Note: Descriptions are shown in the official language in which they were submitted.
212S336
GROWTH MEDIUM
This invention relates to growth media for growing embryogenic tissue and
embryos.
BACKGROUND TO THE INVENTION
A basic plant medium commonly used for ca~Luling and s~lst~inin~ the growth
of plant embryogenic tissue is known as the Murashige Skoog (MS) medium. This medium
is based on the ratios and concentrations of minerals found in tobacco leaves. It has been
possible to successfully grow embryogenic tissue from many plant species in media which
have slight variations from the concçntr~tions/ratio of the minerals in the MS medium.
Unfortunately, the MS medium or variations thereof are not ideal for growing coniferous
tissue.
One growth medium known as the Weyerhaeuser medium (Gupta and Pullman
1990, l991a, l991b) has been developed particularly for coniferous embryogenic tissue,
based on chemical analysis of the composition of pine seeds. This medium is significantly
different from the MS medium in both its ratios and concentrations. Unfortunately the
Weyerh~usPr medium only works in relation to a few conifer genotypes and therefore is too
specific for general conifer propagation. There are other problems associated with the
Weyerhaeuser medium and the media referred to above which will become apparent from the
following discussions.
212~336
With embryogenesis, an aim is to obtain as many embryos as possible from
a single seed. The natural growth of a seed proceeds in six main stages:
a) The first stage has an embryo con~i~ting of between one to three cells
attached to the archegonium and positioned within the corrosion cavity
of the seed.
b) The second stage has a number of embryos multiplying and developing
with each embryo having less than 64 cells. It is usually at this
growth stage (zygotic polyembryogenesis) that embryos are placed onto
or into the growth medium.
c) The third stage is growth of the embryo away from the archegonium
and towards the end of the corrosion cavity. The long axis of the
embryo develops and ~sllmes a cylindrical shape with a complex of
elongated cells, the suspensor, at one end, and with a rounded head at
the other end where the apical meri~tem will eventually develop. This
is known as a bullet stage.
d) The fourth stage is the development of cotyledonary tissue at the shoot
apex of the embryo, at the root end of which is a group of cells known
as the suspensor zone.
212533~
e) The fifth stage is the further development and maturation of embryos,
with the formation and greening of cotyledons, formation of an
epicotyl or shoot apex, and formation of a hypocotyl. This stage of
development ends with emergence of a root (radicle), that is, the
process of germin~tion.
f) The sixth stage is the establi~hm~nt of the germin~ted embryo as a
plant capable of growing in soil.
Prior growth media do not encourage the natural zygotic polyembryogenic state
which occurs in the seeds. Instead, with the previous media it has been necessary to dissect
out embryos at the cotyledonary stage. Plant growth regulators (hormones) are then applied
to the cells of the body of the embryo or at the point of attachment of the suspensor to
encourage the cells to differentiate back to nonspecialized cells which can then be multiplied.
This process, often referred to in the literature as somatic embryogenesis, has been widely
reported for Picea (Spruce) species, and to a lesser degree for other conifers.
One problem with the above process is that there is in effect double handling
involving first the development of spe~ ed tissue, reversion of same to basic cell types and
then re-growth of the tissue to form mature embryos. Another problem is that the growth
hormones used (such as 2,4-D) may induce somaclonal variation. That is, the ideal genotype
which is being cloned may be collupted by the growth hormones and the resultant embryo
may not be true to type.
2125336
It is an object of the present invention to address the above problems, or at
least to provide the public with a useful choice.
Further objects and advantages of the present invention will become a~alent
from the following description.
SUM~RY OF lll~; lNVENTION
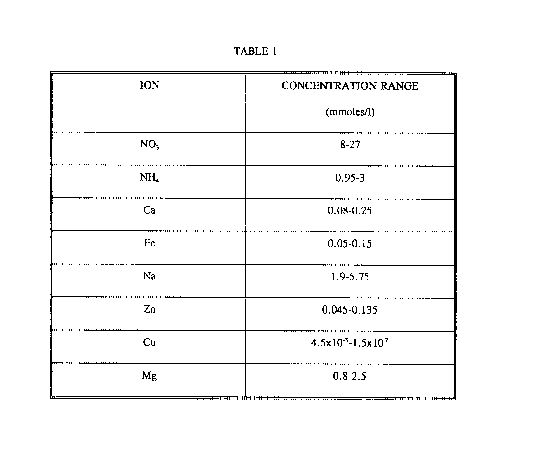
According to one aspect the present invention comprises growth media
effective for capturing and sll~t~ining the growth of embryogenic tissue and for subsequent
development, maturation and germination of conifer embryos, including inorganic ions within
the concentration ranges given in Table 1:
TABLE 1
ION CONCENTRATION RANGE mmoles/l
NO3 8-27
NH4 0.95-3
Ca 0.08-0.25
Fe 0.05-0.15
Na 1.9-5.75
Zn 0.045-0. 135
Cu 4 Sx10-3-l Sxl0~2
2125336
-
ION CONCENTRATION RANGE mmoles/l
Mg 0.8-2.5
It should be appreciated that the growth medium may be prepared in liquid or
solid form.
Reference throughout this specific~tion will be made to the use of the present
invention with respect to embryogenic tissue from conifers such as Pinus radiata, Pinus
taeda, Pinus elliotii, and Pseudotsuga menziesii, however it should be appreciated that the
present invention may be able to be used with embryogenic tissue coming from other
conifers.
