Note: Descriptions are shown in the official language in which they were submitted.
WO93/10281 2 1~ 3 ~ 1 7 PCT/EP92/02666
~ -- 1 --
~T.T. FOR T~F. ~T~CTROTYSIS OF ATUMINA
p~FF.R~RT.Y AT TOW TF~PF~ATURES
Techn; CA 1 Fie1~
The invention relates to a cell for producing
aluminium by electrolysis of alumina dissolved ln a molten
halide electrolyte particularly at temperatures between
680~-880~C.
Background of the Invention
Aluminium is produced by the Hall-Héroult process
which involves the electrolysis of alumina dissolved in
molten cryolite (Na3AlF6) at about 960~C using carbon
anodes which are consumed with the evolution of CO2.
However, the process suffers from major disadvantages. The
high cell temperature is necessary to increase the
solubility of alumina and its rate of dissolution so that
sufficient alumina can be maintained in solution, but
requires heavy expenditure of energy. At the high cell
temperature, the electrolyte and the molten aluminium
aggressively react with most materials including ceramic
and carbonaceous materials, and this creates problems of
contAlnment and cell design. The anode-cathode distance is
critical and has to be maintained high due to the
irregular movement of the molten aluminium cathode pool,
and this leads to loss of energy. Since the anodes are
continually being consumed, this creates problems of
process control. Further, the back oxidation of Al to Al3+
decreases the current efficiency.
WO93/10281 PCT/EP92/02666
2123~17
-2- _
Potentially, the electrolysls of alumina at low
temperatures ~below 880~C) in halide melts has several
distlnct advantages over the conventional Hall-Héroult
process operating at about 960~C. As shown by bench-scale
tests, electrolysis at reduced current densities in low
temperature melts potentially offers a significant
advantage in increasing the stability of electrode
materials, but it has not yet proven possible to implement
the process in a way where this advantage could be
realised in larger scale cells and in commercial cells.
Other potential advantages are higher current and energy
efficiencies and the possibility of designing a completely
enclosed electrolytic cell.
Problems which hindered the practicability of low
temperature electrolysis are the low alumina solubility in
low temperature electrolytes, as well as low alumina
solution rates. Under these conditions, a sufficiently
high transport rate of oxide ion species from the bulk of
the electrolyte to the anode surface cannot be maintained
at the anode current densities normally used in
conventional Hall-Héroult cells. The configuration of
cells presently used does not permit a substantial
increase of the relative surface area of anode to cathode.
This means that a reduction of the current density would
lead directly to a reduction of the cell productivity.
Moreover, the design of presently used cells does not
enable an increase of the electrolyte circulation to
increase the transport rate of oxygen ions to the anode
active surface area and to increase the dissolution rate
of alumina in the electrolyte.
Low temperature alumina electrolysis has been
described in US Patent No 3,951,763 and requires numerous
expedients such as the use of a special grade of water-
containing alumina to protect the carbon anodes, and the
bath temperature had to be 40~C or more above the liquidus
temperature of the Na3AlF6/AlF3 system in an attempt to
avoid crust formation on the cathode. In practice,
WO93/10281 2 1 2 3 '~ 1 7 PCT/EP92/02666
however, the carbon anodes were severely attacked during
anode effects accompanied by excessive CF4 emissions.
Crusts also formed on the cathode up to electrolyte
temperatures of 930~C.
Because of the difficulties encountered with
fluoride-based melts, major efforts to secure the
advantages of low temperature electrolysis were devoted to
different electrolytes, notably chloride based
electrolytes where AlC13 is used as a feed, the anode
reaction being chlorine evolution. See e.g. K. Grjotheim,
C. Krohn and H. 0ye, Aluminium 51, No 11, 1975, pages 697-
699, and US Patent 3,893,899. However, problems related to
the production of pure AlCl3 have hitherto eliminated this
process from commercial application.
Another proposal to produce aluminium in a low
temperature process involved dissolving A12O3 in an
- LiCl/AlC13 electrolyte to form AlOCl which was
electrolyzed at approximately 700~C. However, the rate of
aluminium production was too low for practical commercial
application (see "Light Metal" Vol 1979, p. 356-661).
