Note: Descriptions are shown in the official language in which they were submitted.
? 1~279 ~
TS 7~02
P~STICIDAL ~ETHODS ~ND CO~POUND~
The present invention relates to the combating of
certain insect pests using isothiazole compounds, some of
which are novel. Aspects of the invention relats to the `~
use of such c~mpounds in combating larval pests, to nvvel
compounds, compositions and synthetic processes.
U.S. Patent No. 3541108 discloses pesticidal
isothiazole derivative~ of the formula
Cl CN
N /
S NHR -~
.~
: wherein R may be COR~ where Rl is an alkyl radical
containing 1 to 10 carbon atoms or phenyl.
: Insecticidal and herbicidal activity of the compounds
25 of U.S. Patent No. 3541108 is disclosed, the former being
against Musca domestica (housefly) and Blatta orientalis
(oriental cockroach~.
:
G.B. Patent No. 1226913 discloses a method for the
preparation of pesticidal, especially herbicidal and
insecticidal, compounds of general formula
.
` -' 2~22794L
,
R3 CN
N I .
\ / \ , ~
S NHR
in which Rl is a hydrogen atom or an alkyl radical
containing up to 6 carbon atoms or is a COR2 radical, R2 is
Ar or a straight or branched chain alkyl or alkenyl
radical containing up to 18 carbon atoms, which can be
substituted by at least one halogen atom, nitrile group or
radical Ar and/or in which one or more -CH2- groups can be
replaced by oxygen and/or an -NH- group which may be
substituted and/or CO, and/or in which one or more =CH~
groups are replaced by nitrogen, or R2 is a c~cloalkyl
radical containing 3-6 carbon atoms, or is a fuxyl,
:pyrrolyl or thie~yl radical which can be substituted~by at
least on halogen atom, nitro or sulphonic acid group or
acetyl radical, Ar is a phPnyl or naphthyl radical which
can be substituted by at least one halogen atom, cyano,
nitro, hydroxyl, mercapto or amino qroup or alkyl, alkoxy,
alkylthio, alkylamino or dialkylamino radicals, wherein
; 25 the:alkyl radicals or parts of radicals contain up to 4
~ carbon atoms, and R3 is a chlorine or bromine atom.
; ~ It has now been determined that certain isothiazole
compounds, some of which are novel, show interesting and
unexpected activity in combating larval pests of
lepidoptera species.
: ~ ~
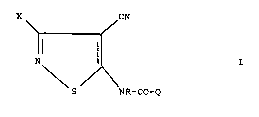
According to one aspect of the present invention
there is provided a method o~ combating caterpillar pests
? 2~22794
- 3 ~
at a locus, which comprises treating the locus with a
compound of general formula
X CN
N~ ¦ - :
: \ /\ .- ~" '''
s NR-CO-Q
'' .'. ~
whPre X represents a halogen atom, R represents a hydroqen -
atom or an optionally su~stituted alkyl group, and Q
represents an optionally substituted aryl or heteroaryl
group.
~15
Unless otherwise stated in this specification, an
: alkyl group may be linear or branched and suitably
co~tains up to lo, preferably up to 6, and mos~ preferably
up to 4,:carbon atomsj preferred examples being methyl and
~ : 20 et~yl.
; ~~ Unless otherwise stat~d in this specification, when
: any groups are designated as being optionally substituted,
the substituent groups which are optionally present may be
~any: of those customarily employed in the development of
pesticidal compounds, andlor the modification of such
: compounds to influence their structure/activity, ;:
p~rsistence, p~netration or other property. In relation to
a halogen atom, this may suita~ly denote iodine or, `: `~
espe~ially, bromine, fluorine or chlorine. In g2lleral
chlorine i5 a pr ferred halogen atom. In relation to an
alkyl group, specific ~xamples of such substituents may
include halo~en atoms, and phenyl, alkoxy, alkylthio,
hydroxy and cyano groups. In relation to an aryl or
heteroaryl moiety, optional substituents may include
.. .~,--. . -, , . , . ~ .
