Note: Descriptions are shown in the official language in which they were submitted.
21 21 361
APPARATUS AND METHOD OF SALIVA COLLECTION AND
VERIFICATION FOR DRIED SALIVA SPOT
DRUG AND HIV ANTIBODY TESTING
BACKGROUND OF THE INVENTION
This invention relates to improvements in an
apparatus and method for collecting a saliva sample from
human subjects. More particulary, the invention provides
indication of the collection of a minimum saliva sample
amount as well as providing verification that the sample
collected is saliva. The indicator further serves to
locate the saliva and to show the sample was properly
applied to an absorbent after the saliva has been dried
for shipping to a laboratory for HIV antibody testing or
drug abuse testing or other viral or bacterial antibody
testing, or testing for other analytes of interest.
In collecting body fluid samples, and in
particular human body fluid samples, it is at times
necessary to collect samples in the field or outside of a
controlled collection environment. Two such cases are
immediately evident, that of law enforcement sample
collection for evidentiary purposes and the collection of
samples by the health insurance industry for health status
determinations on prospective insurance purchasers.
In law enforcement sample collection of human
body fluid samples, the collection of saliva is
potentially useful as a superior indicator of blood
alcohol levels in contrast to simple "breathalyser" types
of testing. Saliva samples can be saved for future
confirmation of the original test results whereas the
"breath-alyser" tests cannot. For the health and life
insurance industry saliva testing is useful as a matter of
convenience for the individual seeking insurance. When the
.~
WO93/11~34 2 1 2 1 3 6 1 PCT/US92/1008
sample collection for testing for preexisting conditions can be
conducted at their home or within their work site, inconvenience
is minimized and there is less resistance to testing.
In the insurance industry it has become common
practice to send out a medical on-site examiner and have the
fluid sample--saliva--collected at a location most convenient
for the subject desiring insurance. Heretofore on-site
examiners have collected saliva by having individual expectorate
into a container and then adding an anti-bacterial agent to the
saliva and shipping it into the laboratory for testing or by
collecting a sample of saliva on a swab and then plunging the
swab into an anti-bacterial agent for shipment into the
laboratory.
Inherent in such "on-site" sample collection
lS situations is that subsequent transport of the sample to a
testing laboratory is required. In many cases it is convenient,
as well as necessary, to ship the sample to the laboratory by
mail or courier in order to minimize the number of laboratory
operations. In such cases it is beneficial if the sample can be
reduced to a solid phase or at least minimized in volume. As a
result it has recently been found advantageous to ship body
fluid samples, blood in particular, as a dried spot on an
absorbent matrix. In this manner the sample volume is reduced
and the sample weight commensurately reduced by evaporation of
the fluid component.
Conveyance of these physiological fluid samples to the
laboratory often occurs under poor conditions and the use of
public and private mail delivery or couriers to transport such
samples increases the potential for careless handling. Damaged
and leaking fluid samples may alarm or endanger courier or mail
system workers who contact the damaged and leaking packages of
fluid physiological materials. Thus it is of substantial
benefit when the fluid component can be reduced or eliminated in
such samples.
However, the application of the fluid sample to an
absorbent upon which the sample can be dried causes certain
problems in the case of saliva. Unlike the on-site examiner,
the receiving laboratory does not have the advantage of actual
2 1 2 1 36 1
~093/ll434 PCT/US92/l(~085
- 3
observation of the subject. Upon receiving an absor~ent upon
which a saliva sample has dried, the laboratory must be able to
locate the saliva thereon (saliva being invisible unlike blood),
determine that a sufficient quantity of the sample has ~een
obtained to perform the tests to be conducted, and verify that
the dried sample is, in fact, saliva and not another body fluid
or a fake specimen. Once the sample is dried it may be easily
transported to a laboratory where a wide range of tests may be
performed on the sample.
A dried saliva spot on an absorbent may be used for
determining drug use such as cocaine and may also be used for
analyzing the health status of the potential insured for HIV
antibodies or analytes of interest. The utility of saliva as a
testing medium is a direct result of its formation in the body.
Saliva is formed in the mouth by the salivary glands of the oral
cavity and adjacent areas. The salivary glands consist of two
parotid, submandibular and sublingual glands in addition to the
labial and lingual glands as well as the small mucous glands of
the soft and hard pallet. The daily saliva output is between
500 and 1,500 milliliters. This flow may be affected by various
factors including age, sex, time of day, time of year,
nutritional state and emotional state of the individual.
The main constituents of saliva are proteins, sodium,
potassium, calcium, magnesium, chloride, bicarbonate, and
inorganic phosphate. However, as the salivary gland ducts are
separated from the human blood circulation by only a layer of
epithelial cells many constituents of the blood are passed into
the saliva. While most drugs and blood constituents seem to
enter the saliva by diffusion across the epithelial cell lipid
membrane, it is also possible that there is some active
secretion into the saliva of drugs such as penicillin and
lithium.
It has been confirmed that intravenous use of cocaine
in addition to oral or intra-nasal administrations of cocaine
may be determined from saliva. Recently, there has been
interest in determining through saliva testing the presence of
human immuno-deficiency virus (HIV) antibodies to assist
W093J11434 2 ~ 2 1 ~ 6 1 PCT/US92/10085
insurance companies in screening out risks to business prior to
offering contracts for health and life insurance.
Generally, samples of saliva for these purposes have
been obtained by having the subject expectorate into a
container. This is assisted by having or by having the subject
suck or chew onto a washed rubber band, a piece of paraffin wax,
chewing gum or a strip of citric acid-impregnated filter paper
and then having the subject expectorate into a container or
allowing the saliva to be absorbed onto an absorbent paper or
sponge.
These saliva collection techniques, however, may be
considered less than optimal for use in a business office
situation where the form of collection may be obtrusive and out
of keeping with the office environment. In addition, the sample
must then be transported bac~ to the testing laboratory and if
the laboratory is some distance from the sample gathering
location, the inconvenience and difficulty of shipping a body
fluid sample is then incurred.
One avenue around these difficulties has been to allow
the subject to place an absorbent swab or paper into the mouth
and allow the absorbent material to become saturated with saliva
within a set time. However, this presents the problem that it
is difficult to determine the degree of saturation of the swab
and if a number of saliva samples are being concurrently taken,
it may not be possible for the on-site examiner to visually
observe each subject and thereby personally witness and
ascertain that it is saliva which has been absorbed onto the
paper.
More importantly, due to the nature of the absorbent
paper, it may be difficult for the on-site examiner to determine
that adequate sample has been placed on the absorbent to allow
proper testing. In this event it is necessary to return to the
subject at a later date to collect an additional sample which is
not only inconvenient but adds additional expense to the
determination being made and may raise questions as to the
competence of the company requesting the test, the competency of
the on-site examiner, as well as the health of the subject.
Therefore, it is an object of the present invention to provide
2121361
WO9~Jll43~ PCT/VSg2/~008S
--5--
an apparatus and method for field saliva sampling which will
avoid the above debilities of the prior apparatus and methods of
saliva sampling.
Another object is to provide an apparatus and method
for obtaining a saliva sample and determining as the sample is
taken that an ade~uate sample has been obtained.
Yet another object of the present invention is to
provide a field saliva sample collection apparatus and method
which can immediately verify that the sample obtained is in fact
saliva.
Another object of the present invention is to provide
an apparatus and method for saliva sample taking which will at
once indicate to the sample collector that a sufficient sample
for testing has been collected and that the collected sample is
in fact saliva.
Yet another object of the present invention is to
provide a rapid and convenient apparatus and method for saliva
sample collection which can be conveniently and easily stored
for law enforcement evidentiary purposes.
It is yet another object of the present invention to
provide an apparatus and method for saliva sample collection
which can be conveniently and easily shipped from one location
to another.
Yet another object of the invention is to provide an
apparatus and method in which the area of application of a clear
fluid sample may be observed after the sample is dried.
Yet another object of the invention is to provide an
apparatus and method that allows determination that the proper
sample auount has been applied at the time of sampling as well
as after the sample has been allowed to dry.
Yet another object of the invention is to provide an
apparatus and method which protects technical personnel working
with a dried clear fluid sample by making apparent the location
of the sample after it is dried.
Another object of the invention is to provide an
apparatus and method that indicates that the sample was
correctly and uniformly applied to the absorbent.
W093/ll434 2 1 2 1 3 6 1 PCT/US92/10085
--6--
Yet another object of the invention is to provide an
apparatus and method which can be made and utilized at a low
cost.
Yet another object of the invention is to provide an
apparatus and method which assists in avoiding the need to take
repeat samples from subjects due to improper sampling
procedures.
Other objects and advantages of this invention will
become apparent from the following description taken in
connection with the accompanying drawings, wherein is set forth
by way of illustration and example, an embodiment of this
invention.