US Patent 4,681,671 proposed an important new
principle for the production of aluminium by electrolysis
of alumina dissolved in a molten fluoride-based
electrolyte in an aluminium reduction cell, at a
temperature below 900~C, by effecting steady-state
electrolysis using an oxygen-evolving anode at an anode
current density at or below a threshold value
corresponding to the ,m~X; ~lm transport rate of oxlde ions
in the electrolyte and at which oxide ions are discharged
preferentially to fluoride ions.
That invention was based on the insight that oxide
ions in low concentrations, as in the case of low
temperature melts, could be discharged efficiently
provided the anode current density did not exceed the
given threshold. Exceeding this value would lead to the
WO93/10281 PCT/EP92/02666
2123417
discharge of fluoride ions which had been observed ln
experiments using carbon anodes.
The electrolytic alumina reduction cell for carrying
out the method contained a molten fluoride-based
electrolyte with dissolved alumina at a temperature below
900~C, an inert oxygen-evolving anode and a cathode. The
anode had an electrochemically active surface area
sufficiently large to allow it to operate with an anode
current density at or below the given threshold. In order
to carry out stable electrolysis under the given
temperature conditions and with the corresponding low
solubility of alumina, the low temperature electrolyte was
circulated from an electrolysis zone to an enrichment zone
and back, to facilitate and speed up the solution rate of
alumina.
The preferred cell design had vertical anodes in
parallel spaced apart relationship above a horizontal
drained cathode having holes for the upward circulation of
electrolyte and through which the produced aluminium could
drain to the bottom of the cell. With this design it was
proposed to lower the anode current density to values
compatible with low temperature operation, usually while
maintaining the cathode current density at conventional
values. The aim was to maintain a satisfactory production
of aluminium per unit floor surface, enabling the process
to operate economically.
A proposal to implement this principle was made in
U.S. Patent No. 5'015'343, for the electrolysis of alumina
in halide melts in conditions of very low solubility (< l
weight percent of alumina) which corresponds also to low
temperature operation. Here, use was made of a carbon
anode or a substantially non-consumable anode, whose lower
surface faced a cathode pool of molten aluminium. The
anode was a massive body provided with a series Of
vertical openings designed on the one hand to increase the
4 '1 ~ ~i
_- -5-
surface area of the anode and on the other hand for the
release of the anodically evolved gas.
This design however suffers the serious drawback that
most of the anode reaction takes place on the lower
horizontal part of the anode surface, opposite the
underlying cathode, which nullifies the attempt to produce
an anode with a high operating surface area. A similar
objection applies, to a lesser extent, to the previously
. mentioned cell.
With these cell designs proposed for low temperature
electrolysis of alumina in a halide melt it has not proven
possible to achieve efficient electrolysis. In particular
it has not been possible with these designs to achieve the
desired production per cell unit floor area in the low
temperature conditions with the corresponding low
solubility of alumina because of the difficulties of
effectively operating the anodes over an extended surface
area compared to the floor area.
With known cells and processes virtually all
materials developed for the anodes inadequately withstand
the operating conditions in the agressive electrolyte at
high temperature and high current density, thus providing
an incentive for operation at lower temperatures.
European Patent Application EP-A-O 126 555, filed on April 25,
1984 and published on November 28, 1984, discloses an aluminum
production cell with spaced monopolar anodes and cathodes joined by
bolted pins. In one embodlment, the anodes and cathodes are
generally vertical with slanted or inclined electrode surfaces.
U.S. Patent S 006 209 discloses an aluminium
production cell with multimonopolar anodes and cathodes
wherein the anodes have protruding bottom parts which
generate bubbles providing a gas-lift effect in the
electrolyte between the anodes and cathodes. Alumina is
fed into a space outside the anodes and cathodes.
A~DFn ~L~tr~
2 ~ 1 2r 3 Q~ 1 7 r r ~
. .
r
-6-
Summary of the Invention
In electrolysis cells for the production of aluminium
by the electrolysis of alumina dissolved in a molten salt
electrolyte containing halogen compounds, the electrolyte
has an electrical resistivity substantially higher than
that of the anode or cathode materials utilizing
carbonaceous or substantially non-consumable material made
of electrically conductive material resistant to the
electrolyte and to the products of electrolysis.