: ` - 212279~
-- 4 --
halogen atoms, and nitro, cyano, alkoxy, haloalkoxy,
hydroxy, amino, alkylamino, alkyl and haloalkyl
(especially CF3~ groups. There may suita~ly be 0-3
substituents.
A preferred halogen atom X is a chlorine atom.
.,
Preferably R represents a hydrogen atom or a
~ubstituted ~ethyl group, suitably a benzyl or a
(C~4alkoxy~methyl group. However, most preferably R
represents a hydrogen atom.
.,. -. ~
A suitable optionally substituted heteroaryl group Q
may include an optionally substituted thienyl yroup and an
optionally substituted pyridyl group. Preferably a
substituted heteroaryl group has a single substituent. A
preferred substituent is a halogen atom.
', ., .';~:
A preferred optionally substituted aryl group is an :
:20 optionally substituted phenyl ~roup. Preferably thiæ may
have 1, 2 or 3 substituents. ~ ~
Preferably, Q as a phenyl group has a substituent at ~- -
the 3-position, and optionally one further substituent, at
25 the 4- or, most preferably, the 5- position, or two ~
further substituents, at the 4- and the 5- positions. ~-
Preferred optional substituent(s~ of a phenyl group
Q include haloalkyl, alkyl, alkoxy, nitro and cyano
group(s) and, most preferably, halogen atom~s).
Particularly preferrPd groups Q include phenyl groups :~
having a halogen substituent at the 3-position, for
example 3,4-dihalophenyl, 3,5-dihalophPnyl groups and~
especially, 3-halophenyl groups. Such compounds of
-~ 21~'~7~4
general formula I, which show particularly interesting
activity may be regarded as a novel selected clas~ of
compounds and accordingly constitute a ~urther aspect of
the present invention. A part~icularly preferred compound
is 3-chloro- 4-cyano-5-(3-chlorobenæoylamino) isothiazole.
A further novel class of compounds is constituted by
compounds of the general formula I in which R represents
an optionally substituted alkyl group and accordingly such
compounds provide a further aspect of the present
invention.
.-: ,
In accordance with a fuxther aspect of the present
invention there is provided a process for the preparation
of a novel compound of the general formula I, which
process comprises reacting together compounds of general
formula II and III
X CN
\r
: 11 11 , ,
N
\ / \
S NHR
Hal C0-Q III
The reaction may suitably be effected under standard
acylation conditions, in the presence of an organic
solvent. Examples include pyridine, dimethylformamide and
acetonitrile. A basic solvent may be employed, and/or an
additional base, for example an alkali metal base, an
212279~
- 6 ~-
example being potassium carbonate. ThP reaction may
suitably be effected at an elevated temperature, for
example from 50C to the reflux temperature. ~;:
When the process is applied t~ prepare a novel
compound o~ general formula I in which R represents an :
optionally substituted alkyl group, the required compound
of general formula II may suitably be prepared via the
corresponding 5-amino isothiazole, reacted with a compound :~ ~
lO R-Hal, suitably in the presence o~ an organic solvent, for :~-
example acetonitrile, and an alkali metal base, for ` ~:
example potassium carbonate, suitably at an elevated ~-~
temperature; or via the corresponding 5-halo isothiazole,
reacted with a compound RNH2, suitably in the presence of
an organic solvent, for Pxa~ple tetrahydrofuran, suitably
: at ambient temperature. Both such methods are described
hereafter in greater detail.
The term Hal preferaby denotes a chlorine atom.
~ ~.
ThP invention also provide~ a pesticidal composition :~
comprising a carrier and, as active ingredient, a novel ~ ~
compound of general formula I. . ~:
~: ~
The invention further provides the use of a compound : :
of general formula II, in combating larvae of lepidoptera
species.
The target pests may be the larval "caterpillar" or
"worm" forms of insects o~ the genus Heliothis, such as
H.zea (corn earworm), cotton bollworm, tomato fruitworm,
and H. virescens ~tobacco budworm); the genus Agrotis,
: such as A. ipsilon (black cutworm); the genus
Tri~hoplusia, such as T.ni (cabbage looper); the genus
Plutella, such as Plutella xylostella (diamond back moth);
: 35 and the genus Spodoptera, such as S. littoralis (Egyptian
r~ 212279~ `:
- 7
cotton leafworm). A preferred aspect of the present
invention therefore relates to the pssticidal treatmen~ of
such pests.