SUMMARY OF THE INVENTION
In broad summary, the apparatus and method of the
present invention takes advantage of the ability of the
peroxidase enzyme in saliva, in the presence of a peroxide, to
oxidize the "leuco" or colorless form of a dye or other
indicator to produce a colored form of the dye or indicator.
The reaction of the saliva peroxidase with the indicator may be
used to determine that the sample is, in fact, saliva and also
that sufficient sample has been placed on the absorbent.
A second enzymatic method for verification of saliva
presence and sample sufficiency utilizes the amylase enzyme of
saliva to effect a color change, from colorless to colored, in a
substrate compound. The compound, a polysaccharide or
oligosaccharide having a chromogenic moiety or molecule attached
thereto, is applied to the absorbent at a location distant from
the zone of application. Upon being contacted by the amylase of
saliva the substrate reacts with the amylase to release the
cnromogen from the polysaccharide or oligosaccharide substrate
to thereby provide a free chromogen which is colored and visible
to an observer.
An alternate apparatus and method relies on the
application of a simple food dye placed at a particular
predetermined distance from the zone of saliva application. The
mixing effect of the saliva with the dye indicates that at least
2 1 2 1 3 6 1
a predetermined amount of saliva has been placed on the
absorbent.
Generally, the determination of adequate saliva
absorption i$ accomplished by first determining the
absorptive capacity of the selected absorbent. Once this is
determined the proper position on the absorbent for
application of the pre-positioned indictor may be selected.
The proper position is that distance from the zone of saliva
application which will require application and absorption of
sufficient sample quantity for testing by the laboratory
prior to the saliva contacting the indicator.
In this manner, once sufficient saliva has been
absorbed so as to allow sufficient saturation of the
absorbent to result in saliva contacting and reacting with
the indicator, the on-site examiner can easily observe the
color change of the indicator or the diffusing or migration
of the indicator in the saliva and be assured that
sufficient sample has been placed on the absorbent.
To insure against tampering with the absorbent and
indicator the portion of the absorbent upon which the
indicator is placed may be sandwiched between layers of a
clear support stick and the layers of the support stick
riveted or sonically welded or joined by adhesive such that
if an attempt is made to open the layers and tamper with the
absorbent or indicator the attempt will be apparent as the
layers cannot be resealed.
In a broad aspect, then, the present invention
relates to a method of collecting and identifying saliva for
analysis, said method comprising the steps of: (a) inserting
a first absorbent element into the mouth of a subject for
application of a fluid saliva specimen thereto, (b)
providing a second absorbent element which is separate from
said first absorbent element and having a zone of
application for transfer of saliva thereto, wherein said
2 1 2 t 3 6 1
- 7(a) -
zone of application comprises an indicator which indicates
the presence of said fluid saliva specimen when contacted
with said specimen, said indicator is spaced a predetermined
distance from said zone of application to require transfer
of a predetermined quantity of saliva to said second
absorbent element prior to said applied saliva contacting
said indicator, said predetermined distance also provides
for collection of a sufficient quantity of specimen which is
uncontacted by said indicator to permit subsequent analysis
of said specimen, (c) transferring the fluid saliva from
said first absorbent element to said zone of application,
and (d) observing said indicator for saliva contact
therewith in order to determine that said predetermined
quantity of saliva has been transferred to said second
absorbent element.
In another broad aspect, the present invention
relates to a method of collecting saliva for analysis, said
method comprising the steps of: (a) providing an absorbent
element having a zone of application thereon for insertion
into a subject's mouth for absorption of saliva, said
absorbent element being sized to extend out of the subject's
mouth to provide an exterior absorbent element portion,
wherein said exterior absorbent portion has an indicating
means thereon which indicates the presence of saliva when
contacted with saliva, said indicating means is positioned
on said exterior absorbent element in sufficient spaced
relationship to said zone of application to require
application of at least a predetermined amount of said
specimen to said absorbent element prior to the presence of
the saliva being indicated, said spaced relationship further
providing for collection of a sufficient quantity of saliva
which is uncontacted by said indicator to permit subsequent
analysis of the collected saliva, (b) inserting said zone of
application into the mouth of a subject, (c)collecting a
.
2 1 ~ ~ 3 6 1
- 7(b) -
fluid specimen of saliva on said zone of application,
(d)allowing said saliva to migrate on said absorbent from
said zone of application to said exterior portion, and (e)
observing said indicating means for saliva contact therewith
for determination that said at least predetermined amount of
saliva has been absorbed.
In still another broad aspect, the present
invention relates to a method of collecting and identifying
saliva for analysis, said method comprising the steps of:
(a) applying a fluid specimen to an absorbent element having
a sample portion and a separable test portion; (b) providing
a means for testing that upon contact with said specimen
gives an indication of the presence of saliva in said
specimen; (c) following said step (a), removing said test
portion from said sample portion; and (d) applying said
testing means to said test portion to contact the applied
specimen, whereby the presence of saliva therein results in
said indication and analysis may proceed utilizing said
sample portion.
In yet another broad aspect, the present invention
relates to a method of collecting from a subject a desired
quantity of saliva sample for analysis comprising the steps
of: (a) providing a saliva collection absorbent comprising a
zone for saliva application adapted for insertion into the
subject's mouth and an absorbent indicator portion
sufficiently spaced from said zone to position said
indicator portion outside the mouth, said collection
absorbent is attached to a handle which allows for
uncontaminated manipulation of the absorbent and permits
insertion of said zone for saliva application into the
subject's mouth, said indicator portion has an indicator
thereon which signals the presence of saliva on said
indicator portion of said absorbent and said indicator
spaced a predetermined distance from said zone to permit
21 21 361
- 7(c) -
collection of a predetermined amount of saliva prior to
contact of the collected saliva with said indicator, said
predetermined distance providing for collection of a
sufficient quantity of specimen which is uncontacted by said
indicator for subsequent analysis of the collected saliva
sample, (b) inserting said application zone into the
subject's mouth for saliva collection, (c) allowing saliva
to absorb onto said zone and migrate toward said indicator,
(d) observing said indicator during saliva collection to
determine saliva contact with said indicator, and (e)
withdrawing said zone from the mouth upon indicator reaction
with the saliva.
Other objects and advantages of this invention
will become apparent from the following description taken in
connection with the accompanying drawings, wherein is set
forth by way of illustration and example, an embodiment of
the invention.
.~
-~- 21 21 361 `,
BRIEF DESCRIPTION OF THE DRAWINGS
Fig. 1 is a plan view of the preferred embodiment showing
an applicator transferring a saliva sample to the sample
application zones of an absorbent layer. Each zone is shown
surrounded by an indicator component, three of the indicators are
shown in the migrated state after having been contacted by the
applied saliva;
Fig. 2 is a plan view of an alternate embodiment showing
an absorbent material bisected by perforations and having tandem
indicator components one which surrounds the sample area and one
isolated indicator for saliva verification;
Fig. 3 is a side elevational view of the embodiment of
Fig. 2;
Fig. 4 is a front elevational view of an alternative
embodiment of the apparatus with an absorbent material affixed to
a handle and having an indicator component as a ring of dots
around the upper most portion of the absorbent material;
Fig. 5 is a front elevational view of another embodiment
of the invention showing a portion of the absorbent partially
detached to allow separate testing with the indicator component;
Fig. 6 is a side elevational view of the absorbent swab
of Fig. 5 with the detachable portion intact and the line of
detachment shown in phantom lines;
Fig. 7 is a side elevational view of yet another
embodiment showing a sampling device within an outer protective
tube;
Fig. 8 shows the sampling device of Fig. 7 withdrawn from
the outer protective tube and showing the absorbent material
extending from the base of the capillary tube connecting the
absorbent to the cap;
Fig. 9 is a perspective view of the capillary tube
showing the internal channel for insertion of the absorbent;
Fig. 10 is a plan view of the indicator component taken
along line 10-10 of Fig. 8 and showing the indicator component on
a one-way barrier on top of the absorbent material; and
2~ 21 361
Fig. 11 is a perspective view of the absorbent
material which is inserted into the capillary tube of the
collection apparatus shown in Fig. 8 and showing on one end
the one-way barrier and the indicator component.
Fig. 12 is a top plan view of an alternative
embodiment of the sample probe showing a portion of the
absorbent captured by the compression lid;
Fig. 13 is a side elevational view of the
embodiment of Fig. 12, and showing in phantom lines the
flattened rivet securing the compression lid;
Fig. 14 is a fragmentary bottom plan view of the
embodiment of Fig. 12;
Fig. 15 is a front and right side perspective view
of the embodiment of Fig. 12 showing the absorbent attached
to the compression lid which is partially detached from the
support stick; and
Fig. 16 is a fragmentary top plan view of the
embodiment of Fig. 12 showing the compression lid opened on
its hinges to receive the absorbent.
