy]-benzaldehyde having a melting
point of 196-198C. As the NMR spectrum shows, the product is a mixture of the E/Z
isomers having the following structure:
CF3 Cl
N~ O ~CN
H2 CN
1.3. Preparation of 2-r2-(chloro)~-(4-trifluoromethyl-6-cvclopropyl-pvrimidin-2
oxv)l-phenvl-4.5-dicvanoimidazole (end product)
9.6 g of the Schiff's base of 2-chloro-4-l4-trifluoromethyl-~cyclopropyl-pyrirnidin-2-yl-
oxy]-benzaldehyde prepared according to Procedure 1.2. and 3.3 g of nicotinic acid amide
are dissolved under a nitrogen atmosphere in 100 ml of DMF; 3.6 g of N-chloro-
succinimide are added in portions, and the mixture is slowly heated in an oil bath. When a
temperature of +52C is reached, the heat source is removed. The internal temperature
nevertheless continues to rise to approximately +68C, whereupon a solid precipitates.
After cooling of the reaction mixture to room temperature, the precipitate is filtered off
and the filtrate is poured onto ice-water and extracted with ethyl acetate. The organic
phase is washed several times with saturated sodium chloride solution and is dried over
magnesium sulfate, and the solvent is removed in vacuo. The crystalline residue is
recrystallised from diethyl ether/hexane. In order to remove final traces of DMF, the
residue is further dried in vacuo for 24 h at +80~C and is then dissolved in ethyl acetate,
and the ester solution is purified with activated carbon. After filtering and evaporating off
W0 94/05652 2 1 ~: ~ 3 ~ 1 PCI/EP93/02232
- 26 ~
:,
the solvent, the residue is chromatographed on silica gel wi~h n-hexane/ethyl acetate lVl] ~-
- with the addition of 5 % v/v acetic acid. The fractions containing the product are
combined and washed with water until neutral, dried over magnesium sulfate and -
concentrated by evaporation. The crystalline residue is stirred in a 2/1 mixture of
n-hexane/diethyl ether and, after filtration, is dried, yielding 5.2 g of 2-[2-(chloro)-4-(4-~
fluoromethyl-~cyclopropyl-pyrimidin-2-yl-oxy)]-phenyl-4,5-dicyanoimid~zole having a
melting point of 194-196C.
The compounds mentioned in the following Tables may also be prepared analogously to
the above-described methods.
WO 94/05652 2 1 2 0 3 4 1 PCI/EP93/02232
Table 1: Compounds of formula Ia
Het--X~CCN
Ra
wher~in Het is ~\ N and hereinafter "cp" denotes cyclopropyl and "tb" denotes
N~
Rb ~ ~
ter~-butyl: :
Comp ~1 R2 R3 Ra Rb Pos.of X m.p.
No. Het- X- in C
1.1 CH3 H H CF3 cp 4 O 107-109
1.2 H H H CF3 cp 4 O 19~200
1.3 H H H CF3 CH3 3 O
1.4 H 3-OCH3 H CF3 cp 4 O 19~198
1.5 H 5-OCH3 2-Cl CF3 cp 4 O 217-219 --
1.6 H 2-Cl H CF3 cp 4 O 194-196 -:.
1.7 C2Hs H H CF3 tb 3 S
1.8 H 3-CN H CF3 cp 4 O
1.9 H 2-CI ~CI CF3 cp 4 O 253-254 : ~-
1.10 H 3-Cl H CF3 cp 4 O 145-148
1.11 H 3-F H CF3 cp 4 O 148-151
1.12 H ! 2-C1 3-CI CF3 cp 4 O :~
1.13 CH2OCH2CH3 H H CF3 cp 4 O
1.14 CH2COOH H H CF3 cp 4 O -:
1.15 CH2CH2CF3 2-C1 5-CI CF3 cp 4 O
1.16 CH2OCH2CH3 3-Cl H CF3 tb 4 O
1.17 CH2OCH2CH3 2-CI H CF3 cp 4 O -:
1.18 CH3 3-CI H CF3 cp 4 O ~ ~
1.19 CH2OCH3 H H CF3 iso- 3 O -
WO 94/056~2 ~ 1 ~ 0 3 4 1 pcr/Eps3/o2232
- 28 -
Comp Rl R2 R3 RaRb Pos.of X m.p. ~ ;
No. Het-X- in C -~ -
propyl
1.20 CH2COOCH2CH3 H H CF3 cp 4 O
1.21 CH2COOC4H9(t) 3-Cl H CF3 cp 4 O
1.22 CH2CH2COOCH3 H H CF3 cp 3 O
1.23 CH2COOH 2-C1 6-CI CF3 cp 4 O ; ~ ;
1.24 CH2COOH
1.25 CH2CH2CH2F 3-CI H CF3 tb 4 O
1.26 CH2CH2CH3 H H CF3 cp 2 O
1.27 CH(CH3)-OC2Hs 3F H CF3 cp 4 O :~1.28 CH2OCH3 3-CI H .~CF3 cp 4 O ~:
1.29 CH2-O-C2Hs H H CF3 cp 3 O
1.30 CH2COO CH(CH3~2 2-F H CF3 cp 4 O
1.31 CH~CH2CF3 3-Cl H CF3 cp 4 O
WO 94J056~2 2 1 2 0 3 4 ~. PCI/EP93/02232
- 29 -
Table 2: Com~ounds of formula Ib
He!--X~N3~ CN
RA I
R
Rd
wherein Het is ~
IN 6 Rc
Comp Rl R2 R3 Rc Rd Pos. of X m.p.