A medium produced in accordance with the present invention provides an
environment for the embryos to grow in and the applicant has found that a number of
distinctive advantages have arisen.
The first advantage is that the present invention provides for sustained zygotic
polyembryogeny in-vitro. Unlike the situation with the previous media, it is generally no
longer necessary to dissect embryos at the late pre-cotyledonary stage out of seed nor is it
necessary to apply growth hormones. When using a medium of the present invention, the
embryogenic tissue grows out of the seed naturally, as illustrated in the examples herein.
212533fi
Another advantage of the present invention is that the mylillm is suitable for
growing a high perce~ ge of genotypes.
According to another aspect the invention provides a method of growing
embryogenic tissue comprising the step of growing the tissue on a growth medium as
described above.
The medium and method of the invention are particularly suitable for capturing
and sl~st~ining the growth in-vitro of embryogenic tissue of conifers in particular, including
Pinus radiata, Pinus taeda, Pinus elliotii and Pseudotsuga menziesii, for example.
The medium and method of the invention are also particularly suitable for the
development, maturation and germination of somatic embryos of conifers in particular
including Pinus radiata, Pinus taeda, Pinus elliotii and Pseudotsuga menziesii, for example.
DETAILED DESCRIPTION OF THE INVENTION
The m~lillm of the invention comprises ions within the concentration ranges
of Table 1 above, which are given again in column A of Table 2 below while column B gives
preferred ranges for the ion concentrations and column C gives ion concentrations of one
particularly plert;lled medium. Ion concentrations within 5 % of those of column C are also
highly plert;ll~d.
212~336
TABLE 2
MEDIA ION CONCENTRATIONS
(mmc)le~/l)
A B C
ION
NO3 8-27 13-23 17.8
NH4 0.95-3 1.5-2.5 1.96
Ca 0.08-0.25 0.12-0.21 0.17
Fe 0.05-0.15 0.07-0.13 0.10
Na 1.9-5.75 2.9-4.9 3.85
Zn 0.045-0.135 0.06-0.12 0.09
Cu 4 sxlo-3-l 5xl0~2 7x10-3-1.2x10-2 9.61x10-3
Mg 0.8-2.5 1.2-2.0 1.62
Table 3 provides a comparison between the ion concentrations of one pl~fe.led
medium of the invention with the Weyerh~ell~Pr medium, the Murashige Skoog medium and
a general tissue culture medium for radiata pine improved at New Zealand Forest Research
Institute Limited, Rotorua, New Zealand and referred to as FRI-LP. It can be seen again
that there are distinct differences between the concentration of ions in the preferred medium
and in the previous media.
Previously it was thought that a relatively high level of calcium was required
in growth media, however the level of c~lcil-m in the pler~lled medium in Table 3 has been
2125336
effective for many conirelous genotypes. Another noticeable difference between the
plerell~d m~ m and the prior art media is the higher level of sodium, copper and zinc in
the preferred medium.
TABLE 3
MEDIA ION CONCENTRATION COMPARISON
(mmoles/1)
ION PREFERRED WEYERHAEUSER MURASHIGE FRI-LP
MEDIUM MEDIUM SKOOG MEDIUM
MEDIUM
NO3 17.80 20.54 39.40 32.96
NH4 1.96 7.54 20.61 5.00
TOTAL19.76 28.09 60.02 37.96
N
P 1.96 1.00 1.25 1.98
K 14.16 10.02 20.05 19.79
Ca 0.17 1.00 2.99 5.08
Mg 1.62 2.50 1.50 1.46
Cl 3.42x10-l 1.00 5-99 2.10x104
Fe 0.10 0.02 0.10 0.11
2125336
'_
ION PREFERRED WEYERHAEUSER MURASHIGE FRI-LP
MEDIUM MEDIUM SKOOG MEDIUM
MEDIUM
S 1.83 1.14 1.73 1.69
Na 3.85 0.06 0.20 0.22
B 0.13 0.25 0.10 0.10
Mn 1.62x10-2 6.21x10-2 1.00x10-l 8.97x10-2
Zn 0.09 0.05 0.03 0.03
Cu 9.61x10-3 5.01x104 1.00x104 1.00x10-3
Mo 8.27x104 5.17x104 1.03x10-3 1.03x10-3
Co 8.41x104 5.25x104 1.05x104 1.05x104
6.02x10-3 2.50x10-2 5.00x10-3 4.82x104
It should be appreciated that the composition of the preferred medium above
is given by way of example only and that other ratios and concentrations may be used, within
the ranges of Table 1 above.
It is the concentrations of the inorganic ions listed in Table 1 which distinguish
media of the invention from other media such that the medium of the invention is able to
sustain the growth and proliferation of embryogenic tissue of a wide range of conifer types.
