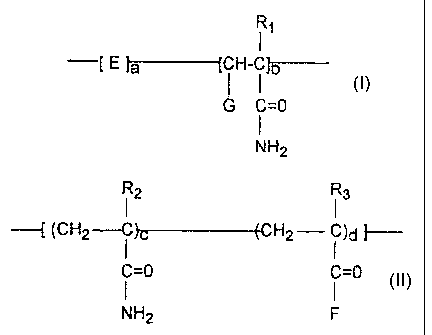Note: Descriptions are shown in the official language in which they were submitted.
L-692~/A-2
WATER SOLUBLE GRAFT COPOLYMERS AND
METHODS OF tJSE THEREOF
FIELD OF THE INlfENTION
The present invention pertains to novel water soluble graft copoly-
mers which are useful for water treatment, such as sludge dewatering
and water clarification. In addition they are also effective as retention
1 ~ and drainage aids in the papermaking process.
BACKGROUND OF ThIE fN~JENTION
There is an increasing usage of water soluble polymers and co-
polymers in wastewater treatment industries. These compounds have
shown desirable utility for the purpose of dewatering sludge and clarifying
contaminated water.
2
The effiicacies of the polymers or copolymers used will vary de-
pending upon the type of monomers chosen to form the polymer or co-
polymer, the molecular weight of the synthesized molecule and, in the
case of a copolymer, the placement of the selected monomers on the
backbone of the copolymer. It is the Batter characteristic that is the focus
of the present invention.
Polymers with long sequences of two monomers can be catego-
rized as block copolymers or graft copolymers. In graft copolymers
sequences of one monomer are °'grafted" onto a "backbone" of the
second monomer type,
__ ~ __p,~__,q~p,__, etc.
B B
t i
B B B
Graft copolymers have unique and highly desirable properties as
compared to random copolymers or the blend of two homopolymers.
Therefore, there is a great interest in preparing them. Few techniques
described in the literature satisfy the need.
Furthermore, with ever increasing usages of water soluble poly-
mers and copolymers i.n industries such as wastewater treatment, cool-
ing, boiler and deposit control, coating, textile, mining, detergency, cos-
metics, and papermaking, etc., there is an urgent need to synthesize
novel water soluble graft copolymers for this broad range of applications.
CA 02127011 2001-07-23
3
This invention prepares distinctive water soluble graft copolymers
for water treatment applications and specifically for papermaking
processes.
5 U.S. Patent 3,869,418 describes a graft copolymer comprising a
polymeric N-vinyl lactam such as N-vinyl pyrrolidone with unsaturated
carboxylic acids, like acrylic acid and methacrylic acid in an emulsion
process. The resulting copolymer is not water soluble and is used for
adhesive and coating applications.
10
U.S. Patent 4,271,053 discloses a quaternary ammonium graft
copolymers prepared by grafting quaternary ammonium ionene-type poly-
meric side chains onto a polymer backbone formed by the reaction of a
difunctional amine and an epihalohydrin or diperoxide. The polymers are
15 different than the present invention.
U.S. Patent 4,400,496 and European Patent Application 0 356 241
teach grafting acrylamide or acrylic acid with starch in the presence of
ceric ions. The product has to be precipitated and separated in acetone
20 prior to use.
Smirnova et al., Journal of Polymer Science, Vol. 29, pp. 139-145
describe a graft copolymerization of methacrylic acid with polycapro-
amide by the persulfate/sulfite redox system in the presence of copper
25 ions. It is a different reaction mechanism and results in a different co-
polymer than the present invention.
U.S. Patent 4,916,191 discloses a gra~'t copolymer prepared from a
macromonomer with hydrophilic and fluorinated monomers for dispersion
30 stabilizer in an emulsion polymerization process.
CA 02127011 2001-07-23
4
Compared to the related art disclosed above, there exists a need
to prepare water soluble graft copolymers in a convenient and economic
process. This is achieved by the present invention. The resulting
copolymers exhibit desired efficacy as paper retention and drainage
aids.