A carrier in a composition according to the invention
is any material with which the active ingredient is
formulated to facilitate application to the l~cus to be
treated, which may for example be a plant, seed or soil,
or to facilitate storage, transport or handling. A carrier
may be a solid or a liquid, including a material which is
normally gaseous but which has been compressed to form a
liquid, and any of the carriers normally used in
formulating pesticidal compositions may be used.
Preferably compositions according to the invention contain
0.5 to 95% by weight of active ingredient.
Suitable solid carriers include natural and synthetic
clays and silicates, for example natural silica~ such as
diatomaceous earths; magnesium silicates, for example
talcs; magnesium aluminium silicates, for example
attapulgites and vermiculites; aluminium silicates, for
example kaolinites, montomorillonites and micas; calcium
carbonate; calcium sulphate; ammonium sulphate; synthetic
hydrated silicon oxides and synthietic calcium or aluminium
~ilicates; elements, for example carbon and sulphur;
:natural and synthetic resins, for example coumarone
resins, polyvinyl chloride, and styrene polymers and
copolymers; solid polychlorophenols; bitumen; waxes; a~d
solid fertilisers, for example superphosphates.
: : 30
Suitable liquid carriers include water; alcohols, for
example isopropanol and glycols; ketones, for example
acetone, methyl ethyl ketone, methyl isobutyl ketone and
cyclohexanone; ethers; aromatic or araliphatic
: 35 hydrocarbons, for ~xample benzene, toluene and xylene;
~r ~
9 ~
- 8
petroleum fractions, for example kerosine and light
mineral oils; chlorinated hydrocarbons, for example carbon
tetrachloride, perchloroethylene and trichloroethane.
Mixtures of different liquids are often sui~able.
Agricultural compositions are often formulated and
transported in a concentrated form which is subsequently
diluted by the user before application. The presence of
small amounts of a carrier which is a surface-active agent
facilitates this process of dilution. Thu5, pre~erably at
least one carrier in a composition according to the
invention is a surface-active agent. For example the
composition may contain at least two carriers, at least
one of which is a surface-active agent.
A surface-active agent may be an emulsifying agent,
a dispersing agent or a wetting agent; it may be nonionic
or ionic. Examples of suitable surfac~-active agents
include the sodium or calcium salts of polyacrylic acids
and lignin sulphonic acids, the condensation products of
fatty acids or aliphatic amines or amides containing at
l~ast 12 carbon atoms in the molecule with ethylene oxide
and/or propylene oxide; fatty acid esters of glycerol,
sorbitan, sucrose or pentaerythritol; condensates of these
with ethylene oxide and/or propylene oxide; condensation
products of fatty alcohol or alkyl phenols, for example ~-
octylphenol or -octylcresol, with ethylene oxide and/or
propylene oxide; sulphates or sulphonates of these
condensation products; alkali or alkaline earth metal
salts, preferably sodium salts, of sulphuric or sulphonic
acid esters containing at least 10 carbon atoms in khe
molecule, for example sodium lauryl sulphate, sodium
secondary alkyl sulphates, sodium salts of sulphonated
castor oil, and sodium alkylaryl sulphonates such as
-
2~2279~
: `
- 9 ~
dodecylbenzene sulphonate; and polymers of ethylene oxide
and copolymers of ethylene oxide and propylene nxide.
The compositions of the invention may for example be
formulated as wettable powders t dusts, granules,
solutions, emulsifiable concentrates, emulsions,
suspension concentrates and aerosols. Wettable powders
usually contain 25, 50 or 75% w of active ingredient and
usually contain in addition to solid inert carrier, 3-10%
w of a dispersing agent and, where necessary, 0-19% w of
stabiliser(s) and/or other additives such as penetrants or
stickers. Dusts are ususally formulated as a dust
concentrate having a similar composition to that of a
wettable powder but without a dispersant, and are diluted
in the field with further solid carrier to give a
composition usually containing l/2-10% w ~f active -~
ingredient. Granules are usually prepared to have a size
between 10 and loo BS mesh (1.676 - 0.152 mm), and may be
manufactured by agglomeration or impregnation techniques.