- ,~ 2121361
DETAILED DESCKl~'rlON
The Collection Apparatus
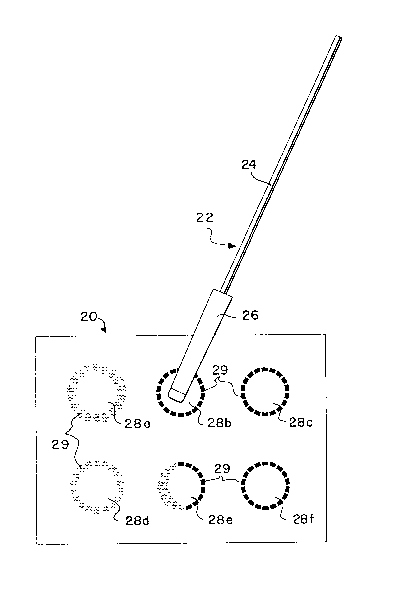
In the embodiment of Fig. 1 sample probe 22 is
utilized to obtain a saliva sample from a subject and then to
apply the sample to an absorbent sheet or layer 20. Sample
probe 22 is comprised of support stick 24 having swab 26 for
saliva collection thereon. In use, swab 26 is placed into the
mouth of a subject and saliva is collected. After collection of
saliva on swab 26 probe 22 is removed from the subject's mouth
and swab 26 is touched to saliva application zones 28a-f on
absorbent sheet or layer 20. In this manner saliva is
transferred from swab 26 onto absorbent layer 20.
In making the transfer of saliva, swab 26 is first
touched to application zone 28a where saliva begins to be
absorbed upon absorbent layer 20. As the saliva passes from
swab 26 to absorbent layer 20 it diffuses through absorbent
layer 20 and reaches indicator component 29. Indicator 29 is
spaced a distance from the center of application zone 28a-f.
The spacing is such that as saliva is absorbed onto absorbent
layer 20 and migrates across absorbent layer 20 to contact
indicator 29, it may be concluded that the proper quantity of
saliva has been applied to absorbent layer 20 when indicator 29
reacts to the presence of the saliva. The on-site examiner can
then proceed to each of the next zones until the complete
sampling has been accomplished.
Indicator 29 of Fig. 1, as well as the other
embodiments, may be either a vegetable dye or a colorless
chemical substrate which acts as a chromogen in the presence of
saliva. Where indicator 29 is a vegetable dye the contact of
saliva with the vegetable dye at indicator 29 will cause some of
the vegetabre dye to begin to diffuse. This migration of the
vegetable dye can be observed by the on-site examiner who is
then assured that sufficient sample quantity has been collected
to allow the testing laboratory to complete analysis of the
saliva. Alternatively, indicator 29 may be one of several
compounds which becomes a colored chromogen in the presence of --
saliva. In this case, the saliva absorbs through layer 20 to
WO 93/1 1 ~1 2 1 2 1 3 6 1 I'c'rt~'s92/10085
--11--
contact indicator 29. Upon contact with the chromogen substrate
in indicator 29 a color change in the indicator occurs. This
color change shows the on-site examiner that the material on
absorbent layer 20 is, in fact, saliva. In addition, the use of
the chromogen at indicator 29 will simultaneously serve to
indicate that sufficient saliva has been absorbed onto absorbent
layer Z0 at zone 28a-f to permit the laboratory to complete
testing of the saliva sample. The chemical substrate enzyme
reaction is more particularly discussed in~a.
Once the sample has been applied to absorbent layer 20
the saliva sample spots are allowed to dry for shipping to the
laboratory. By allowing the saliva spot to dry on the absorbent
layer the saliva sample becomes more stable and can better
withstand elevated temperatures. In addition, the cost of
shipping the sample is substantially lowered as both weight and
volume of the sample are diminished through evaporation of the
excess fluid. The dried sample also offers added protection to
laboratory personnel and shipping personnel as they do not have
to deal with fluid samples which can spill or deal with leaking
packages. The marker dye or chromogen substrate allows the
laboratory technician to ascertain where the proper saliva
sample was located so he or she can avoid contaminating the
sample area and avoid the chance of infection. The sample
obtained from the area within the marker will be uniform,
therefore presenting a more accurate sample for analysis.
At the laboratory, portions of the dried saliva sample
are punched from the absorbent and tested for HIV virus
antibodies or drugs of abuse such as cocaine. Alternatively,
the sample may be tested to identify tobacco smoking subjects
through testing for nicotine and its metabolites or adducts in
the saliva, or the dried saliva spot sample may be tested for
other analytes of interest such as auto-antibodies, hormones,
proteins, or drugs.
In using the simple dye marker method with the
embodiment of Fig. 1, swab 26 contacts application zone 28b and
in the fashion previously described saliva from swab 26 is
~ransferred to application zone 28b. After a period of saliva
transfer the saliva will contact indicator 29. Upon contact of
W~93/ll434 2 1 2 1 3 6 1 rCT/~IS92/10085
the saliva with indicator 29 the dye indicator itself will begin
to migrate in the absorbent layer 20. This is shown in
application zone 28a of Fig. 1 where saliva, previously applied,
has diffused partially into the absorbent layer. It is this
migration or diffusion of indicator 29 which alerts the on-site
examiner that sufficient saliva has been applied to absorbent
layer 20.
Alternatively, where indicator 29 is composed of a
colorless chromogen substrate which changes color upon contact
by saliva, the colorless chromogen is incorporated into the
apparatus at the location of indicator 29. As the substrate is
colorless until it contacts saliva or saliva components, it is
useful to mark the center of application zone 28a-f so the on-
site examiner can apply the sample to the center of the zone
28a-f. Once the applied saliva contacts the indicator a
chemical reaction with the enzymes present in saliva is
initiated and the chromogen substrate is transformed into a
visible colored compound.
The reaction which produces the color change in the
chromogen is specific to the presence of saliva as opposed to
water or other colorless fluids such as soft drinks. Thus it
serves to indicate that the subject has properly saturated
absorbent 20 with saliva and not with some other convenient
fluid such as toilet water which would cause the simple dye
marker indicator to change as described above.
In addition to indicating that the sample is saliva by
the color change of the chromogen substrate, application of the
dye or the chromogen in a pattern can provide additional
information to the laboratory regarding the sample quality. It
is intended that the on-site examiner apply the saliva to the
center of the application zone. When this is the case the
contacting of indicator 29 by the saliva will cause the dye, or
the now colored chromogen, to migrate outwardly as shown in Fig.
1 at reference numeral 28a. The direction and degree of dye or
colored chromogen migration will be a result of two factors:
first, the accuracy with which the on-site examiner applied the
sample to the center of the application zone; and second, the
amount of sample applied to the zone.
W093/ll434 21 213 6 PCTJUS92/l()n~5
-13-
As to application accuracy, if the on-site examiner
applies the sample in the center of zone 28a-f, indicator 29
will be evenly contacted by saliva and the indicator will evenly
migrate outwardly from the center of the application zone. Such
an even outward migration may be observed at 28a or 28d. If the
on-site examiner were to apply the sample outside of the
indicator, the sample would migrate on absorbent 20 such that
the indicator would flow inwardly toward the center of the zone
of application as shown at application zone 28e. In the manner
the laboratory can, by simple inspection determine if the sample
has been properly applied. This opportunity to infer the point
of sample application also imparts added safety to the work of
the laboratory workers who must analyze the samples.
As workers, with the aid of the inventive indicator,
can now see where the sample has been applied, they may avoid
touching that part o~ the absorbent con.aining the sample. In
the prior art absorbents, the laboratory technician was unable
to observe the location of the colorless dried saliva and
therefore could contact those areas containing sample which not
only leads to erroneous results from manual contamination, but
leads to infection of personnel.
The second attribute--observat on of the amount of
sample applied to the zone--is provided by noting the distance
of outward migration of indicator 29. _~ the on-site examiner
has applied the proper quantity of sample to application zone
28a-f a particular distance of migration of the indicator will
result and can be observed by the on-site examiner at the time
of sampling and by the laboratory personnel upon receipt. If
the on-site examiner has applied an improper quantity of sample
~o the application zone the distance of migration by the
indicator will be less than expected and a correction by the on-
site examiner can be made by resampling or that zone can be
avoided by the laboratory analyst at testing.
~eferring now to Figs. 2 and 3, another embodiment of
the present invention is shown. In the embodiment of Fig. 2,
absorbent sheet or layer 30 is bisected by perforations 32 into
- absorbent portions 34, 36. On one of absorbent portions 34, 36
is provided indicator 39 for determination of saliva presence.