No. Het-X- inC
.:
2.1 H 2-F H 5-CF3 3-Cl 4 O
2.2 H H H 5-CF3 3-Cl 4 S
2.3 H H H 4-CF3 H 2 O
2.4 H H H 5-CF3 3-Cl 4 O 208-211
2.5 H 2-Cl H 5-CF3 3-Cl 4 O 130-132
2.6 H 3-Cl 5-Cl 5-CF3 3-Cl 4 O
2.7 H H H 5-CF3 3-Cl 4 O 112-113**
2.8 H 3-Br 5-OCH3 5-CF3 3-Cl 4 O 273-277 - ~-
2.9 H 3-OC~3 H 5-CF3 3-Cl 4 O 212-214
2.10 H 5-OCH3 2-Cl 5-CF3 3-Cl 4 O 198-200 :-
2.11 H 3-Cl 5-OCH3 5-CF3 3-Cl 4 O 254-257 . :~
2.12 H H H 4-CF2Cl ~CH3 4 O solid* ;:-
2.13 CH3 H H 5-CF3 3-Cl 4 O 93-95
2.14 H 2-Cl H 5-CF3 H 4 O ~ -
2.15 H 2-Cl 6-Cl 5-CF3 3-Cl 4 O
2.16 CH2OH H H 6-CF3 H 4 O
2.17 H 2-Cl 6-Cl 5-CF3 3-Cl 4 O 223-225 ;~
2.18 CH3 3-OCH3 H 5-CF3 3-Cl 4 O 163-166 ``
2.19 H 3-Cl H 4-CF3 H 4 O 268-272
2.20 H 3-Cl H 5-CF3 3-Cl 4 O 18~188
WO 94/05652 2 ~L 2 0 3 41 PCI/EP93/02232 ~;
- 30-
ComP Rl R2 ~3 Rc Rd Pos.of X m.p.
No. Het-X- in C
2.~1 H 3-F H S-CF3 3-C1 4 O 18~183 -:-
2.22 CH2-OCH2CH3 2-CI H S-CF3 3-C1 4 O
2.23 CH2-OC~2CH3 2-C1 3-CI S-CF3 3-C1 4 O
2.24 CH2-OCH2CH3 3-F H S-CF3 3-C1 4 O
2.25 CH2-OCH2CH3 H H S-CF3 3-C1 4 O
2~26 CH~-OCH3 3-Cl H S-Cl 3-C1 4 O
2.2~ CH2COOH 2-C1 6-C1 4-CF3 H 4 O
2.28 CH2COOH H H S-CF3 H 3 O -;
2.29 CH2CH2COOH H H 2-CF3 S-CI 2 O :
2.30 CH2COO~3 3-Cl H S-CF3 H 4 O
2.31 CH2COOCH2CH3 H H 5-CF3 3-C1 4 o
2.32 CH2COOCH2CH3 3-F H S-CF3 3-C1 4 O -
2.33 CH2COOC4Hg(t) 3-Cl H S-CF3 3-C1 4 O ~
2.34 CH2CH2OH 3-Cl S-Cl 2-CF3 3-C1 4 O ~:
2.35 CH2CH2OH H H S-CF3 3-C1 4 O
2.36 CH2CH2CF3 3-Cl H S-CF3 3-C1 4 O
2.37 CH3 H H 5-CF3 H 4 O -- ~
2.38 CH2OCOCH3 2-F H 5-Br 3-Br 4 O ~ ~ :
2.39 C(H)CH30-CH2CH3 H H S-CF3 3-C1 4 O
2.40 CH2COOH H H 5-CF3 3-Cl 4 O
2.41 CH2-OCH2CH3 3-Cl H 5-CF3 3-C1 4 O 125-127 :- -
2.42 CH3 3-Cl H 5-CF3 3-C1 4 O 72-80 ~ -
l* = x lH20, ** = x lDMFl
` ,'
wo 94/0s6~2 2 1 2 0 3 4 1 pcr/Eps3/o2232
- 31 -
Table 3: InteImediates of formula IIa (wherein R~ = H)
R3
Het X ~2 -~
~H ~NXCN ;
Ra
wlu:rein Hot is ~N and heleinafter "cp" deno~s cyclopropyl and "tb" denotes
Rb ;
tert-butyl~
,`.''", .