DETAILED DESCRIPTION OF THE INVENTION
The present invention pertains to novel water soluble graft co-
polymers which are useful as retention and drainage aids in paperlpulp
making processes.
Specifically, the graft polymers in the invention contain polymeric
segments obtained from the polymerization of acrylamide and cationic
monomers which are attached or "grafted" to another polymer chain
which is comprised of the repeating units of one or more monomers.
The resulting graft copolymers are soluble in an aqueous medium.
These graft copolymers may be utilized along with bentonite clay
to form an effective retention and drainage aid. When these two com-
pounds are combined, the most desirable weight ratio of copolymer to
bentonite is between about 2 to 1 and 1 to 20.
..........w_.~-,-~... rv...~ _:. . .. ,~,.._..F~,w.~"~-~...~._~.e_....-. ~.. .
._.__~~~..._.-~.. ... _
5
The graft copolymer of the invention has the general structure:
Formula I
11
--t ~ l~- fC~l- i lb
G- C=0
N N2
wherein E in the above formula (Formula !) is the repeat unit ob-
tained after polymerization of an a, (i ethylenically unsaturated com-
pound, preferably carboxylic acid, amide form thereof, alkyl (C1-C8) ester
or hydroxylated alkyl (C1-C8) ester of such carboxylic acid. Compounds
encompassed by E include the repeat unit abtained after polymerization
of acrylamide, methacrylamide, acrylic acid, methacrylic acid, malefic acid
or anhydride, styrene sulfonic acid, 2-acrylamido-2-methylpropyl sulfonic
acid, itaconic acid, and the like. Ester derivatives of the above mentioned
acids such as 2-hydroxypropyl acrylate, methyl methacrylate, and 2-ethyl-
hexyl acryiate, are also within the purview of the invention.
The molar percentage of a:b is from about 95:5 to 5:95, with the
proviso that the sum of a and b equals 100°fo.
2.~~'~~~:~.
6
~G in the above formula (Formula I) is a polyrneric segment com-
prising repeat units having the structure:
Formula 11
I~ 13
-I (CR2- i )c (CHZ_.r i )d ~--
C=0 C=0
~lElz F
wherein R~, R2 and Ra in Formulae I and II are the same or different and
are hydrogen or a lower alkyl group having C~ to C3. F in the above
formula is a salt of an ammonium cation, such as NHR3N~'R(4,5,~) M- or
OR3N+R(a,s,g) M-, wherein R3 is a C~ to C~ linear or branched alkylene
group, and R4, R5 and R6 can be selected from the group consisting of
hydrogen, C~ to C~ linear or branched alkyl, C~ to Cs cycloalkyl, aromatic
or alkylaromatic group; and M is an anion, such as chloride, bromide, or
methyl or hydrogen sulfate. Typical cationic monomers are 2-acryloyl-
oxyethyltrimethylammonium chloride (AETAC), 3-(meth)acrylamido-
propyltrimethylammonium chloride (MAPTAC or APTAC), 2-methacryloyl-
oxyethyltrimethylammonium chloride (METAL) and diallyldimethyl-
ammonium chloride (t7A~MAC), etc.
It is understood that more than ane kind of cationic monomer may
be present in Formula II.
7
The molar percentage c:d in Formula II may vary from 95:5 to 5:95,
with the proviso, however, the sum of c and d equals 100%.
There is no limit to the kind and mole percent of the monomers
chosen so long as the total adds up to 100 mole % and the resulting
copolymers are water soluble.