WO93/ll434 2 1213 61 pcT/us92tl~)n85
-14-
Indicator 39 is situated atop one-way barrier 38. One-way
barrier 38 allows saliva to move upwardly from absorbent sheet
or layer 30 to contact indicator 39, but does not allow the
reversed migration of saliva which has become mixed with
indicator 39 to then mingle with the saliva on absorbent layer
30. Referring to Fig. 3, a front side elevational view of the
embodiment of Fig. 2 is illustrated having perforation 32
bisecting absorbent layer 30 into absorbent portions 34, 36.
In use, the embodiment of Fig. 2 is presented to a
subject who is instructed to expectorate onto absorbent layer 30
in the vicinity of perforations 32. After several saliva
samples are placed onto absorbent layer 30, the saliva will
spread into both absorbent layer halves 34, 36. As saturation
of absorbent layer 30 is completed the saliva will pass through
one-way barrier 38 and contact indicator 39. Upon contact of
indicator 39 with saliva the indicator will show the on-site
examiner that saliva has approached that point on absorbent
layer 30 and that sampling may be terminated.
Alternatively, the indicator in the embodiment of Fig.
2 may be placed at indicator 39 as well as perimeter indicator
37. In this situation it may be useful to utilize the simple
vegetable or fooq dye marker for indicator 37 to indicate to the
on-site examiner that there is a sufficiency of sample quantity
while using the chemical substrate at indicator 29 as a means of
indicating that the sample is in fact saliva.
Referring now to Fig. 4, another embodiment of the
invention is shown. A sample probe 40 comprises a support stick
42 having an absorbent 44 attached to one end of support 42. A
on-site examiner can, by grasping support 42, insert zone of
application 45 into the mouth of a subject. Saliva from the
subject is then wicked upwardly on absorbent 44 and diffuses
through absorbent 44 toward support 42. Upon saturation of
substantially all of absorbent 44, saliva will approach
indicator 48. Again, indicator 48 may be a simple vegetable dye
or it may be a chemical indicator of the type which will undergo
a color change upon contact with saliva or components of saliva.
In this manner the on-site examiner can observe when sufficient
WO93tll43~ 2` ~ 2 I ~ 6`1 l~CT/US92/l00~5
-15-
sample has been absorbed and/or verify that the sample is saliva
by observation of the change in indicator 48.
Referring now to Figs. 5 and 6, an alternate apparatus
similar to that of Fig. 4 will be discussed. In the embodiment
of Fig. 5 the entirety of absorbent 54 may be inserted into the
mouth of a subject by support stick 52 for collection of saliva
thereon. Thus, the entirety of absorbent 54 is the zone of
application. After the collection of saliva, sample probe 50 is
removed from the subject's mouth and testing of the sample can
be accomplished to determine if the sample truly is saliva.
The indicator component of this embodiment is separate
from the absorbent 54. Indicator test portion 58 of absorbent
54 is detached from sample portion 57 along perforations 56.
After separation of test portion 58 from absorbent 54 the
indicator component for saliva presence may be applied to test
portion 58. If the test demonstrates that the sample on
absorbent 54 is in fact saliva, sample portion 57 of absorbent
54, attached to support 52, may be allowed to dry and sent to
the laboratory for testing for HIV antibodies or abuse of drugs
such as cocaine or for testing of other analytes of interest
such as islet cell antigen antibodies.
Referring to Fig. 6, a side elevational view of the
embodiment of Fig. 5 is illustrated showing perforations 56 on
absorbent 54 between test portion 58 and sample portion 57.
Referring now to Fig. 7 another embodiment, collection
device 70, is shown assembled for shipment. Sample probe 72 is
contained within tube 74 to protect the sample probe during
shipment. Probe 72 is secured within tube 74 by cap 76 which
contacts tube 74 by a frictional fit or by registrable threads
on tube 74 and cap 76. It is desirable that tube 74 be
constructed from a light excluding material to protect the
sample, the indicator and other components of collection device
70 from degradation by light exposure.
In Fig. 8, sample probe 72 is shown removed from tube
3~ 74. It may now be appreciated that in this embodiment cap 76 is
joined to sample probe 72 and is utilized for holding sample
probe 72 during sample collection. Attached to cap 76 of sample
probe 72 is capillary tube 78. Capillary tube 78 extends from
WO93~1143~ 2 1 2 1 3 6 1 PCT/US92/1008~
-16-
cap 76 approximately four to six inches. Absorbent or wick 80
is inserted into the end of capillary tube 78 opposite cap 76.
In use sample probe 72 is inserted into the mouth of a subject
and allowed to remain until such time as absorbent or wick 80
becomes saturated with saliva. The absorption of saliva onto
absorbent 80 is assisted by capillary action provided by
capillary tube 78.
Referring now to Fig. 9, a perspective view of
capillary tube 78 is shown to illustrate hollow channel 79 which
extends through tube 78 and into which absorbent 80 is inserted.
Referring now to Fig. lO, a cross-sectional view of
sample probe 72 taken along line l0-l0 of Fig. 8 is presented.
Capillary tube 78 surrounds absorbent 80. On top of absorbent
80 is one-way barrier 82 and on one-way barrier 82 is indicator
component 84. As saliva migrates or is wicked along absorbent
80 it approaches barrier 82 which is situated atop absorbent 80
as shown in Fig. 8. The saliva will pass through one-way
barrier 82 to interact with indicator component 84. However,
once the saliva has passed upwardly through barrier 82 to
contact indicator 84 the saliva is unable to migrate back
through barrier 82. Thus contact between the subject and the
saliva that has interacted with indicator 84 is avoided.
Again indicator component 84 can interact with the
presence of saliva in two ways. First, indicator 84 may be a
simple dye marker which, when contacted by saliva, will become
partially dissolved and then diffuse in a bleeding fashion.
Such a change in the dye would indicate that saliva had entirely
coated absorbent 30 and contacted indicator 84. The change in
the dye marker indicator 84 serves to show the on-site examiner
when a sufficient saliva sample has saturated absorbent 80 to
allow testing to be properly accomplished at the laboratory.
A second function of indicator 84 is accomplished when
a chemical chromogen substrate is incorporated into indicator
84. In this manner the apparatus provides rapid indication, at
the time of taking a saliva sample from a subject, that not only
has sufficient sample been applied to absorbent 80, but that the
sa~ple applied to absorbent 80 is, in fact, saliva. In Fig. ll,
\V093/ll434 _17_ l'CT/US92/lnn~5
a perspective view of absorbent 80 is shown having barrier 82
and indicator 84 in place on an end of absorbent 80.
The Method of Indication of SamPle Sufficiency
As outlined in the Summary of the Invention, the
inclusion of a simple marker or dye which is at least partially
soluble in saliva or which can migrate along the absorbent layer
with the moving saliva front as it is wicked up the absorbent
can provide a means for determining that a sufficient amount of
saliva sample has been applied to the absorbent.
In this method, and referring now to Figure 4, a
simple dye or marker is placed on the absorbent layer as shown
in Figure 4 at reference numeral 48. This colored marker or dye
need only be observable against the background of the absorbent
48. As the saliva saturates absorbent 44 in zone of application
45, the saliva will move along the absorbent and approach marker
or dye 48 as the amount of saliva applied to absorbent 44
increases. This migration of a saliva front along absorbent 44
will eventually reach the point on the absorbent where marker or
dye 48 has been applied. Upon contact of the saliva with marker
or dye 48 an interaction between the saliva and the marker or
dye will occur.
This interaction between the dye or marker and the
saliva may be of various types. In the case where the dye or
marker is soluble or partially soluble in saliva, the dye or
marker will begin to diffuse into the saliva and the bleeding
effect of the diffusing dye will be noted by the on-site
examiner as illustrated in Figure l at zones 28a, 28d.
Alternatively, if the dye or marker is insoluble in
saliva but exhibits mobility with respect to its position on
absorbent 44, 20, 30 such as colored fluorescent plastic micro
particles, it is possible to accomplish the migration of the
marker or dye along the absorbent with the migration of the
saliva front along absorbent 44, 20, 30. This manner of
utilizing a simple dye marker is suitable for indication to the
on-site examiner that a sufficient level of saliva has been
acquired on absorbent 44, 20, 37.
~ 93/ll~3~ 2 1 2 1 3 6 1 l'CT/US92/1008~
-18-
This signaling that sufficient saliva has been applied
is accomplished by positioning marker or dye 48 (Fig. 4) or 29
(Fig. 1) or 37 (Fig. 2) at a predetermined position on the
absorbent sufficiently distant from zone of application 28a-f,
35, 45 that the minimum quantity of saliva required for proper
testing of the sample must be applied to zone of application
28a-f, 35, 45 prior to the saliva having sufficiently wetted
absorbent 44, 20, 30, to come into contact with marker or dye
48, 29, 37. Thus, at the moment the dye or marker diffuses into
the saliva or begins to move with the saliva front along
absorbent 44, 20, 30 the on-site examiner is made immediately
aware that sufficient quantity has been collected and sampling
can cease.