2125330
~Potassium, chloride, phosphate, m~ng~n~se, borate, sulphate, iodide, molybdenum and cobalt
ions are preferably included in the medium. The media of the invention preferably also
contain other nutrients generally used in conifer embryogenesis such as a carbon source. In
the pl~fell~d media vitamins especially thi~mine, nicotinic acid, and pyridoxine are
preferably also incl~lded. Inositol, sucrose, agar and gl~lt~mine and other amino acids
(particularly asparagine, arginine, citrulline, nrnithine, lysine, alanine and proline) are also
preferably present in pl~felled media. The concentrations of some of the other components
can usefully be varied according to the stage of the embryogenesis. For example it is
pl~fell~d to include gl~lt~mine and other amino acids in relatively high concentrations for the
later stages of embryogenesis. Particularly plc~fell~d media include those of the solutions of
Tables A5 to A9 at the end of this disclosure. Also preferred are media of the invention
having concentrations of the nondistinguishing components at levels between 50% and 150%
of those found in Tables A5 to A9. Highly preferred are media with concentrations of the
non-distinguishing components at 75 % to 125 % of those found in the solutions of Tables A5
to A9. The plcf~lled pH for media of the invention is in the range 5.5-5.9. Agar may be
included in plefell~d media at O.S-l.Sg/l (w/v). Sucrose may be included in preferred media
at S-SOg/l (w/v).
According to another aspect of the invention there is provided a process for
conifer embryogenic tissue capture and embryogenic tissue m~inten~nce. In embryogenic
tissue capture it is pl~felled that the seeds are surface stPrili~ed and entire megagametophytes
containing the imm~tllre zygotic embryos are dissected and placed directly onto a medium
of the invention. Media with concentrations of each ion in the lower half of the ranges
shown in Table 1 are preferred for this step. The medium shown in Table AS is particularly
- 10 -
212~3~6
prer~lled for this. Culture is carried out at an appro~liate light intensity. Ambient photo
period is preferably used and the lelllpel~lul~ is preferably about 24~C. It is pl~relled that
plant growth regulators such as auxins and cytokinins are not present. Preferred species for
the practice of the invention include Pseudotsuga menziesii, Pinus taeda, Pinus elliotii and
Pinus radiata.
Embryogenic tissue will grow for up to a month on the capture medium but
for sustained maintenance it is recommPndecl that conifer embryogenic tissue is maintained
on standard embryogenesis medium (Table A6). Again for the maintenance of conifer
embryogenic tissue it is ~ rt;ll~d not to use auxins or cytokinins.
In one embodiment of the present invention a growth medium may be used
having the same ratios of ions as shown for the plerell~d medium in Table 3 but with a
different overall ion concentration. For instance, at the zygotic polyembryogenesis stage of
the embryo development (stage b above) a merlillm may be used which has only one half of
the concentration of each of the ions listed for the pler~lled medium in Table 3 (eg see Table
A5). The tissue may be grown on this medium until a sustained extragametophytic growth
of tissue has been established and can be relocated on full strength medium such as that of
Table A6. Again it is noted that the level of c~lcil-m is an important factor with respect to
a growth m~lillm suitable for growing embryogenic tissue either at stage b or at later stages.
The growth medium may also contain 4-9 gm/l of gellan gum. Media of this
embodiment have been found to be particularly useful for conifer embryo maturation. The
CA 0212F7336 1998-08-14
gellan gum marketed under the name Gelrite ~ has been found to be particularly
useful. Transfer from embryo development medium with conventional Gelrite content
of about 3 gm/l to an embryo maturation medium with 5-7 gm/l (preferably 6 gm/l)
Gelrite followed by subsequent transfer to another embryo maturation medium differing
in that the Gelrite concentration was 4-5 gm/l (preferably 4.5 gm/l Gelrite) gave
surprisingly good yields of embryos. It appears that transient use of very high Gelrite
levels (eg 6 gm/l) stimulates the maturation of somatic embryos.
Water vapour permeable films or filters may be used in conjunction with
the medium of the invention. Using water vapour permeable films or filters allows the
avoidance of buildup of condensation. Thus, problems such as those relating to the
presence of freely available liquid on the surface of solid media are avoided. Also the
films or filters allow a controlled rate of water loss. This is preferably in the range of
90-150 gm/sq meter per day. A rate particularly suitable for Pin~s radiata embryos is
118 gm/sq meter per day. Many types of films may be used provided they have the
desired qualities of being able to seal against microbial infection and are sufficiently
permeable to water vapour. For instance, the film may be made from plastics material
such as polyvinyl chloride (PVC). Among those films suitable are those sold under the
trade mark VITAFILM by the Goodyear Tyre & Rubber Co (Australia) Ltd. In this
range the OMNI, VW, MWT and F10 V/S are all useful with the medium of the
invention for conifer embryogenesis.
212~3~6
When the somatic embryos ~ g on the medium of the invention are in
vessels covered with a water permeable film, evaporation of liquid from the medium makes
it less available to the embryos, mimicking the changes in matric potential that occur during
natural embryo development. As a consequence of the loss of water, the concentration of
ions within the me lil~m increases, however without taking it outside the range useful for
conifer embryogenesis. I have been unable to ~uplic~te the benefici~l effect of the water
permeable film simply by tr~n~ferrin~ embryos to medium with higher concentrations of
medium components equivalent to that attained when water vapour is lost through a water
permeable film. I have concluded therefore that the beneficial effect of the use of water
permeable film is not due to an increase in osmotic potential of the medium, or to the
increase in concentration of substrates such as sucrose. I believe rather that the effect of the
film is to decrease the availability of water to the developing embryo at a precise and critical
stage of maturation.
According to another aspect of the invention there is provided a process for
harvest of mature embryos with developed cotyledons, and subsequently treating them to
allow a high percentage of germin~tion, such that the conversion of cotyledonary stage
embryos to plants growing in soil averages or exceeds 50% . Conversion of somatic embryos
to plants at an efficiency approaching that necessary for the commercial application of
somatic embryogenesis has not been previously reported for conifers other than spruces.