~enerally, granules will contain '/2-75% w active
ingredient and 0-10% w of additives such as stabilisers,
surfactants, slow release modifiers and binding agents.
The so-called ~dry flowable powders" consist of relatively
small granules having a relatively higher concentration of
active ingredientO Emulsifiable concentrates usually
contain, in addition to a solvent and, when necessary, co-
solvent, 10 50~ w/v active ingredient, 2-20% w/v
emulsifiers and 0-20% w/v of other additives such as
stabilisers, penetrants and corrosion inhibitors.
Suspension concentrat2s are ~susally compounded so as to
obtain a stable, non-sedimenting flowable product and
usually contain 10-75~ w actiYe ingredient, 0.5-15% w of
dispersing agents, 0.1-10% w of suspending agents such as
protective colloids and thixotropic agents, 0-10% w of
other additives such as defoamers, corrosion inhibito~s,
: ~:
~ :
~ 212279~ ::
~. ~ .;,
1 0
stabilisers, penetrants and stickers, and water or an
organic liguid in which the active ingredient is
substantially insoluble; certain organic solids or
inorganic salts may be present dissolved in the
formulation to assist in preventing sedimentation or as
antifreeze agents for water.
Aqueous dispersions and emulsions, for example
oompositions obtained by diluting a wettable powder or a
concentrate according to the invention with water, also
lie within the scope of the inventionO The said emulsions
may be of the water-in-oil or of the oil-in-water type,
and may have a thick "mayonnaise"-like consistency.
Compositions in accordance with the invention may
also contain other ingredients, for example other
compounds possessing pesticidal, herbicidal, or fungicidal
properties. Ths rompounds of the invention may be found to
be especially useful when applied in admixture with
insecticides and/or acaricides, e.g. organophosphates,
pyrethroids, ureas and organotin compounds, for example
the commercial products fenvalerate, permethrin,
cypermethrin, deltamethrin, alphacyperm~thrin, fenbutatin
oxide, flufenoxuron diflubenzuron and trefluron.
The invention will be further understood from the
following illustrative examples.
Example 1
Preparation of 3 -chloro-4 -cy~no-5-
chlorobenzoylamino) isothiazole
[X=Cl; R=H; Q=3-Cl-Ph]
.
~t227~
3-Chloro-4-cyano-5-amino isothiazole tO.8g) was
stirred in acetonitrile (50ml), together with potassium
carbonate (0.7g). 3-Chlorobenzoyl chloride was added and
the reaction mixture was heated to 100C, for 3 hours. The
solvent was removed by evaporation and the residue
titurated in water, and acidified to a pH of about 1,
using 2M hydrochloric acid. The solid was filtered and
recrystallised from acetone-methanol (1/1, v/v) to give
the title compound as a cream solid (mp 260C; 1.20g).
Analysis
%C %H %N
Calc. 44.3 1.7 14.1
Found 44.5 2.1 14.4
Examples 2-31
Further compounds as noted in Table 1 were prepared
by prvcesses analogous to the process of Example 1.
Characterising data for the compounds of Examples 2-31 is ::
noted in Tabl- 2, where available.
" " i
, :~
.:
:
L2279~ ;~
- 12 - `
TABLE l
- _ ~_= ' ~:
E~ X ~ Q ~ ~
_ _ ~ _
2 Cl _ H 2-Cl-Ph
3 Cl H 2-F-~h _ _
_ _._. __ _
4 Cl H _4-CF3-Ph :~
5 __ _l 4-Cl-Ph _
6 Cl H _ _ 3-NO2-Ph
7 Cl H 3-F-Ph : ::~
_
8 _ Cl H 3~CF3-Ph
9 Cl H 3-Br-Ph
_ ::
Cl H 3-CH3-Ph
11 Cl H 3-OCH1-Ph
. _ ., , I
12 Cl H 3-CN-Ph _ ¦
13 Cl H 3,4-Cl2-Ph ¦
14 Cl H _ 3,4-F2-Ph-
Cl H 3-Cl,4-F-Ph ¦
16 Cl H 3-Br,4-~-Ph
. . , I :
20 ~7 Cl H 3,5-Cl2-Ph
: 18 Cl H 3,5-F2-Ph
. _. _ : ;`
19 l H 3,5-Br2-Ph :
ClCH2 Ph 3-Cl-Ph _: ~
21 Cl-CH2-O-c2~s 3 Cl-Ph ~ :-
2 5 2 2 Cl H Ph _ -~
. . _ _ . _. ~
23 _ Cl H . 4-F-Ph _
24 Cl H 6-Cl-pyrid-3-yl
Cl H 5-Cl-thien-2-yl ;:
. . . ~ .