In an alternate embodiment, not illustrated, a dye
suitable for human consumption or external contact could be
placed at a position on absorbent 44 (Fig. 4) below the known
position at which sufficient sample is indicated. The dye is
then allowed to migrate along absorbent 44 with the moving front
of absorbed saliva. In this manner when the moving marker or
dye front reaches the known point of sample sufficiency along
absorbent 44 the on-site examiner would be aware that sufficient
saliva sample had been collected.
In this embodiment, any of a number of suitable
vegetable or food dyes may be used as the marker. Examples of
suitable vegetable or food dye markers are the FDA approved dyes
for use as food, drug or cosmetic dyes such as red No. 1 or No.
40 or yellow No. 5 or Blue No. 1 or a combination thereof or any
other colorant which is non-toxic to humans. The dye or marker
need only be easily visible against the absorbent selected and
be safe for human consumption or human external contact.
In using the inventive apparatus it is necessary to
first select the particular absorbent for collecting the saliva
sample. Suitable examples of absorbents include an absorbent
paper, S&S 903~, manufactured by Schleicher & Schuell, Inc. or
equivalent absorbents from other vendors such as Whatman, Inc.
of Clifton, N.J. Also porous plastic absorbent supports formed
from polyethylene, polypropylene, polyvinylidene fluoride,
ethylene-vinyl acetate, styrene-acrylonitrile, or
2 1 2 1 3 6 1 ~'CT/US92JI~)08~
--19--
polytetrafluoroethylene having known absorption characteristics
may be used in place of cellulose or sponge material. As
regards the absorbent, it is principally necessary that the
material selected absorb a known reproducible quantity of saliva
when it is provided with sufficient sample to saturate the
material.
The amount of saliva required for accurate laboratory
testing of the sample is then calculated. The amount of
required sample in milliliters is divided by the number of
milliliters of saliva which can be absorbed per square
centimeter of absorbent material. The dye or marker is then
imprinted upon the absorbent material at a location sufficiently
distant from the area of sample application so as to require
that at least the minimum necessary sample volume will be
applied before the saliva interacts with the indicator. In this
manner the desired degree of accuracy is assured.
~ y way of example and not limitation, it was
determined that 300 microliters of saliva sample (wet volume)
were required for conducting a particular series of laboratory
tests on the saliva sample. The selected absorbent material was
capable of absorbing 50 microliters of saliva per square
centimeter of absorbent material. This resulted in a required
total saturated absorbent area of four cubic centimeters to
accomplish proper testing.
The design of the saliva sample apparatus is therefore
a card of absorbent material provided with six saliva
application zones 28a-f having a diameter of 1.25 cm and
circumscribed by an imprinted dashed indicator line which
encloses 1.23 square centimeters of absorbent area. Thus, if
each zone is properly filled it will contain 61.36 microliters
of saliva and the card will contain approximately 368
microliters of total saliva.
The on-site examiner is to apply the saliva to center
of the application zone. As the saliva absorbs on the absorbent
layer 20 (Fig. 1) it moves outwardly from the application zone
and after application of slightly more than 50 microliters of
- saliva the saliva will contact the indicator. The contact of
the saliva will cause the indicator to migrate outwardly with
2 1 2 1 36 1
~VO93/ll43~ rCT/US92/10085
z c~ -
the moving saliva and will present the diffusion pattern shown
in Fig. 1 at reference numeral 28a. If less than the required
amount of saliva is applied to the card the saliva will only
partially contact the indicator. In this case only a slight
migration of the indicator will be noted and a diffusion pattern
such as shown in Fig. 1 at reference numeral 28d will be
observed.
Should the on-site examiner misapply the sample in
place it outside the application zone this too will be noted.
Such an application outside the zone would result in the saliva
migrating inwardly--toward the center of the application zone as
shown in Fig. 1 reference numeral 28e. In this case the on-site
examiner would immediately note the improper application and a
second application could be made. This therefore avoids the
premature releasing the subject and the need to request that
they return again for additional testing.
In the embodiment of Fig. 1, the application of the
saliva to the absorbent card could be accomplished by having the
subject expectorate into a container and then applying the
saliva with a pipette to the absorbent sheet 20.
It will be apparent to those skilled in the art that a
number of variations on this methodology and apparatus can be
made. It is to be appreciated that such modifications of the
embodiments shown are contemplated in the invention.
Method of Verification of Saliva Presence
As previously discussed, during the course of
obtaining saliva samples either by law enforcement officers or
by an on-site examiner for an insurance company or other
interested party, it may be necessary to provide verification
that the sample was in fact saliva. For law enforcement
officers it may be necessary for evidentiary purposes to be able
to swear that the sample obtained was observed to be saliva at
the time of sampling. For on-site examiners of insurance
companies it may be necessary for the on-site examiner to sample
multiple subjects at one time resulting in diversion of the on-
site examiner's attention from any single subject. The
opportunity presented by non-observation could permit persons so
W093/ll4~ -21- 21213 61 PCT/US92/10()8
disposed to substitute a water sample or some other clear fluid
which would provide negative test results at the laboratory.
The present method of verifying saliva presence avoids such
attempts at substitution by reacting with components present in
saliva to produce a reaction with an indicator which has been
previously placed on the absorbent or which may be added to a
portion of the absorbent after the sample has been collected.
One method of accomplishing the verification of saliva
presence is through use of the salivary peroxidase
(Myeloperoxidase) enzyme and/or catalase in saliva. The
peroxidase of saliva is typical of most peroxidases in that it
catalyzes the oxidation by hydrogen peroxide of a relatively
narrow number of classes of organic compounds. These classes of
compounds include phenols; aromatic, primary, secondary, and
tertiary amines; the leuco-dyes; certain heterocyclic compounds
such as ascorbic ac~l and indole; as well as certain inorganic
ions such as the iodide ion.
It should be noted that the function of peroxidase is
not restricted to hyarogen peroxide in particular, but that any
number of compounds which are of the hydroperoxide type may act
as the oxygen provider according to the following reaction
scheme:
Peroxidase + ~22 ~ Compound I
Compound I + AH2(oxidi~bl~ s~trate) (colorless) ~ Compound II + AH
Compound II + AH2 ~ Peroxidase + AH-
2 AH - Oxidized product(cOlOr~)
Some of the hydrogen peroxide compounds which may be
utilized in this reaction are hydrogen peroxide, cumene
hydroperoxide, benzoyl hydroperoxide, methyl hydrogen peroxide,
ethyl hydrogen peroxide, and butyl hydrogen peroxide.
Alternatively, the peroxide necessary for the reaction
with peroxidase or catalase may be generated in situ on the
absorbent utilizing glucose oxidase on the absorbent to react
with the glucose of the saliva sample to produce hydrogen
peroxide. The generated hydrogen peroxide could then be
utilized in reaction with the catalase or peroxidase of saliva
W~93tll434 2 ~ 21~ 6 t Pcrtuss2/1onss
-22-
to react with the colorless substrate compound to provide the
inventive indicating system of the invention.
In the present invention the peroxidase and/or
catalase of saliva is used to catalyze a reaction upon the
S absorbent which visibly indicates to the on-site examiner that
the fluid sample obtained contains peroxidase and/or catalase
and may be concluded to be saliva. Also, it may be indicated to
the on-site examiner that sufficient sample has been collected
for laboratory testing. The latter attribute is determined by
the specific pre-positioning of the peroxidase/catalase
substrate and reactants at a particular location on the paper as
previously described for the simple marker methodology in
determining sample sufficiency.
In the inventive method utilizing saliva
peroxidase/catalase to verify the presence of saliva in a fluid
sample, it is useful to employ an enzyme substrate which reacts
with peroxidase/catalase to undergo an easily observed color
change. A wide variety of such compounds exist and are well
known in the art. In the present methodology the preferred
class of enzyme substrate compounds for verification of saliva
presence is known generally as the leuco-dyes or leuco
compounds.
Leuco-dyes may be thought of as chromogens in that
they will, in at least one of their molecular forms, become
colored compounds easily observable in the visible spectrum.
The classification "leuco" is due to the fact that these
compounds present one molecular form which is white or colorless
therefore exacting the name "leuco" dye (from the Greek le~s
meaning clear) from observers.
Historically leuco-dyes have received much attention
as vat-dyes for textiles. In this application the leuco form of
the dye is soluble in alkalinic aqueous solutions whereas the
colored or oxidized form of the compound is essentially
insoluble. Once the textile has been saturated with the leuco
form of the dye the textile is removed from the vat and air
oxidized into the colored form of the compound.
