Note: Descriptions are shown in the official language in which they were submitted.
WO 93/13239 PCl /KR92/OOOX I
2~ 2~7~
Exothex~ic Reflexible Glass. ~xothen~ic Transparent Glass and Process of
anufacturing the
. E~C3I;~lIND I~e INVENIION
- ~` Eield of Invention
This Invention relates to the exothermic reflexible ~lass and exothermic
transparent glass as well as to a process for the manufacture of them, whose
:: :
~ surface temperature can be adjusted at vill by means of coating non-conductive
. . .
~ glass either with reflexibilîty-conductive material or transparency-conductive
,
material by use of the sputterin~ technique generally used for plasma.
10~ DescrîPtion of Prior Art
The technique of vacuum evaporation by sputtering, the very technique of
manufacturîng exothexmic reflexible glass and exothenmic transparent glass here,
is performed by a direct evaporation of the m~terial to be evaporated în vacuum
through colliding ionized inert ~as into the surface of the target, that is,
ionization of the inert gas takes place in the area of abnormal glow dîscharge
and the thus ionized ~as, under the influence of the electric field, is made to
beat the surface of a cathode. Thus, in the sputtering technîque, the target is
used as the cathode and the vacuum container or the matrix, as the anode.
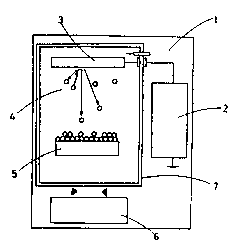
A simplest diode planar sputterin~ technique is shown in Fîg. 1, wherein a low
pressure electric discharge takes place between the target(3), whîch is used as
the cathode, and the anode. The pressure of the inert ~as in co~mon use should,
for the purpose of maintaînin8 electrîc discharge, be over 5 X 10-3 torr, the
working pressure ranging from 2 X 10-2 torr to 10~~ torr. The electric pressu M
. ~
applicable to the cathode can be varied from hundreds of volts to thousands of
W O 93/13239 PC~r/K R92/00081
2t`~6Ç~ 2
volts. while the distance between the cathode and the matrix(vacuum container),
the anode. is so near as about 5cm. The velocity of vacuum evaporation bein~
about 100 A/min, it is decided according to the energy and quantity of the ion
being shot out. That is. the velocity of vacuum evaporation can be increased by
raising the electric pressure and restricted by the decrease of the ionized cross
section.
However,~ even though the ionized electric current can be increased by added
pressure of incrt gas. ~the velocity of-vacu o ev~poration can rather be decreased
in an effect of dispersion of the gas. Thus. the optimun condition as to the
lO~ velocity~of the vacuum evaporation will have to be determined only through
repeated experiments.
To see the basic idea of the sputtering it is known that when a certain
pressure and electric tension are applied to a target(3). which exists within a
;vacu o container, plasma(4) is given rise to around the target and the positive
- 15 -ions existing in the area of the electric discharge come to beat the surface of
. ~
the target by virtue of electric forces. At this time the kinetic energy of the
positive ions is transferred to the atoms which exist on the surface of target,
and if this energy is stron~er than the bonding energy of the atom which are
beaten, the atoms of the target are emitted.
The disadvantage of the sputtering technique lies in the very slow velocity in
the coating formation. and the resultant low productivity. when a diode process
is adopted. To solYe the above problems the triode system has been invented.
which has~a third electrode~for control of both the source of thermion emission.
and ~the~ flow~of the ~emitted ther ion is added to the diode system. where a
W O 93/13239 2 ~ 4 pc~r/K Rg2/0008
tungsten filament is used as the source of emission for the thermions.
~ hen a triode system is adopted the velocity of vacuum evaporation can be
increased, because it is possible to increase the concentration of plasma by the
emission of thermions. When the concentration of the electrons in plasma is
increased by emission of thermions, the probability of ionization is heightened
by brisk action of electrons, the number of ions that beat the target is
increased, and thus the velocity of vacuum evaporation is accelerated.