26 Cl _ H 2! 4 9 5-F3-Ph
27 Cl H 3,4,5-F3-Ph
28 Cl H 3-NO2~4-Cl-Ph
~ ._ _ _ - .
2 ~
- 13 - ~
_ , __ _ _ _ ___, _ ~:
Ex. X R Q
~o.
_ ___ ___ -
29 Cl H 3-NO2, 4-F-Ph
_
3 0 Cl -CH2-O-CH3 3-Cl-Ph
_ .
31 Cl 2_ _3 3-Br, ~-F Ph
.
`''.
'''.'.''
' ~ ~
- -:
7~
.:
- 14 -
TAB~8 2
_ ~ r~ ,_
¦EXA~IPLE ANALYSIS MP/C ¦
IN0.
I ~C %H %N
_ _ _ _ = _.
l 2 calc 213-215 I -
I . . I
¦- - found
3 calc 12g-131 ¦
found
_ _ .
4 calc 43.4 1.5 12.7 300 1
. ~ . I
found 43.6 2.0 ~2.5 I
-- .. .. _ . . _ .. . , . . ~ .11
calc 298-300
.... _ I
found
6 calc42.7 1.6 18.2 301
. _
found42.0 1.9 18.2
_
7 c~lc 274-2~1
found
. _ __ ~ _ _ . I ,~
8 cal_43.4 1.5 12_7 253 ¦
found43.0 2.0 13.3 l l
. .. . _~ ~ . . .:
9 c~ _ 2.1 _ _ 11.1 300
_ _ l
found 35.0 2.4 11.0 I
. _ . . . _ ~
calc 51.g 2.9 15.1 250 ¦ ~1
.. _ _ . I . ,.
found52O4 3~.0 15.3
_ _ __ - _
11 calc 4901 2.7 14.3 240
~ . ~ ,
found 49.0 3.0 14.3
, . . . _ . _ . . I .
12 calc49.9 1.7 19.4 300 ~ -
found 48.9 2.0 19.3 ¦
.. . _ .... _ _ . _
13 calc3g.7 1.2 1206 300 l
~ , l
found 39.5 1.6 12.5 I
. _ .. . . .......
14 calc44.1 1.3 14.0 273 l
- ... _ _ . ._ I
found 44.4 lo9 14~1
_ . I_
calc41~8 1.3 13.3 300 l
._ ... _.. _ . I
found 41.4 1.7 13.4 l
_ _ _ _ _. _ . e- - r
16 ~ 6 ~ 1.1 11.6 278
found 36.9 1.5 11.6 _
~ 15 ~
_
l E~ANPLE _ A~Y8I~ NP/~
I N0. r - _
I %~ I%II %~
_ ___ _
17 calc 39.7 1.2 12.6 294
.
found 39.8 1.5 12.4
.. __ ~ . _ _ . .
18 calc 44.1 1.3 14.0 287
.... _ ._ . . _
l found 43.9 1.8 14.1 l
..._ _ ,.___ . . ~
19 c~lc 31.3 1.0 10.0 30
_ ~ .
found 31.7 1.4 10.1
_ I
l 20 calc 55 7 2.i3 10.8 48
I ___ ~ _. _ _
found 56.1 3.1 10.
. . . . . I
i 21 calc 38u6 3.6 19.3 75
_ ~ . . _ I
found 38.9 3.7 19.2 l
. .__ . 1.