It is this ability of these compounds to become
colored upon oxidation which has been utilized in the inventive
2121361
~'O 93/1 143'1 I'C'r/US92/10(~8
--23--
method to visibly indicate the presence of saliva. This is
accomplished by relying on the reduction-oxidation (redox)
reaction of the leuco-dyes to change from their colorless-
reduced-('leuco') form into their colored-oxidized-form. The
catalyst for performing this reaction is saliva peroxidase or
saliva catalase which in the presence of an oxygen donor
molecule such as a hydroperoxide can accept two hydrogen atoms
from the leuco compound to thereby oxidize the leuco form enzyme
substrate into its colored form.
This reaction is generally presented in the following
equation:
~ Peroxidase ~
HO-C = (--C-C =~ n--C -0~ = C-( -C = C-) n~C = O
H202 2~2o
leuco form (colorless) Oxidized form (colored)
n = 1: indigo, benzo-, naphtho- and anthraquinone
n = 2: anthanthrone, dibenzpyrenequinone, etc.
n = 4: pyranthrone, dibenzanthrone,
dipyrazolanthrone, etc.
2121361
~093/11~W l'CT/US92/1008
-24-
An alternate form of the leuco compounds is present in
the aromatic amines and in particular derivatives of benzidine:
R2 ~ \ Peroxidase / R2
~ / \ ` ' ~
H2N ~ J ~ / NH2 ~ ~ > HN ~\ ~ ~ NH
1 1 H2 2
colorless colored
R1=~=H benzidine
R1=CH3 and Rz=H o-tolidine
Rl=0CH3; ~=H o-dianisidine
~=R -CH tetramethylbenzidine (TMB)
The above reaction between the peroxidase of saliva
and a hydroperoxide in the presence of a leuco dye proceeds
generally as follows. Peroxidase joins with the hydroperoxide
to provide an oxygen atom to the reactants which then acts as a
hydrogen acceptor. The peroxidase-hydroperoxide complex then
extracts two hydrogens from the leuco form of the molecule to
oxidize the molecule to its colored form. In the case of amine
lS compounds a hydrogen is extracted from each amine group to
result in the tertiary amine. In the case of diols the
hydrogen is removed from the two alcohol moieties to form the
colored oxidized form of the compound and a molecule of water.
Where hydrogen peroxide is used, two molecules of water are
formed from the destruction of hydrogen peroxide.
Inventive use of this enzyme catalyzed reaction of
leuco form enzyme substrate compounds into their oxidized
colored form is accomplished by the application of the leuco
form of the substrate compound in solution and application of a
suitable hydroperoxide to a preselected location on an
absorbent.
As previously described for the simple dye or marker
method, the proper position for the leuco compound on the
absorbent is determined by calculating the amount of saliva
sample required for laboratory testing and then calculating the
particular distance from a zone of saliva application for
2121361
~'093/ll434 rcT~us92/1oo~5
-25-
placement of the leuco compound. The proper distance is that
which would require placement of sufficient saliva sample on the
absorbent to saturate the absorption to the preselected point to
accomplish contact between the saliva and the leuco reactants.
The leuco substrate compound and the hydroperoxide
compound are then applied to the absorbent at that location so
that when saliva is wicked from the zone of application on the
absorbent to the point of application of the leuco enzyme
substrate and the hydroperoxide the peroxidase or catalase
enzyme presence in saliva will contact the peroxide and the
leuco compound substrate to oxidize the leuco substrate to its
colored form. Thus is indicated the presence of saliva to the
on-site examiner as well as indicating that a sufficiency of
saliva sample had been applied to the absorbent.
By way of example, a solution of tetramethylbenzidine
(TMB) dissolved in dimethyl formamide (DMF) or dimethyl
sulfoxide (DMS0) to give a final concentration of 660 ~grams/ml
in an acetate buffer (0.05 mol/l) (pH 4.7 to 4.8) and containing
1 mmol/ml EDTA is applied to a preselected position (indicator
29) on absorbent layer 20 in Fig. 1. The TMB solution is then
allowed to dry and a solution of cumene hydroperoxide in DMF
(0.03 percent v/v) is then applied to the same area as was the
TMB. After the peroxide has dried the absorbent layer 80 is
protected from light. Alternatively, cumene hydroperoxide may
be replaced by a 0.03% aqueous hydrogen peroxide solution in an
acetate buffer (0.05 mol/l) containing 1 mmol/ml EDTA at a pH of
4.7 and dried immediately at elevated temperatures (50-C + 5 C).
When an on-site examiner is prepared to take a sample
the absorbent layer 20 (Fig. 1) is removed from the light
protective cover and a sample probe 22 containing saliva from
the subject is applied to the center of zone 28a Fig. l. The
s~ab is left in contact with absorbent layer 20 until the on-
site examiner observes a color change as represented in Fig. 1
at indicator 28a. The swab 26 of probe 22 then may be removed
from zone 28a and moved to zone 28b for application of saliva to
that zone. In this manner the on-site examiner can quickly and
conveniently deliver the required amount of saliva to the
W~93/ll434 21221 3 61 rCT/US92/10085
absorbent and also verify that, in fact, it is saliva which is
being applied to the absorbent.
The saliva which has been applied to zone 28a migrates
until it contacts the peroxide and the TM~. The peroxidase
enzyme in the migrating saliva contacts the peroxide and TMB and
oxidizes the TMB into its colored form whereupon the on-site
examiner can observe the color change due to the reaction and
determine that the sample is saliva and that the sample quantity
was sufficient.
In a second example, a solution of o-phenylenediamine
dissolved in DMSO to give a final concentration of 1 mg/ml in an
acetate buffer (0.05 mol/l) (pH 4.7 to 4.8) and containing 1
mmol/ml EDTA is applied to a preselected position (indicator 29)
on absorbent layer 20 in Fig. 1. The o-phenylenediamine
solution is then allowed to dry and a solution of hydrogen
peroxide in DMF (0.03 percent v/v) is then applied to the same
area as was the o-phenylenediamine. After the peroxide has
dried the absorbent layer 80 is protected from light. This is
then utilized in the manner previously described.
In a third example, a solution of guaiacol dissolved
in DMS0 to give a final concentration of 50% v/v is applied to a
preselected position (indicator 29) on absorbent layer 20 in
Fig. 1. The guaiacol solution is then allowed to dry and a
solution of hydrogen peroxide in acetate buffer, 0.05 mol/l, pH
4.7, 1 mmol/ml EDTA (0.03 percent v/v) is then applied to the
same area as was the guaiacol. After the hydrogen peroxide has
dried the absorbent layer 80 is protected from light. This is
then utilized in the manner previously described.
In like manner this procedure can be accomplished with
solutions of the below listed compounds at a concentration in a
range of between 100 ~grams to 1 milligram per milliliter
dissolved in a suitable solvent such as an acetate buffer and
organic solvent mixture (e.g. DMS0, DMF or alcohol). At this
concentration the reaction of the leuco compound with peroxidase
and the selected hydroperoxide will arrive at a sufficient end
point (color change observation by the on-site examiner) within
- a period of 40 seconds to approximately 1/2 minutes.
WO93~1143~ 2 1 2 1 3 6 1 1'CTtUS92/1()~)X~
-27-
Suitable leuco enzyme substrate and indicator compounds
which can react in accordance with this method are benzidine:
o-tolidine; o-dianisidine; o-phenylenediamine; 2,2'-azino-di(3-
ethylbenzthiazoline sulphonic acid-6) (ABTS); 4-amino
antipyrine; guaiacol; pyrogallol; 4-chloro-l-naphthol; and
nitrotetrazolium blue chloride. It should also be appreciated
that the buffers that can be utilized to maintain the pH of the
peroxidase reaction (pH 4-7) may be citrate, succinate,
phthalate or phosphate.
It will be appreciated by those familiar with the art
of redox reactions that a number of additional compounds will be
effective with the inventive method. It is to be understood
that the substitution of such compounds for those particularly
specified is contemplated within the present invention and will
produce a method equivalent to that described herein.
As an alternative to reliance upon the peroxidase of
saliva, the amylase enzyme of saliva may be utilized to produce
a visible color change in a substrate compound. This color
change is observable to the on-site examiner and indicates that
the substance applied to the absorbent is saliva. Also, by
placing the amylase-reactive compound at a specified distance
from the zone of application, the on-site examiner can determine
that sufficient sample has been applied to the absorbent to
permit proper testing at the laboratory.
Amylase is present in all living organisms and in
mammals is present in high concentration in saliva. Amylase is
a member of the group of hydrolase enzymes which cleave the Q-
l,4 glycoside bonds in oligosaccharides (from trisaccharides
upwards) and polysaccharides such as starch, glycogen and
dextrins. Amylase acts upon the ~-l,4-glucan links in large
linear polysaccharides having three or more ~-l,4-linked D-
glucose units. The high amylase activity in saliva can be used
to drive a reaction which releases a portion of a polysaccharide
substrate. If the released portion of the polysaccharide
contains sufficient conjugated double bonds within its structure
it will absorb light and be visible to an observer as a colored
compound on the absorbent paper.