Germin~tion is preferably carried out on media with inorganic ions at concentrations of 50%
to 100% of those listed in Table 2, column C and Table 3. Particularly preferred for this
is the medium of Table A10.
- 13 -
2125336
-
EXAMPLES
The following Examples further illustrate the invention. The Examples
illustrate the use of the medium in embryogenic tissue capture and in embryogenic tissue
m~inten~nce for capture and ",~inl~n~nce of embryogenic tissue of Pseudotsuga menziesii,
Pinus taeda, Pinus elliotii and Pinus radiata and for subsequent embryo development and
maturation, and subsequent germin~tion and collvel~ion to plants in soil.
Standard procedures for the preparation of plant tissue culture media are
followed. Media as described below are sterili~ed by autoclaving for 20 minutes at 121~C.
Organic components are filter sterili~ed, and are added to the medium after autoclaving.
Culture is carried out in 90 mm petri dishes each col~ 22 ml of medium.
EXAMPLE 1- EMBRYOGENIC TISSUE CAPIURE
Seeds were removed from cones of Pinus radiata, Pinus taeda, Pinus elliotii,
and Pseudotsuga menziesii at an a~?r~liate stage of development of the zygotic embryos
(for instance for Pinus radiata in New 7~ n~, from early December to early January), and
the seeds were surface st~rili~e~l and entire meg~g~metophytes cont~inin~ the imm~tllre
zygotic embryos were dissected and placed directly onto Standard Embryogenic Tissue
Capture Medium (Table A 5). Dishes were cultured at low light intensity (5 microFin~t~in~
m~2 sec~l) under ambient photoperiod. The tellll?eld~ul~ was m~int~ined constant throughout
at 24~C +/- 1~C
- 14 -
2125336
As a variation, zygotic embryos at any stage of development up to the
formation of cotyledonary primordium formation may be dissected and placed directly onto
Standard Embryogenic Tissue Capture Me lil-m (Table A5), or onto Standard Embryogenesis
Medium (Table A6) or Embryo Development Me lillm (Table A7).
Embryogenic tissue grew out onto the m~ m over the next three months, and
tissue pieces greater than 2 millimetres across were transferred to Standard Embryogenesis
Medium.
This protocol differs from that described by other authors, for example in the
patents granted to Gupta et. al (1990, l991a, l991b ), and in the references cited therein.
The major difference is that the mineral salt composition differs m~rk~lly from that used by
the above named authors, and in the references which they cite (see Table 3).
The mineral salt formula of this invention allows the capture of embryogenic
tissue without the need to resort to the use of plant growth regulators such as auxins (eg.
2,4-Dichlorophenoxy acetic acid, Indole -3- acetic acid, l-Napthylacetic acid, or
Indole-butyric-acid) and/or cytokinins ( eg. 6-Benzylamino Purine, Zeatin, or N6-[2
Isopentenyl]~lçnine). While plant growth regulators such as auxins or cytokinins may on
occasions enhance the growth of tissue on the Standard Embryogenic Tissue Capture
Medium, the Standard Embryogenesis Medium or the Embryo Development Medium, their
use is not ess~nti~l when explants are put into culture at the a~ropliate stage of development
of the imm~tllre zygotic embryo.
2125336
The simple protocol described has been succes~fully used to capture
embryogenic tissue of Pseudotsuga men~iesii, Pinus taeda, Pinus elliotii, and Pinus radiata
without need to alter the protocol an any way for each of the above named species. 15500
explants from 10 different control pollin~tPcl cone parents of Pinus radiata were put into
culture on the Standard Embryogenic Tissue Capture M~ m and after adju~tmPnt of results
for cont~min~tion, up to 100% of the whole meg~g~metophyte explants gave rise to
embryogenic tissue, when the tissue placed into culture was developmentally competent to
form embryogenic tissue. The mean response of the best result for each cone parent was
32.9% for P~nus radiata. For Pinus taeda, Pinus elliotii and Pseudotsuga menziesii about
30% of ml-g~m~tophyte explants at an al)~o~?liate stage of development gave rise to
embryogenic tissue.
EXAMPLE 2 - EMBRYOGENIC TISSUE MAINTENANCE
Embryogenic tissue has been found to continue to grow for up to a month on
the Standard Embryogenic Tissue Capture Medium, however this medium is unsuitable for
s~lst~ined tissue m~int~n~nce. The conifer embryogenic tissue was more effectively
m~int~ined on Standard Embryogenesis Me~ium (Table A6).
Tissue development was m~int~in~d in a primitive state on Standard
Embryogenesis Medium, ideally with embryos never developing past the eight-celled stage
before dissociating into simple embryonic initials. These embryos may have a single
suspensor cell attached to the embryo initial - further development of suspensors is not
encouraged on this m~illm, and the tissue does not become "bulky".
- 16 -
2125336
2.1 Plant growth r~ tors
This protocol for the ~ ltP~ lce of conifer embryogenic tissue differs from
that described by other authors, for example in the patents granted to Gupta et. al (1990,
l991a, l991b ), and in the references cited therein. The major difference is that the mineral
salt composition differs m~rk~Aly from that used by the above named authors, and in the
references which they cite.