22 calc 249-253
_ ..
found l :
_ .......... _ __~!
23 calc 272-278 1
I -. ... _ . I -~:
found _ _
24 calc 35.8 2.3 16.7 300 ¦
. . . _ I .
found 36.3 1.9 16.7 l
. . _ l .- ':
l 25 calc 35.5 1.0 13.8 300
I .. _ I :.
found 36.1 1.4 13.8 l
_ .. .. ._ . . . I
26 calc 41.6 l.0 13.2213 215 ¦
I . . I :~'
_ found 41.7 1.3 13u2 l
l l
27 ~ 6 1.0 13u2 300
found 42.0 1.4 13.4
_~ . I
Z8 calc 38.5 1.2 16.3 236
_ , ~_ . _
found 39.0 1.5 16.4 l
. . - l :.
¦ 29 calc 40.4 1.2 17.1 236l - ~;~
r
found 39.8 1.4 16.8
I . .... _ I
1 30 calc 45.6 2u6 12.3 130
l . l
found 46.~ 3.1 12.2 l
I _ _ . __~ . Il
1 31 calc 38 6 2.0 10.4 131
I . . o _ .. ~ -- --~.
found 39.0 2.4 10.2
- ~ ,~ _. ____ _~---_ _ . . _ _
- 16 - -~
Examples 32 and 33 below describe the preparation of
intermediates for the compounds of Example 20 and 21,
respectively.
Example 32
Preparation of 3-chloro-4-cyano-5-~N-benz~lL
isothiazole.
10Benzylamine (l.lg) wa~ added to a stirring solution
of 3,5-dichloro 4-cyano isothiazole (0.9g) in
tetrahydrofuran ~50ml) at 20C. The mixture was stirred at
20C for 3 hours. The mixture was filtered and the
filtrate evaporated. The residue was chromatographed --
using silica gel and chloroform as eluent to give the
title compound as a white solid (1.2g, mp 164C~.
Analysis
-
%C %H %N
CalcO 52.9 3.2 16.8
: Found 53.3 3.3 16.8 ~-
Exam~le 33
Prepara~ion of 3-chloro-5-cyano-5-(N-ethoxymethylL
¦ isothiazole.
:
Potassium carbonate (1.4g) and chloromethyl ethyl
- ; 30 ether ~2g) were added to a stirring solution of 3-chloro
4-cyano-5-amino isothiazole (1.6g) in acetonitrile (50ml).
~he mixt~re was stirred at 100C for 2 hours. The mixture
was filtered and the filtrate evaporated. The residue was
chromatographed using silica gel and dichloromethane as
. ,~ . . . , ~ , . , , - , , . , - - , , ,
212279~
,
eluent to give the ti~le compound as a whîte solid (l.Og,
mp 200C).
Analysis
%C %H %N
Calc. 38.6 3.6 19.3
Found 38.9 3.7 19.2 :~
Example 34 ~-
..... ................................................................... .... -, ,:
Pesticidal Activity - ~-
Pesti~idal activity o~ compounds of the invention was
assess d against larvae of the following pests~
SPodoptera littoralis
Heliothis armi~era
Plutella xylostella
The test methods employed for each species appear `~
below. In each test, unless otherwise stated, solutions
or suspensions of test compound were made up at a
concentration in water of Q.1%w containing 10%w acetone -:
and 0.025%w '~TRITON X-lOO" (trade mark) surface active
agent (the condensation product of ethylene oxide with an
alkyl phenol). These solutions were sprayed at a rate ~-
equivalent to 340 litres per hectare (3.4 x 10~5m3/m2) onto
Petri dishes containing either test species per se or diet : ~-
: onto which test species were subsequently introduced, as
indirated. In some assays leaf discs infested with test
species were sprayed whilst other assays inv~lved the
30 spraying of plants which were infested subseguently with ~:
:test species after the spray solution had dried. The
tes~s were all conducted under normal insectary conditions
~'
- .
.
~ 12279~
- 18 -
[23C + 2C, fluctuating humidity and light). Mortality
assessments were made as indicated below, in terms of
percentage mortality figures.