WO9~/1143~ 2 1 2 1 3 6 1 PCT/US92/10085
-28-
It should be appreciated that in the present
description the term polysaccharide embraces oligosaccharide and
the two are used interchangeably when generally referencing an
amylase substrate to which is attached a chromogen. Therefore
the chromogen derivative reactive to amylase could be a
tetraoside, pentaoside, hexaoside, heptaoside, or other
polysaccharide.
In the inventive method, a colorless form of an
amylase substrate (an oligosaccharide or a polysaccharide
attached to a chromogen) in a proper combination of enzymes and
buffer system is applied to an absorbent and dried. Saliva is
then applied to the absorbent by an on-site examiner. The
substrate is then cleaved by the saliva amylase and enzymes
present in the applied substrate solution to release the
chromogen compound from the oligosaccharide. This results in a
colored area on the absorbent paper to which saliva has been
applied. This colored area is then visible to an on-site
examiner or other observer.
The reactions by which amylase, in combination with
other enzymes, cleaves the substrate molecule to
release the chromogen is:
amylase
ChrOmO-(glucOsel7 chromo-(glucose)4 3 2 + (glUCOSe)5 4 3
(colorless) (colorless)
glucoamylase;
~-glucosidase
chromo-~glucose) 4,3,2 chromo + chromo(glucose) 4 + x-glucose
(colored)
In the above reaction sequence the chromogen
contAini ng oligosaccharide (e.g. silyl-blocked-4-
nitrophenylmaltoheptaoside) is first cleaved at the ~-l,4
linkages to produce the chromogen linked to oligosaccharides of
2, 3 and 4 glucose units and oligosaccharides of 3, 4 and 5
W093/1143~ 21213 G 1 PCT/US92/10085
-29-
glucose units. In the second step of the reaction glucoamylase
and ~-glucosidase further act on the chromogen-linked
oligosaccharides of 2, 3 and 4 glucose units to hydrolase the
chromogen from the 2 and 3 glucose unit oligosaccharides. A few
of the 4 glucose unit oligosaccharides are also hydrolysed, but
at a much slower rate. This hydrolysis results in release of a
sufficient quantity of the chromogen to permit visual
observation of a color presence on the absorbent by the on-site
examiner .
Thus the examiner is able to quickly and easily verify
the presence of saliva on the absorbent. If the chromogen-
containing oligosaccharide is placed at a particular distance
from the zone of application the examiner may also determine
that sufficient sample quantity has been placed on the absorbent
to permit analysis at the laboratory. In this manner the
amylase present in saliva may be utilized within the inventive
method and apparatus to determine both sufficiency of saliva
quantity as applied to the absorbent and also verify that the
substance applied to the absorbent is in fact saliva.
The substrate molecules utilized are generally
composed of a polysaccharide or oligosaccharide chain having a
chromogenic moiety or molecule attached to one end of the chain.
The other end of the chain is either open or has attached a
molecule which inhibits the enzymatic action of glucoamylase or
~-glucosidase and therefore amylase. The purpose of such a
blocking agent is to promote the action of amylase on the
substrate molecule over the action of glucoamylase or ~-
glucosidase.
Examples of polysaccharide or oligosaccharide
molecules having chromogens incorporated include:
~-4-nitrophenylmaltoheptaoside, ~-4-nitrophenylmaltoheptaoside,
4,6, ethylidine-blocked-4-nitrophenylmaltoheptaoside, silyl-
blocked-4-nitrophenylmaltoheptaoside, 3-ketobutylidene-beta-2
chloro-4-nitrophenylmaltopentaoside, and derivatives of the
above wherein Indole moiety is substituted for the p-nitrophenyl
moiety such as Indolyl-~-D-maltoheptaoside. Also, saccharogenic
substrates such as thiazine-labeled starches, amylopectin-azure
and a~ylochrome dissolved in a suitable buffer may be used.
2 1 2 1 3 6 1 PCT/US92/1()085
-30-
However, if these substrates are used the eluate from the
absorbent will require centrifugation to remove the substrate
material.
In use within the inventive method a solution is
composed of the chromogen-containing amylase substrate selected
from the above compounds or their equivalents, ~-glucosidase,
glucoamylase, sodium chloride, calcium gluconate or calcium
chloride dissolved in a suitable buffer. Suitable buffers
include: ACES ~N-2-acetamido-2-aminoethanesulfonic acid), ADA
(N-2-acetamidoiminodiacetic acid), BIS-Tris-Propane (l 3-
bis[ins(hydroxymethyl)methylamino]propane), HEPES (N-2-
hydroxyethylpiperazine-N'-2-ethanesulfonic acid), HEPPS (N-2-
hydroxyethylpiperazine-N'-3'propanesulfonic acid), MES [2-(N-
morpholino)ethanesulfonic acid~, MOPS t3-(N-
morpholino)propanesulfonic acid], PIPES [piperazine-N,N'-bis(2-
ethanesulfonic acid)], TES [2( ~ (tris-
hydroxymethyl)methyl]amino)ethanesulfonic acid].
The following examples of this inventive method are
presented by way of illustration and not limitation. The method
first involves formulation of a solution containing the
chromogen-linked substrate and secondary enzymes for release of
the chromogen after action on the substrate molecule by amylase.
In a first example, the solution applied to the
substrate was composed as follows:
Actual Amount Alternative
Used Per Approximate
l00 ~l Working Ranges
Per l00ml
Silyl-blocked substrate 0.08 nM 0.008-0.8 nM
(4-nitrophenylmalto-
heptaoside)
~-Glucosidase 3.0 units 0.2 - 30 Vnits
Glucoamylase 3.0 units 0.l - 30 Units
Sodium Chloride S.0 nM 0.5 Nm - 25 nM
Calcium Gluconate 0.5 nM 0.05 Nm - 5 nM
prpEs buffer l3.5 nM 3.9 nM - 40.5 nM
2121361
~VO93/ll434 l'CT/~S92/lnO8
-31-
Approximately 100 microliters of this solution was
applied to the absorbent in the area of the saliva zone of
application. The absorbent was allowed to dry prior to
application of saliva thereto. Saliva was then applied to the
absorbent and upon the saliva contacting the area to which the
solution was applied, a yellow color was observed.
In a second example the following concentrations were
used in formulating the substrate solution for application to
the absorbent:
Actual Amount Alternative
Used Per Approximate
lO0 ~l Working Ranges
Per lOOml
Amylase substrate 0.05 nM 0.005 nM - O.5 nM
(4-nitrophenylmalto-
heptaoside PNPG-7)
Sodium Chloride 5.0 nM 0.5 nM - 25 nM
Calcium Chloride 0.5 nM 0.05 nM - 5 nM
~-Glucosidase i.5 Units 0.25 - 25 Units
Glucoamylase 1.0 Units 0.1 - 25 Units
PIPES buffer 13.5 nM 3.9 nM - 40.5 nM
Approximately 100 microliters of this solution are
applied to the absorbent in the area of the saliva zone of
application. The absorbent may be then allowed to dry prior to
the application of saliva thereto. Saliva is then applied to
the absorbent and upon the saliva contacting the area to which
the solution was applied a yellow color will be observed.
Though the foregoing specific examples set forth the
working concentrations of each ingredient, the concentrations
may be varied according to the speed of the visual color
reaction required.
When an on-site examiner is ready to utilize the
absorbent containing the chromogen linked substrate, saliva is
applied to the absorbent by any convenient method. Upon the
amylase of saliva contacting the chromogen-linked substrate, the
W~9~/ll434 21213 61 rCr/~'S92/l0085
-32-
chromogen will be released from the remaining glucose units and
become colored and visible to the examiner.
Referring to Fig. 12, an embodiment of the present
invention is shown which further comprises the addition of
tamper resistant features to sample probe 100 and features to
notify testing personnel of such tampering attempts. Sample
probe 100 is composed of support stick 102 having absorbent 104
attached to an end thereof. Absorbent 104 is affixed within two
registrable portions of support stick 102. The specifics of the
capture of absorbent 104 within support stick 102 are described
herein. However, the purpose of such capture is to prevent
substitution of a false absorbent layer into sample probe 100
and to allow easy and sure identification of any sample probes
subjected to tampering attempts.
lS In Fig. 13 the capture of absorbent 104 within the
portions of support stick 102 is shown. Absorbent 104 is
sandwiched between two registrable portions of support stick
102. An absorbent holder portion 106 on support stick 102 is
provided to receive absorbent 104. A compression lid which fits
into or in registration with holder 106 may then be fitted in
place within holder 106 to securely fasten absorbent 104 within
sample probe 100. In Fig. 15 absorbent 104 is shown already
having been captured in or sandwiched between holder 106 by
compression lid 110 and the lid reopened. This, as Fig. 15
reveals, results in absorbent 104 being attached to spikes 108
of compression lid 110. The capture of absorbent 104 between
holder 106 and compression lid 110 is accomplished in the
following manner.