Besides the way of increasing the concentration of the plasma by supplying
electrons there is another way of increasing the probability of ionization, that
1 o is, by means~of controlling the action of electrons with the use of magnetism.
`The sputtering vith the use of magnetism results in N and S electrodes at the
back of the target changing the action of electrons from rectilinear to spiral.
Thus the probability of electrons to hit neutral atoms and others under the
m ~~ sàEe pressure increases as the distance of electrons' movement is increased, and
15 ~by hei~htening the probability of ionization a greater velocity for vacuum
evaporation is obtained. Fig. 2(A)~B) show the structure of the target. wherein
the highest probability of ionization is to be seen at the point at which a line
of magnetic force intersects another perpendicularly, showing a regional
sputtering taking place forming a belt of high plasma concentration.
20 SUnnnRY Q~ IEE INVeNIIoN
The present invention is intended to provide both the process of manufacturing
exothermic reflexible glass by coating any such metals as Cr, Ni, Au, Ag, Al, Cu
over the~surface of 61ass by the technique of magnetic sputtering as given above,
~ and the process of ~anufacturing exothermic transparent glass by creating a layer
.- ~
,~ , .
,: '
::
;, . ~
W O 93/13239 pc~r/KR92/ooo81
2 .t 2 6 ~ 7 L~
o~ oxidized coating with In(90~)-Sn(10%) alloy in a vacuum container.
Also the present invention is intended to provide both exothermic reflexible
glass and exothermic transparent glass prepared by the above processes.
DESCRIPIION OF I~E DRA~INGS
Fi~. 1 is a sketch of the diode planar sputterin~ apparatus of the present
invention.
, ~
Eig. 2(A) and (B) are sketches to show the structure of a tar~et for the
~N~ ,` sputtering.
Eig. 3 is a graph showing the different transparencies of the respective ITO
and Au thin coating test pieces on glass of different times spent on vacuu~
evaporation.
1 0~ Fig.~4 is a graph showing the different resistivilities of the respective ITO
~, ~
and Au thin coating test pieces of different times spent cn vacuum evaporation.
Figs. 5(A) and (B) atd graphs~showin6 the surface temperatures of ITO and Au
thin coatin~s of 6.000 A and 400 A thicknesses. respectively.
Fig. 6 shows results of the XRD analysis of ITO test piece whose FO2/PAr value
:~
is 0.43.
Indices
1..... gas inlet. 2.... power source.
~, 3..... target, 4.... plasma.
5,11.. ...substrate, 6.... pumping system,
::
7..... chamber. 12... .electron.
13.... .argont 14... .atom sputtered.
15.... .target, 16... .anode.
17.... .cathode. 18... .ma~netic field lines.
: ~
: : ~,
W O 93/13239 PCl`/KR92/00081
212~4
l9substrate carrier.
DEIAILED DESCRIPIION OP I~e INVENIION
., .
In the present invention the direct current supplied by the DC power supply
source was used and, as shown in Eig. 2, magnetron was attached to the back of
S the target to raise the velocity of vacuum evaporation up to 1,000 A/min.
Eor the target an experimental item of 75mm in diameter and 5mm in thickness
vas used, and in the case of Ni a thin coating was formed on the target whose
.. ~
-~ ~ ' thickness was adjusted to lmm - 1.5mm due to its propensity for ~agnetizing by
; magnetrons.
In the case of such metals as Cr, Ni, and Cu the density of the electric power
applied to them is 11.3W/cm3, and at the initial vacuity of 1 X 10-5 torr Ar ~as
of purity 99.995Z was introduced for generation of plasma at the working pressure
~ .~
of 6 X 10-3 torr to produce reflexible glass. In the case of such metals as Au,
Ag, and Al the density of the electric power was lowered to 6.8W/cm3 because
under same conditions, the velocity of vacuum evaporation of these is greater
than that of Cr, Ni, or Cu.