When operating at a temperature substantially below
that of commercial Hall-Héroult cells (much below 860~C)
the solubility of alumina becomes substantially lower
therefore requiring operation at a lower anode current
density, the lower the alumina concentration in order to
have an effective current density substantially below that
corresponding to the resulting lower limiting current
density of preferential oxygen evolution. Therefore such
electrolysis cells, in order to have a productivity per
unit horizontal area comparable to that of a Hall-Héroult
cell, require a substantial increase of the effective
active anode surface.
Such increase can be obtained by increasing,
according to the present invention, that part of the
active surface area of the anode which faces the active
surface area of the cathode and which is substantially
parallel to such surface area. The active surface areas
are positioned preferably substantially upright or at a
slope so that their horizontal projected area is only a
fraction of the active surface areas.
An object of the invention is thus to provide an
electrolysis cell for the production of aluminium by the
electrolysis of alumina dissolved in a molten salt
electrolyte containing halides, preferably at a
temperature below 880~C, using substantially non-
consumable anodes cooperating with a cathode arrangement,
AMEN~ED SHEEJ
3 4 ~ 7
--7--
wherein hlgh ceil productivity can be attained by using
anodes and cathodes in a configuration enabling effective
use of large anode and cathode surfaces-
This is achieved with a design using a multimonopolar
arrangement of interleaved anodes and cathodes have facing
operative surfaces which are upright and are in spaced
subs~antially parallel relationship. In other words, by
ma~ing the active anode surface area substantially
parallel to the active surface area of the cathode, and by
positioning the anodes and cathodes upright or
substantially upright, large active anode and cathode
surface areas can be used and the horizontal projected
area of the anodes and cathodes on the cell floor is only
lS a fraction of the active surface areas. This parallelmultimonopolar configuration provides an optimum current
distribution because of the near homogeneous electric
field between the electrodes.
Previously proposed designs of multipolar cells for
aluminium production by the electrolysis of alumina
dissolved in a halide melt were aimed at increasing the
cell productivity, over that obtainable with Hall-Héroult
cells, through an increase of electrode surface area,
keeping the operating current density referred to the
projected surface cell floor area at the usual value of
0.5 - 1 A/cm2. However, anode and cathode materials with
acceptable technical/ecomomical characteristics are not
available at present and these cell designs remain purely
conceptual.
When adopting the present invention with a vertical
mutipolar configuration and preferably used in a low
temperature bath at 680 - 880~ C, use is made of the large
available active electrode areas to operate at a low
current density compatible with low alumina solubility,
ie. below or at the threshold value for halide evolution,
typically at an anode current density of 0.1 to 0.4 A/cm2,
..,,,-uED SHEET
21-%3~1~7
'~ --8 r
while still attaining an acceptable cell productivity per
cell floor surface area, comparable to that of a Hall-
Héroult cell or possibly even higher.
By using facing electrodes with appropriate large
surface areas, it is also possible to operate with
electrolytes (fluorides or mixed fluoride-chlorides) that
could not hitherto effectively be used as a carrier for
alumina to be electrolysed, on account of the low
solubility.
This new arrangement has the advantage that it can
make use of existing anode and cathode materials that can
withstand the operating conditions at lower current
densities at the same temperature (usually about 940-
960~C) or at lower temperatures (below about 880~C), but
which failed in the more aggressive higher temperature
baths at the usual high current densities necessary to
achieve an acceptable production rate in the conventional
cell designs.
Thus, the arrangement is particularly advantageous at
lower temperatures, but can still be operated
advantageously at higher temperatures, because the low
current density operation enables the use of anode
materials that could not withstand operation at higher
current densities in high temperature molten electrolytes.
By suitably- lowering the anode current density and
maintaining an uniform current distribution over the large
anode surface area with the new ce-ll design, many anode
materials which fail at the usual high current densities
(from 0.5 but usually about 1.0 A/cm2 of the operative
anode surface) can now perform satisfactorily at the
higher temperatures if the anode current density is
lowered sufficiently, possibly down to about a tenth of
the values used heretofore.