The ion composition of the medium that is described here allows the
m~inten~nce of embryogenic tissue of conifers without the need to resort to the use of plant
growth regulators such as auxins (eg. 2,4-Dichlorophenoxy acetic acid, Indole -3- acetic acid,
l-Napthylacetic acid, or Indole-butyric-acid) and/or cytokinins (eg. 6-Benzylamino Purine,
Zeatin, or N6-[2 Isopentenyl]~enine).
Plant growth regulators such as 2,4-D and BAP may stimulate apparent growth
of embryogenic tissue, in part due to formation of suspensor cells, but the use of plant
growth regulators is not necessary, and confers no benefits. While we have recovered sound,
mature somatic embryos from some cell lines Ill~inl~ined on medium with 2,4-D and BAP,
my experience has been that these cell lines lose their plant-forming potential much sooner
than the same cell lines which have been m~int~in~d on the Standard Embryogenesis Medium
without plant growth regulators.
212533~
It is usually the experience of pra~titi~nPrs of the art of somatic embryogenesis
of conifers that cell lines quicl~y lose the ability to form mature embryos, and subsequently
plants.
The ability to retain the plant-forming potential of conifer cell lines for periods
of in excess of one year is a benefit of the use of the unique mineral salt composition which
is described here. This benefit is possibly due to the fact that plant growth regulators are not
required for embryogenic tissue capture and m~inten~nee in the mP~lium described herein.
Most cell-lines grown on the pl~r~ d medium showed ill-thrift or died when placed on the
Weyerh~Pu~Pr medium.
EXAMPLE 3 - EMBRYO DEVELOPMENT
Embryo development was encouraged by transfer of tissue to an Embryo
Development Medium (Table A7). Embryogenic tissue from the maintenance medium was
suspended in liquid medium in a sterile McCartney bottle. Embryogenic tissue was used at
the rate of 1 gm per 4 ml of Cell Suspension Medium (Standard Embryogenesis Medium
with the agar omitted). When the suspension was finely divided, it was dispensed as 0.25
ml aliquots, three per 90 mm. petri dish cont~ining Embryo Development Medium which
consisted generally of Standard Embryogenic Tissue M~intPn~nce Medium to which was
added amino acids including glut~mine at 550 mg/l, and which was gelled with 3.0 gm/l
Gelrite rather than with Difco Bacto agar (see Table A7).
- 18 -
2125336
The first step in somatic embryo development is marked by the continued
multiplication of the cells in an individual embryo to form a compact mass, often referred
to in the lil~ ul~; as the "proembryo" or "proembryonic mass". It is roughly equivalent to
the "globular" stage of development in dicotyledonous angiosperms. As this embryo mass
forms, the single suspensor cell develops into a multi-stranded structure, also called the
suspensor. The embryo head continues to develop, and assumes a cylindrical shape with a
rounded head. This stage is sometimes referred to in the li~ ule as the "bullet" stage.
Culture dishes were incubated under the same conditions as the m~inten~nce
stage. Over approximately 2 to 3 weeks the tissue rapidly increased in bulk, and took on a
"spiky" appearance. Over the next 2 to 3 weeks, "bullet" stage embryos with well defined
suspensors became readily visible to the unaided eye. At this point, the tissue masses were
subdivided, and are transferred to Embryo Maturation Medium.
EXAMPLE 4 - EMBRYO MATURATION
Each "spot" of tissue on the Embryo Development Medium which was judged
to be at a suitable stage of development was subdivided and the pieces transferred to Embryo
Maturation Medium # 1 (Table A8). Embryo Maturation Medium # 1 is similar to Embryo
Development Medillm, but contains higher concentrations of amino acid, such as gl~lt~mine
at 5 - 10 gm/l, Abscisic acid in the range 5 mg/l to 25 mg/l, and Gelrite at a concentration
between 4.5 gm/l and 6 gm/l. A concentration of 6 gm/l, is the pl~fell~d level of Gelrite
for the first transfer from Embryo Development Medium to Embryo Maturation Medium
(refer Table A8).
- 19 -
2125336
The usefulness of manipulating the Gekite levels in this manner is illustrated
by the following experiment~.
Embryogenic tissue of a co~ elellt plant-forming clone of Pinus radiata was
transferred from Embryo Development MeAillm onto Embryo Maturation Medium #l gelled
with 8 grams/litre Difco Bacto agar, or 3 grams/litre gellan gum (Gekite TM) or 6 grams/litre
gellan gum. Each tre~tm~-nt had eight replicate dishes. The yield of harvestable somatic
embryos, capable of germination and conversion to plants was ~sessed when embryo
production ceased after six weeks. Results are shown in Table 4.
TABLE 4
Tre~tmentTotal number of embryosAverage yield per dish
8 g/l Bacto agar 0 0.0
3 g/l GelriteTM 16 2.0
6 g/l GelriteTM 141 17.6
As a refinement to this protocol, I have found that it is useful to further
enhance embryo yield by removing the tissue from Embryo Maturation Medium #1 with 6
grams/litre Gelrite after three weeks, and placing subdivided tissue pieces onto Embryo
Maturation Medium #2 with 4.5 grams/litre Gelrite. Figure 1 illustrates a maturation
protocol taking advantage of these effects and those obtained from the use of water vapour
permeable films.