At present, the preferred water soluble graft copolymer for use as
retention and drainage aids is:
Formula III
-~-CH-CHI iCM-CH~--
2 ~ a ~ ~ b
C=0 G C=0
N~i ~IH
2 2
The molar percentage of a:b is from about 95:5 to 5:'95, with the
proviso that the sum of a and b equals 100%. G in Formula III is:
8
Formula IV
w'E (CH2- i ~--°~°E (CH2"'° i )d W-'
C=0 C=0
NHS O
CH2
CH2
H3C N+ CH3 C1-
CH3
The cationic monomer is 2-acryloyloxyethyltrimethylammonium chloride
(AETAC). The molar percentage c:d in the polymer segment G (Formula
IV) is the ratio of acrylamide:AETAC. It may fall within the range between
95:5 and 5:95. The sum of c and d must add up to 100%.
The number average molecular weight (Mn) of the polymeric seg-
ment G is not critical and may fall within the range of 1,000 to 1,000,000.
Preferably, the number average molecular weight will b~ within the range
of 5,000 to 500,000, with the range of about 10,000 to about 200;000
being even more desirable. The key criterion is that the resulting graft
copolymer be water soluble.
CA 02127011 2001-07-23
9
The bentonite can be any of the materials commercially referred to
as bentonites or bentonite-type clays, i.e., anionic swelling clays such as
sepialite, attapulgite or, preferably, montmorillinite. The montmorillinites
are preferred. Bentonites broadly as described in U.S. Patent No.
5 4,305,781 are suitable. Suitable montmorillinite clays include Wyoming
bentonite or Fullers Earth. The clays may or may not be chemically
modified, e.g., by alkali treatment to convert calcium bentonite to alkali
metal bentonite. The swelling clays are usually metal silicates wherein
the metal comprises a metal selected from aluminum and magnesium,
10 and optionally other metals, and the ratio silicon atoms: metal atoms in
the surface of the clay particles, and generally throughout their structure,
is from about 5:1 to 1:1.
The graft copolymer is prepared via a two-step polymerization
15 process. First, a macromonomer comprised of acrylamide and AETAC is
prepared by a water-in-oil inverse emulsion polymerization method using
peroxide as an initiator. Such processes have been disclosed in U.S.
Patents 3,284,393, Reissue 28,474 and Reissue 28,576. The initiator may
be selected from peroxides, persulfates, bromates, and azo-type initiators
20 such as 2,2'-azobis-(2-amidinopropane) dihydrochloride, 2,2'-azobis-(2,4-
dimethylvaleronitrile). Copper (II) sulfate is added in the process as an
oxidative chain transfer agent to generate a terminal unsaturated double
bond in the polymer chain. It is conceivable that transition metal ions
25 other than copper, such as iron, cobalt, and nickel etc., may be used
in the invention.
10
Ethylenediaminetetraacetic acid or diethylenetriamine pentaacetic
acid and their salts or their amino analogue are used as chelating agents
to chelate or to form complexes with copper prior to the second polymeri-
zation step.
The resulting macromonomer is then copolymerized with acryl-
amide or other monamers to form grafit copolymers by a similar water-
in-oil inverse emulsion technique.
Branching agents such as polyethyleneglycol di(meth)-acrylate,
N,N'-methylenebis(meth)acrylamide, PJ-vinyl acrylamide, allyl glycidyl
ether, giycidyl acrylate and the like may also be added, providing the
resulting graft copolymer is water soluble. Any of tho well known chain
transfer agents familiar to those who are skilled in the art may be> used to
control the molecular weight. Those include, but are not limited to, lower
alkyl alcohols such as isopropanol, amines, mercaptans, phosphites,
thioacids, formats, allyl alcohol and the like.
Conventional initiators such as peroxide, persulfate, along vvith
sulfite/bisultite and azo compounds may be used depending on the
system chosen.
High HLB inverting surfactants such as those described in U.S.
Patent Re. 28,474 are then added to the emulsion to convert the resulting
emulsion to a "self-inverting" emulsion. Using the procedure described
herein, a unique graft copolymer in emulsion form is obtained.
11
The resulting copolymer may also be further isolated by precipitat-
ing it in an organic solvent such as acetone and dried to a powder form.