Moreover, the current efficiency would be at least as
high as in Hall-Héroult cells, usually higher, and the
~.MEN~ED S~E~T
2 1 2 3 4 1 7 r 1
~ r ~
_9_
energy efficiency would be significantly improved by 20 to
30% compared to Hall-Héroult cells particularly because of
the low current density and the reduced anode-cathode
distance at which the multipolar cells according to the
present invention can efficiently operate.
The multimonopolar arrangement of anodes and cathodes
can have means for electrical connection to the anodes at
the top of the cell, and means for electrical connection
to the cathodes at the bottom of the cell. For instance,
the bottom ends of the cathodes dip into a cathodic
aluminium layer on the bottom of the cell, the cell bottom
having a current collector bar or similar means for
providing electrical connection of the aluminium layer to
an external cathodic current supply.
The anodes and cathodes may be substantially vertical
plates with the cathodes separated from the anodes by
spacers of electrically non-conducting material resistant
to the electrolyte and to the products of the
electrolysis, which spacers also act as electrolyte guide
means as explained below.
Preferably, at least the operative surfaces of the
anodes and possibly also of the cathodes are high surface
area structures such as porous or preferably reticulated
skeletal structures. The anodes and possibly also the
cathodes advantageously have a central current feeder
carrying a porous active part on its opposite faces. The
pore sizes of such structures may fo~ example range from 1
to 10 mm with a porosity of from 30 to 60 vol%.
The spacing between the facing active anode and
cathode surfaces is arranged to allow s~lely an upward
circulation of electrolyte in this space by gas lift, and
spaces are provided outside the multimonopolar arrangement
of anodes and cathodes for downward circulation of
electrolyte, and for replenishment of alumina in the
electrolyte. These spaces are conveniently arranged at the
AA~ENDED SffEET
2123'~17
--10--
sides or ends of the multlmonopolar arrangement of anodes
and cathodes, for instance several multimonopolar
arrangements of anodes and cathodes can be arranged slde-
by-side with the spaces therebetween. This electrolyte
recirculatlon arrangement promotes the dlssolutlon of
alumlna. To replenlsh the electrolyte alumlna can be fed
lnto these spaces by any sultable means whlch contlnuously
or lntermittently feed metered amounts of alumina.
To enhance this electrolyte recirculation the cell is
provided with electrolyte circulation guide means
adjacent the edges of the facing anodes and cathodes,
formed by electrically non-conductive spacers between the
edges of the facing anodes and cathodes, or by generally
vertical bars of electrically non-conductlve materlal
adjacent the edges of the faclng anodes and cathodes.
Advantageously, the electrolyte clrculatlon gulde means
comprise plates of electrically non-conductlve material,
possibly of alumina, arranged generally perpendicular to
and on either side of the multimonopolar arrangement of
anodes and cathodes.
In all of the cell designs, the total facing active
surface areas of the anodes and the corresponding facing
active surface areas of the cathodes is many tlmes,
preferably at least 1.5 tlmes and posslbly much greater
than the horlzontal projected area of the anodes and
cathodes onto the cell floor area, l.e. the area of the
cell bottom covered by the vertical shadow on the cell
bottom of an area enclosed by a llne surrounding all of
the anodes and cathodes. In this way, high cell
productivity per unit floor area can be achieved even at
very low current densities.
The electrolyte may be a fluoride melt or a mixed
fluoride-chloride melt. Suitable fluorides are NaF, AlF3,
MgF2, LiF, KF and CaF2 in sultable mixtures.
Ali,LNDED S.~IEET
~12:3~1~7
.~
--11--
The electrolyte may comprise a mixture of 42-63 wt%
AlF3 with up to 48 wt% NaF, and up to 48 wt% LiF, at a
temperature in the range of 680~-880~C, preferably 700~-
860~C
Another example of a fluoride-based molten salt is
about 35 wt% lithium fluoride, about 45 wt% magnesium
fluoride and about 20 wt% calcium fluoride, which melt has
a solidus temperature of approximately 680~C.
Other examples include alkali and alkaline earth
metal chlorides, and Group III metal chlorides, eg.
lithium, sodium and potassium chlorides, magnesium and
calcium chlorides and aluminium chloride mixed with alkali
and alkaline earth metal fluorides, and Group III metal
fluorides, eg. lithium, sodium and potassium fluorides,
magnesium and calcium fluorides and aluminium fluorides.