Comp R2 R3 Ra Rb Pos. of X m.p.
No. Het-X- in C
3.1 H H CF3 cp 4 O 179-180 ~ ~-
3.2 H H CF3 CH3 3 :
3.3 3-OCH3 H CF3 cp 4 O 195-198
3.4 5-OC~I3 2-Cl CF3 cp 4 O -~
3.5 2-Cl H CF3 cp 4 O 19~198 -,`
3.6 H H CF3 tb 3 S ~ -
3.7 3-CN H CF3 cp 4 O
3.8 2-Cl ~Cl CF3 cp 4 O 195-197 i
3.9 3-F H CF3 cp 4 O 212-214
3.10 3-Cl H CF3 cp 4 O 185-188
,
wo 94/05652 212 0 3 41 Pcr/Eps3/o2232
- 32 -
Table 4: Inte~nediates of formula IIb (wherein R~ = H)
R3
~N:XCN
Rd
3~ 4
wherein Het is ~\
lN ~Rc
Comp R2 R3 Rc Rd Pos. of X m.p. -
No. Het-X- in C
' :-,',
4.1 2-F H 5-CF3 3-Cl 4 O -
4.2 H H 5-CF3 3-Cl 4 S
4.3 H H 4-CF3 H 2 O
4.4 H H 5-CF3 3-Cl 4 O 208-213
4.5 2-Cl H 5-CF3 3-Cl 4 O æ6 (dec.)
4.6 3-Cl 5-Cl 5-CF3 3-Cl 4 O
4.7 3-Br 5 OCH35-CF3 3-Cl 4 O 241-243
4.8 3-QCH3 H 5-CF3 3-Cl 4 O 232-233
4.9 5-OC~3 2-C1 5-CF3 ~-Cl 4 O ~-;
4.10 3-C1 5-OCH35-CF3 3-Cl 4 O --
4.11 H H 4-CF2Cl 6-CH3 4 O 195-198
4.12 2-Cl ~Cl 5-CF3 3-Cl 4 O -~
4.13 H H ~CF3 H 4 O 223-224
4.14 3-Cl H 4-CF3 H 4 O 210-214
4.15 3-Cl H 5-CF3 3-Cl 4 O 230-233
4.16 3-F H 5-CF3 3-Cl 4 O æs-230
W094/05652 2 t 2 ~ 3 41 PCI/EP93/02232
Table S: In~ermediates of formula VIIa
.
Het-X~2 H
(VIIa)
R3 ~:
Ra
whe~ein Het is ~=(N and hereinafter "cp" denotes cyclopropyl and "tb" denotes
N~
Rb
tert-butyl~
Comp R2 R3 Ra Rb Pos. of X m.p.
No. H~t-X- in C
5.1 H H CF3 cp 4 O 5~57
5.2 H H CF3 CH3 3 O ;-
5.3 3-OCH3 H CF3 cp 4 . O oil
5.4 5-OCH3 2-Cl CF3 cp 4 O
5.5 2-CI H CF3 cp 4 O 92-95
5.6 H H CF3 tb 3 S
5.7 3-CN H CF3 cp 4 O
5.8 2-Cl 6-Cl CF3 cp 4 O 81-83
5.9 3-F H CF3 cp 4 O 82
5.10 3-Cl H CF3 cp 4 O 78-80
WO 94/056~2 21~ ~ 3 41 pcrJEps3/o2z32
::
- 34 -
Table 6: Intermediates of formula VIIb
Het-X~2 H
(VIIb) ~- ~
~3 ~ ~:
Rd
wherein Het is ~
~N 6 E~C ~ :
.