The powder can be easily dissolved in an aqueous medium for use in the
desired applications.
It is to be understood that the aforementioned polymerization
methods do not in any way limit the synthesis of copolymers according to
this invention.
The resulting emulsion disperses and dissolves rapidly into an
aqueous solution upon addition to water. Within minutes, a maximum
solution viscosity is obtained. The emulsion dissolves well even in water
containing a high level of hardness and it also rotains most of its solution
viscosity in brine water.
The structure of the graft copolymer is substantiated by a conven-
tional solution viscosity study and C~3 lVMR spectroscopy. The molecu-
lar weight of the resulting graft copolymer is not critical, as song as the
polymer is soluble in water. The molecular weight may vary over a wide
range, e.g., 10,000 - 30,000,000 and may be selected depending upon
the desired application.
The graft copolymer is added to the pulp furnish prior to the paper
forming stages. It is added in an amount of from about 0.5 to 25 pounds
per ton of furnish. Preferably about 1.0 to 10 pounds of copolymer per
ton of furnish is used.
~:~~'1~:~ _~
12
When used in conjunction with bentonite, dosage levels for the
graft copolymer range from 0.05 to 10 pounds per ton, and preferably
0.10 to 5 pounds per ton, active polymer to active furnish solids. The
copolymer is best applied to the furnish as a dilute aqueous solution.
Dosage levels for the bentonite clay are in the range of about 0.25 to 25
pounds per ton of active clay to active furnish solids. The preferred
range is about 0.5 to 10 pounds per ton.
Examples
Utilizing the procedure described above, numerous water soluble
graft copolymers were prepared. Table I hereinbelow summarizes the
physical properties of the resulting acrylamide>AETAC graft copolymers.
TAE3LE 6
Physical erties of f9~e olvrtaers
Prop Craft Cop
AETAC Content UL Viscosity
Solids
Example mole % % f cps)
J-2 5 34.4 11.6
J-10 5 34.9 21.0
J-14 5 35.1 12.6
J-19 5 31.3 13.0
J-21 5 31.9 14.1
J-28 5 32.0 11.0
J-30 10 33.2 12.7
J-31 10 35.0 11.6
J-23 10 34.7 4.5
J-25 10 34.5 19.3
J-07 10 37. 9 16.1
J-20 10 34.3 12.6
13
Performance Evaluation
Papermaking retention aids are used to increase the retention of
fiber and filler fine furnish solids in the web during the turbulent process
of draining and forming the paper web. The eaten;>ive use of retention
aids is well documented in the paper industry. For example, see
"Retention Chemistry", Chapter 17, Pulp and Paper, James P. Casey,
Volume 3, 3rd edition, pages 1593 to 1607. Without adequate retention
of the fine solids, they are either lost to the process effluent, which is a
cost consideration for expensive fillers, or accumulate to excessively high
concentrations in the recirculating white water loop and cause production
difficulties including deposit buildup and impaired paper machine drain-
age.
Additionally, insufficient retention of the fine solids and the dispro-
portionate quantity of chemical additives which are adsorbed on the
surface reduces the paper-maker's ability to achieve necessary paper
quality specifications such as opacity, strength, sizing, basis weight, and
formation (sheet uniformity},
In the following tests, the performance of the resulting water sol-
uble graft copolymers as retention and drainage aids in the papermaking
process is demonstrated. The standard ~ritt jar test as well as tests
using a pulp drainage testing device were used for evaluation.
.
14
Laboratory prepared acid and alkaline furnishes were used as the
substrates. Hardwood and softwood market pulp were refined separately
to respective 300 and 500 Canadian Standard Freeness, and mixed at a
50/50 ratio. Alkaline furnish was prepared with the addition of precipi-
tated calcium carbonate at a level of 20% of the total furnish solids. The
resulting pH was 8.5. Acid furnish was prepared with the addition of rosin
size at 10 Ibs/ton and alum at 15 Ibs/ton based upon fiber solids. 20%
clay and 5% Ti02 based upon fiber solids was then added, and the pH
was adjusted to 4.5.