- 20 -
2125336
EXAMPLE 5 - THE E~FECT OF USE OF WATER VAPOUR-PERMEABLE ~ILMS
ON EMBRYO MATURATION
This ~Y~mple illustrates the use of water vapour permeable films on yield of
embryos obtained from culture on the mylillm of the invention. Tissue of a plant-forming
Pinus radiata embryogenic cell line was distributed in equal amounts at random over a
number of petri dishes cont~ining the EMM#l m~lillm as described in Table A8. Some of
the dishes retained plastic lids which were sealed at the margins with impermeable domestic
cling film. Other dishes were instead covered with one of four different plastic films. In
this experim~nt, over a period of ten days cotyledonary stage somatic embryos were
harvested directly from EMM#l (Table A8). The results are shown in Table S below. Each
tr~tment had four dishes of embryo-forming tissue. The use of Vitafilm Omni-film
produced the greatest yield of somatic embryos for this particular cell line in this experiment.
TABLE 5 The Effect of Pe~ eable Films on the Yield of Somatic Embryos
Film/Closure Total number of somatic Average somatic
embryos embryos per dish
Plastic petri dish lid/cling film 28 - 7.0
Vitafilm F10 V/S 51 12.7
Vitafilm VW 62 15.5
Vitafilm Omni-film 97 24.2
Vitafilm MWT 50 12.5
- 21 -
212~336
-
EXAMPLE 6 - THE EFFECT OF FILMS ON WATER LOSS FROM GROWTH
MEDIUM IN PETRI DISH ~.
Petri dishes co~ inin~ solid somatic embryo development medium were
covered with plastic lids, sealed on with cling film, or with one of four different gas
permeable plastic films. Initial weights of the dishes were recorded, and weight loss noted
at intervals of 2-3 days. There were four replicates of each tre~tment, and dishes were
m~int~ined in a 24~C incubator under the same conditions as used for somatic embryo
development.
The mean water loss from dishes after 9 days when covered with lids of
different films was determined. Water loss was correlated with the somatic embryo counts
from ide-nti~l dishes of m~lillm cultured under the same conditions. A correlation between
embryo formation and water loss of 0.994 was detPrmined statistically for the film covered
dishes. Experiment~l results are shown in Table 6.
TABLE 6 - Embryo formation and water loss from media
Lid/Film Number of EmbryosWater Loss (gm/9 days)
Lid 28 0
Vitafilm F10 V/S 51 4. 88
Vitafilm VW 62 5.14
Vitafilm Omni-film 97 6.73
2125~36
Lid/Film Number of Embryos Water Loss (gm/9 days)
Vitafilm MWT 50 4.60
The water loss from dishes giving the highest yield of somatic embryos, that
is those covered with Vitaf~m Omni-film, was determined to be 118 g/m2 dish area/day.
In another experim~nt following approxim~t~ly three weeks of culture on
EMM#l, tissue of four plant-forrning Pinus radiata embr,vogenic cell lines was subdivided
and distributed in equal amounts at random over a number of petri dishes cont~ining the
EMM#2 medium which had Gelrite at 4.5gm/1 as described in Table A9. Some of the dishes
retained plastic lids which were sealed at the margins with impermeable domestic cling film.
Other dishes were instead covered with one of two different plastic films. As an additional
tr~tmPnt lidded, sealed dishes contained an ethylene absorbing agent (potassium
perm~ng~n~te on a matrix of ~ ",i,~ oxide). After a period of eight days, cotyledonary
stage somatic embryos were harvested. The results are shown in Table 7 below. Each
tre~trnent had four dishes of embryo-forming tissue.
TABLE 7 The effect of p~ hle films on yield of cotyledonary stage somatic
embryos.
Cell Line Tre~tm~nt Mean embryos per 5% LSD test
dish
- 23 -
21253~6
I22 Standard dish 14.5 b
Vitafilm Omnifilm 78.0 a
Vitafilm MWT 380 110.0 a
Ethylene absorber 26.5 b
I25 Standard dish 4.0 b
Vitafi1m Omnifilm 17.0 a
Vitafilm MWT 380 14.5 a
Ethylene absorber 5.0 b
A13 Standard dish 1.0 b
Vitafilm Omnifilm 6.5 ab
Vitafilm MWT 380 9.5 a
Ethylene absorber 2.0 ab
A17 Standard dish 1.5 a
Vitafilm Omnifilm 1.0 a
- 24 -
2125336
Vitafilm MWT 380 0.5 a
Ethylene absorber 0.5 a
a,b Difference in results of tre~trnent~ bearing the same letter were not statistically
~ipnific~nt
The results in Table 7 intlic~t~ that the use of water-permeable films in the
EMM#2 stage enhances the production of cotyledonary stage somatic embryos, and also that
there is probably an optimal rate of water loss a~)~r~liate to each plant cell line (clone)
when grown on EMM#2. These results also show that up to more than 100 embryos at the
cotyledonary stage may be obtained from a single petri dish for Pinus radiata. With Pinus
taeda the results of this step are similar with as many as 10-20 embryos at the cotyledonary
stage being produced per dish.
EXAMPLE 7 - RECOVERY OF PLANTS FROM SOMATIC EMBRYOS
Well developed somatic embryos were transferred to NZ FRI Embryo
Germination Medium (Table A10). Dishes were sealed with clingfilm and maintained under
50% shade cloth in standard incubator conditions. Embryos were incubated at 24+/-1
degree Celsius, under a light intensity of approximately 40 micro Fin~t~in~ m~2sec~l, and a
16-hour photoperiod.