Referring to Fig. 16, unassembled support stick 102 is
illustrated with compression lid 110 opened. Breakaway
connectors 112 are attached between compression lid 110 and
holder 106 to secure lid 110 to support stick 102. Breakaway
connectors 112 serve as a hinge-like structure allowing
compression lid 110 to be folded over onto holder 106 and
brought into registration for capture of absorbent 104. It will
be appreciated that both compression lid 110 and absorbent 104
are sized to closely register with or fit within holder 106.
W093/1l434 2 1 2 1 3 6 1 l'CT/US92/l(~085
-33~
Still referring to Fig. 16, absorbent 104 has been
shown in phantom lines to indicate its positioning under the
opened compression lid 110 and placement in holder 106. Once
absorbent 104 is inserted into holder 106, compression lid 110
is then pivoted on breakaway connectors 112 and aligned with
holder 106. A slight pressure on compression lid 110 will press
spikes 108 through absorbent 104 and into spike receptacles 129.
Crimp rivet 114 then enters rivet receptacle 116 on support
stick 102.
When it is desired to securely fasten compression lid
110 to support stick 102, the area of support stick 102 near
holder 106 is placed on a solid surface and struck from above
with a hammer or other blunt instrument. This impact causes the
tip of crimp rivet 114, extending through receptacle 116, to
become expanded into flattened rivet 130 on bottom surface 132
of support stick 102 (Fig. 13). Flattened rivet 130 (Fig. 13)
prevents any reopening of compression lid 110 and withdrawal of
absorbent 104 from holder 106. The only method of opening
compression lid 110 is by resort to prying tools which will
cause deformation of probe 100 structure and tearing of
breakaway connectors 112.
Once compression lid 110 is affixed within holder 106
and rivet 114 compressed into flattened rivet 130, the apparatus
appears as shown in Figs. 12-14. In Fig. 14 flattened crimp
rivet 130 is shown on bottom surface 132 of sample probe 100.
After preparation of sample probe 100 as described above, it may
then be used for obtaining samples of saliva from a subject
without the need for the on-site examiner to observe the subject
at all times during the sampling procedure. This is of
particular importance in relieving the on-site examiner from the
burden of personally verifying the collection of each sample
through observation. This also permits the on-site examiner to
simultaneously make collections from multiple subjects in
different areas. As it requires a few moments for sufficient
saliva to saturate absorbent 104, the on-site examiner is able
to attend to other duties and testing of other personnel as it
- is no longer necessary for the entire saliva collection from
each subject to be actually observed by the on-site examiner.
21 21361
WO 93/1 1434 I'Cl/US92/1~)085
In use, absorbent 104 is placed within the mouth of
the subject and saliva is allowed to saturate absorbent 104.
The entirety of support stick 102 is exterior of the mouth. In
this manner the application of saliva to absorbent 104 will
saturate a first saliva application zone or test area 120 and a
second saliva application zone or archive area 122 prior to
contacting indicator 125. It will be appreciated from the
foregoing discussion that indicator 125 is not contacted by
saliva until saliva has saturated test area 120 and archive area
122. After such saturation saliva is wicked onto covered area
126 (Fig. 15). As saliva migrates along covered area 126 of
absorbent 104 and toward indicator 125 it moves between
compression lid 110 and holder 106. The migrating saliva then
contacts indicator 125 causing either migration of indicator 125
or a color change in the indicator or both. In this manner the
on-site examiner can observe that sufficient saliva quantity has
been applied to the absorbent and/or that the substance applied
to the absorbent is in fact saliva. The type of analysis
enabled will depend upon the type of substance used as indicator
125.
The apparatus provides test security both through the
type of indicator 125 which is utilized and also through the
structural features of compression lid 110 and holder 106 which
evidence attempts to tamper with sample probe 100. As stated
earlier once compression lid 110 has been affixed within holder
106 through the flattening crimp rivet 114 strong pressures are
required to reopen compression lid 110. In such an event, crimp
rivet 114 will be pulled back through receptacle 116, but crimp
rivet 114 will not be recompressed. Therefore, it is not
possible to insert a second time crimp rivet 114 through
receptacle 116. A second tamper resistant feature is provided
by breakaway connectors 112. As substantial pressure is
required to be applied by a blade-type wedge instrument to
remove compression lid 110 from holder 106 and to force crimp
rivet 114 from receptacle 116 the sudden release of compression
lid 110 from holder 106 will be strong and rapid and
- uncontrolled and will cause the rupture of at least one of
breakaway connectors 112. As these cannot be reattached it will
W093/l143~ 2 1 2 1 3 6 1 l'CTtUS92/1~)085
-35-
be obvious to the testing laboratory that sample probe 100 has
been the subject of tampering. Such separated breakaway
connectors 127 are illustrated in Fig. 15.
An additional tamper resistant feature is provided by
spikes 108 which protrude from compression lid 110 and upon
closure of compression lid 110 within holder 106 pierce
absorbent 104 and extend through spike receptacles 129. As
spikes 108 pierce absorbent 104 and secure the absorbent within
support stick 102 it is not possible to slide absorbent 104 out
of support stick 102 when compression lid 110 is closed and
secured within holder 106. If an attempt is made to withdraw
absorbent 104 when compression lid 110 is secured absorbent 104
will be shredded by the attempt. Additionally, it will not be
possible to insert a second absorbent sheet into the closed
holder as spikes 108 are projecting through spike receptacles
129 and blocking the insertion of a bogus absorbent into support
stick 102.
An alternative means of providing secure closure of
compression lid 110 within holder 106 is through the use of an
adhesive applied to holder 106 as shown in Fig. 15 by adhesive
134. Adhesive 134 can serve to seal compression lid 110 within
holder 106 and also serve as evidence of tampering. once
adhesive 134 dries it is in contact with both absorbent holder
106, compression lid 110, and absorbent 104. An attempt to open
compression lid 110 will result in shredding and release of
absorbent 104 which will be clearly obvious upon examination of
sample probe 100.
The embodiment of Figs. 12-16 also provides the
laboratory with a means for both testing the saliva sample upon
its receipt in the laboratory and a method of archiving a
portion of the same exact sample for future confirmation of test
results. This is accomplished through the use of a two-part
absorbent 104. In Fig. 12 absorbent 104 is shown composed of
test area 120 and archive area 122. These two areas are
separated on a line between tear points 136. Tear points 136
permit test area 120 to be separated from archive area 122 so
the desired tests may be performed on test area 120.
W093/ll434 21213 61 rCT/US92tl0085
-36-
In actual use, a sample probe 100 is inserted into a
subject's mouth and absorbent 104 is saturated with saliva. The
saliva migrates into covered area 126 and activates indicator
125. The activation of indicator 125 demonstrates to the on-
site examiner that a saliva sample of sufficient quantity hasbeen obtained. Sample probe 100 is then sent to the laboratory
for analysis of the saliva sample. In the laboratory, the
identification information of sample probe 100, as recorded on
label 138, by writing or by bar code, is noted. Test area 120
is then separated from sample probe 100 by tearing along tear
point 136. The desired tests are conducted on the saliva
present in test area 120. The remainder of sample probe 100,
inclusive of archive absorbent area 122, is then stored under
proper conditions for as long as the laboratory believes
necessary. This provides the laboratory with a convenient
reference sample composed of a specimen in archive area 122
which is identical to the specimen in test area 120 upon which
the initial testing was conducted. If a question arises
regarding the results of the analysis on test area 120, the
laboratory can be contacted, the archive specimen contained in
archive area 122 retrieved, and testing repeated.
In such a retest situation, it also will be clear to
the retest investigator that the sample probe 100 has not been
tampered with during the intervening period between initial
testing and repeat testing as all security indicia remain in
tact. During the course of original testing there is no
occasion to damage break-away connectors 112, or to make any
change to indicator 125, or to open compression lid 110.
Therefore, the integrity and security of the original sample is
maintained by archive area 122 and assurance is derived that no
substitution of absorbent 104 of archive area 122 has occurred
during the intervening time period between original testing and
the retest of archive area 122.
As stated previously indicator 125 may be composed of
a simple vegetable dye or composed of any of the biochemically
reactive indicators discussed herein. When a simple vegetable
dye is utilized indicator 125 indicates only that sufficient
saliva quantity has been applied to absorbent 104. When the
2 1213 61 PCT/US92/1008~
-37-
biologically reactive indicators are utilized indicator 125 will
demonstrate both that sufficient quantity of saliva has been
applied and that the substance applied to absorbent 104 is in
fact saliva.
It is to be understood that while certain forms of
this invention have been illustrated and described, it is not
limited thereto, except insofar as limitations are included in
the following claims.