Fines retention tests were conducted on the Britt jar with thin stock
at 0.5% consistency. Polymers at the specified dosage were added to
stock in the 8ritt jar at 1400 rpm shear speed and allowed to mix for 15
seconds. 100 mls of filtrate was collected and filtered to determine the
fines content per standard procedure. Polymers were tested at 1.5, 2.25,
3.0 and 3.75 Ibs/ton based upon furnish solids, and compared to blank
samples. Drainage tests were conducted with the CSF tester. Thin stock
was prepared at 0.30% consistency. Polymers were added to the stock
at 1400 rpm and mixed for 15 seconds before testing on the CSF. Poly-
mers were tested at 1.5, 2.25, and 3.0 Ibs/ton, and compared to blank
tests.
The results are shown in Tables II and lll.
15
TABLE
II
Polymer Pounds
Dosage (Ibs)
in Polymer
per
ton
(T)
of Furnish
Example 1.51bs/T2.251bs/'f3.OIbs/T3.751bsIT
J-2 55.2 60.0 65.0 66.2
J-10 61.6 71.1 76.5 80.6
J-30 54.5 66.1 70.6 74.0
J-31 55.3 56.5 63.6 68.7
J-23 40.4 46.4 50.0 46.0
J-25 50.7 59.8 62.0 65.3
10J-O7 40.1 46.7 56.0 59.5
J-20 52.3 61.3 66.4 70.0
Blank
16.3%
TABLE
III
15Polymer Pounds
Dosage (Ibs)
in Polymer
per
ton
(T)
of Furnish
Example 1.51bsrf2.251bs~1'3.OIbsl1'3.751bsf'f
J-2 36.9 44.3 58.9 72.4
J-10 40.4 51.8 61.8 74.6
J-30 38.6 47.7 61.1 68.2
20J-31 41.3 52.9 62.8 74.3
J-23 39.6 42.1 50.6 50.9
J-25 39.1 45.3 59.3 65.9
J-07 36.6 42.1 46.6 56.1
J-20 39.8 51.8 60.0 67.5
25 Blank 18.5%
16
A drainage testing device equipped with a rotating hydrofoil (300
rpm), and vacuum capability underneath the wire screen (100 mesh) was
also used to evaluate the graft copolymers of the invention. The device
can be operated for multi-pass of substrate and reach an equilibrium
stage. It is a closer simulation of the actual paperrnaking process than
the conventional Britt jar and CSF tests. Both the retention and drainage
behavior of the tested paper pulp and treatment can be easily character-
ized. The testing conditions are:
A) Foil = 300 rpm (equivalent to a 1500 ft/min machine);
Mixer = 1400 rpm; Wire screen = 100 mush. Vacuum
dewatering starts when the white water drainage (by
gravitylpulsation forces) reaches 80% of the initial
furnish volume.
Couch vacuum = 14 inch-Ng.
B) Furnish: Headbox stock from Hawesville:
Fiber fines = 40.69, CSF = 350.
C) Multi-pass test and white water recirculation: For the first-
pass, the stock (178ccm %con = 0.56) was diluted with
process water to 1000cc before test. For the subsequent
passes, the stock was diluted with the white water generat-
ed from the previous pass (only about 82% of the white
water can be recovered in this process). A total of six
passes were conducted for each tested system. The
results are shown in Table IV.
17
'f~A~f_E I!!