5(i) Spodopte~a littoralis (7 day)(Sl 7D)
Test solutions were sprayed as indicated above onto
Petri dishes containing a nutritious diet for Egyptian
cotton leafworm larvae. When the spray deposit had dried,
each dish was infested with ten 2nd instar larvae.
Mortality assessments were made 7 days after spraying.
(ii) Spodoptera littoralis (foliar)(S1 Fol)
Test solutions were sprayed as described above onto
Petri dishes containing 9cm discs of Chinese cabbage
leaves on filter papers. After drying, each di~h was
infested with ten 2nd instar larvaa. Mortali~y
assessments were made 24 hours after infestation.
(ii3 Plutella xylostella (Px)
20A test solution was sprayed as described above onto
: a 4 cm diameter Chinese cabbage leaf placed ventral side
up in a pot. When the spray deposit had dried the pot was
infested with 10 young instar larvae and a lid added.
: Mortality was assessed after 1 or 2 days, depending on
when the test was carried out.
(iv) Heliothis armi~era (Ha3
; A test solution was sprayed as described above onto
a 4 cm diameter Chinese cabbage leaf placed ventral side
up in a pot. When the spray deposit had dried the pot was
infested with 10 young instar larvae and a lid added.
~ Mortality was assessed after 1 day.
:~:
Results of the assessments d~scribed in Examples 34
:~ 3S for each of the compounds of Examples 1 to 31 are provided
~ 7 9 ~
19
in Table 3. In the Table grades A, B and C indicate
mortalities of 70-100%, 40-69% and 0 39%, respectively, at
the tPst concentratioll of O .1%w ( 1000 ppm) . A blank space ~
denotes that testing was not carried out. ~ ~ ;
~,. ;'
' ~ ' ;''
~` ~''`
- .: .
.. ~
: ~ '. ,:
~ ~ :
,
:
^~ 2122~
-- 20
~ABLE 3
~ __ = ----, - ,. _ . . . ,
Compound ~1 81 P~ Ha :
ol~ 7~ ~ol
3~x No. :
~ _ __ _ ___ : ~.
1 A A A A I
2 . C
_ I
3 A A I ~:
. _ _ A
. __ _ _ I '.:~'.. :.'~
l O 5 A A . ~ ~ `~
6 A A __~
. ,,_ . . _ . I . '
7 A A B l -~ ::
. , ~ . I ~.`
8 C B A I ~::
A A
. . . _ . I :~
A C C I -
. . _ _ I
11 C A C
12 A A . .. .
14 A A A :
__
A A A
.. _ _
16 A A A A
17 A A A A
18 A A A
~ . . . .
19 A A A
... .. . .
B A A
_~ . . T
21 A A A A
. . __
2 2 A .
2 3 A .=
24 B A A
..... _ __ .... ... . . _
3 0 2 5 C A C C
26 A A A
...
27 A A
_ -
~ ~2.~794
- 21 -- :
~ --- --= _ _ . ~ I ~:
¦ Compound 81 81 P~ N~
¦ of 7D Fol ¦
I Ex NoO I
. . _ . _ _ ~
I 28 A A l
~ . ~ ~ .
2 9 A A B ¦ :
3 0- ~ A A A
31 A A A l ::
_ ~ . .... ~ _ --- ~ - -
'' -: .~'
2~22~
Compounds of the present invention were also tested
against other insect pests ~ including Aedes .aPqypti
(yellow fever mosquito), Musca domestica (housefly),
Acyrthosiphon pisum (pea aphid), Megoura viciae (vetch
aphid), Phaedon cochleariae ~mustard beetle), Trialeurodes
vaPorariorum (greenhouse whitefly), Nephotettix cincticeps
(green leaf hopper) and Nilaparvata luaens ~brown rice
plant hopper). However, in most cases this revealed no or
low activity. Preliminary conclusions are that the
activity of the compounds found, in the majority of tests,
against caterpillar pests, is surprising, given the
otherwise unpromising insecticidal activity of the
compounds.
Further glasshouse tests have shown particularly
interestin~ activity against larval pests from ~he
compounds of Examples 1, 7, 9, 13, 14, 15, 16, 17, 18 and
19 .