'~',.-'
Comp R; R3 Rc Rd Pos. of X m.p. ----
No. Het-X- in C
6.1 2-F H 5-CF3 3-C1 4 O
6.2 H H S^CF3 3-C1 4 S ~ -
6.3 H H 4-CF3 H 2 O : -
6.4 H H S-CF3 3-C1 4 O
6.5 2-CI H S-CF3 3-C1 4 O 71-72 -
6.6 3-C1 5-CI S-CF3 3-C1 4 O
6.7 3-Br 5-OCH35-CF3 3-C1 4 O 122-123
6.8 3-OCH3 H 5-CF3 3-C1 4 O 65-67
6.9 S-OCH3 2-Cl S-CF3 3-C1 4 O
6.10 3-C1 5-OCH35-CF3 3-C1 4 O 112-115
6.11 H H 4-CF2Cl 6-CH3 4 O oil
6.12 2-CI H 5-CF3 H 4 O
6.13 H H 6-CF3 H 4 O ;
6.14 2-C1 6-Cl S-CF3 3-C1 4 O 103-104
6.15 3-Cl H 4-CF3 H 4 O oil -
6.16 3-Cl H 5-CF3 3-C1 4 O 46-48
6.17 3-F H S-CF3 3-C1 4 O oil -
WO 94/056~;2 2 1 2 ~ 3 ~ 1 PCI`/EP93/02Z32
.
- 35 -
2. Formula~ion Examples
2.1. Emulsifiable concentrates a) b) c)
acompoundofTable 1 or2 25 % 40 % 50%
calciumdodecylbenzenesulfonate 5 % 8 % 6 % -- ~ -
castor oil polyethylene glycol ether
(36 mol of e~hylene oxide) 5 % - - -
tributylphenol polyethylene glycol
ether (30 mol of ethylene oxide) - 12 % 4 %cyclohexanone - 15 % 20 %xylene mixture 65 % 25 % 20 %
Emulsions of any desired concentration can be produced from such concen~rates bydilution with water.
2.2. Emulsifiable concentrates a) b) c) -
a compound of Table l or 2 10 % 8 % 60 % ~ -
octylphcnol polyethylene ~lycol ether
(~S mo~ of ethylene oxide) 3 % 3 % 2 %
cal~ium dodecylbenzenesulfonate 3 % 4 % 4 %
castor oil polyethylene glycol ether
(35 mol of ethylene oxide) 4 % 5 % 4 %
cyclohexanone 30 % 40 % 15 %
xylenc mixture 50 % 40 % 15 % -
Emulsions of any desired concentration can be produced from such concentrates by ~ -
dilution with water.
2.3. Suspension concentrate
a compound of Table 1 or 2 40 %
ethyleneglycol 10 %
nonylphenol polyethylene glycol ether
(15 mol of ethylene oxide) 6 %
sodium lignosulfonate 10 %
carboxymethylcellulose 1 %
37 % aqueous formaldehyde solution 0.2 %
W094/05652 ~!1 2 ~3 ~:1 PCr/EP93/1)2232
-36- ~ ~
:
silicone oil in the forrn of a 7~ %
aqueous emulsion 0.8 %
water 32 %
The finely ground active in~edient is intimately mixed with the adjuvants, giving a
suspension concentrate from which suspensions of any desired concentration can be
obtained by dilution wi~ water.
2.4. Water di~persible powder mixtures a) b) c) ~ ;
a compound of Table 1 or 2 25 % 50 % 75 % -
sodium lignosulfonate 5 % 5 % -
oleicacid 3 % - 5 %
sodium diisobutylnaphthalenesulfonate - 6 % 10 %
octylphenol polyethylene glycol ether
(7-8 mol of ethylene oxide) - 2 %
highly dispersed silicic acid 5 % 10 % 10 %
kaolin 62 % 27 %
The active ingredient is thoroughly mixed with the adjuvants and the mixture is
thoroughly ground in a suitable mill, affording wettable powders which can be diluted
with water to give suspensions of the desired concentration. ~-
2.5. Dusts a) b)
a compound of Table 1 or 2 2 % 5 %
highly dispersed silicic acid 1 % 5 %
talcum 97 %
kaolin - 90 %
Ready-for-use dusts are obtained by intimately mixing the carriers with the active
ingredient and grinding the mixture.