- 25 -
2125336
~
For somatic embryos from new cell lines, roots appealed as early as 10 days
after transfer to Germin~tion Medium. As cell lines age, roots took longer to emerge but
usually appea~ed within 12 weeks.
New cell lines produced many somatic embryos of high quality. Generally,
these were dark green, had long cotyledons, and norm~lly formed a definite epicotyl while
still in sterile culture. Embryos of this type tend to form roots quickly, and a high
conversion efficiency (percell~ge of harvested mature cotyledonary stage embryos converting
to plants in soil, greater than 50~) is observed. I have observed that the growth and quality
of plants is directly related to the quality of the epicotyl at time of pricking-out of embryos
into propagation medium. Somatic embryos with very short epicotyls do not perform as well
as those which have well developed shoots of 5 mm or longer. It is likely that the cotyledons
of somatic embryos do not function as photosynthetic organs, and that the embryos rely on
the epicotyl for carbon fixation in-vivo.
Setting of Gerrnin~te~ Embryos
Germin~ted somatic embryos were set under ambient glasshouse conditions
into 350 mm x 295 mm x 55 mm nursery flats cont~ining the following propagation medium:
1.5 parts fine peat
1.0 parts perlite
0.5 parts fine pumice
Diazinon, 1/2 teaspoon per tray
- 26 -
2125336
M~gn~ m ammonium pho~hate 30 gm per tray
Normal nursery procedures for the eYfl~cl~ing of tissue cultured plant m~tPri~l are
followed subsequently.
The protocols described above, using the unique mineral salt m~ lm described
in Table 3 have been used to capture embryogenic tissue from whole megagametophyte
explants of Pinus radiata, Pinus taeda, Pinus elliotii, and Pseudotsuga menziesii using the
Standard Embryogenic Tissue Capture Me lium described in Table A5. This medium has
proved to be satisfactory for all the conifer species named above without alteration.
Embryogenic tissue of Pinus radiata, Pinus taeda, Pinus elliotii, and Pseudotsuga menziesii
has been proliferated and m~int~ined, often for periods of several years, by regular 14 day
transfers to fresh dishes of Standard Embryogenesis Medium as described in Table A6. This
medium has proved to be of use for these four species without alteration.
Embryogenic tissue of Pinus radiata, Pinus taeda, Pinus elliotii, and
Pseudotsuga menziesii has been proliferated and the formation of "bullet" stage somatic
embryos has been observed upon transfers to Embryo Development M~ m as described in
Table A7. This m~Aium has proved to be of use for these four species without alteration.
Mature somatic embryos have been observed on EMM#l and EMM#2.
Several thousand mature cotyledonary stage somatic embryos of over 50 clones of Pinus
radiata have been harvested from these media and transferred via standard germination and
nursery procedures to soil. Over 4000 plants from 50 clones of Pinus radiata have been
- 27 -
2125336
grown in soil in the greenhouse, nursery bed, or in field trials. By way of example, for one
collection of Pinus radiata, 28 different cell lines (clones) produced 6066 mature
cotyledonary stage embryos. Of these 2980 plants were successfully established in soil,
giving an average conversion of 49.1 ~o . The best conversion of mature somatic embryos to
plants in soil was 73% (1001 plants from 1367 somatic embryos). Plants from somatic
embryos of Pinus taeda have also been transferred to soil by the same process.
~XAMPLE 8 EFFECTIVE RANGE OF CONCENTRATIONS OF PREFERRED
MEDIUM MINERAL ELEMENTS
Similar experiments to Examples 1-4 and 7 were carried out using ion
concentrations, 0.125, 0.25, 0.5, 1.0, 1.5 and 2.0 times those of the preferred medium of
Table 3 at each developmental stage and also for embryo germin~tion. The results are
presented in Table 8. The concentrations of components other than inorganic ions is the
same for each of the six different strength solutions and a~op~iate for the step being
investiF;~tYI The concentration of the other components are those used in Examples 1-4 and
7 for the particular step being investig~ted.
TABLE 8 Effective range of collc~ ation of preferred me~ m mineral elements
Stage of Development
ETC ETP EDM EM#l EM#2 EG
(1) (2) (3) (4) (5) (6)
1/8
strength*
- 28 -
212~336
ETC ETP EDM EM#l EM#2 EG
(1) (2) (3) (4) (5) (6)
1/4 -- -- --
strength
1/2 +++ ++ ++ + + +++
strength
Full + +++ +++ +++ +++ ++
strength
1.5 x - + + + + +
strength
2 x
strength
ETG = Embryogenic Tissue Capture
ETP = Embryogenic Tissue Proliferation on Standard Embryogenesis Medium
EDM = Embryogenic Development on Development Medium
EM#l = Embryo Maturation #l
EM#2 = Embryo M~tllr~tinn #2
EG = Embryo Germin~tion
* does not gel
- 29 -
2125336
+ + + optimal growth
+ + useful growth
+ slower growth
not effective
This table represents the effective range of concentration of preferred medium
mineral element.e for Pinus radiata. The concentrations giving the best result (+ + +) for
Pinus radiata also gave good results for Pinus taeda at every stage, for Pinus elliotii for the
first 3 stages, and for Pseudotsuga menziesii for the first 4 stages.