Lithium-based low temperature electrolytes are
advantageous because lithium penetrates carbon
preferentially to sodium, thereby reducing damage by
sodium intercalation. Also the lithium may act as dopant
for some ceramic oxides used as anode materials, or to
prevent dissolution of a lithium dopant from a lithium-
doped ceramic oxide used as anode material, and
furthermore lithium increases the electrical conductivity
of the melt.
The alumina can be present in the molten salt at a
concentration of about 0.1 to abou~ 5% by weight, often
from 1% to 4.5~, as compared to 10% for a standard
cryolite bath at the usual Hall-Héroult operating
temperature of about 960~C. Part of the alumina in the low
temperature bath can be present as undissolved, solid
suspension.
Mixtures of chlorides and fluorides may be
advantageous to improve physical properties such as
density and viscosity, and chemical reactivity. Examples
of mixed fluoride-chloride baths include one or more of
A~v'7ENDED S~tEET
4 ~ ;~
the fluorides of sodium, potassium, lithium, calcium and
alumlnium with one or more chlorides of the same elements,
typically with 90-70% by weight of fluorides for 10-30% by
weight of chlorides.
5Brief Descrl~tion of the Dr~win~s
The invention will now be described with reference to
the accompanying schematic drawings in which:
- Figure 1 is a cross-section through part of a
first embodiment of a multimonopolar cell according to the
invention ;
- Figure 2 is a similar view of a second
emboalment of a multimonopolar cell;
- Figure 3 illustrates a possible arrangement of
she c~lls of Figures 1 and 2 to provide for electrolyte
circulatlo.~ and alumina replenishment;
- Figure 4 is a schematic side elevation showing
diffe-ent forms of spacers arranged to promote electrolyte
re-circulation;
- Figure 5 is a schematic plan view showing
different forms of members arranged to promote electrolyte
re-circuLation;
- Figure 6 is a schematic plan view showing
another arrangement for promoting electrolyte re-
circulation; and
25_ Figure 7 is a schematic illustration of the
electrolyte circulation with the arrangement of Figure 6.
Det~iled Description
Fig. 1 shows a cell design with vertical anodes and
cathodes in the form of plates. In this cell, vertical
cathode plates 1 and anode plates 2 are held apart in
spaced parallel relationship by spacers 5. The cathode
-13-
plates 1 extend downwardly from the bottom of the anode
plates 2 and dip in a pool 4 of cathodic aluminium on the
cell bottom 7. This cell bottom 7 contains collector bars
(not shown) for the supply of current to the cathode.
The tops of the cathode plates 1 are located below
the level 6 of electrolyte 3 which advantageously is one
of the aforementioned halide-based electrolytes containing
dissolved alumina at a temperature up to 880~C.
The anode plates 2 extend up from the top of the
cathode plates 1, to above the electrolyte level 6, and
are connected by any convenient means to buswork, not
shown, for supplying anodic current. The level of the
aluminium pool 4 may fluctuate in use, but always remains
below the bottom of anode plates 2.
The spacers 5 occupy only a small part of the facing
anode/cathode surfaces, leaving the main part of these
facing surfaces separated by an electrolysis space
containlng electrolyte 3. Advantageously, the spacers 5
are located along the opposite edges of the facing
anodes/cathodes. The spacers 5 can be made of any suitable
electrically non-conductive material resistant to the
electrolyte and to the products of electrolysis, including
silicon nitride and aluminium nitride. Alumina,
particularly that calcined at high temperature, can also
be used, on account of the low solubility of alumina in
the melt and operation with the dissolved alumina at or
near saturation, with continuo,us or intermittent
replacement of the depleted alumina.