2.6. Granules a) b)
a compound of Table 1 or 2 5 % 10 %
kaolin 94 %
highly dispersed silicic acid 1 %
attapulgite - 90 %
~VO 94/056S2 212 0 3 ~ 1 PCr/EP93/02232
- 37 -
The active ingredient is dissolved in methylene chloride, the solution is sprayed onto the
carrier, and the solvent is subsequently evaporated off in vacuo. Such granules can be
mixed with the animal feed.
2.?. Granules
acompoundofTable 1 or2 10 %
sodium lignosulfonate 2 %
carboxymethylcellulose 1 %
kaolin 87 %
The active ingredient is mixed and ground with the adjuvants, and the mixture ismoistened with water. The mixture is extruded and then dried in a stream of air.
2.8. Granules
a compound of Table 1 or 2 3 %
polyethylene glycol (mol. wt~ 200) 3 %
kaolin 94 %
The finely ground active ingledient is uniformly applied, in a mixer, to the kaolin - -~
moistened with polyethylene glycol. Non-dusty coated granules are obtained in this
manner. -
2.9. Tablcts or boli
a compound of Table l or 2 33.00 %
methylcellulose 0.80 % ~ -~
bighly dispersed silicic acid 0.80 %
maize starch 8.40 % ~-
- :.-
II clystalline lactose 22.50 % ;
maize starch 17.00 % - ;:
microcrystalline cellulose 16.50 %
magnesium stearate 1.00 % ~;
.~. .
The methylcellulose is stirred into water. When the material has swollen, the silicic
WO 94~05652 PCI-/EP93/02232 ~ .
21~3~1
- 38 -
acid is s~rred in and the mixnlre is suspended homogeneously. The active
ingredient and the maize starch are mixed. The aqueous suspension is inco~porated
into that mixture~ and the batch is kneaded to form a dough. The mass so obtained is
granulated through a 12 M sieve and dried.
II All four adjuvants are mixed thoroughly.
III The pre-mixtures obtained according to I and II are mixed and compressed to forrn
tablets or boli.
2.4. Iniectables ; ~ ~
A. O11Y vehicle (slow release) - -
a compound of Table 1 or 2 0.1-1.0 g
groundnut oil ad 100 ml
, ~
a compound of Table l or 2 0.1-1.0 g
sesame oil ad 100 ml
Preparation: The active ingredient is dissolved in some of the oil with stirring and,
optionally, with slight heating. After cooling, the solution is made up to the desired ~-
volume and sterile-filtered through a suitable 0.22 ~m membrane filter.
B. Water-miscible solvent (moderate release rate)
a compound of Table 1 or 2 0.1-1.0 g
4hydroxymethyl-1,3~ioxolane
(glycerol fomlal) 40 g
1,2-propanediol ad 100 ml
a compound of Table 1 or 2 0.1-1~0 g
glycerol dimethyl ketal 40 g
1,2-propanediol ad 100 ml
Preparation: The active ingredient is dissolved in some of the solvent, vith stirring; the
solution is made up to the desired volume and sterile-filtered through a suitable 0.22 ~m
membrane filter.
W094/05652 212 ~ 3 41PCr/EP93/02232
- 39 -
C. Aqueous solubilisate (rapid release)
a compound of Table 1 or 2 0.1-1.0 g
polyethoxylated castor oil
(40 ethylene oxide units) 10 g -
1,2-propanediol 20 g
benzylalcohol 1 g
aqua ad inject. ad 100 ml ;
a compound of Table 1 or 2 0.1-1.0 g
polyethoxylated sorbitan monooleate ; -
(20 ethylene oxide units) 8 g
4-hydroxymethyl- 1 ,3-dioxolane
(glycerol formal) 20 g
benzyl alcohol 1 g - -
aqua ad inject. ad 100 ml
Preparation: The activc ingredient is dissolved in the solvents and the surfactant, and the
solution is made up to the desired volume with water. Sterile filtration through a suitaUe ~ -
membrane filter having a pore diameter of 0.22 ~lm.
The aqueous systems may also be used preferably for oral and/o~ intraruminal
administration.
3. Biological Examples
.....
The anthelmintic activity is demonstrated by means of the following tests: -
3.1. Test on shee~ infested with nematodes such as Haemonchus contortus and
Trichostron~vlus colubriformis
.~ -
The active ingredient is administered in the form of a suspension, by means of a stomach ~;
probe or by rumen injection, to sheep which have previously been ar~ficially infested with
nematodes such as Haemonchus contortus and Trichostrongylus colubriformis. From 1 to
3 animals are used per test or per dose. Each sheep is treated with only a single dose.