The glass used as the matrix was 2mm thick, of 130mm respectively in length
and breadth. lt was placed under a process of cleansing with aloohol, distilled
water, and aceton in that order, drying in an oven of 200~C for 10 minutes and
puttin~ inside the sputter chamber, and then over 1,000 A thick coating was
formed over ItS surface by the sputtering process. Then, when electric wires
were attached by silver paste to the reflexible glass made by the above method
., ~,~,,
electric power of about 0.06~/cm3 was supplied, it was found that a temperature
of over 50~C was obtained on the density of the electric power and a desired
, ~
.,~ ~. -
w O 93/13239 pc~r/KR92/oooxl
212~ 4 6
temperature on the surface was obtained without difficulty.
Exothermic transparent glass is, unlike exothermic reflexible glass. made by
coating glass with transparent oxide instead of usin~ any single metal, and in
the present invention a tar~et of In(90~)-Sn(10%) was used for production of
S exothermic transparent glass, and indium-tin oxide(ITO) was synthesized under the
mixture of Ar and 02 for formation of the oxide. The specific feature of this
material is that even when its thickness is 1,000 A it can allow penetration of
ht by more than 80% so that it can be made wide~use of in production of the
liquid crystal for- TV or other liquid crystal display systems. Hence the
extensive study of this material has been made recently.
Then indium-tin oxide(ITO) in the present invention is made through the
reactional process of DC magnetron, and what is important at this time is the
ratio of Ar and 02 ~as in the mixture. If the ratio of 02 iS lower than the
preferable ratio, the desired oxide is not produced, while if it is higher than
that, the transparency decreases and the transparent conductive coating is not
obtained. Thereupon in the present invention, using a mass flow meter
manufactured by a US company MRS, the flow of Ar gas uas adjusted to 100 SCCM and
the flow of 02 gas to 30-98 SCCM, resulting in formation of a transparent
conductive material of good quality, while settin~ the flow o Ar gas at 100 SCCM
and 02 ~as at 43 SCCM is more preferable.
The density of the electric power can be 1.5 - 8.0W/cm3 and if it is higher,
it takes short time for the vacuum evaporation and if it is lower, it takes lon~
time for it. It is more desirable to set the density of the power at 2.26W/cm3
and the time at 6.5 minutes.
W O 93/l3239 21 2 6 5 7 4 P(~r/K R92/00081
The experimental conditions for said indium-tin oxide(IT0) are given in Table
1 below:
TABLE 1. ExPerimental Conditions for IT0 Vacuum Eva w ration
Coatin~ material Process Parameter
IT0 Reactive Power density : 1.5 - 8.0W/cm3
sputtering ~orking pressure : 6 X 10-3 ~bar
Sputterin~ time : 0.5, 1, 2, 3, 4,
5, 6.5, 8, 10,
12.5, 15 and 20
minutes
F02/FAr = 0, 0.35, 0.43, 0.5. 0.7,
0.92, 0.98
The ~reatest factor that affects the transparency and conductivity at the time
: 5~ of production of the I10 thin coating is the partial pressure of 02. and when the
flow of the~neutral Ar gas supplied for reactive sputtering was indicated by EAr
t that of 02 by E02, when the value of FO2/EAr was 0.39 or lower a coating of
very good conductivity but of very bad transparency was formed, while when the
value was 0.45 or more the transparency was fine but the conductivity fell to M Q
/Gm(mega Q/cm). When the value of EOz/EAr was 0.43 it was possible to form a
coating fitting the purpose of the present invention, and the velocity of the
formation of the coating at the time was ~easured about 1,200 A /min.
Among the materials which have so far been widely used in production of thin
coating layers is Au with the best conductivity, and in the present invention Au
1~ vas vacuum evaporated on glass~by the sputtering technique in order to compare it
with an I D thin coating layer, when the speed of the fonmation of the coating
was set at 40 A /sec. With Cr, Al, Ni, and Cu coating it was found practically
, ~ ~
~ impossible to produce a thin coating of such conductivity and transparency as
W O 93/13239 PC~r/K R92/00081
2.t266~ 8
will justify commercial production. but yet it was also possible to use them in
production of conductive reflexible glass by means of increasing the tickness of
the coating layers.