MEDIA FOR EMBRYOGENIC TISSUE CAPTURE, MAINTENANCE,
DEVELOPMENT, AND MATURATION
TABLE Al - Major Ion Stock:
Compound Weight gm
KN03 14.31
MgS04-7H20
CaCl2.2H2O 0.25
NaNO3 3. 10
NH4H2PO4 2.25
make up to 400 ml
- 30 -
212S3~6
,
TABLE A2 - Minor Ion Stock
Compound Weight mg
MnSO4-4H2O 36.0
H3BO3 80.0
ZnSO4-7H2O 250.0
KI 10.0
CuS04.5H20 24.0
Na2MoO4-2H2o 2.0
CoCl2 6H2O 2.0
make up to 200 ml
TABLE A3 - Iron stock - to make 1 litre
FeSO4.7H2O 1.5 gm
Na2EDTA 2.0 gm
TABLE A4 - Vitamin stock - to make 1 litre
Thi~mine HCl 0.5 gm
Nicotinic acid 0.5 gm
Pyridoxine HCl 0.05 gm
TABLE A5 - Standard Embryogenic Tissue Capture r l~ m
per litre of m~Aillm
major ion stock20 ml
2125336
minor ions stock 10 ml
Iron chelate stock 10 ml
Vitamin stock 5 ml
Inositol 0.5 gm
Sucrose 10.0 gm
Charcoal (Merck, activated) 2.0 gm
Difco Bacto agar 8.0 gm
pH adjust to 5.6-5.8 before addition
of agar and autoclaving
Table A6. Standard Embryogenesis Medium (embryogenic tissue m~inten~nce
medium)
per litre of medium
Major ion stock 40 ml
Minor ion stock 20 ml
Iron chelate stock 20 ml
Vitamin stock 10 ml
Inositol 1.0 gm
Sucrose 30.0 gm
Difco Bacto agar 8.0 gm
pH adjust to 5.6-5.8 before addition of agar and autoclaving
212S336
add the following filter stPrili~e~ amino acids after autoclaving:
major amino acids milli~ram per litre
gl~lt~mine 1 10
asparagine 105
arginine 35
minor amino acids stock 2 ml per litre
Table A6 b Minor amino acid stock
amino acid gm
citrulline 1.58
ornithine 1.52
lysine 1. 10
alanine 0.8
proline 0-7
3.1 Make up to 800 ml with double distilled water.
3.2 Dispense into 40 ml aliquots.
3.3 Freeze immediately, store frozen, and thaw only on day of use.
3.4 Adjust pH to 5.6-5.8 and filter stPrilisP, before use.
- 33 -
212~336
~able A7 - Embryo Development Medium
per litre of mPAillm
Major ion stock 40 ml
Minor ion stock 20 ml
Iron chelate stock 20 ml
Vltamin stock 10 ml
Inositol 1.0 gm
Sucrose 30.0 gm
Kelco Gelrite 3.0 gm
pH adjust to 5.6-5.8 before addition of agar and autoclaving.
Add the following filter st~rili~l amino acids after autoclaving.
major amino acids milligram per litre
glllt~mine 550
asparagine 510
arginine 175
minor amino acids stock 10 ml per litre
(as per Table A6b)
- 34 -
2125336
Table A8 - Embryo ~aturationmP~ -m #l (EMM#l)
To make one litre of m~lillm
Step 1
Major ion stock 40 ml
Minor ion stock 20 ml
Iron chelate stock 20 ml
Vitamin stock 10 ml
Inositol 1 gm
Sucrose 30 gm
Dissolve in double distilled water, and adjust volume to allow for addition of filter sterilised
components.
Adjust pH to 5.7
Add Gelrite 3 gm per 500 ml flask (6 gm per litre) then add pH adjusted liquid. Autoclave.
Step 2
Dissolve with heating to give final volume of 50 ml
Minor amino acid stock 40 ml
Glllt~mine 7.3 gm
Asparagine 2.1 gm
Arginine 0.7 gm
Abscisic Acid 15 mg
- 35 -
2125336
(dissolve in lN NaOH)
Filter st~rili.~e and add to autoclaved m~ lm
TABLE A9 - Embryo Maturation Medium #2 (EMM#2)
Prepare as for EMM#l
Substitute Gelrite 2.25 gm per 500 ml flask (4.5 gm per litre)
TABLE A10 - Embryo Germinqtion Medium (NZRI EGM)
Per litre of medium
Major ion stock 24 ml
Minor ion stock 12 ml
Iron chelate stock 12 ml
Vitamin stock 6 ml
Inositol ~ 0.6 gm
Glucose 30 gm
Gelrite 5.0 gm
Adjust to pH 5.70 and autoclave
Add filter st~rili.~ed amino acids in aqueous solution adjusted to pH 5.70
- 36 -
2125336
Arginine 0.26 gm
t~mine 0.40 gm
Proline 0.02 gm
Aspects of the present invention have been described by way of example only
and it should be appreciated that mo-lific~ti( nc and additions may be made thereto without
departing from the scope of the invention.
References Cited:
Gupta, P.K., Pullman G.S., 1990: Method for reproducing coniferous plants by somatic
embryogenesis. United States Patent number 4,957,866, September 18, 1990.
Gupta, P.K., Pullman G.S., l991a: Method for reproducing coniferous plants by using
somatic embryogenesis using abscisic acid and osmotic potential variation. United States
Patent Number 5,036,007, July 30 1991.
Gupta, P.K., Pullman G.S., l991b: High concentration enrichment of conifer embryonal
cells. United States Patent number 5,041,382, August 20 1991.
- 37 -