1 st Pass 6th ~E uilibrium
Pass
Treatment %FR p~4DF2vDR RES
%~'R
pg~R
vDR
RES
5
J-14 49.5 15.8 1.09 0.1249.7 34.4 2.57 0.27
J-19 60.9 16.4 -___ ____51.3 30.2 ____ ____
J-21 56.8 15.8 ---- ----52.4 28.5 ____ ..___
J-28 51.4 14.6 0.94 0.1143.7 28.9 2.13 0.23
J-10 51.5 14.0 0.88 0.1154.1 34.1 2.65 0.,27
J-2 53.5 15.5 0.99 0.1146.6 29.7 2.09 0.24
J-07 42.1 12.7 0.93 0.1038.0 25.9 2.05 0.23
J-20 52.3 14.9 0.77 0.1046.7 24.8 1.97 0.23
J-23 49.2 14.0 0.82 0.1140.1 28.9 2.10 0.24
J-25 48.8 14. 5 0.93 46.2 34.1 2.51 0.27
0.1 't
J-30 42.7 12.0 0.99 0.1150.7 25.7 2.56 0.28
J-31 48,2 13.3 0.88 0.1148.3 26.0 2.07 0.23
A 57.7 14.8 ---- ----51.4 30.1 ---- ----
blank 49.5 16.5 0.99 0.1344.0 32.2 2.61 0.30
,'ol=Rthe percentage of
= fines retained
in the sheet.
pgDR pulsation/gravity (sec). r the R
= drainage time The pgD is,
smalle
the better; a 5% gnificant.
difference is si
vDR vacuum drainage the etter.
= time (sec). The vDR
smaller is,
the
b
RES air flaw resistance ure The
= of the wet pad, of
a meas formation.
greater the RES
is, the better.
A = a commercial acrylamidelAETAD
copolymer with
a linear
configuration.
2~~ ~~.~~>~
18
Examples: Graft Copolymer plus bentoraite
Canadian Standard Freeness procedures were followed for drain
age results. Dosages of the respective polymers wand bentonite clay are
as shown in Table V.
Test samples consisted of 1000 ml of a synthetic alkaline furnish,
having a consistency of .286%. The samples with polymer were sub-
jected to shearing at 1400 for 15 seconds, and far those samples con-
taining the bentonite clay, followed by shearing at 1400 rpm for 60
seconds which was reduced to 1000 rpm for 15 more seconds.
To demonstrate retention properties, Standard Britt Jar Retention
testing was performed utilizing the same dosages as for drainage testing.
The test samples were 500 m! of synthetic alkaline furnish at .467%
consistency. Shear speed and contact times were as described above.
For calculating retention, 100 ml of effluent was drained, filtered, dried
and weighed.
TAi3l_E V
Dosage CSF % Fines
Treatment Ibs/ton Drainage Retention
Blank ---- 456 11.5
Example 1 1.5 508 65.0
2.25 526 74.7
3.0 528 75.2
19
'TALE ~/ (corat'~~
Dosage CSF % Fines
Treatment Ibs/ton Drainage Ft~tention
5
Example 11 1.5/4 587 74.5
Bentonite 2.5/4 639 88.2
Clay 3.0/4 666 92.3
Example 2 1.5 510 67.7
2.25 527 74.0
3.0 542 77.2
Example 2/ 1.5/4 562 74.3
Bentonite 2.2514 611 88.3
Clay 3.0/4 644 88.6
Comparative 1.5 492 63.3
Example 1 2.25 500 68.5
3.0 507 7'I .6
Comparative 1.5/4 588 78.5
Example 1/ 2.25/4 634 90.3
Bentonite 3.014 666 93.4
Glay
20
T~~L~E ~ (cont'd)
Dosage C,SF % Fines
Treatment Ibs/ton Drainage Retention
Comparative .5 524 80.6
Example 2* .75 542 77.0
1.0 552 78.6
Comparative .5/4 587 75.0
Example 2*/ .75/4 649 90.8
Bentonite Glay 1.0/4 671 91.5
*powder form: dosage different, so that results are based on an equal
actives basis.
While this invention has been described with respect to particular
embodiments thereof, it is apparent that numerous other forms and modi-
fications of this invention will be obvious to those skilled in the art. The
appended claims and this invention generally should be construed to
cover all such obvious forms and modifications which are within the true
spirit and scope of the present invention.