The anode plates 2 may be made of porous,
reticulated, skeletal or multicellular mat~rial, or may be
ribbed, louvered or otherwise configured to increase their __
active surface area relative to their geometrical area. ~
Generally, any substantially non consumable ceramic,
cermet or metal can be used, possibly coated with a
protective layer such as cerium oxyfluoride. The anodes
AMEN~a ~ffl
212:~17
-14-
can for ir.stance be made of SnO2-based materials, nickel
ferrites, metals such as copper and silver or alloys such
as Ni-Cu alloy or INCONEL~, possibly coated with a
protective coating. Composite structures can also be used,
for instance a Ni-Cu alloy on a Ni-Cr substrate, or
composite structures of oxidised copper/nickel on a
substrate which is an alloy of chromium with nickel,
copper or iron and possibly other components, as described
in US Patent N~ 4,960,494.
The cathode plates 1 are normally solid but porous
cathode plates may also be used. The main requirement for
the cathode configuration is that it should ensure
homogeneous current distribution over the entire anode
active surface area. Thus, in most cases, flat facing
anodes and cathodes of equal sizes will be preferred.
The described cell configuration leads to a high
productivity of aluminium per unit area of the cell bottom
at low current densities, because large facing
anode/cathode plates can be used, as more fully explained
below.
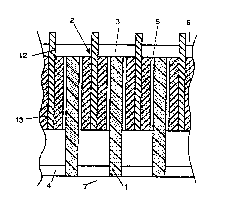
Fig. 2 is a similar view of another multimonopolar
cell, the same parts as before being designated by the
same references. In this cell, the anodes 2 are composite
structures each having a current feeder 12 made of a
suitable metal alloy sandwiched between high surface area
operative anode faces 13, for instance having a porous,
reticulated structure.
These porous anode faces 13 can be made of or coated
with a refractory oxycompound coating. For example, the
current feeder 12 and the reticulated faces 13 can be made
of the same or a similar metallic alloy having an
excellent electrical conductivity, and the reticulated
structure can be coated with a cerium oxyfluoride based
protective layer applied ex situ, or formed in the cell.
In this way, the resistivity of the reticulated faces 13
AMENDED SHEET
212~1 7
-15-
is closer to that of the electrolyte 3, which ensures an
even current distribution throughout the structure over a
high surface area, therefore a very low effective anodic
current density. The current feeder 12 of metallic alloy
S ensures even current distribution all over the active
surface area of the anodes 2, while minimising the voltage
drop across the electrodes.
The cathodes 1 in this cell are porous bodies, for
example of reticulated structure whose bottom ends dip
into the cathodic aluminium pool 4 on the cell bottom 7.
These porous cathode bodies can be made of or coated with
an aluminium-wettable refractory hard material such as
TiB2. It is possible to provide the cathodes 1 with a
central current feeder plate (not shown), like the anodic
current feeders 12.
In use of the cells of Figs. 1 and 2, and
advantageously with the electrolyte at a temperature of
680~-880~C, electrolysis current passes between the facing
operative anode and cathode surfaces which are parallel or
substantially parallel surfaces arranged upright in the
cell. Because of this configuration, the total operative
anode and cathode surface area can be many times greater
than the underlying area of the cell bottom 7. In this
way, it is possible to operate the cell at comparatively
low anodic current densities, compatible with the usual
low operating temperatures and the corresponding low
alumina solubilities, while achieving an acceptable
productivity per unit floor area.
Because of the closely packed arrangement of anodes 2
and cathodes 1 necessary to achieve operation with the
lowest possible voltage drop, constant circulation of the
electrolyte 3 in the anode-cathode gap is necessary,
especially when operating at low temperatures.
This electrolyte circulation is provided by making
use of the gas lift effect. Thus, the anodically released
AME~E~ S~EET
_ -16-
gas (oxygen with an o.Yide-containing electrolyte) entrains
with it an u?ward current of electrolvte 3 between the
anodes 2 and cathodes 2. Because of the small anode-
cathode cap, there is no downward circulation of
electroly~e in the anode-cathode gap. In the cell housing,
on either side of the anodes 2 and cathodes 1, a space is
left for downward recirculation of the electrolyte 3.
Fresh alumina can be supplied to these spaces to
compensate for depletion during electrolysis. The high
electrolyte circulation rate promoted by gas lift enhances
the rzte o alumina dissolution, compared to conventional
cells.