;Fig. 3 is a ~raph to show the different transparencies of the ITO and Au thin
s coating layers obtained by varied times of vacuum evaporation. In the case of:
~;the ITO thin coating layer the penetration of light was about 80% when the thin
~ ~coating layer's thickness was 0.8~um(vacuum evaporation by sputtering for 6.5
-ainutes), and the transparency gradually decreased as the layer's thickness
increased, falling to 40% when the thickness was about 2.4~lm(vacuum evaporation
by sputtering for 20 minutes). On the contrary. in the case of the thin coating
iarer ~of Au. the transparency measured 65% with the thickness 200 A(vacuum
evaporation by the sputtering for 5 seconds) and as the time for the vacuum
evaporation increased the transparency rapidly fell.
The resistive features were measured by calculating the current, as lOV
electric pressure was applied, after placing a Cu electrode each on both ends of
an experimental piece of 72mm by 23mm size. Those showing resistance by M Q
units were measured with ordinary multi meters, the resultsi being shown in Fi~.
4. Fig. 4(A) carries a graph showing the resistive features of an ITO
experimental piece, different as the time for vacuum evaporation by- the
; 20 sputtering varied. The value of resistance was 400 Q when the time for vacuum
evaporation by the sputtering WdS two ~inutes. but it declined rapidly as the
ti~e was protracted to fall, for instance, as low as about 20 Q when the time was
10 minutes(1.2~um).
,~
~Eig. 4(8) carries a graph of the resistive features in the case of an Au
WO 93/13239 PCr/KR92/00081
212~6~
coating layer, and in this, too, as in the case of the IT0 coating layer, the
resistance rapidly declined with rapidity as the time for vaauum evaporation by
the sputterin~ increased.
Ihe object of the present invention is to develop a aaterial as well as the
software for the manufacture of conductive and transparent glass for use in
auto~obiles, and since the change of temperatures of the experimental piece
makes, as well as its transparency, a most i~portant part of the present
invention.~ the temperatures should be measured acculately. Hence, on the surface
of the~experimental piece a K-type thermo-electric band was attached by means of
IO silver paste and~ it was connected to a X-Y recorder in order to ~easure
continuously the te~peratures of the surface of the experimental piece varying at
the change of~the elec *ic ~ressure and the electric current.
Pig. 5(A) is a ~raph sho~ing the measured surfsce temperatures of the IT0 thin
coating layers of 6,000 A in thickness, drawing a curve of (a), (b), (c), (d),
and (e)~ for 0.775W(SV X O.lSSA), 1.512W(7V X 0.216A). 2.466W(9V X 0.274A)~
3.3W(llV X 0.330A), 5.07W(13V X 0.390A), respectively. Moreover, the IT0 thin
coating of 6,000 A having, as shown in Fig. 3, a transparency of 80~, it can be
adopted as a very important material in production of the conductive glass for
automobiles.
Eig. 5(B) shows the cur~e indicating the surface temperatures of the Au thin
coating layers of 400 A in thickness, the electric current being 0.114A when it
was supplied at 13.8V, the surface temperature showing about 45~C 600 seconds
later
Fi~. 6~shows the results of XRD analysis of the IT0 experimental piece with
-, ~ ,
- ~ :
~,,i. ~
WO 93/13239 PCT/KR92/00081
212~ 4 lo
its FO2/FAr value at 0.43. wherein in the case of an experinental piece of good
transparency and conductivity its In2SnOs coating exceedingly well develops at 2~ = 45.36. and it was found that the àbove ITO coating contributed to
i~proveaent of conductivity.
Such exothenmic reflexible glass. coated over the surface of the glass in
automobiles. can be used very preferably to remove the moisture inside. and is
extensively ade use of for liquid crystal display systems also.
,