"
A fLrst evaluation is made by comparing the number of worm eggs excreted in the sheep's
faeces before and after treatment.
WO 94/05652 PCI /EP93/02232
~ ~ f~
- 40 -
Seven to ten days after the treatrnent, the sheep are sacrificed and dissected. Evaluation is
made by counting the number of worms remaining in the intestine after the treatment. :
Untreated sheep infested at the same time and in the same manner are used as control or ~ -
comparison.
In this test, compounds of formula I of Tables 1 and 2 achieve a marked reduction in
nematode infestation. For example, when 20 mg of active ingredient are used per kg of
body weight, compounds 1.5, 1.6, 2.4, 2.5, 2.8, 2.9 and 2.10 achieve a reduction of
approximately 90-100 % in nematode infestation. With some compounds, that result is ~ `
also achieved with an even lower dose, for example with 10 mg of active ingredient per kg ~ .
of body weight or even smaller amounts of active ingredient. -
3.2. Test on sheep infested with Fasciola hepatica
The actîve ingredient is administered in the fo~n of a suspension, by means of a stomach
probe or by rumen injecdon, to sheep which have previously been ar~ficially infested with
Fasciola hcpatica 3 animals are used per test or per dose. Each animal is treated with
only a single dose.
A first evaluation is made by comparing the number of worm eggs excreted in the sheep's
faeces before and after treatment.
Three to four weeks after the treatrnent, the sheep are sacrif~ced and dissectc~ Evaluation
is made by counting the number of liver flukes remaining in the bile duct after the
treatment, Untreated sheep infested at the same time and in the same manner are used as
control or comparison. The difference in the number of liver flukes found in the two
groups indicales the level of activity of the test compound.
In this test, compounds of Tables 1 and 2 exhibit good activity against Fasciola hepatica at
doses of less than S0 mg of active ingredient per kg of body weight. Of those compounds,
compound no. 2.4 proves to be particularly effective against Pasciola hcpatica.
The insecticidal and acaricidal activity of the compounds of formula I is demonstrated by
means of the following tests:
wo 94/056~2 21 2 ~ 3 41 pcr/Eps3~o2232
- 4 1 -
3.3. OvicidaUlarvicidal action a~ainst Heliothis virescens
Egg deposits of Heliothis virescens on cotton are sprayed with an aqueous emulsion
comprising the test compound in a concentration of 400 ppm. 8 days later, the percentage
of eggs which have hatched and the survival rate of the caterpillars are evalua~ed in
comparison with untreated controls (% reduction in the population). - ~-
Compounds of Tables 1 and 2 exhibit good activity against Heliothis virescens in this test. ~-~
For example, compounds 1.1 and 2.13 exhibit especially a pronounced development-inhibiting activity. ~ -~
:':
3.4 Action a~ainst Anthonomus ~randis adults
Young cotton plants are sprayed with an aqueous emulsion comprising the test compound ~ ~ -
in a concentration of 400 ppm. After the spray coating has dried, the cotton plants are -
populated with 10 adults of Anthonomus grandis and placed in a plastics container.
Evaluation is made 3 days later. The percentage reduction in the population and thc -
percentage reduction in feeding damage (% activity) are determined by comparing the - - -
number of dead beetlcs and the feeding damage on the treated plants with that onuntreated plants.
Compounds of Tables 1 and 2 exhibit good activity against Anthonomus grandis in this - - -
test. In particular, compound 2.5 is more than 80 % effective. -~
3.5. Action a~ainst APhis craccivora
. . .
Pez seedlings are infested with Aphis craccivora and then sprayed with a spray mixture
comprising the test compound in a concentration of 400 ppm, and incubated at 20C. -
Evaluation is made 3 and 6 days later. The percentage reduction in the population
(% activity) is determined by comparing the number of dead aphids on the treated plants -
with that on untreated plants.
Compounds of Tables 1 and 2 exhibit good activity against Aphis craccivora in this test.
3.6. Action a~ainst Tetranvchus urticae ;~
Young bean plants are populated with a mixed population of Tetranychus ur~cae and
sprayed one day later with an aqueous emulsion comprising the test compound in aconcentration of 400 ppm. The plants are then incubated for 6 days at 25C and then
evaluated. The percentage reduction in the population (% activity) is deterrnined by
wo 94/0s6s2 pcr/Eps3/o2232
2~203~11
- 42 -
comparing the number of dead eggs, larvae and adults on the treated plants with that on
untreated plants.
Compounds of Tables 1 and 2 are effective against Tetranychus urticae in this test.