Suc.. a.r. arrangement, illustrzted schematically in
Fig. ' ~c- cells of the type shown in Figs. 1 and 2, may
~- have seve-~l multimonopolar rows of anodes 2 and calhodes
1 s?acea a_ross the width or along the length of the cell,
~ith 2 C?ace 20 between the adiacent rows and also
adjacenr -..e sidewalls 21 of the cell. Alternatively, the
cell could have a sin~le row of multimonopolar anodes and
cathodes along its length, with recirculation spaces on
either side and~or at the ends of the cell.
~ y ~-.e gas-lift effect, electrolyte 3 is circulated
as indica~ed by arrows 22 up between the opposite active
surfaces o~ the anodes 2 and cathodes 1, and down in the
spaces 20. If required, the gas lift effect can be
assisted by forced circulation using a pump made of
alumina or other electrolyte-resistant material.
Alumina is fed to the spaces 20 as indicated by
arrows 23 at a rate to compensate for depletion during
electroLvsis. This rate can be calculated from the cell's
current consumption and can, if necessary, be monitored by
measuring the alumina concentration of the cell
pericdically .
B~
A~F~nFn~FFr
21~311.7
. .
-17-
In the schematlc illustration of Fig. 3, current is
supplied to the conductive cell bottom 7 by a cathodic
current feeder 23. However, other arrangements are
possible.
The anodes 2 can if required be provided with
vertical grooves or ribs to assist the gas release.
Circulation of the electrolyte is enhanced by
circulation guide means, possibly formed by the spacers 5,
adjacent the edges of the facing anodes and cathodes of
each multimonopolar stack, as illustrated in Figs. 4 to 7.
Fig. 4 shows in side view several possible forms of
spacers: spacer 5 extends over the entire height of the
anodes/cathodes; spacer 5a extends over a major part of
the height, to near the top and bottom of the
anodes/cathodes 1,2; and spacers 5b are spaced apart from
one another over the height of the anodes/cathodes 1,2.
The plan view of Fig. 5 shows how these spacers 5 are
located between the anodes 2 and cathodes 1 adjacent their
edge. Thus, with this arrangement, the facing electrodes
1,2 are enclosed at their sides like a box, forcing the
electrolyte flow up inside, and down outside. When
discontinuous spacers like 5b are provided, this allows
for some electrolyte intake from the sides.
AMENDED SltEET
WO93/10281 212 3 ~ 1 7 PCT/E~2/02666
Fig. 5 also shows alternative electrolyte guides
which do not act as spacers, namely generally vertical
bars 25 of triangular section, bars 26 of circular section
and bars 27 of square or rectangular section. These bars
are placed outside the anode-cathode space, allowing
maximum use of the facing electrode surfaces. As shown for
25 and 26, the bars can be spaced from the edges of the
facing electrodes 1,2 to allow controlled intake of
electrolyte from outside. Or, as shown for the rectangular
bar 27, the bars can contact the edges of the facing
electrodes 1,2 to close the sides of the multimonopolar
stack. As for the spacers 5, these bars 25,26,27 can
extend over the entire height of the electrodes 1,2, or
only a part of the height.
Figs. 6 and 7 show another arrangement for
controlling the electrolyte flow path, namely plates 28
extending along each side of each multimonopolar stack of
electrodes 1,2 over their entire height or, as shown in
Fig. 7, over the major part of their height to just below
the top and just above the bottom of the stack. These
plates 28 can contact the edges of the electrodes 1,2 or
can be spaced apart by a convenient disance. Fig. 7 shows
the upward electrolyte flow between the electrodes 1,2 and
the downward flow outside the stack, as well as the
alumina feed 23.
The bars 25,26,27 and plates 28 can all be made of
the same electrically-resistant non-conductive materials
as the spacers 5. By making the bars 25,26,27 and the
plates 28 of alumina, which slowly dissolves in the molten
electrolyte, this dissolution contributes to the alumina
feed and the bars/plates can be replaced when necessary.
The feasibility of a multipolar cell according to the
invention is further illustrated in the following
examples.
., . , ~ ~
W093/10281 ~12J 3 117 PCT/E~2/02666
--19--
Fxample I
An experiment was conducted in a laboratory scale
electrolytic cell composed of an alumlna crucible
containing two copper sheet anodes measuring approximately
lOOxlOOxlmm vertically facing opposite sides of a block
cathode of graphite measuring approximately lOOxlOOx8mm.
These electrodes were immersed in an electrolyte composed
of 63% Na3AlF6 (cryolite) and 37% AlF3, by welght,
saturated with alumina. The electrolyte temperature was
750~C; the alumina solubility was approximately 4% by
weight of the electrolyte. Excess alumina powder was
present in the cell, outside the anode-cathode gap.
The gaps between the large faces of the anodes and
cathode were 6mm. Current was supplied at an anode and an
equal cathode current density of 0.2A/cm2, this current
flowing uniformly over the entire surfaces of the facing
anodes and cathode. The cell voltage was approximately
3.2V. The gas lift during electrolysis was sufficient to
circulate electroyte upwardly in the anode-cathode gaps,
the electrolyte flowing down outside the electrodes.
Alumina powder was added outside the electrode during
operation to maintain the alumina concentration in the
anode-cathode gaps. Electrolysis was continued for 200
hours. The current efficiency was >90%. This experiment
demonstrates the advantages of facing vertical anode and
cathode plates in a basic multimonopolar unit, which
readily can be scaled up by multiplying the number of
units and their sizes.
EXAMRLE II
A second experiment using the cell design shown in
Fig. I was carried out in a laboratory cell consisting of
an alumina crucible of 12cm internal diameter heated in an
electrical resistance furnace.
WO 93/10281 212 3 417 PCI/EP92/02666
--2~ ~
Two plates of titanium diboride of 80mm length, 50mm
width and 5mm thickness were used as vertical cathodes.
Three plates of tin oxide of 120mm length, 50mm width and
5mm thickness were used as vertical anodes. Anodes and
5 cathodes were held together at a 5mm interelectrode
distance by means of two alumina plates 60mm high, 55mm
wide and lOmm thick, each fitted with five vertical
grooves into which the vertical edges of the cathodes and
anodes were lodged. The lower end of the cathodes rested
10 on the crucible bottom and were dippping in a molten
aluminum pad of 1 cm thickness which acted as the cathode
current collector. The upper parts of the anodes were held
together by means of an Inconel 600rM block bolted to the
anodes and which also served as the anode electrical
15 contact and mechanical support.
The nominal electrolyte composition was 63% Na3AlF6
(cryolite) and 37% AlF3 by weight saturated with alumina.
The electrolyte temperature was 750~C. The alumina
solubility was approximately 4% by weight of the
20 electrolyte.
The electrochemically active surface area of each
anode and cathode face was 21.50 cm2 and the total active
surface was 86 cm2. The vertically projected surface area
of the anode-cathode assembly was approximately 23 cm2-
Current was supplied to the anodes and cathodes at an
equal current density of 0.2 A/cm2 corresponding to a total
current of approximately 3.8V. This corresponds to a current
density of 0.76 A/cm2 over the projected area of the cell
bottom, which is equivalent to that in convention~l Hall-
30 Héroult cells. The productivity of the cell per unit
projected area of the cell bottom is therefore also
equivalent to that in conventional Hall-Héroult cells.
Efficient electrolyte circulation between the anodes
and cathodes was achieved by the gas lift due to the
35 oxygen evolution at the surface of the anodes. This effect
WO93/10281 PCT/EP92/02666
~ 2 1 2 3 ~l ~1 7 r
-21~ ~3 ~ '; ' '~
was demonstrated by the fact that alumina powder feed was
added outside the electrode system without significant
drop in alumlna concentration in the electrode gaps as
evidenced by a stable voltage during the electrolysis. The
electrolysis was continued for 100 hours. The current
efficiency was about 88%. The cathodes after the
experiment were completely wetted by aluminum indicating
that the metal was drained from the cathode to the bottom
of the cell. The relatively high current efficiency shows
that no significant aluminum reoxidation by the evolving
oxygen did occur.
This experiment demonstrates the feasibility of
operating a vertical multimonopolar anode and cathode
assembly at a low current density while maintaining a cell
productivity equivalent to a conventional Hall-Héroult
cell. Another significant advantage is the considerably
increased electrolyte circulation achieved with the
proposed design which allows for efficient feeding in an
enrichment zone outside the anode-cathode assembly.
~. .